Abstract
Objective—
Pericytes/pericyte precursors produce milk fat globule-associated protein with epidermal growth factor and factor VIII-like domains (MFG-E8) in vivo, and this αv integrin ligand enhances angiogenesis in tumors and in oxygen-induced retinopathy in mice. Inhibition of MFG-E8 production or function attenuates platelet-derived growth factor-BB (PDGF-BB)-induced migration of pericyte/pericyte precursor-like 10T1/2 cells in vitro. Herein, we describe mechanisms by which MFG-E8 modulates PDGF-BB:PDGF receptor β (PDGFRβ) signaling in 10T1/2 cells.
Methods and Results—
Small interfering RNA depletion of MFG-E8 from 10T1/2 cells or antibody inhibition of MFG-E8 action enhanced PDGF-BB-dependent degradation of PDGFRβ and attenuated signaling. Coimmunoprecipitation revealed transient association of MFG-E8 with PDGFRβ in PDGF-BB-treated 10T1/2 cells and reduced PDGFRβ-focal adhesion kinase association in MFG-E8-depleted cells. Confocal microscopy demonstrated that MFG-E8 binding to 10T1/2 cells was RGD motif and αv dependent but PDGF-BB treatment independent, whereas colocalization of MFG-E8 with PDGFRβ was enhanced by PDGF-BB. Ubiquitination of PDGFRβ was also increased in MFG-E8 small interfering RNA-transfected cells.
Conclusion—
Integrin αv-bound MFG-E8 associates with PDGFRβ and focal adhesion kinase after PDGF-BB treatment, results in cell surface retention of PDGFRβ, delays receptor degradation, potentiates downstream signaling, and enhances migration of 10T1/2 cells. MFG-E8 may promote angiogenesis, in part, via cell autonomous actions on pericytes or pericyte precursors that result in enhanced PDGF-BB:PDGFRβ signaling mediated via integrin-growth factor receptor cross-talk.
Keywords: MFG-E8, PDGF receptor β, PDGF-BB, integrin, pericyte
Platelet-derived growth factor (PDGF)-BB:PDGF receptor β (PDGFRβ) signaling is an important regulator of angiogenesis. PDGF-BB is released by endothelial cells (ECs), platelets, vascular smooth muscle cells (VSMCs), and inflammatory cells at sites of neoangiogenesis, and it enhances pericyte (PC) and VSMC migration and proliferation.1–6 PDGF-BB binding to the receptor tyrosine kinase PDGFRβ leads to receptor dimerization, autophosphorylation, and activation of downstream signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway. Ligand-bound receptor undergoes endocytosis and can either recycle to cell surfaces or undergo ubiquitination and proteosomal or lysosomal degradation.1–3,7–9 Pathway activity is influenced by the quantities of receptor that are expressed, and the location of the receptor with respect to ligand and additional proteins that participate in downstream signaling.
Several receptor tyrosine kinases, including vascular endothelial growth factor (VEGF) receptor 2, PDGFRβ, epidermal growth factor receptor, and basic fibroblast growth factor receptor, are known to associate with integrins, and these associations regulate kinase activity.10,11 Integrin avβ3 complexes with PDGFRβ in vascular ECs, fibroblasts and cancer cells that have been treated with PDGF-BB.12–14 Integrin αvβ3-associated PDGFRβ subsequently interacts with signaling molecules, including Src, SHP2, phospholipase Cγ, phosphatidylinositol 3-kinase, and focal adhesion kinase (FAK), triggering downstream signaling.12,15–17
The secreted glycoprotein milk fat globule-associated protein with epidermal growth factor and factor VIII-like domains (MFG-E8) (also termed lactadherin and SED1) is composed of 2 N-terminal epidermal growth factor-like domains and 2 C-terminal discoidin-like domains (C1 and C2) that are homologous to blood coagulation factors V and VIII.18–22 One endothelial growth factor–like domain (E2) contains an RGD consensus integrin-binding motif, and MFG-E8 binds to integrin αvβ3/5.20,21,23,24 Initial reports documented that the carboxy-terminal domains of MFG-E8 bound to negatively charged and oxidized phospholipids,25,26 facilitating opsonization of apoptotic cells for uptake by phagocytes.23,27 Interactions of MFG-E8 with αv integrins have also been implicated in regulation of angiogenesis and mammary gland branching,24,28 whereas interactions mediated via the C1 domain are important for sperm-egg binding and enhancement of collagen turnover.29,30
With respect to angiogenesis, it has been reported that MFG-E8 enhanced VEGF-induced angiogenesis in an αvβ3/β5 integrin–dependent manner using a mouse model of acute hindlimb ischemia.28 Our group subsequently demonstrated that MFG-E8 enhanced tumor-related angiogenesis and tumor growth in the Rip1-Tag2 mouse pancreatic tumor model, a model in which tumor progression is critically dependent on angiogenesis.31 We have additionally characterized the involvement of MFG-E8 in the regulation of angiogenesis in tumors, and in oxygen-induced retinopathy (OIR) in mice.32 We determined that PC/PC precursors are important sources of MFG-E8 in vivo, that MFG-E8 may enhance angiogenesis via actions on PC/PC precursors as well as ECs, and that MFG-E8 can be effectively targeted with therapeutic benefit.32 In melanomas and in retinas of mice with OIR, MFG-E8 colocalized with PC/PC precursors rather than ECs, and PDGFRβ+ PC/PC precursors purified from tumors contained large amounts of MFG-E8 mRNA. Tumor- and retinopathy-associated angiogenesis was diminished in MFG-E8 knockout mice, and PC coverage of neovessels was also reduced.32
To determine whether MFG-E8 could act directly on PDGFRβ+ PC/PC precursors, we studied C3H 10T1/2 cells in vitro as surrogates. 10T1/2 cells share surface markers with PCs but have multilineage differentiation potential and may be more closely related to PC precursors. Inhibition of MFG-E8 production by 10T1/2 cells using small interfering RNAs (siRNAs) and short hairpin RNAs, or inhibition of MFG-E8 action with some anti-MFG-E8 antibodies (Abs), attenuated 10T1/2 cell migration.32 Significantly, systemic injection of Ab to MFG-E8 that inhibited 10T1/2 cell migration in vitro also resulted in inhibition of pathological neoangiogenesis in the OIR model. These results suggested that MFG-E8 derived from PC/PC precursors enhanced PDGF-BB-induced PC/PC precursor function. However, the precise mechanisms of MFG-E8 action on PC/PC precursors and mechanisms by which PDGF-BB:PDGFRβ signaling might be regulated by MFG-E8 have not been determined.
Herein, we describe the results of experiments designed to determine whether MFG-E8 regulates PDGF-BB:PDGFRβ signaling. We found that MFG-E8 that was produced by 10T1/2 cells associated with integrin αv and PDGFRβ on cell surfaces after PDGF-BB treatment, altered the distribution of PDGFRβ within cells, increased the ability of PDGFRβ to associate with FAK, and inhibited degradation of PDGFRβ, thereby enhancing PDGFRβ signaling and cell function (migration).
Methods
Detailed descriptions of additional materials and methods used in this study are available in the supplemental materials, available online at http://atvb.ahajournals.org.
Cells and Culture Conditions
Mouse C3H-derived 10T1/2 cells (ATCC) were maintained in BME medium (Invitrogen) supplemented with 10% heat-inactivated FBS, 2 mmol/L L-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL) and were used before passage 10.
Abs
Abs and their sources were as follows: rat anti-mouse PDGFRβ monoclonal Ab (mAb) (eBioscience), rabbit anti-mouse PDGFRβ mAb (Millipore), rabbit anti-integrin αv polyclonal Ab (pAb) (intracellular C terminus) (Millipore), rat anti-integrin αv mAb (RMV-7) (Chemicon), anti-phosphotyrosine mAb (pTyr100) (Cell Signaling), anti-phospho-FAK (Tyr397) pAb (Abcam), anti-FAK pAb (Millipore), anti-phospho-Akt (Ser473) pAb (Cell Signaling), anti-Akt pAb (Cell Signaling), anti-phospho-p44/42 MAPK (extracellular signal-regulated kinase 1/2 [ERK1/2; Thr202/Tyr204]) pAb (Cell Signaling), anti-p44/42 MAPK (ERK1/2) pAb (Cell Signaling), mouse anti-ubiquitin mAb (P4D1) (Santa Cruz Biotechnology), rabbit anti-Cbl pAb (Santa Cruz Biotechnology), mouse anti-β actin mAb (Sigma), and rabbit anti-collagen types 1 and 4, anti-fibronectin, and anti-laminin pAb (Millipore). Rabbit anti-mouse MFG-E8 pAb and mouse anti-mouse MFG-E8 mAb (clones: 1H6, B10C7) were generated and characterized in our laboratory as described.31,32
MFG-E8 Knockdown Experiments and Quantification of MFG-E8 mRNA
siRNA selective for mouse MFG-E8 mRNA were designed using the Qiagen GeneGlobe Search Center (MFG-E8 siRNA) (5’-AAGCGGTGGAGACAAGGAGTT-3’), and MFG-E8 siRNA and AllStars negative control siRNA were purchased from Qiagen. To inhibit MFG-E8 production, 10T1/2 cells (5X 105 cells per 60-mm plate) were transfected with 10 nmol/L siRNA using HiPerFect Transfection Reagent (Qiagen). After 48 hours, MFG-E8 mRNA levels were assessed by quantitative reverse transcription–polymerase chain reaction (RT-PCR). Total RNA was isolated with RNeasy Kits (Qiagen) and reverse transcribed using the Super-ScriptIII First-Strand Synthesis System for RT-PCR (Invitrogen). Quantitative RT-PCR was performed using the following primers: MFG-E8 (forward, 5’-ATCTACTGCCTCTGCCCTGA-3’; reverse, 5’-ACACAGACGAGGCGGAAATC-3’) and glyceralde-hyde-3-phosphate dehydrogenase (GAPDH) (forward, 5’-ACCCAGAAGACTGTGGATGG-3’; reverse, 5’-CACATTG-GGGGTAGGAACAC-3’). PCR products were generated and then quantified using SYBR Green PCR Master Mix (Applied Biosystems) and a CFX96 Real-Time PCR Detection System (Bio-Rad) equipped with CFX Manager software, version 1.5.
Assessment of PDGF-Related Signaling Activities via Immunoprecipitation and Immunoblotting
Relationships between MFG-E8 levels, and PDGFRβ expression and PDGF-BB-related signaling were probed using MFG-E8 siRNA. In these experiments, 10T1/2 cells were transfected with siRNA oligonucleotides (MFG-E8 siRNA or control siRNA), shifted to low-serum (0.5% FCS) medium after 24 hours, and then stimulated with PDGF-BB (50 ng/mL) for 0 to 120 minutes after an additional 24 hours (48 hours after transfection). After washing with ice-cold PBS, cells were disrupted in lysis buffer (20 mmol/L Tris-HCl [pH 7.6], 140 mmol/L NaCl, 1% Nonidet P-40) containing a protease inhibitor cocktail (Roche) and phosphatase inhibitor (Roche) (complete lysis buffer) on ice. Cell lysates were centrifuged at 10 000g for 15 minutes at 4°C, and the resulting supernatants were subjected to immunoprecipitation using rat anti-PDGFRβ mAb or rat IgG as a control or to SDS-PAGE followed by immunoblot analysis with rabbit anti-PDGFRβ mAb, anti-phosphotyrosine mAb (pTyr100), and anti-ubiquitin mAb (P4D1) as indicated. Supernatants were also subjected to SDS-PAGE, followed by immunoblot analysis using anti-phospho-FAK (Tyr397) pAb, anti-FAK pAb, anti-phospho-Akt (Ser473) pAb, anti-Akt pAb, anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) pAb, anti-44/42 MAPK (Erk1/2) pAb, and anti-p actin mAb. Anti-mouse or anti-rabbit horseradish peroxidase–conjugated secondary Abs (Jackson Laboratory) were used in conjunction with ECL (Pierce) for immunoblotting. Densitometric analysis of exposed films was accomplished using ImageJ software.
To examine the effect of the MEK/ERK kinase inhibitor PD98059 on PDGF-BB-induced phosphorylation of ERK1/2 and FAK and degradation of PDGFRβ, 10T1/2 cells were preincubated with 50 μmol/L PD98059 (Sigma) or the same amount of dimethyl sulfoxide (0.2%, vehicle control) for 30 minutes and then stimulated with PDGF-BB (50 ng/mL) for 30 minutes. Cells were then disrupted in lysis buffer and subjected to SDS-PAGE, followed by immunoblot analysis as described above.
The ability of MFG-E8 to regulate PDGFRβ expression and cell signaling was also assessed using anti-MFG-E8 Abs. In these experiments, 10T1/2 cells were preincubated for 20 minutes with anti-MFG-E8 mAb (B10C7), a rabbit anti-MFG-E8 pAb reactive with the N terminus (including the RGD-containing domain), or corresponding control Abs (all at 20 μg/mL) and then stimulated with PDGF-BB (50 ng/mL) for 0 to 30 minutes. Cells were then disrupted in lysis buffer and subjected to SDS-PAGE, followed by immunoblot analysis as described above.
Signaling Complex Characterization via Coimmunoprecipitation
10T1/2 cells were incubated with 0.5% FCS medium overnight. On the day of the experiment, cells were pretreated with 100 ng/mL recombinant MFG-E8 for 15 minutes and then incubated with PDGF-BB (50 ng/mL) (PeproTech) for 0 to 30 minutes. After being washed with ice-cold PBS, cells were disrupted on ice in complete lysis buffer, lysates were cleared via centrifugation at 10 000g for 15 minutes at 4°C, and supernatants were incubated for 3 hours at 4°C with Ab-coated protein G–Sepharose beads (Amersham Biosciences). Rat anti-PDGFRβ mAb (eBioscience) or rabbit anti-av integrin pAb (C terminus, Intracellular, Millipore) were added as appropriate, and rat IgG or rabbit IgG were used as relevant controls. Beads were then washed 3 times with 1-mL volumes of wash buffer (50 mmol/L HEPES-NaOH [pH 7.6], 150 mmol/L NaCl, 0.1% Triton X-100), suspended in SDS sample buffer, boiled, and subjected to SDS-PAGE followed by immunoblot analysis using the indicated Abs and an ECL detection system (Amersham Bioscience).
Immunofluorescence Microscopy and Image Analysis
To assess colocalization/association of PDGFRβ and MFG-E8, and effects of MFG-E8 on PDGFRβ expression by 10T1/2 cells, cells were stained with anti-PDGFRβ and anti-MFG-E8 after treatment with control siRNA and MFG-E8 siRNA. To accomplish this, 10T1/2 cells were transfected with control siRNA or MFG-E8 siRNA oligonucleotides, seeded in 8-well culture slides (BD Falcon) 24 hours after transfection and incubated with 0.5% FCS medium for an additional 24 hours. Forty-eight hours after siRNA treatment, cells were treated with PDGF-BB (50 ng/mL) for 0 to 120 minutes, and then fixed in 4% paraformaldehyde (PFA) in PBS at room temperature for 30 minutes. After being blocked with 3% dry milk-PBS (Bio-Rad) supplemented with 5% normal goat serum for 1 hour at room temperature, cells were stained with rabbit anti-MFG-E8 pAb and rat anti-PDGFRβ Ab followed by Alexa 488 - or Alexa 568–conjugated secondary Ab and Alexa 647–phalloidin (Invitrogen). Cells were mounted in ProLong Gold antifade reagent (Invitrogen), and immunofluorescence images were collected and visualized with a LSM510 confocal laser-scanning microscope (Zeiss). Quantification of PDGFRβ, MFG-E8, and phosphotyrosine expression levels per cell and extents of colocalization were accomplished with Zeiss AIM (v4.2) software. Each data point represents analysis of 10 randomly chosen cells. Cell borders were determined by visualizing stress fibers with phalloidin as necessary.
Visualization of protein-associated intracellular phosphotyrosine in 10T1/2 cells was achieved via treatment with 0.1% Triton X-100 at room temperature for 30 minutes after fixation with 4% PFA, followed by staining with anti-phosphotyrosine mAb (PY100) and anti-PDGFRβ mAb. To compare images of permeabilized and nonpermeabilized cells, cells were fixed in 4% PFA with or without subsequent 0.1% Triton X-100 treatment before staining with Abs (see Supplemental Figure II). These results strongly support the conclusion that immunofluorescence staining of fixed cells that have not been permeabilized with detergent results in preferential detection of proteins that are extracellular or are located on external cell surfaces.
MFG-E8-Related Proteins and Assessment of Cell Binding
MFG-E8-Ig is a fusion protein that is comprised of the N-terminal 148 amino acids of MFG-E8, including the endothelial growth factor-like domains (E1 and E2) and the linker region (L) fused in frame with the hinge-containing Fc region of human IgG1 (Figure 1B). RGE-MFG-E8-Ig is the corresponding point mutant of MFG-E8-Ig in which aspartic acid (D) in the RGD motif was replaced by glutamic acid (E). cDNA fragments encoding MFG-E8-Ig, and RGE-MFG-E8-Ig were subcloned into pCl-neo vector (Promega), and 293F cells (Invitrogen) were transfected with these plasmids. Fusion proteins produced by 293F cells grown in serum-free medium were purified from culture supernatants via affinity chromatography using protein A–Sepharose 4 Fast Flow (GE Healthcare Biosciences) with acid elution followed by rapid neutralization. All fusion proteins migrated as homogenous dimers in size exclusion columns under nondenaturing conditions, in contrast to recombinant MFG-E8, which formed aggregates of various sizes (data not shown).
Figure 1.
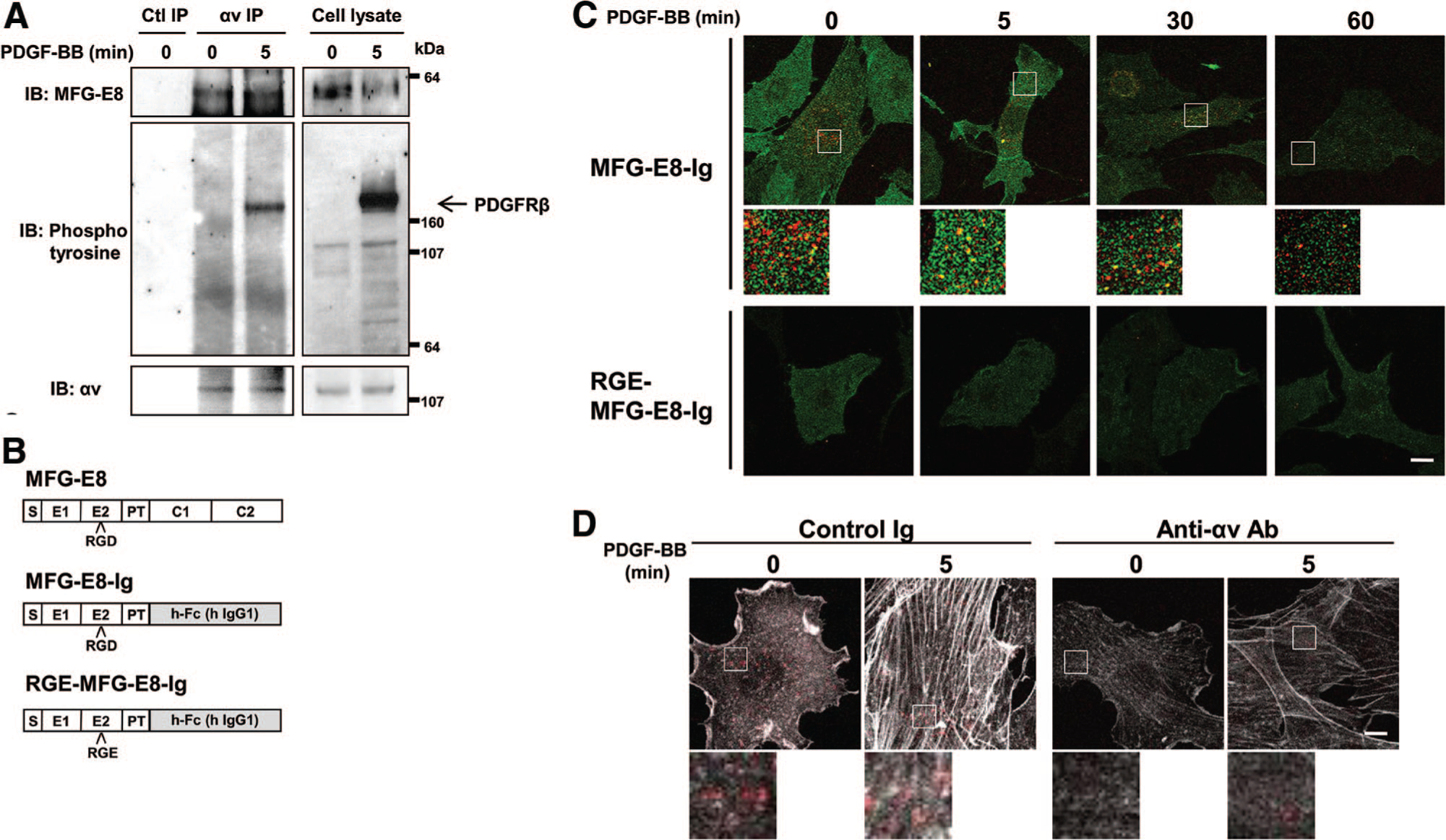
Interactions of milk fat globule-associated protein with epidermal growth factor and factor Vlll-like domains (MFG-E8) and platelet-derived growth factor receptor (PDGFR) β are mediated by αv integrins. A, Association of MFG-E8 with complexes including αv integrin and PDGFRβ. Serum-starved 10T1/2 cells were stimulated with platelet-derived growth factor (PDGF)-BB and subjected to immunoprecipitation with rabbit anti-αv integrin antibody (Ab). SDS-PAGE-resolved proteins were subsequently immunoblotted with mouse anti-MFG-E8 monoclonal Ab (mAb), anti-phosphotyrosine Ab (pTyr100), and anti-αv integrin Ab. Data are representative of n=4 experiments. B, Schematic illustration of the structures of MFG-E8, MFG-E8-Ig, and RGE-MFG-E8-Ig (S indicates signal sequence; E1 and E2, endothelial growth factor-like domain; PT, proline/threonine-rich linker domain; C1 and C2, blood coagulation factors V and VIII homologous [discoidin] domains.) C, Fluorescence photomicrographs depicting MFG-E8-Ig or RGE-MFG-E8-Ig (red) and PDGFRβ (green) associated with PDGF-BB-treated 10T1/2 cells (high-magnification images of the areas indicated by the squares in the top images; scale bar=20 μm). D, Digital fluorescence images depicting MFG-E8-Ig (red) and actin (white) in control Ig or anti-αv Ab-pretreated 10T1/2 cells (high-magnification images of the areas indicated by the squares in the top images; scale bar=20 μm).
To test fusion protein binding, 10T1/2 cells were seeded into 8-well culture slides (BD Falcon) and incubated with 0.5% FCS medium overnight. Cells were subsequently treated with MFG-E8-Ig or RGE-MFG-E8-Ig (20 μg/mL) for 20 minutes and then incubated with PDGF-BB (50 ng/mL) for 0 to 60 minutes, followed by fixation with 4% PFA in PBS. After being blocked with 3% dry milk–PBS (Bio-Rad) supplemented with 5% normal goat serum for 1 hour at room temperature, cells were stained with anti-PDGFRβ Ab followed by Alexa 488–conjugated anti-rat Ig, Alexa 568–conjugated anti-human Ig and Alexa 647–phalloidin (Invitrogen), washed and mounted in ProLong Gold before visualization.
To assess effects of anti-integrin αv blocking Ab on binding of MFG-E8-Ig to 10T1/2 cells, 10T1/2 cells were incubated with rat anti-integrin av blocking mAb (RMV-7) (Chemicon) or control rat Ig (20 μg/mL) for 20 minutes and then treated with MFG-E8-Ig (20 μg/mL) for 20 minutes. After MFG-E8-Ig treatment, cells were incubated with PDGF-BB (50 ng/mL) for 0 or 5 minutes. Cells were subsequently washed, fixed in 4% PFA in PBS, and stained with Alexa 568–conjugated anti-human Ig and Alexa 647–phalloidin (Invitrogen), and immunofluorescence images were collected and analyzed.
Statistics
Probability values were calculated using the Student t test (2-sided) or by analysis of 1-way ANOVA followed by the Bonferroni post test as appropriate. Error bars represent standard errors of the mean, and numbers of experiments (n) are as indicated.
Results
MFG-E8 Enhances PDGF-BB-Induced Phosphorylation of ERK1/2 and FAK
We previously determined that 10T1/2 cells express PC markers, including PDGFRβ; that they produce MFG-E8; and that inhibition of MFG-E8 production using siRNA inhibited PDGF-BB-dependent 10T1/2 cell migration but not proliferation.32 Because PDGF-BB-induced ERK1/2 and FAK activation downstream of PDGFRβ phosphorylation has been reported to be important for VSMC migration, we hypothesized that MFG-E8 might modulate this pathway.1,15,16,33,34 We examined the effect of MFG-E8 on PDGFRβ-stimulated signaling in 10T1/2 cells by assessing phosphorylation of MAPK, FAK, and Akt in MFG-E8 siRNA and control siRNA-transfected cells. MFG-E8 mRNA expression in MFG-E8 siRNA-transfected cells was inhibited by ≈75% relative to that in control siRNA-transfected cells (Figure 2A). We confirmed that the protein level of MFG-E8 was similarly inhibited by MFG-E8 siRNA using immunoblot and ELISA assays (Figure 2A).
Figure 2.
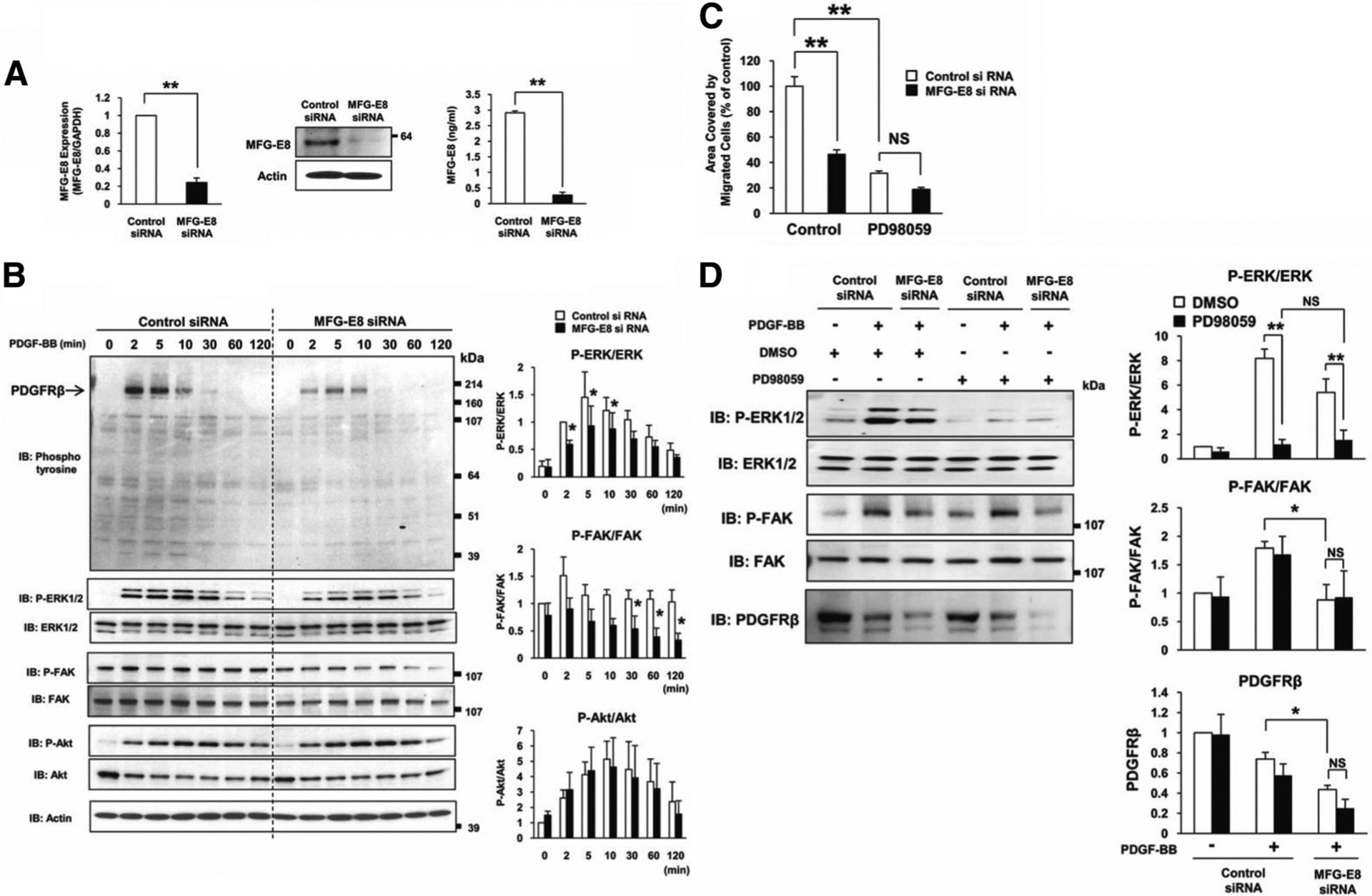
Milk fat globule-associated protein with epidermal growth factor and factor VIII-like domains (MFG-E8) enhances platelet-derived growth factor (PDGF)-BB-induced phosphorylation of extracellular signal-regulated kinase (ERK) and focal adhesion kinase (FAK). A, Small interfering rNa (siRNA)-induced inhibition of MFG-E8 mRNA expression by 10T1/2 cells assessed by quantitative reverse transcription-polymerase chain reaction 48 hours after transfection and immediately before immunoblot (**P<0.01 relative to siRNA control; values determined in 7 independent experiments). siRNA-induced inhibition of MFG-E8 protein by 10T1/2 cells assessed by immunoblot (cell lysate) and ELISA (24 hours of supernatant) 48 hours after transfection (**P<0.01 relative to siRNA control; values determined in 3 independent experiments). B, Control siRNA or MFG-E8 siRNA-transfected 10T1/2 cells were treated with PDGF-BB for the indicated times and then subjected to SDS-PAGE, followed by immunoblotting with anti-phosphotyrosine antibody (Ab), anti-phospho-ERK1/2 and ERK1/2 Ab, anti-phospho-FAK (Tyr397) and fAk Ab, anti-phospho-Akt and Akt Ab, and anti-actin Ab. Quantification of relative phosphorylation levels of ERKl/2, FAK, and Akt was accomplished via densitometry using ImageJ software. The level of ERK1/2 phosphorylation in control siRNA-transfected cells 2 minutes after PDGF-BB stimulation was assigned a value of 1. The levels of FAK and Akt phosphorylation in control siRNA-transfected cells at 0 minutes after PDGF-BB stimulation was assigned a value of 1. (*P<0.05 relative to siRNA control; Values determined in 3 independent experiments). C, Effects of MEK/ERK kinase inhibitor PD98059 on PDGF-BB induced-10T1/2 cell migration. Control siRNA- or MFG-E8 siRNA-transfected 10T1/2 cells were pretreated with PD98059 (50 μmol/L, 30 minutes), and cells adherent to the bottom surfaces of FluoroBlok filters 4 hours after initiation of PDGF-BB-induced migration were quantified using ImageJ (**P<0.01; values determined in 3 independent experiments; NS indicates not significant). D, Effects of PD98059 on PDGF-BB induced-phosphorylation of ERK1/2 and FAK, and degradation of PDGF receptor (PDGFR) β. Control siRNA- or MFG-E8 siRNA-transfected 10T1/2 cells were pretreated with PD98059 (50 μmol/L, 30 minutes), stimulated with PDGF-BB for 30 minutes, and then subjected to SDS-PAGE, followed by immunoblotting with anti-phospho-ERK1/2 and ERK1/2 Ab, anti-phospho-FAK (Tyr397) and FAK Ab, and anti-PDGFRβ Ab. Quantification of relative phosphorylation levels of ERK1/2 and FAK and the expression of PDGFRβ was accomplished via densitometry using ImageJ software. The amount of protein in control siRNA-transfected cells cultured in medium with dimethyl sulfoxide (DMSO) (0.2%) and without PDGF-BB was assigned a value of 1 (*P<0.05 relative to siRNA control; values determined in 3 independent experiments).
In immunoblots of whole cell lysates, MFG-E8 depletion using siRNA significantly reduced phosphorylation of ERK1/2 as early as 2 minutes after PDGF-BB stimulation (Figure 2B). Phosphorylated FAK was also significantly decreased by depletion of MFG-E8 from 10T1/2 cells, but this became evident at later time points (Figure 2B). In contrast, PDGF-dependent Akt phosphorylation was not influenced by modulation of MFG-E8 production, suggesting that MFG-E8 selectively enhances PDGF-BB-induced phosphorylation of ERK1/2 and FAK, consistent with our previous functional data regarding selective effects of MFG-E8 on 10T1/2 cell migration (Figure 2C).32 We also examined the effect of the MEK/ERK kinase inhibitor PD9805934 on the enhancement of PDGF-BB-induced phosphorylation of ERK1/2 and FAK and migration by MFG-E8. As previously demonstrated,32 inhibition of MFG-E8 production using siRNA inhibited PDGF-BB-dependent 10T1/2 cell migration (Figure 2C). The MEK/ERK kinase inhibitor PD98059 effectively inhibited PDGF-BB-induced control and MFG-E8 siRNA-transfected cell migration. As expected, inhibition of PDGF-BB-induced phosphorylation of ERK1/2 in control and MFG-E8 siRNA-transfected cells by PD98059 was almost complete (Figure 2D). In contrast, the MEK/ERK kinase inhibitor had no effect on PDGF-BB-induced phosphorylation of FAK in control and MFG-E8 siRNA-transfected cells. These results suggest that potentiation of MFG-E8-induced activation of ERK1/2 contributes to the enhancement of PDGF-BB-induced migration and that ERK1/2 activation is downstream of MFG-E8-enhanced FAK phosphorylation.
MFG-E8 Enhances PDGF-BB-Induced Phosphorylation of PDGFRβ and Inhibits PDGF-BB-Dependent PDGFRβ Degradation
PDGF-BB-induced PDGFRβ autophosphorylation is required for activation of downstream signaling pathways. In initial anti-phosphotyrosine immunoblots of whole cell lysates, PDGF-BB-induced phosphorylation of a 190-kDa protein, consistent with PDGFRβ based on molecular size (Figure 2B, top panel). Phosphorylation of this protein was significantly attenuated in MFG-E8-depleted cells. To confirm that the 190-kDa protein represented PDGFRβ, we performed immunoprecipitation/immunoblotting experiments with anti-PDGFRβ and anti-phosphotyrosine Ab. We confirmed that phosphorylation of PDGFRβ was increased after PDGF-BB stimulation, peaking at ≈5 minutes after stimulation and gradually decreasing to basal levels by 60 minutes (Figure 3A, top panel). In MFG-E8-depleted 10T1/2 cells, PDGF-BB-dependent PDGFRβ phosphorylation was decreased relative to controls. Differences between control and MFG-E8 siRNA-treated cells were evident from 5 to 30 minutes after PDGF-BB stimulation and continued up to 120 minutes after stimulation (Figure 3A, top panel). FAK is required for both integrin- and growth factor-stimulated cell migration.15,16,33 It has been reported that FAK associates with activated PDGFRβ through its N-terminal domain and with integrins through its C-terminal domain, thereby promoting PDGF-BB-stimulated cell migration.15,16 We also detected PDGFRβ-associated FAK in PDGFRβ immunoprecipitates by immunoblotting with anti-FAK Ab (Figure 3A, bottom panel). Interestingly, PDGF-BB-augmented association of FAK with PDGFRβ was strikingly dependent on MFG-E8 (Figure 3A, bottom panel).
Figure 3.
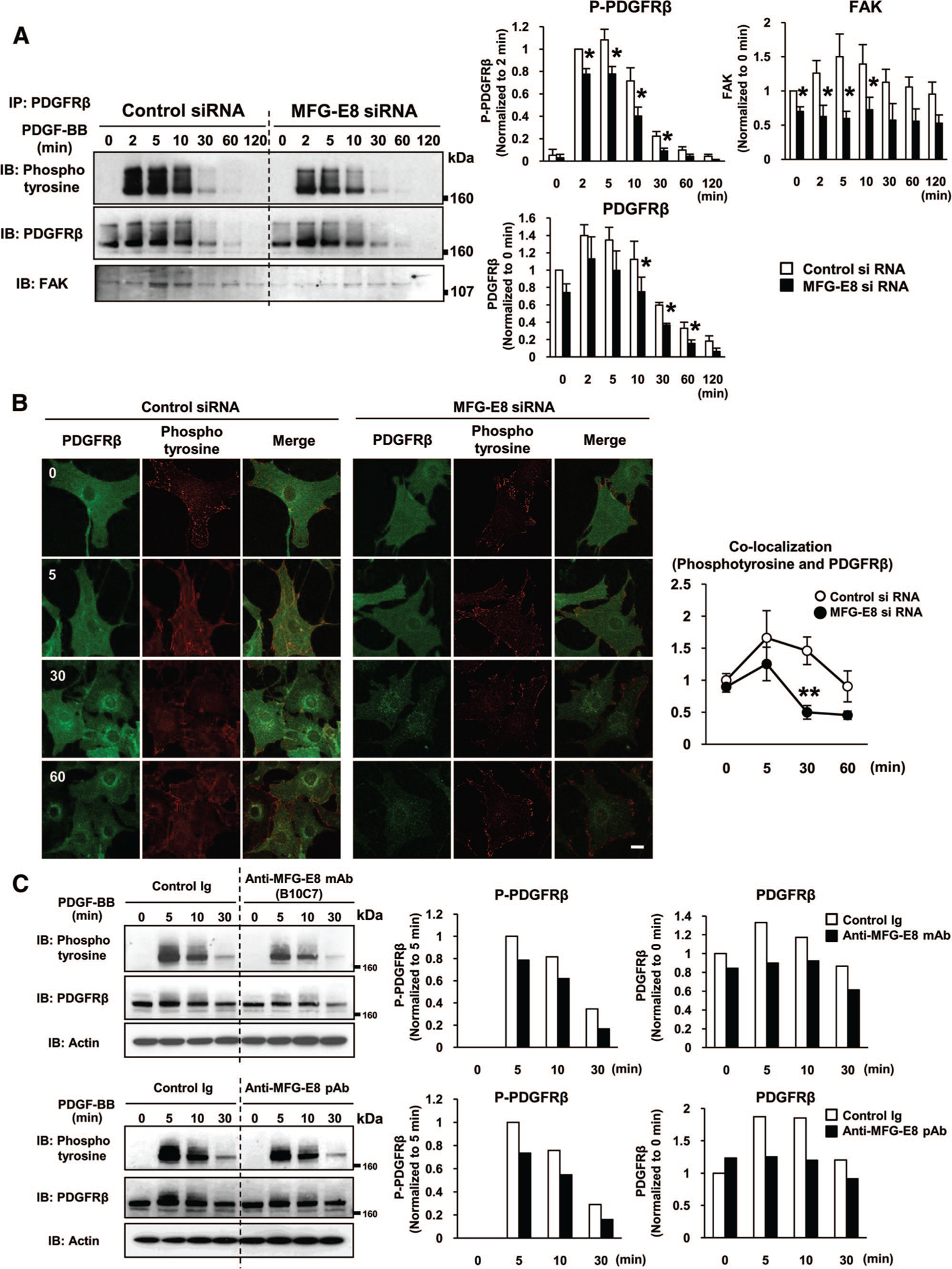
Milk fat globule-associated protein with epidermal growth factor and factor VIII-like domains (MFG-E8) enhances platelet-derived growth factor (PDGF)-BB-induced phosphorylation of PDGF receptor (PDGFR) β and reduces PDGF-BB-dependent PDGFRβ degradation. A, Control small interfering rNa (siRNA)- or MFG-E8 siRNA-transfected 10T1/2 cells were stimulated with PDGF-BB for the indicated times, lysed, and then immunoprecipitated (IP) with rat anti-PDGFRβ antibody (Ab) followed by immunoblotting (IB) with anti-phosphotyrosine (pTyr100), anti-PDGFRβ, and anti-focal adhesion kinase (FAK) Ab. Phosphorylated PDGFRβ, total PDGFRβ, and PDGFRβ-associated FAK expression was quantified as described. The amount of phosphorylated PDGFRβ in control siRNA-transfected cells at 2 minutes after PDGF-BB stimulation was assigned a value of 1. The amount of PDGFRβ and FAK in control siRNA-transfected cells at 0 minutes after PDGF-BB stimulation was assigned a value of 1. (*P<0.05 relative to siRNA control; values determined in 3 independent experiments). B, Digital fluorescence images depicting phosphorylated-tyrosine (red) and PDGFRβ (green) in control siRNA- or MFG-E8 siRNA-transfected 10T1/2 cells after incubation with PDGF-BB for the indicated times (scale bar=20 μm). Colocalization of phosphotyrosine and PDGFRβ was assessed using a Zeiss LSM510 confocal microscope and Zeiss AIM software. C, 10T1/2 cells were incubated with anti-MFG-E8 monoclonal Ab (mAb) (B10C7), rabbit anti-MFG-E8 polyclonal Ab (pAb), or corresponding control Abs (20 μg/mL), and then stimulated with PDGF-BB for the indicated times. Cells were disrupted in lysis buffer, and then subjected to SDS-PAGE, followed by immunoblotting. The amount of phosphorylated PDGFRβ in control Ig treated cells at 5 minutes after PDGF-BB stimulation was assigned a value of 1. The amount of PDGFRβ in control Ig treated cells at 0 minutes after PDGF-BB stimulation was assigned a value of 1.
The amount of total PDGFRβ that was immunoprecipitated from control 10T1/2 cells increased 2 to 10 minutes after PDGF-BB treatment, and decreased by 30 minutes after stimulation (Figure 3A, middle panel). Total PDGFRβ expression in MFG-E8 siRNA-transfected cells was significantly decreased relative to that in control cells from 10 to 60 minutes after PDGF-BB stimulation, suggesting that MFG-E8 might inhibit PDGF-BB-dependent PDGFRβ degradation. Addition of recombinant MFG-E8 to MFG-E8 siRNA-transfected cells reversed the decreases in FAK-PDGFRβ association, and PDGFRβ phosphorylation and levels of PDGFRβ were increased to some extent (Supplemental Figure I). To additionally address the issues of PDGFRβ expression and distribution, we examined effects of MFG-E8 on PDGFRβ and phosphotyrosine levels and assessed colocalization of phosphotyrosine and PDGFRβ in situ via confocal laser immunofluorescence microscopy (Figure 3B). Consistent with the immunoblotting results, PDGFRβ levels were somewhat decreased, and phosphotyrosine levels were dramatically decreased, in MFG-E8 siRNA-transfected cells compared with controls. Colocalization of phosphotyrosine and PDGFRβ (presumably phos-phorylated-PDGFRβ) was also decreased in MFG-E8 siRNA-transfected cells, consistent with our association studies. In aggregate, these results suggest that MFG-E8 inhibits PDGF-BB-dependent PDGFRβ degradation and enhances both phosphorylation of PDGFRβ and association of FAK with activated PDGFRβ.
We previously determined that PDGF-BB-induced 10T1/2 cell migration was inhibited by rabbit anti-MFG-E8 pAb, which recognized the N terminus of MFG-E8, as well as mouse anti-MFG-E8 mAb B10C7, which recognized the RGD motif in the E2 domain, and that injection of these Abs also inhibited angiogenesis in OIR in mice.32 Consistent with results that we obtained with MFG-E8 siRNA, both anti-MFG-E8 neutralizing Abs inhibited PDGF-BB-induced phosphorylation of PDGFRβ and led to decreased receptor levels in PDGF-BB-stimulated cells (Figure 3C). These results are consistent with the concept that at least some MFG-E8 that is secreted by 10T1/2 cells acts extracellularly to potentiate PDGFRβ phosphorylation, to attenuate decreases in PDGFRβ levels that occur in PDGF-BB stimulated cells, and thereby to enhance PDGF-BB-mediated signaling in 10T1/2 cells.
MFG-E8 Associates With PDGFRβ in PDGF-BB-Stimulated Cells
MFG-E8 has been reported to bind to integrin αvβ3/5 via its RGD motif.20,21,23,24 It has also been reported that integrin αvβ3 associates with phosphorylated PDGFRβ in NIH3T3 cells treated with PDGF-BB, and this association enhances downstream signaling.12,13 We therefore hypothesized that PDGF-BB induces association of MFG-E8, integrin αv, and PDGFRβ, providing a possible link between MFG-E8 formation and PDGFRβ-mediated signaling. To test this, we characterized PDGF-BB-induced physical interactions between MFG-E8 and PDGFRβ via coimmunoprecipitation. Serum-starved 10T1/2 cells were stimulated with PDGF-BB, lysed, and immunoprecipitated with anti-PDGFRβ mAb. MFG-E8 was detected in PDGFRβ immunoprecipitates; the association of PDGFRβ and MFG-E8 was increased 5 minutes after PDGF-BB stimulation and decreased by 30 minutes after stimulation (Figure 4A, top panels).
Figure 4.
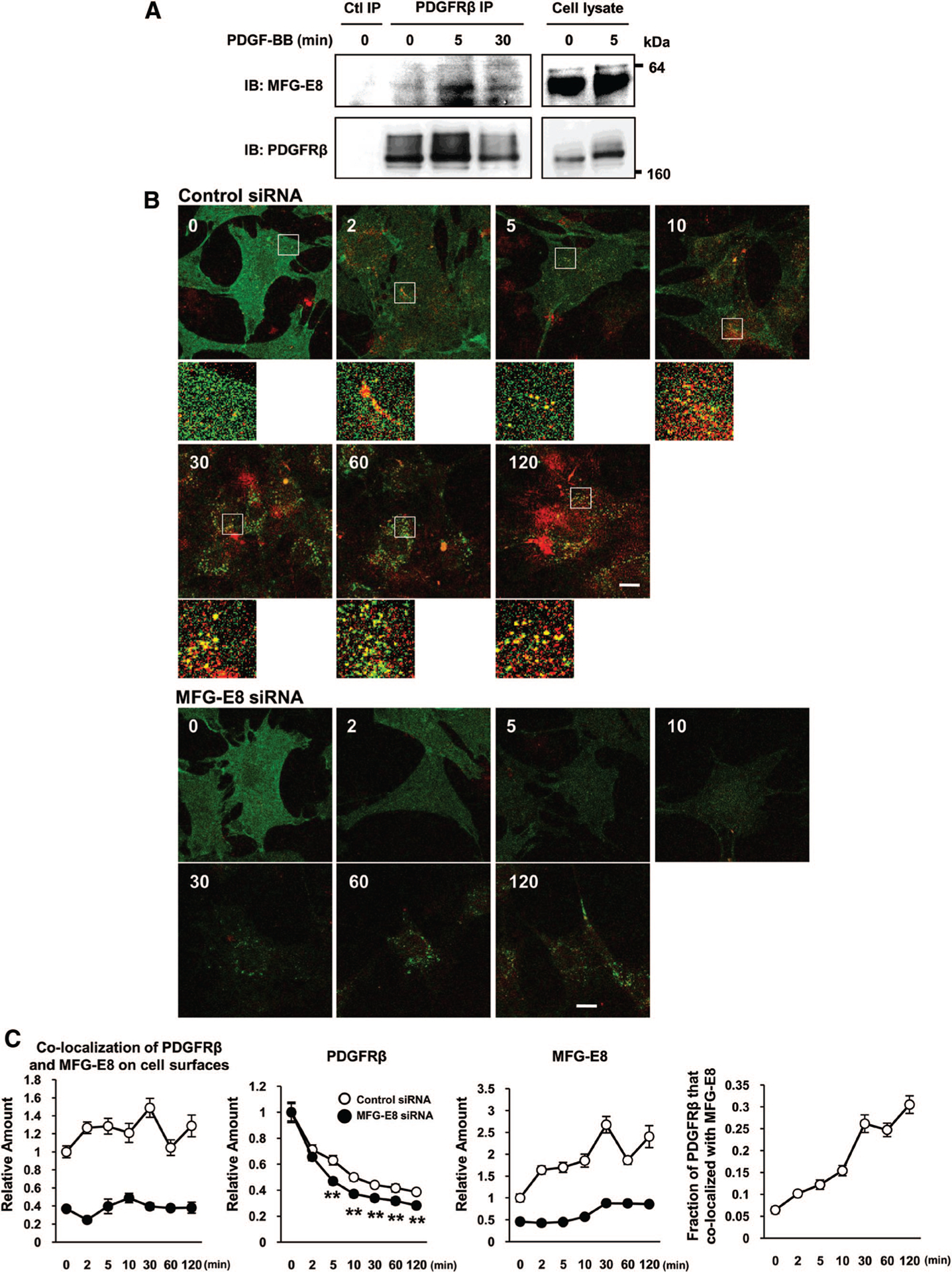
Platelet-derived growth factor (PDGF)-BB induces association of milk fat globule-associated protein with epidermal growth factor and factor VIII-like domains (MFG-E8) with PDGF receptor (PDGFR) β on cell surfaces. A, PDGF-BB enhances association of MFG-E8 and PDGFRβ. Serum-starved 10T1/2 cells were stimulated with PDGF-BB and subjected to immunoprecipitation (IP) with rat anti-PDGFRβ or control antibody (Ab), and SDS-PAGE-resolved proteins were immunoblotted (IB) with rabbit anti-MFG-E8 polyclonal Ab (pAb) and rabbit anti-PDGFRβ monoclonal Ab (mAb). Data are representative of n=6 experiments. B, Digital fluorescence images depicting colocalization of MFG-E8 (red) and PDGFRβ (green) in PDGF-BB-treated control and MFG-E8-depleted 10T1/2 cells (high-magnification images of the areas indicated by squares in the top images; scale bar=20 μm). C, Quantification of colocalization of PDGFRβ and MFG-E8 on cell surfaces and surface expression of PDGFRβ or MFG-E8 in control and MFG-E8-depleted 10T1/2 cells, and fractions of PDGFRp that colocalized with MFG-E8 (O, control small interfering RNA [siRNA]; ●, MFG-E8 siRNA treated cell) (**P<0.01; data are means±SE of values from 10 randomly chosen cells and representative of n=2 experiments). Levels of surface expression are expressed relative to those present on control siRNA-treated cells at time=0.
To examine relationships between MFG-E8 and PDGFRβ in situ, we assessed colocalization of these proteins on the surfaces of 10T1/2 cells. To test the feasibility of our approach, we performed PDGFRβ and phosphotyrosine immunofluorescence staining in both permeabilized (0.1% Triton X-treated) and nonpermeabilized (Triton X-nontreated) PFA-fixed 10T1/2 cells (see Supplemental Figure II). Thirty minutes after PDGF-BB stimulation, PDGFRβ staining of nonpermeabilized cells was significantly reduced compared with that of permeabilized cells and also compared with that of nonpermeabilized unstimulated cells. In contrast, phosphotyrosine staining in permeabilized cells was significantly increased after PDGF-BB stimulation and was minimal in nonpermeabilized cells in the presence or absence of PDGF-BB treatment. We interpret these findings to indicate that in the absence of detergent treatment, immunofluorescence staining of PFA-fixed cells detects primarily external surface-associated proteins.
Having established that our experimental approach could be informative, we examined PDGF-BB-induced association of MFG-E8 with PDGFRβ on the surfaces of PDGF-BB-treated nonpermeabilized 10T1/2 cells via immunofluorescence confocal laser microscopy (Figure 4B), and quantified the colocalization of MFG-E8 and PDGFRβ, as well as levels of expression of MFG-E8 and PDGFRβ on the surfaces of 10T1/2 cells (Figure 4C). Before treatment with PDGF-BB, PDGFRβ was diffusely distributed on 10T1/2 cells, and some MFG-E8 was also present on cell surfaces (Figure 4B and 4C; MFG-E8). After treatment with PDGF-BB, PDGFRp staining became punctate and then decreased dramatically (≈30% by 2 minutes after stimulation and diminishing to ≈40% of initial levels at the end of the 120-minute observation period; Figure 4B and 4C; PDGFRβ). In contrast, surface-associated MFG-E8 increased ≈50% by 2 minutes after PDGF-BB stimulation and continued to increase, reaching levels 2.5 times higher than those on unstimulated cells by 30 minutes after PDGF-BB addition (Figure 4C). At least some of this increase may be attributable to increased MFG-E8 production, because MFG-E8 mRNA levels in 10T1/2 cells were increased within 5 minutes after PDGF-BB was added (Supplemental Figure III).
Interestingly, colocalization of PDGFRβ and MFG-E8 increased after PDGF-BB treatment, and this colocalization persisted for 120 minutes after stimulation (Figure 4B and 4C; colocalization of PDGFRβ and MFG-E8 on cell surfaces). The fraction of PDGFRβ that colocalized with MFG-E8 increased after PDGF-BB stimulation; ≈5% of PDGFRβ colocalized with MFG-E8 before stimulation, increasing to ≈30% colocalization 120 minutes after stimulation (Figure 4C; fraction of PDGFRβ that colocalized with MFG-E8). To determine whether MFG-E8 might modulate PDGFRβ surface levels or distribution, we studied siRNA-treated, MFG-E8-depleted and control siRNA-transfected 10T1/2 cells (Figure 4B and 4C). Surface PDGFRβ expression by control siRNA-treated cells decreased in a time-dependent manner at a slower rate than was observed with MFG-E8 siRNA treated cells (Figure 4C; PDGFRβ). Differences were evident beginning 5 minutes after PDGF-BB treatment and persisted for up to 120 minutes. These results suggest that 10T1/2 cell-derived MFG-E8 potentiates receptor clustering and that physical association of MFG-E8 with PDGFRβ may lead to retention of PDGFRβ at cell surfaces with concordantly augmented signaling.
Interactions of MFG-E8 and PDGFRβ Are Mediated by αv Integrins
We predicted that association of MFG-E8 with PDGFRβ was dependent on integrin αv and the RGD motif present in the second endothelial growth factor-like domain of MFG-E8. To test this, we immunoprecipitated integrin αv from untreated and PDGF-BB-treated 10T1/2 cells and assayed for copurifying MFG-E8 and phosphoproteins of interest by immunoblotting with anti-MFG-E8 and anti-phosphotyrosine, respectively. Association of integrin αv and MFG-E8 was observed before stimulation and was slightly increased at 5 minutes after stimulation (Figure 1A, top panels). Anti-phosphotyrosine immunoblots revealed a 190-kDa tyrosine-phosphorylated protein, consistent with phosphorylated PDGFRβ, that associated with αv integrin after PDGF-BB stimulation (Figure 1A, middle panels, lane 3). These results indicate that MFG-E8 binds integrin αv before PDGF-BB stimulation and that PDGFRβ is recruited into relevant complexes only after PDGF-BB-stimulated receptor autophosphorylation.
To confirm that MFG-E8 binds to cell-associated integrin αv via the RGD consensus integrin-binding motif in the E2 domain of MFG-E8, we compared the binding of several MFG-E8 fusion proteins to the surfaces of 10T1/2 cells. MFG-E8-Ig is a fusion protein composed of the N terminus of MFG-E8 (without the C1 and C2 domains) fused in frame to the Fc portion of human IgG1, and RGE-MFG-E8-Ig represents the corresponding RGE point mutant (Figure 1B). These proteins, rather than recombinant MFG-E8, were used because they can easily be distinguished from residual MFG-E8 that is produced by 10T1/2 cells. In addition, these fusion proteins exist in solution as homogenous dimers as compared with the multiple aggregated species that are present in preparations of recombinant full length MFG-E8, making experimental results potentially easier to interpret. Immunofluorescence staining showed that MFG-E8-Ig bound to the surfaces of 10T1/2 cells with or without PDGF-BB treatment (Figure 1C: PDGFRβ in green, MFG-E8-related proteins in red). After stimulation with PDGF-BB, MFG-E8-Ig colocalized in punctate clusters with PDGFRβ. In contrast, we could not detect RGE-MFG-E8-Ig binding to 10T1/2 cells (Figure 1C). Binding of MFG-E8-Ig to 10T1/2 cells was also readily inhibited by anti-integrin αv blocking Ab (Figure 1D). These results indicate that MFG-E8 binds to αv integrins and that integrin-MFG-E8 binding is RGD dependent, and they suggest that PDGF-BB-induced interactions of MFG-E8 with PDGFRβ are dependent on αv-containing integrins.
MFG-E8 Inhibits PDGF-BB-Dependent Ubiquitination of PDGFRβ
Levels of PDGFRβ and activity of the PDGF-BB:PDGFRβ signaling axis can be regulated by receptor ubiquitination, and polyubiquitination triggers receptor degradation.7–9 To determine whether MFG-E8 depletion of 10T1/2 cells led to increased PDGFRβ ubiquitination, we assayed immunoprecipitated PDGFRβ for associated ubiquitin and polyubiquitin via immunoblotting with anti-ubiquitin Ab. We found that PDGF-BB-induced ubiquitination of PDGFRβ was significantly higher in MFG-E8 siRNA-transfected cells than in control siRNA-transfected cells (Figure 5A, top panel). Quantification of total amounts of PDGFRp and ubiquitinated PDGFRβ 10 minutes after PDGF-BB stimulation revealed that PDGFRβ levels in MFG-E8-depleted cells were ≈40% lower than in control cells (as expected), whereas the proportion of PDGFRβ that was ubiquitinated in MFG-E8-depleted cells was increased by ≈60% compared with that in control cells (Figure 5B). c-Cbl is a ubiquitin E3 ligase that has been implicated as a negative regulator of PDGFRβ, and PDGFRp is a substrate for c-Cbl.35–38 We determined that the association of c-Cbl with PDGFRβ was increased in MFG-E8 siRNA-transfected cells (Figure 5A, middle panel). In addition, we demonstrated that the proteasome inhibitor MG132 inhibited PDGF-BB-induced PDGFRβ degradation in control and MFG-E8 siRNA-transfected cells (Supplemental Figure IV). These results suggest that MFG-E8 reduces the association of c-Cbl with PDGFRβ, PDGFRβ ubiquitination, and receptor degradation via the proteasomal pathway, thereby enhancing cell surface PDGFRβ levels and PDGFRβ-mediated signaling. The MEK/ERK kinase inhibitor PD98059 had no effect on PDGFRp degradation (Figure 2D, bottom panel), indicating that ERK activity did not influence regulation of PDGFRβ degradation by MFG-E8.
Figure 5.
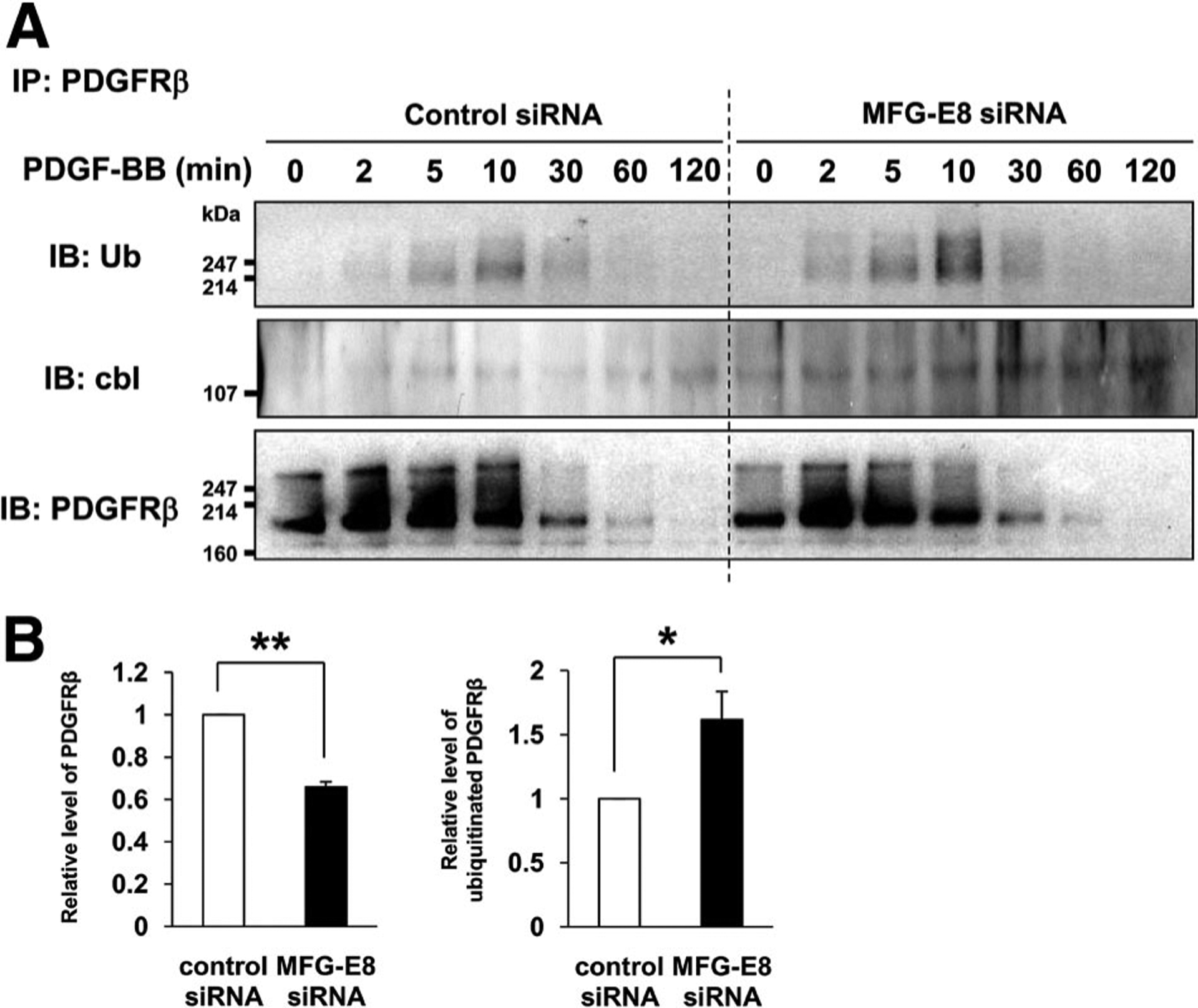
Milk fat globule-associated protein with epidermal growth factor and factor VIII-like domains (MFG-E8) prevents platelet-derived growth factor (PDGF)-BB-dependent ubiquitination of PDGF receptor (PDGFR) β. A, Control small interfering RNA (siRNA)- or MFG-E8 siRNA-transfected 10T1/2 cells were stimulated with PDGF-BB, lysed, and immunoprecipitated (IP) with rat anti-PDGFRβ antibody (Ab), and SDS PAGE-resolved proteins were immunoblotted (IB) with anti-ubiquitin Ab (top panel), anti-cbl Ab (middle panel), and anti-PDGFRβ Ab (bottom panel). B, Total PDGFRβ and ubiquitinated PDGFRβ were quantified 10 minutes after PDGF-BB stimulation as described (**P<0.01, *P<0.05 relative to siRNA control; values determined in 3 independent experiments).
Discussion
MFG-E8 enhances VEGF-induced angiogenesis in a mouse model of acute hindlimb ischemia,28 tumor-associated angiogenesis in pancreatic β-cell tumors in Rip1-Tag2 mice31 and melanomas,32 and angiogenesis in a mouse OIR model as well.32 In a previous study, we demonstrated that PC/PC precursors as well as ECs are relevant sources of angiogenesis-promoting MFG-E8 and determined that PDGF-BB-induced migration of PC/PC precursor 10T1/2 cells was inhibited by MFG-E8 depletion using siRNA and by anti-MFG-E8 neutralizing Abs (Figure 2C).32 In the present study, we sought to characterize the mechanism(s) by which MFG-E8 regulates PDGF-BB-induced alterations in cellular physiology, focusing on alterations downstream of PDGFRβ activation.
Depletion of MFG-E8 from 10T1/2 cells using siRNA led to decreased PDGFRβ and ERK1/2 phosphorylation after PDGF-BB stimulation, and FAK Y397 phosphorylation became transient. These effects were selective in that Akt phosphorylation was not significantly altered. PDGF-BB-induced ERK1/2 activation is known to be required for smooth muscle cell motility.15 Phosphorylation of FAK at Y397 promotes recruitment of SH2 domain-containing adaptor proteins, including Src, phosphatidylinositol 3-kinase, and Shc, and subsequently leads to ERK activation that is important for integrin-stimulated cell migration, including PDGF-BB-induced migration.15,16,33 Laser scanning confocal microscopy confirmed a global attenuation of PDGF-BB-stimulated tyrosine phosphorylation in MFG-E8-depleted cells and suggested that whole cell PDGFRβ levels also decreased more rapidly after stimulation in MFG-E8-depleted cells. Coimmunoprecipitation studies indicated that PDGFRβ entered into complexes with MFG-E8 after receptor ligation, presumably via interactions that are mediated by αv integrin that binds to FAK. This provides a possible link between MFG-E8 production and PDGF-BB:PDGFRβ signaling. Confocal microscopy confirmed colocalization of cell surface-associated MFG-E8 and PDGFRβ that increased over time after PDGF-BB addition, and it demonstrated higher levels of PDGFRβ on surfaces of MFG-E8-producing cells as compared with MFG-E8-depleted cells. Interestingly, ubiquitination of PDGFRβ was also enhanced in MFG-E8-depleted cells. Associations between the E3 ubquitin ligase c-Cbl and PDGFRβ also increased in MFG-E8-depleted 10T1/2 cells, and inhibition of the proteasome prevented ligand-induced PDGFRβ degradation. Finally, we formally demonstrated that MFG-E8-related proteins interact with 10T1/2 cells via the RGD consensus integrin-binding sequence in the E2 domain of MFG-E8 and αv-containing integrins.
PDGF-BB:PDGFRβ binding leads to receptor dimerization, receptor autophosphorylation, recruitment of requisite signaling molecules into supramolecular complexes, and receptor internalization.1–3 Although receptor internalization is likely to ultimately lead to decreased signaling overall, receptors and associated proteins in endosomal compartments may continue to signal. Some internalized receptors may recycle to cell surfaces where they are available to continue to participate in signaling events, whereas others are degraded in proteasomes and lysosomes. Persistent ubiquitination of PDGFRβ has been reported to target this protein for destruction via the proteasome- and lysosome-dependent pathways.7–9 Thus, the activity of the PDGF-BB:PDGFRβ signaling axis can be regulated via multiple mechanisms. Levels of expression of growth factor, receptor, downstream kinases, phosphatases, and scaffolding proteins are all relevant, but the physical and temporal locations of these components in relationship to one another are also critical.
Several recent studies have focused on regulation of PDGFRβ ubiquitination as an important mechanism by which PDGF-BB:PDGFRβ signaling can be modulated. The E3 ligase c-Cbl is thought to be primarily responsible for PDGFRβ ubiquitination, and regulation of c-Cbl expression and activity influences PDGF-BB:PDGFRβ signaling.35–38 Low-density lipoprotein receptor-related protein 1 binds to c-Cbl, preventing c-Cbl:PDGFRβ interaction and PDGFRp ubiquitination. In the absence of lipoprotein receptor-related protein 1, ubiquitination and degradation of PDGFRβ is enhanced and PDGF-BB:PDGFRβ signaling is attenuated.39 Endothelin and smooth muscle–derived neuropilin-like protein regulates PDGF-BB:PDGFRβ signaling via a different mechanism. Knockdown of endothelin and smooth muscle-derived neuropilin-like protein in VSMCs using siRNA causes coordinate increases in PDGFRβ signaling, proliferation, and migration that are accompanied by diminished PDGFRβ ubiquitination and turnover, as well as decreased c-Cbl mRNA and protein expression.40
In the present study, we demonstrate the existence of another route by which PDGFRβ ubiquitination may be regulated and PDGF-BB:PDGFRβ signaling can be augmented. 10T1/2 cells secrete MFG-E8 under basal conditions, and MFG-E8 production (as assessed at the mRNA level) was rapidly increased after PDGF-BB treatment. On the basis of its tendency to aggregate in solution and its predominately extracellular location in tissues29 and because it has previously been reported to bind to collagen,30 we believe that secreted MFG-E8 is readily incorporated into extracellular matrix. Indeed, we detected laminin, fibronectin, and collagen type 1 that was deposited in close proximity to 10T1/2 cells that were propagated in vitro (Supplemental Figure V). Matrix-associated MFG-E8 binds to αv-containing integrins, and these proteins become incorporated into cell surface-associated supramolecular complexes that include phosphorylated FAK and PDGFRβ (and other proteins) in the presence of growth factor. Binding of MFG-E8 to αv-containing integrins promotes retention of PDGFRβ at cell surfaces, reduces receptor ubiquitination by preventing PDGFRβ from becoming accessible to c-Cbl, and potentiates PDGF-BB-mediated signaling that involves FAK Y397 phosphorylation and subsequent activation of downstream signaling molecules, including ERK1/2, resulting in increased PDGFRβ-dependent cellular responses including cell migration.
The scenario that we propose above provides a framework in which to consider the activities of MFG-E8 more globally. Although we have made a strong case for PCs as significant sources of MFG-E8 in situ, other cells (including ECs, VSMCs, macrophages and tumor cells) also have the capacity to produce the protein.20,22,28,31 We have determined that MFG-E8 preferentially regulates PDGF-BB-stimulated migration of PC/PC precursor 10T1/2 cells, whereas others have demonstrated that MFG-E8 augments VEGF-induced EC proliferation and survival.28 Thus, in the setting of reparative angiogenesis, MFG-E8 can be produced by, and act on, several types of cells by potentiating the stimulatory effects of several growth factors. Recent experience with so-called molecular targeting small molecules indicates that drugs that have inhibitory effects on multiple targets (kinases) may be more efficacious than those that are more specific. It is tempting to speculate that, by analogy, inhibition of MFG-E8 production or action may be more advantageous than inhibition of a single signaling pathway, stimulated by VEGF, for example. It will also be interesting to determine whether the mechanisms by which MFG-E8 enhances VEGF signaling are distinct from those that we have described as operative for PDGF-BB.
Supplementary Material
Acknowledgments
We thank Mallorie Heneghan and Joshua Drago for performing mouse husbandry and genotyping; Michael Lu for purification of fusion proteins; and Drs Thomas Hornyak, Chuanjin Wu, and Stan Lipkowitz for helpful discussions.
Sources of Funding
This work was supported by the Intramural Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute. Dr Motegi was supported, in part, by project grants from the Japan Foundation for Aging and Health and by a Japan Society for Promotion of Science Research Fellowship for Japanese Biomedical and Behavioral Researchers at the National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Heldin CH, Ostman A, Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378: F79–F113. [DOI] [PubMed] [Google Scholar]
- 2.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. [DOI] [PubMed] [Google Scholar]
- 3.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. [DOI] [PubMed] [Google Scholar]
- 5.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. CircRes. 2005;97:512–523. [DOI] [PubMed] [Google Scholar]
- 6.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. [DOI] [PubMed] [Google Scholar]
- 7.Mori S, Heldin CH, and Claesson-Welsh L. Ligand-induced polyubiquitination of the platelet-derived growth factor β-receptor. J Biol Chem. 1992;267:6429–6434. [PubMed] [Google Scholar]
- 8.Mori S, Heldin CH, Claesson-Welsh L. Ligand-induced ubiquitination of the platelet-derived growth factor β-receptor plays a negative regulatory role in its mitogenic signaling. J Biol Chem. 1993;268:577–583. [PubMed] [Google Scholar]
- 9.Mori S, Tanaka K, Omura S, Saito Y. Degradation process of ligand-stimulated platelet-derived growth factor β-receptor involves ubiquitin-proteasome proteolytic pathway. J Biol Chem. 1995;270:29447–29452. [DOI] [PubMed] [Google Scholar]
- 10.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. [DOI] [PubMed] [Google Scholar]
- 12.Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodard AS, Garcia-Cardena G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J Cell Sci. 1998;111:469–478. [DOI] [PubMed] [Google Scholar]
- 14.Ding Q, Stewart J Jr, Olman MA, Klobe MR, Gladson CL. The pattern of enhancement of Src kinase activity on platelet-derived growth factor stimulation of glioblastoma cells is affected by the integrin engaged. J Biol Chem. 2003;278:39882–39891. [DOI] [PubMed] [Google Scholar]
- 15.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. [DOI] [PubMed] [Google Scholar]
- 16.Hauck CR, Hsia DA, Schlaepfer DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis migration of vascular smooth muscle cells. J Biol Chem. 2000;275:41092–41099. [DOI] [PubMed] [Google Scholar]
- 17.Salazar EP, Rozengurt E. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J Biol Chem. 1999;274:28371–28378. [DOI] [PubMed] [Google Scholar]
- 18.Stubbs JD, Lekutis C, Singer KL, Bui A, Yuzuki D, Srinivasan U, Parry G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc Natl Acad Sci USA. 1990;87: 8417–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogura K, Nara K, Watanabe Y, Kohno K, Tai T, Sanai Y. Cloning and expression of cDNA for O-acetylation of GD3 ganglioside. Biochem Biophys Res Commun. 1993;225:932–938. [DOI] [PubMed] [Google Scholar]
- 20.Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 1997;16:861–869. [DOI] [PubMed] [Google Scholar]
- 21.Andersen MH, Berglund L, Rasmussen JT, Petersen TE. Bovine PAS-6/7 binds αvβ5 integrins and anionic phospholipids through two domains. Biochemistry. 1997;36:5441–5446. [DOI] [PubMed] [Google Scholar]
- 22.Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. [DOI] [PubMed] [Google Scholar]
- 24.Ensslin MA, Shur BD. The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis. Proc Natl Acad Sci USA. 2007;104:2715–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J, Heegaard CW, Rasmussen JT, Gilbert GE. Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim Biophys Acta. 2004;1667:82–90. [DOI] [PubMed] [Google Scholar]
- 26.Shao C, Novakovic VA, Head JF, Seaton BA, Gilbert GE. Crystal structure of lactadherin C2 domain at 1.7A resolution with mutational and computational analyses of its membrane-binding motif. J Biol Chem. 2008;283:7230–7241. [DOI] [PubMed] [Google Scholar]
- 27.Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. JExp Med. 2004;200:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestre JS, Théry C, Hamard G, Boddaert J, Aguilar B, Delcayre A, Houbron C, Tamarat R, Blanc-Brude O, Heeneman S, Clergue M, Duriez M, Merval R, Lévy B, Tedgui A, Amigorena S, Mallat Z. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499–506. [DOI] [PubMed] [Google Scholar]
- 29.Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405–417. [DOI] [PubMed] [Google Scholar]
- 30.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, Wolters P, Emson CL, Turner SM, Werb Z, Sheppard D. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neutzner M, Lopez T, Feng X, Bergmann-Leitner ES, Leitner WW, Udey MC. MFG-E8/lactadherin promotes tumor growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res. 2007;67:6777–6785. [DOI] [PubMed] [Google Scholar]
- 32.Motegi S, Leitner WW, Lu M, Tada Y, Sardy M, Wu C, Chavakis T, Udey MC. Pericyte-derived MFG-E8 regulates pathologic angiogenesis. Arterioscler Thromb Vasc Biol. 2011;31:2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112: 2677–2691. [DOI] [PubMed] [Google Scholar]
- 34.Graf K, Xi XP, Yang D, Fleck E, Hsueh WA, Law RE. Mitogen-activated protein kinase activation is involved in platelet-derived growth factor-directed migration by vascular smooth muscle cells. Hypertension. 1997; 29:334–339. [DOI] [PubMed] [Google Scholar]
- 35.Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. [DOI] [PubMed] [Google Scholar]
- 36.Miyake S, Mullane-Robinson KP, Lill NL, Douillard P, Band H. Cbl-mediated negative regulation of platelet-derived growth factor receptor-dependent cell proliferation: a critical role for Cbl tyrosine kinase-binding domain. J Biol Chem. 1999;274:16619–16628. [DOI] [PubMed] [Google Scholar]
- 37.Kales SC, Ryan PE, Nau MM, Lipkowitz S. Cbl and human myeloid neoplasms: the Cbl oncogene comes of age. Cancer Res. 2010;70: 4789–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddi AL, Ying G, Duan L, Chen G, Dimri M, Douillard P, Druker BJ, Naramura M, Band V, Band H. Binding of Cbl to a phospholipase Cγ1-docking site on platelet-derived growth factor receptor β provides a dual mechanism of negative regulation. J Biol Chem. 2007;282: 29336–29347. [DOI] [PubMed] [Google Scholar]
- 39.Takayama Y, May P, Anderson RG, Herz J. Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor β (PDGFR β). JBiol Chem. 2005;280:18504–18510. [DOI] [PubMed] [Google Scholar]
- 40.Guo X, Nie L, Esmailzadeh L, Zhang J, Bender JR, Sadeghi MM. Endothelial and smooth muscle-derived neuropilin-like protein regulates platelet-derived growth factor signaling in human vascular smooth muscle cells by modulating receptor ubiquitination. J Biol Chem. 2009; 284:29376–29382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


