Abstract
Common bean (Phaseolus vulgaris L.) is a major source of proteins and one of the most important edible foods for more than three hundred million people in the world. The common bean plants are frequently attacked by spider mite (Tetranychus urticae Koch), leading to a significant decrease in plant growth and economic performance. The use of resistant cultivars and the identification of the genes involved in plant-mite resistance are practical solutions to this problem. Hence, a comprehensive study of the molecular interactions between resistant and susceptible common bean cultivars and spider mite can shed light into the understanding of mechanisms and biological pathways of resistance. In this study, one resistant (Naz) and one susceptible (Akhtar) cultivars were selected for a transcriptome comparison at different time points (0, 1 and 5 days) after spider mite feeding. The comparison of cultivars in different time points revealed several key genes, which showed a change increase in transcript abundance via spider mite infestation. These included genes involved in flavonoid biosynthesis process; a conserved MYB-bHLH-WD40 (MBW) regulatory complex; transcription factors (TFs) TT2, TT8, TCP, Cys2/His2-type and C2H2-type zinc finger proteins; the ethylene response factors (ERFs) ERF1 and ERF9; genes related to metabolism of auxin and jasmonic acid (JA); pathogenesis-related (PR) proteins and heat shock proteins.
Introduction
Common bean (Phaseolus vulgaris L.), is one of the most important edible foods in the world which provides about 50% of the grain legumes for direct human consumption [1–3]. In addition, it is an inexpensive healthy food due to having the richest sources of proteins (20–25%), micronutrients and calories [2]. Common bean is widely distributed around the world. In Asia, most collections exist in India [4], and Iran [5]. Notably, over the last 10 years, the production of common bean has increased ~33% in Asia [2]. Because of high nutrient content and commercial potential, common bean holds great promise for fighting hunger and increasing income. Low yield of this crop is attributed to pest attack, weak soil fertility, drought and salinity, and poor agronomic practices [6]. According to the records, the two spotted spider mite (TSSM), Tetranychus urticae Koch, is the most widespread and the most polyphagous herbivores mites which feed on cell contents of common bean and causes serious substantial economic losses (up to 100% yield losses) in fields and greenhouses [7, 8]. The TSSM damages plant cells by its stylet that pierces the leaf either in between epidermal pavement cells or through a stomatal opening, suck-out their contents and forms the chlorotic lesions at the feeding sites [8–10]. In recent years, it has become evident that insect-resistant crops have brought great benefits, not only in terms of economic, but also because of the reduction of pesticides use and keeping a safe environment. The development of new cultivars is being established as one of the most appropriate methods and the main objective of plant breeding programs for resistance to TSSM [11, 12]. However, lacking information on how plant and mite interact with each other emphasizes the importance of a comprehensive study of the molecular interactions between common bean and T. urticae to understand the mechanisms and potential biological pathways of common bean resistance. Although RNA-Seq has been used to study the expression profiles of stress response genes in model and non-model plants, but there has not been any study of common bean transcriptome changes due to spider mite feeding. In this study we used RNA-Seq analysis to detect differences in gene expression between two cultivars of P. vulgaris (susceptible and resistant), and specify effective genes and pathways in response to T. urticae infestation. Such information could lead to identity resistant mechanisms and genes in common bean and improve the breeding efforts by identifying molecular markers to incorporate resistance into commercial bean varieties.
Materials and methods
Plants and insect infestation
According to our previous study [13], two cultivars, including Akhtar and Naz were selected as susceptible and resistant cultivars to T. urticae, respectively. Seeds were sown in plastic pots (15 cm diameter, 25 cm high) containing soil, peat moss, and perlite (1:1:1), with only a single plant in each pot. The experiment was conducted using a factorial experiment based on completely randomized design with three replicates in greenhouse condition (28 ± 3ºC temperature, 40–50% relative humidity, photoperiod 16h light and 8h darkness). The founder population of mite was collected from a commercial bean farm in Karaj, Iran and colonies were reared on the similar cultivars to be tested for three generations before being used in this experiment. In six-leaf stage (about 30 days after planting) based on Meier [14], 45 same-aged adult female mites were placed on sixth leaf of plants. Since, the number of females is higher than males and they also cause the most damages, only female mites were selected from a mass which had the opportunity for mating. The leaves were collected after 0, 1 and 5 days of infestation. Treated leaves were frozen in liquid nitrogen and kept at −80°C until they were used for RNA extraction.
RNA isolation and transcriptome sequencing
Total RNA was extracted from two biological replicates, which each were pooled samples from at least three plants using TRIzol® Reagent (Invitrogen) as described by the manufacturer's protocol, and then treated with RNase-free DNaseI (Invitrogen). Nanodrop™ 2000 spectrophotometer, agarose gel electrophoresis and Bioanalyzer 2100 (Agilent) were used to check and confirm quantity and quality of RNAs. All RNAs were sent to Beijing Genomic Institute (BGI) in China for library preparation and transcriptome sequencing using the Illumina HiSeq 2500 platform to generate Paired-end (2×150 bp) reads.
Reads preprocessing and differentially expression analysis
The raw reads were downloaded from BGI institute web site in Fastq format and then were subjected to quality control (QC) analysis using Trimmomatic software [15] to trim low quality reads, adapters and other Illumina-specific sequences, minimum length 50 bp and minimum quality 30 determined as quality thresholds. Before and after filtering, the quality of the raw sequences was assessed with FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Clean reads were mapped to the P. vulgaris [16] and T.urticae [17] reference genomes V. 2.0 (https://phytozome.jgi.doe.gov) via RNA-Seq aligner STAR software [18] requiring at least 90% of the read sequence to match with at least 95% identity. The STAR-resultant. bam files were used to estimate the abundance of mapped reads, differential expression analyses, and visualization of analyses results using Cufflinks [19] package coupled with CummeRbund [20]. Cufflinks was used to calculate FPKM values and differential expression analysis was done with Cuffdiff. The analysis focused on genes with statistically significant difference in expression levels between times and cultivars. The genes were considered significantly differentially expressed if false discovery rate (FDR, the adjusted P value) was <0.01 and Log2 FPKM (fold change) was ≥1.0. All RNA-Seq data were deposited in the NCBI SRA database under the project PRJNA482175.
GO terms and KEGG pathways enrichment analysis
The functions of the DEGs were characterized using AgriGO’s Singular Enrichment Analysis (SEA) module to identify the enriched Gene Ontology terms (http://bioinfo.cau.edu.cn/agriGO/analysis.php) with the agriGO database [21]. The enrichment analysis was performed at significance level of 0.05. Enrichment analysis of Kyoto Encyclopedia of Genes and Genomics (KEGG) pathways was also carried out on DEGs using KOBAS 3.0 web server (http://kobas.cbi.pku.edu.cn/). KEGG pathways with corrected p-value ≤0.05 were considered as significantly enriched.
Quantitative real-time PCR validation
To validate candidate differentially expressed genes (DEGs), qRT-PCR was performed for six DEGs and Actin 11 as a reference gene with three replicates. Primers were designed by Primer 3.0 [22] (Table 1), and cDNAs were synthesized by using TaKaRa cDNA Synthesis Kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The 20 μL qRT-PCR solutions contained EvaGreen Master Mix (Solis Biodyne, 5x), 0.3 μL forward and 0.3 μL reverse primers, and 30 ng of cDNA template. qRT-PCR reactions (95 °C, 3 min; 95 °C, 5 s; 60 °C, 34 s; 40 cycles) were carried out on a Bio-Rad iQ5 Optical System (Bio-Rad Laboratories, CA, USA). Finally, relative gene expression was calculated using 2-ΔΔCT formula and REST software [23].
Table 1. Description of the candidate gens and primer sequences for qRT-PCR assay efficiencies.
| Funcional annotation | Gene ID | Forward primer | Reverse prime |
|---|---|---|---|
| Pathogenesis-related protein | Phvul.004G155500 | TGGGATACAGCTACAGCATCGT | ATCTTCATTGGGTGGAGCATCT |
| WRKY transcription factor 50 | Phvul.009G080000 | GTCGCTGAGATCGGAGAATC | GCAAATCCAGCTTTGACCAT |
| Heat shock protein (Molecular chaperone) | Phvul.008G011400 | CTTTCAACACCAACGCCATG | GCTCAAGCTCCGAGTAGG |
| Leucine Rich Repeat | Phvul.008G044600 | CTTGACTATGAGCTTGTCCCC | TGCTTTCTCTGTAAGGTGTCC |
| MYB113 | Phvul.008G038200 | GTCGCTGAGATCGGAGAATC | GCAAATCCAGCTTTGACCAT |
| Xyloglucan endotransglucosylase/hydrolases | Phvul.005G111300 | AGTTCGACGAGCTGTTCCAG | ACGTTGGTCTGCACGCTGTA |
Results and discussion
Quality control and mapping statistics
A total of 12 RNA libraries were sequenced with the number of reads ranging from 26.8–30.2 million paired-end reads (Table 2). Approximately 70–73% of reads passed the quality control and an 84.58–90.30% of the clean reads were mapped to unique location in the common bean reference genome. Alignment of clean reads to T. urticae reference genome was also carried out to determine whether a significant mite RNA contamination exists in our datasets. Assessment of quality of mRNA-Seq data revealed less than 0.1% mapping, indicating a strong enrichment of genes specific for P. vulgaris in all samples.
Table 2. Summary of sample information and transcriptome sequencing output statistics for the RNA-Seq libraries.
| Cultivars | Replicate | Time points | Reads before quality control | Reads after quality control | Removing percent |
|---|---|---|---|---|---|
| Akhtar (susceptible) | Replicate 1 | Control | 30261354 | 21020563 | 30.54 |
| 1 Day | 28834008 | 20871380 | 27.62 | ||
| 5 Day | 26832585 | 19131482 | 28.70 | ||
| Replicate 2 | Control | 31261254 | 22020354 | 29.56 | |
| 1 Day | 27836208 | 20971682 | 24.66 | ||
| 5 Day | 26632382 | 19231180 | 27.79 | ||
| Naz (resistant) | Replicate 1 | Control | 30593227 | 22305624 | 27.09 |
| 1 Day | 27677349 | 19152514 | 30.80 | ||
| 5 Day | 29214322 | 21461892 | 26.54 | ||
| Replicate 2 | Control | 30243118 | 22212456 | 26.55 | |
| 1 Day | 28123454 | 19252314 | 31.54 | ||
| 5 Day | 28876322 | 21863542 | 24.29 |
Differentially expressed genes (DEGs)
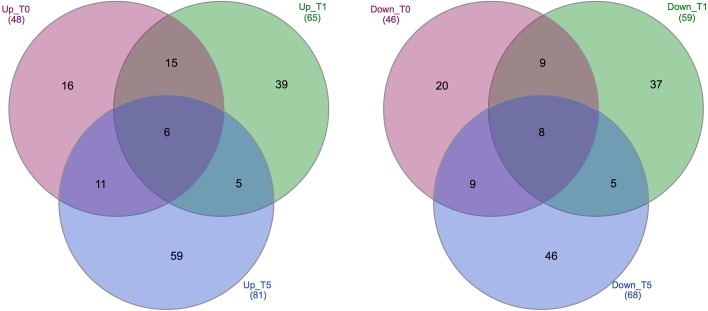
Differentially expressed genes analysis was performed for the pairwise comparisons of twelve libraries. The largest differences in expression occurred among three time points of resistant cultivar. When comparing the different time points for resistant cultivar, 274 differentially expressed genes were identified (S1 Table), almost the same number of DEGs for susceptible cultivar (270 DEGs, S2 Table). The number of up-regulated genes was higher than down-regulated genes in all different time point comparisons in both cultivars. To gain a better understanding, the overlap differentially expressed patterns of DEGs were analyzed between cultivars in each time point and across time points in each cultivars using Venn diagram. The comparison of cultivars in each time points revealed 48, 65 and 81 up-regulated genes along with 46, 59, 68 down-regulated genes in resistant cultivar for control samples and these infested at 1 and 5 days post-feeding, respectively (Fig 1, S3–S5 Tables). The number of DEGs showed a rising trend with the extension of infestation time, So that the smallest and largest differences were observed between resistant and susceptible plants at first and third time points, in which 94 and 146 DEGs were identified, respectively. This result indicates there are probably no significant differences in gene expression patterns during the first attempts of spider mite in both susceptible and resistant reactions. However, gene expression patterns were more different during the second phase of infestation depending on the resistance/susceptibility of the plant.
Fig 1. Venn diagram showing the number of specific and shared DEGs between pair time points in both cultivars.
Up_T0, genes up-regulated in resistant cultivar in comparison with susceptible cultivar at first time point. Down_T0, genes down-regulated in resistant cultivar or up-regulated in susceptible cultivar at first time point. T1 and T3 represent the second and third time points, respectively.
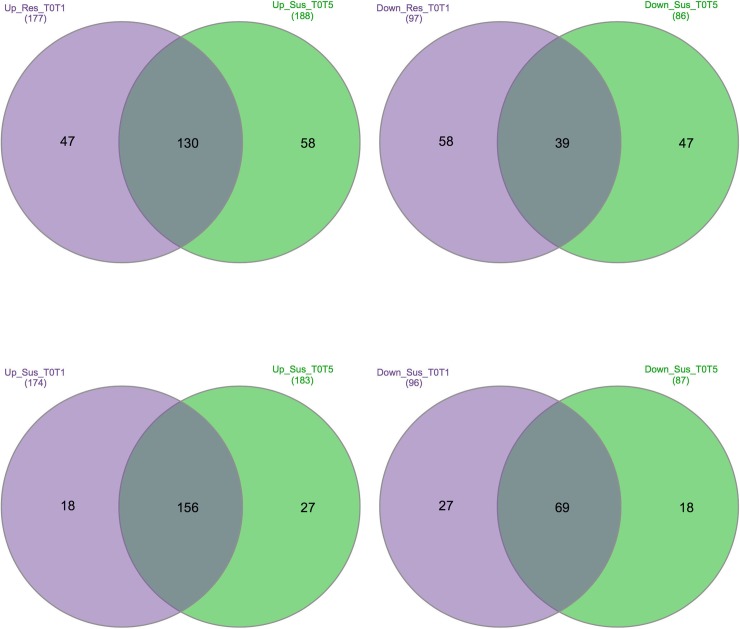
Gene expression patterns were also different with the extension of infestation time depending on the resistance/susceptibility of common bean cultivars. Among DEGs, approximately 44% and 37% of up-regulated genes were common among three time points, while less than 7% of down-regulated genes were shared among times in both cultivars. Interestingly, there was no any common up or down regulated gene between T0T1 (comparison of first and second time points) and T1T3 (comparison of second and third time points) and also any unique gene for T0T3 (comparison of first and third time points). As shown in the Venn diagram in Fig 2, the number of up-regulated DEGs in T0T3 comparison was higher than T0T1 comparison in both cultivars. This analysis indicated that more than 70% and 85% of DEGs were common between T0T1 and T0T3 comparisons, respectively.
Fig 2. Venn diagram representing specific and shared up- and down-regulated genes between two sets in each cultivar.
Pink circle (first set) represents the comparison of first and second time points and green (second set) is the comparison of first and third time points. Up and Down represent up-regulated, down-regulated genes. Res and Sus represent resistant and susceptible cultivars.
Functional classification and GO enrichment analysis of DEGs
To eliminate the effects of genetic differences, DEGs were compared between cultivars at the same time points. GO analysis detected 57, 39 and 46 categories, according to biological process (P), molecular function (F), and cellular component (C) among all up- and down-regulated genes at time 0, 1 and 3, respectively.
Secondary metabolism
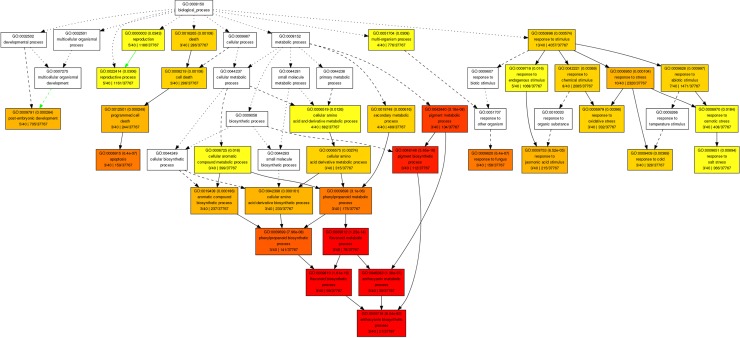
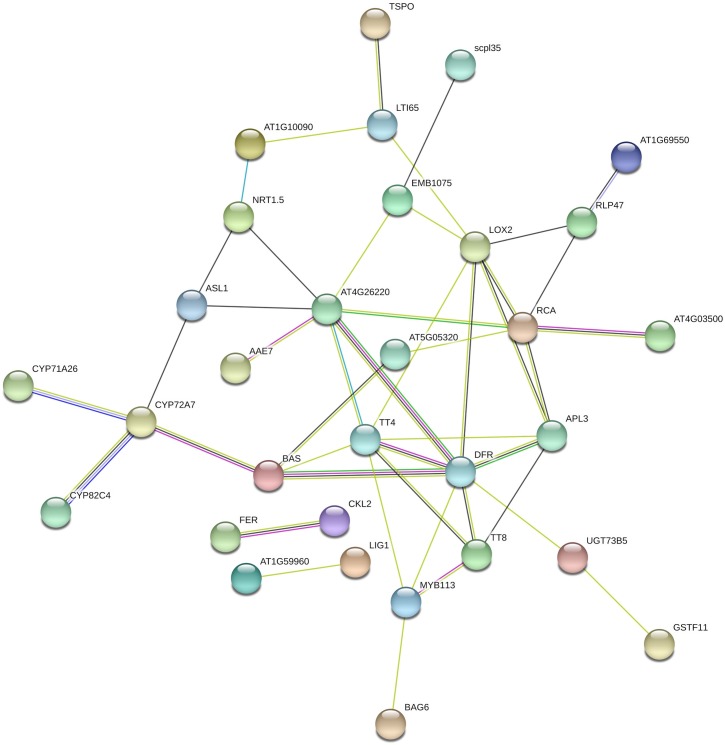
The differences between control samples was determined by the metabolism of phenylpropanoid, a central to produce defense-related compounds [24–26], including anthocyanins and flavonoids (Fig 3). The two most induced genes involved in flavonoid biosynthesis process corresponded to dihydroflavonol reductase (DFR) and chalcone synthase (CHS). We found transcripts of DFR which were up-regulated in resistant cultivar (Fig 4). DFR has been previously reported as induced by Fusarium oxysporum inoculations on Linum usitatissimum [27]. Colletotrichum camelliae on Camellia sinensis [28] and Elsinoe ampelina in grapevine [29]. DFR is also a key regulatory gene belonging to the subgroup of late anthocyanin biosynthesis genes which can be activated by TFs such as MYBs [30, 31]. CHS showed more than 7-fold change increase in resistant cultivar. O-methyltransferases (OMTs), involved in phenylpropanoids, flavonoids, and anthocyanin methylation, was up-regulated in resistant cultivar and showed an 8-fold increase in expression level upon spider mite feeding. O-Methylation plays key roles in plant defense following pathogen attack [32]. Additionally, the interaction network suggests the possible involvement of Cytochrome P450 (CYP) genes (CYP72A7 and CYP71A26) in resistance, previously reported in several studies [33, 34]. In the susceptible genotype, CYP83B1 gene required for the synthesis of indole glucosinolates, was down-regulated during infestation which is not consistent with previous reports in a number of researches [27, 35]. This result demonstrates the role of spider mite effectors in suppressing the defence-related gene of common bean in compatible interaction (susceptible cultivar) but not in an incompatible interaction (resistant cultivar). Previous investigation indicate when a pathogen interacts with plant tissues, more intensive transcriptional changes are found in the compatible interaction and pathogens commonly develope effectors that interfere with signaling pathways to suppress resistance responses. Finally, other secondary metabolism gene (BAS: beta-amyrin synthase) with higher transcript abundance in resistant cultivar was related to sesquiterpenoid and triterpenoid biosynthesis.
Fig 3. GO enrichment analysis for up-regulated genes in resistant cultivar as compared to susceptible cultivar at day 0 (control samples).
Boxes in the graph represent GO IDs, term definitions and statistical information. Significant GO terms (p ≤ 0.05) are marked with color. The degree of color saturation of a box is positively correlated to the enrichment level of the term.
Fig 4. Interaction networks of up-regulated genes identified in resistance cultivar as compared to susceptible cultivar at day 0 (control samples).
Transcriptional regulation
TF families found in our study are widely reported to be involved in plant defense responses, including MYB, WRKY, ethylene responsive factors (ERFs), zinc finger domain proteins and basic helix-loop-helix (bHLH). A key TF that currently appears in the studies of plant-pathogen interactions [27, 36] and had a significant expression in resistant cultivar of our study is MYB113. TRANSPARENT TESTA4 (TT4), a chalcone and stilbene synthase family protein, is a key enzyme involved in the biosynthesis of flavonoids to encode chalcone synthase (CHS), and is required for the accumulation of purple anthocyanins in leaves and stems [37]. TT4 along with TRANSPARENT TESTA8 (TT8), a bHLH DNA-binding superfamily protein which is required for normal expression of DFR [38] associated with MYB113 in the interaction network. An important candidate TF for spider mite resistance is WD40 protein, which was expressed only during mite infestation in the resistant cultivar. Many studies have shown that a conserved MYB-bHLH-WD40 (MBW) regulatory complex control the expression of anthocyanin biosynthesis genes [39]. So, we deduced that MBW regulatory complex has the same function in common bean and is involved in resistance. In addition, another TF for spider mite resistance may be TCP protein, whose expression was increased during spider mite infestation in the resistant cultivar. Recent studies suggested that TCP proteins play an important role in systemic acquired resistance (SAR) which is induced plant immunity, activated by pathogen infection [40–42]. Additionally, we identified a gene encoding WRKY50 which was significantly up-regulated in resistant cultivar during the middle-to-late stages (at day 5) of spider mite feeding. The spider mite infestation also affected the expression levels of Cys2/His2-type and C2H2-type zinc finger proteins as up-regulated genes in resistance and susceptible cultivars at day 5 post-infestation, respectively. The Cys2/His2-type zinc finger proteins are not only related to plant stress responses, but also enhance the resistance against pathogen infection [43]. ERFs, another important group of TFs, which play roles in integrating ET/JA signals [31, 44], activating the phenylpropanoid biosynthetic pathway and expression of resistance genes [45, 46], were activated in resistance cultivar at day 5 post-infestation. We observed increased transcript abundance of two key ERFs, including ERF1 and ERF9. The role of ERF1 as a regulator of ethylene responses after pathogen attack has been documented in Arabidopsis [47] but ERF9 has not proven to be relevant in the defense responses.
Hormone regulation
GO enrichment analysis showed other GO terms that significantly overrepresented among up-regulated genes of resistant cultivar, including “response to stress”, “response to stimulus” and “response to jasmonic acid”. JA signaling has closely been associated with defense mechanisms against pathogens and insects [48, 49]. In our study, 13S-lipoxygenase 2 (LOX2), a JA signaling and biogenesis gene, was detected as up-regulated gene in resistant cultivar, where it had a low transcript abundance in susceptible cultivar. Up-regulation of allene oxide synthase (AOS), needed to JA production, at day 1 post-feeding suggests that common bean resistance to the disease is enhanced by the activation of JA signaling pathways. Our result is corroborated with the previous studies where the expression levels of LOX and AOS significantly increased after infestation [27, 50, 51]. In addition, the expression of two auxin signaling pathway genes, SAUR (small auxin-up RNA) and ARF5 (Auxin response factor 5), were down- and up-regulated in resistant cultivar, respectively. SAUR genes are related to cell division[52, 53] and reportedly regulated by the auxin level, indicating that this process could be impaired by spider mite feeding. The down-regulation of SAUR gene is in agreement with the previous studies on A. thaliana [54] and soybean [55].
Pathogen elicitor perception
In our study, only one disease resistance protein from TIR-NBS-LRRs class (Phvul.005G093400.1) had higher transcript abundance in resistance cultivar, while one TIR-NBS-LRR (Phvul.010G029800.1), two NB-ARC domains-containing (Phvul.002G130666.1, Phvul.010G064700.1), and one CC-NBS-LRR (Phvul.003G247601.1) showed a high level of expression in susceptible cultivar before infestation. During the first stage of mite infestation, five TIR-NBS-LRRs (Phvul.002G323100.1, Phvul.002G323400.2, Phvul.004G046400.1, Phvul.011G140300.1 and Phvul.010G132433.1), three NB-ARC (Phvul.003G002926.1, Phvul.004G013300.1 and Phvul.008G071300.3) and one leucine-rich repeat (LRR) protein kinase (Phvul.007G087550.1) were highly expressed in resistance cultivar, whereas one TIR-NBS-LRR (Phvul.004G058700.1) and three NB-ARC (Phvul.008G031200.10, Phvul.010G064700.1, Phvul.011G195400.1) were found to be up-regulated in susceptible common bean cultivar. But these genes could not fully exert their expression with the extension of infestation time except one NB-ARC (Phvul.010G064700.1) in susceptible cultivar. At the first glance, it seems that the susceptible cultivar has a higher number of up-regulated disease resistance genes than the resistant one before mite infestation. But our results indicated that the response of the resistant plants was more robust than that of the susceptible cultivar upon pathogen attack. This can be elucidated by the role of miRNAs in down-regulating defense-related genes expression in susceptible cultivar [56, 57]. The non-specific defense responses to deter the pathogen can also explain loss of defense-related genes expression at fifth day.
In addition, one gene encoding the Cysteine-rich RLK (CRK10) was highly expressed upon spider feeding. This highly up-regulated CRK gene seems to indicate its potential role in resistance against spider mite. We also observed receptor-like proteins (RLPs) and receptor-like kinase (RLK) genes that have a direct effect on the pathogen in the both cultivars [58].
Antioxidant and detoxification processes
Reactive oxygen species (ROS) are involved in various processes along the plant life, but are best known as a key component of the signaling events involved in abiotic and biotic stress responses, so that are rapidly induced and accumulated after pathogen attack [51, 59]. An important response to control ROS is the induction of scavenging genes. In this respect, heat shock proteins (HSPs) play an important role in supporting ROS scavenging activity and stress tolerance [60]. In our study, HSP 70 was found to be up-regulated during spider mite feeding only in resistant cultivar, which highlight the function of HSPs in plant defense against pathogenic infection and reduce accumulation of ROS. This notion can be supported by the P. vulgaris-Colletotrichum interaction study [61], that HSPs are highly expressed against Colletotrichum lindemuthianum infection. Among detoxification genes, UDP-glycosyltransferase significantly up-regulated in resistant cultivar at all-time points, suggesting this gene may play an important role in the common bean resistance to spider mite feeding. The previous studies conducted on nematode attack in wheat [62] and Fusarium in Brachypodium distachyon [63] reinforces our argument about UDP-glycosyltransferase function.
Cell wall
Xyloglucan endotransglucosylase/hydrolases (XTHs) are a family of enzymes that facilitate cell wall expansion [64] and also have the functions, probably associated with resistance mechanism [65]. In current study, we observed two XTHs (XTH22: Phvul.003G147700 and XTH9: Phvul.005G111300) in resistant cultivar significantly expressed at day 1 post infestation. Another important candidate gene for spider mite resistance may be the malectin-like receptor kinase FERONIA (FER), which was up-regulated and showed increase abundance during mite infestation, although there is no significant information on the role of this gene in response to feeding.
Other genes
There are substantial reports regarding expression of pathogenesis-related (PR) genes under numerous stresses in common bean [61, 66]. Our transcriptome study successfully identified one PR-5 like receptor kinase (Phvul.004G155500) as up-regulated gene in resistant cultivar at day 5, which has been implicated in plant disease resistance, induced by different pathgens and share significant sequence similarity in many species [61, 66] 2-oxoglutarate (2OG) and Fe (II)-dependent oxygenase is another up-regulated gene in resistance cultivar under infestation which make it a potential candidate gene for resistance against spider mite and probably suitable for breeding programs. This gene has been previously described as responsive to pathogens [27].
KEGG pathway functional enrichment analysis of the DEGs
Based on the KEGG pathway enrichment analysis, flavonoid biosynthesis, biosynthesis of secondary metabolites, metabolic pathways, phenylpropanoid biosynthesis and Linoleic acid metabolism were found to be the most changed pathways (S6–S8 Tables). In this assay it was demonstrated that the metabolic pathway biosynthesis of secondary metabolites contained the largest number of DEGs. From the results, we found that KEGG pathways, such as plant–pathogen interaction, plant hormone signaling transduction, and glucosinolate biosynthesis pathways play important roles in defence responses of common bean to spider mite.
Validation of DEGs by using qRT-PCR
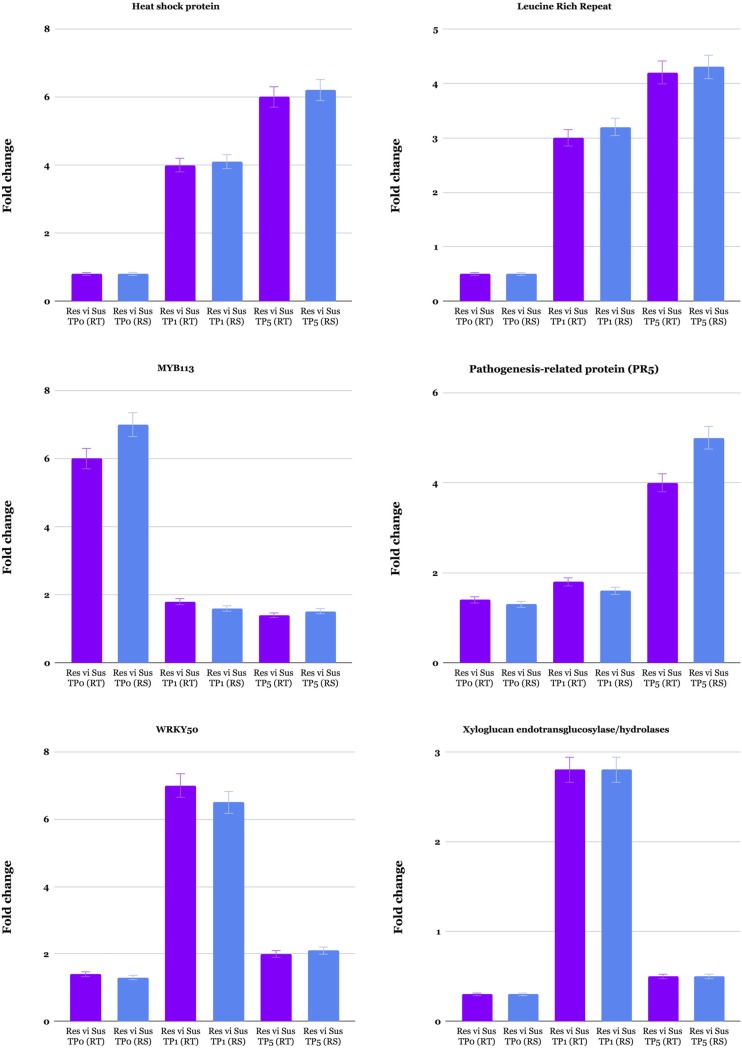
In order to verify gene expression results of transcriptome data analysis, six DEGs having annotations were selected for qRT-PCR analysis. They include the genes encoding pathogenesis-related proteins PR5, heat shock protein, leucine rich repeat, MYB113, XTH and a WRKY50 (Fig 5). Quantitative RT-PCR analysis was conducted on 12 RNA samples that were used in the preparation of sequencing libraries. Relative expression profiles of DEGs in the both resistant and susceptible evaluated using qRT-PCR were in complete agreement with the RNA-Seq data. This is in line with other studies, which showed almost the same level of fold changes between RNA-Seq data and qPCR [67, 68].
Fig 5. qRT-PCR results of genes selected from the RNA-Seq analysis of common bean–spider mite interaction.
Expression levels of tested genes were normalized based on of Actin gene and then compared to relative expression values determined by RNA-Seq. Relative expression values of samples were determined by using the average expression value of all replicates of a particular group. Standard deviation among replicates is represented by error bars. Res and Sus represent resistant and susceptible cultivars. TP0, TP1 and TP5 represent first, second and third time points. RT ans RS in parentheses represent qRT-PCR and RNA-Seq.
Conclusion
To our knowledge, this investigation is the first study to identify molecular mechanisms involved in the common bean resistance to spider mite feeding by using RNA sequencing technology. In summary, DEGs were identified for control samples,1 and 5 days after infestation of spider mite in resistant and susceptible cultivars of common bean. Importantly, we identified secondary metabolism, multiple disease resistance proteins, TFs and genes involved in cell wall expansion and antioxidant processes that were modulated by spider mite attack. Overall, this study extended our understanding of the defense molecular mechanisms of two common bean cultivars with different genetic backgrounds during spider mite infestation. We came to the conclusion that these data provide important and valuable information for future research in common bean.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability
All RNA-Seq data were deposited to the NCBI sequence read archive (SRA) under the project PRJNA482175.
Funding Statement
The authors received no specific funding for this work.
References
- 1.McConnell M, Mamidi S, Lee R, Chikara S, Rossi M, Papa R, et al. Syntenic relationships among legumes revealed using a gene-based genetic linkage map of common bean (Phaseolus vulgaris L.). TAG Theoretical and applied genetics Theoretische und angewandte Genetik. 2010;121:1103–16. 10.1007/s00122-010-1375-9 [DOI] [PubMed] [Google Scholar]
- 2.Castro-Guerrero NA, Isidra-Arellano MC, Mendoza-Cozatl DG, Valdés-López O. Common Bean: A Legume Model on the Rise for Unraveling Responses and Adaptations to Iron, Zinc, and Phosphate Deficiencies. Front Plant Sci. 2016;7:600–. 10.3389/fpls.2016.00600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broughton WJ, Hernández G, Blair M, Beebe S, Gepts P, Vanderleyden J. Beans (Phaseolus spp.)–model food legumes. Plant and Soil. 2003;252(1):55–128. 10.1023/A:1024146710611 [DOI] [Google Scholar]
- 4.Tiwari M, Singh NK, Rathore M, Kumar N. RAPD markers in the analysis of genetic diversity among common bean germplasm from Central Himalaya. Genetic Resources and Crop Evolution. 2005;52(3):315–24. 10.1007/s10722-005-5123-y [DOI] [Google Scholar]
- 5.Ghasemi Pirbalouti A, Golparvar G, Ali Rostampoor S. Evaluation of Seed Yield and Yield Components of Common Bean Iranian Cultivars for Inoculation with Four Strains of Rhizobium legominosarum biovar phaseoli. Journal of Agronomy. 2006;5 10.3923/ja.2006.382.386 [DOI] [Google Scholar]
- 6.Katungi E, Sperling L, Karanja D, Farrow A, Beebe S. Relative importance of common bean attributes and variety demand in the drought area of Kenya. J Dev Agric Econ. 2011;3. [Google Scholar]
- 7.Capinera JL. Greenhouse Whitefly, Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) In: Capinera JL, editor. Encyclopedia of Entomology. Dordrecht: Springer Netherlands; 2008. p. 1723–6. [Google Scholar]
- 8.Bensoussan N, Santamaria ME, Zhurov V, Diaz I, Grbić M, Grbić V. Plant-Herbivore Interaction: Dissection of the Cellular Pattern of Tetranychus urticae Feeding on the Host Plant. Front Plant Sci. 2016;7:1105–. 10.3389/fpls.2016.01105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiełkiewicz M, Barczak-Brzyżek A, Karpińska B, Filipecki M. Unravelling the Complexity of Plant Defense Induced by a Simultaneous and Sequential Mite and Aphid Infestation. Int J Mol Sci. 2019;20(4):806 10.3390/ijms20040806 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochemistry and Molecular Biology. 2010;40(8):563–72. 10.1016/j.ibmb.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 11.Sharma HC, Sharma K, Seetharama N, Ortiz R. Prospects for using transgenic resistance to insects in crop improvement. Electronic Journal of Biotechnology. 2000;3:21–2. 10.4067/S0717-34582000000200001 [DOI] [Google Scholar]
- 12.Smith C. Plant Resistance to Arthropods: Molecular and Conventional Approaches 2005. 1–423 p. [Google Scholar]
- 13.Shoorooei M, Hoseinzadeh AH, Maali-Amiri R, Allahyari H, Torkzadeh-Mahani M. Antixenosis and antibiosis response of common bean (Phaseolus vulgaris) to two-spotted spider mite (Tetranychus urticae). Experimental and Applied Acarology. 2018;74(4):365–81. 10.1007/s10493-018-0240-4 [DOI] [PubMed] [Google Scholar]
- 14.Meier U. Growth stages of mono- and dicotyledonous plants: Berlin: Blackwell Wissenschafts-Verlag; 1997. [Google Scholar]
- 15.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics (Oxford, England). 2014;30(15):2114–20. Epub 2014/04/01. 10.1093/bioinformatics/btu170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmutz J, McClean PE, Mamidi S, Wu GA, Cannon SB, Grimwood J, et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nature Genetics. 2014;46:707 10.1038/ng.3008 https://www.nature.com/articles/ng.3008#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grbić M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature. 2011;479:487 10.1038/nature10640 https://www.nature.com/articles/nature10640#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29(1):15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–78. 10.1038/nprot.2012.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S, Chan C-KK. Analysis of RNA-Seq Data Using TopHat and Cufflinks In: Edwards D, editor. Plant Bioinformatics: Methods and Protocols. New York, NY: Springer New York; 2016. p. 339–61. [DOI] [PubMed] [Google Scholar]
- 21.Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38(Web Server issue):W64–W70. Epub 2010/04/30. 10.1093/nar/gkq310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Untergasser A, Cutcutache I, Koressaar T, Ye J, C Faircloth B, Remm M, et al. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012;40:e115 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36–e. 10.1093/nar/30.9.e36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrer JL, Austin MB, Stewart C, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiology and Biochemistry. 2008;46(3):356–70. 10.1016/j.plaphy.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lea US, Slimestad R, Smedvig P, Lillo C. Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta. 2007;225(5):1245–53. 10.1007/s00425-006-0414-x [DOI] [PubMed] [Google Scholar]
- 26.Kerio LC. The functional significance of anthocyanins in leaves: a review. Tea. 2011;32(1):46–52. [Google Scholar]
- 27.Galindo-González L, Deyholos MK. RNA-seq Transcriptome Response of Flax (Linum usitatissimum L.) to the Pathogenic Fungus Fusarium oxysporum f. sp. lini. Front Plant Sci. 2016;7:1766–. 10.3389/fpls.2016.01766 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao M, Wang Q, Wan R, Fei Z, Wang X. Identification of genes differentially expressed in grapevine associated with resistance to Elsinoe ampelina through suppressive subtraction hybridization. Plant Physiology and Biochemistry. 2012;58:253–68. 10.1016/j.plaphy.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Wang Y, Cao H, Hao X, Zeng J, Yang Y, et al. Transcriptome Analysis of an Anthracnose-Resistant Tea Plant Cultivar Reveals Genes Associated with Resistance to Colletotrichum camelliae. PLoS One. 2016;11(2):e0148535–e. 10.1371/journal.pone.0148535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petroni K, Tonelli C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Science. 2011;181(3):219–29. 10.1016/j.plantsci.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Wang J, Zhang X, Xu L. Comparative transcriptome analysis of Anthurium "Albama" and its anthocyanin-loss mutant. PLoS One. 2015;10(3):e0119027–e. 10.1371/journal.pone.0119027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.C Lam K, Ibrahim R, Behdad B, Dayanandan S. Structure, function, and evolution of plant O -methyltransferases. Genome / National Research Council Canada = Génome / Conseil national de recherches Canada. 2007;50:1001–13. 10.1139/g07-077 [DOI] [PubMed] [Google Scholar]
- 33.Grubor VD, Heckel DG. Evaluation of the role of CYP6B cytochrome P450s in pyrethroid resistant Australian Helicoverpa armigera. Insect Molecular Biology. 2007;16(1):15–23. 10.1111/j.1365-2583.2006.00697.x [DOI] [PubMed] [Google Scholar]
- 34.Cifuentes D, Chynoweth R, Guillén J, De la Rua P, Bielza P. Novel Cytochrome P450 Genes, CYP6EB1 and CYP6EC1, are Over-Expressed in Acrinathrin-Resistant Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Economic Entomology. 2012;105 10.1603/EC11335 [DOI] [PubMed] [Google Scholar]
- 35.Becker M, Zhang X, Walker P, Wan J, Millar J, Khan D, et al. Transcriptome analysis of the Brassica napus—Leptosphaeria maculans pathosystem identifies receptor, signalling and structural genes underlying plant resistance. The Plant journal: for cell and molecular biology. 2017;90 10.1111/tpj.13514 [DOI] [PubMed] [Google Scholar]
- 36.Zhu Q-H, Stephen S, Kazan K, Jin G, Fan L, Taylor J, et al. Characterization of the defense transcriptome responsive to Fusarium oxysporum-infection in Arabidopsis using RNA-seq. Gene. 2013;512(2):259–66. 10.1016/j.gene.2012.10.036 [DOI] [PubMed] [Google Scholar]
- 37.Saslowsky D, D. Dana C, Winkel B. An allelic series for the chalcone synthase locus in Arabidopsis. Gene. 2000;255:127–38. 10.1016/s0378-1119(00)00304-8 [DOI] [PubMed] [Google Scholar]
- 38.Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell. 2000;12(10):1863–78. 10.1105/tpc.12.10.1863 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y, Tan H, Ma Z, Huang J. DELLA Proteins Promote Anthocyanin Biosynthesis via Sequestering MYBL2 and JAZ Suppressors of the MYB/bHLH/WD40 Complex in Arabidopsis thaliana. Molecular Plant. 2016;9(5):711–21. 10.1016/j.molp.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 40.Mukhtar MS, Carvunis A-R, Dreze M, Epple P, Steinbrenner J, Moore J, et al. Independently Evolved Virulence Effectors Converge onto Hubs in a Plant Immune System Network. 2011;333(6042):596–601. 10.1126/science.1203659%JScience [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu ZQ, Dong X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. 2013;64(1):839–63. 10.1146/annurev-arplant-042811-105606 . [DOI] [PubMed] [Google Scholar]
- 42.Weßling R, Epple P, Altmann S, He Y, Yang L, Henz Stefan R, et al. Convergent Targeting of a Common Host Protein-Network by Pathogen Effectors from Three Kingdoms of Life. Cell Host & Microbe. 2014;16(3):364–75. 10.1016/j.chom.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SH, Hong JK, Lee SC, Sohn KH, Jung HW, Hwang BK. CAZFP1, Cys2/His2-type zinc-finger transcription factor gene functions as a pathogen-induced early-defense gene in Capsicum annuum. Plant Molecular Biology. 2004;55(6):883–904. 10.1007/s11103-004-2151-5 [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Shao J, Zhou Z, Davis RE. Comparative Transcriptome Analysis Reveals a Preformed Defense System in Apple Root of a Resistant Genotype of G.935 in the Absence of Pathogen. Int J Plant Genomics. 2017;2017:8950746–. Epub 2017/03/30. 10.1155/2017/8950746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends in Plant Science. 2010;15(5):247–58. 10.1016/j.tplants.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 46.De Geyter N, Gholami A, Goormachtig S, Goossens A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends in Plant Science. 2012;17(6):349–59. 10.1016/j.tplants.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 47.Berrocal-Lobo M, Molina A. Ethylene Response Factor 1 Mediates Arabidopsis Resistance to the Soilborne Fungus Fusarium oxysporum. 2004;17(7):763–70. 10.1094/mpmi.2004.17.7.763 . [DOI] [PubMed] [Google Scholar]
- 48.Guerreiro A, Figueiredo J, Sousa Silva M, Figueiredo A. Linking Jasmonic Acid to Grapevine Resistance against the Biotrophic Oomycete Plasmopara viticola. Front Plant Sci. 2016;7:565–. 10.3389/fpls.2016.00565 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W-W, Zheng C, Hao W-J, Ma C-L, Ma J-Q, Ni D-J, et al. Transcriptome and metabolome analysis reveal candidate genes and biochemicals involved in tea geometrid defense in Camellia sinensis. PLoS One. 2018;13(8):e0201670–e. 10.1371/journal.pone.0201670 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen T, Lv Y, Zhao T, Li N, Yang Y, Yu W, et al. Comparative transcriptome profiling of a resistant vs. susceptible tomato (Solanum lycopersicum) cultivar in response to infection by tomato yellow leaf curl virus. PLoS One. 2013;8(11):e80816–e. 10.1371/journal.pone.0080816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agut B, Pastor V, Jaques JA, Flors V. Can Plant Defence Mechanisms Provide New Approaches for the Sustainable Control of the Two-Spotted Spider Mite Tetranychus urticae? Int J Mol Sci. 2018;19(2):614 10.3390/ijms19020614 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spartz AK, Lee SH, Wenger JP, Gonzalez N, Itoh H, Inzé D, et al. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. The Plant Journal. 2012;70(6):978–90. 10.1111/j.1365-313X.2012.04946.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markakis MN, Boron AK, Van Loock B, Saini K, Cirera S, Verbelen J-P, et al. Characterization of a small auxin-up RNA (SAUR)-like gene involved in Arabidopsis thaliana development. PLoS One. 2013;8(11):e82596–e. 10.1371/journal.pone.0082596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen YC, Wong CL, Muzzi F, Vlaardingerbroek I, Kidd BN, Schenk PM. Root defense analysis against Fusarium oxysporum reveals new regulators to confer resistance. Scientific Reports. 2014;4:5584 10.1038/srep05584 https://www.nature.com/articles/srep05584#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ban Y-W, Samir Roy N, Yang H, Choi H-K, Kim J-H, Basnet P, et al. Comparative transcriptome analysis reveals higher expression of stress and defense responsive genes in dwarf soybeans obtained from the crossing of G. max and G. soja2018. [DOI] [PubMed]
- 56.Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, et al. A Plant miRNA Contributes to Antibacterial Resistance by Repressing Auxin Signaling. 2006;312(5772):436–9. 10.1126/science.1126088%JScience [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Zhang Y, Shi D, Liu X, Qin J, Ge Q, et al. Spatial-temporal analysis of zinc homeostasis reveals the response mechanisms to acute zinc deficiency in Sorghum bicolor. New Phytologist. 2013;200(4):1102–15. 10.1111/nph.12434 [DOI] [PubMed] [Google Scholar]
- 58.Gouveia BC, Calil IP, Machado JPB, Santos AA, Fontes EPB. Immune Receptors and Co-receptors in Antiviral Innate Immunity in Plants. 2017;7(2139). 10.3389/fmicb.2016.02139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mhamdi A, Van Breusegem F. Reactive oxygen species in plant development. 2018;145(15):dev164376 10.1242/dev.164376 [DOI] [PubMed] [Google Scholar]
- 60.Driedonks N, Xu J, Peters JL, Park S, Rieu I. Multi-Level Interactions Between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front Plant Sci. 2015;6:999–. 10.3389/fpls.2015.00999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Padder BA, Kamfwa K, Awale HE, Kelly JD. Transcriptome Profiling of the Phaseolus vulgaris—Colletotrichum lindemuthianum Pathosystem. PLoS One. 2016;11(11):e0165823 10.1371/journal.pone.0165823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao F, Kong L-A, Peng H, Huang W-K, Wu D-Q, Liu S-M, et al. Transcriptional profiling of wheat (Triticum aestivum L.) during a compatible interaction with the cereal cyst nematode Heterodera avenae. Scientific Reports. 2019;9(1):2184 10.1038/s41598-018-37824-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schweiger W, Pasquet J-C, Nussbaumer T, Paris MPK, Wiesenberger G, Macadré C, et al. Functional characterization of two clusters of Brachypodium distachyon UDP-glycosyltransferases encoding putative deoxynivalenol detoxification genes. Molecular Plant-Microbe Interactions. 2013;26(7):781–92. 10.1094/MPMI-08-12-0205-R [DOI] [PubMed] [Google Scholar]
- 64.Sasidharan R, Voesenek LACJ, Pierik R. Cell Wall Modifying Proteins Mediate Plant Acclimatization to Biotic and Abiotic Stresses. Critical Reviews in Plant Sciences. 2011;30(6):548–62. 10.1080/07352689.2011.615706 [DOI] [Google Scholar]
- 65.Jiang Y, Dong J, Chen R, Gao X, Xu Z. Isolation of a novel PP2C gene from rice and its response to abiotic stresses. African Journal of Biotechnology. 2011;10:7143–54. [Google Scholar]
- 66.Jain S, Chittem K, Brueggeman R, Osorno JM, Richards J, Nelson BD Jr. Comparative Transcriptome Analysis of Resistant and Susceptible Common Bean Genotypes in Response to Soybean Cyst Nematode Infection. PLoS One. 2016;11(7):e0159338 10.1371/journal.pone.0159338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, Xu Y, Zhang L, Li W, Cai Z, Li F, et al. De novo assembly and analysis of the transcriptome of Rumex patientia L. during cold stress. PLoS One. 2017;12(10):e0186470–e. 10.1371/journal.pone.0186470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musacchia F, Vasilev F, Borra M, Biffali E, Sanges R, Santella L, et al. De novo assembly of a transcriptome from the eggs and early embryos of Astropecten aranciacus. PLoS One. 2017;12(9):e0184090–e. 10.1371/journal.pone.0184090 . [DOI] [PMC free article] [PubMed] [Google Scholar]