Abstract
Imaging of the living human brain is a powerful tool to probe the interactions between brain, gut and microbiome in health and in disorders of brain–gut interactions, in particular IBS. While altered signals from the viscera contribute to clinical symptoms, the brain integrates these interoceptive signals with emotional, cognitive and memory related inputs in a non-linear fashion to produce symptoms. Tremendous progress has occurred in the development of new imaging techniques that look at structural, functional and metabolic properties of brain regions and networks. Standardisation in image acquisition and advances in computational approaches has made it possible to study large data sets of imaging studies, identify network properties and integrate them with non-imaging data. These approaches are beginning to generate brain signatures in IBS that share some features with those obtained in other often overlapping chronic pain disorders such as urological pelvic pain syndromes and vulvodynia, suggesting shared mechanisms. Despite this progress, the identification of preclinical vulnerability factors and outcome predictors has been slow. To overcome current obstacles, the creation of consortia and the generation of standardised multisite repositories for brain imaging and metadata from multisite studies are required.
INTRODUCTION
Functional brain imaging research in gastroenterology has allowed for improved insight into spontaneous and evoked brain features and into the role of brain–gut interactions in health and disease.1,2 Until recently, the focus of brain imaging research in gastroenterology has been to gain a better understanding of the pathophysiology of disorders of brain-gut interactions (DBGI),3 also known as functional gastrointestinal disorders.1,4,5 DBGIs are defined as a group of disorders classified by the presence of GI symptoms related to any combination of motility disturbance, visceral hypersensitivity, altered mucosal and immune function, altered gut microbiota and altered central nervous system (CNS) processing in the absence of detectable organic disease.3 Common DBGIs include IBS, functional dyspepsia (FD), chest pain of oesophageal origin and functional heartburn. There is considerable overlap of the DBGIs with each other, with other visceral and somatic ‘functional’ pain syndromes (including urological pelvic pain syndromes (UCPPS), vulvodynia, fibromyalgia and chronic back pain3,6) and with psychiatric disorders, in particular anxiety and depression.7,8
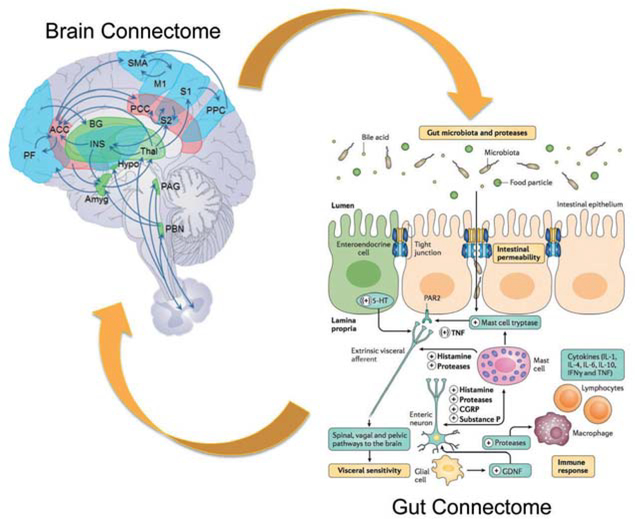
The current diagnostic criteria for DBGIs, as well as illness severity, frequency, duration and treatment efficacy all rely exclusively on subjective patient reports and not on objective biomarkers.3,6 Regardless of the primary aetiology, these subjective symptom reports are generated in part by the brain from interoceptive signals originating in the GI tract, from memories of such signals, and are extensively modulated by emotional (anxiety and depression), cognitive (attention and expectation) and motivational factors. As pointed out for all other chronic pain disorders, this translation of objective gut signals into subjective symptoms is highly non-linear.9,10 Therefore, multimodal assessment of the brain’s structure, function and biochemical and receptor properties has the potential to provide more objective information about pathophysiology, treatment efficacy and biologically based patient subgroups in these conditions by elucidating the contribution of multiple brain networks to the subjective symptom reports. Indeed, numerous studies examining brain processing of visceral sensations have been published in an attempt to identify biomarkers of these disorders (see details in refs 7,11,12) (reviewed in refs 2,9,13). A comprehensive model of brain-gut interactions incorporating reported alterations in brain networks (‘brain connectome’) and networks of interacting systems in the gut (‘gut connectome’)14 is shown in figure 1.
Figure 1.
Proposed integrative model for disorders of gut–brain Interactions. Replacing the conventional focus on individual brain regions and cell types in the gut, this integrative model posits reciprocal interactions between brain networks (brain connectome) and networks made up of multiple cells in the gut, including the gut microbiota (gut connectome). Gut-to-brain communication is mediated by neural, endocrine and inflammatory pathways, while brain-to-gut communication relies mainly on autonomic nervous system output to the gut. Modified with permission from Enck et al.14
In the last two decades, and in particular since the last Rome Working Team report on this topic in 2009,7 multimodal brain imaging research has greatly improved our understanding of the brain–gut interactions in DBGIs and identified commonalities and differences to other functional pain syndromes and psychiatric disorders. However, the ultimate goal of identifying generally agreed on biomarkers for individual syndromes, patient stratification for treatment trials and assessing treatment efficacy has not been fully realised.13,15 This article will review the current literature on the use of brain imaging in DBGI and will provide recommendations for future studies.
Understanding structural and functional brain alterations and their role in pathophysiology of DBGIs
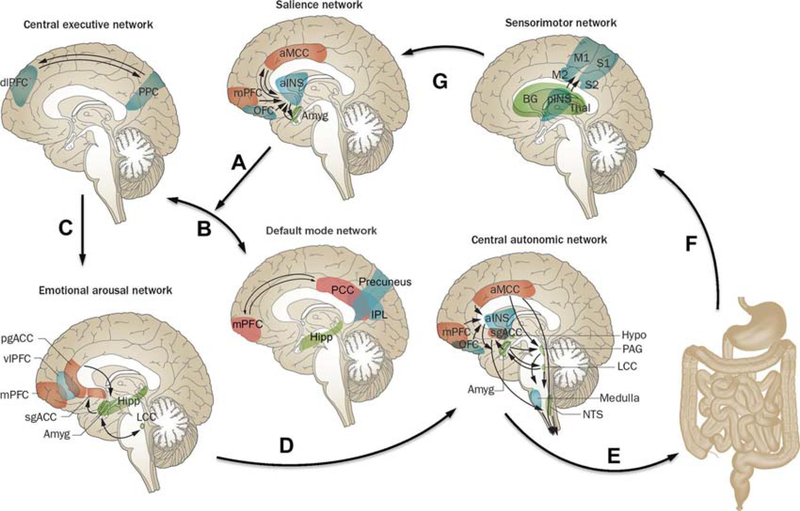
Although it has long been assumed that specific brain functions, such as pain processing, emotion and cognition are attributable to the isolated operations of single brain regions, these processes are now viewed as resulting from the dynamic interactions of distributed brain areas operating in several large-scale networks (figure 2; box 1). These networks and their properties have been assessed by using neuroanatomical and neurophysiological studies in animals,16 as well as different brain imaging techniques and analyses in humans.17–24 In humans, several types of networks have been reported: (A) functional brain networks based on evoked responses7,25 or intrinsic connectivity of the brain during rest,17,19–21,23,26–30 (B) structural networks based on grey matter parameters31,32 and white matter properties and (C) anatomical networks based white matter connectivities.33 Both evoked and resting state studies performed in patients with IBS have demonstrated abnormalities in regions, as well as in resting state and task related networks related to default mode (DMN),34,35 emotional arousal,7,23,36–38 central autonomic control,17,18,20,22 central executive control,17,20,38 sensorimotor processing39–42 and salience detection.43,44 (table 1). IBS-related alterations in these networks have provided plausible neurobiological substrates for several information processing abnormalities reported in patients with IBS, such as biased threat appraisal (‘catastrophising’) and expectancy of outcomes (eg, salience network), autonomic hyperarousal (emotional arousal and central autonomic networks) and symptom-focused attention (central executive network).19
Figure 2.
Brain networks involved in centrai processing and modulation of visceral pain. Shown are the default mode network (DMN) and four task-related brain networks that have been described in the literature, for which structural and functional alterations and correlations with clinical and behavioural measures have been reported in IBS subjects. Correlations of the listed clinical and behavioural measures have been reported for the salience network,43,50,65,145,146 sensorimotor network,46,100,147 emotional arousal network,40,45,47,145,147 central executive network,43 central autonomic network43,45,47 and DMN.146 Arrows indicate: (A) shift of activity from the DMN to the task-related networks in response to input from the salience network; (B) switching between DMN and central executive network depending on input from the salience network; (C) engagement of emotional arousal network in response to central executive network activation; (D) engagement of central autonomic network in response to emotional arousal network activation; (E) central autonomic network activation with output in the form of descending pain modulation and autonomic nervous system activity to GI tract; (F) ascending viscerosensory signals from gut to sensorimotor network; and (G) assessment of information from sensorimotor network by salience network. The functions of these networks are described in detail in the text. Modified with permission from Mayer et al.9
Box 1. Understanding structural and functional brain alterations and their role in the pathophysiology of disorders of brain–gut interactions.
Specific brain functions, including the perception and modulation of visceral pain, can best be understood as the result of multiple interacting brain networks.
Networks most relevant to symptoms of IBS include salience, attentional, emotional arousal, central autonomic and sensorimotor networks.
Sex differences in IBS-related structural and functional brain alterations may relate to known sex differences in prevalence, symptom presentation, comorbidities and response to treatment of patients with IBS.
Several acquisition methods for brain imaging data, including MRI, diffusion tensor imaging, positron emission tomography and MRS are available to provide complementary information on the structure, function and biochemistry of the human brain.
Table 1.
Brain network alterations in IBS
| Default mode network (DMN) | Brain regions | Medial frontal cortex, posterior cingulate or retrosplenial cortex, precuneus, inferior parietal cortex, lateral temporal cortex and hippocampal formation. ► Self-awareness processing. ► Episodic memory. |
| Function | ► Monitoring internal thoughts, external goals and future planning. ► Altered functional connectivity and topological reorganisation in various regions, consistent with the network’s dysregulation in chronic visceral pain.148 |
|
| Alterations in IBS | ► Higher amygdala and dorsal anterior insula (INS) functional connectivities within DMN in hypersensitive IBS.149 | |
| Sex difference | ► No reported sex difference in IBS to date. | |
| Sensorimotor network | Brain regions | Thalamus, basal ganglia, sensorimotor cortex and posterior INS. |
| Function | ► Central processing and modulation of visceral and somatic sensory information. | |
| Alterations in IBS | ► Increased frequency power of spontaneous brain oscillations.40 ► Widespread microstructural white matter changes.150 ► Female IBS greater volume and cortical thickness, correlated with symptom severity.65,147 ► Greater grey matter in posterior INS, correlated with symptom duration.99 ► Anterior cingulate and thalamus are hubs in structural network analysis.65 ► Coupling of cingulate gyrus with thalamus.65 |
|
| Sex difference | ► Higher cortical thickness in sensorimotor cortex in female IBS.147 ► Lower integrity of sensorimotor region tracts in female IBS.150 ► Lower fractional anisotropy and mean diffusivity in globus pallidus in female IBS.150 |
|
| Salience network | Brain regions | Dorsal anterior cingulate cortex (ACC) and anterior INS. |
| Function | ► Response to subjective experience or expectation of any interoceptive and exteroceptive stimulus. ► Coordination of the appropriate attentional, behavioural, affective and visceral responses to such stimuli. |
|
| Alterations in IBS | ► Greater engagement of anterior INS and anterior middngulate cortex in response to actual and expected rectal distension.2,151 ► Increased affective, central, emotional-arousal processes as well as enhanced visceral stimulus perception.130,152,153 ► Alterations in the activity and connectivity of anterior INS in women both during the resting state40,145 and abdominal pain threat.146 |
|
| Sex difference | ► Greater pain-related INS response in male IBS.154,155 ► Greater pain-related ACC response in female IBS156 ► More prominent alterations in the connectivity between INS and DMN in female IBS.43 ► Lower subgenual ACC cortical thickness in female IBS.147 ► Greater mean diffusivity in cingulate white bundles in female IBS.150 |
|
| Emotional arousal network | Brain regions | Amygdala, hippocampus, hypothalamus, posterior ACC and subgenual cingulate (sgACC). |
| Function | ► Activated by perceived or real disruption in homeostasis. ► Generation of rapid feedback inhibition of amygdala, thereby limiting the magnitude and duration of network activity and related activity in the central autonomic network. |
|
| Alterations in IBS | ► Decrease in inhibitory feedback loop104,152,154; also seen in healthy controls whose central serotonin levels were lowered by acute tryptophan depletion.157 ► Increased responsiveness to both expected and delivered visceral stimuli in females.158–167 ► More consistent activation in response to controlled rectal distension.12 ► Reactivity associated with serotonin (5-hydroxytryptamine)-related gene polymorphisms.49 ► Functional alterations are accompanied by structural brain alterations.65 |
|
| Sex difference | ► Greater emotional-arousal reactivity and altered connectivity in female IBS.156,168 ► Greater emotional-arousal reactivity to specific stimuli (faces depicting fear and anger) in male IBS154 |
|
| Central autonomic network | Brain regions | Control centres in the pontine-medulla (including periaqueductal grey (PAG) and hypothalamus), the central nucleus of the amygdala and several cortical regions (including the anterior INS, ACC and prefrontal and motor regions). |
| Function | ► Central control and modulation of the autonomic nervous system. ► Regulation of respiratory, cardiovascular, endocrine and digestive activities during cognitive, affective, and motor tasks and sensations. |
|
| Alterations in IBS | ► Alterations in the corticotropin releasing factor (CRF) and CRF receptor 1104,105 and norepinephrine-adrenergic receptor signalling system.169 | |
| Sex difference | ► Greater activation of dorsolateral prefrontal cortex, INS and dorsal pons/PAG in response to visceral stimulus in male IBS.156 ► Greater activation of ventromedial prefrontal cortex, right anterior cingulate cortex and left amygdala in response to visceral stimulus in female IBS.156 |
|
| Central executive network | Brain regions | Lateral prefrontal cortices and posterior parietal cortex. |
| Function | ► Activated during tasks involving executive functions such as attention, working memory, planning and response selection. ► Often coactivated with regions of the salience network, as the brain attempts to focus its limited processing capacity to only salient information via attention, working memory, planning and response selection. |
|
| Alterations in IBS | ► Deficient activation of inhibitory cortical regions involved in down regulation of pain and emotion as well as attention during expectation and experience of aversive GI stimuli.12 ► Selective recall of negative and GI sensation words, as well as selective attention to threat-related stimuli.170–173 ► Reduced effective connectivity during repeated exposure to the anticipation and experience of a threatening GI stimulus, which was linked to was linked to a reduction in IBS hypersensitivity.174 ► Altered error feedback mechanisms linked to decreased dorsolateral prefrontal cortex activity in Japanese patients with IBS.16 ► Strong negative association between the cortical thickness and grey matter density of the dorsolateral prefrontal cortex and pain catastrophising.85,175 ► Altered prepulse inhibition (a process by which an organism can filter the flow of information from its internal and external environments).41 |
|
| Sex difference | ► No reported sex differences in IBS to date. |
What are the correlations of these networks with non-brain imaging metadata and how can these correlations help to gain insights into DBGI pathophysiology
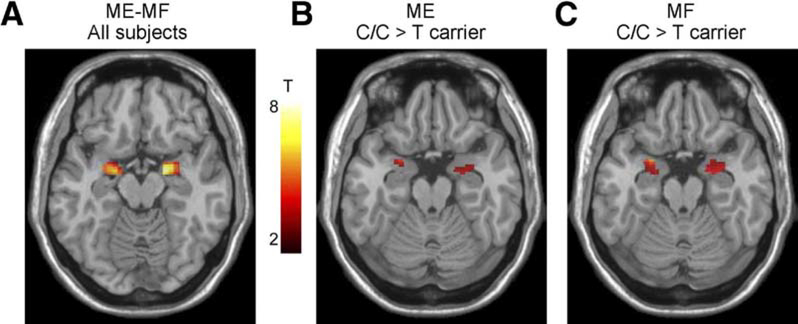
In order to make conclusions about the involvement of structural and functional brain alterations in the generation of clinical symptoms of IBS and other brain–gut disorders, associations with subjective clinical (including symptom severity, abdominal pain and bowel habits) and behavioural measures (including anxiety, depression, stress, early adverse life events) should be correlated with these brain alterations. Even though such associations have been reported in most published cross-sectional reports, effect sizes are generally small, and causality has not been demonstrated for any of these parameters in longitudinal studies. Reported associations of clinical, behavioural and biological measures with brain parameters are shown in figure 2.43,45–47 Several such associations of specific brain parameters with genes related to the hypothalamic-pituitary-adrenal axis,48 catecholamine46 and 5-hydroxytryptamine (5-HT) signalling49 and with gene expression profiles in peripheral blood mononuclear cells50 have been reported. An example of the association of a 5-HT3 receptor polymorphism with amygdala activation is shown in figure 3.49 Preliminary data suggest correlations of regional brain structural differences with gut microbial taxa,51 even though similar to the situation with clinical metadata, it is currently not possible to draw conclusions about causality from these results.
Figure 3.
Effect of the HTR3A polymorphism c. −42C>T on amygdala reactivity to emotional and non-emotional stimuli. C/C genotype subjects displayed greater amygdala responses during an emotion matching and form matching task, suggesting a role of this gene polymorphism in influencing the emotional response to different laboratory tasks. With permission from Kilpatrick et al.49
How do brain signatures differ between male and female subjects and what implications do these differences have for DBGI pathophysiology and treatment
Sex is increasingly being understood as an important basic variable, influencing the quality and generalisability of biomedical research.52 Sex differences in IBS-related structural and functional brain alterations may relate to known sex differences in prevalence, symptom presentation, comorbidities and response to treatment of patients with IBS.53 Although IBS neuroimaging research is predominately female specific or mixed sex,54 an increasing number of studies have examined sex differences in IBS-related brain alterations, demonstrating differences among key regions in the emerging brain networks discussed above (table 1). In addition, several studies have examined the role of variations in female sex hormones (related to menstrual cycle or birth control pills) in neural pain processing, which may explain some of the observed sex related differences.55–58 These findings highlight the importance of taking sex differences into account when reporting brain imaging data in IBS. Reported structural and functional sex differences in several brain networks may explain the greater prevalence of IBS in women and sex differences in individual symptoms. Furthermore, sex differences in brain alterations may play a role in different responsiveness of male and female patients to pharmacological and non-pharmacological therapies.
Acquisition of multimodal brain imaging data
Several acquisition methods (summarised in table 2) are available to provide complementary information on the structure and function of the brain in humans. When applied together, this methodological approach is referred to as multimodal brain imaging.
Table 2.
Brain imagina modalities
| Imaging modality | Description |
|---|---|
| Positron emission tomography | Measures regional glucose utilisation, cerebral blood flow (both measures of regional brain activity) and receptor occupancy. |
| Arterial spin labelling | Cerebral blood flow. |
| Electroencephalogram | Cerebral electrical activity. |
| Magnetoencephalography | Measures magnetic fields produced by electrical activity of the brain. |
| Magnetic resonance spectroscopy | Measures brain concentration of brain metabolites and neurotransmitters. |
| Structural MRI | Provides high spatial resolution and soft tissue contrasts to measure brain morphometry. |
| Functional MRI | Measures brain activity by detecting changes in blood oxygenation and flow during rest or an evoked task. |
| Diffusion tensor imaging | Assesses the microstructure of white matter and anatomical connectivity and integrity. |
Contributions to better understanding of the pathophysiology of DBGIs from positron emission tomography (PET) ligand studies and MRI of brain metabolites (MR spectroscopy)
Radioligand PET and MR spectroscopy studies have made it possible to elucidate the involvement of specific neurotransmitter systems or brain metabolites in DBGI pathophysiology but are only beginning to be applied in this context.
Radioligand PET studies
This technique allows quantification of regional availability of receptor/transporter systems in the brain by injecting radioactively labelled ligands for these systems in sub pharmacological doses. Limitations include the availability of ligands (although they are available for most receptor systems of the major neurotransmitters, and new ones are being developed continuously), the need for a specialised radiopharmaceutical facility in close proximity to the study location and the involvement of radiation burden for the subjects. Some ligands also allow quantification of endogenous release of the corresponding neurotransmitter. For example, using [11C]-carfentanyl, Ly et al demonstrated that prolonged painful gastric stimulation, contrary to a similar somatic pain stimulus, did not provoke endogenous opioid release in pain responsive brain regions in healthy volunteers.59
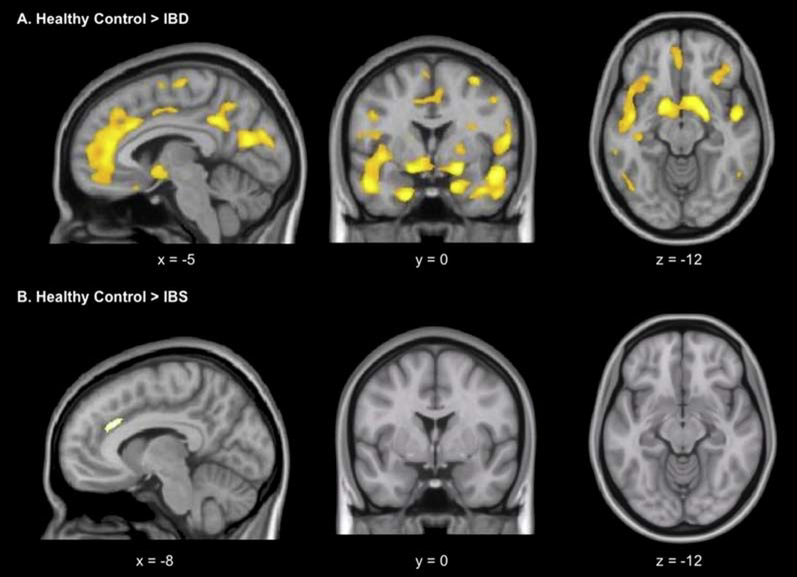
Most studies have compared regional availability of receptor/transporter systems between DBGI patient populations and healthy controls (HCs). Jarcho et al60 studied neurokinin-1 receptor (NK-1R) availability in a small sample of IBS patients compared with age-matched and sex-matched samples of HCs and patients with IBD. As shown in figure 4, patients with gut inflammation showed a widespread reduction in NK-1R availability compared with HCs, particularly in the basal ganglia, hippocampus, amygdala and cingulate subregions. In contrast, in patients with IBS, reductions compared with HCs were only found in the putamen and anterior middle portion of the anterior cingulate cortex (ACC) but did not reach statistical significance. However, effect sizes were large, suggesting the lack of significance could be driven by the small sample size.
Figure 4.
Reduced neurokinin-1 receptor binding in IBD. Whole-brain voxel-wise statistical parametric mapping analysis shows regions with lower levels of neurokinin-1 receptor binding in several brain regions in subjects with IBD (A) and patients with IBS (B), relative to healthy controls (voxel extent threshold p<0.001; cluster extent threshold >20). With permission from Jarcho et al.60
Ly et al found widespread increases in cannabinoid-1 receptor (CB1-R) availability in a small sample of patients with FD compared with age-matched, sex-matched and body mass index-matched HCs.61 More specifically, significant differences surviving multiple testing correction, with large effect sizes, were found in subcortical (basal ganglia, amygdala and brainstem) and cortical (insular, cingulate and prefrontal subregions) areas involved in pain processing and modulation as well as control of appetite, food intake and nutrient tolerance. These increases in CB1-R availability were stable after a naturalistic follow-up of on average 3 years in a subsample of the patients. Tominaga et al62 reported preliminary findings, demonstrating increased serotonin transporter (SERT) availability in the midbrain and the thalamus in patients with FD compared with HCs. In patients with FD, SERT availability in these regions correlated with total GI symptom and abdominal pain levels.
MR spectroscopy studies
This MR-based technique allows quantification of regional metabolite concentrations in brain tissue, including the neurotransmitters glutamate and GABA, and the inflammatory mediator myo-inositol, based on the differential resonance frequency of protons in different molecules, although with a much lower spatial and temporal resolution compared with MRI.63 To the best of our knowledge, only one study used this technique in DBGI. Niddam et al demonstrated a reduction in hippocampal glutamate-glutamine (Glx) in 15 patients with IBS without psychiatric comorbidity compared with 15 well-matched controls. Glx concentrations were inversely associated with stress indicators in IBS patients only, which was interpreted as malfunction of inhibitory hippocampal feedback on the hypothalamo–pituitary–adrenal axis.64
Analsis of multimodal brain imaging data
IBS brain connectome
Until recently characterising and comparing the brain’s wiring in DBGIs has been limited to functional and effective connectivity analyses associated with specific circuitry and functionality of neural subsystems including attention/cognitive control, emotional arousal and homeostatic afferent brain networks. However, using network analysis based on graph theory,22 it has become possible to characterise the architecture of large-scale functional and structural networks in IBS65–67 and to examine how these network properties relate to clinical and other biological parameters (box 2). Network analysis based characterises of the role of brain regions and their connections in the integrity and information flow of brain networks. Network metrics are classified into measures that reflect centrality, integration and segregation.68–70
Box 2. Analysis of multimodal brain imaging data.
Advanced network analysis applied to structural and functional brain imaging data has made it possible to characterise the architecture of large-scale functional and structural networks in IBS and examine relationships of these networks with clinical and other biological data.
Data-driven analysis methods (Big Data approaches) apply supervised and unsupervised machine-learning techniques (also called multivariate pattern analysis and projection methods) to large data sets to find patterns in the data without referring to theories or prior hypotheses.
An essential prerequisite for Big Data approaches is the generation of multisite data repositories for standardised multimodal brain imaging, biological and clinical metadata.
Data repositories
It is clear not all labs have the means to produce a large number of images required to ensure reliability of results. With this in mind, The Pain and Interoception Imaging Network Repository (painrepository.org71) was developed to accelerate scientific discovery regarding brain mechanisms in pain and to provide more rapid benefits to pain patients through the harmonisation of efforts and data sharing. This will serve as an invaluable research for studying central mechanisms in DBGIs.
In sum, the volume and diversity of neuroimaging data available for analyses has increased exponentially. These developments and the urgent need for large, well-phenotyped data sets has become a major limitation to progress in the field. Computational tools in neuroscience are yielding larger and more complex data sets than ever before. However, determining which imaging parameters best answers specific questions (biomarkers, outcome predictors, underlying mechanisms) remains to be determined.
Computational algorithms and tools applied to each type of data acquired via automated and semiautomated processing pipelines can result in a vast amount of subject-specific data at the regional or voxel level. For example, from structural MRI volume, cortical thickness, surface area and mean cortical thickness can be estimated. For diffusion tensor imaging, workflows result in several measures of microstructural integrity (eg, mean diffusivity and fractional anisotropy) and connectivity (eg, tractography). Using resting state data, we can produce measures that reflect oscillatory dynamic and intrinsic connectivity at the voxel or regional level. Time-efficient neuroimaging data processing and analysis pipelines that produce an enormous amount of data reflecting white matter properties, brain topology, grey matter morphometry, anatomical and functional connectivity can be of great benefit.72,73
Big Data approaches to study brain–gut interactions
Improvement in computer storage and processing capacity and efficiency has ushered in the age of ‘Big Data’ (box 2). Nowhere is this more true than in the field of neuroscience and neuroimaging, which has experienced exponential increases in the scale and speed of data collection and generation of complex data sets. Concurrently, there has been a shift from smaller scale, hypothesis-driven science to complementary data-driven methods that apply machine learning techniques to large-scale data sets to identify underlying networks and patterns with little or no reference to existing theories. Multivariate data sets (including data from brain, microbiome, metabolome, symptoms and genetics) permits modelling of complex interactions between the brain, biology and behaviour to inform disease phenotypes, diagnosis, prevention and treatment of functional gastroenterological disorders. In addition to the advantages of generating large-scale data sets, experts in the field strongly encourage multisite studies and open access repositories in order to promote a culture of sharing, collaboration and as a consequence the greatest advancements in the field. The BRAIN Initiative74 and the European Human Brain Project75 mark this paradigm shift and focus on neurological disorders and psychiatric disease. Other important large data repositories include the NIH Human Connectome Project (1200 people)76 and the UK Biobank Imaging (100000 brains imaged from 500000 people who have all their genotype and phenotypes plus lifestyle aspects catalogued).77
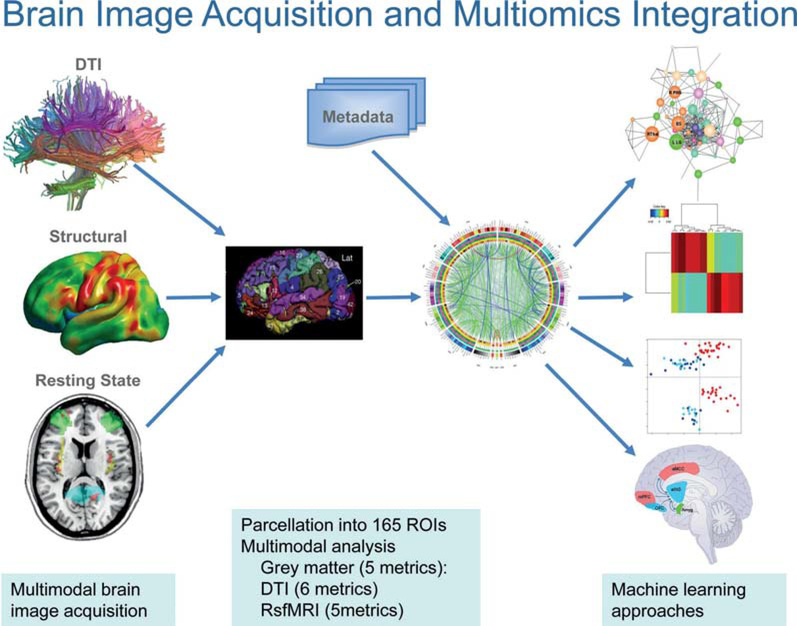
Analyses of multimodal, large-scale neuroimaging data (figure 5)
Figure 5.
Schematic of workflow from multimodal brain image acquisition to multiomics integration of brain and metadata. Acquisition of structural, anatomical (DTI), functional (resting state oscillations) and metabolic (MR spectroscopy, not shown) is followed by image processing and parcellation into multiple regions of interest (ROIs). These parcellated data undergo multiomics integration of different image modalities and clinical, behavioural and non-brain metadata using machine learning approaches. Such data-driven analysis approaches are expected to reveal distinct patters of brain-gut interactions. DTI, diffusion tensor imaging.
Big Data approaches are needed to analyse the high-dimensional neuroimaging data sets. These so-called data-driven methods apply supervised and unsupervised machine learning techniques (also called multivariate pattern analysis and projection methods) to large data sets to find patterns in the data without referring to theories (summarised in table 3). Big Data analysis has provided insights into disease mechanisms that have propelled genomic and metabolic science into the spotlight for unprecedented advances in medical care and having measureable positive influence financially within the USA. Big Data science has brought cutting-edge technological advances for patient diagnosis and care including providing insights into the genetic and immunological underpinning in Alzheimer’s78,79 and Parkinson’s diseases.80
Table 3.
Machine learning approaches in brain imaging analysis
| Supervised algorithms | Unsupervised methods | |
|---|---|---|
| Techniques | Support vector machines, random forest and sparse partial least squares discriminate analysis. | Hierarchical clustering, principal coordinate analysis and sparse k-mean clustering. |
| Rationale | Reduce dimensionality of multimodal large-scale functional, structural and anatomical neuroimaging data by finding a set of brain signatures comprised by selected set of brain features. These brain signatures form the basis of a classification or predictive algorithms that provide insight into the pathophysiological mechanisms. | Integrate and decipher large amounts of multivariate neuroimaging data to subgroups of patients based on objective biological markers and characterise central nervous system alterations for further pathophysiological investigations targeting treatment of chronic pain and other brain disorders. |
| Examples | Functional dyspepsia145 and IBS.20 | Has been applied successfully to clinical, physiological and microbiota data in IBS51,176,177 but not brain data. |
| Outcomes | Identify patterns that discriminate and predict acute pain state, pain diagnosis and pharmacological and non-pharmacological treatment outcomes including longitudinal symptom trajectories. | Future identification of therapeutic targets and development of tailored patient treatment. In combination with other biological data, results may translate into identification of novel therapeutic targets and development of individualised pain therapies based on brain signatures.178–180 |
Identifying the neurobiological basis of treatment effects using neuroimaging and its relevance for dbgi pathophysiology and treatment
Can structural and functional brain signatures be used as biomarkers in treatment prediction and outcome?
There is considerable potential to use neuroimaging-based measures of brain structure and function as predictors (moderators) of treatment selection and outcome (box 3). In addition, brain imaging measures can be used also to estimate a chronic pain or disease trajectory, that is, identifying who might be vulnerable towards getting certain conditions based on their brain functional and structural networks. Outside of the field of DBGI, several studies have identified that patients transiting from acute to chronic back pain show differences in their reward and corticolimbic brain networks (identified functionally and structurally) at baseline that are highly sensitive and specific for predicting the development of chronic pain.81–83 Other areas proposed as conferring vulnerability include the descending pain modulatory system that includes the brainstem’s inhibitory and facilitatory arms.84 A major caveat of these studies to date is their failure to identify causality. For brain–gut related conditions, which are more complex in terms of the beginning of their trajectory (often in childhood), longitudinal studies, Big Data initiatives and consortia alongside the supervised and unsupervised classification methods will be required to generate similar information. Therefore, for now studies are taking a different approach and attempting to characterise whether ‘non-pain’-related features are present in cross-sectional studies that correlate with differential brain activity or structure compared with HCs. For example, identifying potential pre-existing vulnerabilities due to neuroticism, a stable personality trait characterised by a propensity for negative affect has shown a correlation between white matter connectivity strength and neuroticism in IBS.85 Also, IBS patients with a tendency to predict worst outcomes with high likelihood (catastrophising) showed reduced dorsolateral prefrontal cortex (PFC) thickness and increased hypothalamic grey matter.86 These studies suggest that aspects of an individual’s personality might be associated with differential brain structure and connectivity in areas relevant to chronic pain. Such presymptomatic brain alterations in healthy individuals could include the sensorimotor cortex (making healthy individuals more sensitive to visceral and somatic stimuli),87 the PFC and the emotional arousal system (compromising a healthy person’s ability to downregulate emotional circuits), and the endogenous pain modulation system (limiting an individual’s ability to counter regulate acute pain). Interestingly, the research exploring sex differences described in the earlier section might be seen as conferring a differential vulnerability. An alternative hypothesis about chronic pain vulnerability is related to the responsiveness of patients with acute pain to pharmacological intervention. For example, studies in HCs and animals have shown that baseline reward circuitry and normal endogenous opioid activity in the anterior cingulate, respectively, is predictive of and necessary for analgesic outcome with pharmacological agents in somatic pain.88,89 These findings suggest a reinterpretation of the ‘vulnerability’ question from predicting chronic pain development towards perhaps resistance to positive analgesic outcome.90 It remains to be determined if such observations pertain to chronic visceral pain conditions. To date, there is little human data and mostly from non-GI conditions to inform how such brain measures, whether identified as precondition vulnerability or disease relevant factors and responsiveness to treatment, predict treatment outcome. The experimental challenge is getting adequate signal to noise to perform such prediction studies for individual patients—a necessary condition for patient stratification and personalised medicine approaches.
Box 3. Identifying the neurobiological basis of treatment effects using neuroimaging and its relevance for disorders of brain–gut interactions pathophysiology and treatment.
Specific network alterations have the potential to become biomarkers for IBS or for IBS subtypes, as well as predictors (moderators) of treatment outcomes, replacing existing symptom-based classifications.
Specific brain alterations are potential targets for pharmacological and non-pharmacological treatments.
Future treatment goals include modification of altered functional connectivity patterns, the induction of network specific neuroplastic changes and the normalisation of altered metabolite patterns in the brain.
A recent study91 in a small cohort of female fibromyalgia patients highlights how brain imaging may be used to distinguish drug from placebo effects in patients with DBGI. The study showed that glutamate/glutamine (right posterior insula (INS) only), connectivity of anterior and posterior INS to a key region of the DMN and deactivation of some DMN regions to evoked pressure pain were altered by pregabalin but not placebo. Many of the pretreatment baseline levels of these measures correlated with the magnitude of clinical pain at that time. In addition, the study showed that clinical pain changes were predicted by resting connectivity and evoked neural activity (deactivation) in the DMN, whereas glutamate within the posterior INS predicted behavioural changes in evoked pain only. This study highlights the potential for neuroimaging to aid the prediction of treatment outcomes. Together with the baseline reward network/opioid analgesia prediction study,89 it illustrates that neuroimaging might aid treatment selection by identifying networks more amenable to one treatment over another. This principle holds also for non-pharmacological treatment interventions and identifying patients who will benefit most from either a cognitive–behavioural treatment (CBT)-based approach rather than acupuncture, hypnosis or mindfulness-based stress reduction.
How do pharmacological therapeutic interventions affect brain network alterations in DBGIs?
In contrast to many chronic pain disorders that have well-defined peripheral disease mechanisms (eg, neuropathic pain and inflammatory pain), issues of how therapeutic interventions affect brain systems in patients with DBGI remains more challenging due to the lack of agreed on brain92 or other biomarkers for each condition,93 a relatively poor understanding of how pharmacological agents affect brain systems, the multifaceted and complex nature of the disease involving sensory, emotional, cognitive and modulatory networks as well as complex psychosocial issues independent of potential biological processes that may be the target of pharmacological agents. In the following sections we briefly review: (1) brain measures of pharmacological effects and (2) putative mechanisms of drugs on brain networks.
Unlike some brain disorders that are characterised by a major abnormality in a particular neurotransmitter system (eg, the dopamine system in Parkinson’s disease), there is currently no such discrete neurobiological abnormality in DBGIs. The ideal pharmacological agent or combination of agents (or other adjunctive therapies) should normalise an altered brain state. Currently, our best objective measure of such an altered brain state is the normalisation of resting state networks (RSNs) as well as grey matter changes that has been noted to respond to treatment in other chronic pain conditions (see refs 94,95). A few papers have evaluated altered functional networks96–98 and grey matter changes85,99,100 in patients with DBGIs2,9 and have been discussed at the beginning of this review.
Network modules and functional specialisation and grey matter changes
As discussed in detail earlier, brain networks provide an integrated measure of neural systems that define behaviour and are made up of modules. The complexity is intricate given the anatomical connectivity of any specific brain region with multiple local and distant brain regions. These processes will provide targets for pharmacological measures. Numerous processes including sex, comorbidities, age, duration of disease, pain intensity or treatment resistance are issues that need to be defined in the context of potential pharmacological targets given the ongoing changes in the brain connectome (https://www.humanconnectome.org).
Ideally, any pharmacological agent should have the following effects on brain systems: (1) modify functional connectivity towards a ‘normal’ state and (2) induce plastic changes in brain morphology (including white matter connections) or grey matter volume. Pharmacological agents are known to have effects on brain systems as evaluated by functional MRI approaches.101,102 Commonly used pharmacotherapies aimed at the CNS (neuromodulators) include antidepressants (tricyclic antidepressants (mechanisms of action (MOAs): noradrenergic and serotonergic but also antimuscarinic and antihistaminic properties); selective serotonin uptake inhibitors (MOA: blocking uptake of 5-HT); and cyproheptadine (MOA: antihistaminic, anticholinergic and antiserotonergic properties).
Modification of functional connectivity in DBGIs by pharmacological agents (box 4)
Box 4. Clinical implications.
Data driven analyses of large multimodal brain imaging data sets obtained at multiple time points has the potential to identify:
The biological basis of individual disorders of brain-gut interactions, including IBS.
The biological mechanisms underlying common comorbidities with other chronic pain and affective conditions.
The causative role of gut microbial metabolites in IBS symptom generation.
Subgroups of patients responsive to specific pharmacological and non-pharmacological therapeutic interventions.
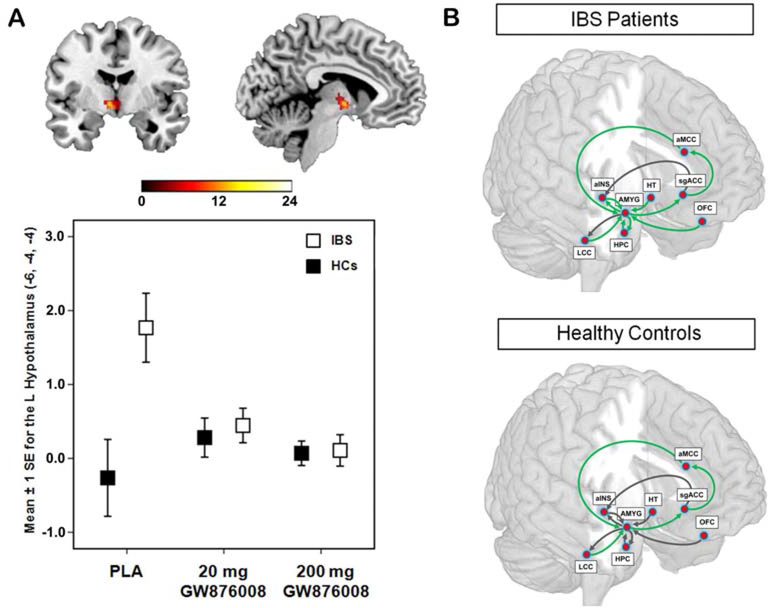
The main brain targets of current pharmacological action of commonly used drugs for DBGIs include: serotonergic, noradrenergic and histaminergic mechanisms. These are well defined in the mammalian brain103 but how changing one affects modular or more diverse brain circuits in DBGIs is not known. Previous brain imaging studies have demonstrated the effects of several candidate compounds for IBS treatment (antagonists for the CRF-R1 receptor,104,105 5-HT3 receptor106,107 and neurokinin 1 (NK1) receptor60,108 which are no longer pursued for IBS drug development. Figure 6 shows the reported effect of a CRF-R1 receptor antagonist on the activity of the hypothalamus and on functional connectivity within the emotional arousal network in IBS and HC subjects. Levels of specific neurotransmitters in one brain region may predict responses in other brain regions using RSN analysis109 or the effects on brain RSNs evaluated by pharmacological manipulation of a specific system, for example, dopamine.110,111 In the case of dopamine, changes have provided insights into specific network changes112 and symptoms (eg, pain).
Figure 6.
Effect of a CRF-R1 antagonist on amygdala response and emotional arousal circuit. (A). Error plot showing standard mean errors for beta contrasts (threat – safe) following placebo (PLA) versus a 20 mg or a 200 mg dose of the CRF-R1 antagonist GW876008 for the left locus coeruleus complex in patients with IBS and healthy controls (HCs) during an experimental pain threat. Results show a dose-dependent reduction in the threat-induced amygdala response by the CRF-R1 antagonist. (B). Path coefficients for the effective connectivity analysis of an emotional-arousal circuit during a pain threat following placebo versus high dose of the CRF-R1 antagonist (200 mg GW876008) In healthy controls and IBS subjects. Significantly different parameter estimates are shown by green arrows, while those not significantly different are shown in black. With permission from Hubbard et al.104 alNS, anterior insula; aMCC, anterior midclngulate cortex; AMYG, amygdala; HPC, hippocampus; HT, hypothalamus; LCC, locus coeruleus complex; OFC, orbitomedial prefrontal cortex; sgACC, subgenual anterior cingulate cortex.
Modification of structural changes in DBGIs by pharmacological agents
Several alterations in brain grey matter volume have been reported in DBGIs (see above). As such, these findings represent an enormous opportunity to target and understand functional changes. Changes in structure are linked to changes in functional connectivity.113 Structural changes in grey matter volume have been considered to reflect levels of dendritic complexity.114 The notion that pharmacotherapies alter morphology is not new (see ref 115) and offers a robust measurable approach to understanding effective treatments.116 Dendritic plasticity may be very rapid to induce remodelling and consequently new connections. How to maintain such changes if effective are still now well defined. However, changes in dendritic complexity may provide insights into drug resistance or disease modification.
In summary, pharmacological approaches may contribute to alterations in brain systems whether their effects are central or peripheral in action. Such changes may reflect processes that are dependent on drug–receptor interactions but may also affect dendritic plasticity acutely or in a more disease modulatory role. Effective pharmacotherapies have the potential to change the brain. Brain pharmacoimaging may help dissect systems that may be targets to recapitulate altered brain morphology and connections to define the development of new pharmacological therapeutic strategies.
How do non-pharmacological therapeutic interventions affect brain network alterations?
Neuroimaging is at its most powerful when it can reveal an insight and understanding to a phenomenon that has been either a mystery or not believed due to the subjectivity of response measures. In terms of lending credence to the efficacy of non-pharmacological therapeutic interventions by identifying the neurophysiological basis, neuroimaging has been a powerful advocate. An early study showed that CBT in IBS was associated with reduced activity in emotion-related brain regions (parahippocampal gyrus and inferior portion of the right ACC, GI-related symptoms and anxiety.117 A more recent study using moxibustion-induced analgesia in IBS with diarrhoea showed improved symptoms and quality of life in the active treatment group compared with sham, with a decrease in the perception of rectal distention and a decreased PFC and ACC activation to rectal distension.118 The majority of studies in this area have been done in HCs or small patient cohorts with very few in DBGI. Several excellent reviews and articles have been written that summarise the findings to date across non-pharmacological interventions (eg, refs 119,120) or focus exclusively on one type of intervention (eg, acupuncture and opioids121; acupuncture and brain connectivity normalisation in chronic pain122; acupuncture and the human brain123). A recent review suggests that brain mechanisms underlying the modulation of pain perception under hypnotic conditions involve cortical as well as subcortical areas including anterior cingulate and prefrontal cortices, basal ganglia and thalami.124It has been suggested that hypnosis modulates pain perception and tolerance by affecting cortical and subcortical activity in brain regions involved in these specific processes with the ACC playing a central role in modulating pain circuitry activity under hypnosis. Most studies also showed that the neural functions of the prefrontal, insular and somatosensory cortices are consistently modified during hypnosis-modulated pain conditions. From these reviews, authors conclude that findings from neuroimaging studies support the clinical use of hypnosis.125 While there have been several reasonably sized studies exploring how mindfulness therapies produce benefits in patients with IBS, none to date have combined this with neuroimaging.126,127 However, several neuroimaging studies aimed to identify brain changes underlying mindfulness interventions and symptom improvement are currently under way.
In conclusion, while no consensus has been reached yet regarding a unified set of mechanisms underpinning how non-pharmacological interventions produce their effects—and certainly not specifically for DBGIs—there is evidence that such interventions have specific neurophysiological effects that can be detected using neuroimaging tools. It appears that different psychological and non-pharmacological treatment modalities are associated with activations of executive-cognitive and affective-motivational brain networks, with some evidence for decreased pain-related activations in afferent pain regions (sensorimotor network) and emotional structures (emotional arousal network), with the descending pain modulatory system as a potential key system recruited by several interventions. Future classification methods employing multivariate pattern analyses will help identify whether common underlying modulatory mechanisms exist or if each therapy relates to a specific brain mechanism.
Identifying gaps in current knowledge and goals for future research (box 5)
Box 5. Identifying gaps in current knowledge and goals for future research.
To realise the full potential of multimodal brain imaging approaches to the study of disorders of brain–gut interactions and to revolutionise the understanding and treatment of IBS, the following goals have been identified:
Longitudinal studies in large patient cohorts with specific pharmacological and non-pharmacological approaches, including medications, diet and mind based therapies.
Developmental studies starting in infancy to identify the aetiology of IBS, including the role of early life experiences (diet, antibiotics and stress) in the development of brain alterations.
Understanding the relative causative role of central and peripheral alterations in children and adults in IBS pathophysiology.
Understanding similarities and differences between different types of chronic pain conditions.
Psychological factors and specificity
There is a general consensus that DBGIs are heterogeneous group of disorders with respect to GI symptoms, and a large proportion of patients are characterised by psychological and behavioural alterations such as psychiatric comorbidity, dysfunctional symptom-related cognitions (catastrophising) and symptom-related anxiety. These cognitive and emotional factors modulate central processing both during expectation and during the actual delivery of visceral stimuli and contribute to altered structural and functional brain connectivity, as well as to the associated alterations in autonomic nervous system outflow to the gut. However, many studies on emotional and cognitive modulation of symptom perception have been carried out in HCs, and more studies are needed to determine if and how psychological modulation of central pain processing is altered in patients with chronic visceral pain. Innovative paradigms involving psychological stress or administration of stress mediators, placebo/nocebo intervention or conditioning studies are emerging and awaiting application in patient studies.
In order to address whether brain alterations are specific to chronic visceral pain rather than to the associated anxiety or depression, future studies should include carefully selected patient control groups, such as patients with chronic somatic pain or patients with a diagnosis of anxiety or depression. Finally, while brain imaging studies in the GI field have already successfully begun to unravel how psychological trait and state factors shape brain structure and function, future work will need to address how trait factors (such as depression or anxiety), interact with state factors (such as negative emotions) and determine how these factors contribute to symptom generation and maintenance. This knowledge may reveal if chronic symptoms are primarily driven by central alterations or peripheral changes in specific patient subgroups, which could be a basis for individualised treatment approaches.
Increased perception of visceral stimuli (visceral hypersensitivity)
Although visceral hypersensitivity (the increased perception or response to visceral stimuli) plays an important role in the pathophysiology of the functional GI disorders, especially IBS, and has inspired much mechanistic work, the number of brain imaging studies addressing visceral hypersensitivity remains very small. Like in other chronic pain condition, there is strong evidence that visceral hypersensitivity is a consequence of altered central pain processing in IBS128 and FD.129 However, altered neural activation in response to visceral stimuli has also been reported in normosensitive IBS,130 and both perceptual ratings and central arousal appear to habituate over time.44 Future brain imaging work is needed to clarify which peripheral and/or central processes may underlie visceral hypersensitivity in the pathophysiology of DBGIs.
Combining central and peripheral measures
One of the greatest challenges of the field will be to conduct innovative and highly interdisciplinary research to address the interactions between peripheral alterations, including gut microbiota and their metabolites, permeability or GI transit and changes at the level of the brain. For example, in healthy subjects, perturbation of the gut microbiota by regular intake of a probiotic mix was shown to result in an altered brain response to an emotion recognition task.131 Preliminary results show correlation of gut microbial taxa with brain structure and function in both HCs132 and IBS subjects.51 Multimodal brain imaging approaches, including MR spectroscopy, with peripheral measures, hold this promise and innovative approaches are emerging in related fields.133 Along the same lines, a combination of structural and functional brain imaging techniques reveal sensitivity of specific brain measures to treatment.134
Understanding similarities and differences between different types of chronic pain conditions
The cerebral processing of clinical pain shares many similarities across different conditions with different sources of nociceptive input. This is not surprising, given that the perception of pain, in acute and potentially even more so in chronic situations, is influenced and shaped to a large extent by supraspinal processes, such as emotions, cognitions and memories. Imaging research has started to tease out contributions of supraspinal modulatory influences to an individual’s subjective experience. Depending on the emotional and cognitive states of a patient, specific modulatory areas might be engaged to a variable extent, relatively independent of the type of pain. Nevertheless, pain characteristics influence how pain is processed supraspinally. For example, pain that is uncontrollable and unpredictable is processed differently than controllable and predictable pain.135 Such pain characteristics vary systematically across different clinical conditions: patients with episodic migraine, for instance, experience frequent unavoidable and unpredictable pain attacks, whereas an osteoarthritis patient who only experiences pain on movement is able to avoid pain and is therefore in control. Therefore, future studies could investigate how pain characteristics shape supraspinal pain processing across different clinical pain conditions. At present, the knowledge on differences in brain processing of controllable and uncontrollable pain stems from several experimental pain studies in HCs and studies in patients are lacking. Furthermore, it is unknown how patient characteristics (degrees of anxiety, depression and sense of control) and pain characteristics interact. It is tempting to speculate that individuals with premorbid high anxiety levels are more vulnerable when faced with unpredictable stressors compared with individuals with low anxiety. Future work could address this for different clinical pain conditions, also because it might impact to what extent ‘controllability’ of pain should be addressed therapeutically and weighted against other (life) goals. In addition to pain characteristics, the type of afferent input influences how the brain processes pain and how the organism copes with the input. For example, activation of superficial A8-fibres promotes active coping, such as escape or avoidance behaviour, whereas activation of C-fibres originating in deeper tissues triggers quiescence and passivity.136 This neurobiological phenomenon becomes apparent every time when a patient with visceral pain curls up and does not move, but it is virtually never taken into account when pain coping is investigated. Given that for most chronic pain conditions, including DBGIs, an active coping style is far more adaptive than passivity,137,138 which is not the neurobiologically ‘innate’ response; it is important that future imaging studies investigate pain coping for different types of afferent input and their interaction with top modulation.
Preclinical brain imaging studies
Significant technical progress has been made in the use of brain imaging modalities in preclinical studies.139–141 The usefulness of such studies is their ability to identify cellular and molecular mechanism underlying the more descriptive findings reported from human studies. For example, such preclinical studies will be required to identify mechanism underlying neuroplastic brain changes observed in human studies and to identify the mechanism by which certain gut microbial metabolites can modulate brain structure and function. A major limitation in performing disease-relevant studies in rodents is the poor homology between mouse and human brain and the absence of a rodent model with great validity for IBS and other DBGIs.
SUMMARY AND CONCLUSIONS
Considerable progress has been made since the last Rome Neuro-imaging Working Group report in 20097 in the characterisation of altered neural mechanisms in the development and maintenance of chronic visceral pain. This progress has been driven by several factors, including the evolution of novel imaging modalities, the development of novel analytical techniques and the study of large, homogeneous patient populations made possible through national funding agencies.142–144 Structural and functional alterations in brain regions and in the network properties that include these regions have been reported (see above and figure 1), and several studies are under way to assess the effect of therapeutic interventions on these alterations. Similarities of some observed brain changes have been identified in other chronic pain populations such as the often comorbid UCPPS interstitial cystitis and chronic prostatitis.2 Despite this progress, challenges remain that include the likely heterogeneous nature of DBGI and its overlap with equally heterogeneous visceral and somatic syndromes; methodological differences in stimulation and recording techniques; and lack of control for psychological, physiological, gut microbial, dietary and genetic factors that are known to influence sensory perception and emotional reactivity. There is clearly a need for standardising brain imaging studies and the acquisition of metadata across different centres, as has happened in other fields.142–144 The growing use of complimentary and multimodal brain imaging modalities such as resting state imaging, arterial spin labelling, brain morphometry, spectroscopy and tractography, and analytical techniques such as connectivity analysis and machine learning approaches coupled with the use of large data sets obtained from standardised studies in homogenous populations from multiple centres has great promise to contribute to a full understanding of the CNS alterations and better treatment outcomes in DBGIs.
Acknowledgements
EAM has been supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK048351, DK064539 and DK096606).
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests EAM Is on the scientific advisory boards of Axial Biotherapeutics, Bloom Science, Danone, Viome, Whole Biome and Mahana Therapeutics.
Correction notice: This article has been corrected since it published Online First. The eighth author’s name has been corrected.
Patient consent for publication Not required.
Provenance and peer review Commissioned; internally peer reviewed.
REFERENCES
- 1.Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology 1998;114:559–78. [DOI] [PubMed] [Google Scholar]
- 2.Mayer EA, Gupta A, Kilpatrick LA, et al. Imaging brain mechanisms in chronic visceral pain. Pain 2015; 156:S50–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016;150:1257–61. [DOI] [PubMed] [Google Scholar]
- 4.Tillisch K, Labus JS. Advances in imaging the brain-gut axis: functional gastrointestinal disorders. Gastroenterology 2011;140:407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Oudenhove L, Aziz Q. Recent insights on central processing and psychological processes in functional gastrointestinal disorders. Dig Liver Dis 2009;41:781–7. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016;150:1262–79. [DOI] [PubMed] [Google Scholar]
- 7.Mayer EA, Bushnell MC. Functional Pain Disorders: Time for a Paradigm Shift? In: Mayer EA, Bushnell MC, eds. Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP Press, 2009:531–65. [Google Scholar]
- 8.Jones MP, Tack J, Van Oudenhove L, et al. Mood and Anxiety Disorders Precede Development of Functional Gastrointestinal Disorders in Patients but Not in the Population. Clin Gastroenterol Hepatol 2017;15:1014–20. [DOI] [PubMed] [Google Scholar]
- 9.Mayer EA, Labus JS, Tillisch K, et al. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 2015;12:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 2007;55:377–91. [DOI] [PubMed] [Google Scholar]
- 11.Derbyshire SWG. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol 2003;98:12–20. [DOI] [PubMed] [Google Scholar]
- 12.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011;140:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Omran Y, Aziz Q. Functional brain imaging in gastroenterology: to new beginnings. Nat Rev Gastroenterol Hepatol 2014; 11:565–76. [DOI] [PubMed] [Google Scholar]
- 14.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobson AR, Aziz Q. Brain imaging and functional gastrointestinal disorders: has it helped our understanding? Gut 2004;53:1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizawa E, Sato Y, Kochiyama T, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology 2012;143:1188–98. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj S, Adhikari BM, Dhamala M. Higher frequency network activity flow predicts lower frequency node activity in intrinsic low-frequency BOLD fluctuations. PLoS One 2013;8:e64466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012;13:336–49. [DOI] [PubMed] [Google Scholar]
- 19.Greicius MD, Supekar K, Menon V, et al. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 2009;19:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo CC, Kurth F, Zhou J, et al. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage 2012;61:1471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchison RM, Womelsdorf T, Gati JS, et al. Resting-state connectivity identifies distinct functional networks in macaque cingulate cortex. Cereb Cortex 2012;22:1294–308. [DOI] [PubMed] [Google Scholar]
- 22.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69. [DOI] [PubMed] [Google Scholar]
- 23.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sporns O Networks of the Brain. Cambridge, Massachussets: The MIT Press, 2010. [Google Scholar]
- 25.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drossman DA. Psychosocial and psychophysiologic mechanisms in GI illness Kirsner JB, ed. The Growth of Gastroenterologic knowledge in the 20th Century. Philadelphia: PA: Lea & Febiger, 1994:419–32. [Google Scholar]
- 27.Kennedy PJ, Clarke G, O’Neill A, O’Neill A, et al. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med 2014;44:1553–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014;146:1500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piché M, Arsenault M, Poitras P, et al. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 2010;148:49–58. [DOI] [PubMed] [Google Scholar]
- 30.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011; 60:1589–99. [DOI] [PubMed] [Google Scholar]
- 31.Tijms BM, Seriès P, Willshaw DJ, et al. Similarity-based extraction of individual networks from gray matter MRI scans. Cereb Cortex 2012;22:1530–41. [DOI] [PubMed] [Google Scholar]
- 32.Barboza JL, Talley NJ, Moshiree B. Current and emerging pharmacotherapeutic options for irritable bowel syndrome. Drugs 2014;74:1849–70. [DOI] [PubMed] [Google Scholar]
- 33.Hagmann P, Kurant M, Gigandet X, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS One 2007;2:e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 2008; 1124:1–38. [DOI] [PubMed] [Google Scholar]
- 35.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U SA 2003;100:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013; 10:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohórguez DV, Liddle RA. The gut connectome: making sense of what you eat. J Clin Invest 2015; 125:888–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farmer AD, Aziz Q, Tack J, et al. The future of neuroscientific research in functional gastrointestinal disorders: integration towards multidimensional (visceral) pain endophenotypes? J Psychosom Res 2010;68:475–81. [DOI] [PubMed] [Google Scholar]
- 39.Fukudo S Stress and visceral pain: focusing on irritable bowel syndrome. Pain 2013; 154(Suppl 1):S63–S70. [DOI] [PubMed] [Google Scholar]
- 40.Hong JY, Kilpatrick LA, Labus J, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci 2013;33:11994–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilpatrick LA, Ornitz E, Ibrahimovic H, et al. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS). Biol Psychol 2010;84:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Oudenhove L, Vandenberghe J, Vos R, et al. Factors associated with co-morbid irritable bowel syndrome and chronic fatigue-like symptoms in functional dyspepsia. Neurogastroenterol Motil 2011; 23:524–e202. [DOI] [PubMed] [Google Scholar]
- 43.Gupta A, Kilpatrick L, Labus J, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med 2014;76:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naliboff BD, Berman S, Suyenobu B, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 2006;131:352–65. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Labus J, Kilpatrick LA, et al. Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females. Brain Struct Puna 2016;221:1667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orand A, Gupta A, Shih W, et al. Catecholaminergic Gene Polymorphisms Are Associated with GI Symptoms and Morphological Brain Changes in Irritable Bowel Syndrome. PLoS One 2015;10:e0135910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman S, Suyenobu B, Naliboff BD, et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage 2012;63:1854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A, Mayer EA, Bonyadi M, et al. NR3C1 and IL-1 β Polymorphisms Interact with Early Adverse Life Events in influencing Gray Matter Variations in Women with and without chronic abdominal pain. Society for Neuroscience. San Diego, California, USA 2013. [Google Scholar]
- 49.Kilpatrick LA, Labus JS, Coveleskie K, et al. The HTR3A polymorphism c. −42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology 2011; 140:1943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta A, Cole S, Labus JS, et al. Gene expression profiles in peripheral blood mononuclear cells correlate with salience network activity in chronic visceral pain: A pilot study. Neurogastroenterol Motil 2017;29:e13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labus JS, Oezguen N, Hollister EB, et al. 752 Regional Brain Morphology Is Associated With Gut Microbial Metabolites in Irritable Bowel Syndrome (IBS). Gastroenterology 2015;148:S-142–S-142. [Google Scholar]
- 52.Clayton JA. Studying both sexes: a guiding principle for biomedicine. Faseb J 2016;30:519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilpatrick L, Tillisch K. Irritable Bowel Syndrome, 2012. [Google Scholar]
- 54.Gupta A, Mayer EA, Fling C, et al. Sex-based differences in brain alterations across chronic pain conditions. J Neurosci Res 2017;95:604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent K, Tracey I. Sex hormones and pain: the evidence from functional imaging. CurrPain Headache Rep 2010;14:396–403. [DOI] [PubMed] [Google Scholar]
- 56.Vincent K, Warnaby C, Stagg CJ, et al. Brain imaging reveals that engagement of descending inhibitory pain pathways in healthy women in a low endogenous estradiol state varies with testosterone. Pain 2013; 154:515–24. [DOI] [PubMed] [Google Scholar]
- 57.Melchior M, Poisbeau P, Gaumond I, et al. Insights into the mechanisms and the emergence of sex-differences in pain. Neuroscience 2016;338:63–80. [DOI] [PubMed] [Google Scholar]
- 58.Choi JC, Park SK, Kim YH, et al. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology 2006; 105:120–7. [DOI] [PubMed] [Google Scholar]
- 59.Hg L, Dupont P, Geeraerts B, et al. Lack of endogenous opioid release during sustained visceral pain: a [11C]carfentanil PET study. Pain 2013;154:2072–7. [DOI] [PubMed] [Google Scholar]
- 60.Jarcho JM, Feier NA, Bert A, et al. Diminished neurokinin-1 receptor availability in patients with two forms of chronic visceral pain. Pain 2013;154:987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hg L, Ceccarini J, Weltens N, et al. Increased cerebral cannabinoid-1 receptor availability is a stable feature of functional dyspepsia: a [FJMK-9470 PET study. Psychother Psychosom 2015;84:149–58. [DOI] [PubMed] [Google Scholar]
- 62.Tominaga K, Tsumoto C, Ataka S, et al. Regional brain disorders of serotonin neurotransmission are associated with functional dyspepsia. Life Sci 2015;137:150–7. [DOI] [PubMed] [Google Scholar]
- 63.Ende G Proton Magnetic Resonance Spectroscopy: Relevance of Glutamate and GABA to Neuropsychology. Neuropsychol Rev 2015;25:315–25. [DOI] [PubMed] [Google Scholar]
- 64.Niddam DM, Tsai SY, Lu CL, et al. Reduced hippocampal glutamate-glutamine levels in irritable bowel syndrome: preliminary findings using magnetic resonance spectroscopy. Am J Gastroenterol 2011; 106:1503–11. [DOI] [PubMed] [Google Scholar]
- 65.Labus JS, Dinov ID, Jiang Z, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain 2014;155:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Labus JS, Hamadani K, Gupta A, et al. Su1571 Functional Network Properties of Brain Regions in Irritable Bowel Syndrome. Gastroenterology 2016;150:S529–529. [Google Scholar]
- 67.Labus JS, Van Horn JD, Torgerson C, et al. 585 Architecture of Anatomical Brain Networks Differs in Irritable Bowel Syndrome Compared to Healthy Controls. Gastroenterology 2014; 146:S–109. [Google Scholar]
- 68.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–98. [DOI] [PubMed] [Google Scholar]
- 69.Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci 2013;15:247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sporns O Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol 2013;23:162–71. [DOI] [PubMed] [Google Scholar]
- 71.Labus JS, Naliboff B, Kilpatrick L, et al. Pain and Interoception Imaging Network (PAIN): A multimodal multisite, brain-imaging repository for chronic somatic and visceral pain disorders. Neuroimage 2016;124:1232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dinov ID, Petrosyan P, Liu Z, et al. The perfect neuroimaging-genetics-computation storm: collision of petabytes of data, millions of hardware devices and thousands of software tools. Brain Imaging Behav 2014;8:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013;80:105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Secretary TWHOotP. Fact Sheet: BRAIN Initiative, 2013.
- 75.Markram H Human Brain Project: Henry Markram plans to spend €1 bn building a perfect model of the human brain. Neuroscience Observer. The Guardian 2013. [Google Scholar]
- 76.Van Essen DC, Smith SM, Barch DM, et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 2013;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allen N, Sudlow C, Downey P, et al. UK Biobank: Current status and what it means for epidemiology. Health Policy Technol 2012;1:123–6. [Google Scholar]
- 78.Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med 2013;368:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swarup V, Geschwind DH. Alzheimer’s disease: From big data to mechanism. Nature 2013;500:34–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nalls MA, McLean CY, Rick J, et al. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol 2015;14:1002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012; 15:1117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. Pain 2013;154:2160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vachon-Presseau E, Tétreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016;139:1958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 2014;17:192–200. [DOI] [PubMed] [Google Scholar]
- 85.Blankstein U, Chen J, Diamant NE, et al. Altered Brain Structure in Irritable Bowel Syndrome: Potential Contributions of Pre-Existing and Disease-Driven Factors. Gastroenterology 2010; 138:1783–9. [DOI] [PubMed] [Google Scholar]
- 86.Chen JY, Blankstein U, Diamant NE, et al. White matter abnormalities in imitable bowel syndrome and relation to individual factors. Brain Res 2011;1392:121–31. [DOI] [PubMed] [Google Scholar]
- 87.Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain 2012;153:1602–9. [DOI] [PubMed] [Google Scholar]
- 88.Navratilova E, Xie JY, Meske D, et al. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 2015;35:7264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wanigasekera V, Lee MC, Rogers R, et al. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc Natl Acad Sci U S A 2012; 109:17705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tracey I A vulnerability to chronic pain and its interrelationship with resistance to analgesia. Brain 2016;139:1869–72. [DOI] [PubMed] [Google Scholar]
- 91.Hanis RE, Napadow V, Huggins JP, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013;119:1453–64. [DOI] [PubMed] [Google Scholar]
- 92.Borsook D, Hargreaves R, Bountra C, et al. Lost but making progress-Where will new analgesic drugs come from? Sci Transi Med 2014;249:sr3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camilleri M Review article: biomarkers and personalised therapy in functional lower gastrointestinal disorders. Aliment Pharmacol Ther 2015;42:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Erpelding N, Simons L, Lebel A, et al. Rapid treatment-induced brain changes in pediatric CRPS. Brain Structure and Function 2016;221:1095–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Becerra L, Sava S, Simons LE, et al. Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage 2014;6:347–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta A, Kilpatrick L, Labus J, et al. Early Adverse Life Events and Resting State Neural Networks in Patients With Chronic Abdominal Pain. Psychosom Med 2014;76:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hong J-Y, Kilpatrick LA, Labus JS, et al. Sex and Disease-Related Alterations of Anterior Insula Functional Connectivity in Chronic Abdominal Pain. Journal of Neuroscience 2014;34:14252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hubbard CS, Becerra L, Heinz N, et al. Abdominal Pain, the Adolescent and Altered Brain Structure and Function. PLoS One 2016; 11 :e0156545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piché M, Chen JI, Roy M, et al. Thicker posterior insula is associated with disease duration in women with imitable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain 2013;14:1217–26. [DOI] [PubMed] [Google Scholar]
- 100.Labus JS, Van Horn JD, Gupta A, et al. Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. Pain 2015;156:1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bourke JH, Wall MB. phMRI: methodological considerations for mitigating potential confounding factors. Front Neurosci 2015;9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. Neuroimage 2012;62:1072–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 1992;72:165–229. [DOI] [PubMed] [Google Scholar]
- 104.Hubbard CS, Labus JS, Bueller J, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci 2011;31:12491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Labus JS, Hubbard CS, Bueller J, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with imitable bowel syndrome. Gastroenterology 2013; 145:1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berman SM, Chang L, Suyenobu B, et al. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology 2002;123:969–77. [DOI] [PubMed] [Google Scholar]
- 107.Mayer EA, Berman S, Derbyshire SW, et al. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther 2002; 16:1357–66. [DOI] [PubMed] [Google Scholar]
- 108.Tillisch K, Labus J, Nam B, et al. Neurokinin-1-receptor antagonism decreases anxiety and emotional arousal circuit response to noxious visceral distension in women with imitable bowel syndrome: a pilot study. Aliment Pharmacol Ther 2012;35:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arrubla J, Tse DH, Amkreutz C, et al. GABA concentration in posterior cingulate cortex predicts putamen response during resting state fMRI. PLoS One 2014;9:e106609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cole DM, Beckmann CF, Oei NY, et al. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. Neuroimage 2013;78:59–67. [DOI] [PubMed] [Google Scholar]
- 111.Cole DM, Beckmann CF, Searle GE, et al. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex 2012;22:2784–93. [DOI] [PubMed] [Google Scholar]
- 112.Cole DM, Oei NY, Soeter RP, et al. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex 2013;23:1509–16. [DOI] [PubMed] [Google Scholar]
- 113.van den Heuvel MP, Mandl RC, Kahn RS, et al. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 2009;30:3127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Borsook D, Erpelding N, Becerra L. Losses and gains: chronic pain and altered brain morphology. Expert Rev Neurother 2013; 13:1221–34. [DOI] [PubMed] [Google Scholar]
- 115.Castrén E, Hen R. Neuronal plasticity and antidepressant actions. Trends Neurosci 2013;36:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Castrén E Neuronal network plasticity and recovery from depression. JAMA Psychiatry 2013;70:983–9. [DOI] [PubMed] [Google Scholar]
- 117.Lackner JM, Lou Coad M, Mertz HR, et al. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav Res Ther 2006;44:621–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y, Wu Z, Ma X, et al. Brain regions involved in moxibustion-induced analgesia in imitable bowel syndrome with diarrhea: a functional magnetic resonance imaging study. BMC Complement Altern Med 2014;14:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flor H Psychological pain interventions and neurophysiology: implications for a mechanism-based approach. Am Psychol 2014;69:188–96. [DOI] [PubMed] [Google Scholar]
- 120.Jensen KB, Berna C, Loggia ML, et al. The use of functional neuroimaging to evaluate psychological and other non-pharmacological treatments for clinical pain. Neurosci Lett 2012;520:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harris RE, Zubieta J-K, Scott DJ, et al. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on μ-opioid receptors (MORs). Neuroimage 2009;47:1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin 2015;9:430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dhond RP, Kettner N, Napadow V. Neuroimaging Acupuncture Effects in the Human Brain. The Journal of Alternative and Complementary Medicine 2007;13:603–16. [DOI] [PubMed] [Google Scholar]
- 124.Vanhaudenhuyse A, Laureys S, Faymonville M-E. Neurophysiology of hypnosis. Neurophysiologie Clinique/Clinical Neurophysiology 2014;44:343–53. [DOI] [PubMed] [Google Scholar]
- 125.Del Casale A, Ferracuti S, Rapinesi C.et al. Pain perception and hypnosis: findings from recent functional neuroimaging studies. Int J Clin Exp Hypn 2015;63:144–70. [DOI] [PubMed] [Google Scholar]
- 126.Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol 2011;106:1678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ljótsson B, Falk L, Vesterlund AW, et al. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome-a randomized controlled trial. Behav Res 7fter 2010;48:531–9. [DOI] [PubMed] [Google Scholar]
- 128.Larsson MBO, Tillisch K, Craig AD, et al. Brain Responses to Visceral Stimuli Reflect Visceral Sensitivity Thresholds in Patients With Irritable Bowel Syndrome. Gastroenterology 2012; 142:463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Van Oudenhove L, Vandenberghe J, Dupont P, et al. Regional brain activity in functional dyspepsia: a H(2)(15)0-PET study on the role of gastric sensitivity and abuse history. Gastroenterology 2010;139:36–47. [DOI] [PubMed] [Google Scholar]
- 130.Elsenbruch S, Rosenberger C, Enck P, et al. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut 2010;59:489–95. [DOI] [PubMed] [Google Scholar]
- 131.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tillisch K, Mayer EA, Gupta A, et al. Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosom Med 2017;79:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haroon E, Fleischer CC, Felger JC, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Čeko M, Shir Y, Ouellet JA, et al. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp 2015;36:2075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bräscher AK, Becker S, Hoeppli ME, et al. Different Brain Circuitries Mediating Controllable and Uncontrollable Pain. J Neurosci 2016;36:5013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keay KA, Bandler R. Distinct central representations of inescapable and escapable pain: observations and speculation. Exp Physiol 2002;87:275–9. [DOI] [PubMed] [Google Scholar]
- 137.Brown GK, Nicassio PM. Development of a questionnaire for the assessment of active and passive coping strategies in chronic pain patients. Pain 1987;31:53–64. [DOI] [PubMed] [Google Scholar]
- 138.Snow-Turek AL, Norris MP, Tan G. Active and passive coping strategies in chronic pain patients. Pain 1996;64:455–62. [DOI] [PubMed] [Google Scholar]
- 139.Akassoglou K, Merlini M, Rafalski VA, et al. In Vivo Imaging of CNS Injury and Disease. J Neurosci 2017;37:10808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Greenwood-Van Meerveld B, Prusator DK, Johnson AC. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 2015;308:G885–G903. [DOI] [PubMed] [Google Scholar]
- 141.Holschneider DP, Bradesi S, Mayer EA. The role of experimental models in developing new treatments for irritable bowel syndrome. Expert Rev Gastroenterol Hepatol 2011;5:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alger JR, Ellingson BM, Ashe-McNalley C, et al. Multisite, multimodal neuroimaging of chronic urological pelvic pain: Methodology of the MAPP Research Network. Neuroimage 2016;12:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clemens JQ, Mullins C, Kusek JW, et al. The MAPP research network: a novel study of urologie chronic pelvic pain syndromes. BMC Urol 2014;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hong JY, Kilpatrick LA, Labus JS, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci 2014;34:14252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hong J-Y, Naliboff BD, Labus JS, et al. Sa2014 IBS Patients Show Altered Brain Responses During Uncertain, but Not Certain Expectation of Painful Stimulation of the Abdominal Wall. Gastroenterology 2015;148::S–384.. [Google Scholar]
- 147.Jiang Z, Dinov ID, Labus J, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS One 2013;8:e73932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qi R, Ke J, Schoepf UJ, et al. Topological Reorganization of the Default Mode Network in Irritable Bowel Syndrome. Mol Neurobiol 2016;53:6585–93. [DOI] [PubMed] [Google Scholar]
- 149.Icenhour A, Witt ST, Elsenbruch S, et al. Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. Neuroimage 2017;15:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ellingson BM, Mayer E, Harris RJ, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 2013;154:1528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mayer EA, Aziz Q, Coen S, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil 2009;21:579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hall GB, Kamath MV, Collins S, et al. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil 2010;22:276–e80. [DOI] [PubMed] [Google Scholar]
- 153.Elsenbruch S, Rosenberger C, Bingel U, et al. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology 2010;139:1310–9. [DOI] [PubMed] [Google Scholar]
- 154.Labus JS, Gupta A, Coveleskie K, et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain 2013;154:2088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Berman S, Munakata J, Naliboff BD, et al. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain 2000;4:157–72. [DOI] [PubMed] [Google Scholar]
- 156.Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology 2003;124:1738–47. [DOI] [PubMed] [Google Scholar]
- 157.Labus JS, Mayer EA, Jarcho J, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut 2011;60:1196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Straube T, Schmidt S, Weiss T, et al. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage 2009;44:975–81. [DOI] [PubMed] [Google Scholar]
- 159.Porro CA, Baraldi P, Pagnoni G, et al. Does anticipation of pain affect cortical nociceptive systems? 7 Neurosci 2002;22:3206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koyama T, McHaffie JG, Laurienti PJ, et al. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA 2005; 102:12950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bornhövd K, Quante M, Glauche V, et al. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal insula and somatosensory cortex: a single-trial fMRI study. Brain 2002;125:1326–36. [DOI] [PubMed] [Google Scholar]
- 162.Verne GN, Himes NC, Robinson ME, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 2003;103:99–110. [DOI] [PubMed] [Google Scholar]
- 163.Seifert F, Schuberth N, De Col R, et al. Brain activity during sympathetic response in anticipation and experience of pain. Hum Brain Mapp 2013;34:1768–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yágüez L, Coen S, Gregory U, et al. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology 2005;128:1819–29. [DOI] [PubMed] [Google Scholar]
- 165.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci 2008;28:349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stephan E, Pardo JV, Faris PL, et al. Functional neuroimaging of gastric distention. J Gastrointest Surg 2003;7:740–9. [DOI] [PubMed] [Google Scholar]
- 167.Lawal A, Kern M, Sanjeevi A, et al. Neurocognitive processing of esophageal central sensitization in the insula and cingulate gyrus. Am J Physiol Gastrointest Liver Physiol 2008;294:G787–G794. [DOI] [PubMed] [Google Scholar]
- 168.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage 2008;41:1032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rubio A, Van Oudenhove L, Pellissier S, et al. Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system - A fMRI study in healthy volunteers. Neuroimage 2014; 107010–22. [DOI] [PubMed] [Google Scholar]
- 170.Afzal M, Potokar JP, Probert CS, et al. Selective processing of gastrointestinal symptom-related stimuli in irritable bowel syndrome. Psychosom Med 2006;68:758–61. [DOI] [PubMed] [Google Scholar]
- 171.Gibbs-Gallagher N, Palsson OS, Levy RL, et al. Selective recall of gastrointestinalsensation words: evidence for a cognitive-behavioral contribution to irritable bowel syndrome. Am J Gastroenterol 2001; 96:1133–8. [DOI] [PubMed] [Google Scholar]
- 172.Phillips K, Wright BJ, Kent S. Irritable bowel syndrome and symptom severity: Evidence of negative attention bias, diminished vigour, and autonomic dysregulation. J Psychosom Res 2014;77:13–19. [DOI] [PubMed] [Google Scholar]
- 173.Tkalcic M, Domijan D, Pletikosic S, et al. Attentional biases in irritable bowel syndrome patients. Clin Res Hepatol Gastroenterol 2014;38:621–8. [DOI] [PubMed] [Google Scholar]
- 174.Labus JS, Naliboff BD, Berman SM, et al. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage 2009;47:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Seminowicz DA, Shpaner M, Keaser ML, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. 7 Pain 2013;14:1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jeffery IB, O’Toole PW, Öhman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- 177.Simrén M IBS with intestinal microbial dysbiosis: a new and clinically relevant subgroup? Gut 2014;63:1685–6. [DOI] [PubMed] [Google Scholar]
- 178.Baliki MN, Schnitzer TJ, Bauer WR, et al. Brain morphological signatures for chronic pain. PLoS One 2011;6:e26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Klöppel S, Abdulkadir A, Jack CR, et al. Diagnostic neuroimaging across diseases. Neuroimage 2012;61:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rosa MJ, Seymour B. Decoding the matrix: benefits and limitations of applying machine learning algorithms to pain neuroimaging. Pain 2014;155:864–7. [DOI] [PubMed] [Google Scholar]