Abstract
Background
Several studies have examined the Controlling Nutritional Status (CONUT) score, which is a screening tool for nutritional status and an effective biomarker for patient survival after cancer treatment. However, its role in soft-tissue sarcoma (STS) remains unknown. Because of the lack of predictive markers for survival in patients with STS, we aimed to determine the CONUT score’s association with survival.
Questions/purposes
(1) Is there a relationship between the CONUT score and clinicopathologic characteristics such as tumor size, tumor location, pathological grade, and advanced stage based on the American Joint Committee on Cancer (AJCC) guidelines? (2) Is the CONUT score associated with disease-free survival (DFS) and overall survival (OS) in patients treated surgically for STS, even when compared with other systemic inflammatory response markers?
Methods
Between 1999 and 2016, 769 patients underwent R0 resection for STS at our institution. Adequate medical records and available followup data were required for inclusion in this study. Exclusion criteria were synchronous inflammatory diseases, unplanned excision, and neoadjuvant therapy. There were 658 patients (86%) who fulfilled all criteria. The minimum followup time was 24 months (median, 103 months; range, 61-147 months). The median age of the patients was 43 years (range, 5-85 years), and 265 patients (40%) were women. All patients had Stage I to IV tumors according to the 8th edition of the AJCC staging system. The grade classification was determined to be G1 in 130 patients (20%), G2 in 304 (46%), and G3 in 201 (31%). The CONUT score was calculated based on the serum albumin concentration, total peripheral lymphocyte count, and total cholesterol concentration. The score ranged from 0 to 12, with higher scores indicating worse nutritional status. The patients were classified into two groups according to a receiver operating characteristic curve analysis: the high (≥ 2) and low (0 or 1) CONUT score groups. There were 435 patients in the low CONUT score group and 223 in the high CONUT score group. We tested for an association between the CONUT scores and gender, age, tumor diameter, tumor depth, tumor grade, and AJCC stage using the chi-square and Fisher’s exact methods. We also compared the strength of the association between postoperative survival and the CONUT scores, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) using multivariate Cox proportional hazard model analyses.
Results
High CONUT scores were associated with large tumor size (odds ratio [OR], 1.47; 95% CI, 1.06-2.04; p = 0.020), deep tumor location (OR, 1.66; 95% CI, 1.17-2.36; p = 0.004), high tumor grade (OR, 2.54; 95% CI, 1.56-4.14; p = 0.001), and advanced AJCC stage (OR, 1.86; 95% CI, 1.14-3.02; p < 0.001). The low CONUT score group exhibited a higher 5-year OS rate and longer OS than the high CONUT score group (82% versus 65%; odds ratio, 2.45; 95% CI, 1.27-4.72; p < 0.001; 81 versus 64 months, Z = -2.56; p < 0.001). A multivariate analysis indicated that an elevated CONUT score was an independent predictor of OS (hazard ratio [HR], 1.86; 95% CI, 1.47-4.14; p < 0.001) and DFS (HR, 1.63; 95% CI, 1.26-2.11; p < 0.001), but the NLR and PLR were not. In an individual subgroup analysis, the CONUT scores were associated with OS and DFS in the tumor diameter (< 5 or ≥ 5 cm) subgroup, tumor depth (superficial or deep) subgroup, tumor grade (G1 and G2) subgroup, and AJCC stage (I/II or III/IV) subgroup, but not in the G3 subgroup (p = 0.051 and p = 0.065).
Conclusion
High CONUT scores were independently associated with aggressive tumor behavior and unfavorable survival for patients with low-grade, but not high-grade, resected STS. If these findings can be substantiated in larger studies, the CONUT score might be useful for predicting survival and help to develop new treatment strategies for nutrition interventions.
Level of Evidence
Level III, therapeutic study.
Introduction
Soft-tissue sarcomas (STS) are rare and heterogeneous malignant neoplasms originating from mesenchymal cells [15]. Surgical resection or amputation is the cornerstone treatment of local or regional STS, and chemotherapy and radiation therapy are widely used as adjuvant and palliative approaches [4]. Despite improvements in the diagnosis and treatment of STS, the survival of these patients remains poor, especially for those with high-grade sarcomas [3, 10]. As such, it is important to find readily available clinical tools, aside from the operative and pathologic ones, to improve the patient’s prognosis and better plan individualized treatment strategies.
Some inflammation- and nutrition-related predictive factors, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), high-sensitivity modified Glasgow prognostic score, and prognostic nutritional index have been proposed as promising prognostic factors in patients with various malignancies [7, 16, 20, 22, 29, 30]. The Controlling Nutritional Status (CONUT) score, which is calculated based on the serum albumin concentration, total peripheral lymphocyte count, and total cholesterol concentration, was developed as a screening tool for the early detection of poor nutritional status [18, 23]. Serum albumin, as a chronic-phase protein, is currently considered an indicator of nutritional and inflammatory status. A low serum albumin level has been found to reduce the patient’s tolerance of treatment toxicities, subsequently resulting in a poorer prognosis [14, 17]. Lymphocytes have a key role in the immune response to tumor defense by inducing cytotoxic cell death and inhibiting tumor cell proliferation and migration [5, 6], reflecting the homeostasis between cancer progression and antitumor activity [19, 28]. As an essential component of cell membranes, total cholesterol is involved in various intracellular metabolism pathways and is regarded as an indicator of a patient’s caloric reserves [2]. Thus, the CONUT score could reflect not only the nutritional status, but also the systemic inflammation and immune response [11]. Recently, the CONUT score has been reported to be a simple and convenient tool to predict the survival of patients with esophageal [26], pancreatic [12], gastric [13], and hepatocellular cancer [8]. However, to our knowledge, the association between clinicopathologic features and the CONUT score as well as the potential importance of this score with respect to survival in patients with STS has not yet been reported.
We therefore asked the following questions: (1) Is there a relationship between the CONUT score and clinicopathologic characteristics such as tumor size, tumor location, pathological grade, and advanced stage based on the American Joint Committee on Cancer (AJCC) guidelines? (2) Is the CONUT score associated with disease-free survival (DFS) and overall survival (OS) in patients treated surgically for STS, even when compared with other systemic inflammatory response markers?
Patients and Methods
Patients and Tumor Characteristics
Between January 1999 and July 2016, 769 patients underwent R0 resection for STS at our institution. R0 was defined as the microscopic absence of malignant cells at the resection margin. Seventy-seven of the 769 patients (10%) were lost to followup. Patients with deficient medical records (five patients) and synchronous inflammatory diseases (five patients with active hepatitis, Crohn's disease, and primary sclerosing cholangitis) were excluded. Other patients were excluded because they had neoadjuvant therapy (13 patients) or additional resection after previously undergoing an unplanned excision (11 patients). The minimum followup time was 24 months (median, 103 months; range, 61-147 months). No patients were recalled specifically for this study, and all data were obtained from medical records. Finally, 658 patients were analyzed (Fig. 1).
Fig. 1.
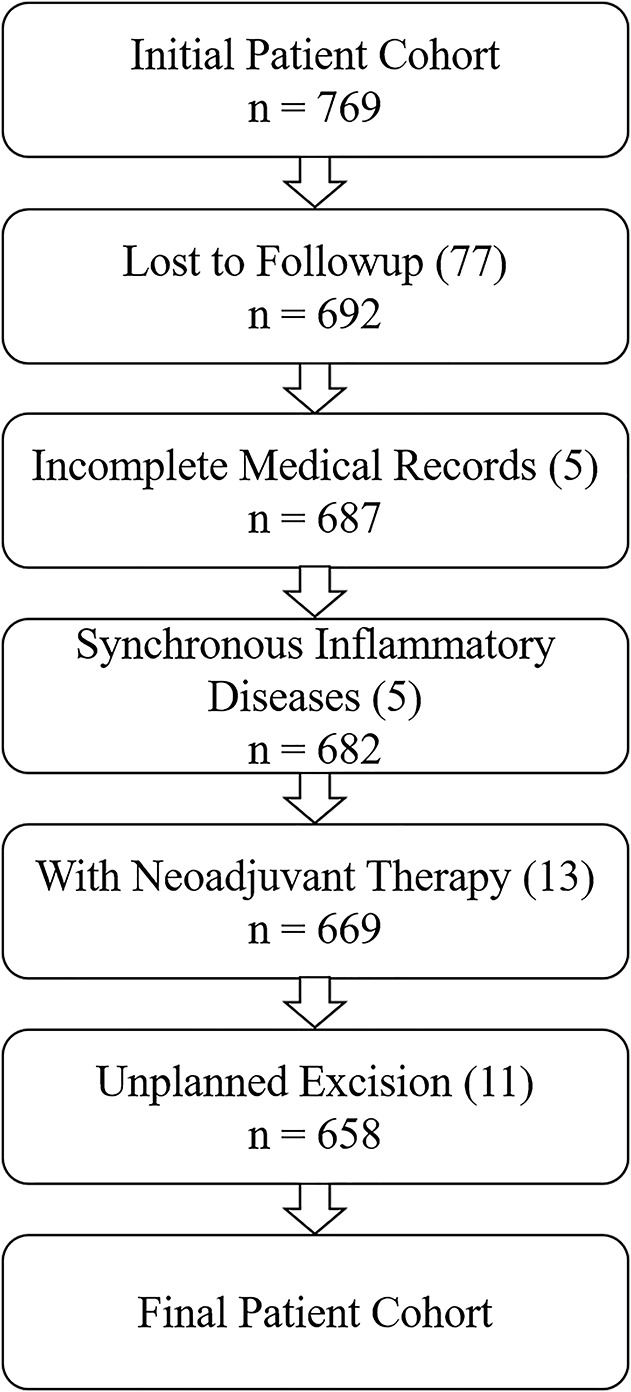
This flowchart shows the exclusion criteria for our patient cohort.
This study was approved by the institutional review board of our institution, the ethics committee decided that obtaining informed consent was unnecessary, and all information was anonymous.
The median age of the patients was 43 years (range, 5-85 years), and there were 393 men and 265 women in a 1.5 to 1 ratio. The different pathologic tumor subtypes were undifferentiated pleomorphic sarcoma (n = 149, 23%), fibrosarcoma (n = 151, 23%), liposarcoma (n = 91, 14%), and synovial sarcoma (n = 77, 12%). The tumor depth was defined as superficial in 235 patients and deep in 423. The grade classification was determined as G1, G2, and G3 in 130 (20%), 304 (46%), and 201 (31%) patients, respectively, according to the French Federation of Cancer Centers Sarcoma Group’s grading system [21]. The clinical stage was determined based on the recommendations of the AJCC, 8th Edition [1]. Most sarcomas were Stage II or III (in 233 [35%] and 207 [32%] patients, respectively), and 133 (20%) and 12 (2%) patients had Stages I and IV disease, respectively. The stage of intraabdominal disease was classified as unknown because a staging system for intraabdominal sarcomas is unavailable. Ninety-six (15%) patients received an adjuvant (postoperative) therapy, including chemotherapy (mostly doxorubicin-based combination chemotherapy), radiotherapy, or chemoradiotherapy. Two hundred fifty (38%) patients had local relapse, 94 (14%) had a metastasis, and 174 (26%) died at the last followup. For the entire cohort, the 5-year OS and DFS rates were 76.6% and 57.4%, respectively (Table 1).
Clinical Data Collection and the CONUT Score
Laboratory data including the total lymphocyte, neutrophil, and platelet counts; total cholesterol; and other basic hematologic parameters were obtained within 1 week before surgical resection. Clinical information such as age at the time of surgery, treatment plan, and histopathologic diagnosis was collected from our electronic medical record system. The NLR was obtained by dividing the neutrophil count by the lymphocyte count. The PLR was obtained by dividing the platelet count by the lymphocyte count. The high-sensitivity modified Glasgow prognostic score was determined as previously described [24]. The CONUT score was also calculated (see Table, Supplemental Digital Content 1, http://links.lww.com/CORR/A184) [11]. The score ranges from 0 to 12, with higher scores indicating a worse nutritional status. The CONUT score was 0, 1, and 2-12 in 227 (35%), 208 (32%), and 223 (34%) participants, respectively (Table 1).
Determination of Cutoff Values
The most-appropriate cutoff value was 2.51 for the NLR, 164.5 for the PLR, and 2 for the CONUT score in a receiver operating characteristic curve analysis. All patients were categorized into the high CONUT score group (score ≥ 2, n = 223, 34%) or low CONUT score group (score = 0 or 1, n = 435, 66%).
Patient Followup
All patients were routinely examined every 3-4 months in the first 2 years after surgery, every 6 months for the next 3 years, and annually thereafter via outpatient visits or telephone interviews via an independent followup program. The final survival followup time was considered the latest followup date of this study (July 1, 2018) or death. OS was defined as the time between the initial surgery and death of any cause or the last followup, while DFS was defined as the time from the initial surgery until recurrence or metastasis.
Statistical Analysis
General characteristics are expressed as a number (%), and variables for each group were compared using the chi-square test, Fisher’s exact test for categorical data, or the Mann-Whitney U test for continuous variables. The optimal cutoff point was determined by the maximum Youden index, based on the receiver operating characteristic curve analysis. Survival curves were analyzed with the Kaplan-Meier method and were compared using the log-rank test. The correlation between tumor size, tumor depth, tumor grade, AJCC stage, NLR, PLR, and the CONUT score and DFS and OS were evaluated with univariate and multivariate analyses. Prognostic variables associated with OS and DFS that were significant in the univariate analyses were selected for multivariate Cox proportional hazard model analyses using the forward stepwise method. Hazard ratios (HRs) estimated from the Cox analysis were reported as relative risks with their corresponding 95% CIs. A p value less than 0.05 was considered statistically significant. All analyses and visualizations were performed using the SPSS software, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Correlation Between the CONUT Score and Clinicopathologic Characteristics
A high CONUT score was associated with a large tumor size (OR, 1.47; 95% CI, 1.06-2.04; p = 0.020), deep tumor location (OR, 1.66; 95% CI, 1.17-2.36; p = 0.004), high tumor grade (OR, 2.54; 95% CI, 1.56-4.14; p = 0.001), advanced AJCC stage (OR, 1.86; 95% CI, 1.14-3.02; p < 0.001), elevated NLR (OR, 5.48; 95% CI, 3.38-7.83; p < 0.001), elevated PLR (OR, 6.67; 95% CI, 4.44-10.02; p < 0.001), and worse survival (p < 0.001) (Table 2).
Survival Analysis
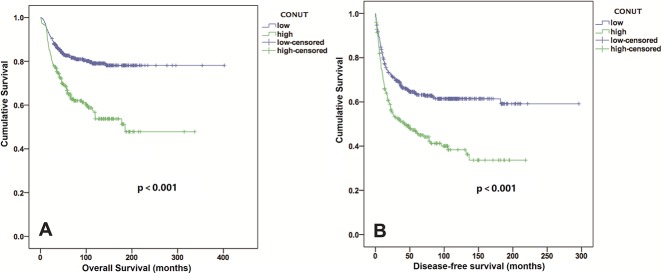
The low CONUT score group exhibited a higher 5-year OS rate and longer OS than the high CONUT score group did (82% versus 65%; OR, 2.45; 95% CI, 1.27-4.72; p = 0.006; 81 versus 64 months; Z = -2.56; p < 0.001) (Fig. 2A). After controlling for potentially confounding variables such as gender, age, and tumor size, we found that the CONUT scores (HR, 1.86; 95% CI,1.32-2.61; p < 0.001) were independently associated with OS. In addition, the tumor depth, tumor grade, and AJCC stage were identified as independent predictors of OS, but the NLR and PLR were not (Table 3). Patients with low CONUT scores had a median DFS period of 48.5 months, whereas those with high CONUT scores had a median DFS period of 24.1 months (Fig. 2B). The incidence of local recurrence or metastatic disease in the low CONUT score group (158 of 435 patients, 36%) was lower than that in the high CONUT score group (123 of 223 patients, 55%) (OR, 0.46; 95% CI, 0.33-0.64; p < 0.001). An elevated CONUT score (HR, 1.63; 95% CI, 1.26-2.11; p < 0.001), but not the PLR or NLR, was independently associated with decreased DFS after controlling for confounding variables such as patient gender, age, tumor size, and AJCC stage (Table 4). In individual subgroup analyses, the CONUT scores were associated with OS and DFS in the < 5 cm subgroup (p < 0.001 and p = 0.005, respectively), ≥ 5 cm subgroup (both p < 0.001), superficial subgroup (p = 0.021 and p = 0.035, respectively), deep subgroup (both p < 0.001), G1 and G2 subgroup (both p < 0.001), early Stage I + II subgroup (p < 0.001 and p = 0.001, respectively), and advanced Stage III + IV subgroup (p = 0.006 and p = 0.007, respectively), but not in the G3 subgroup (p = 0.051 and p = 0.065, respectively) (Fig. 3 A-H and Fig. 4 A-H).
Fig. 2.
Kaplan-Meier curves show the (A) OS and (B) DFS of patients with STS based on the CONUT scores.
Fig. 3.
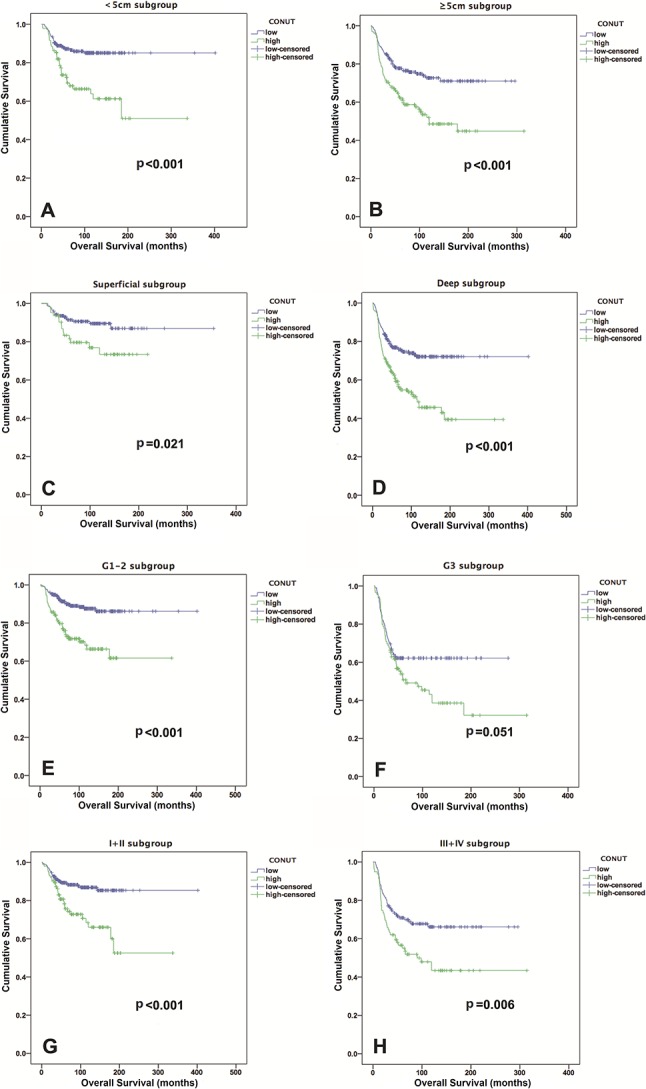
Kaplan-Meier curves show the OS based on the CONUT score in the (A) < 5 cm subgroup; (B) ≥ 5 cm subgroup; (C) superficial subgroup; (D) deep subgroup; (E) G1-2 subgroup; (F) G3 subgroup; (G) Stage I + II subgroup; and (H) Stage III + IV subgroup.
Fig. 4.
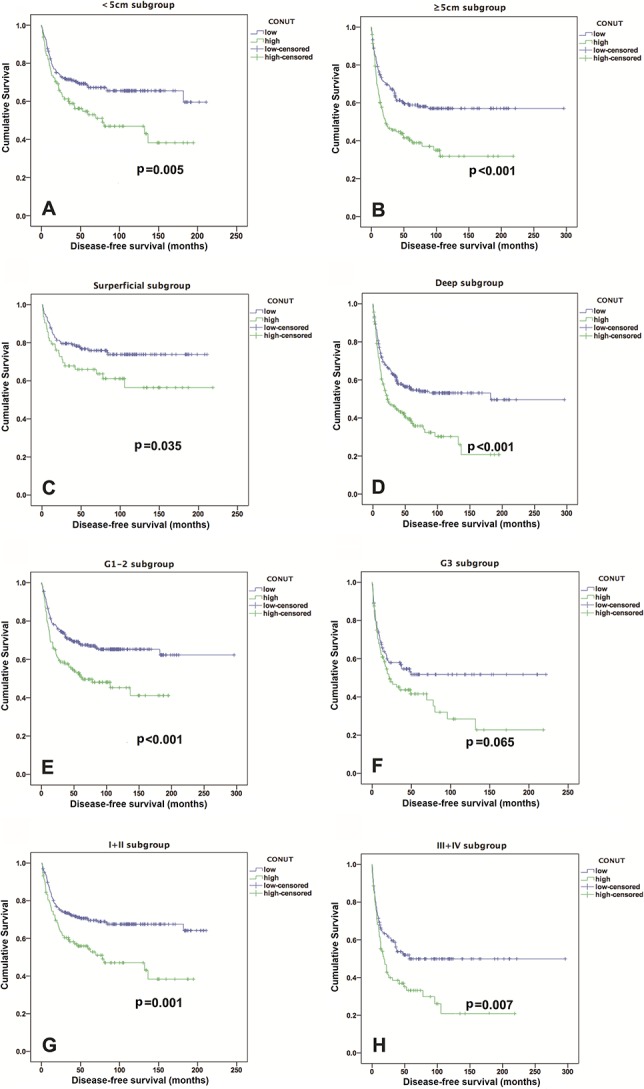
Kaplan-Meier curves show the DFS based on the CONUT score in the (A) < 5 cm subgroup; (B) ≥ 5 cm subgroup; (C) superficial subgroup; (D) deep subgroup; (E) G1-2 subgroup; (F) G3 subgroup; (G) Stage I + II subgroup; and (H) Stage III + IV subgroup.
Discussion
Some studies have indicated that an elevated CONUT score is correlated with poor survival in patients with various types of cancers [8, 12, 13, 25]. However, little is known about the association between the CONUT score and the clinocopathologic features of STS and the outcome of patients with it. Therefore, we assessed the potential of the CONUT score to predict survival and its relationship with disease severity in patients with STS who underwent R0 resection.
The limitations of this study were as follows: first, the retrospective design could have resulted in transfer bias (that is, loss to followup) and selection bias (clinical decision by surgeons based on nutritional status). This may have led to an overrated ‘‘true’’ DFS and OS, to a certain extent, because some of the patients who were lost to followup may have been sicker because of a more-aggressive disease phenotype and thus may have had a higher risk of recurrence and/or death. Although these may have caused subtle differences, we were able to confirm that there were no differences between the patients who were and were not included in terms of gender, age, or the CONUT score, and consecutive patients were selected to reduce the possible effects of selection bias. Second, the heterogeneity in data may have caused some biases. Although there were small numbers of patients with abdominal or retroperitoneal sarcomas (n = 71, 11%)—which may have influenced the patients’ appetite and nutritional status—and patients with an initial diagnosis of metastatic disease but had complete resection of primary and metastatic sarcomas simultaneously, these patients were also included in the final analyses (n = 12, 2%) because we wanted to investigate the relationship between the CONUT score and the survival of patients in these two groups of patients. Because their inclusion in the final analyses may have affected both the CONUT score and survival, to a certain extent, we believe that these biases, which had similar strength, would not substantially influence the observations of this study.
Third, because of the low incidence of STS, the sample size was not large enough to establish a validation group to further verify the observed findings of this study. Therefore, our data must be regarded as preliminary, and the subgroup analyses should be interpreted with caution. We were unable to investigate the effects of adjuvant therapy (n = 96, 15%) on survival in this study because few patients underwent chemotherapy and/or radiotherapy. Some other indicators, including the prognostic nutritional index and high-sensitivity modified Glasgow prognostic score, were not further explored because of incomplete data owing to the 17-year retrospective span. However, we consider our results meaningful as a pilot study. Fourth, cause-specific mortality was not included because only the dates of death, not death certificates, were available. This could potentially bias our OS results because patients could have died of nontumoral causes. This may help to explain the discrepancies observed in our data regarding some patients with Stage I sarcomas who died during the followup period but did not have metastatic disease. Finally, our participants were limited to one population drawn from an urban area of China and all surgeries were performed in a single institution. Therefore, the investigated patients’ characteristics and the study results may not be generalizable to other populations. Despite these limitations, our study was based on a large and heterogeneous group of patients, and as such, the conclusions postulated remain highly plausible.
In our study, we found that an increased CONUT score was associated with a large tumor size, high tumor grade, and advanced AJCC stage, suggesting that a higher CONUT score correlates with a more-aggressive disease phenotype and with more severe disorders in patients with STS. Consistent with our study, Kuroda et al. [13] found that a higher CONUT score was associated with older age, lower BMI, deeper invasion, higher serum carcinoembryonic antigen level, and higher serum carbohydrate antigen 19-9 level in patients with gastric cancer. They demonstrated that a high CONUT score was associated with tumor progression. Therefore, because it is cost-effective and easily assessable, the CONUT score may have important implications for individualized treatment and surveillance. Our findings suggest that clinicians should be cautious when considering surgery in patients with malnutrition who have an elevated preoperative CONUT score. These patients may be considered for more extensive resection and closer followup, rather than marginal resection, and may be considered for additional neoadjuvant therapy or more intense adjuvant chemotherapy to reduce the risk of recurrence.
Furthermore, after controlling for likely confounding variables, we found that the CONUT score was independently associated with OS and DFS, but the NLR and PLR were not. Similar to our findings, a study by Toyokawa et al. [26] indicated that the CONUT score was superior to the NLR and PLR and was associated with the OS of patients with resectable squamous cell carcinomas of the thoracic esophagus. In addition, two other studies have shown that the NLR and PLR were ineffective at predicting the survival of patients with cancer [9, 27], indicating that the predictive role of the NLR and PLR in patients with cancer requires further investigation. The NLR and PLR should be assessed alongside other markers because of the confounding factors introduced by inflammatory conditions. In our study, patients with a high CONUT score had shorter DFS and OS than patients with a low CONUT score did. In the high CONUT score group, the risk of death was 1.86 times higher than that in the low CONUT score group. This may help surgeons identify patients with STS who are at a higher risk of not surviving after surgery before those patients undergo surgery. However, in the subgroup analysis, we were unable to show a relationship between the CONUT score and OS and DFS in the G3 subgroup, suggesting that the CONUT score is associated with survival in patients with low-grade tumors, but not in those with high-grade sarcomas. A possible explanation is that G3 represents biologically aggressive STS. Patients with G3 tumors tend to have larger tumors that are in an anatomically unfavorable location or they have highly infiltrative tumors, which may explain the worse DFS and OS and diminished impact of the CONUT score. Moreover, we found that the tumor depth, which was excluded in the AJCC staging system, 8th Edition, was independently associated with OS and DFS, and that it could be a risk stratification tool for STS staging.
In conclusion, the present study provides the first evidence that the CONUT score was independently associated with DFS and OS in patients with low-grade STS if the tumor could be completely resected. Moreover, compared with the NLR and PLR, the CONUT demonstrated a closer relationship with the severity of STS based on our study population. The CONUT score might be a useful adjunct to counseling patients on their prognosis and guiding treatment options. If substantiated in a larger prospective study, the observations from this pilot study might provide the rationale to enhance perioperative nutrition support as a potential therapeutic protocol.
Acknowledgments
We thank M.G. Chen and W. Xiao for their role in data collection and W.M. Hu for his assistance with the statistical analysis for this research.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Sun Yat-sen University Cancer Center, Guangzhou, China.
References
- 1.Amin MB, Edge SB, Greene FL, Compton CC. AJCC Cancer Staging Manual. 8th ed. New York: Springer International; 2017. [Google Scholar]
- 2.Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond). 2018;38:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94-109. [DOI] [PubMed] [Google Scholar]
- 4.Crago AM, Brennan MF. Principles in management of soft tissue sarcoma. Adv Surg. 2015;49:107-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Kenawi A, Ruffell B. Inflammation, ROS, and mutagenesis. Cancer Cell. 2017;32:727-729. [DOI] [PubMed] [Google Scholar]
- 6.Fontana R, Bregni M, Cipponi A, Raccosta L, Rainelli C, Maggioni D, Lunghi F, Ciceri F, Mukenge S, Doglioni C, Colau D, Coulie PG, Bordignon C, Traversari C, Russo V. Peripheral blood lymphocytes genetically modified to express the self/tumor antigen MAGE-A3 induce antitumor immune responses in cancer patients. Blood. 2009;113:1651-1660. [DOI] [PubMed] [Google Scholar]
- 7.Galun D, Bogdanovic A, Djokic Kovac J, Bulajic P, Loncar Z, Zuvela M. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative-intent surgery for hepatocellular carcinoma: experience from a developing country. Cancer Manag Res. 2018;10:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harimoto N, Yoshizumi T, Sakata K, Nagatsu A, Motomura T, Itoh S, Harada N, Ikegami T, Uchiyama H, Soejima Y, Maehara Y. Prognostic significance of preoperative Controlling Nutritional Status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma. World J Surg. 2017;41:2805-2812. [DOI] [PubMed] [Google Scholar]
- 9.Hirahara N, Matsubara T, Kawahara D, Nakada S, Ishibashi S, Tajima Y. Prognostic significance of preoperative inflammatory response biomarkers in patients undergoing curative thoracoscopic esophagectomy for esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017;43:493-501. [DOI] [PubMed] [Google Scholar]
- 10.Hoang NT, Acevedo LA, Mann MJ, Tolani B. A review of soft-tissue sarcomas: translation of biological advances into treatment measures. Cancer Manag Res. 2018;10:1089-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38-45. [PubMed] [Google Scholar]
- 12.Kato Y, Yamada S, Suenaga M, Takami H, Niwa Y, Hayashi M, Iwata N, Kanda M, Tanaka C, Nakayama G, Koike M, Fujiwara M, Kodera Y. Impact of the Controlling Nutritional Status score on the prognosis after curative resection of pancreatic ductal adenocarcinoma. Pancreas. 2018;47:823-829. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, Baba Y, Sakamoto Y, Yamashita Y, Yoshida N, Chikamoto A, Baba H. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204-212. [DOI] [PubMed] [Google Scholar]
- 14.Lim WS, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY. Pretreatment albumin level predicts survival in head and neck squamous cell carcinoma. Laryngoscope. 2017;127:E437-E442. [DOI] [PubMed] [Google Scholar]
- 15.Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187-202. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Jiang S, Yang X, Li X, Wang N. The significant value of preoperative prognostic nutritional index for survival in pancreatic cancers: a meta-analysis. Pancreas. 2018;47:793-799. [DOI] [PubMed] [Google Scholar]
- 17.Miura K, Hamanaka K, Koizumi T, Kitaguchi Y, Terada Y, Nakamura D, Kumeda H, Agatsuma H, Hyogotani A, Kawakami S, Yoshizawa A, Asaka S, Ito KI. Clinical significance of preoperative serum albumin level for prognosis in surgically resected patients with non-small cell lung cancer: comparative study of normal lung, emphysema, and pulmonary fibrosis. Lung Cancer. 2017;111:88-95. [DOI] [PubMed] [Google Scholar]
- 18.Naito H, Nezu T, Hosomi N, Aoki S, Kinoshita N, Kuga J, Shimomura R, Araki M, Ueno H, Ochi K, Maruyama H. Controlling Nutritional Status score for predicting 3-mo functional outcome in acute ischemic stroke. Nutrition. 2018;55-56:1-6. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Smyth MJ. Targeting cancer-related inflammation in the era of immunotherapy. Immunol Cell Biol. 2017;95:325-332. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Matsumine A, Asanuma K, Matsubara T, Sudo A. The value of the high-sensitivity modified Glasgow prognostic score in predicting the survival of patients with a soft-tissue sarcoma. Bone Joint J. 2015;97:847-852. [DOI] [PubMed] [Google Scholar]
- 21.Neuville A, Chibon F, Coindre JM. Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology. 2014;46:113-120. [DOI] [PubMed] [Google Scholar]
- 22.Peng J, Zhang R, Zhao Y, Wu X, Chen G, Wan D, Lu Z, Pan Z. Prognostic value of preoperative prognostic nutritional index and its associations with systemic inflammatory response markers in patients with stage III colon cancer. Chin J Cancer. 2017;36:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Luo L, Zhao X, Ye P. Controlling Nutritional Status (CONUT) score as a predictor of all-cause mortality in elderly hypertensive patients: a prospective follow-up study. BMJ Open. 2017;7:e015649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeno S, Hashimoto T, Shibata R, Maki K, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87:205-214. [DOI] [PubMed] [Google Scholar]
- 25.Toyokawa G, Kozuma Y, Matsubara T, Haratake N, Takamori S, Akamine T, Takada K, Katsura M, Shimokawa M, Shoji F, Okamoto T, Maehara Y. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis. 2017;9:2942-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Liang D, Xu X, Jin J, Li S, Tian G, Gao Z, Liu C, He Y. The prognostic value of neutrophil to lymphocyte and platelet to lymphocyte ratios for patients with lung cancer. Oncol Lett. 2017;14:6449-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YP, Lei QY. Metabolic recoding of epigenetics in cancer. Cancer Commun (Lond). 2018;38:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, Uchida E. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23:646-654. [DOI] [PubMed] [Google Scholar]
- 30.Zhao QT, Yuan Z, Zhang H, Zhang XP, Wang HE, Wang ZK, Duan GC. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3,720 patients. Int J Cancer. 2016;139:164-170. [DOI] [PubMed] [Google Scholar]