Abstract
Rotaviruses are the most common infectious agents causing severe diarrheal diseases in young children globally. Three rare human rotavirus strains, two G3P[9] and one G3P[6], were detected in stool samples of children under 5 years of age hospitalized for gastroenteritis in Lebanon during the course of a surveillance study. Complete genomes of these strains were sequenced using VirCapSeq-VERT, a capture based high-throughput sequencing method. Genomic sequences were further characterized by using phylogenetic analyses with global RVA G3P[6]/P[9] strains, other vaccine and reference strains. Genetic analysis revealed that the G3P[6] strain emerged as a DS-1/Wa-like mono-reassortant strain with a potential Ethiopian origin. The two G3P[9] strains possessed a mixed DS-1/Wa/AU-1-like origin indicating that these may have evolved via multiple reassortment events involving feline, human and bovine rotaviruses. Furthermore, analysis of these strains revealed high antigenic variability compared to the vaccine strains. Additional studies are essential to fully understand the evolutionary dynamics of G3P[6]/P[9] strains spreading worldwide and their implications on vaccine effectiveness.
Keywords: Human Rotavirus A, G3P[6], G3P[9], Reassortment, Lebanon, VirCapSeq-VERT
1. Introduction
Human group A Rotavirus (RVA) infection is the leading cause of acute gastroenteritis in young children and infants worldwide. RVA infection accounts for approximately 228,000 fatalities annually including 128,500 fatalities in children under five years of age (GBD 2016 Diarrhoeal Disease Collaborators, 2018). Globally, the RVA burden is greatest in children living in low-and middle-income countries, especially due to unhygienic living condition, poor and limited supply of clean drinking water, and inadequate sanitation (Sindhu et al., 2017). In Lebanon, RVA was detected in 27.7–30.3% of children under 5 years of age who were hospitalized for gastroenteritis (Ali et al., 2016; Dbaibo et al., 2013). Approximately 83% of total RVA cases in Lebanon were detected in children under 2 years of age.
Rotaviruses are members of the family Reoviridae, and its genome consists of 11 segments of double stranded RNA (dsRNA) coding for six structural proteins (VP). A binary classification system exists based on sequence similarities of the glycoprotein VP7 and the protease-sensitive VP4 that form the outer capsid of the virus and define the “G” and “P” viral genotypes, respectively. VP7 and VP4 also play major roles in eliciting the production of neutralizing antibodies in the host immune response to rotavirus infection (Nair et al., 2017; Taniguchi et al., 1991). More recently, full genome-based classification system was introduced by which a specific genotype is assigned to each of the 11 genomic segments using the convention Gx-P[x]-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx, where x indicates the genotype number. This classification system established three human RVA genogroups exhibiting the Wa-like constellation (I1-R1-C1-M1-A1-N1-T1-E1-H1), the DS-1-like constellation (I2-R2-C2-M2-A2-N2-T2-E2-H2), or the AU-1-like genotypes (I3, R3, C3, M3, A3, N3, T3, E3 and H3) (Matthijnssens et al., 2008; Matthijnssens and Van Ranst, 2012).
To date, at least 27 G and 37 P genotypes have been reported globally (Desselberger, 2014). G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8] are the predominant strain combinations of genotypes that have been responsible for >90% of human RVA infections worldwide (Bányai et al., 2012a; Kirkwood, 2010). According to the most recent data from Lebanon, G1P[8] is the most prevalently reported genotype (36%) followed by G9P[8] (26.4%), G2P[4] (17.8%) and G4P[8] (15.9%) (Ali et al., 2016). Less common genotypes, such as G12P[8], G12P[6], G2P[8], G4P[6], and G3P[6] were also reported with lower rates of incidences in different countries (EuroRotaNet: Annual Report 2017. Available from: http://www.eurorota.net/, n.d.; László et al., 2012; Stupka et al., 2012; Usonis et al., 2012). Similarly, other rare genotypes were also detected in Lebanon, including G3P[6] and G3P[9] isolates (Ali et al., 2016).
G3 RVA has a very broad range (Martínez-Laso et al., 2009). The G3P[6] genotype has been identified in different parts of the world less frequently (Bourdett-Stanziola et al., 2011; Heylen et al., 2013a; Ianiro et al., 2015). Whole genome characterization of recently identified G3P[6] strains revealed a DS-1-like genotype constellation (Heylen et al., 2013a; Ianiro et al., 2015; Nyaga et al., 2018; Utsumi et al., 2018). G3P[9] strains have been commonly recovered from cats with diarrhea (Jeong et al., 2014a) and occasionally in humans (De Grazia et al., 2010a; Grant et al., 2011a; Hwang et al., 2011; Khananurak et al., 2010). Reassortant strains of feline/human or feline/canine G3P[9] rotaviruses have also been reported in humans (Khamrin et al., 2007; Martella et al., 2011; Theamboonlers et al., 2013; Tsugawa and Hoshino, 2008).
In this study, we sequenced the complete genomes of two G3P[9] and one G3P[6] RVA strains from Lebanon using a capture based, sensitive and targeted viral sequencing method, VirCapSeq-VERT during a prospective, multicenter, hospital-based surveillance study conducted between 2011 and 2013 (Ali et al., 2016). These three genomic sequences were further characterized based on phylogenetic analyses with global RVA and vaccine strains.
2. Materials and methods
2.1. Sample collection:
The study was conducted in seven hospitals distributed across Lebanon (North, Central and South Lebanon) to determine the rate of RVA-associated gastroenteritis in hospitalized children under 5 years of age. During 2011 through 2013, 428 subjects were diagnosed with RVA-associated gastroenteritis (RVGE) by consensus PCR. Three of the 428 positive samples; N235, N566, and N568 revealed rare genotypes with PCR and Sanger sequencing of partial VP4 and VP7 segments as previously described (Ali et al., 2016).
These three samples, N235, N566 and N568, were collected from children hospitalized with severe gastroenteritis as assessed by using the Vesikari scoring system (Table 1) (Lewis, 2011; Ruuska and Vesikari, 1990).
Table 1.
Clinical features of Lebanese children infected with G3 RVA.
| Sample name | Collection year | Origin | Age of Patient | Gender | Vesikari score | Duration of hospitalization | Vaccination status |
|---|---|---|---|---|---|---|---|
| N235 | 2011 | North Lebanon | 6 months | Male | 18 | 4 days | N/A |
| N566 | 2013 | North Lebanon | 23 months | Male | 13 | 3 days | N/A |
| N568 | 2013 | North Lebanon | 37 months | Male | 12 | 6 days | N/A |
N/A: not available
2.2. Ethical considerations:
Subject recruitment and stool specimen collection were approved by the American University of Beirut Institutional Review Board (IRB). Stool samples were de-identified to ensure confidentiality. A written consent was obtained from the subjects’ parents prior to sample collection.
2.3. Nucleic Acid Extraction and VirCapSeq-VERT library preparations-
Forty μl of total nucleic acid was extracted from 240 μl of stool suspension using the NucliSENS easyMAG automated platform (BioMérieux, Boxtel, The Netherlands). Individual VirCapSeq-VERT libraries were prepared using the Hyper Prep kit (KAPA Biosystems, Boston, MA, USA) and unique barcodes. Superscript III and random hexamer primers were used to generate first strand cDNA (Life Technologies, Carlsbad, CA, USA). Second-stranded cDNA synthesis for HTS was carried out by random primer extension with Klenow enzyme (New England Biolabs, Ipswich, MA, USA). Double stranded DNA preparations were sheared (E210 sonicator; Covaris, Woburn, MA, USA) for an average fragment size of 200 base pairs and added to Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) for purification, and libraries were prepared with the Hyper Prep kit (KAPA Biosystems, Wilmington, MA, USA). Libraries were pooled and hybridized with the VirCapSeq-VERT probe set prior to a final PCR and sequencing (Illumina HiSeq 4000).
2.4. VirCapSeq-VERT data analysis and Genotyping:
Illumina adaptor sequences were removed from the raw sequence reads using cutadapt (v 1.8.3) (Martin, 2011). Adaptor trimming was followed by generation of quality reports using FastQC software (v 0.11.5) (Andrew, 2010), which were used to determine filtering criteria based on the average quality scores of the reads, presence of indeterminate nucleotides and homopolymeric reads. The FastQ reads were then imported into Geneious 11.0.2 (https://www.geneious.com) for further processing and sequence assembly.
The reads were trimmed to remove low quality sequences and were assembled using the map-to-reference tool and a reference rotavirus genome obtained from GenBank.
The assembled nucleotide sequences were aligned with reference sequences obtained from the NCBI database using the CLUSTALW multiple alignment tool in BioEdit software (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
The consensus sequence for each genome segment was then manually edited. The Virus Pathogen Database and Analysis Resource (VIPR) (https://www.viprbrc.org/brc/rvaGenotyper.spg?method=ShowCleanInputPage&decorator=reo) was used to determine the genotype of each genome segment.
2.5. Phylogenetic analyses:
Phylogenetic analyses of genome segments were carried out using MEGA6.06 (Tamura et al., 2013). Phylogenetic trees were constructed using maximum likelihood method based on the best-fit nucleotide substitution model. The reliability of the branching order was estimated from 1000 bootstrap replicates (Felsenstein, 1985). The results of phylogenetic analyses were validated using several other genetic distance models, such as Tamura Nei, Tamura 3-parameter and General Time Reversible (GTR) (data not shown).
2.6. Accession Numbers:
The full genome sequences are submitted to the GenBank NCBI under the following accession numbers: N235 (MN029110 - MN029120), N566 (MN029121- MN029131) and N568 (MN029132- MN02914).
3. Results
3.1. Full genome-based classification
Based on the full genome characterization, the N568, N566 and N235 strains had the following genome constellations: G3-P[6]-I2-R2-C2-M2-A2-N2-T1-E2-H2, G3-P[9]-I2-R2-C2-M2-A3-N2-T1-E2-H2 and G3-P[9]-I2-R2-C2-M2-A3-N1-T6-E2-H3, respectively (Table 2). The N568 strain has DS1-like VP1–3, VP6, NSP1, NSP2, NSP4 and NSP5 genes. The N566 strain has same genotypes combination except for NSP1, which belonged to the AU-1-like A3 genotype, and VP4. The NSP3 genes of N566 and N568 strains are of Wa-like genogroup-1 origin. Therefore, N568 strain was classified as a DS-1/Wa-like mono-reassortant strain, while the N566 strain was considered a double reassortant DS-1/Wa/AU-1-like strains.
Table 2.
Genotype constellations of the eleven gene segments of the G3 RVA strains.
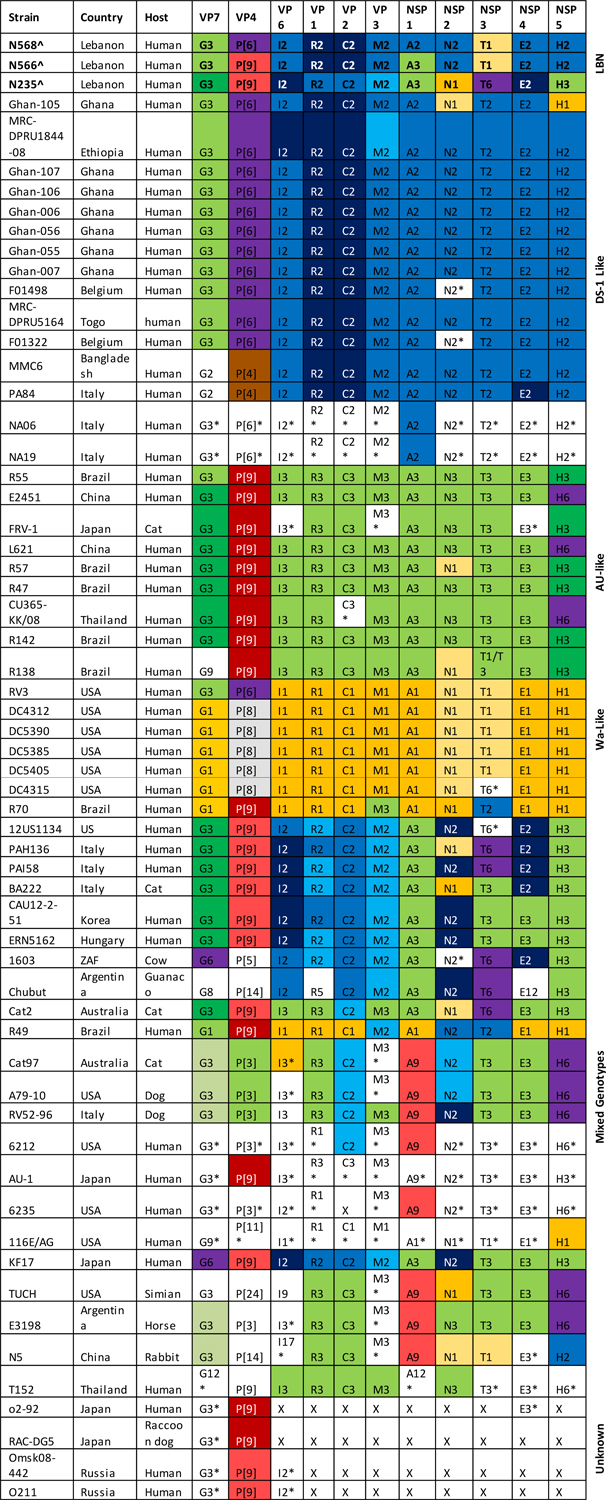
|
The colors on the cells correspond to those used in figure 1 designating the various subclusters in the phylogenetic trees.
Lebanese strains (LBN) detected in this study are in bold.
indicates that no sequence data were available in the GeneBank database.
indicates that the corresponding segment is missing in the trees.
Concerning the N235 strain, the VP1–3, VP6 and NSP4 genes are of DS-1-like genogroup-2 origin. While, the NSP1 and NSP5 genes are related to AU-1-like genogroup-3. The NSP2 gene is related to Wa-like strain. The NSP3 gene has a T6 genotype. Thus, N235 strain can be considered a DS-1/Wa/AU-1-like reassortant further mixing with a fourth strain of genogroup-6 origin.
3.2. Phylogenetic analysis
To further investigate the genetic relationships among the N235, N566, and N568 strains and other RVA strains, phylogenetic trees were constructed based on nucleotide sequences of the entire open reading frame of the 11 gene segments. The nucleotide similarity between the Lebanese and other reference strains was determined (Supplementary Table).
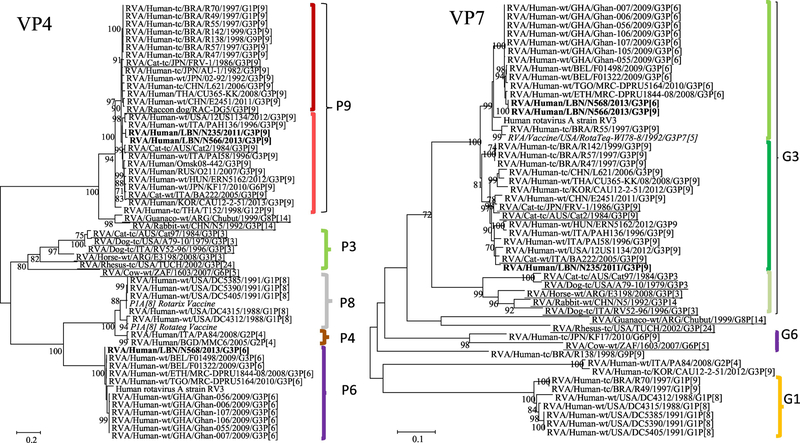
The VP4 gene of the N568 strain clustered with other P[6] human strains, where it exhibited the maximum nucleotide sequence identities (NSId) with those isolated from Belgium (98.7%), Ethiopia (97.8%), Togo (97.5%) and Ghana (95.1%). The VP4 gene of the N235 showed the highest NSId with the Thailand human strain (CU365-KK) (NSId 95.3%) and the Japanese canine strain (RAC-DG5) (95.1%). Whereas the VP4 gene of N566 showed the highest similarity (93.1%, 92.8%) with Japanese human (AU-1) and feline FRV-1 strains, respectively. The N235 and N566 clustered with P[9], harboring a number of animal-origin (feline and canine) rotavirus strains in addition to several human strains from different countries including USA, Brazil, Italy and China (Figure 1 ).
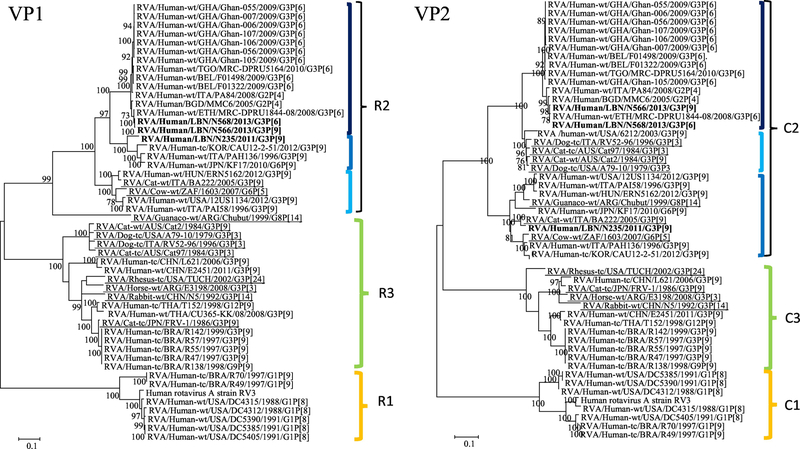
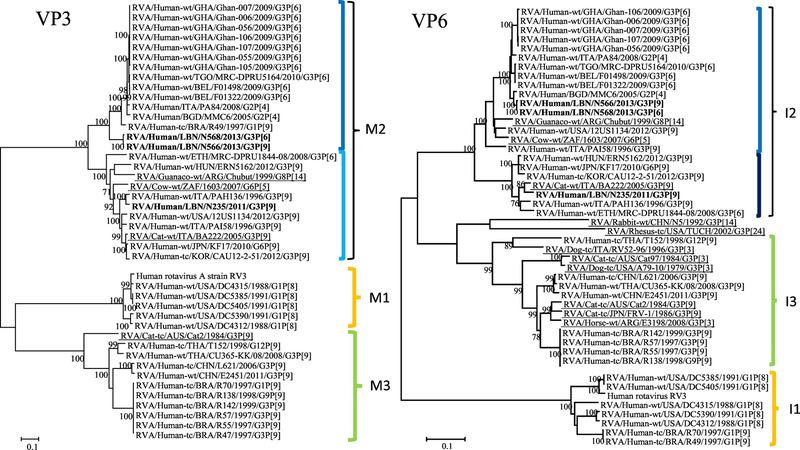
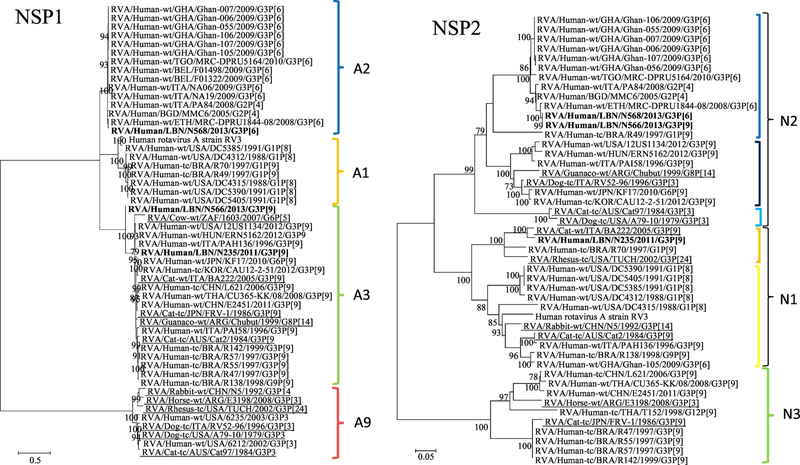
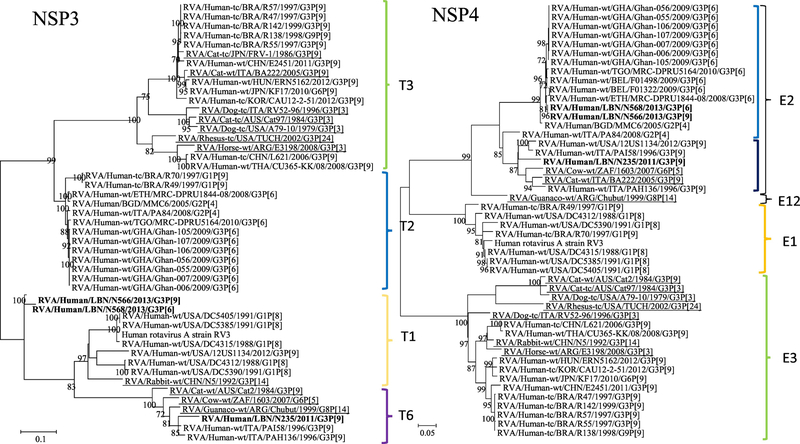
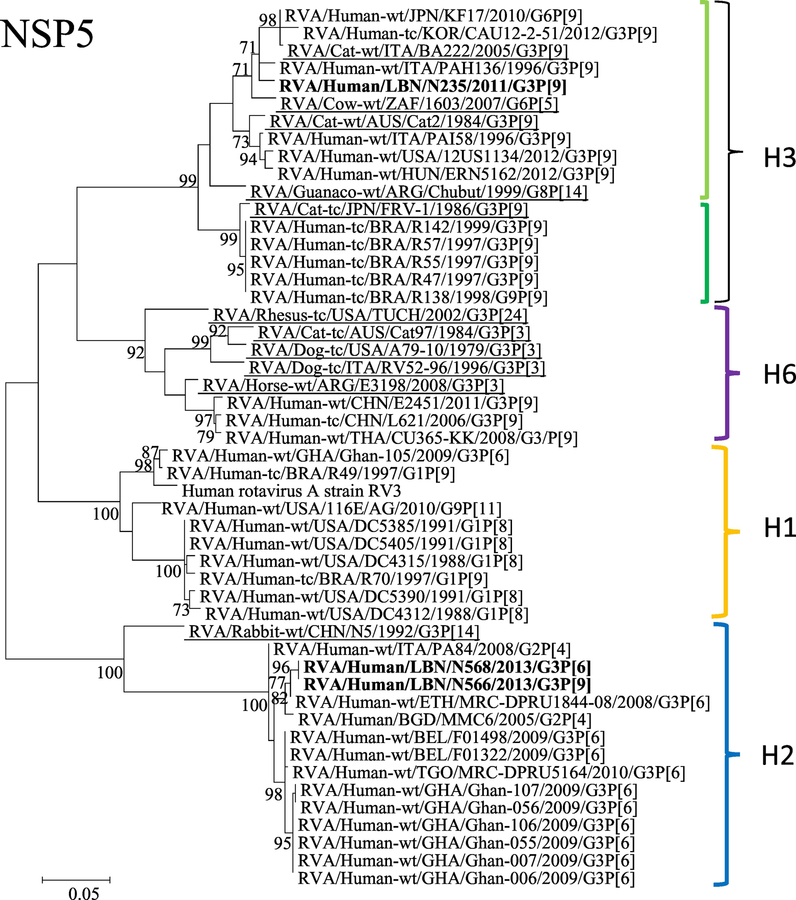
Figure 1.
Phylogenetic trees of the 11 gene segments of the Lebanese G3 RVA strains and representative RVA strains. The phylogenetic trees were inferred using the maximum-likelihood analysis based on the best-fit nucleotide substitution model for each individual gene. The Lebanese strains are bolded, whereas those of the animal and vaccine strains are underlined and italicized, respectively. Brackets and colored tones are used to show the clustering patterns in the phylogenetic trees. Bootstrap values equal or greater than 70% are shown. Scale bars indicate the substitutions per nucleotide.
The VP7 genes showed that the N566 and N568 strains (NSId 99.9%) clustered with other human G3 strains isolated from Belgium (NSId 98.6%, 98.7%), Ethiopia (NSId 95.2%, 95.3%), and Ghana (NSId 91.5%, 91.6%). While the N235 strain clustered with the human (12US1134) (NSId. 96.9%) and feline G3 strains (BA222) (NSId 98%) isolated in USA and Italy, respectively (Figure 1).
The phylogenetic analysis of the VP1, VP2, VP3, and VP6 genes of the G3P[6] and G3P[9] strains revealed that they all clustered within their corresponding genogroup-2 clusters (Figure 1). In the VP1 tree, the Lebanese strains could be assigned to genotype R2. Both N566 and N568 (NSId 99.9%) belonged to an R2 sub-cluster that harbored other G3P[6] human RVA strains from Ethiopia (NSId 98.4%), Belgium (NSId 97%), and Togo (NSId 96.2%). Interestingly, this subcluster included two G2P[4] RVA strains isolated in Italy (NSId 97.2%) and Bangladesh (NSId 98%). The N235 strain fell into an R2 sub-cluster including Italian, Japanese, and Korean human G3P[9] strains (Figure 2 A).
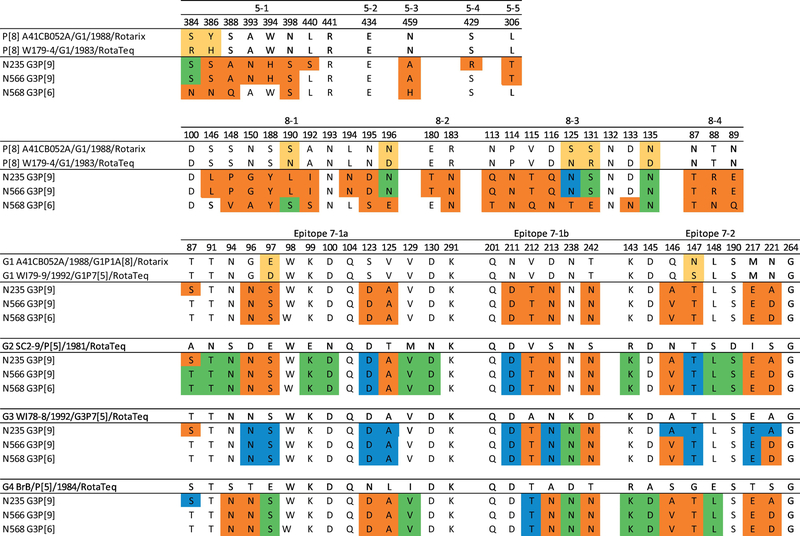
Figure 2. Alignment of the amino acid residues in VP4 and VP7 antigenic epitopes of the Lebanese G3 RVA strains and those of vaccine strains.
Amino acids that differ between Rotarix and RotaTeq are indicated in yellow. Blue colored residues are residues that are different from Rotarix, and green colored residues are different from RotaTeq. Residues colored in orange are different from both Rotarix and RotaTeq.
In the VP2 tree, the Lebanese strains could be assigned to genotype C2. The N566 and N568 strains (NSId 99.4%) belonged to a C2 subcluster that harbored other G3P[6] human RVA strains from Ethiopia (NSId 97.9–98.1%). This subcluster also included two G2P[4] strains PA84 and MMC6 (NSId for N566, 97.2 and 98.2%, respectively; NSId for N568, 97.5% and 98.5%, respectively). The N235 strain belonged to a subcluster including Italian feline strains BA222 (G3P[9] (NSId 97.4%) and was adjacent to a subcluster harboring the bovine strain 1603 (NSId 90.3%) isolated in South Africa (Figure 1).
In the VP3 tree, the Lebanese strains fell in the M2 genotype. The N566 and N568 strains (NSId 99.4%) belonged to a subcluster harboring G3P[6], G3P[9], G2P[4] and G1P[9] RVA strains from Brazil, Italy, Ghana, and other countries (NSId 85.3–88.7%). The N235 strain belonged to the subcluster including the Italian feline strain BA222 (G3P[9]; NSId 92.5%), the Argentinian Guanaco strain (G8P[14]; NSId 86.8%), and the South African bovine strain 1603 (G6P[5]; NSId 89.3%) in addition to human G3P[6], G3P[9], and G6P[9] strains isolated in several countries (Figure 1).
The Lebanese strains VP6 gene belonged to the I2 genotype. The N566 and N568 (NSId 99.9%) strains belonged to the subcluster harboring the Argentinian Guanaco strain (G8P[14]; NSId 92.7–92.8%) and South African bovine strain (G6P[5]; NSId 93.4–93.5%). The N235 strain belonged to the subcluster including the Italian feline strain BA222 (G3P[9]; NSId 96.9%) along with human strains of the G3P[6], G3P[9], and G6P[9] genotypes (Figure 1).
The NSP1 gene of N566 and N235 (NSId 94.9%) clustered within the A3 genotype, which also harbored human (NSId 91.2–96.5%) and feline G3P[9] strains (NSId 90–93.7%), a bovine G6P[5] (NSId 83–85.4%), and a G8P[14] strain (NSId 90.3–94.2%) of Camelidae origin, respectively. The N568 strain belonged to the A2 genotype, which mainly accommodated human G3P[6] isolates from several countries including Ethiopia (Figure 1).
Regarding the NSP2, the N235 strain harboring N1 genotype closely clustered with the feline strain BA222 (G3P[9]; NSId 96.6%). Moreover, both the N566 and N568 strains (NSId 99.9%) were tightly clustered within N2 along the Bangladeshi strain MMC6 (NSId 98.3–98.4%), the Italian strain PA84 (NSId 98–98.1%), and the Ethiopian strain MRC-DPRU1844-08 (G3P[6]; NSId 94%–94.1%) (Figure 1).
NSP3 gene analysis showed that the N235 strain with other human Italian G3P[9] strains (NSId 94.5–94.6%) in the T6 genotype, which included artiodacyl-like RVA strain of camelid (G8P[14]; NSId 92.4%) and bovine (G6P[5]; NSId 90.2%) origins and feline G3P[9] strain (NSId 85.5%) (Figure 1). On the other hand, the N566 and N568 strains (NSId 97.2%) clustered distantly within the T1 genotype that was mainly comprised of human G1P[8] stains (NSId 80–86.1%).
The Lebanese RVA strains NSP4 clustered in the E2 genotype. N235 clustered among the bovine strain 1603 (G6P[5]; NSId 92.2%) from South Africa and the feline strain BA222 (G3P[9]; NSId 92.6%) from Italy. This E2 subcluster also included other human G3P[9] strains from Italy and USA (NSId 93.4%). The N566 and N568 (NSId 99.7%) fell within the E2 subcluster that consisted mainly of human G3P[6] strains from other countries such as Belgium (NSId 98.9–99.3%), Ethiopia (NSId 92.9–93%), Italy (NSId 94.4–95%), and G2P[4] strain (NSId 97.6–97.8%) from Bangladesh (Figure 1).
N235 belonged to an NSP5 subcluster of the H3 genotype, harboring human G3P[9] strains from Italy (NSId 98.2%), USA (NSId 95.3%), Korea (NSId 96.7%), and a Japanese G6P[9] strain (NSId 97.7%). This H3 subcluster also accommodated bovine 1603 (G6P[5]) (NSId 97.3%) and feline BA222 G3P[9] (NSId 97.7%) strains. The N566 and N568 strains clustered closely with the H2 genotype along with G3P[6] strains from Belgium (NSId 92.5%) and Ethiopia (NSId 91.5%), G2P[4] strains from Bangladesh (NSId 93.3%) and Italy (NSId 92.7%) (Figure 1).
3.3. Comparison of the circulating Lebanese G3 strains with RotaTeq and Rotarix vaccines
The VP4 and VP7 proteins constitute the outer capsid proteins of the virus and are thus the key targets for the host immune response (Zeller et al., 2012). The VP4 is cleaved into VP8* (globular head) and VP5* (stalk domain), containing four (8–1 to 8–4) and five (5–1 to 5–5) surface-exposed antigenic epitopes (37 amino acids), respectively. Seven out of the 25 VP8* and 6–8 out of 12 VP5* antigenic residues were conserved in the N568 P[6] strain compared to RotaTeq and Rotarix VP4 component. In total, 35% and 40% of the VP4 antigenic residues of N568 were identical to RotaTeq and Rotarix, respectively. N235 VP4 displayed the greatest antigenic divergence compared to the P[8] VP4 of the vaccines, with 30 amino acids (81%) being different from RotaTeq and 27 amino acid (73%) changes compared to Rotarix. N566 possessed 28 amino acid (75.6%) differences in the VP4 antigenic sites compared to RotaTeq and 25 amino acid (67.5%) changes compared to Rotarix (Figure 2).
The VP7 epitopes of the Lebanese G3 strains compared to vaccine strains (G1, G2, G3, and G4) showed that 5 amino acids were fully conserved among them (Figure 2). Because the G3 VP7 is not included in Rotarix, the comparison is only described for RotaTeq. The N235 G3P[9] strain contain four residues (14%) in the VP7 epitopes that differ from those of RotaTeq (WI78–8 strain), distributed within the 7–1a and 7–1b epitopes (residues 87, 212, 238, and 242). The N566 G3P[9] and N568 G3P[6] strains have five changes (17%) compared to RotaTeq (WI78–8) distributed within the 7–1b and 7–2 epitopes (residues 212, 238, 242, 146, and 221). All three strains possessed a K238N change, which creates a potential N-linked glycosylation site that is absent in the RotaTeq G3 strain (Umair et al., 2018).
4. Discussion
In this study, we report the full genome characterization of three rotavirus strains, one G3P[6] (N568) and two G3P[9] (N235 and N566), that were sporadically detected in Lebanon between 2011–2013 (Ali et al., 2016). G3 is one of the most frequently detected RVAs worldwide. It presents the broadest host range in combination with P[4], P[6], P[9], and P[8] (Degiuseppe et al., 2014; Santos and Hoshino, 2005). G3P[8] is the most prevalent G3 genotype infecting humans worldwide (Bányai et al., 2012b; Heylen et al., 2013b; Martínez-Laso et al., 2009). Human G3 with P[3] and P[9] types sharing ancestry with feline or canine strains have been reported (Grant et al., 2011b). G3P[6] strains were identified in different parts of the world at a considerable rate following the Rotarix vaccine introduction (Abebe et al., 2018, 2014; da Silva Soares et al., 2014; Heylen et al., 2013c; Lartey et al., 2018; Ndombo et al., 2017; Seheri et al., 2014). In Lebanon, only one G3P[6] and two G3P[9] isolates were detected among 428 rotavirus-positive specimens collected over a period covering three RVA seasons (Ali et al., 2016).
G3P[6] strains are often reported to possess a Wa-like genogroup-1 constellation, speculated to be of porcine origin (Kaneko et al., 2018; Zhou et al., 2015). Nonetheless, G3P[6] strains with a DS1-like genogroup-2 constellation were first reported during the 2008–2009 season in Belgium (Heylen et al., 2013a). The VP4 P[6] of these strains is very similar to those isolated in Africa (mainly Ethiopia), suggesting an African origin. The Belgian strains also share a high degree of similarity with a G2P[6] strain isolated during an American outbreak, indicating a reassortant background (Heylen et al., 2013a). Interestingly, the Lebanese N568 strain (G3P[6]) exhibited a high degree of similarity with two Belgian strains, namely F01498 and F01322 in all genes, except the NSP3. Moreover, N568 is closely related to the Ethiopian strain MRC-DPRU1844-08 (G3P[6]) in all gene segments except for the VP6 and NSP3 genes. Our strain had a T1 NSP3 on a DS-1 backbone. The Ethiopian G3P[6] was shown to have a pure DS1-like genogroup-2 constellation same as the Belgian and Ghanaian G3P[6] strains. Both N568 and MRC-DPRU1844-08 possessed an I2 VP6, but subclustered separately. The Lebanese strains might have emerged from a DS1-like G3P[6] strain of Ethiopian origin where Ethiopians represent over 70% of the migrant domestic workers in Lebanon, that acquired a Wa-like NSP3 gene.
In contrast to P[6] RVA strains, P[9] strains are not frequently detected and do not show a specific pattern of geographical distribution. Human G3P[9] strains frequently show common origins with feline RVAs, particularly AU-1-like strain (Jeong et al., 2014b). Other strains, including the Lebanese N235 G3P[9] strain, did not display a pure AU-1-like genogroup-3. The VP4, VP7, NSP1, and NSP5 genes were AU-1-like genotype, while VP1, VP2, VP3, VP6, and NSP4 display a DS-1 like genotype. Intriguingly, N235 had an artiodactyl-like T6 NSP3 genotype. Overall, the Lebanese N235 strain shows the highest degree of similarity to the Italian feline strain BA222 (G3P[9]). This feline G3P[9] strain revealed a constellation of G3-P[9]-I2-R2C2-M2-A3-N1-T3-E2-H3, and share common origins with animal and zoonotic human RVAs. Several other G3P[9] RVA strains of feline-or human-origin with similar mosaic genomic constellations have been reported to have a relatively stable assortment of genes, G3-P[9]-I2-R2-C2-M2-A3-(N1/N2)-(T1/T6)-E2-H3, differentiating them from G3P[9] strains with a pure genogroup-3 backbone (Mijatovic-Rustempasic et al., 2014). Two Italian G3P[9] (PAI58/96 and PAH136/96) that clustered close to N235 possess high resemblance for G6/G8P[14] human or bovine/other artiodactyl-like rotaviruses and human/feline AU-1–like RVAs (De Grazia et al., 2010b, 2008). Their VP7 and VP4 genes were also highly similar to the Cat2 G3P[9] strain (G3-P[9]-I3-R3-C2-M3-A3-N1-T6-E3-H3). These findings suggest that such G3P[9] strains might be the result of reassortment events among feline/human AU-1–like rotaviruses, feline Cat2-like rotaviruses, and G6/G8P[14] human or artiodactyl-like rotaviruses. This hypothesis is reinforced in our study as the N235 strain clustered with the bovine strain 1603 (G6P[5]) in the VP1–3, VP6, NSP1, andNSP3–5 trees, with the feline strain BA222 (G3P[9]) in all gene trees except NSP3, and with the feline strain Cat2 (G3P[9]) in the VP4, VP7, NSP1, NSP2, NSP3, and NSP5 trees.
N566, another G3P[9] strain was more closely related to the G3P[6] N568 than to the G3P[9] N235 strain. N566 and N568 has a similar genomic constellation and cluster together in all the genes except for VP4 and NSP1. N566 and N568 also share the T1 genotype, as opposed to the T6 genotype of N235 strain. Thus, N566 might be a result of an intergenotypic reassortment event by which an N568-like G3P[6] strain obtained its VP4 (P[9]) and NSP1 (A3) genes from a G3P[9] strain. Interestingly, all G3P[9] strains of animal and human origins consistently possess an A3 genotype. The unique combination of VP4 and NSP1 genes might provide these viruses with a competitive replicative capacity in various hosts (Feng et al., 2011).
Currently, there are two globally approved vaccines for rotavirus: RotaTeq, a pentavalent, live bovine-human reassortant vaccine containing the G1, G2, G3, G4, and P[8] genotypes; and Rotarix, a single, live attenuated human G1P[8] rotavirus strain (Soares-Weiser et al., 2012). Analysis of the Lebanese G3P[6] and G3P[9] strains revealed high antigenic variability compared to the vaccine strains. The N568, N566, and N235 strains displayed 65%, 75.6%, and 81%, respectively, divergence in the antigenic epitopes from RotaTeq’s P[8] components, and 60%, 67.5%, and 73% divergence from Rotarix P[8] components. When compared to RotaTeq, four to five amino acid changes were observed within the 7–1b and 7–2 VP7 antigenic epitopes of N568, N566, and N235 strains. These strains showed a K238N substitution similarly to all Pakistani G3 strains in contrast to the G3 strain of RotaTeq (Umair et al., 2018). This substitution introduces a potential N-linked glycosylation site in the vicinity of the 7–1a epitope that has been previously shown to reduce neutralization of animal RVA strains by hyperimmune sera and alter serotype reactivity with monoclonal antibodies (Ciarlet et al., 1997). Further investigation of the antigenic variability and their impact is required to understand the significance of these differences and their influence on vaccine efficacy.
In conclusion, the present study provides important insights into the genetic background of G3 RVA and their antigenic relatedness to the vaccine strains. Further studies are essential to fully understand the evolutionary dynamics of G3P[6]/P[9] strains spreading worldwide and its implications on vaccine effectiveness. Finally, the “One Health” approach necessitates simultaneous monitoring of RVA strains in humans and animals for a better understanding of RVA ecology.
Supplementary Material
Supplementary Table. Nucleotide sequence identity matrix of the Lebanese G3P[6]/P[9] RVA and representative strains
Highlights:
The genetic background of Lebanese G3P[6]/P[9] RVAs was elucidated.
VirCapSeq-VERT was used to recover full RVA genomes.
G3P[6]/P[9] RVAs possess a mixed DS-1/Wa-like or DS-1/Wa/AU-1-like backgrounds.
Lebanese G3P[6] and Ethiopian G3P[6] RVAs share common ancestry.
Acknowledgments:
We thank Chun Kiat Lee for his support help with sequence analysis.
Funding: Funding support to CII team (NM, RT, CG, WIL) and financial support for VirCapSeq-VERT analysis was provided by National Institutes of Health award U19AI109761 [CETR: Center for Research in Diagnostics and Discovery]. Sample collection was originally funded by an MSD physician-initiated study grant to Ghassan Dbaibo.
The funder had no role in the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- RVA
Human group A Rotavirus
- VP
structural proteins
- NSP
nonstructural proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare that they have no competing interests.
Conflicts of Interest
The authors declare that they have no conflicts of interest pertaining to the submitted work.
References
- Abebe A, Getahun M, Mapaseka SL, Beyene B, Assefa E, Teshome B, Tefera M, Kebede F, Habtamu A, Haile-Mariam T, Jeffrey Mphahlele M, Teshager F, Ademe A, Teka T, Weldegebriel GG, Mwenda JM, 2018. Impact of rotavirus vaccine introduction and genotypic characteristics of rotavirus strains in children less than 5 years of age with gastroenteritis in Ethiopia: 2011–2016. Vaccine 36, 7043–7047. 10.1016/j.vaccine.2018.09.048 [DOI] [PubMed] [Google Scholar]
- Abebe A, Teka T, Kassa T, Seheri M, Beyene B, Teshome B, Kebede F, Habtamu A, Maake L, Kassahun A, Getahun M, Mitiku K, Mwenda JM, 2014. Hospital-based surveillance for rotavirus gastroenteritis in children younger than 5 years of age in Ethiopia: 2007–2012. Pediatr. Infect. Dis. J 33 Suppl 1, S28–33. 10.1097/INF.0000000000000048 [DOI] [PubMed] [Google Scholar]
- Ali Z, Harastani H, Hammadi M, Reslan L, Ghanem S, Hajar F, Sabra A, Haidar A, Inati A, Rajab M, Fakhouri H, Ghanem B, Baasiri G, Gerbaka B, Zaraket H, Matar GM, Dbaibo G, 2016. Rotavirus Genotypes and Vaccine Effectiveness from a Sentinel, Hospital-Based, Surveillance Study for Three Consecutive Rotavirus Seasons in Lebanon. PLoS ONE 11 10.1371/journal.pone.0161345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew S, 2010. FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- Bányai K, László B, Duque J, Steele AD, Nelson EAS, Gentsch JR, Parashar UD, 2012a. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine, Rotavirus Vaccines for Children in Developing Countries 30, Supplement 1, A122–A130. 10.1016/j.vaccine.2011.09.111 [DOI] [PubMed] [Google Scholar]
- Bányai K, László B, Duque J, Steele AD, Nelson EAS, Gentsch JR, Parashar UD, 2012b. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine 30 Suppl 1, A122–130. 10.1016/j.vaccine.2011.09.111 [DOI] [PubMed] [Google Scholar]
- Bourdett-Stanziola L, Ortega-Barria E, Espinoza F, Bucardo F, Jimenez C, Ferrera A, 2011. Rotavirus genotypes in Costa Rica, Nicaragua, Honduras and the Dominican Republic. Intervirology 54, 49–52. 10.1159/000318863 [DOI] [PubMed] [Google Scholar]
- Ciarlet M, Hoshino Y, Liprandi F, 1997. Single point mutations may affect the serotype reactivity of serotype G11 porcine rotavirus strains: a widening spectrum? J. Virol 71, 8213–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Soares L, de Fátima Dos Santos Guerra S, do Socorro Lima de Oliveira A, da Silva Dos Santos F, de Fátima Costa de Menezes EM, Mascarenhas J. d’Arc P., Linhares AC, 2014. Diversity of rotavirus strains circulating in Northern Brazil after introduction of a rotavirus vaccine: high prevalence of G3P[6] genotype. J. Med. Virol 86, 1065–1072. 10.1002/jmv.23797 [DOI] [PubMed] [Google Scholar]
- Dbaibo G, Rajab M, Inati A, Mikhael R, Choueiry E, Al-Tannir M, Salam O, Ramakrishnan G, DeAntonio R, 2013. Hospital-based surveillance study of rotavirus gastroenteritis in children under 5 years of age in Lebanon. Trials Vaccinol 2, 25–30. 10.1016/j.trivac.2013.08.002 [DOI] [Google Scholar]
- De Grazia S, Giammanco GM, Martella V, Ramirez S, Colomba C, Cascio A, Arista S, 2008. Rare AU-1-Like G3P[9] Human Rotaviruses with a Kun-Like NSP4 Gene Detected in Children with Diarrhea in Italy. J. Clin. Microbiol 46, 357–360. 10.1128/JCM.01593-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grazia S, Giammanco GM, Potgieter CA, Matthijnssens J, Banyai K, Platia MA, Colomba C, Martella V, 2010a. Unusual assortment of segments in 2 rare human rotavirus genomes. Emerg. Infect. Dis 16, 859–862. 10.3201/eid1605.091826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grazia S, Giammanco GM, Potgieter CA, Matthijnssens J, Banyai K, Platia MA, Colomba C, Martella V, 2010b. Unusual assortment of segments in 2 rare human rotavirus genomes. Emerg. Infect. Dis 16, 859–862. 10.3201/eid1605.091826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degiuseppe JI, Parra GI, Stupka JA, 2014. Genetic diversity of G3 rotavirus strains circulating in Argentina during 1998–2012 assessed by full genome analyses. PloS One 9, e110341 10.1371/journal.pone.0110341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger U, 2014. Rotaviruses. Virus Res 190, 75–96. 10.1016/j.virusres.2014.06.016 [DOI] [PubMed] [Google Scholar]
- EuroRotaNet: Annual Report. 2017 Available from: http://www.eurorota.net/, n.d.
- Felsenstein J, 1985. CONFIDENCE LIMITS ON PHYLOGENIES: AN APPROACH USING THE BOOTSTRAP. Evol. Int. J. Org. Evol 39, 783–791. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- Feng N, Sen A, Wolf M, Vo P, Hoshino Y, Greenberg HB, 2011. Roles of VP4 and NSP1 in determining the distinctive replication capacities of simian rotavirus RRV and bovine rotavirus UK in the mouse biliary tract. J. Virol 85, 2686–2694. 10.1128/JVI.02408-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Diarrhoeal Disease Collaborators, 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis 18, 1211–1228. 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Esona M, Gentsch J, Watt J, Reid R, Weatherholtz R, Santosham M, Parashar U, O’Brien K, 2011a. Detection of G3P[3] and G3P[9] rotavirus strains in American Indian children with evidence of gene reassortment between human and animal rotaviruses. J. Med. Virol 83, 1288–1299. 10.1002/jmv.22076 [DOI] [PubMed] [Google Scholar]
- Grant L, Esona M, Gentsch J, Watt J, Reid R, Weatherholtz R, Santosham M, Parashar U, O’Brien K, 2011b. Detection of G3P[3] and G3P[9] rotavirus strains in American Indian children with evidence of gene reassortment between human and animal rotaviruses. J. Med. Virol 83, 1288–1299. 10.1002/jmv.22076 [DOI] [PubMed] [Google Scholar]
- Heylen E, Zeller M, Ciarlet M, De Coster S, Van Ranst M, Matthijnssens J, 2013a. Complete genetic characterization of human G2P[6] and G3P[6] rotavirus strains. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 13, 27–35. 10.1016/j.meegid.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Heylen E, Zeller M, Ciarlet M, De Coster S, Van Ranst M, Matthijnssens J, 2013b. Complete genetic characterization of human G2P[6] and G3P[6] rotavirus strains. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 13, 27–35. 10.1016/j.meegid.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Heylen E, Zeller M, Ciarlet M, De Coster S, Van Ranst M, Matthijnssens J, 2013c. Complete genetic characterization of human G2P[6] and G3P[6] rotavirus strains. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 13, 27–35. 10.1016/j.meegid.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Hwang K-P, Huang Y-C, Bányai K, Wu H-S, Chang F-Y, Yang DC-F, Hsiung CA, Lin J-S, Jiang B, Gentsch JR, Wu F-T, 2011. Severe gastroenteritis associated with G3P[9] rotavirus in Taiwan. Infection 39, 271–275. 10.1007/s15010-011-0098-4 [DOI] [PubMed] [Google Scholar]
- Ianiro G, Delogu R, Fiore L, Ruggeri FM, 2015. Genomic characterization of uncommon human G3P[6] rotavirus strains causing diarrhea in children in Italy in 2009. Infect. Genet. Evol 33, 143–149. 10.1016/j.meegid.2015.04.022 [DOI] [PubMed] [Google Scholar]
- Jeong S, Than VT, Lim I, Kim W, 2014a. Whole-Genome Analysis of a Rare Human Korean G3P Rotavirus Strain Suggests a Complex Evolutionary Origin Potentially Involving Reassortment Events between Feline and Bovine Rotaviruses. PLoS ONE 9 10.1371/journal.pone.0097127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Than VT, Lim I, Kim W, 2014b. Whole-genome analysis of a rare human Korean G3P rotavirus strain suggests a complex evolutionary origin potentially involving reassortment events between feline and bovine rotaviruses. PloS One 9, e97127 10.1371/journal.pone.0097127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Do LP, Doan YH, Nakagomi T, Gauchan P, Agbemabiese CA, Dang AD, Nakagomi O, 2018. Porcine-like G3P[6] and G4P[6] rotavirus A strains detected from children with diarrhoea in Vietnam. Arch. Virol 163, 2261–2263. 10.1007/s00705-018-3836-4 [DOI] [PubMed] [Google Scholar]
- Khamrin P, Maneekarn N, Peerakome S, Tonusin S, Phan TG, Okitsu S, Ushijima H, 2007. Molecular characterization of rare G3P[9] rotavirus strains isolated from children hospitalized with acute gastroenteritis. J. Med. Virol 79, 843–851. 10.1002/jmv.20840 [DOI] [PubMed] [Google Scholar]
- Khananurak K, Vutithanachot V, Simakachorn N, Theamboonlers A, Chongsrisawat V, Poovorawan Y, 2010. Prevalence and phylogenetic analysis of rotavirus genotypes in Thailand between 2007 and 2009. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 10, 537–545. 10.1016/j.meegid.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, 2010. Genetic and Antigenic Diversity of Human Rotaviruses: Potential Impact on Vaccination Programs. J. Infect. Dis 202, S43–S48. 10.1086/653548 [DOI] [PubMed] [Google Scholar]
- Lartey BL, Damanka S, Dennis FE, Enweronu-Laryea CC, Addo-Yobo E, Ansong D, Kwarteng-Owusu S, Sagoe KW, Mwenda JM, Diamenu SK, Narh C, Binka F, Parashar U, Lopman B, Armah GE, 2018. Rotavirus strain distribution in Ghana pre-and post-rotavirus vaccine introduction. Vaccine 36, 7238–7242. 10.1016/j.vaccine.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- László B, Kónya J, Dandár E, Deák J, Farkas Á, Gray J, Grósz G, Iturriza-Gomara M, Jakab F, Juhász Á, Kisfali P, Kovács J, Lengyel G, Martella V, Melegh B, Mészáros J, Molnár P, Nyúl Z, Papp H, Pátri L, Puskás E, Sántha I, Schneider F, Szomor K, Tóth A, Tóth E, Szűcs G, Bányai K, 2012. Surveillance of human rotaviruses in 2007–2011, Hungary: exploring the genetic relatedness between vaccine and field strains. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol 55, 140–146. 10.1016/j.jcv.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Lewis K, 2011. Vesikari clinical severity scoring manual. PATH
- Martella V, Potgieter AC, Lorusso E, De Grazia S, Giammanco GM, Matthijnssens J, Bányai K, Ciarlet M, Lavazza A, Decaro N, Buonavoglia C, 2011. A feline rotavirus G3P[9] carries traces of multiple reassortment events and resembles rare human G3P[9] rotaviruses. J. Gen. Virol 92, 1214–1221. 10.1099/vir.0.027425-0 [DOI] [PubMed] [Google Scholar]
- Martin M, 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10–12. 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- Martínez-Laso J, Román A, Rodriguez M, Cervera I, Head J, Rodríguez-Avial I, Picazo JJ, 2009. Diversity of the G3 genes of human rotaviruses in isolates from Spain from 2004 to 2006: cross-species transmission and inter-genotype recombination generates alleles. J. Gen. Virol 90, 935–943. 10.1099/vir.0.007807-0 [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gómara M, Maes P, Patton JT, Rahman M, Van Ranst M, 2008. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol 82, 3204–3219. 10.1128/JVI.02257-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Van Ranst M, 2012. Genotype constellation and evolution of group A rotaviruses infecting humans. Curr. Opin. Virol 2, 426–433. 10.1016/j.coviro.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Mijatovic-Rustempasic S, Roy S, Sturgeon M, Rungsrisuriyachai K, Esona MD, Degroat D, Qin X, Cortese MM, Bowen MD, 2014. Full-Genome Sequence of a Rare Human G3P[9] Rotavirus Strain. Genome Announc 2 10.1128/genomeA.00143-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair N, Feng N, Blum LK, Sanyal M, Ding S, Jiang B, Sen A, Morton JM, He X-S, Robinson WH, Greenberg HB, 2017. VP4-and VP7-specific antibodies mediate heterotypic immunity to rotavirus in humans. Sci. Transl. Med 9 10.1126/scitranslmed.aam5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndombo PK, Ndze VN, Fokunang C, Ashukem TN, Boula A, Kinkela MN, Ndode CE, Seheri ML, Bowen MD, Waku-Kouomou D, Esona MD, 2017. Pre-vaccine circulating group a rotavirus strains in under 5 years children with acute diarrhea during 1999–2013 in Cameroon. Virology 1 10.15761/VRR.1000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyaga MM, Tan Y, Seheri ML, Halpin RA, Akopov A, Stucker KM, Fedorova NB, Shrivastava S, Duncan Steele A, Mwenda JM, Pickett BE, Das SR, Jeffrey Mphahlele M, 2018. Whole-genome sequencing and analyses identify high genetic heterogeneity, diversity and endemicity of rotavirus genotype P[6] strains circulating in Africa. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 63, 79–88. 10.1016/j.meegid.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska T, Vesikari T, 1990. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand. J. Infect. Dis 22, 259–267. 10.3109/00365549009027046 [DOI] [PubMed] [Google Scholar]
- Santos N, Hoshino Y, 2005. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol 15, 29–56. 10.1002/rmv.448 [DOI] [PubMed] [Google Scholar]
- Seheri M, Nemarude L, Peenze I, Netshifhefhe L, Nyaga MM, Ngobeni HG, Maphalala G, Maake LL, Steele AD, Mwenda JM, Mphahlele JM, 2014. Update of rotavirus strains circulating in Africa from 2007 through 2011. Pediatr. Infect. Dis. J 33 Suppl 1, S76–84. 10.1097/INF.0000000000000053 [DOI] [PubMed] [Google Scholar]
- Sindhu KN, Babji S, Ganesan S, 2017. Impact of rotavirus vaccines in low and middle–income countries. Curr. Opin. Infect. Dis 30, 473–481. 10.1097/QCO.0000000000000397 [DOI] [PubMed] [Google Scholar]
- Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N, 2012. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst. Rev 11, CD008521 10.1002/14651858.CD008521.pub3 [DOI] [PubMed] [Google Scholar]
- Stupka JA, Degiuseppe JI, Parra GI, 2012. Increased frequency of rotavirus G3P[8] and G12P[8] in Argentina during 2008–2009: Whole-genome characterization of emerging G12P[8] strains. J. Clin. Virol 54, 162–167. 10.1016/j.jcv.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S, 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol 30, 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Urasawa T, Kobayashi N, Ahmed MU, Adachi N, Chiba S, Urasawa S, 1991. Antibody response to serotype-specific and cross-reactive neutralization epitopes on VP4 and VP7 after rotavirus infection or vaccination. J. Clin. Microbiol 29, 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theamboonlers A, Maiklang O, Thongmee T, Chieochansin T, Vuthitanachot V, Poovorawan Y, 2013. Complete genome analysis of a rare human G3P[9] rotavirus posing as an AU-1 like strain. SpringerPlus 2, 569 10.1186/2193-1801-2-569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa T, Hoshino Y, 2008. Whole genome sequence and phylogenetic analyses reveal human rotavirus G3P[3] strains Ro1845 and HCR3A are examples of direct virion transmission of canine/feline rotaviruses to humans. Virology 380, 344–353. 10.1016/j.virol.2008.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umair M, Abbasi BH, Sharif S, Alam MM, Rana MS, Mujtaba G, Arshad Y, Fatmi MQ, Zaidi SZ, 2018. High prevalence of G3 rotavirus in hospitalized children in Rawalpindi, Pakistan during 2014. PloS One 13, e0195947 10.1371/journal.pone.0195947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usonis V, Ivaskeviciene I, Desselberger U, Rodrigo C, 2012. The unpredictable diversity of co-circulating rotavirus types in Europe and the possible impact of universal mass vaccination programmes on rotavirus genotype incidence. Vaccine 30, 4596–4605. 10.1016/j.vaccine.2012.04.097 [DOI] [PubMed] [Google Scholar]
- Utsumi T, Wahyuni RM, Doan YH, Dinana Z, Soegijanto S, Fujii Y, Juniastuti, null, Yamani LN, Matsui C, Deng L, Abe T, Soetjipto, null, Lusida MI, Ishii K, Shimizu H, Katayama K, Shoji I, 2018. Equine-like G3 rotavirus strains as predominant strains among children in Indonesia in 2015–2016. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 61, 224–228. [DOI] [PubMed] [Google Scholar]
- Zeller M, Patton JT, Heylen E, De Coster S, Ciarlet M, Van Ranst M, Matthijnssens J, 2012. Genetic Analyses Reveal Differences in the VP7 and VP4 Antigenic Epitopes between Human Rotaviruses Circulating in Belgium and Rotaviruses in Rotarix and RotaTeq. J. Clin. Microbiol 50, 966–976. 10.1128/JCM.05590-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang Y-H, Ghosh S, Tang W-F, Pang B-B, Liu M-Q, Peng J-S, Zhou D-J, Kobayashi N, 2015. Genomic characterization of G3P[6], G4P[6] and G4P[8] human rotaviruses from Wuhan, China: Evidence for interspecies transmission and reassortment events. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 33, 55–71. 10.1016/j.meegid.2015.04.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table. Nucleotide sequence identity matrix of the Lebanese G3P[6]/P[9] RVA and representative strains