Abstract
Background
Sudden cardiac death (SCD) is a major global health problem, accounting for up to 20% of deaths in Western societies. Clinical quality registries have been shown in a range of disease conditions to improve clinical management, reduce variation in care and improve outcomes.
Aim
To identify existing cardiac arrest (CA) and SCD registries, characterising global coverage and methods of data capture and validation.
Methods
Biomedical and public search engines were searched with the terms ‘registry cardio*’; ‘sudden cardiac death registry’ and ‘cardiac arrest registry’. Registries were categorised as either CA, SCD registries or ‘other’ according to prespecified criteria. SCD registry coordinators were contacted for contemporaneous data regarding registry details.
Results
Our search strategy identified 49 CA registries, 15 SCD registries and 9 other registries (ie, epistries). Population coverage of contemporary CA and SCD registries is highly variable with registries densely concentrated in North America and Western Europe. Existing SCD registries (n=15) cover a variety of age ranges and subpopulations, with some enrolling surviving patients (n=8) and family members (n=5). Genetic data are collected by nine registries, with the majority of these (n=7) offering indefinite storage in a biorepository.
Conclusions
Many CA registries exist globally, although with inequitable population coverage. Comprehensive multisource surveillance SCD registries are fewer in number and more challenging to design and maintain. Challenges identified include maximising case identification and case verification.
Trial registration number
CRD42019118910.
Keywords: epidemiology, emergency medicine, resuscitation, sudden cardiac death
Key questions.
What is already known about this subject?
Many cardiac arrest and sudden cardiac death registries have been established around the world. This is a comprehensive review using geographic mapping to provide a contemporary picture of the sudden cardiac death registry landscape.
What does this study add?
This study provides a resource of existing registries, highlights areas of geographic inequity, and compares international metrics of sudden cardiac death identification and adjudication. It identifies unique challenges in registry design that merit further discussion.
How might this impact on clinical practice?
Clinical registries are now recognised to deliver clinical benefits. Comparison of existing national registries is important to highlight variations in clinical practice. It may also standardise cardiac death case evaluations to facilitate international collaborations and benchmarking.
Introduction
Sudden cardiac death (SCD) is a major global public health issue, accounting for up to 20% of deaths in Western societies.1 The WHO defines SCD as a sudden unexpected death within 1 hour of symptom onset or within 24 hours of having been last seen well.1 For patients aged under 35 years old, no cause is identified in up to 30% of cases after forensic analysis.2 Unexplained SCD is frequently associated with devastating psychological and economic impact on both the family and community. Furthermore, genetic relatives may inherit conditions associated with SCD risk that requires characterisation and management.
A clinical quality registry is an organised system using prospective observational study methods to collect data to evaluate specific outcomes for a population.3 Well-designed registries are cost-effective, typically delivering approximately a 4:1 return on investment.4 Cardiac arrest (CA) and SCD registries have been shown internationally to assist epidemiological analysis and improve care linkage between hospital, forensic and ambulance services. Additionally, SCD registries provide ongoing clinical care and may help initiate critical testing for survivors and family members of SCD victims.5
We sought to provide a comprehensive description and analysis of current global resources dedicated to CA and SCD registries, in the context of designing Australia’s first multisource surveillance SCD registry, the Unexplained Cardiac Death Project (UCDP Registry). Key aims were to identify reported challenges experienced by other registries, design data collection to maximise clinical utility and novelty and identify gaps in existing population coverage.
Methods
Search strategy
Identification of current CA and SCD registries was performed via PubMed search (search terms=registry cardio*, registry+sudden+cardiac+death, cardiac+arrest+registry, search filter=Title/Abstract for all searches, results=1395+448+1328, respectively, as of 10 December 2018), citation tracking and interrogation of the internet search engine Google for registries with public websites or published annual reports. A separate search was also conducted in Google using the search term ‘cardiac+arrest+registry+country’, with ‘country’ being each of the recorded member countries of the United Nations (figure 1). All searches were performed in the English language. The search strategy was performed by two authors (EDP and LR) independently, then results were compared. Discrepancies were discussed and resolved between authors, with referral to a third author for adjudication if required. All coauthors on this publication were requested to further nominate any registries recognised as being missed by the search strategy. The search strategy and review structure were preregistered with PROSPERO as a systematic review.
Figure 1.
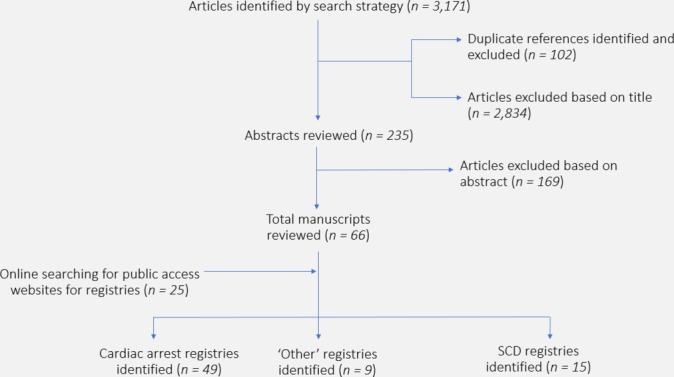
Flow diagram of registry identification, selection and inclusion.
Inclusion/exclusion criteria and characterisation of registries
Only registries collecting data prospectively were considered for inclusion. We included national registries or the four largest state registries for analysis. Exclusion criteria included retrospective data analysis or inactivity for more than 10 years. Genetic heart disease registries (eg, long QT syndrome registries and hypertrophic cardiomyopathy registries) were also excluded, as the majority of these registries enrol living patients and primarily evaluate disease-specific management rather than CA outcomes.
Registries were categorised as CA registries if they primarily evaluated resuscitation outcomes using Utstein style templates.6 Registries were required to appear independently in search results (not only as part of a referring data source to a higher level epistry) and have issued either research publications or an annual report of clinical volume to qualify for inclusion.
Registries were categorised as SCD clinical quality registries if they used more than one data source (ie, ambulance and forensic data) to validate SCD case reporting and undertook ongoing data collection and clinical follow-up.7
Registries collecting data relating to SCD not meeting the above criteria were categorised as ‘other’. Registries categorised as ‘other’ included higher level epidemiological registries or ‘epistries’ collecting a minimum data set of CA variables from participating smaller registries, intermittent collection registries collecting snapshot data periodically, large-scale ‘deep-phenotyping’ registries archiving genetic data of patients only without any clinical correlation or clinical care provided and registries limited to single centres.
As this is a systematic review of existing CA and SCD registries, patient/public involvement was not feasible.
Outcomes assessed
We compiled a list of all existing CA, SCD and ‘other’ registries, with geographic mapping to identify areas of global under-representation.
Listed coordinators of SCD registries were individually contacted to confirm details of case identification and obtain contemporary enrolment numbers. We identified age groups enrolled, type of patient group enrolled (deceased, survivors and/or family members) and reported strategies for genetic material collection and storage.
From methodology published by existing SCD registries, we also derived a table of potential data sources that may be employed for multisource surveillance when creating an SCD registry. We incorporated consideration of published experiences, with particular awareness of real-world implementation difficulties acknowledged by registry leaders.
Results
We identified 49 CA registries and 15 SCD registries, including the newly formed Australian UCDP Registry8–17 (table 1). Nine registries were categorised as ‘other’. The global population coverage of registries is patchy, with the majority being densely located in Western Europe and North America (figure 2). There were no CA or SCD registries identified in any of Russia, India or China, and no out-of-hospital CA or SCD registries identified in South America. There are currently no active registries in Africa.
Table 1.
Existing CA, SCD and other registries identified
| Cardiac arrest registry | Other | Multisource sudden cardiac death registry | |
| Definition | Prehospital metrics primarily collected, using standard Utstein templates. Index cases only. No in-person follow-up offered: data collection±phone calls only. |
Inclusion criteria may include any of: epidemiological registry (epistry) receiving minimum data set from referring community registries. Genetic data only without clinical care. Single-centre registry – no network-level data from either hospital or ambulance service. |
Multisource data surveillance and sampling. Family members may be screened. Clinical services offered: in-person meeting and further assessment. Results of hospital tests, forensic data collected. Genetic data may be collected and stored. |
| Function | Benchmarking of prehospital and in-hospital performance outcomes primarily using Utstein-type variables to improve system response and management of cardiac arrest with an aim to improve outcomes. | A registry not incorporating either standard prehospital metrics nor a multisource surveillance approach. May provide a snapshot of cardiac arrest care from receiving hospital or intermittent data collection or may collect higher level non-clinical data such as genetic data only. | To use multiple sources of cardiac arrest reporting to establish the most accurate community rates of SCD. To provide a centralised clinical quality and research registry that provides families and survivors of SCD with access to state-of-the-art ongoing clinical care and further investigations. |
| Registries identified |
USA and Canada
South America
Middle East and Africa
Asia
Australia and New Zealand
|
Epistries
Intermittent data capture (epistry):
Single-centre registry:
Genetic data only:
Subgroups:
|
|
CA, cardiac arrest; FIFA-SDR, federation internationale de football association - sudden death registry; OHCA, out of hospital cardiac arrest; SCD, sudden cardiac death; SCDY, sudden cardiac death in the young.
Figure2.
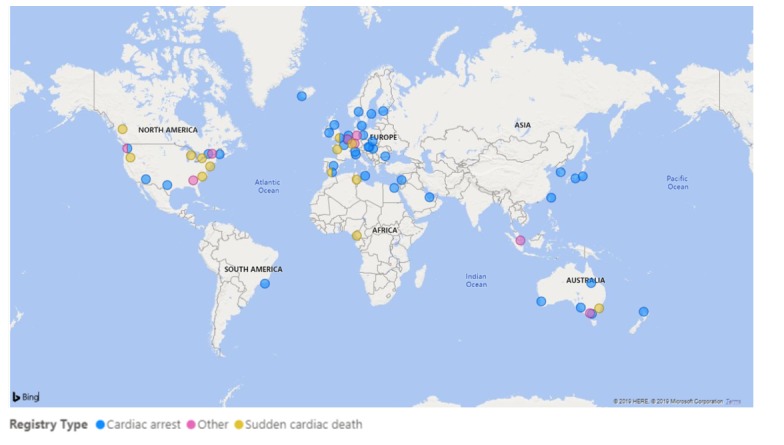
Current global distribution of cardiac arrest, sudden cardiac death and other registries.
CA registries
Forty-nine CA registries were identified using the predefined search strategy. Within low-income or middle-income countries, the Egypt Cardiac Arrest Project was the only identified independent registry; however, multiple other low-income or middle-income countries contribute data to the Pan-Asian Resuscitation Outcomes Study (PAROS), a higher level epistry. Forty-five registries (91.8%) function predominantly as out-of-hospital CA registries, with the Get With the Guidelines Registry, UK National Cardiac Arrest Audit, Danish In-hospital Cardiac Arrest Registry and Brazilian CODE registry collecting exclusively in-hospital CA data.18
There is a degree of data overlap and shift between the registries. For example, the National Register of Cardiopulmonary Resuscitation evolved into the Get with the Guidelines Registry. Multiple local CA registries share data sets with higher level epidemiological registries or ‘epistries’ such as the PAROS,19 Resuscitation Outcomes Consortium or Cardiac Arrest Registry to Enhance Survival.
Data collection is largely focused on patient characteristics and resuscitation outcomes for CA registries, with Utstein-type variables being collected by emergency medical services. Some registries incorporate limited follow-up data, for example, to identify what proportion of ambulance-reported cardiac deaths were true cardiac arrests on postmortem examination.20
SCD registries
Eleven SCD registry coordinators (73.3%) provided direct details regarding data collection strategies and contemporary enrolment (table 2). Worldwide, SCD registries have currently enrolled approximately 19 000 clinical cases of SCD deceased and survivors and 234 family units (numbers drawn from published results and direct contact with registry coordinators). Two registries (Pan-Africa SCD and Canadian Sudden Cardiac Arrest Network) have not yet commenced recruitment or data collection. The Tunisian SCD registry has ceased data collection since 2013.
Table 2.
Details of sudden cardiac death registries
| Site | Commenced | Age (years) | Case identification and data collected | Numbers enrolled | |
| Unexplained Cardiac Death Project | Australia | 2019 | 1–50 | Case reporting via ambulance services, with correlation with forensic and hospital results. Enrolling victims, survivors and genetically relevant next of kin. Collecting demographic, clinical and genetic material. Genetic material also stored for future retesting and return of results. |
300 |
| SCD Germany9 | Germany | 2012 | 10–79 | Systematic media monitoring (~70% case identification). Online reporting of cases by family members, athletes and coaches (~30% case identification). Enrols athletes only: victims and survivors of SCD. Demographic, clinical data and autopsy information collected. |
~200 |
| FIFA-SDR Registry in Football36 | Global coverage but administered by same German institute as SCD Germany | 2014 | All | Systematic media monitoring (~70% case identification). Online reporting of cases by family members, athletes and coaches (~30% case identification). Enrols athletes only: victims and survivors of SCD. Demographic, clinical data and autopsy information collected. |
~100 |
| Nantes Centre for the Prevention of SCD37 | France | 2009 | <45 | Referral from medical centres in France to coordinating registry. Familial screening of first-degree relatives of patients with SCD aged <45 years old. Demographic, clinical data collected. Electrophysiological challenges and genetic testing performed. |
64 families |
| Andalusian Registry of Out-of-Hospital Cardiac Arrest Clinical and Pathological Registry of Tarragona38 |
Catalonia, Spain | 2014 | All | Initial referral from ambulance services, linked with forensic and in-hospital results and ongoing clinical care. Deceased and survivors of SCA. Demographic and clinical data collected. DNA samples collected in cases of unexplained cardiac death after other investigations conducted but no ongoing storage in biorepository. |
4072 as of 2016 |
| Cardiac Risk in the Young (CRY)10 | UK | 1995 | <35 | Autopsy-based case identification: cases referred from across the UK. Deceased patients only enrolled directly and forensic testing subsidised and expedited for this group. Referral of first-degree relatives possible by medical practitioners or self-contact by families. General ECG screening events also coordinated and offered by CRY. Clinical, demographic and autopsy information. Tissue and genetic samples stored indefinitely when consent provided. |
5200 |
| Pan-Africa SCD study15 | ’15 African countries’ | 2015 | >15 | Case identification primarily via hospitals, local newspapers and screening of death certificates. Will enrol all OHCA – victims and survivors. Demographic and clinical information collected. |
0 – awaiting financing still |
| Douala-SCD study16 30 | Douala, Cameroon | 2014 | >15 | Case identification via local reporting and screening of wakes, then obtaining medical reports. Enrolled all OHCA – deceased only, no survivors identified. All cases adjudicated by a cardiologist as to whether they are true SCD. |
2–304 |
| Tunisian Sudden Cardiac Death Registry27 | Tunisia | 2012 | >20 | Prehospital and hospital data combined with autopsy results. No survivors or family members enrolled. |
542 |
| Sudden Death in the Young Registry11 | Georgia, Tennessee, New Jersey, Minnesota, Nevada, Delaware, New Hampshire, Virginia, Wisconsin, USA | 2014 | <20 | Primary case identification via autopsies. Population-based surveillance of all sudden deaths including SUDEP and SCD. Excluded if known terminal illness, homicide, suicide, accident or clearly drugs. Enrolling patients deceased from SCD only. Demographics from index cases. Blood samples from subset of cases. Collection and storage of genetic material. |
As of 2016: 562 cases entered 64 DNA samples |
| Oregon Sudden Unexplained Death Study12 | Oregon, USA | 2002 | all | Multisource notification: ambulance services, emergency departments and/or coroner. Adjudication of all cases to determine if true SCD. Enrols deceased patients with SCD. Demographic and clinical data collected. Genetic material collected and stored in biorepository. |
353 |
| Sudden Unexplained Death in North Carolina13 | North Carolina, USA: Wake County Emergency Medical Services | 2013 | 18–65 | Electronic death certificate screening for OHCA in previous year in appropriate age range. Patient data then linked to ambulance reports of OHCA and autopsy reports where available. Will enrol OHCA deceased. All cases adjudicated to agree that is a true SCD. Genetic material collected at autopsy: family offered opportunity to be notified of positive return of results but not otherwise captured in cardiac screening process. |
399 as of 201539 |
| Sudden Unexplained Death in Childhood40 | USA, and accepts referrals from external to the USA (has received case details from 16 other countries) | 2014 | 11 months–18 years | Self-referral of patients (typically by parents) through website and social media outreach. Self-referral internationally also accepted. Enrolling deceased patients, biological parents and any clinically relevant first or second degree relatives. Survivors of SCA not enrolled. Genetic material collected and option for DNA storage in a biorepository explicitly offered. |
~170 families |
| Cardiac Arrest Survivors with Preserved Ejection Fraction14 | 14 centres across Canada | 2004 | >2 | Enrolling survivor patients and first-degree family members. Family members of deceased patients also included. Survivors have normal coronary arteries, normal LVEF and normal resting ECG and must have experienced VT or VF and received defibrillation. Collect demographics and clinical information. DNA and plasma collected for stratified whole exome sequencing and biobanking. |
1350 |
| Canadian Sudden Cardiac Arrest Network41 | Canadian national registry | 2018 | 2–85 | Case identification via ambulance services and coronial reporting linked with administrative databases. Deceased and surviving patients enrolled. Will collect demographic and clinical data. Genetic analysis performed only when performed in standard clinical work-up; no genetic analysis for research purposes or ongoing storage in a biorepository. |
Commencing from 2018 |
LVEF, left ventricular ejection fraction; SCA, sudden cardiac arrest; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
Several registries enrol only specified subgroups experiencing SCD: for example, two registries (SCD Germany and FIFA-SDR) recruit only SCDs during competitive sport. Elsewhere, the North American Sudden Death in the Young Registry (SDY) and Sudden Unexplained Death in Childhood (SUDC) registries are exclusively for paediatric patients, while four other registries (Pan-Africa SCD, Douala-SCD, Tunisian SCD and SUDDEN) enrol adult cases (>15 years old) only.
All registries capture basic clinical and demographic information. Eight registries (53.3%) explicitly state mechanisms to identify and enrol sudden cardiac arrest (SCA) survivors, and five (33.3%) enrol family members of SCD patients, with varying degrees of inclusion and screening. Nine registries (60.0%) perform genetic analysis on enrolled patients. Of these, seven (46.6%) collect and store specimens in a biorepository with the potential for future return of clinically important analyses such as new diagnoses made in the context of new discoveries in cardiac genetics.
The registries use multiple data surveillance techniques to refine data collection (figure 3). From described methods and experiences, potential data sources are presented in table 3. Four registries (26.7%) rely primarily on ambulance-based referrals, four (26.7%) on forensic referrals and seven (46.6%) on medical practitioner and family referrals. The majority of registries use all these sources of referral with varying emphasis. Seven registries (Unexplained Cardiac Death Project, SDY, SUDC, Cardiac Risk in the Young, Oregon SUDS, SCD Germany and FIFA-SDR) also have publicly accessible websites explaining registry structure and recruitment methods, with referral options for medical practitioners and families to nominate cases of SCD.
Figure 3.
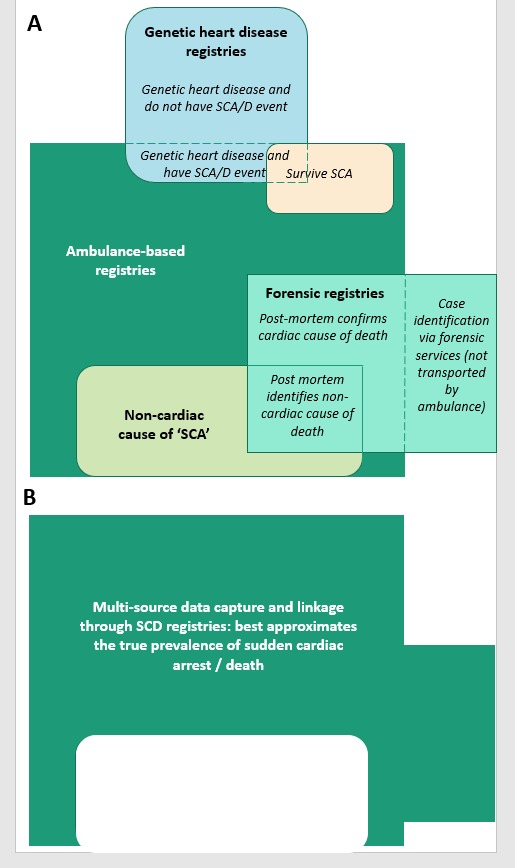
Multi-source data surveillance and capture provides the optimum mechanism of case adjudication and refining case inclusion in SCD registries.
Table 3.
Avenues for data sourcing in sudden cardiac death registries
| Benefits | Limitations | UCDP Registry implementation | |
| Ambulance-based out-of-hospital cardiac arrest (OHCA) registries | Many countries have well-established OHCA registries (figure 2) and use comparable Utstein variables. | Rates of first-responder reported OHCA have been shown to have significant mismatch when correlated with autopsy (ie, drugs may be responsible for cardiac arrest).25 Over-reporting of true rates will occur if limited to ambulance-reported OHCA only. | Ambulance data will be a key component of the UCDP Registry and a vital source of case detection. Data will be cross-referenced from other sources to enhance internal case verification and data quality. |
| In-hospital cardiac arrest registers | Most hospitals maintain a ‘Code Blue’ or cardiac arrest registry that can be accessed. | There are over 1300 public and private hospitals in Australia.42 Contacting each hospital and arranging ethical approval for release on in-hospital audits will be prohibitive. | The registry will be an OHCA registry only. The impact of this is discussed further in the article. |
| Forensic institutes: autopsy data | Autopsy is the gold standard investigation in verifying that a sudden death is cardiac in aetiology. | Rates of autopsy are falling.37 Autopsy-focused registries will capture only a subset of total sudden cardiac deaths. | Will be used to verify rates of sudden cardiac death and cross-reference with ambulance data. |
| Death certificate tracking | Should represent the broadest way of capturing the denominator as the endpoint. | Reported rates of death due to ‘sudden cardiac death’, ‘cardiac arrest’ and ‘heart failure’ are notoriously high: the positive predictive value of a death certificate stating ‘sudden cardiac death’ is only 19%.26 | Needs to be correlated against autopsy data as part of a multiple source surveillance protocol to reduce over-reporting. |
| Genetic heart disease registries or disease-specific registries (ie, Brugada syndrome) | There will be overlap between patients with genetic heart conditions (and their families) and sudden cardiac death. | Patients already known to have genetic heart disease and enrolled with disease-specific registries will receive appropriate therapy/devices and be anticipated to have a low rate of sudden cardiac death. | Primary utility may be in data linkage or dual referral to both UCDP Registry and disease-specific registry when a death occurs and culprit is identified. |
UCDP, Unexplained Cardiac Death Project.
Discussion
Our review identifies that there are many existing CA registries operating in the developed world. Ideally, CA registries can function both as stand-alone large registries of prehospital data and also collaborate as major data sources for a local SCD registry.
When creating a SCD registry, we have identified three primary challenges with regards to data capture. These are comprehensive case capture, case adjudication to assess the true burden of SCD and ‘close the loop’ on SCD diagnosis for referring CA registries and the ongoing challenge of achieving equitable global coverage.
Optimising case capture into existing SCD registries
Maximising data inclusion
For characterisation of SCD on a population level, maximal case capture is key. There are multiple potential sources of data (table 3), and integrating multisource data is a key distinguishing feature of SCD registries compared with CA registries.
Although ambulance-reported data are an important source for identifying OHCA cases, not all SCD patients are transported by ambulance for a variety of reasons. For example, patients may be found clearly deceased and transported directly to coronial services. More unusually, availability of ambulance services in some areas of the world may mean that even active CAs are not always transported to hospital by ambulance, as in the Douala-SCD registry where 67% of CA patients arrived at hospital via private taxi cab.21
The option of capturing all SCDs via postmortem examination is attractive due to the specificity of the SCD diagnosis, and postmortem data have been employed in several major studies as a primary method of data capture.22 However, in some regions, there are significant cultural barriers to gaining consent for postmortem.16 Additionally, while postmortem examination is strongly recommended in all SCD victims aged under 40 years old, rates of postmortem examination are reduced in older age groups.23 Obviously, a postmortem-focused method of recruitment also precludes enrolment of survivors and their families.
Accurate rate reporting
Reported rates of OHCA reported around the world vary widely1 due to a combination of true cultural and genetic variation and discrepancies in the recognition of SCD. Even when strict international criteria are used to define SCD, rates of ‘true SCD’ show further variation.24
Limiting data collection to ambulance-reported OHCA is logistically very straightforward and highly appealing. This underpins the function of most CA registries and generates high-quality data that can be used to refine outcomes in the prehospital management of CA.
However, an ambulance-defined SCD does not equate to a true SCD. The WHO definition of an SCD is highly sensitive and thus has the potential to capture many non-cardiac causes of death. Tseng and colleagues25 identified that of 630 out-of-hospital deaths meeting WHO criteria for classification as an SCD, around one in five were identified at autopsy to be due to drug overdose or neurological causes. In Victoria, Australia,20 a similarly designed study showed even higher rates of misdiagnosis, with 38.6% of WHO-defined SCDs identified to be non-cardiac on subsequent autopsy. Death certificate screening is an even more inaccurate method of identifying cases of SCD, with a positive predictive value of only 19%.26
It is thus vital to have mechanisms of internal validation within the registry (figure 3), to provide confirmation of definite SCD, and quality control feedback. The majority of multisource SCD registries use an adjudication panel to review cases.
The challenge of equitable global coverage
Geographic coverage of existing SCD registries is highly variable. Existing registries are highly concentrated in Western Europe and North America, with low-income and middle-income regions of the world lacking surveillance of SCD. There were no OHCA or SCD registries identified in any of South America, Russia, India or China; together these countries comprise just under half the world’s population. In Africa, the Pan-Africa SCD study is still awaiting funding in order to be able to commence, the Douala-SCD registry was ceased after 1 year of recruitment due to financial issues and logistic challenges with patient recruitment (personal communication) and the Tunisian-SCD registry has closed.27
Under-representation of low-income and middle-income countries is important for several reasons. Preliminary reports suggest that patterns of SCD and their management may vary with geography and ethnicity. From several Asian CA registries,19 28 29 it would appear that Asian countries may have lower rates of VT/VF as presenting rhythm in OHCA and lower rates of survival to hospital discharge.28 The Douala-SCD registry reported that there were no survivors of OHCA during the entire recruitment period, reflecting the challenges of CA management in low-resource settings. Although 86.2% of OHCAs were witnessed, cardiopulmonary resuscitation by bystanders was provided in only 7.4% of cases.30
Although well-designed clinical registries deliver benefits in care and a positive return on financial investment,4 initial costs may be preclusive for many poorer national health systems. Additionally, the need for specialist expertise to make an accurate diagnosis of SCD (ie, specialist cardiac investigations periarrest and autopsy performed by a qualified specialist postmortem) may be impractical. In Cuba, for example, SCD has been recognised to be grossly under-reported due to confusion around the WHO definition of SCD.31 Strategies to improve representation of low-income and middle-income countries in SCD registries include training of community health workers with questionnaires to facilitate performance of a ‘verbal autopsy’ where specialist facilities are not available.32 33 Automated case identification tools have also been trialled in a range of disease conditions, but currently have only moderate sensitivity in identifying deaths as ‘cardiac’, let alone any specific cause of SCD or case adjudication.34
Low-income and middle-income countries contain the bulk of the world’s population, and their residents carry an increasing burden of cardiovascular risk factors placing them at risk of SCD.35 Preliminary data suggest that the majority of SCD is occurring in regions without SCD registries, accurate methods of codifying cause of death and very poor clinical outcomes.35 Further capturing this information and undertaking quantitative analysis to drive advocacy is essential.
Limitations
The search strategy used the English language only, and thus may not have captured registries that have been published on only in non-English languages. Geolocation of the researchers may also have impacted search strategy results compared with results that would be obtained elsewhere in the world. Response from SCD registry coordinators was incomplete.
Conclusion
Constructing a multisource surveillance SCD registry including data from existing CA registries provides comprehensive care likely to benefit patients and their families, as well as a crucial research platform for the comprehension of true incidence and aetiology of CA. However, existing CA and SCD registries are highly concentrated in developed countries, and case enrolment and verification are challenging. There is scope to learn from existing registry designs to maximise clinical utility. Furthermore, given the recent advances in genetic causes of SCD, collection and storage of biospecimens may be considered a priority.
Footnotes
Twitter: @pretzeldr
Contributors: All authors contributed to study design, study write-up and approved the final draft. EDP and LR conducted the data search, with adjudication by ALG, DS or KS. EDP is the corresponding author and responsible for overall content.
Funding: The work of the Unexplained Cardiac Death Project Registry is supported by funds from the Ross Dennerstein Foundation. EDP is supported by an NHMRC/NHF (National Health and Medical Research Council / National Heart Foundation) cofunded Postgraduate Scholarship, RACP JJ Billings Scholarship and PSA Cardiovascular Scholarship. CS is supported by an NHMRC Australia Practitioner Fellowship. ALG is supported by an NHF Future Leadership Fellowship. DS is supported by an NHF Future Leadership Fellowship and Viertel Foundation Grant.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Our search strategy is detailed in the article. We are happy to share spreadsheets with details of search results on any reasonable request.
References
- 1. Wong CX, Brown A, Lau DH, et al. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ 2019;28:6–14. 10.1016/j.hlc.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 2. Semsarian C, Ingles J, Wilde AAM. Sudden cardiac death in the young: the molecular autopsy and a practical approach to surviving relatives. Eur Heart J 2015;36:1290–6. 10.1093/eurheartj/ehv063 [DOI] [PubMed] [Google Scholar]
- 3. Gliklich RDN, Center OD. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville, Maryland, 2010. [Google Scholar]
- 4. ACSQHC Economic evaluation of clinical quality registries: final report. Sydney, 2016. [Google Scholar]
- 5. Campbell RM, Berger S, Ackerman MJ, et al. Call for a sudden cardiac death registry: should reporting of sudden cardiac death be mandatory? Pediatr Cardiol 2012;33:471–3. 10.1007/s00246-011-0085-7 [DOI] [PubMed] [Google Scholar]
- 6. Baert V, Escutnaire J, Nehme Z, et al. Development of an online, universal, Utstein registry-based, care practice report card to improve out-of-hospital resuscitation practices. J Eval Clin Pract 2018;24:431–8. 10.1111/jep.12880 [DOI] [PubMed] [Google Scholar]
- 7. Care ACoSaQiH Framework for Australian clinical quality registries. Sydney, NSW, 2014. [Google Scholar]
- 8. Schupp T, Behnes M, Weiß C, et al. Beta-Blockers and ACE inhibitors are associated with improved survival secondary to ventricular tachyarrhythmia. Cardiovasc Drugs Ther 2018;32:353–63. 10.1007/s10557-018-6812-z [DOI] [PubMed] [Google Scholar]
- 9. Bohm P, Scharhag J, Meyer T. Data from a nationwide registry on sports-related sudden cardiac deaths in Germany. Eur J Prev Cardiol 2016;23:649–56. 10.1177/2047487315594087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raju H, Behr ER. Unexplained sudden death, focussing on genetics and family phenotyping. Curr Opin Cardiol 2013;28:19–25. 10.1097/HCO.0b013e32835b0a9e [DOI] [PubMed] [Google Scholar]
- 11. Burns KM, Bienemann L, Camperlengo L, et al. The sudden death in the young case registry: collaborating to understand and reduce mortality. Pediatrics 2017;139:e20162757 10.1542/peds.2016-2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aro AL, Rusinaru C, Uy-Evanado A, et al. Syncope and risk of sudden cardiac arrest in coronary artery disease. Int J Cardiol 2017;231:26–30. 10.1016/j.ijcard.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nanavati PP, Mounsey JP, Pursell IW, et al. Sudden unexpected death in North Carolina (sudden): methodology review and screening results. Open Heart 2014;1:e000150 10.1136/openhrt-2014-000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herman ARM, Cheung C, Gerull B, et al. Outcome of apparently unexplained cardiac arrest: results from investigation and follow-up of the prospective cardiac arrest survivors with preserved ejection fraction registry. Circ Arrhythm Electrophysiol 2016;9:e003619 10.1161/CIRCEP.115.003619 [DOI] [PubMed] [Google Scholar]
- 15. Bonny A, Ngantcha M, Amougou SN, et al. Rationale and design of the Pan-African Sudden Cardiac Death survey: the Pan-African SCD study : cardiovascular topic. Cardiovasc J Afr 2014;25:176–84. 10.5830/CVJA-2014-035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonny A, Noah DN, Ngantcha M, et al. Epidemiology of sudden cardiac death in Cameroon: rationale and design of the Douala-SUD survey. Arch Cardiovasc Dis 2014;107:433–42. 10.1016/j.acvd.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 17. Empana J-P, Blom MT, Bӧttiger BW, et al. Determinants of occurrence and survival after sudden cardiac arrest–A European perspective: the ESCAPE-NET project. Resuscitation 2018;124:7–13. 10.1016/j.resuscitation.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 18. Goldberger ZD, Nichol G. Registries to measure and improve outcomes after cardiac arrest. Curr Opin Crit Care 2013;19:208–13. 10.1097/MCC.0b013e328360ad06 [DOI] [PubMed] [Google Scholar]
- 19. Doctor NE, Ahmad NS, Pek PP, et al. The Pan-Asian resuscitation outcomes study (PAROS) clinical research network: what, where, why and how. Singapore Med J 2017;58:456–8. 10.11622/smedj.2017057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deasy C, Bray JE, Smith K, et al. Out-Of-Hospital cardiac arrests in young adults in Melbourne, Australia-adding coronial data to a cardiac arrest registry. Resuscitation 2011;82:1302–6. 10.1016/j.resuscitation.2011.05.031 [DOI] [PubMed] [Google Scholar]
- 21. Bonny A. Cardiac arrhythmias in Africa: prospect, challenges and perspectives. Khartoum, Sudan: PASCAR, 2017. [Google Scholar]
- 22. Bagnall RD, Weintraub RG, Ingles J, et al. A prospective study of sudden cardiac death among children and young adults. N Engl J Med 2016;374:2441–52. 10.1056/NEJMoa1510687 [DOI] [PubMed] [Google Scholar]
- 23. Sanchez O, Campuzano O, Fernández-Falgueras A, et al. Natural and undetermined sudden death: value of post-mortem genetic investigation. PLoS One 2016;11:e0167358 10.1371/journal.pone.0167358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol 2011;57:794–801. 10.1016/j.jacc.2010.09.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tseng ZH, Olgin JE, Vittinghoff E, et al. Prospective Countywide surveillance and autopsy characterization of sudden cardiac death. Circulation 2018;137:2689–700. 10.1161/CIRCULATIONAHA.117.033427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol 2004;44:1268–75. 10.1016/j.jacc.2004.06.029 [DOI] [PubMed] [Google Scholar]
- 27. Ahmed HB, Boussaid H, Zoghlami B, et al. 0201: symptoms before sudden cardiac death (the Northern Tunisian sudden cardiac-death registry). Archives of Cardiovascular Diseases Supplements 2015;7:89 10.1016/S1878-6480(15)71741-6 [DOI] [Google Scholar]
- 28. Lin Y-N, Chang S-S, Wang L-M, et al. Prehospital predictors of initial Shockable rhythm in out-of-hospital cardiac arrest: findings from the Taichung sudden unexpected death registry (THUNDER). Mayo Clin Proc 2017;92:347–59. 10.1016/j.mayocp.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 29. Kim JY, Hwang SO, Shin SD, et al. Korean cardiac arrest research Consortium (KoCARC): rationale, development, and implementation. Clin Exp Emerg Med 2018;5:165–76. 10.15441/ceem.17.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonny ANM, Saka C, Pouth CN, et al. Incidence of sudden cardiac death in sub-Saharan Africa: the Douala-SCD registry. Archives Cardiovasc Dis 2016;8:98. [Google Scholar]
- 31. Vilches E, Ochoa LA, Ramos L. The debate in Cuba's scientific community on sudden cardiac death. MEDICC Rev 2015;17:48–52. [DOI] [PubMed] [Google Scholar]
- 32. Ganapathy SS, Yi Yi K, Omar MA, et al. Validation of verbal autopsy: determination of cause of deaths in Malaysia 2013. BMC Public Health 2017;17:653 10.1186/s12889-017-4668-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abbas SM, Alam AY, Majid A. To determine the probable causes of death in an urban slum community of Pakistan among adults 18 years and above by verbal autopsy. J Pak Med Assoc 2011;61:235–8. [PubMed] [Google Scholar]
- 34. Hazard RH, Alam N, Chowdhury HR, et al. Comparing tariff and medical assistant assigned causes of death from verbal autopsy interviews in Matlab, Bangladesh: implications for a health and demographic surveillance system. Popul Health Metr 2018;16:10 10.1186/s12963-018-0169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vedanthan R, Fuster V, Fischer A. Sudden cardiac death in low- and middle-income countries. Glob Heart 2012;7:353–60. 10.1016/j.gheart.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scharhag J, Bohm P, Dvorak J, et al. F-MARC: the FIFA sudden death registry (FIFA-SDR). Br J Sports Med 2015;49:563–5. 10.1136/bjsports-2015-094770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quenin P, Kyndt F, Mabo P, et al. Clinical yield of familial screening after sudden death in young subjects: the French experience. Circ Arrhythm Electrophysiol 2017;10:e005236 10.1161/CIRCEP.117.005236 [DOI] [PubMed] [Google Scholar]
- 38. Azeli Y, Barbería E, Jiménez-Herrera M, et al. The ReCaPTa study - a prospective out of hospital cardiac arrest registry including multiple sources of surveillance for the study of sudden cardiac death in the Mediterranean area. Scand J Trauma Resusc Emerg Med 2016;24:127 10.1186/s13049-016-0309-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel S, Conover MM, Joodi G, et al. Medication use in women and men with sudden unexpected death. Ann Pharmacother 2018;52:868–75. 10.1177/1060028018771061 [DOI] [PubMed] [Google Scholar]
- 40. Foundation S Sudden unexplained death in childhood Roseland. New Jersey USA, 2018. https://sudc.org/ [Google Scholar]
- 41. Lin S. Canadian sudden cardiac arrest network (C-SCAN), 2018. Available: https://clinicaltrials.gov/ct2/show/NCT03642587
- 42. Welfare AIoHa Hospital resources 2015-2016: Australian hospital statistics (health services series No. 78). Canberra, Australia: Welfare AIoHa, 2017. [Google Scholar]


