Abstract
Kaposi sarcoma herpesvirus (KSHV), also known as human herpesvirus-8, is associated with 1 form of multicentric Castleman disease (MCD) and is the etiologic agent for most MCD in human immunodeficiency virus (HIV)–infected patients. Diagnosis is usually determined by lymph node biopsy. Bone marrow findings in KSHV-MCD are not well characterized. We conducted histomorphologic and immunohistochemical evaluation of bone marrow biopsy specimens in HIV-infected patients with KSHV-MCD, including evaluation for KSHV latency-associated nuclear antigen. Findings were correlated with clinical features and KSHV viral load. Reactive plasmacytosis was the predominant feature. Lymphoid aggregates were less common and not diagnostic of KSHV-MCD. Forty-eight percent of cases contained scattered KSHV-infected mononuclear cells. Although patients were generally cytopenic, bone marrow biopsy specimens were normocellular to hypercellular except in patients receiving hematotoxic therapy. Bone marrow biopsy specimens in KSHV-MCD patients recapitulate findings of interleukin-6 excess. In patients with HIV, unexplained cytopenias, and bone marrow plasmacytosis, evaluation for KSHV-MCD is warranted.
Keywords: Kaposi sarcoma herpesvirus, Human herpesvirus-8, Multicentric Castleman disease, Human immunodeficiency virus, Bone marrow, Bone marrow examination, Plasmacytosis
Multicentric Castleman disease (MCD) is a group of rare B-cell lymphoproliferative disorders. One form is caused by Kaposi sarcoma–associated herpesvirus (KSHV),1,2 otherwise known as human herpesvirus-8, which is also the etiologic agent of Kaposi sarcoma (KS)3–5 and primary effusion lymphoma.6 KSHV-associated MCD (KSHV-MCD) accounts for nearly all MCD in human immunodeficiency virus (HIV)–infected individuals. KSHV-MCD is characterized clinically by severe inflammatory symptoms attributable largely to cytokine disarray, particularly elevations in human interleukin-6 (IL-6), a KSHV-encoded viral homolog of IL-6 (vIL-6), and human interleukin-10 (IL-10).7–12 In KSHV-MCD lymph node biopsy specimens, vIL-6 is noted in a proportion of KSHV-infected cells, and immunohistochemical evaluation for vIL-6 is useful in diagnosis. In addition, KSHV-MCD is associated with high levels of circulating KSHV. Historically, patients with KSHV-MCD had a poor clinical outcome, with deaths generally resulting from uncontrolled MCD-associated inflammatory syndromes, concurrent infections, or progressive chemotherapy-resistant KSHV-associated lymphoma.2,13,14 However, recent studies suggest prognosis is improving with a better understanding of disease pathophysiology and improved therapies.15–17 Unlike KS, whose incidence has decreased dramatically with the availability of effective anti-HIV therapy,18 the incidence of MCD may be increasing19 and often arises in the setting of well-controlled HIV.12,15
Thrombocytopenia and anemia are common hematologic abnormalities in patients with symptomatic KSHV-MCD, and a bone marrow examination is sometimes performed to evaluate the presence of marked cytopenias in patients with known or suspected KSHV-MCD. However, bone marrow findings in KSHV-MCD are not well characterized. One report of bone marrow findings in 13 patients with KSHV-MCD (11 of whom were HIV infected)20 noted the frequent presence of interstitial KSHV-positive cells, whereas a minority of cases were characterized by lymphoid aggregates containing plasmablasts, recapitulating the lymph node morphology. A similar finding was described in the bone marrow of a patient with MCD and no evidence of KSHV,21 suggesting that in rare cases, bone marrow biopsy may have a diagnostic role in patients with HIV and KSHV coinfection and inflammatory syndromes or cytopenias of unclear etiology. Similar Castleman-like lymphoid aggregates also have been described in bone marrow biopsy specimens of untreated patients with polyneuropathy, organo-megaly, endocrinopathy, monoclonal protein, and skin changes syndrome and associated KSHV-negative Castleman disease.22 We performed a study of bone marrow biopsy specimens with clinicopathologic correlation in a well-characterized cohort of patients with KSHV-MCD, with the goal of establishing morphologic and immunophenotypic features of bone marrow biopsy specimens in this disorder.
Materials and Methods
Patients with a tissue diagnosis of KSHV-MCD (nodal or splenic biopsy) enrolled in a National Cancer Institute (NCI) natural history study of KSHV-MCD () between 2004 and 2010 with at least 1 bone marrow biopsy specimen available for examination were included. The Institutional Review Board of the NCI approved this study. Written informed consent was obtained from all patients. Symptoms attributed to MCD flares were scored as absent or present based on the following definition: at least 1 symptom grade less than or equal to the NCI Common Terminology Criteria for Adverse Events 3.0 (CTCAE) grade 1 attributed to MCD, including but not limited to fever, fatigue, rigors/chills, diaphoresis, weight loss, anorexia, and weight gain/edema, and at least 1 of 5 laboratory abnormalities attributed to MCD from the following list: less than or equal to NCI CTCAE grade 1 anemia, thrombocytopenia, hyponatremia, hypoalbuminemia, and/or C-reactive protein (CRP) elevated above the upper limit of normal. Indication for bone marrow biopsy was considered clinical if performed to specifically evaluate cytopenias or stage a concurrent non-Hodgkin lymphoma. Otherwise, it was noted as a research biopsy. Pertinent laboratory studies included complete blood count, serum albumin, sodium, CRP, quantitative serum HIV viral load, and peripheral blood mononuclear cell (PBMC) KSHV viral load23 obtained near the time of bone marrow biopsy. Prior therapy for KS or KSHV-MCD and its temporal relationship to the bone marrow biopsy were noted.
Histologic Examination
Bone marrow core biopsy specimens and/or clot sections were stained with H&E for morphologic examination. Specific morphologic features were lymphoid aggregates, plasmablasts, plasmacytosis, serous atrophy, morphologic dysplasia in hematopoietic elements, and fibrosis. Immu-nostains for CD20 (L26; Dako, Carpinteria, CA), CD3 (PS-1; Cell Marque, Rocklin, CA), CD79a (clone 11E3; Dako), CD38 (SPC32; Leica Microsystems, Buffalo Grove, IL), CD138 (B-A38; Cell Marque), vIL-6 (Abbiotec, San Diego, CA), and κ/λ by immunohistochemistry (Dako) and/or in situ hybridization (Ventana Medical Systems, Tucson, AZ) were examined in most cases with available material. Plasmacytosis was estimated as a percentage of CD138 staining cells out of a total number of hematopoietic cells in the bone marrow. For KSHV latency-associated nuclear antigen (LANA) staining, we performed hot start antigen retrieval for 6 minutes in a pressure cooker in citrate buffer at pH 6.0 with a 2-hour incubation in primary antibody (clone LN53/ORF-73; Advanced Biotechnologies, Columbia, MD). Detection was performed on an Ultra View DAB detection kit (Ventana Medical Systems). Marrows and/or aspirates were considered KSHV positive if LANA was detected in a speckled nuclear pattern in mononuclear cells. Double immunohistochemical staining for CD138/LANA, CD20/LANA, CD3/LANA, CD68/LANA, λ/LANA, and κ/LANA was performed on a Ventana BenchMark Ultra automated stainer. Microphotographic images were obtained using an Olympus BX51 microscope and Olympus DP70 digital camera and acquisition software (Olympus America, Center Valley, PA).
KSHV Viral Load and Human IL-6 Assays
KSHV viral load was evaluated by extracting DNA from PBMCs using the QIAamp DNA blood mini kit (Qiagen, Valencia, CA). DNA quality and concentration were assessed by optical density using the NanoDrop 1000 (Thermo Scientific, Wilmington, DE). DNA concentration was adjusted to 250 ng/10 μL for 2 quantitative real-time polymerase chain reaction (PCR) assays developed using TaqMan (Applied Biosystems, Foster City, CA). Negative control wells were run in triplicate on each assay plate. KSHV DNA was detected using previously reported primers for the K6 gene region.24 The number of cellular equivalents was determined using a quantitative assay for human endogenous retrovirus 3.25 Samples were tested in triplicate for both assays, averaged, and reported as viral DNA copies per million PBMCs. Human IL-6 was measured using the MSD 96-well Multiarray Proinflammatory 7-plex Assay and the Sector Imager (Meso Scale Discovery, Gaithersburg, MD).
Statistical Analysis
Correlation between percent plasmacytosis and circulating KSHV viral load and serum human IL-6 was evaluated by Spearman rank correlation, with evaluation limited to patients who had not received hematotoxic therapy in 30 days prior to the bone marrow biopsy. The statistical significance of the difference in circulating log10 KSHV viral load according to LANA positivity in bone marrow samples was determined by an exact Wilcoxon rank sum test. All P values are 2-tailed.
Results
The study population Table 1 consisted of 19 patients with available bone marrow biopsy specimens (9 white, 5 African, 3 African American, 2 Hispanic; 17 men and 2 women); 6 other patients with KSHV-MCD who entered the protocol did not have bone marrow biopsy specimens. All were HIV infected at diagnosis of KSHV-MCD. Eighteen of 19 (95%) patients had anemia, 8 of 19 (42%) had leukopenia, and 13 of 19 (68%) had thrombocytopenia at the time of bone marrow biopsy. Sixteen patients had inflammatory symptoms attributed to KSHV-MCD, and 12 of the 15 tested had an elevated CRP level at the time of marrow examination. In 4 of the cases, patients with cytopenias in the setting of treatment had bone marrow biopsies performed with the goal of evaluating whether the cytopenias were due to therapy or to disease. Two patients had concurrent lymphoma in addition to KSHV-MCD at the time of bone marrow biopsy; 1 patient had untreated diffuse large B-cell lymphoma, and 1 had an extracavitary variant of primary effusion lymphoma. Neither of these patients was receiving chemotherapy at the time of the biopsy. An additional patient was diagnosed with primary effusion lymphoma 5 months after the bone marrow biopsy. Twelve (63%) had a history of either prior or concurrent KS, and 3 of these 12 (25%) had concurrent involvement by KS spindle cell prolif-eration and KSHV-MCD in the same lymph node.
Table 1.
Patient Characteristics at Time of Bone Marrow Biopsy
| Characteristic | Value (n = 19) |
|---|---|
| Age, median (range), y | 43 (27 to 56) |
| Sex, male/female, No. | 17/2 |
| Duration of HIV, median (IQR), y | 5 (2 to 15) |
| HIV viral load, median (IQR), copies/mL | <50 (<50 to 2,599) |
| CD4 count, median (IQR), cells/mL | 226 (136 to 439) |
| History of Kaposi sarcoma, No. (%) | 12 (66) |
| KSHV-MCD flare at time of biopsy, No. (%) | 15 (82) |
| KSHV viral load, median (IQR), copies/106 PBMCs | 12,227 (1,923 to 369,231) |
| Serum human IL-6, median (IQR), pg/mL | 15.9 (11.2 to 45.4)a |
| Serum IL-10, median (IQR), pg/mL | 591 (19 to >10,000) |
| KSHV-MCD treatment within 30 days of marrow, No. (%) | |
| None | 12 (63) |
| High-dose zidovudine and valganciclovir | 4 (21) |
| Valganciclovir | 2 (11) |
| Cytotoxic therapy | 2 (11) |
| Rituximab | 1 (5) |
| Laboratory parameters, median (IQR) | |
| Hemoglobin, g/L | 89 (79 to 103) |
| Platelets, ×109/L | 75 (42 to 206) |
| White blood cells, ×109/L | 4.7 (2.8 to 6.7) |
| Serum immunoglobulins, mg/dL | |
| IgG | 2,880 (1,750 to 4,390) |
| IgA | 335 (270 to 460) |
| IgM | 101 (81 to 148) |
| IgE | 393 (114 to 633) |
| CRP, mg/Lb | 84.3 (11.2 to 136.7) |
| Sodium, mmol/L | 134 (131 to 138) |
| Serum albumin, g/L | 23 (20 to 28) |
CRP, C-reactive protein; HIV, human immunodeficiency virus; IL-6, interleukin-6; IL-10, interleukin-10; IQR, interquartile range; KSHV, Kaposi sarcoma herpesvirus; MCD, multicentric Castleman disease; PBMC, peripheral blood mononuclear cell.
Available for 17 patients; normal range for human IL-6 in healthy volunteers is 2.3 ± 1.1 pg/mL.
CRP was assayed using Siemens Dimension Vista platform (Siemens AG, Munich, Germany); from study inception until May 2009, a standard sensitivity assay (sCRP) was used, which was subsequently replaced by a high-sensitivity CRP (hsCRP) assay. Correction of CRP for values obtained before May 2009 was performed using a formula (hsCRP = sCRP/0.93 + 0.56) that was validated by the National Institutes of Health Clinical Center Department of Laboratory Medicine. Normal CRP was less than 3 mg/L.
Histopathologic Findings: Plasma Cells, Light Chain Restriction, and Lymphoid Aggregates
Cases showed varying plasmacytosis, with a median of 10.5% (range, <3%–25%; n = 16) plasma cells as seen with CD38 and/or CD138 stains. A summary of morphologic findings is presented in Table 2. Plasmacytosis was often extreme in patients with severe inflammatory symptoms Image 1. The degree of plasmacytosis correlated with circulating PBMC-associated KSHV viral load, a marker of MCD disease activity (Spearman r = 0.71, P = .01), as well as with serum human IL-6 (Spearman r = 0.77, P = .006). In most cases (12/16), plasma cells were polytypic with interstitial and perivascular clusters of mature-looking plasma cells. Four of 16 cases in which this was assessed showed skewed light chain expression (2 λ and 2 κ). One case showed λ light chain–restricted plasma cells at the periphery of several lymphoid aggregates Image 2. Another case showed focal clusters of λ-restricted plasma cells without lymphoid aggregates Image 3. Two other cases showed predominance of scattered k light chain–positive plasma cells without formation of aggregates. In each of the cases with k light chain predominance, histology of the MCD in the corresponding lymph node showed typical λ restriction of the KSHV-infected plasmablasts and polytypic plasmacytosis of the surrounding KSHV-uninfected cells. In addition, in 1 of the κ-restricted bone marrow cases in a patient with extracavitary primary effusion lymphoma, bone marrow showed no KSHV-positive cells, and the clonal extracavitary primary effusion lymphoma was λ restricted.
Table 2.
Summary of Bone Marrow Pathology
| Case No. | Patient Age, y | Lymphoid Aggregates | Light Chain (ISH or IHC) | KSHV LANA IHC |
|---|---|---|---|---|
| 1 | 41 | Present | κ Predominant | − |
| 2 | 43 | Absent | ND | ND |
| 3 | 40 | Present | Polytypic | + |
| 4 | 43 | Present | Polytypic | + |
| 5 | 44 | Absent | Polytypic | + |
| 6 | 38 | Absent | Polytypic | − |
| 7 | 54 | Absent | Polytypic | − |
| 8 | 55 | Absent | λ Clusters | − |
| 9 | 42 | Absent | ND | − |
| 10 | 38 | Absent | Polytypic | − |
| 11 | 42 | Absent | Polytypic | + |
| 12 | 47 | Present | Polytypic | + |
| 13 | 35 | Absent | ND | ND |
| 14 | 45 | Absent | Polytypic | + |
| 15 | 27 | Absent | Polytypic | + |
| 16 | 47 | Present | λ Predominant | + |
| 17 | 56 | Present | κ Predominant | − |
| 18 | 43 | Absent | Polytypic | − |
| 19 | 47 | Absent | Polytypic | − |
IHC, immunohistochemistry; ISH, in situ hybridization; KSHV, Kaposi sarcoma herpesvirus; LANA, latency-associated nuclear antigen; ND, not done; −, negative; +, positive/detected.
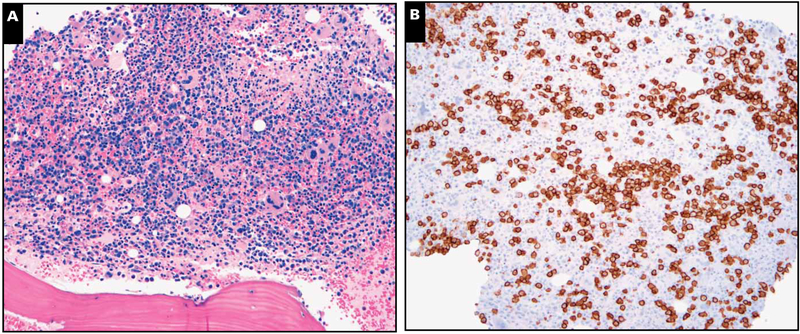
Image 1.
Markedly hypercellular marrow with plasmacytosis. A representative case with (A) marked marrow hypercellularity (H&E stain, magnification ×100) and (B) plasmacytosis (CD138 immunoperoxidase, magnification ×100) that was polytypic by light chain stains. This case did not show any lymphoid aggregates or stainable Kaposi sarcoma herpesvirus latency-associated nuclear antigen in the marrow biopsy specimen.
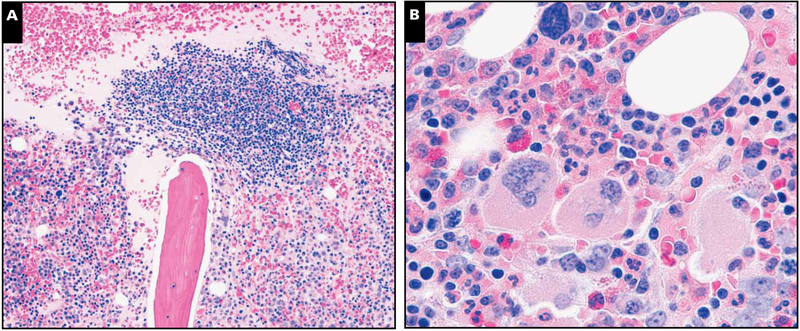
Image 2.
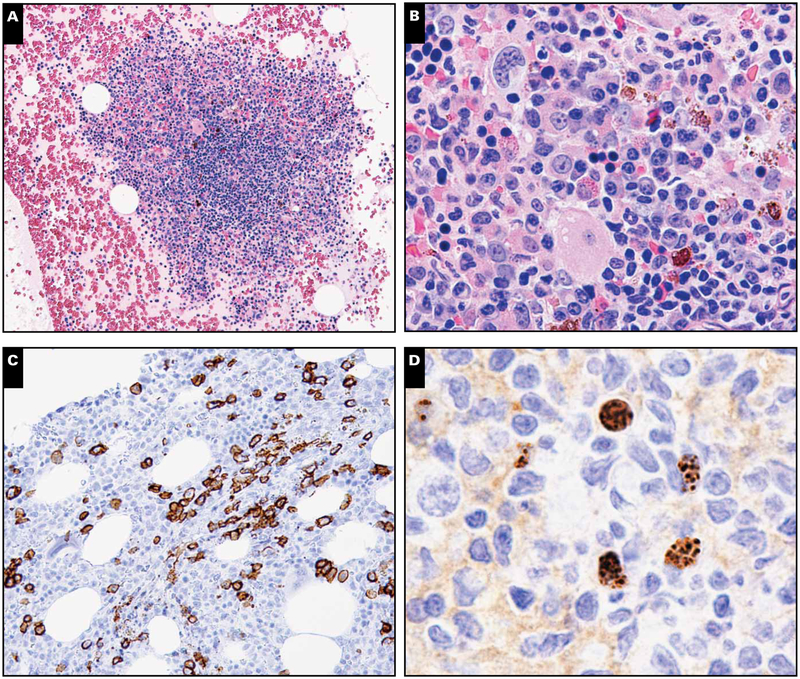
Illustration of a representative case with lymphoid aggregates and light chain–restricted plasma cells. A, Core biopsy specimen depicting paratrabecular lymphoid aggregate composed of lymphocytes, plasma, and few histiocytes with surrounding normocellular marrow with plasmacytosis (H&E stain, magnification ×40). B, Focal areas showed megakaryocytic clustering with mild fibrosis, but significant dyspoiesis was lacking in all 3 hematopoietic cell elements (H&E stain, magnification ×400).
C, CD138 reveals marked interstitial plasmacytosis (magnification ×40). D, Plasma cells expressing monotypic λ light chain are seen rimming the edges of the lymphoid aggregate (magnification ×40). E, κ Light chain is largely negative (magnification ×40). F, Occasional lymphoid aggregates without well-formed germinal centers were noted to contain rare Kaposi sarcoma herpesvirus latency-associated nuclear antigen–positive intermediate-sized mononuclear cells with a characteristic stippled pattern of nuclear staining (inset magnification ×400). Most cells did not have the morphology of plasmablasts.
Image 3.
Case with “grape-like” clusters of light chain–restricted plasma cells. A, Clusters of atypical λ light chain–restricted plasma cells in an interstitial and perivascular location (in situ hybridization, magnification ×200). B, Corresponding κ light chain shows only singly scattered plasma cells (in situ hybridization, magnification ×200). C, D, High-power view depicting atypical perivascular and interstitial grape-like clusters of plasma cells possessing abnormal cytomorphology (in situ hybridization, magnification ×400). This case, however, was negative for Kaposi sarcoma herpesvirus (KSHV) latency-associated nuclear antigen and had no lymphoid aggregates. This patient died less than 1 year after KSHV–multicentric Castleman disease diagnosis with evidence of primary effusion lymphoma diagnosed at autopsy.
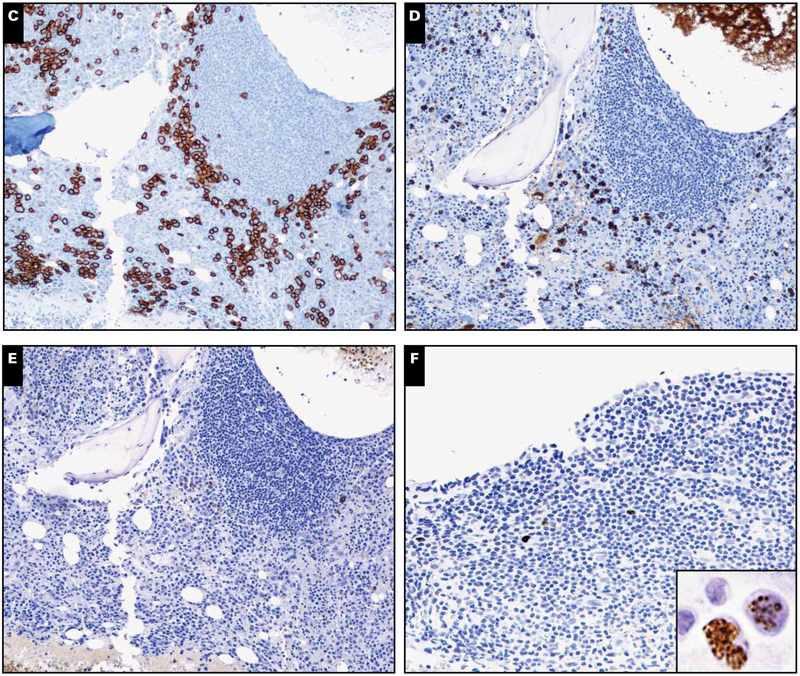
Six of 19 cases showed scattered lymphoid aggregates composed of an admixture of B and T cells without prominent plasmablasts (Image 2) Image 4. Both interstitial and paratrabecular lymphoid aggregates were present. Two of these cases showed interstitial lymphoid aggregates that were sharply demarcated and composed of small lymphoid cells with compact chromatin. The other 4 cases showed polymorphous lymphoid aggregates composed of lymphocytes, plasma cells, and histiocytes. There were no follicular structures or evidence of reactive germinal centers with vascularization recapitulating the morphology of MCD in nodal tissues. None of our cases had features diagnostic of MCD.
Image 4.
Lymphoid aggregates and Kaposi sarcoma herpesvirus (KSHV) latency-associated nuclear antigen (LANA)–positive cells (case 4). A, Clot section depicting large lymphoid aggregates (H&E stain, magnification ×40). B, These aggregates comprise a polymorphous population of cells, including many plasma cells (H&E stain, magnification ×200). No plasmablasts are apparent. C, CD138 marks interstitial plasmacytosis that was polytypic by light chains (not shown) (magnification ×100). D, Many larger interstitial KSHV LANA-positive mononuclear cells are present (magnification ×400).
KSHV LANA Immunostaining
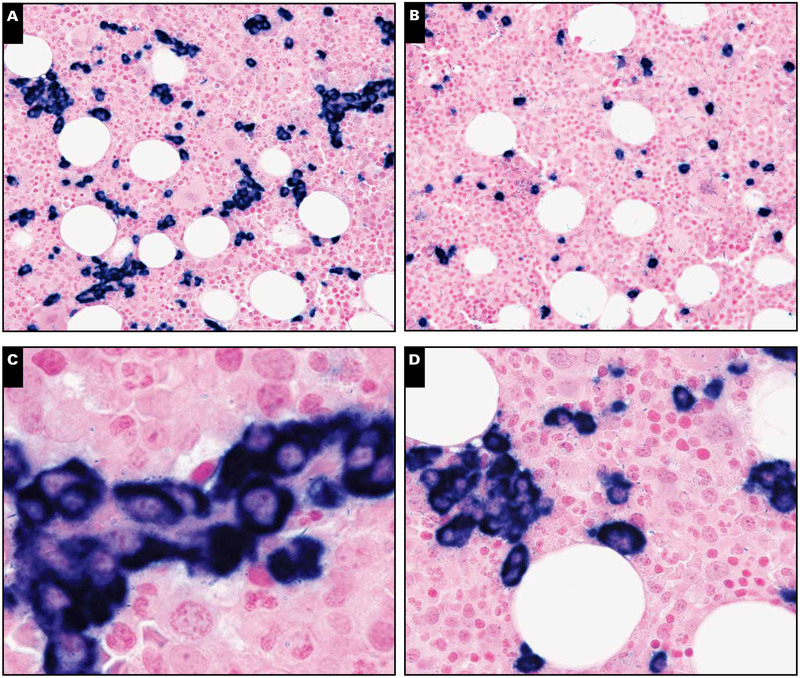
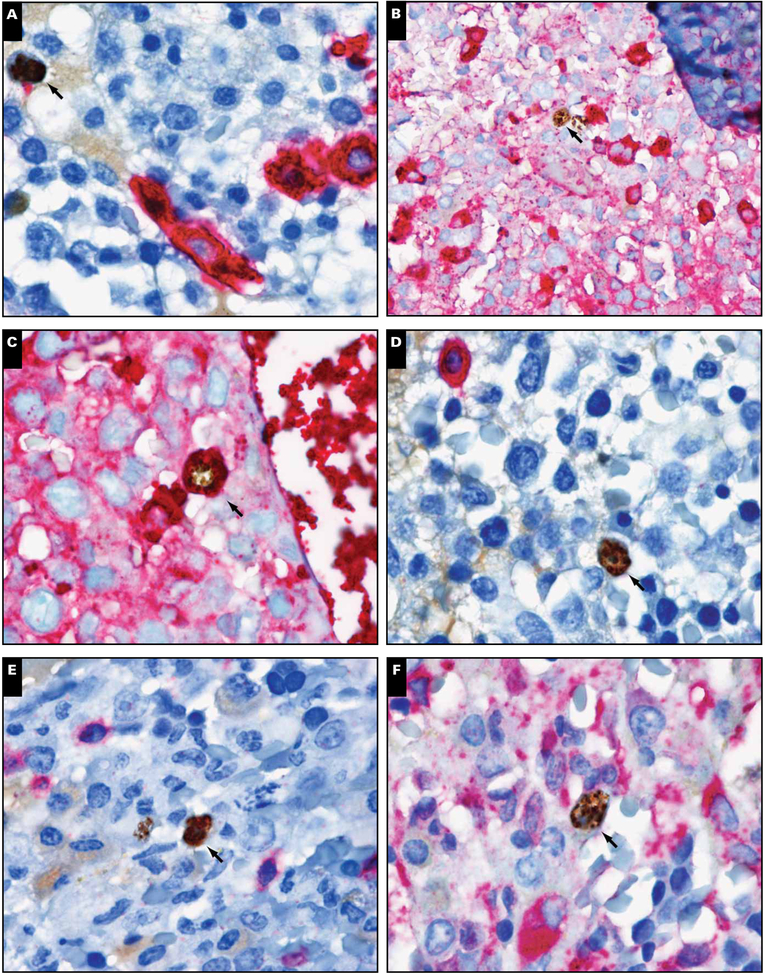
Seventeen of 19 biopsy specimens were tested for KSHV LANA by immunohistochemistry. LANA was detectable in 8 of 17 (48%) bone marrow specimens in either the core biopsy specimen or clot sections. KSHV LANA positivity in the bone marrow did not appear to be associated with circulating PBMC-associated KSHV viral load (Wilcoxon rank sum, P = .51). Generally, LANA-positive cells were scattered interstitial mononuclear cells; only 1 case showed rare positive cells associated with benign lymphoid aggregates (Image 2). Only occasional LANA-positive cells had plasmacytoid features Image 5. In 2 cases with LANA-positive interstitial cells, double immunostaining showed that the LANA-positive cells did not express CD20, CD138, CD3, CD68, or κ, but many of the LANA-positive cells were positive for λ light chain (Image 5). vIL-6 staining was negative in all cases. There was no association between KSHV immunopositivity and the presence of lymphoid aggregates.
Image 5.
Immunohistochemical characterization of Kaposi sarcoma herpesvirus latency-associated nuclear antigen (LANA)–positive cells. Double staining of LANA-positive cells (brown; arrows) with (A) CD138, (B) κ, (C) λ, (D) CD20, (E) CD3, and (F) CD68 (all in red) revealed that LANA-positive cells stained only with λ (magnification ×1,000).
Other Hematopoietic Elements
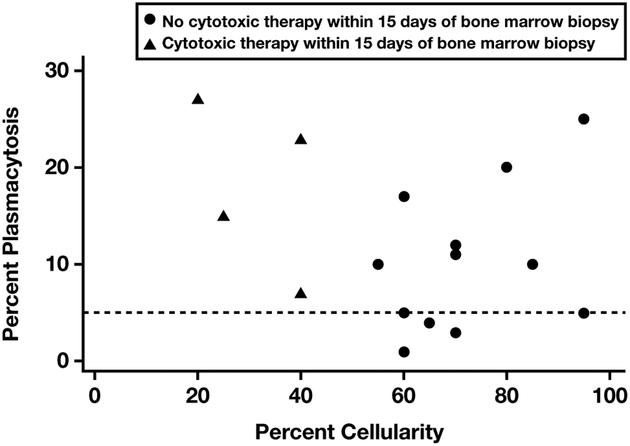
Bone marrow cellularity varied widely (20%−95%). In most patients (15/17), it was normocellular or hypercellular, despite associated cytopenias. Four of the 19 patients had received high-dose zidovudine combined with valganciclovir within 2 weeks of the bone marrow biopsy, and 2 of these 4 had hypocellular bone marrow biopsy specimens Figure 1. This group also demonstrated significant plasmacytosis, suggesting evidence of both disease activity and drug effect. Hypocellular marrow may be a sign of drug effects in patients with KSHV-MCD.
Figure 1.
Scatterplot depicting the relationship between the bone marrow cellularity and plasma cell percentage. Patients received myelosuppressive/marrow toxic therapy (black triangles) or did not receive cytotoxic therapy (black circles) at least 15 days prior to multicentric Castleman disease diagnosis. Dashed line indicates the upper limit of normal bone marrow plasmacytosis.
Mild megakaryocytic clustering (Image 2B) was apparent only in a minority of cases, as well as occasional naked megakaryocytes. Reticulin staining revealed minimal and patchy grade 1 to 2 of 4 fibrosis in most cases. Scattered histiocytes were seen admixed amid other hematopoietic elements. All bone marrow aspirates were routinely examined for signs of hemophagocytosis. Mild hemophagocytosis was noted in 1 case, but this patient had no clinical evidence of hemophagocytic syndrome. Three cases showed marked paucity of erythroid precursors. Four cases showed left-shifted granulopoiesis. Two of 3 cases with erythroid paucity and 3 of 4 with left-shifted granulopoiesis were on high-dose zidovudine combined with valganciclovir for treatment of KSHV-MCD. There was no significant dysplasia in erythroid or myeloid precursors. Serous atrophy previously described in marrows of patients with AIDS and uncontrolled HIV was lacking. Also, there was no evidence of involvement by KS or lymphoma in any of the bone marrow specimens.
Discussion
Our evaluation of bone marrow biopsy specimens of 19 patients with HIV and KSHV-MCD suggests the predominant findings are reactive changes related to elevated IL-6 and associated elevated circulating KSHV viral load. Reactive plasmacytosis was noted in most cases and appears to be the predominant feature in KSHV-MCD. In addition, KSHV-positive mononuclear cells were noted in about 50% of the cases. The KSHV LANA-positive cells were commonly positive for λ light chains, raising the possibility that KSHV-infected plasmablasts in the bone marrow might contribute to disease pathophysiology in some cases. However, morphologic features diagnostic of KSHV-MCD were not evident. Although lymphoid aggregates were present in 30% of cases, we did not encounter reactive germinal centers and λ light chain–expressing plasmablasts as usually observed in lymph node biopsy specimens of patients with MCD, and the KSHV-positive cells usually were not associated with lymphoid aggregates. Furthermore, despite mild to moderate plasmacytosis noted in all cases, only a minority had skewed light chain expression. Last, vIL-6 immunostaining did not show the presence of vIL-6.
Our findings did not confirm unusual bone marrow features previously described in patients with KSHV-MCD. In contrast to a previous report, in which lymphoid aggregates typically seen in MCD (regressed germinal centers associated with scattered λ light chain–restricted KSHV-positive plasmablasts within the mantle zones) were described in marrow biopsy specimens of 3 of 13 patients,20 we did not observe findings diagnostic of KSHV-MCD. One possible explanation of this is that although 82% of patients had a disease flare at the time of biopsy, 39% had received some therapy for KSHV-MCD or KS within 4 weeks prior to the biopsy. Likewise, we did not observe evidence of KSHV-associated hemophagocytic syndrome, previously reported in KSHV-MCD9; only 1 case showed evidence of occasional hemophagocytic histiocytes, and this patient did not have clinical evidence of a hemophagocytic syndrome. This suggests that bone marrow findings sufficient to make a diagnosis of KSHV-MCD or hemophagocytic syndrome appear to be rare in patients with KSHV-MCD. One unique finding in our series was a case with λ light chain–restricted “grape-like” clusters of atypical plasma cells, which are similar to those described in HIV-negative KSHV-MCD marrows reported by Kreft et al.21
Comparison with historical descriptions of bone marrow morphology in patients with uncontrolled HIV suggests that bone marrow plasmacytosis is not simply related to underlying HIV. Prior to the availability of combination antiretro-viral therapy, Karcher and Frost26 described the effect of uncontrolled HIV infection on bone marrow pathology from 216 bone marrow biopsy specimens and/or clot sections in a cohort of 178 HIV-positive patients. In their series, plasmacytosis was present in 55 (25%) of 216 evaluable biopsy specimens. This is considerably lower than the frequency of plasmacytosis observed in our patient cohort (>5% plasma cells observed in 11/16 [69%] cases), despite the fact that many (9/19; 47%) of the KSHV-MCD patients had undetect-able HIV. Furthermore, dysplastic changes, seen in 69% of patients in the Karcher and Frost series, were not prominent in our patients with KSHV-MCD but generally controlled HIV. On the other hand, the presence of lymphoid aggregates, observed in 35% of patients in the Karcher and Frost series and 32% of patients with KSHV-MCD in this study, does not appear to distinguish uncontrolled HIV from KSHV-MCD in HIV-infected patients.
Despite a lack of diagnostic germinal centers, bone marrow biopsy specimens showed abnormalities that were informative about disease activity. Prominent interstitial and perivascular plasmacytosis was common, with 60% of patients having greater than 10% plasmacytosis. Furthermore, evidence of KSHV-infected mononuclear cells in the bone marrow was commonly observed through evaluation of LANA in our biopsy and aspirate samples. KSHV-infected cells were mainly identified as interstitial mononuclear cells with stippled nuclear positivity (Image 5). Only some of the positive cells had a plasmacytoid appearance with relatively abundant dense cyto-plasm. KSHV-infected cells were negative for CD20, CD138, CD3, CD14, CD68, and κ light chains but positive for λ light chain. The number of LANA-positive cells was variable, with most cases showing only occasional cells.
To evaluate whether immunohistochemically identified KSHV-positive mononuclear cells in the bone marrow were indicative of a higher KSHV burden, we evaluated the association between plasma PBMC-associated KSHV viral loads and marrow immunopositivity for KSHV and did not find a statistically significant association. The significance of LANA-staining mononuclear cells in bone marrow specimens remains unclear. Indeed, this finding is not specific for KSHV-MCD and may be seen in a spectrum of KSHV-associated diseases. For example, HIV-infected patients with CD4 lymphopenia and KS without evidence of KSHV-MCD have been shown to harbor KSHV-positive mononuclear cells in their marrows by PCR and immunohistochemistry for both LANA and vIL-6.27 KSHV viral loads can vary widely over time in patients with HIV and KSHV-MCD, depending on the disease activity. It is of some interest that the detection of KSHV in bone marrow samples may be associated with several KSHV-associated diseases, suggesting that the spectrum of these conditions should be considered in the differential diagnosis of abnormal plasmacytosis in diagnostic bone marrow samples of HIV-infected patients with unexplained cytopenias or inflammatory syndromes.12
Plasmacytosis in KSHV-MCD may to be due to a para-crine effect of KSHV-infected cells in the bone marrow and/or systemic cytokine disarray in this disease. Patients with symptomatic KSHV-MCD, including those in this study, have markedly increased circulating IL-6 and IL-10,7 and they often have detectable circulating vIL-6, a KSHV lytic gene product with 25% homology to human IL-6 that can signal through the common cytokine receptor gp130. Mouse models of IL-6 and vIL-6 excess are instructive.8,28 Deliberate overexpression of IL-6 in mice leads to a syndrome with similarities to MCD, and pathologic evaluation of organs is remarkable for plasmacytic infiltrates. Interestingly, these animals demonstrate transient leukocytosis rather than leukopenia.28 Likewise, when vIL-6–expressing fibroblasts are inoculated in athymic mice, a similar syndrome is encountered, with evidence of plasmacytosis and increased myeloid hematopoiesis.8 Although the plasmacytosis in our series appears to recapitulate findings in mouse models of IL-6 excess, the relatively common finding of leukopenia suggests that other cytokines may also play an important role in hematologic abnormalities in KSHV-MCD. In this regard, the IL-10 excess common in symptomatic KSHV-MCD is an interesting candidate. IL-10 is a growth factor for activated B lymphocytes and, moreover, induces secretion of IgG, IgA, and IgM.29,30 IL-10 secretion is particularly upregulated in KSHV or Epstein-Barr virus–infected B cells and associated B-cell lymphomas.30,31 IL-10 reduces viable bone marrow cells in IL-3/stem cell factor (SCF) models, perhaps through downregulation of SCF and c-kit and increased apoptosis of myeloid precursors.32,33 In a phase I study of recombinant human IL-10 in patients with Crohn disease, thrombocytopenia and lymphocytopenia were frequent findings.34 Further studies will be required to determine the relative effects of the cytokines on bone marrow findings.
In summary, there is a spectrum of histologic changes in the bone marrow biopsy specimens of documented KSHV-MCD cases in the setting of controlled HIV. Notably, marrow involvement generally does not recapitulate the morphologic findings typically seen in the lymph nodes. However, KSHV-infected mononuclear cells are detectable in a significant proportion of patients, and these cells appear similar to KSHV-positive λ-restricted plasmablasts noted in KSHV-MCD lymph nodes. Furthermore, reactive plasmacytosis appears to be the most common finding. Diagnostic bone marrow samples of HIV-infected patients with unexplained inflammatory syndromes or cytopenias should warrant evaluation for KSHV through immunohistochemical staining for LANA. Furthermore, evaluation of bone marrow cellularity and plasmacytosis is clinically useful when bone marrow biopsies are performed to evaluate persistent cytopenias in patients receiving hematotoxic agents for the treatment of KSHV-MCD. Additional studies are needed to expand upon the spectrum of KSHV-associated disease and may clarify the utility of bone marrow examination in patients with HIV infection and unexplained inflammatory syndromes, cytopenias, or known KSHV-associated malignancies.
Acknowledgments:
We thank the patients who participated in this study, as well as the clinical staff of the HIV and AIDS Malignancy Branch, the Laboratory of Pathology, and the Department of Laboratory Medicine for their help.
Supported by the Intramural Research Program of the National Institutes of Health. Additional funding comes from the National Cancer Institute, National Institutes of Health (contract No. HHSN261200800001E).
References
- 1.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma–associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 2.Oksenhendler E, Duarte M, Soulier J, et al. Multicentric Castleman’s disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10:61–67. [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. [DOI] [PubMed] [Google Scholar]
- 4.Whitby D, Howard MR, Tenant-Flowers M, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. [DOI] [PubMed] [Google Scholar]
- 5.Moore PS, Kingsley LA, Holmberg SD, et al. Kaposi’s sarcoma–associated herpesvirus infection prior to onset of Kaposi’s sarcoma. AIDS. 1996;10:175–180. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma–associated herpesvirus-like DNA sequences in AIDS-related body-cavity–based lymphomas. N Engl J Med. 1995;332:1186–1191. [DOI] [PubMed] [Google Scholar]
- 7.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood. 2000;96:2069–2073. [PubMed] [Google Scholar]
- 8.Aoki Y, Jaffe ES, Chang Y, et al. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma–associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034–4043. [PubMed] [Google Scholar]
- 9.Bower M, Veraitch O, Szydlo R, et al. Cytokine changes during rituximab therapy in HIV-associated multicentric Castleman disease. Blood. 2009;113:4521–4524. [DOI] [PubMed] [Google Scholar]
- 10.Aoki Y, Tosato G, Fonville TW, et al. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood. 2001;97:2526–2527. [DOI] [PubMed] [Google Scholar]
- 11.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum Immunol. 1999;60:921–927. [DOI] [PubMed] [Google Scholar]
- 12.Uldrick TS, Wang V, O’Mahony D, et al. An interleukin-6–related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma–associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis. 2010;51:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma–associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99:2331–2336. [DOI] [PubMed] [Google Scholar]
- 14.Collins LS, Fowler A, Tong CY, et al. Multicentric Castleman’s disease in HIV infection. Int J STD AIDS. 2006;17:19–24; quiz 25. [DOI] [PubMed] [Google Scholar]
- 15.Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus–associated multicentric Castleman disease: a pilot study of virus–activated cytotoxic therapy. Blood. 2011;117:6977–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147:836–839. [DOI] [PubMed] [Google Scholar]
- 17.Gerard L, Berezne A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus–associated multicentric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25:3350–3356. [DOI] [PubMed] [Google Scholar]
- 18.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. [DOI] [PubMed] [Google Scholar]
- 19.Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann Oncol. 2009;20:775–779. [DOI] [PubMed] [Google Scholar]
- 20.Bacon CM, Miller RF, Noursadeghi M, et al. Pathology of bone marrow in human herpes virus-8 (HHV8)–associated multicentric Castleman disease. Br J Haematol. 2004;127:585–591. [DOI] [PubMed] [Google Scholar]
- 21.Kreft A, Weber A, Springer E, et al. Bone marrow findings in multicentric Castleman disease in HIV-negative patients. Am J Surg Pathol. 2007;31:398–402. [DOI] [PubMed] [Google Scholar]
- 22.Dao LN, Hanson CA, Dispenzieri A, et al. Bone marrow histopathology in POEMS syndrome: a distinctive combination of plasma cell, lymphoid, and myeloid findings in 87 patients. Blood. 2011;117:6438–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma–associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–355. [DOI] [PubMed] [Google Scholar]
- 24.de Sanjose S, Marshall V, Sola J, et al. Prevalence of Kaposi’s sarcoma–associated herpesvirus infection in sex workers and women from the general population in Spain. Int J Cancer. 2002;98:155–158. [DOI] [PubMed] [Google Scholar]
- 25.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods. 2001;91:109–117. [DOI] [PubMed] [Google Scholar]
- 26.Karcher DS, Frost AR. The bone marrow in human immunodeficiency virus (HIV)–related disease: morphology and clinical correlation. Am J Clin Pathol. 1991;95:63–71. [DOI] [PubMed] [Google Scholar]
- 27.Meggetto F, Cesarman E, Mourey L, et al. Detection and characterization of human herpesvirus-8–infected cells in bone marrow biopsies of human immunodeficiency virus-positive patients. Hum Pathol. 2001;32:288–291. [DOI] [PubMed] [Google Scholar]
- 28.Brandt SJ, Bodine DM, Dunbar CE, et al. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in mice. J Clin Invest. 1990;86:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1890–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burdin N, Rousset F, Banchereau J. B-cell–derived IL-10: production and function. Methods. 1997;11:98–111. [DOI] [PubMed] [Google Scholar]
- 31.Jones KD, Aoki Y, Chang Y, et al. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus–associated infected primary effusion lymphoma cells. Blood. 1999;94:2871–2879. [PubMed] [Google Scholar]
- 32.Bailey DP, Kashyap M, Bouton LA, et al. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J Leukoc Biol. 2006;80:581–589. [DOI] [PubMed] [Google Scholar]
- 33.Mirmonsef P, Shelburne CP, Fitzhugh Yeatman C II, et al. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3’-kinase.J Immunol. 1999;163:2530–2539. [PubMed] [Google Scholar]
- 34.Schreiber S, Fedorak RN, Nielsen OH, et al. , for the Crohn’s Disease IL-10 Cooperative Study Group. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Gastroenterology. 2000;119:1461–1472. [DOI] [PubMed] [Google Scholar]