Abstract
Background
Systemic corticosteroids are used routinely in the management of children with severe acute asthma. There is a lack of consensus regarding the agent, dose and route of corticosteroid administration.
Objectives
To determine the benefit of systemic corticosteroids (oral, intravenous, or intramuscular) compared to placebo and inhaled steroids in acute paediatric asthma.
Search methods
All controlled trials were identified from the Cochrane Airways Review Group Register, hand searching of respiratory journals, reference lists and contacts with experts and pharmaceutical companies.
Selection criteria
Studies were included if they described a randomised controlled trial (RCT) involving children aged 1‐18 years with severe acute asthma who received oral, inhaled, intravenous or intramuscular corticosteroids. Only studies in which patients required hospital admission were included.
Data collection and analysis
Two reviewers using a standard form extracted all data. All data, numeric calculations and graphic extrapolations were independently confirmed.
Main results
Seven trials were included with a total of 426 children studied (274 with oral prednisone vs. placebo, 106 with intravenous steroids vs placebo and 46 with nebulised budesonide vs prednisolone). A significant number of steroid treated children were discharged early after admission (>4 hours) with an OR of 7.00 (95% CI: 2.98 to 16.45) and NNT of 3 (95%CI: 2 to 8). The length of stay was shorter in the steroid groups with a WMD of ‐8.75 hours (95% CI: ‐19.23 to 1.74). There were no significant differences between groups in pulmonary function or oxygen saturation measurements. Children treated with steroids in hospital were less likely to relapse within one to three months with OR 0.19 (95%CI: 0.07 to 0.55) and NNT of 3 (95%CI: 2 to 7). The single small study that compared nebulised budesonide to oral prednisone failed to demonstrate equivalence or a difference between each therapy.
Authors' conclusions
Systemic corticosteroids produce some improvements for children admitted to hospital with acute asthma. The benefits may include earlier discharge and fewer relapses. Inhaled or nebulised corticosteroids cannot be recommended as equivalent to systemic steroids at this time. Further studies examining differing doses and routes of administration for corticosteroids will clarify the optimal therapy.
Plain language summary
Corticosteroids for hospitalised children with acute asthma
An acute asthma attack in a child often results in a trip to the hospital. In the emergency department steroid drugs are given which may improve the child's condition and allow them to be sent home after a few hours observation. However, some children require continued treatment in hospital. This review asked the question "do steroid drugs help children admitted to hospital with asthma?" We found that steroids given by mouth or through an intravenous tube help children recover from acute asthma. The benefits may include earlier discharge or a shorter stay in hospital. Children were less likely to come back to hospital in the one to three months following the admission. However, the evidence was not overwhelming due to the limited number of studies available and different medicines used. Further research needs to concentrate on the best medications to use and the best route of administration.
Background
Despite the significant improvements in asthma treatment over the past several decades, children are commonly hospitalised with acute flare‐ups of this chronic disease (Homer 1996; To 1996). Corticosteroids are one of the cornerstones of therapy for acute asthma; however, the corticosteroid doses, delivery and agents used in the therapy of acute paediatric asthma vary considerably.
For many years, corticosteroids in acute childhood asthma were considered controversial (Weinburger 1988). Much of the research in this area suffered with methodological problems such as inadequate study design and differing populations. However, recent clinical trials in children have shown a beneficial effect of corticosteroids when they are used early in acute asthma. Much of the research in acute asthma has been performed in the emergency setting. The main benefit reported in these studies is up to a 27% reduction in hospitalisation (Storr 1987). In this study of 140 patients, prednisone was compared with a placebo and resulted in a discharge rate of 30% from the emergency department compared with 3% in the placebo group. Intramuscular methylprednisolone has also been found to be useful in very young children with acute asthma (Tal 1990). The benefits of this steroid were observed within 3 hours of administration. Similar benefits were also seen in a study completed by Scarfone 1993 with a range of paediatric age groups receiving oral prednisolone or placebo. In this study there was an 18% reduction in admission rate in the treated group. When combined, these studies suggest that steroid treatment in the emergency department results in a reduced hospital admission as well as improved symptom scores (Rowe 2000a). Furthermore, another Cochrane review that included both adults and children concluded that a short course of systemic corticosteroids after an emergency visit for acute asthma reduces relapses and decreases beta‐agonist use (Rowe 2000b). Finally, inhaled corticosteroids in the emergency setting have also been shown to provide some reduction in admissions in severe asthma (Edmonds 2000).
In ambulatory clinic settings, oral corticosteroids have been used to avoid progression to severe asthma (Horowitz 1994; Shapiro 1983). In addition, there has been considerable work on using inhaled steroids to prevent viral induced asthma attacks (Connett 1994; Doull 1997; Svedmyr 1999). These studies demonstrate that in mild, episodic asthma presenting to a clinic, high dose inhaled steroids minimise wheezing episodes and possibly reduce the need for oral steroids. It is unclear whether, these medications reduce the hospitalisation rate of acute asthma (McKean 2000).
Research into the pathophysiology of acute asthma has clarified the significant role of inflammation (Taylor 1993). Corticosteroids reduce the production of many mediators released during the inflammatory process and inhibit the many cells that are activated including macrophages, monocytes, T‐lymphocytes, eosinophils, basophils and airway epithelial cells (Taylor 1993; Djukanovic 1992). Corticosteroids may also decrease microvascular leakage and mucus secretion in irritated airways (Boschetto 1991). Additionally, corticosteroids improve the effectiveness of beta‐2 agonists (Svedmyr 1990).
Although it is generally accepted and recommended that corticosteroids be initiated or continued when a child is hospitalised with acute asthma, there are still questions regarding the extent of the clinical benefit derived from this therapy. There is a lack of consensus regarding the optimal dose, frequency and route of steroid for those children who are affected most severely by this disease. It would therefore be useful to examine the studies that have assessed steroid treatment in children beyond the initial assessment in the emergency room or ambulatory setting. Only one previous review has been completed in this topic area, and that addressed a similar question in adults (Manser 2000).
Objectives
The overall objective was to determine the clinical outcomes of children treated with corticosteroids when hospitalised with severe acute asthma. The two specific objectives are: 1. Compare systemic corticosteroids (oral, intravenous, or intramuscular) with placebo; 2. Compare systemic corticosteroids with inhaled steroids.
Methods
Criteria for considering studies for this review
Types of studies
Studies that were described as randomised controlled trials (RCT) were considered for inclusion in the review.
Types of participants
Studies involving children aged 1‐18 years with severe acute asthma defined by history, doctor's diagnosis, response to initial treatment, spirometry or peak flow were considered. Only studies where patients were treated in an emergency or outpatient department and required hospital admission have been included. Studies that included patients on pre‐existing oral corticosteroids were not included.
Types of interventions
Studies that reported results of patient's who were randomised to receive oral, inhaled, intravenous or intramuscular corticosteroids were divided into the following comparisons: 1. Systemic corticosteroids versus placebo (and include standard care with bronchodilators); 2. Systemic corticosteroids versus inhaled steroids.
Data on co‐interventions were collected including information regarding additional therapy such as beta‐agonists, anti‐cholinergics, theophylline compounds, antibiotics, oxygen.
Types of outcome measures
Given the limitations of paediatric studies, any of the following clinically relevant outcomes were included: 1. length of stay 2. symptom scores; 3. pulmonary function testing (Peak expiratory flows {PEF} and forced expiratory volume in 1 second {FEV1}; absolute and percent predicted) when performed; 4. duration of oxygen therapy; 5. relapse rates following discharge; 6. bronchodilator use (number of treatments per 24 hour period); 7. adverse effects.
To be included, all studies required a minimum follow‐up of 24 hours.
Search methods for identification of studies
Electronic searches
Firstly, all controlled trials were identified from a Cochrane Airways Review Group ASTHMA AND WHEEZ* Register, which is a compilation of systematic searches of CINAHL, EMBASE, MEDLINE and CENTRAL and hand searching of 20 respiratory journals. The computerised search was completed using the following terms:
[Acute OR status OR exacerbation* OR hospitalization] AND [Infusion OR multi‐dose OR bolus OR intravenous OR administration OR dosage OR oral OR PO OR inhaled OR nebulized] AND [Prednisolone OR Prednisone OR methyl‐prednisolone OR MP OR methylprednisolone OR corticosteroid OR hydrocortisone OR glucocorticoids OR solucortef OR solu‐cortef OR solumedrol OR dexamethasone OR triamcinolone OR betamethasone OR budesonide OR fluticasone OR flunisolide OR flovent OR pulmicort OR beclofort OR flixotide OR decadron OR becotide OR solucortef OR precortisyl forte OR prednesol OR solu‐medrone OR depo‐medrone]
Searching other resources
Secondly, primary authors and content experts have been contacted to identify appropriate studies. Thirdly, reference lists from all retrieved studies and reviews as well as textbooks were also searched for relevant studies. Finally, contacts with pharmaceutical companies, trialists, and known authors in the field have also be contacted to seek other studies relevant to the review.
Data collection and analysis
Selection of studies
Two reviewers (MS, SI) independently screened the initial search of all the databases and reference lists to identify citations with potential relevance.
The full text of selected articles (translated into English where required) were obtained and using defined eligibility criteria, two independent reviewers (MS, SI) decided on trial inclusion. Reviewers were not blinded to authors, journal, results, etc. Discussion, and a third party resolved disagreements when necessary.
Data extraction and management
Two reviewers (TN'D, SI) independently extracted data using a standard form. All data, numeric calculations and graphic extrapolations were independently confirmed. Reviewers have attempted to contact authors to obtain some missing data.
To use the data in some of the trials a number of data conversions were necessary as follows: Ho reported mean + 95% CI for all outcome measures 95% CI's have been converted to SE's: 95% CI for continuous data =Xbar ‐ 1.96*SE(Xbar ) to Xbar + 1.96*SE(Xbar ) if 95% CI is (LL to UL) then SE(Xbar ) can be calculated thus SE(Xbar ) = (Xbar ‐ LL) / 1.96orSE(Xbar ) = (UL ‐ Xbar) / 1.96 where LL and UL = lower and upper limit of CI
Assessment of risk of bias in included studies
Two independent reviewers (TE, SI) using two approaches, independently subjected included trials to quality assessments: 1. Allocation concealment. Using the Cochrane approach to assessment of allocation concealment, all trials were scored and entered using the following principles: Grade A: Adequate concealment Grade B: Uncertain Grade C: Clearly inadequate concealment
2. Quality assessment. Quality was assessed using a 5 part score (Jadad 1996) and summarised as follows: Was the study described as randomised (1=yes; 0=no) Was the study described as double‐blind (1=yes; 0=no) Was there a description of withdrawals and dropouts (1=yes; 0=no) Was the method of randomisation well described and appropriate (1=yes; 0=no) Was the method of double blinding well described and appropriate (1=yes; 0=no) Points were deducted for either inappropriate randomisation or blinding.
Dealing with missing data
When the standard deviation (SD) for length of stay was missing from a study, an estimate was imputed (Gleeson 1990). The estimate was based on the weighted average (by sample size) of the deviations from other included studies for that category. Data from one study (Younger 1987) were not reported in tables but demonstrated in charts. To extract these data values scaled grids were produced on acetate and date points traced onto these; results were checked for reliability twice. Graphs displayed % predicted measure with standard errors of the mean (SEM). SEM's for these trials and in two others (Gleeson 1990; Connett 1994) were converted to SD's thus: SD(Xbar) = SEM(Xbar)*sqrt(n).
Data synthesis
Data from trials were entered into Review Manager. Only one outcome (oral prednisolone vs placebo) and comparison (discharge at first exam (4h)) was suitable for meta‐analysis. Two trials (Connett 1994; Storr 1987) used this outcome measure. Consideration was given to the possibility of heterogeneity between these studies using chi‐square test for heterogeneity. One study (Connett 1994) further randomised their sample into two groups those taking salbutamol every 30 minutes and those taking salbutamol every 1‐4 hours. These sub‐groups have been analysed individually and combined for an overall effect using fixed effect models.
Results
Description of studies
Results of the search
The initial search yielded 127 papers. Following full text review, 18 studies were considered to be relevant and subjected to phase one of the assessment.
Included studies
The included trials were single‐centre RCT's from the UK (Connett 1994; Gleeson 1990; Storr 1987), US (Younger 1987), Canada (Kattan 1980) and Australia (Ho 1994) and one trial (Matthews 1999) was a multi‐center study encompassing 9 centres from around the UK.
POPULATION STUDIED: Some of the studies examined children with a mean age of 5 years (Ho 1994; Connett 1994; Storr 1987; Gleeson 1990) and the others (Younger 1987; Matthews 1999; Kattan 1980) examined children predominantly in the school age. This is an important distinction as the younger ages are unlikely to provide pulmonary function data and the outcomes are more often determined by clinical assessment. All studies dealt with acute asthma; however, many did not include children who were seriously ill with asthma who were likely to go on to require intensive care. Some studies were explicit in this exclusion (Ho 1994; Matthews 1999) and for others this was inferred from the clinical information.
Previous corticosteroid use was reported in most studies to a varying degree. All studies excluded patients currently using oral corticosteroids. Inhaled steroids were an excluding characteristic for some studies (Younger 1987; Gleeson 1990; Ho 1994; Kattan 1980). The remaining studies allowed prophylactic inhaled steroids in their study and this ranged from 3% (Ho 1994) to 33% (Connett 1994) of subjects. This possibly reflects the variability in acceptance and use of prophylactic inhaled steroids in this time period.
All studies included children who were initially assessed in an emergency setting and admitted with acute severe asthma. All had been treated with an initial protocol (which varied with each institution) and admitted because of their failure to improve. Some of these protocols were very specific and admission was based on a poor response to therapy (Connett 1994; Gleeson 1990; Younger 1987; Kattan 1980) and others indicated that admission was based largely on clinical grounds. None of the studies used peak flow as a guide to admission.
INTERVENTIONS: Three studies used oral prednisolone in three different doses (Ho 1994; Connett 1994; Storr 1987). In each of these studies, only one dose was given on admission. In four studies intravenous steroids were administered. The Kattan 1980 study used hydrocortisone 7 mg/kg/day. In the Gleeson 1990 study intravenous hydrocortisone was given initially as a loading dose (6 mg/kg) followed by 2 mg/kg for at least 24 hours before being converted to oral prednisolone 2 mg/kg/day. In Younger 1987 intravenous methylprednisolone 2 mg/kg was given initially followed by 1 mg/kg every 6 hours for the duration of the intravenous therapy. Only one study was identified (Matthews 1999) that investigated the use of nebulised budesonide (2 mg every 8 hours) and compared this with two days of oral prednisolone (2 mg/kg/day).
CO‐INTERVENTIONS: All studies used nebulised bronchodilators as part of the treatment of acute asthma. The usual medication was salbutamol either as 0.15 mg/kg or a set 5 mg dose. Terbutaline was used in one study (Matthews 1999). The dose interval varied according to the clinical response and ranged between every 2 hours up to 3 times per day. In Connett 1994 study salbutamol was given frequently (every 30 minutes with prednisolone or placebo) or infrequently (every 1‐4 hours with prednisolone or placebo). Some studies used intravenous aminophylline (Ho 1994; Gleeson 1990; Younger 1987; Kattan 1980) and other bronchodilators (Younger 1987; Kattan 1980).
OUTCOMES: Precise outcome measurement of acute asthma in children is difficult. Therefore, it is not surprising that the outcomes were reported variably in these trials; there are no consistent outcomes for a combined analysis. In two studies the percentage of children discharged at the first assessment (4‐5 hours) was used as a measure of the steroid effect (Connett 1994; Storr 1987). Length of stay in hospital was used in the remainder of the studies. Most studies (Ho 1994; Connett 1994; Storr 1987; Younger 1987; Matthews 1999; Kattan 1980) used symptom scores or pulmonary indexes as a measure to determine outcome. Unfortunately many of the scores differed in content or were assessed at different time points. Pulmonary function testing was limited by the ages of the patients studied and therefore only completed in a portion of the participants in all of the studies. Other proxy measures of respiratory distress (e.g., oxygen saturation, heart rate and blood gas analysis) were reported sporadically.
DESIGN: All of the studies were randomised‐controlled trials with information on withdrawal of participants except in one study (Matthews 1999), where this information was not stated.
Refer to the table of included studies section for further details.
Excluded studies
Eleven studies (Barnett 1997; Becker 1999; Chavez 1992; Daugbjerg 1993; Devidayal 1999; Gonzalez 1994; Langton‐Hewer 1998; Lin 1991; Loren 1980; Pierson 1974; Sano 2000) were removed for one or more of the following reasons:
not a randomised trial (n = 4);
no appropriate comparison arm ‐either placebo or drug (n = 3);
not located in a hospital setting (n = 1);
included children <12 months (n = 4);
emergency department study only ‐ not hospitalised (n = 2).
Risk of bias in included studies
The methodological quality of the included studies was generally good. The results for the initial quality assessments are listed below.
The Jadad quality scores were as follows: 5 points: (Connett 1994; Storr 1987) 4 points: (Ho 1994) 3 points: (Matthews 1999) 2 points: (Gleeson 1990) 0 points: (Kattan 1980)
Allocation concealment was adequate in three studies (Connett 1994; Storr 1987; Ho 1994). In all other studies the allocation concealment was unclear (Gleeson 1990; Matthews 1999;Younger 1987; Kattan 1980).
All studies were randomised and double blind but in some the description of randomisation (Matthews 1999) and the method of double blinding (Gleeson 1990; Ho 1994) were not described. One study (Kattan 1980) was a single‐blind study.
Effects of interventions
Three comparisons have been used by the seven studies. 1. oral prednisolone vs placebo (Ho 1994; Connett 1994;Storr 1987); 2. oral prednisolone vs nebulised budesonide (Matthews 1999); 3. IV steroids vs placebo (Younger 1987; Kattan 1980; Gleeson 1990).
To maximise the usefulness of this review all corticosteroid comparisons have been considered in two parts 1. systemic steroids vs placebo (placebo vs oral or IV corticosteroids); 2. inhaled corticosteroids vs systemic corticosteroids.
Not all of the data could be extracted from the papers because of unreported parameters and although it has been possible to extrapolate some of the results as previously described, it remains impossible to assess some information. Authors have been contacted with requests for all missing data (see characteristics of included studies for detail).
A number of different outcome measures were reported in the studies but only two outcomes were suitable for meta‐analysis: discharge at first re‐examination and relapse after discharge. Using results from two trials (Storr 1987;Connett 1994) involving a total of 210 patients, discharge at first re‐examination was evaluated. Both Storr 1987 and Connett 1994 report outcomes at or after four hours and no heterogeneity has been detected between the populations of these trials (p=0.14). The second outcome suitable for meta‐analysis was relapse rate, using the trial results of Younger 1987 and Gleeson 1990 involving a total of 84 patients. Although the test for heterogeneity on this outcome was non significant (p=0.98), the studies use slightly different measurements; one measured relapse four weeks after discharge (Younger 1987) whereas the other measured it three months following discharge (Gleeson 1990).
Other relevant trial results have been reported individually.
SYSTEMIC STEROIDS VS PLACEBO
1. LENGTH OF STAY
Discharge at first re‐exam (>4h)
This outcome was reported in two studies (Connett 1994; Storr 1987) as a measure of effectiveness. Both Storr 1987(OR =3.83; 95% CI: 1.28 to 11.44) and Connett 1994 (OR=15.11; 95% CI: 3.37 to 67.67) reported a significant difference between treatment and control groups, indicating that treatment groups were more likely to be discharged at first re‐examination than the control groups. The pooled results of these studies was also significant (OR = 7.00; 95% CI: 2.98 to 16.45) in favour of treatment. The NNT for this outcome indicates that 3 (95%CI: 2 to 8) children would need to be treated with systemic corticosteroids to allow one to be discharged after the four hour assessment. The test for heterogeneity between these studies was non significant (p=0.14).
Hospital length of stay
There was no significant difference in the length of stay in Ho 1994 (2.18 d vs 2.50 d). The median length of stay in the Gleeson 1990 trial for the steroid group (54 hours; range 41 to 100 hours) vs (64 hours; range 39 to 176 hours) in the placebo group. This was not statistically significant. The length of stay in hours in the Younger 1987 study was also less in the corticosteroid treatment group (mean difference ‐9.00 hours; 95% CI: ‐27.07 to 9.07); however, this was not statistically significant. The pooled result of the above studies was (WMD = ‐8.75 hours; 95% CI: ‐19.23 to 1.74) in favour of treating with corticosteroids.
2. PULMONARY FUNCTION TESTING
% predicted PEFR
There was no significant difference between the control and treatment groups in % predicted PEFR at 24 hours according to the Younger 1987 and Kattan 1980 data (WMD=7.21; 95% CI: ‐7.01 to 21.25).
3. RELAPSE RATE FOLLOWING DISCHARGE
Both Connett 1994 and Storr 1987 found there was no re‐referrals with acute asthma in the two weeks following the study treatment. Ho 1994 did not find exacerbations in either group in the one week following the treatment. Younger (Younger 1987) investigated relapse rate (number of patients) within four weeks and Gleeson (Gleeson 1990) counted relapsed patients within three months. Younger (Younger 1987) showed that the treatment group were less likely to relapse within this time and although this result is not significant it is extremely close to significance (OR=0.19; 95% CI: 0.03 to 1.01). Gleeson 1990's results also show that patients in the treatment group were significantly less likely to relapse within three months of discharge (OR=0.19; 95% CI: 0.05 to 0.76). The combined effect of these two studies shows relapse rate to be significantly lower in the treatment groups (OR=0.19; 95% CI: 0.07 to 0.55). The NNT for this outcome indicates that 3 (95% CI: 2 to 7) children need to be treated with systemic steroids to prevent one relapse at one to three months.
NEBULISED STEROIDS VS SYSTEMIC STEROIDS
Matthews 1999 reported that the severity of shortness of breath decreased more in the patients who received budesonide than those receiving prednisolone (WMD=‐0.77; 95% CI: ‐1.34 to ‐0.20). However, there were no significant differences in cough or wheeze, or in measures of pulmonary function such as FEV1, PEFR, FVC or oxygen saturation between the nebulised budesonide and prednisolone groups.
Discussion
This systematic review examined the best available evidence for the use of systemic or nebulised corticosteroids in the management of acute asthma in children. Several important points arise from this meta‐analysis. First, for such an important clinical question, we were surprised at the limited number and small sample sizes of available trials upon which recommendations are based. Second, the data from the included trials were sparse and pooled results were not possible for many important outcomes. Third, the use of systemic corticosteroids seems to favour early discharge in the first 4‐6 hours, but does not appear to significantly reduce hospital length of stay. Fourth, improved clinical severity scores were noted in the patients treated with these agents, yet corticosteroid therapy did not improve the measures of pulmonary function or oxygen saturation. Fifth, relapses were less common in the 1‐3 months after discharge in the groups treated with systemic corticosteroids. Finally, while adverse effects were rarely reported, these agents seem to be well tolerated.
While the systemic corticosteroids appear to improve some outcomes, there was insufficient research on nebulised agents and firm conclusions regarding their use cannot be offered at this time. The studies included in the review excluded patients requiring intensive care or status asthmaticus, so results cannot be generalised to such patients. None of the studies included patients on regular oral corticosteroids, so the findings may not be generalisable to these patients as well. In chronic asthma, some patients have been reported to require relatively high doses of oral maintenance corticosteroids and others are classified as steroid resistant (Barnes 1995; Payne 1998). Consequently, these results may not apply to these subsets of patients.
CORTICOSTEROID TYPE The studies comparing systemic steroids with placebo can be broadly divided into oral and intravenous groups. In the oral corticosteroid comparison, three studies assessed the effect of a single dose of prednisolone (Ho 1994; Connett 1994; Storr 1987). These patients were obviously well enough to tolerate medication and it was assumed that one dose was adequate for the treatment of their exacerbation. Prednisolone is known to take effect within 1‐4 hours and has a physiological half life of 12‐30 hours (Ziment 1986). Therefore it is likely that any benefits of this regimen would be seen initially and then dissipate over the first day. In the intravenous group, all studies used multiple doses for the duration of the stay thus sustaining a continuous steroid effect (Younger 1987; Gleeson 1990; Kattan 1980). Intravenous medications do not depend on patient compliance or severity. There are greater costs, risks and discomfort of this type of therapy; however, given the between‐study comparisons and the small numbers of patients involved, no specific conclusions can be drawn about the comparative efficacy of the two routes of systemic corticosteroid administration. Finally, only one study compared nebulised medication with oral medication in hospital (Matthews 1999)
SETTING These results are applicable to children hospitalised with acute moderate to severe asthma and should be generalisable in developed countries.
SIDE EFFECTS None of these studies formally addressed the issue of safety although they all suggested that short courses of steroids were safe when used to treat acute exacerbations of asthma; however, formal safety measurements were generally not part of the evaluations. Storr 1987 reported that most children disliked the taste of the oral medicine.
STRENGTH OF EVIDENCE The evidence from this review suggests that treating children with acute severe asthma with systemic steroids may result in earlier discharge and slightly shorter hospital stays when compared with bronchodilator treatment alone. These benefits were not reflected by improved oxygen saturation measurement. Furthermore there was no substantial evidence that pulmonary function tests improve with inpatient steroid therapy. PEFR improved early on in two studies but this was not corroborated by other measures of pulmonary obstruction. There was a trend towards decreasing relapse rates within one to three months in patients who received steroid therapy during their hospitalisation.
Only one study compared nebulised budesonide with oral prednisone showed two small improvements in the nebulised group but the majority of clinical and pulmonary function measures favoured prednisone. This study concluded that the two steroids were equivalent but since the power to detect significant differences was not adequate there is the possibility of a beta error.
METHODOLOGICAL LIMITATIONS
DESIGN OF STUDIES: The majority of included studies were constructed to assess whether corticosteroids were of benefit when used in the treatment of exacerbations of asthma requiring hospitalisation. The studies used a variety of outcome measures making it difficult to aggregate data for any outcome other than duration of admission and some measures of pulmonary function. There were no studies comparing the effect of different dosages of steroids on the outcome measures. This has been addressed in another review in adults (Manser 2000).
There is a possibility of publication bias in this meta‐analysis. For example, by missing unpublished negative trials we may be over‐estimating the effect of corticosteroid treatment. However, a comprehensive search of literature for potentially relevant studies was conducted, using a systematic strategy to avoid bias. This was followed by attempts to contact corresponding and first authors. Although no published trials were identified, some negative trials were found and we recognise that more of these types of trials may exist. There is also the possibility of study selection bias. However we employed two independent reviewers, and feel confident that the studies excluded were done so for consistent and appropriate reasons. Our search was comprehensive and so it is unlikely that there were trials in publication which were missed.
Authors' conclusions
Implications for practice.
In children hospitalised with severe acute asthma, emergency department treatment of an asthma exacerbation with systemic corticosteroids will result in an earlier recovery of the illness. The trials suggests that a variety of corticosystemic steroids can be used including oral prednisolone, intravenous methylprednisone and intravenous hydrocortisone. All of these have approximately similar benefits including earlier discharge and improving symptom scores. This review does not support the use of nebulised corticosteroids as a substitute for systemic therapy at this time.
Implications for research.
Despite the findings of this review there are many questions arising from the analysis. Given the differing treatments and outcomes it was difficult to make substantial conclusions regarding the effect size of corticosteroid treatment. The multiple types, doses and routes make clear recommendations of one steroid over another impossible. Consequently, the results indicate the following research is required:
A high‐quality, multi‐center RCT is required examining different routes, doses and duration of systemic corticosteroid therapy.
All future studies involving children with asthma require clearly defined and validated outcome measures which are appropriate for age and reported in a standardised fashion.
The role of co‐interventions such as the frequency and dose of beta agonists must be considered when addressing outcome measures such as lung function.
Lack of methodology for assessing potential long term effects of single and repeated courses of systemic steroids is a major limitation to full assessment of this type of intervention; and side‐effects must be formally collected in future studies.
Further research that includes patients requiring intensive care might be useful to clarify the risk‐benefit of different doses and frequency of systemic corticosteroids in this sub group needs to be conducted.
Further research on the possible additive beneficial effects of inhaled corticosteroids needs to be conducted.
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 30 October 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to gratefully acknowledge the assistance provided by the members of the Cochrane Airways Group who helped with the protocol development, literature searches, and obtaining the studies (Steve Milan, Toby Lasserson and Karen Blackhall). We would also like to gratefully acknowledge the assistance of Dr. Chris Cates (ARG Technical and Criticism Editor) and Dr Paul Jones (ARG Co‐ordinating Editor).
Data and analyses
Comparison 1. All Steroids vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discharge at first re‐examination (4h) | 2 | 210 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.0 [2.98, 16.45] |
| 2 Length of stay (hours) | 3 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐8.75 [‐19.23, 1.74] |
| 3 % predicted PEFR (24h) | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | 7.12 [‐7.01, 21.25] |
| 4 Measurements of FEV1 (24h) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Predicted FEV1 | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 % predicted FEF 25‐75 (24h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 % predicted FVC (24h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Change in SaO2 (24h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Clinical score (12h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Clinical score (24h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Clinical score (48h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Change in pulmonary index (12h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Change in pulmonary index (24h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Change in pulmonary index (48h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Total severity score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Salbutamol every 30 min | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Salbutamol every 1‐4 h | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Respiratory rate (breaths/min) at 4h | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Salbutamol every 30 min | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Salbutamol every 1‐4 h | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Requirement for supplementary therapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17 Wheeze free on auscultation @ discharge | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18 Relapse rate | 2 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.55] |
1.1. Analysis.
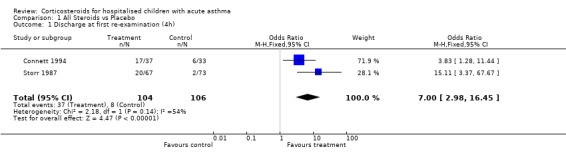
Comparison 1 All Steroids vs Placebo, Outcome 1 Discharge at first re‐examination (4h).
1.2. Analysis.
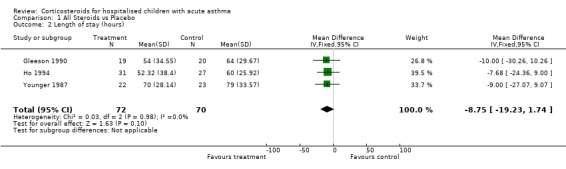
Comparison 1 All Steroids vs Placebo, Outcome 2 Length of stay (hours).
1.3. Analysis.
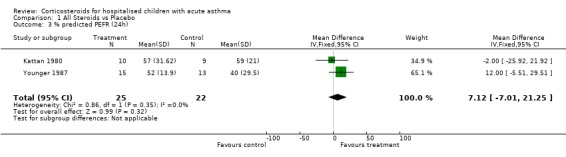
Comparison 1 All Steroids vs Placebo, Outcome 3 % predicted PEFR (24h).
1.4. Analysis.
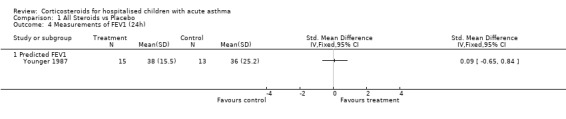
Comparison 1 All Steroids vs Placebo, Outcome 4 Measurements of FEV1 (24h).
1.5. Analysis.
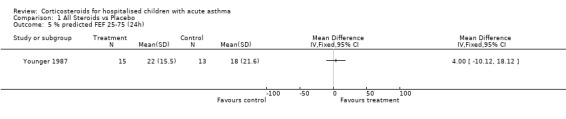
Comparison 1 All Steroids vs Placebo, Outcome 5 % predicted FEF 25‐75 (24h).
1.6. Analysis.
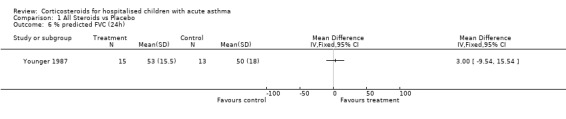
Comparison 1 All Steroids vs Placebo, Outcome 6 % predicted FVC (24h).
1.7. Analysis.
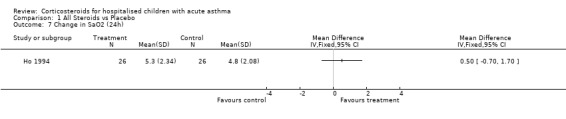
Comparison 1 All Steroids vs Placebo, Outcome 7 Change in SaO2 (24h).
1.8. Analysis.
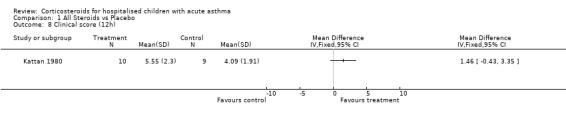
Comparison 1 All Steroids vs Placebo, Outcome 8 Clinical score (12h).
1.9. Analysis.
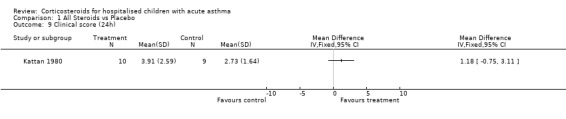
Comparison 1 All Steroids vs Placebo, Outcome 9 Clinical score (24h).
1.10. Analysis.
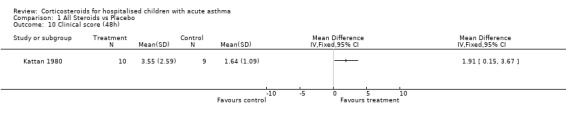
Comparison 1 All Steroids vs Placebo, Outcome 10 Clinical score (48h).
1.11. Analysis.
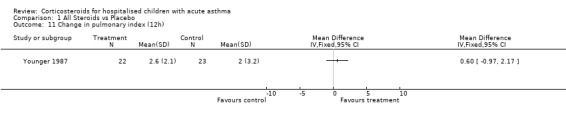
Comparison 1 All Steroids vs Placebo, Outcome 11 Change in pulmonary index (12h).
1.12. Analysis.
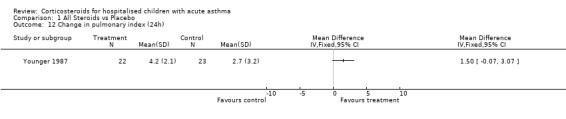
Comparison 1 All Steroids vs Placebo, Outcome 12 Change in pulmonary index (24h).
1.13. Analysis.
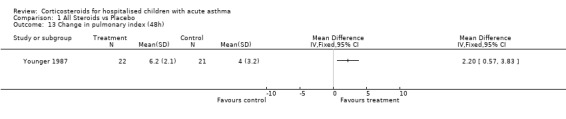
Comparison 1 All Steroids vs Placebo, Outcome 13 Change in pulmonary index (48h).
1.14. Analysis.
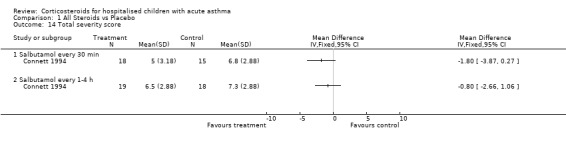
Comparison 1 All Steroids vs Placebo, Outcome 14 Total severity score.
1.15. Analysis.
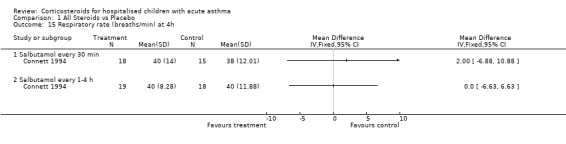
Comparison 1 All Steroids vs Placebo, Outcome 15 Respiratory rate (breaths/min) at 4h.
1.16. Analysis.
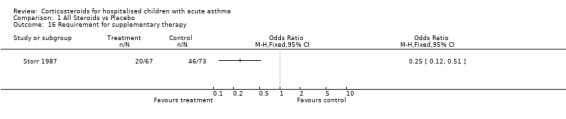
Comparison 1 All Steroids vs Placebo, Outcome 16 Requirement for supplementary therapy.
1.17. Analysis.
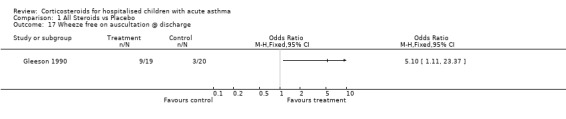
Comparison 1 All Steroids vs Placebo, Outcome 17 Wheeze free on auscultation @ discharge.
1.18. Analysis.
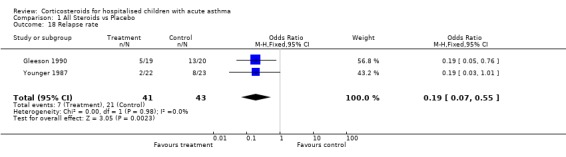
Comparison 1 All Steroids vs Placebo, Outcome 18 Relapse rate.
Comparison 2. Nebulised budesonide vs Oral prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % change in FEV1 (24h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Increase in cough | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Increase in wheeze | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Increase in shortness of breath | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Increase in PEFR | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Increase in FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Increase in pulse (beats/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Increase in SaO2 (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Increase in respiratory rate (breaths/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
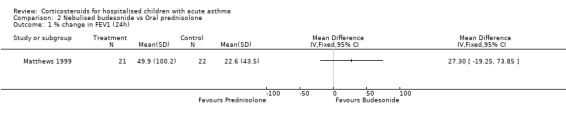
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 1 % change in FEV1 (24h).
2.2. Analysis.
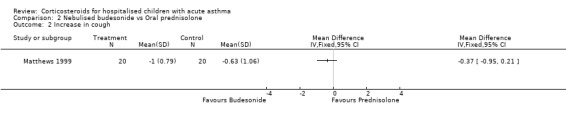
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 2 Increase in cough.
2.3. Analysis.
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 3 Increase in wheeze.
2.4. Analysis.
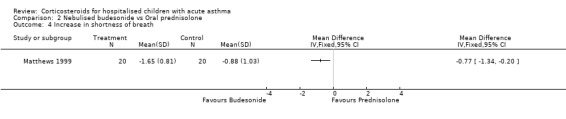
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 4 Increase in shortness of breath.
2.5. Analysis.
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 5 Increase in PEFR.
2.6. Analysis.
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 6 Increase in FVC.
2.7. Analysis.
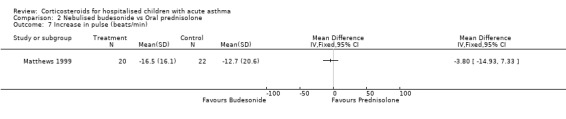
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 7 Increase in pulse (beats/min).
2.8. Analysis.
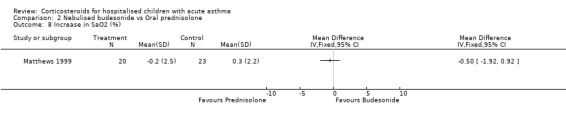
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 8 Increase in SaO2 (%).
2.9. Analysis.
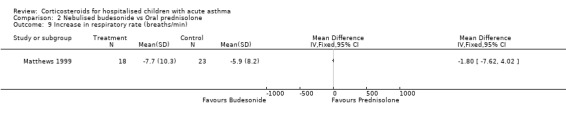
Comparison 2 Nebulised budesonide vs Oral prednisolone, Outcome 9 Increase in respiratory rate (breaths/min).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Connett 1994.
| Methods | setting: Childrens hospital, Brighton, England design: randomised double‐blind controlled trial, parallel design length of intervention: duration of hospital stay masking: double blind excluded: not stated withdrawals: stated baseline characteristics: similar in all treatment groups | |
| Participants | 70 children median age: 4.9y: M 49, F 21 prednisone arm: n=37 control arm: n=33 inclusion criteria: children aged > 18m requiring admission to hospital with acute asthma. Each child seen within 30 min of admission and given 5mg of salbutamol and assessed 10 min before and after. exclusion criteria: Not mentioned but 12 who got "steroids" were excluded, as were those with croup | |
| Interventions | Salbutamol 0.15mg/kg in 2 mls saline q30 + placebo
OR
Salbutamol 0.15mg/kg in 2 mls saline q30 + prednisolone 2 mg/kg po
OR
Salbutamol 5mg in 2 mls saline q1‐4 prn + prednisolone 2mg/kg po all children got standard therapy of salbutamol 5mg in 2 mls saline q1‐4h prn |
|
| Outcomes | Discharge at first re‐examination (4h) % best PEFR SaO2 (4h) Total severity score Respiratory rate |
|
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gleeson 1990.
| Methods | setting: Children's hospital, London, England design: randomised double‐blind controlled trial, parallel design length of intervention: duration of hospital stay masking: double blind excluded: stated withdrawals: not stated baseline characteristics: similar in both treatment groups | |
| Participants | 39 children median age: 5 y: M 29, F 9 hydrocortisone/prednisone arm: n=19 control arm: n=20 inclusion criteria: children age 2 ‐ 11 admitted to hospital with acute asthma who were unresponsive to two doses of nebulised salbutamol exclusion criteria: Previous regular inhaled/oral steroid use in previous year or in previous month a short course of oral steroid. Liver disease, hypothyroidism, drug interaction potential. | |
| Interventions | IV Hydrocortisone 6mg/kg initially then 2mg/kg q 4h for at least 24h then po prednisone 1mg/kg bd
OR
placebo all children got standard therapy of Nebulised salbutamol 0.15mg/kg/dose made up to 4mls saline q2‐4h IV aminophylline 5mg/kg load then 1mg/kg/hr then switched to oral theophylline Ipratropium was also used if above did not work |
|
| Outcomes | SaO2 <95% at discharge Wheeze free on ausculation at discharge Relapse rate < 3 months |
|
| Notes | Jadad score 2 One patient treated twice |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ho 1994.
| Methods | setting: Paediatric emergency department, Perth, Australia design: randomised, double‐blind controlled trial, parallel design length of intervention: length of hospitalisation up to 5 days masking: double blind excluded: stated withdrawals: stated baseline characteristics: similar in both treatment groups | |
| Participants | 58 children mean age: 4 y: M 24, F 34 prednisolone arm: n=31 control arm: n=27 inclusion criteria: children age 2 ‐ 14 attending hospital emergency department with acute asthma with saturation <= 93% and requiring admission exclusion criteria: Seriously ill with asthma, previous steroids used (any oral or inhaled steroids >400 mcg/d) Child < 2yrs excluded | |
| Interventions | 1mg/kg oral prednisolone given at admission
OR
placebo all children got standard therapy of regular beta 2 agonists. Some received theophylline and ipratropium bromide |
|
| Outcomes | Length of stay Change in SaO2 (12, 24 hrs) Change in predicted FEV1 (12, 24, 36hrs) Change in predicted PEFR (12, 24, 36 hrs) |
|
| Notes | Jadad score 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kattan 1980.
| Methods | setting: Children's hospital, Toronto, Canada design: Randomised, controlled trial, parallel design length of intervention: 48 hours masking: none excluded: stated withdrawals: stated baseline characteristics: certain blood values were stated and were similar | |
| Participants | 19 children mean age 11.5 y : gender unknown hydrocortisone arm: n=10 control arm: n=9 inclusion criteria: children (age 1‐15y) admitted to hospital with acute asthma having received emergency room treatment of 2 salbutamol inhalations and one IV bolus of aminophylline exclusion criteria: long term steroid therapy either inhaled or oral. PCO2 level > 45 mm Hg. | |
| Interventions | 7mg/kg intravenous hydrocortisone every 6h all children got standard therapy of IV fluids, IV aminophylline 5mg/kg q 6h and oxygen |
|
| Outcomes | clinical score PEFR | |
| Notes | Jadad score 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Matthews 1999.
| Methods | setting: 9 UK centres of child health design: randomised double‐blind controlled trial, parallel design length of intervention: length of hospitalisation masking: double‐blind excluded: stated withdrawals: not stated baseline characteristics: similar characteristics in both treatment and control groups | |
| Participants | 46 children median age: unknown (range 5‐16 y) gender unknown budesonide arm: n=23 control arm: n=23 inclusion criteria: children age 5 ‐ 16 admitted to hospital with severe asthma exacerbations and who demonstrated evidence of tachypnoea and tachycardia exclusion criteria: Oral steroids in the last 7 days. Life threatening disease as defined in BTS guidelines (bradycardia, hypotension, O2sat<90, requiring IV aminophylline or salbutamol), pregnancy or severe allergy | |
| Interventions | Inhaled budesonide 2mg every 8 hours and placebo prednisolone tablets
OR
Prednisolone 2mg/kg (up to 40mg) at entry and at 24h and placebo inhalation all children got Inhaled terbutaline 2.5 mg as needed. Following the 24 hr phase, all received budesonide 800mcg/day and terbutaline as needed for 24 days |
|
| Outcomes | FEV1 clinical symptoms | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Storr 1987.
| Methods | setting: children's hospital, Brighton, England design: randomised double‐blind controlled trial, parallel design length of intervention: 36 hours masking: double‐blind excluded: stated withdrawals: stated baseline characteristics: similar in both treatment groups | |
| Participants | 140 children mean age: 5.3 y: M 97, F 43 prednisone arm: n=67 control arm: n=73 inclusion criteria: children hospitalised with moderate or severe asthma exclusion criteria: Other lung illnesses such as croup, pneumonia, pertussis. Vomited drink or previous "steroids" in the most recent 48h | |
| Interventions | Oral prednisolone3mg/kg prednisolone in water (one dose)
(children<5y got 30mg and others got 60mg)
OR
grapefruit juice all children got standard therapy of nebulised salbutamol 5mg in 2 ml saline on admission and >3 times daily as needed |
|
| Outcomes | Discharge at first re‐examination (4h) % expected PEFR (6h) Requirement for suplimentary therapy |
|
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Younger 1987.
| Methods | setting: children's hospital, Memphis, Tennesee, USA design: randomised, double‐blind controlled trial, parallel design length of intervention: 48 hours masking: double‐blind excluded: stated withdrawals: stated baseline characteristics:similar in both treatment groups | |
| Participants | 45 children mean age: 9.5 y: gender unknown methylprednisolone arm: n=22 control arm: n=23 inclusion criteria: All children (6‐16y) hospitalised with status asthmaticus after failure to improve with epinephrine injection and isoetharine inhalations exclusion criteria: No inhaled steroids in the previous 4 weeks or systemic steroids in previous 8 weeks | |
| Interventions | IV methyl‐prednisolone 2mg/kg followed by 1mg/kg q 6h for duration of IV therapy1mg/kg/6h IV prednisolone
OR
placebo all children got standard therapy of IV fluids, IV aminophylline, Isoetharine inhalations q4h and oxygen as needed |
|
| Outcomes | Pulmonary index (12, 24, 36 hrs) % predicted FEV25‐75 (12, 24, 36) % predicted FEV1 (12, 24, 36) % predicted FVC (12, 24, 36) % predicted PEFR (12, 24, 36) Length of stay Relapse < one month |
|
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnett 1997 | Emergency department study Comparing 2 different types of steroids (no placebo) |
| Becker 1999 | Comparing 2 different types of steroids (no placebo) |
| Chavez 1992 | Not RCT Included patients < 12 months |
| Daugbjerg 1993 | Included patients <12 months |
| Devidayal 1999 | Emergency department study |
| Gonzalez 1994 | Included patients < 12 months |
| Langton‐Hewer 1998 | Comparing 3 different types of steroids (no placebo) |
| Lin 1991 | Not RCT Included patients < 12 months |
| Loren 1980 | not located in hospital (residential school) |
| Pierson 1974 | At the onset of this trial randomisation and blinding were carried out adequately. However, at 3 hours into the trial 7 of the control group (almost half) were reassigned to the steroid group because of changes in arterial gasses. These were not reported as withdrawals and were treated as randomised units |
| Sano 2000 | Included patients < 12 months Not RCT |
DBRCT = double‐blind, randomized controlled trial; RCT = randomized controlled trial.
Contributions of authors
Smith MBH: Protocol development, study selection and quality assessment, data entry and analysis, report writing and editing. Primary author. Iqbal S: Protocol development, study selection and quality assessment, report writing and editing. Elliott TM: Study selection and quality assessment, data entry and statistical analysis, report writing. Everard M: Protocol development and study selection Rowe BH: Protocol development, report writing and editing.
Sources of support
Internal sources
Division of Emergency Medicine, University of Alberta, Edmonton, Alberta, Canada.
Department of Paediatrics, Craigavon Area Hospital Group Trust, National Health Service, Craigavon, Northern Ireland, UK.
External sources
Canada Institute of Health Research (CIHR), Ottawa, Canada.
Declarations of interest
MS has received funding from 3M and Allen and Hanburys to attend peer‐reviewed respiratory conferences and fees from Astra Zeneca and Merck, Sharpe and Dohme for lectures on asthma therapy. BHR has received funding from GSK and Astra and has been paid fees to lecture at respiratory educational conferences by GSK, Astra, Merck, and Boehringer‐Ingelheim.
Edited (no change to conclusions)
References
References to studies included in this review
Connett 1994 {published data only}
- Connett GJ, Warde C, Wooler E, Lenney W. Prednisolone and salbutamol in the hospital treatment of acute asthma. Archives of Disease in Childhood 1994;70(3):170‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gleeson 1990 {published data only}
- Gleeson JGA, Loftus BG, Price JF. Placebo controlled trial of systemic corticosteroids in acute childhood asthma. Acta paediatrica Scandinavica 1990;79:1052‐8. [DOI] [PubMed] [Google Scholar]
Ho 1994 {published data only}
- Ho L, Landau LI, Souef PNL. Lack of efficacy of single‐dose prednisolone in moderately severe asthma. Medical Journal of Australia 1994;160:170‐3. [PubMed] [Google Scholar]
Kattan 1980 {published data only}
- Kattan M, Gurwitz D, Levison H. [Corticosteroids in status asthmaticus]. The Journal of Pediatrics 1980;96(3):596‐9. [DOI] [PubMed] [Google Scholar]
Matthews 1999 {published data only}
- Matthews E, Curtis P, McLain B, Morris L, Turbitt M. Nebulized budesonide versus oral steroid in severe exacerbations of childhood asthma. Acta Paediatrica 1999;88:841‐3. [DOI] [PubMed] [Google Scholar]
Storr 1987 {published data only}
- Storr J, Barry W, Barrell E, Lenney W. Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet 1987;8538(1):879‐82. [DOI] [PubMed] [Google Scholar]
Younger 1987 {published data only}
- Younger RE, Gerber PS, Herrod HG, Cohen RM, Crawford LV. Intravenous methylprednisolone efficacy in status asthmaticus of childhood. Pediatrics 1987;80:225‐30. [PubMed] [Google Scholar]
References to studies excluded from this review
Barnett 1997 {published data only}
- Barnett P, Caputo G, Baskin M, Kuppermann. Intravenous versus oral corticosteroids in the management of acute asthma in children. Annals of Emergency Medicine 1997;29(2):212‐7. [DOI] [PubMed] [Google Scholar]
Becker 1999 {published data only}
- Becker J, Arora A, Scarfone R, Spector N, Fontana‐Penn M, Gracely E, et al. Oral versus intravenous corticosteroids in children hospitalized with asthma. Journal of Allergy and Clinical Immunology 1999;103:586‐90. [DOI] [PubMed] [Google Scholar]
Chavez 1992 {published data only}
- Chavez EE, Muniz LOG, Chagoyan OT, Corte GF. [Steroids and inhalation therapy in the management of acute asthma in children]. Current Therapeutic Research 1992;52(1):7‐12. [Google Scholar]
Daugbjerg 1993 {published data only}
- Daugbjerg P, Brenoe E, Forchhammer H, Frederiksen B, Glazowski MJ, Ibsen KK, et al. A comparison between nebulized terbutaline, nebulized corticosteroid and systemic corticosteroid for acute wheezing in children up to 18 months of age. Acta Paediatrica 1993;82:547‐51. [DOI] [PubMed] [Google Scholar]
Devidayal 1999 {published data only}
- Devidayal, Singhi S, Kumar L, Jayshree M. [Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma]. Acta Paediatrica 1999;88:835‐40. [DOI] [PubMed] [Google Scholar]
Gonzalez 1994 {published data only}
- Gonzalez‐Perez‐Yarza E, Benito AR, Aranzadi JG, Ibarra CR, Calvo JB, Arana LA, et al. [Estudio multicentrico randomizado, abierto, grupos paralelos, para comparar la efiicacia de tres regimenes terapeuticos en lactantes hospitalizados por bronquitis aguda siblante]. Anales espanoles de pediatria 1994;41:315‐9. [Google Scholar]
Langton‐Hewer 1998 {published data only}
- Langton‐Hewer S, Hobbs J, Reid F, Lenney W. Prednisolone in acute childhood asthma: responses to three dosages. Respiratory Medicine 1998;92:541‐6. [DOI] [PubMed] [Google Scholar]
Lin 1991 {published data only}
- Lin Y‐Z, Hsieh K‐H, Chen W, Wu K‐W. Clinical trial of corticosteroid and beta‐2 bronchodilator in acute wheezing infants. Acta paediatrica sinica 1991;32:333‐40. [PubMed] [Google Scholar]
Loren 1980 {published data only}
- Loren ML, Chai H, Leung P, Rohr C, Brenner AM. [Corticosteroids in the treatment of acute exacerbations of asthma]. Annals of Allergy 1980;45:67‐71. [PubMed] [Google Scholar]
Pierson 1974 {published data only}
- Pierson WE, Bierman CW, Kelley VC. [A double‐blind trial of corticosteroid therapy in status asthmaticus]. Pediatrics 1974;54(3):282‐8. [PubMed] [Google Scholar]
Sano 2000 {published data only}
- Sano F, Cortez G, Sole D, Naspitz C. Inhaled budesonide for the treatment of acute wheezing and dyspnea in children up to 24 months old receiving intravenous hydrocortisone. Journal of Allergy and Clinical Immunology 2000;105:699‐703. [DOI] [PubMed] [Google Scholar]
Additional references
Barnes 1995
- Barnes PJ, Adcock IM. Steroid resistance in asthma. Quarterly Journal of Medicine 1995;88:455‐68. [PubMed] [Google Scholar]
Boschetto 1991
- Boschetto P, Rogers D, Fabbri L, Barnes P. Corticosteroid inhibition of airway microvascular leakage. American Review of Respiratory Disease 1991, (143):605‐9. [DOI] [PubMed] [Google Scholar]
Connett 1993
- Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Archives of Diseases in Childhood 1993, (68):85‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Djukanovic 1992
- Djukanovic R, Wilson J, Britten K, Wilson S, Walls A, Roche W, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. American Review of Respiratory Disease 1992;145(669‐74). [DOI] [PubMed] [Google Scholar]
Doull 1997
- Doull I, Lampe F, Smith S, Schreiber J, Freezer N, Holgate S. Effect of inhaled corticosteroids on episodes of wheezing associated with viral infection in school age children: randomised double blind placebo controlled trial. BMJ 1997, (315):858‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edmonds 2000
- Edmonds ML, Camargo CA Jr, Pollack CV Jr, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. The Cochrane Library 2000, Issue 3. [DOI] [PubMed] [Google Scholar]
Homer 1996
- Homer C, Szilagyi P, Rodewald L, Bloom S, Greenspan P, 1. Yazdgerdi S, et al. Does quality of care affect rates of hospitalization for childhood asthma?. Pediatrics 1996;98:18‐23. [PubMed] [Google Scholar]
Horowitz 1994
- Horowitz L, Zafir O, Gilboa S, Berger I, Wolach B. Acute asthma. Single dose oral steroids in paediatric community clinics. European Journal of Pediatrics 1994, (153):526‐30. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Littenberg 1986
- Littenberg B, Gluck E. A controlled trial of methylprednisolone in the emergency treatment of acute asthma. New England Journal of Medicine 1986, (314):150‐2. [DOI] [PubMed] [Google Scholar]
Manser 2000
- Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. The Cochrane Library 2000, Issue 1. [DOI] [PubMed] [Google Scholar]
McFadden 1994
- McFadden E. Are there risks associated with beta‐agonists? The data reviewed. International Respiratory Forum 1994, (1):27‐33. [Google Scholar]
McKean 2000
- McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. The Cochrane Library 2000, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Payne 1998
- Payne DNR, Hubbard M, MacKenzie SA. Corticosteroid unresponsiveness in asthma: primary or acquired?. Pediatric Pulmonology 1998;25:59‐61. [DOI] [PubMed] [Google Scholar]
Rowe 2000a
- Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. The Cochrane Library 2000, Issue 1. [DOI] [PubMed] [Google Scholar]
Rowe 2000b
- Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. The Cochrane Library 2000, Issue 3. [DOI] [PubMed] [Google Scholar]
Scarfone 1993
- Scarfone R, Fuchs S, Nager A, Shane S. Controlled trial of oral prednisone in the emergency department treatment of children with asthma. Pediatrics 1993, (92):513‐8. [PubMed] [Google Scholar]
Shapiro 1983
- Shapiro G, Furukawa C, Pierson W, Gardinier R, Bierman W. Double‐blind evaluatioon of methylprednisolone versus placebo for acute asthma episodes. Pediatrics 1983, (71):510‐4. [PubMed] [Google Scholar]
Storr 1987
- Storr J, Barry W, Barrell E, Lenney W. Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet 1987;8538(1):879‐82. [DOI] [PubMed] [Google Scholar]
Svedmyr 1990
- Svedmyr N. Action of corticosteroids on beta‐adrenergic receptors. American Review of Respiratory Disease 1990, (141 (suppl 2)):S31‐S38. [PubMed] [Google Scholar]
Svedmyr 1999
- Svedmyr J, Nyberg E, Thunqvist P, Asbrink‐Nilsson E, Hedlin G. Prophylactic intermittent treatment with inhaled corticosteroids of asthma exacerbations due to airway infections in toddlers. Acta Paediatrica 1999, (88):42‐7. [DOI] [PubMed] [Google Scholar]
Tal 1990
- Tal A, Levy N, Bearman J. Methylprednisone therapy for acute asthma in infants and toddlers; a controlled clinical trial. Pediatrics 1990, (86):350‐60. [PubMed] [Google Scholar]
Taylor 1993
- Taylor I, Shaw R. The mechanism of action of corticosteroids in asthma. Respiratory Medicine 1993, (87):261‐7. [DOI] [PubMed] [Google Scholar]
To 1996
- To T, Dick P, Feldman W, Hernandez R. A cohort study on childhood asthma admissions and readmissions. Pediatrics 1996, (98):191‐5. [PubMed] [Google Scholar]
Weinburger 1988
- Weinberger M. Corticosteroids for exacerbations of asthma: Current status of the controversy. Pediatrics 1988, (81):726‐9. [PubMed] [Google Scholar]
Ziment 1986
- I Ziment. Steroids. Clinics in Chest Medicine 1986;7(3):341‐54. [PubMed] [Google Scholar]


