Abstract
Background
Antitumour antibiotics are used in the management of metastatic breast cancer. Some of these agents have demonstrated higher tumour response rates than non‐antitumour antibiotic regimens, however a survival benefit has not been established in this setting.
Objectives
To review the randomised evidence comparing antitumour antibiotic containing chemotherapy regimens with regimens not containing an antitumour antibiotic in the management of women with metastatic breast cancer.
Search methods
The Specialised Register maintained by the Cochrane Breast Cancer Group was searched on 3rd October, 2006 using the codes for 'advanced breast cancer' and 'chemotherapy'. Details of the search strategy and coding applied by the Group to create the register are described in the Group's module on The Cochrane Library.
Selection criteria
Randomised trials comparing antitumour antibiotic containing regimens with regimens not containing antitumour antibiotics in women with metastatic breast cancer.
Data collection and analysis
Data were collected from published trials. Studies were assessed for eligibility and quality, and data were extracted by two independent reviewers. Hazard Ratios (HRs) were derived from time‐to‐event outcomes where possible, and a fixed effect model was used for meta‐analysis. Response rates were analysed as dichotomous variables. Quality of life and toxicity data were extracted where present. A primary analysis was conducted for all trials and by class of antitumour antibiotic.
Main results
Thirty‐four trials reporting on 46 treatment comparisons were identified. All trials published results for tumour response and 27 trials published time‐to‐event data for overall survival. The observed 4244 deaths in 5605 randomised women did not demonstrate a statistically significant difference in survival between regimens that contained antitumour antibiotics and those that did not (HR 0.96, 95% CI 0.90 to 1.02, P = 0.22) and no significant heterogeneity. Antitumour antibiotic regimens were favourably associated with time‐to‐progression (HR 0.84, 95% CI 0.77 to 0.91) and tumour response rates (odds ratio (OR) 1.33, 95% CI 1.21 to 1.48) although statistically significant heterogeneity was observed for these outcomes. These associations were consistent when the analysis was restricted to the 30 trials that reported on anthracyclines. Patients receiving anthracycline containing regimens were also more likely to experience toxic events compared to patients receiving non‐antitumour antibiotic regimens. No statistically significant difference was observed in any outcome between mitoxantrone containing and non‐antitumour antibiotic‐containing regimens.
Authors' conclusions
Compared to regimens without antitumour antibiotics, regimens that contained these agents showed a statistically significant advantage for tumour response and time to progression in women with metastatic breast cancer but were not associated with an improvement in overall survival. The favourable effect on tumour response and time to progression observed in anthracycline containing regimens was also associated with greater toxicity.
Plain language summary
Antitumour antibiotic containing regimens for metastatic breast cancer
Advanced (metastatic) breast cancer is cancer that has spread beyond the breast. Treatment for metastatic disease usually involves some type of chemotherapy (anti‐cancer drugs) to try to reduce the cancer. Chemotherapy drugs can either be given as a single agent or in combination with other chemotherapy drugs. This is done according to a plan or a course of the drug referred to as a regimen. There are many types of chemotherapy drugs which work in various ways. Antitumour antibiotics work by damaging the cancer cells thereby preventing those cells from multiplying. Chemotherapy in general produces a range of side effects or adverse events related to the treatment. The known side effects of antitumour antibiotics include nausea, vomiting, a reduction in the number of white blood cells (known as leukopenia), and in some cases a toxic reaction which alters the working of the heart (called cardiotoxicity).
This review sought to identify and review the randomised evidence comparing courses of chemotherapy containing antitumour antibiotics against courses not containing antitumour antibiotics. This review identified 34 eligible trials involving 5605 women. This review found that for women with advanced breast cancer, taking antitumour antibiotics did not result in better survival than women who took other types of chemotherapy drugs. Despite the lack of evidence of survival benefit, this review demonstrated that women taking these drugs had an advantage in time to progression (the length of time it takes for the cancer to progress after taking the drug) and tumour response (shrinking of the tumour) compared to women who did not take the antitumour antibiotic drugs. In addition however, the risks of side effects including cardiotoxicity, leukopenia and nausea/vomiting were all significantly increased in the women taking the antitumour antibiotics. Given that this review failed to show a benefit in survival for women taking this group of drugs but a higher rate of side effects, the use of these drugs in the management of metastatic breast cancer must be carefully weighed against the risk of these side effects.
Background
Breast cancer is the most common type of cancer in women and the most common cause of cancer death in that group. In 2002, there were over 1 million new cases and approximately 410,000 deaths from breast cancer worldwide; an age standardised death rate (ASR) of 13.2 (per 100,000). ASRs of 25 or greater were recorded that same year by Barbados (25.5), Belgium (27.7), Botswana (25.0), Cyprus (29.6), Denmark (27.8), Georgia (25.1), Ireland (25.7), Malta (29.6), The netherlands (27.5) and The Philippines (27.1). (Ferlay 2002).
The stage of breast cancer at the time of diagnosis is an important indicator of prognosis. Median survival in women with metastatic breast cancer (MBC) is around 18 to 24 months although this could range from a few weeks to several years (Stockler 2000). Although there is no randomised evidence comparing chemotherapy with observation in women with metastatic breast cancer, it is widely accepted that women with metastatic disease should receive some form of systemic therapy at some time during the course of their disease. Chemotherapy is considered by many to be the appropriate first treatment option for women with multiple sites of recurrence or where visceral disease is not easily treated by local modalities (Hayes 1995). Chemotherapy is also considered to be useful in women whose cancer is hormone refractory or expected to be hormone resistant (Hortobagyi 1996).
As a class, antitumour antibiotics are agents that have been isolated, or synthetically derived, from a variety of fungal organisms for their cytotoxic properties. They damage the DNA template by a variety of mechanisms including intercalation into DNA and RNA, alkylation of DNA and the generation of oxygen free radicals to produce single‐ and double‐strand DNA breaks (Perry 1997). Antitumour antibiotics include the anthracyclines (for example doxorubicin and epirubicin); anthracenediones (mitoxantrone/mitozantrone); and mitomycin‐C. This class of drugs, in particular anthracycline‐based FAC (cyclophosphamide, 5‐fluorouracil and doxorubicin) and FEC (cyclophosphamide, 5‐fluorouracil and epirubicin) regimens, have been used in chemotherapy for the management of metastatic breast cancer for the last three decades (Hortobagyi 2003). Several large randomised trials have supported their use over standard CMF (cyclophosphamide, 5‐fluorouracil, methotrexate) regimens but despite yielding higher response rates, the evidence for survival benefit has not been conclusive (Fossati 1998). Common side effects of these agents are nausea, vomiting, hair loss and leucopenia. Rarely, anthracyclines may cause cardiomyopathy and these agents should be used with care in patients with known cardiac disease.
Objectives
The objective of this review was to compare antitumour antibiotic containing chemotherapy regimens with regimens not containing antitumour antibiotics in the management of women with MBC.
Antitumour antibiotics were classified as anthracyclines, anthracendiones or other antitumour antibiotics for the purposes of this review. Pre‐specified subquestions within the review for each of these classes were: a) regimen A plus antitumour antibiotic versus regimen A; b) regimen A plus antitumour antibiotic versus regimen B; c) single agent antitumour antibiotic versus regimen C.
Methods
Criteria for considering studies for this review
Types of studies
Properly randomised controlled clinical trials.
Types of participants
1. Women diagnosed with advanced breast cancer a. advanced breast cancer was defined as metastatic disease; b. women with locoregional disease only were excluded*; c. both newly diagnosed and recurrent cases were included. 2. Women randomised to receive chemotherapy for advanced disease as first line treatment (ie. no previous chemotherapy given except as adjuvant therapy)** 3. No age restrictions were applied *Trials which included both women with metastatic disease and women with locoregionally recurrent disease only were included if women with locoregional recurrence were less than 20% of the total group. **Trials reporting on antitumour antibiotics for advanced disease were excluded if more than 50% of participants had received prior cytotoxic chemotherapy for MBC.
Types of interventions
Intervention group: any chemotherapy regimen containing an antitumour antibiotic. Comparator: any chemotherapy regimen not containing an antitumour antibiotic. See Table 1 for classification of chemotherapeutic agents; Table 2 for classification of antitumour agents (Perry 1997). Endocrine therapy may also have been given to both treatment groups. Trials may or may not specify recommended treatment upon disease progression or initial treatment failure. Trials where patients crossed over to the other treatment arm at the time of progression or received other treatment off‐study were included in this review and analysed according to the treatment they were originally randomised to receive. Sequential trials where patients were allocated to receive a set number of cycles of one treatment and then crossed over to the other treatment arm (not at the time of progression but upon completion of the first treatment) were excluded from this review.
1. Chemotherapeutic Agents (adapted from Table 1.1 in The Chemotherapy Source Book).
| Type of Agent | Action | Includes |
| Agents that damage the DNA template | by alkylation: nitrogen mustards | cyclophosphamide, melphalan, ifosfamide, chlorambucil |
| by alkylation: nitrosureas | carmustine (BCNU), lomustine (CCNU) | |
| by alkylation: other agents | thiotepa, mitomycin C | |
| by platinum coordination cross‐linking | cisplatin, carboplatin | |
| antibiotics | doxorubicin, daunorubicin, mitoxantrone, idarubicin, epirubicin, amsacrine | |
| podophyllotoxins | etoposide, teniposide | |
| by intercalation | dactinomycin, mithramycin | |
| by uncertain mechanisms | bleomycin | |
| Spindle poisons | vinca alkaloids | vincristine, vinblastine, vendesine, vinorelbine |
| taxanes | taxol, taxotere | |
| Antimetabolites | thymidylate synthase | 5‐fluorouracil |
| dihydrofolate reductase | methotrexate |
2. Antitumour antibiotics.
| Generic Name | Other names |
| doxorubicin hydrochloride | adriamycin, caelyx, doxorubicin, rubex |
| daunorubicin hydrochloride | cerubidine |
| dactinomycin | actinomycin D, cosmogen |
| mitomycin C | mitomycin, mitomycin C, mitomycin‐C, mutamucin |
| mitozantrone | novantrone, mitoxantrone |
| epirubicin hydrochloride | ellence, epirubicin, pharmorubicin |
| plicamycin | mithramycin, mithracin |
| bleomycin sulfate | blenoxane |
Types of outcome measures
1. Overall survival 2. Time to progression (or progression‐free survival) 3. Response 4. Quality of life measures (trial‐specific instruments) 5. Toxicity
For the purpose of this review, the following outcome definitions applied: 1. Overall survival (OS): time from date randomised to date of death (any cause); 2. Time to progression (TTP): time from date randomised to date of progression or death (any cause); 3. Response rate (RR): the proportion of patients with a complete or partial response as defined by the National Cancer Institute's Response Evaluation Criteria in Solid Tumors (NCI 2002).
This review also attempted to investigate treatment‐related death which, for the purpose of this review, was defined as death due to the toxicity of the drug and not to disease progression. If an individual trial did not include the definition used by that trial but used the terms 'toxic death' or 'lethal toxicity', or indicated that death was due to treatment, then the information was included in the review.
Time‐to‐treatment failure was a planned outcome for this review and was defined as time from date randomised to date of progression, death (any cause), withdrawal due to adverse event, patient refusal or further anti‐cancer therapy for documented progression. Eight trials reported data on time to treatment failure, however, not all these trials used definitions in alignment with our pre‐specified definition and this outcome was not included in this review.
Search methods for identification of studies
The specialised register maintained by the Secretariat of the Cochrane Breast Cancer Group (CBCG) was searched (3rd October 2006). Details of the search strategy applied by the Group to create the register, and the procedure used to code references, are described in the Group's module on The Cochrane Library. The register includes both published and unpublished (including ongoing) trials identified from searches of electronic databases including MEDLINE, EMBASE and the Cochrane Controlled Trials Register, and handsearching of journals and conference proceedings. All references that had been assigned the CBCG codes 'advanced' and 'chemotherapy' as applied to the specialised register and the abstracts were screened in an attempt to determine if the reference pertained to a randomised trial in women with metastatic breast cancer comparing one chemotherapy combination with another. The complete article was obtained for references that were definitely eligible, or where it was not possible to determine eligibility based only on information in the abstract.
The reference lists of other related literature reviews were also searched. The reviews searched included Fossati 1998 and Stockler 2000 as well as review articles identified by the search strategy.
Data collection and analysis
At least two individuals applied the selection criteria (including the quality of randomisation) to each reference identified by the search strategy, masked to the study results. A third reviewer resolved any discrepancies regarding eligibility or quality.
The hazard ratio (HR) and associated variances for overall survival and time to progression were extracted directly from the trial publication/s. If not reported, this data was obtained indirectly using the methods described by Parmar 1998 et al using either other available summary statistics or from data extracted from published Kaplan‐Meier curves. To allow for immature follow up, the numbers at risk were adjusted based on estimated minimum and maximum follow‐up times. If these were not reported in any of the reports available, minimum follow up was estimated using the estimated time taken to complete treatment, and maximum follow up was estimated using the last event reported in the relevant time‐to‐event curve. These follow‐up estimates are recorded in the Characteristics of Included Studies table under 'Notes'.
A pooled HR was obtained from the derived observed (O) less expected (E) number of events and the variance for each trial, using the fixed effect model (Yusuf 1985). The pooled HR represents the overall risk of an event on chemotherapy regimens containing antitumour antibiotics versus those not containing antitumour antibiotics.
All outcomes available from the individual studies were included in the meta‐analysis with heterogeneity reported using chi‐square tests (see the Cochrane Reviewers' Handbook).
Ratios of treatment effects for time‐to‐event outcomes were reported so that HRs less than 1.0 favour regimens containing antitumour antibiotics and values greater than 1.0 favour regimens that do not contain antitumour antibiotics. The plots for overall survival and progression‐free survival are hazard ratio (HR) plots, although they are labelled as odds ratio (OR) plots in the default mode of meta‐view.
Response rates were analysed as dichotomous variables (complete or partial response versus stable disease or no response) and a pooled odds ratio was derived. Response has been reported based on assessable (not randomised) patients as most of the trials included in this review only reported response in this way. Ratios of treatment effects for response were reported so that ORs less than 1.0 favour regimens containing antitumour antibiotics and values greater than 1.00 favour regimens that do not contain antitumour antibiotics.
If all arms in a multi‐arm trial were included in the meta‐analysis and one treatment arm was included in more than one of the treatment comparisons then the number of events and the number of participants in that arm were divided by the number of treatment comparisons made. This method was used to avoid the multiple use of participants in the pooled estimate of treatment effect while retaining information from each arm of the trial and is likely to compromise the precision of the pooled estimate slightly.
Quality of life data were collected using a variety of instruments across trials. These data were not statistically synthesised but were summarised and evaluated qualitatively.
Toxicity data was extracted for Grade III or Grade IV events of leukopenia, nausea or vomiting, alopecia and cardiotoxicity. This data was not consistently reported across the included trials and the analysis was limited to the calculation a single odds ratio (with 95% confidence intervals) using the total number events and number at risk added up across trials.
As specified in the protocol, each of these outcomes was reported, where available, for all trials combined and by class of antitumour antibiotic (primary analyses). Two secondary analyses were also performed as pre‐specified: 1. analysis by type of antitumour antibiotic‐containing regimen (single, additional or replacement agent to comparator regimen); and 2. sensitivity analysis using studies with adequate concealment clearly stated. Planned subgroup analysis by menopausal status, hormone receptor status and stage of disease was not undertaken due to the lack of data available in the included trials for these subgroups.
Post‐hoc subgroup analyses were conducted for the subclass of anthracycline containing antitumour antibiotic regimens by type of comparator regimen. These analyses were planned after identification of the eligible trials, and prior to the pooling of results. Comparator regimens used in each trial were classified into four subgroups to allow analyses of antitumour antibiotic efficacy by comparator class. These subgroups were selected on the basis that they each represented classes of agents of similar activity: 1. CMF; 2. CMF based, with addition of other cytoxics for example Vincristine; 3. other C‐ or CF‐based (no methotrexate); 4. taxanes. Chlorambucil was considered equivalent to cyclophosphamide and regimens that included prednisone were considered equivalent to those that did not for the purpose of this classification.
Overall this review tested 40 comparisons for each of the six outcome variables.
Results
Description of studies
On the 3rd October 2006, the Cochrane Breast Cancer Group Specialised Register contained 6,176 references of which 829 were coded as references to studies of chemotherapy and advanced breast cancer (see Figure 1 for the quorum flow chart). Of these, 502 were references that reported the comparison of two different chemotherapy combinations in metastatic breast cancer, of which 421 were not eligible based on information in the abstract. The complete paper was obtained for 100 references leading to the exclusion of a further 48 references. The remaining 52 references reported the results of 48 randomised trials, 15 of which were excluded from the meta‐analysis: 15 were not considered to be eligible for the review (see Characteristics of excluded studies). The 34 eligible trials reported on 46 treatment comparisons: 42 comparisons of anthracyclines, and four comparisons of mitoxantrone with non‐antitumour antibiotic containing regimens. The regimens used in each trial are summarised in Figure 2 and Figure 3. Where a trial included more than one comparison these were labelled alphabetically (a, b, c). The trials included in the forest plots were labelled by trial name or primary author and date of publication.
1.

2.
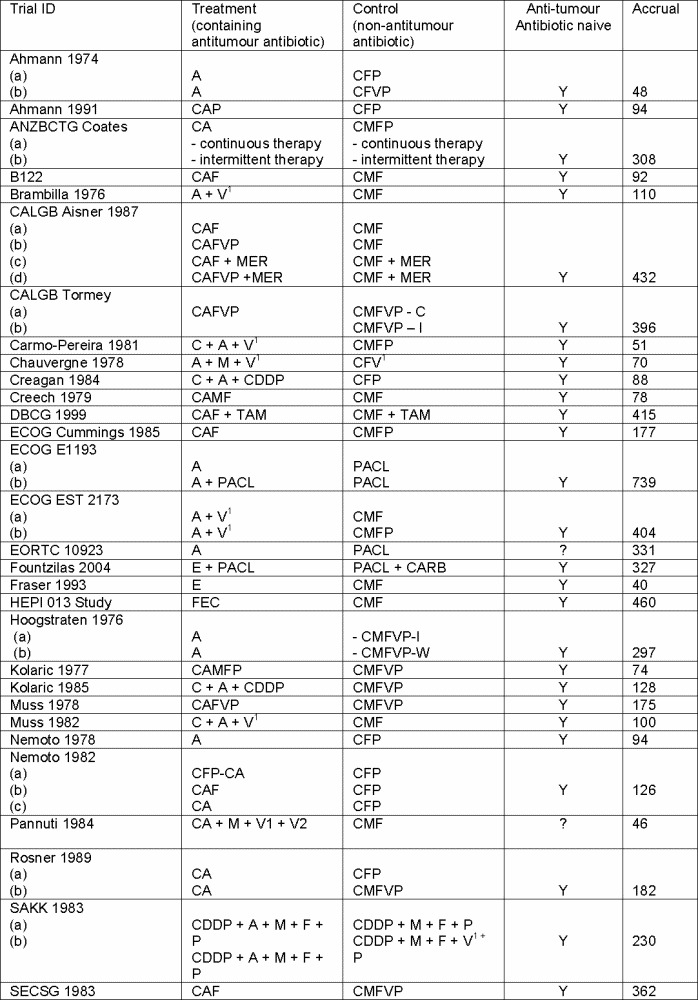
Summary of regimens included in the analyses
a) Treatment arm = Anthracycline containing regimens
3.

Risk of bias in included studies
Each study was reviewed, according to its design and by how the study was conducted, to assess the potential for bias. Trial quality was assessed based on: ‐ quality of randomisation; ‐ comparability between groups (treatment arms) at the baseline; ‐ inclusion of all randomised participants in the analysis.
The quality of randomisation was assessed based on generation and concealment of the allocation sequence. This was graded as A ‐ clearly adequate, B ‐ possibly adequate, C ‐ clearly inadequate (see Characteristics of Included Studies). It was not possible to accurately assess the quality of randomisation used in most studies due to lack of information in the published articles. The following sensitivity analyses were conducted.
Sensitivity analysis A Trials graded as having clearly adequate allocation concealment (grade A). In this review these trials were: ANZ BCTG 8614B122; CALGB Aisner; Coates 1987; EORTC 10923; HEPI; SECSG.
Sensitivity analysis B Trials graded as having possibly adequate allocation concealment and having good comparability of baseline characteristics and adequate reporting of outcomes. In this review, eligible trials that did not meet the quality criterion (A) satisfied quality criterion (B).
Sensitivity analysis C Trials graded as having inadequate allocation concealment, for example the use of alternation, case record numbers or other open lists of random numbers. No studies included in this review were graded at this level.
Trials were considered to have adequate reporting of time‐to‐event outcomes if: i) they included all patients in the analysis; or ii) patients were excluded from analysis and reasons were given for excluding patients, and the exclusions were not of a number that could lead to a misleading conclusion. See the Characteristics of Included Studies table for details.
Effects of interventions
The 34 trials (46 treatment comparisons) included in this review randomised 7237 women; of these, 6474 (89%) were randomised to 30 trials (42 treatment comparisons) comparing anthracycline based therapies to non‐antitumour antibiotic based regimens, and 763 were randomised to four trials (four treatment comparisons) comparing mitoxantrone containing regimens with non‐antitumour antibiotic based regimens. Time‐to‐event data was extractable for overall survival from 27 trials (35 treatment comparisons, 76% of all patients randomised) and progression‐free survival from 12 trials (treatment comparisons, 36% of all patients randomised). Tumour response rates based on assessable patients were available for all trials and treatment comparisons.
The observed 4244 deaths in 5605 randomised women did not demonstrate a statistically significant difference in survival between regimens that contained antitumour antibiotics and those that did not contain these agents, with an overall HR of 0.96 (95% CI 0.90 to 1.02, P = 0.22) and no statistically significant heterogeneity (Forest plot 1.1). Antitumour antibiotic containing regimens were favourably associated with time‐to‐progression (HR 0.84, 95% CI 0.77 to 0.91) and tumour response rates (OR 1.33, 95% CI 1.21 to 1.48 ) although statistically significant heterogeneity was observed for these outcomes across the trials (Forest plots 1.2, 1.3). Treatment related deaths were reported in 2% of participants across the 19 trials (treatment comparisons) reporting on this outcome. Of the 81 treatment related deaths reported, 46 occurred in the antitumour antibiotic arm compared to 35 in the non‐antitumour antibiotic arm (OR 1.18, 95% CI 0.77 to 1.83).
The results for the analyses by class of antitumour antibiotic (anthracycline containing or mitoxantrone containing) are presented below.
1. Anthracycline‐containing regimens versus non‐antitumour antibiotic regimens Twenty‐seven trials (39 treatment comparisons) reported on doxorubicin and three trials (three treatment comparisons) reported on epirubicin (HEPI 013 2001; Fraser 1993; Fountzilas 2004). The majority of comparator regimens were CMF (14 trials, 18 treatment comparisons), CMFVP (eight trials, ten treatment comparisons) or other CF‐based regimens (seven trials, ten treatment comparisons). Two trials (three treatment comparisons) compared doxorubicin or a doxorubicin/paclitaxel combination regimen with single agent paclitaxel (ECOG E1193a; ECOG E1193b; EORTC 10923). Figure 4 and Figure 5 lists the outcomes extracted from each trial.
4.
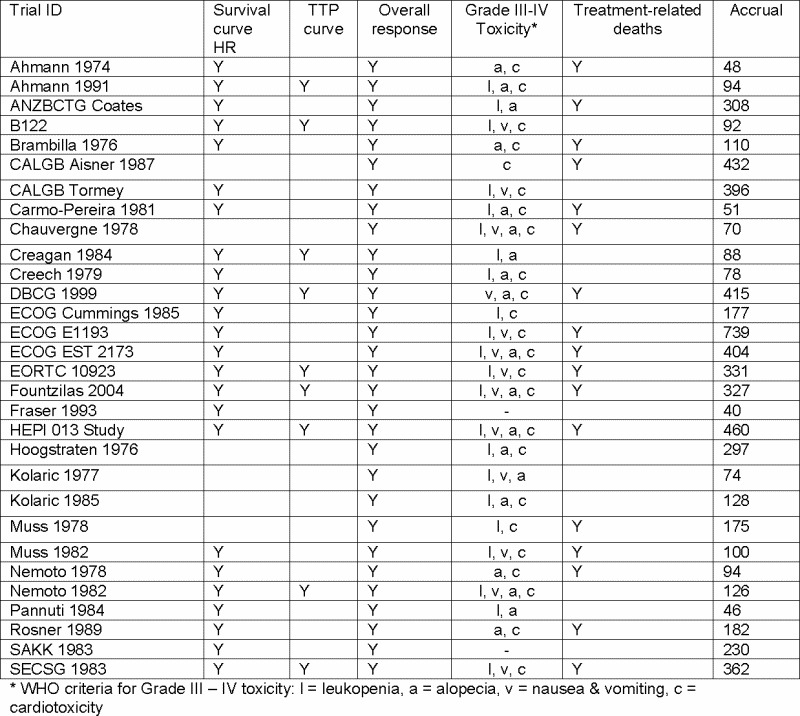
Summary of outcomes included in the analyses
a) Treatment arm = Anthracycline containing regimen
5.

Overall survival Sufficient data was available for 23 of the 30 eligible trials (31 treatment comparisons) to calculate an HR for overall survival (Forest plot 2.1). No survival advantage was observed for anthracycline containing regimens, with an HR of 0.97 (95% CI 0.91 to 1.04). A null effect was also observed within each of the three subquestions where anthracyclines were used as an additional agent (question a: three trials, four treatment comparisons); replacement agent in combination therapy (question b: 18 trials, 22 treatment comparisons); or single agent (question c: five trials, five treatment comparisons). Heterogeneity of survival effect across the 31 treatment comparisons was not statistically significant (heterogeneity chi‐square 34.12, df = 30, P = 0.28).
A statistically significant survival advantage for anthracycline containing regimens was observed when a sensitivity analysis was undertaken (Forest plot 3.1). Four trials (five treatment comparisons) addressing Question (b) reported randomisation methods of high quality (B122; Coates 1987a; Coates 1987b; HEPI 013 2001; SECSG 1983). Each of these studies suggested a survival advantage for regimens where an anthracycline was used to replace methotrexate, or methotrexate in combination with fluorouracil or vincristine (comparator CMF or CMFVP), with an overall HR of 0.86 (95% CI 0.75 to 0.98). One other study reported clearly adequate allocation concealment (EORTC 10923). This trial addressed question (c) and compared doxorubicin with paclitaxel as single agent therapy. No statistically significant difference in survival was observed between these two single agents (HR 0.93, 95% CI 0.73 to 1.19).
Time to progression Data on time to progression was available from ten trials reporting on 12 treatment comparisons (Forest plot 2.2). There was a statistically significant difference in favour of anthracycline containing regimens for time to progression, with an HR of 0.76 (95% CI 0.69 to 0.83).
This beneficial effect was consistently observed in each of the three categories of anthracycline regimens. Nine trials (nine treatment comparisons) compared anthracycline containing regimens with alternate non‐anthracycline combination therapies (question b), with an HR of 0.82 (95% CI 0.74 to 0.91) for time to progression. One of these trials also reported on the addition of an anthracycline to a regimen (Nemoto 1982a; Nemoto 1982b), which also showed a favourable effect but this did not reach statistical significance (HR 0.67, 95% CI 0.37 to 1.24). Another trial investigating the use of anthracyclines as a single agent (EORTC 10923) reported that anthracycline was favoured over paclitaxel in time to progression, with an HR of 0.55 (95% CI 0.44 to 0.68). Heterogeneity across the 12 treatment comparisons was statistically significant (heterogeneity chi‐square 22.73, df = 11, P = 0.02) but not within each of the three subquestions.
Overall response All of the 30 eligible trials (42 treatment comparisons) reporting on anthracycline containing regimens versus non‐antitumour antibiotic containing regimens provided information about response rates based on assessable patients (Forest plot 02.3). The odds ratio for overall response showed a statistically significant difference in favour of anthracycline containing regimens, with an OR of 1.40 (95% CI 1.26 to 1.56).
Four trials (5 treatment comparisons) addressed question (a), the addition of an anthracycline to a regimen, to demonstrate a benefit in favour of anthracyclines, with an OR of 1.90 (95% CI 1.33 to 2.72). Each of the five comparisons favoured the addition of an anthracycline and a test for heterogeneity was not statistically significant (heterogeneity chi‐square 2.08, df = 4, P = 0.72).
Twenty three trials (29 treatment comparisons) addressed question (b), the replacement of a non‐antitumor antibiotic with an anthracycline in a combination therapy, demonstrating a statistically significant difference in overall response in favour of anthracycline containing regimens, with an OR of 1.44 (95% CI 1.27 to 1.63). Heterogeneity of effect was statistically significant across these treatment comparisons (heterogeneity chi‐square 49.49 df = 28, P = 0.007).
Five trials (eight treatment comparisons) addressed question (c), the use of anthracyclines as a single agent versus non‐anthracycline containing regimens. Overall, no statistically significant difference in response rates was observed (OR 1.09, 95% CI 0.85 to 1.41), although there was significant heterogeneity across treatment comparisons (heterogeneity chi‐square 16.41, df = 7, P = 0.02).
Toxicity Leukopenia (white blood cell count (WCC) less than 2000 x 10‐9/litre) was the commonest Grade III to IV toxic event in both anthracycline and comparator arms (Table 3). Twenty‐two trials reported 1540 events in 4425 participants, with an OR of 1.25 (95% CI 1.10 to 1.41), to show a significantly increased risk for anthracycline containing regimens. Twenty‐three trials provided data on Grade III to IV cardiotoxicity, with 110 events in 4777 participants for an OR of 5.17 (95% CI 3.16 to 8.48) in patients receiving anthracyclines. Patients receiving anthracycline based regimens also were more likely to experience moderate to severe nausea/vomiting (OR 1.98, 95% CI 1.62 to 2.41) and alopecia (OR 3.87, 95% CI 3.31 to 4.52) compared to patients receiving non‐antitumour antibiotic regimens.
3. Acute toxicity Grade III‐IV.
| Site of toxicity | No. of trials | AA events (pts)/n | Ctrl events (pts)/n | OR (95% CI) |
| ASSESSABLE PTS | ||||
| All Trials | ||||
| leukopenia* | 26 | 931/2621 | 774/2527 | 1.25 (1.11‐1.41) |
| nausea or vomiting** | 17 | 341/2150 | 197/1945 | 1.71 (1.42‐2.06) |
| alopecia | 22 | 1177/1800 | 854/1934 | 2.36 (2.07‐2.70) |
| cardiac toxicity | 25 | 94/2572 | 18/2452 | 5.91 (3.56‐9.80) |
| Anthracycline Trials | ||||
| leukopenia* | 22 | 846/2265 | 694/2160 | 1.25 (1.10‐1.41) |
| nausea or vomiting** | 14 | 327/1852 | 170/1642 | 1.98 (1.62‐2.41) |
| alopecia | 18 | 1097/1447 | 705/1569 | 3.87 (3.31‐4.52) |
| cardiac toxicity | 23 | 92/2451 | 18/2326 | 5.17 (3.16‐8.48) |
| Anthracycline vs CMF based Trials | ||||
| leukopenia* | 19 | 478/1622 | 531/1851 | 1.04 (0.90‐1.20) |
| nausea or vomiting** | 12 | 236/1359 | 180/1478 | 1.52 (1.23‐1.87) |
| alopecia | 16 | 847/1410 | 600/1585 | 2.47 (2.13‐2.86) |
| cardiac toxicity | 17 | 49/1565 | 7/1710 | 7.86 (3.55‐17.41) |
| Mitoxantrone Trials | ||||
| leukopenia* | 4 | 85/365 | 80/367 | 1.17 (0.84‐1.63) |
| nausea or vomiting** | 3 | 14/298 | 27/303 | 0.64 (0.34‐1.18) |
| alopecia | 4 | 100/353 | 149/365 | 0.66 (0.49‐0.90) |
| cardiac toxicity | 2 | 2/121 | 0/126 | 5.29 (0.25‐111.39) |
| * data on grade II or IV neutropenia was included if data on leucopenia not reported | ||||
| ** if data on nausea and vomiting was reported separately data on vomiting was included. |
Seventeen anthracycline trials provided information about treatment related deaths (Forest plot 2.4). Overall, there was no statistically significant difference in risk of treatment related deaths (OR 1.16, 95% CI 0.74 to 1.82).
Quality of life Information about quality of life (QoL) was only available from two trials comparing anthracycline containing regimens with non‐antitumour antibiotic (Fraser 1993; EORTC 10923). No difference in global QoL scores were reported between the two treatment groups in these two trials (Table 4).
4. Quality of life.
| Trial ID | Instruments used | Summary of findings |
| ANZ BCTG 8614 | Patients completed 14 linear analogue self‐assessment scales. Clinicians used the Spitzer QL index. | Completion rates for each instrument are not available. No significant differences in patient or clinician rated overall QOL were reported between the treatment groups at 3 months. Patients on CMFP rated significantly higher for mood, pain, feeling sick, vomiting, appetite/taste and sexual interest, but worse for hair loss that patients on MTZ. |
| Harper Wynne 1999 | HADS and RSCL (plus 3 satisfaction questions) pre‐treatment and at weeks 12 and 24 (or on withdrawal) | Only 35 (30%) completed all 3 assessments. Reported no evidence of a difference between treatment groups. |
| IDBBC EORTC 10923 | Patients completed EORTC QLQ‐C30 and Rotterdam Symptom Checklist | 64% of randomised patients completed baseline QLQ‐C30 and 61% completed baseline RSCL. QOL comparisons were only performed for the first 3 cycles. Doxorubicin was associated with significantly more nausea/vomiting, loss of appetite and burden of disease and treatment, but less bone pain and rash. |
| Fraser 1993 | Patients completed 3 quality of life instruments: 4 weekly Nottingham Health Profile (NHP ‐ emotional state, energy, pain, physical mobility, sleep and social factors ) and Linear Analogue Self‐Assessment (LASA) at the start of treatment and four weekly thereafter and the Qualitator daily dairy card throughout treatment which measured the domains of physical symptoms, social factors, emotional factors and physical performance. | Of the 40 patients randomised, compliance for the 29 who started the Qualitator, the 37 who started the NHP and 36 who started the LASA respectively were 88%, 89% and 92%. Quality of life measures only recorded a significant difference in energy and pain, influenced primarily by the non responders in each treatment group but with no difference in overall global scores. Scores for responders (58% for CMF, 29% for epirubicin P>0.05), irrespective of treatment were better to start with (LASA P=0.001); at 12 weeks, scores had improved (Qualitator P<0.05; NHP P<0.05) . Scores in non responders showed no change. |
Subgroup analyses Anthracycline containing regimens demonstrated a statistically significant difference in survival compared to CMFVP (four trials, five treatment comparisons; HR 0.84, 95% CI 0.72 to 0.99). This benefit was not observed in the 11 trials (13 treatment comparisons) using CMF as the comparator (HR 0.95, 95% CI 0.86 to 1.05) nor in the other comparator subclasses (Forest plot 4.1). Anthracycline containing regimens showed a statistically significant advantage in time to progression compared to CMF regimens (four trials, four treatment comparisons; HR 0.82, 95% CI 0.72 to 0.93) and CMFVP regimens (one treatment comparison; HR 0.72, 95% CI 0.57 to 0.90) (Forest plot 4.2).
Anthracycline containing regimens demonstrated a statistically significant increase in tumour response compared to CMF regimens (14 trials, 18 comparisons; OR 1.41, 95% CI 1.20 to 1.66), CMFVP regimens (eight trials, nine treatment comparisons; OR 1.42, 95% CI 1.13 to 1.78), taxanes (two trials, three treatment comparisons; OR 1.57, 95% CI 1.20 to 2.06). No difference was observed in comparison with regimens based on cyclophosphamide and 5‐fluorouracil that did not include methotrexate for example CFP, CFVP (seven trials, ten comparisons; OR 1.23, 95% CI 0.87 to 1.76) (Forest plot 4.3).
2. Mitoxantrone‐containing regimens versus non‐antitumour antibiotic regimens Four trials reported on four treatment comparisons of mitoxantrone containing regimens versus CMF regimens as first line chemotherapy for metastatic breast cancer (Forest plot 5). No statistically significant difference in overall survival (HR 0.95, 95% CI 0.81 to 1.12) or response (HR 0.88, 95% CI 0.64 to 1.19) was observed and heterogeneity was statistically significant for response (heterogeneity chi‐square 17.06, df = 3, P = 0.0007). A statistically significant benefit for time to progression was observed in one of these trials (ANZ BCTG 8614) comparing single agent mitoxantrone to CMFP (HR 0.79, 95% CI 0.65 to 0.94); but response favoured the comparator regimen with an OR of 0.56 (95% CI 0.36 to 0.86). The three other trials did not show a statistically significant difference in response for regimens containing mitoxantrone versus non‐antitumour antibiotic containing regimens.
There was no statistically significant association between the use of mitoxantrone and toxic events or treatment related deaths compared to the comparator regimens (treatment‐related deaths: OR 1.58, 95% CI 0.26 to 9.44). Of the two trials reporting treatment related deaths, both addressing Question (b), one trial reported a toxic death in the control group; the other trial reported two toxic deaths in the mitoxantrone group. Two trials reported on QoL and demonstrated no difference in global QoL scores between the treatment groups (Table 4) (ANZ BCTG 8614; Harper‐Wynne 1999).
Discussion
This review did not identify a statistically significant benefit in overall survival for antitumour antibiotic containing regimens over non‐antitumour antibiotic containing regimens in the first line management of metastatic breast cancer. Despite the lack of evidence of survival benefit, this review demonstrated that anthracycline containing regimens provided a statistically significant advantage in time to progression and tumour response compared to non‐antitumour antibiotic containing regimens. Only ten anthracycline trials provided data on time to progression (2226 randomised patients; HR 0.76, 95% CI 0.69 to 0.83). All eligible anthracycline trials provided response data for 6,538 assessable patients, for an OR of 1.33 (95% CI 1.21 to 1.48). The favourable effect on time to progression and response was consistent and statistically significant in regimens where an anthracycline was used as a replacement agent (29 treatment comparisons; 4439 patients). Fewer trials reported on comparisons between an anthracycline as an additional agent (five treatment comparisons; 604 patients) or an anthracycline as a single agent (eight treatment comparisons; 1104 patients). In these trials we observed a response benefit for regimens that included an anthracycline as an additional agent (OR 1.90, 95% CI 1.33 to 2.72), but not for regimens that included anthracyclines as a single agent (OR 1.09, 95% CI 0.85 to 1.41).
An exploratory subgroup analysis by class of comparator demonstrated that anthracycline containing regimens were statistically significantly associated with improved tumour response compared to CMF, CMFVP and taxane regimens and showed no difference to other cyclophosphamide based regimens. We also observed a statistically significant survival benefit for anthracycline containing regimens compared to CMFVP regimens (HR 0.84, 95% CI 0.72 to 0.99, P = 0.04). It is unclear why anthracycline containing regimens would be more effective compared to CMFVP regimens and not regimens containing CMF alone. One possible explanation is that the dose or scheduling of CMF varied between these regimens or that patients allocated to CMF regimens received the potential benefit of anthracyclines as second line therapy earlier or more frequently than those allocated CMFVP regimens. Further investigation of the type of regimens and protocols used in the individual trials is required to address this question. It is also possible that this modest finding represents a type I error, and we note that this finding is not statistically significant after correction for the multiple comparisons performed. A pre‐specified sensitivity analysis of the four anthracycline trials that reported clearly adequate methods of allocation concealment also indicated a modest survival benefit for anthracycline containing regimens (HR 0.86, 95% CI 0.75 to 0.98) compared to CMF (two trials) or CMFVP regimens (two trials) to suggest that anthracyclines dominated CMF regimens, however as one of multiple comparisons performed in this review this result must also be interpreted with caution.
This review did not demonstrate a difference in overall survival or tumour response for mitoxantrone containing regimens versus non‐antitumour antibiotic regimens, although a benefit was observed for time to progression (HR 0.84, 95% CI 0.72 to 0.98). Statistically significant heterogeneity was observed for tumour response (P heterogeneity = 0.0007). Similarly, there was statistically significant heterogeneity of effect across the anthracycline trials for response (P heterogeneity = 0.0009). In contrast, no significant heterogeneity was observed in the survival estimates for trials within each class of antitumour antibiotic trials (anthracyclines and mitoxantrone). The heterogeneity in the response estimates most likely reflects the different activity of the wide range of different regimens and protocols represented in the included trials, however, specific factors have not been explored in this review.
The interpretation of treatment effect on response is also problematic in this review due to the lack of information provided in some of the trials about the reasons for excluding patients from the analysis of response rates and it is possible that the definition of 'assessable' varied across the trials.
Given the lack of survival benefit, the use of anthracyclines in the management of metastatic breast cancer must be carefully weighed against the risk of toxicities associated with these agents. The risks of cardiotoxicity, leukopenia and nausea/vomiting were all significantly increased in anthracycline containing regimens (cardiac toxicity OR 5.91, 95% CI 3.56 to 9.80; leukopenia OR 1.25, 95% CI 1.11 to 1.41; nausea/vomiting OR 1.71, 95% CI 1.42 to 2.06).
Our results are consistent with an earlier review of 30 trials (5241 patients) of first and second line therapy for metastatic breast cancer, which found no overall survival benefit for combination therapies that included anthracyclines compared to those that did not (Fossati 1998). A recent large trial comparing the efficacy of doxorubicin and paclitaxel as single and combined agents has recently reported similar findings that antitumour response activity did not confer an advantage in overall survival (Sledge 2003).
The tumour response associated with these agents appeared to offer a poor surrogate for overall survival gain, however, some correlation between these outcomes cannot be ruled out. One possible explanation is that while anthracyclines provide a highly active initial anti‐tumour response, 'catch‐up' occurs after tumour progression when a subsequent and less active regimen is used as salvage therapy. Conversely, the initial use of less active combinations may be rectified with the subsequent use of anthracyclines.
Authors' conclusions
Implications for practice.
Regimens that contain antitumour antibiotics do not offer any additional benefit in overall survival over regimens that do not contain these agents in the first line management of metastatic breast cancer. Anthracycline containing regimens do provide advantages in tumour response and time to progression over standard non‐antitumour antibiotic containing regimens but these benefits need to be weighed against the increased risk of toxicity before consideration as palliative therapy. There is insufficient evidence to determine the relative efficacy of other non‐anthracycline antitumour antibiotic regimens compared to non‐antitumour antibiotics.
Implications for research.
A review of trials comparing overall survival, quality of life and toxicity for anthracyclines as a first line agents in regimens of sequential therapy is warranted to further investigate the optimal use of these agents.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2018 | Review declared as stable | It is internationally accepted that anthracycline‐containing regimens are part of standard clinical practice. Today, however, chemotherapy decisions are based on the specific molecular subtype of the breast cancer. As this Cochrane Review contains trials that are unselected for breast cancer subtype, it is no longer feasible to update this Review. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 7 August 2008 | Amended | Converted to new review format. |
Notes
This review was updated for Issue 3, 2007. A new search was conducted October 2006. One new trial was added (Fountzilas 2004) to the meta‐analysis, one new trial was exluded from the analysis (Hori 2001) and one ongoing trial was removed (Leiden Uni Centre) without results being available for inclusion.
This review was also copyeditied and a plain language summary included to replace the previous synopsis.
There were no statistically significant changes resulting from the inclusion of Fountzilas 2004 and the recommendations of the authors remain unchanged.
Acknowledgements
We would like to thank Nicole Holcroft for her work in the identification of studies through the Cochrane Breast Cancer Group's specialised register. We also acknowledge the contribution made to the original concept for this review by I. Craig Henderson, Kathleen Pritchard, Martin Tattersall, Martin Stockler, Christine Brunswick, Roldano Fossati and Alessandro Liberati.
Data and analyses
Comparison 1. Antitumour antibiotic containing regimens vs not: all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 35 | 5605 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.96 [0.90, 1.02] |
| 1.1.1 Regimen A plus antitumour antibiotic vs Regimen A | 4 | 547 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 1.1.2 Regimen A plus antitumour antibiotic vs Regimen B | 25 | 3818 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 1.1.3 Single agent antitumour antibiotic vs Regimen C | 6 | 1240 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.03 [0.92, 1.16] |
| 1.2 Time to progression | 14 | 2815 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.84 [0.77, 0.91] |
| 1.2.1 Regimen A plus antitumour antibiotic vs Regimen A | 2 | 78 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.67 [0.37, 1.24] |
| 1.2.2 Regimen A plus antitumour antibiotic vs Regimen B | 10 | 2015 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.83 [0.75, 0.92] |
| 1.2.3 Single agent antitumour antibiotic vs Regimen C | 2 | 722 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.86 [0.74, 1.00] |
| 1.3 Overall response (assessable patients) | 45 | 6538 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.21, 1.48] |
| 1.3.1 Regimen A plus antitumour antibiotic vs Regimen A (assessable patients) | 5 | 604 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.33, 2.72] |
| 1.3.2 Regimen A plus antitumour antibiotic vs Regimen B (assessable patients) | 32 | 4439 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.27, 1.62] |
| 1.3.3 Single agent antitumour antibiotic vs Regimen C (assessable patients) | 9 | 1495 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.15] |
| 1.4 Treatment‐related death | 20 | 4364 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.77, 1.83] |
| 1.4.1 Regimen A plus antitumour antibiotic vs Regimen A | 1 | 345 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.18, 5.54] |
| 1.4.2 Regimen A plus antitumour antibiotic vs Regimen B | 15 | 3242 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.61, 1.67] |
| 1.4.3 Single agent antitumour antibiotic vs Regimen C | 4 | 777 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.80, 6.41] |
1.1. Analysis.

Comparison 1: Antitumour antibiotic containing regimens vs not: all trials, Outcome 1: Overall survival
1.2. Analysis.

Comparison 1: Antitumour antibiotic containing regimens vs not: all trials, Outcome 2: Time to progression
1.3. Analysis.

Comparison 1: Antitumour antibiotic containing regimens vs not: all trials, Outcome 3: Overall response (assessable patients)
1.4. Analysis.

Comparison 1: Antitumour antibiotic containing regimens vs not: all trials, Outcome 4: Treatment‐related death
Comparison 2. Antitumour antibiotic regimens containing anthracyclines vs non antitumour antibiotic containing regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 31 | 4846 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 2.1.1 Regimen A plus anthracycline vs Regimen A | 4 | 553 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.01 [0.83, 1.24] |
| 2.1.2 Regimen A plus anthracycline vs Regimen B | 22 | 3445 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 2.1.3 Single agent anthracycline vs Regimen C | 5 | 848 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 2.2 Time to progression | 12 | 2226 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.76 [0.69, 0.83] |
| 2.2.1 Regimen A plus anthracycline vs Regimen A | 2 | 78 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.67 [0.37, 1.24] |
| 2.2.2 Regimen A plus anthracycline vs Regimen B | 9 | 1817 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.82 [0.74, 0.91] |
| 2.2.3 Single agent anthracycline vs Regimen C | 1 | 331 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.55 [0.44, 0.68] |
| 2.3 Overall response (assessable patients) | 42 | 5786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.26, 1.56] |
| 2.3.1 Regimen A plus anthracycline vs Regimen A (assessable patients) | 5 | 604 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.33, 2.72] |
| 2.3.2 Regimen A plus anthracycline vs Regimen B (assessable patients) | 29 | 4078 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.27, 1.63] |
| 2.3.3 Single agent anthracycline vs Regimen C (assessable patients) | 8 | 1104 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.41] |
| 2.4 Treatment‐related death | 18 | 4117 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.74, 1.82] |
| 2.4.1 Regimen A plus anthracycline vs Regimen A | 1 | 345 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.18, 5.54] |
| 2.4.2 Regimen A plus anthracycline vs Regimen B | 13 | 2995 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.57, 1.64] |
| 2.4.3 Single agent anthracycline vs Regimen C | 4 | 777 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.80, 6.41] |
2.1. Analysis.

Comparison 2: Antitumour antibiotic regimens containing anthracyclines vs non antitumour antibiotic containing regimens, Outcome 1: Overall survival
2.2. Analysis.

Comparison 2: Antitumour antibiotic regimens containing anthracyclines vs non antitumour antibiotic containing regimens, Outcome 2: Time to progression
2.3. Analysis.

Comparison 2: Antitumour antibiotic regimens containing anthracyclines vs non antitumour antibiotic containing regimens, Outcome 3: Overall response (assessable patients)
2.4. Analysis.

Comparison 2: Antitumour antibiotic regimens containing anthracyclines vs non antitumour antibiotic containing regimens, Outcome 4: Treatment‐related death
Comparison 3. Sensitivity analysis: anthracyclines vs not, using studies with clearly described allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Overall survival | 6 | 1439 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.88 [0.78, 0.99] |
| 3.1.1 Regimen A plus anthracycline vs Regimen A | 0 | 0 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | Not estimable |
| 3.1.2 Regimen A plus anthracycline vs Regimen B | 5 | 1108 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.86 [0.75, 0.98] |
| 3.1.3 Single agent anthracycline vs Regimen C | 1 | 331 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 3.2 Time to progression | 4 | 1134 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.71 [0.63, 0.80] |
| 3.2.1 Regimen A plus anthracycline vs Regimen A | 0 | 0 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | Not estimable |
| 3.2.2 Regimen A plus anthracycline vs Regimen B | 3 | 803 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.80 [0.69, 0.92] |
| 3.2.3 Single agent anthracycline vs Regimen C | 1 | 331 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.55 [0.44, 0.68] |
| 3.3 Overall response (assessable patients) | 10 | 1765 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.35, 1.98] |
| 3.3.1 Regimen A plus anthracycline vs Regimen A (assessable patients) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3.2 Regimen A plus anthracycline vs Regimen B (assessable patients) | 9 | 1434 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.26, 1.94] |
| 3.3.3 Single agent anthracycline vs Regimen C (assessable patients) | 1 | 331 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.26, 3.22] |
3.1. Analysis.

Comparison 3: Sensitivity analysis: anthracyclines vs not, using studies with clearly described allocation concealment, Outcome 1: Overall survival
3.2. Analysis.

Comparison 3: Sensitivity analysis: anthracyclines vs not, using studies with clearly described allocation concealment, Outcome 2: Time to progression
3.3. Analysis.

Comparison 3: Sensitivity analysis: anthracyclines vs not, using studies with clearly described allocation concealment, Outcome 3: Overall response (assessable patients)
Comparison 4. Subgroup analysis: anthracyclines vs not, by class of comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Overall survival | 29 | 4438 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.95 [0.89, 1.02] |
| 4.1.1 Anthracyclines vs C+ comparator | 8 | 506 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.25 [0.99, 1.57] |
| 4.1.2 Anthracyclines vs CMF comparator | 13 | 2136 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.95 [0.86, 1.05] |
| 4.1.3 Anthracyclines vs CMF+ comparator | 5 | 767 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.84 [0.72, 0.99] |
| 4.1.4 Anthracyclines vs taxane comparator | 3 | 1029 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.96 [0.83, 1.10] |
| 4.2 Time to progression | 11 | 1899 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.76 [0.69, 0.83] |
| 4.2.1 Anthracyclines vs C+ comparator | 5 | 304 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 4.2.2 Anthracyclines vs CMF comparator | 4 | 999 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.82 [0.72, 0.93] |
| 4.2.3 Anthracyclines vs CMF+ comparator | 1 | 265 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.72 [0.57, 0.90] |
| 4.2.4 Anthracyclines vs taxane comparator | 1 | 331 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.55 [0.44, 0.68] |
| 4.3 Overall response (assessable patients) | 41 | 5493 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.26, 1.58] |
| 4.3.1 Anthracyclines vs C+ comparator | 10 | 567 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.87, 1.76] |
| 4.3.2 Anthracyclines vs CMF comparator | 18 | 2515 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.20, 1.66] |
| 4.3.3 Anthracyclines vs CMFVP comparator | 10 | 1396 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.11, 1.72] |
| 4.3.4 Anthracyclines vs taxane comparator | 3 | 1015 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.20, 2.06] |
4.1. Analysis.

Comparison 4: Subgroup analysis: anthracyclines vs not, by class of comparator, Outcome 1: Overall survival
4.2. Analysis.

Comparison 4: Subgroup analysis: anthracyclines vs not, by class of comparator, Outcome 2: Time to progression
4.3. Analysis.

Comparison 4: Subgroup analysis: anthracyclines vs not, by class of comparator, Outcome 3: Overall response (assessable patients)
Comparison 5. Mitoxantrone containing regimens vs non antitumour antibiotic containing regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Overall survival | 4 | 763 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.95 [0.81, 1.12] |
| 5.1.1 Regimen A plus mitoxantrone vs Regimen A | 0 | 0 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | Not estimable |
| 5.1.2 Regimen A plus mitoxantrone vs Regimen B | 3 | 372 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 5.1.3 Single agent mitoxantrone vs Regimen C | 1 | 391 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.02 [0.83, 1.25] |
| 5.2 Time to progression | 3 | 635 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.84 [0.72, 0.98] |
| 5.2.1 Regimen A plus mitoxantrone vs Regimen A | 0 | 0 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | Not estimable |
| 5.2.2 Regimen A plus mitoxantrone vs Regimen B | 2 | 244 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.02 [0.75, 1.39] |
| 5.2.3 Single agent mitoxantrone vs Regimen C | 1 | 391 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.79 [0.65, 0.94] |
| 5.3 Overall response (assessable patients) | 4 | 752 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.19] |
| 5.3.1 Regimen A plus mitoxantrone vs Regimen A (assessable patients) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.3.2 Regimen A plus mitoxantrone vs Regimen B (assessable patients) | 3 | 361 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.90, 2.18] |
| 5.3.3 Single agent mitoxantrone vs Regimen C (assessable patients) | 1 | 391 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.86] |
| 5.4 Treatment‐related death | 2 | 247 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.26, 9.44] |
| 5.4.1 Regimen A plus mitoxantrone vs Regimen A | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.4.2 Regimen A plus mitoxantrone vs Regimen B | 2 | 247 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.26, 9.44] |
| 5.4.3 Single agent mitoxantrone vs Regimen C | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
5.1. Analysis.

Comparison 5: Mitoxantrone containing regimens vs non antitumour antibiotic containing regimens, Outcome 1: Overall survival
5.2. Analysis.

Comparison 5: Mitoxantrone containing regimens vs non antitumour antibiotic containing regimens, Outcome 2: Time to progression
5.3. Analysis.

Comparison 5: Mitoxantrone containing regimens vs non antitumour antibiotic containing regimens, Outcome 3: Overall response (assessable patients)
5.4. Analysis.

Comparison 5: Mitoxantrone containing regimens vs non antitumour antibiotic containing regimens, Outcome 4: Treatment‐related death
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmann 1974a.
| Study characteristics | ||
| Methods | Single centre randomised controlled trial. 3 arm trial. Method of randomisation and allocation concealment not reported. Stratification by disease free interval, time since menopause and dominant site of metastases. Other baseline comparability not reported. | |
| Participants | 48 pts. Women with metastatic breast cancer. No prior cytotoxic chemotherapy. | |
| Interventions | Comparison 1: A vs CFP Arm A: A doxorubicin 60mg/m2 iv day 1 and repeated 3‐4 weeks Maximum culmulative dose of doxorubicin = 550mg/m2 Arm B: CFP cyclophosphamide 150mg/m2 iv day 1‐5; 5‐fluorouracil 300mg/m2 day 1‐5; and prednisone po at a dose level of 30mg/d for 2 wks, 20mg/day for 1 week and a maintenance dose of 10mg/d. 4 week cycle Arm C: CVFP cyclophosphamide 150mg/m2 iv day 1‐5; 5‐fluorouracil 300mg/m2 day 1‐5; vincristine was given 1.4mg/m2 on days 1 and 5 and prednisone was given orally at a dose level of 30mg/d for 2 wks, 20mg/day for 1 week and a maintenance dose of 10mg/d; |
|
| Outcomes | Response Overall survival Toxicity | |
| Notes | ITT analysis. 47/48 pt followed up until death. Est min f/up 2 months (2 x 4 week cycles), est max 102 months (from OS curve). Overall survival for doxorubicin vs polychemotherapy regimens extracted for meta‐analysis from follow‐up publication (Ahmann, 1987) Cross over to alternate regimen on disease progression. 1 possible treatment‐related death due to cardiac failure in a pt on Doxorubicin. Another pt withdrawn from doxorubicin arm due to early cardiac failure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ahmann 1974b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: A vs CVFP Arm A: A doxorubicin 60mg/m2 iv day 1 and repeated 3‐4 weeks Maximum culmulative dose of doxorubicin = 550mg/m2 Arm C: CVFP cyclophosphamide 150mg/m2 iv day 1‐5; 5‐fluorouracil 300mg/m2 day 1‐5; vincristine was given 1.4mg/m2 on days 1 and 5 and prednisone was given po at a dose level of 30mg/d for 2 wks, 20mg/day for 1 week and a maintenance dose of 10mg/d; |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ahmann 1991.
| Study characteristics | ||
| Methods | Single centre randomised controlled trial. Pts were stratified by menopausal age, ECOG performance score, hormonal treatment and dominant disease status. Patients randomised according to a dynamic allocation scheme. Method of allocation concealment not reported. Dates of accrual not reported. Baseline comparability achieved. | |
| Participants | 94pts (93 eligible) Women with histologically confirmed BC, and progressive metastatic disease not amenable to standard surgical or radiotherapeutic techniques. No patients had prior treatment with the chemotherapy agents used in this study. Age range: 36‐75 yrs in CAP arm; 33‐78 yrs in CFP arm. Median age 58 yrs in CAP arm, 56 years in CFP arm | |
| Interventions | CAP vs CFP Arm A: CAP cyclophosphamide 400mg/m2 iv over 30min; doxorubicin 40mg/m2 by iv push every 4 weeks in stable or responding patients; prednisone 30mg po on days 2‐14, 20mg orally on days 15‐21 and 10mg orally therafter Arm B: CFP cyclophosphamide 150mg/m2 iv by push over 30min on each of 5 sucessive days every 5 weeks if stable or responding, 5‐flurouracil 300mg/m2 by iv push every 30mins on each 5 successive days every 5 weeks, prednisone 30mg po on days 2‐14, 20mg orally on days 15‐21 and 10mg po thereafter. Regimens continued until objective evidence of progressive disease ‐ or a maximum culminative dose of doxorubicin was 450mg/m2 was reached‐ then pts just received CP |
|
| Outcomes | Response Overall Survival Time to progression Toxicity | |
| Notes | One pt was ineligible due to heart disease, 93 eligible pts were included in the analyses. Follow‐up details not reported. Estimated min = 5.5 months (median time to progression, averaged over both arms ), est. max 0S =66 months (from curve), est max PFS= 34.5 months (from curve). Toxic deaths not reported. Cardiac toxicity was observed in 4 pts on the CAP arm but it was not clinically severe. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ANZ BCTG 8614.
| Study characteristics | ||
| Methods | Multi‐centre international randomised controlled trial. Accrual January 1988 to June 1993. Pts stratified by performance status, metastatic site & institution. Randomisation via centralised office. Baseline comparisons of pt characteristics not available | |
| Participants | 391 pts Advanced/metastatic breast cancer No prior chemotherapy for metastatic disease | |
| Interventions | MZA vs CMFP Arm A: MZA mitoxantrone 14mg/m2 iv. day 1 of 21 day cycle Arm B: CMFP cyclophosphamide 100mg/m2 po. day 1‐14; methotrexate 40mg/m2 iv day 1, 8; 5‐fluorouracil 600mg/m2 i.v. day 1, 8 prednisone 40mg/m2 p.o. day 1‐14. 28 day cycle. |
|
| Outcomes | Response Overall survival Time to Treatment failure Time to first disease progression. Toxicitiy Quality of Life (self‐assessment, spitzer QL index) | |
| Notes | Analysis by ITT. 9 pts excluded: ineligible, reasons given (8); did not receive protocol treatment (1) Min reported follow‐up 9 months, max reported follow‐up 74 months. Toxic deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
B122.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial Pts were stratified according to menstrual status, diseae free survival, number of disease sites, and dominant disease sites. Centralised randomisation used for 3 hospitals (Pocock method), randomisation using sealed envelopes used for 1 hospital. Dates of accrual not reported. Pre‐treatment pt characteristics balanced across the 2 treatment arms. | |
| Participants | 92 pts (78 evaluable) Women with evidence of metastatic disease, no prior chemotherapy. 56% of pts were age 50yrs or older. | |
| Interventions | CAF vs CMF Arm A: CAF cyclophosphamide 100mg/m2 po as a single daily dose on days 1‐14; doxorubicin 30mg/m2 iv days 1, 8; 5‐fluorouracil 500mg/m2 iv 1, 8. Arm B: CMF cyclophosphamide 100mg/m2 po on days 1‐14; methotrexate 40mg/m2 iv day 1 and 8 each cycle; 5‐fluorouracil 600mg/m2 i. days 1, 8 Both regimens were given at 4 week cycles with 2 weeks on drug and 2 weeks off therapy until evidence of disease progression ‐ or a maximum culminative dose of doxorubicin was 450mg/m2 was reached‐ then pts received CMF |
|
| Outcomes | Overall Survival Progression free survival Reponse Toxicity | |
| Notes | Did not report as ITT. 92 pts were enrolled, 78 pts were evaluable. Of the 14pts excluded from the analysis 10pts did not meet eligibility criteria (reasons stated), 3pts refused therapy after randomisation, one pt refused further therapy after day 1 of cycle. Follow‐up time not reported. Est min follow‐up =2 months (minimum 2 cycles followed to assess response), est max f/up from curve 29 months (OS), 23 months (PFS). Treatment‐related deaths not reported. No cardiovascular toxicity observed in any pt. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Brambilla 1976.
| Study characteristics | ||
| Methods | Randomised controlled trial. Accrual dates: March 1973 to June 1974 Before randomisation pts were stratified according to menopausal status, disease free interval and site of dominant lesion. Method of randomisation and allocation concealment not stated. Baseline comparability in pt age, prior therapy and disseminated osseous metastases noted between each arm. | |
| Participants | 110 pts (105 evaluable) Advanced/metastatic breast cancer. Excluded if the only manifestation of disease was either pleural effusion, osteoblastic or mixed osteoblastic‐osteolytic lesions, or a previously irradiated lesion of the breast. No pts had received prior chemotherapy. Age range: 25 ‐69 yrs in AV arm; 29‐70 yrs in CMF arm. Median age: 49 yrs in AV arm; 54 yrs in CMF arm. | |
| Interventions | AV vs CMF Arm A: AV doxorubicin 75mg/m2 iv every three weeks; vincristine 1.4mg/m2 iv days 1, 8. x 8 cycles Arm B: CMF cyclophosphamide 100mg/m2 po days 1‐14; methotrexate 40mg/m2 iv days 1, 8; 5‐fluorouracil 600mg/m2 iv days 1 and 8. x 8 cycles |
|
| Outcomes | Response Overall Survivial Toxicity | |
| Notes | ITT not followed. 110 pts randomised, 5 pts were not considered evaluable as lost to follow‐up (1), or died early of progressive disease after the first cycle of treatment (4). Efficacy analyses conducted using 105 evaluable pts. Pts cross‐over on disease progression. Pts with complete or partial remission after 8 cycles AV crossed over to CMF for the next 8 cycles to avoid cardiotoxicity. Pts over 60 or with widespread metastases had an initial dose reduction. Follow‐up time not reported. Est min=5 months (8 cycles x 2‐3 weeks), est max=32 months (from survival curve) The dominant site of disease was in the soft tissues (breast, skin, lymph nodes) in 56%, in viscera in 22% and in bones in 22% in AV Arm and 51%, 24% and 24% respectively in the CMF arm. Response reported overall and by site of metastases. One treatment‐related death reported due to cardiac toxicity in a pt who had completed 8 full cycles of doxirubicin (total 600mg/m2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
CALGB Aisner 1987a.
| Study characteristics | ||
| Methods | Multi‐centre national randomised controlled trial. 6 arm trial Pts were stratified by disease free interval & dominant site of disease. Dates of accrual: 11 October, 1976 to 1 February, 1980. Randomisation was by sealed envelope using a Latin square design balancing across and within institutions. Baseline comparability between chemo‐immunotherapy and chemotherapy trials and between intervention arms achieved. | |
| Participants | 432 pts (395 evaluable). Women with recurrent, progressive or metastatic disease. Over 80% of patients has visceral or osseous metastatic disease. No pts had been treated with cytotoxic chemotherapy. Median age: 57 yrs in CAF arm; 55 yrs in CAFVP arm and CMF arm Median age across chemo‐immunotherapy arms: 56 yrs. | |
| Interventions | Comparison 1: CAF vs CMF Arm A: CAF cyclophosphamide 100mg/m2/d po, days 1‐14; doxorubicin 25mg/m2 iv, days 1, 8 (after total dose of 450mg/m2 replaced with methotrexate 40mg/m2 iv, days 1 & 8); 5‐fluorouracil 500mg/m2 iv, days 1 & 8. 28 day cycle. Arm B: CMF cyclophosphamide 100mg/m2/d po days 1‐14; methotrexate 40mg/m2 iv days 1, 8; 5‐fluorouracil 500mg/m2 iv days 1, 8. 28 day cycle. |
|
| Outcomes | Response Overall survival (from the date of intiating therapy) Time to treatment failure Toxicity | |
| Notes | 6 arm trial. 4 comparisons used for this meta‐analysis. Randomisation to chemoimmunotherapy ceased after an interim evaluation showed no benefit & increased toxicity. 432 pts were enrolled. 37 pts were unevaluable: ineligible (20), protocol violoations, early deaths (4), inadequate records (2), improper randomisation(1). Analyses was conducted using 395 evaluable patients (260/283 patients randomised to chemotherapy, 135/149 patients randomised to chemoimmunotherapy). Time‐to‐event data not extracted from published curves for inclusion in this meta‐analysis as unable to do so accurately to replicate reported study findings. Overall survival benefit reported for the CAF arm versus the CMF arm (p=0.04). A three way comparison between time to progression in the 3 chemotherapy arms showed a statistically significantly difference (p=0.01) favouring the CAF arm. No statistically significant differences in survival or time to progression reported between the 3 chemoimmunotherapy arms. Follow‐up times not reported. Estimated min = 2 months (2 cycles), est. max = 36 months (from survival curve). 8 treatment‐related deaths: 5 due to infection in arms CAF+MER, CAFVP+MER, CAF, CAFVP (2); 2 due to haemorrhage in arms CAF+MER, CMF; 1 due to cardiac toxicity in CAF arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
CALGB Aisner 1987b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: CAFVP vs CMF Arm C: CAFVP cyclophosphamide 100mg/m2/d po, days 1‐14; doxorubicin 25mg/m2 iv, days 1, 8 (after total dose 450mg/m2 replaced with methotrexate 40mg/m2 iv, days 1 & 8); vincristine 1.0mg/m2 iv, days 1, 8; prednisone 40mg/m2/d po, days 1‐14. 28 day cycle Arm A: CMF cyclophosphamide 100mg/m2/d po days 1‐14; methotrexate 40mg/m2 iv days 1, 8; 5‐fluorouracil 500mg/m2 iv days 1, 8. 28 day cycle |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
CALGB Aisner 1987c.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 3: CAF + MER vs CMF + MER Arm A: CAF + MER cyclophosphamide 100mg/m2/d po, days 1‐14; doxorubicin 25mg/m2 iv, days 1, 8 (after total dose of 450mg/m2 replaced with methotrexate 40mg/m2 iv, days 1, 8); 5‐fluorouracil 500mg/m2 iv, days 1, 8. 28 day cycle Arm B: CMF + MER cyclophosphamide 100mg/m2/d po days 1‐14; methotrexate 40mg/m2 iv days 1, 8; 5‐fluorouracil 500mg/m2 iv days 1, 8. 28 day cycle MER 200µg days 1 & 8. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
CALGB Aisner 1987d.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 4: CAFVP + MER vs CMF + MER Arm C: CAFVP + MER cyclophosphamide 100mg/m2/d po, days 1‐14; doxorubicin 25mg/m2 iv, days 1, 8 (after total dose 450mg/m2 replaced with methotrexate 40mg/m2 iv, days 1, 8); vincristine 1.0mg/m2 iv, days 1, 8; prednisone 40mg/m2/d po, days 1‐14. 28 day cycle Arm A: CMF + MER cyclophosphamide 100mg/m2/d po days 1‐14; methotrexate 40mg/m2 iv days 1, 8; 5‐fluorouracil 500mg/m2 iv days 1, 8. 28 day cycle MER 200µg days 1 & 8. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
CALGB Tormey 1984a.
| Study characteristics | ||
| Methods | Multi‐centre international randomised controlled trial. 3 arm trial. Method of randomisation and allocation concealment not reported. Accrual commenced in 1974. Baseline comparability achieved. | |
| Participants | 396 randomised (302 evaluable). Women with progressive metastatic breast carcinoma. No prior chemotherapy Median age of entry 54‐57 yrs across each arm. | |
| Interventions | Comparison 1: CAFVP vs CMFVP‐Continuous Arm A: CAFVP cyclophosphamide 80mg/m2 po q day, adriamycin 25mg/m2 iv q week, 5‐fluorouracil 500mg/m2 i.v. q week, vincristine 1.0mg/m2 iv q week, prednisone 40mg/m2 po day 1‐14. 28 day cycles. After 6 cycles of CAFVP, cross‐over to CMFVP‐I. Arm B: CMFVP‐C cyclophosphamide 80mg/m2 po.daily, methotrexate 40mg/m2 iv weekly, 5‐fluorouracil 500mg/m2 iv weekly, vincristine 1.0mg/m2 iv weekly, prednisone 30mg/m2 days 1‐21 then tapering to zero over 7 days. 12 weeks of therapy then a 2 week break followed by maintenance therapy with cyclophosphamide 80mg/m2 po daily, methotrexate 40mg/m2 i. q 3weeks, 5‐fluorouracil 500mg/m2 iv q 3weeks, vincristine 1.0mg/m2 iv q6weeks, and from week 18 additional prednisone 30mg/m2 po days 1‐7 |
|
| Outcomes | Response Overall survival Time to treatment failure Toxicity | |
| Notes | 3 arm trial. 2 comparisons used in this meta‐analysis: CAFVP vs CMFVP‐C, CAFVP vs CMFVP‐I. ITT analysis not followed. Time to event data based on 302/396 patients. Follow‐up time not reported. Est min f/up =9.3 months (median time to treat failure, average over 3 arms), est max f/up = 48 months (from survival curve). The median time for overall survival was 19 months for CAFVP compared to 13 months for CMFVP‐I (p=0.01) and 16 months for CMFVP‐I (p=0.24). The time to treatment failure for CAFVP was also statistically significantly longer than CMFVP‐I (p=0.01) but not CMFVP‐C (p=0.09). The CR+PR median remission duration was 14 months for CAFVP compared to 7 months for CMFVP‐I (p<0.01) and 9 months for CMFVP‐C (p=0.07). Using reported table percentages, 10% of deaths associated with toxicities in CAFVP arm (cardiac toxicity 1%, sepsis 4%, leukopenia 3%, thrombocytopenia 1%, GI toxicity 1%); 10% in the CMFVP‐C arm (cardiac toxicity 1%, sepsis 5%, leukopenia 3%, thrombocytopenia 1%); and 18% in the CMFVP‐I arm (cardiac toxicity 1%, sepsis 8%, leukopenia 7%, thrombocytopenia 1%, GI toxicity 1%). Total number of treatment‐related deaths not estimable from table. One death associated with cardiac toxicity in each arm, and 2 additional pts with severe non‐fatal cardiac toxicity and 4 pts with mild CHF observed in CAFVP arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
CALGB Tormey 1984b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: CAFVP vs CMFVP‐Intermittent Arm A: CAFVP cyclophosphamide 80mg/m2 po q day, adriamycin 25mg/m2 iv q week, 5‐fluoruouracil 500mg/m2 iv q week, vincristine 1.0mg/m2 iv q week, prednisone 40mg/m2. 28 day cycles. Arm C: CMFVP ‐I cyclophosphamide 100mg/m2 po days 1‐4, methotrexate 40mg/m2 iv days 1 and 8, 5‐fluorouracil 500mg/m2 iv days 1 and 8, vincristine 1.0mg/m2 iv days 1 and 8, prednisone 40mg/m2 days 1‐14. 28 day cycles After 6 cycles of CAFVP, cross‐over to CMFVP‐I. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Carmo‐Pereira 1981.
| Study characteristics | ||
| Methods | Randomised controlled trial Pts stratified by disease‐free interval, menopausal status, site of dominant lesion Method of randomisation and allocation concealment not reported. Dates of accrual: March 1976 to June 1979 Baseline comparability achieved. | |
| Participants | 51 pts. Women with progressive, histologically proven metastatic breast cancer, refractory to endocrine therapy and irradiation No pt had prior cytoxic chemotherapy Age range: 29‐64 yrs in VAC arm; 28‐62 yrs in CMFP arm. Median age: 49 yrs in VAC arm; 50.5yrs in CMFP arm. | |
| Interventions | VAC vs CMFP Arm A: VAC vincristine 1.4mg/m2 iv on days 1, 8; doxorubicin 40mg/m2 iv on day 1; cyclophosphamide 500mg/m2 iv on day 1 with the cycle repeated every 21 days Arm B: CMFP cyclophosphamide 100mg/m2 p.o. day 1‐15; methotrexate 20mg/m2 iv; 5‐fluorouracil 500mg/m2 iv weekly for 20 weeks; prednisone 20mg/m2 po daily with diminishing doses. Maintenance regimen: cyclophosphamide 100mg/m2 po day 1‐15; methotrexate 20mg/m2 iv on days 1,8 and 15; 5‐fluorouracil 500mg/m2 iv on days 1,8 and 15; prednisone 20mg/m2 po daily on days 1‐15 with a 3 week rest period between the courses. |
|
| Outcomes | Response (UICC) Overall survival (lifetable method) Toxicity | |
| Notes | ITT analysis followed. CMFP regime had a maintenance phase Pts crossed‐over on progression. There was no statistically significant difference in median duration of response (12 months for each arm) or median survival between the VAC (median = 22.4 months) and CMFP (median=18 months) arms. Minimum follow‐up of 6 months reported. Estimated maximum follow‐up of 48 months (from lifetable plot of survival). 1 treatment‐related death in VAC arm due to sepsis. Cardiac toxicity was observed in 1 pt in VAC arm (symptomatic CHF). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Chauvergne 1978.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial Method of randomisation and allocation concealment not stated. Dates of accrual: October 1975 to April 1976 Baseline comparability achieved. | |
| Participants | 70pts All pts had advanced breast cancer. 69/70 patients had metastatic disease. All patients had no prior cytotoxic chemotherapy. Age range: 24‐71 yrs in DVM arm; 31‐85 yrs in VCF arm Median age reported: 58‐62 yrs in DVM arm; 60‐64 yrs in VCF arm. | |
| Interventions | AMV vs CVF Arm A: AMV doxorubicin 50mg/m2 iv day 1; vincristine 1mg/m2 iv day 2; methotrexate 6mg/m2 day 3,4,5. 20 day cycle Arm B: CVF cyclophosphamide 300mg/m2 iv days 3,4,5,6; 5‐fluorouracil 500mg/m2 iv 3,4,5; vincristine 0.6mg/m2 iv day 1,2. 31 day cycle. |
|
| Outcomes | Response Toxicity | |
| Notes | ITT analysis not followed, some pts not considered evaluable due to discontinuation of treatments. 61/70 patients completed at least 2 cycles and were included in evaluation of efficacy. Overall survival not reported. 1 treatment ‐related death reported in the DVM arm (leukothrombocytopenia) and nil in the VCF arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Coates 1987a.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial, two‐by‐two factorial design. 4 arm trial. Accrual: June 1982 ‐ June 1985 Pts were stratified by institution, performance score and history of adjuvent chemotherapy. Randomisation was conducted through a central telephone centre Baseline comparability achieved. | |
| Participants | 308 Pts (305 evaluable). 154 evaluable pts allocated to the 2 continuous therapy arms. 307 women and 1 man with histologically confirmed breast cancer and recurrent or metastatic disease. No prior use of cytotoxic agents. The majority of pts had received endocrine therapy for metastatic disease. 69% of pts were aged 50 yrs of age or over | |
| Interventions | Comparison 1: DC vs CMFP ‐ Continuous therapy Arm A: DC ‐ Continuous therapy doxorubicin 50mg/m2; cyclophosphamide 750mg/m2 iv each for 21 days Arm B: CMFP ‐ Continuous therapy cyclophosphamide 100mg/m2 orally daily for 14 days; methotrexate 40mg.m2 iv; 5‐fluorouracil on days 1, 8; prednisone 40mg/m2 daily for 14 days. 28‐day cycles. In each arm 3 cycles repeated continuously until disease progression occurred. |
|
| Outcomes | Response (WHO) Survival Time to Progression Quality of life | |
| Notes | 4 arm trial with 2 comparisons, both DC vs CMFP. Comparison 1= Continuous therapy: 3 cycles given and repeated until evidence of disease progression occurred. Comparison 2 = Intermittent therapy: 3 cycles given and repeated until there was evidence of disease progression. 308 subjects randomized, 2 subjects ineligible and one patient lost to follow‐up. Time‐to‐event analyses were performed on the 305 remaining patients. Pts in whom disease progressed during treatment or within six weeks of the last day of therapy were withdrawn from the study and followed only for survival. Minimum reported follow‐up=12 months, max reported f/up=48 months. Overall, 6 treatment related deaths in the CMFP arm and 2 in the DC arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Coates 1987b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: DC vs CMFP ‐ Intermittent therapy Arm A: DC ‐ Intermittent therapy doxorubicin 50mg/m2; cyclophosphamide 750mg/m2 iv each for 21 days. 3 cycles Arm B: CMFP ‐ Intermittent therapy cyclophosphamide 100mg/m2 orally daily for 14 days; methotrexate 40mg.m2 iv, 5‐fluorouracil on days 1, 8; prednisone 40mg/m2 daily for 14 days. 28‐day cycles, 3 cycles In each arm the 3 cycles were repeated when evidence of disease progression |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creagan 1984.
| Study characteristics | ||
| Methods | Randomised controlled trial. Treatments assigned according to a dynamic allocation scheme. Method of allocation concealment not reported. Stratification by ECOG performance score, yrs since menopause, site of dominant metastases and prior chemotherapy used. Baseline comparability achieved. | |
| Participants | 88 pts (86 eligible) Histologically confirmed, progressive metastatic breast cancer unable to be managed by standard surgical or therapeutic techniques. No prior use of the chemotherapeutic agents used in this study. Median age: 58 yrs in both arms | |
| Interventions | CA + CDDP vs CFP Arm A: CA + CDDP Single‐day i.v. infusion of cyclophosphamide 400mg/m2 and doxorubicin 40mg/m2, cisplatin 40mg/m2 was delievered over one‐hour. Repeated every 4 weeks. cross‐over to CFP after 4 cycles Arm B: CFP cyclophosphamide 150mg/m2 and 5‐fluorouracil 300mg/m2 administred by iv on days 1‐ 4; prednisone po 30mg/d days 1 through to 14, 20mg/d during days 15 to 21. Repeated every 5 weeks until progression. |
|
| Outcomes | Response Overall survival Time to progression Toxicity | |
| Notes | 86/88 pts included in the efficacy analyses. 1 pt was ineligible due to prior treatment, 1 pt was ineligible due to concurrent treatment for CNS metatstases. Fixed cross‐over from CAP to CFP after 4 treatment cycles. Patients failing CAP early entered a pilot study using 5‐FU, dibromodulcitol and prednisone. Follow‐up times not reported. Estimated minimum = 7.5 months (average median time to progression over both arms), est. max = 55 months (OS) and 44 months (PFS). Treatment related deaths and cardiotoxicity not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Creech 1979.
| Study characteristics | ||
| Methods | Randomised controlled trial. No information on randomisation provided. Pts were stratified according to 'poor' or 'good' risk Baseline comparability achieved. | |
| Participants | 78 pts. Women with visceral metastatic breast cancer. No prior cytotoxic chemotherapy Age range: 34‐79 yrs in CAMF arm; 32‐87 yrs in CMF arm. Median age: 56 yrs both arms | |
| Interventions | CAMF vs CMF Arm A: CAMF cyclophosphamide 50mg/m2 po, days 1‐14; doxorubicin 20mg/m2 iv, days 1, 8; methotrexate 20mg/m2 iv, days 1, 8; 5‐fluorouracil 300mg/m2 iv, days 1, 8. 28 day cycle. Arm B: CMF cyclophasphamide 50mg/m2 po, days 1‐14; methotrexate 20mg/m2 iv, days 1, 8; 5‐fluoruoracil 300mg/m2 iv, days 1, 8. 28 day cycle. Cycles continued until progression. CMF arm received doxorubicin 20mg/m2, iv days 1,8 after progression. Doxorubicin ceased after a maximum culmulative dose of 550 mg/m2 reached. |
|
| Outcomes | Response (ECOG) Survival (reported by response status) Progression‐free survival (reported by response status) Toxicity | |
| Notes | Randomised controlled trial of 2 low dose regimens. CMF pts crossed over to low dose Doxorubicin on progression. ITT analysis followed. Estimated min f/up = Estimated min f/up = 5months (median time to progression), max follow‐up = 39 months (from survival curve). Overall survival and time to progression were reported for subsets of pts by response status and not extracted for meta‐analysis. No statistically significant differences between CAMF and CMF were reported for time to event data. Median survival for PR/CR pts was 20 months in CAMF arm vs 19 months in CMF arm. Treatment related deaths were not reported. No cardiotoxicity was observed in either arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
DBCG 1999.
| Study characteristics | ||
| Methods | Multi‐centre national randomised controlled trial Randomisation occurred within department by a sealed envelope system Dates of accrual: April 1980‐ August 1984 Pre‐treatment characteristics of eligible pts balanced across treatment arms. | |
| Participants | 415 pts (341 eligible) Postmenopausal women with locally advanced (9%) or metastatic (91%) histiologically confirmed breast cancer. Age range: 45‐65yrs in CAF arm; 43‐65 yrs in CMF arm. Median age: 58 yrs in both arms No prior cytotoxic therapy for recurrent disease. Prior adjuvent treatment with tamoxifen if over one year ago since completion. | |
| Interventions | CAF + tamoxifen vs CMF + tamoxifen Arm A: CAF +Tamoxifen cyclophosphamide 400mg/m2, doxorubicin 25mg/m2 and 500mg/m2 5‐fluorouracil iv days 1, 8. Repeated every 4 weeks. + tamoxifen 30mg p.o. daily doxorubicin was replaced by methotrexate at a culmulative dose of 550mg/m2. Arm B: CMF + Tamoxifen cyclophosphamide 400mg/m2, and 40mg/m2 methotrexate; 500mg/m2 5‐fluorouracil iv days 1, 8. Repeated every 4 weeks + tamoxifen 30mg po daily |
|
| Outcomes | Response (WHO) Survival Time to Progression Toxicity | |
| Notes | 74/415 women were ineligible (reasons provided). Distribution of ineligible pts not equal across treatment arms (p=0.008). 6 pts in each arm not evaluable (protocol violation, missing data, lost to follow‐up). Time‐to‐event analysis used 341 eligible pts. Minimum follow‐up reported: 48 months (PFS), 132 months (OS). Maximum f/up reported: 108 months (PFS), 180 months (OS). There were no treatment‐related deaths. 1 pt in CAF arm developed CHF at a culmulative dose of Doxorubicin of 346mg/m2 and treatment was stopped. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ECOG Cummings 1985.
| Study characteristics | ||
| Methods | Randomised controlled trial. Method of randomisation not reported. Pts stratified by performance status and site of metastases. Dates of accrual: May 1978 to November 1979. Baseline imbalance in disease free interval (DFI). 41% of pts had a DFI <1month in CAF arm vs 29% in CMFP arm. 10% of pts had a DFI of 5+ yrs in CAF arm, 20% in CMFP arm. | |
| Participants | 177 pts (155 evaluable for CAF vs CMFP comparison arms) Women with histologically documented recurrent or metastic breast cancer aged 65 yrs or younger. Age distribution: 84% aged 50+ yrs in CAF arm; 75% aged 50+ yrs in CMFP arm. No prior cytotoxic chemotherapy. | |
| Interventions | CAF vs CMFP Arm A: CAF cyclophosphamide 100mg/m2/d orally on days 1, 14: adriamycin30mg/m2 and 5‐fluorouracil 500mg/m2 given iv on days 1, 8. 4 week cycles x 8 cycles Arm B: CMFP cyclophosphamide 100mg/m2 orally on days 1 ‐14; methotrexate 40mg/m2 iv on days 1 and 8; 5‐fluorouracil 600mg/m2 iv. on days 1, 8; prednisone 40mg/m2 orally on days 1‐ 14. 4 week cycles x 6 cycles. |
|
| Outcomes | Response Overall survival Time to treatment failure Toxicity | |
| Notes | 177 women randomised to CAF (82) CMFP (83) or CAF + Cp immunotherapy (12). CAF+Cp arm dropped at 6 months due to poor accrural and not included in this analysis. 10 pts not evaluated: ineligibility (6), transfered hospitals (1), pt refusal(1), reason not reported (2). 155 evaluable pts included in efficacy analysis. Min follow‐up = 30 months, Max f/up=48 months. There was no statistically significant difference between median response duration, times to treatment failure and survival times between the 2 arms. Treatment‐related deaths were not reported. 1 case of severe cardiotoxicity was reported in a pt in the CAF+Cp arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ECOG E1193a.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial. 3 arm trial. Accrual dates: Feb 1993 ‐ Sept 1995. Pts stratified by institution. Randomisation method not described. Baseline comparability of pt pre‐treatment characteristics achieved.. | |
| Participants | 739 pts (683 evaluable) Women with histologically confirmed breast adenocarcinoma with progressing regional (13‐19%) or metastatic disease No prior cyctotoxic chemotherapy for metastatic disease. Prior adjuvent chemotherapy eligible if ceased >=6 months prior and the regimen did not include anthracyclines or taxanes. Age range: 25‐79 yrs in doxorubicin arm; 27‐78yrs in combined agent arm; 27‐76 yrs in paclitaxel arm. Median age: 58 yrs in doxorubicin arm; 56 yrs in paclitaxel and combined agent arms. | |
| Interventions | Comparison 1: A vs T Arm A: A doxorubicin 60mg/m2 iv day 1. 3 week cycle x 8. Arm B: T paclitaxel 175mg/m2 iv day 1. 3 week cycle until disease progression. |
|
| Outcomes | Response Survival Time to treatment failure Toxicity QoL | |
| Notes | Pts on single agent arms were crossed over to the alternate single agent at progression. ITT not followed, data on 683 pts included in time‐to‐event analyses. 41 pts cancelled or excluded from analysis due to ineligibility (reasons stated), additonal 15 pts excluded with reasons not given. Estimated min follow‐up = 6 months (3 week cycle x 8), estimated max f/up = 75 months from curve. Treatment‐related deaths were reported in 2.5% of pts assigned to doxorubicin and 1.6% of pts assigned to the other 2 arms. Moderate‐severe cardiac complications were reported in 8.6‐8.7% of pts assigned to the 2 doxorubicin‐containing arms and 3.7% of pts assigned to paclitaxel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ECOG E1193b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: A T vs T. Arm C: AT doxorubicin 50mg/m2 iv day 1; paclitaxel 150mg/m2 iv, day 1. 3 week cycle x 8 Arm B: T paclitaxel 175mg/m2 iv day 1. 3 week cycle until disease progression. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ECOG EST 2173a.
| Study characteristics | ||
| Methods | Multi‐centre national randomised controlled trial. 3 arm trial. Pts stratified by performance status, disease‐free interval and dominant metastatic site. Method of randomisation and allocation concealment not reported Dates of accrual: October 1973 ‐ April 1974 | |
| Participants | 404 pts (331 evaluable) Women had recurrent or metastatic breast cancer. No prior cytotoxic chemotherapy. Age distribution: 61% aged 50‐65, 39% <50yrs. | |
| Interventions | Comparison 1: AV vs CMF Arm A: AV adriamycin 60mg/m2 iv and vincristine 1.2 mg/m2 iv (maximum dose of 2mg) day 1 of each 21 day cycle. 8 cycles. Arm B: CMF cyclophosphamide 100mg/m2 po. days 1‐14; methotrexate 40mg/m2 iv days 1‐8 and 5‐FU 600mg/m2 iv days 1, 8 of each 28 day cycle. 6 cycles. Responding patients were subsequently randomised to a maintenance program. |
|
| Outcomes | Response Survival Time to treatment failure Toxicity | |
| Notes | 19 pts cancelled prior to treatment, 53 pts ineligible (reasons not stated), 1 pt lost to follow‐up, 331 evaluable pts included in efficacy analysis. Cross‐over to alternate arm if disease progression. Responders were randomised to CMF or CMF + fluoxymesterone maintenance therapy after completing 6 month AV/CMF induction phase. Estimated minimum f/up = 6 months (time for completion of induction), est. max f/up = 48 months (from survival curve). Median time to treatment failure: AV = 5.7 months, CMF = 5.3 months, CMFP = 9.1 months (p=0.04). Median survival: AV = 13.7 months, CMF = 14.5 months, CMFP = 16.4 months (p=0.03). x treatment‐related deaths. AV arm: leukopenia (1%), infection(2%), cardiotoxicity (1%). CMF arm: infection (1%). CMFP arm: leukopenia (4%), infection (2%), other (1%). Mod‐severe, non‐fatal cardiotoxicity observed in 1% of pts in each arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ECOG EST 2173b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: AV vs CMFP Arm A: AV adriamycin 60mg/m2 iv and vincristine 1.2 mg/m2 iv (maximum dose of 2mg) day 1 of each 21 day cycle. 8 cycles. Arm C: CMFP cyclophosphamide 100mg/m2 po days 1‐14; methotrexate 40mg/m2 iv days 1‐8 and 5‐FU 600mg/m2 iv days 1 and 8 of each 28 day cycle, Prednisone 40mg/m2 orally days 1‐14 of each 28 day cycle. 6 cycles. Responding patients were subsequently randomised to a maintenance program. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
EORTC 10923.
| Study characteristics | ||
| Methods | International, multi‐centre randomised trial. Opened to accrual Aug 1993 and closed May 1996. Pts were stratified according to institution and prior adjuvant CT and randomised using centralised ranodimsation centre. | |
| Participants | 331 pts. Women with histologically proven metastatic breast cancer. No prior cytotoxic chemotherapy for advanced BC. Age range: 26‐75yrs. Median age: 54‐55 yrs. | |
| Interventions | Arm A: A
doxorubicin 75mg/m2 iv every 3 weeks. Arm B: T paclitaxel 200mg/m2 iv every 3 weeks. |
|
| Outcomes | Survival Progression‐free survival Response Toxicity QoL. | |
| Notes | 331 pts randomised, 4 pts never started treatment. If pts progressed within first 7 cycles they were crossed over to the alternate therapy. F/up details not reported ‐ est min = 5 months, est max = 46 months. All randomized patients included in time to event analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Fountzilas 2004.
| Study characteristics | ||
| Methods | Multi centre national, randomised Phase III trial | |
| Participants | 327 histologically proven ABC | |
| Interventions | Arm A:
epirubicin 80mg/m2 iv followed by paclitaxel 175mg/m2 iv every 3 weeks Arm B: T paclitaxel 175mg/m2 iv followed by carboplatin 180mg for 6 cycles |
|
| Outcomes | Overall response Survival Time to treatment failure Toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fraser 1993.
| Study characteristics | ||
| Methods | Single centre randomised controlled trial. Method of randomisation and allocation concealment not stated. Dates of accrual: October 1988 and March 1990. 11 year difference in median age between the 2 arms (not statistically significant). Menopausal status and site of disease balanced across the 2 arms. | |
| Participants | 40pts Pts with locally advanced or metastatic breast cancer. Failure to respond to hormone therapy. No prior use of non‐adjuvent cytotoxic chemotherapy. Age range: 26‐80 yrs in Epirubicin arm; 39‐84 yrs in CMF arm. Median age: 52yrs in Epirubicin arm; 63 yrs in CMF arm. | |
| Interventions | Epirubicin vs CMF Arm A: epirubicin 20mg iv every 7 days for 6 months or until disease progression. Arm B: CMF cyclophosphamide 100mg/m2 po on days 1‐14; methotrexate 35mg/m2 iv on days 1 and 8; 5‐fluorouracil 600mg/m2 iv on days 1, 8 on a 28 day cycle for 6 months or until disease progression. |
|
| Outcomes | Reponse (UICC) Survival Time to treatment failure Toxiticity (WHO) Quality of Life (NHP, LASA) | |
| Notes | ITT analysis. Different number of pts evaluable for different QoL instruments. 3 pt did not receive treatment and were not assessed for QoL. 1 pt did not complete the LASA correctly. Estimated minimum follow‐up time = 6 months (completion of course), est max f/u = 26 months (from survival curve). No treatment‐related deaths were observed. Cardiotoxicity was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hainsworth 1997.
| Study characteristics | ||
| Methods | Multi‐centre phase 2 randomised controlled trial.
Pts stratified by age & site of metastases.
Randomisation by a random card system, method of allocation concealment not stated.
Accrual dates: July 1991 ‐ Nov 1994.
Baseline comparability reported. 128 pts entered, 126 were evaluable for response (1 death, 1 PE). All evaluable for survivial & toxicity. |
|
| Participants | 128 pts Biopsy proved metastatic BC No prior cytotoxic chemotherapy for metastatic disease. Age range: 34‐81 yrs in NFL arm; 35‐78yrs in CMF arm. Median age: 57 yrs in NFL arm; 59 yrs in CMF arm. | |
| Interventions | MZA + FL vs CMF Arm A: MZA + FL mitoxantrone 12mg/m2 iv, day 1; 5‐fluorouracil 350mg/m2 iv bolus, days 1‐3; leucovorin 300mg iv, days 1‐3. 21 day cycle x 8 cycles Arm B: CMF cyclophosphamide 600mg/m2 iv, day 1; methotrexate 40mg/m2 iv, day 1; 5‐fluorouracil 600mg/m2 iv, day 1. 21 day cycle x 8 cycles |
|
| Outcomes | Response Survival Toxicity | |
| Notes | ITT followed for time‐to‐event analysis. 2 patients in CMF arm unevaluable for response (reasons stated). Estimated minimum follow‐up time = 6 months (21 day cycles x 8), estimated max f/up = 45 months (from survival curve). 1 treatment‐related death reported in CMF arm (neutropenic sepsis). No clinically significant cardiotoxicity observed. Pts with bone metastases who had only abnormal bone scans were included but stratified and analysed seperately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Harper‐Wynne 1999.
| Study characteristics | ||
| Methods | Single‐centre randomised controlled trial. Method of randomisation and allocation concealment not reported. Dates of accrual: January 1992 and December 1996 Baseline comparability achieved. | |
| Participants | 116 pts Locally advanced or metastatic disease Age range: 28‐84yrs in MM arm; 28‐84 yrs in CMF arm. Median age: 61 yrs in MM arm; 58 yrs in CMF arm 1 (2%) pt in each arm had prior chemotherapy for advanced disease. 10% of all pts had prior adjuvent chemotherapy. | |
| Interventions | MZA + MX vs CMF Arm A: MZA + MX methotrexate 30mg/m2, mitoxantrone 6.5mg/m2 iv day 1. 3 week cycles x 8. Arm B: CMF cyclophosphamide 600mg/m2 day 1 and 8 iv; methotrexate 40mg/m2 day 1 and 8 iv monthy; 5‐fluorouracil 600mg/m2 day 1, 8 iv. 4 week cycle x 6 cycles. |
|
| Outcomes | Response (UICC criteria) Time to progressive disease Toxicity (WHO criteria) Quality of Life (HADS, RSCL) | |
| Notes | ITT followed for time‐to‐event analyses. 21 pts (18%) not evaluable for response assessment (ineligible, unevaluable, or protocol deviations ‐ reasons stated). Estimated min f/up = 6 months (completion of regimen), est. max f/up for overall survival = 24 months (from OS curve). Treatment‐related deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
HEPI 013 2001.
| Study characteristics | ||
| Methods | Multi‐centre, international randomised controlled trial Dates of accrual: September 1990 and November 1992. Pts were stratified and a separate randomisation code was computer generated and centrally assigned for each stratum. Baseline comparability achieved. | |
| Participants | 460 pts (454 eligible) Women with histologically proven breast cancer metastatic progression No prior cytotoxic chemotherapy for metastatic BC. No prior anthracyclines. Age range: 22‐71 yrs in CEF arm; 26‐71 yrs in CMF arm Median age: 56 yrs in CEF arm; 55 yrs in CMF arm | |
| Interventions | FEC vs CMF Arm A: FEC cyclophosphamide 400mg/m2 iv; epirubicin 50mg/m2 iv and 5‐fluorouracil 600mg/m2 on days 1, 8. 3‐4 week cycles x 6‐9 (according to response) Arm B: CMF cyclophosphamide 400mg/m2 iv; methotrexate 40mg/m2 iv and 5‐fluorouracil 600mg/m2 on days 1, 8. 3‐4 week cycles x 6‐9 (according to response) |
|
| Outcomes | Response (UICC criteria) Survival Time to progression Time to treatment failure Toxicity | |
| Notes | ITT analysis for all efficacy analyses. Tumour response measured for randomised (460) and assessable pt (389). F/up time not reported. Est min = 5 months (6 cycles of 3‐4weeks), est max for OS = 72 months (from curve). Treatment ‐related deaths: CEF (7) vs CMF (9). 3 pts in CEF arm developed congestive heart failure due to cardiotoxicity (no deaths). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Hoogstraten 1976a.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial. 3 arm trial. Initial randomisation into three treatment groups with non compulsory 'crossover' following relapse or failure to respond Accrual dates: Jan 1972 ‐ Feb 1974 | |
| Participants | 297 pt (283 evaluable)
Women with measurable metastatic BC
No prior chemotherapy (except for hormones) Randomised no's ‐ Not provided Of remaining 283 evaluable pts, 97 were crossed over to phase 11 Evaluable numbers Phase 1 1) n = 79 2) n = 98 3) n = 106 |
|
| Interventions | Comparison 1: A vs CMFVP‐Intermittent 1) doxorubicin 60 mg/m2 iv, 3 week cycle 2) Intermittent ‐ vincristine 0.625 mg/m2/ iv days 1 and 5 + methotgrexate 4 mg/m2/ iv dx5 + 5‐flurouracil 180 mg/m2/ iv dx5 + cyclophosphamide 120 mg/m2 iv dx5 + prednisone 40 mg/m2/day X 5 28 day cycle then crossover |
|
| Outcomes | Response (Phase 1) Toxicity | |
| Notes | ITT not followed. 14 were invaluable and not analysed due to protocol violations and lack of adequate data. Duration of follow‐up not reported. Treatment‐related deaths were reported in the CMFVP arms due to sepsis (4), haemorrhage (2) and pulmonary embolism with associated thrombocytopenia (1). No treatment‐related deaths were reported in the doxorubicin arm, 1 patient in this arm was reported to have grade III cardiotoxicity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hoogstraten 1976b.
| Study characteristics | ||
| Methods | As above | |
| Participants | As above | |
| Interventions | Comparison 2: A vs CMFVP‐ weekly 1) doxorubicin 60 mg/m2 iv, 3 week cycle 3) Weekly vincristine 0.625 mg/m2/week iv + methotrexate 15 mg/m2/wk iv + 5‐fluorouracil 300 mg/m2/wk iv + cyclophosphamide 60 mg/m2/day po + prednisone 30 mg/m2/day X 14, 20 mg/m2/day X 14, 10 mg/m2/day then crossover |
|
| Outcomes | As above ‐ All outcomes documented in (A) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kolaric 1977.
| Study characteristics | ||
| Methods | Randomised controlled trial Method of randomisation and allocation concealment not reported. Age distribution and prior treament were similar in both arms, bone metastases more common in CMFAP (63%) vs CMFVP (50%) | |
| Participants | 74pts All metastatic breast cancer No prior cytotoxic chemotherapy. Age range: 28‐70 yrs (average 48 yrs) | |
| Interventions | CAMFP vs CMFVP Arm A: CAMFP cyclophosphamide 5mg/kg iv 1‐5 day; 5‐fluorouracil 8mg/kg iv day 1,3,5; methotrexate 0.4mg/k iv days 2‐4; doxorubicin 40mg/m2 iv 1 day; prednisolone 40mg po daily 1‐5 days Arm B: CMFVP cyclophosphamide 5mg/kg iv days 1‐5; 5‐fluorouracil 8mg/kg iv 1, 3,5; methotrexate 0.4mg/kg iv 2,4 days; vincristine 0.025mg/kg iv 1‐5 days; prednisolone 40mg po daily 1‐5 day |
|
| Outcomes | Response Toxicity | |
| Notes | Response and toxicity data reported for 74 pts. There was no signif difference between the overall response rate or average remission duration in both arms. Treatment‐related deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kolaric 1985.
| Study characteristics | ||
| Methods | Randomised controlled trial Method of randomisation and allocation concealment not reported. Stratification by dominant metastatic site. Slight imbalance with 56% of postmenopausal women in CMFVP arm vs 44% in CAP arm. | |
| Participants | 128 pts (123 evaluable) Metastatic breast cancer. No prior cytotoxic chemotherapy. Age range was 30‐70 yrs. | |
| Interventions | CA + CDDP vs CMFVP Arm A: CA + CDDP cisplantin, 30mg/m2 daily as an iv days 1, 3, 5; doxorubicin 40mg/m2 on day 1; cyclophosphamide 200mg/m2 iv daily on days 1, 3, 5. 3‐4 week cycles x 10 cycles Arm B: CMFVP cyclophosphamide 200mg/m2 iv daily 1,2,3,4 and 4; methotrexate 20mg/m2 iv days 2, 4; 5‐fluorouracil 500mg/m2 ivdaily days 1, 3, 5, vincristine 1mg/m2 iv days 1 and 5 and prednisolone 40mg po daily days 1, 2, 3, 4, 5. 3‐4 week cycles x 10 cycles. |
|
| Outcomes | Response Progression Toxicity (WHO/UICC) | |
| Notes | 123/128 pts considered to be evaluable (received more than 2 chemotherapy cycles. Minimum reported f/up = 6 months, maximum reported f/up=33 months. Overall response and complete remission rate favoured the CAP regimen (p<0.01) All pts who had no response with CAP underwent treatment with FIVB + P | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lorusso 1993.
| Study characteristics | ||
| Methods | A multicentre randomised controlled trial. Method of randomisation and allocation concealment not reported. Dates of accrual: April 1988 to December 1990 Pretreatment characteristics showed some imbalance of metastatic site by treatment arm. Visceral metastases CNF = 53% vs CMF = 38%. Soft tissue metastases: CNF = 19% vs CMF = 31%. | |
| Participants | 128 pts (119 considered evaluated) Locally advanced or metastatic breast cancer No prior chemotherapy for metastatic disease, no anthracycline‐containing adjuvent chemotherapy. Age range: 37‐67yrs in CNF arm; 34‐71 yrs in CMF arm Median age: 56 yrs in CNF arm; 57 yrs in CMF arm | |
| Interventions | C+ MZA + F vs CMF Arm A: C + MZA + F 5‐fluorouracil 600mg/m2 iv, cyclophosphamide 600mg/m2 iv and mitoxantrone 10mg/m2 iv. 3 week cycle x 8 cycles Arm B: CMF 5‐fluorouracil 600mg/m2 iv, cyclophosphamide 600mg/m2 iv and methotrexate 40mg/m2 iv. 3 week cycle x 8 cycles |
|
| Outcomes | Response Overall survival Progression free survival Toxicity | |
| Notes | ITT not followed. 119/128 pts completed a minimum of 2 cycles and included in the efficacy analysis (3 pt refused treatment, 6 pt lost to follow‐up). Estimated minimum follow‐up = 1.5 months (2 cycles), est max f/up = 21 months (from survival curve). Non‐responding pts were treated with an anthracycline every 3 weeks. 1 treatment‐related death reported in the CNF arm (sepsis). 2 pts ceased treatment in the CNF arm due to MUGA scan signs of cardiotoxicity, but did not demonstrate clinical or ECG signs of heart failure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Muss 1978.
| Study characteristics | ||
| Methods | Randomised controlled trial Method of randomisation and allocation concealment not stated Dates of accrual: May 1975 ‐ August 1976. No imbalance in the pre‐treatment characteristics in each arm. | |
| Participants | 175 pts (148 evaluable) All pts had a histologically confirmed diagnosis of breast cancer and metastatic disease. 148/175 evaluable pts. 27 pts were cancelled due to protocol violations. Mean age of 55yrs in each arm 93% of evaluable pt had not received prior chemotherapy. 8% of pt in CVFP + ADR arm and 6% of pt in CFVP + MTX arm had received prior single agent chemotherapy (not doxirubicin or methotrexate). | |
| Interventions | CAFVP vs CMFVP Arm A: CAFVP cyclosphamide 2mg/kg/po day 1‐14 then 100mg daily; vincristine 25 mcg/kg/iv day 1, then Q2 wk X 3, then Q4 wk; fluorouracil, 12 mg/kg/iv, day 1‐3, Q2 wk; prednisone 0.75 mg/kg/po day 1‐14, then 10mg daily; doxorubicin 20mg/m2/iv day 1 then Q2 wk Arm B: CMFVP cyclosphamide 2mg/kg/po day 1‐14 then 100mg daily; vincristine 25 mcg/kg/iv day 1, then Q2 wk X 3, then Q4 wk; fluorouracil, 12 mg/kg/iv, day 1‐3, Q2 wk; prednisone 0.75 mg/kg/po day 1‐14, then 10mg daily; methotrexate 0.2mg/kg/iv day 1 then Q2 wk Phase 1 intervention: induction and maintenance cycles, 30 weeks for completion Phase 2 intervention: maintenance cycles of doxirubicin (Arm A) or methotrexate (Arm B) every for weeks for a minimum of 40 wks. After completion of Phase 2, pts crossed over to alternate arm of Phase 2 maintenance. Maximum culmulative dose of doxorubicin was 500mg/m2, after which it was discontinued. |
|
| Outcomes | Response Overall Survival (for complete and partial responders) Toxicity | |
| Notes | ITT not followed. 175 subjects randomized, 13 pts were ineligible. Another 14 pts were cancelled due to other protocol violations (reasons stated). Analyses used 148 evaluable pts: Arm A: 76/92 pts Arm B: 72/83 pts. Duration of f/up not reported. Survival for each treatment arm only reported for partial and complete responders. Cross‐over design was used after maintenance phase 2 (at 70 weeks). The median survival for CR and PR patients with CMFVP was 20.2 months compared to 33 month for CAFVP (p=0.07). No treatment‐related deaths reported. 1 pt with prior atherosclerotic heart disease developed congestive heart failure after receiving 200mg/m2 of Doxorubicin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Muss 1982.
| Study characteristics | ||
| Methods | Randomised controlled trial Pts stratified according to estrogen receptor status. Method of randomisation and allocation concealment not reported. Baseline imbalance in dominant site of metastaic disease: CMF 52% visceral, 34% bone; VAC 24% visceral, 58% bone Dates of accrual: June 1979‐March 1981 | |
| Participants | 100pts (89 evaluable). Advanced breast cancer, recurrent or metastatic disease. No prior use of chemotherapeutic agents used in study protocol. | |
| Interventions | VAC vs CMF Arm A: VAC vincristine 1mg/m2 day 1 iv; doxorubicin 40mg/m2 day 1 iv; cyclophosphamide 200mg/m2 day 3‐6 po. 3 week cycle When culmulative maximum dose of doxorubicin = 450 mg/m2 reached, maintenance regimen CVMF. Arm B: CMF cyclophosphamide 350mg/m2; methotrexate 20mg/m2; fluorouracil 350mg/m2 iv. 3 week cycle for 1 year then 4 week cycle. |
|
| Outcomes | Response (IUCC) CR, PR Survival Toxicity | |
| Notes | ITT not followed. 11 eligible pts not considered evaluable (reasons stated) and not included in analyses. Estimated minimum follow‐up = 6 months (from last accrual to publication acceptance), estimated maximum follow‐up = 13 months (from survival curve). 3 pts on VAC arm developed symptomatic cardiotoxicity. Treatment‐related deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nemoto 1978.
| Study characteristics | ||
| Methods | Multi‐centre national randomised controlled trial. Pts stratified according to dominant site of matastasis, disease free interval & menopausal status. Description of randomisation not given. Dates of accrual: Nov 1972 ‐ May 1974. Baseline comparability achieved | |
| Participants | 94 pts. Postmenopausal women with metastatic BC No prior cytotoxic or hormonal agents. Median age: 57 yrs in Adriamycin arm; 56 years in CFP arm. | |
| Interventions | A vs CFP Arm A: A doxorubicin 40mg/m2 iv; 4 week cycles. culmulative maximum dose of doxorubicin = 500mg/m2. Arm B: CFP cyclophosphamide 150mg/m2 iv, days 1‐5; 5‐fluorouracil 300mg/m2 iv, days 1‐5; prednisone 40 to 10mg po, daily. 5 week cycle. |
|
| Outcomes | Survival Response Toxicity | |
| Notes | ITT analysis. Cross‐over to alternate regimen on disease progression. Three sequences were: 1)ADX, CFP, ADR 2)CFP, ADR, ADX 3)ADR, ADX, CFP. Time‐to‐event data extracted for pts initially allocated to Adriamycin and CFP arms for meta‐anlaysis. Minimum reported f/up = 18 months, max estimated f/up = 36 months (from OS curve). 2 treatment‐related deaths within one month of CFP combination therapy observed. 3 treatment‐related death in Adriamycin arm due to hepatic failure (1) and delayed cardiac toxicity (2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nemoto 1982a.
| Study characteristics | ||
| Methods | Multi‐centre national randomised controlled trial. Dates of accrual; July 1974 ‐ Oct 1975. 4 arm study Randomisation by closed‐envelope at one institution. Pts stratified by menopausal status, DFI and dominant site of metastasis. Baseliine comparability achieved. | |
| Participants | 126 pts. Postmenopausal women with metastatic breast cancer. No prior cytotoxic chemotherapy. Age distribution: 80% pts 50yrs + | |
| Interventions | Comparison 1: CFP‐CA vs CFP Arm A: CFP‐CA alternating regimens: Cyclophosphamide 150mg/m2 iv, days 1‐5; 5‐fluorouracil 300mg/m2 iv, days 1‐5; prednisone 30mg/d po for 1 week, then 10mg/d po. 5 week cycle then cyclophosphamide 500mg/m2 iv and doxorubicin 40mg/m2 iv every nine weeks. Arm B: CFP cyclophosphamide 150mg/m2 iv, days 1‐5; 5‐fluorouracil 300mg/m2 iv, days 1‐5; prednisone 30mg/d po for 1 week, then 10mg/d po. 5 week cycles. |
|
| Outcomes | Survival Response Toxicity | |
| Notes | ITT analysis. 9 pts were nonevaluable due to early deaths or withdrawal from study. Cumulative dose of adriamycin did not exceed 500mg/m2. Pts rerandomised to tamoxifen or adrenalectomy at progression. One pt on CA arm developed moderate cardiotoxicity. Treatment‐related deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nemoto 1982b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: CAF vs CFP Arm C: CAF cyclophosphamide 400mg/m2 iv, day 1; 5‐fluorouracil 200mg/m2 iv, days 1‐3; doxorubicin 40mg/m2 iv, day 1. Arm B: CFP cyclophosphamide 150mg/m2 iv, days 1‐5; 5‐fluorouracil 300mg/m2 iv, days 1‐5; prednisone 30mg/d po for 1 week, then 10mg/d po |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nemoto 1982c.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 3: CA vs CFP Arm D: CA cyclophosphamide 500mg/m2 iv, day 1; doxorubicin 40mg/m2 iv, day 1 Arm B: CFP cyclophosphamide 150mg/m2 iv, days 1‐5; 5‐fluorouracil 300mg/m2 iv, days 1‐5; prednisone 30mg/d po for 1 week, then 10mg/d po |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pannuti 1984.
| Study characteristics | ||
| Methods | Randomised controlled trial Method of randomisation and allocation concealment not reported. Baseline comparability achieved. | |
| Participants | 46pts Advanced breast cancer (87% with metastatic disease of viscera or bone). 17% pts had prior hormone and cytotoxic chemotherapy. Age range: 37‐74yrs R14; 36‐74yrs CMF. Median age: 55yrs both arms | |
| Interventions | R14 vs CMF Arm A: R14 cyclophosphamide 2mg/kg iv vincristine 0.01mg/kg iv, vinblastine 0.1mg/kg iv Day 1 and methotrexate 0.7mg/kg iv, doxorubicin 0.5mg/kg iv Day 2. 21 day cycle. Arm B: CMF cyclophosphamide 100mg/m1 days 1‐14; methotrexate 40mg/m2 day 1, 8; 5‐fluorouracil 600mg/m2 day 1, 8. 28 day cycle. |
|
| Outcomes | Response (CR, PR, P, NC) Survival Toxicity | |
| Notes | 46 pts included in all analyses. Estimated min f/up = 5.5 months (median time to progression), estimated max f/up = 45 months (from survival curve). treatment‐related deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rosner 1989a.
| Study characteristics | ||
| Methods | Randomised controlled trial. 3 arm trial. Method of randomisation and allocation concealment not reported. Stratification by receptor status & dominant site of disease before randomisation. Estrogen receptor‐positive or receptor‐unknown pts received hormonotherapy and randomised on disease progression. Estrogen receptor‐negative pts were randomised at enrolment. Baseline comparability achieved. Dates of accrual: Sept 1981 ‐ Dec 1987 | |
| Participants | 182 pts Progressive, metastatic breast cancer No prior chemotherapy. Age range (first 141 pts): 26‐71 yrs in CA arm; 34‐73 yrs in CFP arm; 32‐72 yrs in CFPMV arm. Median age (first 141 pts ): 54 yrs in CA arn; 57 yrs in CFP arm; 56 yrs in CFPMV arm. | |
| Interventions | Comparison 1: CA vs CFP Arm A: CA doxorubicin 40mg/m2 iv; cyclophosphamide 400mg/m2 iv. 4 week cycles. culmulative maximum dose of doxorubicin = 500mg/m2. Arm B: CFP cyclophosphamide 150mg/m2 iv, days 1‐5; 5‐fluorouracil 300mg/m2 iv, days 1‐5; prednisone 40 to 10mg po, daily. 5 week cycle. |
|
| Outcomes | Response Overall survival (from date of first chemotherapy) Toxicity | |
| Notes | Randomised trial of sequential CT. 108/182 pts were ER‐positive and received hormonotherapy prior to randomisation. Pts were crossed over upon disease progression. ITT analysis. Reported minimum follow‐up = 6 months, maximum f/up = 81 months. 6 treatment‐related deaths reported in the preliminary report of 141/182 pts. 3 deaths in the CFPMV arm (GI bleed and bronchopneumonia(1), pulmonary embolism (2)) and 3 deaths in the CA arm reported due to cardiotoxicity. 3 additional pts in the CA arm were also reported with non‐fatal CHF. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rosner 1989b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: CA vs CFPMV Arm A: CA doxorubicin 40mg/m2 iv; cyclophosphamide 400mg/m2 iv. 4 week cycles. culmulative maximum dose of doxorubicin = 500mg/m2. Arm B CFPMV cyclophosphamide 50mg po twice daily; 5‐fluorouracil 500mg iv, weekly; methotrexate 25mg iv, weekly; vincristine 1mg iv, weekly; prednisone 40 to 10mg po, daily. 5 week cycles. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
SAKK 1983a.
| Study characteristics | ||
| Methods | Multi‐centre national randomised controlled trial. 3 arm trial. Pts were stratified according to menopausal status and risk group. Method of randomisation and allocation concealment not described. Dates of accrual: Sept 1975 ‐ Dec 1980. Baseline comparability reported for evaluable pts. | |
| Participants | 230 pts randomised (216 evaluable). Women with measurable metastatic breast cancer. No prior cytoxic chemotherapy or hormonotherapy. Median age: 57.2 ‐57.9 yrs in each arm | |
| Interventions | Comparison 1: CLB + AMFP vs CLB + MFP Arm A: CLB + AMFP chlorambucil 5/mg/m2/d po, days 1‐14; methotrexate 40mg/m2/w iv, days 1, 8; 5‐fluorouracil 600mg/m2/w iv, days 1, 8; prednisone 30mg/m2/d, days 1‐14 (then decreasing); doxorubicin 60mg/m2, day 28. 8 week cycles for 6 months maximum culmulative dose of doxorubicin = 450mg/m2. Arm B: CLB + MFP chlorambucil 5mg/m2/d po, days 1‐14; methotrexate 10mg/m2/w po, days 1 & 8; 5‐fluorouracil 500mg/m2/w po, days 1 & 8; prednisone 30mg/m2/d, days 1‐14 (then decreasing). 4 week cycles for 6 months |
|
| Outcomes | Response (UICC) Survival Time to progression (median) Toxicity | |
| Notes | 464 pts were entered in study and randomised to receive tamoxifen/oophorectomy or tamoxifen/oophorectomy with concurrent chemotherapy. The 230 pts assigned to the chemotherapy arm were further randomised to 3 different chemotherapy regimens. 216/230 pts were reported as evalubale: 14 were excluded from analysis due to major protocol violations (10 pts), 2 poorly evaluable tumour parameters & 2 early deaths. Minimum reported follow‐up = 17 months, maximum reported follow‐up = 80 months. Median time to progression was 19.5 months (Adrimaycin arm) vs 20.5 months (CLB, Mtx, 5‐FU, Pred) and 19.5 months (CLB, Mtx, 5‐FU, Vcr, Pred). Treatment‐related deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
SAKK 1983b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | Comparison 2: CLB + AMFP vs CLB + MFVP Arm A: CLB + AMFP chlorambucil 5/mg/m2/d po, days 1‐14; methotrexate 40mg/m2/w iv, days 1, 8; 5‐fluorouracil 600mg/m2/w iv, days 1, 8; prednisone 30mg/m2/d, days 1‐14 (then decreasing); doxorubicin 60mg/m2, day 28. 8 week cycles for 6 months maximum culmulative dose of doxorubicin = 450mg/m2. Arm C: CLB + MFVP chlorambucil 5/mg/m2/d po, days 1‐14; methotrexate 15mg/m2/w po, days 1‐3 & 8‐10; prednisone 30mg/m2/d, days 1‐14; 5‐fluorouracil 500mg/m2/w iv, days 15 & 22; vincristine 1.2mg/m2/w iv, days 15 & 22; prednisone 30mg/m2/d days 5‐28 (then decreasing). 4 week continuous cycle for 6 months. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
SECSG 1983.
| Study characteristics | ||
| Methods | Multi‐centre randomised trial. Randomised by telephone without stratification Accrual dates: April 1974 ‐ July 1977 Baseline comparability of number of metastatic sites and prior use of hormone therapy achieved. | |
| Participants | 362 pts (265 pts were considered evaluable) Pts with metastatic or recurrent breast carcinoma. No prior cytotoxic chemotherapy. No age restrictions or age ranges reported. | |
| Interventions | CAF vs CMFVP Arm A: CAF cyclophosphamide 500mg/m2, doxorubicin 50mg/m2; 5‐fluorouracil 500mg/m2 iv. 3 week cycle, 9‐10 cycles. to a maximum dose of 500mg/m2 doxorubicin. Arm B: CMFVP cyclophosphamide 400mg/m2 iv. day 1, methotrexate 30mg/m2 iv. day 1, 8, 5‐fluorouracil 400mg/m2 iv. days 1, 8; vincristine 1mg days 1 and 8 iv.; prednisone 20mg days 1‐7 po. 28 day cycle X 6. |
|
| Outcomes | Response (SECSG) Survival Progression free survival Toxicity | |
| Notes | During the last six months of accrual eligibility was limited to patients with viseral metastases. ITT not followed. 265/362 patients were considered evaluable due to: ineligibility (41); protocol violations (25); or incomplete data (31). Estimated minimum follow‐up time = 7 months (from protocol), est max f/up = 60 months (from survival curve). No treatment‐related deaths observed. Cardiotoxicity observed in one pt who received the maximum dose of 500mg/m2 of doxorubicin ( CHF responsive to treatment). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| 303 Study Group | 2nd line chemotherapy trial. All pts had received previous alkalylating agent chemotherapy eg. CMF or variants for adjuvant (47%) and/or advanced disease (53%). |
| 304 Study Group | 2nd line chemotherapy trial. 81% pts had previous anthracycline chemotherapy for advanced disease or disease progression within 12 months of anthracycline based adjuvent therapy. |
| Ahmann 1978 | Subjects were re‐randomized after 2 cycles (10 weeks) if stable disease or regression. Complete time‐to‐event data on subsets of subjects not available. |
| Bezwoda 1979 | 15 (12%) of patients failing treatment prior to 3 month evaluation not included in efficacy analyses. Admission of scientific fraud by principal investigator noted in a later trial. |
| Dieras 1995 | 2nd line chemotherapy trial. All pts had prior metastatic or adjuvant chemotherapy, 98% had prior anthracycline treatment. |
| Erkisi 1997 | 2nd line chemotherapy trial. All pts had prior CMF chemotherapy, 52% for advanced disease. |
| Falkson 1988 | 2nd line chemotherapy trial. 85% of pts had prior chemotherapy. |
| GOIRC 1990 | 2nd line chemotherapy trial. 198 pts underwent 6 cycles of CMF, 96pts showed no progression and were randomised to continuation of CMF or AV with CF, CM or MF 'intensification‐discontinuation' arm. 96 pts were not randomised due to progression within first 6 cycles. |
| Hori 2001 | Patients were found not to have ABC following randomistaion. |
| Legha 1979 | 30 patients received combination of hexamethylamine, vincristine, mitomycin C (HOM) V 23 patients received hexamethylamine(HMM). The HMM arm was closed early. Therefore, it is unclear whether patients were automatically allocated to HOM after the closure of the HMM arm compromising randomisation. |
| Leiden Uni Centre | Thsi trial was previously listed as "Ongoing". Clarification was sought from authors regarding status of trial. Trial is closed. No further information available. |
| Porzsolt 1990 | 99 subjects entered the trial and received 2 cycles of anthracycline. 66 Subjects who remained stable or responded were eligible for randomisation to one of the 2 regimens. |
| SWG Gottlieb 1974 | Not properly randomised trial, patients with prior doxorubicin or nitrosurea use assigned to alternate arm. |
| Venturino 2000 | 2nd line chemotherapy trial. Pts had received one course of prior chemotherapy for metatstatic disease (including CMF, CEF). |
| Zekan 1984 | Of the 51 patients recruited into the study, 24 had been allocated to treatment arms using an 'alternating sequence' recorded on unsealed cards. The remaining 27 were allocated according to a random number sequence generated by random number tables recorded on sealed cards. Results are presented on entire sample of 51 patients and hence we could not identify results attributable to those 27 patients appropriately randomised. |
Characteristics of ongoing studies [ordered by study ID]
Butler 2004.
| Study name | A study of docetaxel monotherapy or DOXIL/CAELYX and doxetaxel in patients with advanced breast cancer |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
DG designed the review and wrote the protocol. MG, JB, SW, CT, LB and SL collected the data for the review. SL and CT wrote the results and discussion for the review in collaboration with NW, DG and JS.
Sources of support
Internal sources
NHMRC Clinical Trials Centre, Australia
External sources
U.S. Army Medical Research Acquisition Activity, USA
Declarations of interest
None
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ahmann 1974a {published data only}
- Ahmann DL, Bisel HF, Eagan RT, Edmonson JH, Hahn RG. Controlled evaluation of adriamycin (NSC-123127) in patients with disseminated breast cancer. Cancer Chemotherapy Reports 1974;58:877-82. [PubMed] [Google Scholar]
- Ahmann DL, Schaid DJ, Bisel HF, Hahn RG, Edmonson JH, Ingle JN. The effect on survival of initial chemotherapy in advanced breast cancer: polychemotherapy versus single drug.. Journal of Clinical Oncology 1987;5:1928-32. [DOI] [PubMed] [Google Scholar]
Ahmann 1974b {published data only}
- Ahmann DL, Bisel HF, Eagan RT, Edmonson JH, Hahn RG. Controlled evaluation of adriamycin (NSC-123127) in patients with disseminated breast cancer. Cancer Chemotherapy Reports 1974;58:877-82.. [PubMed] [Google Scholar]
- Ahmann DL, Schaid DJ, Bisel HF, Hahn RG, Edmonson JH, Ingle JN. The effect on survival of initial chemotherapy in advanced breast cancer: polychemotherapy versus single drug... Journal of Clinical Oncology 1987;5:1928-32. [DOI] [PubMed] [Google Scholar]
Ahmann 1991 {published data only}
- Ahmann DL, Schaid DJ, Ingle JN, Bisel HF, Schutt AJ, Buckner JC, et al. A randomized trial of cyclophosphamide, doxorubicin, and prednisone versus cyclophosphamide, 5-fluorouracil, and prednisone in patients with metastatic breast cancer. American Journal of Clinical Oncology 1991;14(3):179-83. [DOI] [PubMed] [Google Scholar]
ANZ BCTG 8614 {published data only}
- Simes RJ. Mitozantrone vs CMFP in advanced breast cancer: A quality of life study. Proceedings of ASCO 1994 2001.
B122 {published data only}
- Bull JM, Tormey DC, Li SH, Carbone PP, Falkson G, Blom J, et al. A randomised comparative trial of adriamycin versus methotrexate in combination drug therapy. Cancer 1978;41:1649-57. [DOI] [PubMed] [Google Scholar]
- Falkson G, Tormey DC, Carey P, Witte R, Falkson HC. Long-term survival of patients treated with combination chemotherapy for metastatic breast cancer. Eur J Cancer 1991;27(8):973-7. [DOI] [PubMed] [Google Scholar]
Brambilla 1976 {published data only}
- Brambilla C, De Lena M, Rossi A, Valagussa P, Bonadonna G. Response and survival in advanced breast cancer after two non- cross-resistant combinations. British Medical Journal 1976;1(6013):801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lena M, Brambilla C, Morabito A, Bonadonna G. Adriamycin plus vincristine compared to and combined with cyclophosphamide, methotrexate, and 5-flurouracil for advanced breast cancer. Cancer 1975;35(4):1108-15. [DOI] [PubMed] [Google Scholar]
CALGB Aisner 1987a {published data only}
- Aisner J, Weinberg V, Perloff M, Weiss R, Perry M, Korzun A, et al. Chemotherapy versus chemoimmunotherapy (CAF v CAFVP v CMF each +/- MER) for metastatic carcinoma of the breast: a CALGB study. Cancer and Leukemia Group B. Journal of Clinical Oncology 1987;5(10):1523-33. [DOI] [PubMed] [Google Scholar]
CALGB Aisner 1987b {published data only}
- Aisner J, Weinberg V, Perloff M, Weiss R, Perry M, Korzun A, et al. Chemotherapy versus chemoimmunotherapy (CAF v CAFVP v CMF each +/- MER) for metastatic carcinoma of the breast: a CALGB study. Cancer and Leukemia Group B. Journal of Clinical Oncology 1987;5(10):1523-33. [DOI] [PubMed] [Google Scholar]
CALGB Aisner 1987c {published data only}
- Aisner J, Weinberg V, Perloff M, Weiss R, Perry M, Korzun A, et al. Chemotherapy versus chemoimmunotherapy (CAF v CAFVP v CMF each +/- MER) for metastatic carcinoma of the breast: a CALGB study. Cancer and Leukemia Group B. Journal of Clinical Oncology 1987;5(10):1523-33. [DOI] [PubMed] [Google Scholar]
CALGB Aisner 1987d {published data only}
- Aisner J, Weinberg V, Perloff M, Weiss R, Perry M, Korzun A, et al. Chemotherapy versus chemoimmunotherapy (CAF v CAFVP v CMF each +/- MER) for metastatic carcinoma of the breast: a CALGB study. Cancer and Leukemia Group B. Journal of Clinical Oncology 1987;5(10):1523-33. [DOI] [PubMed] [Google Scholar]
CALGB Tormey 1984a {published data only}
- Tormey DC, Weinberg VE, Leone LA, Glidewell OJ, Perloff M. A comparison of intermittent versus continous and of adriamycin versus methotrexate 5-drug chemotherapy for advanced breast cancer. American Journal of Clinical Oncology Cancer Clinical Trials 1984;7(3):231-9. [DOI] [PubMed] [Google Scholar]
CALGB Tormey 1984b {published data only}
- Tormey DC, Weinberg VE, Leone LA, Glidewell OJ, Perloff M. A comparison of intermittent versus continous and of adriamycin versus methotrexate 5-drug chemotherapy for advanced breast cancer. American Journal of Clinical Oncology Cancer Clinical Trials 1984;7(3):231-9. [DOI] [PubMed] [Google Scholar]
Carmo‐Pereira 1981 {published data only}
- Carmo-Pereira J, Costa FO, Henriques E. Chemotherapy of advanced breast cancer: a randomized trial of vincristine, adriamycin, and cyclophosphamide (VAC) versus cyclophosphamide, methotrexate, 5-fluorouracil, and prednisone (CMFP). Cancer 1981;48(7):1517-21. [DOI] [PubMed] [Google Scholar]
Chauvergne 1978 {published data only}
- Chauvergne J, Berlie J, Clavel B, Guerrin J, Gary-Bobo J, Brule G, Klein T, Pommatau E, Carton M, Gary Bobo J. Chemotherapy of advanced breast cancer. Results of a controlled trial comparing two three-drug regimens. European Journal of Cancer 1978;14(9):911-17. [DOI] [PubMed] [Google Scholar]
Coates 1987a {published data only}
- Coates A, Gebski V, Bishop JF, Jeal PN, Woods RL, Snyder R, et al. Improving the quality of life during chemotherapy for advanced breast cancer. A comparison of intermittent and continuous treatment strategies. N Engl J Med 1987;317(24):1490-5. [DOI] [PubMed] [Google Scholar]
Coates 1987b {published data only}
- Coates A, Gebski V, Bishop JF, Jeal PN, Woods RL, Snyder R, et al. Improving the quality of life during chemotherapy for advanced breast cancer. A comparison of intermittent and continuous treatment strategies. N Engl J Med 1987;317(24):1490-5. [DOI] [PubMed] [Google Scholar]
Creagan 1984 {published data only}
- Creagan ET, Green SJ, Ahmann DL, Ingle JN, Edmonson JH, Marschke RF-J. A phase III clinical trial comparing the combination cyclophosphamide, adriamycin, cisplatin with cyclophosphamide, 5- fluorouracil, prednisone in patients with advanced breast cancer. Journal of Clinical Oncology 1984;2(11):1260-5. [DOI] [PubMed] [Google Scholar]
Creech 1979 {published data only}
- Creech RH, Catalano RB, Harris DT, Engstrom PF, Grotzinger PJ. Low dose chemotherapy of metastatic breast cancer with cyclophosphamide, adriamycin, methotrexate, 5-fluorouracil (CAMF) versus sequential cyclophosphamide, methotrexate, 5-fluorouracil (CMF) and adriamycin. Cancer 1979;43:51-9. [DOI] [PubMed] [Google Scholar]
DBCG 1999 {published data only}
- Andersson M, Madsen EL, Overgaard M, Dombernowsky P, Mouridsen HT. CAF vs CMF with tamoxifen in postmenopausal patients with advanced breast cancer - A randomized study with more than 10 years follow-up from the Danish breast cancer cooperative group. European Journal of Cancer 1997;33(Suppl 8):S146. [DOI] [PubMed]
- Andersson M, Madsen EL, Overgaard M, Rose C, Dombernowsky P, Mouridsen HT. Doxorubicin versus methotrexate both combined with cyclophosphamide, 5-fluorouracil and tamoxifen in postmenopausal patients with advanced breast cancer -a randomised study with more than 10 years follow-up from the Danish Breast Cancer Cooperative Group. Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer 1999;35(1):39-46. [DOI] [PubMed] [Google Scholar]
ECOG Cummings 1985 {published data only}
- Cummings FJ, Gelman R, Horton J. Comparison of CAF versus CMFP in metastatic breast cancer: analysis of prognostic factors. Journal of Clinical Oncology 1985;3(7):932-40. [DOI] [PubMed] [Google Scholar]
ECOG E1193a {published data only}
- Sledge GW Jr, Neuberg D, Ingle J, Martino S, Wood W. Phase III trial of doxorubicin (A) vs paclitaxel (T) vs doxorubicin + paclitaxel (A + T) as first-line therapy for metastatic breast cancer (MBC): an intergroup trial. Proceedings of the American Society of Clinical Oncology 1997;16. [DOI] [PubMed]
- Sledge GW Jr. Paclitaxel doublets in metastatic breast cancer: Eastern Cooperative Oncology Group and Hoosier Oncology Group studies. Seminars in Oncology 1996;23(5 SUPPL. 11):57-59. [PubMed] [Google Scholar]
- Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, Wood WC. Phase III Trial of Doxorubicin, Paclitaxel, and the Combination of Doxorubicin and Paclitaxel as Front-Line Chemotherapy for Metastatic Breast Cancer: An Intergroup Trial (E1193). Journal of Clinical Oncology 15-2-2003;21(4):588-92. [DOI] [PubMed] [Google Scholar]
- Sledge GW Jr, Robert N, Sparano JA, Cobeligh M, Goldstein LJ, Neuberg, D, et al. Paclitaxel (taxol)/doxorubicin combinations in advanced breast cancer: the Eastern Cooperative Oncology Group experience. Seminars in Oncology 1994;21(5 Suppl 8):15-8. [PubMed] [Google Scholar]
- Sledge JGW, et al. Doxorubicin/paclitaxel combination chemotherapy for metastatic breast cancer: The Eastern Cooperative Oncology Group experience. Seminars in Oncology 1995;22(5 Supp 12):123-9. [PubMed] [Google Scholar]
ECOG E1193b {published data only}
- Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, Wood WC. Phase III Trial of Doxorubicin, Paclitaxel, and the Combination of Doxorubicin and Paclitaxel as Front-Line Chemotherapy for Metastatic Breast Cancer: An Intergroup Trial (E1193). Journal of Clinical Oncology 15-2-2003;21(4):588-92. [DOI] [PubMed] [Google Scholar]
ECOG EST 2173a {published data only}
- Tormey DC, Gelman R, Band PR, Sears M, Bauer M, Arseneau JC, Falkson G, Falkson G. A prospective evaluation of chemohormonal therapy remission maintenance in advanced breast cancer. Breast Cancer Research & Treatment 1981;1(2):111-9. [DOI] [PubMed] [Google Scholar]
- Tormey DC, Gelman R, Band PR, Sears M, Rosenthal SN, DeWys W, et al. Comparison of induction chemotherapies for metastatic breast cancer. An Eastern Cooperative Oncology Group Trial. Cancer 1982;50(7):1235-44. [DOI] [PubMed] [Google Scholar]
ECOG EST 2173b {published data only}
- Tormey DC, Gelman R, Band PR, Sears M, Rosenthal SN, DeWys W, et al. Comparison of induction chemotherapies for metastatic breast cancer. An Eastern Cooperative Oncology Group Trial. Cancer 1982;50(7):1235-44. [DOI] [PubMed] [Google Scholar]
EORTC 10923 {published data only}
- Paridaens R, Biganzoli L, Bruning P, Klijn JG, Gamucci T, Houston S, et al. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. Journal of Clinical Oncology 2000;18(4):724-33. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Bruning P, Gamucci T, Klijn J, Roy JA, Awada A, et al. An ongoing European organization for research and treatment of cancer crossover trial comparing single-agent paclitaxel and doxorubicin as first- and second-line treatment of advanced breast cancer. Seminars in Oncology 1996;23(5 Suppl 11):11-5. [PubMed] [Google Scholar]
Fountzilas 2004 {published data only}
- Fountzilas G, Kalofonos HP, Dafni U, Papadimitrou C, Bafaloukos D, Papakostas P, Kalogera-Fountzila A, Gogas H, Aravantinos G, Moulopoulos LA, Economopoulos T, Pectasides D, Maniadakis N, Siafaka V, briasoulis E, Christodoulou C, Tasavaridis D, Makrantonakis P, Razis E, Kosmidis P, Skarlos D, Dimopoulos MA. Paclitaxel and epirubicin versus paclitaxel and carboplatin as first-line chemotherapy in patients with advanced breast cancer: a pahse III study conducted by the Hellenic Cooperative Oncology Group. Annals of oncology 2004;15:1517-1526. [7950] [DOI] [PubMed] [Google Scholar]
Fraser 1993 {published data only}
- Anonymous. Combination versus mild single agent chemotherapy for advanced breast cancer. UKCCCR Register of Cancer Trials 2002.
- Fraser SC, Dobbs HJ, Ebbs SR, Fallowfield LJ, Bates T, Baum M. Combination or mild single agent chemotherapy for advanced breast cancer? CMF vs epirubicin measuring quality of life. British Journal of Cancer 1993;67(2):402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SC, Ramirez AJ, Ebbs SR, Fallowfield LJ, Dobbs HJ, Richards, MA, et al. A daily diary for quality of life measurement in advanced breast cancer trials. British Journal of Cancer 1993;67(2):341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hainsworth 1997 {published data only}
- Hainsworth, JD. Mitoxantrone, 5-fluorouracil, and high dose leucovorin (NFL) in the treatment of metastatic breast cancer: randomised comparison to cyclophosphamide, methotrexate, and 5- fluorouracil (CMF) and attempts to improve efficacy by adding pacliataxel. European Journal of Cancer Care 1997;6(Supplement 1):4-9. [DOI] [PubMed] [Google Scholar]
- Hainsworth JD, Jolivet J, Birch R, Hopkins LG, Greco FA. Mitoxantrone, 5-fluorouracil, and high dose leucovorin (NFL) versus intravenous cyclophosphamide, methotrexate, and 5- fluorouracil (CMF) in first-line chemotherapy for patients with metastatic breast carcinoma. A randomized phase II trial. Cancer 1997;79(4):740-8. [PubMed] [Google Scholar]
Harper‐Wynne 1999 {published data only}
- Harper-Wynne C, English J, Meyer L, Bower M, Archer C, Sinnett HD, et al. Randomized trial to compare the efficacy and toxicity of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) with methotrexate mitoxantrone (MM) in advanced carcinoma of the breast. British Journal of Cancer 1999;81(2):316-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
HEPI 013 2001 {published data only}
- Ackland SP, Anton A, Breitbach GP, Colajori E, Tursi JM, Delfino C, Efremidis A, Ezzat A, Fittipaldo A, Kolaric K, Lopez M, Viaro D, HEPI. Dose-intensive epirubicin-based chemotherapy is superior to an intensive intravenous cyclophosphamide, methotrexate, and fluorouracil regimen in metastatic breast cancer: a randomized multinational study. Journal of Clinical Oncology 2001;19(4):943-53. [DOI] [PubMed] [Google Scholar]
Hoogstraten 1976a {published data only}
- George SL, Hoogstraten B. Prognostic factors in the initial response to therapy by patients with advanced breast cancer. Journal of the National Cancer Institute 1978;60:731-6. [DOI] [PubMed] [Google Scholar]
- Hoogstraten B, George SL, Samal B, Rivkin SE, Costanzi JJ, Bonnet JD, et al. Combination chemotherapy and adriamycin in patients with advanced breast cancer. A. Cancer 1976;38:13-20. [DOI] [PubMed] [Google Scholar]
- Hoogstraten B George S. Adriamycin and combination chemotherapy breast cancer: a SouthWest Oncology Group study. Proceedings of the American Association for Cancer Research 1974;15:70.
Hoogstraten 1976b {published data only}
- Hoogstraten B, George SL, Samal B, Rivkin SE, Costanzi JJ, Bonnet JD, Thigpen T, Braine H. Combination chemotherapy and adriamycin in patients with advanced breast cancer.. Cancer 1976;38:13-20. [DOI] [PubMed] [Google Scholar]
Kolaric 1977 {published data only}
- Kolaric K, Nola P, Roth A, et al. The value of adriamycin in combination chemotherapy of metastatic breast cancer. A comparative study. LIBRI ONCOL 1977;6:5-10. [Google Scholar]
Kolaric 1985 {published data only}
- Kolaric K, Vukas D, Roth A, Potrebica V, Cervek J, Cerar O. Cyclophosphamide, adriamycin and platinum (CAP) combination chemotherapy, a new effective approach in the treatment of disseminated breast cancer. Preliminary report. Tumori 1985;71:159-65. [DOI] [PubMed] [Google Scholar]
Lorusso 1993 {published data only}
- Lorusso V, Vici P, Bianco AR, Lopez M, De PS, Piano A, et al. Combination chemotherapy with CMF (cyclophosphamide, methotrexate, 5-fluorouracil) versus CNF (mitoxantrone, 5- fluorouracil, cyclophosphamide) in advanced breast cancer: a multicenter randomized study. International Journal of Oncology 1993;2:531-5. [DOI] [PubMed] [Google Scholar]
Muss 1978 {published data only}
- Muss HB, White DR, Richards F, Cooper MR, Stuart JJ, Jackson DV, et al. Adriamycin versus methotrexate in five-drug combination chemotherapy for advanced breast cancer: a randomized trial. Cancer 1978;42:2141-8. [DOI] [PubMed] [Google Scholar]
Muss 1982 {published data only}
- Muss H, Richards FI, Jackson D V, et al. A randomized trial of vincristine (V), adriamycin (A) and cyclophosphamide (C) versus low dose cyclophosphamide, methotrexate (M), and fluorouracil (F). In: Proceedings of the American Association of Cancer Research. 1981:584.
- Muss HB, Richards F, Jackson DV, Cooper MR, White DR, Stuart JJ, et al. Vincristine, doxorubicin, and cyclophosphamide versus low-dose intravenous cyclophosphamide, methotrexate, and 5-fluorouracil in advanced breast cancer: a randomized trial of the Piedmont Oncology Association. Cancer 1982;50:2269-74. [DOI] [PubMed] [Google Scholar]
Nemoto 1978 {published data only}
- Nemoto T, Rosner D, Diaz R, Dao T, Sponzo R, Cunningham T, et al. Combination chemotherapy for metastatic breast cancer: comparison of multiple drug therapy with 5-fluorouracil, cytoxan and prednisone with adriamycin or adrenalectomy. Cancer 1978;41:2073-7. [DOI] [PubMed] [Google Scholar]
Nemoto 1982a {published data only}
- Nemoto T, Horton J, Simon R, Dao TL, Rosner D, Cunningham T, et al. Comparison of four-combination chemotherapy programs in metastatic breast cancer: comparison of multiple drug therapy with cytoxan, 5-FU and prednisone versus cytoxan and adriamycin, versus cytoxan, 5-FU and adriamycin, versus cytoxan, 5-FU and prednisone alternating with cytoxan and adriamycin. Cancer 1982;49(10):1988-93. [DOI] [PubMed] [Google Scholar]
Nemoto 1982b {published data only}
- Nemoto T, Horton J, Simon R, Dao TL, Rosner D, Cunningham T, et al. Comparison of four-combination chemotherapy programs in metastatic breast cancer: comparison of multiple drug therapy with cytoxan, 5-FU and prednisone versus cytoxan and adriamycin, versus cytoxan, 5-FU and adriamycin, versus cytoxan, 5-FU and prednisone alternating with cytoxan and adriamycin. Cancer 1982;49(10):1988-93. [DOI] [PubMed] [Google Scholar]
Nemoto 1982c {published data only}
- Nemoto T, Horton J, Simon R, Dao TL, Rosner D, Cunningham T, et al. Comparison of four-combination chemotherapy programs in metastatic breast cancer: comparison of multiple drug therapy with cytoxan, 5-FU and prednisone versus cytoxan and adriamycin, versus cytoxan, 5-FU and adriamycin, versus cytoxan, 5-FU and prednisone alternating with cytoxan and adriamycin. Cancer 1982;49(10):1988-93. [DOI] [PubMed] [Google Scholar]
Pannuti 1984 {published data only}
- Pannuti F, Iafelice G, Martoni A, Fruet F, Angelelli B, Casadei M. A new six-drug antiblastic regimen (R 14) at low doses (micropolychemotherapy) compared to CMF in the treatment of metastatic breast cancer: phase III study. Chemioterapia 1984;3:216-9. [PubMed] [Google Scholar]
Rosner 1989a {published data only}
- Rosner D, Lane WW, Nemoto T. Differential response to chemotherapy in metastatic breast cancer in relation to estrogen receptor level. Results of a prospective randomized study. Cancer 1989;64(1):6-15. [DOI] [PubMed] [Google Scholar]
- Rosner D, Nemoto T, Lane WW. A randomized study of intensive versus moderate chemotherapy programs in metastatic breast cancer. Cancer 1997;59(5):874-83. [DOI] [PubMed] [Google Scholar]
Rosner 1989b {published data only}
- Rosner D, Nemoto T, Lane WW. A randomized study of intensive versus moderate chemotherapy programs in metastatic breast cancer. Cancer 1997;59(5):874-83. [DOI] [PubMed] [Google Scholar]
SAKK 1983a {published data only}
- Cavalli F, Pedrazzini A, Martz G, Jungi WF, Brunner KW, Goldhirsch A, et al. Randomized trial of 3 different regimens of combination chemotherapy in patients receiving simultaneously a hormonal treatment for advanced breast cancer. European Journal of Cancer Clinical Oncology 1983;19(11):1615-24. [DOI] [PubMed] [Google Scholar]
SAKK 1983b {published data only}
- Cavalli F, Pedrazzini A, Martz G, Jungi WF, Brunner KW, Goldhirsch A, et al. Randomized trial of 3 different regimens of combination chemotherapy in patients receiving simultaneously a hormonal treatment for advanced breast cancer. European Journal of Cancer Clinical Oncology 1983;19(11):1615-24. [DOI] [PubMed] [Google Scholar]
SECSG 1983 {published data only}
- Smalley RV, Carpenter J, Bartolucci A, Vogel C, Krauss S. A comparison of cyclophosphamide, adriamycin, 5-fluorouracil (CAF) and cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, prednisone (CMFVP) in patients with metastatic breast cancer: a Southeastern Cancer Study Group project. Cancer 1977;40:625-32. [DOI] [PubMed] [Google Scholar]
- Smalley RV, Lefante J, Bartolucci A, Carpenter J, Vogel C, Krauss S. A comparison of cyclophosphamide, adriamycin, and 5-fluorouracil (CAF) and cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, and prednisone (CMFVP) in patients with advanced breast cancer. Breast Cancer Research and Treatment 1983;3:209-20. [DOI] [PubMed] [Google Scholar]
- Smalley RV, Lefante J, Liu C, Bartolucci AA. Multivariate prognostic factor analysis evaluating dose received in patients with advanced breast cancer treated with doxorubicin combination. Breast Cancer Research & Treatment 1981;1(1):162. [Google Scholar]
References to studies excluded from this review
303 Study Group {published data only}
- Aapro M. Docetaxel versus doxorubicin in patients with metastatic breast cancer who have failed alkylating chemotherapy: a preliminary report of the randomized phase III trial. 303 Study Group. Seminars in Oncology 1998;25(5 Suppl 12):7-11. [PubMed] [Google Scholar]
- Chan S, Friedrichs K, Noel D, Pinter T, Van Belle S, Vorobiof D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. John Crown for the 303 Study Group. Journal of Clinical Oncology 1999;17(8):2341-5. [DOI] [PubMed] [Google Scholar]
- Chan S. Docetaxel (Taxotere) vs doxorubicin in patients with metastic breast cancer (MBC) who have failed alkylating chemotherapy. Randomized multicenter phase III trial. Proceedings of American Society of Clincial Oncology 1997;16:154 Abstract. [Google Scholar]
- Chan S. Docetaxel vs doxorubicin in metastatic breast cancer resistant to alkylating chemotherapy. Oncology (Huntington) 1997;11(8 Suppl 8):19-24. [PubMed] [Google Scholar]
- Nabholtz JM, Crown J. Phase III studies of single-agent docetaxel in patients with metastatic breast cancer who have progressed despite previous chemotherapy regimens: preliminary results. Seminars in oncology 1998;25(6, Supp 13):4-9. [PubMed] [Google Scholar]
304 Study Group {published data only}
- Nabholtz, JM, Crown, J. Phase III studies of single-agent docetaxel in patients with metastatic breast cancer who have progressed despite previous chemotherapy regimens: preliminary results. Semin Oncol 1998;25(6, Supp 13):4-9. [PubMed] [Google Scholar]
- Nabholtz JM, Crown J. Phase III studies of single-agent docetaxel in patients with metastatic breast cancer who have progressed despite previous chemotherapy regimens: preliminary results. Seminars in Oncology 1998;25(6 Suppl 13):4-9. [PubMed] [Google Scholar]
- Nabholtz JM, Senn HJ, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. Journal of Clinical Oncology 1999;17(5):1413-24. [DOI] [PubMed] [Google Scholar]
- Nabholtz J M, Thuerlimann B, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, et al. Docetaxel vs mitomycin plus vinblastine in anthracycline- resistant metastatic breast cancer. Oncology (Huntington) 1997;11(8 Suppl 8):32-7. [PubMed] [Google Scholar]
- Nabholtz JM. Docetaxel (Taxotere) vs mitomycin C + vinblastine in patients with metastatic breast cancer (MBC) who have failed an anthracycline-containing regimen. Preliminary evaluation of a randomized phase III study. Proceedings of American Society of Clincial Oncology 1997;16:148a. [Google Scholar]
Ahmann 1978 {published data only}
- Ahmann DL, O'Fallon J, O'Connell MJ, Bisel HF, Hahn RG, Frytak S, et al. Evaluation of a fixed alternating treatment in patients with advanced breast cancer. Cancer Clinical Trials 1978;1:219-26. [PubMed] [Google Scholar]
Bezwoda 1979 {published data only}
- Bezwoda WR, De Moor NG, Derman D, Lange M, Saner R, Dando R, et al. Combination chemotherapy of metastatic breast cancer: a randomized trial comparing the use of adriamycin to that of Vinblastine. Cancer 1979;44:392-7. [DOI] [PubMed] [Google Scholar]
Dieras 1995 {published data only}
- Dieras V, Marty M, Tubiana N, Corette L, Morvan F, Serin D, et al. Phase II randomised study of paclitaxel versus mitomycin in advanced breast cancer. Seminars in Oncology 1995;22(4 Suppl 8):33-9. [PubMed] [Google Scholar]
Erkisi 1997 {published data only}
- Erkisi M, Bilkay BC, Seyrek E, Hazar B, Burgut R. Refractory breast cancer: a comparison of two different chemotherapy regimens. Journal of Chemotherapy 1997;9:442-5. [DOI] [PubMed] [Google Scholar]
Falkson 1988 {published data only}
- Falkson CI, Falkson HC, Falkson G. High-dose medroxyprogesterone acetate plus mitomycin-C or vindesine in the treatment of advanced breast cancer. American Journal of Clinical Oncology 1988;11(4):431-4. [DOI] [PubMed] [Google Scholar]
GOIRC 1990 {published data only}
- Cocconi G, Bisagni G, Bacchi M, Buzzi F, Canaletti R, Carpi A, et al. A comparison of continuation versus late intensification followed by discontinuation of chemotherapy in advanced breast cancer. A prospective randomized trial of the Italian Oncology Group for Clinical Research (G.O.I.R.C.). Annals of Oncology 1990;1(1):36-44. [DOI] [PubMed] [Google Scholar]
Hori 2001 {published data only}
- Hori T, Kodama H, Nishimura S, Hatano T, Okamura R, Fujii K, Inamoto T, Sawai K, Kobayashi M, Ogawa H, Yoshimura N, Hiraoka M. A randomised study comparing oral and standard regimens for metastatic breast cancer. Oncology Reports 2001;8:1067-1071. [DOI] [PubMed] [Google Scholar]
Legha 1979 {published data only}
- Legha, SS, Benjamin, RS, Buzdar, AU, Hortobagyi, GN, Blumenschein, GR. Rubidazone in metastatic breast cancer. Cancer Treatment Reports 1979;63:135-136. [PubMed] [Google Scholar]
Leiden Uni Centre {published data only (unpublished sought but not used)}
- Leiden Uni Centre. Phase II randomised study of cyclophosphamide/methotrexate/fluorouracil (CMF) vs mitoxantrone in elderly patients with advanced breast cancer.. Clinical Trials website - www.clinicaltrials.gov.
Porzsolt 1990 {published data only}
- Porzsolt F, Kreuser ED, Meuret G, Mende S, Buchelt L, Redenbacher M, et al. High-intensity therapy versus low-intensity therapy in advanced breast cancer patients. Cancer Treatment Reviews 1990;17(2-3):287-92. [DOI] [PubMed] [Google Scholar]
SWG Gottlieb 1974 {published data only}
- Gottlieb JA, Rivkin SE, Spigel SC, et al. Superiority of adriamycin over oral nitrosoureas in patients with advanced breast carcinoma. A Southwest Cancer Chemotherapy Study Group study. Cancer 1974;33:519-26. [DOI] [PubMed] [Google Scholar]
Venturino 2000 {published data only}
- Venturino A, Comandini D, Simoni C, Merlini L, Naso C, Palumbo R, et al. Is salvage chemotherapy for metastatic breast cancer always effective and well tolerated? A phase II randomized trial of vinorelbine versus 5-fluorouracil plus leucovorin versus combination of mitoxantrone, 5-fluorouracil plus leucovorin. Breast Cancer Research and Treatment 2000;60(3):195-200. [DOI] [PubMed] [Google Scholar]
Zekan 1984 {published data only}
- Zekan PJ, Muss HB, Capizzi RL, et al. gh-dose cyclophosphamide and 5-fluorouracil versus vincristine, doxorubicin, and cyclophosphamide in advanced carcinoma of the breast. A phase II study of the piedmont oncology association (POA. Cancer 1984;54:2338-43. [DOI] [PubMed] [Google Scholar]
- Zekan PJ, Muss HB, Pope E, Cooper MR, White DR, Jackson DV, et al. High dose cyclophosphamide and fluorouracil (CF) versus vincristine (V), doxorubicin (A), and cyclophosphamide (C) as first line therapy for advanced breast carcinoma. Breast Cancer Research and Treatment 1982;2(3):295.
References to ongoing studies
Butler 2004 {unpublished data only}
- A study of docetaxel monotherapy or DOXIL/CAELYX and doxetaxel in patients with advanced breast cancer. Ongoing study. Starting date of trial not provided. Contact author for more information.
Additional references
Ferlay 2002
- J Ferlay, F Bray, P Pisani, DM Parkin. Cancer Incidence, Mortality and Prevalence Worldwide. GLOBOCAN, Version 2.0. IARC CancerBase No. 5. Lyon, IARCPress, 2004:http://www-dep.iarc.fr/globocan/globocan.html.
Fossati 1998
- Fossati R, Confalonieri C, Torri V, Ghislandi E, Penna A, Pistotti V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomised trials involving 31,510 women.. Journal of Clinical Oncology 1998;16(10):3439-60. [DOI] [PubMed] [Google Scholar]
Hayes 1995
- Hayes DF, Henderson IC, Shapiro CL. Treatment of Metastatic Breast Cancer: Present and Future Prospects. Seminars in Oncology 1995;22(Suppl 5):5-21. [PubMed] [Google Scholar]
Hortobagyi 1996
- Hortobagyi GN, Piccart-Gebhart MJ. Current Management of Advanced Breast Cancer. Seminars in Oncology 1996;23(Suppl 11):1-5. [PubMed] [Google Scholar]
Hortobagyi 2003
- Hortbagyi GN. In: ASCO Conference. 2003.
NCI 2002
- National Cancer Institute. Response Evaluation Criteria in Solid Tumours. www3.cancer.gov 2002.
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
Perry 1997
- Michael C Perry (Ed). The Chemotherapy Source Book. Vol. Second Edition. Williams and Wilkins, 1997. [ISBN 0-683-06868-7] [Google Scholar]
Sledge 2003
- Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, Wood WC. Phase III Trial of Doxorubicin, Paclitaxel, and the Combination of Doxorubicin and Paclitaxel as Front-Line Chemotherapy for Metastatic Breast Cancer: An Intergroup Trial (E1193). J Clin Oncol 15-2-2003;21(4):588-592. [DOI] [PubMed] [Google Scholar]
Stockler 2000
- Stockler M, Wilcken NRC, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treatment Reviews 2000;26:151-68. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S Peto R Lewis J Collins R Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials.. Progress in Cardiovascular Diseases. 1985;27(5):335-371. [DOI] [PubMed] [Google Scholar]


