Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disease characterized by the accumulation of amyloid plaques and neurofibrillary tangles in the brain. The AD pathophysiology entails chronic inflammation involving innate immune cells including microglia, astrocytes, and other peripheral blood cells. Inflammatory mediators such as cytokines and complements are also linked to AD pathogenesis. Despite increasing evidence supporting the association between abnormal inflammation and AD, no well-established inflammatory biomarkers are currently available for AD. Since many reports have shown that abnormal inflammation precedes the outbreak of the disease, non-invasive and readily available peripheral inflammatory biomarkers should be considered as possible biomarkers for early diagnosis of AD. In this mini-review, we introduce the peripheral biomarker candidates related to abnormal inflammation in AD and discuss their possible molecular mechanisms. Furthermore, we also summarize the current state of inflammatory biomarker research in clinical practice and molecular diagnostics. We believe this review will provide new insights into biomarker candidates for the early diagnosis of AD with systemic relevance to inflammation during AD pathogenesis.
Keywords: Alzheimer’s disease, Cytokine, Immune Cell, Inflammation, Peripheral blood biomarker
INTRODUCTION
Alzheimer’s disease (AD) is one of the most prevalent neurodegenerative diseases with progressive and irreversible pathogenesis. Numerous studies have suggested that AD pathogenesis is associated with not only abnormal cerebral function but also changes in central and peripheral immune system. Specifically, it is characterized by neuroinflammation (1, 2). Activated microglia are one of the early hallmarks of AD pathogenesis (3). Numerous reports have shown over-activation of microglia (4, 5), activated complement system (6), increased production of cytokines (7) and chemokines during AD pathogenesis, and some of them were suggested as diagnostic biomarkers (8, 9). Recently, immune-related pathophysiological mechanisms were discovered during AD pathogenesis, including innate immune cell infiltration and altered brain-gut axis as well as microglial and astrocyte activation (10, 11) which play a role in peripheral immune dynamics and act as possible biomarkers for disease.
Various inflammatory molecules are affected by AD pathogenesis, with abnormal levels in different brain regions (12, 13). Amyloid beta (Aβ) and amyloid precursor protein (APP) induce cytokine and chemokine release from microglia, astrocytes, and neurons; chemokine and cytokines also promote the expression and deposition of amyloid beta, which activates the vicious cycle (14). Furthermore, neuroinflammation may be an early event and plays a critical role in tau pathology, characterized by microglial activation preceding tau tangle formation. Immunosuppression ameliorated tau pathology in P301S transgenic mice (15). Also, peripheral levels of inflammation-related cytokines change during AD pathogenesis and are significantly correlated with disease progression (16–18). Therefore, the crosstalk between abnormal events in the central compartment, involving neuronal dysfunction and microglial activation, and peripheral immune system is critical for the understanding of the mechanism of AD pathogenesis (19).
In this review, we discuss various inflammation-related molecules, especially those altered at the peripheral level, and suggested their potential role as risk factors, diagnostic markers and therapeutic targets in AD. Even though it is ambiguous whether inflammatory cytokines are related to the cause or effect of AD pathogenesis, a better understanding of these molecules during AD pathogenesis can lead to a therapeutic breakthrough against AD.
PRO-INFLAMMATORY CYTOKINES IN AD
IL-1
IL-1 is a pro-inflammatory cytokine, which elicits diverse systemic effects (20). IL-1 was the first cytokine whose actions on the brain were identified (21, 22). The two isoforms of IL-1 were identified as IL-1α and IL-1β encoded by two different genes IL-1A and IL-1B, respectively (23).
Elevated IL-1 was reported not only in acute injuries but also in a number of chronic neurodegenerative disorders (24, 25). IL-1 levels are markedly overexpressed in the brains of patients with AD (26), mainly in microglia (26). Increased IL-1 level is related to Aβ plaque formation, tau phosphorylation, and neurofibrillary tangle formation via activation of MAPK-P38 (27). Also, IL-1 has been reported to modulate neurons in hippocampus (28) and trigger age-related impairment of long-term potentiation (29). In vitro and in vivo experiments demonstrated that the secreted βAPP induced IL-1 overexpression from microglia, which suppressed cholinergic function by enhancing the expression and activity of acetylcholinesterase (AChE) (30), suggesting a possible mechanistic event relevant to cholinergic dysfunction in AD. In a recent report, peripheral changes in blood AChE level were also detected in cognitively normal individuals with cerebral amyloid deposition (31). This phenomenon might be possibly associated with altered cytokine release, including IL-1, during AD pathogenesis. Furthermore, several genetic variants in IL-1 are associated with an increased risk for AD depending on the ethnic groups or ApoE genotypes (32, 33), which reinforce the strong relationship between IL-1 and AD pathogenesis. Additionally, IL-1 interacts with several products of known genetic risk factors for AD, including ApoE, α2-macroglobulin, α1-antichymotrypsin and βAPP (33), suggesting implications for AD pathogenesis.
Increased IL-1β was detected in the blood of patients with AD (7, 34–38). Increased levels of both IL-1α and IL-1β have also been reported in the serum of AD patients; however, no changes in cytokine levels were detected in the serum of patients with mild cognitive impairment (MCI), compared with healthy control (39). Further, evidence is not sufficient to suggest that the elevated plasma IL-1 level in AD was caused by peripheral immune activation during AD pathogenesis (35). Instead, the increased levels of circulating IL-1 might be attributed to the central alteration during AD (35). Unfortunately, in other studies, no differences in blood IL-1 levels have been detected between controls and AD patients (40–43). Results of blood IL-1 level in MCI are also disputed, suggesting an increase compared with healthy controls in a few studies but not others (37, 39). These contradictory results might be attributed to differences in the sensitivity of diagnostic and screening tools, low levels of IL-1 in the blood or altered IL-1 level in only a subpopulation or specific stage of AD patients.
IL-6
IL-6 levels are elevated in the brains of AD patients (44) and are linked to diffuse plaques representing the early stage of plaque formation (45). Aβ has been shown to induce IL-6 expression in microglia and astrocyte culture system (46). Significant correlations between IL-6 levels in CSF and in matched blood samples were observed (47, 48), and the elevated level of plasma IL-6 was detected in mild cases of AD (48). AD-related increase in blood IL-6 level has been reported in other studies as well (7, 35, 38, 48, 49).
However, other studies failed to show consistent results of increased IL-6 levels in the blood (40, 43, 50, 51), CSF (7, 40, 52, 53) or brain (40). Further, decreased IL-6 levels were reported in serum (54) and in CSF (54, 55) of AD patients. In addition, lipopolysaccharide-induced IL-6 release from blood cells was significantly reduced in moderate AD (36). These conflicting results involving peripheral cytokine levels, including levels of IL-6 and IL-1, dispute the role of cytokines in AD pathogenesis. Nonetheless, reports suggesting the modulation of AD susceptibility based on specific IL-1 or IL-6 gene polymorphism demonstrate the important role played by these cytokines during AD pathogenesis (56–58). Especially, specific IL-1 gene polymorphisms are strongly correlated with the increased risk for AD possibly via altered modulation of IL-1-induced neurodegeneration (58).
TNF-α
The pro-inflammatory cytokine tumor necrosis factor (TNF) α is a critical mediator of inflammatory response in diverse tissues and is secreted by diverse cell types including astrocytes and microglia in brain (53). This cytokine is secreted during both acute and chronic systemic inflammation involving immune system and the brain (59).
TNF-α is one of the most well-defined cytokines during AD pathogenesis because TNF-α level is strongly correlated with cognitive decline, neuronal toxicity and cerebral apoptosis. Increased TNF-α levels were found in different anatomic locations of brain in AD patients (60). Also, mild-to-severe AD patients with increased serum TNF-α level showed a 2-fold increase in the rate of cognitive decline and a high baseline TNF-α level was related to a 4-fold increase in cognitive decline (16).
Evidence suggests that the pathological hallmark Aβ induces microglial activation and TNF-α release (61) and this TNF-α-related inflammation plays a deleterious role in neuronal death (62). TNF-α release from microglia, astrocytes and neurons during AD pathogenesis is a chronic event (63) and altered levels of TNF-α in the brain might induce changes in the peripheral levels of TNF-α. Numerous reports suggest evidences for alteration of TNF-α level in biological fluids from AD patients (64). In CSF, an average 25-fold increase of TNF-α level has been reported in AD compared with control (65). In the periphery, the increased levels of blood TNF-α have been widely reported in various studies including MCI and AD patients and were strongly associated with the rate of cognitive decline (7, 16, 43, 66–68). Meanwhile, no changes in TNF-α level were reported in another study using serum (42, 53) or CSF (7, 40, 52). Instead, a lower TNF-α level has been reported in the blood of AD patients compared with age-matched control subjects in a study of patients with early-as well as late-onset AD (34). Also, TNF-α release from blood cell following lipopolysaccharide exposure was shown to decrease significantly in moderate AD (36), Probably, the blood TNF-α level may be associated with a wide range of individual variation and reflect disease-dependent alteration (64).
IL-12
IL-12 is a proinflammatory cytokine secreted by activated macrophages, monocytes, and glial cells via Toll-like receptor activation (69, 70) and its dual potential for pro- and anti-inflammation activity has been shown in a previous study (71). During AD pathogenesis, the IL-12 level in CSF is reduced in AD patients compared with neurological control patients (72). Also, another study reported increased IL-12 levels in the blood of patients with mild and moderate AD (7) while severe AD suppressed plasma IL-12 levels (18), which might possibly suggest the potential for dual activities. A recent report demonstrated the inhibition of IL-12/IL-23 pathway via genetic ablation or pharmacological manipulation resulting in a drastic reduction of cerebral amyloid pathology and cognitive deficit (73). Indirect evidence supporting the role of IL-12 during AD pathogenesis is based on Aβ immunization in a mouse model of AD with reduced amyloid burden along with decreased expression of the IL-12Rβ1 receptor in T cells (73, 74). All these results suggest the potential role of IL-12 in neurodegenerative disease including AD.
ANTI-INFLAMMATORY CYTOKINES IN AD
IL-4
IL-4 is an anti-inflammatory cytokine neutralizing the pro-inflammatory action. IL-4 inhibits the secretion of IL-1β, IL-6 and TNF-α by activated monocytes (75). Despite the absence of differences between AD and control subjects in terms of peripheral concentration of IL-4 (7), the blood IL-4 level is closely associated with cognitive decline in AD (17). In this study, AD patients with rapid cognitive decline showed a significant increase in blood IL-4 level compared with those representing a slow decline. More interestingly, the peripheral levels of IL-4 and IL-1β are altered by treatment with acetylcholinesterase inhibitor (AChEI), showing increased IL-4 and decreased IL-1β (76, 77). Recently, it has been reported that AChEI-treated AD patients showed an increase in the peripheral levels of AChE, demonstrating the connection between central AChEI action and peripheral synchronization (31). Therefore, it is possible that the beneficial effects of AChEI treatment in AD patients may not be limited to the central compartment but also mediated via peripheral changes in inflammation mediated by IL-1 via IL-4.
IL-10
IL-10 is an anti-inflammatory cytokine, which reduces inflammation during AD pathogenesis. Increased IL-10 level has been reported in the brains of patients with neurological diseases including Alzheimer’s disease (78) and also in the serum of patients with AD and vascular dementia compared with healthy controls (38) while no change was detected in AD in another study (7, 43). Peripheral IL-10 is another candidate biomarker of disease progression and is strongly associated with clinical status and imaging parameters of disease severity (17). Similar to IL-4, AD patients with increased levels of blood IL-10 exhibit rapid cognitive decline compared with those demonstrating a slow decline (17). Also, the IL-10 level was associated with brain atrophy in MRI measurements during AD (17). Possibly, the anti-inflammatory cytokines, including IL-4 and IL-10, may be regarded as a compensatory mechanism during pathogenesis. A strong compensatory mechanism may imply severe pathological lesions.
THE ROLE OF INTESTINAL CYTOKINES (BRAIN-GUT AXIS) IN AD
Gut microbiota play an important role in host metabolism and physiology and influences not only the immune system but also the nervous system related to brain development and cognitive function (79, 80). Several recent studies have demonstrated the importance of gut microbiota composition in AD pathogenesis and the role of gut microbiota has emerged as the new field in AD-related neurodegeneration (81). Furthermore, microbiota in gut play a key role in peripheral immune cell activation and cytokine secretion during the inflammatory cascade in the periphery and central nervous system-related disruption of gut barrier and blood-brain barrier (81). Therefore, neuroscientists have expanded the scope of studies investigating neuroinflammation in brain to include systemic inflammation involving the brain-gut axis during AD pathogenesis.
The mouse model of AD indicates altered composition of gut microbiota compared with that of healthy wild-type mice, in addition to loss of gut epithelial barrier integrity, chronic systemic and intestinal inflammation (11). A previous study demonstrated that the direct transfer of fecal microbiota from wild-type to AD mouse models ameliorated not only amyloid and tau pathology but also cognitive dysfunction, and the underlying mechanism entailed recovery of intestinal macrophage activity and circulating Ly6C+ monocytes (11). Another study showed that the altered composition of gut microbiota via probiotic formulation in AD mouse models reduced the plasma levels of pro-inflammatory cytokines, IL-1α, IL-1β, IL-2, IL-12, IFNγ and TNF-α, which were higher in AD mouse models compared with levels in healthy wild-type mice (82). Also, probiotic treatment increased the levels of anti-inflammatory cytokines, including IL-4, IL-6, G-CSF, GM-CSF, and downregulated inflammatory cytokines. Partial restoration of impaired neuronal proteolytic pathways and decreased cognitive impairment suggest that inflammation-related cytokines are important modulators of brain gut axis during AD pathogenesis.
POTENTIAL USE OF BLOOD IMMUNE CELLS AS BIOMARKERS FOR AD
Peripheral changes in blood circulation have been known to impact brain function. However, due to the failure of various protein-based circulating blood biomarkers, recent studies investigated cell-based biomarkers. Blood immune cells are potential biomarkers in AD. These white blood cells are classified according to cell lineage (myeloid or lymphoid cells) and are further divided into specific cell types such as T lymphocytes, B lymphocytes, and natural killer cells derived from the common lymphoid progenitor cells, and monocytes, eosinophils, neutrophils, basophils, erythrocytes, mast cells, and thrombocytes originating from common myeloid progenitor cells (83). Accumulating evidence suggests that these immune cells play an important role in AD pathogenesis, for e.g., migration of neutrophils traversing the BBB into the brain targeting amyloid plaques (10), recruitment of monocytes/macrophages via CCL2 or CX3CL1 to the sites of inflammatory response (84), and changes in regulatory T lymphocyte profiles (85) and amyloid-beta peptide autoantibodies from B lymphocytes (86). We discuss the potential immune cell-associated blood biomarkers to provide novel insight into blood-based biomarkers beyond the circulating biomarker candidates reported previously.
Changes in the peripheral profile of blood immune cells
The changes in blood cell profile of AD have yet to be fully investigated; however, several reports showed altered peripheral profile of blood cells in AD. Chen et al. (2017) reported that the blood cell population was associated with AD pathogenesis in 92 AD patients and 84 cognitively normal controls (age and sex matched). The population of lymphocytes and basophils decreased in AD patients compared with normal controls; however, there were no changes in the levels of other white blood cells such as neutrophils, monocytes, and eosinophils (87). Another study reported similar results suggesting that the population of resting regulatory T lymphocytes (Treg) decreased significantly in the AD group compared with that of cognitively normal group (85). Although these studies did not reveal any changes in myeloid cell profile except for basophils, other studies showed an increase in the number of monocytes in the blood (88) and decreased CCR2+ monocytes (89) in AD brain, suggesting that monocytes are possible AD biomarker candidates. Furthermore, Richartz-Salzburger et al. (2007) also reported that T and B lymphocytes are significantly downregulated in subjects with clinically diagnosed AD compared with cognitively normal participants (90). Even if few studies reported the diagnostic sensitivity or specificity of blood immune cell population for AD, the published studies demonstrate the possible role of peripheral immune cells as one of the candidate biomarkers for AD.
COMPLEMENTS AS BIOMARKERS FOR AD
The complement system plays an important role in innate immune response (91) and in the regulation of AD risk factors inducing neuronal death (92). A preponderance of evidence derived from genetic, proteomic, and immunological studies suggests that the system is relevant to AD pathogenesis. Genetic profiles determined using genome-wide association studies (GWAS) reveal the relationship between AD risk and genes associated with the complement cascade, such as single nucleotide polymorphisms (SNP) involving clusterin (CLU) and complement receptor 1 (CR1) (93, 94). C1q and C3 components bind to amyloid beta plaques and activate the classical and alternative complement pathways (92, 95). Previous studies have shown that the levels of complement system components in plasma in AD were altered compared with that of normal controls, such as complement factor H (FH), clusterin, plasma factor I (FI), C4d, iC3b, Bb, and iC3b (96–99). Here, we discuss the role of reliable blood-based biomarkers using the complement cascade components.
Genetic profile of the complement system
Emerging evidence derived from genetic studies suggest that the components of the complement cascade are strongly associated with AD pathophysiology (100–102). Recent GWAS demonstrated the existence of many SNPs for genes associated with the complement pathway related to AD, such as CLU, CR1, SERPINA3, CRP, C2, CFH, C3, and C4 (91, 102–105). Especially, the CLU, also known as apolipoprotein J (ApoJ), and CR1 genes are the major risk genes associated with late-onset AD (LOAD) (102, 106). The three representative SNPs in the Clu gene include rs11136000, rs2279590, and rs9331888 (105). Interestingly, the rs11136000 SNP is observed in Asian and Caucasian populations, and the two populations exhibit similar genetic risk (Asian, OR = 0.85; Caucasian, OR = 0.82) (107, 108). The rs2279590 SNP showed significant correlation with the degree of brain amyloid plaques (109), and the rs9331888 polymorphism is linked to the levels of CLU in blood plasma (110). The CR1 gene, which is mainly expressed in human erythrocytes, encodes CR1 protein to facilitate amyloid-beta clearance mediated via C3b (111). The major polymorphisms of CR1 gene include rs6656401, rs4844609, and rs3818361 (102, 112). Several studies have investigated the specific roles of these SNPs.
Plasma levels of complement cascade-related molecules
Since both CLU and CR1 are the major risk factors involved in AD, they play key roles in neuro-inflammation and innate immunity (91). The relationship between CLU protein and amyloid-beta peptide has been reported, such as the ability of CLU in amyloid beta clearance (113). Blood CLU levels were drastically increased in AD patients compared with cognitively normal controls (114), and similar results were published by another group suggesting that the plasma levels of CLU increased in participants with cognitive decline and in AD patients in longitudinal follow-up, but not in CSF samples (115). Plasma CR1 levels were not related to the clinical diagnosis of AD. However, the levels of rs4844609 and rs6656401 SNP carriers were slightly increased compared with that of CR1 without the SNPs (112, 116). Cerebrospinal fluid (CSF) levels of CR1 also did not show any differences between the different cognitive stages of AD (117). Plasma levels of C3 and C4 were not much in evidence; however, Bennett el al., (2012) showed that the levels of C4a protein were enhanced in AD participants compared with cognitively normal subjects. Thambisetty et al. (2011) reported the association of C3 and C3a protein with brain atrophy in AD subjects (99, 118). Plasma C-reactive protein (CRP) levels are relatively well known, but there have been some contradictory results suggesting the reduced levels of plasma CRP in AD (119). However, other studies suggest higher plasma levels of CRP in AD or subjects with cognitive decline (120–122).
CONCLUSION
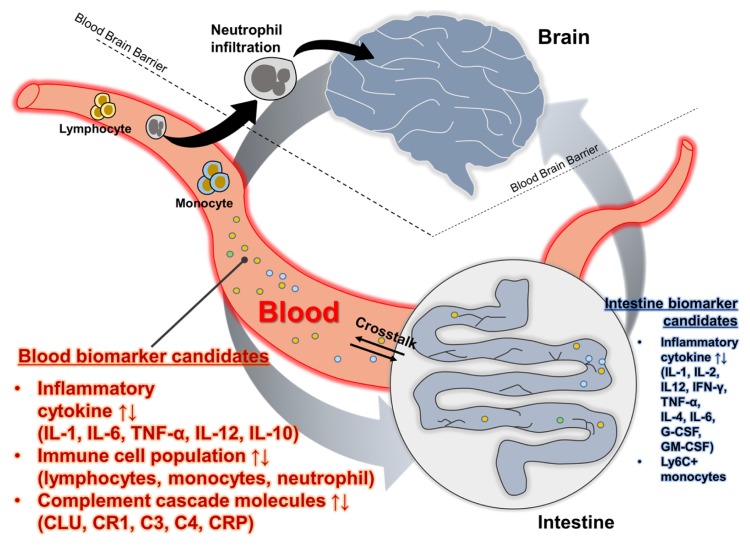
Here, we discussed a variety of inflammatory mediators such as pro- and anti-inflammatory cytokines, intestinal cytokines, peripheral immune cells, and complement cascade-related molecules (Fig. 1 and Table 1). Reports regarding peripheral cytokine levels show very inconsistent results in various studies. The studies involving IL-1, IL-6 and TNF-α need to be validated using large-scale population cohorts. Recently, a combination of protease and phosphatase inhibitors has been used to detect low levels of blood biomarkers in AD (123). Effective detection of low levels of blood biomarkers might greatly facilitate the detection of low levels of cytokines in the blood. Furthermore, AD pathogenesis can alter diverse physiological systems, including neuronal function, brain structure, immune dynamics, brain-gut axis, and metabolism. Therefore, a single biomarker may not be adequate to delineate the pathophysiology of AD completely. Instead, a combination of multiple markers representing different stage of disease progression is the best strategy. Immune cell profiles and complement cascade play a potential role in providing novel insight into blood-based biomarkers compared with circulating protein biomarkers reported previously, although further validation is needed. Although the use of inflammatory mediators as peripheral AD biomarkers has yet to be established, further studies are needed given the link between inflammation and AD.
Fig. 1.
Graphical summary of peripheral inflammatory biomarker candidates.
Table 1.
Altered levels of candidate biomarkers
| Biomarker (blood) | Up regulation (reference no.) | Down regulation (reference no.) | No regulation (reference no.) | Superiority |
|---|---|---|---|---|
| IL-1 | (7, 34–39) | - | (40–43) | Up + No |
| IL-6 | (7, 35, 38, 48, 49) | (54) | (43, 50, 51) | Up + No + Down |
| TNF-α | (7, 16, 43, 66–68) | (34) | (42, 53) | Up + No + Down |
| IL-12 | (7) | (18) | - | Up + Down |
| IL-4 | (7) | No | ||
| IL-10 | (7, 43) | No | ||
| Lymphocyte | - | (85, 90) | - | Down |
| Monocyte | (88) (89) | - | (87) | Up + No |
| CLU | (114, 115) | - | - | Up |
| CR1 | - | - | (112, 116) | No |
| C3 | (99) | - | - | Up |
| C4 | (118) | - | - | Up |
| CRP | (120–122) | (119) | - | Up + Down |
|
| ||||
| Biomarker (intestine) | Up & Down changes based on probiotic formulation or FMT in AD mouse model | Reference no. | ||
|
| ||||
| IL-1 | Down | (82) | ||
| IL-2 | Down | (82) | ||
| IL-12 | Down | (82) | ||
| IFN-γ | Down | (82) | ||
| TNF-α | Down | (82) | ||
| IL-4 | Up | (82) | ||
| IL-6 | Up | (82) | ||
| G-CSF | Up | (82) | ||
| GM-CSF | Up | (82) | ||
| Ly6C+ monocyte | Down | (11) | ||
Abbreviations: up, up regulation; down, down regulation; no, no regulation; FMT, fecal microbiota transplant.
ACKNOWLEDGEMENTS
This work was supported by a grant of the Korea Health technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0630 and HI19C1132) for I. Mook-Jung and a grant from NRF (NRF-2019R1I1A1A01063525) for S. Han.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han SH, Park JC, Mook-Jung I. Amyloid beta-interacting partners in Alzheimer’s disease: From accomplices to possible therapeutic targets. Prog Neurobiol. 2016;137:17–38. doi: 10.1016/j.pneurobio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cagnin A, Brooks DJ, Kennedy AM, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 5.Baik SH, Kang S, Lee W, et al. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019;30:493–507 e496. doi: 10.1016/j.cmet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Pathol. 1999;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Correa JD, Starling D, Teixeira AL, Caramelli P, Silva TA. Chemokines in CSF of Alzheimer’s disease patients. Arq Neuropsiquiatr. 2011;69:455–459. doi: 10.1590/S0004-282X2011000400009. [DOI] [PubMed] [Google Scholar]
- 9.Sui X, Liu J, Yang X. Cerebrospinal fluid biomarkers of Alzheimer’s disease. Neurosci Bull. 2014;30:233–242. doi: 10.1007/s12264-013-1412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baik SH, Cha MY, Hyun YM, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35:1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MS, Kim Y, Choi H, et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69:283–294. doi: 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 14.Solfrizzi V, D’Introno A, Colacicco AM, et al. Circulating biomarkers of cognitive decline and dementia. Clin Chim Acta. 2006;364:91–112. doi: 10.1016/j.cca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe MJ, Figueiredo-Pereira ME. Relationship between tau pathology and neuroinflammation in Alzheimer’s disease. Mt Sinai J Med. 2010;77:50–58. doi: 10.1002/msj.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung R, Proitsi P, Simmons A, et al. Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PLoS One. 2013;8:e64971. doi: 10.1371/journal.pone.0064971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motta M, Imbesi R, Di Rosa M, Stivala F, Malaguarnera L. Altered plasma cytokine levels in Alzheimer’s disease: correlation with the disease progression. Immunol Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Dionisio-Santos DA, Olschowka JA, O’Banion MK. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation. 2019;16:74. doi: 10.1186/s12974-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 21.Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 22.Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicklin MJ, Weith A, Duff GW. A physical map of the region encompassing the human interleukin-1 alpha, interleukin-1 beta, and interleukin-1 receptor antagonist genes. Genomics. 1994;19:382–384. doi: 10.1006/geno.1994.1076. [DOI] [PubMed] [Google Scholar]
- 24.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 25.Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Ann N Y Acad Sci. 2003;992:39–47. doi: 10.1111/j.1749-6632.2003.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 26.Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng JG, Jones RA, Zhou XQ, et al. Interleukin-1 promotion of MAPK-p38 overexpression in experimental animals and in Alzheimer’s disease: potential significance for tau protein phosphorylation. Neurochem Int. 2001;39:341–348. doi: 10.1016/S0197-0186(01)00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Liu L, Kang J, et al. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–155. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SH, Park JC, Byun MS, et al. Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol Aging. 2019;73:21–29. doi: 10.1016/j.neurobiolaging.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Su F, Bai F, Zhang Z. Inflammatory Cytokines and Alzheimer’s Disease: A Review from the Perspective of Genetic Polymorphisms. Neurosci Bull. 2016;32:469–480. doi: 10.1007/s12264-016-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mrak RE, Griffin WS. Interleukin-1 and the immunogenetics of Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:471–476. doi: 10.1093/jnen/59.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez XA, Franco A, Fernandez-Novoa L, Cacabelos R. Blood levels of histamine, IL-1 beta, and TNF-alpha in patients with mild to moderate Alzheimer disease. Mol Chem Neuropathol. 1996;29:237–252. doi: 10.1007/BF02815005. [DOI] [PubMed] [Google Scholar]
- 35.Licastro F, Pedrini S, Caputo L, et al. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/S0165-5728(99)00226-X. [DOI] [PubMed] [Google Scholar]
- 36.De Luigi A, Pizzimenti S, Quadri P, et al. Peripheral inflammatory response in Alzheimer’s disease and multiinfarct dementia. Neurobiol Dis. 2002;11:308–314. doi: 10.1006/nbdi.2002.0556. [DOI] [PubMed] [Google Scholar]
- 37.Forlenza OV, Diniz BS, Talib LL, et al. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;28:507–512. doi: 10.1159/000255051. [DOI] [PubMed] [Google Scholar]
- 38.Angelopoulos P, Agouridaki H, Vaiopoulos H, et al. Cytokines in Alzheimer’s disease and vascular dementia. Int J Neurosci. 2008;118:1659–1672. doi: 10.1080/00207450701392068. [DOI] [PubMed] [Google Scholar]
- 39.Italiani P, Puxeddu I, Napoletano S, et al. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J Neuroinflammation. 2018;15:342. doi: 10.1186/s12974-018-1376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzrein AS, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1beta, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-alpha, the soluble tumor necrosis factor receptors I and II, and alpha1-antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12:215–227. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Pirttila T, Mehta PD, Frey H, Wisniewski HM. Alpha 1-antichymotrypsin and IL-1 beta are not increased in CSF or serum in Alzheimer’s disease. Neurobiol Aging. 1994;15:313–317. doi: 10.1016/0197-4580(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 42.Yasutake C, Kuroda K, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2006;256:402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- 43.Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer’s disease patients. J Neuroimmunol. 2008;193:183–187. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Bauer J, Strauss S, Schreiter-Gasser U, et al. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-N. [DOI] [PubMed] [Google Scholar]
- 45.Huell M, Strauss S, Volk B, Berger M, Bauer J. Interleukin-6 is present in early stages of plaque formation and is restricted to the brains of Alzheimer’s disease patients. Acta Neuropathol. 1995;89:544–551. doi: 10.1007/BF00571510. [DOI] [PubMed] [Google Scholar]
- 46.Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- 47.Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16:136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 48.Wu YY, Hsu JL, Wang HC, Wu SJ, Hong CJ, Cheng IH. Alterations of the Neuroinflammatory Markers IL-6 and TRAIL in Alzheimer’s Disease. Dement Geriatr Cogn Dis Extra. 2015;5:424–434. doi: 10.1159/000439214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh VK, Guthikonda P. Circulating cytokines in Alzheimer’s disease. J Psychiatr Res. 1997;31:657–660. doi: 10.1016/S0022-3956(97)00023-X. [DOI] [PubMed] [Google Scholar]
- 50.Angelis P, Scharf S, Mander A, Vajda F, Christophidis N. Serum interleukin-6 and interleukin-6 soluble receptor in Alzheimer’s disease. Neurosci Lett. 1998;244:106–108. doi: 10.1016/S0304-3940(98)00136-0. [DOI] [PubMed] [Google Scholar]
- 51.van Duijn CM, Hofman A, Nagelkerken L. Serum levels of interleukin-6 are not elevated in patients with Alzheimer’s disease. Neurosci Lett. 1990;108:350–354. doi: 10.1016/0304-3940(90)90666-W. [DOI] [PubMed] [Google Scholar]
- 52.Garlind A, Brauner A, Hojeberg B, Basun H, Schultzberg M. Soluble interleukin-1 receptor type II levels are elevated in cerebrospinal fluid in Alzheimer’s disease patients. Brain Res. 1999;826:112–116. doi: 10.1016/S0006-8993(99)01092-6. [DOI] [PubMed] [Google Scholar]
- 53.Tarkowski E, Liljeroth AM, Minthon L, Tarkowski A, Wallin A, Blennow K. Cerebral pattern of pro-and anti-inflammatory cytokines in dementias. Brain Res Bull. 2003;61:255–260. doi: 10.1016/S0361-9230(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 54.Richartz E, Stransky E, Batra A, et al. Decline of immune responsiveness: a pathogenetic factor in Alzheimer’s disease? J Psychiatr Res. 2005;39:535–543. doi: 10.1016/j.jpsychires.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Yamada K, Furusawa S, Saito K, et al. Concurrent use of granulocyte colony-stimulating factor with low-dose cytosine arabinoside and aclarubicin for previously treated acute myelogenous leukemia: a pilot study. Leukemia. 1995;9:10–14. [PubMed] [Google Scholar]
- 56.Papassotiropoulos A, Bagli M, Jessen F, et al. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer’s disease. Ann Neurol. 1999;45:666–668. doi: 10.1002/1531-8249(199905)45:5<666::AID-ANA18>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 57.Grimaldi LM, Casadei VM, Ferri C, et al. Association of early-onset Alzheimer’s disease with an interleukin-1alpha gene polymorphism. Ann Neurol. 2000;47:361–365. doi: 10.1002/1531-8249(200003)47:3<361::AID-ANA12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Nicoll JA, Mrak RE, Graham DI, et al. Association of interleukin-1 gene polymorphisms with Alzheimer’s disease. Ann Neurol. 2000;47:365–368. doi: 10.1002/1531-8249(200003)47:3<365::AID-ANA13>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 60.Zhao M, Cribbs DH, Anderson AJ, et al. The induction of the TNFalpha death domain signaling pathway in Alzheimer’s disease brain. Neurochem Res. 2003;28:307–318. doi: 10.1023/A:1022337519035. [DOI] [PubMed] [Google Scholar]
- 61.Lue LF, Walker DG, Rogers J. Modeling microglial activation in Alzheimer’s disease with human postmortem microglial cultures. Neurobiol Aging. 2001;22:945–956. doi: 10.1016/S0197-4580(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 62.Janelsins MC, Mastrangelo MA, Park KM, et al. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAlpine FE, Tansey MG. Neuroinflammation and tumor necrosis factor signaling in the pathophysiology of Alzheimer’s disease. J Inflamm Res. 2008;1:29–39. doi: 10.2147/jir.s4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol. 2014;50:534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tarkowski E, Blennow K, Wallin A, Tarkowski A. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol. 1999;19:223–230. doi: 10.1023/A:1020568013953. [DOI] [PubMed] [Google Scholar]
- 66.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–364. doi: 10.1093/gerona/54.7.M357. [DOI] [PubMed] [Google Scholar]
- 68.Fillit H, Ding WH, Buee L, et al. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett. 1991;129:318–320. doi: 10.1016/0304-3940(91)90490-K. [DOI] [PubMed] [Google Scholar]
- 69.Aliberti J, Reis e Sousa C, Schito M, et al. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat Immunol. 2000;1:83–87. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 70.Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/S1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 71.Chang HD, Radbruch A. The pro- and anti-inflammatory potential of interleukin-12. Ann N Y Acad Sci. 2007;1109:40–46. doi: 10.1196/annals.1398.006. [DOI] [PubMed] [Google Scholar]
- 72.Rentzos M, Paraskevas GP, Kapaki E, et al. Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. J Neurol Sci. 2006;249:110–114. doi: 10.1016/j.jns.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 73.Vom Berg J, Prokop S, Miller KR, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med. 2012;18:1812–1819. doi: 10.1038/nm.2965. [DOI] [PubMed] [Google Scholar]
- 74.Town T, Vendrame M, Patel A, et al. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer’s beta-amyloid(1-42) J Neuroimmunol. 2002;132:49–59. doi: 10.1016/S0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 75.te Velde AA, Huijbens RJ, Heije K, de Vries JE, Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990;76:1392–1397. doi: 10.1182/blood.V76.7.1392.1392. [DOI] [PubMed] [Google Scholar]
- 76.Gambi F, Reale M, Iarlori C, et al. Alzheimer patients treated with an AchE inhibitor show higher IL-4 and lower IL-1 beta levels and expression in peripheral blood mononuclear cells. J Clin Psychopharmacol. 2004;24:314–321. doi: 10.1097/01.jcp.0000125683.74595.2f. [DOI] [PubMed] [Google Scholar]
- 77.Lugaresi A, Di Iorio A, Iarlori C, et al. IL-4 in vitro production is upregulated in Alzheimer’s disease patients treated with acetylcholinesterase inhibitors. Exp Gerontol. 2004;39:653–657. doi: 10.1016/j.exger.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Strle K, Zhou JH, Shen WH, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. doi: 10.1615/CritRevImmunol.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 79.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kowalski K, Mulak A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J Neurogastroenterol Motil. 2019;25:48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonfili L, Cecarini V, Berardi S, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7:2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arosio B, D‘Addario C, Gussago C, et al. Peripheral blood mononuclear cells as a laboratory to study dementia in the elderly. Biomed Res Int. 2014;2014 doi: 10.1155/2014/169203. 169203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 85.Ciccocioppo F, Lanuti P, Pierdomenico L, et al. The Characterization of Regulatory T-Cell Profiles in Alzheimer’s Disease and Multiple Sclerosis. Sci Rep. 2019;9:8788. doi: 10.1038/s41598-019-45433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rezai-Zadeh K, Gate D, Szekely CA, Town T. Can peripheral leukocytes be used as Alzheimer’s disease biomarkers? Expert Rev Neurother. 2009;9:1623–1633. doi: 10.1586/ern.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen SH, Bu XL, Jin WS, et al. Altered peripheral profile of blood cells in Alzheimer disease: A hospital-based case-control study. Medicine (Baltimore) 2017;96:e6843. doi: 10.1097/MD.0000000000006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lunnon K, Ibrahim Z, Proitsi P, et al. Mitochondrial dysfunction and immune activation are detectable in early Alzheimer’s disease blood. J Alzheimers Dis. 2012;30:685–710. doi: 10.3233/JAD-2012-111592. [DOI] [PubMed] [Google Scholar]
- 89.Naert G, Rivest S. A deficiency in CCR2+ monocytes: the hidden side of Alzheimer’s disease. J Mol Cell Biol. 2013;5:284–293. doi: 10.1093/jmcb/mjt028. [DOI] [PubMed] [Google Scholar]
- 90.Richartz-Salzburger E, Batra A, Stransky E, et al. Altered lymphocyte distribution in Alzheimer’s disease. J Psychiatr Res. 2007;41:174–178. doi: 10.1016/j.jpsychires.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Aiyaz M, Lupton MK, Proitsi P, Powell JF, Lovestone S. Complement activation as a biomarker for Alzheimer’s disease. Immunobiology. 2012;217:204–215. doi: 10.1016/j.imbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 92.Kolev MV, Ruseva MM, Harris CL, Morgan BP, Donev RM. Implication of complement system and its regulators in Alzheimer’s disease. Curr Neuropharmacol. 2009;7:1–8. doi: 10.2174/157015909787602805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamboh MI, Demirci FY, Wang X, et al. Genome-wide association study of Alzheimer’s disease. Transl Psychiatry. 2012;2:e117. doi: 10.1038/tp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kok EH, Luoto T, Haikonen S, Goebeler S, Haapasalo H, Karhunen PJ. CLU, CR1 and PICALM genes associate with Alzheimer’s-related senile plaques. Alzheimers Res Ther. 2011;3:12. doi: 10.1186/alzrt71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J Exp Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hakobyan S, Harding K, Aiyaz M, et al. Complement Biomarkers as Predictors of Disease Progression in Alzheimer’s Disease. J Alzheimers Dis. 2016;54:707–716. doi: 10.3233/JAD-160420. [DOI] [PubMed] [Google Scholar]
- 97.Weinstein G, Beiser AS, Preis SR, et al. Plasma clusterin levels and risk of dementia, Alzheimer’s disease, and stroke. Alzheimers Dement (Amst) 2016;3:103–109. doi: 10.1016/j.dadm.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams MA, Haughton D, Stevenson M, Craig D, Passmore AP, Silvestri G. Plasma Complement factor H in Alzheimer’s Disease. J Alzheimers Dis. 2015;45:369–372. doi: 10.3233/JAD-142742. [DOI] [PubMed] [Google Scholar]
- 99.Thambisetty M, Simmons A, Hye A, et al. Plasma biomarkers of brain atrophy in Alzheimer’s disease. PLoS One. 2011;6:e28527. doi: 10.1371/journal.pone.0028527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haure-Mirande JV, Wang M, Audrain M, et al. Integrative approach to sporadic Alzheimer’s disease: deficiency of TYROBP in cerebral Abeta amyloidosis mouse normalizes clinical phenotype and complement subnetwork molecular pathology without reducing Abeta burden. Mol Psychiatry. 2019;24:431–446. doi: 10.1038/s41380-018-0255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Efthymiou AG, Goate AM. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol Neurodegener. 2017;12:43. doi: 10.1186/s13024-017-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 103.Belbin O, Dunn JL, Chappell S, et al. A SNP in the ACT gene associated with astrocytosis and rapid cognitive decline in AD. Neurobiol Aging. 2008;29:1167–1176. doi: 10.1016/j.neurobiolaging.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 104.Toral-Rios D, Franco-Bocanegra D, Rosas-Carrasco O, et al. Evaluation of inflammation-related genes polymorphisms in Mexican with Alzheimer’s disease: a pilot study. Front Cell Neurosci. 2015;9:148. doi: 10.3389/fncel.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morgan BP. Complement in the pathogenesis of Alzheimer’s disease. Semin Immunopathol. 2018;40:113–124. doi: 10.1007/s00281-017-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin C, Li W, Yuan J, Xu W, Cheng Z. Association of the CR1 polymorphism with late-onset Alzheimer’s disease in Chinese Han populations: a meta-analysis. Neurosci Lett. 2012;527:46–49. doi: 10.1016/j.neulet.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 107.Liu G, Wang H, Liu J, et al. The CLU gene rs11136000 variant is significantly associated with Alzheimer’s disease in Caucasian and Asian populations. Neuromolecular Med. 2014;16:52–60. doi: 10.1007/s12017-013-8250-1. [DOI] [PubMed] [Google Scholar]
- 108.Zhu R, Liu X, He Z. Association between CLU gene rs11136000 polymorphism and Alzheimer’s disease: an updated meta-analysis. Neurol Sci. 2018;39:679–689. doi: 10.1007/s10072-018-3259-8. [DOI] [PubMed] [Google Scholar]
- 109.Tan L, Wang HF, Tan MS, et al. Effect of CLU genetic variants on cerebrospinal fluid and neuroimaging markers in healthy, mild cognitive impairment and Alzheimer’s disease cohorts. Sci Rep. 2016;6:26027. doi: 10.1038/srep26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xing YY, Yu JT, Cui WZ, et al. Blood clusterin levels, rs9331888 polymorphism, and the risk of Alzheimer’s disease. J Alzheimers Dis. 2012;29:515–519. doi: 10.3233/JAD-2011-111844. [DOI] [PubMed] [Google Scholar]
- 111.Zhou J, Fonseca MI, Pisalyaput K, Tenner AJ. Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer’s disease. J Neurochem. 2008;106:2080–2092. doi: 10.1111/j.1471-4159.2008.05558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fonseca MI, Chu S, Pierce AL, et al. Analysis of the Putative Role of CR1 in Alzheimer’s Disease: Genetic Association, Expression and Function. PLoS One. 2016;11:e0149792. doi: 10.1371/journal.pone.0149792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Foster EM, Dangla-Valls A, Lovestone S, Ribe EM, Buckley NJ. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons From Other Pathologies. Front Neurosci. 2019;13:164. doi: 10.3389/fnins.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thambisetty M, Simmons A, Velayudhan L, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jongbloed W, van Dijk KD, Mulder SD, et al. Clusterin Levels in Plasma Predict Cognitive Decline and Progression to Alzheimer’s Disease. J Alzheimers Dis. 2015;46:1103–1110. doi: 10.3233/JAD-150036. [DOI] [PubMed] [Google Scholar]
- 116.Winston CN, Goetzl EJ, Schwartz JB, Elahi FM, Rissman RA. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer’s disease dementia. Alzheimers Dement (Amst) 2019;11:61–66. doi: 10.1016/j.dadm.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Daborg J, Andreasson U, Pekna M, et al. Cerebrospinal fluid levels of complement proteins C3, C4 and CR1 in Alzheimer’s disease. J Neural Transm (Vienna) 2012;119:789–797. doi: 10.1007/s00702-012-0797-8. [DOI] [PubMed] [Google Scholar]
- 118.Bennett S, Grant M, Creese AJ, et al. Plasma levels of complement 4a protein are increased in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2012;26:329–334. doi: 10.1097/WAD.0b013e318239dcbd. [DOI] [PubMed] [Google Scholar]
- 119.Yarchoan M, Louneva N, Xie SX, et al. Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J Neurol Sci. 2013;333:9–12. doi: 10.1016/j.jns.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Locascio JJ, Fukumoto H, Yap L, et al. Plasma amyloid beta-protein and C-reactive protein in relation to the rate of progression of Alzheimer disease. Arch Neurol. 2008;65:776–785. doi: 10.1001/archneur.65.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watanabe Y, Kitamura K, Nakamura K, et al. Elevated C-Reactive Protein Is Associated with Cognitive Decline in Outpatients of a General Hospital: The Project in Sado for Total Health (PROST) Dement Geriatr Cogn Dis Extra. 2016;6:10–19. doi: 10.1159/000442585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vintimilla R, Hall J, Johnson L, O‘Bryant S. The relationship of CRP and cognition in cognitively normal older Mexican Americans: A cross-sectional study of the HABLE cohort. Medicine (Baltimore) 2019;98:e15605. doi: 10.1097/MD.0000000000015605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park JC, Han SH, Cho HJ, et al. Chemically treated plasma Abeta is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Ther. 2017;9:20. doi: 10.1186/s13195-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]