Abstract
Objective
Rheumatoid arthritis (RA) is a known risk factor for developing coronary artery disease (CAD). The influence of RA on the prognosis after initial CAD diagnosis and treatment is however largely unknown. We examined the risk of major cardiovascular events among RA and non-RA patients with chest pain referred to cardiac CT.
Methods
This was a follow-up study, using data from the Western Denmark Heart Registry, containing data on CT angiography examinations (Cardiac CT). Information on RA diagnosis and covariates were identified through nationwide administrative registers. The primary outcome was a combined outcome including, myocardial infarction, ischaemic or unspecified stroke, coronary artery bypass grafting, percutaneous coronary intervention, and all-cause mortality. Median time until events or censoring was 3.5 years (min/max: 0.0: 9.2). Cox proportional hazard models were used to examine the association between RA/non-RA patients and outcomes.
Results
Among 42 257 patients, referred between 2008 and 2016, we identified 358 (0.8%) with RA. An increased risk was seen in RA compared with non-RA (adjusted HR 1.35, 95% CI 0.93 to 1.96). Among patients who had received flare treatment more than once prior to cardiac CT the adjusted HR 1.80 (95% CI 1.08 to 3.00), and among patients with seropositive RA the adjusted HR 1.42 (95% CI 0.93 to 2.16).
Conclusion
In patients referred to cardiac CT due to chest pain, we found a trend of an association between RA and the combined primary outcome, supporting that RA per se, but in particular seropositive and active RA, may increase the risk of CAD even after initial CAD diagnosis and treatment.
Keywords: rheumatoid arthritis, cardiovascular disease, epidemiology
Key messages.
What is already known about this subject?
Evidence of an increased risk of cardiovascular disease (CVD) in rheumatoid arthritis (RA) is well established.
What does this study add?
· In patients with RA referred to cardiac CT due to chest pain, there is an association between RA and major adverse cardiovascular events. Our findings support that in particular seropositive and active RA may increase the risk of CVD.
How might this impact on clinical practice?
Patients with seropositive RA, who have had disease flares within the past 3 years, have an additional risk of CVD, which emphasises the importance of treating to target. Not only for the sake of joint protection, but also in order to prevent the risk of CVD.
Background
The prevalence of cardiovascular (CV) disease is increased among patients with rheumatoid arthritis (RA).1 Recent data suggest that patients with early-onset RA, receiving consistent RA medication, have no increased risk of CV mortality compared with the general population, at least in the early years of the disease.2 However, many patients with RA still receive suboptimal or no CV risk management.3
Substantial evidence suggests an association between impaired endothelial function and the presence of rheumatoid factor (RF) and anticyclic citrullinated peptide (ACPA) independent of other CV risk factors.4–6 Furthermore, a clear link has been described between chronic inflammation and coronary artery disease (CAD).4 Additionally, a recent study has demonstrated that residual disease activity based on Disease Activity Score 28 (DAS28) was associated with the presence of high-risk non-calcified plaques and plaques with mixed non-calcified and calcified content in patients with RA.7 The same study demonstrated that patients with RA without known CAD had a higher prevalence, extent and severity of coronary plaques compared with healthy controls.7
Due to the association between increased CAD and RA,7 the CV disease (CVD) risk profile in RA tends to be underestimated by conventional clinical CV risk algorithms.8 9 Cardiac CT is an accurate method to evaluate CAD in patients with angina pectoris.10 The CT angiography (cardiac CT) provides measurements of the Coronary Artery Calcium Score (CACS), which is a strong predictor of future events,11 and an independent estimate of all-cause mortality, providing independent prognostic information in addition to traditional risk factors.12 In combination with the contrast-enhanced CT angiography, CACS is considered suitable for the diagnostics of patients with low-to-intermediate pretest probability of CAD. CACS has consistently been reported to be the strongest known risk marker of CV risk compared with other risk assessments.13 Therefore, this very low-dose imaging tool is now recommended in the new European Society of Cardiology guideline.14
Many cohort studies have investigated the risk of major CV events and death among patients with RA compared with the background population,1 15 and guidelines recommended screening for incident CVD at least every 5th year.16 Still, despite improved treatment of patients with RA with the treat-to-target strategy17 implying escalation with disease-modifying antirheumatic drugs (DMARDs) and glucocorticoid (GC) flare treatment (either per oral or as intra-articular injection) our knowledge regarding the effect of RA on the prognosis after an initial CAD diagnosis is limited.
The Western Denmark Cardiac Computed Tomography Registry (Western Denmark Heart Registry (WDHR)) contains data on approximately 63 000 cardiac CT examinations with registration of up to 40 variables for each procedure, making it possible to investigate the consequences of the increased risk of CAD among patients with RA.10
The aim of the present study was to examine the risk of major CV events and death among RA and non-RA patients with chest pain referred to cardiac CT after the initial diagnosis and treatment for CVD.
Methods
Design and study population
This registry-based follow-up study was completed and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology recommendations.18
The study used data from the WDHR database. Data were collected at nine cardiac centres in Denmark between 1 January 2008 and 31 December 2016 covering a population of approximately 3.3 million inhabitants, about 55% of the Danish population. WDHR data were linked to Danish nationwide registers using the unique personal identification number from the Civil Registration System (CRS), assigned to all Danish citizens at birth.10
Patients were only included into the study if the reason for performing the CT was suspicion of CAD. According to Danish guidelines, patients suspected of CAD with an a priori low-to-intermediate risk profile of CAD (15%–85%) may be referred to a cardiac CT.
Patients had their first cardiac CT due to chest pain and suspicion of CAD between 1 January 2008 and 31 December 2016; follow-up ended on 30 June 2017. Events that occurred within 90 days after the cardiac CT were considered a result of the cardiac CT findings, and not the exposure (RA); the first 90 days after cardiac CT were thus omitted.
Patients were excluded if they had a history of vascular disease, acute myocardial infarction (AMI), percutaneous coronary intervention (PCI), heart surgery, known CAD or artrial fibrillation, prior to or up to 90 days after cardiac CT. Furthermore, patients were excluded if they had another indication for cardiac CT than CAD suspicion, age <18 years or follow-up <90 days.
Exposure
Identification of patients with RA
In the WDHR, patients diagnosed with RA were identified through the Danish National Patient Registry (DNPR).19 NPR includes data on all somatic hospital admissions for inpatients since 1977 and outpatients since 1995, including information on discharge diagnoses, hospital department and dates of admission and discharge. Data on patients admitted to privately practising rheumatologists are not included in the NPR.
Since 1994, discharge diagnoses in the NPR have been classified according to the ICD-10 classification.19 The following codes for RA were used: M05.3, M05.9, M05.8 and M06.9. For diagnosis before 1994, the ICD-8 codes 712.19, 712.39 and 712.59 were used.
To increase the specificity of the RA diagnosis, we combined the RA ICD-10 codes with relevant treatment codes in the DNPR using the Anatomical Therapeutic Chemical (ATC) Classification codes for conventional synthetic DMARDs (csDMARDs): methotrexate (ATC: L01BA01, L04A×03), sulfasalazine (ATC: A07EC01), azathioprine (ATC: L04A×01), chloroquine phosphate (ATC: P01BA02) and leflunomide (ATC: L04AA13).20 Patients with an RA diagnosis any time before and within 90 days after the date of cardiac CT were considered to be exposed.
RA treatment escalation
According to the treat-to-target strategy, escalation to treatment with biological DMARDS (bDMARDs) or targeted synthetic DMARDs is offered to patients with RA when disease activity has lasted for >3 months or treatment goal or remission has not been achieved after 6 months.17 Hence, information about escalation of treatment can be seen as a surrogate marker for disease severity, although there may be other explanations such as intolerance and side effects. We defined two treatment levels: csDMARD (treatment with csDMARD alone) and bDMARD (treatment with bDMARD at any time during the disease course with or without a combination with csDMARDs).
Information on csDMARDs was retrieved through the DNPR using the ACT codes mentioned above. Information on bDMARDs was identified through the NPR using the following treatment procedure codes: etanercept (ICD-10: BOHJ18A2), infliximab (ICD-10: BOHJ18A1), adalimumab (ICD-10:BOHJ18A3), golimumab (ICD-10: BOHJ18A4), certolizumab (ICD-10: BOHJ18A5), tocilizumab (ICD-10: BOHJ18B2), abatacept (ICD-10: BOHJ18C1) and rituximab (ICD-10: BOHJ11).
RA disease activity
In RA, the disease fluctuates between long periods of remission and short periods with disease flares. In Denmark, RA flares are generally controlled by intra-articular GCIs into the swollen joints and/or administration of intramuscular GC injections (GCIs).21 Therefore, we used information about GCIs as a surrogate marker for disease activity. The following treatment codes were retrieved from the NPR: BLHN0 (injections in joints and tissue), BLHN00 (injections in upper or lower extremities) and BLHO01 (injection in temporomandibular joints). Information was retrieved up to 3 years prior to the cardiac CT examination. We defined two variables: >1 GCI during the past 3 years and 0 GCIs during the past 3 years. As patients often receive multiple GCI injections during the first year following a diagnosis, the first year was ignored.
RA serology
Data on RA seropositivity are considered robust. However, misclassification often occurs among patients with a diagnosis of seronegative RA.20 Therefore, we defined three serological subtypes: ‘overall RA’, ‘seropositive RA’ and ‘other RA’. Patients registered with one of the ‘M05’ diagnostic codes were defined as having ‘seropositive RA’ and patients registered with one of the ‘M06’ diagnostic codes as having ‘other RA’. In the registres, we were unable to distinguish between patients who were ACPA positive and IgM rheumatoid factor positive.
RA treatment escalation, disease activity and serology were assessed up to 90 days after cardiac CT.
Covariates
Comorbidity and traditional CVD risk factors
The Charlson Comorbidity Index22 was applied based on data from the NPR, at any time before date of cardiac CT.
Traditional CVD risk factors such as smoking and obesity were retrieved from WDHR. Data on diabetes were defined as follows: the patients were etiher registered with diabetes in WDHR or were identified with one of the following International Classification of Diseases -10 (ICD-10) codes: E-10-E14, O24 (but not O24.4) and H36.0. For ICD-8 codes, we used 249–250. Patients were considered diabetic if they had been identified as users of the following drugs in the DNPR: insulin and analogues (ATC: A10A) or blood glucose-lowering drugs, except insulin (ATC: A10B).
Users were patients who filled in a prescription within 180 days before the cardiac CT examination.
Concurrent drugs
Information on concurrent medications was collected from both DNPR and WDHR. Treatment with lipid-lowering drugs was defined as either a registration of treatment in the WDHR or as statin user in the DNPR (ATC: C10AA). Treatment with oral GC therapy was retrieved from DNPR (ATC: H02, H02AB).
In Denmark, most patients are diagnosed and treated for hypertension by their general practitioner, and therefore, data on this diagnosis are not valid in NPR. Thus, instead we used data on treatment with blood pressure medications to identify patients with hypertension. This information was retrieved through DNPR; angiotensin-converting enzyme or angiotensin II inhibitors (ATC: C09A, C09B, C09C), beta-blockers (ATC: C07A), calcium channel blockers (ATC: C08CA) and bendroflumethiazide (ATC: C03AB01).
Identification of CACS
CACSs were identified through the WDHR, and divided into four levels: 0; 1–99; 100–399 and >400.23
Outcome
We abstained from including data on CV endpoints such as heart failure due to low validity of these diagnosis in NPR.24 Thus, the outcome of this study was major adverse cardiovascular events (MACE). In order to take potential heterogeneity in using a composite endpoint into account, we included death in our primary outcome and presented MACE without death as our secondary outcome as a sensitivity analysis.25
Primary outcome
We conducted a combined outcome calculation including: AMI (ICD-10: multiple imputation 2 (MI2)), ischaemic stroke (ICD-10: I63, unspecified stroke (ie, not specified as haemorrhage or infarction (ICD-10: I64), coronary artery bypass grafting (ICD-10 code: I25.810), PCI (ICD-10 code: I21.A9) and all-cause mortality.
Secondary outcome
As secondary outcome we looked at the combination of myocardial infarction (ICD-10: MI2), ischaemic stroke (ICD-10: I63) and unspecified stroke (ICD-10: I64), and coronary artery bypass grafting (ICD-10 code: I25.810) and PCI (ICD-10 code: I21.A9).
Diagnostic information was retrieved from the NPR and information on death from the CRS.
Statistical analysis
We compared patients with RA as well as patients with RA stratified by serology, treatment level and disease activity with non-RA patients (reference group). For the primary outcome, patients were followed until myocardial infarction, ischaemic stroke, unspecified stroke, coronary artery bypass grafting, PCI, death, emigration or end of follow-up, whichever came first.
For the secondary outcome, patients were followed until myocardial infarction, ischaemic stroke, unspecified stroke, emigration or end of follow-up, whichever came first.
We tabulated all relevant, available characteristics of the patients according to RA. Associations between each RA exposure and the two outcomes were examined using Cox Proportional hazards regression models with delayed entry of 90 days. We computed HRs and 95% CIs in crude and adjusted models, adjusting for: sex, age, comorbidity (0, 1, 2+), hypertension (yes/no), lipid-lowering treatment (yes/no), body mass index (BMI) (<18, 18.5–24.9, 25–29.9, >30) and smoking (never, former current).
Model fit was assessed graphically and found adequate in spite of the limited number of events among patients with RA.
We used MI by chained equation to handle missing values for BMI and smoking. The procedure relies on the missing at random assumption. We independently analysed 50 copies of the data, each with missing values imputed with a proper imputation model, adjusting for all plausible measured covariates. The continuous variables height (15% missing) and weight (14% missing) were imputed by a regression method. A multivariate logistic regression was used for imputation of smoking (8% missing). The estimates from the 50 datasets were combined according to Rubin’s rule.26 Sensitivity analyses were performed for smoking and calcium score both with respect to the MI model and the regression model.
We generated cumulative incidence function (CIF) plots to show the cumulative failure rates over time due to primary and secondary outcome with follow-up starting 90 days after cardiac CT.
Results
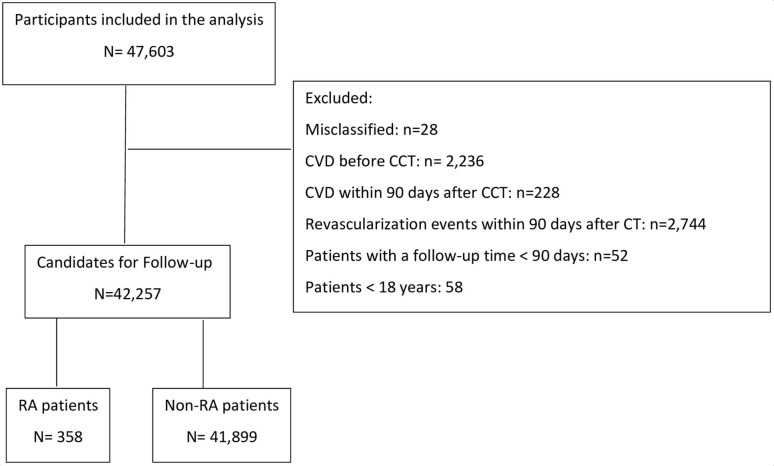
We identified 47 603 patients, and 42 257 eligible patients were included during the 8-year study period. A total of 5346 patients were excluded. Figure 1 gives a full overview of eligible, excluded and included patients.
Figure 1.
Study flow chart. CVD, cardiovascular disease; RA, rheumatoid arthritis. CCT: Cardiac CT
Among the included patients, n=358 (0.8%) were identified with RA. The median RA duration was 6.5 years (min-max: 0.1–14.0). The incidence rate for revascularisation in RA and non-RA patients was 3.4 (95% CI 1.3 to 9.0) vs 3.7 (95% CI 3.7 to 4.1) per 1000 person-years. Baseline characteristics among RA and non-RA patients are listed in table 1. Patients with RA tended to be older (median age 67.7 (IQR: 55.8–70.1) vs 57.2 (IQR: 49.1–65.1)), more often female (76% vs 56%) and to have more comorbidities (prevalence of comorbidity score >2 was 22.7% vs 12.5%). No differences were seen concerning smoking, obesity, diabetes or non-obstructive CAD. However, more patients with RA had a calcium score >0 (49.7 vs 40.2%), and were more likely to have hypertension (55.9% vs 45.8%), and to be treated with lipid-lowering drugs (40.0% vs 33.7%) and oral GC treatment (22.3% vs 4.3 %). CACS >399 was seen among 6.5% of the non-RA patients versus 10.3% among patients with RA. The median time until censoring or primary outcome was 3.2 (IQR 0.0–8.6) vs 3.5 (IQR 0.0–9.2) years for RA and non-RA patients, respectively.
Table 1.
Baseline characteristics among 42 257 patients admitted to their first cardiac CT procedure due to chest pain from 1 January 2008 to 31 December 2016 in Denmark
| Non-RA patients | Patients with RA | |||
| N | % | N | % | |
| No of patients | 41 899 | 99.2 | 358 | 0.8 |
| Age, median (IQR)* | 57.24 (49.1–65.1) | 62.7 (55.7–70.1) | ||
| Gender | ||||
| Male | 18 438 | 44.0 | 84 | 23.5 |
| Female | 23 461 | 56.0 | 274 | 76.5 |
| Comorbidity according to Charlson | ||||
| No comorbidity | 29 989 | 71.6 | 204 | 57.0 |
| Moderate comorbidity | 6675 | 15.9 | 75 | 20.9 |
| Severe comorbidity | 5235 | 12.5 | 79 | 22.1 |
| Diabetes, N (%) | 3308 | 7.9 | 31 | 8.7 |
| BMI (kg/m2) | ||||
| <18.5 | 455 | 1.1 | 6 | 1.7 |
| 18.5≤25 | 13 568 | 32.4 | 132 | 36.9 |
| 25≤30 | 14 463 | 34.5 | 122 | 34.1 |
| ≤30 | 7278 | 17.4 | 61 | 17.0 |
| Missing | 6136 | 14.6 | 37 | 10.3 |
| Hypertension, N (%) | 19 203 | 45.8 | 200 | 55.9 |
| Treatment with lipid-lowing drugs N (%) | 14 115 | 33.7 | 143 | 39.9 |
| Smoking | ||||
| Current smoker | 8766 | 20.9 | 57 | 15.9 |
| Former smoker | 13 362 | 31.9 | 147 | 41.0 |
| Never smoker | 16 410 | 39.2 | 120 | 33.5 |
| Missing | 2361 | 8,2 | 34 | 9.5 |
| Level of stenosis | ||||
| Non-obstructive CAD | 12 657 | 30.2 | 122 | 34.1 |
| 1-vessel obstructive CAD | 4014 | 9.6 | 37 | 10.3 |
| 2-vessel obstructive CAD | 959 | 2.3 | 13 | 3.6 |
| 3-vessel/LM obstructive CAD | 246 | 0.6 | 5 | 1.4 |
| Missing | 63 | 0.2 | 1 | 0.3 |
| Calcium score | ||||
| 0 | 19 690 | 47.0 | 132 | 36.9 |
| 1–99 | 9789 | 23.4 | 101 | 28.2 |
| 100–399 | 4331 | 10.3 | 41 | 11.5 |
| >399 | 2706 | 6.5 | 37 | 10.3 |
| Missing | 5384 | 12.8 | 47 | 13.1 |
*Age at time for first cardiac CT due to chest pain.
BMI, body mass index; CAD, coronary artery disease; LM, Left Main; RA, rheumatoid arthritis.
Primary outcome
Table 2 displays the number of events, incidence rates and HRs for the primary outcome in RA and non-RA patients. The incidence rate in RA and non-RA patients was 24.4 vs 14.0 per 1000 person-years, corresponding to an adjusted HR 1.35 (95% CI 0.93 to 1.96).
Table 2.
Crude, adjusted and imputed HR rate with 95% CI for the combined outcome: acute myocardial infarction (ICD-10: MI2), ischaemic stroke (ICD-10: I63), unspecified stroke (ICD-10: I64), coronary artery bypass grafting (ICD-10 code: I25.810), percutaneous coronary intervention (ICD-10 code: I21.A9) and all-cause mortality among 42 257 patients undergoing their first non-contrast-enhanced cardiac CT examination due to incident chest pain in Denmark, 2008–2016
| Exposure | N | Events in follow-up | Rate per 1000 person-years (95% CI) |
HR, crude (95% CI) |
HR, adjusted* (95% CI) |
Imputed† 95% CI |
| Overall | ||||||
| Not RA | 41 899 | 2052 | 14.0 (13.4 to 14.6) | 1 | 1 | 1 |
| RA | 358 | 28 | 24.4 (16.8 to 35.3) | 1.75 (1.21 to 2.54) | 1.31 (0.90 to 1.91) | 1.35 (0.93 to 1.96) |
| Serology | ||||||
| Not RA | 41 899 | 2052 | 14.0 (13.4 to 14.6) | 1 | 1 | 1 |
| Seropositive RA | 280 | 22 | 24.9 (16.4 to 37.8) | 1.79 (1.20 to 2.72) | 1.41 (0.92 to 2.14) | 1.42 (0.93 to 2.16) |
| Other RA | 78 | 6 | 22.7 (10.2 to 50.4) | 1.62 (0.73 to 3.61) | 1.35 (0.60 to 3.02) | 1.15 (0.51 to 2.56) |
| Flare treatment | ||||||
| Not RA | 41 899 | 2052 | 14.0 (13.4 to 14.6) | 1 | 1 | 1 |
| No joint injection‡ | 215 | 13 | 18.9 (11.0 to 32.6) | 1.35 (0.79 to 2.34) | 1.22 (0.69 to 2.15) | 1.05 (0.61 to 1.81) |
| ≥1 joint injections§ | 143 | 15 | 32.4 (19.6 to 53.8) | 2.34 (1.41 to 3.89) | 1.62 (0.92 to 2.85) | 1.80 (1.08 to 3.00) |
| Treatment escalation | ||||||
| Not RA | 41899 | 2052 | 14.0 (13.4 to 14.6) | 1 | 1 | 1 |
| csDMARD¶ | 257 | 22 | 26.7 (17.6 to 40.6) | 1.92 (1.26 to 2.91) | 1.42 (0.91 to 2.24) | 1.38 (0.91 to 2.11) |
| bDMARD** | 101 | 6 | 18.4 (8.30 to 41.0) | 1.33 (0.59 to 2.95) | 1.27 (0.53 to 3.05) | 1.25 (0.56 to 2.79) |
*Adjusted for sex, age, comorbidity, hypertension, lipid-lowing treatment, BMI, smoking
† Imputation of missing values for BMI and smoking.
‡0 joint injection within 3 years prior to cardiac CT.
§≥1 joint injection within 3 years prior to cardiac CT.
¶Patients exclusively treated with csDMARDs.
**Patients treated with a bDMARD at any time during the course of disease.
bDMARDs, biological disease-modifying antirheumatic drugs; BMI, body mass index; csDMAD, conventional synthetic disease-modifying antirheumatic drugs; RA, rheumatoid arthritis.
Patients who had received flare treatment 3 years prior to cardiac CT had an incidence rate of 19.3 per 1000 person-years, corresponding to an HR 1.80 (95% CI 1.08 to 3.00). Patients with seropositive RA had an incidence rate of 24.9 per 1000 person-years, corresponding to an adjusted HR 1.42 (0.93 to 2.16).
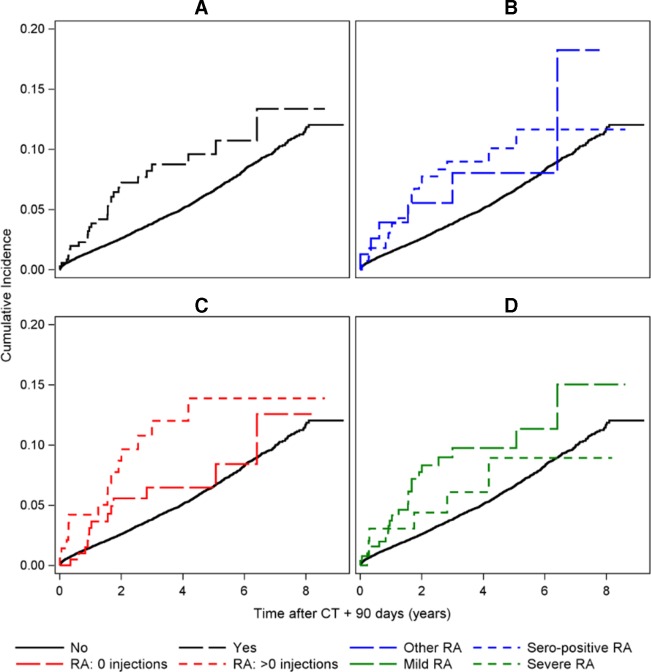
The risk of developing CV events did not depend on the RA treatment. Patients treated with csDMARD had an incidence rate of 14.4 per 1000 person-yers, corresponding to an adjusted HR 1.38 (95% CI 0.91 to 2.11). Patients treated with bDMARDs at any time during the course of disease had an incidence rate of 18.4 per 1000 person-years, corresponding to an adjusted HR 1.25 (95% CI 0.56 to 2.79). Figure 2 shows the CIF curves for each exposure.
Figure 2.
CIF curves for the primary outcome: myocardial infarction, ICD-10: MI2, ischaemic stroke, ICD-10: I63, unspecified stroke, ICD-10: I64, coronary artery bypass grafting, ICD-10 code: I25.810, percutaneous coronary intervention, ICD-10 code: I21.A9 and all-cause mortality. For the exposures (A) RA versus non-RA, (B) seropositive RA versus other RA, (C) flare treatment versus no flare treatment and (D) sDMARD versus bDMARD treatment. bDMARD, biological disease-modifying antirheumatic drug; CIF, cumulative incidence function; MI2, multiple imputation 2; RA, rheumatoid arthritis; sDMARD, synthetic DMARD.
Secondary outcome
For the secondary outcome, we found similar but stronger associations. The incidence rate in RA and non-RA patients was 15.7 vs 8.2 per 1000 person-years, corresponding to an adjusted HR 1.58 (95% CI 0.99 to 2.52). Patients who had received flare treatment 3 years prior to cardiac CT had an incidence rate of 21.6 per 1000 person-years vs 11.7 for patients, who did not, corresponding to an HR 2.21 (95% CI 1.18 to 4.13). The incidence rate for seropositive and other RA was 18.1 vs 7.6 per 1000 person-years, corresponding to an adjusted HR 1.79 (95% CI 1.03 to 3.10). No differences were seen in DMARD treatment. All results are shown in table 3.
Table 3.
Crude, adjusted and imputed HR rate with 95% CIs for the secondary outcome: myocardial infarction (ICD-10: MI2), ischaemic stroke (ICD-10: I63) and unspecified stroke (ICD-10: I64) and coronary artery bypass grafting (ICD-10 code: I25.810) and percutaneous coronary intervention (ICD-10 code: I21.A9) among 42 257 patients undergoing their first non-contrast-enhanced cardiac CT examination due to incident chest pain in Denmark, 2008–2016
| Exposure | N | Events in follow-up | Rate per 1000 person-years (95% CI) | HR, crude (95% CI) | HR, adjusted* (95% CI) |
Imputed† 95% CI |
| Overall | ||||||
| Not RA | 41899 | 1208 | 8.2 (7.8 to 8.7) | 1 | 1 | 1 |
| RA | 358 | 18 | 15.7 (9.9 to 24.9) | 1.40 (0.70 to 2.81) | 1.88 (1.08 to 3.26) | 1.58 (0.99 to 2.52) |
| Serology | ||||||
| Not RA | 41 899 | 208 | 8.2 (7.8 to 8.7) | 1 | 1 | 1 |
| Seropositive RA | 280 | 16 | 18.1 (11.1 to 29.6) | 1.88 ((1.33 to 3.58) | 1.42 (0.93 to 2.16) | 1.79 (1.03 to 3.10) |
| Other RA | 78 | 2 | 7.6 (1.9 to 30.2) | 0.92 (0.22 to 3.66) | 1.15 (0.51 to 2.56) | 0.82 (0.20 to 3.32) |
| Flare treatment | ||||||
| Not RA | 41 899 | 1208 | 8.2 (7.8 to 8.7) | 1 | 1 | 1 |
| No joint injection‡ | 215 | 8 | 11.7 (5.8 to 23.3) | 1.40 ((0.77 to 2.81) | 1.27 (0.60 to 2.67) | 1.17 (0.58 to 2.34) |
| ≥1 joint injections§ | 143 | 10 | 21.6 (11.6 to 40.2) | 2.62 (1.40 to 4.89) | 1.92 (0.95 to 3.86) | 2.21 (1.18 to 4.13) |
| Treatment escalation | ||||||
| Not RA | 41 899 | 1208 | 8.2 (7.8 to 8.7) | 1 | 1 | 1 |
| csDMARD¶ | 257 | 14 | 17.0 (10.1 to 22.7) | 2.05 (1.21 to 3.47) | 1.64 (0.93 to 2.91) | 1.63 (0.96 to 2.76) |
| bDMARD** | 101 | 4 | 12.3 (4.6 to 32.8) | 1.48 (0.55 to 3.96) | 1.26 (0.40 to 3.93) | 1.43 (0.54 to 3.83) |
*Adjusted for sex, age, comorbidity, hypertension, lipid-lowing treatment, BMI, smoking
†Imputation of missing values for BMI and smoking.
‡0 joint injection within 3 years prior to cardiac CT.
§>1 joint injection within 3 years prior to cardiac CT.
¶Patients exclusively treated with csDMARDs.
**Patients treated with a bDMARD at any time during the disease course.
bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; MI2, multiple imputation 2; RA, rheumatoid arthritis.
Discussion
In patients referred to cardiac CT due to chest pain, there is a trend of an association between RA and major adverse events. Our findings support that in particular seropositive and active RA may increase the risk of CVD among patients with RA.
Evidence of an increased risk of CVD in RA is well established; however, most epidemiological studies have estimated the CVD risk in RA compared with the general population. A meta-analysis of observational studies found an overall increased risk of AMI and cerebrovascular incidents of 68%.27 In a large Danish cohort study, Lindhardsen et al found that patients with RA had a 71% increased risk of developing AMI compared with the general population.1 We found a risk among patients with RA of only 35%, which is probably due to the fact that we excluded patients who were treated with revascularisation within 90 days after the cardiac CT scan, and further, patients who were diagnosed with CAD, were treated medically following the cardiac CT scan. Furthermore, we compared our results with a population with an increased risk of CAD, which may also have resulted in lower estimates. Finally, our study was smaller, leading to a higher degree of imprecision and wider CIs. Therefore, the absolute magnitude of the estimates must be interpreted with some caution.
The link between inflammation in RA and atherosclerosis plaques is well decribed,4 5 and we know that patients with seropositive RA tend to have more severe joint damage and extra-articular manifestations, including CVD.28
In line with this, we saw a tendency towards an increased risk of developing major CV events among patients with seropositive RA, and patients who had experienced episodes of flares prior to the cardiac CT scan. Evidence also suggests that treatment with bDMARDs may be associated with a reduced risk of CV events.29 We found the risk to be almost the same for patients treated with csDMARDs and bDMARDS. However, due to a few events, the estimates for bDMARDs presented with 95% CIs ranging from a decreased risk to a high risk.
In our cohort, approximately 10% of patients having RA referred to cardiac CT due to chest pain, had a calcium score >399. This is in line with a study by Rollefstad et al, showing that chest pain in it self was weakly associated with coronary atherosclerosis.30 However, the predicted CVD risk by several risk calculators was highly associated with the presence of coronary atherosclerosis. We abstained from including CACS in the analysis, as this might act as a mediator. Sensitivity analysis where performed, where we tried two strategies: including CACS as a confounder, and stratified according to CACS=0 vs CACS >0. All analyses showed the same trend in the results but did, however, have large CIs, and in particular, the stratified analysis mainly made sense for overall RA as it became critical with the low number of events in the other analysis.
Our study has some limitations. First of all, we had relatively few events in our dataset, leading to borderline or non-significant results. Ideally, we could have included other CV endpoints, such as, for example, heart failure. The DNPR is widely used for research purposes, however, it is an administrative database, and the validity of diagnosis varies between different diseases. Thus, it has been recommended not to use the information on heart failure if not validated.24 Therefore, in order to avoid misclassification and overestimation of the effect, we chose to only include information on diagnosis with known high validity.
The use of multiple composite end points in CV research can be debated.31 We decided to present two different definitions as a sensitivity analysis—one including death and one without this endpoint. Overall, the findings in the two definitions were similar, however, the inclusion of all-cause mortality into the definition (our primary outcome) resulted in less precise estimates. We only have access to all-cause mortality, and the use of CV death might enhance specificity. However, in the Danish Cause of Death register, a lack of accuracy in mortality from ischaemic heart disease has been discovered, mainly due to discontinuity in rules for the classification of ill-defined conditions as ‘unknown cause of death’ versus prioritising of ischaemic heart disease as the underlying cause of death.32
Second, more patients in the RA group were treated with oral GC, which may have a negative impact on the cardiovascular system.33 However, the risk of harm is considered low for the majority of patients with RA receiving dosages <5 mg/day.34 Furthermore, non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with an increased risk of CAD,16 but in Denmark, many NSAIDs are over-the-counter drugs, and therefore, we had no access to this information through the registres. Consequently, we were unable to control for this confounder. Lowering disease activity in RA may, however, also have beneficial effects on the CVD risk, and Denmark is following the use of NSAIDs and corticosteroids according to treatment-specific guidelines16 34 Therefore, we do not believe that the difference in oral GC and NSAIDs use have had any substantial effect in the development of CVD in our study.
We had no access to a continuous measure of disease activity in RA such as the DAS28.35 Instead we used GCI flare treatment prior to the cardiac CT as a surrogate marker.21
Thus, some patients who presented with high disease activity, but were not offered a GCI might have been wrongly classified. This bias would have made us underestimate the influence of disease activity on the risk of developing CV events. However, as we found that patients who had had >1 GCI 3 years prior to cardiac CT had an increased risk, this bias is probably not substantial in our study. It is well known that flares should be seen as an accumulation over time.36 Therefore, we have used a time window of 3 years before the index date (ie, the occurrence of a CV event) to define flares. To make the assessment as precise as possible, we ignored the first year following RA diagnosis, as patients often receive multiple GCI injections during the first year following an RA diagnosis. This might potentially have made us underestimate the number of patients with high disease activity. However, since the median disease duration was 6.5 years, we believe this potential bias would have had a minimal effect on the overall estimate.
The selection of patients in our cohort was restricted to patients with symptoms of CVD, and limited to a median follow-up period of 3.5 (IQR 0.0 to 9.2). Therefore, we cannot make generalisations to the general RA population, and we are unable to evaluate the long-term risk of cardiovascular events in RA.
Finally, patients seeking private practising rheumatologists are expected to have a milder disease course than patients referred to hospital, and would thus be expected to have a lower risk of major CV events, which could lead to an overestimation of the risk in our study. However, the vast majority of rheumatology specialists in Denmark are hospital based, thus, we expect any potential selection bias to have little impact on our study estimates.
We consider it to be a strength of our study that all data in WDHR were prospectively collected, and that we had almost complete coverage and validated information on both exposure and outcome. A large international cohort among 5638 patients with RA has shown that RA risk factors were attributable for 30.3% of CVD risk, and that lifestyle factors such as BMI and smoking explained 69.9% of the risk.8 Therefore, we consider it to be a strength of our study that the WDHR contains information on these important confounders, which is usually not included in administrative databases. The inclusion of these variables increases the reliability of the adjusted estimates. Further, in Denmark, cardiac CT is the primary and preferred investigation offered to patients with low risk of CAD. Only patients with very high risk (>85%) are referred directly to invasive coronary angiography. Therefore, we believe that the population is representative to all patients with RA and chest pain. Patients with very high CAD risk (>85%) are, however, referred directly to invasive coronary angiography and are not included in this present study. Finally, it has been shown that patients with RA are generally underdiagnosed, and undertreated for their CVD.3 In this cohort, all patients have, however, been seen by a cardiologist, and subsequently treated according to their CAD diagnosis, making this potential misclassification unlikely.
Conclusion
We found a trend of an association between RA and the development of CVD in this cohort of patients with an a priori low-intermediate risk of CAD, even after initial diagnosis and medical treatment, supporting that RA per se, and in particular seropositive and active RA, may increase the risk of CAD even after initial CAD diagnosis and treatment.
Acknowledgments
This paper was funded by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Regions.
Footnotes
Contributors: All authors included in the paper fulfil the criteria of authorship. The final manuscript has been seen and approved by all the authors.
Funding: This paper was funded by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Region.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: In Denmark, register based studies do not need either ethics approval.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementary information. All data are deidentified participant data avaliable from the corresponding author on reasonable request. Reuse is not permitted
References
- 1. Lindhardsen J, Ahlehoff O, Gislason GH, et al. . The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011;70:929–34. 10.1136/ard.2010.143396 [DOI] [PubMed] [Google Scholar]
- 2. Kerola AM, Nieminen TV, Virta LJ, et al. . No increased cardiovascular mortality among early rheumatoid arthritis patients: a nationwide register study in 2000-2008. Clin Exp Rheumatol 2015;33:391–8. [PubMed] [Google Scholar]
- 3. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. . Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology 2016;55:1210–6. 10.1093/rheumatology/kew039 [DOI] [PubMed] [Google Scholar]
- 4. Sen D, González-Mayda M, Brasington RD. Cardiovascular disease in rheumatoid arthritis. Rheum Dis Clin North Am 2014;40:27–49. 10.1016/j.rdc.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 5. Hjeltnes G, Hollan I, Førre Ø, et al. . Anti-CCP and rf IgM: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol 2011;40:422–7. 10.3109/03009742.2011.585350 [DOI] [PubMed] [Google Scholar]
- 6. Navarro-Millán I, Yang S, DuVall SL, et al. . Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans health administration. Ann Rheum Dis 2016;75:341–7. 10.1136/annrheumdis-2013-204987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karpouzas GA, Malpeso J, Choi T-Y, et al. . Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis 2014;73:1797–804. 10.1136/annrheumdis-2013-203617 [DOI] [PubMed] [Google Scholar]
- 8. Crowson CS, Rollefstad S, Ikdahl E, et al. . Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:48–54. 10.1136/annrheumdis-2017-211735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arts EEA, Popa CD, Den Broeder AA, et al. . Prediction of cardiovascular risk in rheumatoid arthritis: performance of original and adapted score algorithms. Ann Rheum Dis 2016;75:674–80. 10.1136/annrheumdis-2014-206879 [DOI] [PubMed] [Google Scholar]
- 10. Nielsen LH, Nørgaard BL, Tilsted HH, et al. . The Western Denmark cardiac computed tomography registry: a review and validation study. Clin Epidemiol 2015;7:53–64. 10.2147/CLEP.S73728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown ER, Kronmal RA, Bluemke DA, et al. . Coronary calcium coverage score: determination, correlates, and predictive accuracy in the multi-ethnic study of atherosclerosis. Radiology 2008;247:669–75. 10.1148/radiol.2473071469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Budoff MJ, Dowe D, Jollis JG, et al. . Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter accuracy (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol 2008;52:1724–32. 10.1016/j.jacc.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 13. Nissen L, Winther S, Westra J, et al. . Diagnosing coronary artery disease after a positive coronary computed tomography angiography: the Dan-NICAD open label, parallel, head to head, randomized controlled diagnostic accuracy trial of cardiovascular magnetic resonance and myocardial perfusion scintigraphy. Eur Heart J Cardiovasc Imaging 2018;19:369–77. 10.1093/ehjci/jex342 [DOI] [PubMed] [Google Scholar]
- 14. Knuuti J, Wijns W, Saraste A, et al. . Esc guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019;2019. [DOI] [PubMed] [Google Scholar]
- 15. Solomon DH, Kremer J, Curtis JR, et al. . Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis 2010;69:1920–5. 10.1136/ard.2009.122226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agca R, Heslinga SC, Rollefstad S, et al. . EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]
- 17. Smolen JS, Breedveld FC, Burmester GR, et al. . Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international Task force. Ann Rheum Dis 2016;75:3–15. 10.1136/annrheumdis-2015-207524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, et al. . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 19. Mosbech J, Jorgensen J, Madsen M, et al. . The National patient registry. evaluation of data quality. Ugeskrift for laeger 1995;157:3741–5. [PubMed] [Google Scholar]
- 20. Linauskas A, Overvad K, Johansen MB, et al. . Positive predictive value of first-time rheumatoid arthritis diagnoses and their serological subtypes in the Danish national patient registry. Clin Epidemiol 2018;10:1709–20. 10.2147/CLEP.S175406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hetland ML, Hørslev-Petersen K. The CIMESTRA study: intra-articular glucocorticosteroids and synthetic DMARDs in a treat-to-target strategy in early rheumatoid arhtritis. Clin Exp Rheumatol 2012;30:S44–9. [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 23. Abdulla J, Pedersen KS, Budoff M, et al. . Influence of coronary calcification on the diagnostic accuracy of 64-slice computed tomography coronary angiography: a systematic review and meta-analysis. Int J Cardiovasc Imaging 2012;28:943–53. 10.1007/s10554-011-9902-6 [DOI] [PubMed] [Google Scholar]
- 24. Delekta J, Hansen SM, AlZuhairi KS, et al. . The validity of the diagnosis of heart failure (I50.0-I50.9) in the Danish national patient register. Dan Med J 2018;65. [PubMed] [Google Scholar]
- 25. McCoy C. Understanding the use of composite endpoints in clinical trials. WestJEM 2018;19:631–4. 10.5811/westjem.2018.4.38383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donders ART, van der Heijden GJMG, Stijnen T, et al. . Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59:1087–91. 10.1016/j.jclinepi.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 27. Aviña-Zubieta JA, Choi HK, Sadatsafavi M, et al. . Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7. 10.1002/art.24092 [DOI] [PubMed] [Google Scholar]
- 28. Myasoedova E, Chandran A, Ilhan B, et al. . The role of rheumatoid arthritis (rA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis 2016;75:560–5. 10.1136/annrheumdis-2014-206411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barnabe C, Martin B-J, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 2011;63:522–9. 10.1002/acr.20371 [DOI] [PubMed] [Google Scholar]
- 30. Rollefstad S, Ikdahl E, Hisdal J, et al. . Association of chest pain and risk of cardiovascular disease with coronary atherosclerosis in patients with inflammatory joint diseases. Front. Med. 2015;2:80 10.3389/fmed.2015.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kip KE, Hollabaugh K, Marroquin OC, et al. . The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol 2008;51:701–7. 10.1016/j.jacc.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 32. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39:26–9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 33. Myasoedova E, Crowson CS, Nicola PJ, et al. . The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol 2011;38:1601–6. 10.3899/jrheum.100979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strehl C, Bijlsma JWJ, de Wit M, et al. . Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR Task force. Ann Rheum Dis 2016;75:952–7. 10.1136/annrheumdis-2015-208916 [DOI] [PubMed] [Google Scholar]
- 35. Prevoo ML, van 't Hof MA, Kuper HH, et al. . Modified disease activity scores that include twenty-eight-joint counts. development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 36. Solomon DH, Reed GW, Kremer JM, et al. . Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449–55. 10.1002/art.39098 [DOI] [PMC free article] [PubMed] [Google Scholar]