Abstract
Objective
The American College of Radiology Imaging Network Trial 6667 showed that MRI can detect cancer in the contralateral breast that is missed by mammography and clinical examination at the time of the initial breast cancer diagnosis, based on 1-year follow-up. This study is a continuation of the trial that evaluates the diagnostic accuracy of MRI for contralateral breast cancer after 2 years of follow-up.
Methods
In total, 969 women with a diagnosis of unilateral breast cancer and no clinical or imaging abnormalities in the contralateral breast underwent breast MRI. The cancer status of all participants was monitored for 2 years after the initial MRI. Follow-up included documentation of any clinical, imaging, or interventional procedures performed. A study participant was considered positive for cancer if she had a tissue diagnosis of in situ or invasive breast cancer in the contralateral breast within 730 days of her initial MRI.
Results
Three additional cancers were diagnosed in the study population in the second year of the trial. The diagnostic yield for MRI for the 2-year period was 3% (31/969). After 2 years of follow-up, breast MRI has a sensitivity of 86% and specificity of 88% for detection of contralateral breast cancer. Its negative predictive value was 99%, and its positive predictive value was 22%. These values did not change significantly from the 1-year data.
Conclusion
A negative contralateral breast MRI has a very high and reliable negative predictive value over 2 years, and, therefore, is helpful in managing and counseling patients during the period of initial diagnosis and early treatment.
Keywords: contralateral breast cancer, breast MRI, ACRIN trial
Key Messages.
After 2 years of follow-up, breast MRI has a sensitivity of 86%, specificity of 88%, negative predictive value of 99%, and positive predictive value of 22% for detection of contralateral breast cancer, indicating no significant change from the first year data.
A negative contralateral breast MRI has a very high and reliable negative predictive value over 2 years, and, therefore, is helpful in managing and counseling patients during the period of initial diagnosis and early treatment.
Introduction
Women with a diagnosis of unilateral breast cancer carry a 2- to 6-fold increased risk of developing a second primary compared with the general population (1). Conventional diagnostic methods using mammography, sonography, and clinical breast exam (CBE) identify synchronous contralateral cancer in 1%–3% of patients (2–7). There is increasing evidence in the literature for the use of MRI to screen the contralateral breast in those with newly diagnosed cancer (8–12). However, few reports describe the long-term follow-up of these patients. A meta-analysis of 22 studies evaluating screening MRI of the contralateral breast reports an incremental cancer detection rate of 4.1%, but only 5 of the 22 studies confirmed the absence of cancer in those negative on MRI with 1-year clinical or imaging follow-up (13).
In 2007, Lehman et al published the results of American College of Radiology Imaging Network (ACRIN) Trial 6667, a prospective multi-institutional MRI screening trial of the contralateral breast in women with a recent diagnosis of breast cancer (14). The primary study sought to determine if MRI could improve on CBE and mammography in detecting contralateral breast cancer. During the first year after the initial breast cancer diagnosis, MRI detected mammographically and clinically occult contralateral breast cancer in 3.1% (30 of 969) of participants. The purpose of this paper is to report on the second-year follow-up of this cohort of women and to evaluate the diagnostic accuracy of the initial MRI among these high-risk patients at 2-year follow-up.
Methods
The trial was conducted by the ACRIN, funded by the National Cancer Institute, and monitored by a data and safety monitoring board. It was open to all interested sites that were approved by ACRIN through a general qualifying application and a protocol-specific application. Between 1 April 2003 and 10 June 2004, 1007 women with a recent diagnosis of unilateral breast cancer were enrolled at 25 sites, including seven private practices and 18 academic institutions (see the Appendix). Each institution obtained institutional review board approval before patient accrual and was compliant with the Health Insurance Portability and Accountability Act regulations. Informed consent was obtained from all patients.
Patients aged at least 18 years, of any ethnic background, with a recent history (within 60 days of the initial MRI) of biopsy-proven ductal carcinoma in situ (DCIS) or invasive cancers of the breast were eligible for inclusion. In addition, a negative or benign mammogram and a normal CBE of the contralateral breast were required within 90 days of the initial MRI. The following women were excluded: those with a current or recent history of chemotherapy or hormonal therapy for cancer, those with a previous biopsy in the study breast, those with a remote history of breast cancer in either breast, those with a history of an MRI exam of the study breast within 12 months, and those with new breast symptoms within the past 60 days that utilized MRI as part of the evaluation.
All participants underwent dynamic contrast-enhanced breast MRI. The standard criteria for each MRI study performed were as follows: a 1.5-T or stronger magnet, a dedicated breast surface coil, one sequence obtained before and two sequences obtained after the administration of contrast material, with 3-D, fat-suppressed T1 weighted, gradient-echo sequences (repetition time of less than 60 msec; echo time of less than 20 msec). Initial and delayed images were obtained within 4 and 8 minutes after the injection of contrast material. Spatial-resolution criteria included voxels smaller than 0.9 mm in the frequency-encoding direction, smaller than 1.8 mm in the phase encoding direction, and 3 mm or smaller in the slice direction, providing full coverage of the breast.
The cancer status of all participants was followed for 730 days after the study MRI. Follow-up included documentation of any breast procedures (CBE, imaging, or interventions) performed in the months following the initial MRI. For all procedures with results that were positive, additional follow-up for 6 months (in total, 30 months following enrollment) data were collected, including additional imaging and any available pathology results. The study participants were classified as positive for cancer if a contralateral breast cancer was histologically verified within 730 days after the initial MRI, and negative for cancer if the study records at the end of the 2-year follow-up period showed no diagnosis of contralateral cancer within that period. The participants who did not have any follow-up imaging nor CBE, and even those lost to follow-up, were considered negative if the ACRIN had not received confirmation of a diagnosis of cancer from participating institutions.
All examinations were interpreted according to the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) (15).The initial MRI assessment was classified on a six-point scale (0, “needs additional imaging evaluation”; 1, “negative”; 2, “benign”; 3, “probably benign”; 4, “suspicious abnormality”; and 5, “highly suggestive of malignancy”). For examinations scored as 0 or 3, additional imaging was performed to determine the final BI-RADS assessment. For receiver-operating-characteristic (ROC) curve analysis, readers rated the study MRI on a five-point malignancy scale (with a score of 1 indicating “definitely not malignant,” and a score of 5 indicating “definitely malignant”). A diagnosis of cancer was based on histological examination of a biopsy specimen and included all cases of invasive carcinoma or DCIS.
The Center for Statistical Sciences at Brown University (Providence, RI) analyzed the data for the study. The aim of the second-year follow-up study was to report the results of 2 years of clinical and imaging follow-up using a new reference standard: cancer status as reported anytime up to 2 years from initial contralateral MRI examination. The final BI-RADS MRI assessment score was used to derive estimates of the diagnostic yield, sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and associated positive biopsy rate. Participants with an initial score of 0 or 3 were assigned a final score that took into account the results of the workup after the initial MRI when performed. Not all women underwent recommended additional workup. For estimation of the diagnostic yield, sensitivity, and specificity, a final BI-RADS score of 1, 2, or 3 was considered to be negative, whereas a final score of 0, 4, or 5 was considered to be positive. The same classification of the test results was used to estimate the PPV of a final positive score. The PPV was defined as the percentage of positive examinations that resulted in a histological diagnosis of cancer within 2 years. Diagnostic test data from all sites were pooled for the analysis. Exact 95% confidence intervals (CIs) for the binary test measure (diagnostic yield, sensitivity, specificity, NPV, and PPV) were constructed.
Results
The ACRIN 6667 trial recruited 1007 women; 969 women comprised the final study group. Of the 1007 recruited women, 20 were deemed ineligible; 4 patients withdrew consent; and 14 did not undergo the study MRI. Subsequently, 77 patients had a contralateral mastectomy: 7 of those procedures were in patients with a diagnosis of contralateral breast cancer, and 70 were prophylactic. The remaining 892 participants were followed for 730 days, and most had a combination of conventional imaging (mammography and/or sonography), MRI, CBE, and intervention when indicated. Although MRI follow-up was not a requirement per protocol, 120 patients received an MRI examination as part of their follow-up during the second year. Eighteen patients had no imaging assessment recorded but were known to be alive. Two patients died. The cause of death in each case was not related to the initial diagnosis of breast cancer. No information was available on clinical or imaging follow-up for 107 patients by the end of the second year. Based on the study design, these participants were categorized as negative for contralateral breast cancer in the statistical analysis.
In total, the study discovered 36 contralateral breast cancers in 2 years, 33 within year 1 and 3 in year 2. Of the 36, 31 were associated with a positive MRI, and 5 with a negative MRI. Of the 5 false negative MRI, 3 were interpreted as BI-RADS 1, and 1 each was interpreted as BI-RADS 2 and 3. Of these 5 patients, 3 (2 with a BI-RADS 1 study and 1 with a BI-RADS 3 study) opted for a prophylactic mastectomy during year 1 of the trial with cancers identified in the mastectomy specimens. These 3 tumors were pure ductal carcinomas in situ and were 1, 3, and 4 mm in diameter, respectively (14). The 2 remaining patients were diagnosed with breast cancer during the second year of follow-up, as discussed in details below.
In the first cancer case, the patient carried no family history of breast cancer. She was diagnosed with DCIS in the index right breast after stereotactic biopsy of microcalcifications found on routine screening mammography. She was treated with a lumpectomy and radiation therapy. The initial MRI of the contralateral left breast was interpreted as BI-RADS 1 (negative). She returned for a routine follow-up mammogram 322 days later, which was assessed as BI-RADS 4 because of two new groups of microcalcifications, 1 cm in total area, in the contralateral left breast. Stereotactic needle biopsy showed 2 foci of DCIS. An MRI performed after the DCIS diagnosis in the contralateral left breast was assessed as BI-RADS 5 for 2 foci of enhancement separate from the biopsy-proven sites. A left simple mastectomy was performed confirming multiple foci of DCIS 452 days after the initial study MRI examination. The extent of DCIS was not reported by the pathologist. For statistical analysis, this case was categorized as true negative in year 1 and false negative in year 2.
The second cancer, a DCIS, was discovered 457 days after the initial study MRI, based on the pathology review of the tissue obtained during a mastopexy procedure. The size of the DCIS was not reported. The initial MRI was interpreted as BI-RADS 3. A short-interval follow-up MRI 220 days after the initial MRI was performed and was considered benign (BI-RADS 2). Additional MRI performed after the diagnosis of DCIS was nonrevealing, but was graded as BI-RADS 6 (ie, biopsy-proven malignancy).
A total of 3 cancers were diagnosed during the second year of follow-up. In addition to the two cases described above, a third cancer was discovered in a participant whose study MRI was interpreted as BI-RADS 4 (ie, suspicious). She underwent an MR-guided core needle biopsy. Biopsy results indicated sclerosing adenosis with clusters of microcalcifications, but no evidence of cancer. The pathologic and MRI results were deemed concordant, as sclerosing adenosis can have patterns of enhancement that cause false positive MRI interpretations. Routine follow-up was advised. One year later, the patient’s CBE was abnormal, and she was referred for imaging. Mammography and ultrasound findings were suspicious for malignancy. A second MRI was again interpreted as BI-RADS 4, with an abnormality identified at the same site as the previously biopsied finding. A second core biopsy revealed malignancy, and the patient underwent mastectomy. The mastectomy was performed 379 days from the study MRI. The pathology specimen showed invasive cancer with intraductal spread, measuring 60 mm in maximal dimension with the invasive and intraductal disease combined. For statistical analysis, this case was categorized as false positive in year 1 and true positive in year 2.
Of the 120 additional MRIs performed during the second year, 110 studies were interpreted as BI-RADS 1 or 2, with no contralateral cancer identified. Seven patients were assessed as BI-RADS 0 or 3. Of these 7 patients, 4 underwent further evaluation with a combination of CBE and mammogram or CBE and ultrasound. The remaining 3 of the 7 patients did not have additional imaging study. No contralateral cancer was identified in these 7 patients either. Three of the 120 additional MRIs were interpreted as BI-RADS 4 or 5. One was followed with core needle biopsy, revealing fibrocystic changes. One was evaluated with CBE and chest computed tomography with normal results. One participant underwent mastectomy, with pathology revealing atypical ductal hyperplasia, not cancer.
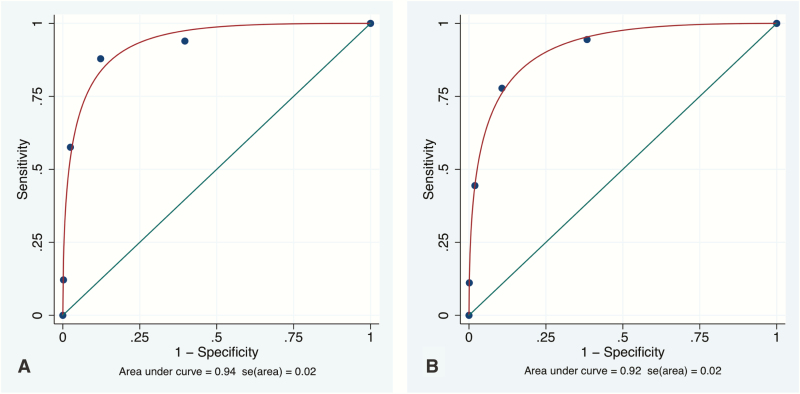
For the entire population at 2 years, the diagnostic yield of MRI for contralateral mammographically and clinically occult cancers was 3% (31/969) (Table 1). The year-2 estimated sensitivity of breast MRI was 86% (95% CI: 71–95), and the specificity was 88% (95% CI: 86–90). The NPV of MRI was 99% (95% CI: 99–100). The estimated PPV of MRI was 22% (95% CI: 15–29). The estimated mean area under the ROC curve (+/- standard error (S.E.)) was 0.92+/-0.2 (95% CI: 0.88–0.94) (Table 1; Figure 1). Because only 3 additional cancers were diagnosed in the second year, these values do not significantly vary from the year-1 data (14).
Table 1.
Estimates of Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, and Diagnostic Yield for the Study MRI at 1- and 2-Year Follow-Up
| Cancers Detecteda | Sensitivityb | Specificityc | NPVd | PPVe | Fitted AUC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | No./Total (%) | S.E. (95% CI) | No./Total (%) | S.E. (95% CI) | No./Total (%) | S.E. (95% CI) | No./Total (%) | S.E. (95% CI) | No./Total (%) | S.E. (95% CI) | AUC | S.E. (95% CI) |
| 1 | 30/969 | 0.6 | 30/33 | 5 | 822/936 | 1 | 822/825 | 0.2 | 30/144 | 3 | 0.94 | 2 |
| (3) | (2–5) | (91) | (81–100) | (88) | (86–90) | (100) | (99–100) | (21) | (14–27) | (90–98) | ||
| 2 | 31/969 | 0.6 | 31/36 | 6 | 820/933 | 1 | 820/825 | 0.2 | 31/144 | 3 | 0.92 | 2 |
| (3) | (3–5) | (86) | (71–95) | (88) | (86–90) | (99) | (99–100) | (22) | (15–29) | (88–94) |
aThe first number is the number of cancers detected; the second is the total number of patients.
bThe first number is the number of true positive results; the second is the total number of patients with tumors in the contralateral breast.
cThe first number is the number of true negative results; the second is the total number of patients without tumors in the contralateral breast.
dThe first number is the number of true negative results; the second is the total number of true negative and false negative results.
eThe first number is the number of true positive results; the second is the total number of true positive and false positive results.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; AUC, area under the ROC curve; S.E., standard error.
Figure 1.
Fitted ROC Curves for Initial Study MRI. A: ROC curve for year 1. B: ROC curve for year 2.
Discussion
In our study, the estimated sensitivity and NPV of the initial MRI did not change between 1 and 2 years of follow-up. Of the 3 contralateral cancers found in 3 patients in the second year, diagnosis was made by means other than MRI: 1 was diagnosed by biopsy of mammographic microcalcifications; 1 was detected on the tissue removed during mastopexy, and 1 was detected by abnormal CBE and confirmed by mammogram and ultrasound. The MRIs during the second year for these 3 patients were performed for the purpose of staging and evaluation of extent of disease.
Among the 120 participants undergoing MRI during the second year, no contralateral breast cancers were identified. One of the MRIs was assessed as BI-RADS 5, leading to a mastectomy that revealed only atypical ductal hyperplasia. Another MRI interpreted as BI-RADS 4 led to a core needle biopsy with a benign result (fibrocystic changes). Therefore, the additional MRI in year 2 provided no cancer yield and probably caused some harm to the participants because of the unnecessary intervention.
The 2-year NPV of MRI remained extremely high at 99%. This is likely in part related to the systemic therapy the patients were receiving. In the clinical setting, an initial negative contralateral breast MRI with 1-year surveillance using CBE, mammography, and sonography for assessment of cancer status is likely adequate. These patients may return to screening mammogram safely after 1 year of close surveillance post treatment, which will result in cost savings and reduced anxiety. However, this should not supplant or supersede the need for follow-up if there are recommendations for MRI based on clinically suspicious findings or abnormalities identified on other imaging modalities. There has been increasing utilization of breast MRI for staging of newly diagnosed breast cancer patients since numerous articles and recommendations from the American Cancer Society were published 16–19). However, there are few guidelines for follow-up of women with a history of breast cancer. According to the American Cancer Society, annual MRI screening is recommended for women with a greater than 20% lifetime risk of developing breast cancer, such as those with sufficient genetic or family history. Women with a moderate lifetime risk, 15%–20%, such as those with a personal history of breast cancer, a history of lobular neoplasia, or extremely dense breasts on mammography, “are advised to speak with their clinicians to discuss the benefits of yearly breast MR in addition to mammography” (20). This conversation has been difficult as there is no real consensus in the breast imaging community for its use. Because of this, the practice is varied and sometimes sporadic. The most recently published ACR appropriateness criteria for breast cancer screening notes that the use of screening MRI in this group continues to be an area of debate (21).There are some published reports that support the use of MRI as an adjunct to mammographic screening in this group (22, 23). The most recent guidelines from the American College of Radiology support long-term surveillance of these high-risk women for recurrence and late metachronous cancers with yearly supplemental breast MRI, because a modeling study suggests that additional risk factors such as dense breast tissue and premenopausal diagnosis push them into the over 20%-lifetime-risk group (24). Given the lasting diagnostic accuracy of contralateral breast MRI shown in our study, such MRI surveillance may not need to begin until 2 years after the initial negative MRI at the time of diagnosis. Because the scope of our study was limited to the first 2 years post breast cancer diagnosis, there is a definite need for prospective long-term studies to determine the long-term appropriate breast MRI protocol for surveillance of women with a personal history of breast cancer.
Recently, an increasing number of women, (even those with sporadic unilateral breast cancer), are choosing contralateral prophylactic mastectomy (CPM), despite the lack of demonstrable survival advantage in multiple studies (25–29). Our data may reassure women that their risk for an occult contralateral breast cancer 2 years after a negative MRI and mammogram is extremely small (0.5%). Women and their surgeons choose CPM for many reasons (30–32). However, our findings may help to reduce the number of women choosing a prophylactic mastectomy based on fear of contralateral disease.
The strength of this study is that it is a multi-institutional prospective study involving a large number of participants. Furthermore, all of the participating sites used the same standard MRI protocol, and this ensured uniform performance of the breast MRIs across the institutions.
This study has some limitations. Because breast centers at other locations may use a different MRI protocol, the outcome of this study may not be applicable to other institutions. By study design, the 107 participants that were lost to follow-up were considered negative for contralateral breast cancer at the end of 2 years, if ACRIN did not receive a report from the participating institutions of a cancer diagnosis. Some of these participants may have developed contralateral breast cancer by the end of 2 years, and this may decrease the accuracy of our data, although the number of such cases is likely not significant. Our “lost to follow-up” rate of 107 participants (12.3%) is comparable with rates reported in similar studies. For example, Benndorf et al report a “lost to follow-up” rate of 10.8 % in 216 consecutive patients referred for breast MRI as an adjunct to mammography where negative findings were followed for a mean time of 26.7 months (33).
Conclusion
A negative contralateral breast MRI at the time of initial cancer diagnosis has a very high and reliable NPV, and, therefore, is very helpful in managing and counseling patients during the period of initial diagnosis and early treatment. We demonstrate that patients do not benefit from additional MRI of the contralateral breast during the second year of surveillance following the initial diagnosis.
Supplementary Material
Acknowledgments
Funding statement: The study was supported by the ACRIN, which receives funding from the National Cancer Institute through the grants U01 CA079778 and U01 CA080098. The authors gratefully acknowledge the ACRIN 6667–participating institutions, site principal investigators, and research coordinators (see the Appendix S1).
Conflict of interest statement
None declared.
References
- 1. Hankey BF, Curtis RE, Naughton MD, Boice JD Jr, Flannery JT. A retrospective cohort analysis of second breast cancer risk for primary breast cancer patients with an assessment of the effect of radiation therapy. J Natl Cancer Inst 1983;70:797–804. [PubMed] [Google Scholar]
- 2. Carmichael AR, Bendall S, Lockerbie L, Prescott R, Bates T. The long-term outcome of synchronous bilateral breast cancer is worse than metachronous or unilateral tumours. Eur J Surg Oncol 2002;28:388–391. [DOI] [PubMed] [Google Scholar]
- 3. Takahashi H, Watanabe K, Takahashi M, Taguchi K, Sasaki F, Todo S. The impact of bilateral breast cancer on the prognosis of breast cancer: a comparative study with unilateral breast cancer. Breast Cancer 2005;12:196–202. [DOI] [PubMed] [Google Scholar]
- 4. Kollias J, Ellis IO, Elston CW, Blamey RW. Prognostic significance of synchronous and metachronous bilateral breast cancer. World J Surg 2001;25:1117–1124. [DOI] [PubMed] [Google Scholar]
- 5. Donovan AJ. Bilateral breast cancer. Surg Clin North Am 1990;70:1141–1149. [DOI] [PubMed] [Google Scholar]
- 6. Hungness ES, Safa M, Shaughnessy EA, et al. Bilateral synchronous breast cancer: mode of detection and comparison of histologic features between the 2 breasts. Surgery 2000;128:702–707. [DOI] [PubMed] [Google Scholar]
- 7. Heron DE, Komarnicky LT, Hyslop T, Schwartz GF, Mansfield CM. Bilateral breast carcinoma: risk factors and outcomes for patients with synchronous and metachronous disease. Cancer 2000;88:2739–2750. [PubMed] [Google Scholar]
- 8. Lee SG, Orel SG, Woo IJ, et al. MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: preliminary results. Radiology 2003;226:773–778. [DOI] [PubMed] [Google Scholar]
- 9. Pediconi F, Catalano C, Roselli A, et al. Contrast-enhanced MR mammography for evaluation of the contralateral breast in patients with diagnosed unilateral breast cancer or high-risk lesions. Radiology 2007;243:670–680. [DOI] [PubMed] [Google Scholar]
- 10. Lehman CD, Blume JD, Thickman D, et al. Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: results from the International Breast Magnetic Resonance Consortium (IBMC) trial. J Surg Oncol 2005;92:9–15; discussion 15. [DOI] [PubMed] [Google Scholar]
- 11. Slanetz PJ, Edminster WB, Yeh ED, Talele AC, Kopans DB. Occult contralateral breast carcinoma incidentally detected by breast magnetic resonance imaging. Breast J 2002;8:145–148. [DOI] [PubMed] [Google Scholar]
- 12. Ozaki S, Tozaki M, Fukuma E, et al. Bilateral breast MR imaging: is it superior to conventional methods for the detection of contralateral breast cancer? Breast Cancer 2008;15:169–174. [DOI] [PubMed] [Google Scholar]
- 13. Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol 2009;27:5640–5649. [DOI] [PubMed] [Google Scholar]
- 14. Lehman CD, Gatsonis C, Kuhl CK, et al. ; ACRIN Trial 6667 Investigators Group MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007;356:1295–1303. [DOI] [PubMed] [Google Scholar]
- 15. Kuhl CK, Weinreb JC, Morris EM, et al. ACR BI-RADS® Magnetic Resonance Imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 16. Orel SG, Schnall MD, Powell CM, et al. Staging of suspected breast cancer: effect of MR imaging and MR-guided biopsy. Radiology 1995;196:115–122. [DOI] [PubMed] [Google Scholar]
- 17. Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999;213:881–888. [DOI] [PubMed] [Google Scholar]
- 18. Orel SG, Reynolds C, Schnall MD, Solin LJ, Fraker DL, Sullivan DC. Breast carcinoma: MR imaging before re-excisional biopsy. Radiology 1997;205:429–436. [DOI] [PubMed] [Google Scholar]
- 19.Breast Cancer Stages n.d., American Cancer Society. Available at: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-staging Accessed 29 August 2019.
- 20. Saslow D, Boetes C, Burke W, et al. ; American Cancer Society Breast Cancer Advisory Group American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75–89. [DOI] [PubMed] [Google Scholar]
- 21. Mainiero MB, Lourenco A, Mahoney MC, et al. ACR Appropriateness Criteria Breast Cancer Screening. J Am Coll Radiol 2016;13:R45–R49. [DOI] [PubMed] [Google Scholar]
- 22. Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol 2010;195:510–516. [DOI] [PubMed] [Google Scholar]
- 23. Kalish G, Demartini W, Peacock S, Eby P, Gutierrez R, Lehman C. Screening MRI for high risk women: should patients with a treated personal history of breast cancer be screened? Presented at: Radiological Society of North America 2010 Scientific Assembly and Annual Meeting; 28 November–3 December 2010, Chicago, IL. [Google Scholar]
- 24. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018;15:408–414. [DOI] [PubMed] [Google Scholar]
- 25. Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203–5209. [DOI] [PubMed] [Google Scholar]
- 26. Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol 2010;17:2554–2562. [DOI] [PubMed] [Google Scholar]
- 27. Rahbar H, Hanna LG, Gatsonis C, et al. Contralateral prophylactic mastectomy in the American college of radiology imaging network 6667 trial: effect of breast MR imaging assessments and patient characteristics. Radiology 2014;273:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pesce C, Liederbach E, Wang C, Lapin B, Winchester DJ, Yao K. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol 2014;21:3231–3239. [DOI] [PubMed] [Google Scholar]
- 29. Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg 2017;265:581–589. [DOI] [PubMed] [Google Scholar]
- 30. Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol 2009;16:2697–2704. [DOI] [PubMed] [Google Scholar]
- 31. Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Presented at: 2009 Annual Meeting of the Society of Clinical Oncology; 29 May–2 June 2009. [Google Scholar]
- 32. Recht A. Contralateral prophylactic mastectomy: caveat emptor. J Clin Oncol 2009;27:1347–1349. [DOI] [PubMed] [Google Scholar]
- 33. Benndorf M, Baltzer PA, Vag T, Gajda M, Runnebaum IB, Kaiser WA. Breast MRI as an adjunct to mammography: does it really suffer from low specificity? A retrospective analysis stratified by mammographic BI-RADS classes. Acta Radiol 2010;51:715–721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.