Abstract
Background
Topical fluoride therapy (TFT) in the form of toothpastes, mouthrinses, varnishes and gels are effective caries preventive measures. However, there is uncertainty about the relative value of these interventions when used together.
Objectives
To compare the effectiveness of two TFT modalities combined with one of them alone (mainly toothpaste) when used for the prevention of dental caries in children.
Search methods
We searched the Cochrane Oral Health Group's Trials Register (May 2000), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2000, Issue 2), MEDLINE (1966 to January 2000), plus several other databases. We handsearched journals, reference lists of articles and contacted selected authors and manufacturers.
Selection criteria
Randomized or quasi‐randomized controlled trials with blind outcome assessment, comparing fluoride varnish, gel, mouthrinse, or toothpaste in combination with each other in children up to 16 years during at least 1 year. The main outcome was caries increment measured by the change in decayed, missing and filled tooth surfaces (D(M)FS).
Data collection and analysis
Inclusion decisions, quality assessment and data extraction were duplicated in a random sample of one third of studies, and consensus achieved by discussion or a third party. Authors were contacted for missing data. The primary measure of effect was the prevented fraction (PF) that is the difference in mean caries increments between the 'treatment' and 'control' groups expressed as a percentage of the mean increment in the control group. Random‐effects meta‐analyses were performed where data could be pooled.
Main results
Eleven of the 12 included studies contributed data for the meta‐analyses. For the nine trials that provided data for the main meta‐analysis on the effect of fluoride mouthrinses, gels or varnishes used in combination with toothpaste (involving 4026 children) the D(M)FS pooled PF was 10% (95% CI, 2% to 17%; P = 0.01) in favour of the combined regimens. Heterogeneity was not substantial in these results (I2 = 32%). The separate meta‐analyses of fluoride gel or mouthrinse combined with toothpaste versus toothpaste alone favour the combined regimens, but differences were not statistically significant; the significant difference in favour of the combined use of fluoride varnish and toothpaste accrues from a very small trial and appears likely to be a spurious result. Not all other combinations of possible practical value were tested in the included studies. The only other statistically significant result was in favour of the combined use of fluoride gel and mouthrinse in comparison to gel alone (pooled DMFS PF 23%; 95% CI, 4% to 43%; P = 0.02), based on two trials. No other combinations of TFT were consistently superior to a single TFT.
Authors' conclusions
Topical fluorides (mouthrinses, gels, or varnishes) used in addition to fluoride toothpaste achieve a modest reduction in caries compared to toothpaste used alone. No conclusions about any adverse effects could be reached, because data were scarcely reported in the trials.
Plain language summary
Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents
Additional forms of topical fluoride can reduce tooth decay in children and adolescents more than fluoride toothpaste alone, but the extra benefit is not great. Tooth decay (dental caries) is painful, expensive to treat and can seriously damage teeth. Fluoride is a mineral that prevents tooth decay. Fluoride is added to the water supply in many areas. It can also be applied in the form of toothpastes, mouthrinses, gels or varnishes. The review of trials found that children and adolescents who used another form of topical fluoride in addition to fluoride toothpaste experienced some additional reduction in tooth decay compared with children who only used fluoride toothpaste. However, the additional benefit was not great, and the trials did not provide data about adverse effects.
Background
Dental caries and its consequences pose important and uncomfortable problems in all industrialized societies and in a large number of developing countries. Although the prevalence and severity of dental caries in most industrialized countries have decreased substantially in the past 2 decades, reaching averages as low as 1.1 decayed, missing and filled teeth (DMFT) in 12 year olds, nearly half of those without any tooth decay or fillings (Marthaler 1996), this largely preventable disease is still common, increases significantly with age, and remains a public health problem for a significant proportion of the world population (Burt 1998). In the United Kingdom, 30% of 3.5 to 4.5 year olds (Moynihan 1996), and 50% of 12 year olds (Downer 1995) had experienced caries in 1993. In 2000, the figures were 40% for 5 year olds in Great Britain (Pitts 2001) and 38% for 12 year olds in England and Wales (Pitts 2002). These findings demonstrate the continuing need for effective preventive strategies and treatment services for these age groups in a country that has experienced a substantial caries decline. In general, dental caries levels vary considerably between and within different countries, but children in the lower socio‐economic status (SES) groups have higher caries levels than those in the upper SES groups, and these differences are consistent in industrialized and in urbanized developing countries (Chen 1995).
Fluoride therapy has been the cornerstone of caries‐preventive strategies since the introduction of water fluoridation schemes over 5 decades ago (Murray 1991). Fluoride controls the initiation and progression of carious lesions. Intensive laboratory and epidemiological research on the mechanism of action of fluoride in preventing caries indicates that fluoride's predominant effect is topical, which occurs mainly through promotion of remineralization of early caries lesions and by reducing sound tooth enamel demineralization (Featherstone 1988). Various modes of fluoride use have evolved, each with its own recommended concentration, frequency of use, and dosage schedule. The use of topically applied fluorides in particular, which are much more concentrated than the fluoride in drinking water, has increased over recent decades and fluoride containing toothpastes (dentifrices), mouthrinses, gels and varnishes are the modalities most widely used at present, either alone or in different combinations. By definition, the term 'topically applied fluoride' describes those delivery systems which provide fluoride to exposed surfaces of the dentition, at elevated concentrations, for a local protective effect and are therefore not intended for ingestion. Fluoride gels and varnishes are typical methods of professional topical fluoride application and both delivery systems have been used in preventive programs. Fluoride gels have also been used as a self‐applied intervention in such programs. Fluoride mouthrinses and toothpastes are the main forms of self‐applied fluoride therapy. The intensive use of fluoride mouthrinsing in school programs has been discontinued in many developed countries because of doubts regarding its cost‐effectiveness at a low prevalence of dental caries and are being replaced by selective fluoride therapy directed to high risk children. Such procedures usually involve the combined use of fluoride toothpastes with gels or varnishes. Toothpaste is by far the most widespread form of fluoride usage (Murray 1991a; Ripa 1991) and the decline in the prevalence of dental caries in developed countries has been mainly attributed to its increased use (Glass 1982; Rolla 1991; Marthaler 1994; O'Mullane 1995; Marthaler 1996).
However, there is currently a debate regarding the appropriate use of fluorides. The lower caries prevalence now prevailing in many countries and the widespread availability of fluoride from multiple sources have raised the question of whether topically applied fluorides are still effective in reducing caries, and safe, mainly in terms of the potential risk of fluorosis (mottled enamel) (Ripa 1991). In this context, even the need for selective professional fluoride applications has been questioned (Seppa 1998). The persistence of this debate and the variations in the use of the main forms of topically applied fluorides suggest the need to search for meaningful ways to summarize the empirical findings on this topic systematically.
If topical fluorides remain effective it will then become relevant to assess which form is best by directly comparing the various treatments currently used and to assess how much extra benefits topical fluoride treatments used together may actually have, and whether the likely benefits are worth the effort considering potential negative effects such as fluorosis. Because the use of fluoride toothpaste is widespread in fluoridated and non‐fluoridated areas, and supported by researchers and public health authorities as the method of choice among all topical fluoride interventions, there would be little justification for the use of professionally‐applied or supervised self applied fluoride interventions if their combined use with toothpastes results in a marginal enhancement of effectiveness. The unanswered question today, of how much extra caries protection comes from a professionally‐applied fluoride or a fluoride rinsing program on top of that provided from the regular use of fluoride toothpaste, is of clear importance and needs to be formally investigated.
Over the past half‐century, numerous clinical trials have investigated the anti‐caries effect of each topical fluoride intervention. It appears that most of the trials have focused on topical fluoride in one form or another and that a small number of such trials have directly investigated increased effectiveness when two or more fluoride interventions are topically applied. Although the results of studies investigating the cariostatic efficacy of the combined use of various fluorides have been assessed before (Marthaler 1971; Horowitz 1980; Marthaler 1990), there has been no systematic review of the available evidence.
With regard to the clinical effectiveness of topical fluoride therapy (TFT) in the form of toothpastes, mouthrinses, gels and varnishes three basic questions can be asked: (1) Is TFT effective in preventing dental caries in children and adolescents? (2) Is one of these forms of TFT more effective than another? (3) Are combinations of these TFT forms more effective than one form used alone? This review attempts to answer the third question; the other two questions are addressed in separate reviews.
Objectives
The primary objective of this systematic review is: (1) to determine whether there is a beneficial effect of adding topical fluoride therapy (TFT) in the form of mouthrinse, gel or varnish to fluoride toothpaste. As secondary objectives we: (2) evaluated the addition of each TFT modality to toothpaste separately; (3) evaluated all other combinations of two TFT modalities compared to one of them.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials using or indicating blind outcome assessment, in which one form of topical fluoride therapy (TFT) (toothpaste, mouthrinse, varnish or gel) is compared concurrently with another in combination with it, during at least 1 calendar or school year. Randomized or quasi‐randomized controlled trials using within group paired comparison designs (e.g. split‐mouth trials involving fluoride varnish, as the effect of the varnish could spread across the mouth leading to contamination of control sites), or with open outcome assessment or no indication of blind assessment, or lasting less than 1 calendar or school year, or controlled trials where random or quasi‐random allocation was not used or indicated were excluded.
Types of participants
Children or adolescents aged 16 or less at the start of the study (irrespective of initial level of dental caries, background exposure to fluorides, dental treatment level, nationality, setting where intervention is received or time when it started). Studies where participants were selected on the basis of special (general or oral) health conditions were excluded.
Types of interventions
Topical fluoride therapy (TFT) in the form of toothpastes, mouthrinses, gels or varnishes only, using any fluoride agent (which may be formulated with any compatible abrasive system, in the case of fluoride toothpastes), at any concentration (ppm F), amount or duration of application, and with any technique or method of application, provided the frequency of application was at least once a year. The following comparisons are of relevance (combined TFT compared with single TFT): Fluoride toothpaste plus any topical fluoride (varnish, gel, mouthrinse) compared with toothpaste alone, and any other combination of two of these modalities compared with one modality alone. Studies where the intervention consisted of any caries preventive agent/procedure (e.g. other fluoride‐based measures, anti‐plaque or anti‐calculus agents, sealants, oral hygiene interventions, xylitol chewing gums, glass ionomers) used in addition to any form of TFT described above were excluded.
Types of outcome measures
The primary outcome measure in this review is caries increment, as measured by change from baseline in the decayed, (missing) and filled surface (D(M)FS) index, in all permanent teeth erupted at start and erupting over the course of the study. For studies in younger children the outcome measure of interest is caries increment in deciduous tooth surfaces, as measured by change in the decayed, (missing/extraction indicated), and filled surface d(e/m)fs index. Dental caries is defined here as being clinically and radiographically recorded at the dentin level of diagnosis. (SeeMethods for the different ways of reporting the decayed, (missing) and filled teeth or surfaces (D(M)FT/S) scores in clinical trials of caries preventives.)
The following outcomes were considered relevant: coronal dental caries and dental fillings, in both the permanent and the deciduous dentitions; tooth loss; proportion of children developing new caries; dental pain/discomfort; specific side effects (fluorosis, tooth staining/discoloration, oral allergic reactions, adverse symptoms such as nausea, vomiting); unacceptability of preventive treatment as measured by drop outs during the trial (in non‐placebo controlled studies); use of health service resources (such as visits to dental care units, length of dental treatment time). Studies reporting only on changes in plaque/calculus formation, plaque regrowth/vitality, plaque/salivary bacterial counts, or gingival bleeding/gingivitis, dentin hypersensitivity or fluoride physiological outcome measures (fluoride uptake by enamel or dentin, salivary secretion levels, etc.) were excluded.
Search methods for identification of studies
With a comprehensive search, we attempted to identify all relevant studies irrespective of language, from 1965 onwards.
Electronic searching
Up to 1998
Relevant studies were identified (for the series of topical fluoride reviews) by searching several databases from date of inception: MEDLINE (1966 to 1997), EMBASE (1980 to 1997), SCISEARCH (1981 to 1997), SSCISEARCH (1981 to 1997), ISTP (1982 to 1997), BIOSIS (1982 to 1997), CINAHL (1982 to 1997), ERIC (1966 to 1996), DISSERTATION ABSTRACTS (1981 to 1997) and LILACS/BBO (1982 to 1997). Two overlapping but complementary subject search phrases (Appendix 1) with low specificity (but high sensitivity), using 'free text 'and 'controlled vocabulary', were formulated within Silverplatter MEDLINE around two main concepts, fluoride and caries, and combined with all three levels of the Cochrane Optimal Search Strategy for Randomized Controlled Trials (RCTs). These subject search phrases were customised for searching EMBASE and the other databases.
RCT filters were also adapted to search EMBASE, BIOSIS, SCISEARCH, DISSERTATION ABSTRACTS, and LILACS/BBO. All the strategies (subject search and methodological filters) developed to search each database are fully described in a report produced for the Systematic Reviews Training Unit (Marinho 1997), and are available on request. These were used for the development of a register of topical fluoride clinical trials for the systematic reviews, as the Cochrane Oral Health Group's Trials Register was not yet developed in 1997/98.
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 1997, Issue 1), the Community of Science database (1998), which included ongoing trials funded by the National Institute of Dental Research (NIDR), the System for Information on Grey Literature in Europe (SIGLE) database (1980 to 1997), and OLDMEDLINE (1963 to 1965) were searched using the terms 'fluor' and 'carie' truncated. (Grey literature search had also been carried out by searching the Index to Scientific and Technical Proceedings (ISTP) and DISSERTATION ABSTRACTS.)
From 1999 to 2001
The strategy included in Appendix 2 was used to search LILACS/BBO in 1999 (1982 to 1998), where free text subject search terms were combined with a methodological filter for RCTs.
Four supplementary and more specific subject search phrases (including 'free text' and 'controlled vocabulary' terms), refined exclusively for the reviews on the effects of individual fluoride modalities, formulated around three concepts each (the relevant topical fluoride therapy (TFT), fluoride and caries) were used to search Silverplatter MEDLINE (up to January 2000) without methodological filters (Appendix 3). These strategies were adapted to search the Cochrane Oral Health Group's Trials Register (up to May 2000), and have also been run on CENTRAL (The Cochrane Library 2000, Issue 2) to double check.
The metaRegister of Current Controlled Trials was searched in October 2001 for ongoing RCTs using the terms 'fluoride' and 'caries'.
Reference searching
All eligible trials retrieved from the searches, meta‐analyses and review articles located up to January 2000 were checked for relevant references. Reviews had been identified mainly by a MEDLINE search strategy specifically carried out to provide information on available systematic reviews or meta‐analyses and on the scope of the literature on the topic, when the Cochrane Database of Systematic Reviews (CDSR), and the Database of Abstracts of Reviews of Effects (DARE) and NHS Economic Evaluation Database (NHSEED), were also searched. Reference lists of relevant chapters from preventive dentistry textbooks on topically applied fluoride interventions were also consulted.
Full text searching
Prospective handsearching of the seven journals identified as having the highest yield of eligible RCTs/controlled clinical trials (CCTs) was carried out, from January 1999 until January 2000: British Dental Journal, Caries Research, Community Dentistry and Oral Epidemiology, Journal of the American Dental Association, Journal of Dental Research, Journal of Public Health Dentistry and European Journal of Oral Sciences. The handsearch of Community Dentistry and Oral Epidemiology was undertaken (1990 to December 1999), as this was the journal with the highest yield of eligible reports.
Personal contact
Searching for unpublished studies (or 'grey' literature such as technical reports and dissertations, or studies published in languages other than English which may not have been indexed to major databases) started by contacting experts in the field of preventive dentistry. A letter was sent to the author(s) of each included study published during the last 2 decades in order to obtain information on possible unpublished studies eligible for inclusion. All the authors of studies who had been contacted in order to clarify reported information to enable assessment of eligibility or obtain missing data were also asked for unpublished studies.
Based on information extracted mainly from included studies, a list of manufacturers of fluoride toothpastes, mouthrinses, gels and varnishes was created for locating unpublished trials. Letters to manufacturers were sent out by the Cochrane Oral Health Group, in the hope that companies might be more responsive to contact from the editorial base than from individual reviewers. Fourteen manufacturers were contacted (October 2000) and information on any unpublished trials requested: Bristol‐Myers Co, Colgate‐Palmolive, Davies Rose‐Hoyt Pharmaceutical Division, Gaba AG, Ivoclar North America, John O Butler Company, Johnson & Johnson, Oral‐B Laboratories, Pharmascience, Procter & Gamble, Smithkline Beecham, Synthelabo, Unilever/Gibbs, Warner‐Lambert.
Data collection and analysis
Management of records produced by the searches
Because multiple databases were searched, the downloaded set of records from each database, starting with MEDLINE, was imported to the bibliographic software package Reference Manager and merged into one core database to remove duplicate records and to facilitate retrieval of relevant articles. The records yielded from LILACS, BBO, CENTRAL, SIGLE and NIDR databases were not imported to Reference Manager and were checked without the benefit of eliminating duplicates. The records produced by OLDMEDLINE and by the specific MEDLINE search performed without methodological filter were imported to Reference Manager for inspection, in a database separate from the core database. The records produced by searching the Cochrane Oral Health Group's Trials Register and the metaRegister of Current Controlled Trials were also checked outside Reference Manager. In order to facilitate inspection of all records located from searching other (non‐electronic) sources (reference lists of relevant studies, review articles and book chapters, journal handsearch, personal contact), we also tried to locate them in MEDLINE and to import them to Reference Manager. Those references that could not be downloaded in this way were entered manually.
Relevance assessment
All records identified by the searches were printed off and checked on the basis of title first, then by abstract (when this was available in English or in languages known by the reviewer) and/or keywords by one reviewer, Valeria Marinho (VM). Records that were obviously irrelevant were discarded and the full text of all remaining records was obtained. Records were considered irrelevant according to study design/duration, participants, or interventions/comparisons (if it could be determined that the article was not a report of a randomized/quasi‐randomized controlled trial; or the trial was of less than 6 to 8 months duration; or the trial was exclusively in adults; or the trial did not address at least two of the relevant topical fluoride treatments; or the trial did not compare one topical fluoride with topical fluoride used in combination).
Data extraction
Data from all included studies were extracted by one reviewer (VM) using a pilot tested data extraction form. A second reviewer (Julian Higgins (JH)) extracted data from a random sample of approximately one third of included studies. Again, data that could not be coded by the first reviewer were independently coded by the second, any disagreements were discussed and a third reviewer consulted to achieve consensus where necessary. (In future updates all reports will be data extracted and quality assessed in duplicate.) Data presented only in graphs and figures were extracted whenever possible, but were included only if two reviewers independently had the same result. Attempts were made to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. Papers in languages not known by the reviewers were data extracted with help from appropriate translators.
Additional information related to study methodology or quality that was extracted included: study duration (years of follow up); comparability of baseline characteristics: methods used pre‐randomization in sizing/balancing (stratification based on relevant variables) or used post‐randomization in analysing/adjusting for possible differences in prognostic factors between groups; objectivity/reliability of primary outcome measurement (diagnostic methods and thresholds/definitions used and included, and monitoring of diagnostic errors); any co‐intervention and/or contamination. Information on sponsoring institutions and manufacturers involved was also recorded.
Characteristics related to participants that were extracted included: age (range) at start, caries severity at start (average DMFS, DFS, or other measure), background exposure to fluoride sources other than the study option(s) (in water, topical applications, etc., year study began, place where study was conducted (country), setting where participants were recruited, and dental treatment level (F/DMF). Characteristics of the interventions that were extracted included: fluoride modality(s), mode of application (how the intervention was delivered), methods (technique/device) of application, prior‐ and post‐application, fluoride active agents and concentrations used, frequency and duration of application, and amount applied.
Different ways of assessing/reporting caries increment in the trials (change from baseline as measured by the DMF index) were recorded separately and/or combined according to the components of the index chosen and units of measurement (DMFT/S, or DFT/S, or DT/S, or FT/S), types of tooth/surface considered (permanent/deciduous teeth/surfaces, first molar teeth, approximal surfaces, etc), state of tooth eruption considered (erupted and/or erupting teeth or surface), diagnostic thresholds used (cavitated/dentin lesions, non‐cavitated incipient lesions), methods of examination adopted (clinical and/or radiographic), and approaches to account or not for reversals in caries increment adopted (in a net or observed/crude caries increment respectively). In addition, caries increments have been recorded whenever the authors reported them (various follow ups), and where assessments of caries increments were made during a post‐intervention follow‐up period, the length of time over which outcomes were measured after the intervention ended was noted.
As we were aware that caries increment could be reported differently in different trials we developed a set of a priori rules to choose the primary outcome data for analysis from each study: data on surface level would be chosen over data on tooth level; DFS data would be chosen over DMFS data, and this would be chosen over DS or FS; data for 'all surface types combined' would be chosen over data for 'specific types' only; data for 'all erupted and erupting teeth combined' would be chosen over data for 'erupted' only, and this over data for 'erupting' only; data from 'clinical and radiological examinations combined' would be chosen over data from 'clinical' only, and this over 'radiological' only; data for dentinal/cavitated caries lesions would be chosen over data for enamel/non‐cavitated lesions; net caries increment data would be chosen over crude (observed) increment data; and follow up nearest to 3 years (often the one at the end of the treatment period) would be chosen over all other lengths of follow up, unless otherwise stated. When no specification was provided with regard to the methods of examination adopted, diagnostic thresholds used, groups of teeth and types of tooth eruption recorded, and approaches for reversals adopted, the primary choices described above were assumed.
All other relevant outcomes assessed/reported in the trials were also recorded/listed.
Quality assessment
The methodological quality of the included studies was assessed according to the criteria for concealment of treatment allocation described in the Cochrane Reviewers' Handbook (Clarke 2000) used in the Cochrane Review Manager software (RevMan). Allocation concealment for each trial was rated as belonging to one of three categories. (A) Adequately concealed (an adequate method to conceal allocation is described). (B) Concealment unclear ('random' allocation stated/indicated but the actual allocation concealment method is not described or an apparently adequate concealment scheme is reported but there is uncertainty about whether allocation is adequately concealed). (C) Inadequately concealed (an inadequate method of allocation concealment is described). Excluded: random (or quasi‐random) allocation clearly not used in the trial, or 'random' allocation not stated and not implied/possible.
Blinding of main outcome assessment was also rated according to the following three categories defined for the topical fluoride reviews. (A) Double‐blind (blind outcome assessment and use of placebo/blinding of participants described). (B) Single‐blind (blind outcome assessment stated and no placebo used/participants not blind). (C) Blinding indicated (blind outcome assessment not stated but likely in any element/phase of outcome assessment, e.g. clinical and/or radiographic examinations performed independently of previous results, or radiographic examinations performed independently of clinical examinations with results reported separately/added later, or examiners clearly not involved in giving treatment, or use of placebo described) or reported but unclear (blind outcome assessment reported but there is information that leads to suspicion/uncertainty about whether the examination was blind). Excluded: clearly open outcome assessment used or blind outcome assessment not reported and unlikely (no description of an examination performed independently of previous results, of x‐rays registered independently of clinical examination, of use of a placebo, and of examiners clearly not involved in giving treatment).
One reviewer (VM) assessed the quality of all included studies. A second reviewer (JH) duplicated the process for a random sample of approximately one third. Any disagreement was discussed and where necessary a third reviewer was consulted to achieve consensus. Where uncertainty could not be resolved an effort was made to contact authors directly to clarify the method used to conceal allocation or whether assessment of the main outcome had been carried out blind.
Checking of interobserver reliability was limited to these validity assessments.
Other methodological characteristics of the trials such as completeness of follow up (proportion excluded) and handling of exclusions (extent to which reasons for attrition are explicitly reported, or losses are independent of treatment allocated) were not used as thresholds for inclusion. However, all assessments of study quality are described in the Characteristics of included studies table, and were coded for possible use in metaregression/sensitivity analyses. (For example, sensitivity analyses could be performed to assess the impact of blind outcome assessment and concealment of allocation, since studies where blinding is not clearly stated (but likely) and studies reporting inadequate allocation concealment are also included in this review.)
Data analyses
Handling of missing main outcome data
It was decided that missing standard deviations for caries increments that were not revealed by contacting the original researchers would be imputed through linear regression of log standard deviations on log mean caries increments. This is a suitable approach for caries prevention studies since, as they follow an approximate Poisson distribution, caries increments are closely related (similar) to their standard deviations (van Rijkom 1998).
Handling of results (main outcome) of studies with more than one treatment arm
For studies with more than two‐arms, where the same topical fluoride therapy (TFT) form(s) is(are) compared in two or more 'experimental' groups (for example, different active agents or concentrations of fluoride ion are compared for the same modality(ies) of TFT to a common 'control' group), raw results (the numbers, mean caries increments and standard deviations) from all relevant 'experimental' groups were combined in order to obtain a measure of treatment effect (this enables the inclusion of all relevant data for each form/combined forms of TFT in the meta‐analyses). In the studies comparing more than two relevant combined modalities of TFT with a common fluoride toothpaste group, the toothpaste group was divided into approximately equally sized smaller groups to provide a pairwise comparison for each combination of modalities. Means and standard deviations were unchanged.
Choice of measure of effect and meta‐analyses of main outcome
The chosen measure of treatment effect was the prevented fraction (PF), that is (mean increment in the 'controls' minus mean increment in the 'treated' group) divided by mean increment in the 'controls'. For an outcome such as caries increment (where discrete counts are considered to approximate to a continuous scale and are treated as continuous data) this measure was considered more appropriate than the mean difference or standardised mean difference, since it allows combination of different ways of measuring caries increment and a meaningful investigation of heterogeneity between trials. It is also simple to interpret.
The meta‐analyses were conducted as inverse variance weighted averages. Within‐study variances were estimated using the formula presented in Dubey 1965 which was more suitable for use in a weighted average, and for large sample sizes the approximation should be reasonable. Random‐effects meta‐analyses were performed throughout in RevMan/RevMan Analyses.
Deciduous and permanent teeth were analysed separately throughout.
For illustrative purposes, when overall results were significant, the results were also presented as the number of children needed to treat (NNT) to prevent one carious teeth/surface. These were calculated by combining the overall prevented fraction with an estimate of the caries increment in the control groups of the individual studies.
Assessment of heterogeneity and investigation of reasons for heterogeneity
Heterogeneity in the results of the trials was assessed by inspection of a graphical display of the estimated treatment effects from the trials along with their 95% confidence intervals and by formal tests of homogeneity (Thompson 1999).
Statistically significant heterogeneity was investigated using metaregression when a meta‐analysis included a sufficiently large number of studies. In addition to aspects of study quality, potential sources of heterogeneity investigated would include baseline levels of caries severity and exposure to fluoride sources other than the study options. The association of these factors with estimated effects (D(M)FS PFs) would be examined by performing random‐effects metaregression analyses in Stata version 6.0 (Stata Corporation, USA) using the program Metareg (Sharp 1998).
Investigation of publication and other biases
A funnel plot (plots of treatment effect estimates versus the inverse of their standard errors) was drawn. Asymmetry of the funnel plot may indicate publication bias and other biases related to sample size, though may also represent a true relationship between trial size and effect size. A formal investigation of the degree of asymmetry was performed using the method proposed by Egger et al (Egger 1997).
Measures of effect and meta‐analysis of other outcomes
For outcomes other than caries increment, continuous data would be analysed according to differences in mean treatment effects and their standard deviations. Dichotomous outcome data were analysed by calculating risk ratios (RR) or, for adverse effects of fluoride treatment, risk differences (RD). RevMan was used for estimation of overall treatment effects. Again, a random‐effects model was used to calculate a pooled estimate of effect. NNT was calculated when overall results were significant. As a general rule only (relevant) outcomes with useable data were shown in the analyses tables.
Results
Description of studies
Search results
Our initial multiple database search (1997/98) produced the following total number of records, according to database searched: MEDLINE, 4599; EMBASE, 5052; BIOSIS, 421; SCISEARCH, 514; SSCISEARCH, 169; ISTP, 66; CINAHL, 133; ERIC, 60; DISSERTATION ABSTRACTS, 95; LILACS, 48; BBO, 47; CENTRAL, 86; SIGLE, 6. Searching OLDMEDLINE produced 545 records, and the Community of Science database, 24 records. In the second stage of searches (1999), searching LILACS and BBO with a modified search strategy produced 210 records (142 and 68 records respectively). The more specific MEDLINE searches (by individual modalities of topical fluoride therapy (TFT)) performed without a randomized controlled trial (RCT) filter produced 2441 records, and the searches performed in the Cochrane Oral Health Group's Trials Register (May 2000) produced 479 records. Searching the metaRegister of Current Controlled Trials for ongoing studies produced 5 records. Many records retrieved through electronic search were duplicates merged later in the core database, and many appeared more than once in different databases and/or searches performed (overlapped).
Searching other non‐electronic sources (reference lists of potentially relevant reports, review articles or book chapters, relevant journals, and contacting authors) produced 171 additional records for inspection. (Any search results produced by contacting manufacturers will feature in updates of this review.)
Results of relevance assessment
When all the records produced by the searches above were screened, a total of 713 reports were identified as potentially eligible and further assessment was sought.
Study selection results
One full text report could not be obtained (this was an incomplete reference of an unpublished study/grey literature). Six hundred and ninety (690) reports were considered immediately irrelevant for this review, largely as a result of the types of intervention compared with (or used in addition to) the relevant topical fluorides (including all placebo or no treatment control trials without a relevant comparison of topical fluorides in combination with each other), and due to the types of study design described.
Thus, 16 studies (22 reports) are considered/cited in this review. These comprise 15 reports relating to 12 included studies, 6 reports relating to three excluded studies, and one report relating to one study waiting assessment (because it requires translation, but look unlikely to either be a randomised trial or to add to data already acquired). There are no reports of ongoing studies. Two non‐English reports in Portuguese (one study) listed under included studies have been fully assessed.
Excluded studies
SeeCharacteristics of excluded studies table for the description of reasons for rejecting each study.
We have excluded two studies comparing fluoride mouthrinse plus toothpaste with mouthrinse alone (one of which also compared fluoride toothpaste plus mouthrinse with toothpaste alone) and one study comparing fluoride mouthrinse plus gel with mouthrinse alone. These three studies were excluded for the following reasons: Two studies did not mention or indicate random (or quasi‐random) allocation and blind outcome assessment; and one study did not mention or indicate random or quasi‐random allocation but described blind outcome assessment (attempts to contact the authors of this study were unsuccessful and it was excluded).
Included studies
SeeCharacteristics of included studies table for details of each study.
There are 12 trials included. The study conducted by Marthaler 1970 was treated as two independent trials because the results for the two age groups involved were reported separately as distinct studies. The 12 trials were conducted between 1966 and 1985: two during the 1960s (in Switzerland), nine in the 1970s (two in Sweden, two in USA, three in UK), and one in the 1980s (in Brazil). Three studies had more than one publication, one of these had four published reports. All 15 reports were published between 1969 and 1995. Of a total of three studies whose authors were sent request letters for unpublished information, reply related to one study was obtained.
Design and methods
All the 12 included studies used parallel group designs and with one exception (Arcieri 1988), all had more than two relevant arms. In one of the 11 multiple arm trials (Triol 1980) there was one group (study arm) of the single topical fluoride modality (toothpaste) and three groups of toothpaste and mouthrinse combined (where different concentrations of the same fluoride agent in the mouthrinse was tested); in another (Mainwaring 1978) there were two toothpaste study‐arms (testing different flavours of toothpaste) and one group of gel and toothpaste combined; and in another (Ringelberg 1979) there were two groups of each, toothpaste or mouthrinse, and of these tested in combination (using different active fluoride agents). It should be noted that two of the included studies (Arcieri 1988; Triol 1980) had only one single fluoride modality being compared with this combined with another; i.e. each study had one relevant comparison only; eight studies compared two different single topical fluoride modalities to a common group where both modalities were combined; i.e. there were two relevant comparisons (with a common group) in each; and one study (Axelsson 1987) with three relevant comparisons, where both the single fluoride group and the combined fluoride group were alternatively common to two comparisons. This study has therefore been entered as two distinct studies (Axelsson 1987; Axelsson 1987a) because mouthrinses or varnishes tested in combination with toothpaste, each combined regimen in a separate arm, were to be compared to a common toothpaste group in the main meta‐analysis. All but one study (Arcieri 1988) used inactive/placebo interventions for the single fluoride arm of the relevant comparisons. Study duration ranged from 2 to 3 years. Studies were generally large with only three allocating less than 200 children to relevant study groups; all but one study recruited children from school settings.
Interventions
There are five trials comparing fluoride toothpaste plus mouthrinse with toothpaste alone (Ashley 1977; Axelsson 1987; Blinkhorn 1983; Ringelberg 1979; Triol 1980) ‐ and four comparing mouthrinse plus toothpaste with mouthrinse alone (Ashley 1977; Axelsson 1987; Blinkhorn 1983; Ringelberg 1979), followed by three comparing toothpaste plus gel with toothpaste alone (Mainwaring 1978; Marthaler 1970; Marthaler 1970a) ‐ and the same three comparing fluoride gel plus toothpaste with gel alone, two comparing toothpaste plus varnish with toothpaste alone (Axelsson 1987a; Petersson 1985) ‐ and one comparing fluoride varnish plus toothpaste with varnish alone (Petersson 1985), two comparing gel plus mouthrinse with gel alone (Arcieri 1988; DePaola 1980) ‐ and one comparing mouthrinse plus gel with mouthrinse alone (DePaola 1980). In all but one trial testing fluoride toothpastes, the fluoride concentrations in the toothpastes were similar, ranging from 1000 to 1250 ppm F, and in three of these trials toothbrushing was performed under supervision at school. In one of the trials testing fluoride varnish, the application frequency was semi‐annual (concentration 22,600 ppm F) and in the other, testing a 22,600 ppm F (Duraphat) varnish, the frequency of application was four times a year. The fluoride concentration in all five trials testing a fluoride gel was also similar (12,300/12,500 ppm F), but frequency of gel application varied from twice (operator‐applied) to 22 times a year (self applied). There was variation in the fluoride concentration (100, 30/250,900 ppm F) in the trials testing fluoride mouthrinsing, but frequency of application was either daily (in two trials) or weekly (in the other five trials).
Participants
Participants were aged 14 or less at the start (in all trials), with similar numbers from both sexes (where these data were reported). The majority of trials included children who were around 12 years at start, and only one trial (Petersson 1985) involved pre‐school children. Caries prevalence at baseline, reported in all but two of the studies, ranged from 1 to 10 D(M)FS (and was 0.9 dfs in the study by Petersson). All studies reported exposure or not to water fluoridation, and only one was conducted in a fluoridated community.
Outcome measures
Caries increment: all trials reported caries increment data (or data from which these could be derived) at the tooth surface level (D(M)FS was reported in 11 trials, and defs in one), and three trials reported caries increment at the tooth level (D(M)FT). With regard to the components of the DMFS index used (and types of teeth/surfaces assessed), six trials reported DFS data (for all tooth surface types), three trials reported DMFS data (for all tooth surface types) and two trials reported DS data (for approximal surfaces of premolars and molars only). No choice had to be made between DMFS or DFS data in any one trial. Trials presented results using one caries grade only (usually CA/ER or CA/DR), or did not report the grade, or reported caries increment data at both levels of diagnosis (in which case CA was chosen). Data on the state of tooth eruption considered were not clearly specified in most trials.
The table Characteristics of included studies provides a description of all the main outcome data reported from each study with the primary measure chosen featuring at the top.
Other dental caries data reported: caries incidence rate (one trial), caries progression (two trials), and proportion of children developing new caries (two trials, one for the permanent dentition and another for the deciduous).
Data on adverse effects: stain score (one trial), any side effects (one trial, without complete or useable data, and with the following statement: "no side effects observed in both groups"). Fluorosis data have not been reported in any of the trials.
Data for unacceptability of treatment (as measured by drop outs/exclusions) were completely reported in six trials.
Risk of bias in included studies
Based on 28 studies included in the topical fluoride reviews and randomly selected for assessment of reproducibility and agreement between two reviewers, interrater reliability was excellent (89%) for both allocation concealment and blinding, and agreement was good for allocation (Kappa = 0.61) and very good for blinding (Kappa = 0.73).
There was variation in the quality of the studies in this review (using the reported information and additional information obtained from investigators).
Allocation concealment
Ten trials were described as randomized but provided no description on how the 'random' allocation was done and were coded B, two trials were considered to be quasi‐randomized and were coded C. None of the trials which described the randomization process or whose investigators provided further information in answer to our enquiry could be assigned code A (adequate concealment of allocation fully described).
Blinding
Double‐blinding was described in seven trials (code A), single‐blinding (blind outcome assessment described but no placebo used) was described in three trials (code B), and blind outcome assessment was indicated in three trials (code C) which described the use of placebo. It may be noted that the study by Axelsson 1987 was performed double‐blind and Axelsson 1987a single‐blind (i.e. there were two relevant comparisons double‐blind and one single‐blind in this study which was treated as two studies, one double‐blind and another single‐blind).
Loss to follow up
Seventy‐four per cent (74%) of the participants originally enrolled in the studies were included in the final analysis (3386 analysed out of 4556 initially randomized). These data exclude five of the 12 included studies, which provided no information on the number of participants randomized to relevant groups. Drop‐out rates were obtained from all but one study and ranged from 5% at 2 years to 40% at 2.5 years. The most common reason for attrition was that participants were not available for follow‐up examination at the end of the study.
Other methodological features
Individuals were allocated to study arms in all trials, where each participant's caries incidence, over a period of time was used as the unit of analysis.
Type of randomization: stratified randomization was reported in five trials (but there was no mention of use of blocking).
Baseline comparisons and handling of any differences: one trial described as 'balanced' (for which randomization may have succeeded to produce nearly exact balance) did not report any of the actual values for the baseline characteristics (such as initial caries levels).
Objectivity/reliability of primary outcome measurement: diagnostic methods used (clinical or radiographic) were described in all studies, but thresholds/definitions used for caries and monitoring of diagnostic errors were not always reported (see 'Notes' in the Characteristics of included studies table for methodological features assessed).
Effects of interventions
Effect on dental caries increment
Pooled estimates of the relative effects of topical fluoride therapy (TFT) are presented for caries increment in the permanent dentition as Decayed, (Missing) and Filled Surfaces Prevented Fraction (D(M)FS PF). Estimates for caries increment in the deciduous dentition are presented as decayed, (missing/extraction indicated), and filled surfaces Prevented Fraction (d(m/e)fs PF).
Eleven studies contributed data suitable for meta‐analysis. Standard deviations (SD) of mean caries increment data (new D(M)FS) were missing in three of the 11 studies (Arcieri 1988; Axelsson 1987; Axelsson 1987a). From the analysis of the 179 available treatment arms for the topical fluoride reviews with complete information (as of October 1999) we derived a regression equation log (SD caries increment) = 0.64 + 0.55 log (mean caries increment), (R2 = 77%). This equation was used to estimate missing standard deviations from mean D(M)FS increments for the meta‐analyses. The single study reporting caries increment in deciduous tooth surfaces (Petersson 1985) did not provide standard deviations of mean caries increment (new dfs) either, and is not included in the analysis of D(M)FS PF (no caries increment data for the permanent dentition).
The results are reported separately here for the following main comparisons: (1) Adjuncts to toothpaste tested against toothpaste alone (Any topical fluoride plus toothpaste versus toothpaste alone) Subgroup 1: Fluoride mouthrinse plus toothpaste versus toothpaste alone Subgroup 2: Fluoride gel plus toothpaste versus toothpaste alone Subgroup 3: Fluoride varnish plus toothpaste versus toothpaste alone
(2) Other combinations of topical fluorides tested against any single modality Subgroup 1: Fluoride mouthrinse plus gel versus mouthrinse alone Subgroup 2: Fluoride mouthrinse plus gel versus gel alone Subgroup 3: Fluoride mouthrinse plus toothpaste versus mouthrinse alone Subgroup 4: Fluoride gel plus toothpaste versus gel alone Subgroup 5: Fluoride mouthrinse plus gel versus gel alone Subgroup 6: Fluoride varnish plus toothpaste versus varnish alone
Objective 1 is addressed in comparison (1), in the meta‐analysis which pools data across all subgroups and includes nine trials, while each subgroup in comparison (1) in effect addresses Objective 2. It may be noted that there was one included study that had a common fluoride toothpaste group and tested two different relevant combinations of topical fluoride with toothpaste, mouthrinse plus toothpaste and varnish plus toothpaste. Due to the meta‐analysis addressing Objective 1, this has been entered as two comparisons/studies (Axelsson 1987/ Axelsson 1987a) in this review (dividing up the group of the fluoride toothpaste arm into approximately equally sized smaller groups to provide a pairwise comparison for each combination of the two modalities with fluoride toothpaste; means and standard deviations were unchanged). In comparison (2), each subgroup addresses a relevant comparison for Objective 3.
As mentioned before, relatively few trial reports provided data able to contribute to meta‐analysis and with the exception of three comparisons from three trials (Arcieri 1988; Axelsson 1987a, Marthaler 1970), all reported equivocal results for caries reductions, i.e. no demonstrated differential effect. Apart from the division of trials into the subsets comparing fluoride toothpaste in combination with gel, varnish or mouthrinse in comparison (1), no subgroup analyses were performed due to the lack of an appropriate volume of data. No metaregression and funnel plot analyses were performed either, on the grounds of insufficient data.
(1) Fluoride toothpaste plus any TFT versus toothpaste alone
For all nine trials combined (one comparing fluoride toothpaste with varnish plus toothpaste, three comparing toothpaste with gel plus toothpaste, and five comparing toothpaste with mouthrinse plus toothpaste; n = 4026), the D(M)FS prevented fraction pooled estimate from the random effects meta‐analysis was 0.10 (95% CI, 0.02 to 0.17; P = 0.01), i.e. a significant difference was detected in favour of toothpaste used in combination with other topical fluorides. Heterogeneity in results was not detected statistically (Chi2 = 11.75 on 8 degrees of freedom, P = 0.16), although some inconsistency in treatment effects can be observed graphically, and confirmed by the I2 heterogeneity statistic (I2 = 32%). Nevertheless, the largest variation in D(M)FS PF (‐0.15 and 0.48) accrues from the trials that carry the lowest weight in the meta‐analysis.
Numbers of children needed to treat (NNT) to prevent one D(M)FS were calculated based on the pooled D(M)FS prevented fraction and on the caries increments in the single toothpaste groups of the nine trials in the meta‐analysis. The overall caries‐inhibiting effect (% PF) derived from the pooled results of the trials was 10% (95% CI, 2% to 17%); the caries increments in the included trials ranged from 0.8 to 2.5 D(M)FS per year. In populations with a caries increment of 0.8 D(M)FS per year (at the lowest end of the results seen in the included studies), this implies an absolute caries reduction of 0.08 D(M)FS per year, equivalent to an NNT of 13 (95% CI, 8 to 63): i.e. 13 children need to use topical fluorides in combination to avoid one D(M)FS. In populations with a caries increment of 2.5 D(M)FS per year (at the highest range of the results seen in the included studies), this implies an absolute caries reduction of 0.25 D(M)FS per year, equivalent to an NNT of 4 (95% CI, 3 to 20): i.e. 4 children need to use combined TFT to avoid one D(M)FS.
Results for the separate subsets comparing fluoride toothpaste with this in combination with varnish, gel, or mouthrinse are as follows:
Fluoride toothpaste plus mouthrinse versus toothpaste alone
Five trials (Ashley 1977; Axelsson 1987; Blinkhorn 1983; Ringelberg 1979; Triol 1980) compared fluoride toothpaste in combination with mouthrinse versus toothpaste alone (n = 2738). The D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis of all five trials combined was 0.07 (95% CI, 0.00 to 0.13; P = 0.06), a just non‐significant effect in favour of the combined regimen within a relatively narrow confidence interval for the pooled estimate of effect. Heterogeneity in the results could not be observed graphically nor statistically (Chi2 = 1.42 on 4 degrees of freedom, P = 0.84; I2 = 0%).
Fluoride toothpaste plus gel versus toothpaste alone
Three trials (Mainwaring 1978; Marthaler 1970; Marthaler 1970a) compared fluoride toothpaste in combination with fluoride gel versus toothpaste alone (n = 1217). The D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis of the three trials combined was 0.14 (95% CI, ‐0.09 to 0.38; P = 0.23), a non‐significant effect in favour of the combined regimen within a relatively large confidence interval. Although no significant heterogeneity was detected (Chi2 = 5.12 on 2 degrees of freedom, P = 0.08), since the test would have minimal power to detect heterogeneity in this meta‐analysis involving very few trials, the inconsistency in treatment effects is in fact large according to the I2 statistic (I2 = 61%).
Fluoride toothpaste plus varnish versus toothpaste alone
There was one small trial (Axelsson 1987a) for this comparison (n = 71), estimating the relative effects in the permanent dentition, which showed a large and highly significant effect in favour of fluoride varnish in combination with toothpaste, and very wide confidence interval for the estimate of effect. The D(M)FS prevented fraction for this trial was 0.48 (95% CI, 0.12 to 0.84; P = 0.009).
Numbers of children needed to treat (NNT) to prevent one D(M)FS were calculated based on the D(M)FS PF and on the caries increment in the toothpaste group of this trial. In populations with a caries increment of 0.8 D(M)FS per year (seen in this study), this implies an absolute caries reduction of 0.38 D(M)FS per year, equivalent to an NNT of 3 (95% CI, 2 to 11): i.e. 3 children need to use the combined regimen (rather than toothpaste alone) to avoid one D(M)FS.
Another trial (Petersson 1985) comparing fluoride varnish combined with toothpaste versus toothpaste alone (n = 173) assessed the relative effect in terms of caries increment in deciduous surfaces only and provided no standard deviations or data from which these could be derived. It reported a dfs PF of 0.15 in favour of the combined therapy (CI not obtainable).
(2) Other combinations of topical fluorides tested against any single modality
Fluoride mouthrinse plus gel versus fluoride mouthrinse alone
Only one trial (DePaola 1980) compared fluoride gel in combination with mouthrinse versus mouthrinse alone (n = 252). It showed non‐significant differences in effect. The D(M)FS prevented fraction was 0.02 (95% CI, ‐0.20 to 0.24; P = 0.86) suggesting that there is insufficient evidence from this trial to confirm or refute a differential effect in caries reduction.
Fluoride gel plus mouthrinse versus fluoride gel alone
Two trials (Arcieri 1988; DePaola 1980) compared fluoride gel in combination with mouthrinse versus mouthrinse alone (n = 497). The D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis of the two trials combined was 0.23 (95% CI, 0.04 to 0.43; P = 0.02), a significant effect in favour of the combined regimen. Although heterogeneity in the results could not be detected by the standard Chi2 test (Chi2 = 2.05 on 1 degree of freedom, P = 0.15), this was not due to homogeneity but to the smaller number of studies (I2 = 51%).
Numbers of children needed to treat (NNT) to prevent one D(M)FS were calculated based on the pooled D(M)FS PF and on the caries increments in the gel groups of the trials that contributed data to the meta‐analysis. The caries increments were 1.56 and 5.09 D(M)FS per year. In populations with a caries increment of 1.56 D(M)FS per year, this implies an absolute caries reduction of 0.36 D(M)FS per year, equivalent to an NNT of 3 (95% CI, 2 to 16): i.e. 3 children need to use the combined regimen (rather than fluoride gel alone) to avoid one D(M)FS. In populations with a caries increment of 5.09 D(M)FS per year, this implies an absolute caries reduction of 1.17 D(M)FS per year, equivalent to an NNT of 1 (95% CI, 1 to 5): i.e. one child need to use the combined regimen to avoid one D(M)FS.
Fluoride mouthrinse plus toothpaste versus mouthrinse alone
Four trials (Ashley 1977; Axelsson 1987; Blinkhorn 1983; Ringelberg 1979) compared fluoride toothpaste in combination with mouthrinse versus mouthrinse alone (n = 1678). The D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis of the four trials combined was 0.05 (95% CI, ‐0.05 to 0.15; P = 0.33), a non‐significant effect in favour of the combined regimen. Heterogeneity in the results could not be observed graphically nor statistically (Chi2 = 3.38 on 3 degrees of freedom, P = 0.34; I2 = 11%).
Fluoride gel plus toothpaste versus gel alone
Three trials (Mainwaring 1978; Marthaler 1970; Marthaler 1970a) compared fluoride toothpaste in combination with fluoride gel versus gel alone (n = 759). The D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis of the three trials combined was 0.10 (95% CI, ‐0.01 to 0.21; P = 0.06), a just non‐significant effect in favour of the combined regimen. Heterogeneity in the results could not be observed graphically nor statistically (Chi2 = 0.17 on 2 degrees of freedom, P = 0.92; I2 = 0%).
Fluoride varnish plus toothpaste versus varnish alone
The single trial (Petersson 1985) comparing fluoride varnish combined with fluoride toothpaste versus varnish alone (n = 186) assessed the relative effect in terms of caries increment in deciduous surfaces only and provided no standard deviations or data from which these could be derived. It reported a dfs PF of 0.19 in favour of the combined therapy (CI not obtainable).
Effect on other outcomes
Data for unacceptability of treatment were reported in six trials that reported dropouts fully. Each of the six trials reported equivocal results for this outcome, i.e. no demonstrated differential effect. Meta‐analysis results for these are described below.
(1) Fluoride toothpaste plus any TFT versus toothpaste alone
The pooled estimate (random‐effects meta‐analysis) of the risk ratio (RR) of dropping out from the fluoride toothpaste group as opposed to the group where other fluoride treatment is in combination with toothpaste in the five trials that reported drop outs was 1.06 (95% CI, 0.96 to 1.21), a non‐significant effect (P = 0.37) slightly in favour of fluoride toothpaste. Heterogeneity was not detected in these results (Chi2 = 2.66 on 4 degrees of freedom, P = 0.62; I2 = 0%). Using alternative measures of effect has given similar results (odds ratio (OR) 1.09, CI 0.88 to 1.34; risk difference (RD) 0.00, CI ‐0.03 to 0.03).
Fluoride toothpaste plus mouthrinse versus toothpaste alone
The pooled estimate (random‐effects meta‐analysis) of the risk ratio (RR) of dropping out from the fluoride toothpaste group as opposed to the combined mouthrinse‐toothpaste arm in the three trials (Axelsson 1987; Blinkhorn 1983; Ringelberg 1979) that reported drop outs was 1.03 (95% CI, 0.84 to 1.26). Heterogeneity was not detected in these results (Chi2 = 2.15 on 2 degrees of freedom, P = 0.34), and the amount present was negligible (I2 = 8%). Using alternative measures of effect has given similar results (OR 1.02, CI 0.74 to 1.40; RD 0.00, CI ‐0.05 to 0.05).
Fluoride toothpaste plus varnish versus toothpaste alone
The pooled estimate (random‐effects meta‐analysis) of the risk ratio (RR) of dropping out from the fluoride toothpaste group as opposed to the combined varnish‐toothpaste arm in the two trials (Axelsson 1987a; Petersson 1985) that reported drop outs was 1.29 (95% CI, 0.61 to 2.71). Heterogeneity was not detected in these results (Chi2 = 0.24 on 1 degree of freedom, P = 0.62; I2 = 0%). Using alternative measures of effect has given similar results (OR 1.31, CI 0.57 to 3.05; RD 0.01, CI ‐0.05 to 0.06).
(2) Other combinations of topical fluorides tested against any single modality
Fluoride mouthrinse plus toothpaste versus mouthrinse alone
Pooled estimates of the risk ratio (RR) of dropping out from the fluoride toothpaste group as opposed to the combined TFT arm could be obtained for the three trials comparing fluoride mouthrinse plus toothpaste versus mouthrinse alone. Results are again consistent with no difference in effect: 0.88 (95% CI, 0.67 to 1.17), and heterogeneity is low (I2 = 24%).
Discussion
Topical fluorides in the form of toothpastes, mouthrinses, varnishes and gels are effective caries preventive interventions. The effectiveness of each of these has been fully assessed in four previous systematic reviews in this series (Marinho 2002; Marinho 2002a; Marinho 2003; Marinho 2003a). In these and in a subsequent review which compiles the evidence from the previous four and exploits power with additional investigation of covariates across all topical fluoride therapies (TFTs), we found no evidence that the effect of topical fluorides was dependent on background exposure to fluoridated water (Marinho 2003b). The main question addressed by this review is how effective the simultaneous use of combined topical fluoride therapy (TFT) for the prevention of caries in children is compared to one topical fluoride treatment used alone. The 11 studies included in the seven meta‐analyses (or in the nine comparisons) have not tested all combinations of possible practical value, and there is a small number of trials in each relevant comparison/meta‐analysis. However, the randomized evidence that we have brought together is, as far as we can ensure, the totality of the available randomized evidence comparing the combined use of any two topical fluoride modalities with one of them used alone. Although there is a suggestion of a modest caries inhibiting effect with the combined use of topical fluorides in the permanent dentition for most of the comparisons, a general lack of statistical significance is apparent. Further, in a few comparisons, the confidence intervals are relatively wide and the variation among the results of the studies can be substantial. This calls for a cautious interpretation of the data.
Thus, for the primary objective of the review, there is evidence showing that simultaneous use of a topical fluoride treatment with fluoride toothpaste results in an enhanced caries inhibiting effect compared with the use of toothpaste alone. Over 4000 children were included in the trials, and for the majority of children the combined topical fluoride regimen they used at the same time was toothpaste and mouthrinse, followed by toothpaste and gel, and toothpaste and varnish. The random‐effects meta‐analysis of the nine studies assessing the effect of fluoride mouthrinses, gels or varnishes used in combination with fluoride toothpaste on the permanent dentition suggests that their combined use is associated on average with a 10% (95% CI, 2% to 17%) reduction in decayed, missing and filled tooth surfaces. It may be noted that whilst there is evidence that additional caries protection accrues from their combined use, the size of the estimated benefit, of the order of 10%, is not substantial. As to the practical value of the combined regimens tested against fluoride toothpaste alone, the caries reduction would correspond to a number needed to treat (NNT) of 4 to avoid one decayed, filled or missing tooth surface (DMFS) per year in a child population with a caries increment of 2.5 D(M)FS per year (at the highest range of toothpaste group rates for included studies), or an NNT of 13 for children from a population with a caries increment of 0.8 D(M)FS per year (at the lowest end of the observed range). There was only one trial assessing the effect of the combined use of topical fluorides with toothpaste on the deciduous dentition. This compared varnish plus toothpaste versus toothpaste alone only and suggests a 15% reduction in decayed and filled tooth surfaces in favour of the combined therapy, but it is unclear whether the effect was significant.
To what extent statistically significant caries reductions in the order of 10% should be considered important? Some authorities have advocated the use of arbitrary thresholds that indirectly define clinical significance for anticaries products. For example, the American Dental Association produced guidelines proposing that a toothpaste cannot be claimed to be superior to another unless it provides a 10% difference in effect (just the size of the difference for the simultaneous use of TFT and fluoride toothpaste in this review) (CDT‐ADA 1988). The trials in a review may give a power calculation that specifies the size of effect the trialists considered to be important, which may be preferred to the use of arbitrary thresholds. In this review this was provided in the trial by Blinkhorn 1983, which had an 80% power to detect a 25% difference between the combined TFT group (toothpaste and mouthrinse in this trial) and the fluoride toothpaste group. Taking this as the clinically important difference indicates that the combined use of toothpaste with other TFTs had no greater effect than toothpaste used alone.
A secondary objective of this review was to examine whether there was a beneficial effect in terms of caries prevention from the addition of each TFT modality to toothpaste separately compared to toothpaste alone or from the combined use of any other two TFT modalities separately compared to one of them alone. We were unable to detect a clear differential effect from all but two of the seven available comparisons.
Thus, a differential treatment effect for each relevant subset in the main meta‐analysis, which assessed the effect of fluoride gel plus toothpaste and toothpaste alone and of fluoride mouthrinse plus toothpaste and toothpaste alone on the permanent dentition, could not be clearly detected, whereas the evidence from one single small trial, which was not carried out double‐blind, of a significant differential effect in caries reduction favouring the combined use of fluoride varnish and toothpaste over fluoride toothpaste alone should be viewed with caution, as this is far from definite.
Turning to the combined use of gels or mouthrinses with toothpaste when compared with gels or mouthrinses used alone respectively the general observation is that there is indication of an increased benefit with the use of the combined topical fluoride regimens, although, again, results are not conclusive and the magnitude of any possible differential effect seems to be small.
Among the other relevant combined regimens analysed there is evidence of an increased benefit with the use of fluoride gel and mouthrinse compared to fluoride gel alone, and no suggestion of a significant beneficial effect with the use of fluoride mouthrinse and gel compared to mouthrinse alone. This finding may in fact indirectly suggest that larger caries reductions may be achieved with fluoride mouthrinse used singly, as opposed to the single use of fluoride gel.
As regards the acceptance of combinations of topical fluoride treatments, as measured by the proportion of children dropping out from the trials, there is no suggestion of significant differences in effect. We found little useful information about the effects of combined topical fluorides on other clinically important outcomes such as caries incidence in the deciduous dentition, and on outcomes such as the proportion of children remaining caries‐free. We also found no useful information on adverse effects such as fluorosis, oral allergic reactions, or tooth staining. Although the lack of data on enamel fluorosis in particular is likely in part to reflect the type of studies considered, the age ranges of the participants in such trials (children under five were included in only one trial), and their usual duration of 2 to 3 years, this lack of evidence about adverse effects makes it more difficult for clinicians and policy makers to weigh the benefits of using topical fluorides in different combinations that appeared to be effective for the prevention of caries in children against possible shortcomings of the combined procedures. In general, even with additional caries protection accruing from some of the combined preventive regimens, the additional cariostatic effect may be slight and not worth the extra effort with the use of a second intervention.
Authors' conclusions
Implications for practice.
This review has found that compared with fluoride toothpaste used alone, topical fluorides (mouthrinses, gels, or varnishes) used in addition to fluoride toothpaste reduce caries by 10% on average. In terms of acceptability, there is no suggestion of differences in effect between topical fluorides used in combination and fluoride toothpaste used alone. Because the size of the caries preventive effect is relatively small and as none of the trials consistently compared other important outcomes such as possible side effects from the combined use of topical fluorides and toothpaste, it is not possible to make a clear recommendation on the superiority of using another topical fluoride in addition to toothpaste. Current clinical practice includes an additional TFT (over toothpaste) for children at higher risk of developing dental caries. Since increased effectiveness of topical fluorides is to be expected in children with higher initial D(M)FS scores, this practice may be considered in populations with a caries increment of around 2 D(M)FS per year or more. However, it should be recognised that such an approach reinforces targeting preventive care to high‐risk sub‐populations. That high‐risk approach fails to deal with the majority of new caries which occurs in the majority who are at lower risk. Therefore applying the results relating to additional TFT (over toothpaste) from this review needs to be made with the aforementioned caveat. As for all other combined regimens tested separately against one single topical fluoride, there is an indication that the additional cariostatic effects that may accrue from the combined topical fluorides are slight, and most results are not significant.
Implications for research.
There is a general lack of randomized trial evidence evaluating the use of different combinations of topical fluorides for the prevention of dental caries in children, and, therefore, a modest treatment effect may have been missed for most relevant comparisons. However, the lack of a clear suggestion of significant benefits from the data analysed in the majority of the comparisons may not indicate priority for the performance of new studies.
What's new
| Date | Event | Description |
|---|---|---|
| 29 August 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors wish to thank the Systematic Reviews Training Unit (UCL) and the Cochrane Oral Health Group for the continuous support. The synopsis was provided by the Cochrane Consumer Network, for which we express our thanks. Many thanks are due to the investigators who have kindly provided additional information about their trials or supplied reports of trials, as well as to those who helped with the translations or provided comments and editorial input to this review.
Appendices
Appendix 1. MEDLINE search strategy
(a) [("DENTAL‐CARIES" explode all subheadings or "DENTAL‐CARIES‐ACTIVITY‐TESTS" all subheadings or "DENTAL‐CARIES‐SUSCEPTIBILITY" all subheadings or CARIE* or DMF*) and (("FLUORIDES" explode all subheadings or "FLUORIDES,‐TOPICAL" explode all subheadings or FLUOR* or AMF or AMINE F OR SNF2 OR STANNOUS F OR NAF OR SODIUM F OR APF OR SMFP OR MFP OR MONOFLUOR*) or ("CARIOSTATIC‐AGENTS" explode all subheadings or "DENTAL‐PROPHYLAXIS" explode all subheadings or "DENTIFRICES" explode all subheadings or "MOUTHWASHES" explode all subheadings or CARIOSTA* or PROPHYLA* or ANTICARI* or ANTI CARI* or VARNISH* or LACQUER* or DURAPHAT or GEL* or TOOTHPASTE* or TOOTH PASTE* or PASTE* or DENTIFRIC* or MOUTHRINS* or MOUTH RINS* or RINS* or MOUTHWASH* or MOUTH WASH*))]. (b) [((explode FLUORIDES/all subheadings) or (explode FLUORIDES‐TOPICAL/ALL SUBHEADINGS) or (FLUOR*) or (AMF or AMINE F OR SNF2 OR STANNOUS F OR NAF OR SODIUM F OR APF OR MFP OR SMFP OR MONOFLUOR* OR DURAPHAT)) and ((CARI*) or (DMF*) or (TOOTH*) or (TEETH*) or (DENT* in TI, in AB, in MESH)) or ((explode CARIOSTATIC‐AGENTS/all subheadings) or (ANTICARI* or ANTI CARI*) or (explode MOUTHWASHES/all subheadings) or (MOUTHWASH* or MOUTH WASH*) or (MOUTHRINS* or MOUTH RINS*) or (VARNISH* or LACQUER*))]
Appendix 2. LILACS/BBO search strategy
[(fluor$ or ppmf or ppm f or amf or snf or naf or apf or mfp or smfp or monofluor$ or duraphat$) and (carie$ or dmf$ or cpo$ or tooth$ or teeth$ or dent$ or dient$ or anticarie$ or cario$ or mouthrins$ or mouth rins$ or rinse$ or bochech$ or enjuag$ or verniz$ or varnish$ or barniz$ or laca$ or gel or gels)] and [random$ or aleatori$ or acaso$ or azar$ or blind$ or mask$ or cego$ or cega$ or ciego$ or ciega$ or placebo$ or (clinic$ and (trial$ or ensaio$ or estud$)) or (control$ and (trial$ or ensaio$ or estud$))].
Appendix 3. Supplementary MEDLINE search strategy
[(CARIE* or (DENT* near CAVIT*) or TOOTH* DECAY* or DMF* or (explode "DENTAL‐CARIES"/ALL SUBHEADINGS)) and (FLUOR* or APF* or NAF* or AMINE F OR SNF* or ACIDULATED* PHOSPHATE* FLUORID* or ACIDULATED* FLUORID* or PHOSPHATE* FLUORID* or SODIUM* FLUORID* or AMINE* FLUORID* or STANNOUS* FLUORID* or (explode "FLUORIDES"/ ALL SUBHEADINGS)) and (1) (TOOTHPASTE* or TOOTH* PASTE* or DENTIFRICE* or PASTE*) or (explode "DENTIFRICES"/all subheadings)]. (2) ((RINS* or MOUTH* RINS* or WASH* or MOUTH* WASH*) or (MOUTHRINS* or MOUTHWASH*)) or (explode "MOUTHWASHES"/ all subheadings)]. (3) (FLUOR* or ELMEX* or (explode "FLUORIDES"/ALL SUBHEADINGS)) and (GEL* or TRAY*)]. (4) (FLUOR* or (DURAPHAT* or FLUOR PROTECTOR*) or (explode "FLUORIDES"/ALL SUBHEADINGS)) and (VARNISH*) or (LACQUER* or LAQUER*) or (VERNIZ*) or (LACKER*) or (LAKK*) or (SILANE* or POLYURETHANE*)].
Data and analyses
Comparison 1. Fluoride toothpaste plus others (varnish, gel or rinse) versus toothpaste alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years (9 trials) | 9 | 4026 | Prevented Fraction (Random, 95% CI) | 0.10 [0.02, 0.17] |
| 1.1 Fluoride toothpaste plus mouthrinse versus Fluoride toothpaste | 5 | 2738 | Prevented Fraction (Random, 95% CI) | 0.07 [‐0.00, 0.13] |
| 1.2 Fluoride toothpaste plus gel versus Fluoride toothpaste | 3 | 1217 | Prevented Fraction (Random, 95% CI) | 0.14 [‐0.09, 0.38] |
| 1.3 Fluoride toothpaste plus varnish versus Fluoride toothpaste | 1 | 71 | Prevented Fraction (Random, 95% CI) | 0.48 [0.12, 0.84] |
| 2 d(e)fs increment (PF) ‐ nearest to 3 years (1 trial) | 1 | 173 | Prevented Fraction (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Fluoride toothpaste plus varnish versus Fluoride toothpaste | 1 | 173 | Prevented Fraction (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Unacceptability of treatment as measured by leaving study early (5 trials) | 5 | 1998 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.22] |
| 3.1 Fluoride toothpaste plus mouthrinse versus Fluoride toothpaste | 3 | 1704 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.84, 1.26] |
| 3.2 Fluoride toothpaste plus varnish versus Fluoride toothpaste | 2 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.61, 2.71] |
1.1. Analysis.
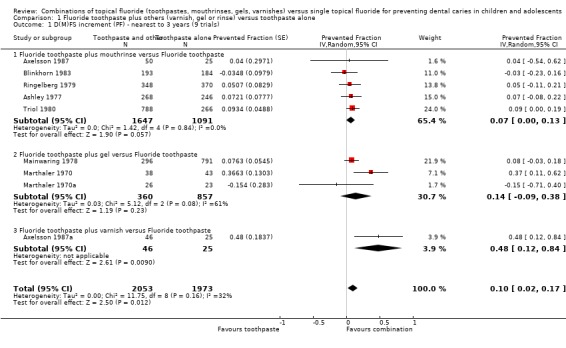
Comparison 1 Fluoride toothpaste plus others (varnish, gel or rinse) versus toothpaste alone, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years (9 trials).
1.2. Analysis.
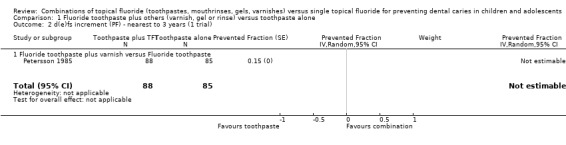
Comparison 1 Fluoride toothpaste plus others (varnish, gel or rinse) versus toothpaste alone, Outcome 2 d(e)fs increment (PF) ‐ nearest to 3 years (1 trial).
1.3. Analysis.
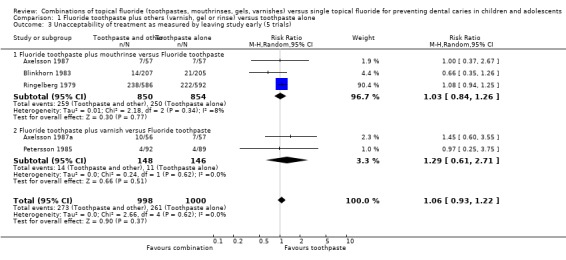
Comparison 1 Fluoride toothpaste plus others (varnish, gel or rinse) versus toothpaste alone, Outcome 3 Unacceptability of treatment as measured by leaving study early (5 trials).
Comparison 2. Other combinations of topical fluoride versus one topical fluoride alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years | 9 | Prevented Fraction (Random, 95% CI) | Subtotals only | |
| 1.1 Fluoride mouthrinse plus gel versus Fluoride mouthrinse (1 trial) | 1 | 252 | Prevented Fraction (Random, 95% CI) | 0.02 [‐0.20, 0.24] |
| 1.2 Fluoride gel plus mouthrinse versus Fluoride gel (2 trials) | 2 | 497 | Prevented Fraction (Random, 95% CI) | 0.23 [0.04, 0.43] |
| 1.3 Fluoride mouthrinse plus toothpaste versus Fluoride mouthrinse (4 trials) | 4 | 1678 | Prevented Fraction (Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 1.4 Fluoride gel plus toothpaste versus Fluoride gel (3 trials) | 3 | 759 | Prevented Fraction (Random, 95% CI) | 0.10 [‐0.01, 0.21] |
| 2 d(e)fs increment (PF) ‐ nearest to 3 years | 1 | Prevented Fraction (Random, 95% CI) | Subtotals only | |
| 2.1 Fluoride varnish plus toothpaste versus Fluoride varnish (1 trial) | 1 | 186 | Prevented Fraction (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Unacceptability of treatment as measured by leaving study early | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoride gel plus mouthrinse versus Fluoride gel (1 trial) | 1 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.72, 1.40] |
| 3.2 Fluoride mouthrinse plus toothpaste versus Fluoride mouthrinse (3 trials) | 3 | 1697 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.17] |
| 3.3 Fluoride varnish plus toothpaste versus Fluoride varnish (1 trial) | 1 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.22, 2.59] |
2.1. Analysis.
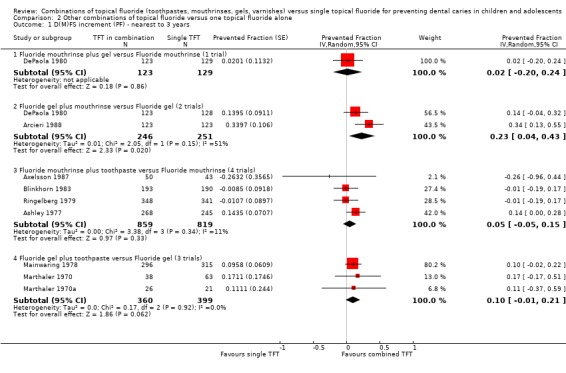
Comparison 2 Other combinations of topical fluoride versus one topical fluoride alone, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years.
2.2. Analysis.
Comparison 2 Other combinations of topical fluoride versus one topical fluoride alone, Outcome 2 d(e)fs increment (PF) ‐ nearest to 3 years.
2.3. Analysis.
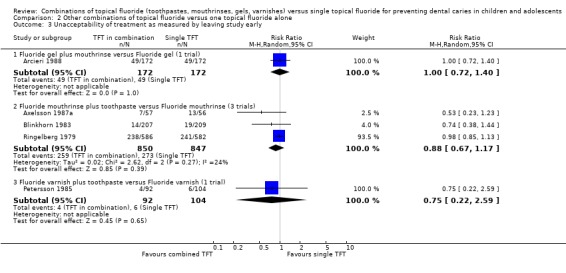
Comparison 2 Other combinations of topical fluoride versus one topical fluoride alone, Outcome 3 Unacceptability of treatment as measured by leaving study early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arcieri 1988.
| Methods | Stratified quasi‐random allocation; blind caries assessment stated but unclear (C); non‐placebo‐controlled; 28.5% drop out after 2 years (study duration = 2 years). Reason(s) for attrition NR; no differential group losses. | |
| Participants | 246 children analysed at 2 years (available at final examination). Age range at start: 7‐11 years. Surfaces affected at start: 4.5 DMFS. Exposure to other fluoride: water. Year study began: 1983. Location: Brazil. | |
| Interventions | FG versus FG+FR. FG = APF 12,300 ppm F. Operator‐applied, with cotton‐paint, twice a year (prior tooth‐cleaning with fluoride‐free paste performed). FR = NaF 900 ppm F. School mouthrinsing/supervised, weekly (approximately 35 sessions in 2 years). |
|
| Outcomes | 2yDMFS increment.
Reported at 1 and 2 years follow ups. Proportion of children remaining caries‐free. Drop out. Side effects (incomplete data). |
|
| Notes | Participants randomized (N = 344). Baseline characteristics (dental age, DMFS) 'balanced'. Clinical (VT) caries assessment by two examiners; diagnostic threshold NR; state of tooth eruption included NR; diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Ashley 1977.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 12% drop out (for all study groups combined) after 2 years (study duration = 2 years). Natural losses; any differential group losses not assessable. | |
| Participants | 514 children analysed at 2 years (available at final examination). Average age at start: 12 years. Surfaces affected at start: 9.1 DFS. Exposure to other fluoride: no. Year study began: 1973. Location: UK. | |
| Interventions | FR+PLT versus FR+FT versus FT+PLR. FR = NaF 100 ppm F. School mouthrinsing/supervised, daily, 20 ml applied for 1 min (after toothbrushing with appropriate toothpaste at school). FT = SMFP 1000 ppm F. School toothbrushing/supervised, daily, 1 g applied for 1 min (followed by mouthrinsing with appropriate solution); non‐fluoride toothpaste provided to all for home use. Abrasive system: IMP (main abrasive). |
|
| Outcomes | 2yNetDFS increment ‐ (E+U)(NCA)cl+(ER)xr.
Reported at 2 years follow up. PF‐DFS. MD‐BL‐DFS. MD‐DFS. DFS (U). |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, DFS, DMFS, DMFT) 'balanced'. Clinical (V) caries assessment by one examiner (FOTI used); diagnostic threshold = NCA. Radiographic assessment (postBW) by one examiner; diagnostic threshold = ER. State of tooth eruption included = E/U. Intra‐examiner reproducibility checks for incremental caries data (icc for clinical 0.95, for radiographic 0.8); reversal rate beween 12% and 7% of observed DFS increment in study groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Axelsson 1987.
| Methods | Random allocation; double‐blind (A); placebo‐controlled; 16% drop out after 3 years (study duration = 3 years). Reasons for attrition described with respective total numbers by group; no differential group losses. | |
| Participants | 143 children analysed at 3 years (available at final examination). Age range at start: 13‐14 years. Surfaces affected at start: relevant data NR. Exposure to other fluoride: no. Year study began: 1977. Location: Sweden. | |
| Interventions | FR+PLT versus FR+FT versus FT+PLR. FR = NaF 230 ppm F. School mouthrinsing/supervised, weekly. FT = NaF 1000 ppm F. Home toothbrushing/unsupervised, daily frequency assumed (instructed to brush twice a day). Abrasive system: silica. FV (Fluor Protector®) = NaF 7000 ppm F. Applied 4 times a year. |
|
| Outcomes | 3ypostMD‐DS increment ‐ (ER)xr.
Reported at 3 years follow up. Caries progression. Drop out. |
|
| Notes | Participants randomized (N = 170). Baseline characteristics (DS) 'balanced'. Radiographic assessment (4 postBW) by two examiners; diagnostic threshold = ER. State of tooth eruption included NR. Examiner reproducibility checks for incremental caries data performed ('consistency of duplicate examination reached 94% for scores 1&2 combined'). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Axelsson 1987a.
| Methods | Random allocation; single‐blind (B); placebo‐controlled; 15% drop out after 3 years (study duration = 3 years). Reasons for attrition described with respective total numbers by group; no differential group losses. | |
| Participants | 96 children analysed at 3 years (available at final examination). Age range at start: 13‐14 years. Surfaces affected at start: relevant data NR. Exposure to other fluoride: no. Year study began: 1977. Location: Sweden. | |
| Interventions | FT+PLR* versus FT+FV. FT = NaF 1000 ppm F. Home toothbrushing/unsupervised, daily frequency assumed (instructed to brush twice a day). Abrasive system: silica. FV (Fluor Protector®) = NaF 7000 ppm F. Applied 4 times a year. |
|
| Outcomes | 3ypostMD‐DS increment ‐ (ER)xr.
Reported at 3 years follow up. Caries progression. Drop out. |
|
| Notes | Participants randomized (N = 113). Baseline characteristics (DS) 'balanced'. Radiographic assessment (4 postBW) by two examiners; diagnostic threshold = ER. State of tooth eruption included NR. Examiner reproducibility checks for incremental caries data performed (''consistency of duplicate examination reached 94% for scores 1 & 2 combined''). *FT+PLR is the same group as in Axelsson 1987. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Blinkhorn 1983.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 9% drop out after 3 years (study duration = 3 years). Reasons for attrition described with respective total numbers; no differential group losses. | |
| Participants | 567 children analysed at 3 years (available at final examination). Age range at start: 11‐12 years. Surfaces affected at start: 8.4 DMFS. Exposure to other fluoride: no. Year study began: 1972. Location: UK. | |
| Interventions | FR+PLT versus FR+FT versus FT+PLR. FR = NaF 230 ppm F. School mouthrinsing/supervised, daily, for 0.5 min (after toothbrushing with appropriate toothpaste at school). FT = SMFP 1000 ppm F. School toothbrushing/supervised, daily, for 1 min (followed by mouthrinsing with appropriate solution); appropriate toothpaste provided for home use. Abrasive system: IMP (main abrasive). |
|
| Outcomes | 3yNetDFS increment ‐ (E+U)(CA)cl+(DR)xr.
Reported at 3 years follow up. PF‐DFS. MD‐BL‐DFS. MD‐DFS. postMD‐DFS. DMFT (E/U). anterior DMFT. posterior DMFT. DFS (U). Drop out. |
|
| Notes | Participants randomized (N = 621). Baseline characteristics (DMFS, DMFT, SAR) 'balanced' (DFS baseline data NR). Clinical (V) caries assessment by one examiner, diagnostic threshold = CA. Radiographic assessment (1 postBW) by one examiner; diagnostic threshold = DR. State of tooth eruption included = E/U. Intra‐examiner reproducibility checks for incremental clinical and radiographic caries data in 10% sample (icc score 0.9). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
DePaola 1980.
| Methods | Random allocation; double‐blind (A); placebo‐controlled; drop‐out rate NR nor obtainable (study duration = 2 years + 1 year post‐study period). Exclusions based on compliance and presence in all follow‐up examinations; any differential group losses not assessable. | |
| Participants | 380 children analysed at 1* year (after exclusions, present for entire study period). Age range at start: 12‐14 years (average = 13). Surfaces affected at start: NR. Exposure to other fluoride: toothpaste assumed. Year study began: in/before 1977. Location: USA. | |
| Interventions | FG+PLR versus FG+FR versus FR+PLG. FG = APF 12,300 ppm F. Self‐applied under supervision at school, with tray, 10 consecutive applications (days) in 1st year, applied for 5 minutes. FR = NaF 230 ppm F. School use/supervised, daily, 10 ml applied for 1 min. |
|
| Outcomes | 1y*NetDFS increment ‐ (CA)cl+xr. Reported at 1 and 2 years follow ups (and 1 year post‐treatment). | |
| Notes | Participants randomized (numbers NR). Baseline characteristics (age, dental age, DFS) described as 'balanced' (values NR). Clinical (VT) caries assessment by two examiners; diagnostic threshold = CA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by two examiners (diagnostic threshold NR); diagnostic errors NR. *Intervention (gel) applied during 1st year of study only (thus, final 2 years results not considered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mainwaring 1978.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 18% drop out (for all study groups combined) after 3 years (study duration = 3 years). Natural losses; any differential group losses not assessable. | |
| Participants | 1402 children analysed at 3 years (available at final examination). Age range at start: 11‐12 years. Surfaces affected at start: 8 DFS. Exposure to other fluoride: no. Year study began: in/before 1974. Location: UK. | |
| Interventions | FG+PLT versus FG+FT versus FT+PLG (2 groups). FG = APF 12,300 ppm F. Operator‐applied, with tray, twice a year, applied for 4 minutes (prior toothbrushing with non‐fluoride toothpaste performed). FT1 & FT2 = SMFP 1000 ppm F (but of different flavours). Home use/unsupervised, for 1 min, daily frequency assumed. Abrasive system: Ca carbonate. |
|
| Outcomes | 3yNet/CrudeDFS increment ‐ (CA)(E)cl+(ER)xr.
Reported at 3 years follow up. PF‐DFS cl. postMD‐DFS xr. DFS (U) cl+xr. CIR. |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, SAR, DFS) 'balanced'. Clinical (VT) caries assessment by one examiner; diagnostic threshold = CA; state of tooth eruption included = E. Radiographic assessment (2 postBW) by one examiner; diagnostic threshold = ER. Intra‐examiner reproducibility checks for DFS in 10% sample (icc for VT/XR over 0.95); error variance less than 5% of total variance; reversal rate less than 4% of observed DFS increment in all groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marthaler 1970.
| Methods | Random allocation; indication of blind caries assessment (C); placebo‐controlled; 18% drop out (for all study groups combined) after 3 years (study duration = 3 years). Exclusions based on use of orthodontic bands and presence in all follow‐up examinations; any differential group losses not assessable. | |
| Participants | 144 children analysed at 3 years (present for all examinations). Age range at start: 6‐7 years. Surfaces affected: 1 DMFS. Exposure to other fluoride: salt. Year study began: 1966. Location: Switzerland. | |
| Interventions | FG+PLT versus FG+FT versus FT+PLG. FG = AmF/NaF 12,500 ppm F. Self‐applied under supervision at school, with toothbrush, 20 times a year, 1 g applied for 6 minutes. FT = AmF 1250 ppm F. Home use/unsupervised, twice/three times a day/680 times a year estimated. Abrasive system: IMP. |
|
| Outcomes | 3yNetDFS increment ‐ (CA)cl+(DR)xr.
Reported at 1 and 3 years follow ups. 1stmPF‐DFS. 1stmMD‐DFS. |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, DFMS, 1stmDMFS) 'balanced'. Clinical (V) caries assessment by two examiners; diagnostic threshold = CA and NCA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by two examiners; diagnostic threshold = DR and ER; partial recording. ''Sufficient agreement of the two examiners known from earlier work''. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marthaler 1970a.
| Methods | Random allocation; indication of blind caries assessment (C); placebo‐controlled; 30% drop out (for all study groups combined) after 4 years (study duration = 4 years). Exclusions based on use of orthodontic bands, and presence in all follow‐up examinations; any differential group losses not assessable. | |
| Participants | 70 children analysed at 2 & 4* years (present for all examinations). Age range at start: 7‐9 years. Surfaces affected: 1.9 DMFS. Exposure to other fluoride: salt. Year study began: 1966. Location: Switzerland. | |
| Interventions | FG+PLT versus FG+FT versus FT+PLG. FG = AmF/NaF 12,500 ppm F. Self‐applied under supervision at school, with toothbrush, 22 times a year, 1 g applied for 6 minutes. FT = AmF 1250 ppm F. Home use/unsupervised, twice/three times a day/800 times a year estimated. Abrasive system: IMP. |
|
| Outcomes | 2y*NetDFS increment ‐ (CA)cl+(DR)xr.
Reported at 2 and 4 years follow ups. 1stmPF‐DFS (CA) cl. 1stmMD‐DFS (DR) xr. |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, DMFS, 1stmDMFS) 'balanced'. Clinical (V) caries assessment by two examiners; diagnostic threshold = CA and NCA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by two examiners; diagnostic threshold = DR and ER; partial recording. ''Sufficient agreement of examiners known from earlier work''. *F solution used by all children after 2 years (final 4 years results not considered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Petersson 1985.
| Methods | Quasi‐random allocation; single‐blind (B); non‐placebo‐controlled; 5% drop out after 2 years (study duration = 2 years). Reason(s) for attrition NR; no differential group losses. | |
| Participants | 271 children analysed at 2 years (present for entire trial period). Average age at start: 3 years. Surfaces affected at start: 0.9 dfs (data from original sample only). Exposure to other fluoride: none assumed. Year study began: 1978. Location: Sweden. | |
| Interventions | FV+PLT versus FV+FT versus FT alone. FV (Duraphat®) = NaF 22,600 ppm F. Applied twice a year. FT = NaF 250 ppm F. Home use/unsupervised, daily frequency assumed. |
|
| Outcomes | 2ydfs increment ‐ (E) (CA)cl+(DR)xr.
Reported at 2 years follow up. O‐defs. MD‐defs. BL‐defs. Proportion of children with one or more new defs (at CA level). Drop out. |
|
| Notes | Participants randomized (N = 285). Baseline characteristics (dfs) 'balanced'. Clinical (VT) caries assessment by two examiners; diagnostic threshold = CA; radiographic assessment (2 postBW) by two examiners; diagnostic threshold = DR. Diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Ringelberg 1979.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 40% drop out after 2.5 years (study duration = 2.5 years). Reason(s) for attrition NR; no differential group losses. | |
| Participants | 1059 children analysed at 2.5 years (available at final examination). Average age at start: 11 years. Surfaces affected at start: 4.3 DMFS. Exposure to other fluoride: no. Year study began: 1973. Location: USA. | |
| Interventions | FR+PLT (2 groups) versus FR+FT (2 groups) versus FT+PLR (2 groups). FR1 = AmF 250 ppm F, FR2 = NaF 250 ppm F. School use/supervised, daily, 10 ml applied for 1 min. FT1 = AmF 1250 ppm F, FT2 = SnF2 1000 ppm F. Home use/unsupervised, daily frequency assumed. Abrasive system: Ca pyrophosphate in SnF2 toothpaste, NR for AmF toothpaste. |
|
| Outcomes | 2.5yNetDMFS increment ‐ (CA)cl + (DR)xr.
Reported at 2.5 years follow up. DMFT. Stain score. Drop out. |
|
| Notes | Participants randomized (N = 1760). Baseline characteristics (DMFS, DMFT) 'balanced'. Clinical (VT) caries assessment by two examiners, diagnostic threshold = CA. Radiographic assessment (5 BW) by two examiners; diagnostic threshold = DR. State of tooth eruption included NR. Reversal rate between 4 and 9% of observed caries increment in the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Triol 1980.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 20% drop out after 2.5 years (study duration = 2.5 years). Natural losses; any differential group losses not assessable. | |
| Participants | 1054 children analysed at 2.5 years (available at all examinations). Age range at start: 10‐13 years (average = 11.5). Surfaces affected at start: 9.9 DFS. Exposure to other fluoride: toothpaste assumed. Year study began: in/before 1977. Location: USA. | |
| Interventions | FT+PLR versus FR+FT (3 groups). FR1 = NaF 110 ppm F, FR2 = NaF 230 ppm F, FR3 = NaF 450 ppm F. School mouthrinsing/supervised, daily, 7.5 ml applied for 0.5 min (after toothbrushing with fluoride toothpaste at school). FT = SMFP 1000 ppm F (in all groups). School toothbrushing/supervised, daily, 1 g applied for 1 min (followed by water rinse and mouthrinsing with appropriate solution); Fluoride toothpaste provided to all for home use. Abrasive system: IMP (main abrasive). |
|
| Outcomes | 2.5yDMFS increment ‐ cl+xr.
Reported at 1.5 and 2.5 years follow ups. MD‐DMFS. DMFT. Drop out (no data by group). |
|
| Notes | Participants randomized (N= 1320); numbers by group NR. Baseline characteristics (age, SAR, DFS, D, F, TAR, DFT) 'balanced' (DMFS baseline data NR). Clinical (VT) caries assessment; diagnostic threshold NR; state of tooth eruption included NR. Radiographic assessment (2 postBW); diagnostic threshold NR; diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Drop‐out rate based only on groups relevant to review, on relevant follow ups, unless otherwise stated. Baseline caries experience averaged among relevant study arms, and based on the study sample analysed at the end of study period (final sample), unless otherwise stated. Age range (average age when reported) at the time the study started based on all study participants (or on groups relevant to the review when data were available). 1stm = first permanent molar; AmF = amine fluoride; APF = acidulated phosphate fluoride; BW = bite‐wing x‐ray assessment; Ca = calcium; Ca carbonate = CaCO3; CA = lesions showing loss of enamel continuity that can be recorded clinically (undermined enamel, softened floor/walls) or showing frank cavitation; CAR = caries attack rate; CIR = caries incidence rate; cl = clinical examination; d(e)ft/s = decayed, (extracted) and filled deciduous teeth or surface; dmft/s = decayed, missing (or extracted) and filled deciduous teeth or surface; D(M)FS/T = decayed, (missing ) and filled permanent surfaces or teeth; DR = radiolucency into dentin; E = teeth erupted at baseline; ER = any radiolucency in enamel/enamel‐dentin junction; F = fluoride; FG = fluoride gel; FR = fluoride mouthrinse; FT = fluoride toothpaste; FV = fluoride varnish; icc = intra‐class correlation coefficient (for inter‐rater reliability); IMP = insoluble Na metaphosphate; M = missing permanent teeth; MD = mesio and distal surfaces; N = numbers; Na = sodium; NaF = sodium fluoride; NCA = non‐cavitated enamel lesions visible as white spots or discoloured fissures; NR = not reported; NS = not significant; NT = no treatment control; O = occlusal surfaces; PF = pit and fissure surfaces; PL = placebo; postBW = posterior bite‐wing x‐ray assessment; ppm F = parts per million of fluoride; ptc = prior tooth‐cleaning performed with or without a non‐fluoride paste; Silica = silicon dioxide (SiO2); SMFP = sodium monofluorophosphate; SnF2 = stannous fluoride; U = teeth unerupted at baseline; VT = visual‐tactile assessment; xr = radiographic examination.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bohannan 1985 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated or indicated (FR plus FG versus FR). |
| Louw 1995 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated or indicated (FR plus FT versus FR). |
| Wilson 1978 | Random or quasi‐random allocation not stated (FR plus FT versus FR; FT plus FR versus FT). |
FG = fluoride gel; FR = fluoride mouthrinse; FT = fluoride toothpaste; FV = fluoride varnish
Contributions of authors
All authors contributed to the development of the protocol and execution of the review. Valeria Marinho (VM) wrote the protocol, designed and implemented the search strategies, contacted authors, selected studies, assessed validity, and extracted data. Julian Higgins (JH) duplicated study selection, quality assessment, and data extraction in a sample of studies and Stuart Logan (SL) or Aubrey Sheiham (AS) were consulted where necessary. VM entered and analysed the data in consultation with JH. VM prepared the full review. All authors contributed to its revision, interpretation of results and approval.
Sources of support
Internal sources
Department of Epidemiology and Public Health (UCL), UK.
Systematic Reviews Training Unit, Institute of Child Health (UCL), UK.
Medical Research Council, UK.
External sources
CAPES ‐ Ministry of Education, Brazil.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Arcieri 1988 {published data only}
- Arcieri RM, Carvalho M‐d‐L, Goncalves LM, Almeida HA, Marra EM, Ferreira AL. Reduction of dental caries after two years with a combination of mouthwashes and topical fluorides [Reducao da carie dentaria apos dois anos da associacao de bochechos e aplicacoes topicas com fluor]. Revista do Centro de Ciencias Biomedicas da Universidade Federal de Uberlandia 1988;4:58‐65. [PubMed] [Google Scholar]
- Arcieri RM, Lourdes Carvalho M, Goncalves LM, Afonso de Almeida H, Pereira AL, Oliveira EM. Incidence of dental caries in students after topical application of acidulated phosphate fluoride with or without fluoride mouthwashes; comparative study [Incidencia de carie dentaria em escolares apos aplicacoes topicas de fluor fosfato acidulado precedida ou nao de bochechos fluoretados; estudo comparativo]. Revista do Centro de Ciencias Biomedicas da Universidade Federal de Uberlandia 1985;1:43‐9. [PubMed] [Google Scholar]
Ashley 1977 {published data only}
- Ashley FP, Mainwaring PJ, Emslie RD, Naylor MN. Clinical testing of a mouthrinse and a dentifrice containing fluoride. A two‐year supervised study in school children. British Dental Journal 1977;143:333‐8. [DOI] [PubMed] [Google Scholar]
Axelsson 1987 {published data only}
- Axelsson P, Paulander J, Nordkvist K, Karlsson R. Effect of fluoride containing dentifrice, mouthrinsing, and varnish on approximal dental caries in a 3‐year clinical trial. Community Dentistry and Oral Epidemiology 1987;15:177‐80. [DOI] [PubMed] [Google Scholar]
Axelsson 1987a {published data only}
- Axelsson P, Paulander J, Nordkvist K, Karlsson R. Effect of fluoride containing dentifrice, mouthrinsing, and varnish on approximal dental caries in a 3‐year clinical trial. Community Dentistry and Oral Epidemiology 1987;15:177‐80. [DOI] [PubMed] [Google Scholar]
Blinkhorn 1983 {published and unpublished data}
- Blinkhorn AS, Holloway PJ, Davies TG. Combined effects of a fluoride dentifrice and mouthrinse on the incidence of dental caries. Community Dentistry and Oral Epidemiology 1983;11:7‐11. [DOI] [PubMed] [Google Scholar]
DePaola 1980 {published data only (unpublished sought but not used)}
- DePaola PF, Soparkar M, Leeuwen M, DeVelis R. The anticaries effect of single and combined topical fluoride systems in school children. Archives of Oral Biology 1980;25:649‐53. [DOI] [PubMed] [Google Scholar]
- Lu KH, Porter DR, Pickles TH. Separate and combined cariostatic effects of fluoride gel and rinse [abstract No 239]. Journal of Dental Research 1980;59:947. [Google Scholar]
Mainwaring 1978 {published data only}
- Mainwaring PJ, Naylor MN. A three‐year clinical study to determine the separate and combined caries‐inhibiting effects of sodium monofluorophosphate toothpaste and an acidulated phosphate‐fluoride gel. Caries Research 1978;12:202‐12. [DOI] [PubMed] [Google Scholar]
Marthaler 1970 {published data only}
- Marthaler TM, Konig KG, Muhlemann HR. The effect of a fluoride gel used for supervised toothbrushing 15 or 30 times per year. Helvetica Odontologica Acta 1970;14:67‐77. [PubMed] [Google Scholar]
Marthaler 1970a {published data only}
- Marthaler TM, Konig KG, Muhlemann HR. The effect of a fluoride gel used for supervised toothbrushing 15 or 30 times per year. Helvetica Odontologica Acta 1970;14:67‐77. [PubMed] [Google Scholar]
Petersson 1985 {published data only}
- Petersson LG, Koch G, Rasmusson CG, Stanke H. Effect on caries of different fluoride prophylactic programs in preschool children. A two year clinical study. Swedish Dental Journal 1985;9:97‐104. [PubMed] [Google Scholar]
Ringelberg 1979 {published data only}
- Ringelberg ML, Webster DB. Effects of an amine fluoride mouthrinse and dentifrice on the gingival health and the extent of plaque of school children. Journal of Periodontology 1977;48:350‐3. [DOI] [PubMed] [Google Scholar]
- Ringelberg ML, Webster DB, Dixon DO. Effects of an amine fluoride dentifrice and mouthrinse on the dental caries of school children after 18 months. Journal of Preventive Dentistry 1978;5:26‐30. [PubMed] [Google Scholar]
- Ringelberg ML, Webster DB, Dixon DO, Fairchild S, Driscoll WS. Results of gingival, plaque, and stain assessments after 30 months use of amine fluorides and their inorganic counterparts. Pharmacology and Therapeutics in Dentistry 1979;4:27‐31. [PubMed] [Google Scholar]
- Ringelberg ML, Webster DB, Dixon DO, LeZotte DC. The caries‐preventive effect of amine fluorides and inorganic fluorides in a mouthrinse or dentifrice after 30 months of use. Journal of the American Dental Association 1979;98:202‐8. [DOI] [PubMed] [Google Scholar]
Triol 1980 {published data only (unpublished sought but not used)}
- Triol CW, Kranz SM, Volpe AR, Frankl SN, Alman JE, Allard RL. Anticaries effect of a sodium fluoride rinse and a MFP dentifrice in a nonfluoridated water area: a thirty‐month study. Journal of Clinical Preventive Dentistry 1980;2:13‐5. [Google Scholar]
References to studies excluded from this review
Bohannan 1985 {published data only}
- Preventing tooth decay: results from a four‐year national study. Princetown (NJ): The Robert Wood Johnson Foundation; 1983. Special Report No. 2.
- Bell RM, Klein SP, Bohannan HM, Disney JA, Graves RC, Madson R. Treatment effects in the national preventive dentistry demonstration program. Santa Monica (CA): The Rand Corporation; 1984. Report No. R‐3072‐RWJ.
- Bohannan HM, Klein SP, Disney JA, Bell RM, Graves RC, Foch CB. A summary of the results of the National Preventive Dentistry Demonstration Program. Journal of the Canadian Dental Association 1985;51:435‐41. [PubMed] [Google Scholar]
- Klein SP, Bohannan HM, Bell RM, Disney JA, Foch CB, Graves RC. The cost and effectiveness of school‐based preventive dental care. American Journal of Public Health 1985;75:382‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Louw 1995 {published data only}
- Louw AJ, Carstens IL, Hartshorne JE, Blignaut RJ. Effectiveness of two school‐based caries preventive programmes. Journal of the Dental Association of South Africa 1995;50:43‐9. [PubMed] [Google Scholar]
Wilson 1978 {published data only (unpublished sought but not used)}
- Wilson CJ, Triol CW, Volpe AR. The clinical anticaries effect of a fluoride dentifrice and mouthrinse [abstract]. Journal of Dental Research 1978;57:276. [Google Scholar]
References to studies awaiting assessment
Torell 1969 {published data only}
- Torell P. The use of fluoride toothpaste combined with fluoride rinsing every two weeks [Bruk av fluortandkram i samband med fluorskoljning varannan vecka]. Sver Tandlakarforb Tidningen 1969;61:873‐5. [Google Scholar]
Additional references
Burt 1998
- Burt BA. Prevention policies in the light of the changed distribution of dental caries. Acta Odontologica Scandinavica 1998;56:179‐86. [DOI] [PubMed] [Google Scholar]
CDT‐ADA 1988
- Anonymous. Report of workshop aimed at defining guidelines for caries clinical trials: superiority and equivalency claims for anticaries dentifrices. Council on Dental Therapeutics. Journal of the American Dental Association 1988;117:663‐5. [DOI] [PubMed] [Google Scholar]
Chen 1995
- Chen MS. Oral health of disadvantaged populations. In: Cohen LK, Gift CH editor(s). Disease Prevention and Oral Health Promotion. Copenhagen: Munksgaard, 1995:152‐212. [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Assessment of study quality. Cochrane Reviewers' Handbook version 4.1 [updated June 2000]; Section 6. The Cochrane Collaboration, 2000. [Google Scholar]
Downer 1995
- Downer MC. The 1993 national survey of children's dental health. British Dental Journal 1995;178:407‐12. [DOI] [PubMed] [Google Scholar]
Dubey 1965
- Dubey SD, Lehnhoff RW, Radike AW. A statistical confidence interval for true per cent reduction in caries‐incidence studies. Journal of Dental Research 1965;44:921‐3. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Featherstone 1988
- Featherstone JDB, Cate JM. Physicochemical aspects of fluoride‐enamel interactions. In: Ekstrand J, Fejerskov O, Silverstone LM editor(s). Fluoride in Dentistry. Copenhagen: Munksgaard, 1988:125‐49. [Google Scholar]
Glass 1982
- Glass RL. The first international conference on the declining prevalence of dental caries. Journal of Dental Research 1982;61:1301‐83. [Google Scholar]
Horowitz 1980
- Horowitz HS. Combinations of caries preventive agents and procedures. Journal of Dental Research 1980;59:2183‐9. [Google Scholar]
Marinho 1997
- Marinho VC. A systematic review of the effectiveness of topical fluoride therapy in the prevention of dental caries in children and adolescents [dissertation]. London: University of London 1997.
Marinho 2002
- Marinho VCC, Higgins J, Logan S, Sheiham A. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 2. [Art. No.: CD002280. DOI: 10.1002/14651858.CD002280] [DOI] [PubMed] [Google Scholar]
Marinho 2002a
- Marinho VCC, Higgins J, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 3. [Art. No.: CD002279. DOI: 10.1002/14651858.CD002279] [DOI] [PubMed] [Google Scholar]
Marinho 2003
- Marinho VCC, Higgins J, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 1. [Art. No.: CD002278. DOI: 10.1002/14651858.CD002278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2003a
- Marinho VCC, Higgins J, Logan S, Sheiham A. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD002284. DOI: 10.1002/14651858.CD002284] [DOI] [PubMed] [Google Scholar]
Marinho 2003b
- Marinho VCC, Higgins J, Logan S, Sheiham A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD002782. DOI: 10.1002/14651858.CD002782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marthaler 1971
- Marthaler TM. Confidence limits of results of clinical caries tests with fluoride administration. Caries Research 1971;5:343‐72. [DOI] [PubMed] [Google Scholar]
Marthaler 1990
- Marthaler TM. Cariostatic efficacy of the combined use of fluorides. Journal of Dental Research 1990;69 Spec No:797‐800. [DOI] [PubMed] [Google Scholar]
Marthaler 1994
- Marthaler TM, Steiner M, Menghini G, Bandi A. Caries prevalence in Switzerland. International Dental Journal 1994;44:393‐401. [PubMed] [Google Scholar]
Marthaler 1996
- Marthaler TM, O'Mullane DM, Vrbic V. The prevalence of dental caries in Europe 1990‐1995. ORCA Saturday afternoon symposium 1995. Caries Research 1996;30:237‐55. [DOI] [PubMed] [Google Scholar]
Moynihan 1996
- Moynihan PJ, Holt RD. The national diet and nutrition survey of 1.5 to 4.5 year old children: summary of the findings of the dental survey. British Dental Journal 1996;181:328‐32. [DOI] [PubMed] [Google Scholar]
Murray 1991
- Murray JJ, Rugg‐Gunn AJ, Jenkins GN. A history of water fluoridation. In: Murray JJ, Rugg‐Gunn AJ, Jenkins GN editor(s). Fluorides in caries prevention. Oxford: Wright, 1991:7‐37. [Google Scholar]
Murray 1991a
- Murray JJ, Rugg‐Gunn AJ, Jenkins GN. Fluoride toothpastes and dental caries. In: Murray JJ, Rugg‐Gunn AJ, Jenkins GN editor(s). Fluorides in caries prevention. Oxford: Wright, 1991:127‐60. [Google Scholar]
O'Mullane 1995
- O'Mullane DM. Contribution of fluoride toothpastes to oral health. In: Bowen WH editor(s). Relative efficacy of sodium fluoride and sodium monofluorophosphate as anti‐caries agents in dentifrices. The Royal Society Of Medicine Press Limited, 1995:3‐7. [Google Scholar]
Pitts 2001
- Pitts NB, Evans DJ, Nugent ZJ. The dental caries experience of 5‐year‐old children in Great Britain. Surveys coordinated by the British Association for the Study of Community Dentistry in 1999/2000. Community Dental Health 2001;18(1):49‐55. [PubMed] [Google Scholar]
Pitts 2002
- Pitts NB, Evans DJ, Nugent ZJ, Pine CM. The dental caries experience of 12‐year‐old children in England and Wales. Surveys coordinated by the British Association for the Study of Community Dentistry in 2000/2001. Community Dental Health 2002;19(1):46‐53. [PubMed] [Google Scholar]
Ripa 1991
- Ripa LW. A critique of topical fluoride methods (dentifrices, mouthrinses, operator‐applied, and self‐applied gels) in an era of decreased caries and increased fluorosis prevalence. Journal Of Public Health Dentistry 1991;1:23‐41. [DOI] [PubMed] [Google Scholar]
Rolla 1991
- Rolla G, Ogaard B, Cruz R‐d‐A. Clinical effect and mechanism of cariostatic action of fluoride‐containing toothpastes: a review. International Dental Journal 1991;41:171‐4. [PubMed] [Google Scholar]
Seppa 1998
- Seppa L, Karkkainen S, Hausen H. Caries frequency in permanent teeth before and after discontinuation of water fluoridation in Kuopio, Finland. Community Dentistry and Oral Epidemiology 1998;26:256‐62. [DOI] [PubMed] [Google Scholar]
Sharp 1998
- Sharp S. Meta‐analysis regression: statistics, biostatistics, and epidemiology 23 (sbe23). Stata Technical Bulletin 1998; Vol. 42:16‐22.
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
van Rijkom 1998
- Rijkom HM, Truin GJ, 't Hof MA. A meta‐analysis of clinical studies on the caries‐inhibiting effect of fluoride gel treatment. Caries Research 1998;32:83‐92. [DOI] [PubMed] [Google Scholar]


