Figure 6.
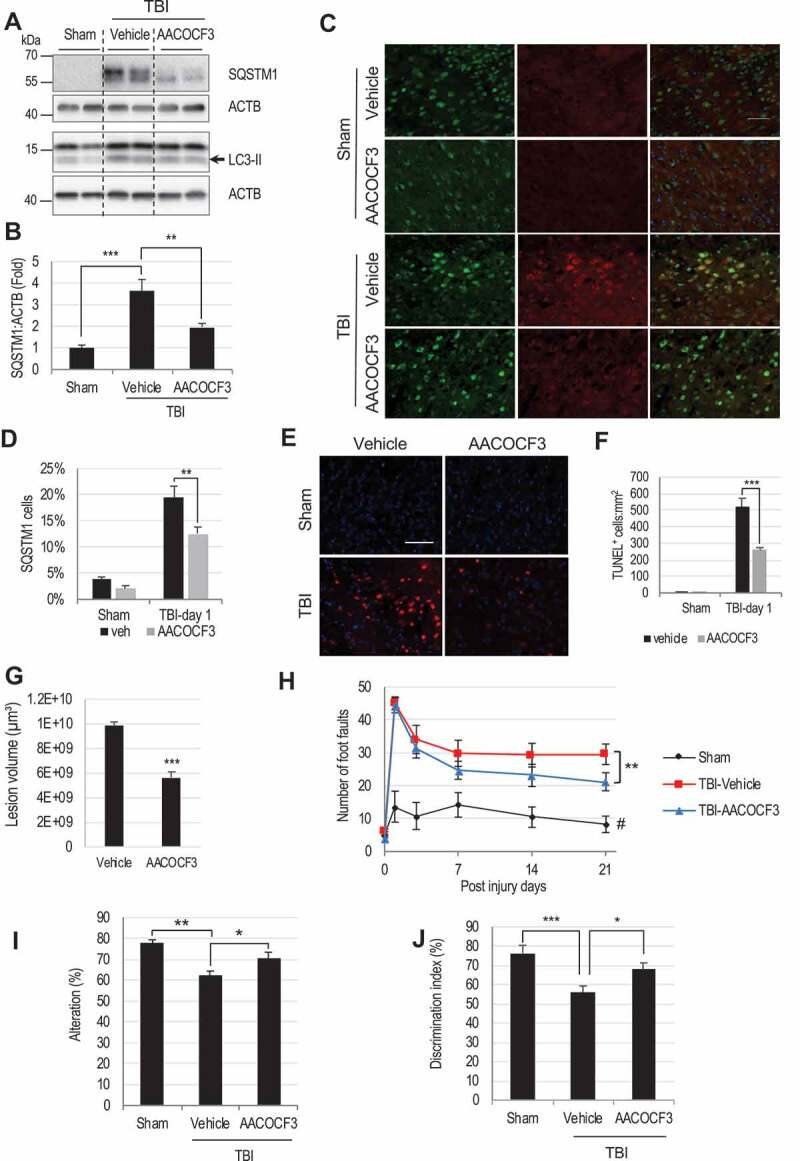
PLA2G4A inhibition restores autophagy flux, attenuates cortical cell death and improves motor and cognitive function in mice after TBI.
(A) Western blot of SQSTM1 and LC3 in sham, TBI and TBI+AACOCF3 cortical lysates and (B) Quantification of SQSTM1 level. Data are mean ± SEM, n = 6 for sham and 10 for vehicle or AACOCF3 treated TBI mice; **p < 0.01, ***p < 0.001, (One-way ANOVA with Turkey’s multiple comparison test). (C and D) IF analysis demonstrating decreased SQSTM1 accumulation in AACOCF3 treated TBI cortical neurons as compared to TBI controls. (C) Images (20×) of cortical brain sections of sham, sham+AACOCF3, TBI and TBI+AACOCF3 mice stained with antibodies against neuronal marker RBFOX3/NeuN and SQSTM1. Scale bar: 50 μm. (D) Quantification of SQSTM1 positive cells. Data are mean ± SEM, n = 3 for vehicle or AACOCF3 treated sham and 5 for vehicle or AACOCF3 treated TBI mice; *p < 0.05, ***p < 0.001, (Two-way ANOVA with Bonferroni posttests). (E) Images (20×) demonstrating decreased cell death (TUNEL) in cortical brain sections from TBI+AACOCF3 as compared to TBI mice. Scale bar: 50 μm (F) Quantification of TUNEL positive cells. Data are mean ± SEM, n = 3 for vehicle or AACOCF3 treated sham and 5 for vehicle or AACOCF3 treated TBI mice; ***p < 0.001, (Two-way ANOVA with Bonferroni posttests). (G) Quantification of lesion volume (Cavalieri method) at 28 days post injury in TBI and TBI+AACOCF3 mouse cortices. Representative images are included in the Fig. S6N. Data are mean ± SEM, n = 7 for vehicle treated and 8 for AACOCF3 treated TBI mice; ***p < 0.001, vs. vehicle treated TBI group. (Students’ t-test). (H-J) AACOCF3 treatment leads to improved functional outcomes after TBI. (H) Assessment of sensorimotor function of sham, TBI+vehicle and TBI+AACOCF3 mice at days 1, 3, 7, 14 and 21 post injury using the beam-walk test. Day 0 represents baseline prior to injury. AACOCF3 treatment of TBI mice led to improvement in motor function vs. TBI+vehicle controls, starting from day 14 (p = 0.0513) and increasing through day 21 (**p = 0.0047). Data are mean ± SEM, n = 11 sham, 14 TBI+vehicle and 14 TBI+AACOCF3 mice. Significant effects of injury and AACOCF3 treatment [F(2,26) = 18.41; p < 0.0001] were detected across all time points [F(5,65) = 105.8, p < 0.0001] except day 0. (Two-way repeated-measures ANOVA with Turkey’s multiple comparison test). (I) Spatial memory assessment using Y-maze spontaneous alternation test at 7 day after TBI. TBI led to significantly reduced percentages of spontaneous alternation (**p < 0.01 vs. sham). AACOCF3 treatment significantly increased percentage of spontaneous alternation compared with the vehicle treated TBI group (*p < 0.05). Data are mean ± SEM, n = 11 sham and 14 TBI and TBI+AACOCF3 mice, (One-way ANOVA, with Turkey’s multiple comparison test). (J) Memory retention assessment by novel object recognition (NOR) in mice at day 22 after TBI. All groups spent equal time with the two identical objects during the sample phase (Fig. S6O). AACOCF3 treated TBI mice spent significantly more time with the novel vs. familiar object as compared with vehicle treated TBI group (*P < 0.05). Significant differences were also observed between the sham and vehicle treated TBI group (**P < 0.01). Data are mean ± SEM, n = 11 sham and 14 TBI and TBI+AACOCF3 mice, (One-way ANOVA with Turkey’s multiple comparison test).
