Abstract
Dendritic cell (DC)-derived IL-1α/β plays a critical role in the induction of T helper type 1 (Th1)-dependent immunity against Leishmania. DCs from susceptible BALB/c mice produce less IL-1α/β when compared with resistant C57BL/6 mice, contributing to aberrant Th2 development and ultimate death of infected mice. We have extended our studies of the role of IL-1 in leishmaniasis using IL-1RA−/− BALB/c mice that are characterized by upregulated IL-1 receptor signaling. Unexpectedly, infection of IL-1RA−/− mice led to significantly worsened disease outcome with larger lesions, dramatically higher parasite burdens, and decreased IFN-γ production by antigen-specific T cells. We determined that IL-1RA−/− DCs were more mature already in the steady state, exhibited less phagocytotic capacity, and IL-12 production in response to various stimuli was impaired. Our data suggest that in addition to effects on Th education, IL-1α/β signaling also modulates DC homeostasis with increased signaling, leading to downmodulation of IL-12 synthesis and worsened disease outcome after infection with Leishmania major. Thus, the complex regulation of various members of the IL-1 cytokine family mediated through effects on both DCs and T cells critically contributes to disease outcome against this important human pathogen.
INTRODUCTION
Leishmaniasis is a parasitic disease transmitted by the bite of a sand fly. The disease ranges from cutaneous leishmaniasis with skin sores characterized by ulcerating, painful skin nodules associated with enlarged lymph nodes (LNs) to visceral leishmaniasis affecting internal organs of the body (e.g., liver, spleen, and bone marrow (BM)). Murine models of cutaneous leishmaniasis have been used to study resulting immune responses. In leishmaniasis, control of Leishmania infections in resistant C57BL/6 mice relies on IL-12-dependent production of T helper type 1 (Th1)/T-cytotoxic type 1 (Tc1)-derived IFN-γ that activates infected macrophages (MΦs) to eliminate parasites (Reiner and Locksley, 1995; Belkaid et al., 2002; Sacks and Noben-Trauth, 2002; von Stebut and Udey, 2004). Skin dendritic cells (DCs) infected with Leishmania major represent important sources of IL-12 (von Stebut et al., 1998). In contrast, BALB/c mice respond to infection with preferential production of Th2-type cytokines such as IL-4 and IL-10, which are associated with disease progression and susceptibility (Sacks and Noben-Trauth, 2002). BALB/c mice also produce increased levels of IL-17A after infection with L. major, and lesion sizes in IL-17−/− BALB/c mice are smaller when compared with those in wild-type mice (Lopez Kostka et al., 2009). Improved disease outcome is associated with decreased neutrophil immigration into lesions of infected IL-17−/− mice (Lopez Kostka et al., 2009).
DC-derived factors that influence disease susceptibility of BALB/c mice include elevated levels of inhibitory IL-12p80 (Nigg et al., 2007) and decreased release of IL-1α/β (Filippi et al., 2003; von Stebut et al., 2003a). Previously, we demonstrated that DC-derived IL-1α/β facilitates Th1 induction in inflammatory disease models (von Stebut et al., 2003a; Lopez Kostka et al., 2006; Caucig et al., 2010). Treatment of BALB/c mice with IL-1α during T-cell priming inhibited progressive disease by shifting the immune response toward Th1 predominance (von Stebut et al., 2003a). However, prolonged administration of IL-1α promoted Th2 expansion in already established infections and worsened disease outcome (Lopez Kostka et al., 2006).
IL-1 is a key mediator of inflammation and is produced by various cell types (Dinarello, 1996, 2009). IL-1α and IL-1β exert similar biological functions by binding to the IL-1 type I receptor (IL-1RI) (Sims et al., 1993). IL-1 receptor antagonist (RA) is an endogenous inhibitor of IL-1. IL-1RA is structurally homologous to IL-1β and binds tightly to IL-1RI, thereby blocking access of IL-1 to the receptor. Thus, IL-1RA−/− mice exhibit excessive IL-1α/β signaling due to a lack of an antagonist. Deficiency of IL-1RA causes autoimmunity and arthritis in mice, emphasizing the importance of the IL-1/IL-1RA balance for the immune system homeostasis (Horai et al., 1998, 2000). Additionally, IL-17 is required for IL-1RA-deficient BALB/c mice to develop arthritis (Nakae et al., 2003).
To additionally characterize the role of IL-1 for resulting immune responses in cutaneous leishmaniasis, we studied IL-1RA−/− BALB/c mice. Surprisingly, and contrary to our expectations, infected IL-1RA−/− BALB/c mice displayed enhanced lesion progression when compared with wild-type controls. IL-1RA−/− DCs were markedly impaired in IL-12 production and displayed a more mature phenotype with a reduced phagocytotic capacity. Reconstitution of IL-1RA−/− DCs with recombinant IL-1RA restored inflammatory mediator production (including IL-12). In summary, we demonstrate that apart from effects of IL-1α/β on T-cell priming toward Th1, excessive IL-1 signaling in vivo due to the genetic deficiency of IL-1RA leads to alterations in DC function, even under steady-state conditions, and results in pathological activation of DCs.
RESULTS
L. major-infected IL-1RA−/− mice exhibit increased disease susceptibility associated with decreased IL-12 levels
We previously showed that substitution of IL-1α during T-cell priming in vivo protected BALB/c mice from progressive disease and abrogated aberrant Th2 development (von Stebut et al., 2003a). To further characterize the role of IL-1 in leishmaniasis, we studied IL-1RA−/− mice on a genetically susceptible BALB/c background. IL-1RA−/− BALB/c and wild-type BALB/c mice were infected intradermally with physiologically relevant low doses (1,000 parasites) of L. major metacyclic promastigotes, and lesion development was monitored over the course of 3 months. Surprisingly, IL-1RA−/− BALB/c mice displayed significantly enhanced lesion progression when compared with wild-type mice and had to be killed in week 10, whereas control mice progressed more slowly (Figure 1a). Lesion progression in IL-1RA−/− and wild-type BALB/c mice correlated with lesional parasite burdens (Figure 1b, left panel). At 6 weeks after infection, lesions of BALB/c mice contained ~1.9 × 106 parasites, whereas infected ears of IL-1RA−/− BALB/c mice harbored ~1.6 × 107 parasites (8-fold difference), and 8 weeks after infection, lesions of these mice contained ~4.5 × 108 parasites, which was 58-fold higher as in control mice. Significantly increased parasite numbers in spleens of IL-1RA−/− mice were also detected at week 6 and, to a lesser degree, at week 8 (Figure 1b, right panel).
Figure 1. BALB/c mice deficient in IL-1 receptor antagonist (IL-1RA) exhibit increased susceptibility in cutaneous leishmaniasis because of decreased levels of IL-12 and IFN-γ.
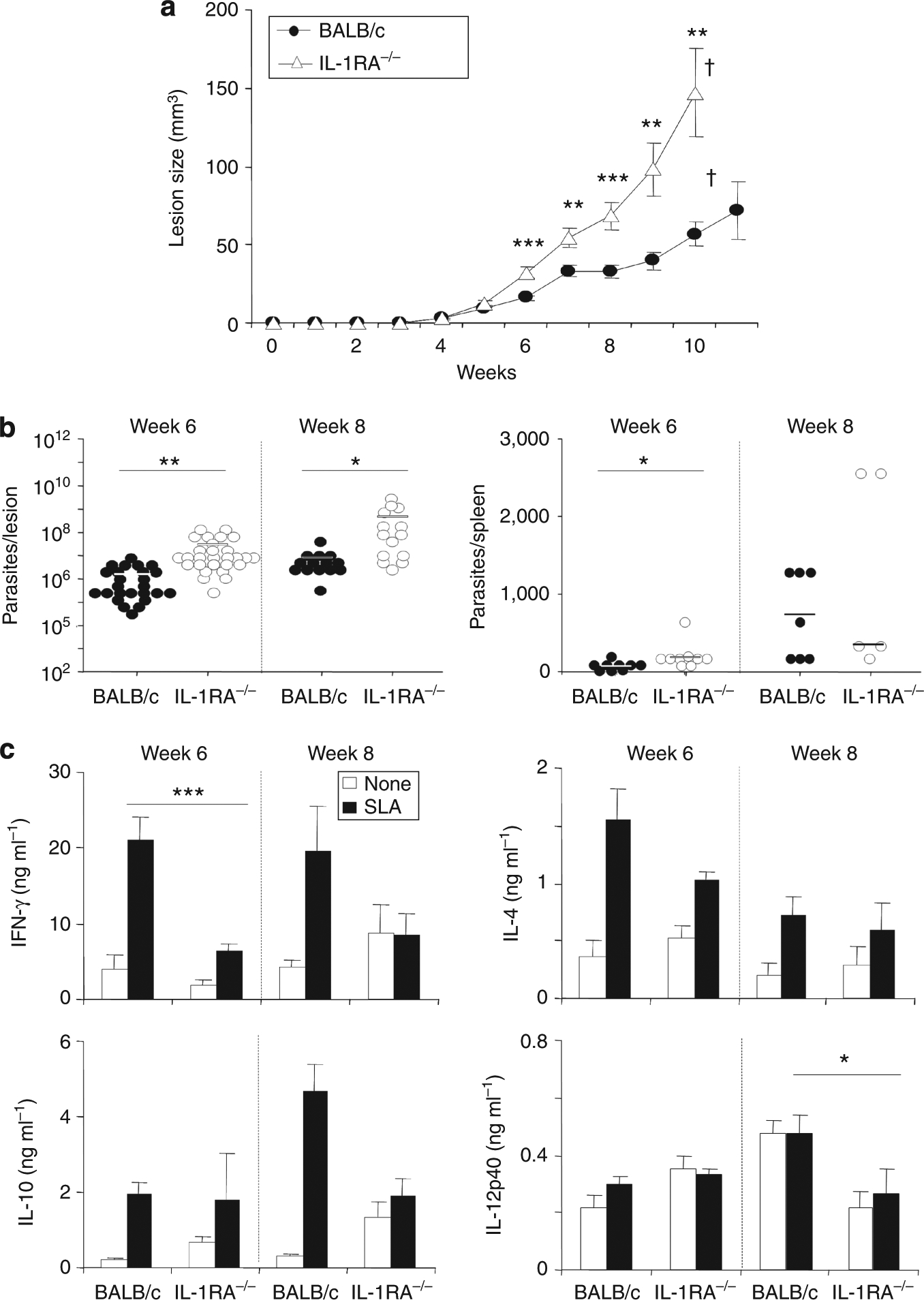
Groups of ⩾5 IL-1RA−/− or BALB/c mice were infected with 103 Leishmania major. (a) Lesion development was assessed weekly. (b) In weeks 6 and 8, lesional and splenic parasite burdens were determined using limiting dilution. Dots represent parasites in individual ears; bars indicate means. (c) Draining lymph nodes were plated at 1 × 106 cells per well and restimulated in the presence of soluble Leishmania antigen (SLA, 25 μg ml−1). All data are expressed as mean±SEM (n⩾8 from seven independent experiments; †mice were killed, *P⩽0.05, **P⩽0.005, and ***P⩽0.002).
Additionally, cytokine profiles of LN cells restimulated with soluble Leishmania antigen were determined in weeks 6 and 8 (Figure 1c). We detected significantly decreased levels of IFN-γ (6.5 ± 0.9 vs. 20.9 ± 3.1 ng ml−1 in week 6) and IL-12p40 (267 ± 82 vs. 474 ± 63 pg ml−1 in week 8) in IL-1RA−/− mice when compared with control mice (Figure 1c). Interestingly, levels of IL-4 and IL-10 were unaltered in IL-1RA−/− mice.
To confirm that differences in cytokine production were because of different T-cell priming in vivo and not differences in antigen-presenting cell dysfunction in vitro, CD4+ T cells were isolated from 6-week L. major-infected IL-1RA−/− and BALB/c mice and restimulated with BALB/c DCs for 48 hours. Here, IL-1RA−/− CD4+ T cells co-cultured with unpulsed or L. major amastigote-infected DCs produced significantly less IFN-γ, and more IL-10 and IL-13 (Figure 2a). Surprisingly, under these conditions, leaving only T cells as the cytokine source (when compared with crude LN restimulations as shown in Figure 1c), IL-4 production from BALB/c CD4+ T cells was significantly higher in comparison with T cells from IL-1RA−/− mice. Intracellular FACS analysis of LN cells also revealed that there were fewer IFN-γ+ and more IL-10 + CD4+ T cells in IL-1RA−/− mice compared with BALB/c mice (Figure 2b). Differences in IL-4 levels were not significant.
Figure 2. Th cells from IL-1RA−/− and BALB/c mice are differentially primed in vivo.
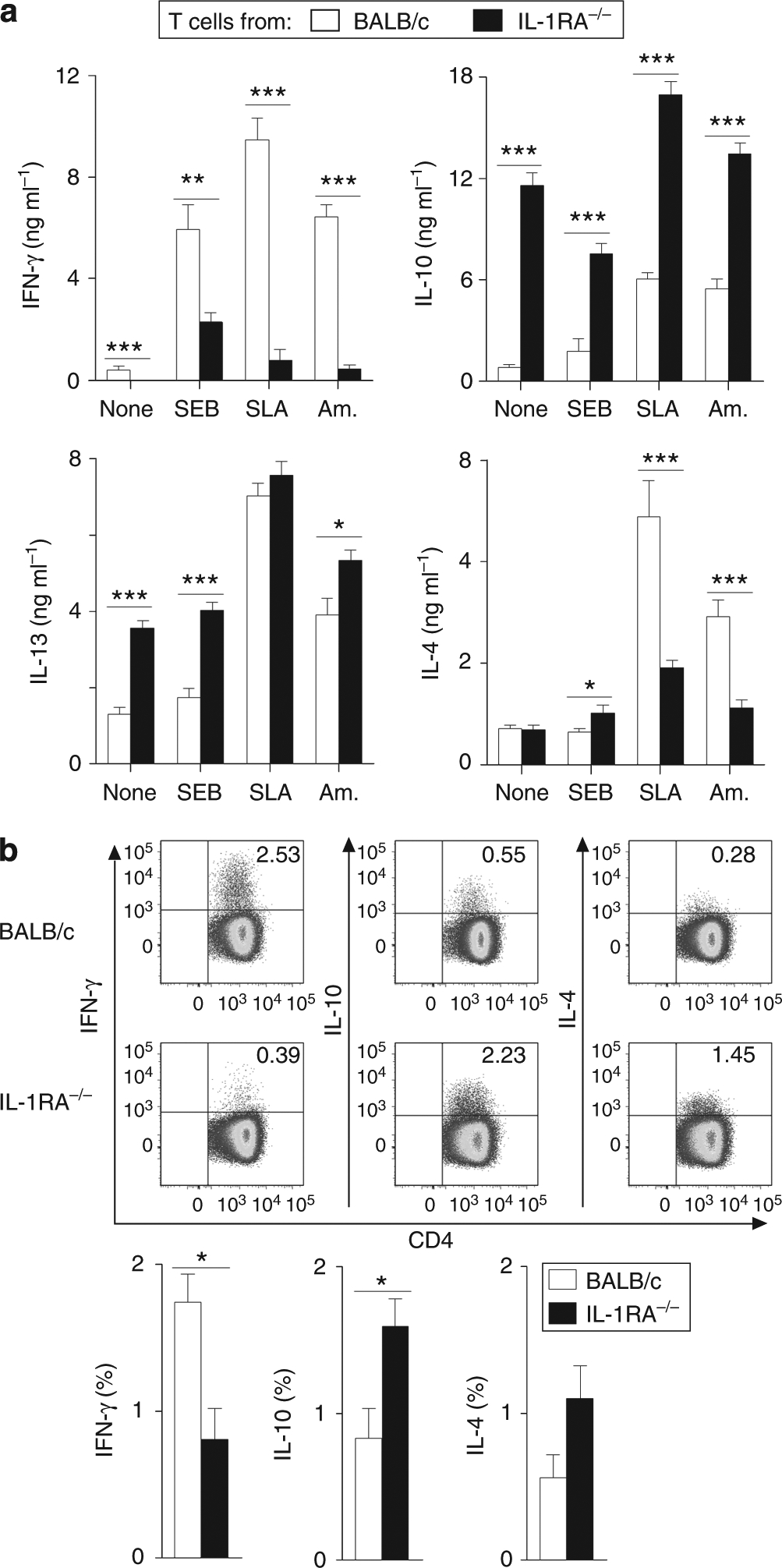
(a) Bone marrow-derived dendritic cells (DCs) from BALB/c mice were plated at 2 × 105 per ml and incubated for 18 hours with soluble Leishmania antigen (SLA), Leishmania major amastigotes (Am., five parasites per cell), or Staphylococcus enterotoxin B (SEB, 20 μg ml−1), or left untreated. CD4+ T cells from lymph nodes (LNs) from 6-week-infected IL-1RA−/− and BALB/c mice were enriched and co-cultured with DCs for 48 hours. Supernatants were analyzed for cytokine production by ELISA. Pooled data from two independent experiments are shown (mean±SEM, n = 8 different mice). (b) For intracellular cytokine analysis, LN cells from infected mice were restimulated using phorbol 12-myristate 13-acetate (PMA)/ionomycin in the presence of Brefeldin A for 4–6 hours, followed by extracellular staining for CD3, CD45, and CD4 and intracellular cytokine staining. Representative plots of cells pregated on CD3, CD45, and CD4 are shown (upper panel). Pooled data from two independent experiments are shown (lower panel) (mean±SEM, n = 8 different mice). (a, b) *P⩽0.05, **P⩽0.005, and ***P⩽0.002.
Previous studies have demonstrated that in L. major infections, enhanced recruitment of neutrophils is associated with disease susceptibility in BALB/c mice (Ribeiro-Gomes et al., 2004; Jacobs et al., 2005). We analyzed the lesional inflammatory cell infiltrates in IL-1RA−/− and control BALB/c mice in detail (Supplementary Figure S1 online). The numbers of lesional inflammatory neutrophils (NIMP-R14+), DCs (CD11c+), and MΦs (F4/80+) were significantly elevated in IL-1RA−/− mice in comparison with BALB/c mice at 5 weeks after infection.
Impaired IL-12 release from IL-1RA−/− bone marrow-derived dendritic cells (BMDCs) upon infection with L. major
To investigate the mechanism underlying the reduced IL-12 (and IFN-γ) levels in IL-1RA−/− mice, BMDCs or skin-derived MΦs were generated from IL-1RA−/− or BALB/c mice. Cells were stimulated with lipopolysaccharide (LPS) ± IFN-γ (100 ng per 1,000 U ml−1) or infected with amastigotes or promastigotes of L. major (five parasites per cell) and cytokine production was analyzed by ELISA. IL-12p40 production by MΦs was similar in both mouse groups. In addition, infection rates and the ability of IL-1RA−/− MΦs to release nitric oxide did not differ from those of controls (Supplementary Figure S2 online). However, IL-1RA−/−-derived DCs produced significantly less IL-12p40 in comparison with wild-type DCs (e.g., 0.8 ± 0.3 vs. 16.5 ± 5.4 ng ml−1 and 0.8 ± 0.3 vs.18.4 ± 6.4 ng ml−1 after incubation with amastigotes or amastigotes + IFN-γ, respectively; Figure 3a). IL-10 production was not altered in IL-1RA−/− mice. Importantly, production of the bioactive IL-12p70 heterodimer was also significantly reduced in IL-1RA−/−-derived DCs after incubation with amastigotes in comparison with controls (591 ± 205 vs. 1,193 ± 447 pg ml−1; Figure 3b). Infection rates of DCs were equal in both mouse strains (Figure 3c), but the number of amastigotes per single cell was significantly reduced in IL-1RA-deficient DCs (e.g., 2.8 ± 0.3 vs. 4.1 ± 0.6 after incubation with amastigotes, P⩽0.05). Taken together, IL-1RA−/− DCs were significantly impaired in their IL-12 production and their ability to phagocytose L. major.
Figure 3. IL-1RA−/− dendritic cells (DCs) are more mature and impaired in IL-12 production and in their ability to phagocytose Leishmania major.
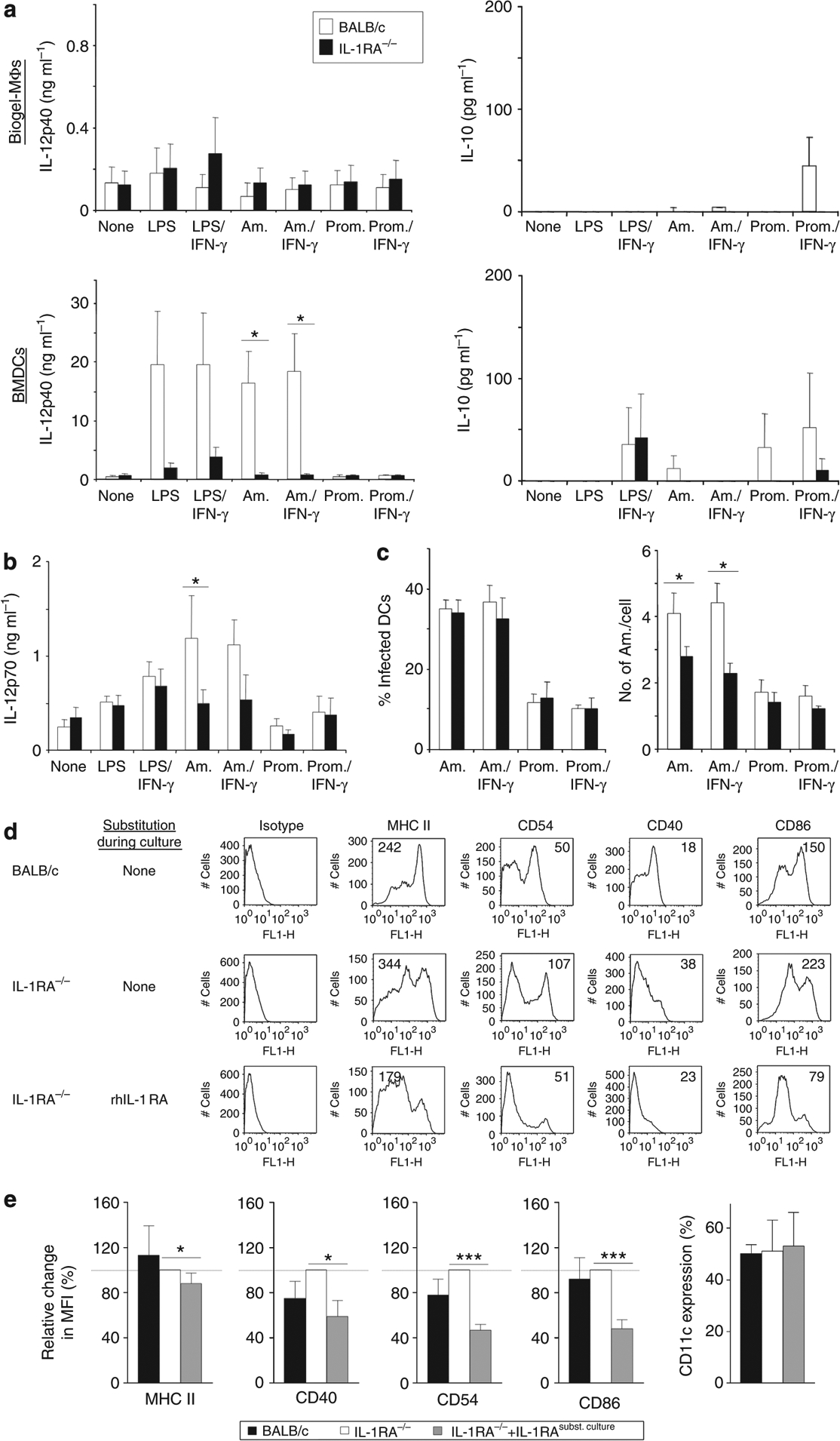
Skin-derived macrophages (MΦs) and bone marrow-derived DCs (BMDCs) were plated at 2 × 105 per ml and incubated for 18 hours with lipopolysaccharide (LPS; 100 ng ml−1), IFN-γ (1,000 U ml−1), amastigotes (Am.), or promastigotes (Prom.) of L. major (five parasites per cell) as indicated. (a, b) Cytokine content in supernatants was determined by ELISA. (c) After 18 hours, cells were harvested and infection rates as well as the number of parasites/cell were determined on DiffQuick-stained cytospins. All data are expressed as mean±SEM (n⩾4; *P⩽0.05). (d, e) DCs were generated from bone marrow using rGM-CSF and IL-4 (10 ng ml−1). In some cultures, rhIL-1RA was added at 1 μg ml−1. On day 6, cells were harvested and analyzed for expression of major histocompatibility complex (MHC) class II, CD40, CD54, and CD86 by flow cytometry. (d) One representative staining with mean fluorescence intensity (MFI) is shown. (e) Pooled data from n⩾4 independent experiments are shown. IL-1RA−/− MFI values were normalized to 100% and relative changes in other groups calculated (mean±SEM; *P⩽0.05, and ***P⩽0.002).
Next, we analyzed the expression of major histocompatibility complex (MHC) class II and the co-stimulatory molecules CD40, CD54, and CD86 on DCs, asking if IL-1RA deficiency led to an altered maturation status of the DCs. DCs from IL-1RA−/− and BALB/c mice were generated using IL-4 and GM-CSF. A third group of IL-1RA−/− BMDCs were treated with rhIL-1RA (1 mg ml−1) during culture. On day 6, DCs were harvested and analyzed for expression of surface molecules by flow cytometry. Data were analyzed by determining the mean fluorescence intensity of the specific marker on CD11c-gated cells. Individual histograms of one representative experiment are shown in Figure 3d. DCs generated from IL-1RA−/− mice showed a more mature phenotype in comparison with wild-type DCs. Correspondingly, expression of MHC class II, CD40, CD54, and CD86 by IL-1RA−/− DCs was significantly reduced and restored to normal (or even below normal) levels, when cells were incubated with rhIL-1RA during culture (Figure 3e).
Substitution of IL-1RA leads to normalization of IL-12 production by IL-1RA−/− DCs
As shown in Figure 3a, IL-1RA−/− DCs were severely impaired in their IL-12 production. To determine if the lack of IL-RA could be responsible for the reduced IL-12p40 levels, IL-1RA−/− DCs were treated with rhIL-1RA during culture. BMDCs from IL-1RA−/− and BALB/c mice were stimulated with LPS, IFN-γ, and L. major amastigotes. Additionally, some cultures were incubated with rhIL-1RA during DC generation (1 μg ml−1), during stimulation (50 ng ml−1), and both (DC generation + stimulation period). IL-12p40 release into culture supernatants by DCs was determined by ELISA. Treatment with rhIL-1RA during culture of IL-1RA−/− DCs resulted in restoration of their ability to produce IL-12p40 (Figure 4a). In LPS-stimulated cells, levels of IL-12p40 were significantly increased when IL-1RA−/− DCs were treated with rIL-1RA during DC generation (6.9 ± 0.5 and 7.6 ± 0.7 ng ml−1 by IL-1RA−/− DC + rhIL-1RA during DC generation and during DC generation + DC stimulation, respectively, in comparison with 3.9 ± 0.5 ng ml−1 by untreated IL-1RA−/− DCs, P⩽0.005). In LPS/IFN-γ-stimulated cells, similar results were obtained (8.7 ± 1.1 by IL-1RA−/− DCs incubated with rhIL-1RA during DC generation + DC stimulation in comparison with 6.1 ± 1.1 ng ml−1 by untreated IL-1RA−/− DCs). No effect was observed when cells received rhIL-1RA only during stimulation. Importantly, L. major uptake was also restored when IL-1RA−/− DCs were treated with rhIL-1RA during DC generation (3.0 ± 0.3 vs. 2.3 ± 0.1 amastigotes per cell in untreated IL-1RA−/− DCs; Figure 4b). Taken together, incubation with rhIL-1RA during propagation of DCs in vitro resulted in the generation of DCs capable of normal L. major uptake and restored their ability to respond by production of inflammatory cytokines, including IL-12p40.
Figure 4. Restoration of a normal immature phenotype of IL-1RA−/− BALB/c dendritic cells (DCs) with recombinant IL-1RA.
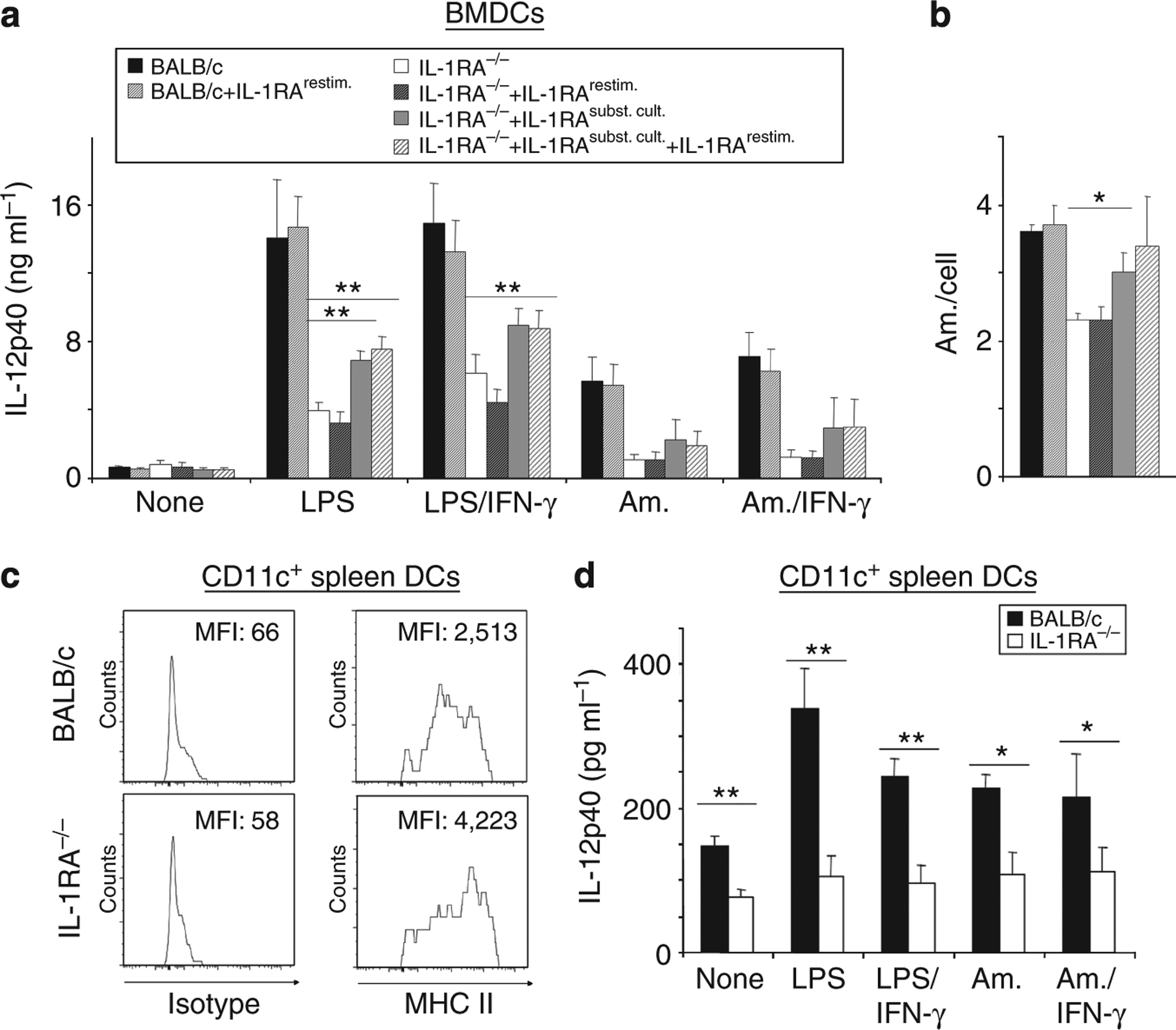
(a, b) Bone marrow-derived DCs (BMDCs) were generated with rGM-CSF and IL-4 (10 ng ml−1 each) and harvested as immature DCs on day 6. (c, d) CD11c+ DCs were isolated ex vivo from lymph nodes (LNs) and spleens using MACS beads. All cells were plated at 2 × 105 cells per ml and stimulated with lipopolysaccharide (LPS; 100 ng ml−1), IFN-γ (1,000 U ml−1), and Leishmania major amastigotes (Am., 1:5 cells/parasites). (a, b) In addition, some co-cultures were treated with rhIL-1RA during culture (IL-1RAsubst. cult., 1 μg ml−1) and/or restimulation (IL-1RArestim, 50 ng ml−1). (a, d) IL-12p40 release was determined by ELISA. (b) Infection rates of DCs were assessed on cytospins. (c) Spleen DCs were analyzed for their expression of major histocompatibility complex (MHC) class II using flow cytometry. One representative of two independent experiments is shown, numbers in the upper right corner represent mean fluorescence intensity (MFI) values. (a, b, d), Pooled data from n⩾5 independent experiments are shown (mean±SEM; *P⩽0.05, and **P⩽0.005).
Finally, to confirm our findings with primary cells, IL-1RA−/− DCs isolated from LN and spleen ex vivo expressed dramatically increased levels of MHC class II (Figure 4c) and produced significantly less IL-12p40 after stimulation with LPS/IFN-γ, amastigotes, and even spontaneously without stimulation in comparison with wild-type BALB/c DCs (Figure 4d).
The phenotype of IL-1RA−/− mice relies on BM-derived cells
As shown in Figure 1, IL-1RA−/− mice showed progressive lesion development and reduced production of IFN-γ when compared with BALB/c mice. To determine if BM-derived cells represented the relevant source of IL-1RA, we generated hematopoietic BM chimeras. BALB/c and IL-1RA−/− donor BM was used to reconstitute lethally irradiated (7 Gy) BALB/c and IL-1RA−/− host mice. The resulting chimeric mice were allowed to reconstitute their hematopoietic compartments for at least 6 weeks before analysis. Subsequently, mice were infected with physiologically relevant low-dose inocula of L. major and lesion development was assessed weekly. As expected, IL-1RA−/− mice reconstituted with IL-1RA−/− BM revealed progressive lesion development, but lesion sizes were significantly reduced when IL-1RA−/− mice received BM from control mice instead (23 ± 2 vs. 68 ± 8 mm3 in week 6; Figure 5a). This was accompanied by reduced parasite numbers per lesion (5.6 × 106 vs. 27.7 × 106 parasites in week 6). Consistent with this result, BALB/c mice reconstituted with IL-1RA−/− BM showed more severe lesion progression than BALB/c mice reconstituted with BALB/c BM (118 ± 17 vs. 36 ± 6 mm3 in week 6).
Figure 5. Leishmania susceptibility of IL-1RA−/− BALB/c mice is determined by bone marrow (BM)-derived cells.
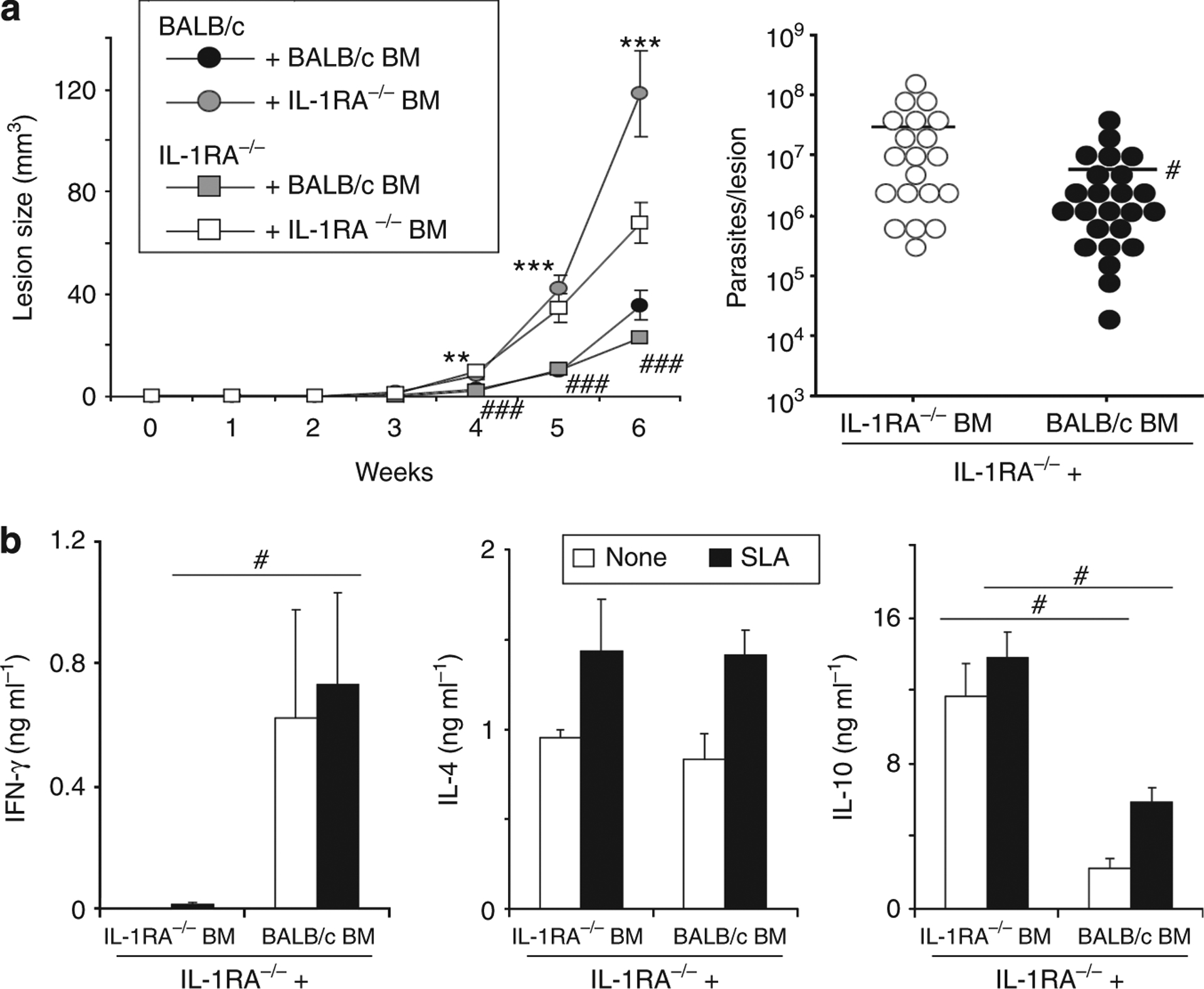
IL-1 receptor antagonist (IL-1RA)-deficient or wild-type mice were lethally irradiated with 7 Gy, adoptively transferred with either wild-type or IL-1RA−/− BM (5 × 106 cells per mouse intravenously (i.v.)), and rested for 6 weeks. Subsequently, mice were infected with physiologically relevant low-dose inocula of Leishmania major (103 metacyclic promastigotes). (a) Lesion development was assessed in three dimensions. In week 6, lesional parasite burdens were determined using a limiting dilution assay. Dots represent parasites in individual ears, bars indicate means. (b) In week 6, draining lymph nodes were harvested and restimulated with soluble Leishmania antigen (SLA; 1 × 106 per ml). Cytokine release into 48 hours of supernatants was determined by ELISA. Pooled data from n = 2 independent experiments are shown (mean±SEM; n⩾8, *P⩽0.05, **P⩽0.005, and ***P⩽0.002). * Differences between BALB/c recipient mice; #differences between IL-1RA−/− recipients.
We next analyzed supernatants of antigen-specifically restimulated LN cell cultures for the production of IFN-γ, IL-4, and IL-10. Interestingly, IL-1RA−/− mice reconstituted with BALB/c BM produced significantly more IFN-γ when compared with controls (731 ± 304 vs. 11 ± 11 pg ml−1; Figure 5b). On the other hand, IL-10 production was significantly reduced in these mice (2.2 ± 0.6 vs.11.7 ± 1.8 ng ml−1 and 5.8 ± 0.8 vs. 13.9 ± 1.4 ng ml−1 in unstimulated and soluble Leishmania antigen-stimulated cells, respectively). No difference in IL-4 release was observed.
DISCUSSION
DCs are essential for activating adaptive immune responses and initiating the differentiation of T cells (Th1/Th2) (Shortman and Liu, 2002; Boonstra et al., 2003). Genetically determined differences in DC functions are associated with resistance or susceptibility to infection with L. major (Moll, 2003), and different cytokines released by DCs may thus be important for Th cell differentiation. Development of protective Th1 responses requires IL-12 during priming of naive T cells (Reiner and Locksley, 1995; Berberich et al., 2003), and DCs may be an important early source of IL-12. Additionally, production of IL-1 influences the outcome of infection in BALB/c and C57BL/6 mice.
IL-1 is a highly active proinflammatory cytokine that is expressed in two forms: IL-1α and IL-1β (Dinarello, 1996, 2009). The physiological role of IL-1α and IL-1β in the development of Th cell responses remains controversial. It has been demonstrated that IL-1 promotes proliferation of Th2 cells in vitro (Lichtman et al., 1988; Weaver et al., 1988; Huber et al., 1998). Other reports have suggested that increased Th2 responses develop in IL-1-deficient mice (Satoskar et al., 1998). IL-1 is also required for IL-12-induced proper Th1 cell development in BALB/c CD4 cells (Shibuya et al., 1998). In leishmaniasis, we and others previously demonstrated that BALB/c DC produced less IL-1 after being stimulated with L. major when compared with DCs from C57BL/6 mice (Filippi et al., 2003; von Stebut et al., 2003a). In this study we investigated the role of IL-1 in more detail using IL-1RA−/−-susceptible BALB/c mice that exhibit enhanced IL-1 action that we anticipated would compensate for the relative dysbalance that we observed earlier.
Previously, using the model of cutaneous leishmaniasis, we demonstrated that the timing of IL-1 release is critical for resulting T cell-dependent immune responses. In BALB/c mice, administration of IL-1α during T-cell priming induced effective protection of the host against progressive leishmaniasis, whereas administration of IL-1α at later time points with already established Th2 immunity worsened disease outcome (von Stebut et al., 2003a). The IL-1 family includes members that suppress inflammation, including the IL-1 type II receptor that binds IL-1, but does not transduce a signal and functions as a decoy for IL-1 (Colotta et al., 1993). In contrast, IL-1RA binds to IL-1RI and blocks the activity of IL-1α/β (Dinarello, 1996, 2005). The outcome of an inflammatory process is likely to be affected by the relative amounts of IL-1 and IL-1RA. In this study, we studied IL-1RA−/− BALB/c mice, which exhibit excessive IL-1 signaling due to the lack of its antagonist.
We analyzed the influence of IL-1RA on lesion development after physiological low-dose L. major infection. IL-1RA−/− mice infected with L. major showed worsened disease outcome correlating with decreased IL-12p40 and IFN-γ production, whereas levels of IL-10 and IL-4 were unaltered. Increased lesion development was accompanied by elevated numbers of lesional inflammatory neutrophils, DCs, and MΦs in IL-1RA−/− mice in comparison with BALB/c mice. Additionally, IL-12p40 production in response to L. major was significantly impaired by IL-1RA−/− DCs ex vivo. Analysis of isolated CD4 T cells from infected BALB/c and IL-1RA−/− mice revealed that Th cells from IL-1RA−/− mice produced higher amounts of IL-10, IL-13, and less IFN-γ than BALB/c T cells, underlining the enhanced Th2 response. Interestingly, DCs from IL-1RA-deficient mice were more mature and thus exhibited less phagocytic activity. We suggest that the unopposed high levels of IL-1α/β in IL-1RA−/− leads to pathological activation of DCs, even under steady-state conditions, which in turn renders DCs less able to properly respond to stimuli such as L. major infection with upregulation of activation markers and IL-12 production. Incubation with rIL-1RA during culture of DCs in vitro restored an immature DC phenotype and resulted in DCs that were capable of normal L. major uptake and IL-12p40 production upon infection.
Our data also indicate that the phenotype of IL-1RA−/− mice in leishmaniasis relies on BM-derived cells. To generate BM chimeras, IL-1RA−/− mice were lethally irradiated and reconstituted with either syngeneic IL-1RA−/− or BALB/c wild-type BM. After transferring wild-type BM into IL-1RA−/− mice, lesion sizes and parasite burdens were significantly reduced to the level of wild-type mice upon L. major challenge. These findings were accompanied by increased levels of IFN-γ and significantly decreased production of IL-10.
Horai et al. (2000) reported previously that deficiency of IL-1RA in BALB/cA mice causes autoimmunity and arthritis. Additionally, proinflammatory cytokine expression (IL-1β, IL-6, and tumor necrosis factor-α) was enhanced in these mice, suggesting that the IL-1 activity is suppressed by IL-1RA under physiological conditions. Overproduction of these cytokines (because of unbalanced IL-1 signaling) can also activate the immune system by enhancing recruitment of immune cells and induce inflammation (Dinarello, 1996; Horai et al., 2000). Previously, restimulation of antigen-specific CD4+ T cells was shown to be decreased upon co-culture with IL-1RA−/− DCs, but not IL-1α/β−/− DCs (Nambu et al., 2006), suggesting alterations in the phenotype of IL-1RA−/− DCs. In addition, Iizasa et al. (2005) showed that IL-1RA controlled optimal activation and migration of inflammatory DCs within the liver with decreased expression of CCR7 (chemokine (C-C motif) receptor 7) and IL-12p40 by IL-1RA−/− DCs in a Propionibacterium acnes-induced murine granulomatous liver disease model. Our finding of dysfunctional DCs in this genetically altered mouse may have broad implications regarding autoimmune diseases that develop in IL-1RA−/− mice or for patients continuously treated with IL-1RA for therapeutic reasons.
In summary, our study provides evidence that the tightly regulated balance between IL-1 and IL-1RA is crucial for DC homeostasis and thus disease outcome in leishmaniasis and the lack of IL-1RA leads to worsening of the disease. In IL-1RA−/− BALB/c mice, this effect was reversible by substitution with rIL-1RA. To our knowledge, the importance of IL-1RA during Leishmania infections is previously unreported, as the antagonist influences the maturation status of the DCs and thereby directs the immune response. IL-1RA is approved for treatment of rheumatoid arthritis and several systemic and local inflammatory diseases (Fitzgerald et al., 2005; Pascual et al., 2005) and thus might be of benefit in treatment of intracellular infections such as with Leishmania sp.
MATERIALS AND METHODS
Animals
BALB/c mice, 6–8 weeks old, or IL-1RA-deficient BALB/c mice (Horai et al., 1998) were obtained from the Central Animal Facility of the University of Mainz. All animals were housed and used in experiments in accordance with the institutional guidelines.
Propagation of L. major parasites and infections
Infectious-stage metacyclic promastigotes were isolated from stationary cultures of L. major clone VI (MHOM/IL/80/Friedlin) by positive selection using a biphasic Ficoll gradient (10/20%). Amastigotes of L. major were enriched from infected BALB/c footpads as described previously (Belkaid et al., 1998; von Stebut et al., 1998). Isolated parasites were opsonized with 5% BALB/c normal mouse serum and washed before in vitro infections.
In vivo infections were initiated intradermally in ear skin of mice using physiological low-dose inocula of 1 × 103 metacyclic promastigotes of L. major per ear. Lesion volumes were measured weekly in three dimensions and are reported as ellipsoids ((a/2 × b/2 × c/2) × 4/30π).
Determination of lesional parasite burden and cytokine production
Parasites present in lesional tissue were enumerated using a limiting dilution assay in Schneider’s Drosophila medium (BioWhittaker, Taufkirchen, Germany), 2% human urine, 10% fetal calf serum, 2% glutamine, 1% penicillin/streptomycin, and 0.5% 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid as described (Belkaid et al., 2000).
For measurement of cytokine production, 1 × 106 retroauricular LN cells per 200 μl were added to 96-well plates in the presence of soluble Leishmania antigen (25 μg ml−1). Antigen-specific IFN-γ, IL-4, IL-10, and IL-12p40 production were determined after 48 hours by ELISA (R&D Systems, Wiesbaden, Germany, and BD Pharmingen, Heidelberg, Germany).
Generation of DCs and skin-MΦs, phagocytosis assays
Skin-derived MΦs from IL-1RA−/− or wild-type mice were elicited by injection of Biogel into skin pouches (von Stebut et al., 2003b). The gel was removed after 5 days and MΦs were enriched to high purity by plate adherence. DCs were generated from BM using GM-CSF and IL-4 (10 ng ml−1). Immature DCs were harvested on day 6. In some experiments, cultures were incubated with recombinant crossreactive human IL-1RA (1 μg ml−1 and 50 ng ml−1, respectively; Kineret, Amgen Europe B.V., Breda, Netherlands).
All cells were plated at 2 × 105 cells per ml and stimulated with LPS (100 ng ml−1), IFN-γ (1,000 U ml−1), amastigotes, and promastigotes of L. major (parasite/cell ratio 5:1). The DC surface phenotype was determined using flow cytometry and staining with Fc-Block (2.4G2) and CD11c (N418), MHC II (2G9), CD40 (3/23), CD54 (ICAM-1), and CD86 (GL1) (BD Pharmingen, eBioscience, Frankfurt, Germany). Supernatants were harvested after 18 hours and IL-12p40, IL-12p70, and IL-10 content was determined by ELISA (R&D Systems, BD Pharmingen). Cells were harvested after 18 hours, cytospins prepared, and DiffQuick-stained cells were analyzed for the presence of intra- and extra-cellular parasites by a blinded investigator. At least 200 cells were counted per sample (× 400 magnification).
For measurements of nitric oxide production, supernatants of 48 hours of MΦ cultures were treated with Gries reagent (1% sulfanilamide (Sigma, Steinheim, Germany), 0.1% naphtylethylenediamine (Sigma), and 2–5% 1 m H3PO4) and the relevant product was quantified at 550–570 nm.
CD11c+ DCs were enriched ex vivo from naive BALB/c or IL-1RA−/− mice using microbeads (Miltenyi, Bergisch Gladbach, Germany) using the manufacturer’s protocol. The level of MHC class II expression was determined using flow cytometry as described above for BMDCs. DCs were plated at 106 per ml and stimulated as indicated with LPS, IFN-γ, and/or L. major amastigotes. After 18 hours, super-natants were assayed for the presence of IL-12p40 by ELISA.
Intracellular cytokine staining
CD4+ T cells were enriched from LN cells from 6-week-infected mice using CD4 microbeads (Miltenyi). Cells were restimulated with phorbol 12-myristate 13-acetate (50 ng ml−1), ionomycin (500 ng ml−1), and Brefeldin A (1 μgml−1) for 4–6 hours at 37 °1C. Surface staining was performed using the following antibodies: CD4 (L3T4 RM4–5) (BD Pharmingen), CD3 (eBio500A2), and CD45 (30-F11) (eBioscience). After fixation and permeabilization, cytokine accumulation was assessed with appropriate mAb: IL-4 (11B11), IL-10 (JES5–16E3), and IFN-γ (XMG1.2) (eBioscience). Cells were analyzed using a LSRII cytometer (BD Biosciences) and Flowjo software, Ashland, OR.
BM chimeras
BALB/c and IL-1RA-deficient BALB/c donor BM was used to reconstitute lethally irradiated (7 Gy) host mice (IL-1RA-deficient BALB/c mice and BALB/c, respectively). The resulting chimeric mice were allowed to reconstitute the hematopoietic compartment for at least 6 weeks before analysis. Subsequently, mice were infected with physiologically relevant low-dose inocula of L. major and lesion development was assessed weekly.
Statistical analysis
Statistical significance was determined using the unpaired Student’s t-test using StatView for Windows (Cary, NC).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Klaus Griewank for help with bone marrow chimeras and Dr Kurt Reifenberg and staff for excellent assistance in animal experimentation. This work was supported by grants from the Deutsche Forschungsge-meinschaft (DFG, SFB490, GK1043, and STE833/6-1, to EvS).
Abbreviations:
- BM
bone marrow
- BMDC
bone marrow-derived dendritic cell
- DC
dendritic cell
- IL-1RA
IL-1 receptor antagonist
- LN
lymph node
- LPS
lipopolysaccharide
- MΦ
macrophage
- MHC
major histocompatibility complex
- Th1
T helper type 1
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Belkaid Y, Butcher B, Sacks DL (1998) Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur J Immunol 28:1389–400 [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Mendez S, Lira R et al. (2000) A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol 165:969–77 [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Von Stebut E, Mendez S et al. (2002) CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol 168:3992–4000 [DOI] [PubMed] [Google Scholar]
- Berberich C, Ramirez-Pineda JR, Hambrecht C et al. (2003) Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J Immunol 170:3171–9 [DOI] [PubMed] [Google Scholar]
- Boonstra A, Asselin-Paturel C, Gilliet M et al. (2003) Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med 197:101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caucig P, Teschner D, Dinges S et al. (2010) Dual role of interleukin-1alpha in delayed-type hypersensitivity and airway hyperresponsiveness. Int Arch Allergy Immunol 152:303–12 [DOI] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M et al. (1993) Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 261:472–5 [DOI] [PubMed] [Google Scholar]
- Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87:2095–147 [PubMed] [Google Scholar]
- Dinarello CA (2005) Blocking IL-1 in systemic inflammation. J Exp Med 201:1355–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–50 [DOI] [PubMed] [Google Scholar]
- Filippi C, Hugues S, Cazareth J et al. (2003) CD4+ T cell polarization in mice is modulated by strain-specific major histocompatibility complex-independent differences within dendritic cells. J Exp Med 198:201–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald AA, Leclercq SA, Yan A et al. (2005) Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum 52:1794–803 [DOI] [PubMed] [Google Scholar]
- Horai R, Asano M, Sudo K et al. (1998) Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med 187:1463–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai R, Saijo S, Tanioka H et al. (2000) Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med 191:313–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Beuscher HU, Rohwer P et al. (1998) Costimulation via TCR and IL-1 receptor reveals a novel IL-1alpha-mediated autocrine pathway of Th2 cell proliferation. J Immunol 160:4242–7 [PubMed] [Google Scholar]
- Iizasa H, Yoneyama H, Mukaida N et al. (2005) Exacerbation of granuloma formation in IL-1 receptor antagonist-deficient mice with impaired dendritic cell maturation associated with Th2 cytokine production. J Immunol 174:3273–80 [DOI] [PubMed] [Google Scholar]
- Jacobs T, Andra J, Gaworski I et al. (2005) Complement C3 is required for the progression of cutaneous lesions and neutrophil attraction in Leishmania major infection. Med Microbiol Immunol 194:143–9 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Chin J, Schmidt JA et al. (1988) Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci USA 85:9699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Kostka S, Dinges S, Griewank K et al. (2009) IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol 182:3039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Kostka S, Knop J, Konur A et al. (2006) Distinct roles for IL-1 receptor type I signaling in early versus established Leishmania major infections. J Invest Dermatol 126:1582–9 [DOI] [PubMed] [Google Scholar]
- Moll H (2003) Dendritic cells and host resistance to infection. Cell Microbiol 5:493–500 [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R et al. (2003) IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA 100:5986–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Nakae S, Iwakura Y (2006) IL-1beta, but not IL-1alpha, is required for antigen-specific T cell activation and the induction of local inflammation in the delayed-type hypersensitivity responses. Int Immunol 18:701–12 [DOI] [PubMed] [Google Scholar]
- Nigg AP, Zahn S, Ruckerl D et al. (2007) Dendritic cell-derived IL-12p40 homodimer contributes to susceptibility in cutaneous leishmaniasis in BALB/c mice. J Immunol 178:7251–8 [DOI] [PubMed] [Google Scholar]
- Pascual V, Allantaz F, Arce E et al. (2005) Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 201:1479–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner SL, Locksley RM (1995) The regulation of immunity to Leishmania major. Annu Rev Immunol 13:151–77 [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Otero AC, Gomes NA et al. (2004) Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol 172:4454–62 [DOI] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N (2002) The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2:845–58 [DOI] [PubMed] [Google Scholar]
- Satoskar AR, Okano M, Connaughton S et al. (1998) Enhanced Th2-like responses in IL-1 type 1 receptor-deficient mice. Eur J Immunol 28:2066–74 [DOI] [PubMed] [Google Scholar]
- Shibuya K, Robinson D, Zonin F et al. (1998) IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J Immunol 160:1708–16 [PubMed] [Google Scholar]
- Shortman K, Liu YJ (2002) Mouse and human dendritic cell subtypes. Nat Rev Immunol 2:151–61 [DOI] [PubMed] [Google Scholar]
- Sims JE, Gayle MA, Slack JL et al. (1993) Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci USA 90:6155–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut E, Belkaid Y, Jakob T et al. (1998) Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med 188:1547–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut E, Ehrchen JM, Belkaid Y et al. (2003a) Interleukin 1alpha promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med 198:191–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut E, Metz M, Milon G et al. (2003b) Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1alpha/beta released from neutrophils recruited by mast cell-derived TNFalpha. Blood 101:210–5 [DOI] [PubMed] [Google Scholar]
- von Stebut E, Udey MC (2004) Requirements for Th1-dependent immunity against infection with Leishmania major. Microbes Infect 6:1102–9 [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hawrylowicz CM, Unanue ER (1988) T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci USA 85:8181–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


