Abstract
Zerumbone is a sesquiterpene ketone with potent anti‐cancerogenic activities, produced in several ginger species of the Zingiberaceae familiy. We have investigated the biotechnological production of α‐humulene, a precursor of zerumbone. By implementing a heterologous mevalonate pathway in combination with the α‐humulene synthase expression, we effectively synthesized α‐humulene from glucose in Escherichia coli. In this study, we developed a practical and efficient in situ separation method for α‐humulene by comparison of extractive and adsorptive strategies. By the in situ adsorption of the product to the hydrophobic resin Amberlite® XAD4 we were able to increase α‐humulene yield by 2310% to 60.2 mg/L. Furthermore we present an easy applicable, short subsequent chemical process for the conversion of α‐humulene to zerumbone by using transition metal catalysis. To reduce process steps, the chemical reaction was carried out in the same solvent as the eluting solvent that was used to elute α‐humulene from the adsorbent resin. By allylic oxidation of α‐humulene with manganeseII chloride as a catalyst and tert.‐butylhydroperoxide as an oxidizing agent we were able to synthetize zerumbone with a selectivity of 51.6%. Product and byproducts of the oxidation reaction were identified by GC‐MS.
Keywords: Allylic oxidation, In situ product recovery, Integrated bioprocess, Semi‐synthesis, Zerumbone
Abbreviations
- AU
absorbance unit
- DCM
dichloromethane
- FID
flame ionization detector
- GC
gas chromatography
- HUM
α‐humulene synthase
- IPTG
isopropyl‐β‐d‐galactopyranoside
- MS
mass spectrometry
- MVA
mevalonate
- TBHP
tert.‐butylhydroperoxide
1. Introduction
Terpenes continue to be the most important source of lead compounds for the flavor, fragrance and pharmaceutical industry 1. Isolation of terpenes from their natural resources is the major production route to date—a process that can be time consuming, tedious and expensive, as well as being potentially unsustainable for the resource. Due to their structural complexity, chemical total synthesis of these natural compounds is rather difficult and ineffective. Major developments in biotechnology have given access to novel production strategies of many natural substances and their derivatives 2, 3, 4. Biotransformation strategies using metabolically engineered microorganisms offer possibilities of developing alternative manufacturing processes for terpene production 5, 6, 7, 8. Therefore, semi‐synthetic strategies, combining both strength of biotechnological and organic synthesis, offer more flexibility and efficiency to produce terpenoids.
The sesquiterpenoid zerumbone was first isolated from the rhizome of tropical wild shampoo ginger (Zingiber zerumbet Smith) 9. In previous studies zerumbone has been extensively studied for its effectiveness in a broad range of biological activities including antimicrobial 10, antioxidant 11, antidiabetic 12, antitumor 13, anti‐inflammatory 14, antiangiogenic 15 and antiallergic properties 16. Zerumbone is a highly potential anticangerogenic agent.
The biosynthesis of zerumbone in Z. zerumbet Smith from the sesquiterpene precursor farnesyl diphosphate (FDP) is analogous to other known plant sesquiterpene ketones. In the initial step, the reaction of FDP is catalyzed by α‐humulene synthase (HUM) giving α humulene as the major product (95%) and β‐caryophyllene as the minor product (5%) 9, 17. In a subsequent reaction by α‐humulene‐8‐hydroxylase, α‐humulene is converted to 8‐hydroxy‐α‐humulene 18, which is finally oxidized by the dehydrogenase zerumbone synthase to yield zerumbone 19.
In this work, we present an alternative semi‐synthetic production route for zerumbone (Fig. 1). We describe the heterologous expression of α‐humulene in metabolically optimized E. coli. Furthermore, we present a subsequent synthetic strategy to directly oxidize α‐humulene to zerumbone without solvent change. Although several syntheses for zerumbone were published previously 20, 21, efficient direct oxidation of α‐humulene at C8‐position was not described to date.
Figure 1.
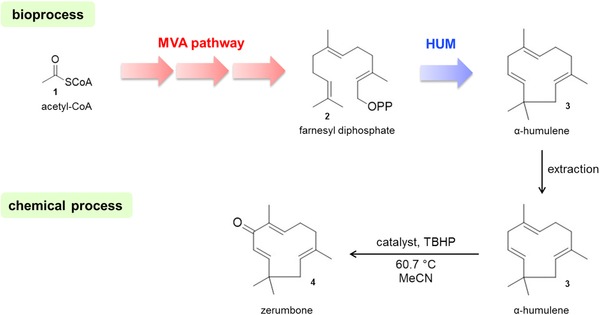
Production strategy for semi‐synthetic zerumbone using metabolically engineered E. coli and transition metal catalysis.
2. Materials and methods
2.1. Extractive and adsorptive separation of α‐humulene from aqueous solution
Ethyl acetate, dichloromethane (DCM), pentane, isooctane and nonane were each evaluated as prospective solvents for in situ α‐humulene extraction. The product recovery rate of a definied α‐humulene solution in ZYP31‐media was determined according to a series of model studies. 1 mL of stock solution containing 5 μg/mL α‐humulene was contacted with 400 μL of each solvent. These biphasic samples were shaken vigorously at 20°C for 30 s, after which the phases were separated by centrifugation for 1 min at 14 800 rpm. Samples of the organic phase were removed for analysis by gas chromatography (GC). All extractive experiments were performed in triplicates to provide an estimate of standard error.
After measuring solute concentrations before and after sample equilibration with particular organic solvents partition coefficients (K ex) were determined by the following relationship:
| (1) |
where c ex represents the concentration of α‐humulene in the solvent fraction and is the solute concentration in the aqueous phase after equilibration.
The polyaromatic adsorbent Amberlite® XAD4 (Sigma Aldrich, Germany) was tested for α‐humulen uptake. Acetonitrile, ethyl acetate, ethanol, acetone, dichloromethane and isooctane were evaluated as eluting solvents. An experimental study was conducted to determine the product recovery rate of a defined 5 μg/mL α‐humulene solution in ZYP31‐media. 50 mg of the adsorbent were washed three times with 1 mL water and conditioned with the eluting solvent. The resin was added to 1 mL of α‐humulene solution and the mixture was allowed to equilibrate at 20°C for 30 min at 400 rpm. The adsorbent was separated from the aqueous solution and suspended in 1 mL of the respective eluting solvent. The elution of the bound product was performed by ultrasonication for 1 min, followed by 4 min shaking at 400 rpm. The elution step was repeated three times and the eluted fractions were combined. Samples were analyzed by GC. All adsorptive experiments were performed in triplicates to provide an estimate of standard error.
After measuring solute concentrations before and after sample equilibration with a particular eluting organic solvent, partition coefficients (K ad) were determined by the following relationship:
| (2) |
where c ad represents the concentration of α‐humulene eluted from the adsorbent.
A calibration curve using commercially available α‐humulene (Sigma Aldrich, Germany) was generated for the purpose of quantifying sesquiterpenes. α‐Humulene standards were made in isooctane at concentrations of 0.05, 0.5, 1, 2.5, 5 and 10 μg/mL. Samples were measured by GC‐FID method. A calibration curve was generated by plotting the average peak area of each sample against its concentration.
2.2. Microorganism, media and growth conditions
The host strain used in this study was E. coli BL21(DE3), purchased from Merck Millipore, Germany. Cells were transformed with plasmids pET16b::hum_co, containing the codon optimized α‐humulene synthase gene as described before 17, and pBbA5c‐MevT(CO)‐T1‐MBIS(CO, ispA), carrying a synthetic mevalonate pathway for terpene precursor synthesis. The plasmid pBbA5c‐MevT(CO)‐T1‐MBIS(CO, ispA) was a gift from Jay Keasling & Taek Soon Lee (Addgene plasmid # 35152) 22. The production strain will be referred to as E. coli HUM1510.
The heterologous production of α‐humulene in E. coli HUM1510 was carried out in defined ZYP31‐media (6.507 g/L KH2PO4, 6.795 g/L Na2HPO4, 3.159 g/L (NH4)2SO4, 10 g/L glucose, 4.920 g/L MgSO4·7H2O, 0.160 g/L FeCl3, 0.650 mg/L ZnCl2, 0.200 mg/L H3BO3, 0.089 mg/L MnCl2·4H2O, 0.050 mg/L CoCl2·6H2O, 0.100 mg/L CuSO4·2H2O, 0.100 mg/L NiSO4, 0.525 g/L Na2MoO4·2H2O. The starter culture was set up in 25 mL of LB‐Miller medium supplemented with carbenicillin (100 μg/mL) and chloramphenicol (34 μg/mL). The medium was inoculated with glycerol stocks of cells and incubated at 37°C and 150 rpm for 10 h in an orbital shaker. Precultures in ZYP31‐medium containing the same concentration of antibiotics were inoculated with starter culture to give an initial optical density of OD600 0.1 and grown under the same conditions as above for 12 h. Then it was directly used to inoculate the bioreactor.
2.3. Bioreactor cultivation
Bioreactor cultivations were carried out in a 2 l bioreactor (BIOSTAT® A, Sartorius Stedim Biotech GmbH, Göttingen, Germany). ZYP31‐media was used as production medium in 1.8 l working volume. Foam production was controlled by the addition of antifoam. The pH value was maintained at pH 7.5 by adding 1 M ammonia solution. In order to achieve and keep concentration of dissolved oxygen constant at 20% air saturation, the culture was stirred in cascade mode (stirrer speed range from 0 to 1700 rpm) at an aeration rate of 1 vvm and an initial stirring speed of 100 rpm. The polyaromatic adsorbent Amberlite® XAD was added to the media prior sterilization.
The reactor was inoculated with preculture to give an initial OD600 of 0.1. Fermentation was carried out at 37°C. Gene expression was induced at early exponential phase at an OD600 of 1.0 by adding isopropyl‐β‐d‐thiogalactopyranoside (IPTG) at a final concentration of 0.1 mM, and growth was continued at 20°C for 24 h. Cells were sampled at different times to monitor optical density at 600 nm and biomass. Culture samples for α‐humulene production analysis were normalized according to their optical density at 600 nm.
2.4. General procedure for allylic oxidation of α‐humulene
In a typical reaction, to a solution of α‐humulene (445 mg, 2.18 mmol) in acetonitrile (13.2 mL) under air, catalyst (0.0159 mmol) and 70% aq. tert.‐butylhydroperoxide (6.5 mL, 10.12 mmol) were added. The progress of the reaction was followed by GC‐FID. After 24 h under magnetic stirring at 60.7°C, the solution was poured into 10% aq. sodium sulfite solution and extracted with diethyl ether. The extract was dried over magnesium sulfate and concentrated by evaporation. The reaction products were then separated by preparative thin layer chromatography using a 6:1 ratio of n‐hexane/ethyl acetate. Zerumbone was located by UV visualization at 254 nm and the silica containing the product was scraped off the plate. The silica was washed with ethyl acetate, filtered and the filtrate analyzed by GC.
2.5. Gas chromatography
Sample preparation for α‐humulene analysis of culture samples was done as followed: Samples were centrifuged at 4000 × g, 4°C for 15 min, the supernatant was separated and sesquiterpenes isolated by the extractive or adsorptive methods described above and directly applied to GC analysis. Cell pellets were resuspended in extraction buffer (50 mM MOPS pH 7.0, 5 mM MgCl2, 5 mM DTT, 10% (v/v) glycerol) and sonicated during 6 × 15 s time intervals (0.6 s cycle, 100% amplitude) while the cell suspension was kept on ice to prevent heating‐up. Cell extracts were centrifuged (14 000 × g, 4°C, 45 min), and the supernatant was collected. After extractive or adsorptive separation of the sesquiterpene products in the aqueous phase as described above, the organic phase was applied in GC analysis.
Capillary gas chromatography was used to assay the terpene production. Analysis was performed on a Shimadzu GC‐2001 plus gas chromatograph coupled to a flame ionization detector. Separation was done on a Phenomenex Zebron ZB‐Wax Plus column (length, 30 m; inner diameter, 0.25 mm; film thickness, 0.25 μM). Samples of 2 μL were injected via autosampler (splitless mode; split temperature, 240°C). Oven temperature was set to 40°C for 20 s, ramped up at 10°C/min to 200°C and held for 0.5 min, then raised to 230°C (30°C/min) and held for 2 min. The FID was heated to 300°C.
GC‐MS analysis was performed on a Fisons GC 8000 gas chromatograph connected to a Fisons MD 800 mass selective detector. Separation was done on an Agilent VF‐WAXms column (length, 30 m; inner diameter, 0.25 mm; film thickness, 0.25 μM). Samples were injected by cool on‐column injection. Helium was the carrier gas, set at a constant flow rate of 15 mL/min. Oven temperature was set to 40°C for 3 min, ramped up at 3°C/min to 230°C and held for 10 min. The ion source and quadrupole temperature were set at 230°C and 150°C, respectively. Electron impact (EI) mode with an ionization potential of 70 eV was used with a scanning over the mass range of 33–300 amu. MassHunter Qualitative Analysis software (Agilent Technologies, USA) was used to process and compare data.
Kovats indices (I) for temperature programmed chromatography were determined by the following relationship:
| (3) |
where tx represents the retention time of the compound of interest, n the number of carbon atoms of the smaller n‐alkane eluting directly before and n+1 he number of carbon atoms of the smaller n‐alkane eluting directly after the compound of interest.
3. Results and discussion
3.1. Selection of in situ recovery method
α‐Humulene was produced by E. coli HUM1510 harboring two different plasmids: pET16b::hum_co, containing the α‐humulene synthase gene which was previously described 17, and pBbA5c‐MevT(CO)‐T1‐MBIS(CO, ispA), containing a synthetic mevalonate pathway 22. For co‐expression, the genes of interest in both plasmids are under the control of a lac promoter and possess resistance against ampicillin or chloramphenicol respectively. In preliminary cultivation experiments, α‐humulene accumulation in the biomass was observed. However, after an initial increase, the α‐humulene content decreased during cultivation. To investigate, if α‐humulene is degraded by E. coli, additional experiments were performed. A definied α‐humulene concentration was added to the culture media with cells, but expression of the recombinant genes responsible for α‐humulene production was not induced. Thus, the sesquiterpene content measured during culture period results from the initial concentration added. Again, a decrease in α‐humulene concentration was observed, proving degradation by E. coli HUM1510.
To overcome production difficulties associated with α‐humulene degradation, we investigated the effect of recovering the sesquiterpene by in situ product recovery methods. Such strategies circumvent the effects of product degradation by employing an appropriate and biocompatible separation mechanism to selectively remove the target directly from the culture during production.
Although unexplored to date specifically with respect to α‐humulene production, the use of adsorption and solvent extraction with respect to the in situ recovery of other bioproducts from growing cultures has been previously investigated. Appropriate solvent and adsorber selection is a key consideration and, among other attributes ideal solvents and adsorbents should be biocompatible yet non‐bioavailable and display high equilibrium partitioning of the target compound over water 23, 24. In this study, dichloromethane (DCM), pentane, isooctane, nonane and ethyl acetate were selected as prospective solvents for in situ α‐humulene extraction. For the adsorptive product recovery, the polyaromatic adsorbent Amberlite® XAD4 was applied. In addition to a high affinity, effective elution of the bound compound was investigated using different elution solvents, namely acetonitrile, ethyl acetate, ethanol, acetone, DCM and isooctane.
The selected extraction solvents and adsorber were screened for their ability to uptake α‐humulene from an aqueous solution with an initial concentration of 5 μg/mL. Recovery rate and partition coefficients of α‐humulene between each solvent and aqueous phase were evaluated from batch experiments (Table 1).
Table 1.
Experimental partition coefficients and recovery rates of selected solvents measured from a model test mixture with defined α‐humulene content
| Extraction | ||||||
|---|---|---|---|---|---|---|
| Solvent | Ethyl acetate | DCM | Isooctane | Pentane | Decane | Nonane |
| (μg/mL) | 0.846 | 0.157 | 0.193 | 0.246 | 0.006 | 0.078 |
| (μg/mL) | 2.709 | 4.320 | 4.192 | 4.014 | 4.971 | 4.637 |
| (μg/mL) | 2.291 | 0.680 | 0.808 | 0.986 | 0.029 | 0.363 |
| Recovery rate (%) | 45.8 ± 18.5 | 13.6 ± 9.2 | 16.2 ± 15.5 | 19.7 ± 16.5 | 0.1 ± 0.1 | 7.3 ± 7.2 |
| Elution after adsorption to Amberlite® XAD4 | ||||||
|---|---|---|---|---|---|---|
| Solvent | Acetonitrile | Ethyl acetate | Ethanol | Acetone | DCM | Isooctane |
| K ex (μg/mL) | 3.541 | 1.594 | 0.193 | 0.482 | 0.004 | 0.009 |
| (μg/mL) | 1.101 | 1.927 | 4.188 | 3.374 | 4.981 | 4.953 |
| c ex (μg/mL) | 3.899 | 3.073 | 0.813 | 1.626 | 0.019 | 0.047 |
| Recovery rate (%) | 78.0 ± 17.3 | 61.5 ± 0.9 | 16.2 ± 5.4 | 32.5 ± 4.5 | 0.4 ± 0.3 | 0.9 ± 0.4 |
In all cases of solvent extraction less than 50% of the total α‐humulene initially present in each flask existed in the solvent phase in the equilibrium. In comparison, α‐humulene uptake with the hydrophobic Amberlite® XAD4 resin using polar eluting solvents was more successful. Elution of the sesquiterpene with acetonitrile yielded a maximal recovery rate of approximately 80%.
Biocompatibility of the polymeric resin was evaluated with respect to the specific host strain of interest to this study. A growth challenge assay was performed to evaluate its growth characteristics in the presence of 10% (w/v) of Amberlite® XAD4. Consistent with several prior reports 25, 26, no reduction in growth rate was observed relative to a control (data not shown), confirming the biocompatibility of the adsorbent.
3.2. α‐Humulene biosynthesis with in situ adsorption
The hydrophobic adsorbent was next investigated in bioreactor cultivations of the production strain E. coli HUM1510 to assess its potential for promoting enhanced α‐humulene biosynthesis as a result of the in situ adsorption. Here, an adsorbent weight fraction of 0.05 was used in each case, also to ensure that adsorption capacity would not be limiting. Optimal expression conditions, regarding inducer concentration and induction time, were previously determined to be 0.1 mM IPTG at an optical density of OD600 = 1.0. The results of the integrated bioprocess are shown in Fig. 2.
Figure 2.
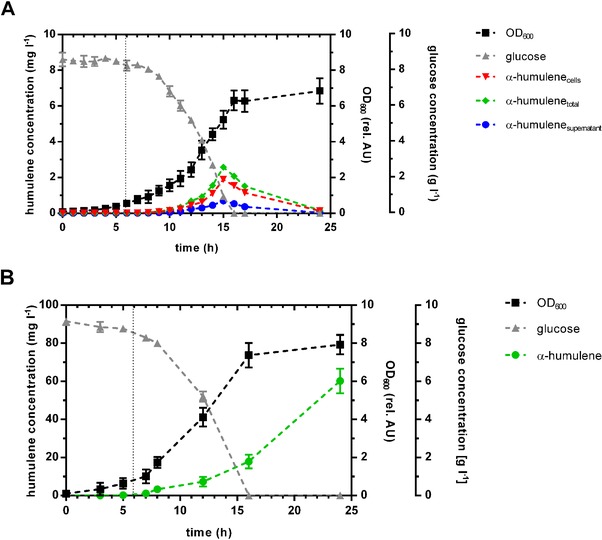
Growth and production by E. coli HUM1510 strain using conventional (A) and in situ product removal (B) by adsorption on the polymeric resin Amberlite® XAD4. Production and glucose consumption of α‐humulene producing strain in a batch fermentation process. Cell growth was monitored by measuring optical density at 600 nm. α‐humulene concentration was measured by elution of adsorped product with acetonitrile and GC‐FID analysis.
When applying a batch bioprocess with conventional product recovery by extraction after end of the batch process, α‐humulene concentration in the biomass and in the culture medium were determined. A consistently increasing α‐humulene concentration is observed until 16 h of batch cultivation, where product titer reached 2.6 mg/L. In the following cultivation period, product titer rapidly decreased until no α‐humulene was detectable, indicating that the heterologous product is degraded by the production strain and used as an alternative carbon source as soon as glucose is fully consumed after 16 h. It is known that E. coli activates the expression of catabolic functions under carbon limited conditions. Although microbial degradation of larger isoprenoids is well described 27, the degradation pathway of α‐humulene in E. coli is not known to date. The sesquiterpene possibly undergoes sequential oxidative degradation before being used for energy generation via tricarboxylic acid cycle.
The application of an integrated bioprocess using Amberlite® XAD4 for in situ product removal, no α‐humulene could be detected in the aqueous culture phase and in the biomass by conventional extractive product removal strategies. The selective concentration of α‐humulene on the adsorbent and following elution with acetonitrile allowed volumetric α‐humulene productions of 60.2 mg/L, representing a 2310% improvement relative to the highest α‐humulene titer measured in a single‐phase culture. By binding on the polymeric adsorbent, the sesquiterpene is not easily accessible for the microbial cells anymore. This prevents degradation of the product when glucose depletion occurs, thus leading to a concentration of the sesquiterpene on the resin surface and higher product titers. Gas chromatographic analysis of the eluted fraction showed two peaks that could be identified as α‐humulene and β‐caryophyllene, the minor product of α‐humulene synthase (Fig. 3) 9, 17. As α‐humulene was isolated with a purity of ∼95%, an additional purification step was not applied.
Figure 3.
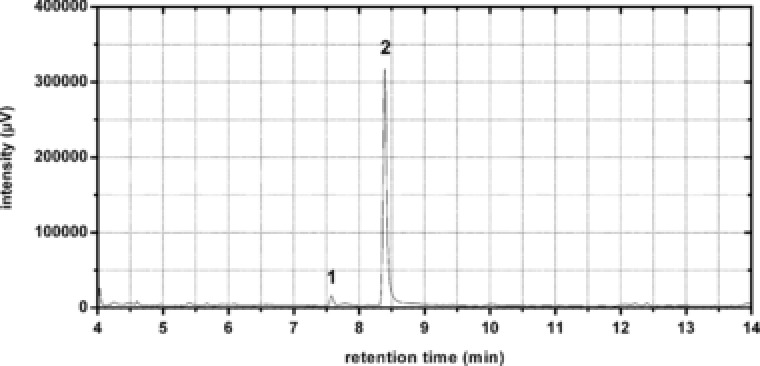
In situ isolation of α‐humulene from E. coli HUM1510 growth culture. GC‐FID chromatogram of eluted compounds from hydrophobic adsorber Amberlite® XAD4. β‐Caryophyllene (1) and α‐humulene (2) were identified by comparison with standards and GC‐MS analysis.
3.3. Chemical conversion of α‐humulene to zerumbone
Here, we report a synthetic strategy starting from isolated α‐humulene. For this route to be practical and scalable, we adapted a simple one‐step allylic oxidation reaction previously published 28. In contrast to oxidation reactions in the literature 28, 29, we carried out the reaction under air and not under oxygen atmosphere as we observed that an oxygen excess in the reaction atmosphere leads to an increased oxidation degree, thus resulting in several undesired byproducts and not the product of interest. After an extensive study, using a statistical design of experiments by Box‐Behnken, optimal reaction conditions for the oxidation reaction were determined to be a reaction temperature of 60.7°C, a catalyst concentration of 0.0073 molar equivalents and an oxidizing agent concentration of 4.64 molar equivalents (data not shown). In order to select the more efficient catalytic system toward the formation of zerumbone, a screening of various transition metal salts was carried out. Catalysts were chosen by their track records in similar oxidation reactions and by their solubility in acetonitrile. Acetonitrile was chosen to further reduce process steps by unnecessary solvent changes or rebuffering as it was the most effective eluent from Amberlite® XAD4. Reactions were performed with copperI iodide, copperII chloride, cobaltII chloride, ironIII chloride and manganeseII chloride. The results are summarized in Table 2.
Table 2.
Allylic oxidation with transition metal salts. Educt conversion and product selectivity was quantified by GC‐FID analysis
| Catalyst | CuI | CuCl2 | FeCl3 | CoCl2 | MnCl2 |
|---|---|---|---|---|---|
| Conversion (%) | 100 | 100 | 100 | 100 | 100 |
| Selectivity (%) | 42.8 | 35.9 | 46.4 | 35.9 | 51.6 |
A kinetic study was carried out, which depicts the evolution of zerumbone versus time. Total conversion of α‐humulene could be achieved with every catalyst tested, but the transition metal salts show differences in reaction kinetics and selectivity (Fig. 4).
Figure 4.
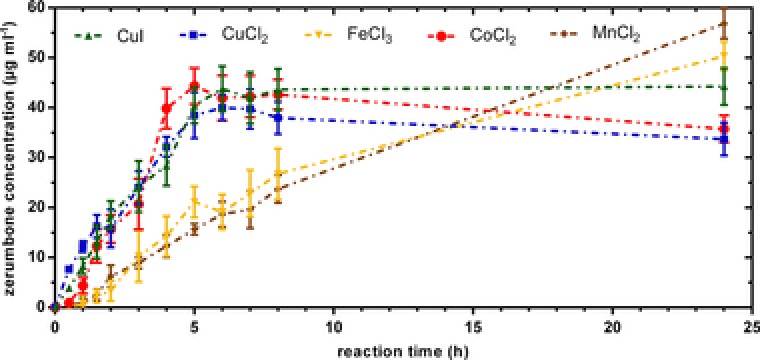
Catalyst screening for the allylic oxidation of α‐humulene to zerumbone. Zerumbone formation over reaction time. Product was quantified by GC‐FID analysis.
It is observed that copper and cobalt catalysts accelerate zerumbone formation, whereas reaction takes place at a slower rate when using manganese and iron salts. However, best selectivity of 51.6% was obtained with manganeseII chloride, which is consistent with literature for allylic oxidations with transition metal salts 28.
Side‐product formation of the oxidation reaction was analyzed by GC‐MS (Fig. 5). Reaction of α‐humulene with TBHP leads to one major product, zerumbone (peak 3, I = 2084), and three notable side products, from which two could be identified as humulene‐6,7‐epoxide (peak 2, I = 1803, selectivity: 21.0%) and viridiflorol (peak 4, I = 2113, selectivity: 19.5%).
Figure 5.
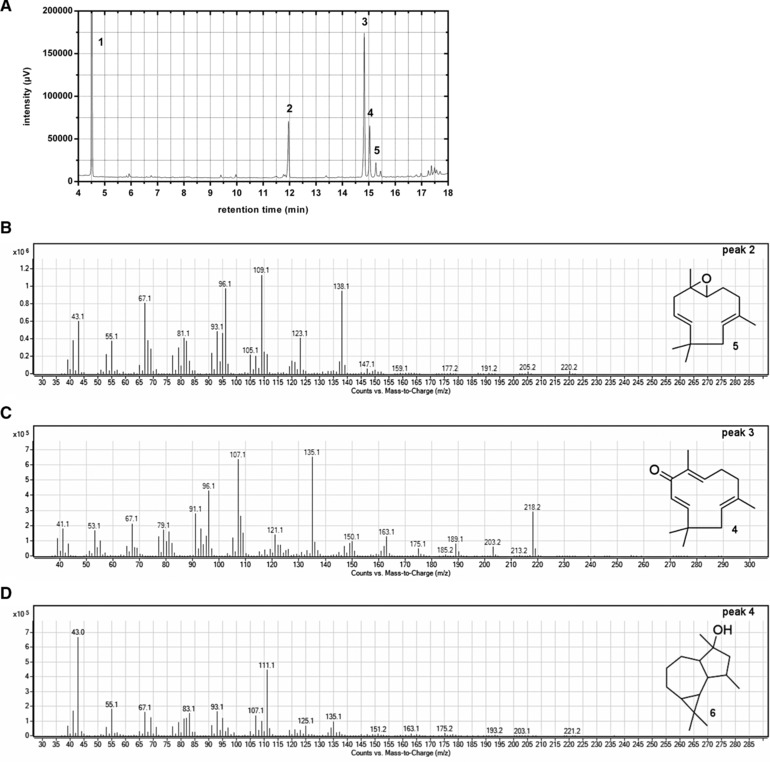
Chemical conversion of α‐humulene. Gas chromatography‐mass spectrometric analysis of reaction products. Peaks in the chromatogram (A) could be identified as TBHP (peak 1), humulene‐6,7‐epoxide (5, peak 2), zerumbone (4, peak 3) and viridiflorol (6, peak 4). Peak (5) could not be identified by GC‐MS. Mass spectra of humulene‐6,7‐epoxide (B), zerumbone (C) and viridiflorol (D).
Humulene‐6,7‐epoxide is a common product seen in previous studies to selectively oxidize α‐humulene in C8‐Position. However, formation of viridiflorol was not reported yet. As methylic groups in viridiflorol are at matching positions to α‐humulene, we assume that under the reaction conditions described above, the humulene carbon skeleton undergoes several rearrangements to form the aromadendrene backbone for viridiflorol (see supporting information).
4. Concluding remarks
In this study, we have successfully developed an integrated bioprocess for α‐humulene production in metabolically optimized E. coli using in situ adsorption techniques. In further experiments, we oxidized α‐humulene at C8‐position by allylic oxidation. This is the first time to report an efficient synthetic strategy to directly oxidize α‐humulene to zerumbone. Since the synthesis of the macrocyclic sesquiterpenoid zerumbone is chemically challenging, semi‐synthetic strategies can provide an efficient and practical route for large‐scale production.
Practical application
Zerumbone is a highly potential chemotherapeutic agent. To date, zerumbone production is still based on extractive methods from the natural source. Total synthesis strategies are often too inefficient as well as time and labor consuming due to the size and complexity of the molecule. With a semi‐synthetic strategy, combing a metabolically optimized E. coli strain for precursor production and a simple one‐step chemical oxidation reaction, we offer more flexibility and efficiency to produce zerumbone.
The authors have declared no conflicts of interest.
Nomenclature
| OD600 | [rel. AU] | optical density at 600 nm | |
| K ex | [‐] | partition coefficient for aqueous/organic extraction system | |
| c ex | [μg/mL] | solute concentration in organic fraction | |
|
|
[μg/mL] | solute concentration in aqueous fraction after equilibration | |
| K ad | [‐] | partition coefficient for adsorptive system | |
| c ad | [μg/mL] | solute concentration in eluted fraction after adsorption | |
| I | [‐] | kovats indices | |
| tx | [min] | retention time of compound of interest | |
| n | [‐] | number of carbon atoms in n‐alkane |
Indices
| ex | [‐] | extraction solvent |
| aq | [‐] | aqueous phase |
| ad | [‐] | adsorber |
| t eq | [‐] | after equilibration |
Supporting information
Supplementary material
Acknowledgments
This study was funded by the European Regional Development Fund (EFRE): Innovation Network “Refinement of plant resources” (ZW‐8‐80130940). Special thanks to Daniel Sandner and Dr. Ulrich Krings (both Institute of Food Chemistry, Leibniz University Hannover) for performing GS‐MS measurements. Jana Schellenberg is gratefully acknowledged for assistance in the laboratory work.
5 References
- 1. Webster, N.S. , Wilson, K. J. , Blackall, L. L. , Hill, R. T. , Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeides odorabile . Appl. Environ. Microbiol. 2011, 67, 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chisti, Y. , Moo‐Young, M. , Bioprocess intensification through bioreactor engineering. Chem. Eng. Res. Des. 1996, 74, 575–583. [Google Scholar]
- 3. Sanchez, S. , Demain, A. L. , Metabolic regulation of fermentation processes. Enzyme. Microb. Technol. 2002, 71, 895–906. [Google Scholar]
- 4. Wilke, D. , Chemicals from biotechnology: Molecular plant genetics will challenge the chemical and the fermentation industry. Appl. Microbiol. Biotechnol. 1999, 52, 135–145. [DOI] [PubMed] [Google Scholar]
- 5. Paddon, C. J. , Keasling, J. D. , Semi‐synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 35–367. [DOI] [PubMed] [Google Scholar]
- 6. Schwab, W. , Fuchs, C. , Huang, F.‐C. , Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Technol. 2012, 115, 3–8. [Google Scholar]
- 7. Martin, V. J. J. , Pitera, D. J. , Withers, S. T. , Newmann, J. D. et al., Engineering the mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [DOI] [PubMed] [Google Scholar]
- 8. Wriessnegger, T. , Augustin, P. , Engleder, M. , Leitner, E. et al., Production of the sesquiterpenoid (+)‐nootkatone by metabolic engineering of Pichia pastoris . Metab. Eng. 2014, 24, 18–29. [DOI] [PubMed] [Google Scholar]
- 9. Yu, F. , Okamto, S. , Nakasone, K. , Adachi, K. et al., Molecular cloning and functional characterization of α‐humulene synthase, a possible key enzyme of zerumbone biosynthesis in shampoo ginger (Zingiber zerumbet Smith). Planta 2008, 227, 1291–1299. [DOI] [PubMed] [Google Scholar]
- 10. Kader, G. , Nikkon, F. , Rashid, M.A. , Yeasmin, T. , Antimicrobial activities of the rhizome extract of Zingiber zerumbet Linn. Asian Pac. J. Trop. Biomed. 2011, 1, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habsah, M. , Amran, M. , Mackeen, M. M. , Lajis, N. H. et al., Screening of Zingiberaceae extracts for antimicrobial and antioxidant activities. J. Ethnopharmacol. 2000, 72, 403–410. [DOI] [PubMed] [Google Scholar]
- 12. Tzeng, T. F. , Liou, S. S. , Chang, C. J. , Liu, I. M. , The ethanol extract of Zingiber zerumbet attenuates streptozotocin‐induced diabetic nephropathy in Rats. Evid.‐Based Complementary Altern. Med. 2013, Article ID 340645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahab, S. I. A. , Abdul, A. B. , Yeel, H. C. , Alzubain, A. S. et al., Anti‐tumor activities of analogues derived from the bioactive compound of Zingiber zerumbet . Int. J. Cancer Res. 2008, 4, 154–159. [Google Scholar]
- 14. Murakami, A. , Miyamoto, M. , Ohigashi, H. , Zerumbone, an anti‐inflammatory phytochemical, induces expression of proinflammatory cytokine genes in human colon adenocarcinoma cell lines. Biofactors 2004, 21, 95–101. [DOI] [PubMed] [Google Scholar]
- 15. Shamoto, T. , Matsuo, Y. , Shibata, T. , Zerumbone inhibits angiogenesis by blocking NF‐κB activity in pancreatic cancer. Pancreas 2014, 43, 396–404. [DOI] [PubMed] [Google Scholar]
- 16. Tewtrakul, S. , Subhadhirasakul, S. , Anti‐allergic activity of some selected plants in the Zingiberaceae family. J. Ethnopharmacol. 2007, 109, 535–538. [DOI] [PubMed] [Google Scholar]
- 17. Alemdar, S. , Hartwig, S. , Frister, T. , König, J. C. et al., Heterologous expression, purification and biochemical characterization of α‐humulene synthase from Zingiber zerumbet Smith. Appl. Biochem. Biotechnol. 2016, 178, 474–489. [DOI] [PubMed] [Google Scholar]
- 18. Yu, F. , Okamoto, S. , Harada, H. , Yamasaki, K. et al., Zingiber zerumbet CYP71BA1 catalyzes the conversion of alpha‐humulene to 8‐hydroxy‐alpha‐humulene in zerumbone biosynthesis. Cell. Mol. Life. Sci. 2011, 68, 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okamoto, S. , Yu, F. , Harada, H. , Okajima, T. et al., A short‐chain dehydrogenase involved in terpene metabolism from Zingiber zerumbet . FEBS J. 2011, 27, 2892–2900. [DOI] [PubMed] [Google Scholar]
- 20. Shirahama, H. , Chhabra, B. R. , Matsumoto, T. , Conversion of humulene to zerumbone. Chem. Lett. 1981, 10, 717–718. [Google Scholar]
- 21. Kodama, M. , Shiobara, Y. , Sumitomo, H. , Mitani, K. , Ueno, K. , Synthesis of macrocyclic terpenoids by intramolecular cyclization. XI. Total synthesis of zerumbone. Chem. Pharm. Bull. 1987, 35, 4039–4042. [Google Scholar]
- 22. Peralta‐Yahya, P. P. , Ouellet, M. , Chan, R. , Mukhopadhyay, A. et al., Identification and microbial production of a terpene‐based advanced biofuel. Nat. Commun. 2011, 2, 483, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MnKenna, R. , Moya, L. , McDaniel, M. , Nielsen, D. R. , Comparing in situ removal strategies for improving styrene bioproduction. Bioprocess Biosyst. Eng. 2015, 38, 165–174. [DOI] [PubMed] [Google Scholar]
- 24. Ghosal, A. K. , Manjare, S. D. , Selection of appropriate adsorption technique for recovery of VOCs: An analysis. J. Loss Prev. Process Ind. 2002, 15, 413–421. [Google Scholar]
- 25. Asada, M. , Shuler, M. L. , Stimulation of ajmalicine production and excretion from Catharanthus roseus: Effects of adsorption in situ, elicitors and alginate immobilization. Appl. Microbiol. Biotechnol. 1989, 30, 475–481. [Google Scholar]
- 26. Yan, Q. , Hu, Z. , Tan, R. X. , Wu, J. , Efficient production and recovery of diterpenoid tanshinones in Salvia miltiorrhiza hairy root cultures with in situ adsorption, elicitation and semi‐continuous operation. J. Biotechnol. 2005, 119, 416–424. [DOI] [PubMed] [Google Scholar]
- 27. García, J. L. , Uhía, I. , Galán, B. , Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb. Biotechnol. 2012, 5, 679–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salvador, J. A. R. , Clark, J. H. , The allylic oxidation of unsaturated steroids by tert‐butylhydroperoxide using surface functionalised silica supported metal catalysts. Green Chem. 2002, 4, 352–356. [Google Scholar]
- 29. Allal, B. A. , Firdoussi, L. E. , Allaoud, S. , Karim, A. et al., Catalytic oxidation of α‐pinene by transition metal using t‐butyl hydroperoxide and hydrogen peroxide. J. Mol. Catal. A: Chem. 2003, 200, 177–184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


