Abstract
Background
Beclomethasone dipropionate (BDP) is available in a wide range of daily doses for the treatment of long‐term asthma.
Objectives
To assess the evidence for a dose response relationship for BDP in the treatment of long‐term asthma.
Search methods
We searched the Cochrane Airways Group trial register, Cochrane Controlled Trials Register (The Cochrane Library issue 1 1999) and references lists of articles. Authors and Glaxo Wellcome UK were contacted to identify eligible studies. We also hand searched the proceeding from relevant respiratory society meetings, the British Journal of Clinical Research and the European Journal of Clinical Research for studies.
Selection criteria
Prospective, randomised trials comparing two or more daily doses of BDP in patients over the age of two years with long‐term asthma.
Data collection and analysis
Trials were selected for inclusion and scored for quality by two reviewers. Data were extracted by one reviewer. Authors were contacted to clarify details of study design and retrieve missing data.
Main results
15 trials were included. Methodological quality was variable. Studies rarely gave a clear indication of the degree of asthma control at baseline. Less than two‐fold to five‐fold dose differences were assessed by different studies. The results are reported as weighted mean differences (WMD) with 95% confidence limits (95% CI). The number of trials (N) contributing to each outcome is stated. In non‐oral steroid treated asthmatics a small advantage of BDP 800 mcg/d over 400 mcg/d was apparent for improvement in morning peak expiratory flow rate (PEFR) compared to baseline, WMD 11 L/min (95% CI 4 to 19 L/min) N=2; improvement in forced expired volume in one second (FEV1) compared to baseline, WMD 9 ml (95% CI 3 to 140) N=1; and reduction in night‐time symptom score compared to baseline, WMD 0.13 (95% CI 0.04 to 0.22) N=1. Studies that assessed BDP 1000 v 500 mcg/d and BDP 1600 v 400 mcg/d demonstrated significant advantage of higher dose over lower dose for histamine bronchial hyper‐responsiveness (BHR) and percentage improvement in FEV1 compared to baseline. No differences between higher and lower daily doses of BDP were apparent for daytime symptoms, withdrawals due to asthma exacerbation, oropharyngeal side effects or measures of hypothalamo‐pituitary‐adrenal (HPA) function. No difference in prednisolone sparing effect was apparent when comparing high dose and low dose BDP in oral corticosteroid (OCS) dependent patients.
Authors' conclusions
BDP appears to demonstrate a shallow dose response effect in long‐term asthma for a small number of efficacy outcomes over range of daily doses from 400 mcg/d to 1600 mcg/d, although the clinical significance of the improvements afforded by higher doses is questionable.
Keywords: Child; Child, Preschool; Humans; Administration, Inhalation; Anti-Asthmatic Agents; Anti-Asthmatic Agents/administration & dosage; Asthma; Asthma/drug therapy; Beclomethasone; Beclomethasone/administration & dosage; Dose-Response Relationship, Drug; Prospective Studies; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
Beclomethasone at different doses for chronic asthma
Inhaled steroids help control inflammation in the airways of the lung. There are numerous different preparations available, and we have assessed how varying the dose of beclomethasone (BDP) affects asthma in this review. There was a limited amount of evidence that 800mcg/d was superior to 400mcg/d in improving morning and evening peak flow. More research into the effects of different doses of BDP is required.
Background
Inhaled beclomethasone dipropionate (BDP) was introduced in the early 1970's for the treatment of chronic asthma. Extensive experience has been gained in its use on a world‐wide basis; it is generally acknowledged to be safe and effective. BDP is available in a range of formulations by metered dose inhaler (MDI) and various dry powder inhaler (DPI) devices. The nominal daily dose that a patient can be prescribed falls over a 40 fold difference in dose from 50 mcg/d to 2000 mcg/d. The term 'dose response' refers to significant improvements in the size of a given outcome with increasing dose. When considering inhaled corticosteroids (ICS) the issue of whether an important dose response effect exists can be framed as two clinical questions: a) Do patients with poorly controlled asthma gain additional benefit by starting ICS treatment at higher as opposed to lower doses? b) For patients in whom asthma is not adequately controlled on a given dose of ICS, are additional benefits gained by increasing the dose? Current asthma guidelines (BTS 1997, GINA 1995, NHLBI 1997) recommend higher starting doses in patients with more severe symptoms and increasing the daily dose if symptoms are uncontrolled. This advice is based on the assumption that beneficial effects increase with dose. However, a systematic review recently undertaken comparing BDP to placebo did not provide evidence for a dose response effect that would support this approach (Adams 2000). This review concluded that BDP was significantly more effective than placebo in improving measures of airway calibre, bronchial hyper‐responsiveness and symptoms in patients who were not receiving oral corticosteroid (OCS) tablets such as prednisolone. BDP also had a significant OCS sparing effect in OCS treated asthmatics when compared to placebo. However higher daily doses of BDP (1000 mcg/d or greater) appeared to be no more effective than lower doses (400 mcg/d or less) for any measure of efficacy. Inferences concerning dose response effect in this review need to be interpreted with caution however because the studies included did not directly compare different doses of BDP. Dose response is ideally assessed by trials in which patients are randomised to two (or more) doses of inhaled corticosteroid within the same trial. The purpose of this review was to systematically assess the studies in which two or more doses of BDP were compared.
Objectives
1. To assess efficacy and safety outcomes in studies that compared inhaled BDP at different nominal daily doses in the treatment of chronic asthma.
2. To test for the presence of a dose response effect.
Methods
Criteria for considering studies for this review
Types of studies
Only prospective randomised studies were considered. Double, single or unblinded studies were eligible for inclusion. Studies could either be of parallel group or crossover design.
Types of participants
Studies including children and/or adults with a clinical diagnosis of asthma. Only patients over the age of 2 years were included. Studies that recruited patients with both asthma and chronic obstructive pulmonary disease (COPD) were considered if the data for asthmatic patients were available separately. Studies conducted in primary care, hospital outpatient or institutional care setting were considered.
Types of interventions
BDP at one nominal daily dose delivered by oral inhalation compared to at least one other daily dose of BDP. Treatment periods had to be for at least one week. Delivery could be by either pressurised‐metered dose inhaler (MDI) with or without holding chamber/spacer, breath‐actuated metered dose inhaler or dry powder inhaler. Studies using nebuliser were excluded. Any co‐intervention was acceptable, including the use of oral corticosteroids.
Types of outcome measures
Most outcome measures were considered. Those of particular interest included: 1. Measures of airway calibre: forced expired volume in one second (FEV1), morning and evening diary card peak expiratory flow rate (PEFR), diurnal variability in diary card PEFR, clinic PEFR 2. Symptom scores 3. Rescue beta2 agonist use 4. Bronchial hyper‐responsiveness (BHR): using either methacholine or histamine challenge 5. Health status/Quality of life assessment 6. Asthma exacerbations: hospital admission rates, days off work or school, unscheduled doctor visits or emergency department attendance due to exacerbation. 7. Safety outcomes: hypothalamic‐pituitary‐adrenal (HPA) axis function assessed by serum and urinary cortisol measures. 8. Oropharyngeal side effects: hoarseness, sore or dry mouth/throat, oropharyngeal Candidiasis
Outcomes that were not considered included growth assessment in children, bone densitometry and biochemical markers of bone turnover.
Search methods for identification of studies
Electronic searches
A search was carried out of the Cochrane Airways Group Trial Register. The following search terms were applied:
steroid* OR glucocorticoid* OR corticosteroid* OR beclomethasone OR budesonide OR fluticasone OR triamcinolone OR flunisolide OR Becotide OR Becloforte OR Pulmicort OR Flixotide
The electronic abstracts of citations resulting from this search were then imported into a bibliographic database termed the Inhaled Steroid Register. This was hand searched by two reviewers (NPA and JB) for duplicate publications, which were removed.
Stage 2: the inhaled steroid register was searched using the following terms:
beclomethasone OR Becotide OR Becloforte
Electronic abstracts were exported to a new database and termed the Beclomethasone Register. Citations were initially excluded if it was clear that the study was:
a) Not concerned with treatment of chronic asthma in humans b) Not a randomised controlled trial (RCT) c) Not include a treatment arm with an inhaled corticosteroid
Where uncertainty existed, the publication was retrieved in full text version
Searching other resources
The bibliographies of all papers retrieved in full text form and relevant narrative reviews were searched for additional publications. The British Journal of Clinical Research and the European Journal of Clinical Research (journals not currently indexed on Medline or EMBASE), were hand searched. Authors of included studies were contacted and asked if they were aware of further studies that had been missed. The UK headquarters of Glaxo Wellcome, manufacturers of Becotide and Becloforte were contacted to obtain details of studies that had been sponsored. Finally, the proceedings of meetings of the European Respiratory Society (1997/1998), British Thoracic Society (1997/1998) and American Thoracic Society (1997 to 1999) were searched for relevant trials. This was undertaken in an attempt to identify relevant trials that may have been completed but not yet reached peer reviewed journal publication.
Data collection and analysis
Selection of studies
The decision to exclude studies prior to full paper retrieval was made by two reviewers (NPA and JB). Disagreement was resolved by consensus. The full text papers retrieved were reviewed independently by two authors (NPA and JB). Disagreement as to which papers to include was resolved by consensus. Two reviewers (NPA and JB) who were blinded to the author's names, institution and funding sources independently assessed each study.
Data extraction and management
One reviewer (NPA) extracted data for each outcome from the published results of included trials.
Authors were written to (by mail, fax and/or electronic mail) on at least two occasions to clarify details of randomisation and/or request missing outcome data. Attempts were made to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital web sites for up‐to‐date contact details. Glaxo Wellcome was approached for data concerning those trials in which contact authors did not initially reply or when authors suggested doing so. Incomplete numerical data that were not available for inclusion in the meta‐analysis have been listed in Table 1.
1. Outcome data not included in meta‐analysis.
| Study | Missing data |
| Carmichael 1978 | FEV1 FVC Morning PEFR, Evening PEFR Daytime wheeze, dyspnoea and cough score Night‐time wheeze, dyspnoea and cough score Use of beta2 agonist (total over treatment period) Short tetracosactrin test (no details of dose or timing) No standard deviation values available for above outcomes |
| Carpentiere 1990 | FEV1 Propranolol bronchial responsiveness (PC20 FEV1) Cough, wheeze, breathlessness, dyspnoea score Beta2 agonist use No standard deviation values available for above outcomes |
| Chatterjee 1980 | FEV1 FVC FEV1/FVC ratio Morning PEFR Evening PEFR No numerical data available |
| Drepaul 1989 | Change in FEV1compared to baseline Change in FVC compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in daytime symptom score compared to baseline Change in night‐time symptom score compared to baseline Change in daily beta2 agonist use compared to baseline No standard deviation values available for above outcomes |
| Lal 1980 | FEV1 FVC FEV1/FVC ratio Morning PEFR Evening PEFR Daily use of beta2 agonists Mid‐morning plasma cortisol No numerical data available for above outcomes |
| Molema 1988 | Plasma cortisol 30 min post 250 mcg tetracosactrin No standard deviation values available |
| Nathan 1997 | % change in FEV1 compared to baseline FEF 25‐50 FVC Morning PEFR Evening PEFR Asthma symptom score Use of rescue beta2 agonist No numerical data available for above outcomes |
| Smith 1986 | FEV1 FVC No numerical data presented for above outcomes |
Assessment of risk of bias in included studies
The trials were scored for methodological quality using the Cochrane approach:
Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
Studies was also assessed using a 5 point scoring instrument (Jadad 1996):
a) Was the study described as randomised? (yes=1 no=0) b) Was the study described as double blind? (yes=1 no=0) c) Was there a description of withdrawals and dropouts? (yes=1 no=0) d) Was the method of randomisation well described and appropriate? (yes=1 no=0) e) Was the method of double blinding well described and appropriate? (yes=1 no=0) f) deduct 1 point if method of randomisation or blinding inappropriate
Inter‐rater agreement was measured using the kappa statistic. Disagreement was resolved by consensus.
Measures of treatment effect
Studies were categorised based on the presence or absence of treatment with a regular OCS such as prednisolone tablets at the point of enrolment. It was expected that most trials in patients receiving a regular OCS would use a 'steroid‐sparing' design in which the daily dose was progressively reduced. In such studies the principal outcome is the daily dose OCS required to maintain asthma control unchanged. Conversely, studies in which patients were not treated with a regular OCS are more likely to have a design aimed at detecting improvements in asthma control. It would be inappropriate to combine trials with these different designs and aims.
Unit of analysis issues
The results of parallel and crossover trials were not pooled.
Assessment of heterogeneity
Heterogeneity of effect size across studies pooled was calculated, with p< 0.05 used as the cut‐off level for significance.
Data synthesis
A weighted treatment effect across trials was calculated using the Cochrane statistical package RevMan 5. For continuous outcomes, a weighted mean difference (WMD) or standardised mean difference (SMD) was calculated as appropriate. For dichotomous outcomes a Peto odds ratio (OR) was calculated. Pooled treatments effects are expressed with their 95% confidence intervals (95% CI).
Sensitivity analysis
Sensitivity analyses were planned, based on methodological quality. Subgroup analyses based upon patient age, study duration and asthma severity were planned. However, these were not undertaken because of the limited number of studies.
Results
Description of studies
Results of the search
Stage 1: 6494 citations retrieved, 2162 unique records
Stage 2: 1149 citations retrieved 379 not RCT 190 not concerned with long‐term asthma in humans 177 no inhaled corticosteroid treatment arm 113 not concerned with a comparison of two or more daily doses of BDP
292 papers retrieved in full text form 159 not concerned with a comparison of two or more daily doses of BDP 89 not RCT 16 delivery device comparison 4 propellant comparison 2 dose scheduling comparison 3 other reasons (see Excluded studies 'notes' section)
3 studies awaiting translation 14 studies selected for inclusion
Other sources: one included study (Hampel 1997) was identified as a result of hand searching respiratory society meeting abstracts.
Total number of included studies: 15
Agreement between the two independent assessments of study quality were as follows:
Randomisation: kappa=1 Double‐blind:kappa=1 Withdrawal/dropout: kappa=0.8 Method of randomisation: kappa=0.6 Method of double‐blinding: kappa=0.4
Included studies
15 studies met the criteria for inclusion. See Characteristics of included studies for details. Three studies require translation and are awaiting assessment. One study (Majima 1993) appeared to be an RCT from the English language abstract. In the case of two studies (Kudo 1995, Pol'ner 1997) it was not clear from the abstract if patients were randomised to intervention groups. No studies were excluded based on language of publication.
Populations
Studies were mainly undertaken in Western Europe (Denmark, Italy, The Netherlands and the UK), North America (USA and Canada). One study (So 1986) was conducted in Hong Kong. Only one study was conducted in primary care (Drepaul 1989); all others were conducted in a hospital outpatient setting. Two studies (Verberne 1998, Wolthers 1993) were conducted in children, the remainders were in adults.
Study design
Six studies (40%) were of parallel group design, nine (60%) of crossover design. Nine studies had a treatment period of 1‐4 weeks, three (20%) a treatment period of 1‐6 months. Two studies (Hummel 1992, Verberne 1998) had treatment periods of between six and 12 months.
Interventions
A range of nominal daily doses was compared, ranging from a less than two‐fold dose difference to five‐fold dose difference. In 11 studies, different daily doses of BDP were administered using identical delivery device (MDI, MDI+spacer or DPI). In four studies (Chatterjee 1980, Drepaul 1989, Lal 1980, So 1986) different delivery devices were used, however the lower comparison dose of BDP was consistently delivered via MDI, higher dose via DPI.
Diagnosis of asthma
In a single study (Verberne 1998), the diagnosis of asthma was made according to American Thoracic Society criteria. In one study (De Marzo 1988) patients had occupational asthma defined by sensitivity to toluene diisocyanate (TDI). In seven studies (47%), diagnosis was supported by significant reversibility of FEV1 or PEFR in response to inhaled beta2 agonist. In six studies (40%) enrolled patients were stated to have asthma but no further details were given regarding the criteria for a diagnosis of asthma.
Prior treatment with corticosteroids
In two parallel group design studies (Hummel 1992, Tarlo 1988) dependence on oral prednisolone for asthma control was an inclusion criterion. In both studies, an attempt was made to reduce daily prednisolone dose throughout the study. In a single crossover study (Chatterjee 1980) a proportion of patients were receiving oral steroids at enrolment but no attempt was made to reduce steroid dose in these patients during the trial. In 12 studies, patients were not receiving regular systemic steroids at the time of enrolment. In seven of these, (Carpentiere 1990, Drepaul 1989, Lal 1980, Molema 1988, Smith 1986, So 1986, Wolthers 1993) prior regular systemic steroid use was an exclusion criterion. In five studies (Carmichael 1978, De Marzo 1988, Hampel 1997, Nathan 1997, Verberne 1998) it was clear from the baseline characteristics that no patients were receiving oral steroids at the time of enrolment.
In four studies (Carpentiere 1990, De Marzo 1988, Hampel 1997, Wolthers 1993) patients had not received a regular ICS prior to the study. In eight studies, some or all patients were receiving a regular ICS at the time of enrolment. In all cases this was discontinued at the point of randomisation.
Risk of bias in included studies
The overall quality of included studies was variable. All were randomised, but in only six (40%) was allocation concealment clearly employed. Twelve studies (80%) were double blind. Numbers of patients withdrawn following randomisation and the reasons for withdrawal were clearly stated in 12 studies (80%). Only one study (Wolthers 1993) achieved a Jadad score of five; 10 studies (67%) achieved a score of three or four. Four studies (27%) were scored two or less.
Effects of interventions
NON ORAL STEROID TREATED ASTHMATICS
A wide range of BDP dose comparisons were made. Only two studies of parallel group design (Drepaul 1989, Verberne 1998) compared the same two daily doses of BDP (400 mcg/d v 800 mcg/d). The results for these studies have been pooled (Comparison 02). Two crossover studies (So 1986, Wolthers 1993) also compared the same pair of daily doses of BDP, the results of these studies have also been pooled separately (Comparison 05). All other studies evaluated different BDP dose comparisons. Numerical data for outcomes reported in these studies, when available, has been included in the Comparison and Data table and can be plotted visually in Metaview.
The results of BDP dose comparisons are discussed according to 'fold difference' in doses of BDP compared.
LESS THAN TWO‐FOLD DIFFERENT BDP DOSE COMPARISONS:
BDP 400 v 500 mcg/d
Two crossover design studies, both conducted in adult asthmatic patients who were already being treated with inhaled corticosteroid compared less than two‐fold BDP dose differences. Smith 1986 assessed the relative efficacy of 400 mcg/d v 500 mcg/d. The primary aim of this study was to compare low and high concentration formulations of BDP aerosol, however slightly different nominal daily doses were used. This four week study was of high methodological quality (Jadad score 4) but did not have a washout period between doses. No statistically significant differences in FEV1, forced vital capacity (FVC), morning or evening PEFR were apparent between treatment groups. Carmichael 1978 assessed the relative efficacy of BDP 400 mcg/d v 600 mcg/d . This four week study was of low methodological quality (Jadad score 2) and did not have a washout period. No significant differences between treatment groups were apparent for FEV1, FVC, diary card morning PEFR or evening PEFR. Symptoms were assessed by day and night‐time wheeze, dyspnoea and cough scores. Due to the way in which the results were presented it is not possible to assess if any statistically significant differences between treatment groups were apparent for these outcomes.
TWO‐FOLD DIFFERENT BDP DOSE COMPARISONS:
BDP 100 v 200 mcg/d
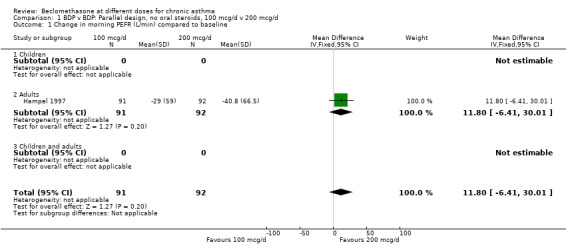
A single parallel group design study of six weeks duration in ICS naïve adult asthmatics (Hampel 1997) assessed BDP 100 mcg/d v 200 mcg/d. No significant difference for change in morning PEFR compared to baseline was apparent between treatment groups: mean difference 12 L/min (95% CI ‐6 to 30 L/min).
BDP 300 v 600 mcg/d
A single crossover design study of four weeks duration in adult asthmatics (Lal 1980) assessed BDP 300 mcg/d v 600 mcg/d. This study was of fair methodological quality (Jadad score 3) but did not have a washout between treatment periods. No statistically significant differences between nominal daily doses were apparent for FEV1, FVC, morning and evening PEFR, mid‐morning plasma cortisol or rescue beta2 agonist use.
BDP 400 v 800 mcg/d
Two parallel group design studies, one in children (Verberne 1998), and one in adults (Drepaul 1989) assessed BDP 400 mcg/d v 800 mcg/d. Both studies were of fair methodological quality (Jadad score 3 or 4) and conducted in asthmatic patients already receiving between 200 and 800 mcg/d of inhaled steroid. Verberne 1998 had a 12 month treatment period and was carried out in a hospital outpatient setting, doses were administered using the Diskhaler DPI. Drepaul 1989 had a six week treatment period and was a large study (365 subjects) carried out in primary care. Although the main objective of this study was to evaluate different delivery devices (MDI v Diskhaler dry powder inhaler), different nominal daily doses were delivered (400 mcg/d via MDI, 800 mcg/d via Diskhaler). Results from these two studies were pooled. A statistically significant but small advantage of BDP 800 mcg/d over BDP 400 mcg/d was apparent for change in morning PEFR compared to baseline: WMD 11 L/min (95% CI 4 to 19 L/min). There was no heterogeneity in effect size between studies. Higher dose BDP also resulted in a small improvement over the lower dose for change in evening PEFR compared to baseline: WMD 8 L/min (95% CI 0 to 16 L/min).
Other outcomes were assessed in individual studies. No significant difference between treatment groups was apparent for change in FEV1 (% predicted), change in methacholine BHR PD20 FEV1 (log doubling dose) or withdrawals due to asthma exacerbation (Verberne 1998). Drepaul 1989 found a small treatment advantage of BDP 800 mcg/d over BDP 400 mcg/d for improvement in FEV1 compared to baseline: mean difference 9 ml (95% CI 3 to 140 ml); reduction in night‐time symptom score compared to baseline: mean difference 0.13 (95% CI 0.04 to 0.22) and reduction in daytime use of beta2 agonist compared to baseline: mean difference 0.49 puffs/d (95% CI 0.02 to 0.96 puffs/d). No difference in the likelihood of oral Candidiasis was seen.
Two crossover design studies (So 1986, Wolthers 1993) assessed the relative effects of BDP 400 mcg/d v 800 mcg/d, outcomes were pooled. No significant difference between treatments were apparent for FEV1, FVC, morning PEFR, evening PEFR, rescue beta2 agonist use, night and daytime asthma symptom scores and percentage of symptom free days and nights. HPA axis function was assessed in a single study (Wolthers 1993). No difference between treatments was apparent for plasma cortisol or plasma cortisol post synthetic adrenocorticotrophic hormone (ACTH).
BDP 500 v 1000 mcg/d
Two crossover design studies (Carpentiere 1990, Molema 1988) assessed BDP 500 mcg/d v BDP 1000 mcg/d. Each study reported different outcomes. Carpentiere 1990 was a small study (10 subjects) of low quality (Jadad score 2) conducted in ICS naive asthmatics. Following three weeks of treatment a statistically significant improvement in histamine BHR was found for BDP 1000 mcg/d compared to 500 mcg/d (geometric mean PC20 FEV1 1.93 mg/ml v 0.86 mg/ml). No significant differences were apparent between doses for FEV1 (% predicted), symptom scores or rescue beta2 agonist use. Molema 1988 was also small study (16 subjects) of low methodological quality (Jadad score 2) and did not employ a washout period. FEV1, morning PEFR, evening PEFR, dyspnoea score, rescue beta2 agonist use and basal morning plasma cortisol were evaluated: no significant differences between intervention groups were apparent. This study also employed a treatment arm using BDP 2000 mcg/d. No significant differences in treatment effect were evident for any outcome when comparing any of the three doses (500, 1000 or 2000 mcg/d).
FOUR‐FOLD DIFFERENT BDP DOSE COMPARISONS:
BDP 400 v 1600 mcg/d
A single parallel group study (Nathan 1997) of four weeks duration assessed BDP 400 v 1600 mcg/d . This was a large study (423 subjects) of fair quality (Jadad score 3) undertaken in a group of asthmatic patients who were currently treated with regular ICS. The percentage improvement in FEV1 compared to baseline was significantly greater in the 1600 mcg/d group (+16%) compared to the 400 mcg/d group (+7%) (p < 0.03). FEF25‐75, FVC, morning PEFR, evening PEFR, symptom scores, rescue beta2 agonist use and withdrawals due to clinical asthma exacerbation were also reported: no significant differences between intervention groups were found.
FIVE‐FOLD DIFFERENT DOSE COMPARISONS:
BDP 400 v 2000 mcg/d
A single crossover study (De Marzo 1988) with one week treatment periods assessed BDP 400 v 2000 mcg/d in asthmatics specifically sensitised to toluene diisocyanate. This was a study of high quality (Jadad score 4) but of small patient numbers (9 adults). No significant differences between treatments were apparent for FEV1, methacholine BHR (log 10 PD20 FEV1), or 8 am plasma cortisol.
ORAL CORTICOSTEROID TREATED ASTHMATICS
Oral corticosteroid sparing studies
Two parallel group studies of fair quality (Jadad score 3‐4), each of 6 months duration assessed the relative efficacy of different daily doses of BDP for their OCS sparing effect in adult asthmatics. Hummel 1992 assessed 148 subjects who were randomised to BDP 300 mcg/d or 1500 mcg/d. During a three month run‐in phase adjustments to prednisolone dose were made to obtain 'optimum' asthma control. Following randomisation daily prednisolone dose was tapered as much as possible whilst maintaining symptom scores, rescue beta2 agonist use and PEFR values as close as possible to those at end of run‐in. No significant difference in prednisolone dose reduction (mg/d), number of patients able to reduce prednisolone dose or the incidence of oropharyngeal side effects/oral Candidiasis were apparent between BDP 300 mcg/d and 1500 mcg/d. In the second smaller study (Tarlo 1988) 40 patients were randomised to BDP 800 mcg/d or 2000 mcg/d. During a variable run‐in phase of between two and 18 weeks the daily dose of prednisolone was tapered down to the lowest level possible before losing symptomatic control of asthma. Following randomisation the daily dose of prednisolone was reduced whilst maintaining symptomatic control. No significant differences between intervention groups were apparent for absolute end of trial daily oral prednisolone dose (mg/d).
Non oral corticosteroid sparing studies
A single crossover design study (Chatterjee 1980) of 8 weeks duration assessed the relative efficacy of BDP 400 mcg/d via MDI v 800 mcg/d via Rotahaler DPI in adult asthmatics, a proportion of whom were treated with regular prednisolone tablets for asthma control at the time of enrolment. No attempt to reduce the daily dose of prednisolone was made. No significant difference between treatment groups was apparent for diary card assessed morning or evening PEFR. FEV1 and FVC were reported, but it is not clear from the presentation of the results whether there were any differences between groups.
Discussion
This review has evaluated the small number of RCT's that have assessed the relative efficacy of BDP at different doses in long‐term asthma. Interpretation is complicated by the fact that a wide range of dose comparisons were made in studies, spanning less than two‐fold to five‐fold dose differences. The majority of studies assessing different doses of BDP in non‐oral steroid treated asthmatics were crossover studies, with small patient numbers. No significant differences between doses compared were found for most outcomes assessed by these trials. Because almost all assessed different daily dose combinations of BDP and could not be pooled in a meta‐analysis, these studies do not allow any firm conclusions to be drawn regarding dose response effect. However, the following conclusions can be made when considering the parallel design studies.
In non‐oral steroid treated asthmatics BDP does appear to exhibit a shallow dose response effect for a number of clinically relevant outcomes. Evidence for this comes from two large, fair quality parallel group studies, one undertaken in children, one in adults. Both were undertaken in subjects already receiving an ICS. Higher dose BDP appears to be more efficacious than lower dose BDP when assessed by a number of outcomes. When two fold (800 v 400 mcg/d) and four fold (1600 v 400 mcg/d) dose differences were compared, a small improvement in favour of higher dose was evident for change in FEV1 compared to baseline, WMD 9ml (95% CI 3 to 140ml), and percentage change in FEV1 compared to baseline (+16% v +7% p<0.03) respectively. BDP 800 mcg/d also resulted in small advantages over BDP 400 mcg/d for improvement in morning PEFR compared to baseline: WMD 11 L/min (95% CI 4 to 19 L/min); evening PEFR: WMD 8 L/min (95% CI 0 to 16 L/min); reduction in night‐time symptoms compared to baseline: WMD 0.13 (95% CI 0.04 to 0.22), and reduction in daytime beta2 agonist use, WMD 0.49 puffs/d (95% CI 0.02 to 0.96 puffs/d). Current asthma management guidelines (BTS 1997, GINA 1995, NHLBI 1997) recommend an increase in dose of BDP when control of symptoms, exacerbations and lung function cannot be achieved at lower daily doses. This is based on the assumption that higher doses lead to improved asthma control. These results provide some support for this approach, although the improvements seen in FEV1 and PEFR are of questionable clinical benefit. These studies did not provide any evidence for dose dependent differences in the experience of daytime symptoms or withdrawal rates due to asthma exacerbation. A dose response effect for histamine BHR was demonstrated in a single low quality crossover study where BDP 1000 mcg/d led to significant improvements compared to BDP 500 mcg/d (Carpentiere 1990). This outcome was not reported in any other trial.
The evidence concerning the relative efficacy of BDP as an oral steroid sparing agent in oral steroid dependent asthma comes from two relatively large parallel group studies of fair methodological quality. Because different doses of BDP were compared and different outcomes were reported, results could not be pooled. Different dose comparisons were made in individual studies (300 v 1500 mcg/d, 800 v 2000 mcg/d), but throughout these ranges no significant difference in oral prednisolone use (reduction compared to baseline or absolute daily dose) or number of patients able to reduce their oral prednisolone dose were apparent.
Methodological limitations:
Few studies met the inclusion criteria for review and individual studies assessed different BDP dose comparisons. The results of most studies could not therefore be pooled in a meaningful way. The majority of studies were crossover trials of small numbers of patients and may not have had sufficient power to detect clinically meaningful treatment differences. Many did not have ICS free washout periods between doses and significant carryover effects cannot be excluded. The strength of evidence from these individual studies is therefore low, and conclusions regarding relative effectiveness of the different BDP doses compared in these trials cannot be made.
Because few trials could be pooled subgroup analyses to explore the influence of asthma severity, prior inhaled corticosteroid use, patient age, delivery device and treatment duration could not be undertaken.
Authors' conclusions
Implications for practice.
A previous systematic review (Adams 2000) has shown that BDP results in clinically significant improvements in FEV1, PEFR and symptoms with reductions in rescue beta2 agonist use and likelihood of exacerbation when compared to placebo, although no evidence for a dose response was found for doses above 400 mcg/d. The evidence available from this review in which doses of BDP were directly compared does suggests a shallow dose response effect exists over a dose range of 400 to 1600 mcg/d in children and adults with non‐oral steroid treated asthma. Current guidelines recommend increasing the dose of BDP when asthma control is inadequate on lower daily doses. The findings of this review are consistent with the rationale for such an approach. However the improvements seen in FEV1 and PEFR are small, of questionable clinical significance and there is no evidence that doses over 400 mcg/d lead to reductions in symptoms or the likelihood of asthma exacerbations.
Implications for research.
There is a place for further studies assessing the relative benefits of different doses of BDP in the treatment of long‐term asthma. Drawing meaningful conclusions from this review was hampered by the fact that small, under‐powered crossover studies that could not be aggregated accounted for a high proportion of included trials. Further studies are needed to confirm the findings of the few studies that could be evaluated . A number of clinically important outcome measures were not reported in any of the studies included in this review, including health status/quality of life assessment, days lost from work/school due to exacerbations, GP attendance rates due to exacerbation and hospital admissions. These types of outcome can only be assessed in longer‐term (> 6 months) parallel group studies with sufficiently large numbers of patients such that clinically meaningful differences can be detected, if they truly exist. There is a place for such trials in the future.
In considering the issue of dose response two distinct questions can be posed. Firstly, do patients with poorly controlled asthma achieve greater benefit from higher as opposed to lower starting doses of BDP. Secondly, do patients with suboptimally controlled asthma already treated with BDP achieve better control if dose is increased. In order to fully answer these questions studies comparing different doses need to be conducted in both ICS naive and ICS treated asthmatics with clearly defined levels of baseline control in terms of FEV (% predicted), PEFR variability, symptoms, rescue beta2 agonist use and frequency of exacerbations. Although this review provides some evidence of a relative difference in efficacy between higher and lower doses, further studies with these design considerations are needed in order to gain a clearer picture of dose response in these separate clinical situations. Such studies should also allow an assessment as to whether dose response effects vary significantly with the degree of underlying asthma severity.
What's new
| Date | Event | Description |
|---|---|---|
| 21 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 23 July 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the support staff of the Cochrane Airways Group (Anna Bara, Jane Dennis and Steve Milan) for assistance with the electronic search and help in retrieving papers. We would also like to thank Dr Broder, Dr de Marzo, Dr Lal, Dr Molema and Dr So who were kind enough to provide additional information regarding their studies and Veronica Prentice of Glaxo Wellcome UK who searched company records for additional studies. Finally, we would like to thank Dr M Partridge who acted as an external referee and provided helpful comment and criticism.
Data and analyses
Comparison 1. BDP v BDP: Parallel design, no oral steroids, 100 mcg/d v 200 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in morning PEFR (L/min) compared to baseline | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 11.80 [‐6.41, 30.01] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 11.80 [‐6.41, 30.01] |
| 1.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 BDP v BDP: Parallel design, no oral steroids, 100 mcg/d v 200 mcg/d, Outcome 1 Change in morning PEFR (L/min) compared to baseline.
Comparison 2. BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (litres) compared to baseline | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.03, 0.15] |
| 1.2 Adults | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.03, 0.15] |
| 1.3 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in FEV1 (% predicted) compared to baseline | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐2.15, 5.15] |
| 2.1 Children | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐2.15, 5.15] |
| 2.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in FVC (litres) compared to baseline | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.00, 0.20] |
| 3.2 Adults | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.00, 0.20] |
| 3.3 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Change in Morning PEFR (litres/min) compared to baseline | 2 | 355 | Mean Difference (IV, Fixed, 95% CI) | 11.21 [3.60, 18.82] |
| 4.2 Adults | 1 | 238 | Mean Difference (IV, Fixed, 95% CI) | 10.4 [1.68, 19.12] |
| 4.3 Children | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 13.8 [‐1.79, 29.39] |
| 5 Change in Evening PEFR (litres/min) compared to baseline | 2 | 354 | Mean Difference (IV, Fixed, 95% CI) | 8.12 [0.20, 16.04] |
| 5.2 Adults | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | 6.80 [‐2.19, 15.79] |
| 5.3 Children | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 12.70 [‐4.06, 29.46] |
| 6 Change in daytime symptom score compared to baseline | 1 | 230 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.06, 0.18] |
| 6.2 Adults | 1 | 230 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.06, 0.18] |
| 6.3 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Change in night‐time symptom score compared to baseline | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.04, 0.22] |
| 7.2 Adults | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.04, 0.22] |
| 7.3 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Change in daytime use of beta2 agonist (pfs/d) compared to baseline | 1 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.02, 0.96] |
| 8.2 Adults | 1 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.02, 0.96] |
| 8.3 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in methacholine bronchial responsiveness (log doubling dose PD20 FEV1) compared to baseline | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.24, 1.24] |
| 9.1 Children | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.24, 1.24] |
| 9.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.79 [0.15, 393.02] |
| 10.1 Children | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.79 [0.15, 393.02] |
| 10.2 Adults | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Children and adults | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Oropharyngeal Candidasis (No. of patients) | 1 | 365 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.27 [0.14, 366.41] |
| 11.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 1 | 365 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.27 [0.14, 366.41] |
2.1. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 1 Change in FEV1 (litres) compared to baseline.
2.2. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 2 Change in FEV1 (% predicted) compared to baseline.
2.3. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 3 Change in FVC (litres) compared to baseline.
2.4. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 4 Change in Morning PEFR (litres/min) compared to baseline.
2.5. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 5 Change in Evening PEFR (litres/min) compared to baseline.
2.6. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 6 Change in daytime symptom score compared to baseline.
2.7. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 7 Change in night‐time symptom score compared to baseline.
2.8. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 8 Change in daytime use of beta2 agonist (pfs/d) compared to baseline.
2.9. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 9 Change in methacholine bronchial responsiveness (log doubling dose PD20 FEV1) compared to baseline.
2.10. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 10 Withdrawal due to asthma exacerbation (No. of patients).
2.11. Analysis.

Comparison 2 BDP v BDP: Parallel design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 11 Oropharyngeal Candidasis (No. of patients).
Comparison 3. BDP v BDP: Parallel design, no oral steroids, 400mcg/d v 1600 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [0.26, 9.00] |
| 1.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [0.26, 9.00] |
| 1.3 Adults and children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Oropharyngeal side effects (No. of patients) | 1 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.34, 1.79] |
| 2.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.34, 1.79] |
| 2.3 Children and adults | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 BDP v BDP: Parallel design, no oral steroids, 400mcg/d v 1600 mcg/d, Outcome 1 Withdrawal due to asthma exacerbation (No. of patients).
3.2. Analysis.

Comparison 3 BDP v BDP: Parallel design, no oral steroids, 400mcg/d v 1600 mcg/d, Outcome 2 Oropharyngeal side effects (No. of patients).
Comparison 4. BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 500 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morning PEFR (litres/min) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐82.26, 72.26] |
| 1.1 Adults | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐82.26, 72.26] |
| 1.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Evening PEFR (litres/min) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐77.87, 49.87] |
| 2.1 Adults | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐77.87, 49.87] |
| 2.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 500 mcg/d, Outcome 1 Morning PEFR (litres/min).
4.2. Analysis.

Comparison 4 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 500 mcg/d, Outcome 2 Evening PEFR (litres/min).
Comparison 5. BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
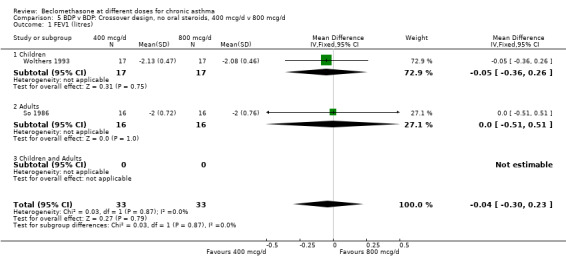
| 1 FEV1 (litres) | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.30, 0.23] |
| 1.1 Children | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.36, 0.26] |
| 1.2 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.51, 0.51] |
| 1.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FVC (litres) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.51, 0.51] |
| 2.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.51, 0.51] |
| 2.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Morning PEFR (litres/min) | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [‐35.23, 40.18] |
| 3.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [‐55.07, 89.07] |
| 3.2 Children | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐47.25, 41.25] |
| 3.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Evening PEFR (litres/min) | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | 3.87 [‐33.50, 41.25] |
| 4.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 14.0 [‐57.05, 85.05] |
| 4.2 Children | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐43.95, 43.95] |
| 4.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Daily beta2 agonist use (pfs/d) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.87, 1.47] |
| 5.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.87, 1.47] |
| 5.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
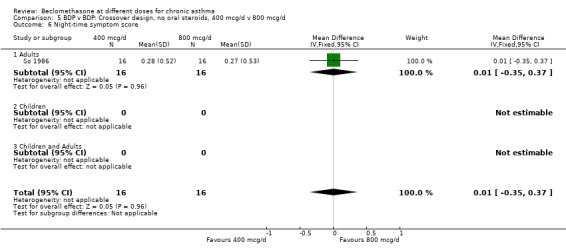
| 6 Night‐time symptom score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.35, 0.37] |
| 6.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.35, 0.37] |
| 6.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Daily cough score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.38, 0.26] |
| 7.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.38, 0.26] |
| 7.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 % symptom free days | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐25.20, 21.20] |
| 8.1 Children | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐25.20, 21.20] |
| 8.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 % symptom free nights | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐10.78, 10.78] |
| 9.1 Children | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐10.78, 10.78] |
| 9.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Plasma cortisol, timing not specified (micromol/litre) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.14, 0.10] |
| 10.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.14, 0.10] |
| 10.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Plasma cortisol 30 mins post 250 mcg tetracosactrin (micromol/litre) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
| 11.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
| 11.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 1 FEV1 (litres).
5.2. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 2 FVC (litres).
5.3. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 3 Morning PEFR (litres/min).
5.4. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 4 Evening PEFR (litres/min).
5.5. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 5 Daily beta2 agonist use (pfs/d).
5.6. Analysis.
Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 6 Night‐time symptom score.
5.7. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 7 Daily cough score.
5.8. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 8 % symptom free days.
5.9. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 9 % symptom free nights.
5.10. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 10 Plasma cortisol, timing not specified (micromol/litre).
5.11. Analysis.

Comparison 5 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 800 mcg/d, Outcome 11 Plasma cortisol 30 mins post 250 mcg tetracosactrin (micromol/litre).
Comparison 6. BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 1000 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.49, 0.49] |
| 1.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.49, 0.49] |
| 1.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
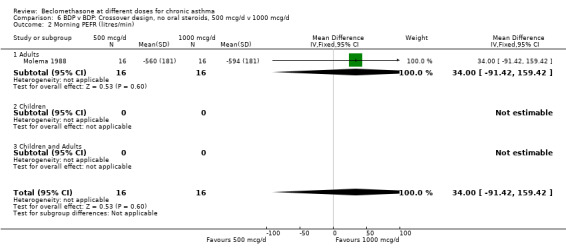
| 2 Morning PEFR (litres/min) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 34.0 [‐91.42, 159.42] |
| 2.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 34.0 [‐91.42, 159.42] |
| 2.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Evening PEFR (litres/min) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐104.46, 130.46] |
| 3.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐104.46, 130.46] |
| 3.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Daily inhaled beta2 agonist use (pfs/d) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐2.06, 2.38] |
| 4.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐2.06, 2.38] |
| 4.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Daily dysponea score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.23, 0.55] |
| 5.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.23, 0.55] |
| 5.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.

Comparison 6 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 1000 mcg/d, Outcome 1 FEV1 (litres).
6.2. Analysis.
Comparison 6 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 1000 mcg/d, Outcome 2 Morning PEFR (litres/min).
6.3. Analysis.

Comparison 6 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 1000 mcg/d, Outcome 3 Evening PEFR (litres/min).
6.4. Analysis.

Comparison 6 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 1000 mcg/d, Outcome 4 Daily inhaled beta2 agonist use (pfs/d).
6.5. Analysis.

Comparison 6 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 1000 mcg/d, Outcome 5 Daily dysponea score.
Comparison 7. BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 2000 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.45, 0.55] |
| 1.1 Adults | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.45, 0.55] |
| 1.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Methacholine bronchial responsiveness (log 10 PD20 FEV1) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.40, 0.54] |
| 2.1 Adults | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.40, 0.54] |
| 2.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 8am plasma cortisol (nmol/litre) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐15.0 [‐145.39, 115.39] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐15.0 [‐145.39, 115.39] |
| 3.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.

Comparison 7 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 2000 mcg/d, Outcome 1 FEV1 (litres).
7.2. Analysis.
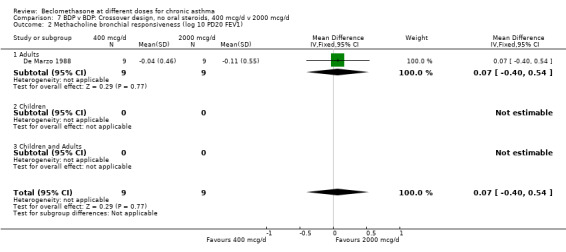
Comparison 7 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 2000 mcg/d, Outcome 2 Methacholine bronchial responsiveness (log 10 PD20 FEV1).
7.3. Analysis.

Comparison 7 BDP v BDP: Crossover design, no oral steroids, 400 mcg/d v 2000 mcg/d, Outcome 3 8am plasma cortisol (nmol/litre).
Comparison 8. BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 2000 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.45, 0.45] |
| 1.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.45, 0.45] |
| 1.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Morning PEFR (litres/min | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 52.0 [‐73.42, 177.42] |
| 2.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 52.0 [‐73.42, 177.42] |
| 2.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Evening PEFR (litres/min) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 19.0 [‐94.36, 132.36] |
| 3.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 19.0 [‐94.36, 132.36] |
| 3.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Daily inhaled beta2 agonist use (pfs/d) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐0.99, 3.11] |
| 4.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐0.99, 3.11] |
| 4.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Daily dyspnoea score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.22, 0.56] |
| 5.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.22, 0.56] |
| 5.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Morning plasma cortisol (micromol/litre) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.11, 0.05] |
| 6.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.11, 0.05] |
| 6.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.

Comparison 8 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 2000 mcg/d, Outcome 1 FEV1 (litres).
8.2. Analysis.

Comparison 8 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 2000 mcg/d, Outcome 2 Morning PEFR (litres/min.
8.3. Analysis.

Comparison 8 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 2000 mcg/d, Outcome 3 Evening PEFR (litres/min).
8.4. Analysis.

Comparison 8 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 2000 mcg/d, Outcome 4 Daily inhaled beta2 agonist use (pfs/d).
8.5. Analysis.

Comparison 8 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 2000 mcg/d, Outcome 5 Daily dyspnoea score.
8.6. Analysis.

Comparison 8 BDP v BDP: Crossover design, no oral steroids, 500 mcg/d v 2000 mcg/d, Outcome 6 Morning plasma cortisol (micromol/litre).
Comparison 9. BDP v BDP: Crossover design, no oral steroids, 1000 mcg/d v 2000 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.45, 0.45] |
| 1.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.45, 0.45] |
| 1.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Morning PEFR (litres/min) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐107.42, 143.42] |
| 2.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐107.42, 143.42] |
| 2.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Evening PEFR (litres/min) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐107.72, 119.72] |
| 3.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐107.72, 119.72] |
| 3.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Daily inhaled beta2 agonist use (pfs/d) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.04, 2.84] |
| 4.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.04, 2.84] |
| 4.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Daily dyspnoea score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.34, 0.36] |
| 5.1 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.34, 0.36] |
| 5.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.

Comparison 9 BDP v BDP: Crossover design, no oral steroids, 1000 mcg/d v 2000 mcg/d, Outcome 1 FEV1 (litres).
9.2. Analysis.

Comparison 9 BDP v BDP: Crossover design, no oral steroids, 1000 mcg/d v 2000 mcg/d, Outcome 2 Morning PEFR (litres/min).
9.3. Analysis.

Comparison 9 BDP v BDP: Crossover design, no oral steroids, 1000 mcg/d v 2000 mcg/d, Outcome 3 Evening PEFR (litres/min).
9.4. Analysis.

Comparison 9 BDP v BDP: Crossover design, no oral steroids, 1000 mcg/d v 2000 mcg/d, Outcome 4 Daily inhaled beta2 agonist use (pfs/d).
9.5. Analysis.

Comparison 9 BDP v BDP: Crossover design, no oral steroids, 1000 mcg/d v 2000 mcg/d, Outcome 5 Daily dyspnoea score.
Comparison 10. BDP v BDP: Parallel design, on oral steroids, 300 mcg/d v 1500 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in daily dose of oral prednsiolone (mg/d) | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐3.09, 2.89] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐3.09, 2.89] |
| 2 Able to reduce daily dose of oral prednisolone (No. of patients) | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.39, 2.07] |
| 2.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.39, 2.07] |
| 3 Oropharyngeal side effects (No of patients) | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.53, 2.02] |
| 3.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.53, 2.02] |
| 4 Oral Candidiasis (No. of patients) | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.18, 2.31] |
| 4.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.18, 2.31] |
10.1. Analysis.

Comparison 10 BDP v BDP: Parallel design, on oral steroids, 300 mcg/d v 1500 mcg/d, Outcome 1 Reduction in daily dose of oral prednsiolone (mg/d).
10.2. Analysis.

Comparison 10 BDP v BDP: Parallel design, on oral steroids, 300 mcg/d v 1500 mcg/d, Outcome 2 Able to reduce daily dose of oral prednisolone (No. of patients).
10.3. Analysis.

Comparison 10 BDP v BDP: Parallel design, on oral steroids, 300 mcg/d v 1500 mcg/d, Outcome 3 Oropharyngeal side effects (No of patients).
10.4. Analysis.

Comparison 10 BDP v BDP: Parallel design, on oral steroids, 300 mcg/d v 1500 mcg/d, Outcome 4 Oral Candidiasis (No. of patients).
Comparison 11. BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily dose of oral prednisolone (mg/) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 4.30 [‐0.42, 9.02] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 4.30 [‐0.42, 9.02] |
| 2 FEV1 (litres) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.43, 0.99] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.43, 0.99] |
| 3 FVC (litres) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.16, 0.38] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.16, 0.38] |
| 4 Morning PEFR (L/min) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐80.70, 92.70] |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐80.70, 92.70] |
| 5 Evening PEFR (L/min) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐92.56, 84.56] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐92.56, 84.56] |
| 6 Daytime asthma symptom score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.04, 1.04] |
| 6.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.04, 1.04] |
| 7 Night‐time asthma symptom score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.54, 0.54] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.54, 0.54] |
11.1. Analysis.

Comparison 11 BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d, Outcome 1 Daily dose of oral prednisolone (mg/).
11.2. Analysis.

Comparison 11 BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d, Outcome 2 FEV1 (litres).
11.3. Analysis.

Comparison 11 BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d, Outcome 3 FVC (litres).
11.4. Analysis.

Comparison 11 BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d, Outcome 4 Morning PEFR (L/min).
11.5. Analysis.

Comparison 11 BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d, Outcome 5 Evening PEFR (L/min).
11.6. Analysis.

Comparison 11 BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d, Outcome 6 Daytime asthma symptom score.
11.7. Analysis.
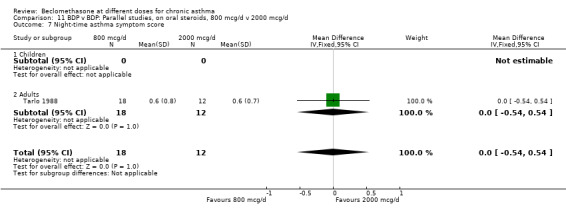
Comparison 11 BDP v BDP: Parallel studies, on oral steroids, 800 mcg/d v 2000 mcg/d, Outcome 7 Night‐time asthma symptom score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carmichael 1978.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind, double dummy Design: crossover, no washout period Excluded: not stated Withdrawals: not stated Baseline characteristics: demographic characteristics by treatment sequence not presented Jadad score: 2 | |
| Participants | 20 adults: 11M 9F
Age range: 30‐65 years
Inclusion criteria:
Patients with chronic asthma already receiving BDP (not further defined). Requiring inhaled salbutamol on most days of week to control symptoms
Exclusion criteria:
None stated Asthma control Baseline FEV1: not stated Symptom frequency: needing salbutamol inhaler on most days of week to control symptoms |
|
| Interventions | 1. BDP 100 mcg 1 pf 4xdaily (400 mcg/d) 2. BDP 150 mcg 1 pf 4xdaily (600 mcg/d) Delivery device: Rotahaler DPI 3. 100 mcg 1 pf 4xdaily (400 mcg/d) via MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daytime wheeze, dyspnoea and cough score Night‐time wheeze, dyspnoea and cough score Use of beta2 agonist (total over treatment period) Short tetracosactrin test | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Carpentiere 1990.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 3 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, 3 week washout period Masking: single blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 10 adults: 6M 4F
Age range: 26‐45 years
Inclusion criteria:
Adult patients with clinically stable asthma
15% or greater improvement in FEV1 after inhaled beta2 agonist
Exclusion criteria:
Oral corticosteroid use within previous 6 months Asthma control: Baseline FEV1: 63 ‐92 (% predicted) Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 250 mcg 1 pf 2xdaily (500 mcg/d) 2. BDP 250 mcg 2 pfs 2xdaily (1000 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Histamine bronchial responsivness (PC20 FEV1) mg/ml Cough score Wheeze score Dyspnoea score Beta2 agonist use | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Chatterjee 1980.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout period Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 65 adults: 49M 16F
Age range: 20‐79 years
Inclusion criteria:
Adults patients with asthma (not further defined)
Requiring treatment with inhaled BDP 400 mcg/d for at least two months
Exclusion criteria:
None stated Asthma control: Baseline FEV1: not stated Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 100 mcg 1 pf 4xdaily (400 mcg/d) via MDI 2. BDP 200 mcg 1 pf 4xdaily (800 mcg/d) via Rotahaler DPI |
|
| Outcomes | FEV1FVCFEV1/FVC ratioMorning PEFREvening PEFR | |
| Notes | No reply from author to clarify details of randomisation method. A proportion of patients were receiving oral steroids at enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
De Marzo 1988.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 1 week Randomised: yes (computer generated random number sequence) Allocation concealment: yes Design: crossover, 1 week washout period Masking: double blind Excluded: stated (none) Withdrawals: stated (none) Baseline characteristics: demographic characteristics by treatment sequence not presented Jadad score: 4 | |
| Participants | 9 adults: 8M 1F
Age range: 20‐48 years
Inclusion criteria:
Subjects TDI induced asthma
Demonstrable dual or late only asthmatic reaction following TDI exposure
No exposure to TDI for at least 2 weeks
Exclusion criteria:
Respiratory tract infection within last 8 weeks Asthma control: Baseline FEV1: 78 ‐115 (% predicted) Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 50 mcg 4 pfs 2xdaily (400mcg/d) 2. BDP 250 mcg 4 pfs 2xdaily (2000mcg/d) 3. Placebo: 4 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 Methacholine BHR (PD20 FEV1) Inhalation challenge with TDI Serum cortisol | |
| Notes | Reply from author confirming method of random order generation and use of allocation concealment. Study examines the effect of low and high dose inhaled steroids and placebo on airway responsiveness to methacholine in TDI sensitised subjects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number sequence |
| Allocation concealment? | Unclear risk | Information not available |
Drepaul 1989.
| Methods | Setting: UK multicentre study, primary care Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 365 adults: 196M 169F
Mean (SD) age: 800 mcg/d group 43.1(17.9)
yrs 400 mcg/d group 41.2 (18.3) yrs
Inclusion criteria:
Over 12 year of age
Using a MDI to deliver BDP 400 mcg/d or less
15% or greater improvement in FEV1
following inhaled beta2 agonist
Exclusion criteria:
PEFR (% predicted) < 30
Use or oral steroids
Recent respiratory tract infection
Chronic non‐pulmonary disease
Use of delivery device other than MDI
Pregnancy Asthma control: Baseline FEV1: not stated Baseline symptom frequency: not stated |
|
| Interventions | BDP 100 mcg 2 pfs 2xdaily (400 mcg/d) via MDI BDP 200 mcg 2 pfs 2xdaily (800 mcg/d) via Diskhaler DPI |
|
| Outcomes | FEV1
FVC
Morning PEFR
Evening PEFR
Daytime symptom score
Night‐time symptom score
Daily beta2 agonist use All outcomes reported as change compared to baseline |
|
| Notes | Reply from author but unable to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Hampel 1997.
| Methods | Setting: multicentre study USA, secondary care Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: unclear Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic data presented Jadad score: 1 | |
| Participants | 270 adults
Age range: not stated
Inclusion criteria:
Patients with mild to moderate asthma: no further details
Exclusion criteria:
not stated Asthma control: Baseline FEV1: not stated Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 50 mcg 2xdaily (100 mcg/d) 2. BDP 100 mcg 2xdaily (200 mcg/d) Delivery device: HFA propellent MDI |
|
| Outcomes | Change in morning PEFR compared to baseline | |
| Notes | Study presented in abstract form only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Hummel 1992.
| Methods | Setting: multicentre study Germany, hospital outpatient clinic Length of intervention period: 6 months Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 143 adults: 65M 78F
Age range: 18 to 65 years
Inclusion criteria:
Prior to run‐in requiring prednisolone 10‐40 mcg/d for control of asthma symptoms for 6 months or longer
Severe symptoms: no daytime symptom free intervals, frequent night‐time wakening, limited exercise capacity.
At end of 3 month run in period on BDP 300 mcg/d: treatment 'optimised' i.e. symptoms at most 'mild or moderate', requiring < 10 puffs rescue beta2 agonist daily, PEFR > 60 (% predicted)
Exclusion criteria:
Asthma treatment not 'optimised' at end of run‐in phase
Serious co‐existent disease Asthma control: Baseline FEV1: not stated Symptom frequency: no symptom free intervals, regular night time asthma attacks |
|
| Interventions | 1. BDP 100 mcg 3xdaily (300 mcg/d) 2. BDP 500 mcg 3xdaily (1500 mcg/d) Delivery device: MDI+spacer |
|
| Outcomes | Reduction in daily dose or oral prednisolone (mg) Able to reduce daily dose of oral prednisolone (No. of patients) Oropharyngeal side effects | |
| Notes | All patients were treated with BDP 300 mcg/d during a three month run‐in period prior to randomisation. In patients on a starting prednisolone dose of 20 mg/d or greater, dose lowering by 5mg per week was done until there was a deterioration in PEFR values, increase in rescue beta2 agonist use > 10 puffs/d, or worsening asthma symptoms. For patients reaching this dose level and those starting on less than 20 mg/d dose adjustments were made by 1mg intervals 1‐2 weekly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Lal 1980.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes (computer generated random number sequence) Allocation concealment: yes (coded, sealed envelopes) Design: crossover, no washout period Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 20 adults: 6M 14F
Age range: 16‐58 years
Inclusion criteria:
Patients with asthma (not otherwise defined)
Regularly using inhaled BDP
Exclusion criteria:
Current use of oral steroids Asthma control: Baseline FEV1: not stated Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 50 mcg 2 pfs 3xdaily (300 mcg/d) via MDI 2. BDP 200 mcg 1 actuation 3xdaily (600 mcg/d) via Rotahaler DPI |
|
| Outcomes | FEV1 FVC FEV1/FVC ratio Morning PEFR Evening PEFR Daily use of beta2 agonists Mid‐morning plasma cortisol Oral Candidiasis | |
| Notes | Reply from author confirming method of random order generation and use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number sequence |
| Allocation concealment? | Low risk | Coded, sealed envelopes |
Molema 1988.
| Methods | Setting: The Netherlands, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: yes Design: crossover, no washout period Masking: single blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: demographic characteristics by treatment sequence not presented Jadad score: 2 | |
| Participants | 16 adults: 14M 2F
Age range: 25‐58 years
Inclusion criteria:
Clinical diagnosis of asthma (not otherwise defined) sufficiently severe to require treatment with inhaled steroids
FEV1 of 1 litre or greater
FEV1 (% predicted) 50 or greater
Histamine bronchial responsiveness (PC20 FEV1) 8mg/ml or less
Exclusion criteria:
Use of systemic steroids within last 12 months Asthma control: Baseline FEV1: > 50 (% predicted) Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 250 mcg 2 pfs daily (500 mcg/d) 2. BDP 250 mcg 4 pfs daily (1000mcg/d) 3. BDP 250 mcg 8 pfs daily (2000 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Daily dyspnoea score Daily cough score Daily expectoration score Daily beta2 agonist use Fasting morning plasma cortisol Plasma cortiol post tetracosactrin Blood eosinophil count | |
| Notes | Reply from author confirming use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Nathan 1997.
| Methods | Setting: USA, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 423 patients: 170M 251F
Age range: 12‐65 years
Inclusion criteria:
Clinical diagnosis of asthma of at least 2 years duration
15% or greater improvement in FEV1 after inhaled beta2 agonist
FEV1 (% predicted) 50‐80
Absence of other significant illness
Requirement of at least a minimum dose of ICS for asthma control
Exclusion criteria:
None stated Asthma control: Baseline FEV1: 50 ‐80 (% predicted) Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 42mcg 4 pfs 2xdaily (336mcg/d) 2. BDP 84mcg 2 pfs 2xdaily (336mcg/d) 3. BDP 84mcg 8 pfs 2xdaily (1344mcg/d) Delivery device: MDI |
|
| Outcomes | % change in FEV1 compared to baseline FEF 25‐50 FVC Morning PEFR Evening PEFR Asthma symptom score Use of rescue beta2 agonist | |
| Notes | Reply from author but unable to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Smith 1986.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout period Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: No separate sequence demographic data Jadad score: 4 | |
| Participants | 21 adults: 10M 11F
Age range: 23‐71 years
Inclusion criteria:
Diagnosis of asthma (not otherwise defined)
20% or greater improvement in PEFR or FEV1 after inhaled beta2 agonist
Current requirement for inhaled BDP 400 mcg/d
No asthma exacerbations within preceding 2 months
Exclusion criteria:
Current use of oral steroids Asthma control: Baseline FEV1: not stated Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 50 mcg 2 pfs 4xdaily (400 mcg/d) 2. BDP 250 mcg 1 pf 2xdaily (500 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
So 1986.
| Methods | Setting: Hong Kong, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes (computer generated random number sequence) Allocation concealment: yes (coded, sealed envelopes) Design: crossover, no washout period Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 16 patients: 6M 10F
Age range: 15‐54 years
Inclusion criteria:
Patients older than 15 years with a diagnosis of asthma (not otherwise defined)
Receiving inhaled BDP 400 mcg/d
15% or greater improvement in spirometry following inhaled beta2 agonist
Exclusion criteria:
Current use of oral steroids Asthma control: Baseline FEV1: not stated Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 100 mcg 1 pf 4xdaily (400 mcg/d) via MDI 2. BDP 200 mcg 2 pfs 2xdaily (800 mcg/d) via Rotahaler DPI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Night‐time symptom score Daily cough score Daily beta2 agonist use Plasma cortisol Plasma cortisol 30min post 250 mcg IM Synathsen | |
| Notes | Reply from author confirming method of random order generation and use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number sequence |
| Allocation concealment? | Low risk | Coded, sealed envelopes |
Tarlo 1988.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 6 months Randomisation: yes, method not stated Allocation concealment: yes (coded, sealed envelopes) Design: parallel Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 40 adults: 22M 18F
Mean (SD) age: 52 (15) years
Inclusion criteria:
Clinical diagnosis of asthma based on a history of episodic airways obstruction/wheeze 15% or greater improvement in FEV1 following inhaled beta2 agonist
Requiring maintenance oral steroids for asthma control
Exclusion criteria:
< 16 years of age
Pregnancy
Co‐existent significant illness Asthma control: Baseline FEV1: not stated Baseline symptom frequency: not stated |
|
| Interventions | 1. BDP 800 mcg/d 2. BDP 2000 mcg/d Delivery device: MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daily dose oral prednisolone Daily beta2 agonist use Daytime asthma symptom score Night‐time asthma symptom score | |
| Notes | Reply from author confirming use of allocation concealment. During run‐in period oral prednisolone dose was reduced at twice weekly interval by 2.5mg/d to the lowest level possible before losing symptomatic control of asthma, or reduced PEFR values. During trial changes in prednisolone dose were based on diary card recorded symptoms, PEFR and pulmonary function tests'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Low risk | Coded, sealed envelopes |
Verberne 1998.
| Methods | Setting: multicentre study The Netherlands, paediatric outpatient clinic Length of intervention period: 12 months Randomisation: yes (computer generated random number sequence) Allocation concealment: yes (independent randomisation centre with coded schedule) Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 177 children: 112M 65F
Age range: 6‐16 years
Inclusion criteria:
Mild to moderate asthma (ATS criteria 1987)
FEV1 55‐90 (% predicted)
Methacholine BHR (PD20 FEV1) 150 mcg or less
Free of asthma exacerbations during previous month
Requiring 200‐800 mcg/d of inhaled corticosteroid for 3 months or longer
Exclusion criteria:
Respiratory tract infection in last month Asthma control: Baseline FEV1: 55‐90 (% predicted) Symptom frequency: not stated |
|
| Interventions | 1. BDP 200 mcg 2xdaily (400 mcg/d) 2. BDP 400 mcg 2xdaily (800 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | Outcomes reported as change compared to baseline: FEV1 (% predicted) Methacholine BHR (PD20 FEV1) Morning PEFR Evening PEFR Withdrawal due to asthma exacerbation |
|
| Notes | Study also included a treatment arm with BDP 400+salmeterol: results not considered in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number sequence |
| Allocation concealment? | Low risk | Independent randomisation centre with coded schedule |
Wolthers 1993.
| Methods | Setting: Denmark, paediatric outpatient clinic Length of intervention period: 2 weeks Randomisation: yes (computer generated random number sequence) Allocation concealment: yes Design: 3 way crossover, 2 week washout periods Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 19 children: 15M 4F
Age range: 7‐14 years
Inclusion criteria:
Mild asthma requiring treatment with as needed beta2 agonists only
Exclusion criteria:
Use of inhaled or oral corticosteroids in the preceding 2 months Asthma control: Baseline FEV1: not stated Symptom frequency: not stated |
|
| Interventions | 1. BDP 200 mcg 1 actuation 2xdaily (400 mcg/d) 2. BDP 400 mcg 1 actuation 2xdaily (800 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR % symptom free days % symptom free nights Lower leg growth rate by knemometry | |
| Notes | A fluticasone (200 mcg/d) treatment group was also included: results not considered in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number sequence |
| Allocation concealment? | Unclear risk | Information not available. |
Abreviations
ATS: American Thoracic Society; BDP: beclomethasone dipropionate; BHR: bronchial hyper‐responsiveness; DPI: dry powder inhaler; FEV1: forced expired volume in one second; FVC: forced vital capacity; HFA: hydrofluroalkane; MDI: metered dose inhaler mcg/d: micrograms/day; PEFR: peak expiratory flow rate; PC20 FEV1: inhalant concentration (mg/ml) required to produce a 20% fall in FEV1 from baseline; PD20 FEV1: cumulative inhalant dose (mg) required to produce a 20% fall in FEV1 from baseline; TDI: toluene diisocyanate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 1998 | Delivery device comparison (Clickhaler DPI versus MDI+Volumatic spacer) |
| Ayres 1998 | Delivery device comparison (Clickhaler DPI versus MDI+Volumatic spacer) |
| Bisgaard 1984 | Delivery device comparison (MDI versus Rotahaler DPI), equal nominal daily dose of BDP |
| Brown 1993 | Delivery device comparison (MDI versus MDI+Volumatic spacer), equal nominal daily dose of BDP |
| Catena 1993 | Delivery device comparison (MDI versus Rotahaler DPI), equal nominal daily dose of BDP |
| D'Arcais 1998 | Not an RCT |
| Dahl 1997 | MDI propellent comparison (CFC versus HFA‐134a), equal nominal daily dose of BDP |
| Davies 1998 | Patients randomised to either BDP HFA‐134a 800 mcg/d or BDP CFC 1500 mcg/d. Designed as propellent equivalence study |
| Edmunds 1979 | Nominal doses of inhaled beclomethasone not stated |
| Gaddie 1973 | Not an RCT |
| Girbino 1996 | Delivery device comparison (MDI versus Jet spacer), equal nominal daily dose of BDP |
| Gross 1997 | Propellent comparison (HFA‐134a versus CFC) |
| Koskela 1998 | Delivery device comparison (Easyhaler DPI versus MD+Volumatic spacer), equal nominal daily dose BDP |
| Laurikainen 1994 | Comparison of different BDP formulations via MDI (Becotide versus Beclomet), with a further phase concerning delivery device comparison (Volumatic spacer versus InspirEase collapsible spacer), same nominal daily dose of BDP |
| Magnussen 1998 | Propellent comparison (HFA134a versus CFC) |
| Mairs 1995 | Delivery device comparison (MDI+Volumatic spacer versus MDI+Integra spacer), equal nominal daily dose of BDP |
| Matthys 1998 | Dose schedule comparison: patients randomised to 50 mcg 4 pfs 2xdaily (400 mcg/d) via HFA propellent MDI or 100 mcg 2pfs 2xdaily (400 mcg/d) via HFA propellent MDI |
| Mecoy 1980 | Dose schedule comparison: patients randomised to receive equal nominal dialy doses of BDP via either twice or four times daily regimen |
| Nell 1998 | Comparisons of two formulations of BDP (Beclate versus Becotide), equal nominal daily dose BDP |
| Nieminen 1998 | Delivery device comparison (Beclomet Easyhaler versus MDI+Volumatic spacer), equal nominal daily dose of BDP |
| Poukkula 1998 | Delivery device comparison (Easyhaler versus MDI+Volumatic spacer), equal nominal daily dose of BDP |
| Salzman 1988 | Delivery device comparison (MDI versus MDI+Aerochamber spacer), equal nominal daily dose of BDP |
| Soria 1998 | Single dose comparison of the bioavailability of oral versus inhaled BDP |
| Stradling 1998 | Delivery device comparison (Clickhaler DPI versus MDi+Volumatic spacer), nominal daily dose used with each device not stated |
| Vidgren 1994 | Delivery device comparison (MDI+Volumatic spacer versus Diskhaler DPI versus Easyhaler DPI), equal nominal daily dose of BDP |
| Wijngaarden 1998 | Delivery device comparison (Rotahaler DPI versus Cyclohaler DPI ), equal nominal daily dose of BDP |
| Woodman 1993 | Delivery device comparison (conventional MDI versus breath‐actuated Autohaler MDI), equal nominal daily dose BDP |
Contributions of authors
Nick Adams retrieved papers identified by electronic search, handsearched additional sources for relevent studies, assessed trials for methodological quality, contacted authors to clarify details of trial design and/or request missing data, extracted data from included trials and wrote text of review. Janine Bestall retrieved papers identified by search, assessed trials for methodological quality, contacted authors for clarification or trial details and/or request missing data. Paul Jones provided editorial support .
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Carmichael 1978 {published data only}
- Carmichael J, Duncan D, Crompton GK. Beclomethasone dipropionate dry‐powder inhalation compared with conventional aerosol in chronic asthma. British Medical Journal 1978;2(6138):657‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpentiere 1990 {published data only}
- Carpentiere G, Marino S, Castello F, Baldanza C, Bonanno CT. Dose‐related effect of beclomethasone dipropionate on airway responsiveness in asthma. Respiration 1990;57(2):100‐3. [DOI] [PubMed] [Google Scholar]
Chatterjee 1980 {published data only}
- Chatterjee SS, Butler AG. Beclomethasone dipropionate in asthma: a comparison of two methods of administration. British Journal of Diseases of the Chest 1980;74(2):175‐9. [DOI] [PubMed] [Google Scholar]
De Marzo 1988 {published data only}
- Marzo N, Fabbri LM, Crescioli S, Plebani M, Testi R, Mapp CE. Dose‐dependent inhibitory effect of inhaled beclomethasone on late asthmatic reactions and increased responsiveness to methacholine induced by toluene diisocyanate in sensitised subjects. Pulmonary Pharmacology 1988;1(1):15‐20. [DOI] [PubMed] [Google Scholar]
Drepaul 1989 {published data only}
- Drepaul BA, Payler DK, Qualtrough JE, Perry LJ, Bryony F, Reeve A, et al. Becotide or becodisks? A controlled study in general practice. Clinical Trials Journal 1989;26(5):335‐44. [Google Scholar]
Hampel 1997 {published data only}
- Hampel F, Lisberg E, Vanden Burgt J, Henon C, Stampone P. 50 mcg twice daily of ultrafine HFA‐beclomethasone dipropionate aerosol improves asthma control in adult patients. American Journal of Respiratory and Critical Care Medicine. 1997; Vol. 155, issue 4:A666.
Hummel 1992 {published data only}
- Hummel S, Lehtonen L. Comparison of oral‐steroid sparing by high‐dose and low‐dose inhaled steroid in maintenance treatment of severe asthma. Lancet 1992;340(8834‐5):1483‐7. [DOI] [PubMed] [Google Scholar]
Lal 1980 {published data only}
- Lal S, Malhotra SM, Gribben MD, Butler AG. Beclomethasone dipropionate aerosol compared with dry powder in the treatment of asthma. Clinical Allergy 1980;10(3):259‐62. [DOI] [PubMed] [Google Scholar]
Molema 1988 {published data only}
- Molema J, Lammers JW, Herwaarden CL, Folgering HT. Effects of inhaled beclomethasone dipropionate on beta 2‐receptor function in the airways and adrenal responsiveness in bronchial asthma. European Journal of Clinical Pharmacology 1988;34(6):577‐83. [DOI] [PubMed] [Google Scholar]
Nathan 1997 {published data only}
- Nathan RA, Nolop KB, Cuss FM, Lorber RR. A comparison of double‐strength beclomethasone dipropionate (84 microg) MDI with beclomethasone dipropionate (42 microg) MDI in the treatment of asthma [see comments]. Chest 1997;112(1):34‐9. [DOI] [PubMed] [Google Scholar]
Smith 1986 {published data only}
- Smith MJ, Hodson ME. Twice daily beclomethasone dipropionate administered with a concentrated aerosol inhaler: efficacy and patient compliance. Thorax 1986;41(12):960‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
So 1986 {published data only}
- So SY, Lam WK. Twice daily administration of beclomethasone dipropionate dry‐powder in the management of chronic asthma. Asian Pacific Journal of Allergy & Immunology 1986;4(2):129‐32. [PubMed] [Google Scholar]
Tarlo 1988 {published data only}
- Tarlo SM, Broder I, Davies GM, Leznoff A, Mintz S, Corey PN. Six‐month double‐blind, controlled trial of high dose, concentrated beclomethasone dipropionate in the treatment of severe chronic asthma. Chest 1988;93(5):998‐1002. [DOI] [PubMed] [Google Scholar]
Verberne 1998 {published data only}
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. American Journal of Respiratory & Critical Care Medicine 1998;158(1):213‐9. [DOI] [PubMed] [Google Scholar]
Wolthers 1993 {published data only}
- Wolthers OD, Pedersen S. Short term growth during treatment with inhaled fluticasone propionate and beclomethasone diproprionate. Archives of Disease in Childhood 1993;68(5):673‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 1998 {published data only}
- Andrews B, Morice AH, Taylor M. Beclomethasone dipropionate (BDP) delivered via a novel dry powder inhaler (DPI) reduces bronchial hyperresponsivness. European Respiratory Journal 1998;12(Suppl 28):67S. [Google Scholar]
Ayres 1998 {published data only}
- Ayres J, Laszlo G. Efficacy and safety of a novel beclomethasone dipropionate (BDP) dry powder inhaler (DPI). European Respiratory Journal 1998;12(Suppl 28):67S. [Google Scholar]
Bisgaard 1984 {published data only}
- Bisgaard H, Andersen JB, Bach‐Mortensen N, Bertelsen A, Friis B, Koch C, et al. A clinical comparison of aerosol and powder administration of beclomethasone dipropionate in childhood asthma. Allergy: European Journal of Allergy & Clinical Immunology 1984;39(5):365‐9. [DOI] [PubMed] [Google Scholar]
Brown 1993 {published data only}
- Brown PH, Greening AP, Crompton GK. Large volume spacer devices and the influence of high dose beclomethasone dipropionate on hypothalamo‐pituitary‐adrenal axis function. Thorax 1993;48(3):233‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catena 1993 {published data only}
- Catena E, Girbino G, Gunella G, Cantini L, Monici Preti PA. Beclomethasone dipropionate administered via two powder inhalers in adult patients affected by stable bronchial asthma. European Journal of Clinical Research 1993;4:107‐115. [Google Scholar]
D'Arcais 1998 {published data only}
- D'Arcais AF, Esposto G, Mariani E, Franco M. Inhaled steroids and adrenal function in asthmatic children. Pediatric Asthma Allergy & Immunology 1998;12(2):117‐21. [Google Scholar]
Dahl 1997 {published data only}
- Dahl R, Ringdal N, Ward SM, Stampone P, Donnell D. Equivalence of asthma control with new CFC‐free formulation HFA‐134a beclomethasone dipropionate and CFC‐beclomethasone dipropionate. British Journal of Clinical Practice 1997;51(1):11‐5. [PubMed] [Google Scholar]
Davies 1998 {published data only}
- Davies R, O'Connor BO, Donnel D, Stampone P. Pulmonary function in moderately severe asthma controlled at a significantly lower total daily dose of steroid from new CFC‐free inhaler. American Journal of Respiratory and Critical Care Medicine. 1997; Vol. 155, issue 4:A666.
- Davies RJ, Stampone P, O'Connor BJ. Hydrofluoroalkane‐134a beclomethasone dipropionate extrafine aerosol provides equivalent asthma control to chlorofluorocarbon beclomethasone dipropionate at approximately half the total daily dose.. Respiratory Medicine 1998;92(Suppl A):23‐31. [DOI] [PubMed] [Google Scholar]
Edmunds 1979 {published data only}
- Edmunds AT, McKenzie S, Tooley M, Godfrey S. A clinical comparison of beclomethasone dipropionate delivered by pressurised aerosol and as a powder from a rotahaler. Archives of Disease in Childhood 1979;54(3):233‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaddie 1973 {published data only}
- Gaddie J, Petrie GR, Reid IW, Skinner C, Sinclair DJ, Palmer KN. Aerosol beclomethasone dipropionate: a dose‐response study in chronic bronchial asthma. Lancet 1973;2(7824):280‐1. [DOI] [PubMed] [Google Scholar]
Girbino 1996 {published data only}
- Girbino G, Lauriello G, Ando F, Cantini L. Beclomethasone dipropionate given to adult asthmatics through a new spacer device: Effects of high‐dose administration. Advances in Therapy 1996;13(4):220‐9. [PubMed] [Google Scholar]
Gross 1997 {published data only}
- Gross G, Chervisnsky P, Ramsdell J, Vanden Burgt J. Half the daily dose of new CFC‐free formulation steroid achieves equivalent pulmonary function in moderate asthma. American Journal of Respiratory and Critical Care Medicine. 1998; Vol. 155, issue 4:A666.
Koskela 1998 {published data only}
- Koskela T, Toivanen P, Silvasti M, Kauppinen R, Tukiainen H. A new multidose powder inhaler (MPI) is clinically equivalent to a metered dose inhaler (MDI) with spacer in the treatment of steroid‐naïve asthmatic patients with beclomethasone. American Journal of Respiratory and Critical Care Medicine. 1998; Vol. 157, issue 3:A637.
Laurikainen 1994 {published data only}
- Laurikainen K, Poukkula A, Korhonen P, Lehtonen L, Vidgren M, Silvasti M. Comparison of two beclomethasone dipropionate inhalation aerosol spacer combinations in the treatment of asthma. International Journal of Clinical Pharmacology & Therapeutics 1994;32(6):293‐8. [PubMed] [Google Scholar]
Magnussen 1998 {published data only}
- Magnussen H. In moderate stable asthma 400 mcg/d beclomethasone dipropionate (BDP) delivered by metered dose inhaler (MDI) with HFA 134A propellant is as effective as 1000 mcg/d BDP inhaled from MDI containing CFC. European Respiratory Journal. 1998; Vol. 12, issue Suppl 28:61S.
Mairs 1995 {published data only}
- Mairs ML. Clinical evaluation of a new compact spacer device for the administration of beclomethasone dipropionate to adult asthmatics. British Journal of Clinical Research 1995;6:31‐44. [Google Scholar]
Matthys 1998 {published data only}
Mecoy 1980 {published data only}
- Mecoy RJ, Laby B. Beclomethasone diproprionate in twice daily treatment of asthma. Australian Family Physician 1980;9(10):721‐6. [PubMed] [Google Scholar]
Nell 1998 {published data only}
- Nell H, Cyster H, Louw C, Williams Z, Bardin PJ, Joubert JR. Therapeutic equivalence of two formulations of beclomethasone dipropionate (BDP) in adult asthmatic patients. European Respiratory Journal 1998;12(Suppl 28):64S. [PubMed] [Google Scholar]
Nieminen 1998 {published data only}
Poukkula 1998 {published data only}
- Poukkula A, Alanko K, Kilpio K, Knuuttila A, Koskinen S, Laitinen J, et al. Comparison of a multidose powder inhaler containing beclomethasone dipropionate (BDP) with a BDP metered dose inhaler with spacer in the treatment of asthmatic patients. Clinical Drug Investigation 1998; Vol. 16, issue 2:101‐10. [DOI] [PubMed]
Salzman 1988 {published data only}
- Salzman GA, Pyszczynski DR. Oropharyngeal candidiasis in patients treated with beclomethasone dipropionate delivered by metered‐dose inhaler alone and with Aerochamber. Journal of Allergy & Clinical Immunology 1988;81(2):424‐8. [DOI] [PubMed] [Google Scholar]
Soria 1998 {published data only}
- Soria I, Harrison LI, Machacek JH, Cline AC, Stampone PA. Beclomethasone relative availability of oral versus inhaled beclomethasone dipropionate from an HFA‐134A metered dose inhaler. Biopharmaceutics & Drug Disposition 1998;19(5):297‐302. [DOI] [PubMed] [Google Scholar]
Stradling 1998 {published data only}
- Stradling JR, Pearson MG. Efficacy of beclomethasone dipropionate delivered via a novel dry powder inhaler (Clickhaler). American Journal of Respiratory and Critical Care Medicine. 1998; Vol. 157, issue 3:A639.
Vidgren 1994 {published data only}
- Vidgren P, Silvasti M, Poukkula A, Laasonen K, Vidgren M. Easyhaler powder inhaler ‐ A new alternative in the anti‐inflammatory treatment of asthma. Acta Therapeutica 1994;20(3‐4):117‐31. [Google Scholar]
Wijngaarden 1998 {published data only}
- Vink‐van Wijngaarden T, Blom‐Ross ME, Lansdorp D, Goedhart DM, Eelhart J, Guelen PJM, et al. Clinical efficacy and safety of beclomethasone dipropionate inhalation capsules inhaled by Cyclohaler compared with Becotide Rotacaps inhaled by Rotahaler. International Journal of Clinical Pharmacology & Therapeutics 1998;36(9):510‐5. [PubMed] [Google Scholar]
Woodman 1993 {published data only}
- Woodman K, Bremner P, Burgess C, Crane J, Pearce N, Besley R. A comparative study of the efficacy of beclomethasone dipropionate delivered from a breath activated and conventional metered dose inhaler in asthmatic patients. Current Medical Research And Opinion 1993;13:61‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kudo 1995 {published data only}
- Kudo K, Hojo M, Kabe J. [Inhaled beclomethasone in long‐term management of asthma: optimal dose and optimal duration of treatment]. Nippon Kyobu Shikkan Gakkai Zasshi 1995;33(9):956‐65. [PubMed] [Google Scholar]
Majima 1993 {published data only}
- Majima T, Katoh H, Yoshida N, Akiyama Y, Hashimoto N, Yamaguchi M, et al. [Effects of high doses of beclomethasone dipropionate in bronchial asthma]. Arerugi 1993;42(4):534‐40. [PubMed] [Google Scholar]
Pol'ner 1997 {published data only}
- Pol'ner SA. [Effects of becotide and becodisk glucocorticoid drugs on bronchial reactivity in patients with bronchial asthma]. Klinicheskaia Meditsina 1997;75(6):44‐6. [PubMed] [Google Scholar]
Additional references
Adams 2000
- Adams NP, Bestall JB, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma (Cochrane Review). The Cochrane Library 2000, Issue 4. [DOI] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. The British guidelines on asthma management 1995 review and postion statement. Thorax 1997;52(Suppl 1):S1‐20. [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global strategy for asthma management and prevention NHBLI/WHO workshop report. National Institute of Health, Bethseda, MD 1995, issue NIH Publication No. 95‐3659.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
NHLBI 1997
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethseda MD: NIH/National Heart, Lung and Blood Institute 1997, issue Publication No. 97‐4051.


