Abstract
Membrane bioreactors (MBRs) augmented with terrestrial white‐rot basidiomycetes have already been tested for the removal of pharmaceutically active compounds (PhACs) from wastewaters. Within the present study, an aquatic ascomycete (Phoma sp.) was initially demonstrated to efficiently remove several PhACs at their real environmental trace concentrations from nonsterile municipal wastewater on a laboratory scale. Then, a pilot MBR was bioaugmented with Phoma sp. and successively operated in two configurations (first treating full‐scale MBR effluent as a posttreatment, and then treating raw municipal wastewater). Treatment of influent wastewater by the Phoma‐bioaugmented pilot MBR was more efficient than influent treatment by a concomitantly operated full‐scale MBR lacking Phoma sp and posttreatment of full‐scale MBR permeate using the pilot MBR. A stable removal of the PhACs carbamazepine (CBZ) and diclofenac (DF) (39 and 34% on average, respectively) could be achieved throughout the pilot MBR influent treatment period of 51 days, without the need for additional nutrient supplementation (full‐scale MBR: on average, 15% DF but no CBZ removed during 108 days). The long‐term presence of Phoma sp. in the pilot MBR could be demonstrated using fluorescence in situ hybridization analysis, but still open questions regarding its long‐term activity maintenance remain to be answered.
Keywords: Bioaugmentation, Carbamazepine, Diclofenac, Fungi, Membrane bioreactor (MBR)
Abbreviations
- CBZ
carbamazepine
- COD
chemical oxygen demand
- DF
diclofenac
- EDC
endocrine disrupting chemical
- FISH
fluorescence in situ hybridization
- HRT
hydraulic retention time
- MBR
membrane bioreactor
- MLSS
mixed liquor suspended solids
- PhAC
pharmaceutically active compound
- WWTP
wastewater treatment plant
1. Introduction
Pharmaceutically active compounds (PhACs) comprise a wide range of synthetic compounds, and have been detected in secondary treated effluents, surface water bodies, and even in drinking water 1. PhACs may occur in wastewater treatment plant (WWTP) effluents due to only little or sometimes missing sorption onto activated sludge, and/or due to their biodegradation that is too slow to become effective within the time frames defined by the hydraulic residence times (HRT) of WWTPs 2. Conventional activated sludge processes were originally not specifically designed to remove PhACs, and the efficiency of PhAC removal by activated sludge can vary significantly 3. Carbamazepine (CBZ) and diclofenac (DF) represent prominent examples of very frequently detected PhACs in wastewaters. Increased concentrations of these compounds and other PhACs observed during the passage through WWTPs have been attributed to their release from the related conjugates 2, 4, 5, 6.
Different approaches currently target the biological removal of PhACs. Enzymatic membrane reactors tackle PhAC degradation but suffer from a gradual loss of enzymatic activity due to various physical, chemical, and biological inhibitors in waste‐water 7, thus necessitating periodical renewal of the enzyme(s). By contrast, whole‐cell reactors may provide the advantage of a continuous enzyme production and thus maintain sufficient levels of active enzymes 7. Membrane bioreactors (MBRs) and other bioreactor types augmented with terrestrial wood‐rotting (white‐rot) fungi have been tested with respect to the efficient removal of contaminating PhACs and other micropollutants–e.g. endocrine disrupting chemicals (EDCs) and further compounds used in daily life–from waters mostly on a laboratory and thus far only rarely on a pilot scale 8, 9, 10. For instance, an effective elimination of carbamazepine (CBZ) of about 60–80% under nonsterile conditions could be achieved with a lab‐scale plate bioreactor containing a white‐rot fungus grown on polyether foam in synthetic medium, and a high CBZ elimination was also achieved in real WWTP effluent 5. For a MBR based on a mixed culture of bacteria and white‐rot fungi, a moderate removal of CBZ and DF (20 and 40%, respectively) from synthetic wastewater has been reported 7. Removal of DF (by more than 90%) and CBZ (in the range of 50–80%) from nonsterile hospital wastewater, upon application of T. versicolor in a continuous fluidized bed bioreactor coupled to a coagulation‐flocculation pretreatment step, has also been described 11.
Phoma is a genus of common coelomycetous fungi belonging to the phylum Ascomycota. Ascomycetes are the predominant fungal group in aquatic habitats 12. Potentially, fungi that have adapted to the living conditions of aquatic ecosystems could be more suitable for wastewater treatment purposes than fungi dwelling in terrestrial habitats 13. The ascomycetous river isolate Phoma sp. strain UHH 5‐1‐03 (DSM 22425) has previously been demonstrated to efficiently attack numerous environmental pollutants such as PhACs, EDCs, and synthetic dyes in lab‐scale experiments 14, 15, 16. Moreover, this fungus possesses a considerable metabolic robustness toward potentially inhibiting organic and inorganic water contaminants, and also with respect to its pH range of activity 16. These characteristics render Phoma sp. particularly suitable for wastewater treatment applications. This fungus produces an extracellular oxidoreductase (laccase) previously demonstrated to be active and stable also at neutral to slightly alkaline pHs 14, which are typical for municipal wastewaters. So far, extracellular peroxidases have not been found in Phoma sp. 14. Extracellular laccase is involved in the biotransformation of a range of PhACs and EDCs by Phoma sp., along with cell‐associated (very likely cytochrome P450) enzyme systems 15. Phoma sp. has already been successfully employed for the treatment of a synthetic dye‐contaminated model wastewater mimicking textile dyeing industry effluents, using a 10‐L airlift bioreactor under nonsterile conditions 16. However, its application for the removal of environmental trace concentrations of PhACs from nonsterile real municipal waste‐waters has not been reported before. Moreover, we are not aware of studies describing the application of fungi, other than white‐rot basidiomycetes, for this purpose.
In general, the present study aimed to test and evaluate the applicability of Phoma sp. for the removal of the PhAC representatives DF and CBZ from nonsterile wastewater using a pilot MBR, which was implemented in a WWTP operated at full scale. More precisely, the targeted objectives were (1) to initially verify the fungal capability to act on PhACs and further micropollutants present in a municipal wastewater matrix, using lab‐scale experiments; and (2) to evaluate whether and to which extent, the target micropollutants DF and CBZ could be removed at their environmentally relevant trace concentrations by Phoma‐augmented pilot‐scale activated sludge MBR systems over extended time periods, hereby testing whether Phoma sp. would remain effective under the nonoptimal conditions of an activated sludge environment.
2. Materials and methods
2.1. Fungal strain
The origin, identification, and maintenance of Phoma sp. strain UHH 5‐1‐03 (available as Phoma sp. DSM 22425 from the German Culture Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) has been previously reported 13.
2.2. Micropollutant degradation experiments employing Phoma sp. in municipal wastewater on a laboratory scale
Fungal precultures were established in 300 mL flasks containing 100 mL of a 2% w/v malt extract medium (pH 5.7) 15. A part of these cultures was additionally supplemented with 50 μM CuSO4 and 1 mM vanillic acid to stimulate laccase production 14, 17. The flasks were inoculated with 2.0 mL of a mycelial suspension prepared as previously described 17, and then incubated on a rotary shaker (Infors HT, Infors, Bottmingen, Switzerland) at 14°C and at 120 rpm in the dark 15. After 8 days of incubation, fungal biomass was harvested by sterile filtration using Whatman no. 1 paper filters (GE Healthcare, Freiburg, Germany), washed with 100 mL sterile distilled water, and transferred to new 300 mL flasks for degradation experiments. These flasks also contained 100 mL of raw influent wastewater (pH 7.5) previously collected at the full‐scale WWTP of Schilde (Schilde, Belgium). The wastewater was filtered using Whatman glass microfiber filters (0.45 μm pore size) prior to application in degradation experiments, in order to remove particles and for hygienic reasons. Amounts of micropollutants as detected at the beginning of the degradation experiments are shown in Supporting Information Table S1. The following batch tests were performed in order to study micropollutant degradation: batch experiments employing active Phoma sp. with stimulated laccase production (see above) (B1); batch experiments containing active Phoma sp. without stimulated laccase production (B2); batch (control) experiments employing Phoma sp. together with 1 g/L NaN3, in order to inactivate any biological degradation mechanism (B3); and batch experiments in the absence of Phoma sp. that were not inactivated with NaN3 (B4). Flasks containing different wastewater treatment variants were incubated on a rotary shaker (Infors) at 120 rpm and 25°C in the dark. Experiments were consistently performed in triplicate. An increase in the pH values above 8.0 was observed after 8 days of incubation and therefore the pH was adjusted to 6.0 throughout this time. The flasks were harvested and analysed for PhACs and further micropollutants, as described in the Supporting Information at the time points indicated in the text.
2.3. Bioreactor set‐up and pilot scale trials
Bioreactor experiments were performed at the full‐scale Schilde WWTP (Schilde, Belgium), which is operated by Aquafin NV and treats 5500 m³/day of municipal wastewater. The Schilde WWTP consists of an anoxic tank, an aerobic tank and a MBR unit equipped with hollow fiber membranes. Fine bubble aeration in the aerobic tank is provided through diffusers and controlled via a fixed dissolved oxygen set‐point.
Two consecutive bioaugmentation experiments with Phoma sp. were performed using a pilot MBR (Fig. 1), which consisted of two separated biological compartments (i.e. anoxic and aerobic), and had a total volume of 1000 L. A submerged membrane was placed into the aerobic compartment, and air scouring was applied at 1.8 Nm3/h to prevent clogging of the membranes. Fine bubble aeration was provided through plate diffusers, and the pH was controlled by automatic dosing of concentrated NaOH and HCl solutions. In addition, internal temperature control was applied to the reactor.
Figure 1.
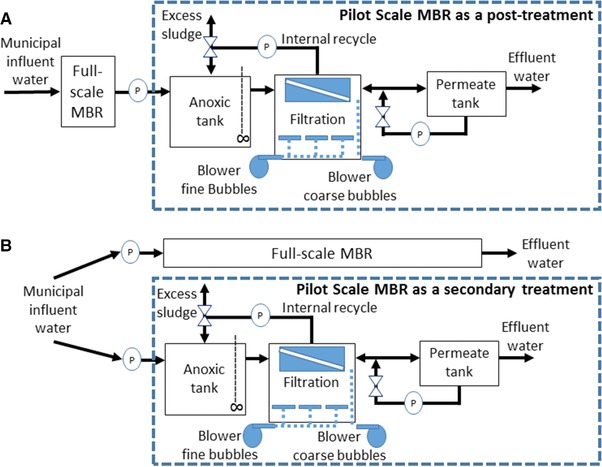
Schematic representation of the pilot scale MBR configurations used for the experiments. (A) Pilot MBR used to treat full‐scale MBR effluent in a posttreatment regime; (B) pilot MBR used to treat raw municipal influent wastewater in a secondary treatment regime.
Biomass of Phoma sp. for pilot MBR bioaugmentation was produced under sterile conditions by inoculating 3 L of 2% malt extract broth (pH 5.4; Biolife, Milano, Italy) in 5 L Pyrex flasks with 50 Phoma‐containing agar cubes (edge length about 5 mm), which were derived from 10 days‐old Phoma sp. cultures pregrown on agar plates (35.6 g/L malt extract agar; Biolife) at 25°C. The flasks were incubated at 25°C under agitation (magnetic stirring at 250 rpm) for 2 days, followed by an increased stirring rate of 400 rpm for another 2 days. After harvesting and a total cultivation time of 4 days, culture suspensions were added to the pilot MBR to yield the Phoma sp. biomass amounts specified in Table 1.
Table 1.
Operational parameters for the full‐scale and pilot MBRs
| Parameter | Full‐scale MBR | Pilot MBR | |
|---|---|---|---|
| Posttreatment | Influent treatment | ||
| Inflowa (m3/h) | 220 ± 10 | 0.037 ± 0.005 | 0.045 ± 0.005 |
| Chemical oxygen demand (COD) (mg/L)a | 196 ± 55 | 22 ± 8 | 196 ± 55 |
| Mixed liquor suspended solids (MLSS)a (g/L) | 9.75 ± 0.75 | 0.75 ± 0.3 | 3.1 ± 0.9 |
| Total reactor volume (m3) | 1200 | 1 | 1 |
| Anoxic to aerobic volume ratio (L/L) | 500/650 | 550/450 | 550/450 |
| Membrane Type | GE ZW 500 | GE ZW 10 | GE ZW 10 |
| Membrane surface (m2) | 10 000 | 2 | 2 |
| Membrane pore size (μm) | 0.04 | 0.04 | 0.04 |
| Recirculation: influent flow ratio | 6:1 | 6:1 | 6:1 |
| T (°C) | 8–19 | 22.6 ± 1.9 | 22.9 ± 1.4 |
| pHa | 7.9 ± 0.4 | 5.5 ± 0.5 | 5.5 ± 0.5 |
| Bioaugmented Phoma sp. biomass (% of MLSSb) | 0 | 43 | 1 |
| Hydraulic retention time (HRT) (h) | 7 | 27 | 22 |
Mean ± standard deviation over the time period of operation indicated in Fig. 4.
On a dry weight basis.
At first, the Phoma‐augmented pilot MBR was used to treat a full‐scale MBR effluent in a posttreatment regime (first experiment) (Fig. 1). The MBR was operated in posttreatment mode from the 1st of April to the 27th of May 2013, and bioaugmentation with Phoma sp. was carried out on the 24th of April 2013. A single dose of 50 μM CuSO4 and 1 mM vanillic acid (final concentrations in the pilot MBR, respectively) to stimulate laccase production 14, 17 was added right after bioaugmentation. Further operational parameters are specified in Table 1, along with those of the full‐scale MBR process. The HRT, temperature and pH values chosen for the pilot MBR operations, which differed from the corresponding parameters of the full‐scale MBR, were inspired by previously established knowledge of activity ranges and degradation abilities of Phoma sp. 15, 16, 17, 18 and intended to support the successful establishment of the fungus. A rationale for the chosen amounts of fungal inoculum (Table 1) is given in the results and discussion section. Due to a comparatively low organic carbon load of the full‐scale MBR permeate, as could be deduced from the corresponding chemical oxygen demand (COD) and mixed liquor suspended solids (MLSS) values (Table 1), sterile liquid malt extract medium (see above) was added until a load of 0.05 kg biological oxygen demand (BOD)/kg MLSS/day was reached and was intended to nourish Phoma sp. in the pilot MBR when operated in posttreatment regime.
Thereafter, the pilot MBR was applied to treat raw municipal influent wastewater in a secondary treatment regime (second experiment) from the 27th of May to the 17th of July 2013. A second bioaugmentation with Phoma sp. was carried out at the beginning of this second experiment (27th May 2013). A single dose of 50 μM CuSO4 and 1 mM vanillic acid (final concentrations in the pilot MBR, respectively) to stimulate laccase production 14, 17 was again added immediately following bioaugmentation, as described for the first experiment above. Further operational parameters are specified in Table 1. Due to higher COD and MLSS values of the influent wastewater compared to the full‐scale effluent (Table 1), Phoma sp. was not additionally supplemented with malt extract during influent treatment.
During MBR operation, grab samples were taken on a regular basis and analyzed for NH4‐N, NO2‐N, NO3‐N, COD, and MLSS according to 19. Target pollutant (DF and CBZ) analysis is described below. The MBRs were further equipped with on‐line dissolved oxygen, nitrate (effluent), pH, and temperature sensors.
2.4. DF and CBZ analysis in the water phases of MBR samples
Influent and effluent samples of the full‐scale and pilot MBRs were collected by flow composite automatic samplers in glass containers. All samples were stored at –20°C.
For DF analysis, aqueous samples were filtered through 0.45 μm Whatman glass microfiber filters and then adjusted to pH 2.8 using 3.5 mM sulfuric acid. DF‐d4 was added as a surrogate standard. The samples (50 mL) were enriched on prepacked Oasis MCX SPE cartridges (60 mg; Waters, Baden‐Dättwil, Switzerland). The cartridges were preconditioned with 2 mL n‐heptane, 2 mL acetone, 3 × 2 mL methanol and 4 × 2 mL noncarbonated mineral water (pH 2.8). After extraction, cartridges were dried under a gentle nitrogen stream for 1 h. The dry cartridges were eluted with 4 mL acetone, and eluents were concentrated to 100 μL with a gentle nitrogen stream. After the addition of 300 μL methanol, eluents were again concentrated to 100 μL under a nitrogen stream. Finally, eluents were diluted with methanol to 1 mL for analysis.
For CBZ analysis, aqueous samples were filtered through 0.45 μm Whatman glass microfiber filters and then adjusted to pH 7.5. CBZ‐C13 was added as a surrogate standard. Samples (500 μL) were enriched on prepacked Oasis HLB cartridges (200 mg; Waters). The cartridges were preconditioned using 2 mL n‐heptane, 2 mL acetone, 3 × 2 mL methanol, and 4 × 2 mL noncarbonated mineral water (pH 7.5). After extraction, the cartridges were dried under a gentle nitrogen stream for 1 h. The dry cartridges were eluted using 2 × 4 mL methanol. The eluents were dried under a gentle nitrogen stream, and resuspended in 1 mL acetonitrile for analysis.
DF and CBZ were analyzed using an Agilent 1260 infinity series HPLC coupled to a single‐quadrupole Agilent 6120 mass‐sensitive detector system (Agilent Technologies, Basel, Switzerland). Compounds in samples were separated on an Agilent Eclipse C8 column (50 × 3 mm id, 1.8 μm particle size), using the eluents A (95% acetonitrile and 5% H2O) and B (5% acetonitrile and 0.02% formic acid in H2O) at a flow of 400 μL/min. For DF analysis, the linear gradient was programmed as follows: 0–1 min 50% A, 3–5 min 90% A, and after 5.1 min 50% A. For CBZ analysis, the linear gradient was programmed as follows: 0–1 min 10% A, after 15 min 60% A, 17–19 min 90% A, and after 20 min 10% A.
ESI was applied to ionize the target compounds. The negative ionization mode was applied for the acidic DF and the positive ionization mode was used for the neutral CBZ. A SIM was employed to acquire the respective target ions. External standards were prepared at different concentrations in blank matrix (mineral water), and extracted with SPE using the procedures described earlier for wastewater samples. Equal amounts of surrogate standards (DF‐d4, CBZ‐C13) were added to both external standards and wastewater samples, which were applied to correct compound quantifications for possible matrix effects, preparation errors, and variations in the detection sensitivity. The relative recoveries of DF and CBZ in the wastewater samples were determined to be 106 and 99%, respectively.
2.5. DF and CBZ analysis in the solid phases of MBR samples
Solid sludge fractions were obtained by filtration of grab sludge water samples through 1.5 μm glass‐fibre filters (TJ Environmental, Hilversum, The Netherlands) (samples had been stored at –20°C prior to filtration). The obtained solid fractions were directly spiked by means of appropriate surrogate standards (isoproturon d6/tribromophenol/fenacetine), and successively extracted with 5 mL acetonitrile and 5 mL methanol (for 30 min, respectively; using a shaker at 160 rpm) at room temperature. Thereafter, the sludge samples were centrifuged at 1840 x g for 5 min. The obtained supernatants were combined and evaporated close to dryness under a gentle nitrogen stream, and the residue was subsequently dissolved in 1 mL methanol.
An HPLC‐MS/MS instrument consisting of an Agilent 1260 infinity series HPLC coupled to an Agilent 6430 triple quadrupole LC‐MS system (Agilent Technologies, Amstelveen, The Netherlands) was used for the analysis of the methanol extracts. Compounds in samples were separated on an Atlantis T3 column (100 × 4.6 mm id, 3 μm particle size; Waters, Etten‐Leur, The Netherlands), using the eluents A (0.3 g/L ammoniumformate in 95: 5 Milli‐Q water: methanol) and B (methanol) at a flow of 500 μL/min. The linear gradient was programmed as follows: start 100% A, after 5 min 60% A, after 10 min 30% A, 20–30 min 0% A, and from 30.5–34 min 100% A. The column oven was set at 40°C, and 10 μL of the sample extract were injected.
The mass spectrometer was operated in a positive and negative ionization mode, using an ESI source. The ion with the highest intensity was used for SRM and quantification in MS/MS mode. Product identification was based on retention times of the analytes and on MS/MS detection. Blank and quality control samples were analyzed to check the analytical equipment. The blank background noise was less than 30% of the quantification limit. Analyte recoveries in quality control samples were in the range of 80–120%.
2.6. Detection and quantification of Phoma sp. and bacteria using fluorescence in situ hybridization (FISH)
Biomass‐containing samples were fixed by adding equal volumes of pure ethanol (>99.9%) in 15 mL vials, and stored at –20°C until analysis.
The presence of Phoma sp. UHH 5‐1‐03 was quantified using the commercial FISH kit VIT® Phoma sp. kit (Vermicon, Munich, Germany). Probe design based on 26S rDNA and in‐silico specificity testing were carried out by using the software package ARB 20. A special approach was developed for the quantification of viable Phoma hyphae by FISH techniques. After specific FISH labeling using the above‐mentioned kit, a microscopic analysis was carried out (Fig. 2). Per microscopic view, 100 square areas were assigned. These square areas served as a basis for the frequency determination of Phoma sp., with a percentage of 1% corresponding to a presence of Phoma hyphae in only one out of 100 square areas. This frequency determination procedure was repeated 20 times, hereby yielding the reported mean values of the viable Phoma hyphae.
Figure 2.
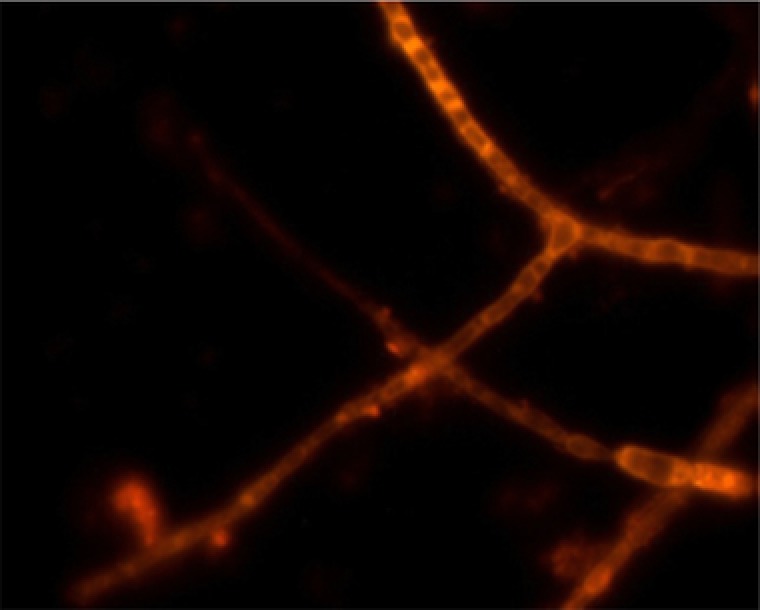
Image of viable Phoma sp. hyphae using FISH staining.
Quantitative bacterial FISH of whole fixed cells 21 was carried out using probe mix EUB 22 and DAPI (4′,6‐diamidino‐2‐phenylindole) DNA staining for total cell counts.
2.7. Determination of laccase activity
In laboratory scale batch degradation tests, laccase activity was routinely determined following the oxidation of 2 mM ABTS in 0.1 M citrate‐phosphate buffer (pH 4.0) at 420 nm, as previously described 15. The activities are expressed in international units (U), where 1 U is defined as the amount of laccase capable of oxidising 1 μmol ABTS per minute.
3. Results and Discussion
3.1. Micropollutant removal by Phoma sp. in municipal wastewater on a laboratory scale
Batch degradation experiments were performed in order to assess the removal of micropollutants by Phoma sp., thereby verifying the activity of the fungus toward target pollutants at their environmentally relevant trace concentrations, and previously reported removal mechanisms involved 15. Time courses of relative concentrations (initial = 100%; please refer to Supporting Information Table S1 for the corresponding absolute value) of DF, which is known to be directly oxidized by laccase 15, 23, 24, in batch tests employing active Phoma sp. with stimulated laccase production and in experiments containing the active fungus without stimulated laccase production are depicted in Fig. 3A. The corresponding extracellular laccase activities are shown in Fig. 3B. The initially clearly higher laccase activities in experiments with stimulated laccase production well correlate with an initially higher DF removal rate observed in these tests, as compared to experiments without stimulated laccase production. These results indicate that laccase along with cell‐associated enzymes have contributed to the fungal DF removal from wastewater, thereby corroborating previous results obtained with Phoma sp. using artificial media 15. Application of a simple exponential decay model without offset to the data shown in Fig. 3A yielded an about 1.8‐fold higher initial DF removal rate for experiments applying Phoma sp. with stimulated laccase production compared to those employing the fungus without laccase stimulation (data not shown). A resulting crude estimate not considering DF concentration effects on enzyme kinetics, compound uptake, and potential sorption as a removal mechanism (see below) suggests that laccase and cell‐associated enzymes accounted for approximately 44 and 56% of the initial removal rate of the compound, respectively. Increasing laccase activities in experiments without stimulation of laccase production at later stages of the experiment (i.e. at 9 and 13 days of incubation; Fig. 3B) could be attributed to laccase expression triggered by increasing nutrient depletion in the batch tests over time, as is known for laccases 25. DF completely disappeared in experiments with active Phoma sp. after 13 days of incubation, irrespective of whether laccase was stimulated or not (Fig. 3A). At the same time, remaining DF amounts of about 88 and 100% of its initial concentration were observed in NaN3‐inactivated control batch tests containing Phoma sp. (any biodegradation impeded) and in noninactivated experiments omitting the fungus, respectively. These results demonstrate that Phoma sp. does not exhibit a striking sorption capability toward DF, and that aerobic wastewater conditions alone (i.e. in the absence of active Phoma sp. or activated sludge) are not sufficient for DF removal. They are further in line with previous work reporting a negligible biosorption of DF onto mycelia of Phoma sp. 15 and a typically only poor removal of the compound in conventional WWTPs 26.
Figure 3.
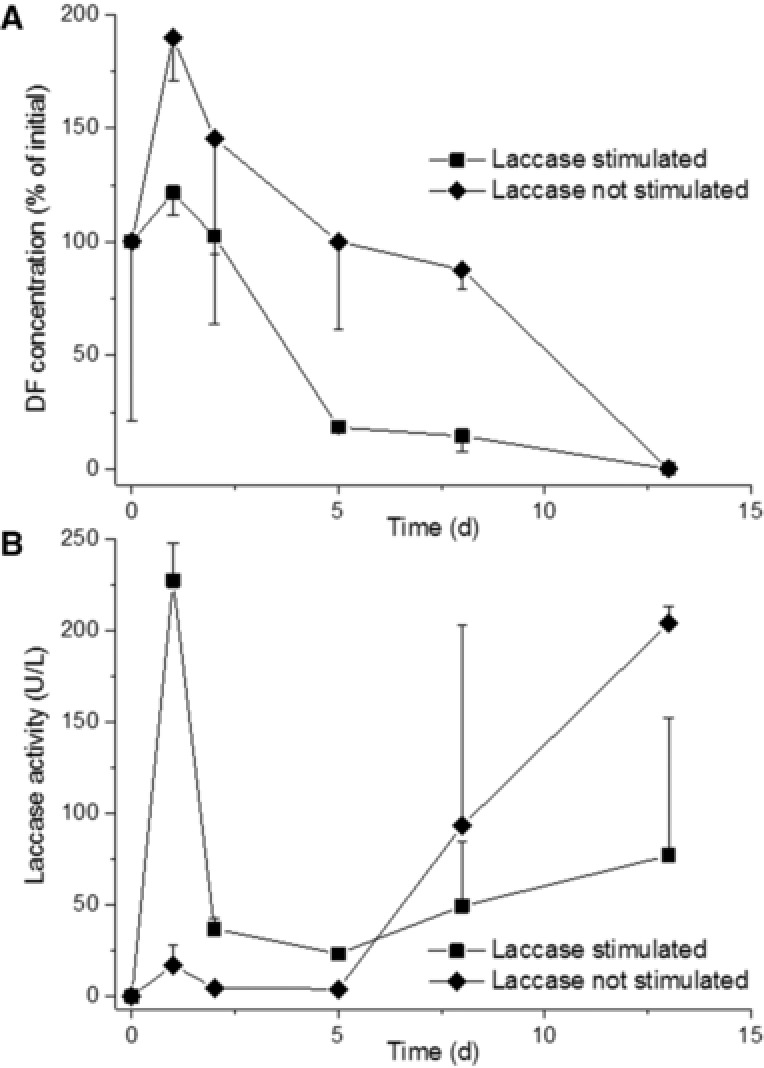
(A) Relative DF concentrations (% of initial) and (B) extracellular laccase activities in batch tests employing active Phoma sp. with stimulated laccase production (squares) and active Phoma sp. without stimulated laccase production (diamonds) in wastewater. Symbols represent means ± standard deviations from triplicate experiments.
Relative wastewater concentrations (% of the initial amounts shown in Supporting Information Table S1) of further detected PhACs (CBZ, ketoprofen, naproxen, ibuprofen, ciprofloxacin) and other micropollutants (caffeine and the artificial sweetener acesulfame) after lab scale treatment of wastewater with Phoma sp. are shown in Supporting Information Fig. S1. These compounds are known to be insusceptible to direct laccase oxidation, and thus cell‐associated enzymes have to primarily be considered to be responsible for their fungal biotransformation 15, 23, 24, 27. In experiments employing active fungal cultures, CBZ was removed below the detection limit already within two days regardless of laccase stimulation (Supporting Information Fig. S1). By contrast, it was not at all removed in experiments with inactivated Phoma sp. as well as in the absence of fungal mycelia; indicating insignificant biosorption and a high persistence of the compound, as previously described 15, 26. Fungal degradation of further PhACs and caffeine (but not acesulfame) is suggested by the results obtained with active fungal cultures (Supporting Information Fig. S1), and has also partly been described in the past for other fungal systems 8. A higher removal rate observed for ketoprofen in the presence of active Phoma sp. with stimulated laccase production compared to fungal cultures without laccase stimulation could be attributed to spontaneous follow‐up reactions between DF radicals formed by laccase and not directly by laccase‐oxidizable ketoprofen. Such effects have been previously described for other micropollutants 18. The results from experiments with inactivated fungal mycelia indicate biosorption of naproxen, ibuprofen, ciprofloxacin, caffeine, and acesulfame to varying extents (Supporting Information Fig. S1). The respective removals of naproxen, ibuprofen, and ciprofloxacin observed in the nonsterile batch tests without Phoma sp. suggest the development of degradative microbial communities in the course of the experiment, which could arise from those microbes not removed during initial wastewater filtration (please also refer to Subsection 2.2 of the materials and methods). Overall, the observed biodegradation/‐transformation and biosorption patterns of the investigated PhACs and other micropollutants (Supporting Information Fig. S1) qualitatively essentially follow those of typical WWTPs 26. A ketoprofen concentration remarkably exceeding the initial value, which was observed in experiments omitting the fungus after 20 days (Supporting Information Fig. S1), could be attributed to a release from the corresponding conjugates contained in the wastewater used for experiments 2.
3.2. Removal of CBZ and DF in the full‐scale MBR
In the period from the 1st of April 2013 to the 17th of July 2013, CBZ and DF were analyzed at the full‐scale MBR (Fig. 4). The influent and effluent DF concentrations as averaged over time were 1163 ± 459 and 989 ± 255 ng/L, respectively; corresponding to an average DF removal of 15% (Fig. 4A). These results are in good agreement with typical DF removal rates reported for conventional WWTPs, with biodegradation representing the clearly dominating removal mechanism of this compound (about 80% DF biodegradation compared to around 20% DF removed by sorption; 26). The influent and effluent concentrations of CBZ averaged over time amounted to 569 ± 345 and 691 ± 570 ng/L, respectively. Thus, no significant removal of CBZ was observed during the sampling campaign (Fig. 4B), in accordance with the widely reported very poor removal of this compound in WWTPs 26 and its observed persistence in laboratory scale batch experiments employing municipal wastewater in the absence of Phoma sp. (Supporting Information Fig. S1). Partly higher CBZ concentrations in the effluent than in the influent of the pilot MBR (of up to 2641 ng/L; not visible in Fig. 4B) may point to a conversion of CBZ glucuronides and other conjugated metabolites back to parent CBZ by enzymatic processes taking place in the treatment plant 2, 5, 6.
Figure 4.
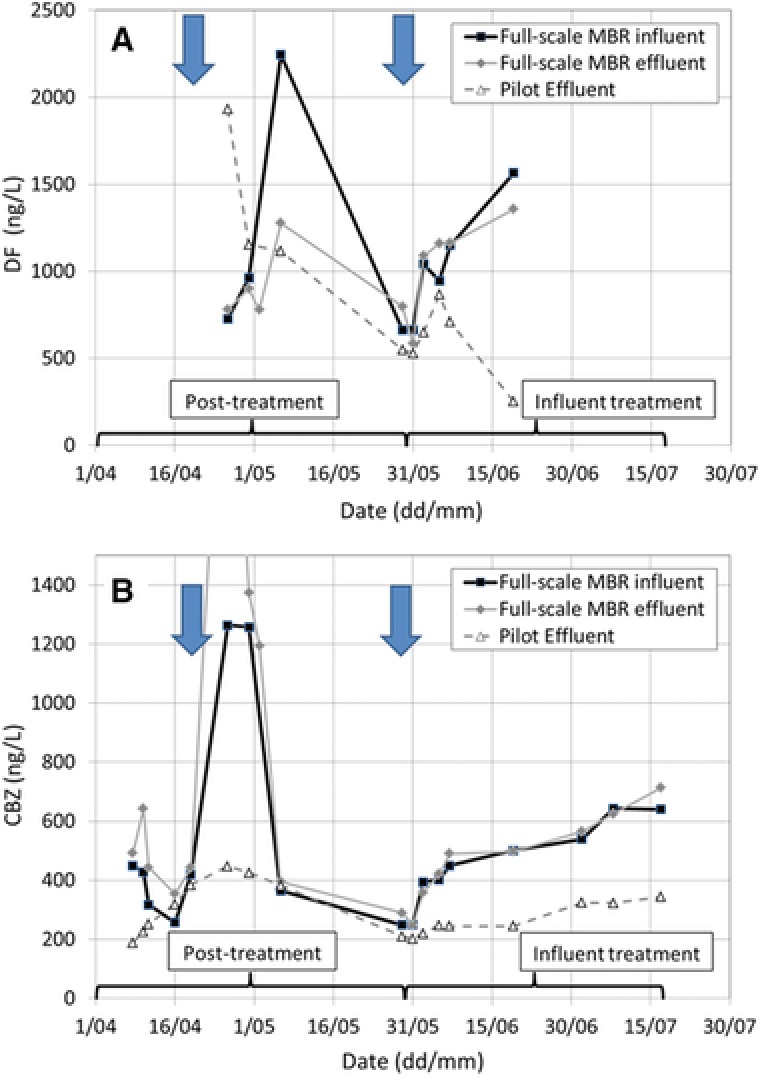
(A) DF and (B) CBZ concentrations in the full‐scale MBR influent, full‐scale MBR effluent, and pilot‐scale MBR effluent during the time course of the experiment. The time periods corresponding to the use of the pilot MBR for the treatment of full‐scale MBR effluent in a posttreatment regime (posttreatment), and the use of the MBR for the treatment of raw municipal influent wastewater in a secondary treatment regime (influent treatment) are labeled. Time points of bioaugmentation of the pilot MBR with Phoma sp. are indicated by the blue arrows.
3.3. Removal of CBZ and DF in the pilot MBR treating full‐scale MBR permeate
The pilot MBR was operated in a posttreatment regime for the treatment of full‐scale MBR effluent from the 1st of April 2013 to the 27th of May 2013 (Fig. 4). The following points were considered to favor the survival and activity of Phoma sp. after bioaugmentation: First, the number of potentially competing microorganisms in the MBR permeate should be rather low 28. Second, the COD in the permeate was about one order of magnitude lower than that of raw influent water (Table 1) and consisted mainly of recalcitrant soluble microbial products 29. Therefore, a comparatively high amount of inoculum (Table 1) was used for the bioaugmentation of the MBR with Phoma sp. on the 24th of April 2013 (Fig. 4), and malt extract was dosed daily according to the load specified in Subsection 2.3 of the materials and methods.
During the operational period, 22 ± 8 mg COD/L and 0.2 ± 0.1 mg NH4‐N/L were recorded in the pilot MBR inflow. The MLSS concentration in the pilot MBR inflow rose from 0.15 g MLSS/L to 0.53 g MLSS/L.
The DF concentration in the pilot MBR effluent was initially (26th of April 2013) rather high, and dropped massively thereafter (Fig. 4A). Starting from the 6th of May 2013, a net DF removal became established in the pilot MBR (i.e. the pilot effluent DF concentrations were lower than the full‐scale MBR effluent DF concentrations feeding the pilot MBR) and was maintained until the end of the operational period (on average, corresponding to an approx. 22% DF removal).
At the beginning of April 2013, the CBZ concentrations in the pilot effluent were lower than in the pilot influent. By diluting the reactor volume with influent wastewater, the influent and the effluent concentrations reached the same range in the samples from the 14th of April and 19th of April 2013 (Fig. 4B). After bioaugmentation with Phoma sp., the CBZ effluent concentrations of the pilot MBR rose only moderately while they had considerably increased in the pilot influent; corresponding to an about 40% CBZ removal for 12 days. However, this successful performance abruptly got lost and no CBZ removal could be observed further on (Fig. 4B). For the entire posttreatment period, an average CBZ removal of about 15% was thus achieved. It is currently difficult to unambiguously conclude on the observed instability of the CBZ removal performance. A sufficient supply of fungi with nutrients is crucial for the maintenance of their cometabolic degradative activities 5, 7, 11. For instance, a supplementation of the white‐rot fungus Phanerochaete chrysosporium with glucose and ammonium nitrogen was necessary to restore its CBZ removal activity during the continuous treatment of effluent from a municipal WWTP 5. The malt extract dosage regime applied within the present study did perhaps not sufficiently support the activity maintenance of Phoma sp., which may have been a reason for the comparatively low and partly unstable removal efficiencies of the two investigated PhACs.
A semi‐quantitative FISH‐based method and FISH‐based DAPI staining was applied to monitor Phoma sp. and total bacterial cell counts, respectively (Fig. 5). A considerable fluctuation of the corresponding values (Fig. 5) can be explained by the highly variable amounts of MLSS in the samples taken for determination. Macroscopically, the samples frequently contained varying amounts of dark lumps, which could not be resuspended neither by mechanical homogenization nor by different enzymatic treatment trials (data not shown). These lumps did largely bias FISH analysis of Phoma sp. as was evident from the microscopic observation of also nonfluorescent fungal hyphae, in addition to those showing red fluorescence. Such large MLSS inhomogeneities in the pilot MBR are partly attributable to the tendency of fungal hyphae to stick to and colonize surfaces, the growth of filamentous fungi in the form of mycelial aggregates (pellets) in suspension 30, and bacteria apparently partly overgrowing Phoma sp. as was evident from microscopic examination. Therefore, the obtained Phoma sp. counts do not accurately reflect the fungal concentration in the MBR. A similar ratio of total bacterial (DAPI) cell counts and relative Phoma sp. counts essentially maintained throughout the monitoring period (with the exception of the last data point; Fig. 5) suggests that the DAPI cell counts were also largely affected by the aforementioned MLSS inhomogeneities, in support of bacteria associated with fungal hyphae. Together these results illustrate the necessity for more reliable methods for the quantification of pollutant degraders, especially of those growing in the form of mycelia and related aggregates. An interpretation of the obtained cell counts in terms of quantitative statements seems not feasible. However, along with the observed effects on target pollutant removal (Fig. 4), the proven presence of Phoma sp. throughout the observation period (Fig. 5) suggests that the fungus remained at least partly alive and active in the pilot MBR.
Figure 5.
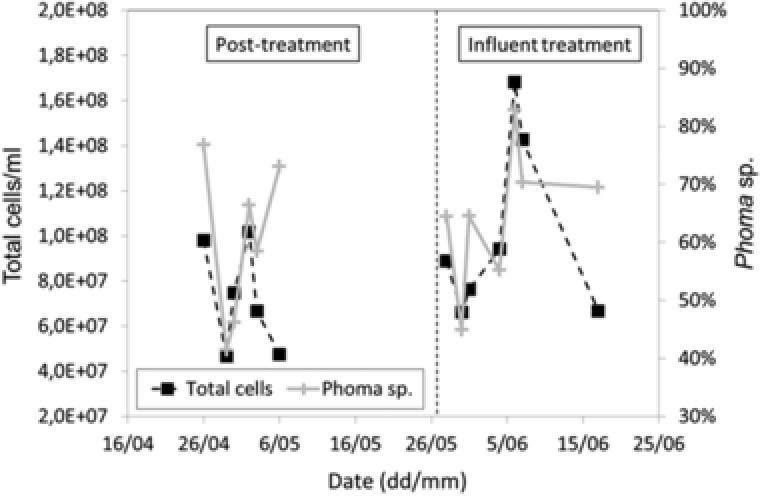
Total bacterial cell counts (DAPI straining; left y‐axis) and relative Phoma sp. counts (FISH analysis; right y‐axis) in the pilot MBR during the time course of the experiment. The time periods corresponding to the use of the pilot MBR for the treatment of full‐scale MBR effluent in a posttreatment regime (posttreatment), and the use of the MBR for the treatment of raw municipal influent wastewater in a secondary treatment regime (influent treatment) are separated by the vertical dashed line.
3.4. Removal of CBZ and DF in the pilot MBR treating raw municipal wastewater
The pilot MBR was fed with municipal influent wastewater and operated in a secondary treatment regime between the 27th of May and the 17th of July 2013. Due to considerably higher COD and MLSS loads in the raw wastewater influent compared to the previous posttreatment regime, which could potentially partly serve as fungal carbon and energy sources, the amount of Phoma sp. inoculum used for bioaugmentation of the MBR on the 27th of May 2014 (Fig. 4) was reduced (Table 1), and malt extract dosing was omitted.
During the pilot MBR operation for influent wastewater treatment, NH4‐N was completely removed and NO3‐N was kept between 1–4 mg NO3‐N/L. The removal of total nitrogen and COD reached 93 and 55%, respectively.
Averaged pilot MBR influent and effluent DF concentrations of 1073 and 593 ng/L were observed during the operational period (Fig. 4A). While the full‐scale MBR showed only occasional DF removals, the pilot MBR bioaugmented with Phoma sp. achieved 34% DF removal overall. As to investigate whether any sorption had contributed to the observed DF removal, the DF concentration in the sludge was determined at the end of the operational period. An average DF concentration in the sludge of 0.03 mg/gg MLSS was measured (in the absence of sludge waste). Considering the average influent DF concentration at 45 L/h and 15 days of operation, sorption onto sludge did not significantly contribute to DF removal. These results are in line with essentially no biosorption of DF onto Phoma sp. mycelia as observed in the laboratory scale batch tests (see Subsection 3.1), and corroborate previous results obtained with Phoma sp. in synthetic media 15.
Full‐scale influent, effluent, and pilot effluent displayed comparable CBZ concentrations at the beginning of the influent treatment (Fig. 4B). While the CBZ concentrations considerably increased in the full‐scale MBR one week after inoculation of the pilot MBR with Phoma sp., they only moderately rose in the pilot MBR effluent. A stable CBZ removal (39% on average) was established (Fig. 4B). Sludge samples were analyzed in order to check whether any sorption had contributed to the observed CBZ removal. During the 15 days of operation, an average influent CBZ concentration of 477 ng/L at 45 L/h resulted in an influent load of 7.7 mg CBZ. The measured average CBZ concentration in sludge (after 15 days of potential accumulation) was 0.02 mg/kg MLSS. Thus, CBZ sorbed to sludge had accounted for only 0.65% of the CBZ removed during the same period. The observed CBZ removal is therefore overwhelmingly attributable to biodegradation or–transformation and not to sorption processes, in agreement with the results of the laboratory scale batch tests described in Subsection 3.1 and previously published data 15.
Total bacterial (DAPI) and Phoma sp. cell counts were continued to be monitored also during influent treatment at the pilot MBR (Fig. 5). The difficulties and implications related to this kind of data have already been described and discussed in Subsection 3.3 above. Nevertheless, together with the observed target pollutant removals (Fig. 4), the demonstrated presence of Phoma sp. during the influent treatment period (Fig. 5) suggests a successful establishment of the fungus in the pilot MBR.
4. Concluding remarks
Within the present study, the aquatic ascomycete Phoma sp. UHH 5‐5‐03 was first demonstrated to efficiently remove several PhACs at their real environmental trace concentrations from nonsterile municipal wastewater in lab‐scale experiments. Phoma sp. was then used for the bioaugmentation of a pilot MBR implemented at a full‐scale wastewater treatment plant site. The pilot MBR was successively used for the treatment of full‐scale MBR permeate in a posttreatment regime, followed by treating raw municipal wastewater. The removal of the PhAC representatives diclofenac (DF) and carbamazepine (CBZ) was followed over a total period of 3 months. Treatment of raw influent wastewater by the Phoma‐bioaugmented pilot MBR was clearly more efficient than influent treatment by a concomitantly operated full‐scale MBR without Phoma sp., and posttreatment full‐scale MBR effluent using the pilot MBR. A stable CBZ and DF removal (39 and 34% on average, respectively) could be achieved throughout a pilot MBR influent treatment period of 51 days, without the need for supplementation with additional nutrients (full‐scale MBR: on average, 15% DF but no CBZ removed during 108 days). Along with the demonstrated long‐term presence of Phoma sp. in the pilot MBR using FISH analysis, the obtained results suggest that the fungus remained active under the nonsterile conditions of the activated sludge environment of the pilot MBR over the investigated period of time, and has contributed to the DF and CBZ removal at their real environmental concentrations. However, our results also demonstrate an urgent need for reliable methods enabling the unbiased quantification of mycelia‐ and pellet‐forming pollutant degraders such as filamentous fungi and other microorganisms adding spatial and/or temporal heterogeneities to technical systems. The peculiarity of fungal organisms to degrade most environmental pollutants in a cometabolic manner 15, 31 necessitates the maintenance of fungal activity through the provision of suitable nutrients. In this context, the maintenance of the PhAC removal performance without external nutrient addition, as observed for the Phoma‐bioaugmented pilot MBR throughout the entire influent treatment period, is promising with respect to practical applications. It further raises the question inasmuch the dissolved organic carbon or total organic carbon of wastewater could support fungal growth and/or activity maintenance, which remains to be elucidated. By contrast, posttreatment of effluents with only low organic loads appears to be rather unfavorable with respect to fungal nutrition and activity maintenance.
Practical application
A pilot scale membrane bioreactor (MBR) was bioaugmented with the aquatic fungal isolate Phoma sp. UHH 5‐1‐03, and the removal of the water‐polluting pharmaceutically active compounds (PhACs) diclofenac and carbamazepine from nonsterile municipal wastewater was successfully demonstrated over several weeks. Concomitantly, the long‐term presence of Phoma sp. in the pilot MBR could be demonstrated. The bioaugmentation of wastewater treatment systems like MBRs with filamentous fungi may hence represent a strategy to cope with the problem of incomplete removal of many PhACs and other micropollutants during conventional wastewater treatment, provided that a range of bottlenecks associated with the implementation of fungal technologies could be overcome. These include technological challenges related to mycelial growth and pellet formation, the related need for reliable methods enabling the unbiased quantification of filamentous fungi in technical wastewater treatment systems, and the necessity to maintain cometabolic fungal degradation activities through the provision of a suitable cosubstrate.
The authors have declared no conflict of interest.
Supporting information
Supporting material
Acknowledgments
We are thankful to J. Svojitka (Institute for Ecopreneurship, School of Life Sciences, University of Applied Sciences Northwestern Switzerland—FHNW, Muttenz, Switzerland) and D. van Brunschot—Wiersma (Eurofins Omegam B.V., Amsterdam‐Duivendrecht, The Netherlands) for their help regarding DF and CBZ analyses in water and solid phases of MBR samples, respectively. This work was supported by the European Commission as a part of the MINOTAURUS project (EC grant agreement no. 265946) and by the Helmholtz Association of German Research Centres under the research programme “Chemicals in the Environment” (CITE) conducted at the Helmholtz Centre for Environmental Research—UFZ.
Compiled in honour of the 80th birthday of Professor Wolfgang Babel.
5 References
- 1. Snyder, S. A., Occurrence, treatment, and toxicological relevance of EDCs and pharmaceuticals in water. Ozone Sci. Eng. 2008, 30, 65–69. [Google Scholar]
- 2. Gros, M. , Petrović, M. , Ginebreda, A. , Barceló, D., Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [DOI] [PubMed] [Google Scholar]
- 3. Hai, F. I. , Yamamoto, K. , Nakajima, F. , Fukushi, K., Factors governing performance of continuous fungal reactor during non‐sterile operation—The case of a membrane bioreactor treating textile wastewater. Chemosphere 2009, 74, 810–817. [DOI] [PubMed] [Google Scholar]
- 4. Zhang, Y. , Geißen, S.‐U. , Gal, C., Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [DOI] [PubMed] [Google Scholar]
- 5. Zhang, Y. , Geissen, S. U., Elimination of carbamazepine in a non‐sterile fungal bioreactor. Bioresour. Technol. 2012, 112, 221–227. [DOI] [PubMed] [Google Scholar]
- 6. Vieno, N. , Tuhkanen, T. , Kronberg, L., Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007, 41, 1001–1012. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen, L. N. , Hai, F. I. , Yang, S. , Kang, J. et al. Removal of trace organic contaminants by an MBR comprising a mixed culture of bacteria and white‐rot fungi. Bioresour. Technol. 2013, 148, 234–241. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed, M. B. , Zhou, J. L. , Ngo, H. H. , Guo, W. et al., Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [DOI] [PubMed] [Google Scholar]
- 9. Cecconet, D. , Molognoni, D. , Callegari, A. , Capodaglio, A. G., Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–603. [Google Scholar]
- 10. Křesinová, Z. , Linhartová, L. , Filipová, A. , Ezechiáš, M. et al., Biodegradation of endocrine disruptors in urban wastewater using Pleurotus ostreatus bioreactor. N. Biotechnol. 2018,. 43, 53–61. [DOI] [PubMed] [Google Scholar]
- 11. Mir‐Tutusaus, J. A. , Parladé, E. , Llorca, M. , Villagrasa, M. et al., Pharmaceuticals removal and microbial community assessment in a continuous fungal treatment of non‐sterile real hospital wastewater after a coagulation‐flocculation pretreatment. Water Res. 2017, 116, 65–75. [DOI] [PubMed] [Google Scholar]
- 12. Shearer, C. A. , Descals, E. , Kohlmeyer, B. , Kohlmeyer, J. et al., Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar]
- 13. Junghanns, C. , Krauss, G. , Schlosser, D., Potential of aquatic fungi derived from diverse freshwater environments to decolourise synthetic azo and anthraquinone dyes. Bioresour. Technol. 2008, 99, 1225–1235. [DOI] [PubMed] [Google Scholar]
- 14. Junghanns, C. , Pecyna, M. J. , Bohm, D. , Jehmlich, N. et al., Biochemical and molecular genetic characterisation of a novel laccase produced by the aquatic ascomycete Phoma sp. UHH 5‐1‐03. Appl. Microbiol. Biotechnol. 2009, 84, 1095–1105. [DOI] [PubMed] [Google Scholar]
- 15. Hofmann, U. , Schlosser, D., Biochemical and physicochemical processes contributing to the removal of endocrine‐disrupting chemicals and pharmaceuticals by the aquatic ascomycete Phoma sp. UHH 5‐1‐03. Appl. Microbiol. Biotechnol. 2016, 100, 2381–2399. [DOI] [PubMed] [Google Scholar]
- 16. Junghanns, C. , Neumann, J. F. , Schlosser, D., Application of the aquatic fungus Phoma sp (DSM22425) in bioreactors for the treatment of textile dye model effluents. J. Chem. Technol. Biot. 2012, 87, 1276–123. [Google Scholar]
- 17. Junghanns, C. , Parra, R. , Keshavarz, T. , Schlosser, D., Towards higher laccase activities produced by aquatic ascomycetous fungi through combination of elicitors and an alternative substrate. Eng. Life Sci. 2008, 8, 277–285. [Google Scholar]
- 18. Jahangiri, E. , Thomas, I. , Schulze, A. , Seiwert, B. et al., Characterisation of electron beam irradiation‐immobilised laccase for application in wastewater treatment. Sci. Total Environ. 2018, 624, 309–322. [DOI] [PubMed] [Google Scholar]
- 19. American Public Health Association . Standard methods for the examination of water and wastewater 20th ed. Washington, DC: American Public Health Association/American Water Works Association/Water Environment Federation; 1999. [Google Scholar]
- 20. Ludwig, W. , Strunk, O. , Westram, R. , Richter, L. et al. ARB: A software environment for sequence data. Nucleic Acids Res. 2004, 32, 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snaidr, J. , Amann, R. , Huber, I. , Ludwig, W. et al., Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl. Environ. Microbiol. 1997, 63, 2884–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daims, H. , Brühl, A. , Amann, R. , Schleifer, K.‐H. et al., The domain‐specific probe EUB338 is insufficient for the detection of all bacteria: Development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 1999, 22, 434–444. [DOI] [PubMed] [Google Scholar]
- 23. Arca‐Ramos, A. , Ammann, E. M. , Gasser, C. A. , Nastold, P. et al. Assessing the use of nanoimmobilized laccases to remove micropollutants from wastewater. Environ. Sci. Pollut. Res. Int. 2016, 23, 3217–3228. [DOI] [PubMed] [Google Scholar]
- 24. Touahar, I. E. , Haroune, L. , Ba, S. , Bellenger, J. P. et al., Characterization of combined cross‐linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [DOI] [PubMed] [Google Scholar]
- 25. Piscitelli, A. , Giardina, P. , Lettera, V. , Pezzella, C. et al., Induction and transcriptional regulation of laccases in fungi. Curr. Genomics 2011,. 12, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Margot, J. , Rossi, L. , Barry, D. A. , Holliger, C., A review of the fate of micropollutants in wastewater treatment plants. WIREs Water 2015, 2, 457–487. [Google Scholar]
- 27. Gao, N. , Liu, C.‐X. , Xu, Q.‐M. , Cheng, J.‐S. et al., Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co‐producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus . Chemosphere 2018, 195, 146–155. [DOI] [PubMed] [Google Scholar]
- 28. Hirani, Z. M. , Bukhari, Z. , Oppenheimer, J. , Jjemba, P. et al., Characterization of effluent water qualities from satellite membrane bioreactor facilities. Water Res. 2013, 47, 5065–5075. [DOI] [PubMed] [Google Scholar]
- 29. Fenu, A. , Wambecq, T. , Thoeye, C. , De Gueldre, G. et al., Modelling soluble microbial products (SMPs) in a dynamic environment. Desalination Water Treat. 2011, 29, 210–217. [Google Scholar]
- 30. Zhang, J. , Zhang, J., The filamentous fungal pellet and forces driving its formation. Crit. Rev. Biotechnol. 2016, 36, 1066–1077. [DOI] [PubMed] [Google Scholar]
- 31. Harms, H. , Schlosser, D. , Wick, L. Y., Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material


