Abstract
Cervical cancer (CC) is the fourth most common cause of cancer death in women. The most important risk factor for the development of CC is cervical infection with human papilloma virus (HPV). Inflammation is a protective strategy that is triggered by the host against pathogens such as viral infections that acts rapidly to activate the innate immune response. Inflammation is beneficial if it is brief and well-controlled, however, if the inflammation is excessive or it becomes of chronic duration, it can produce detrimental effects. HPV proteins are involved, both directly and indirectly, in the development of chronic inflammation, which is a causal factor in the development of CC. However, other factors may also have a potential role in stimulating chronic inflammation. MicroRNAs (miRNAs) (a class of non-coding RNAs) are strong regulators of gene expression. They have emerged as key players in several biological processes, including inflammatory pathways. Abnormal expression of miRNAs may be linked to the induction of inflammation that occurs in CC. Exosomes are a subset of extracellular vesicles shed by almost all types of cells, which can function as cargo transfer vehicles. Exosomes contain proteins and genetic material (including miRNAs) derived from their parent cells and can potentially affect recipient cells. Exosomes have recently been recognized to be involved in inflammatory processes and can also affect the immune response. In this review, we discuss the role of HPV proteins, miRNAs and exosomes in the inflammation associated with CC.
Keywords: Cervical cancer, carcinogenesis, inflammation, HPV, MicroRNAs, Exosomes
1. Introduction
Worldwide, cervical cancer (CC) ranked as the fourth most commonly diagnosed cancer and the fourth cause of cancer death in women. In 2018, there were an estimated 570,000 cases diagnosed globally, resulting in 311,000 deaths. Almost 85% of these deaths occurred in developing or underdeveloped countries 1, 2. The majority of CC cases result from infection with certain sub-types of the human papilloma virus (HPV). The HPV genome has been identified in nearly 95% of CC lesions. Most HPV infections will be cleared spontaneously and are transient in nature. However, persistent infection with certain HPV strains may result in the development of the premalignant condition known as cervical neoplasia 3, 4. The integration of the HPV E7 and E6 oncogenes into the host genome is considered to be the key step in the development of CC 5. Immune evasion is an important cause of persistent HPV infection. Because there is no overt viremia or cytolysis after the initial HPV infection in the cervix, there is no inflammation at the early stages and no activation of the innate immune system. The virus completes its life cycle in actively dividing cells employing the host cellular machinery 6. Once established, the persistent infection triggers changes in the secretion of inflammatory cytokines, which in turn leads to immune cell infiltration. In older patients with persistent HPV infection, alterations in the responsiveness of the immune system and increased systemic levels of inflammatory cytokines have been found 7. In clinical studies, this age group is more likely to be diagnosed with CC 8 and is more likely to show sustained elevation of cytokines, which contributes to the tumorigenesis of HPV 5.
Inflammation is one part of the complicated biological response mounted by body tissues to dangerous stimuli, including pathogens, irritants, or injured cells. Inflammation is designed to be a protective response, which involves blood vessels, immune cells, and molecular mediators 9. Inflammation acts rapidly to release chemokines and cytokines, which activate the host innate immune response. These mediators operate in concert and facilitate the recruitment of effector cells to the site of injury or infection 10–12. After the elimination of the triggering stimulus and the resolution of the injury, inflammation should be disabled in a programmed manner. However, if the stimulus is persistent, the acute inflammation becomes chronic in nature and evidence suggests that chronic inflammation is strongly associated with cancer 10, 13, 14. About 20% of human cancers are associated with chronic inflammation caused by infectious agents, autoimmune diseases and long-term exposure to irritants and other noxious agents. Common causes of persistent inflammation that lead to cancer development, include infection with hepatitis C or B viruses that lead to hepatocellular carcinoma, inflammatory bowel disease that leads to colorectal cancer and infection with Helicobacter pylori that leads to gastric cancer 15. However, the role of HPV infection in the induction of chronic inflammation and the link between chronic inflammation and HPV-induced CC carcinogenesis remains controversial.
Multiple factors are needed for the development of CC, such as the interaction of the virus, host-dependent and environmental factors 16. Furthermore, there is evidence that the epigenetic regulatory machinery can lead to dysregulation of tumor-suppressor genes and activation of oncogenes, triggering the malignant phenotype in cancer cells 17, 18. In this regard, miRNAs (miRNAs) play a major role as regulators of cell cycle progression, apoptosis, metastasis and chemoresistance 18–20. miRNAs are small non-coding RNAs which regulate the expression of genes at a post-transcriptional level, by incomplete sequence pairing to the 3’UTR region of their target mRNAs 19. Recent studies have identified those miRNAs which have different expression profiles and play an important role in diverse physiological and pathological processes, such as carcinogenesis, viral infections and oncogenesis 19, 21. miRNAs have also been implicated in the regulation of both adaptive and innate immune responses, as well as inflammatory networks in various tissues and cell types 21. Inflammatory mediators cause dysregulation of miRNAs and vice versa certain miRNAs operate as inflammatory mediators. Dysregulation of miRNAs has been observed during CC development and several dysregulated miRNAs have the ability to modulate the initiation and development of inflammation-induced cervical carcinogenesis 2, 22.
Exosomes are nanovesicles about 30–150 nm in sizeand are released by almost all cell types, under both pathological and physiological conditions. These nanovesicles are involved in intercellular communication at both local and systemic levels 23–25. Exosomes are vehicles that are able to transfer nucleic acids, proteins and lipids from donor cells to recipient cells, thereby influencing the target cell metabolism 26, 27. Multiple studies have assessed the effect of exosomes in neurodegenerative diseases, sepsis, arthritis, inflammatory bowel disease, atherosclerosis and diabetes, and taken together, suggest that exosomes may participate in the development of several inflammatory diseases 28. Although inflammatory mediators (chemokines and cytokines) are the major players in the initiation of the inflammatory response, increasing evidence supports the longer-term connection between inflammation and exosomes 28. Immunosuppression and inflammation are well-established factors that govern the progression of cancer, while exosomes derived from tumor cells are also involved in the modulation of inflammation and immune system to promote tumorigenesis. Exosomes originating from gastric cancer or breast cancer cells have been demonstrated to trigger the activation of nuclear factor kappa light chain enhancer of activated B cells (NF-κB), which causes the production and release of pro-inflammatory cytokines, including C-C motif chemokine ligand 2 (CCL2), granulocyte colony stimulating factor (GCSF), tumor necrosis factor-α (TNF-α), and interleukin 6 (IL-6) 28–30. Furthermore, macrophages treated with exosomes can cooperatively increase the invasive and migratory properties of tumor cells 28. However, the role of CC-derived exosomes in the modulation of inflammation and how they contribute to CC progression has not yet been fully studied. In the present review, we will discuss the role of HPV proteins, miRNAs, exosome-mediated inflammatory responses and how they contribute to the development of CC.
2. Inflammation and Cervical Cancer
Inflammation means, “to set on fire” and is a protective mechanism triggered by exposure to a myriad of factors, including diseases, infections and trauma. Not surprisingly, the failure of the normal inflammatory program can lead to considerable harmful effects. The inflammatory environment involves a complex network of soluble molecular messengers and biological players, including plasma proteins, cytokines, chemokines, innate and adaptive immune cells, extracellular matrix, stromal fibroblasts and the vascular blood and lymphatic networks. Inflammation can be classified as either acute or chronic depending on the time course. Acute inflammation persists for only a couple of days or weeks at the most. It involves three primary processes: 1) alterations in the vascular diameter; 2) structural alterations in the microcirculation; 3) adhesion and transmigration of leukocytes from the microcirculation into the tissue. After eliminating the infection or controlling the initial injury, mechanisms should be immediately activated which limit any type of damage to the host and initiate the tissue repair processes. If for some reason the inflammation continues too long, chronic inflammation can become established, which can persist over months or even years 31. Chronic inflammation takes place for two main reasons: 1) the host is unable to eliminate the original stimulus; or 2) the host cannot carry out the resolution of inflammation program 32. Chronic inflammation involves progressive changes in several kinds of inflammatory cells and can participate in the causation of chronic diseases, such as cardiovascular, arthritis, diabetes, neurological diseases and cancer 33.
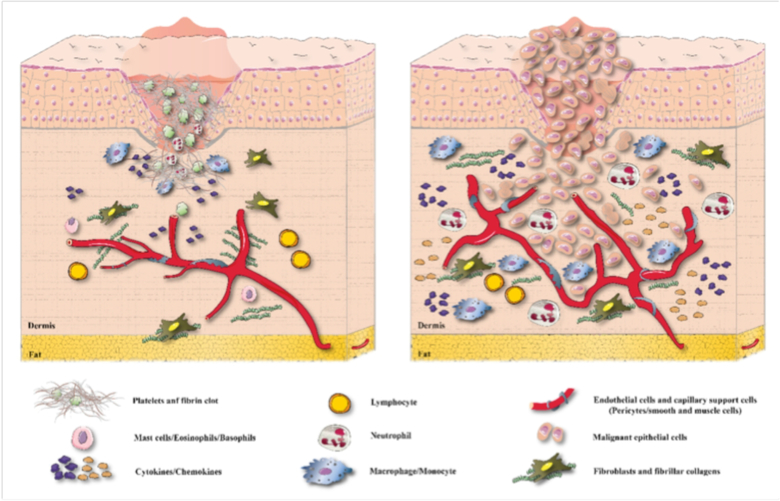
Researchers have suggested that there is a relationship between inflammation and cancer, but this suggestion is not a thoroughly novel idea, because Rudolf Virchow (1863) demonstrated that inflammation and chronic irritation could lead to cancers 34. Nowadays, inflammation is accepted as a significant cancer hallmark (Figure 1). It is estimated that an excessive inflammatory response is associated with at least 15–20% of all cancer deaths worldwide 35. Persistent infection with Helicobacter pylori, hepatitis virus B or C (HVB or HVC), or HPV can cause chronic inflammation in the specific affected organs and increases the risk of cancer 34.
Figure 1.
Role of inflammation in (a) wound healing and (b) invasive tumor growth
Chronic inflammation may be also be caused by long-term exposure to noxious chemicals or the presence of chronic autoimmune disease. Cancer-related inflammation (CRI) promotes tumor growth that is independent of growth factors, has unlimited replicative potential, shows escape from programmed cell death, resistance to growth inhibition, promotes angiogenesis, cancer cell extravasation and metastasis 36. Some cancer therapies such as radiotherapy or chemotherapy can cause necrotic cell death, which can increase tumor-associated inflammation, which can lead on one hand to induction of antitumor immunity, or on the other hand can lead to the development of resistance to therapy 37. The involvement of chronic inflammation has been considered to be important in several cancers, including colon, lung, gastric and cervical malignancies 38, 39.
Intrinsic and extrinsic pathways may directly or indirectly activate inflammatory cells, such as macrophages and T cells. Hyperactivation of inflammatory pathways plays a significant role in tumorigenesis, promoting progression from low-grade lesions to high grade invasive CC lesions 40. NF-κB is a transcription factor implicated in immune cell activation and chronic inflammatory responses. HPV-16 E5, E6 and E7 oncoproteins can trigger activation of the NF-κB pathway, which is correlated with the progression of carcinogenesis in CC. COX-2 is also activated in response to inflammatory stimuli, including cytokines, growth factors and mitogens. NF-κB has been identified as a positive regulator of COX-2. On the other hand, caspase-1 that cleaves pro-IL-1β to mature IL-1β, is an activator of NF-κB. Interestingly, the NF-κB-COX-2/caspase-1 pathway has been demonstrated to be responsible for cell growth, anti-apoptosis and inflammation in CC cells 41. Toll-like receptor 4 (TLR4) is a pattern recognition (or Toll-like) receptor, which is also associated with tumor growth and inflammation in CC. TLR4 signaling initiates cell activation, inflammation and tumor growth through MyD88-dependent and NF-κB-associated signaling pathways. TLR4 down-regulation has been demonstrated to be a potential cause of apoptosis in SiHa CC cells 42. Furthermore, EGFR contributes to extensive crosstalk between other signaling pathways, which are involved in the release of growth factors, cytokines and inflammatory mediators. EGFR over-expression has been found to be related with the induction of inflammation and with poor prognosis in CC patients 42.
Cytokines are small, secreted proteins that are released by cells. Cytokine is a general term for secreted mediators of cell-cell communication. Other terms are lymphokines (cytokines secreted by lymphocytes), monokines (cytokines secreted by monocytes), chemokines (cytokines with chemotactic activity), and interleukins (cytokines secreted by one type of leukocyte, which act on other leukocytes) 43. Cytokine expression by tumor cells attracts inflammatory cells that result in further tumor growth and progression. Various lines of evidence support this mechanisms occurring in CC 44. Recent publications have shown a correlation between dysregulation of several cytokines, and the occurrence of cervical pre-cancerous lesions (LSIL, HSIL), progression from pre-cancer to ‘in situ’ cancer, further invasion and the final phase of metastasis 45. For some time, researchers had assumed that cytokines were mainly messenger molecules in the immune system, which guide leucocytes to inflamed sites45. Researchers now believe in an association between dysregulated cytokines and a majority of cancers, in which they contribute to cell transformation, rapid growth, survival, angiogenesis, invasion and metastasis. Over 33 cytokines have been already identified, and a number of cytokines or their receptors are significantly altered in the carcinogenesis and metastasis of cervical cancer 45.
Cytokines such as IL-6, IL-8, IL12, IL-4R, vascular endothelial growth factor (VEGF), IL-4, IL-10, etc have been used as biomarkers for assessing the risk of invasive cancer and metastasis 46, 47. Many cytokines are significantly altered in cervical precancer and cancer, more so in advanced cancer with metastasis. Excessive expression of a number of cytokines; for example, IL-6, IL-17, and IL-8 show a relationship with tumor growth. On the other hand, different cytokines are associated with the inhibition of HPV replication and tend to suppress tumors; for example, IL-1, TNF-α, TGF-β and IFN-α in the early phases of tumor development 45.
Infection with HPV is recognized to be the key cause of CC. High expression levels of IFN-γ mRNA have been found in CC patients infected by HPV 48. Furthermore, it was shown that high levels of TNF-α contributed to monocyte differentiation into mature DCs in CC. TNF-α is the most important proinflammatory cytokine which is produced by macrophages and monocytes, playing both anti-carcinogenic and pro-carcinogenic roles 49, 50. Mutations in the A allele and the GA/AA SNP in TNF-> j308 have been demonstrated as risk factors for CC development 50. Another cytokine, IL-6, is associated with persistent HPV infection and with the progression of CC. IL-6 activates the signal transducer and activator of transcription 3 (STAT3) oncogene, which contributes to chronic inflammation in CC. Abnormal STAT3 signaling promotes tumor cell growth, invasion, metastasis and inflammation. Its persistent activation has been proposed to be a marker of poor prognosis in CC 40. Moreover, Th2 type inflammatory cells are elevated during the progression of HPV induced cervical lesions. One of the best studied Th2 type cytokines, IL-10, has been found to be increased in cervical tissues and sera from HPV-infected patients and is correlated with high-grade lesions 51. In addition to IL-10, IL‐8 plays a causative role in acute inflammation. It has been suggested that elevated production of IL-8 triggered by cell cycle and apoptosis regulator 2 (CCAR2) under conditions of oxidative stress, has a role in inflammation and progression of CC 52. IL-12 is a heterodimeric proinflammatory cytokine with antitumor activity because it promotes cytotoxic T cell (CTL) responses and Th1 adaptive immunity. The polymorphisms in the IL12 gene, IL12Brs3212227 and IL12Ars568408 contribute to the risk of CC 53. Moreover, CCL2 (MCP‐1), is another chemokine that regulates infiltration and migration of macrophages/monocytes. It has been suggested that CCL2 expression by CC cells is correlated with an enhanced infiltration by inflammatory macrophages, and reduced disease‐free survival in CC patients 49.
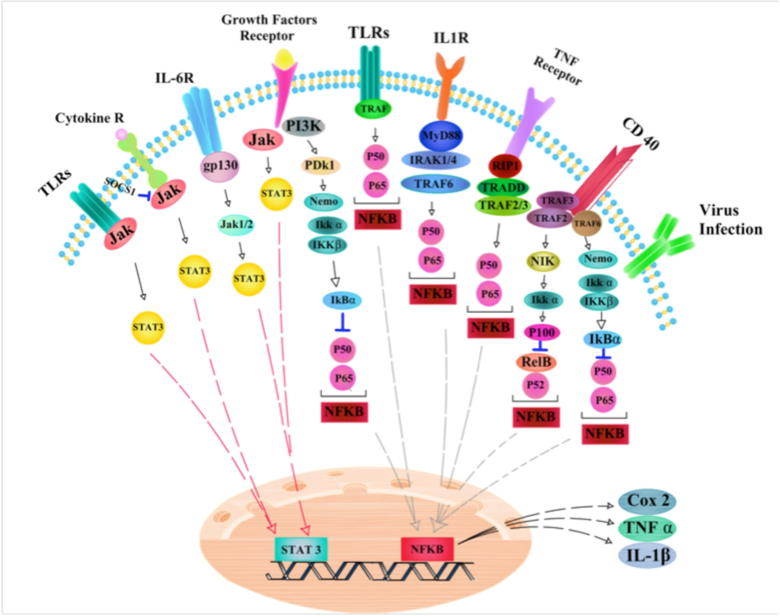
Taken together, these lines of evidence suggest the role of chronic inflammation and its mediators in CC progression and development (Figure 2). Therefore, more complete understanding of the type and function of CC‐related inflammation, may provide new ways to improve on recent successful immunotherapy approaches and suggest new biomarkers for diagnostic assays and targets for therapy.
Figure 2. Inflamation-related signaling pathways in cervical cancer.
CD40: Cluster of differentiation 40; Gp130: Glycoprotein 130; Ikkα: IκB kinase α; Jak: Janus kinase; MyD88:Myeloid differentiation primary response 88; NFĸβ:Nuclear factor kappa beta; NIK: Nuclear Factor κB-inducing Kinase; PDk1: 3-phosphoinositide-dependent protein kinase-1; STAT3: Signal Transducer And Activator Of Transcription 3; TLRs: Toll-like receptors; TNF: tumor necrosis factor; TRAF3: TNF receptor-associated factor
3. HPV and inflammation in cervical cancer
Long-term chronic inflammation due to persistent HPV infection is one potential cause for the development of cervical cancer. This is a complex process involving the participation of reactive oxygen and reactive nitrogen species, cytokines, chemokines, growth and cell survival factors, enzymes (including cyclooxygenase (COX) and metalloproteinases), prostaglandins and specific types of miRNAs 54. The collective action of these mediators induces changes in the processes of proliferation, senescence and cell death and also causes mutation and methylation of DNA, and stimulates angiogenesis contributing to development of HPV-induced CC 54. Inflammation is activated when pattern recognition receptors (PRRs) expressed by innate immune system cells recognize pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs). Once PRRs have detected DAMPs or PAMPs, signaling pathways are triggered, NF-κB is activated and transported to the nucleus. Transcription of several NF-κB-responsive genes leads to expression of proteins that mediate the inflammatory response (e.g. TNFα, IL-6, COX-2 and ICAM), proliferation (CDK2), cell survival (XIAP, cIAP, BCL2 and BCL-xL) and angiogenesis (VEGF) 55–58. NF-ĸB is the key player that connects inflammation and cancer 58. Transcription factors such as STAT3 and NF-κB are activated by inflammatory cytokines, including TNF-α, IL-1β and IL-6. The activation of NF-κB in normal keratinocytes inhibits proliferation and decreases the tendency towards malignant transformation; however in immortalized keratinocytes deregulation of NF-κB can support malignant traits both in vivo and in vitro 59. The low levels of NF-κB activation found in low-grade intraepithelial lesions of cervical squamous tissue was shown by IκB-α phosphorylation, and STAT3 was activated in both the suprabasal and basal layers of the uterine cervix infected with HPV-16 60, 61. Prabhavathy and colleagues reported that HPV-16 E2 protein potentiated the activation of STAT3 and NF-κB through proinflammatory cytokines, and also decreased the E2 protein-mediated apoptotic effects in the HEK 293 cell line. They suggested that cells infected with HPV-16 E2 might have a survival advantage in the presence of chronic inflammation. This is probably a beneficial strategy for the virus life cycle, in which escape from the cell-death process encourages carcinogenesis in epithelial cells infected with HPV 62. Cell sensitivity to apoptosis mediated by TNF-α is regulated via several factors, such as NF-κB and viral infections. Activation of NF-κB plays an important role in regulating cellular sensitivity to TNF-α, by regulating the expression of multiple anti-apoptotic genes 63. Increased tumorigenicity of human keratinocytes transformed with HPV-16 was related to TNF-α resistance acquired during HPV infection 64. Prabhavathy et al studied the effects of TNF-α-mediated NF-κB activation on the senescence induced in response to E2 in HPV-16-integrated SiHa cells. They observed that E2 suppressed the expression of the endogenous E6 gene, and sensitized SiHa cells to TNF-α-induced NF-κB activation. In addition, the expression of the senescence proteins, p16, p21, p27 and p53 was increased, and senescence-associated (SA)-β-galactosidase activity demonstrated that TNF-α enhanced E2-mediated senescence. In this study, re-expression of the E2 gene after TNF-α treatment led to up-regulation of anti-apoptotic Bcl2 protein and other pro-survival genes, including cyclin D1, human telomerase reverse transcriptase (hTERT) and survivin. Concomitantly, the combination of TNF-α and E2 increased cell survival, proliferation and colony formation. Another observation showed the over-expression of key senescence factors (such as HMGA1, HMGB1, IL-16 and IL-8) was regulated by NF-κB, in cells treated with TNF-α and simultaneously transfected with E2 65.
Many studies have demonstrated that chronic inflammation, and its mediators (IL-1, IL-6, IL-8, IL-18, COX, TNF-α etc) can make a significant contribution to the progression of CC. Various studies have focused on the interaction between HPV proteins and the inflammatory pathways in HPV‐mediated cervical carcinogenesis. The pro-inflammatory cytokines, TNF-α and IL-1α are released after macrophage infiltration of tissue in response to infection or injury; these cytokines are also secreted by human cervical epithelial cells 66–68. Some studies have demonstrated that TNF-α and IL-1α have opposite effects on the growth of immortalized vs. normal cervical cells. These cytokines inhibited the proliferation of normal ectocervical or endocervical epithelial-derived cells. In contrast, both TNF-α and IL-1α stimulated the proliferation of several cervical cell lines derived from CC, or cell lines immortalized by HPV-16/18 69, 70. Woodworth et al, suggested that TNF-α and IL-1α were mitogenic for immortalized and malignant cervical epithelial cells via the epidermal growth factor receptor (EGFR)-dependent signaling pathway, and that release of cytokines may contribute to CC carcinogenesis by providing a growth advantage for the cervical cells in vivo 71.
Recent studies have shown that chronic inflammation probably interferes with cellular senescence 72, a normal phenomenon in which a cell stops dividing because of decreased telomere length. Senescent cells release a large amount of inflammatory cytokines such as IL-18, IL-6 and IL-1 73, 74, that have been reported to lead to tumorigenesis and metastasis 75, 76. A high level of IL-6 expression has been linked to cellular senescence and carcinogenesis 77, 78. Ren et al, showed that HPV-16/18 E6 protein promoted fibroblast senescence through IL-6 and STAT3 signaling. Based on the results of this study, cervical epithelial cells infected with HPV-16/18 can stimulate senescence of fibroblasts by IL-6/STAT3-regulated paracrine and autocrine signaling pathways. This alteration of the microenvironment leads to the development of cervical intraepithelial neoplasia I–II (CIN I–II), progressing to CIN III and finally to CC after a prolonged period of latency 78.
IL-10 has been found to be locally over-expressed in biopsies taken from patients with CC or premalignant lesions, and is possibly a marker of a locally immunosuppressed state 79–82. The expression of IL-10 is directly correlated to the grade of cervical lesions associated with HPV infection 83, 84. This suggests that IL-10 is expressed by cervical epithelial cells and can modulate cellular immunity and inflammation in the cervical mucosa. Bermúdez-Morales et al 85 reported that the HPV E2 protein could bind to a regulatory region of the IL-10 gene and increase the promoter activity. Furthermore, after expression of HPV-E2 protein, transformed cells also exhibited elevated levels of IL-10 mRNA after HPV infection 85. The increased expression of IL-10 might encourage virus persistence, transformation of cervical cells, and tumor development.
Researchers showed that up-regulated secretion of IL-10 could possibly suppress immune response against HPV infection in the initial cervical lesions, while up-regulated TNF-α and disrupted cytokine secretion (imbalance between Th1 & Th2 cytokines) could be responsible for the poor immune response in late stage lesions. Thus, measuring IL-10 and TNF-α in cervical secretions could be a helpful indicator of the local immune response against HPV lesions. In addition, Clerici et al. found that systemic immunosuppression (even though it was not specific to HPV infection) was characterized by lower production of IL-2, and the Th2 cytokines IL-4 and IL-10 were higher in CIN compared to normal cervix 86. Researchers suggested there was an association between higher secretion of Th2 cytokines (and lower secretion of Th1 cytokines) and the development of CIN 86. El-Sherif et al. used quantitative reverse transcription-polymerase chain reaction (qRT-PCR) to show IL-10 (Th2) was increased, while INF-γ (Th1) was decreased depending on the grade of the CIN lesions 87. Researchers used micro-dissection to measure epithelial and sub-epithelial levels of IFN-γ and IL-10 mRNAs in 11 specimens from normal cervix and 25 HPV-16 positive CIN specimans. IFN-γ mRNA was reduced in CIN compared to normal cervix (p = 0.04). The IL-10 mRNA levels in CIN were elevated compared to normal cervix. Epithelial IFN-γ mRNA showed a decline in each grade of CIN in comparison normal cervix. Sub-epithelial IFN-γ mRNA significantly declined in CIN 1, CIN 2, and CIN 3 in comparison to normal cervix. Moreover, sub-epithelial IFN-γ mRNA was lower in CIN 2 and CIN 3 as compared to CIN 1. Epithelial IL-10 was detected in just 1 out of 11 normal, and 1 out of 25 CIN specimens; however, sub-epithelial IL-10 was increased in CIN 2 and CIN 3 compared to normal cervix. In conclusion, a Th2-dominant cytokine secretion pattern (increased IL-6, IL-10 and decreased INF-γ, TNF-α) can suppress the immune response against HPV, resulting in persistent infection and lesion progression 87.
COX-2 is an inducible enzyme, which mediates many inflammatory processes. Over-expression of COX-2 is found in the pathophysiology of many types of cancer and inflammatory disorders. Recent studies have shown that NF-κB is involved in the regulation of COX-2 expression, and blocking activation of NF-κB leads to inhibition of COX-2 expression 88. The HPV E6 and E7 oncoproteins can lead to up-regulation of COX-2 in both carcinogenesis and cancer progression 88, 89. Kim and colleagues investigated the effect of HPV-16 E5 protein on COX-2 expression. Their results revealed that E5 increased COX-2 expression through the EGFR-signaling pathway, and that NF-κB and activator protein-1 (AP-1) were involved in the increase in COX-2 expression caused by E5. Thus, the E5 oncoprotein could mediate carcinogenesis of CC (at least partly) by over-expression of COX-2 89. Table 1 shows a list of HPV proteins, which are involved in inflammation and CC pathogenesis.
Table 1:
The Role of HPV Proteins in Cervical Cancer Inflammation
| HPV type | Viral protein | Target | Effect on Inflammation | Note | Ref |
|---|---|---|---|---|---|
| 16 18 |
E6 | CD40 | Induction | The expression level of CD40 was increased in E6 positive CC suggesting the presence of CD40 is related to lymph node metastasis and neovascularization. | 90 |
| 16 | E7 | IL-32 COX-2/PGE2 |
Induction | The high-risk variant of HPV promotes IL-32 expression by E7-mediated COX-2 stimulation. | 91 |
| 16 | E2 | NF-kB STAT3 |
Induction | E2 enhanced STAT3 and NF-kB gene expression and potentiated NF-kB activation via inflammatory mediators. | 92 |
| 16 | E2 | NF-kB | Induction | Re-expression of E2 combined with TNF-α in cervical cells affected NF-kB and and increased IL-6, IL-8, HMGB1, cyclin D1, hTERT, Bcl2 and survivin, promoting cell proliferation and survival. | 93 |
| 16 18 |
- | IL-1α TNF-α |
Induction | HPV virus stimulated IL-1α and TNF-α. Proinflammatory cytokines enhanced amphiregulin expression and proliferation through an EGFR-dependent pathway. | 94 |
| 16 | E6+E7 E7 |
NF-kB | Inhibition | E7 and/or E6 inhibited NF-kB activity in cervical epithelial cells and induced immortalization and cell growth | 95 |
| 16 | E2 | IL-10 | Inhibition | The expression of IL-10 was regulated by E2 protein; over-expressed IL-10 may be related to virus persistence, cancer development and cervical epithelial cell transformation. | 96 |
| 16 | E6 | IL-1β | Inhibition | E6 altered the release of IL-1β in NALP3 inflammasomes and decreased lysosomal or autophagy activity. | 97 |
| 16 | E6 E7 |
TGF-β1 | Inhibition | HPV-E6/E7 induced activation of TGF-beta1 | 98 |
| 16 | E7 | NF-kB Bp50–p65 Erα |
Inhibition | E7 protein induced TLR9 down-regulation affecting IFN responses that negatively regulate viral infection. | 99 |
| 16 | E6 E7 |
IFN-γ | - | The level of IFN-γ was significantly associated with HPV viral load | 100 |
| 16 | E5 | COX-2 | Induction | E5 protein mediated cervical carcinogenesis least in part by over-expression of COX-2 via AP-1 and NF-kB. | 101 |
| 16 18 |
E6 | STAT3 | Induction | Over-expression of HPV-E6 activated STAT3, elevated IL-6 expression and increased tumor burden in mice with ME180 and C33A tumors. | 102 |
4. The role and function of microRNAs in cervical cancer inflammation
Since the initial discovery of miRNAs in 1993, these short non-coding RNA oligonucleotides (~22 nucleotides in length) have been demonstrated to be critical regulatory elements of gene expression at post-transcriptional levels in animals, plants and humans 103. miRNAs can control various biological processes, including cell proliferation and differentiation, cell death, organ function and homeostasis 104. miRNAs repress or degrade target messenger RNA mediated by the RNA-induced silencing complex (RISC) by binding to complementary sites in the 3′ untranslated region (less commonly, in the 5′ UTR) of target mRNAs 103. They have been isolated from the body fluids, cells and tissues of many mammalian species. Around 60% of the human genome has been predicted to be regulated by miRNAs 20. The number of miRNA sequences deposited in the miRBase database is steadily increasing, so that now a single gene may be regulated by several miRNAs, and conversely a single miRNA probably targets several genes 105. In humans, the maturation of the miRNA containing transcript (pri-miRNA) is facilitated through cleavage in the nucleus, mediated by DROSHA and DGCR8 (DiGeorge syndrome critical region 8) complex. This cleavage generates an ~85 nucleotide long precursor miRNA (pre-miRNA) which is transported into the cytoplasm by exportin 5. Subsequently, the pre-miRNA is cleaved by the DICER/TRBP complex to yield a 22 nucleotide miRNA duplex. This duplex is then loaded into the RISC, where miRNAs and their mRNA targets are recruited by members of the Argonaute family. Eventually, the mature miRNA strand is preferentially retained in the functional miRISC complex and negatively regulates its target genes through promoting their degradation or inhibiting their translation 20, 103.
Even a small change in the expression of miRNAs results in dysregulation of several target genes, so it is not surprising that disruption of miRNA functions has been implicated in the pathogenesis of a broad-spectrum of human diseases, such as cancer, cardiovascular diseases, autoimmune diseases and neurological disorders 103, 106. In cancer, miRNAs may function as tumor suppressors or else as oncogenes (onco-miRNAs), based on the function of their target genes 107. The loss of function of tumor suppressor miRNAs contributes to cancer development and progression, because of their function in controlling cell cycle checkpoints and apoptotic pathways. On the other, a number of onco-miRNAs have been recognized to stimulate key steps in the tumor metastatic process 19.
In 2002, the first report of miRNAs being involved in cancer emerged, which reported down-regulation of miR-16 and miR-15 (at chromosome 13q14) in chronic lymphocytic leukemia (CLL) 108. Numerous investigations since then, using microarray methods have addressed the impact of miRNAs in several solid tumors, including hepatocellular carcinoma, colorectal, breast, lung, ovarian, genito-urinary and cervical malignancies 109. A high variability in miRNA expression has been demonstrated in CC and the next section summarizes the miRNAs that play tumor suppressive roles, or are down-regulated in CC.
Fan et al. found that miR-429 expression was down-regulated in CC tissues. They also identified IKKβ (the primary kinase mediating NF-κB activation) to be a new target of miR-429. It was shown that miR-429 down-regulation led to IKKβ/NF-κB pathway activation, IL-6 and IFN-β production and consequently enhancement of inflammation and tumor progression 110. Further studies showed that miR-101 was down-regulated in CC. COX-2 is considered to be an important functional target of miR-101 in HeLa cells. Due to the role of COX-2 in inflammation and tumor development, miR-101 is probably a tumor suppressor by reducing COX-2 and attenuating cell proliferation, invasion and inflammation 111.
It is interesting that high mobility group box 1 (HMGB1) is regulated and targeted by three different miRNAs: miR-34a, miR-1284and miR-142. HMGB1 is an oncogene that induces inflammation and facilitates tumorigenesis and metastasis in CC. There is a reverse association between miR-34a, miR-1284and miR-142 and HMBG1 expression. Therefore, down-regulation of miR-34a, miR-1284and miR-142 can enhance CC proliferation, invasion and inflammation by up-regulating HMGB1 112–114. HMGB1 expression is one cause of chemoresistance, so that miR-1284 down-regulation (a tumor suppressor in CC) increases the resistance of CC cells to cisplatin and inhibits apoptosis via HMGB1 up-regulation 113. HMGB1 plays a significant role in the lymphatic metastasis in CC patients and it has been demonstrated that down-regulation of miR-142 augments lymphatic metastasis in CC patients 114.
Other miRNAs (miR-24, miR-451, let-7a and miR-125a) have been shown to be down-regulated in CC and can induce inflammation via up-regulating their target genes. The first investigation into the relationship between miRNAs and YKL-40 (also known as chitinase-3-like protein 1) in cancer was reported in 2016 by Sun et al. YKL-40 is an inflammatory marker, which is targeted by miR-24. In their report, YKL-40 over-expression along with miR-24 under-expression were detected in CC cells and were proposed to be responsible for cell proliferation, metastasis, invasion and inflammation in CC 115. Another miRNA, miR-451, has an essential function in cell growth and differentiation. Down-regulation of miR-451 increased the expression of its target gene, IL-6R (an inflammatory cytokine) in both RKO and HeLa cells. It was discovered that miR-451 has a tumor suppressor activity by targeting IL-6R, so that its down-regulation can induce inflammation, invasion, angiogenesis and proliferation in CC cells 116.
Analysis of let-7 expression from malignant cervical tissues and in CaSki, SiHa and HeLa cell lines revealed that let-7 was markedly reduced during cervical carcinogenesis. Let-7a has been identified as a significant regulator of STAT3 levels. STAT3, as mentioned previously, plays a key role linking inflammation and CC. HPV oncoprotein E6 may directly or indirectly lead to let-7a down-regulation and promote STAT3 expression. The induction of STAT3 has an effect on the E6 upstream regulatory region. Therefore, elevated levels of E6 and STAT3 are inversely correlated with reduction of let-7a expression in HPV-positive CC lesions 117. In addition to let-7, STAT3 has been demonstrated to be a miR-125a target gene. miR-125a acts as a tumor suppressor and regulates cell growth by promoting cell cycle arrest. In order to do this, miR-125a affects G2/M checkpoint proteins and c-myc oncogene expression by binding to STAT3 3′-UTR and inhibiting its expression. Moreover, miR-125a decreases the expression of N-cadherin, matrix metalloproteinase-2 (MMP-2) and MMP-9 (which all regulate inflammation and weaken tissue barriers to facilitate invasion and metastasis) through suppression of STAT3, so that it promotes CC cell invasion and metastasis. However, down-regulation of miR-125a has been shown in CC tissues, compared to adjacent normal tissues and leads to increases in STAT3, MMP-9, MMP-2 and N-cadherin activities, inducing inflammation and eventually blocking G2/M cell cycle arrest. HPV-16 E6/E7 and HPV-18 E6/E7 proteins probably suppress the expression of miR-125a in CC cells 118. Intriguingly, STAT3 also functions as a target gene for miR‐125b in CC. It has been suggested that miR‐125b is down-regulated by Lnc-SNHG12. Due to the function of STAT3 in inducing inflammation and increasing proliferation, up-regulation of Lnc-SNHG12 indirectly leads to STAT3 activation and enhancement of proliferation, invasion and inflammation in HeLa and SiHa cells 119.
While some miRNAs act as tumor suppressors, other onco-miRNAs are typically up-regulated during tumor progression in CC. miR-21 may be implicated in the proinflammatory process in cervicitis. Diverse target genes have been identified for miR-21, including PDCD4, PTEN, tissue inhibitor of metalloproteinases-3 (TIMP-3), TNF‑ α and ANXA1 117, 120–122. miR-21 up-regulation increased IL-6 (which is involved in inflammation and tumor development) and α-SMA (a fibroblast marker) in HeLa conditioned media-treated fibroblasts, while it significantly diminished programmed cell death protein 4 (PDCD4) expression in CC tissues 120. Alternative downstream targets of miR-21 are PTEN and TIMP-3, which have key functions as STAT3 pathway and MMP-2/MMP-9 negative regulators, respectively. MMP-2 and MMP-9 are downstream target genes of the STAT3 pathway. Taking into account the under-expression of PTEN and TIMP-3 caused by up-regulated miR-21 in CC cells, the enhancement of STAT3 activity leads to MMP-2 and MMP-9 up-regulation. Up-regulation of miR-21 therefore induces inflammation and promotes invasion in CC cells 117. Another report described annexin A1 (ANXA1) as a miR-21 target gene which is reduced when miR-21 is up-regulated in CC. ANXA1 is involved in the resolution of inflammation, thus its down-regulation by miR-21 increases inflammation and proliferation in CC cells 122.
TNF‑ α is one of the most important miR-21 target genes, as a proinflammatory cytokine and a regulator of cell growth. miR-21 may cause inflammation in CC by up-regulating TNF-α 121. Interestingly, TNF-α can play both anticancer and pro-cancer roles and can be negatively regulated by miR-130a in CC. Low levels of TNF-α can stimulate NF-κB expression to up-regulate miR-130a expression. High levels of TNF-α can destroy tumors. The TNF-α/NF-κB/miR-130a feedback signaling pathway may regulate progression and inflammation in CC 123.
Recent investigations have revealed that miR-155 is over-expressed in peripheral blood and tissues from CC patients and may be involved in CC progression and inflammation by targeting SOSC1. SOCS1 inhibits inflammatory pathways such as STAT3 and promotes Th17 differentiation. The STAT3 pathway contributes to the proliferation of Th17 cells. Foxp3 and RORγt are the main transcription factors of Treg and Th17 cells respectively and are both up-regulated in the PBMC isolated from patients with CC, who also have elevated Treg and Th17 populations. On the other hand, IL-17 is a pro-inflammatory cytokine, which is induced by RORγt. Thus, these observations collectively suggest that up-regulation of miR-155 inhibits SOCS1 expression and enhances STAT3, RORγt and IL-17, leading to increased Th17 and Treg cells, resulting in more inflammation in CC 124. Likewise, up-regulation of Lnc-IL7R, an inflammation-related LncRNA, has been identified in tissue samples from CC and CIN III (cervical intraepithelial neoplasia) patients, more so than lower grades, CIN I and CIN II. Apoptosis is also reduced by up-regulation of Lnc-IL-7R in CC 125.
Surprisingly, one recent investigation demonstrated that up-regulation of miR-146a could inhibit inflammation. Over-expression of miR-146a reduces NF-κB activation via targeting TNF receptor associated factor (TRAF6) and interleukin-1 receptor-associated kinase 1 (IRAK1) in CC cells. TRAF6 and IRAK1 directly regulate NF-κB activity. In addition to the function of NF-κB in inflammation, TRAF6 and IRAK1 are implicated in acute inflammation and maintaining cell viability. Accordingly, over-expression of miR-146a inhibits inflammation and promotes cell viability in CC by down-regulating TRAF6 and IRAK1.
miRNAs are an important class of gene regulators that have recently been recognized to play key roles in both the innate and adaptive immune system. In CC, some miRNAs are under-expressed whereas other are over-expressed, implying that miRNAs can function either as tumor suppressor genes or alternatively as oncogenes (Table 2). Our knowledge about the regulation of miRNAs in CC has recently expanded and further discoveries about its role in inflammation may provide new opportunities to discover therapeutic approaches to the treatment of CC.
Table 2:
The role of non-coding RNAs in cervical cancer inflammation
| microRNA / Lnc | Expression | Target | Effect on inflammation | Type of HPV-virus | Notes | Ref |
|---|---|---|---|---|---|---|
| miR-429 | Down | IKKβ | Induction | - | The activity of NF-kB induced by the reduction of miR-429 expression led to the production of IFN-β and IL-6. | 110 |
| miR-21 | Up | PDCD4 | Induction | - | Enhancement of expression levels of IL-6 and α-SMA through up-regulation of miR-21 | 120 |
| miR-130a | UP | TNF-α | NA | - | Created a negative feedback loop between TFFFα, NF-κB, miR-130aand TNF-α. This feedback may regulate TNF-α production at low concentration and cause NF-kB to be activated leading to up-regulation of miR-130a and induced growth of CC cells |
126 |
| miR-155 | Up | SOCS1 | Induction | - | Enhancement of Th17 and Treg cells in PBMC and tissue of patients which leads to increased levels of RORγt, STAT3 and IL-17 | 127 |
| miR-101 | Down | COX-2 | Induction | - | Reduction of apoptosis in CC cells | 128 |
| miR-34a | Down | HMGB1 | Induction | - | Proliferation, migration and invasion elevated via down-regulation of miR-34a | 129 |
| miR-24 | Down | YKL-40 | Induction | - | Metastasis, invasion, angiogenesis and epithelial-mesenchymal transition of CC cells induced by over-expression of YKL-40 gene expression. | 115 |
| miR-451 | Down | IL-6R | Induction | - | Invasion, angiogenesis and cell proliferation increased by decreased miR-451 expression. | 130 |
| let-7a | Down | STAT3 | Induction | 16 18 |
Down-regulation of let-7a by E6 leads to stimulation of STAT3 expression. STAT3 has induction effects on the upstream regulatory region of the gene E6. | 131 |
| miR-21 | UP | PTEN | Induction | 16 18 |
The up-regulation of miR-21 decreased PTEN gene expression and then by up-regulation of MMP-2 and MMP-9 via STAT3 the invasion of CC cells was elevated. | 131 |
| miR-21 | UP | TNF-α | Induction | - | Increased TNF-α expression by miR-21 affects cell proliferation, which ultimately leads to CC development. | 132 |
| miR-21 | UP | ANXA1 | Induction | 16 | Down-regulation of ANXA1 by miR-21 promotes cellular proliferation. | 122 |
| miR-1284 | Down | HMGB1 | Induction | - | MiR-1284 by down-regulating HMGB1 enhances the sensitivity of CC cells to cisplatin. | 133 |
| miR-125a | Down | STAT3 | Induction | 16 18 |
E6 and E7 reduced miR-125a expression that leads to elevated of MMP-9, MMP-2and N-cadherin activities and also blocked G2/M cell cycle arrest. | 134 |
| miR-142 | Down | HMGB1 | Induction | - | MiR-142 by targeting HMGB1 suppresses the growth of CC cells. | 135 |
| miR-146a | Up | TRAF6 IRAK1 |
Inhibiton | 18 | miR-146a by targeting TRAF6 and IRAK1 increases viability of CC cells. | 136 |
| Lnc-SNHG12 | Up | miR–125b | Induction | - | miR-125b was down-regulated by Lnc- SNHG12 and led to STAT3 over-expression which led to enhancement of migration, proliferation and invasion in CC. | 137 |
5. The relationship between exosomes, inflammation and cervical cancer
At the present time, there have been reports of this exchange of information by exosomes, involving interactions between tumor and stromal cells, immune cells and immune cells, or between virally infected cells and interferon secreting cells 26. Identification of the macromolecular components of exosomes originating from cancer or infected cells and their direct or indirect role in inflammation, modulation of immune response and angiogenesis is an important task facing researchers.
A relationship between tumorigenesis and the synthesis, release, and function of extra-cellular vesicles (EVs) has been reported, and the contribution of EVs to HPV-induced malignancy pathogenesis is summarized in Table 3. The first confirmation of the contribution of EVs in HPV pathogenesis appeared in 2009, when the presence of extra-cellular survivin in HPV-18 positive HeLa cells was confirmed 138. Cell medium containing survivin showed anti-apoptotic, pro-proliferative, and metastatic properties based on an inactive T34A mutant 138. The same authors then showed the presence of extra-cellular survivin within exosomes. They found that proton irradiation of the cells led to the synthesis and release of the exosomes 139. Survivin-positive exosomes were further examined to detect stress-induced proteins within the cargo. In this study inhibitor of apoptosis proteins (IAPs), including XIAP, c-IAP1, c-IAP2 and livin/ML-IAP were demonstrated 140, 141. The presence of IAPs was dependent on HPV oncoproteins, because the exosomes derived from HeLa cells in which the E6- and E7-proteins had been silenced had less IAPs, although these silenced cells showed greater overall exosome secretion compared to control cells 140. The cargo contents of HeLa-derived survivin-positive exosomes were examined for levels of miRNAs 142. Overall different 52 miRNAs were de-regulated, and E6/E7 silencing influenced the expression of 23 of these. A majority of the up-regulated miRNAs played antiapoptotic, pro-proliferative, and anti-senescence roles. Down-regulated miRNAs had the opposite functions. Since 11 of 46 miRNAs within the exosomes were not de-regulated in the whole cells, this implies the presence of mechanisms to incorporate specific miRNAs into the exosomes. The HPV-16-positive cell line SiHa with was also examined with similar results. This suggests that deregulation of miRNA expression is not specific to HPV genotype 142. Examination of the miRNAs in exosomes derived from primary keratinocytes transduced with E6 and E7 from HPV-16 or HPV-38 confirmed Honegger et al.’s findings, and they also showed that these miRNAs could be transferred to non-infected keratinocytes 143. Harden and Muller focused on a panel of tumor-related miRNAs, and found corresponding expression profiles between cell-derived and exosome-derived miRNAs144. Other authors have shown the presence of long non-coding RNAs (lncRNAs) within HeLa-derived exosomes, specifically lincRNA-p21, CCNDA1-ncRNA, HOTAIR, TUG1, and GAS5 145. It should be noted that lincRNA-p21 (an inhibitory factor of p53-dependent transcription) was the lncRNA with the highest over-expression in exosomes compared to parental cells. The horizontal transfer of lincRNA-p21 can affect gene expression in acceptor cells 146.
As described above, inflammation plays a critical role in cancer progression and immune response 37. Many studies have shown that exosomes play a critical role in the inflammatory microenvironment in tumors 147. For example, Wu et al investigated the biological effect of exosomes derived from gastric cancer cells in macrophage activation. They showed that these exosomes led to cancer progression by triggering the activation of the NF-κB pathway 30. In many tumors, the NF-κB signaling pathway is constitutively activated and recently it has been shown that palmitoylated proteins present on the surface of exosomes derived from breast cancer cells were involved in the activation of this pathway. Through this mechanism, breast cancer exosomes could stimulate macrophages to release pro-inflammatory mediators such as TNF-α, IL-6, CCL2 and GCSF 148.
Recently, it was reported that exosomes derived from bacteria-infected macrophages had proinflammatory properties 148. Essandoh et al examined the effect of blocking exosome production on protection against sepsis (the inflammatory response triggered by bacteria. They showed that inhibition of the exosome generation process limited the sepsis-induced inflammatory response 148. Exosomes originating from HPV-infected cells were also able to carry out horizontal transfer of mRNA, miRNA and cytokines between cells. Therefore, exosomes probably play an immunomodulatory role in the CC microenvironment. The proinflammatory cytokine, IL-36γ, has the ability to induce inflammation in keratinocytes via the Wnt signaling pathway 149, 150. Rana and colleagues demonstrated the presence of IL-36γ inside exosomes derived from poly(I:C)-treated keratinocytes. Previous studies have shown that HPV-16 inhibited the poly(I:C)-stimulated expression of some pro-inflammatory genes. It is therefore conceivable that IL-36γ inside exosomes is inhibited by HPV 151. In accordance with this hypothesis, the deregulated expression of mRNAs for several proinflammatory chemokines and cytokines was measured in E7/E6- transduced keratinocytes.
6. Use of microRNAs and exosomes for treatment of HPV-positive cervical cancer
Determining which miRNAs have relationships with different kinds of HPV-mediated cancers is of high importance, because they could be used as HPV-specific biomarkers. Additionally, it is possible that further individualized treatment approaches and novel targeted treatments could be developed. A majority of HPV-positive cancers show differential expression of miRNAs measured in body fluids and or in tumor tissue. Our knowledge of miRNA dysregulation in HPV positive cancers and how this affects their respective target genes, is steadily expanding. For instance, expression of the miR-17–92 cluster had a close relationship with the persistent endogenous expression of virally encoded genes 142, which implies that HPV-16 viral integration may interfere with the blockage of miR-17/92 cluster expression. Anti-miRNA therapy or miRNA replacement therapy, and some alternative/complementary natural product-based therapies could be used to target such miRNAs, and therefore tackle HPV-related cancers.
Some miRNAs possess tumor inhibitory functions. miRNA down-regulation in HPV infected cancer cells has been examined in terms of potential treatment possibilities. Badder revealed the contribution of miR-34a to the repression of oncogenic transformation 152. miR-34a is a tumor suppressing factor, which increases the survival of abnormally transformed cells and leads to G1/G2 cell cycle arrest 89. Additionally, Wang et al. showed the down-regulation of miR-34a in HPV positive cancers 153. Thus, replacing miR-34a may be a possible treatment for HPV-positive cancers 154. Ibrahim et al. showed that miR-143/145 could act as anti-oncogenic miRNAs against colon cancer 155. Recent research has shown a correlation between the down-regulation of miR-34a or miR-125 and the invasiveness of cervical cancer in HPV infected patients 156. miRNA-based therapies depend on administering miRNA mimetics that may effectively compensate for the missing tumor inhibitory or cell cycle regulatory functions of miRNAs in normal cells. However up to now, only a few tumor inhibitory miRNAs (such as miR-34a, miR-143/ and miR-14593) have been proposed as treatment options. These miRNAs would require an efficient drug delivery approach. Another limitation may be non-specific or off-target effects, which could affect the treatment efficacy.
In common with conventional gene therapy, the delivery of miRNA mimetics requires an efficient vector system for delivering the intended gene. A variety of nanoparticles (NPs) have been investigated as gene delivery vehicles. These may be administered by intratumoral injection or systematically via parenteral injection. Chen et al. and Wiggins et al. explored the possible use of NPs for miRNA delivery using intra-venous tail vein injections in mouse models 157, 158. Effective delivery of miR-34a mimetics loaded into poly-cationic liposome-hyaluronic acid based NPs conjugated to a modified GC4 single chain anti-body fragment was tested in mice 155. As more candidate miRNA mimetics emerge, the rational for miRNA replacement treatment is gradually advancing from bench to bedside. miR-34a mimetics have already been tested in clinical trials, and other candidates are being proposed 154. Therefore, miR-34a, miR-143/145, miR-125, and other anti-oncogenic miRNAs would be candidates for miRNA replacement therapy against HPV-related cancers.
Exosomes are being increasingly examined as delivery vehicles for small molecule drugs, and large biological medications and in some disease situations. Exosomes have advantages of stability, bio-compatibility, low immunogenicity and the ability to be externally loaded with a cargo 159. Exosomes have acceptable stability for storage and shipping, which are basic features required for drug delivery vehicles, and represent a cell-free and manageable system. Recently, researchers have explored exosomes for the delivery of siRNAs, miRNAs, shRNAs, and antiinflammatory or anti-cancer drugs such as curcumin, paclitaxel or doxorubicin 159. Studies in a mouse model showed that exosomes with HPV-specific activity could be helpful in cervical cancer immunotherapy 160. Proteins expressed on the surface membrane of exosomes, could be used as carriers to deliver the remaining proteins within the exosomes. This applies for the HIV-1 negative regulatory factor (Nef) protein. A naturally defective Nef mutant (Nefmut) of HIV-1 virus, expresses a Nef variant that accumulates in an abnormal amount in the lipid-raft membrane of exosomes, in comparison with the wild-type protein 161. This 27 kDa Nefmut (both myristylated and palmitoylated) has been explored as a carrier for additional proteins (up to 630 amino acids long) when conjugated to its COOH terminus. These fusion products may be incorporated into genome-free viral particles of HIV-1, which could function as a vaccine, and also into exosomes derived from cells transfected with DNA constructs 162. HPV-16-E7 and HPV-16-E6 tumor-specific antigens have been conjugated to Nefmut to develop a therapeutic vaccine against HPV-16-associated tumors. These antigens were expressed in HEK 293 T cells where they were incorporated into exosomes anchored to the membrane by Nefmut. Mice bearing subcutaneous HPV-16-specific tumors, which were immunized with recombinant Nefmut-E7 or Nefmut-E6 exosomes, generated a cell-mediated immune response with the ability to block the growth and reduce the tumor burden in mice that had been challenged by injection of TC-1 tumor cells 163, 164. However, the production of recombinant exosomes for therapeutic vaccine development is a multi-step procedure, whose scale-up may be difficult. Nefmut expression in exosomes has been achieved using Nefmut-E7 or Nefmut-E6 DNA plasmids in vitro and in vivo. The findings provided support for an HPV-16 therapeutic vaccine 165. Cross-presentation of Nef-fused antigens to T-cells, showed that the exosomes were taken up by dendritic cells as a basis for CTL vaccines 166. Nefmut has been examined to deliver intra-cellular anti-bodies that recognize the HPV-E6 and HPV-E7 oncoproteins. Previous studies had shown that single-chain anti-HPV-16-E6 and anti-HPV-16-E7 antibodies had anti-proliferative activities against HPV-positive cells in vitro, and therapeutic efficiency against HPV-positive tumors in animal models 167. Initial testing showed that the exosomes derived from cells transfected with plasmids expressing an Nefmutanti-16E7 scFv fusion protein, contained the antibody in an active E7-binding conformation which performed the same as scFv delivered as DNA or protein 167, 168. These antibodycontaining exosmes could be used to treat lesions situated not only on the cervix, but also on the anogenital region or in the oropharynx. Although these findings are promising, more widespread clinical application will require improved standardized techniques to purify the exosomes and monitor the exosomal contents.
7. Conclusions
In addition to the known role of persistent infection with high-risk HPV sub-types, long-term chronic inflammation is another important cause of the development of invasive CC. The role of chronic inflammation involves the participation of cytokines, chemokines, cell growth and survival factors, reactive oxygen/nitrogen species and other mediators including metalloproteinase, prostaglandins, COX2 and finally specific types of miRNAs. The collective effects of these mediators, promotes alterations in proliferation, cell death, senescence and also, mutation and angiogenesis. All these factors operating together may contribute to the progression of HPV-induced CC. Persistent infection with high-risk HPV leads to the integration of HPV-DNA into the hosts genome, producing the over-expression of the viral oncogenes E6 and E7. Accumulating evidence suggests that the expression levels of E6/E7 are increased in cervical inflammation and therefore may play a crucial role in HPV carcinogenesis. Although it is not clear precisely which miRNAs could link inflammation with CC, more mechanistic studies are required to elucidate the contribution of miRNAs to the inflammatory tumor microenvironment and cervical carcinogenesis. It is known that miRNAs are implicated in the regulation of both adaptive and innate immune responses and modulate inflammatory networks in several cells and tissue types. Furthermore, the steadily increasing interest and number of publications on the role of exosomes, suggests that this research field will continue to grow. Current knowledge about the role of exosomes in human health and disease is complex, but is still rapidly expanding. Analysis of exosomes can provide information about the state of their parental cells and exosomes are being investigated as biomarkers for prognosis or diagnosis of diseases. Based on the precise exosome contents, they can exhibit both anti-inflammatory and pro-inflammatory effects. The role of exosomes has been studied in the onset, mediation and treatment of several inflammatory diseases, however up to the present time, there have been few studies on their role in inflammation in CC. Considering the multifunctional role of exosomes, further investigation is required to analyze the exosome contents in CC, and to understand how exosomes mediate the process of inflammation in CC.
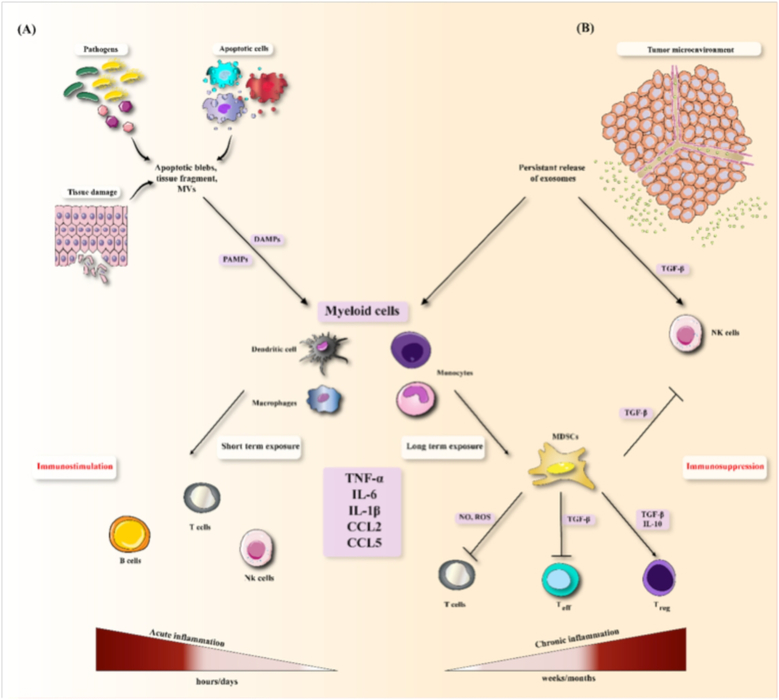
Figure 3. Immunomodulatory effects of exosomes via exposure to inflammatory mediators.
(A) Molecular patterns released by tissue damage, apoptotic cells and pathogens stimulate cytokine generation in myeloid cells. Short-term exposure to these factors results in induction of adaptive immune responses (immmunostimulation) and acute inflammation. (B) Exosomes released from the tumor microenvironment result in persistent induction of myeloid cells that secrete similar inflammatory factors (chronic inflammation).
Acknowledgments
Funding
MRH was supported by US NIH Grants R01AI050875 and R21AI121700.
Abbreviations
- 3′UTR
three prime untranslated region
- ANXA1
annexin A1
- BCL-xL
B-cell lymphoma-extra large
- BCL2
B-cell lymphoma 2
- CC
cervical cancer
- CCAR2
cell cycle and apoptosis regulator 2
- CCL2
C-C motif chemokine ligand 2
- CDK2
cyclin-dependent kinase 2
- cIAP
cellular inhibitors of apoptosis 1
- CIN
cervical intraepithelial neoplasia
- COX2
cyclooxygenase 2
- CRI
cancer-related inflammation
- CTL
cytotoxic T cell
- DAMPs
damage-associated molecular patterns
- DCs
dendritic cells
- DGCR8
DiGeorge syndrome critical region 8
- DICER
endoribonuclease Dicer with RNase motif
- DROSHA
Drosophila ribonuclease III human
- EGFR
pidermal growth factor receptor
- Evs
extra-cellular vesicles
- FOXP3
forkhead box P3
- GCSF
granulocyte colony stimulating factor
- HMGA1
high mobility group AT-hook 1
- HMGB1
high mobility group box protein 1
- HPV
human papilloma virus
- hTERT
human telomerase reverse transcriptase
- HVB
hepatitis virus B
- ICAM
intercellular adhesion molecule
- IFN-α
interferon alpha
- IKKβ
inhibitor of NFkB kinase beta
- IL
interleukin
- IRAK1
interleukin-1 receptor-associated kinase 1
- Let-7a
lethal 7a
- lncRNAs
long non-coding RNAs
- LSIL
low-grade squamous intraepithelial lesion
- MCP1
macrophage chemoattractant protein 1
- miRNA
micro ribonucleic acid
- MMP
matrix metalloproteinase
- Nef
HIV-1 negative regulatory factor
- NF-κB
nuclear factor kappa light chain enhancer of activated B cells
- NPs
nanoparticles
- PAMPs
pathogen-associated molecular patterns
- PBMC
peripheral blood mononuclear cells
- PDCD4L
programmed cell death 4
- poly(I:C)
polyinosinic:polycytidylic acid
- PRRs
pattern recognition receptors
- PTEN
phosphatase and tensin homolog
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- RISC
RNA-induced silencing complex
- RORγt
RAR-related orphan receptor gamma
- SA-β-gal
senescence-associated-β-galactosidase
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SNP
single nucleotide polymorphism
- STAT3
signal transducer and activator of transcription 3
- TGF-β
transforming growth factor beta
- Th2
T-helper lymphocytes type 2
- TIMP
tissue inhibitor of metalloproteinase
- TLR4
toll like receptor 4
- TNF-α
tumor necrosis factor alpha
- TRAF6
TNF receptor associated factor
- TRBP
HIV-1 TAR RNA binding protein
- Treg
regulatory T cells
- VEGF
vascular endothelial growth factor
- Wnt
Wingless/integrase 1
- XIAP
X-linked inhibitor of apoptosis protein
- YKL-40
chitinase-3-like protein 1
Footnotes
Conflict of interest
MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE.
8. References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018;68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Nahand JS, Taghizadeh-Boroujeni S, Karimzadeh M, Borran S, Pourhanifeh MH, Moghoofei M, Bokharaei-Salim F, Karampoor S, Jafari A, Asemi Z, Tbibzadeh A, Namdar A, et al. microRNAs: New prognostic, diagnostic, and therapeutic biomarkers in cervical cancer 2019. [DOI] [PubMed] [Google Scholar]
- 3.Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017;123: 2219–29. [DOI] [PubMed] [Google Scholar]
- 4.Shafabakhsh R, Pourhanifeh MH, Mirzaei HR, Sahebkar A, Asemi Z, Mirzaei H. Targeting regulatory T cells by curcumin: A potential for cancer immunotherapy. Pharmacological research 2019;147: 104353. [DOI] [PubMed] [Google Scholar]
- 5.Sales KJ, Katz AA. Inflammatory pathways in cervical cancer-the University of Cape Town’s contribution. SAMJ: South African Medical Journal 2012;102: 493–6. [DOI] [PubMed] [Google Scholar]
- 6.Stanley M, Pett M, Coleman N. HPV: from infection to cancer: Portland Press Limited, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Kemp TJ, Hildesheim A, García-Piñeres A, Williams MC, Shearer GM, Rodriguez AC, Schiffman M, Burk R, Freer E, Bonilla J. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiology and Prevention Biomarkers 2010;19: 1954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yost S, Hoekstra A. Cervical cancer in women over 65: An analysis of screening. Gynecologic oncology reports 2018;25: 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clinical and experimental immunology 2007;147: 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes JV, Fernandes TAAdM, De Azevedo JCV, Cobucci RNO, De Carvalho MGF, Andrade VS, De Araujo JMG. Link between chronic inflammation and human papillomavirus-induced carcinogenesis. Oncology letters 2015;9: 1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454: 428. [DOI] [PubMed] [Google Scholar]
- 12.Mulvihill N, Foley J. Inflammation in acute coronary syndromes. heart 2002;87: 201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khodabandehlou N, Mostafaei S, Etemadi A, Ghasemi A, Payandeh M, Hadifar S, Norooznezhad AH, Kazemnejad A, Moghoofei M. Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC cancer 2019;19: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghoofei M, Mostafaei S, Nesaei A, Etemadi A, Sadri Nahand J, Mirzaei H, Rashidi B, Babaei F, Khodabandehlou N. Epstein–Barr virus and thyroid cancer: The role of viral expressed proteins. Journal of cellular physiology 2019;234: 3790–9. [DOI] [PubMed] [Google Scholar]
- 15.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nature reviews Clinical oncology 2015;12: 584. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Han Y, Song C, Wei H, Chen Y, Huang K, Li S, Ma D, Wang S, Wang J. Systematic review and meta-analysis of the prognostic significance of microRNAs in cervical cancer. Oncotarget 2018;9: 17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaweed M, Hartmann P, Geddes D, Kakulas F. MicroRNAs in breastmilk and the lactating breast: potential immunoprotectors and developmental regulators for the infant and the mother. International journal of environmental research and public health 2015;12: 13981–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer MicroRNA cancer regulationed.: Springer, 2013: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman S, Ambros VR. MicroRNAs: from basic science to disease biologyed.: Cambridge University Press, 2008. [Google Scholar]
- 20.Laffont B, Rayner KJ. MicroRNAs in the pathobiology and therapy of atherosclerosis. Canadian Journal of Cardiology 2017;33: 313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson-Moncada J, Papavasiliou FN, Tam W. MicroRNAs of the immune system: roles in inflammation and cancer. Annals of the New York Academy of Sciences 2010;1183: 183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardini B, De Maria D, Francavilla A, Di Gaetano C, Ronco G, Naccarati A. MicroRNAs as markers of progression in cervical cancer: a systematic review. BMC cancer 2018;18: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PloS one 2017;12: e0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alenquer M, Amorim M. Exosome biogenesis, regulation, and function in viral infection. Viruses 2015;7: 5066–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Rai AJ, DeCastro GJ, Zeringer E, Barta T, Magdaleno S, Setterquist R, Vlassov AV. An optimized procedure for exosome isolation and analysis using serum samples: application to cancer biomarker discovery. Methods 2015;87: 26–30. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nature immunology 2013;14: 793. [DOI] [PubMed] [Google Scholar]
- 27.Mirzaei H, Sahebkar A, Jaafari MR, Goodarzi M, Mirzaei HR. Diagnostic and Therapeutic Potential of Exosomes in Cancer: The Beginning of a New Tale? Journal of cellular physiology 2017;232: 3251–60. [DOI] [PubMed] [Google Scholar]
- 28.Chan BD, Wong WY, Lee MML, Cho WCS, Yee BK, Kwan YW, Tai WCS. Exosomes in inflammation and inflammatory disease. Proteomics 2019: 1800149. [DOI] [PubMed] [Google Scholar]
- 29.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci Rep 2014;4: 5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun Y, Pan Z, Qian H, Xu W. Exosomes derived from gastric cancer cells activate NF-κB pathway in macrophages to promote cancer progression. Tumor Biology 2016;37: 12169–80. [DOI] [PubMed] [Google Scholar]
- 31.Abudukelimu A, Barberis M, Redegeld FA, Sahin N, Westerhoff HV. Predictable irreversible switching between acute and chronic inflammation. Frontiers in immunology 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nature Reviews Immunology 2017;17: 248. [DOI] [PubMed] [Google Scholar]
- 33.Qu X, Tang Y, Hua S. Immunological Approaches towards Cancer and Inflammation: A Cross Talk. Frontiers in immunology 2018;9: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nature Reviews Immunology 2018. [DOI] [PubMed] [Google Scholar]
- 35.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The lancet 2001;357: 539–45. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454: 436. [DOI] [PubMed] [Google Scholar]
- 37.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140: 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castle PE, Giuliano AR. Chapter 4: Genital tract infections, cervical inflammation, and antioxidant nutrients—assessing their roles as human papillomavirus cofactors. Jnci Monographs 2003;2003: 29–34. [DOI] [PubMed] [Google Scholar]
- 39.Shacter E, Weitzman SA. Chronic inflammation and cancer. ONCOLOGY-WILLISTON PARK THEN HUNTINGTON- 2002;16: 217–29. [PubMed] [Google Scholar]
- 40.Taniguchi K, Karin M: IL-6 and related cytokines as the critical lynchpins between inflammation and cancer Seminars in immunology 2014; 26:54–74. [DOI] [PubMed] [Google Scholar]
- 41.Zeng L, Zhen Y, Chen Y, Zou L, Zhang Y, Hu F, Feng J, Shen J, Wei B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF‑κB/COX‑2‑caspase-1 pathway in HeLa cervical cancer cells. International journal of oncology 2014;45: 1929–36. [DOI] [PubMed] [Google Scholar]
- 42.Yang X, Chen GT, Wang YQ, Xian S, Zhang L, Zhu SM, Pan F, Cheng YX. TLR4 promotes the expression of HIF-1α by triggering reactive oxygen species in cervical cancer cells in vitro-implications for therapeutic intervention. Molecular medicine reports 2018;17: 2229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JM, An J. Cytokines, inflammation, and pain. International anesthesiology clinics 2007;45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nature Reviews Cancer 2002;2: 59. [DOI] [PubMed] [Google Scholar]
- 45.Paradkar PH, Joshi JV, Mertia PN, Agashe SV, Vaidya RA. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pacific journal of cancer prevention : APJCP 2014;15: 3851–64. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Zhang J, Cui ZM, Zhao J, Zheng Y. Expression of the CXCL12/CXCR4 and CXCL16/CXCR6 axes in cervical intraepithelial neoplasia and cervical cancer. Chinese journal of cancer 2013;32: 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayshree RS, Sreenivas A, Tessy M, Krishna S. Cell intrinsic & extrinsic factors in cervical carcinogenesis. The Indian journal of medical research 2009;130: 286–95. [PubMed] [Google Scholar]
- 48.Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, MartínezBarnetche J, Cortina-Ceballos B, López-Estrada G, Delgado-Romero K, Burguete-García AI, Cantú D. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PloS one 2016;11: e0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A. Role of tumor-derived proinflammatory cytokines GM‐CSF, TNF‐α, and IL‐12 in the migration and differentiation of antigen-presenting cells in cervical carcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society 2007;109: 556–65. [DOI] [PubMed] [Google Scholar]
- 50.Ding B, Fu S, Wang M, Yue C, Wang W, Zhou D, Zhang Z, Han S. Tumor Necrosis Factor α−308 G> A polymorphisms and cervical cancer risk: a meta-analysis. International Journal of Gynecological Cancer 2012;22: 213–9. [DOI] [PubMed] [Google Scholar]
- 51.Feng Q, Wei H, Morihara J, Stern J, Yu M, Kiviat N, Hellstrom I, Hellstrom KE. Th2 type inflammation promotes the gradual progression of HPV-infected cervical cells to cervical carcinoma. Gynecologic oncology 2012;127: 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim W, Pyo J, Noh B-J, Jeong J-W, Lee J, Kim J-E. CCAR2 negatively regulates IL-8 production in cervical cancer cells. Oncotarget 2018;9: 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Han S, Wang S, Zhou X, Zhang M, Dong J, Shi X, Qian N, Wang X, Wei Q. Interactions of IL-12A and IL-12B polymorphisms on the risk of cervical cancer in Chinese women. Clinical Cancer Research 2009;15: 400–5. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes JV, DEMF TA, DEA JC, Cobucci RN, DEC MG, Andrade VS, DEA JM. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncology letters 2015;9: 1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124: 783–801. [DOI] [PubMed] [Google Scholar]
- 56.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical microbiology reviews 2009;22: 240–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedman R, Hughes AL. Molecular evolution of the NF-κB signaling system. Immunogenetics 2002;53: 964–74. [DOI] [PubMed] [Google Scholar]
- 58.DA COSTA RMG, Bastos MM, Medeiros R, Oliveira PA. The NFκB signaling pathway in papillomavirus-induced lesions: friend or Foe? Anticancer research 2016;36: 2073–83. [PubMed] [Google Scholar]
- 59.Ren Q, Kari C, Quadros MR, Burd R, McCue P, Dicker AP, Rodeck U. Malignant transformation of immortalized HaCaT keratinocytes through deregulated nuclear factor κB signaling. Cancer research 2006;66: 5209–15. [DOI] [PubMed] [Google Scholar]
- 60.Shukla S, Shishodia G, Mahata S, Hedau S, Pandey A, Bhambhani S, Batra S, Basir SF, Das BC, Bharti AC. Aberrant expression and constitutive activation of STAT3 in cervical carcinogenesis: implications in high-risk human papillomavirus infection. Molecular cancer 2010;9: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-κB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene 2003;22: 50. [DOI] [PubMed] [Google Scholar]
- 62.Prabhavathy D, Vijayalakshmi R, Kanchana MP, Karunagaran D. HPV16 E2 enhances the expression of NF-κB and STAT3 target genes and potentiates NF-κB activation by inflammatory mediators. Cellular immunology 2014;292: 70–7. [DOI] [PubMed] [Google Scholar]
- 63.Klefstrom J, Arighi E, Littlewood T, Jäättelä M, Saksela E, Evan GI, Alitalo K. Induction of TNF‐sensitive cellular phenotype by c‐Myc involves p53 and impaired NF‐κB activation. The EMBO journal 1997;16: 7382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu ZH, Miyamoto S. Induction of a pro-apoptotic ATM–NF‐κB pathway and its repression by ATR in response to replication stress. The EMBO journal 2008;27: 1963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prabhavathy D, Subramanian CK, Karunagaran D. Re-expression of HPV16 E2 in SiHa (human cervical cancer) cells potentiates NF-κB activation induced by TNF-α concurrently increasing senescence and survival. Bioscience reports 2015;35: e00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. New England Journal of Medicine 1993;328: 106–13. [DOI] [PubMed] [Google Scholar]
- 67.Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annual review of cell biology 1993;9: 317–43. [DOI] [PubMed] [Google Scholar]
- 68.Pollard JW. Lymphohematopoietic cytokines in the female reproductive tract. Current opinion in immunology 1991;3: 772–7. [DOI] [PubMed] [Google Scholar]
- 69.Schmauz R, Okong P, De Villiers EM, Dennin R, Brade L, Lwanga S, Owor R. Multiple infections in cases of cervical cancer from a high-incidence area in tropical Africa. International Journal of Cancer 1989;43: 805–9. [DOI] [PubMed] [Google Scholar]
- 70.Koutsky LA, Holmes KK, Critchlow CW, Stevens CE, Paavonen J, Beckmann AM, DeRouen TA, Galloway DA, Vernon D, Kiviat NB. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. New England journal of medicine 1992;327: 1272–8. [DOI] [PubMed] [Google Scholar]
- 71.Craig D, Woodwor T, Mcmullin E. Interleukin la and tumor necrosis factor a stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells. Proc Natl Acad Sci USA 1995;92: 2840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer cell 2010;18: 448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008;133: 1019–31. [DOI] [PubMed] [Google Scholar]
- 74.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proceedings of the National Academy of Sciences 2009;106: 17031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X-J, Peng L-X, Shao J-Y, Lu W-H, Zhang J-X, Chen S, Chen Z-Y, Xiang Y-Q, Bao Y-N, Zheng F-J. As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial–mesenchymal transition and activation of AKT signaling. Carcinogenesis 2012;33: 1302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee Y, Choi I, Ning Y, Kim N, Khatchadourian V, Yang D, Chung H, Choi D, LaBonte M, Ladner R. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. British journal of cancer 2012;106: 1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kojima H, Kunimoto H, Inoue T, Nakajima K. The STAT3-IGFBP5 axis is critical for IL-6/gp130-induced premature senescence in human fibroblasts. Cell Cycle 2012;11: 730–9. [DOI] [PubMed] [Google Scholar]
- 78.Ren C, Cheng X, Lu B, Yang G. Activation of interleukin-6/signal transducer and activator of transcription 3 by human papillomavirus early proteins 6 induces fibroblast senescence to promote cervical tumourigenesis through autocrine and paracrine pathways in tumour microenvironment. European Journal of Cancer 2013;49: 3889–99. [DOI] [PubMed] [Google Scholar]
- 79.Azar KK, Tani M, Yasuda H, Sakai A, Inoue M, Sasagawa T. Increased secretion patterns of interleukin-10 and tumor necrosis factor-alpha in cervical squamous intraepithelial lesions. Human pathology 2004;35: 1376–84. [DOI] [PubMed] [Google Scholar]
- 80.Bhairavabhotla RK, Verma V, Tongaonkar H, Shastri S, Dinshaw K, Chiplunkar S. Role of IL-10 in immune suppression in cervical cancer 2007. [PubMed]
- 81.Alcocer-González JM, Berumen J, Taméz-Guerra R, Bermúdez-Morales V, Peralta-Zaragoza O, Hernández-Pando R, Moreno J, Gariglio P, Madrid-Marina V. In vivo expression of immunosuppressive cytokines in human papillomavirus-transformed cervical cancer cells. Viral immunology 2006;19: 481–91. [DOI] [PubMed] [Google Scholar]
- 82.M El‐Sherif A, Seth R, J Tighe P, Jenkins D. Quantitative analysis of IL‐10 and IFN‐γ mRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. The Journal of pathology 2001;195: 179–85. [DOI] [PubMed] [Google Scholar]
- 83.Bermudez-Morales VH, Gutiérrez LX, Alcocer-González JM, Burguete A, Madrid-Marina V. Correlation between IL-10 gene expression and HPV infection in cervical cancer: a mechanism for immune response escape. Cancer investigation 2008;26: 1037–43. [DOI] [PubMed] [Google Scholar]
- 84.Torres-Poveda K, Bahena-Román M, Madrid-González C, Burguete-García AI, Bermúdez-Morales VH, Peralta-Zaragoza O, Madrid-Marina V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World journal of clinical oncology 2014;5: 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bermúdez-Morales V, Peralta-Zaragoza O, Alcocer‑González J, Moreno J, Madrid-Marina V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Molecular medicine reports 2011;4: 369–75. [DOI] [PubMed] [Google Scholar]
- 86.Clerici M, Merola M, Ferrario E, Trabattoni D, Villa ML, Stefanon B, Venzon DJ, Shearer GM, De Palo G, Clerici E. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. Journal of the National Cancer Institute 1997;89: 245–50. [DOI] [PubMed] [Google Scholar]
- 87.El-Sherif AM, Seth R, Tighe PJ, Jenkins D. Quantitative analysis of IL-10 and IFN-gamma mRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. The Journal of pathology 2001;195: 179–85. [DOI] [PubMed] [Google Scholar]
- 88.Surh Y-J, Chun K-S, Cha H-H, Han SS, Keum Y-S, Park K-K, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2001;480: 243–68. [DOI] [PubMed] [Google Scholar]
- 89.Kim S-H, Oh J-M, No J-H, Bang Y-J, Juhnn Y-S, Song Y-S. Involvement of NF-κB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis 2009;30: 753–7. [DOI] [PubMed] [Google Scholar]
- 90.Huang Q, Qu QX, Xie F, Zhang T, Hu JM, Chen YG, Zhang XG. CD40 is overexpressed by HPV16/18-E6 positive cervical carcinoma and correlated with clinical parameters and vascular density. Cancer epidemiology 2011;35: 388–92. [DOI] [PubMed] [Google Scholar]
- 91.Lee S, Kim JH, Kim H, Kang JW, Kim SH, Yang Y, Kim J, Park J, Park S, Hong J, Yoon DY. Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene. Immunology 2011;132: 410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prabhavathy D, Vijayalakshmi R, Kanchana MP, Karunagaran D. HPV16 E2 enhances the expression of NF-kappaB and STAT3 target genes and potentiates NF-kappaB activation by inflammatory mediators. Cellular immunology 2014;292: 70–7. [DOI] [PubMed] [Google Scholar]
- 93.Prabhavathy D, Subramanian CK, Karunagaran D. Re-expression of HPV16 E2 in SiHa (human cervical cancer) cells potentiates NF-kappaB activation induced by TNF-alpha concurrently increasing senescence and survival. Bioscience reports 2015;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woodworth CD, McMullin E, Iglesias M, Plowman GD. Interleukin 1 alpha and tumor necrosis factor alpha stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells. Proceedings of the National Academy of Sciences of the United States of America 1995;92: 2840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vandermark ER, Deluca KA, Gardner CR, Marker DF, Schreiner CN, Strickland DA, Wilton KM, Mondal S, Woodworth CD. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology 2012;425: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bermudez-Morales VH, Peralta-Zaragoza O, Alcocer-Gonzalez JM, Moreno J, Madrid-Marina V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Molecular medicine reports 2011;4: 369–75. [DOI] [PubMed] [Google Scholar]
- 97.Niebler M, Qian X, Hofler D, Kogosov V, Kaewprag J, Kaufmann AM, Ly R, Bohmer G, Zawatzky R, Rosl F, Rincon-Orozco B. Post-translational control of IL-1beta via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS pathogens 2013;9: e1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peralta-Zaragoza O, Bermudez-Morales V, Gutierrez-Xicotencatl L, Alcocer-Gonzalez J, Recillas-Targa F, Madrid-Marina V. E6 and E7 oncoproteins from human papillomavirus type 16 induce activation of human transforming growth factor beta1 promoter throughout Sp1 recognition sequence. Viral immunology 2006;19: 468–80. [DOI] [PubMed] [Google Scholar]
- 99.Hasan UA, Zannetti C, Parroche P, Goutagny N, Malfroy M, Roblot G, Carreira C, Hussain I, Muller M, Taylor-Papadimitriou J, Picard D, Sylla BS, et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. The Journal of experimental medicine 2013;210: 1369–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song SH, Lee JK, Seok OS, Saw HS. The relationship between cytokines and HPV-16, HPV-16 E6, E7, and high-risk HPV viral load in the uterine cervix. Gynecologic oncology 2007;104: 732–8. [DOI] [PubMed] [Google Scholar]
- 101.Kim SH, Oh JM, No JH, Bang YJ, Juhnn YS, Song YS. Involvement of NF-kappaB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis 2009;30: 753–7. [DOI] [PubMed] [Google Scholar]
- 102.Ren C, Cheng X, Lu B, Yang G. Activation of interleukin-6/signal transducer and activator of transcription 3 by human papillomavirus early proteins 6 induces fibroblast senescence to promote cervical tumourigenesis through autocrine and paracrine pathways in tumour microenvironment. European journal of cancer (Oxford, England : 1990) 2013;49: 3889–99. [DOI] [PubMed] [Google Scholar]
- 103.Alsaweed M, Hartmann PE, Geddes DT, Kakulas F. MicroRNAs in breastmilk and the lactating breast: potential immunoprotectors and developmental regulators for the infant and the mother. International journal of environmental research and public health 2015;12: 13981–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. miR-122–a key factor and therapeutic target in liver disease. Journal of hepatology 2015;62: 448–57. [DOI] [PubMed] [Google Scholar]
- 105.Griffiths-Jones S The microRNA registry. Nucleic acids research 2004;32: D109–D11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mirzaei H, Gholamin S, Shahidsales S, Sahebkar A, Jaafari MR, Mirzaei HR, Hassanian SM, Avan A. MicroRNAs as potential diagnostic and prognostic biomarkers in melanoma. European journal of cancer (Oxford, England : 1990) 2016;53: 25–32. [DOI] [PubMed] [Google Scholar]
- 107.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Advances in experimental medicine and biology 2013;774: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America 2002;99: 15524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Z, Han Y, Song C, Wei H, Chen Y, Huang K, Li S, Ma D, Wang S, Wang J, Lu Q. Systematic review and meta-analysis of the prognostic significance of microRNAs in cervical cancer. Oncotarget 2018;9: 17141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan JY, Fan YJ, Wang XL, Xie H, Gao HJ, Zhang Y, Liu M, Tang H. miR-429 is involved in regulation of NF-kappaBactivity by targeting IKKbeta and suppresses oncogenic activity in cervical cancer cells. FEBS letters 2017;591: 118–28. [DOI] [PubMed] [Google Scholar]
- 111.Huang F, Lin C, Shi YH, Kuerban G. MicroRNA-101 inhibits cell proliferation, invasion, and promotes apoptosis by regulating cyclooxygenase-2 in Hela cervical carcinoma cells. Asian Pacific journal of cancer prevention : APJCP 2013;14: 5915–20. [DOI] [PubMed] [Google Scholar]
- 112.Chandrasekaran KS, Sathyanarayanan A, Karunagaran D. Downregulation of HMGB1 by miR-34a is sufficient to suppress proliferation, migration and invasion of human cervical and colorectal cancer cells. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2016;37: 13155–66. [DOI] [PubMed] [Google Scholar]
- 113.Chen J, Li G. MiR-1284 enhances sensitivity of cervical cancer cells to cisplatin via downregulating HMGB1. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2018;107: 997–1003. [DOI] [PubMed] [Google Scholar]
- 114.Jiang D, Wang H, Li Z, Li Z, Chen X, Cai H. MiR-142 inhibits the development of cervical cancer by targeting HMGB1. Oncotarget 2017;8: 4001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun L, Wang D, Li H, She H, Yang Y, Zhang H, Miao G. Significance of high YKL-40 expression regulated by miR-24 in cervical cancer progression and prognosis. INTERNATIONAL JOURNAL OF CLINICAL AND EXPERIMENTAL PATHOLOGY 2016;9: 5128–37. [Google Scholar]
- 116.Liu D, Liu C, Wang X, Ingvarsson S, Chen H. MicroRNA-451 suppresses tumor cell growth by down-regulating IL6R gene expression. Cancer epidemiology 2014;38: 85–92. [DOI] [PubMed] [Google Scholar]
- 117.Shishodia G, Shukla S, Srivastava Y, Masaldan S, Mehta S, Bhambhani S, Sharma S, Mehrotra R, Das BC, Bharti AC. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signaling and downstream effects during cervical carcinogenesis. Molecular cancer 2015;14: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang L, Han B, Meng J, Yan Z, Yan X, Jiao S. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget 2015;6: 25266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jin XJ, Chen XJ, Zhang ZF, Hu WS, Ou RY, Li S, Xue JS, Chen LL, Hu Y, Zhu H. Long noncoding RNA SNHG12 promotes the progression of cervical cancer via modulating miR-125b/STAT3 axis. Journal of cellular physiology 2018. [DOI] [PubMed] [Google Scholar]
- 120.Bumrungthai S, Ekalaksananan T, Evans MF, Chopjitt P, Tangsiriwatthana T, Patarapadungkit N, Kleebkaow P, Luanratanakorn S, Kongyingyoes B, Worawichawong S, Pientong C. Up-Regulation of miR-21 Is Associated with Cervicitis and Human Papillomavirus Infection in Cervical Tissues. PLoS One 2015;10: e0127109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu L, Xu Q, Li X, Zhang X. MicroRNA-21 regulates the proliferation and apoptosis of cervical cancer cells via tumor necrosis factor-alpha. Mol Med Rep 2017;16: 4659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McBee W Jr, Gardiner AS, Edwards RP, Lesnock J, Bhargava R. MicroRNA analysis in human papillomavirus (HPV)-associated cervical neoplasia and cancer. J Carcinogene Mutagene 2011;1. [Google Scholar]
- 123.Zhang J, Wu H, Li P, Zhao Y, Liu M, Tang H. NF-kappaB-modulated miR-130a targets TNF-alpha in cervical cancer cells. Journal of translational medicine 2014;12: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, Wang ZC, Zhang ZS, Chen F. MicroRNA-155 regulates cervical cancer via inducing Th17/Treg imbalance. European review for medical and pharmacological sciences 2018;22: 3719–26. [DOI] [PubMed] [Google Scholar]
- 125.Fan Y, Nan Y, Huang J, Zhong H, Zhou W. Up-regulation of inflammation-related LncRNA-IL7R predicts poor clinical outcome in patients with cervical cancer. Bioscience reports 2018;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang J, Wu H, Li P, Zhao Y, Liu M, Tang H. NF-κB-modulated miR-130a targets TNF-α in cervical cancer cells. journal of translational medicine 2014;12: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y, Wang Z, Zhang Z, Chen F. MicroRNA-155 regulates cervical cancer via inducing Th17/Treg imbalance. European review for medical and pharmacological sciences 2018;22: 3719–26. [DOI] [PubMed] [Google Scholar]
- 128.Huang F, Lin C, Shi Y-H, Kuerban G. MicroRNA-101 inhibits cell proliferation, invasion, and promotes apoptosis by regulating cyclooxygenase-2 in Hela cervical carcinoma cells. Asian Pacific Journal of Cancer Prevention 2013;14: 5915–20. [DOI] [PubMed] [Google Scholar]
- 129.Chandrasekaran KS, Sathyanarayanan A, Karunagaran D. Downregulation of HMGB1 by miR-34a is sufficient to suppress proliferation, migration and invasion of human cervical and colorectal cancer cells. Tumor Biology 2016;37: 13155–66. [DOI] [PubMed] [Google Scholar]
- 130.Liu D, Liu C, Wang X, Ingvarsson S, Chen H. MicroRNA-451 suppresses tumor cell growth by down-regulating IL6R gene expression. Cancer epidemiology 2014;38: 85–92. [DOI] [PubMed] [Google Scholar]
- 131.Shishodia G, Shukla S, Srivastava Y, Masaldan S, Mehta S, Bhambhani S, Sharma S, Mehrotra R, Das BC, Bharti AC. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signaling and downstream effects during cervical carcinogenesis. Molecular cancer 2015;14: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu L, Xu Q, Li X, Zhang X. MicroRNA-21 regulates the proliferation and apoptosis of cervical cancer cells via tumor necrosis factor-α. Molecular medicine reports 2017;16: 4659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen J, Li G. MiR-1284 enhances sensitivity of cervical cancer cells to cisplatin via downregulating HMGB1. Biomedicine & Pharmacotherapy 2018;107: 997–1003. [DOI] [PubMed] [Google Scholar]
- 134.Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang L, Han B, Meng J, Yan Z, Yan X. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget 2015;6: 25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang D, Wang H, Li Z, Li Z, Chen X, Cai H. MiR-142 inhibits the development of cervical cancer by targeting HMGB1. Oncotarget 2017;8: 4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu Q, Song J, Ding B, Cui Y, Liang J, Han S. miR-146a promotes cervical cancer cell viability via targeting IRAK1 and TRAF6. Oncology reports 2018;39: 3015–24. [DOI] [PubMed] [Google Scholar]
- 137.Jin XJ, Chen XJ, Zhang ZF, Hu WS, Ou RY, Li S, Xue JS, Chen LL, Hu Y, Zhu H. Long noncoding RNA SNHG12 promotes the progression of cervical cancer via modulating miR‐125b/STAT3 axis. Journal of cellular physiology 2018. [DOI] [PubMed] [Google Scholar]
- 138.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S, De Leon M, Langridge WH, Wall NR. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. British journal of cancer 2009;100: 1073–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis : an international journal on programmed cell death 2011;16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Honegger A, Leitz J, Bulkescher J, Hoppe-Seyler K, Hoppe-Seyler F. Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. International journal of cancer 2013;133: 1631–42. [DOI] [PubMed] [Google Scholar]
- 141.Valenzuela MM, Ferguson Bennit HR, Gonda A, Diaz Osterman CJ, Hibma A, Khan S, Wall NR. Exosomes Secreted from Human Cancer Cell Lines Contain Inhibitors of Apoptosis (IAP). Cancer microenvironment : official journal of the International Cancer Microenvironment Society 2015;8: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Honegger A, Schilling D, Bastian S, Sponagel J, Kuryshev V, Sultmann H, Scheffner M, Hoppe-Seyler K, Hoppe-Seyler F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS pathogens 2015;11: e1004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chiantore MV, Mangino G, Iuliano M, Zangrillo MS, De Lillis I, Vaccari G, Accardi R, Tommasino M, Columba Cabezas S, Federico M, Fiorucci G, Romeo G. Human papillomavirus E6 and E7 oncoproteins affect the expression of cancer-related microRNAs: additional evidence in HPV-induced tumorigenesis. Journal of cancer research and clinical oncology 2016;142: 1751–63. [DOI] [PubMed] [Google Scholar]
- 144.Harden ME, Munger K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology 2017;508: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell biology international 2014;38: 1076–9. [DOI] [PubMed] [Google Scholar]
- 146.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010;142: 409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clinica Chimica Acta 2018. [DOI] [PubMed] [Google Scholar]
- 148.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Scientific reports 2014;4: 5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bridgewood C, Stacey M, Alase A, Lagos D, Graham A, Wittmann M. IL‐36γ has proinflammatory effects on human endothelial cells. Experimental dermatology 2017;26: 402–8. [DOI] [PubMed] [Google Scholar]
- 150.Wang W, Yu X, Wu C, Jin H. IL-36γ inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. International journal of medical sciences 2017;14: 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rana AA, Lucs AV, DeVoti J, Blanc L, Papoin J, Wu R, Papayannakos CJ, Abramson A, Bonagura VR, Steinberg BM. Poly (I: C) induces controlled release of IL-36γ from keratinocytes in the absence of cell death. Immunologic research 2015;63: 228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Frontiers in genetics 2012;3: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang X, Tang S, Le S-Y, Lu R, Rader JS, Meyers C, Zheng Z-M. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PloS one 2008;3: e2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X, Wang HK, McCoy JP, Banerjee NS, Rader JS, Broker TR, Meyers C, Chow LT, Zheng ZM. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA (New York, NY) 2009;15: 637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ibrahim AF, Weirauch U, Thomas M, Grunweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer research 2011;71: 5214–24. [DOI] [PubMed] [Google Scholar]
- 156.Ribeiro J, Marinho-Dias J, Monteiro P, Loureiro J, Baldaque I, Medeiros R, Sousa H. miR-34a and miR-125b Expression in HPV Infection and Cervical Cancer Development. BioMed research international 2015;2015: 304584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer research 2010;70: 5923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Molecular therapy : the journal of the American Society of Gene Therapy 2010;18: 1650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mangino G, Chiantore MV, Iuliano M, Capriotti L, Accardi L, Di Bonito P, Fiorucci G, Romeo G. Role of Extracellular Vesicles in Human Papillomavirus-Induced Tumorigenesis Current Perspectives in Human Papillomavirused.: IntechOpen, 2018. [Google Scholar]
- 160.Chen S, Lv M, Fang S, Ye W, Gao Y, Xu Y. Poly(I:C) enhanced anti-cervical cancer immunities induced by dendritic cells-derived exosomes. International journal of biological macromolecules 2018;113: 1182–7. [DOI] [PubMed] [Google Scholar]
- 161.Lattanzi L, Federico M. A strategy of antigen incorporation into exosomes: comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles. Vaccine 2012;30: 7229–37. [DOI] [PubMed] [Google Scholar]
- 162.Di Bonito P, Grasso F, Mochi S, Petrone L, Fanales-Belasio E, Mei A, Cesolini A, Laconi G, Conrad H, Bernhard H, Dembek CJ, Cosma A, et al. Anti-tumor CD8+ T cell immunity elicited by HIV-1-based virus-like particles incorporating HPV-16 E7 protein. Virology 2009;395: 45–55. [DOI] [PubMed] [Google Scholar]
- 163.Manfredi F, Di Bonito P, Arenaccio C, Anticoli S, Federico M. Incorporation of Heterologous Proteins in Engineered Exosomes. Methods in molecular biology (Clifton, NJ) 2016;1448: 249–60. [DOI] [PubMed] [Google Scholar]
- 164.Di Bonito P, Ridolfi B, Columba-Cabezas S, Giovannelli A, Chiozzini C, Manfredi F, Anticoli S, Arenaccio C, Federico M. HPV-E7 delivered by engineered exosomes elicits a protective CD8(+) T cell-mediated immune response. Viruses 2015;7: 1079–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Di Bonito P, Chiozzini C, Arenaccio C, Anticoli S, Manfredi F, Olivetta E, Ferrantelli F, Falcone E, Ruggieri A, Federico M. Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation. International journal of nanomedicine 2017;12: 4579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anticoli S, Manfredi F, Chiozzini C, Arenaccio C, Olivetta E, Ferrantelli F, Capocefalo A, Falcone E, Ruggieri A, Federico M. An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity Against Viral Antigens. Biotechnology journal 2018;13: e1700443. [DOI] [PubMed] [Google Scholar]
- 167.Accardi L, Paolini F, Mandarino A, Percario Z, Di Bonito P, Di Carlo V, Affabris E, Giorgi C, Amici C, Venuti A. In vivo antitumor effect of an intracellular single-chain antibody fragment against the E7 oncoprotein of human papillomavirus 16. International journal of cancer 2014;134: 2742–7. [DOI] [PubMed] [Google Scholar]
- 168.Verachi F, Percario Z, Di Bonito P, Affabris E, Amici C, Accardi L. Purification and Characterization of Antibodies in Single-Chain Format against the E6 Oncoprotein of Human Papillomavirus Type 16 2018;2018: 6583852. [DOI] [PMC free article] [PubMed]