Abstract
Background
Peripheral blood inflammation factor neutrophil-lymphocyte ratio (NLR), platelet count (PLT) and nutritional factor serum albumin (ALB) have been proposed as prognostic markers of head and neck squamous carcinoma cancer (HNSCC) in recent years. In the current study, nomogram predict models based on pre-treatment hematological parameters and a modified risk-stratified score system have been built.
Methods
A total of 197 patients with oropharyngeal, hypopharyngeal and laryngeal cancers receiving multimodality treatment between 2012 and 2014 were included. The pre-treatment ALB, neutrophil, lymphocyte and platelet count (PLT) were detected. Cancer-specific survival and locoregional recurrence (LRC) by 5 years’ follow-up in the cases were obtained. To integrate clinical characteristics, we propose a modified risk-stratified score system. Kaplan–Meier method, proportional hazards COX model, logistic models were used to establish nomograms within external validation.
Results
Five-year LRC was decreased (p=0.004) for 140 patients with pre-treatment NLR <2.77. Five-year LRC and 5-year cancer-specific survival were decreased (p=0.031, p=0.021) with pre-treatment PLT ≥248×109/L. Comparison of univariate parametric models demonstrated that pre-treatment NLR evaluation and PLT>248×109/L were better among tested models. On Bayesian information criteria (BIC) analysis, the optimal prognostic model was then used to develop nomograms predicting 3- and 5-year LRC. The external validation of this predictive model was confirmed in 57 patients from another hospital.
Conclusion
Pre-treatment NLR elevation and PLT>248×109/L are promising predictors of prognosis in patients with operable HNSCC. Nomograms based on the pre-treatment hematological markers and modified risk-stratified score system provide distinct risk stratifications. There results provided the feasibility of anti-inflammatory and antiplatelet treatments for HNSCC patients.
Keywords: head and neck squamous cell carcinoma, neutrophil-lymphocyte ratio, platelet count, albumin, nomogram
Background
Head and neck squamous cell cancer (HNSCC) are diagnosed in approximately 500,000 people per year worldwide.1 The treatment for locally advanced head and neck cancers is multimodal, with either definitive chemoradiation or surgery followed by adjuvant chemoradiation based on pathological features.2–4 Unfortunately, about 60% of HNSCCs are already moderately advanced (regional stage) or metastatic at the time of diagnosis.5 The 5-year survival rates for oropharyngeal cancer, laryngeal cancer and hypopharyngeal cancer are 65%, 61% and 26%, respectively.6,7 The major causes of HNSCC-related deaths are the locoregional recurrence (LRC) and distant metastasis.8–10 Considering distinct patterns of local control and survival of HNSCC, it is urgent to identify effective indicators for the prognosis prediction.
Based on solid evidence from numerous observational studies, clinical parameters (Gender, AJCC TNM Staging, smoking status, age, and sub-site of tumor) have comparatively limited cancers predictive utility for HNSCC.11 Pre-treatment prognostic indicators of therapy response and subsequent survival are of increasing importance.12,13 The process of inflammatory is precancerous phase of HNSCC. Cigarette is well-known established risk factor for the development of HNSCC originating from the oral cavity, pharynx, and larynx with their chronic inflammatory effect. Multiple inflammatory markers are emerging as surrogate biomarkers.14,15 Inflammation may contribute to tumor growth, progression and metastasis through inflammatory mediators and cytokine up-regulation, and immune regulatory cytokines aberrant activation.16 Therefore, inflammatory is extraordinarily essential in HNSCC carcinogenic process.
In fact, multiple inflammatory markers have been long since emerging as surrogate biomarkers and as prognostic indicators for patients with oral cavity/oropharyngeal carcinoma and other solid cancers,14,15 such as neutrophil, lymphocyte, monocyte, platelet counts, either as individual values or ratios to each other.17–20 As it is well known, platelet count (PLT) is critical in coagulation and inflammation. Moreover, activated platelets are linked to increased cancer risk through various mechanisms.21 NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. NLR has been proven to predict better than individual parameters and other ratios. It is increasingly recognized as an important role in carcinogenesis and tumor progression.22–24
Besides inflammatory parameters, various indicators containing nutritional variables were previously introduced to predict the surgical risks and prognosis for head and neck cancer as well as the esophagus cancer patients.25 Serum albumin (ALB), the most acceptable marker of nutrition, has been used to predict survival among patients with cancer who received chemotherapy or radiotherapy. Combination nutritional with inflammatory variables is used to assess immune-nutritional status, such as serum ALB concentration and lymphocyte count. The prognostic value is higher than that of either index alone.20
Despite the recognized contribution of hematologic, inflammatory and nutritional parameters,7,17,26 several crucial puzzles were remained to be unsolved: so far, there was limited data in hypopharyngeal and laryngeal cancer. Besides, most research focused on patients with inoperative head and neck cancer or patients received definitive chemoradiation therapy.14,18,20,26 What’ more, it is still unclear whether a single indicator has the highest predictive value, or whether a combination of multiple indicators has better performance.
In current study, we evaluated the potential associations between NLR, ALB, PLT and oncologic outcomes in HNSCC including hypopharynx and larynx patients receiving surgical treatments, based on database from two large medical centers. We aimed to
- Risk-stratify patient using the pre-treatment clinical characteristics data and oncologic outcomes, then determine the additive value of NLR, ALB, platelet as potential covariates of LRC.
- Develop a visualized reliable prediction model for LRC.
- Be supportive for the hypothesis, that is, combination of anti-inflammatory and anti-platelet treatment and nutrition management could modify the recurrence risk of HNSCC patients.
Materials and Methods
Setting, Design, and Data Sources
This study was in accordance with Declaration of Helsinki and approved by the Research Ethic Committees of The Affiliated Sir Run Run Shaw Hospital, Zhejiang University medical college and The Second Affiliated Hospital of Nanchang University Medical College. Both the institutional review board approved data extraction from an existing HNSCC.
Series treated with radical resection and adjuvant chemoradiation between 2012 and 2014 were selected for analysis, and personal and therapeutic demographics were tabulated. The detailed inclusion criteria were listed as follows: (a) primary cancers of the oropharynx, hypopharynx, and larynx; (b) patients receiving radical surgical ablation with/without reconstruction (elective or therapeutic neck dissection as indicated); (c) patient informed consent signed.
Dahlstrom-Sturgis risk schema (post-1994 part) was modified for stratifying patients into four risk groups.18 The survival stratification was established based on recursive partitioning analysis and had been proven to be much better than traditional TNM staging.
Matched data were extracted via a custom script from the medical records of Sir Run Run Shaw hospital, Zhejiang University Medical College and The Second Affiliated Hospital of Nanchang University Medical College. All pre-treatment platelet, neutrophil, lymphocyte counts, hemoglobin and ALB were extracted. Cases were excluded from the study, if evidences listed as follows in medical records (a) hemorrhage; (b) massive transfusions; (c) cardiopulmonary resuscitation. Patients that received induction chemotherapy were also excluded.
Measures
Ordinal and nominal data were performed using χ-square analysis for proportional agreement between cohorts. Continuous predictors were modelled as a linear association in a regression mode. Locoregional control (coding recurrent or persistent disease in the primary tumor field or cervical lymph node as an event. Data for patients without disease progression or who were lost to follow-up were censored at the time of last tumor imaging for estimation of progression-free survival) and cancer-specific survival (coding death as an event, data for patients who were alive or lost to follow-up were censored at the time of last contact for estimation of overall survival) were calculated using the Kaplan–Meier method for specified hematologic covariates, log-rank for differences in actuarial data.
Statistical Analysis
Univariate and multivariate Cox proportional hazards assessments were performed to determine whether the following variables were correlated with LRC and Cancer-specific surviving: age, sex, AJCC TNM Staging, smoking status, age, tumor sub-site, pre-treatment NLR and platelet evaluation (as a binary variables and continuous variables). In initial assessment, continuous predictors were dichotomized (categorization or a certain cut-off). The second simplified multivariate model specifying dichotomized binary parameters was constructed for the following variables: risk criteria cohorts (as four categorizations), PLT (as continuous variables), NLR (as a cut-off), ALB (as continuous variables).
Furthermore, to evaluate the optimal cut-off value of the platelet, NLR and ALB, ROC (receiver operating characteristic) curves were generated and the area under ROC curve (AUC) was measured and compared. NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count.
After multivariate analysis, best categorical model selected based on experimental Bayesian information (BIC) for model optimization and selection were converted into visual nomograms of 3- and 5-year LRC using the open-source statistical software R (http://www.R-project.org) with the aid of Harrell’s Regression Modeling Strategies package (http://biostat.mc.vanderbilt.edu/rms).
Results
Patients, Clinical Characteristics and Risk Criteria Cohorts
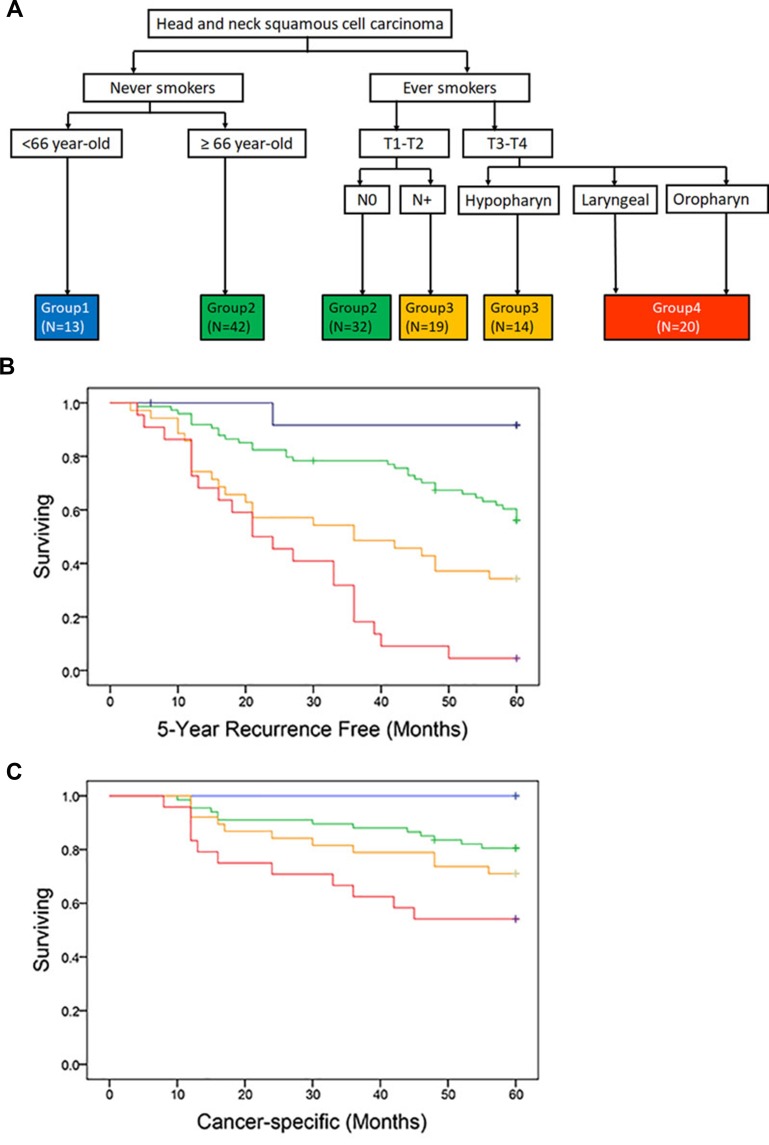
Of the 197 patients included in this study, 140 cases were used for validity of the prediction model, while 57 cases from another hospital for external calibration. All patients had surgical resection of a primary HNSCC lesion and individualized neck dissection, followed by adjuvant radiotherapy/chemoradiotherapy. The median follow-up time was 51.7±0.34 months. Clinical parameters and treatment for the entire HNSCC cohort are summarized separately in Table 1 and Supplementary Table 1. Risk criteria cohorts are illustratively categorized into four groups (Figure 1A) and presented with 5-year LRC and 5-year cancer-specific survival in Figure 1B and C. The difference in the risk was statistically significant between all groups, of which the p-value is 0.000,0.005, 0.008 by Log-Rank by Log-Rank (Mantel-Cox). Patients in group 1 has better prognosis compared with the others. Those in Group 2 had one time the risk of 5-year LRC (HR,1.1; 95% CI,0.9–10.3), those in group 3 had almost 3 times the risk of 5-year LRC (HR,2.7; 95% CI,1.7–13.2), and those in group 4 had 7 times (HR,6.9; 95% CI,5.9–21.2).
Table 1.
Patient, Clinical Characteristics and Treatments
| Characteristic | No. of Patients (%) |
|---|---|
| Sex | |
| Female | 10(7.1) |
| Male | 130(92.9) |
| Age | |
| Median (range) | 62.15 |
| Clinical T stage | |
| T1 | 64(45.7) |
| T2 | 20(14.3) |
| T3 | 16(11.4) |
| T4 | 40(28.6) |
| Clinical N stage | |
| N0 | 75(53.6) |
| N1 | 30(21.4) |
| N2a | 2(1.4) |
| N2b | 21(15.0) |
| N2c | 12(8.6) |
| Smoking history at diagnosis | |
| Never | 55(39.3) |
| Smoker (current/former) | 85(60.7) |
| Disease site | |
| Hypopharyngeal | 34(24.2) |
| Laryngeal | 95(67.9) |
| Oropharyngeal | 11(7.9) |
| Surgical procedure | |
| Without reconstruction | 122(87.1) |
| With reconstruction | 18(12.9) |
| Concomitant therapy | |
| None | 90(64.3) |
| Chemoradiation | 32(22.9) |
| Radiation | 18(12.8) |
| 3-year locoregional recurrence | |
| No | 92(65.7) |
| Yes | 48(34.2) |
| 5-year locoregional recurrence | |
| No | 67(47.9) |
| Yes | 73(52.1) |
| 5-year cancer-specific survival | |
| Survive | 105(75.0) |
| Death | 35(25.0) |
Figure 1.
Risk criteria cohorts (A), with 5-year LRC free (B), and 5-year cancer-specific survival (C), stratified by said cohorts. Solid lines represent Kaplan–Meier curves and short vertical lines represent censored data.
Abbreviation: LRC, locoregional recurrence.
Cut-Off Value of NLR and PLT
By using ROC curve analysis, the optimal cut-off value of continue NLR and PLT for LRC was determined. For 5-year LRC, the area under the ROC curves for NLR was 0.69 (95% CI, 0.60–0.77), and 0.56 (95% CI, 0.46–0.66) for PLT. ROC plots revealed that NLR was a superior prognosis marker to PLT and ALB as measured by AUC (Supplementary Figure S1). An NLR cut-off value of 2.77 showed a sensitivity of 67.1% and a specificity of 65.7% for LRC. It was associated with the strongest Youden index (sensitivity 1+ specificity –1) (Supplementary Figure S2). A platelet cut-off value of 248×109 /L showed a sensitivity of 30.1% and a specificity of 96.6% for LRC (Supplementary Figure S3). The ROC curve as well as the cut-off value are obtained from the online-tool easyROC (Goksuluk D, Korkmaz S, Zararsiz G, Karaağaoğlu AE (2016). easyROC: An Interactive Web-tool for ROC Curve Analysis Using R Language Environment. The R Journal, 8(2):213–230).
The Correlation of NLR and PLT with the Prognostic Parameters
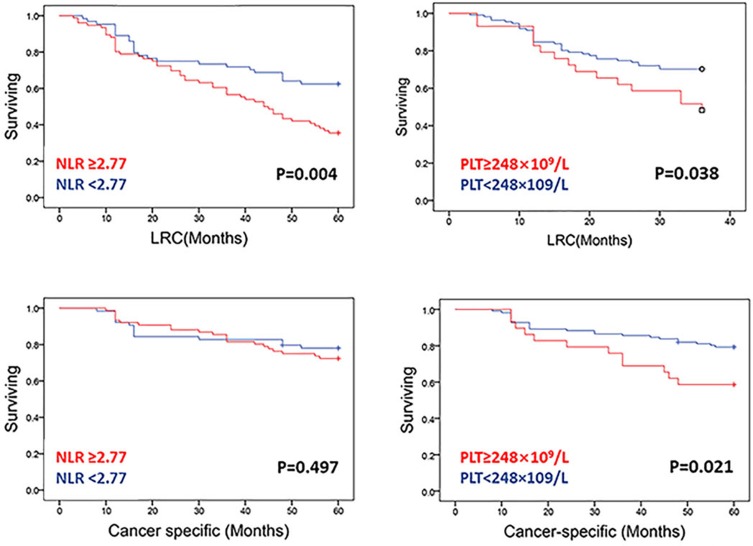
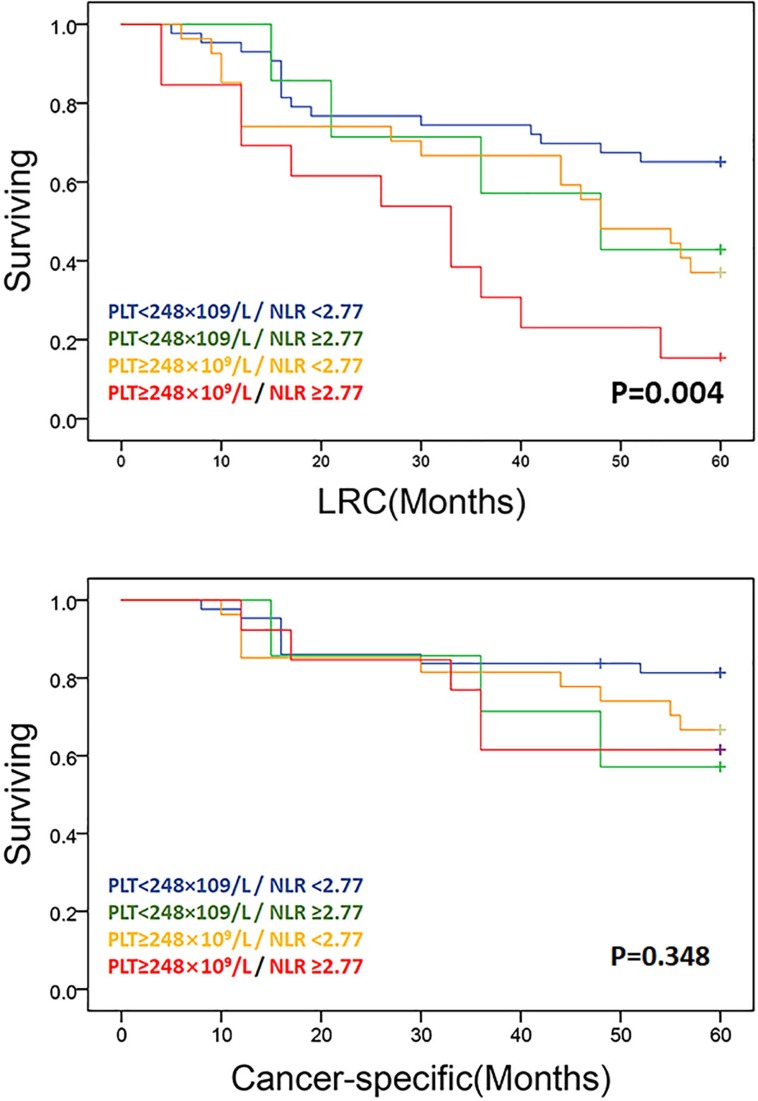
Log-rank testing of actuarial curves demonstrated that 5-year LRC (p=0.004) was significantly decreased for patients with NLR ≥2.77. However, there was no statistical difference between cancer-specific surviving and NLR ≥2.77. Likewise, patients with PLT ≥248×109 /L exhibited comparatively decreased 5-year LRC (p=0.038) and OS (p=0.021) (Figure 2). Patients with concurrent PLT ≥248×109 /L and NLR ≥2.77 had substantively worse oncologic outcomes for 5-year LRC (p=0.004) than those with NLR ≥2.77 or PLT ≥248×109 /L alone or those with NLR <2.77 and PLT <248×109 /L(Figure 3, above). However, there was no statistical difference between cancer-specific survival (Figure 3, below).
Figure 2.
LRC and cancer-specific survival in patients with PLT<248×109/L versus patients with PLT >248×109/L and in patients with NLR <2.77 versus patients with NLR >2.77. Solid lines represent Kaplan–Meier curves and short vertical lines represent censored data.
Abbreviations: PLT, platelets; NLR, neutrophil–lymphocyte ratio; LRC, locoregional recurrence.
Figure 3.
LRC free and cancer-specific survival in composite NLR-platelet cohorts: pre-treatment PLT<248×109/L/ NLR <2.77, PLT<248×109/L/ NLR ≥2.77, PLT≥248×109/L/ NLR <2.77, PLT≥248×109/L/ NLR ≥2.77. Solid lines represent Kaplan–Meier curves and short vertical lines represent censored data.
Prognostic Nomogram for 3-Year LRC and 5-Year LRC
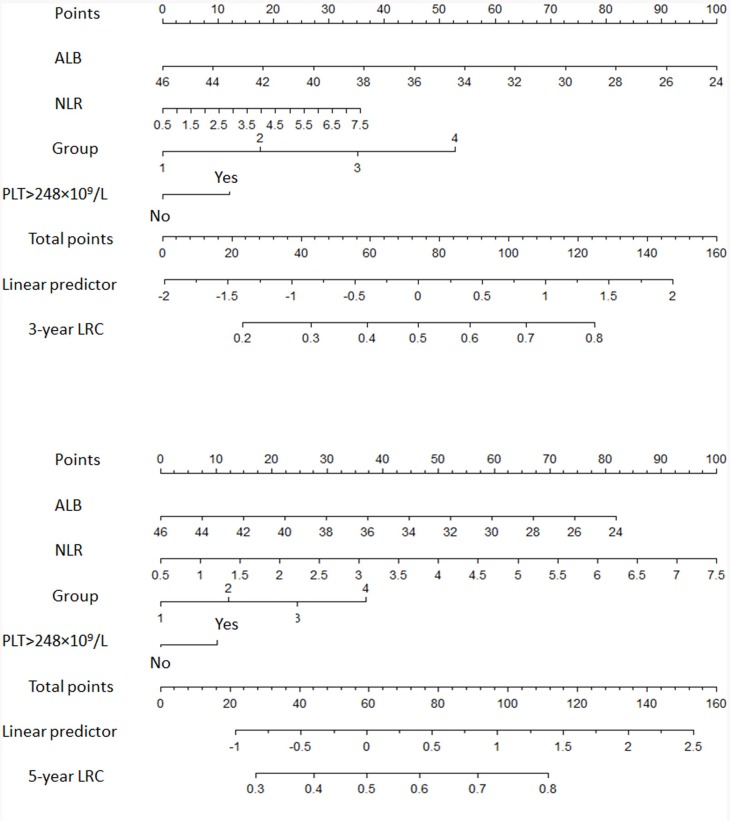
Table 2 shows the hazard ratios (HRs) for all studied variables in the univariate Cox regression analysis. Multivariate analysis showed that NLR (HR 2.16; 95% CI 1.30–3.5; p=0.003) and PLT ≥248×109 /L (HR 2.16; 95% CI 1.30–3.5; p=0.003) were independent predictor of poor prognosis in addition to ALB value and risk criteria cohorts. Validation plots for assessing the predictive ability of model through split-sample internal validation (data not shown) and external validation (data from The Second Affiliated Hospital of Nanchang University Medical College). C-statistic values were incredible excellent 0.95 (Supplementary Figure S4, Supplementary Figure S5). Figure 4 shows 3-year and 5-year LRC nomograms based on the lowest BIC model (Supplementary Table 2). PLT≥248×109 /L and NLR evaluation as potential covariate of LRC for pre-treatment risk stratifications. What is needed to be paid attention is that, on post hoc Bayesian model selection, a resultant univariate BIC was inferior to presented for the current model within continues NLR evaluation.
Table 2.
Univariate Analysis
| Covariate | Strata | Hazard Ratio | 95% CI | P value |
|---|---|---|---|---|
| Age | Continues | 1.0 | 0.9–1.0 | 0.124 |
| Age (binary) | <66 | – | – | – |
| ≥66 | 1.0 | 0.7–1.7 | 0.870 | |
| Disease site | Laryngeal | |||
| Oropharyngeal | 1.2 | 0.5–2.8 | 0.64 | |
| Hypopharyngeal | 1.5 | 0.9–2.5 | 0.11 | |
| Surgery procedure | Without reconstruction | – | – | – |
| With reconstruction | 0.7 | 0.1–1.3 | 0.017 | |
| Smoking | Never | – | – | – |
| Smoker | 1.5 | 0.9–2.1 | 0.01 | |
| Concomitant therapy | None | – | – | – |
| Radiation | 0.1 | −0.4–0.6 | 0.690 | |
| Chemoradiation | 1.3 | 0.8–2.2 | 0.307 | |
| Clinical T stage | T1 | – | – | – |
| T2 | 1.5 | 0.9–2.4 | 0.001 | |
| T3 | 1.9 | 1.4–2.6 | 0.001 | |
| T4 | 1.4 | 0.8–2.2 | 0.001 | |
| Clinical N stage | N0 | – | – | – |
| N1 | 0.4 | 0.2–0.9 | 0.18 | |
| N2a | 1.2 | 0.6–2.3 | 0.01 | |
| N2b | 0.2 | 0.2–0.9 | 0.48 | |
| N2c | 0.4 | 0.5–1.3 | 0.35 | |
| NLR | Continues | 2.2 | 1.3–3.6 | 0.003 |
| NLR (binary) | <2.77 | – | – | 0.001 |
| ≥2.77 | 1.1 | 1.0–1.1 | ||
| PLT | Continues | 1.0 | 0.9–1.0 | 0.245 |
| PLT (binary) | <248×109 | – | – | 0.011 |
| ≥248×109 | 1.9 | 1.2–3.1 | ||
| Risk criteria | Group 1 | – | – | |
| Group 2 | 1.1 | 0.9–10.3 | 0.001 | |
| Group 3 | 2.7 | 1.7–13.2 | 0.001 | |
| Group 4 | 6.9 | 5.1–21.2 | 0.001 | |
| ALB | Continues | 0.96 | 0.92–0.99 | 0.038 |
Note: P value <0.05 are in bold type.
Figure 4.
Nomograms for LRC. A vertical line is drawn from each of the covariates to the “Points” line. The sum of the points from all covariates determines the “Total Points” score. Another vertical line is drawn from the “Total Points” line to the bottommost line to give the probability of individual HNSCC patient 3-year, and 5-year LRC.
Discussion
Recently varieties of evidence suggested proinflammatory mediators associated with the different stages of HNSCC carcinogenesis and contribute to HNSCCs proliferation, neo-angiogenesis, metastasis, and resistance to cancer therapy, regardless of HNSCC subsite.12,27,28 The important role of unresolved inflammation in HNSCC progression and metastasis is well established in previous studies.12,17,27,28
Firstly, elevated blood neutrophil counts in an inflammatory microenvironment have been shown to contribute to tumor angiogenesis. It induces the resistance to anti-vascular endothelial growth factor (anti-VEGF) therapy.20 Secondly, the decreased lymphocyte count plays important roles in inflammatory reaction to tumor.8 This can explain why increased NLR acts as an independent prognostic factor. There were evidence that showed an early diminution in NLR may be associated with more favorable outcomes and higher response rates, whereas an increase in NLR in the first weeks of treatment had the opposite effect.8 Thirdly, platelets direct tumor cell growth, vascular invasion, hematogenous dissemination, immune system evasion and establishment of a metastatic niche. Fourthly, nutrition parameters must be taken into consideration. Many head and neck cancer patients have difficulties in oral food intake due to dysphagia and/or aspiration.25,29 Ablative surgery or radiation-induced mucositis may cause iatrogenic exacerbation of these problems.20
The biochemistry research in this filed is strongly supportive for a laboratory-clinical transition; meanwhile, the reliable clinical data well statically categorified are urgently needed. That’s why we aim to establish prediction model combing NLR, PLT and ALB for operable HNSCC patients with external validation. In this study, an elevated pre-treatment NLR was associated with a shorter LRC but not with disease survival. Different from Sara et al and Yoshie et al reported data18,20 which were anatomically consistent (only oropharyngeal cancers) and homogenous therapeutic regimens (chemotherapy/Radiation/radiochemotherapy),11 our dataset is comprised of oropharyngeal, laryngeal and hypopharyngeal with surgical and concomitant systemic therapy.
Modifying the post-1994 Dahlstrom-Sturgis risk schema, we established a risk criterion score system for integrating novel clinical characteristics, which including primary tumor site (oropharyngeal, hypopharynx, and larynx), age, cigarette history, clinical T stage and N stage. At later model developing phase, we categorized them into four groups. Pre-treatment NLR and PLT in addition to ALB and Risk categories led to the development of nomogram with superior performance.
The results from current study demonstrated that HNSCC patients could be benefited from low NLR value and platelet count. The combination of baseline PLT with NLR value emerged as a promising biomarker for noninvasive detection of cancer surveillance. Aspirin has been demonstrated to have a significant effect protecting against cancer through diverse mechanisms such as blocking the formation of metastatic intravascular.21,30 Antiplatelet agents that also impact inflammatory pathways would be more intensively recommended, such as purinergic antagonists.31 The mechanisms by which platelets participate in inflammation are diverse and offer numerous opportunities for future drug intervention.32 Combination aspirin with other non-steroidal anti-inflammatory drugs (NSAIDs) has been associated with reduced risk of breast cancer, endometrial cancer and cholangiocarcinoma.33–36 Furthermore, nutritional screening should be carried out for each HNSCC patient. Peri-operative individualized balanced nutrition plans should be made accordingly.
Our data have several limitations. In order to meet “standard multimodality protocols”, cases receiving surgery and adjuvant therapy (chemoradiation and radiation) were included. To avoid the impact of tumor pathological heterogeneity on inflammatory measures,36,37 this model is specific for head and neck cancer patients pathologically diagnosed with squamous cell carcinoma. Reasonably, patients using thrombolytic agents (like aspirin), NSAIDs could not be included. Consequently, the study has a small sample size of 198 patients. Finally, long-term follow-up of the patients was not available.
Nonetheless, the prediction model represents, to our knowledge, is the double-center series ever to evaluate the relationship between pre-treatment NLR, PLT, ALB and oncologic outcomes in mix-subsite HNSCC patients receiving radical surgical ablation and chemoradiation. It serves as a benchmark for outcome estimation, as well as a steppingstone to further efforts. The analyses performed show pre-treatment hematological inflammatory parameters provide distinct risk stratification indicators for HNSCC patients. Continuing efforts will be made to accelerate the laboratory-clinical transition by assessing comprehensive systemic inflammatory factors and optimizing the survivorship program.
Conclusion
Pre-treatment NLR elevation, PLT >248×109/L and ALB are promising predictors of prognosis in patients with operable HNSCC. The analyses performed show that nomograms including pre-treatment hematological parameters provide distinct risk stratifications. The results provided the feasibility of anti-inflammatory, antiplatelet treatments and nutrition management in modifying recurrence risk of HNSCC patients.
Funding Statement
This study is sponsored by grants from Medical Health Science and Technology Project of Hangzhou municipal Health Commission (Grant No. OO20190775). Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant No.2019336033 and Grant No. 2020367813). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Abbreviations
AUC, area under ROC curve; BIC, Bayesian information criteria; HNSCC, head and neck squamous carcinoma cell cancer; LRC, locoregional recurrence; NLR, neutrophil-lymphocyte ratio; 4NQO, 4-nitroquinoline-1 oxide; PLT, platelets.
Ethical Approval and Consent to Participate
This study was in accordance with Declaration of Helsinki and approved by the Research Ethic Committees of The Affiliated Sir Run Run Shaw Hospital, Zhejiang University medical college and The Second Affiliated Hospital of Nanchang University Medical College. Both the institutional review board approved data extraction from an existing HNSCC.
Data Sharing Statement
Materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Kim HS, Kwon HJ, Jung I, et al. Phase II clinical and exploratory biomarker study of dacomitinib in patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Clin Cancer Res. 2015;21(3):544–552. doi: 10.1158/1078-0432.CCR-14-1756 [DOI] [PubMed] [Google Scholar]
- 3.Rambeau A, Licaj I, Gery B, et al. Platinum rechallenge in recurrent head and neck squamous cell carcinoma after primary chemoradiation. Eur Ann Otorhinolaryngol Head Neck Dis. 2019. doi: 10.1016/j.anorl.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 4.Tinhofer I, Stenzinger A, Eder T, et al. Targeted next-generation sequencing identifies molecular subgroups in squamous cell carcinoma of the head and neck with distinct outcome after concurrent chemoradiation. Ann Oncol. 2016;27(12):2262–2268. doi: 10.1093/annonc/mdw426 [DOI] [PubMed] [Google Scholar]
- 5.Cohen EEW, Cohen E, Soulières D, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, Phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 6.Faraji F, Eisele DW, Fakhry C. Emerging insights into recurrent and metastatic human papillomavirus-related oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2017;2(1):10–18. doi: 10.1002/lio2.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marur S, Forastiere AA. Head and neck squamous cell carcinoma update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386–396. [DOI] [PubMed] [Google Scholar]
- 8.Ding D, Stokes W, Eguchi M, et al. Association between lymph node ratio and recurrence and survival outcomes in patients with oral cavity cancer. JAMA Otolaryngol Head Neck Surg. 2019;145(1):53. doi: 10.1001/jamaoto.2018.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian K, Di L, Guo K, Zheng X, Ji Q, Wang Z. Cervical lymph node metastatic status and adjuvant therapy predict the prognosis of salivary duct carcinoma. J Oral Maxil Surg. 2018;76(7):1578–1586. doi: 10.1016/j.joms.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 10.Pilborough AE, Lambert DW, Khurram SA. Extranodal extension in oral cancer: a role for the nodal microenvironment? J Oral Pathol Med. 2019. doi: 10.1111/jop.12870 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Bai S, Carroll W, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7(3):211–223. doi: 10.1007/s12105-012-0412-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonomi M, Patsias A, Posner M, Sikora A. The role of inflammation in head and neck cancer. Adv Exp Med Biol. 2014;816:107–127. [DOI] [PubMed] [Google Scholar]
- 13.Bojaxhiu B, Templeton AJ, Elicin O, et al. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol. 2018;13(1). doi: 10.1186/s13014-018-1159-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St JM. Inflammatory mediators drive metastasis and drug resistance in head and neck squamous cell carcinoma. Laryngoscope. 2015;125(Suppl 3):S1–S11. doi: 10.1002/lary.24998 [DOI] [PubMed] [Google Scholar]
- 15.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- 16.Joisten N, Rademacher A, Bloch W, et al. Influence of different rehabilitative aerobic exercise programs on (anti-) inflammatory immune signalling, cognitive and functional capacity in persons with MS - study protocol of a randomized controlled trial. BMC Neurol. 2019;19(1):37. doi: 10.1186/s12883-019-1267-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diao P, Wu Y, He H, et al. Preoperative circulating platelet, neutrophil, and lymphocyte counts predict survival in oral cancer. Oral Dis. 2019;25:1057–1066. [DOI] [PubMed] [Google Scholar]
- 18.Shoultz-Henley S, Garden AS, Mohamed ASR, et al. Prognostic value of pretherapy platelet elevation in oropharyngeal cancer patients treated with chemoradiation. Int J Cancer. 2016;138(5):1290–1297. doi: 10.1002/ijc.29870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lordan R, Tsoupras A, Zabetakis I. The potential role of dietary platelet-activating factor inhibitors in cancer prevention and treatment. Adv Nutr. 2019;10(1):148–164. doi: 10.1093/advances/nmy090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234(2):1794–1802. doi: 10.1002/jcp.27052 [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Wei Y, Zhang R, et al. Elevated platelet count appears to be causally associated with increased risk of lung cancer: a mendelian randomization analysis. Cancer Epidemiol Biomarkers Prev. 2019;28(5):935–942. doi: 10.1158/1055-9965.EPI-18-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ari A, Gunver F. Comparison of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with thyroiditis and papillary tumors. J Int Med Res. 2019;47(5):2077–2083. doi: 10.1177/0300060519838392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inamoto S, Kawada K, Okamura R, Hida K, Sakai Y. Prognostic impact of the combination of neutrophil-to-lymphocyte ratio and Glasgow prognostic score in colorectal cancer: a retrospective cohort study. Int J Colorectal Dis. 2019;34:1303–1315. doi: 10.1007/s00384-019-03316-z [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Zhao G, Chen F, Xia J, Jiang L. The prognostic significance of the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio in giant cell tumor of the extremities. BMC Cancer. 2019;19(1):329. doi: 10.1186/s12885-019-5511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Wang X, Lai Y, Zhou K, Tang Y, Che G. The prognostic value of Pre-treatment prognostic nutritional index in esophageal squamous cell carcinoma: a meta-analysis. Medicine (Baltimore). 2019;98(22):e15280. doi: 10.1097/MD.0000000000015280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano Y, Kogashiwa Y, Araki R, et al. Correlation of inflammatory markers, survival, and COX2 expression in oral cancer and implications for prognosis. Otolaryngol Head Neck Surg. 2018; 158:667–676. [DOI] [PubMed] [Google Scholar]
- 27.Dong GW, DO NY, Lim SC. Relation between proinflammatory mediators and epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Exp Ther Med. 2010;1(5):885–891. doi: 10.3892/etm.2010.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4(1):66–83. doi: 10.7150/jca.5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa MD, Vieira DMC, Amorim AC, Cipriano TDO, Dos SA. Association between nutritional status, inflammatory condition, and prognostic indexes with postoperative complications and clinical outcome of patients with gastrointestinal neoplasia. Nutr Cancer. 2016;68(7):1108–1114. doi: 10.1080/01635581.2016.1206578 [DOI] [PubMed] [Google Scholar]
- 30.Ma B, Duan X, Zhou Q, et al. Use of aspirin in the prevention of colorectal cancer through TIGIT-CD155 pathway. J Cell Mol Med. 2019. doi: 10.1111/jcmm.14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitchford SC. Novel uses for anti-platelet agents as anti-inflammatory drugs. Br J Pharmacol. 2007;152(7):987–1002. doi: 10.1038/sj.bjp.0707364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):449–458. doi: 10.1160/TH14-12-1067 [DOI] [PubMed] [Google Scholar]
- 33.Lapumnuaypol K, Tiu A, Thongprayoon C, et al. Effects of aspirin and non-steroidal anti-inflammatory drugs on the risk of cholangiocarcinoma: a meta-analysis. QJM. 2019;112(6):421–427. doi: 10.1093/qjmed/hcz039 [DOI] [PubMed] [Google Scholar]
- 34.Webb PM, Na R, Weiderpass E, et al. Use of aspirin, other nonsteroidal anti-inflammatory drugs and acetaminophen and risk of endometrial cancer: the Epidemiology of Endometrial Cancer Consortium. Ann Oncol. 2019;30(2):310–316. doi: 10.1093/annonc/mdy541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandeya N, Olsen CM, Thompson BS, et al. Aspirin and nonsteroidal anti-inflammatory drug use and keratinocyte cancers: a large population-based cohort study of skin cancer in Australia. Br J Dermatol. 2019. doi: 10.1111/bjd.17938 [DOI] [PubMed] [Google Scholar]
- 36.Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daley SK, Witte MH, Washington J, et al. Role of lymphatic deficiency in the pathogenesis and progression of inflammatory bowel disease to colorectal cancer in an experimental mouse model. Inflamm Bowel Dis. 2019;25:1919–1926. doi: 10.1093/ibd/izz112 [DOI] [PMC free article] [PubMed] [Google Scholar]