Abstract
Background
Topical fluorides in the form of toothpaste, mouthrinse, varnish and gel are effective caries preventive measures. However, there is uncertainty about the relative value of these interventions.
Objectives
To compare the effectiveness of one form of topical fluoride intervention with another when used for the prevention of dental caries in children.
Search methods
We searched the Cochrane Oral Health Group's Trials Register (May 2000), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2000, Issue 2), MEDLINE (1966 to January 2000), plus several other databases. We handsearched journals, reference lists of articles and contacted selected authors and manufacturers.
Selection criteria
Randomized or quasi‐randomized controlled trials with blind outcome assessment, comparing fluoride varnish, gel, mouthrinse, or toothpaste with each other in children up to 16 years during at least 1 year. The main outcome was caries increment measured by the change in decayed, missing and filled tooth surfaces (D(M)FS).
Data collection and analysis
Inclusion decisions, quality assessment and data extraction were duplicated in a random sample of one third of studies, and consensus achieved by discussion or a third party. Authors were contacted for missing data. The primary measure of effect was the prevented fraction (PF) that is the difference in mean caries increments between the 'experimental' and 'control' groups expressed as a percentage of the mean increment in the control group. Random‐effects meta‐analyses were performed where data could be pooled.
Main results
There were 17 studies included, and 15 contributed data for the meta‐analyses. Fluoride toothpaste was not significantly different from mouthrinse (pooled DMFS PF 0%; 95% CI, ‐18% to 19%; P = 0.94), or gel (pooled DMFS PF 0%; 95% CI, ‐21% to 21%; P = 1), or both gel and mouthrinse (pooled DMFS PF 1%; 95% CI, ‐13% to 14%; P = 0.94); heterogeneity was substantial. Results from the single trial comparing toothpaste with varnish (in deciduous teeth) were inconclusive (dfs PF 5%; CI not obtainable). The pooled results from the comparisons of fluoride varnish with mouthrinse was a non‐significant difference favouring varnish (DMFS PF 10%; 95% CI, ‐12% to 32%; P = 0.40), but this result was not robust to sensitivity analysis performed, and heterogeneity was considerable. Results from the single trial comparing varnish with gel (14%, 95% CI, ‐12% to 40%; P = 0.30) and the single trial comparing gel with mouthrinse (‐14% DMFS PF; 95% CI, ‐40% to 12%; P = 0.30) were inconclusive (favoured varnish and mouthrinse respectively).
Authors' conclusions
Fluoride toothpastes in comparison to mouthrinses or gels appear to have a similar degree of effectiveness for the prevention of dental caries in children. There is no clear suggestion that fluoride varnish is more effective than mouthrinses and the evidence for the comparative effectiveness of fluoride varnishes and gels, and mouthrinses and gels is inconclusive. No conclusions about adverse effects could be reached, because no data were reported on in the trials. Acceptance is likely to be greater for fluoride toothpaste.
Plain language summary
One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents
Topical fluorides such as mouthrinses and gels do not appear to be more effective at reducing tooth decay in children and adolescents than fluoride toothpaste. Tooth decay (dental caries) is painful, expensive to treat and can seriously damage teeth. Fluoride is a mineral that prevents tooth decay. Fluoride is added to the water supply in many areas. It can also be applied in the form of toothpastes, mouthrinses, gels or varnishes. The review of trials found that fluoride toothpastes, mouthrinses and gels reduce tooth decay in children and adolescents to a similar extent. However, toothpastes are more likely to be regularly used. There is no strong evidence that varnishes are more effective than other types of topical fluoride.
Background
Dental caries and its consequences pose important and uncomfortable problems in all industrialized societies and in a large number of developing countries. Although the prevalence and severity of dental caries in most industrialized countries have decreased substantially in the past 2 decades, reaching averages as low as 1.1 decayed, missing and filled teeth (DMFT) in 12 year olds, nearly half of those without any tooth decay or fillings (Marthaler 1996), this largely preventable disease is still common, increases significantly with age, and remains a public health problem for a significant proportion of the world population (Burt 1998). In the United Kingdom, 30% of 3.5 to 4.5 year olds (Moynihan 1996), and 50% of 12 year olds (Downer 1995) had experienced caries in 1993. In 2000, the figures were 40% for 5 year olds in Great Britain (Pitts 2001) and 38% for 12 year olds in England and Wales (Pitts 2002). These findings demonstrate the continuing need for effective preventive strategies and treatment services for these age groups in a country that has experienced a substantial caries decline. In general, dental caries levels vary considerably between and within different countries, but children in the lower socio‐economic status (SES) groups have higher caries levels than those in the upper SES groups, and these differences are consistent in industrialized and in urbanized developing countries (Chen 1995).
Fluoride therapy has been the cornerstone of caries‐preventive strategies since the introduction of water fluoridation schemes over 5 decades ago (Murray 1991). Fluoride controls the initiation and progression of carious lesions. Intensive laboratory and epidemiological research on the mechanism of action of fluoride in preventing caries indicates that fluoride's predominant effect is topical, which occurs mainly through promotion of remineralization of early caries lesions and by reducing sound tooth enamel demineralization (Featherstone 1988). Various modes of fluoride use have evolved, each with its own recommended concentration, frequency of use, and dosage schedule. The use of topically applied fluorides in particular, which are much more concentrated than the fluoride in drinking water, has increased over recent decades and fluoride containing toothpastes (dentifrices), mouthrinses, gels and varnishes are the modalities most widely used at present, either alone or in different combinations. By definition, the term 'topically applied fluoride' describes those delivery systems which provide fluoride to exposed surfaces of the dentition, at elevated concentrations, for a local protective effect and are therefore not intended for ingestion. Fluoride gels and varnishes are typical methods of professional topical fluoride application and both delivery systems have been used in preventive programs. Fluoride gels have also been used as a self‐applied intervention in such programs. Fluoride mouthrinses and toothpastes are the main forms of self‐applied fluoride therapy. The intensive use of fluoride mouthrinsing in school programs has been discontinued in many developed countries because of doubts regarding its cost‐effectiveness at a low prevalence of dental caries and are being replaced by selective fluoride therapy directed to high risk children. Such procedures usually involve the combined use of fluoride toothpastes with gels or varnishes. Toothpaste is by far the most widespread form of fluoride usage (Murray 1991a; Ripa 1991) and the decline in the prevalence of dental caries in developed countries has been mainly attributed to its increased use (Glass 1982; Rolla 1991; Marthaler 1994; O'Mullane 1995; Marthaler 1996).
However, there is currently a debate regarding the appropriate use of fluorides. The lower caries prevalence now prevailing in many countries and the widespread availability of fluoride from multiple sources have raised the question of whether topically applied fluorides are still effective in reducing caries, and safe, mainly in terms of the potential risk of fluorosis (mottled enamel) (Ripa 1991). In this context, even the need for selective professional fluoride applications has been questioned (Seppa 1998). The persistence of this debate and the variations in the use of the main forms of topically applied fluorides suggest the need to search for meaningful ways to summarize the empirical findings on this topic systematically.
If topical fluorides remain effective it will then become relevant to assess which form is best by directly comparing the various treatments currently used, since no consensus on which one, if any, is the most effective can be found in the literature. It is of clinical importance not only to assess the relative effectiveness of the modalities used for professional topical fluoride applications (varnishes and gels), but also to compare the effect of fluoride mouthrinses with that of professionally‐applied fluoride gels or varnishes, and the effect of fluoride toothpastes with that of any other commonly used topical fluoride intervention, since toothpaste use is the most popular method of fluoride application. If the various topical fluoride treatments are shown to be equally effective in controlled trials, the choice of modality will then depend on safety, acceptance and ease of application (and cost).
Over the past half‐century, numerous clinical trials have investigated the anti‐caries effect of each topical fluoride intervention, and their effectiveness has been widely recognised. It appears that most of the trials have focused on topical fluoride in one form or another and that a small number of such trials have investigated the relative effectiveness of the main topical fluoride modalities. Although the evidence on the effect of topical fluorides on the prevention of dental caries in children has been extensively reviewed in a number of reviews, there has been no systematic investigation directly comparing the different topical fluoride interventions currently used in caries prevention.
With regard to the clinical effectiveness of topical fluoride therapy (TFT) in the form of toothpastes, mouthrinses, gels and varnishes three basic questions can be asked: (1) Is TFT effective in preventing dental caries in children and adolescents? (2) Is one of these forms of TFT more effective than another? (3) Are combinations of these TFT forms more effective than one form used alone? This review attempts to answer the second question; the other two questions are addressed in separate reviews.
Objectives
The primary objective of this systematic review is to assess the evidence on the comparative effectiveness of topical fluoride therapy (TFT) in the form of toothpastes, mouthrinses, gels and varnishes in the prevention of dental caries in children and adolescents. The specific objectives are. (1) To determine whether there is a differential effect between any two forms of TFT described above (how each intervention compares with the other). (2) To determine whether there is a differential effect between professionally‐applied topical fluoride varnishes and professionally‐applied gels. (3) To determine whether there is a differential effect between fluoride mouthrinses and professionally‐applied TFT (varnishes or gels). (4) To determine whether there is a differential effect between fluoride toothpastes and any other modality of TFT (mouthrinses, gels or varnishes).
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials using or indicating blind outcome assessment, in which one form of topical fluoride therapy (TFT) (either as toothpaste, mouthrinse, gel or varnish) is compared with another (head to head), during at least 1 calendar or school year. Randomized or quasi‐randomized controlled trials using within group paired comparison designs (e.g. split‐mouth trials involving fluoride varnish, as the effect of the varnish could spread across the mouth leading to contamination of control sites), or with open outcome assessment or no indication of blind assessment, or lasting less than 1 calendar or school year, or controlled trials where random or quasi‐random allocation was not used or indicated were excluded.
Types of participants
Children or adolescents aged 16 or less at the start of the study (irrespective of initial level of dental caries, background exposure to fluorides, dental treatment level, nationality, setting where intervention is received or time when it started). Studies where participants were selected on the basis of special (general or oral) health conditions were excluded.
Types of interventions
Topical fluoride therapy in the form of toothpastes, mouthrinses, gels or varnishes only, using any fluoride agent (which may be formulated with any compatible abrasive system, in the case of fluoride toothpastes), at any concentration (ppm F), amount or duration of application, and with any technique or method of application, provided the frequency of application was at least once a year. Any of the six possible pair‐wise comparisons of the four modalities are eligible for inclusion in the review. Studies where the intervention consisted of any caries preventive agent/procedure (e.g. same or other fluoride‐based measures, anti‐plaque or anti‐calculus agents, sealants, oral hygiene interventions, xylitol chewing gums, glass ionomers) used in addition to any form of TFT described above were excluded.
Types of outcome measures
The primary outcome measure in this review is caries increment, as measured by change from baseline in the decayed, (missing) and filled surface (D(M)FS) index, in all permanent teeth erupted at start and erupting over the course of the study. For studies in younger children the outcome measure of interest is caries increment in deciduous tooth surfaces, as measured by change in the decayed, (missing/extraction indicated), and filled surface d(e/m)fs index. Dental caries is defined here as being clinically and radiographically recorded at the dentin level of diagnosis. (SeeMethods for the different ways of reporting the decayed, (missing) and filled teeth or surfaces (D(M)FT/S) scores in clinical trials of caries preventives.)
The following outcomes were considered relevant: coronal dental caries and dental fillings, in both the permanent and the deciduous dentitions; tooth loss; proportion of children developing new caries; dental pain/discomfort; specific side effects (fluorosis, tooth staining/discoloration, oral allergic reactions, adverse symptoms such as nausea, vomiting); unacceptability of preventive treatment as measured by drop outs during the trial (in non‐placebo controlled studies); use of health service resources (such as visits to dental care units, length of dental treatment time). Studies reporting only on changes in plaque/calculus formation, plaque regrowth/vitality, plaque/salivary bacterial counts, or gingival bleeding/gingivitis, dentin hypersensitivity or fluoride physiological outcome measures (fluoride uptake by enamel or dentin, salivary secretion levels, etc.) were excluded.
Search methods for identification of studies
With a comprehensive search, we attempted to identify all relevant studies irrespective of language, from 1965 onwards.
Electronic searching
Up to 1998
Relevant studies were identified (for the series of topical fluoride reviews) by searching several databases from date of inception: MEDLINE (1966 to 1997), EMBASE (1980 to 1997), SCISEARCH (1981 to 1997), SSCISEARCH (1981 to 1997), ISTP (1982 to 1997), BIOSIS (1982 to 1997), CINAHL (1982 to 1997), ERIC (1966 to 1996), DISSERTATION ABSTRACTS (1981 to 1997) and LILACS/BBO (1982 to 1997). Two overlapping but complementary subject search phrases (Appendix 1) with low specificity (but high sensitivity), using 'free text 'and 'controlled vocabulary', were formulated within Silverplatter MEDLINE around two main concepts, fluoride and caries, and combined with all three levels of the Cochrane Optimal Search Strategy for Randomized Controlled Trials (RCTs). These subject search phrases were customised for searching EMBASE and the other databases.
RCT filters were also adapted to search EMBASE, BIOSIS, SCISEARCH, DISSERTATION ABSTRACTS, and LILACS/BBO. All the strategies (subject search and methodological filters) developed to search each database are fully described in a report produced for the Systematic Reviews Training Unit (Marinho 1997), and are available on request. These were used for the development of a register of topical fluoride clinical trials for the systematic reviews, as the Cochrane Oral Health Group's Trials Register was not yet developed in 1997/98.
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 1997, Issue 1), the Community of Science database (1998), which included ongoing trials funded by the National Institute of Dental Research (NIDR), the System for Information on Grey Literature in Europe (SIGLE) database (1980 to 1997), and OLDMEDLINE (1963 to 1965) were searched using the terms 'fluor' and 'carie' truncated. (Grey literature search had also been carried out by searching the Index to Scientific and Technical Proceedings (ISTP) and DISSERTATION ABSTRACTS.)
From 1999 to 2001
The strategy included in Appendix 2 was used to search LILACS/BBO in 1999 (1982 to 1998), where free text subject search terms were combined with a methodological filter for RCTs.
Four supplementary and more specific subject search phrases (including 'free text' and 'controlled vocabulary' terms), refined exclusively for the reviews on the effects of individual fluoride modalities, formulated around three concepts each (the relevant topical fluoride therapy (TFT), fluoride and caries) were used to search Silverplatter MEDLINE (up to January 2000) without methodological filters (Appendix 3). These strategies were adapted to search the Cochrane Oral Health Group's Trials Register (up to May 2000), and have also been run on CENTRAL (The Cochrane Library 2000, Issue 2) to double check.
The metaRegister of Current Controlled Trials was searched in October 2001 for ongoing RCTs using the terms 'fluoride' and 'caries'.
Reference searching
All eligible trials retrieved from the searches, meta‐analyses and review articles located up to January 2000 were checked for relevant references. Reviews had been identified mainly by a MEDLINE search strategy specifically carried out to provide information on available systematic reviews or meta‐analyses and on the scope of the literature on the topic, when the Cochrane Database of Systematic Reviews (CDSR), and the Database of Abstracts of Reviews of Effects (DARE) and NHS Economic Evaluation Database (NHSEED), were also searched. Reference lists of relevant chapters from preventive dentistry textbooks on topically applied fluoride interventions were also consulted.
Full text searching
Prospective handsearching of the seven journals identified as having the highest yield of eligible RCTs/controlled clinical trials (CCTs) was carried out, from January 1999 until January 2000: British Dental Journal, Caries Research, Community Dentistry and Oral Epidemiology, Journal of the American Dental Association, Journal of Dental Research, Journal of Public Health Dentistry and European Journal of Oral Sciences. The handsearch of Community Dentistry and Oral Epidemiology was undertaken (1990 to December 1999), as this was the journal with the highest yield of eligible reports.
Personal contact
Searching for unpublished studies (or 'grey' literature such as technical reports and dissertations, or studies published in languages other than English which may not have been indexed to major databases) started by contacting experts in the field of preventive dentistry. A letter was sent to the author(s) of each included study published during the last 2 decades in order to obtain information on possible unpublished studies eligible for inclusion. All the authors of studies who had been contacted in order to clarify reported information to enable assessment of eligibility or obtain missing data were also asked for unpublished studies.
Based on information extracted mainly from included studies, a list of manufacturers of fluoride toothpastes, mouthrinses, gels and varnishes was created for locating unpublished trials. Letters to manufacturers were sent out by the Cochrane Oral Health Group, in the hope that companies might be more responsive to contact from the editorial base than from individual reviewers. Fourteen manufacturers were contacted (October 2000) and information on any unpublished trials requested: Bristol‐Myers Co, Colgate‐Palmolive, Davies Rose‐Hoyt Pharmaceutical Division, Gaba AG, Ivoclar North America, John O Butler Company, Johnson & Johnson, Oral‐B Laboratories, Pharmascience, Procter & Gamble, Smithkline Beecham, Synthelabo, Unilever/Gibbs, Warner‐Lambert.
Data collection and analysis
Management of records produced by the searches
Because multiple databases were searched, the downloaded set of records from each database, starting with MEDLINE, was imported to the bibliographic software package Reference Manager and merged into one core database to remove duplicate records and to facilitate retrieval of relevant articles. The records yielded from LILACS, BBO, CENTRAL, SIGLE and NIDR databases were not imported to Reference Manager and were checked without the benefit of eliminating duplicates. The records produced by OLDMEDLINE and by the specific MEDLINE search performed without methodological filter were imported to Reference Manager for inspection, in a database separate from the core database. The records produced by searching the Cochrane Oral Health Group's Trials Register and the metaRegister of Current Controlled Trials were also checked outside Reference Manager. In order to facilitate inspection of all records located from searching other (non‐electronic) sources (reference lists of relevant studies, review articles and book chapters, journal handsearch, personal contact), we also tried to locate them in MEDLINE and to import them to Reference Manager. Those references that could not be downloaded in this way were entered manually.
Relevance assessment
All records identified by the searches were printed off and checked on the basis of title first, then by abstract (when this was available in English or in languages known by the reviewer) and/or keywords by one reviewer, Valeria Marinho (VM). Records that were obviously irrelevant were discarded and the full text of all remaining records was obtained. Records were considered irrelevant according to study design/duration, participants, or interventions/comparisons (if it could be determined that the article was not a report of a randomized/quasi‐randomized controlled trial; or the trial was of less than 6 to 8 months duration; or the trial was exclusively in adults; or the trial did not address at least two of the relevant topical fluoride treatments; or the trial did not compare one topical fluoride with another).
Selection of studies for inclusion
With the inclusion criteria form previously prepared and pilot tested, one reviewer (VM) assessed all studies for inclusion in the review, and a second reviewer, Julian Higgins (JH), independently duplicated the process for a sample of those (approximately 30%). In addition, any study that could not be classified by the first reviewer was independently assessed by the second. A third reviewer was consulted, Stuart Logan (SL) or Aubrey Sheiham (AS), to resolve any disagreement. It was decided in advance to exclude any trial where agreement could not be reached (but this did not occur). Trial reports thought to be potentially relevant in languages not known by the reviewers were translated and the reviewer (VM) completed the inclusion criteria form with reference to the translator. Attempts were made to contact authors of trials that could not be classified in order to ascertain whether inclusion criteria were met.
It was considered essential to identify and check all reports related to the same study; in case of any discrepancy, authors were contacted.
Data extraction
Data from all included studies were extracted by one reviewer (VM) using a pilot tested data extraction form. A second reviewer (JH) extracted data from a random sample of approximately one third of included studies. Again, data that could not be coded by the first reviewer were independently coded by the second, any disagreements were discussed and a third reviewer consulted to achieve consensus where necessary. (In future updates all reports will be data extracted and quality assessed in duplicate.) Data presented only in graphs and figures were extracted whenever possible, but were included only if two reviewers independently had the same result. Attempts were made to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. Papers in languages not known by the reviewers were data extracted with help from appropriate translators.
Additional information related to study methodology or quality that was extracted included: study duration (years of follow up); comparability of baseline characteristics: methods used pre‐randomization in sizing/balancing (stratification based on relevant variables) or used post‐randomization in analysing/adjusting for possible differences in prognostic factors between groups; objectivity/reliability of primary outcome measurement (diagnostic methods and thresholds/definitions used and included, and monitoring of diagnostic errors); any co‐intervention and/or contamination. Information on sponsoring institutions and manufacturers involved was also recorded.
Characteristics related to participants that were extracted included: age (range) at start, caries severity at start (average DMFS, DFS, or other measure), background exposure to fluoride sources other than the study option(s) (in water, topical applications, etc.), year study began, place where study was conducted (country), setting where participants were recruited, and dental treatment level (F/DMF). Characteristics of the interventions that were extracted included: fluoride modality(s), mode of application (how the intervention was delivered), methods (technique/device) of application, prior‐ and post‐application, fluoride active agents and concentrations used, frequency and duration of application, and amount applied.
Different ways of assessing/reporting caries increment in the trials (change from baseline as measured by the DMF index) were recorded separately and/or combined according to the components of the index chosen and units of measurement (DMFT/S, or DFT/S, or DT/S, or FT/S), types of tooth/surface considered (permanent/deciduous teeth/surfaces, first molar teeth, approximal surfaces, etc.), state of tooth eruption considered (erupted and/or erupting teeth or surface), diagnostic thresholds used (cavitated/dentin lesions, non‐cavitated incipient lesions), methods of examination adopted (clinical and/or radiographic), and approaches to account or not for reversals in caries increment adopted (in a net or observed/crude caries increment respectively). In addition, caries increments have been recorded whenever the authors reported them (various follow ups), and where assessments of caries increments were made during a post‐intervention follow‐up period, the length of time over which outcomes were measured after the intervention ended was noted.
As we were aware that caries increment could be reported differently in different trials we developed a set of a priori rules to choose the primary outcome data for analysis from each study: data on surface level would be chosen over data on tooth level; DFS data would be chosen over DMFS data, and this would be chosen over DS or FS; data for 'all surface types combined' would be chosen over data for 'specific types' only; data for 'all erupted and erupting teeth combined' would be chosen over data for 'erupted' only, and this over data for 'erupting' only; data from 'clinical and radiological examinations combined' would be chosen over data from 'clinical' only, and this over 'radiological' only; data for dentinal/cavitated caries lesions would be chosen over data for enamel/non‐cavitated lesions; net caries increment data would be chosen over crude (observed) increment data; and follow up nearest to 3 years (often the one at the end of the treatment period) would be chosen over all other lengths of follow up, unless otherwise stated. When no specification was provided with regard to the methods of examination adopted, diagnostic thresholds used, groups of teeth and types of tooth eruption recorded, and approaches for reversals adopted, the primary choices described above were assumed.
All other relevant outcomes assessed/reported in the trials were also recorded/listed.
Quality assessment
The methodological quality of the included studies was assessed according to the criteria for concealment of treatment allocation described in the Cochrane Reviewers' Handbook (Clarke 2000) used in the Cochrane Review Manager software (RevMan). Allocation concealment for each trial was rated as belonging to one of three categories. (A) Adequately concealed (an adequate method to conceal allocation is described). (B) Concealment unclear ('random' allocation stated/indicated but the actual allocation concealment method is not described or an apparently adequate concealment scheme is reported but there is uncertainty about whether allocation is adequately concealed). (C) Inadequately concealed (an inadequate method of allocation concealment is described). Excluded: random (or quasi‐random) allocation clearly not used in the trial, or 'random' allocation not stated and not implied/possible.
Blinding of main outcome assessment was also rated according to the following three categories defined for the topical fluoride reviews. (A) Double‐blind (blind outcome assessment and use of placebo/blinding of participants described). (B) Single‐blind (blind outcome assessment stated and no placebo used/participants not blind). (C) Blinding indicated (blind outcome assessment not stated but likely in any element/phase of outcome assessment, e.g. clinical and/or radiographic examinations performed independently of previous results, or radiographic examinations performed independently of clinical examinations with results reported separately/added later, or examiners clearly not involved in giving treatment, or use of placebo described) or reported but unclear (blind outcome assessment reported but there is information that leads to suspicion/uncertainty about whether the examination was blind). Excluded: clearly open outcome assessment used or blind outcome assessment not reported and unlikely (no description of an examination performed independently of previous results, of x‐rays registered independently of clinical examination, of use of a placebo, and of examiners clearly not involved in giving treatment).
One reviewer (VM) assessed the quality of all included studies. A second reviewer (JH) duplicated the process for a random sample of approximately one third. Any disagreement was discussed and where necessary a third reviewer was consulted to achieve consensus. Where uncertainty could not be resolved an effort was made to contact authors directly to clarify the method used to conceal allocation or whether assessment of the main outcome had been carried out blind.
Checking of interobserver reliability was limited to these validity assessments.
Other methodological characteristics of the trials such as completeness of follow up (proportion excluded) and handling of exclusions (extent to which reasons for attrition are explicitly reported, or losses are independent of treatment allocated) were not used as thresholds for inclusion. However, all assessments of study quality are described in the table of included studies, and were coded for possible use in metaregression/sensitivity analyses. (For example, sensitivity analyses could be performed to assess the impact of blind outcome assessment and concealment of allocation, since studies where blinding is not clearly stated (but likely) and studies reporting inadequate allocation concealment are also included in this review.)
Analyses
Handling of missing main outcome data
It was decided that missing standard deviations for caries increments that were not revealed by contacting the original researchers would be imputed through linear regression of log standard deviations on log mean caries increments. This is a suitable approach for caries prevention studies since, as they follow an approximate Poisson distribution, caries increments are closely related (similar) to their standard deviations (van Rijkom 1998).
Handling of results (main outcome) of studies with more than one treatment arm
For studies with more than two‐arms, where the same topical fluoride therapy (TFT) form is compared in two or more 'experimental' groups (for example, different active agents or concentrations of fluoride ion are compared for the same modality of TFT to a common 'control' group), raw results (the numbers, mean caries increments and standard deviations) from all relevant 'experimental' groups were combined in order to obtain a measure of treatment effect (this enables the inclusion of all relevant data for each form of TFT in the meta‐analyses).
Choice of measure of effect and meta‐analyses of main outcome
The chosen measure of treatment effect was the prevented fraction (PF), that is (mean increment in the 'controls' minus mean increment in the 'experimental' group) divided by mean increment in the 'controls'. For an outcome such as caries increment (where discrete counts are considered to approximate to a continuous scale and are treated as continuous data) this measure was considered more appropriate than the mean difference or standardised mean difference, since it allows combination of different ways of measuring caries increment and a meaningful investigation of heterogeneity between trials. It is also simple to interpret.
The meta‐analyses were conducted as inverse variance weighted averages. Within‐study variances were estimated using the formula presented in Dubey 1965 which was more suitable for use in a weighted average, and for large sample sizes the approximation should be reasonable. Random‐effects meta‐analyses were performed throughout in RevMan/RevMan Analyses.
Deciduous and permanent teeth were analysed separately throughout.
For illustrative purposes, when overall results were significant, the results were also presented as the number of children needed to treat (NNT) to prevent one carious teeth/surface. These were calculated by combining the overall prevented fraction with an estimate of the caries increment in the 'control' groups of the individual studies.
Assessment of heterogeneity and investigation of reasons for heterogeneity
Heterogeneity in the results of the trials was assessed by inspection of a graphical display of the estimated treatment effects from the trials along with their 95% confidence intervals and by formal tests of homogeneity (Thompson 1999).
Statistically significant heterogeneity was investigated using metaregression when a meta‐analysis included a sufficiently large number of studies. In addition to aspects of study quality, potential sources of heterogeneity investigated would include baseline levels of caries severity and exposure to fluoride sources other than the study options. The association of these factors with estimated effects (D(M)FS PFs) would be examined by performing random‐effects metaregression analyses in Stata version 6.0 (Stata Corporation, USA) using the program Metareg (Sharp 1998).
Investigation of publication and other biases
A funnel plot (plots of treatment effect estimates versus the inverse of their standard errors) was drawn. Asymmetry of the funnel plot may indicate publication bias and other biases related to sample size, though may also represent a true relationship between trial size and effect size. A formal investigation of the degree of asymmetry was performed using the method proposed by Egger et al (Egger 1997).
Measures of effect and meta‐analysis of other outcomes
For outcomes other than caries increment, continuous data would be analysed according to differences in mean treatment effects and their standard deviations. Dichotomous outcome data were analysed by calculating risk ratios (RR) or, for adverse effects of fluoride treatment, risk differences (RD). RevMan was used for estimation of overall treatment effects. Again, a random‐effects model was used to calculate a pooled estimate of effect. NNT was calculated when overall results were significant. As a general rule only (relevant) outcomes with useable data were shown in the analyses tables.
Results
Description of studies
Search results
Our initial multiple database search (1997/98) produced the following total number of records, according to database searched: MEDLINE, 4599; EMBASE, 5052; BIOSIS, 421; SCISEARCH, 514; SSCISEARCH, 169; ISTP, 66; CINAHL, 133; ERIC, 60; DISSERTATION ABSTRACTS, 95; LILACS, 48; BBO, 47; CENTRAL, 86; SIGLE, 6. Searching OLDMEDLINE produced 545 records, and the Community of Science database, 24 records. In the second stage of searches (1999), searching LILACS and BBO with a modified search strategy produced 210 records (142 and 68 records respectively). The more specific MEDLINE searches (by individual modalities of topical fluoride therapy (TFT)) performed without a randomized controlled trial (RCT) filter produced 2441 records, and the searches performed in the Cochrane Oral Health Group's Trials Register (May 2000) produced 479 records. Searching the metaRegister of Current Controlled Trials for ongoing studies produced 5 records. Many records retrieved through electronic search were duplicates merged later in the core database, and many appeared more than once in different databases and/or searches performed (overlapped).
Searching other non‐electronic sources (reference lists of potentially relevant reports, review articles or book chapters, relevant journals, and contacting authors) produced 171 additional records for inspection. (Any search results produced by contacting manufacturers will feature in updates of this review.)
Relevance assessment results
When all the records produced by the searches above were screened, a total of 713 reports were identified as potentially eligible and further assessment was sought.
Study selection results
Two (2) full text reports could not be obtained (these were incomplete references of unpublished studies/grey literature). Six hundred and seventy‐five (675) reports were considered immediately irrelevant for this review, largely as a result of the types of intervention compared with (or used in addition to) a relevant TFT (including placebo or no treatment control trials without a relevant head to head comparison(s) of one TFT with another), and due to the types of study design described.
Thus, 28 studies (37 reports) are considered/cited in this review. These comprise 24 reports relating to 17 included studies, 10 reports relating to nine excluded studies, and three reports relating to two studies waiting assessment (either because they require translation (Polish) or because additional information has not been obtained for one study in abstract form). There are no reports of ongoing studies. Six non‐English reports (five studies) listed either under included or excluded studies have been fully assessed: two in French (by a French translator, with the contact reviewer), one in Portuguese (by the contact reviewer), and three in Russian (by a translator, with the reviewer).
Excluded studies
SeeCharacteristics of excluded studies table for the description of reasons for rejecting each study.
We have excluded two studies comparing fluoride varnish with gel, three comparing varnish with mouthrinse, three comparing toothpaste with mouthrinse, and one comparing fluoride varnish, gel and mouthrinse (there were no excluded studies comparing varnish with toothpaste or gel with toothpaste).
These nine studies were excluded for a variety of reasons. One study used open outcome assessment. One study randomized three clusters, each to one of the three groups compared. Four studies did not mention or indicate random or quasi‐random allocation and blind outcome assessment; one of these also did not report main outcome data for one group and another reported post‐treatment effects only. One study did not mention or indicate random or quasi‐random allocation (but described blind outcome assessment); attempts to contact the authors of this study were unsuccessful and it was excluded. Two studies had other intervention in addition to one of the relevant TFTs, and one of these did not describe the use of blind outcome assessment.
Included studies
SeeCharacteristics of included studies table for details of each study.
There are 17 trials included. The study conducted by Marthaler 1970 was treated as two independent trials because the results for the two age groups involved were reported separately as distinct studies. The study by Koch 1967 presented results by age group for one treatment arm but not the other, so we treated it as a single study, combining the age subgroups for the toothpaste arm. The 17 trials were conducted between 1962 and 1994: four during the 1960s, nine in the 1970s, three in the 1980s and one in the 1990s. Two trials were conducted in USA, three in UK, two in Switzerland, five in Sweden, two in Denmark, two in Finland, and one in Israel. Four studies had more than one publication, two of these had four published reports each. All 24 reports were published between 1965 and 1995. Five studies acknowledged assistance (product provision, etc.) and/or financial support from manufacturers. Of a total of four studies whose authors were sent request letters for unpublished information, replies related to two studies were obtained.
Design and methods
All the 17 included studies used parallel group designs. Five studies had more than two arms; in three of these there were two groups of one of the modalities of TFT being compared, and in the other two studies there were two groups (study arms) of each of the two modalities being compared. There was no study comparing more than two relevant modalities of TFT. Ten trials used inactive/placebo interventions for the head to head comparisons (i.e. were organized on a double‐blind basis) and the remaining seven used no treatment (simple head to head comparisons of the TFTs only). Study duration ranged from 1 to 3 years, with only two studies lasting less than 2 years. Studies were generally large with only four allocating less than 190/200 children to relevant study groups (and eight studies involving over 300 participants); all but one study recruited children from school settings.
Interventions
There are six trials comparing fluoride toothpaste with mouthrinse, four comparing toothpaste with gel (one involving operator‐applied gel, and three self‐applied gel), one comparing toothpaste with varnish, four comparing varnish with mouthrinse, one comparing varnish with gel (operator‐applied gel), and one comparing mouthrinse with gel (self‐applied gel). The fluoride concentration in the 11 trials with a toothpaste arm was similar (ranged from 1000 to 1200 ppm F in the toothpastes), and in four of these toothbrushing was performed under supervision (at school). In all six trials with a fluoride varnish arm the varnish application was semi‐annual, and all but one tested a 22,600 ppm F (Duraphat). The fluoride concentration in all six trials with a fluoride gel arm was also similar (12,300/12,500 ppm F), but frequency of gel application varied from twice (operator‐applied) to 25 times a year (self‐applied). There was variation in both the fluoride concentration (100,230/250,900 ppm F) and frequency of application (daily, weekly, fortnightly) in the trials involving fluoride mouthrinsing.
Participants
Participants were aged 14 or less at the start (in all trials), with similar numbers from both sexes (where these data were reported). At least 10 trials included children who were around 12 at the start, and only one trial (Petersson 1985) involved pre‐school children. Caries prevalence at baseline, reported in all but two of the studies, ranged from 0.9 to 15 D(M)FS and was 0.9 dfs in the study by Petersson. Fifteen studies reported exposure or not to water fluoridation, and only one of these was conducted in a fluoridated community. Background exposure to fluoride toothpaste (or other sources of topical fluoride) was not clearly reported in the majority of studies.
Outcome measures
Caries increment: all trials reported caries increment data (or data from which these could be derived) at the tooth surface level (D(M)FS was reported in 16 trials, and defs in one trial), and three trials reported caries increment at the tooth level (D(M)FT). With regard to the components of the DMFS index used (and types of teeth/surfaces assessed), six trials reported DMFS data (for all tooth surface types), 10 trials reported DFS data (for all tooth surface types) and one trial reported DS data (for approximal surfaces of premolars and molars only). No choice had to be made between DMFS or DFS data in any one trial. Eight trials presented D(M)FS data at more than one follow‐up time. In one trial, assessment of D(M)FS increments were also made during a post‐intervention follow‐up period. Many trials presented results using one caries grade only (usually CA/ER or CA/DR), others either did not report the grade, or reported caries increment data at both levels of diagnosis, in which case CA was chosen. Data on the state of tooth eruption considered were not clearly specified in many trials.
The table Characteristics of included studies provides a description of all the main outcome data reported from each study with the primary measure chosen featuring at the top.
Other dental caries data reported: caries incidence/attack rate (two trials), caries progression (one trial), and proportion of children developing new caries (two trials).
Data on adverse effects: stain score (one trial), signs of sensitivity in oral soft tissue (one trial), any side effects (one trial, without complete or useable data, and with the following statement: "no side effects observed in both groups"). Fluorosis data have not been reported in any of the trials.
Data for unacceptability of treatment (as measured by drop outs/exclusions) were reported in eight trials.
Risk of bias in included studies
Based on 28 studies included in the topical fluoride reviews and randomly selected for assessment of reproducibility and agreement between two reviewers, interrater reliability was excellent (89%) for both allocation concealment and blinding, and agreement was good for allocation (Kappa = 0.61) and very good for blinding (Kappa = 0.73).
There was a considerable variation in the quality of the studies in this review (using the reported information and additional information obtained from investigators).
Allocation concealment
Fourteen trials were described as randomized but provided no description on how the 'random' allocation was done and were coded B, two trials were considered to be quasi‐randomized and were coded C, in one trial allocation concealment was considered adequate by consensus (coded A).
Blinding
Double‐blinding was described in eight trials (code A), single‐blinding (blind outcome assessment described but no placebo used) was described in seven trials (code B), and blind outcome assessment was indicated in two trials (code C) which described the use of placebo.
Loss to follow up
Seventy‐three per cent (73%) of the participants originally enrolled in the studies were included in the final analysis (3243 analysed out of 4423 initially randomized). These data exclude six of the 17 included studies, which provided no information on the number of participants randomized to relevant groups. Drop‐out rates were obtained from all but one of the 17 included studies and ranged from 2% at 2 years to 39% at 3 years. The most common reason for attrition was that participants were not available for follow‐up examination at the end of the study.
Other methodological features
Cluster randomization was used in three trials which compared fluoride varnishes to fluoride mouthrinses (Bruun 1985; Kirkegaard 1986; Seppa 1987) where school classes were used as units of randomization and children used as units of analysis. Individuals were allocated to study arms in all other trials, and each participant's caries incidence, over a period of time was used as the unit of analysis.
Baseline comparisons and handling of any differences: one trial described as 'balanced' (for which randomization may have succeeded to produce nearly exact balance) did not report any of the actual values for the baseline characteristics (such as initial caries levels). Some degree of imbalance was reported in a few trials (for characteristics considered most influential, usually initial caries levels) and generally either described as not significant or that adjustment had resulted in trivial differences in effect estimates.
Objectivity/reliability of primary outcome measurement: diagnostic methods used (clinical or radiographic) were described in all studies, but thresholds/definitions used for caries and monitoring of diagnostic errors were not always reported (see 'Notes' in the Characteristics of included studies table for methodological features assessed).
Effects of interventions
Effect on dental caries
Pooled estimates of the relative effects of topical fluoride therapy (TFT) are presented for caries increment in the permanent dentition as Decayed, (Missing) and Filled Surfaces Prevented Fraction (D(M)FS PF). Estimates for caries increment in the deciduous dentition are presented as decayed, (missing/extraction indicated), and filled surfaces Prevented Fraction (d(m/e)fs PF).
Sixteen studies provided data suitable for meta‐analysis. Standard deviations (SD) of mean caries increment data (new D(M)FS) were missing in two of the 16 studies (Axelsson 1987; Ran 1991). From the analysis of the 179 available treatment arms for the topical fluoride reviews with complete information (as of October 1999) we derived a regression equation log (SD caries increment) = 0.64 + 0.55 log (mean caries increment), (R2 = 77%). This equation was used to estimate missing standard deviations from mean D(M)FS increments for the meta‐analyses. The single study reporting caries increment in deciduous tooth surfaces (Petersson 1985) did not provide standard deviations of mean caries increment (new dfs) either, and is not included in the analysis of D(M)FS PF (no caries increment data for the permanent dentition).
We have decided to exclude the trial of Ran 1991 (comparing fluoride toothpaste with gel) from any analysis because the DMFS increment in the fluoride gel arm of the trial was very small, resulting in a poor estimate of PF. Thus, 15 studies are included in the meta‐analyses.
The results are reported separately here for the following main comparisons: (1) Fluoride varnish versus gel (1 trial) (2) Fluoride varnish versus mouthrinse (4 trials) (3) Fluoride varnish versus toothpaste (1 trial, incomplete data, for deciduous tooth surfaces only) (4) Fluoride toothpaste versus gel (3 trials) (5) Fluoride toothpaste versus mouthrinse (6 trials) (6) Fluoride gel versus mouthrinse (1 trial) (7) Fluoride toothpaste versus any TFT ‐ D(M)FS data available for comparisons with fluoride gel (3 trials) and fluoride mouthrinse (6 trials), but not for comparison with fluoride varnish.
Objective 1 (comparative effect of any two forms of TFT) is addressed in comparisons (1) to (6). The only comparison of fluoride gel versus varnish involved professional application of both, so comparison 1 (of fluoride varnish versus gel) under Objective 1 in effect addresses Objective 2 as well (comparative effect of operator‐applied varnish and operator‐applied gel). Because the only comparison of fluoride gel versus mouthrinse involved self‐application of fluoride gel, comparison 2 (of fluoride mouthrinse versus varnish) under Objective 1 is the only comparison, which, in effect, addresses Objective 3 (comparative effect of fluoride mouthrinse and professionally‐applied TFT). Finally, Objective 4 (comparative effect of fluoride toothpaste and other TFTs) is addressed in comparison 7.
Relatively few trial reports provided data able to contribute to meta‐analysis and with the exception of three trials, which were not carried out on a double‐blind basis (Torell 1965; Koch 1967, Seppa 1987), all reported equivocal results for caries reductions, i.e. no demonstrated differential effect. Apart from the division of trials into those comparing fluoride toothpaste with gel or mouthrinse in comparison (7), no subgroup analyses were performed due to the lack of an appropriate volume of data. No metaregression and funnel plot analyses were performed either, on the grounds of insufficient data.
(1) Fluoride varnish versus fluoride gel (Objectives 1 and 2)
Only one trial (Seppa 1995) compared fluoride varnish with fluoride gel (n = 254). Analysis of this trial showed a non‐significant effect in favour of fluoride varnish and a wide confidence interval for the estimate of effect. The D(M)FS prevented fraction was 0.14 (95% CI, ‐0.12 to 0.40; P = 0.30), suggesting that there is insufficient evidence from this trial to confirm or refute a differential effect in caries reduction.
(2) Fluoride varnish versus fluoride mouthrinse (Objectives 1 and 3)
Four trials compared fluoride varnish with fluoride mouthrinse (n = 952). The D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis of all four trials combined was 0.10 (95% CI, ‐0.12 to 0.32; P = 0.40), a non‐significant effect in favour of fluoride varnish and a wide confidence interval for the pooled estimate of effect. Heterogeneity in the results could be observed graphically and statistically (Chi2 = 11.13 on 3 degree of freedom, P = 0.01) and according to the I2 heterogeneity statistic the extent of heterogeneity (or lack of consistency) in results is indeed large (I2 = 73%). If a fixed‐effect meta‐analysis is performed, however, the result becomes statistically significant and the two models still come out with similar answers, even though the differential effect is larger in the fixed‐effect meta‐analysis. The D(M)FS prevented fraction pooled estimate from the fixed‐effect meta‐analysis was 0.15 (95% CI, 0.04 to 0.26; P = 0.007).
We performed further sensitivity analysis to take account of the additional uncertainty we should have about the three cluster randomized trials (Bruun 1985; Kirkegaard 1986; and Seppa 1987) in this comparison. We inflated the variance of the prevented fraction estimates in these trials by an amount equal to (1 + (m‐1) * ICC), where m is the average cluster size and ICC the intraclass correlation coefficient. A conservative value of 0.1 for the ICC was used since we could not find an ICC from these or any similar trials. The D(M)FS PF pooled estimate (random‐effects meta‐analysis) was 0.14 (95% CI, ‐0.06 to 0.34; P = 0.16). It may be noted that although heterogeneity in these results could not be detected by the standard Chi2 test (Chi2 = 5.86 on 3 degrees of freedom, P = 0.12), this was not due to homogeneity (I2 = 49%). Nevertheless, these results are similar to the analysis ignoring the cluster randomized design (though not identical, since the estimates for these trials differ from the meta‐analysis result), showing a non‐significant differential effect in favour of fluoride varnishes, but less heterogeneity in results. Again, if a fixed‐effect meta‐analysis is performed the result becomes statistically significant and the two models still come out with similar answers (and a larger differential effect is shown again). The D(M)FS prevented fraction pooled estimate from the fixed‐effect meta‐analysis was 0.19 (95% CI, 0.06 to 0.32; P = 0.005).
Double‐blinding (use of placebo rather than no treatment comparisons) may represent a valid indicator of study quality and source of heterogeneity in the topical fluoride reviews (Marinho 2003). If further sensitivity analysis is carried out (in the original data, ignoring the cluster randomized design) excluding the two trials which are not double‐blind, no significant differences are detected. For the two double‐blind trials combined (Bruun 1985; Kirkegaard 1986) the D(M)FS prevented fraction pooled estimate (random‐effects meta‐analysis) was ‐0.12 (95% CI, ‐0.32 to 0.08; P = 0.23), a non‐significant difference in the opposite direction, in favour of fluoride mouthrinse. Heterogeneity in the results could not be observed graphically nor statistically (Chi2 = 0.50 on 1 degree of freedom, P = 0.48; I2 = 0). The revised meta‐analysis yielded an estimate of effect which differed from the overall estimate, indicating the results are not robust and may be distorted by the lesser quality trials. Similar findings are obtained when we use the inflated variances for these two trials: the D(M)FS prevented fraction pooled estimate (random‐effects meta‐analysis) was ‐0.12 (95% CI, ‐0.40 to 0.17; P = 0.43), a non‐significant difference in favour of fluoride mouthrinse. Heterogeneity in the results was not detected.
(3) Fluoride varnish versus fluoride toothpaste (Objective 1)
The single trial (Petersson 1985) comparing fluoride varnish with fluoride toothpaste (n = 183) assessed the relative effect in terms of caries increment in deciduous surfaces and provided no standard deviations or data from which these could be derived. It reported a negligible dfs PF of ‐0.05 (CI not obtainable).
It may be noted that this trial also reported on the proportion of children developing one or more new caries in deciduous tooth surfaces. Exactly the same proportions were reported in both groups (no evidence of a difference).
(4) Fluoride toothpaste versus fluoride gel (Objective 1)
Three trials compared fluoride toothpaste with fluoride gel (n = 1256). The D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis of the three trials combined was 0.00 (95% CI, ‐0.21 to 0.21; P = 1.00), i.e. absolutely no differences in effect and a relatively wide confidence interval. Heterogeneity was not significant according to the standard Chi2 test, but the test would have minimal power to detect heterogeneity (Chi2 = 3.34 on 2 degree of freedom, P = 0.19), which is actually indicated graphically and shown to be moderately large according to the I2 heterogeneity statistic (I2 = 40%).
(5) Fluoride toothpaste versus fluoride mouthrinse (Objective 1)
The six trials comparing fluoride toothpaste with mouthrinse (n = 2545) showed no differences in effect and substantial heterogeneity in results (I2 = 85%). The D(M)FS prevented fraction was 0.00 (95% CI, ‐0.18 to 0.19; P = 0.97) and the Chi2 test for heterogeneity was 32.35 on 5 degrees of freedom (P < 0.00001).
There was one trial reporting on the proportion of children developing one or more new caries in permanent tooth surfaces (Torell 1965). It reported a non‐significant risk ratio (RR) of 0.90 (95% CI, 0.60 to 1.37) in favour of toothpaste.
(6) Fluoride gel versus fluoride mouthrinse (Objective 1)
Only one trial (DePaola 1980) compared fluoride gel with fluoride mouthrinse (n = 257). It showed a non‐significant effect in favour of fluoride mouthrinse and a wide confidence interval for the estimate of effect. The D(M)FS prevented fraction was ‐0.14 (95% CI, ‐0.40 to 0.12; P = 0.30) suggesting that there is insufficient evidence from this trial to confirm or refute a differential effect in caries reduction.
(7) Fluoride toothpaste versus any TFT (Objective 4)
For all nine trials combined (three comparing fluoride toothpaste with gel and six with mouthrinse; n = 3801), the D(M)FS prevented fraction pooled estimate from the random‐effects meta‐analysis was 0.01 (95% CI, ‐0.13 to 0.14; P = 0.94), i.e., no significant differences were detected. Heterogeneity in results was significant (Chi2 = 36.28 on 8 degrees of freedom, P < 0.0001), and substantial (I2 = 78%). Results for the separate subsets comparing toothpaste with either gel or mouthrinse (described above) are consistent with no evidence of a differential effect.
Effect on other oucomes
Data for unacceptability of treatment were reported in eight trials that reported drop outs. Each of the eight trials reported equivocal results for this outcome, i.e. no demonstrated differential effect. Meta‐analysis results for these are described below.
Fluoride varnish versus fluoride mouthrinse
The pooled estimate (random‐effects meta‐analysis) of the risk ratio (RR) of dropping out from the fluoride varnish group as opposed to the mouthrinse arm in the two trials that reported drop outs was 1.19 (95% CI, 0.86 to 1.65) in favour of fluoride mouthrinse, but no significant differences were detected. Using alternative measures of effect has given similar results (OR 1.26, CI 0.82 to 1.94). Heterogeneity was not detected in these results (Chi2 = 0.15 on 1 degree of freedom, P = 0.70; I2 = 0%).
Fluoride varnish versus fluoride toothpaste
There was one trial only reporting drop outs in this comparison. The risk ratio (RR) of dropping out from the fluoride varnish group as opposed to the toothpaste arm of the trial was 1.28 (95% CI, 0.37 to 4.41) in favour of fluoride toothpaste, but no significant differences were detected and the confidence interval was wide.
Fluoride toothpaste versus fluoride mouthrinse
The pooled estimate (random‐effects meta‐analysis) of the risk ratio (RR) of dropping out from the fluoride toothpaste group as opposed to the fluoride mouthrinse arm in the five trials that reported drop outs was 0.89 (95% CI, 0.78 to 1.00; P = 0.05) in favour of fluoride toothpaste. Heterogeneity was not detected in these results (Chi2 = 2.61 on 4 degrees of freedom, P = 0.63; I2 = 0%). Using alternative measures of effect has given similar results (OR 0.83, CI 0.70 to 1.00; RD ‐0.03, CI ‐0.06 to 0.00).
None of the trials in the other pairwise comparisons reported drop outs fully.
Fluoride toothpaste versus any TFT
The pooled estimate (random‐effects meta‐analysis) of the risk ratio (RR) of dropping out from the fluoride toothpaste group as opposed to the other TFT arm (fluoride varnish (one trial), mouthrinse (five trials)) in the six trials that reported drop outs was 0.88 (95% CI, 0.78 to 1.00; P = 0.05) in favour of fluoride toothpaste. Heterogeneity was not detected in these results (Chi2 = 2.65 on 5 degrees of freedom, P = 0.75; I2 = 0%). Using alternative measures of effect has given similar results (OR 0.83, CI 0.70 to 0.99; RD ‐0.02, CI ‐0.05 to 0.00).
Discussion
Topical fluorides in the form of toothpastes, mouthrinses, varnishes and gels are effective caries preventive interventions. The effectiveness of each of these has been assessed fully in four previous systematic reviews in this series (Marinho 2002; Marinho 2002a; Marinho 2003; Marinho 2003a). Compared with placebo or no treatment the average DMFS prevented fractions ranged from 24% for fluoride toothpaste, through 26% for mouthrinses and 28% for gels, to 46% for fluoride varnish. These conclusions were made on a clearer basis in placebo controlled trials (Marinho 2003b). For example, the first review in the series focused on the effectiveness of fluoride gels and reported a pooled PF on permanent tooth surfaces of 21% based on 14 placebo‐controlled studies (Marinho 2002). In terms of absolute caries reductions per year in D(M)FS increments (in populations with D(M)FS increments of around 2), these ranged from 0.46 for fluoride gel to 0.74 for fluoride varnish (mouthrinses 0.56, toothpaste 0.62). There is uncertainty, however, about the relative value of the various topical fluoride treatments.
The main question addressed by this review is how effective the use of one type of topical fluoride therapy (TFT) for the prevention of caries in children is compared to another. The 15 studies included in the meta‐analyses in this review covered nearly all the range of direct head to head comparisons of possible practical value between fluoride toothpastes, mouthrinses, gels and varnishes. Yet, there is a relatively small number of trials in each main comparison/meta‐analysis. The randomized evidence that we have brought together is, as far as we can ensure, the totality of the available randomized evidence comparing the relevant topical fluoride modalities directly. There is a general lack of statistical significance for virtually all meta‐analyses' results in this review. Further, for the great majority of comparisons, the confidence intervals are relatively wide and the variation among the results of the studies can be substantial. This calls for a cautious interpretation of the data.
Our second objective was to assess the relative effectiveness of professionally‐applied gels and varnishes. Based on the results from the single trial comparing fluoride varnish to fluoride gel there is insufficient evidence to confirm or refute a differential effect in caries reduction between these two interventions. Analysis of this trial showed a non‐significant effect in favour of fluoride varnish and a wide confidence interval for the estimate of effect.
A third objective of the review was to examine whether there was a beneficial effect in terms of caries prevention from the use of fluoride mouthrinses (a self‐applied TFT) compared with professionally‐applied TFTs (varnishes or gels). The only available comparison of fluoride gel and mouthrinse, based on a single study, involved self‐application of fluoride gel, and showed a non‐significant effect in favour of fluoride mouthrinse and a wide confidence interval for the estimate of effect. Therefore, the question above is in effect addressed exclusively by the meta‐analysis of five trials of fluoride mouthrinse compared with fluoride varnish. We were unable to detect a clear differential effect from these data. In addition, although the random‐effects meta‐analysis of the five trials produced a non‐significant result (of small magnitude) in favour of varnishes, when the analysis was restricted to the subset of two double‐blind trials (both cluster randomized trials comparing the same fluoride varnish product used semi‐annually with the same fluoride mouthrinse used fortnightly), the difference in effect was reverted in favour of mouthrinses, but still not statistically significant.
It is interesting to compare these results with those of Strohmenger 2001 that carried out a systematic review on the anti‐caries efficacy of fluoride varnishes, using a different and restrictive set of inclusion criteria, which resulted in the analysis of only three studies. All three of these studies were comparisons of fluoride varnishes with fortnightly fluoride mouthrinses at school, and were included in our analyses. Again, although the pooled estimate of the treatment effect in the meta‐analyses by Strohmenger 2001 favoured fluoride varnish, the results were not statistically significant at the 0.05 level.
The final objective of the review was a comparison between fluoride toothpastes and any other modality of TFT (mouthrinses, gels or varnishes). The general observation is that there is no indication of an increased benefit with the use of either, toothpaste or the other TFTs. Again, data (for the permanent dentition) were available from trials involving direct comparisons of toothpaste with fluoride mouthrinse and with fluoride gel; relevant comparisons with useable data of fluoride toothpaste and varnish were lacking. Nevertheless, results for the separate subsets and for all the data combined comparing toothpaste with either gel or mouthrinse are consistent with no evidence of an important differential effect.
The results above, based on head to head comparisons in this review, are generally in line with those from a previous systematic review in this series (Marinho 2003b), based on adjusted indirect comparisons from meta‐analyses of all four TFT types in which a large amount of data from placebo/no treatment trials were considered. Results from the adjusted indirect comparisons suggested no significant differences in treatment effects between gel, mouthrinse and toothpaste, but significantly lower D(M)FS prevented fractions for fluoride gel, mouthrinse or toothpaste in comparison with fluoride varnish. However, relatively few fluoride varnish trials were included in the indirect comparison analyses and very few among these were placebo‐controlled trials, making it difficult to rule out the possibility of an overestimation of the size of the differential effect, due to the preponderance of no treatment control fluoride varnish studies of lower methodological quality in the review. Nevertheless, empirical evidence indicates that in most cases results of adjusted indirect comparisons are not significantly different from those from direct comparisons (Song 2003), and when direct evidence is available but insufficient, the adjusted indirect comparison may provide supplementary information (Higgins 1996). Thus, the data available for indirect comparisons could usefully strengthen conclusions based on the pooled results from the direct comparisons in this review, especially when there are concerns about the methodological quality of a few randomized trials. Methods are being developed to formally combine data from direct and indirect evidence (Higgins 2003) and we hope to be able to apply the new methods in future updates of this review.
As was generally the case for other reviews in this series, we found no useful information in the trials about potential adverse effects such as fluorosis, tooth staining, or oral allergic reactions. However, if children are allocated to fluoride toothpaste they appear to be more likely to stay in the study than if they are given alternative forms of topical fluoride therapy.
Authors' conclusions
Implications for practice.
This review has found that compared with each other, fluoride toothpaste and mouthrinse, and toothpaste and gel appear to be effective to a similar degree in the prevention of dental caries in children; the benefits in terms of caries reduction from fluoride mouthrinse compared with gel, fluoride varnish compared with gel, and varnish compared with toothpaste (deciduous teeth only) are unclear. In addition, there is no clear indication from this review that any increased cariostatic effect may accrue from the use of fluoride varnish in comparison to mouthrinse. The general acceptability for fluoride toothpastes is unquestioned by these results. Arguably, fluoride varnishes lead to less fluoride ingestion, which may be of importance in young children, and require less time for application (usually at semi‐annual intervals), but the general lack of data on potential adverse effects and on acceptance for virtually all topical fluoride therapies (TFTs), makes it more difficult to weigh the benefits of using any given topical fluoride against possible shortcomings of the procedure.
Implications for research.
There is a general lack of randomized trial evidence evaluating the comparative effectiveness of the various topical fluorides for the prevention of dental caries in children, and, therefore, a modest difference in treatment effect may have been missed for most relevant comparisons. However, the lack of a clear suggestion of significant benefits from the data analysed from most direct comparisons may not indicate priority for the performance of new studies. Nevertheless, taking the available results from indirect evidence into account as well, there may be a suggestion for the performance of additional larger studies of higher methodological quality to determine whether fluoride varnishes are more effective in caries prevention than other topical fluorides, gels and mouthrinses in particular, since varnishes are already perceived to present some empirical advantages over these treatments.
What's new
| Date | Event | Description |
|---|---|---|
| 29 August 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors wish to thank the Systematic Reviews Training Unit (UCL) and the Cochrane Oral Health Group for the continuous support. The synopsis was provided by the Cochrane Consumer Network, for which we express our thanks. Many thanks are also due to the investigators who have kindly provided additional information about their trials or supplied reports of trials, as well as to those who helped with the translations or provided comments and editorial input to this review.
Appendices
Appendix 1. MEDLINE search strategy
(a) [("DENTAL‐CARIES" explode all subheadings or "DENTAL‐CARIES‐ACTIVITY‐TESTS" all subheadings or "DENTAL‐CARIES‐SUSCEPTIBILITY" all subheadings or CARIE* or DMF*) and (("FLUORIDES" explode all subheadings or "FLUORIDES,‐TOPICAL" explode all subheadings or FLUOR* or AMF or AMINE F OR SNF2 OR STANNOUS F OR NAF OR SODIUM F OR APF OR SMFP OR MFP OR MONOFLUOR*) or ("CARIOSTATIC‐AGENTS" explode all subheadings or "DENTAL‐PROPHYLAXIS" explode all subheadings or "DENTIFRICES" explode all subheadings or "MOUTHWASHES" explode all subheadings or CARIOSTA* or PROPHYLA* or ANTICARI* or ANTI CARI* or VARNISH* or LACQUER* or DURAPHAT or GEL* or TOOTHPASTE* or TOOTH PASTE* or PASTE* or DENTIFRIC* or MOUTHRINS* or MOUTH RINS* or RINS* or MOUTHWASH* or MOUTH WASH*))]. (b) [((explode FLUORIDES/all subheadings) or (explode FLUORIDES‐TOPICAL/ALL SUBHEADINGS) or (FLUOR*) or (AMF or AMINE F OR SNF2 OR STANNOUS F OR NAF OR SODIUM F OR APF OR MFP OR SMFP OR MONOFLUOR* OR DURAPHAT)) and ((CARI*) or (DMF*) or (TOOTH*) or (TEETH*) or (DENT* in TI, in AB, in MESH)) or ((explode CARIOSTATIC‐AGENTS/ all subheadings) or (ANTICARI* or ANTI CARI*) or (explode MOUTHWASHES/all subheadings) or (MOUTHWASH* or MOUTH WASH*) or (MOUTHRINS* or MOUTH RINS*) or (VARNISH* or LACQUER*))].
Appendix 2. LILACS/BBO search strategy
[(fluor$ or ppmf or ppm f or amf or snf or naf or apf or mfp or smfp or monofluor$ or duraphat$) and (carie$ or dmf$ or cpo$ or tooth$ or teeth$ or dent$ or dient$ or anticarie$ or cario$ or mouthrins$ or mouth rins$ or rinse$ or bochech$ or enjuag$ or verniz$ or varnish$ or barniz$ or laca$ or gel or gels)] and [random$ or aleatori$ or acaso$ or azar$ or blind$ or mask$ or cego$ or cega$ or ciego$ or ciega$ or placebo$ or(clinic$ and (trial$ or ensaio$ or estud$)) or (control$ and (trial$ or ensaio$ or estud$))].
Appendix 3. Supplementary MEDLINE search strategy
[(CARIE* or (DENT* near CAVIT*) or TOOTH* DECAY* or DMF* or (explode "DENTAL‐CARIES"/ALL SUBHEADINGS)) and (FLUOR* or APF* or NAF* or AMINE F OR SNF* or ACIDULATED* PHOSPHATE* FLUORID* or ACIDULATED* FLUORID* or PHOSPHATE* FLUORID* or SODIUM* FLUORID* or AMINE* FLUORID* or STANNOUS* FLUORID* or (explode "FLUORIDES"/ALL SUBHEADINGS)) and (1) (TOOTHPASTE* or TOOTH* PASTE* or DENTIFRICE* or PASTE*) or (explode "DENTIFRICES"/all subheadings)]. (2) ((RINS* or MOUTH* RINS* or WASH* or MOUTH* WASH*) or (MOUTHRINS* or MOUTHWASH*)) or (explode "MOUTHWASHES"/all subheadings)]. (3) (FLUOR* or ELMEX* or (explode "FLUORIDES"/ALL SUBHEADINGS)) and (GEL* or TRAY*)]. (4) (FLUOR* or (DURAPHAT* or FLUOR PROTECTOR*) or (explode "FLUORIDES"/ALL SUBHEADINGS)) and (VARNISH*) or (LACQUER* or LAQUER*) or (VERNIZ*) or (LACKER*) or (LAKK*) or (SILANE* or POLYURETHANE*)].
Data and analyses
Comparison 1. Fluoride varnish versus Fluoride gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years (1 trial) | 1 | 254 | Prevented Fraction (Random, 95% CI) | 0.14 [‐0.12, 0.40] |
1.1. Analysis.
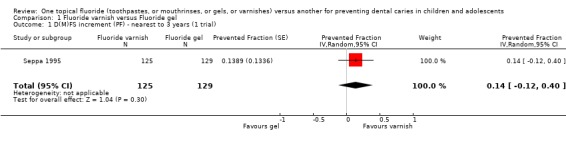
Comparison 1 Fluoride varnish versus Fluoride gel, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years (1 trial).
Comparison 2. Fluoride varnish versus Fluoride mouthrinse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years (4 trials) | 4 | 952 | Prevented Fraction (Random, 95% CI) | 0.10 [‐0.12, 0.32] |
| 2 Unacceptability of treatment as measured by leaving study early (2 trials) | 2 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.85, 1.64] |
2.1. Analysis.
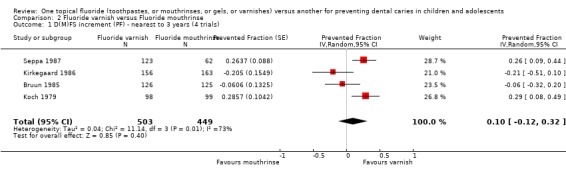
Comparison 2 Fluoride varnish versus Fluoride mouthrinse, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years (4 trials).
2.2. Analysis.
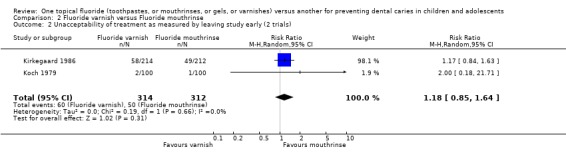
Comparison 2 Fluoride varnish versus Fluoride mouthrinse, Outcome 2 Unacceptability of treatment as measured by leaving study early (2 trials).
Comparison 3. Fluoride varnish versus Fluoride toothpaste.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 d(e)fs increment (PF) ‐ nearest to 3 years (1 trial) | 1 | 183 | Prevented Fraction (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Unacceptability of treatment as measured by leaving study early (1 trials) | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.37, 4.41] |
3.1. Analysis.
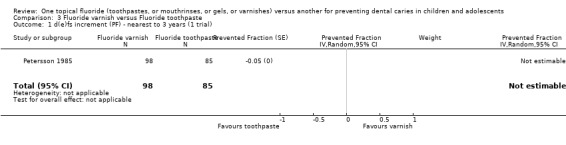
Comparison 3 Fluoride varnish versus Fluoride toothpaste, Outcome 1 d(e)fs increment (PF) ‐ nearest to 3 years (1 trial).
3.2. Analysis.
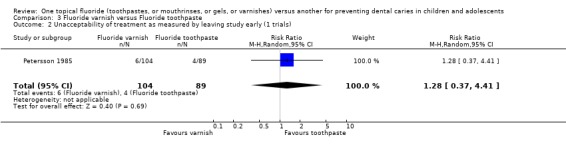
Comparison 3 Fluoride varnish versus Fluoride toothpaste, Outcome 2 Unacceptability of treatment as measured by leaving study early (1 trials).
Comparison 4. Fluoride toothpaste versus Fluoride gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years (3 trials) | 3 | 1256 | Prevented Fraction (Random, 95% CI) | 0.00 [‐0.21, 0.21] |
4.1. Analysis.
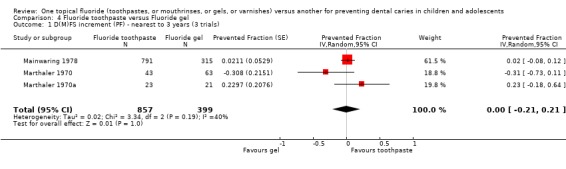
Comparison 4 Fluoride toothpaste versus Fluoride gel, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years (3 trials).
Comparison 5. Fluoride toothpaste versus Fluoride mouthrinse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years (6 trials) | 6 | 2545 | Prevented Fraction (Random, 95% CI) | 0.00 [‐0.18, 0.19] |
| 2 Unacceptability of treatment as measured by leaving study early (5 trials) | 5 | 2752 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.00] |
5.1. Analysis.
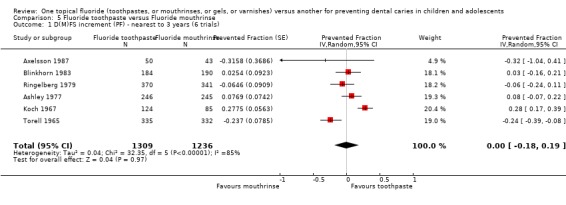
Comparison 5 Fluoride toothpaste versus Fluoride mouthrinse, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years (6 trials).
5.2. Analysis.
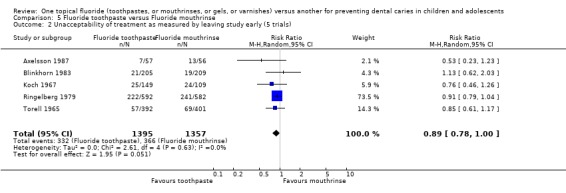
Comparison 5 Fluoride toothpaste versus Fluoride mouthrinse, Outcome 2 Unacceptability of treatment as measured by leaving study early (5 trials).
Comparison 6. Fluoride gel versus Fluoride mouthrinse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years (1 trial) | 1 | 257 | Prevented Fraction (Random, 95% CI) | ‐0.14 [‐0.40, 0.12] |
6.1. Analysis.
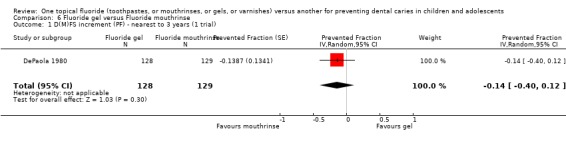
Comparison 6 Fluoride gel versus Fluoride mouthrinse, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years (1 trial).
Comparison 7. Fluoride toothpaste versus Other topical fluoride.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS increment (PF) ‐ nearest to 3 years (9 trials) | 9 | 3801 | Prevented Fraction (Random, 95% CI) | 0.01 [‐0.13, 0.14] |
| 1.1 Fluoride toothpaste versus fluoride gel (3 trials) | 3 | 1256 | Prevented Fraction (Random, 95% CI) | 0.00 [‐0.21, 0.21] |
| 1.2 Fluoride toothpaste versus fluoride mouthrinse (6 trials) | 6 | 2545 | Prevented Fraction (Random, 95% CI) | 0.00 [‐0.18, 0.19] |
| 2 Unacceptability of treatment as measured by leaving study early (6 trials) | 6 | 2945 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 1.00] |
| 2.1 Fluoride toothpaste versus fluoride varnish (1 trial) | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.23, 2.67] |
| 2.2 Fluoride toothpaste versus fluoride mouthrinse (5 trials) | 5 | 2752 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.00] |
7.1. Analysis.
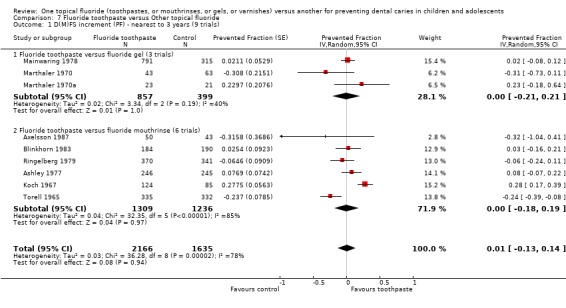
Comparison 7 Fluoride toothpaste versus Other topical fluoride, Outcome 1 D(M)FS increment (PF) ‐ nearest to 3 years (9 trials).
7.2. Analysis.
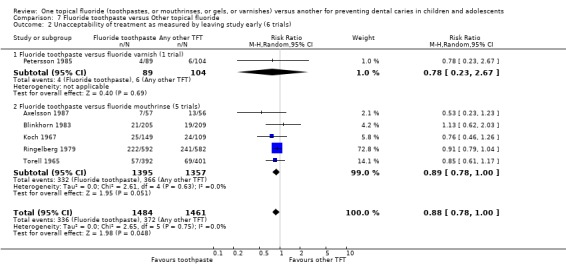
Comparison 7 Fluoride toothpaste versus Other topical fluoride, Outcome 2 Unacceptability of treatment as measured by leaving study early (6 trials).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashley 1977.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 12% drop out (for all study groups combined) after 2 years (study duration = 2 years). Natural losses; any differential group losses not assessable. | |
| Participants | 491 children analysed at 2 years (available at final examination). Average age at start: 12 years. Surfaces affected at start: 8.8 DFS. Exposure to other fluoride: no. Year study began: 1973. Location: UK. | |
| Interventions | FR+PLT versus FT+PLR. NaF group (FR) = 100 ppm F. School mouthrinsing/supervised, daily, 20 ml applied for 1 min (after toothbrushing with placebo toothpaste at school). SMFP group (FT) = 1000 ppm F. School toothbrushing/supervised, daily, 1 g applied for 1 min (followed by rinse with placebo mouthrinse); non‐fluoride toothpaste provided to all for home use. Abrasive system: IMP (main abrasive). |
|
| Outcomes | 2yNetDFS increment ‐ (E+U)(NCA)cl+(ER)xr.
Reported at 2 years follow up. PF‐DFS. MD‐BL‐DFS. MD‐DFS. DFS (U). |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, DFS, DMFS, DMFT) 'balanced'. Clinical (V) caries assessment by one examiner (FOTI used); diagnostic threshold = NCA. Radiographic assessment (postBW) by one examiner; diagnostic threshold = ER. State of tooth eruption included = E/U. Intra‐examiner reproducibility checks for incremental caries data (icc for clinical 0.95, for radiographic 0.8); reversal rate beween 12% and 7% of observed DFS increment in study groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Axelsson 1987.
| Methods | Random allocation; double‐blind (A); placebo‐controlled; 18% drop out after 3 years (study duration = 3 years). Reasons for attrition described with respective total numbers by group; no differential group losses. | |
| Participants | 93 children analysed at 3 years (available at final examination). Age range at start: 13‐14 years. Surfaces affected at start: relevant data NR. Exposure to other fluoride: no. Year study began: 1977. Location: Sweden. | |
| Interventions | FR+PLT versus FT+PLR. NaF group (FR) = 230 ppm F. School mouthrinsing/supervised, weekly. NaF group (FT) = 1000 ppm F. Home toothbrushing/unsupervised, daily frequency assumed (instructed to brush twice a day). Abrasive system: silica. |
|
| Outcomes | 3ypostMD‐DS increment ‐ (ER)xr.
Reported at 3 years follow up. Caries progression. Drop out. |
|
| Notes | Participants randomized (N = 113). Baseline characteristics (DS) 'balanced'. Radiographic assessment (4 postBW) by two examiners; diagnostic threshold = ER. State of tooth eruption included NR. Examiner reproducibility checks for incremental caries data performed (''consistency of duplicate examination reached 94% for scores 1&2 combined''). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Blinkhorn 1983.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 10% drop out after 3 years (study duration = 3 years). Reasons for attrition described with respective total numbers: 57 left school, 12 withdrawn by parents, 6 absent at final examination; no differential group losses. | |
| Participants | 374 children analysed at 3 years (available at final examination). Age range at start: 11‐12 years. Surfaces affected at start: 8.3 DMFS. Exposure to other fluoride: no. Year study began: 1972. Location: UK. | |
| Interventions | FR+PLT versus FT+PLR. NaF group (FR) = 230 ppm F. School mouthrinsing/supervised, daily, for 0.5 min (after toothbrushing with placebo toothpaste at school). SMFP group (FT) = 1000 ppm F. School toothbrushing/supervised, daily, for 1 min (followed by rinse with placebo mouthrinse); appropriate toothpaste provided to all for home use. Abrasive system: IMP (main abrasive). |
|
| Outcomes | 3yNetDFS increment ‐ (E+U)(CA)cl+(DR)xr.
Reported at 3 years follow up. PF‐DFS. MD‐BL‐DFS. MD‐DFS. postMD‐DFS. DMFT (E/U). anterior DMFT. posterior DMFT. DFS (U). Drop out. |
|
| Notes | Participants randomized (N = 414). Baseline characteristics (DMFS, DMFT, SAR) 'balanced' (DFS baseline data NR). Clinical (V) caries assessment by one examiner, diagnostic threshold = CA. Radiographic assessment (1 postBW) by one examiner; diagnostic threshold = DR. State of tooth eruption included = E/U. Intra‐examiner reproducibility checks for incremental clinical and radiographic caries data in 10% sample (icc score 0.9). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bruun 1985.
| Methods | Cluster quasi‐random allocation; double‐blind (A); placebo‐controlled; 30% drop out after 3 years (study duration = 3 years). Natural losses only; any differential group losses not assessable. | |
| Participants | 251 children analysed at 3 years (available at final examination). Average age at start: 11 years. Surfaces affected at start: 5.2 DFS. Exposure to other fluoride: toothpaste assumed. Year study began: in/before 1981. Location: Denmark. | |
| Interventions | FV+PLR versus FR+PLV. NaF group (Fluor Protector® varnish) = 7000 ppm F. Applied twice a year, with soft brush (prior tooth‐cleaning performed). NaF group (FR) = 900 ppm F. School use/supervised, fortnightly, 10 ml applied. |
|
| Outcomes | 3yDFS increment ‐ (CA)(E+U)cl.
Reported at 3 years follow up. DFS(xr). |
|
| Notes | School‐classes randomized (24) and children taken as units for caries increment analyses (N = 359); numbers by group NR. Baseline characteristics (DMFS, erupted surfaces, age, gender) 'balanced'. Clinical (VT) caries assessment by one examiner, diagnostic threshold CA. Radiographic assessment (2 postBW) by one examiner; diagnostic threshold = DR. Diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
DePaola 1980.
| Methods | Random allocation; double‐blind (A); placebo‐controlled; drop‐out rate NR nor obtainable (study duration = 2 years + 1 year post‐study period). Exclusions based on compliance and presence in all follow‐up examinations; any differential group losses not assessable. | |
| Participants | 257 children analysed at 1* year (after exclusions, present for entire study period). Age range at start: 12‐14 years (average = 13). Surfaces affected at start: NR. Exposure to other fluoride: toothpaste assumed. Year study began: in/before 1977. Location: USA. | |
| Interventions | FG+PLR versus FR+PLG. APF group (FG) = 12,300 ppm F. Self‐applied under supervision at school, with tray, 10 consecutive applications (days) in 1st year, applied for 5 min. NaF group (FR) = 230 ppm F. School use/supervised, daily, 10 ml applied for 1 min. |
|
| Outcomes | 1y*NetDFS increment ‐ (CA)cl+xr. Reported at 1 and 2 years follow ups (and 1 year post‐treatment). | |
| Notes | Participants randomized (numbers NR). Baseline characteristics (age, dental age, DFS) described as 'balanced' (values NR). Clinical (VT) caries assessment by two examiners; diagnostic threshold = CA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by two examiners (diagnostic threshold NR); diagnostic errors NR. *Intervention (gel) applied during 1st year of study only (thus, final 2 years results not considered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kirkegaard 1986.
| Methods | Cluster random allocation; double‐blind (A); placebo‐controlled; 25% drop out after 3 years (study duration = 5 years). Natural losses; no differential group losses. | |
| Participants | 319 children analysed at 3 years (available at 3 years examination). Average age at start: 10 years. Surfaces affected at start: 3 DMFS. Exposure to other fluoride: toothpaste assumed. Year study began: 1978. Location: Denmark. | |
| Interventions | FV+PLR versus FR+PLV. NaF group (Duraphat® varnish) = 22,600 ppm F. Applied twice a year, with soft brush, left to dry for 4 min (prior tooth‐cleaning performed). NaF group (FR) = 900 ppm F. School use/supervised, fortnightly, 10 ml applied. |
|
| Outcomes | 3yNetDMFS increment ‐ (E)cl.
Reported at 3 and 5 years follow ups. DMFS (U)(xr). Drop out. |
|
| Notes | School‐classes randomized (22) and children taken as units for caries increment analyses (N = 426). Baseline characteristics (DMFS, erupted surfaces, age) 'balanced'. Clinical (VT) caries assessment by one examiner, diagnostic threshold NR. Radiographic assessment (2 postBW) by one examiner; diagnostic threshold = DR. State of tooth eruption included (E/U). Reproducibility of diagnosis assessed by duplicate clinical and radiographic examination of 10% sample (reliability coefficient 0.90). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Koch 1967.
| Methods | Stratified random allocation***; single‐blind (B); non‐placebo‐controlled; 19% drop out after 3 years (study duration = 3 years + 2 years post‐intervention period). Natural losses; no differential group losses. | |
| Participants | 209 children analysed at 3 years (present for entire trial period). Age range at start: 8‐12 years (average = 10). Surfaces affected at start: 14.6 DFS. Exposure to other fluoride: no. Year study began: 1962. Location: Sweden. | |
| Interventions | FR versus FT. NaF group (FR) = 2250 ppm F. School use/supervised, fortnightly, 10 ml applied for 2 min. NaF group (FT) = 1000 ppm F. School use/supervised, daily, 1 g applied for 2 min; non‐fluoride toothpaste provided to all for home use. Abrasive system: methacrylate polymer (acrylic). |
|
| Outcomes | 3yDFS increment ‐ (CA)(E)cl.
Reported at 1 and 3 years follow ups (and 2 years post‐treatment). DFT. O‐DFS. MD‐DFS. BL‐DFS. CAR (annual). Secondary caries. Oral tissue inflammation (incomplete data). Drop out |
|
| Notes | Participants randomized (N = 258). Baseline characteristics (DFS, DFT, SAR) 'balanced'. Clinical (VT) caries assessment by one examiner; diagnostic threshold = CA; radiographic assessment (2 postBW) used as an aid but not reported; state of tooth eruption included = E. Intra‐examiner reproducibility checks for DFS in 10% sample (icc over 0.98); reversals very small in both groups and equally common. *** Allocation concealment considered adequate by consensus. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Koch 1979.
| Methods | Random allocation; single‐blind (B); non‐placebo‐controlled; 2% drop out after 2 years (study duration = 2 years). Natural losses (all but 3 children completed the study, moved away from the area); no differential group losses. | |
| Participants | 197 children analysed at 2 years (present for entire trial period). Average age at start: 14 years. Surfaces affected at start: 13.1 DFS. Exposure to other fluoride: toothpaste and 0.5 ppm F in water. Year study began: in/before 1976. Location: Sweden. | |
| Interventions | FV versus FR. NaF group (Duraphat® varnish) = 22,600 ppm F. Applied twice a year (0.3 to 0.5 ml), with small brush (prior tooth‐cleaning performed). NaF group (FR) = 900 ppm F. School use/supervised, weekly, 7 ml applied for 1 min. |
|
| Outcomes | 2yDFS increment ‐ (CA)xr.
Reported at 1and 2 years follow ups. DFS(cl). Drop out. |
|
| Notes | Participants randomized (N = 200). Baseline characteristics (DFS) 'balanced'. Clinical (VT) caries assessment by one examiner; diagnostic threshold = CA; radiographic assessment (2 postBW) by two examiners; diagnostic threshold = DR. State of tooth eruption included NR. Diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mainwaring 1978.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 18% drop out (for all study groups combined) after 3 years (study duration = 3 years). Natural losses; any differential group losses not assessable. | |
| Participants | 1106 children analysed at 3 years (available at final examination). Age range at start: 11‐12 years. Surfaces affected at start: 8.1 DFS. Exposure to other fluoride: no. Year study began: in/before 1974. Location: UK. | |
| Interventions | FG+PLT versus FT+PLG (2 groups). APF group (FG) = 12,300 ppm F. Operator‐applied, with tray, twice a year, applied for 4 min (prior toothbrushing with non‐fluoride toothpaste performed). SMFP groups (FT1 & FT2) = 1000 ppm F (but flavours were different). Home use/unsupervised, for 1 min, daily frequency assumed. Abrasive system: Ca carbonate. |
|
| Outcomes | 3yNet/Crude DFS increment ‐ (CA)(E)cl+(ER)xr.
Reported at 3 years follow up. PF‐DFS cl. postMD‐DFS xr. DFS (U) cl+xr. CIR. |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, SAR, DFS) 'balanced'. Clinical (VT) caries assessment by one examiner; diagnostic threshold = CA; state of tooth eruption included = E. Radiographic assessment (2 postBW) by one examiner; diagnostic threshold = ER. Intra‐examiner reproducibility checks for DFS in 10% sample (icc for VT/XR over 0.95); error variance less than 5% of total variance; reversal rate less than 4% of observed DFS increment in all groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marthaler 1970.
| Methods | Random allocation; indication of blind caries assessment (C); placebo‐controlled; 18% drop out (for all study groups combined) after 3 years (study duration = 3 years). Exclusions based on use of orthodontic bands and presence in all follow‐up examinations; any differential group losses not assessable. | |
| Participants | 106 children analysed at 3 years (present for all examinations). Age range at start: 6‐7 years. Surfaces affected: 0.9 DMFS. Exposure to other fluoride: salt. Year study began: 1966. Location: Switzerland. | |
| Interventions | FG+PLT versus FT+PLG. AmF/NaF group (FG) = 12,500 ppm F. Self‐applied under supervision at school, with toothbrush, 20 times a year, 1 g applied for 6 min. AmF group (FT) = 1250 ppm F. Home use/unsupervised, twice/three times a day/680 times a year estimated. Abrasive system: IMP. |
|
| Outcomes | 3yNetDFS increment ‐ (CA)cl+(DR)xr.
Reported at 1 and 3 years follow ups. 1stmPF‐DFS. 1stmMD‐DFS. |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, DFMS, 1stmDMFS) 'balanced'. Clinical (V) caries assessment by two examiners; diagnostic threshold = CA and NCA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by two examiners; diagnostic threshold = DR and ER; partial recording. ''Sufficient agreement of the two examiners known from earlier work''. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marthaler 1970a.
| Methods | Random allocation; indication of blind caries assessment (C); placebo‐controlled; 30% drop out (for all study groups combined) after 4 years (study duration = 4 years). Exclusions based on use of orthodontic bands, and presence in all follow‐up examinations; any differential group losses not assessable. | |
| Participants | 44 children analysed at 2 & 4* years (present for all examinations). Age range at start: 7‐9 years. Surfaces affected: 2.1 DMFS. Exposure to other fluoride: salt. Year study began: 1966. Location: Switzerland. | |
| Interventions | FG+PLT versus FT+PLG. AmF/NaF group (FG) = 12,500 ppm F. Self‐applied under supervision at school, with toothbrush, 22 times a year, 1 g applied for 6 min. AmF group (FT) = 1250 ppm F. Home use/unsupervised, twice/three times a day/800 times a year estimated. Abrasive system: IMP. |
|
| Outcomes | 2y*NetDFS increment ‐ (CA)cl+(DR)xr.
Reported at 2 and 4 years follow ups. 1stmPF‐DFS (CA) cl. 1stmMD‐DFS (DR) xr. |
|
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (age, DMFS, 1stmDMFS) 'balanced'. Clinical (V) caries assessment by two examiners; diagnostic threshold = CA and NCA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by two examiners; diagnostic threshold = DR and ER; partial recording. ''Sufficient agreement of examiners known from earlier work''. *F solution used by all children after 2 years (final 4 years results not considered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Petersson 1985.
| Methods | Quasi‐random allocation; single‐blind (B); non‐placebo‐controlled; 5% drop out after 2 years (study duration = 2 years). Reason(s) for attrition NR; no differential group losses. | |
| Participants | 183 children analysed at 2 years (present for entire trial period). Average age at start: 3 years. Surfaces affected at start: 0.9 dfs (data from original sample only). Exposure to other fluoride: none assumed. Year study began: 1978. Location: Sweden. | |
| Interventions | FV+PLT versus FT alone. NaF group (Duraphat® varnish) = 22,600 ppm F. Applied twice a year. NaF group (FT) = 250 ppm F. Home use/unsupervised, daily frequency assumed. |
|
| Outcomes | 2ydfs increment ‐ (E) (CA)cl+(DR)xr.
Reported at 2 years follow up. O‐defs. MD‐defs. BL‐defs. Proportion of children with one or more new defs (at CA level). Drop out. |
|
| Notes | Participants randomized (N = 193). Baseline characteristics (dfs) 'balanced'. Clinical (VT) caries assessment by two examiners; diagnostic threshold = CA; radiographic assessment (2 postBW) by two examiners; diagnostic threshold = DR. Diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Ran 1991.
| Methods | Random allocation; single‐blind (B); non‐placebo‐controlled; 20% drop out (for all study groups combined) after 1.5 years (study duration = 1.5 years + 0.5 year post‐intervention period). Reasons for attrition/ handling of exclusions NR; any differential group losses not assessable. | |
| Participants | 86 children analysed at 1.5 years; all male. Average age at start: 13 years. Surfaces affected at start: 6.0 DMFS. Exposure to other fluoride: data not obtained for home use of dentifrice. Year study began: in/before 1989. Location: Israel. | |
| Interventions | FG (2 groups) versus FT. AmF group (FG1) = 4000 ppm F, AmF group (FG2) = 12,500 ppm F. Self‐applied under supervision at school, with toothbrush, 25 times a year, 1 g applied for 4 min. AmF group (FT) = 1250 ppm F. School use/supervised, fortnightly/20 times a year, 1 g applied for 4 min (no post‐brush rinsing done and no provision of any toothpaste for home use reported). Abrasive system: NR. |
|
| Outcomes | 1.5yNetDMFS increment ‐ (CA). Reported at 0.5 and 1.5 years follow ups (and 0.5 year post‐treatment). | |
| Notes | Participants randomized (numbers for relevant groups NR). Baseline characteristics (DFMS) with some imbalance (reported as NS difference). Clinical (VT) caries assessment by one examiner; diagnostic threshold = CA; state of tooth eruption included NR; intra‐examiner reproducibility checks for DMFS (icc reaching 0.97). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ringelberg 1979.
| Methods | Stratified random allocation; double‐blind (A); placebo‐controlled; 39% drop out after 2.5 years (study duration = 2.5 years). Reason(s) for attrition NR; no differential group losses. | |
| Participants | 711 children analysed at 2.5 years (available at final examination). Average age at start: 11 years. Surfaces affected at start: 4.0 DMFS. Exposure to other fluoride: no. Year study began: 1973. Location: USA. | |
| Interventions | FR+PLT (2 groups) versus FT+PLR (2 groups). AmF group (FR1) = 250 ppm F, NaF group (FR2) = 250 ppm F. School use/supervised, daily, 10 ml applied for 1 min. AmF group (FT1) = 1250 ppm F, SnF2 group (FT2) = 1000 ppm F. Home use/unsupervised, daily frequency assumed. Abrasive system: Ca pyrophosphate in SnF2 toothpaste, NR for AmF toothpaste. |
|
| Outcomes | 2.5yNetDMFS increment ‐ (CA)cl + (DR)xr.
Reported at 2.5 years follow up. DMFT. Stain score. Drop out. |
|
| Notes | Participants randomized (N = 1174). Baseline characteristics (DMFS, DMFT) 'balanced'. Clinical (VT) caries assessment by two examiners, diagnostic threshold = CA. Radiographic assessment (5 BW) by two examiners; diagnostic threshold = DR. State of tooth eruption included NR. Reversal rate between 4 and 9% of observed caries increment in the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Seppa 1987.
| Methods | Cluster random allocation; single‐blind (B); non‐placebo‐controlled; 9% drop out after 2 years (study duration = 2 years). Reasons for attrition/ handling of exclusions NR; any differential group losses not assessable. | |
| Participants | 185 children analysed at 2 years (available at final examination). Age range at start: 10‐13 years. Surfaces affected at start: 8.9 DMFS. Exposure to other fluoride: toothpaste assumed. Year study began: in/before 1984. Location: Finland. | |
| Interventions | FV (2 groups) versus FR. NaF group 1 (Duraphat® varnish) = 22,600 ppm F. NaF group 2 (Fluor Protector® varnish) = 7000 ppm F. Both applied twice a year (0.3 to 0.5 ml), with small brush, (prior tooth‐cleaning performed). NaF group (FR) = 900 ppm F. School use/supervised, fortnightly, 10 ml applied. |
|
| Outcomes | 2yDMFS increment ‐ cl+xr. Reported at 2 years follow up. | |
| Notes | School‐classes randomized (24) and children taken as units for caries increment analyses (N = 204); numbers by group NR. Baseline characteristics (DMFS, age) slightly 'unbalanced'. Clinical (VT) caries assessment by one examiner; diagnostic threshold = CA; radiographic assessment (2 postBW) by one examiner; diagnostic threshold = DR. State of tooth eruption included NR. Diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Seppa 1995.
| Methods | Random allocation; single‐blind (B); non‐placebo‐controlled; 12% drop out after 3 years (study duration = 3 years). Reasons for attrition/ handling of exclusions NR; any differential group losses not assessable. | |
| Participants | 254 children analysed at 3 years (available at final examination). Age range at start: 10‐12 years. Surfaces affected at start: 7.4 DMFS. Exposure to other fluoride: toothpaste. Year study began: in/before 1991. Location: Finland. | |
| Interventions | FV versus FG. NaF group (Duraphat® varnish) = 22,600 ppm F. Operator‐applied twice a year, with small brush (prior tooth‐cleaning not performed). APF group (FG) = 12,300 ppm F. Operator‐applied twice a year, with tray, applied for 4 minutes (prior tooth‐cleaning not performed). |
|
| Outcomes | 3yDMFS increment ‐ (CA) cl+xr.
Reported at 3 years follow up. Side effects (incomplete data). |
|
| Notes | Participants randomized (N = 289); numbers by group NR. Baseline characteristics (DMFS, MD‐DMFS) 'balanced'. Clinical (VT) caries assessment by one examiner, diagnostic threshold = CA; radiographic assessment (BW) by one examiner; diagnostic threshold = DR. State of tooth eruption included NR. Diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Torell 1965.
| Methods | Random allocation; single‐blind (B); non‐placebo‐controlled; 17% drop out rate after 2 years (study duration = 2 years). Natural losses mainly; no differential group losses. | |
| Participants | 667 children analysed at 2 years (available at final examination). Average age at start: 10 years. Surfaces affected at start: 14.6 DMFS (from sample randomized). Exposure to other fluoride: none assumed. Year study began: 1962. Location: Sweden. | |
| Interventions | FR (2 groups) versus FT (2 groups). NaF group (FR1): 230 ppm F, 10 ml applied daily, home use/unsupervised (instructed to be done after toothbrushing). NaF group (FR2): 900 ppm F, 10 ml applied fortnightly, school use/supervised. SnF2 group (FT1) = 1000 ppm F, NaF group (FT2) = 1100 ppm F. Home use/unsupervised, daily frequency assumed (brushing twice a day and post‐brushing water rinse instructed). Abrasive system: Ca pyrophosphate in SnF2 toothpaste, Na bicarbonate in NaF toothpaste. |
|
| Outcomes | 2yDMFS increment ‐ (CA)cl.
Reported at 1 and 2 years follow ups. MD‐DMFS. FS. Proportion of children with new carious lesions ‐ (U)xr. Drop out. |
|
| Notes | Participants randomized (N = 793). Baseline characteristics (DMFS, MD‐DMFS) 'balanced'. Clinical (VT) caries assessment by two examiners, diagnostic threshold = CA; radiographic assessment (BW) by two examiners; diagnostic threshold = DR. State of tooth eruption included NR. Inter‐ and intra‐examiner reproducibility checks done for clinical caries in 4 and 2% sample respectively; duplicate examination of x‐rays records done and any discrepancies discussed before final diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Drop‐out rate based only on groups relevant to review, on relevant follow ups, unless otherwise stated. Baseline caries experience averaged among relevant study arms, and based on the study sample analysed at the end of study period (final sample), unless otherwise stated. Age range (average age when reported) at the time the study started based on all study participants (or on groups relevant to the review when data were available). 1stm = first permanent molar; AmF = amine fluoride; APF = acidulated phosphate fluoride; BW = bite‐wing x‐ray assessment; Ca = calcium; Ca carbonate = CaCO3; CA = lesions showing loss of enamel continuity that can be recorded clinically (undermined enamel, softened floor/walls) or showing frank cavitation; CAR = caries attack rate; CIR = caries incidence rate; cl = clinical examination; d(e)ft/s = decayed, (extracted) and filled deciduous teeth or surface; dmft/s = decayed, missing (or extracted) and filled deciduous teeth or surface; D(M)FS/T = decayed, (missing ) and filled permanent surfaces or teeth; DR = radiolucency into dentin; E = teeth erupted at baseline; ER = any radiolucency in enamel/enamel‐dentin junction; F = fluoride; FG = fluoride gel; FR = fluoride mouthrinse; FT = fluoride toothpaste; FV = fluoride varnish; icc = intra‐class correlation coefficient (for inter‐rater reliability); IMP = insoluble Na metaphosphate; M = missing permanent teeth; MD = mesio and distal surfaces; N = numbers; Na = sodium; NaF = sodium fluoride; NCA = non‐cavitated enamel lesions visible as white spots or discoloured fissures; NR = not reported; NS = not significant; NT = no treatment control; O = occlusal surfaces; PF = pit and fissure surfaces; PL = placebo; postBW = posterior bite‐wing x‐ray assessment; ppm F = parts per million of fluoride; ptc = prior tooth‐cleaning performed with or without a non‐fluoride paste; Silica = silicon dioxide (SiO2); SMFP = sodium monofluorophosphate; SnF2 = stannous fluoride; U = teeth unerupted at baseline; VT = visual‐tactile assessment; xr = radiographic examination.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnaud 1984 | Only three clusters (schools), each randomized to one of the three interventions compared (FR versus FT1 versus FT2). |
| Hamp 1984 | Additional fluoride‐based intervention associated to fluoride mouthrinse. Blind outcome assessment not stated (FV versus FR). |
| Ivanova 1990 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely (FV versus FG/FV versus FR/FG versus FR). |
| Ivanova 1990a | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely (FV versus FG). |
| Karjalainen 1994 | Additional non fluoride‐based intervention associated to fluoride mouthrinse (supervised FR versus unsupervised FT). |
| Ramos 1995 | Open outcome assessment (FV versus FR). |
| Shobha 1987 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely (FV versus FG). Note ‐ Main outcome data not reported in control group (and not obtainable). |
| Suntsov 1991 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely (FV versus FR). Note ‐ Only post‐treatment effects reported. |
| Wilson 1978 | Random or quasi‐random allocation not stated (FR versus FT). |
FG = fluoride gel; FR = fluoride mouthrinse; FT = fluoride toothpaste; FV = fluoride varnish
Contributions of authors
All authors contributed to the development of the protocol and execution of the review. Valeria Marinho (VM) wrote the protocol, designed and implemented the search strategies, tracked down all full articles, contacted authors, selected studies, assessed validity, and extracted data. Julian Higgins (JH) duplicated study selection, quality assessment, and data extraction in a sample of studies and Stuart Logan (SL) or Aubrey Sheiham (AS) were consulted where necessary. VM entered and analysed the data in consultation with JH. VM prepared the full review. All authors contributed to its revision, interpretation of results and approval.
Sources of support
Internal sources
Department of Epidemiology and Public Health (UCL), UK.
Systematic Reviews Training Unit, Institute of Child Health (UCL), UK.
Medical Research Council, UK.
External sources
CAPES ‐ Ministry of Education, Brazil.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ashley 1977 {published data only}
- Ashley FP, Mainwaring PJ, Emslie RD, Naylor MN. Clinical testing of a mouthrinse and a dentifrice containing fluoride. A two‐year supervised study in school children. British Dental Journal 1977;143:333‐8. [DOI] [PubMed] [Google Scholar]
Axelsson 1987 {published data only}
- Axelsson P, Paulander J, Nordkvist K, Karlsson R. Effect of fluoride containing dentifrice, mouthrinsing, and varnish on approximal dental caries in a 3‐year clinical trial. Community Dentistry and Oral Epidemiology 1987;15:177‐80. [DOI] [PubMed] [Google Scholar]
Blinkhorn 1983 {published and unpublished data}
- Blinkhorn AS, Holloway PJ, Davies TG. Combined effects of a fluoride dentifrice and mouthrinse on the incidence of dental caries. Community Dentistry and Oral Epidemiology 1983;11:7‐11. [DOI] [PubMed] [Google Scholar]
Bruun 1985 {published data only}
- Bruun C, Bille J, Hansen KT, Kann J, Qvist V, Thylstrup A. Three‐year caries increments after fluoride rinses or topical applications with a fluoride varnish. Community Dentistry and Oral Epidemiology 1985;13:299‐303. [DOI] [PubMed] [Google Scholar]
DePaola 1980 {published data only (unpublished sought but not used)}
- DePaola PF, Soparkar M, Leeuwen M, DeVelis R. The anticaries effect of single and combined topical fluoride systems in school children. Archives of Oral Biology 1980;25:649‐53. [DOI] [PubMed] [Google Scholar]
- Lu KH, Porter DR, Pickles TH. Separate and combined cariostatic effects of fluoride gel and rinse [abstract No 239]. Journal of Dental Research 1980;59:947. [Google Scholar]
Kirkegaard 1986 {published data only}
- Kirkegaard E, Petersen G, Poulsen S, Holm SA, Heidmann J. Caries‐preventive effect of Duraphat varnish applications versus fluoride mouthrinses: 5‐year data. Caries Research 1986;20:548‐55. [DOI] [PubMed] [Google Scholar]
Koch 1967 {published data only}
- Hollender L, Koch G. Influence of topical application of fluoride on rate of progress of carious lesions in children. A long‐term roentgenographic follow‐up. Odontologisk Revy 1969;20:37‐41. [PubMed] [Google Scholar]
- Koch G. Effect of sodium fluoride in dentifrice and mouthwash on incidence of dental caries in school‐children. Odontologisk Revy 1967;18(Suppl. 12):1‐125. [Google Scholar]
- Koch G, Lindhe J. The effect of supervised oral hygiene on the gingiva of children. The effect of sodium fluoride. Journal of Periodontal Research 1967;2:64‐9. [DOI] [PubMed] [Google Scholar]
- Koch G, Lindhe J. The state of the gingivae and the caries‐increment in school‐children during and after withdrawal of various prophylactic measures. In: McHugh WD editor(s). Dental Plaque. A symposium held at the University of Dundee, 22 to 24 September, 1969. Edinburgh and London: E. & S. Livingstone, 1970:271‐81. [Google Scholar]
Koch 1979 {published data only}
- Koch G, Petersson LG, Ryden H. Effect of flouride varnish (Duraphat) treatment every six months compared with weekly mouthrinses with 0.2 per cent NaF solution on dental caries. Swedish Dental Journal 1979;3:39‐44. [PubMed] [Google Scholar]
Mainwaring 1978 {published data only}
- Mainwaring PJ, Naylor MN. A three‐year clinical study to determine the separate and combined caries‐inhibiting effects of sodium monofluorophosphate toothpaste and an acidulated phosphate‐fluoride gel. Caries Research 1978;12:202‐12. [DOI] [PubMed] [Google Scholar]
Marthaler 1970 {published data only}
- Marthaler TM, Konig KG, Muhlemann HR. The effect of a fluoride gel used for supervised toothbrushing 15 or 30 times per year. Helvetica Odontologica Acta 1970;14:67‐77. [PubMed] [Google Scholar]
Marthaler 1970a {published data only}
- Marthaler TM, Konig KG, Muhlemann HR. The effect of a fluoride gel used for supervised toothbrushing 15 or 30 times per year. Helvetica Odontologica Acta 1970;14:67‐77. [PubMed] [Google Scholar]
Petersson 1985 {published data only}
- Petersson LG, Koch G, Rasmusson CG, Stanke H. Effect on caries of different fluoride prophylactic programs in preschool children. A two year clinical study. Swedish Dental Journal 1985;9:97‐104. [PubMed] [Google Scholar]
Ran 1991 {published data only (unpublished sought but not used)}
- Ran F, Gedalia I, Fried M, Hadani P, Tved A. Effectiveness of fortnightly tooth brushing with amine fluorides in caries‐prone subjects. Journal of Oral Rehabilitation 1991;18:311‐6. [DOI] [PubMed] [Google Scholar]
Ringelberg 1979 {published data only}
- Ringelberg ML, Webster DB. Effects of an amine fluoride mouthrinse and dentifrice on the gingival health and the extent of plaque of school children. Journal of Periodontology 1977;48:350‐3. [DOI] [PubMed] [Google Scholar]
- Ringelberg ML, Webster DB, Dixon DO. Effects of an amine fluoride dentifrice and mouthrinse on the dental caries of school children after 18 months. Journal of Preventive Dentistry 1978;5:26‐30. [PubMed] [Google Scholar]
- Ringelberg ML, Webster DB, Dixon DO, Fairchild S, Driscoll WS. Results of gingival, plaque, and stain assessments after 30 months use of amine fluorides and their inorganic counterparts. Pharmacology and Therapeutics in Dentistry 1979;4:27‐31. [PubMed] [Google Scholar]
- Ringelberg ML, Webster DB, Dixon DO, LeZotte DC. The caries‐preventive effect of amine fluorides and inorganic fluorides in a mouthrinse or dentifrice after 30 months of use. Journal of the American Dental Association 1979;98:202‐8. [DOI] [PubMed] [Google Scholar]
Seppa 1987 {published data only}
- Seppa L, Pollanen L. Caries preventive effect of two fluoride varnishes and a fluoride mouthrinse. Caries Research 1987;21:375‐9. [DOI] [PubMed] [Google Scholar]
Seppa 1995 {published data only}
- Seppa L, Leppanen T, Hausen H. Fluoride varnish versus acidulated phosphate fluoride gel: a 3‐year clinical trial. Caries Research 1995;29:327‐30. [DOI] [PubMed] [Google Scholar]
Torell 1965 {published data only}
- Torell P. The Goteborg studies of methods of applying fluorides topically. In: Hardwick JL, Held HR, Konig KG editor(s). Advances in fluorine research and dental caries prevention. Vol. 3, Oxford: Pergamon Press, 1965:255‐8. [PubMed] [Google Scholar]
- Torell P, Ericsson Y. Two year clinical tests with different methods of local caries‐preventive fluorine application in Swedish school‐children (Part I: The Goteborg study). Acta Odontologica Scandinavica 1965;23:287‐312. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barnaud 1984 {published data only}
- Barnaud J, Finidori C. The preventive effectiveness of a toothpaste with a high fluoride content [Etude de l'efficacite preventive d'une pate a haute teneur en fluorures]. Journal of the International Association of Dentistry for Children 1984;15:21‐31. [PubMed] [Google Scholar]
- Barnaud J, Finidori C, Texier T, Suen A. Dentifrice pastes with high fluoride contents [Les pates dentifrices a hautes teneurs en fluor]. Chirurgien Dentiste de France 1984;54:50‐1. [PubMed] [Google Scholar]
Hamp 1984 {published data only}
- Hamp SE, Johansson LA, Karlsson R. Clinical effects of preventive regimens for young people in their early and middle teens in relation to previous experience with dental prevention. Acta Odontologica Scandinavica 1984;42:99‐108. [DOI] [PubMed] [Google Scholar]
Ivanova 1990 {published data only (unpublished sought but not used)}
- Ivanova EN. The comparative efficacy of local anticaries agents [Sravnitel'naia effektivnost' mestnykh protivokarioznykh sredtsv]. Stomatologiia Moskva 1990;69:60‐1. [PubMed] [Google Scholar]
Ivanova 1990a {published data only (unpublished sought but not used)}
- Ivanova EN, Frolova GI, Postoiuk SN, Liakh GA. The status of the oral cavity and results of the use of fluorine‐containing preparations in preventing dental caries in children [Sostoianie polosti rta i rezul'taty primeneniia ftorsoderzhashchikh preparatov v profilaktike kariesa zubov u detei]. Stomatologiia Moskva 1990;69:69‐70. [PubMed] [Google Scholar]
Karjalainen 1994 {published data only}
- Karjalainen S, Eriksson AL, Ruokola M, Toivonen A. Caries development after substitution of supervised fluoride rinses and toothbrushings by unsupervised use of fluoride toothpaste. Community Dentistry and Oral Epidemiology 1994;22:421‐4. [DOI] [PubMed] [Google Scholar]
Ramos 1995 {published data only}
- Ramos SB. Efeito de bochechos fluoretados (NaF ‐ 0,2 por cento) e da aplicacao do verniz com fluor (NaF ‐ 5 por cento) na prevencao da carie dentaria: estudo comparativo em escolares da Regiao Norte da cidade de Sao Paulo, 1992‐1993 [Effect of fortnightly rinsing with a 0.2 percent NaF solution and a fluoride varnish with 5 percent NaF in dental caries prevention: comparative study in students of the northern region of Sao Paulo city, Brazil, 1992‐ 1993] [dissertation]. Sao Paulo: Universidade de Sao Paulo (SP), 1995. [Google Scholar]
Shobha 1987 {published data only (unpublished sought but not used)}
- Shobha T, Nandlal B, Prabhakar AR, Sudha P. Fluoride varnish versus acidulated phosphate fluoride for schoolchildren in Manipal. Journal of the Indian Dental Association 1987;59:157‐60. [PubMed] [Google Scholar]
Suntsov 1991 {published data only}
- Suntsov VG, Distel' VA, Zhorova TN, Buiankina RG, Toropov VN, Bulanova EL. The trace efficacy of dental caries prevention in children [Sledovaia effektivnost' profilaktiki kariesa zubov u detei]. Stomatologiia Moskva 1991;2(2):69‐71. [PubMed] [Google Scholar]
Wilson 1978 {published data only (unpublished sought but not used)}
- Wilson CJ, Triol CW, Volpe AR. The clinical anticaries effect of a fluoride dentifrice and mouthrinse [abstract]. Journal of Dental Research 1978;57:276. [Google Scholar]
References to studies awaiting assessment
Autio 1999 {published data only}
- Autio J, Courts F. Feasibility of a fluoride varnish program in Head Start Children [abstract]. Journal of Dental Research 1999;78:170. [Google Scholar]
Cichocka 1981 {published data only}
- Cichocka E. Economic evaluation of selected methods of contact fluoridation of the teeth [Ocena ekonomiczna wybranych metod kontaktowego fluorkowania zebow]. Czasopismo Stomatologiczne 1981;34:245‐50. [PubMed] [Google Scholar]
- Cichocka E. Oral hygiene and the efficacy of selected methods of contact tooth fluoridation [Higiena jamy ustnej a skutecznosc wybranych metod kontaktowego fluorkowania zebow]. Czasopismo Stomatologiczne 1981;34:145‐53. [PubMed] [Google Scholar]
Additional references
Burt 1998
- Burt BA. Prevention policies in the light of the changed distribution of dental caries. Acta Odontologica Scandinavica 1998;56:179‐86. [DOI] [PubMed] [Google Scholar]
Chen 1995
- Chen MS. Oral health of disadvantaged populations. In: Cohen LK, Gift CH editor(s). Disease Prevention and Oral Health Promotion. Copenhagen: Munksgaard, 1995:152‐212. [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD (editors). Assessment of study quality. Cochrane Reviewers' Handbook version 4.1 [updated June 2000]; Section 6. The Cochrane Collaboration, 2000. [Google Scholar]
Downer 1995
- Downer MC. The 1993 national survey of children's dental health. British Dental Journal 1995;178:407‐12. [DOI] [PubMed] [Google Scholar]
Dubey 1965
- Dubey SD, Lehnhoff RW, Radike AW. A statistical confidence interval for true per cent reduction in caries‐incidence studies. Journal of Dental Research 1965;44:921‐3. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Featherstone 1988
- Featherstone JDB, Cate JM. Physicochemical aspects of fluoride‐enamel interactions. In: Ekstrand J, Fejerskov O, Silverstone LM editor(s). Fluoride in Dentistry. Copenhagen: Munksgaard, 1988:125‐49. [Google Scholar]
Glass 1982
- Glass RL. The first international conference on the declining prevalence of dental caries. Journal of Dental Research 1982;61:1301‐83. [Google Scholar]
Higgins 1996
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta‐analysis. Statistics in Medicine 1996;15:2733‐49. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins J. Personal communication August 4 2003.
Marinho 1997
- Marinho VC. A systematic review of the effectiveness of topical fluoride therapy in the prevention of dental caries in children and adolescents [dissertation]. London: University of London 1997.
Marinho 2002
- Marinho VCC, Higgins J, Logan S, Sheiham A. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 2. [Art. No.: CD002280. DOI: 10.1002/14651858.CD002280] [DOI] [PubMed] [Google Scholar]
Marinho 2002a
- Marinho VCC, Higgins J, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 3. [Art. No.: CD002279. DOI: 10.1002/14651858.CD002279] [DOI] [PubMed] [Google Scholar]
Marinho 2003
- Marinho VCC, Higgins J, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 1. [Art. No.: CD002278. DOI: 10.1002/14651858.CD002278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2003a
- Marinho VCC, Higgins J, Logan S, Sheiham A. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD002284. DOI: 10.1002/14651858.CD002284] [DOI] [PubMed] [Google Scholar]
Marinho 2003b
- Marinho VCC, Higgins J, Logan S, Sheiham A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD002782. DOI: 10.1002/14651858.CD002782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marthaler 1994
- Marthaler TM, Steiner M, Menghini G, Bandi A. Caries prevalence in Switzerland. International Dental Journal 1994;44:393‐401. [PubMed] [Google Scholar]
Marthaler 1996
- Marthaler TM, O'Mullane DM, Vrbic V. The prevalence of dental caries in Europe 1990‐1995. ORCA Saturday afternoon symposium 1995. Caries Research 1996;30:237‐55. [DOI] [PubMed] [Google Scholar]
Moynihan 1996
- Moynihan PJ, Holt RD. The national diet and nutrition survey of 1.5 to 4.5 year old children: summary of the findings of the dental survey. British Dental Journal 1996;181:328‐32. [DOI] [PubMed] [Google Scholar]
Murray 1991
- Murray JJ, Rugg‐Gunn AJ, Jenkins GN. A history of water fluoridation. In: Murray JJ, Rugg‐Gunn AJ, Jenkins GN editor(s). Fluorides in caries prevention. Oxford: Wright, 1991:7‐37. [Google Scholar]
Murray 1991a
- Murray JJ, Rugg‐Gunn AJ, Jenkins GN. Fluoride toothpastes and dental caries. In: Murray JJ, Rugg‐Gunn AJ, Jenkins GN editor(s). Fluorides in caries prevention. Oxford: Wright, 1991:127‐60. [Google Scholar]
O'Mullane 1995
- O'Mullane DM. Contribution of fluoride toothpastes to oral health. In: Bowen WH editor(s). Relative efficacy of sodium fluoride and sodium monofluorophosphate as anti‐caries agents in dentifrices. The Royal Society Of Medicine Press Limited, 1995:3‐7. [Google Scholar]
Pitts 2001
- Pitts NB, Evans DJ, Nugent ZJ. The dental caries experience of 5‐year‐old children in Great Britain. Surveys coordinated by the British Association for the Study of Community Dentistry in 1999/2000. Community Dental Health 2001;18(1):49‐55. [PubMed] [Google Scholar]
Pitts 2002
- Pitts NB, Evans DJ, Nugent ZJ, Pine CM. The dental caries experience of 12‐year‐old children in England and Wales. Surveys coordinated by the British Association for the Study of Community Dentistry in 2000/2001. Community Dental Health 2002;19(1):46‐53. [PubMed] [Google Scholar]
Ripa 1991
- Ripa LW. A critique of topical fluoride methods (dentifrices, mouthrinses, operator‐applied, and self‐applied gels) in an era of decreased caries and increased fluorosis prevalence. Journal Of Public Health Dentistry 1991;1:23‐41. [DOI] [PubMed] [Google Scholar]
Rolla 1991
- Rolla G, Ogaard B, Cruz R‐d‐A. Clinical effect and mechanism of cariostatic action of fluoride‐containing toothpastes: a review. International Dental Journal 1991;41:171‐4. [PubMed] [Google Scholar]
Seppa 1998
- Seppa L, Karkkainen S, Hausen H. Caries frequency in permanent teeth before and after discontinuation of water fluoridation in Kuopio, Finland. Community Dentistry and Oral Epidemiology 1998;26:256‐62. [DOI] [PubMed] [Google Scholar]
Sharp 1998
- Sharp S. Meta‐analysis regression: statistics, biostatistics, and epidemiology 23 (sbe23). Stata Technical Bulletin 1998; Vol. 42:16‐22.
Song 2003
- Song F, Altman DG, Glenny A‐M, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ 2003;326(7387):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strohmenger 2001
- Strohmenger L, Brambilla E. The use of fluoride varnishes in the prevention of dental caries: a short review. Oral Diseases 2001;7:71‐80. [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
van Rijkom 1998
- Rijkom HM, Truin GJ, 't Hof MA. A meta‐analysis of clinical studies on the caries‐inhibiting effect of fluoride gel treatment. Caries Research 1998;32:83‐92. [DOI] [PubMed] [Google Scholar]


