Abstract
BACKGROUND & AIMS:
Based largely on results from preclinical studies, the concept of a brain gut microbiome axis has been established, mediating bidirectional communication between the gut, its microbiome, and the nervous system. Limited data obtained in human beings suggest that alterations in these interactions may play a role in several brain gut disorders.
METHODS:
We reviewed the preclinical and clinical literature related to the topic of brain gut microbiome interactions.
RESULTS:
Well-characterized bidirectional communication channels, involving neural, endocrine, and inflammatory mechanisms, exist between the gut and the brain. Communication through these channels may be modulated by variations in the permeability of the intestinal wall and the blood-brain barrier. Brain gut microbiome interactions are programmed during the first 3 years of life, including the prenatal period, but can be modulated by diet, medications, and stress throughout life. Based on correlational studies, alterations in these interactions have been implicated in the regulation of food intake, obesity, and in irritable bowel syndrome, even though causality remains to be established.
CONCLUSIONS:
Targets within the brain gut microbiome axis have the potential to become targets for novel drug development for brain gut disorders.
Keywords: Irritable Bowel Syndrome, Early Life Influences, Diet
Based largely on studies with experimental animals, significant progress has been made in the past decade in illuminating the role of bidirectional interactions between the nervous system, the gastroin-testinal tract, and the gut microbiome. Studies performed using experimental animal models have confirmed the role of the gut microbiome in modulating affective, social, nociceptive, and ingestive behaviors. However, causality and translation of these findings into healthy human beings and patients with gastrointestinal or psychiatric disorders has been limited, and the effectiveness of specific gut microbiome-targeted treatments remains to be established. Despite these limitations, the new brain gut microbiome (BGM) science has spawned a considerable effort in academia and industry to determine if prebiotic, probiotic, and postbiotic interventions may be beneficial either as primary or adjuvant therapy in disorders such as irritable bowel syndrome (IBS) or obesity. Such therapies could be in the form of special diets, dietary supplements (prebiotics and probiotics), or novel molecules targeting or mimicking gut microbial signals (postbiotics). Here, we review key findings that show the existence of bidirectional signaling between the brain and gut microbiota, explore early life influences on brain and microbiota development, and then briefly discuss the potential role of BGM communication channels in 2 common gastrointestinal disorders.
Gut Microbiota to Brain Signaling
Communication from the gut microbiome to the central nervous system (CNS) primarily occurs through microbial-derived intermediates, with the best described examples including short-chain fatty acids (SCFAs), secondary bile acids (2BAs), and tryptophan metabolites.1–3 Although some of these intermediates interact directly with enteroendocrine cells, enterochromaffin cells, and the mucosal immune system to propagate bottom-up signaling, other intermediates are able to cross the intestinal barrier to enter systemic circulation, and may even cross the blood-brain barrier.3–5 It remains unclear whether these microbial-derived intermediates reach brain sites directly in sufficient regional concentrations to modify distinct brain circuits. Alternatively, microbial signals may communicate via neural pathways involving vagal and/or spinal afferents.6,7 Table 13,8–18 outlines some of the best-characterized signaling channels driving bottom-up communication.
Table 1.
Selected Sample of Gut Microbiota to Brain Signaling Channels
| Input | Output | Study |
|---|---|---|
| 2BAs | Improved central regulation of glucose metabolism via production of FGF19 | Marcelin et al8 |
| Ryan et al9 | ||
| Suppression of HPA axis via production of FGF19 | Perry et al10 | |
| GLP-1 and PYY release from L cells via TGR5 receptor | Bala et al11 | |
| Synthesis and release of 5-HT from ECCs | Yano et al3 | |
| SCFAs | PYY, GLP-1, and GLP-2 release from L cells | Cani et al12 |
| Leptin production from adipocytes via GPR41 | Xiong et al13 | |
| Synthesis and release of 5-HT from ECCs | Yano et al3 | |
| Indole | GLP-1 secretion from L cells via interaction with voltage-gated potassium channels and mitochondrial NADH dehydrogenase | Chimerel et al14 |
| Kynurenine synthesis via activation of AhR | Vogel et al15 | |
| TLR ligands: LPS, flagellin, and so forth | CCK synthesis from EECs via TLRs | Palazzo et al16 |
| PYY expression in vitro from L cells via TLRs | Larraufie et al17 | |
| 5-HT release in vitro from ECCs via TLRs | Kidd et al18 | |
NOTE. The table outlines well-characterized signaling channels driving bottom-up communication along the brain gut microbiota axis. Each input represents a microbiota-derived intermediate that results in a physiological output by interacting with host cells or host-derived signaling molecules.
5-HT, serotonin; CCK, cholecystokinin; ECC, enterochromaffin cell; EEC, enteroendocrine cell; FGF19, fibroblast growth factor 19; GLP, glucagon-like peptide; HPA, hypothalamic pituitary adrenal; LPS, lipopolysaccharide; NADH, nicotinamide adenine dinucleotide; PYY, peptide YY; TGR5, Takeda G-protein-coupled receptor 5; TLR, Toll-like receptor
Microbiota Neuroimmune Interactions During Brain Development
A growing body of evidence has shown an important role for the gut microbiota in neuroimmune signaling. Preclinical models involving germ-free (GF) mice or mice exposed to broad-spectrum antibiotics consistently show deleterious effects on neurodevelopment and neurode-generative disease processes, often secondary to disrupted neuromodulatory signaling involving the gut microbiota.19,20
Perhaps the best-characterized example of the interaction between the microbiota and CNS involves microglial development. Comprising 10% to 15% of all glial cells, microglia are tissue macrophages of the brain, representing the most abundant resident innate immune cell of the CNS. These cells take on a diverse role because they are involved with CNS development early on, and with antigen presentation, phagocytosis, and modulating inflammation throughout life.21 Microglia also maintain homeostatic function by continuously scanning the environment of the CNS and directly communicating with neurons, astrocytes, and blood vessels through processes extending from the cell body.21,22
Microbial-derived SCFAs have been shown to have an integral role in promoting microglial maturity and proper functioning.23 GF and antibiotic-treated mice show an increased proportion of immature microglia, characterized by longer processes with more branching, in addition to molecular markers associated with an immature phenotype.23 Although an important role for SCFAs certainly has been implicated in modulation of microglial development and function, the exact mechanisms driving these changes, and the roles of potentially other microbial mediators, still are unclear. This is exemplified by a failure of microglial abnormalities to correct in response to GF colonization with a limited microbial community known to produce SCFAs.23,24 Studies of microglial development also underscore the importance of the gut microbiome in developmental timing. Although GF mice show decreases in both microglial maturity and number, antibiotic-treated mice show decreased microglial maturity only.23 These findings align with research showing differences in gene expression profiles of microglia between adult and newborn GF mice compared with controls.25
Another well-characterized interaction between the microbiota and CNS involves astrocytes. Astrocytes represent a functionally diverse group of glial cells, whose roles include ion homeostasis, neurotransmitter clearance, glycogen storage, maintenance of the blood brain barrier (BBB), and support of neuronal signaling, in addition to their prominent role in neuroinflammation.26 Microbial metabolites can activate aryl hydrocarbon receptors (AhRs) to attenuate inflammation via regulation of type I interferon signaling in astrocytes.27 Although many diverse mediators function as AhR modulators, including xenobiotics, indoles represent an important group of microbial-derived AhR agonists.28,29 Most undigested dietary tryptophan in the gut lumen is converted to indole by the exclusively microbial enzyme tryptophanase.30 Indoles then can be metabolized or modified further by microbial and hepatic enzymes, producing indole derivatives of varying affinities for AhR.30–32
Barriers to Bottom-Up Signaling
Signaling within the BGM axis is regulated by 2 dynamic barriers: first, the intestinal barrier, consisting of a basal monolayer of epithelial cells interconnected by tight junctions and a dynamic mucus layer containing secretory IgA and antimicrobial peptides33; and, second, the BBB consisting of cerebral endothelial cells interconnected by tight junctions.34
Intestinal Barrier
In response to specific microbial products, pattern recognition receptors in the gastrointestinal (GI) mucosa can activate enhanced antimicrobial defense, intestinal inflammation, and immunologic tolerance.35,36 The intestinal epithelial barrier also plays an important role during healthy homeostatic conditions because micro-organisms and macromolecules are able to gain entry through microfold cells of the gut-and mucosa-associated lymphoid tissue, allowing for constant sampling of the gut luminal environment by immune cells.37 The mucus layer, the outer layer of which is inhabited by commensal microorganisms, represents a dynamic barrier that maintains a glycoprotein-rich biofilm.38 This protective biofilm can be degraded by microbes during periods of low dietary fiber, thereby increasing pathogen susceptibility.39 The permeability of the intestinal barrier also can be influenced by inflammatory mediators and by sympathetic nervous system activity.40,41
Blood-Brain Barrier
The BBB represents a diffusion barrier between the circulatory system and the cerebrospinal fluid of the CNS. The gut microbiota can influence the permeability of this barrier by modulating expression of tight junction proteins.34 Preclinical evidence suggests that SCFAs may act as a key signaling metabolite, regulating microbiota-influenced BBB development and maintenance through epigenetic modification.42,43 Lipopolysaccharides also may play a role, although likely a more limited one, in disrupting the BBB through systemic immune activation.44
Brain to Gut Signaling
The CNS can influence the gut microbiota directly, through luminal secretion of endocrine mediators that interact with microbial receptors, and indirectly through modulation of the gut environment. Direct signaling often involves catecholamines, whose concentrations can be influenced by physical and psychological stress, whereas indirect signaling involves both branches of the autonomic nervous system (ANS).45–47 The ANS can induce changes in gut physiology, thereby affecting microbial composition and function. As an example of this, changes in intestinal transit times influence water content, nutrient availability, and even bacterial clearance rates. Impaired migrating motor complex regularity can result in bacterial overgrowth, whereas increased intestinal transit times strongly correlate with stool microbial richness and composition.48,49The ANS also regulates the integrity of the intestinal mucus layer by modulating goblet cell function, as well as intercellular epithelial permeability. In a mouse model of brain injury, increased norepinephrine release contributed to decreased goblet cell abundance and mucoprotein production.50 This resulted in changes to the gut microbiota, which correlated with the extent of injury.50
Because the majority of brain and brain gut disorders are characterized by enhanced stress responsiveness and altered ANS function, top-down modulation of the gut microbiome by the brain is likely to be an important contributor to the observed gut microbial signatures.
Early Programming of Gut Microbiome Brain Interactions
The first 3 years of life represent a particularly important developmental period for the CNS, with extensive synaptogenesis and myelination taking place.51 In parallel, early life, including the prenatal period, also represents an important developmental period for the gut microbiota, and it has been suggested that calories harvested by microbes play an important part in brain development.52 During this time, exposure to different microbes, diets, stressors, antibiotics, and other factors shape microbiota architecture and function, in addition to influencing communication with the developing CNS (Figures 1 and 2). In this way, multiple influences during early life events play a pivotal role in programing the gut microbiota and the brain, and may contribute to the etiology of several neurodevelopmental disorders.
Figure 1.
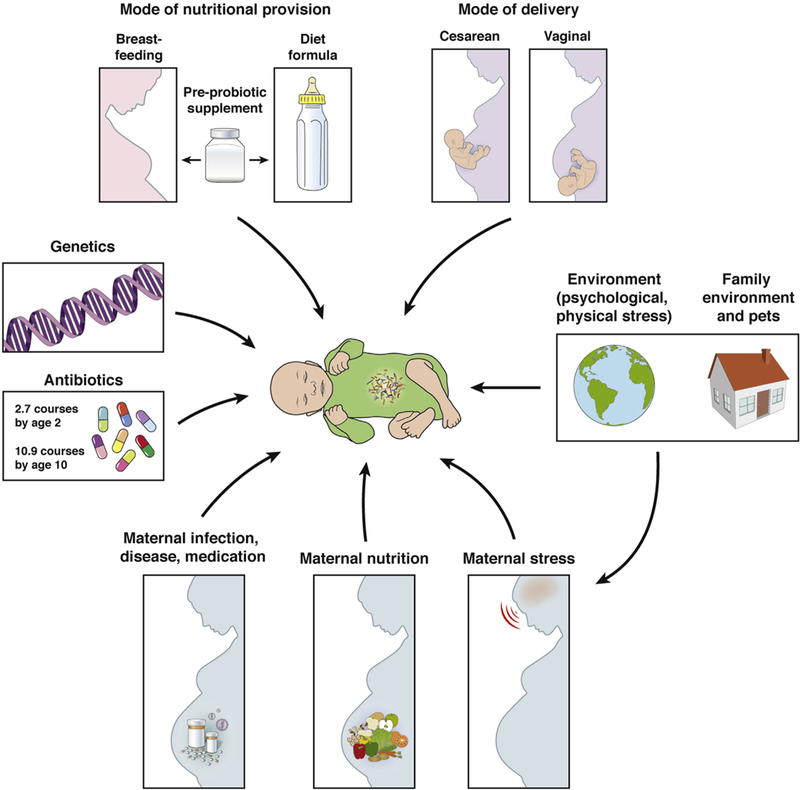
Early life events and the development of the infant gut microbiota. Early life represents a particularly vulnerable period for the infant gut microbiome because it is highly responsive to numerous factors. In addition to genetics, prenatal influences (maternal nutrition, stress, overall health), mode of delivery, early life nutrition (breastfeeding, formula feeding), physical and psychological environment, and antibiotic use all influence the infant gut microbiome. Modified from Borre et al.135
Figure 2.
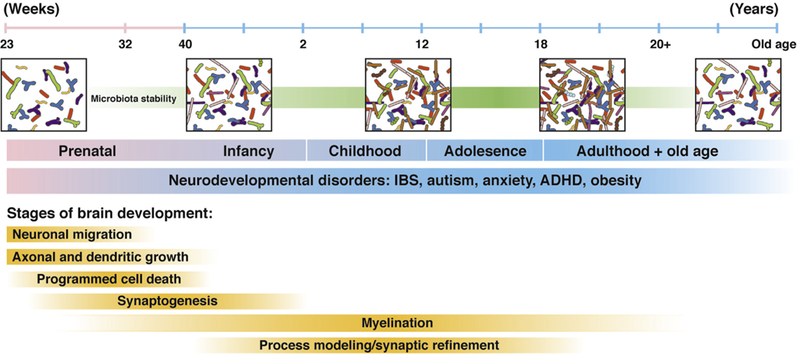
The developing gut microbiome and brain. Gut microbiota and brain development begins during the prenatal period and continues throughout adulthood, with the first 3 years of life representing a particularly important developmental period. Disruptions in development can influence communication between these 2 systems and may contribute to the pathogenesis of neurodevelopmental disorders such as IBS, autism, anxiety, attention-deficit hyperactivity disorder (ADHD), and obesity. Modified from Borre et al.135
Mode of Delivery
The infant microbiota is highly dependent on the mode of delivery because this represents the initial colonization of the gut.53 During vaginal delivery, the neonate is colonized by bacteria closely resembling the maternal vaginal microbiome (enriched in Lactobacillus and Prevotella species), as well as some fecal microbes.53 The vaginal microbiome is dynamic and changes in response to maternal stress, which has been shown to influence the newborn gut microbiome and, more importantly, gut metabolome.54 Maternal stress (infections, psychosocial stress) also has been shown to increase the risk for schizophrenia, autism, and attention deficit hyper-activity disorder in the newborn.55,56 The neonate delivered by Cesarean section (C-section) instead is colonized by microbes enriched in Staphylococcus and Corynebacterium.53,57 Remarkably, differences in skin, gut, and naso-pharyngeal microbiome composition between vaginally delivered and C-section born infants exists up until the age of 2 years, during a period of intense brain development.58,59 In a study of 2 million Danish term children, delivery by C-section was associated with an increased risk for the development of asthma, inflammatory bowel disease, immune deficiencies, and other chronic immune disorders.60
Early Nutrition
The gut microbiota also is influenced by whether the infant is breastfed or formula-fed, with breastfed infants showing better neurodevelopmental outcomes and a more complex Bifidobacterium microbiota relative to formula-fed infants.61–63 A crucial factor in the development of the gut microbial architecture are the group of complex carbohydrates called human milk oligosaccharides.64,65 These molecules are too large to be absorbed by the infant small intestine, and exclusively target the developing gut microbiome. Other factors shown to influence the development of the infant microbiota include genetics and gestational age.66,67
Early Brain Development
During the first 3 years of life,68 extensive changes in brain architecture occur in parallel with the programming of the gut microbiome, with the end of the second year marking the establishment of an adult pattern of myelination51 (Figure 2). A preclinical model of myeli-nation suggests that early life commensal microbes are important in regulating proper myelination of the pre-frontal cortex. In this model, GF mice showed up- regulation of genes coding for structural components of myelin, contributing to the hypermyelination seen in these animals, relative to controls.69 In addition, a study of 89 infants found that the gut microbial composition at 1 year of age was associated with cognitive performance a year later, further underscoring the importance of this early period in the interaction of the gut microbiome and the developing brain.70
Modulation of Brain Gut Microbiome Interactions in the Adult
Over time, the mature gut microbial architecture becomes more stable and relatively resistant to long-term perturbations.71,72 Similarly, the basic architecture of neural networks also stabilizes, although continuous synaptic pruning and myelination continue throughout adulthood.73 Despite the increased stability of the gut microbial community structure, the functional output to the CNS, via metabolites and signaling molecules, can be altered significantly throughout adult life by antibiotics, diet, prebiotics and probiotics, and by chronic stress.
Antibiotics
In adult rodents, long-term, broad-spectrum anti-biotic treatment was associated with changes in brain chemistry and behavior, in addition to the structure of the microbiome.74 Antibiotic-treated mice showed decreases in serum concentrations of tryptophan and kynurenine, brain concentrations of serotonin metabolites, and hypothalamic concentrations of vasopressin and oxytocin.74 These changes in signaling molecules likely contributed to the observed changes in anxiety, memory, and neurocognitive function in these animals.74 Of note, a series of case-control studies from a large UK database found an association between recurrent anti-biotic exposure and an increased risk of depression and anxiety.75 This may be related to an inability of the human gut microbiome, in some individuals, to completely recover after repeated antibiotic perturbation.76
Diet
Several human studies have shown transient diet-induced changes in the gut microbiome and gene expression patterns in adult subjects, whereas evidence has suggested that long-term changes are not observed.77 It recently was shown in the Hadza hunter-gatherers of East Africa that seasonal variations in dietary patterns were associated with changes in the diversity, structure, and function of the gut microbiome.78 These diet-associated changes in the gut microbiome also may be related to changes in brain structure. A preclinical study using machine learning classifiers found that diet-dependent changes in the gut microbiome were associated with changes in white matter architecture.79 Long-term consumption of a low-fiber diet can have deleterious consequences on microbiota diversity and abundance, which is transferred over several generations, and cannot be reversed by a high-fiber diet.80 Similarly, the lower-gut microbial diversity and SCFA production observed in North American infants can be reversed only partially by an increased fiber intake as an adult.81
Prebiotics and Probiotics
Several studies, mostly preclinical or a limited group of small clinical studies, have shown that prebiotic and probiotic ingestion in adults can modulate brain function and behavior, an observation that has led to the term psychobiotics. Table 282–95 highlights the increasing literature related to the effects of these psychobiotics, and underscores the sometimes conflicting data associated with these agents. These findings also are reflected in the similarly conflicting results of recent meta-analyses on the use of these agents in clinical trials for depression, anxiety, and stress.96,97
Table 2.
Selected Sample of Studies Investigating Psychobiotics
| Study | Design | Disease | Intervention | Conclusions |
|---|---|---|---|---|
| Tillisch et al82 | Clinical; RCT | Healthy women | Probiotic | Probiotic changes functional connectivity of an emotional recognition network in the brain |
| Slykerman et al83 | Clinical; RCT in pregnancy | Anxiety and depression | Probiotic | Probiotic significantly decreases postpartum anxiety and depression |
| Pinto-Sanchez et al84 | Clinical; RCT in IBS | Depression | Probiotic | Probiotic reduces depression, increases quality of life; associated with changes in brain activation patterns |
| Romijn et al85 | Clinical; RCT | Depression | Probiotic | No significant effect of probiotic on low mood or inflammatory biomarkers |
| Akkasheh et al86 | Clinical; RCT | Depression | Probiotic | Probiotic reduces depression scores and improves insulin sensitivity |
| Takada et al87 | Clinical; RCT | Stress | Probiotic | Probiotic suppresses cortisol hypersecretion and physical symptoms associated with stress |
| Allen et al88 | Clinical; within-participant placebo-controlled trial | Stress | Probiotic | Probiotic reduces stress and improves memory |
| Kelly et al89 | Clinical; RCT | Stress | Probiotic | No significant effect of probiotic on stress |
| Ostlund-Lagerstrom et al90 | Clinical; RCT in older adults | Anxiety and stress | Probiotic | No significant effect of probiotic on stress |
| Schmidt et al91 | Clinical; RCT | Anxiety | Prebiotic | Prebiotic is associated with anxiolytic properties |
| Wang et al92 | Clinical; RCT | Stress | Rifaximin | Rifaximin shows stress-reducing effects |
| Burokas et al93 | Preclinical | Stress | Prebiotic | Prebiotic improves stress-related behaviors |
| Desbonnet et al94 | Preclinical | Depression | Probiotic | Probiotic normalizes markers associated with a rat model of depression |
| Tarr et al95 | Preclinical | Anxiety | Prebiotic | Prebiotic improves stressor-induced anxiety behavior |
NOTE. The table underscores the numerous studies, often with limited sample sizes, investigating the role of prebiotics, probiotics, and antibiotics to modulate anxiety, depression, stress, and other behavioral measures.
RCT, randomized controlled trial.
In summary, the basic gut microbial composition (including diversity and abundance of certain taxa), as well as BGM interactions, are established early in life, and once established are fairly stable, even in the presence of perturbations by antibiotics, gastrointestinal infections, or dietary changes. However, diet can influence both relative abundances as well as gut microbial functions in the adult within a certain bandwidth. When interpreting studies that explore perturbations in gut microbial structure, it is important to appreciate that the same protective (or deleterious) functional profiles can be generated by different microbial architectures.98
Clinical Implications and Brain Gut Disorders
Although preclinical studies clearly implicate the gut microbiome as a factor in modulating brain development, structure, function, and behavior in rodents, the demonstration of causal relationships in human beings remains challenging. Although no population-based studies have been reported on this topic, antibiotic treatment or total colectomies in the clinic are not known to be associated with significant changes in mood and affect. In addition, the effectiveness of prebi- otic and probiotic intake in the treatment of anxiety and depression remains to be determined by large, well-designed, randomized controlled trials. Largely unexplored is the role of early life influences on the evolving gut microbiome-brain communication network and its impact on gastrointestinal disorders with a strong developmental component. Based predominantly on preclinical studies, alterations in BGM interactions have been proposed as possible disease mechanisms in autism spectrum disorders,19,99 attention-deficit hyperactivity disorder,100 Parkinson’s disease,101 Alzheimer’s disease,102–104 stroke,105,106 and epilepsy.107 In addition, recent translational studies have shown that fecal microbiota transplantation from human donors with anxiety and depression can transmit some features of these conditions to recipient GF mice.108–110
BGM interactions likely also play an important role in healthy individuals, with 1 study identifying bacterial genus-based clusters in healthy females that were associated with functional brain profiles related to emotional regulation regions of the brain.111 In the current review, we focus on 2 brain gut disorders with relevance to gastroenterology.
Irritable Bowel Syndrome
A large number of studies (n = 22 in a total of 827 subjects) have reported significant microbial shifts in fecal microbial community composition between healthy controls and IBS patients, based on disease subtypes (diarrhea-predominant IBS, constipation-predominant IBS, and IBS mixed subtype), age (pediatric vs adult), and compartment (mucosa vs stool).112 Recent studies investigating gut microbial community structure have identified at least 2 subgroups of patients who meet Rome criteria for IBS. One subgroup, a eubiotic group, did not differ from healthy controls despite similar GI symptoms.113,114 The dysbiotic IBS subgroup differed in regional brain volumes from the eubiotic group,113 suggesting a relationship between microbial community structure and brain structure. Another recent study did not find a group difference in microbial composition between healthy controls and IBS, even though IBS symptom severity was correlated with dysbiosis.115 Based on an analysis of fecal samples, regardless of analytical methodology used, a number of studies reported decreased relative abundance of the genera Bifidobacterium and Lactobacillus, and increased firmi-cutes:bacteroidetes ratios at the phylum level.116,117 Because stress has been associated with a reduction in Lactobacilli in preclinical and clinical studies,118–120 the reported IBS-related changes in community structure and resulting metabolism may represent alterations of ANS modulation of the gut, as described earlier.
Obesity
A dysregulation of feeding behavior (referred to as food addiction or hedonic eating behavior) plays a significant role in the current obesity epidemic.121 By interacting with enteroendocrine cells in the distal gut, the gut microbiota and its metabolites modulate satiety signals (see earlier) and eating behaviors.1,122–124 In preclinical studies, fecal transplantation from hyperphagic obese mice to GF mice successfully induced hyperphagic behavior and weight gain in the recipients.125,126 The gut microbiome also has been associated with changes in brain microstructure in obesity, with distinct microbial brain signatures capable of differentiating obese and lean subjects.127 A handful of studies have pointed to a dramatic change in gut microbial composition after bariatric surgery.128–132 Remarkably, fecal transplantation from subjects after bariatric surgery was able to transmit the weight loss effects of bariatric surgery to a GF nonoperated recipient, inducing weight loss and reduced food intake.133,134
Conclusions
Based on available, largely preclinical data, the emerging BGM science has the potential to improve conventional therapies for several brain gut disorders, including IBS and obesity. Although experimental animal studies have suggested a possible therapeutic role for certain probiotics (psychobiotics), well-controlled clinical trials in human beings are needed to confirm the therapeutic value of currently available microbiome-targeted therapies in brain gut disorders. Efforts are underway to identify unique gut microbial fingerprints in several GI disorders that may lead to personalized therapies, including diet, as well as prebiotics and probiotics based on individual patterns of dysbiosis. Similarly, there is a search to identify the role of individual gut microbial signaling molecules (postbiotics), which may be targeted for therapeutic benefits. Based on these efforts, novel personalized interventions may become useful as prophylactic or adjuvant therapies for common brain gut disorders. Finally, interventions during early life such as colonization with certain microbes or fecal microbial transplants may become a therapeutic strategy to reduce the risk for the development of disorders such as IBS, anxiety, and even autism spectrum disorders.
Acknowledgments
Funding
Supported by grants DK048351, DK064539, and DK096606 from the National Institute of Diabetes and Digestive and Kidney Diseases (E.A.M.).
Abbreviations used in this paper:
- AhR
aryl hydrocarbon receptor
- ANS
autonomic nervous system
- BBB
blood-brain barrier
- BGM
brain gut microbiome
- C-section
Cesarean section
- CNS
central nervous system
- GF
germ-free
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- SCFA
short-chain fatty acid
- 2BA
secondary bile acid
Footnotes
Conflicts of interest
This author discloses the following: Emeran A. Mayer is a member of the scientific advisory boards of Danone, Viome, Amare, Prolacta, Pharmavite, Axial Biotherapeutics, Bloom Science, Whole Biome, Ubiome, and Mahana, and a consultant for General Mills, Host Therabiotics, Kelloggs, Nestle, and Kevita. The remaining authors disclose no conflicts.
References
- 1.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012;61:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 2009;106:3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A 2008;105:16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haghikia A, Jorg S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015;43:817–829. [DOI] [PubMed] [Google Scholar]
- 6.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goehler LE, Gaykema RP, Opitz N, et al. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun 2005;19:334–344. [DOI] [PubMed] [Google Scholar]
- 8.Marcelin G, Jo YH, Li X, et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab 2014;3:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan KK, Kohli R, Gutierrez-Aguilar R, et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 2013;154:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry RJ, Lee S, Ma L, et al. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Commun 2015;6:6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bala V, Rajagopal S, Kumar DP, et al. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-epsilon pathway and modulated by endogenous H2S. Front Physiol 2014;5:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009; 90:1236–1243. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y, Miyamoto N, Shibata K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A 2004; 101:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chimerel C, Emery E, Summers DK, et al. Bacterial metabolite indole modulates incretin secretion from intestinal enter- oendocrine L cells. Cell Rep 2014;9:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel CF, Goth SR, Dong B, et al. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 2008;375:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palazzo M, Balsari A, Rossini A, et al. Activation of enter-oendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol 2007;178:4296–4303. [DOI] [PubMed] [Google Scholar]
- 17.Larraufie P, Dore J, Lapaque N, et al. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol 2017;19. [DOI] [PubMed] [Google Scholar]
- 18.Kidd M, Gustafsson BI, Drozdov I, et al. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil 2009; 21:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–712. [DOI] [PubMed] [Google Scholar]
- 20.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015;17:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol 2014;32:367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nayak D, Zinselmeyer BH, Corps KN, et al. In vivo dynamics of innate immune sentinels in the CNS. Intravital 2012;1:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matcovitch-Natan O, Winter DR, Giladi A, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016;353:aad8670. [DOI] [PubMed] [Google Scholar]
- 26.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 2015; 18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothhammer V, Mascanfroni ID, Bunse L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 2016;22:586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbard TD, Murray IA, Bisson WH, et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep 2015;5:12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramadoss P, Marcus C, Perdew GH. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin Drug Metab Toxicol 2005;1:9–21. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy PJ, Cryan JF, Dinan TG, et al. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017;112:399–412. [DOI] [PubMed] [Google Scholar]
- 31.Jin UH, Lee SO, Sridharan G, et al. Microbiome-derived tryp-tophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 2014;85:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen MK, Balaguer P, Ekstrand B, et al. Skatole (3-methylindole) is a partial aryl hydrocarbon receptor agonist and induces CYP1A1/2 and CYP1B1 expression in primary human hepatocytes. PLoS One 2016;11:e0154629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014;6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Round JL, Lee SM, Li J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human micro-biota. Science 2011;332:974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014;146:1500–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macfarlane S, Dillon JF. Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol 2007;102:1187–1196. [DOI] [PubMed] [Google Scholar]
- 39.Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber- deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016; 167:1339–1353 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 2007; 178:4641–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos J, Saunders PR, Hanssen NP, et al. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol 1999;277: G391–G399. [DOI] [PubMed] [Google Scholar]
- 42.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003;278:11312–11319. [DOI] [PubMed] [Google Scholar]
- 43.Lambertsen CJ, Wright WB. Multiday exposure of men to high nitrogen pressure and increased airway resistance at natural expired oxygen tension: a 14-day continuous exposure to 5.2 per cent O 2 in N 2 at 4.0 atmospheres absolute pressure. Aerosp Med 1973;44:826–827. [PubMed] [Google Scholar]
- 44.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun 2017;60:1–12. [DOI] [PubMed] [Google Scholar]
- 45.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 2011;12:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilera M, Vergara P, Martinez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neuro-gastroenterol Motil 2013;25:e515–e529. [DOI] [PubMed] [Google Scholar]
- 47.Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun 1974;9:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Felius ID, Akkermans LM, Bosscha K, et al. Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol Motil 2003; 15:267–276. [DOI] [PubMed] [Google Scholar]
- 49.Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016; 65:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houlden A, Goldrick M, Brough D, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun 2016;57:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz Heijtz R Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med 2016; 21:410–417. [DOI] [PubMed] [Google Scholar]
- 52.Goyal MS, Venkatesh S, Milbrandt J, et al. Feeding the brain and nurturing the mind: linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci U S A 2015; 112:14105–14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial micro-biota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010;107:11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jasarevic E, Howerton CL, Howard CD, et al. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 2015;156:3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malaspina D, Corcoran C, Kleinhaus KR, et al. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry 2008;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ronald A, Pennell CE, Whitehouse AJ. Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front Psychol 2010;1:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 2016;22:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Backhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015;17:690–703. [DOI] [PubMed] [Google Scholar]
- 59.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014;63:559–566. [DOI] [PubMed] [Google Scholar]
- 60.Sevelsted A, Stokholm J, Bonnelykke K, et al. Cesarean section and chronic immune disorders. Pediatrics 2015; 135:e92–e98. [DOI] [PubMed] [Google Scholar]
- 61.Thum C, Cookson AL, Otter DE, et al. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J Nutr 2012; 142:1921–1928. [DOI] [PubMed] [Google Scholar]
- 62.Fan W, Huo G, Li X, et al. Impact of diet in shaping gut micro-biota revealed by a comparative study in infants during the six months of life. J Microbiol Biotechnol 2014;24:133–143. [DOI] [PubMed] [Google Scholar]
- 63.Roger LC, Costabile A, Holland DT, et al. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 2010; 156:3329–3341. [DOI] [PubMed] [Google Scholar]
- 64.Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 2012; 18(Suppl 4):12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr 2011;30:215–220. [DOI] [PubMed] [Google Scholar]
- 66.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrett E, Kerr C, Murphy K, et al. The individual-specific and diverse nature of the preterm infant microbiota. Arch Dis Child Fetal Neonatal Ed 2013;98:F334–F340. [DOI] [PubMed] [Google Scholar]
- 68.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med 2016;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016; 6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlson AL, Xia K, Azcarate-Peril MA, et al. Infant gut micro-biome associated with cognitive development. Biol Psychiatry 2018;83:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palmer C, Bik EM, DiGiulio DB, et al. Development of the human infant intestinal microbiota. PLoS Biol 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajilic-Stojanovic M, Heilig HG, Tims S, et al. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol 2012. Epub ahead of print. October 15, 2012. 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- 73.Sowell ER, Peterson BS, Thompson PM, et al. Mapping cortical change across the human lifespan. Nat Neurosci 2003;6:309–315. [DOI] [PubMed] [Google Scholar]
- 74.Desbonnet L, Clarke G, Traplin A, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun 2015;48:165–173. [DOI] [PubMed] [Google Scholar]
- 75.Lurie I, Yang YX, Haynes K, et al. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry 2015;76:1522–1528. [DOI] [PubMed] [Google Scholar]
- 76.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 2011; 108(Suppl 1):4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017;357:802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ong IM, Gonzalez JG, McIlwain SJ, et al. Gut microbiome populations are associated with structure-specific changes in white matter architecture. Transl Psychiatry 2018;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sonnenburg ED, Smits SA, Tikhonov M, et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016;529:212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394–1401, 401 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine 2017;24:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic Bifido-bacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 2017;153:448–459 e8. [DOI] [PubMed] [Google Scholar]
- 85.Romijn AR, Rucklidge JJ, Kuijer RG, et al. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry 2017;51:810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 2016; 32:315–320. [DOI] [PubMed] [Google Scholar]
- 87.Takada M, Nishida K, Kataoka-Kato A, et al. Probiotic Lacto-bacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol Motil 2016;28:1027–1036. [DOI] [PubMed] [Google Scholar]
- 88.Allen AP, Hutch W, Borre YE, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 2016;6:e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 2017;61:50–59. [DOI] [PubMed] [Google Scholar]
- 90.Ostlund-Lagerstrom L, Kihlgren A, Repsilber D, et al. Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutr J 2016;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt K, Cowen PJ, Harmer CJ, et al. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl) 2015; 232:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H, Braun C, Enck P. Effects of rifaximin on central responses to social stress-a pilot experiment. Neurotherapeutics 2018;15:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burokas A, Arboleya S, Moloney RD, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 2017;82:472–487. [DOI] [PubMed] [Google Scholar]
- 94.Desbonnet L, Garrett L, Clarke G, et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010;170:1179–1188. [DOI] [PubMed] [Google Scholar]
- 95.Tarr AJ, Galley JD, Fisher SE, et al. The prebiotics 3’Sialyllactose and 6’Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun 2015; 50:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng QX, Peters C, Ho CYX, et al. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord 2018;228:13–19. [DOI] [PubMed] [Google Scholar]
- 97.McKean J, Naug H, Nikbakht E, et al. Probiotics and subclinical psychological symptoms in healthy participants: a systematic review and meta-analysis. J Altern Complement Med 2017; 23:249–258. [DOI] [PubMed] [Google Scholar]
- 98.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012; 489:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry 2017;81:411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aarts E, Ederveen THA, Naaijen J, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One 2017;12:e0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016;167:1469–1480 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang C, Li G, Huang P, et al. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis 2017;58:1–15. [DOI] [PubMed] [Google Scholar]
- 103.Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep 2017;7:13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 2017;49:60–68. [DOI] [PubMed] [Google Scholar]
- 105.Winek K, Engel O, Koduah P, et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 2016;47:1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wen SW, Wong CHY. An unexplored brain-gut microbiota axis in stroke. Gut Microbes 2017;8:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olson CA, Vuong HE, Yano JM, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018; 173:1728–1741 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelly JR, Borre Y, O’Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neuro-behavioural changes in the rat. J Psychiatr Res 2016; 82:109–118. [DOI] [PubMed] [Google Scholar]
- 109.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–796. [DOI] [PubMed] [Google Scholar]
- 110.De Palma G, Lynch MD, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med 2017;9. [DOI] [PubMed] [Google Scholar]
- 111.Tillisch K, Mayer EA, Gupta A, et al. Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom Med 2017;79:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013; 62:159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Labus JS, Hollister EB, Jacobs J, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jeffery IB, O’Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- 115.Tap J, Derrien M, Tornblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017;152:111–123 e8. [DOI] [PubMed] [Google Scholar]
- 116.Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther 2012; 35:828–838. [DOI] [PubMed] [Google Scholar]
- 117.Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011;141:1792–1801. [DOI] [PubMed] [Google Scholar]
- 118.Bailey MT, Dowd SE, Galley JD, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011;25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Galley JD, Nelson MC, Yu ZT, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 2014; 14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knowles SR, Nelson EA, Palombo EA. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biol Psychol 2008;77:132–137. [DOI] [PubMed] [Google Scholar]
- 121.Pedram P, Wadden D, Amini P, et al. Food addiction: its prevalence and significant association with obesity in the general population. PLoS One 2013;8:e74832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin HV, Frassetto A, Kowalik EJ Jr, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 2012;7:e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011;60:2775–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osadchiy V, Labus JS, Gupta A, et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS One 2018; 13:e0201772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]
- 126.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010;328:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fernandez-Real JM, Serino M, Blasco G, et al. Gut microbiota interacts with brain microstructure and function. J Clin Endo-crinol Metab 2015;100:4505–4513. [DOI] [PubMed] [Google Scholar]
- 128.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 2009; 106:2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011; 60:1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int 2015;2015:806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010;59:3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 2013; 13:514–522. [DOI] [PubMed] [Google Scholar]
- 133.Liou AP, Paziuk M, Luevano JM Jr, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 2015;22:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Borre YE, O’Keeffe GW, Clarke G, et al. Microbiota and neuro- developmental windows: implications for brain disorders. Trends Mol Med 2014;20:509–518. [DOI] [PubMed] [Google Scholar]


