Summary
Triple combination therapy with an antifungal triazole, echinocandin and amphotericin B (AmB) is used in some centres to treat refractory aspergillosis. The objective of this study was to investigate the effect of subinhibitory concentrations of AmB on the double combinations of caspofungin (CAS) + voriconazole (VOR) or ravuconazole (RAV) against Aspergillus fumigatus, Aspergillus flavus and Aspergillus terreus. Isolates were studied in triplicate against CAS/VOR and CAS/RAV combinations by chequerboard broth microdilution. AmB was added to each double combination at concentrations of 0, 0.1 and 0.2 μg ml−1. The fractional inhibitory concentration (FIC) index was calculated for the double and triple combinations. Comparative analysis was performed by repeated measures analysis followed by Dunnett’s post-test. The double combinations of CAS/RAV and CAS/VOR were synergistic or additive in most conditions. Addition of AmB to the double combinations resulted in increased FIC indices for A. fumigatus and A. flavus. By contrast, AmB increased the synergism of the double combinations decreasing FIC indices for A. terreus (P < 0.05). RAV and VOR displayed similar synergistic activity with CAS. The addition of sub-inhibitory amphotericin B concentrations reduced but did not eliminate the synergistic interaction between the echinocandin and triazole against A. fumigatus and A. flavus, while it increased the synergy against A. terreus.
Keywords: Echinocandins, triazoles, combination therapy, antifungal susceptibility
Introduction
The surge in cases of invasive aspergillosis along with poor clinical outcome raises the need for new more potent antifungal therapeutic strategies. Combination therapy holds the promise to increase efficacy and enhance fungal killing of antifungal agents. The introduction of new antifungal compounds with different mechanisms of action has made antifungal combination therapy feasible.1,2 Antifungal agents target specific cell targets, such as cell-membrane integrity (polyenes), ergosterol biosynthesis (triazoles) and cell-wall integrity (echinocandins). The class of echinocandins includes agents such as caspofungin (CAS), micafungin and anidulafungin, which inhibit (1 → 3)-b-D-glucan biosynthesis but differ in their physicochemical properties. The family of triazoles has been expanded with new agents, which include voriconazole (VOR), ravuconazole (RAV) and posaconazole that inhibit erogosterol biosynthesis. The availability of many agents from each class of antifungal agents increases the possibility of drug combinations and requires careful study to choose the combination that would maximise antifungal efficacy.
Combination therapy with two or more agents may improve clinical outcome of this devastating infection.1,2 Previous in vitro studies of double combination therapy with triazoles + echinocandins against invasive aspergillosis have shown additive or synergistic interaction.3–6 Alternatively, the combination of a triazole + polyene may be indifferent or antagonistic in vitro and in vivo against Aspergillus fumigatus.7,8
Triple antifungal combination therapy has been used to treat invasive aspergillosis especially in critically ill patients with refractory infections. However, there is limited information about the interactions of triple combinations among triazoles, echinocandins and amphotericin B (AmB) against A. fumigatus and non-fumigatus Aspergillus spp. We recently found that the synergistic interactions of the double combination between VOR and CAS were reduced in vitro when AmB was added, and antagonistic interactions increased with increasing concentrations of AmB.4 This effect was explained by the antagonistic interactions between AmB and VOR that overwhelmed the synergistic effects within the triple combination.
Amphotericin B-azole interaction may differ for different triazoles, such that the use of another triazole could provide a triple combination regimen that produces no antagonistic effects and increases the activity of each of the three drugs when they are used alone. Ravuconzole is an extended-spectrum triazole with activity against Aspergillus spp. and a long plasma half-life,9,10 which is being studied in phase I trials. As RAV and VOR are structurally similar, and available in parental formulations, they may be used in settings of critically ill patients where triple therapy may be used. As the antagonistic interactions of the previously studied triple combination of AmB + CAS + VOR were observed at high concentrations of AmB,4 this study describes the effects of sub-inhibitory AmB concentrations on the combinations of CAS with VOR or RAV by the use of two-drug interaction models.
Materials and methods
Organisms
Clinical isolates were obtained from the National Institutes of Health Warren Grant Magnusom Clinical Center and the University of Texas San Antonio, Health Science Center. Nine isolates of A. fumigatus (#4215, #2025, #2350), Aspergillus flavus (#8B, #10B, #50) and Aspergillus terreus (#644, #1290, #2624) were studied. The nine isolates were grown on potato dextrose slants at 30 °C for 5–7 days. Inoculum preparation was based on the CLSI M38-A broth microdilution guidelines for mould susceptibility testing. Conidia were obtained by scraping agar slants with a sterile pipette, the inoculum was prepared in sterile normal saline and adjusted spectrophotometrically. The inoculum was further diluted 1 : 50 in RPMI 1640 containing 0.165 mol l−1 MOPS buffer (BioWhittaker, Walkerville, MD, USA), to a 2× concentration of 0.4–5 × 104 CFU ml−1.
Antifungal compounds
Caspofungin (Merck and Co, Rahway, NJ, USA) and RAV (BMS 207147; Bristol-Myers Squibb Institute for Medical Research, Princeton, NJ, USA) were obtained as reagent grade powders. VOR (Pfizer Pharmaceuticals, New York, NY, USA) was obtained as 10 mg ml−1 vial for injection. Stock solutions of RAV were initially diluted in dimethyl sulfoxide, while VOR and CAS were diluted in sterile saline. The antifungal compounds were further diluted in serial twofold dilutions in RPMI 1640 containing 0.165 mol l−1 MOPS buffer to yield final concentrations of 0.06–4.0 μg ml−1 for RAV, 0.03–2.0 μg ml−1 for VOR and 0.5–256 μg ml−1 for CAS. AmB deoxycholate powder (Bristol-Myers Squibb) was initially diluted to a stock concentration of 10 μg ml−1 in sterile water and further diluted in RPMI to the final concentration of 0.1 and 0.2 μg ml−1.
Chequerboard assay
Three 96-well microtitre plates (Corning Inc., Corning, NY, USA) were used for each isolate. Each experiment involving VOR and CAS with or without AmB was repeated up to three times for each Aspergillus isolate. RAV or VOR and CAS were added to the first microtitre plate using a two-dimensional chequerboard technique. This plate contained only two antifungal combinations of either VOR/CAS or RAV/CAS. The second and third plate contained triple antifungal combinations, one of the two drug combinations as well as either 0.1 or 0.2 μg ml−1 of AmB. Positive control wells contained only organisms. A negative control well contained only RPMI. Microtitre plates were incubated at 35 °C for 48 h. Plates were read visually after incubation for 24 and 48 h.
Quality control
All antifungal compounds were tested against Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 for quality control purposes.
Analysis of antifungal drug interactions
The minimum inhibitory concentration at zero (MIC0) was defined as the concentration of drug with no visible growth. Off-scale MIC values were converted to the next highest twofold MIC. Antifungal drug interactions were analysed based on the fractional inhibitory concentration index (FIC).
Fractional inhibitory concentration
For each well with a numerical score 0 adjacent to a well with growth, the sum of FIC is calculated as
where A, B and C are the concentrations of the drugs A, B and C in combination and MICA, MICB and MICC are the MICs of the drugs A, B and C alone. Among all ΣFICs, the FIC index (FICi) was defined as the lowest ΣFIC (ΣFICmin). Drug interactions were classified based on the FICi as synergistic, additive and antagonistic when FICi was significantly lower, equal or higher than 1, respectively, as previously described.4 Deviation of the FICis of all replicates and isolates from 1 was assessed for each species using the t-test after log-transformation of FICis to approximate normal distribution. To assess the effect of sub-inhibitory AmB concentrations on the double combinations, CAS + VOR and CAS + RAV, logFICs at 0.1 and 0.2 μg ml−1 of AmB were compared with logFICs at 0 μg ml−1 of AmB using an ANOVA (repeated measures analysis) followed by Dunnett’s post-test.
Comparative pharmacodynamic interaction analysis
To compare the effect of sub-inhibitory concentrations of AmB on the CAS + VOR with the effect on the CAS + RAV, the difference of logFICi at 0.1 and 0.2 μg ml−1 from logFICis at 0 μg ml−1 of AmB was calculated for CAS + VOR and compared with the corresponding differences for CAS + RAV using the anova for repeated measures followed by Dunnett’s multiple comparison test, i.e. logFICi difference of CAS + VOR at 0.1 μg ml−1 of AMB vs. logFICi difference of CAS + RAV at 0.1 μg ml−1 of AmB and similarly at 0.2 μg ml−1 of AmB for each species.
Results
Minimum inhibitory concentrations
The MICs for all Aspergillus isolates are presented in Table 1 by species. These MICs ranged from 0.25 to 1 μg ml−1 (median 0.50 μg ml−1) for VOR, 128–512 μg ml−1 (median 256 μg ml−1) for CAS, 0.5–2 (median 1 μg ml−1) for AmB and 0.5–2.0 μg ml−1 (median 1.0 μg ml−1) for RAV.
Table 1.
Median minimum inhibitory concentrations (MICs)1
| Median (range) MICs (μg ml−1) | |||
|---|---|---|---|
| Antifungal agent | Aspergillus fumigatus | Aspergillus flavus | Aspergillus terreus |
| Voriconazole | 0.50 (0.25–1.0) | 0.50 (0.25–1.0) | 1.0 (0.25–1.0) |
| Ravuconazole | 2.0 (0.50–2.0) | 1.0 (0.5–2.0) | 1.0 (0.50–2.0) |
| Caspofungin | 128 (128–256) | 512 (512–512) | 256 (128–512) |
| Amphotericin B | 0.50 (0.50–0.50)* | 1.0 (1.0–2.0) | 2.0 (2.0–2.0) |
Each median value represents 6−−10 different experiments performed on three strains of each species.
Comparing FIC index to 1.0 (P <0.05).
Combination studies
The results of the FIC index for the double combinations of RAV and VOR with CAS and for the triple combinations with 0.1 and 0.2 μg ml−1 of AmB are shown in Table 2 for each Aspergillus species after incubation for 24 and 48 h.
Table 2.
Median fractional inhibitory concentration index of voriconazole (VOR) or ravuconazole (RAV) plus caspofungin (CAS) without and with amphotericin B.
| Median (range) FIC indices | ||||
|---|---|---|---|---|
| Species (no. isolates) | Incubation (h) | 0 μg ml−1 AmB | 0.1 μg ml−1 AmB | 0.2 μg ml−1 AmB |
| CAS + RAV | ||||
| Aspergilluis fumigatus (3) | 24 | 0.50 (0.37–0.52)* | 0.57 (0.48–0.70)* ** | 0.59 (0.49–0.71)* ** |
| 48 | 0.50 (0.26–0.75)* | 0.70 (0.46–0.73)* ** | 0.77 (0.59–0.92)* ** | |
| Aspergilluis flavus (3) | 24 | 0.26 (0.25–0.38)* | 0.34 (0.18–0.37)* | 0.33 (0.22–0.47)* |
| 48 | 0.29 (0.25–0.50)* | 0.35 (0.30–0.43)* | 0.45 (0.36–0.51)* ** | |
| Aspergilluis terreus (3) | 24 | 0.37 (0.13–0.53)* | 0.30 (0.18–0.36)* | 0.23 (0.22–0.38)* |
| 48 | 0.34 (0.26–0.75)* | 0.32 (0.17–0.42)* | 0.29 (0.17–0.41)* | |
| CAS + VOR | ||||
| A. fumigatus (3) | 24 | 0.88 (0.51–1.00) | 0.70 (0.48–0.95)* | 0.71 (0.49–0.92)* |
| 48 | 0.51 (0.5–1.00)* | 0.70 (0.51–0.95)* | 0.90 (0.68–1.15)** | |
| A. flavus (3) | 24 | 0.50 (0.48–1.00) | 0.32 (0.27–0.44)* ** | 0.54 (0.52–0.64)* |
| 48 | 0.76 (0.48–1.06) | 0.83 (0.56–1.10) | 0.90 (0.61–1.20) | |
| A. terreus (3) | 24 | 1.05 (0.37–2.00) | 0.60 (0.60–0.61)* | 0.54 (0.38–0.60)* |
| 48 | 1.50 (0.75–2.00) | 0.62 (0.60–1.55)** | 0.70 (0.70–0.95)* ** | |
(P < 0.05); comparing FIC index to 1.0.
(P < 0.05); comparing FIC index of the double to the triple combination.
RAV + CAS + AmB
The FIC indices after 24 and 48 h were significantly <1.0 (P < 0.05) indicating synergistic interactions for the double combination RAV + CAS (0.26–0.50). Although the triple combination of RAV + CAS + AmB was synergistic (0.23–0.77) for all Aspergillus species, the addition of AmB reduced but did not eliminate the synergistic interaction for A. fumigatus and for A. flavus. There were significant differences of FIC indices between the double and triple combinations for A. fumigatus in which the median FIC index of double combination (0.50) increased at 0.1 μg ml−1 of AmB (0.57 after 24 h and 0.70 after 48 h) and at 0.2 μg ml−1 of AmB (0.59 after 24 h and 0.77 after 48 h) (Table 2). Thus, while the addition of AmB antagonised the synergistic effects of the double combination against A. fumigatus, the FIC index remained synergistic.
VOR + CAS + AmB
The patterns of interaction between the double and triple combinations for VOR, CAS and AmB were similar to those of RAV, CAS and AmB (Table 2). Increasing concentrations of AmB for A. fumigatus and A. flavus at 48 h tended to decrease the synergistic effect of CAS and VOR. For example, the median FIC index for A. fumigatus increased from 0.51 to 0.70 to 0.90 with increasing concentrations of AmB added to the VOR + CAS combination. A similar tendency was observed with A. flavus. However, a synergistic pattern occurred with A. terreus at 48 h, where the FIC index decreased from 1.50 to 0.7 μg ml−1 with the addition of 0.2 μg ml−1 of AmB.
Comparative pharmacodynamic analysis of the effect of dierent triazoles in the triple combination
The effects of VOR vs. RAV on the triple combination at a given AmB concentration are shown in Fig. 1. The increase in the FIC indices at given AmB concentrations was significantly greater for RAV + CAS than for VOR + CAS for all Aspergillus species after incubation for 24 h. The comparative pharmacodynamic differences at 48 h were not significant for most comparisons; however, the overall trends of antagonism of the triazole-echinocandin synergistic interaction by AmB were similar to those of 24 h.
Figure 1.
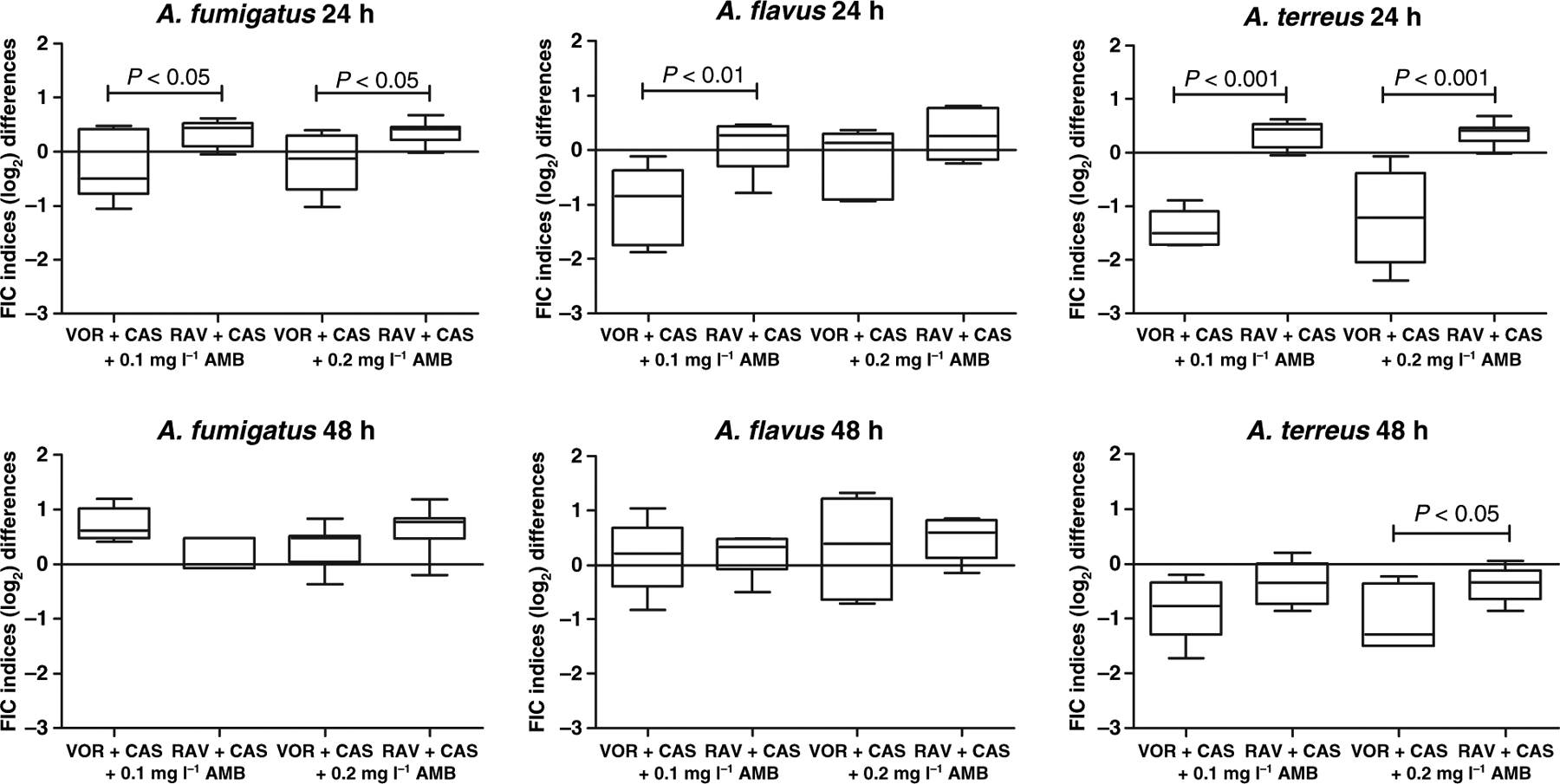
Comparative pharmacodynamic analysis of the effect of different triazoles in the triple combinations of VOR + CAS + AmB and RAV + CAS + AmB. Studies are performed at near MIC concentrations using the fractional inhibitory concentration (FIC) index analysis. Box-plots depict differences between FIC indices at 0.1 and 0.2 μg ml−1 of AMB with the FIC indices at 0 μg ml−1 of AMB for VOR + CAS and RAV + CAS combinations. P-values were analysed by anova followed by Dunnett’s post-test for repeated measures.
Discussion
The double combination of VOR + CAS or RAV + CAS displayed predominantly synergistic interactions. These synergistic interactions were reduced when AmB was added to the triazole/echinocandin combination. Although the interactions remained synergistic, the addition of AmB raised the FIC indices in a dose-dependent manner. This antagonism was observed against A. fumigatus and A. flavus but not against A. terreus.
For patients with refractory invasive mycoses, often all three classes of agents are used in order to eradicate a life-threatening infection. The antifungal triazoles, VOR and RAV, inhibit ergosterol biosynthesis, while AmB directly binds to ergosterol creating the potential for antagonism. However, the echinocandins, such as CAS, inhibit (1 → 3)-b-D-glucan synthase of the fungal cell wall providing the possibility of an additive or synergistic effect.
Earlier studies demonstrated a positive in vitro and in vivo interaction between echinocandins and poly-enes,11–14 as well as between echinocandins and triazoles,3,6,14–17 against Aspergillus species. However, the combination of polyenes with azoles, whether in vivo or in vitro, are antagonistic to indifferent.4,7,8,18 The potential for triple therapy to augment antifungal effect needs to be balanced against the potential for antagonism. Our previous in vitro studies demonstrated that AmB increases the antagonistic interactions and reduces the synergistic interactions when it is combined with VOR and CAS.4,19 Whether this antagonistic effect pertains to other triazoles, remains unknown.
The echinocandin + triazole combination, whether CAS + RAV or CAS + VOR, resulted in a synergistic interaction. Notably, the synergistic interaction was stronger with the combination of CAS + RAV with lower FICis at most time points against A. fumigatus, A. flavus and A. terreus. The addition of AmB to the echinocandin + triazole combination demonstrates no apparent advantage of enhancing this interaction. Instead, there is a trend in A. fumigatus and A. flavus of antagonising the synergistic effect of the echinocandin + triazole combination against A. fumigatus and A. flavus. For example, at 24 and 48 h with increasing concentration of AmB, especially at 0.2 μg ml−1, the FIC index is higher at most time-points for A. fumigatus and A. flavus with a triple combination. The effect is less striking at 0.1 μg ml−1 demonstrating a concentrationdependent reduction of additive/synergistic interaction with the double combination of triazole + echinocandins.
The reduction of the triazole-echinocandin synergistic interaction by AmB may be as a result of the inhibition of the ergosterol biosynthesis by the triazole, thus depleting a potential target for AmB. Another possible mechanism may be that AmB disrupts the cell membrane surrounding transmembrane domains of the (1 → 3)-b-D-glucan synthase complex, thereby diminishing its activity. Alternatively, AmB may increase the cell-membrane permeability of triazoles and potentially increase the inhibition of ergosterol within the cell membrane, subsequently depriving ergosterol as a target for AmB. An alternative hypothesis for the antagonistic effect of AmB on the triazole-echinocandin interaction may be that polyene binds to the cell membrane with steric hindrance between AmB and the triazole at the level of the cell membrane. This effect would depend less on the depletion of ergosterol, but still would reflect a dose-dependent effect.20
In contrast to the effects against A. fumigatus and A. flavus, the effect of the addition of AmB to the echinocandin + triazole combination against A. terreus appears to demonstrate a different pattern. The increasing concentration of AmB appears to result in a decreased FIC index under most conditions at 24 and 48 h for A. terreus but not for other Aspergillus spp. The pattern for A. terreus appears to be concentrationdependent with the effect being seen more prominently at 0.2 μg ml−1. The mechanism for this difference may be related to the intrinsic polyene resistance of A. terreus. Previous studies have demonstrated that A. terreus is resistant to AmB with higher MICs and minimum lethal concentrations, which correlates with in vivo resistance to AmB.21 In A. terreus, the ergosterol content appears to be inversely related to the MIC; i.e. the lower the ergosterol content, the higher the MIC against AmB. These data would suggest that the triple combination of AmB with the combination of triazole + echinocandins may be beneficial against A. terreus. Further, in vivo studies are warranted to substantiate this potentially positive interaction. At the same time, however, the in vitro data would indicate that the triple combination may not be beneficial against A. fumigatus and A. flavus. Perhaps the mechanism of AmB against A. terreus may be an interaction with non-ergosterol sterols, allowing potentially increased permeability or enhancing increased permeability to echinocandins or triazoles or altering the configuration of the trans-membrane domains of (1 → 3)-b-D-glucan synthase to more favourably bind to the echinocandin.
The triple drug combinations with RAV demonstrated greater antagonism than those with VOR. As illustrated in Fig. 1 for all three fungi, A. fumigatus, A. flavus and A. terreus, there was an overall pattern of more likelihood for RAV to be associated with a higher FICI index than for VOR within the triple combination. A mechanism for this may be related to structural differences between VOR and RAV. The thiazolyl cyanophenyl side chain that distinguishes RAV from the fluoropyrimidine of VOR may contribute to a greater degree of hydrophobicity that potentially could confer steric hindrance at the level of the cell membrane and ergosterol.
In summary, the addition of AmB to an echinocandin-triazole combination reduces but does not eliminate the synergistic interaction of the double combination. This effect occurs when either VOR or RAV is used as the triazole in combination with CAS. These effects are species-dependent with the changes occurring in A. fumigatus and A. flavus. By comparison, the triple combination yields a net increase in synergistic effect on A. terreus.
References
- 1.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. Combination antifungal therapy. Antimicrob Agents Chemother 2004; 48: 693–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson MD, Perfect JR. Combination antifungal therapy: what can and should we expect? Bone Marrow Transplant 2007; 40: 297–306. [DOI] [PubMed] [Google Scholar]
- 3.Manavathu EK, Alangaden GJ, Chandrasekar PH. Differential activity of triazoles in two-drug combinations with the echinocandin caspofungin against Aspergillus fumigatus. J Antimicrob Chemother 2003; 51: 1423–5. [DOI] [PubMed] [Google Scholar]
- 4.O’Shaughnessy EM, Meletiadis J, Stergiopoulou T, Demchok JP, Walsh TJ. Antifungal interactions within the triple combination of amphotericin B, caspofungin and voriconazole against Aspergillus species. J Antimicrob Chemother 2006; 58: 1168–76. [DOI] [PubMed] [Google Scholar]
- 5.Patterson TF. Combination antifungal therapy. Pediatr Infect Dis J 2003; 22: 555. [DOI] [PubMed] [Google Scholar]
- 6.Perea S, Gonzalez G, Fothergill AW, Kirkpatrick WR, Rinaldi MG, Patterson TF. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob Agents Chemother 2002; 46: 3039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meletiadis J, Petraitis V, Petraitiene R et al. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: in vitro and in vivo correlation.J Infect Dis 2006; 194: 1008–18. [DOI] [PubMed] [Google Scholar]
- 8.Schaffner A, Bohler A. Amphotericin B refractory aspergillosis after itraconazole: evidence for significant antagonism. Mycoses 1993; 36: 421–4. [DOI] [PubMed] [Google Scholar]
- 9.Boucher HW, Groll AH, Chiou CC, Walsh TJ. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs 2004; 64: 1997–2020. [DOI] [PubMed] [Google Scholar]
- 10.Pasqualotto AC, Denning DW. New and emerging treatments for fungal infections. J Antimicrob Chemother 2008; 61(Suppl. 1): i19–30. [DOI] [PubMed] [Google Scholar]
- 11.Arikan S, Lozano-Chiu M, Paetznick V, Rex JH. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob Agents Chemother 2002; 46: 245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemons KV, Espiritu M, Parmar R, Stevens DA. Comparative efficacies of conventional amphotericin b, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother 2005; 49: 4867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kontoyiannis DP, Hachem R, Lewis RE et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 2003; 98: 292–9. [DOI] [PubMed] [Google Scholar]
- 14.Dannaoui E, Lortholary O, Dromer F. In vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob Agents Chemother 2004; 48: 970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkpatrick WR, Perea S, Coco BJ, Patterson TF. Efficacy of caspofungin alone and in combination with voriconazole in a Guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother 2002; 46: 2564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petraitis V, Petraitiene R, Sarafandi AA et al. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J Infect Dis 2003; 187: 1834–43. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Limaye AP, Forrest G et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation 2006; 81: 320–6. [DOI] [PubMed] [Google Scholar]
- 18.Scheven M, Schwegler F. Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob Agents Chemother 1995; 39: 1779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meletiadis J, Stergiopoulou T, O’Shaughnessy EM, Peter J, Walsh TJ. Concentration-dependent synergy and antagonism within a triple antifungal drug combination against Aspergillus species: analysis by a new response surface model. Antimicrob Agents Chemother 2007; 51: 2053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugar AM. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob Agents Chemother 1995; 39: 1907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh TJ, Petraitis V, Petraitiene R et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis 2003; 188: 305–19. [DOI] [PubMed] [Google Scholar]


