Abstract
Background
Diagnosing periprosthetic joint infection (PJI) represents a challenge that relies on multiple clinical and laboratory criteria that may not be consistently present. The synovial alpha-defensin-1 (AD-1) test has been shown to correlate accurately with the Musculoskeletal Infection Society (MSIS) criteria for the diagnosis of PJI, however, its association with persistent PJI has not been elucidated in the setting of patients receiving antibiotic spacers during second-stage reimplantation. Applying a Delphi-based consensus to define successful eradication of PJI offers an opportunity to test the utility of AD-1 in this setting.
Questions/purposes
(1) Can the AD-1 test determine whether infection has been controlled using the Delphi criteria for persistent PJI as a surrogate for infection eradication during two-stage revision for PJI treatment with a spacer? (2) How does the performance of the AD-1 test compare with the MSIS criteria?
Methods
This was a multicenter analysis of retrospectively collected data on patients who underwent a two-stage revision arthroplasty between May 2014 and July 2016. We included patients who had a previously confirmed PJI and received a cement spacer, underwent the second stage, had MSIS criteria data and a synovial fluid AD-1 test, and had a minimum followup of 1 year. We were unable to determine for all study sites how many patients had the test but did not meet all the criteria and so could not be studied; however, we were able to identify 69 patients (43 knees, 26 hips) who met all criteria. During the period in question, indications for use of AD-1 varied by surgeon; however, during that time, in general if a surgeon ordered it as part of the initial workup, the test would have been repeated before the second-stage reimplantation procedure. To assess the validity of AD-1 against persistence of PJI criteria at 1 year, the following were calculated using the Delphi criteria for persistent PJI as the gold standard: sensitivity, specificity, positive and negative predictive values, accuracy, and area under the curve (AUC) with 95% confidence intervals (CIs). Concordance index (c-index) and its Wald 95% CI with receiver operating characteristic (ROC) curve were calculated in relation to Delphi criteria for persistent PJI using AD-1 and then MSIS criteria. The two c-indices of AD-1 and MSIS were compared using the DeLong nonparametric approach.
Results
The AD-1 test showed poor sensitivity (7%; 95% CI, 0.2–34), and poor overall accuracy (73%; 95% CI, 60–83; AUC = 0.5; 95% CI, 0.3–0.6) in detecting infection eradication at 1 year. The c-index for AD-1 versus Delphi criteria for persistent PJI was 0.519 (95% CI, 0.44–0.60), and the c-index for MSIS criteria versus Delphi criteria for persistent PJI was 0.518 (95% CI, 0.49–0.54), suggesting the weak diagnostic abilities of these models. The contrast estimate between MSIS criteria and AD-1 were not different from one another at -0.001 (95% CI%, -0.09 to 0.09; p = 0.99).
Conclusions
We found that a positive synovial fluid AD-1 test correlated poorly with the presence of persistent infection 1 year after two-stage revision arthroplasty for PJI. For this reason, we recommend against the routine use of AD-1 in patients with cement spacers, until or unless future studies demonstrate that the test is more effective than we found it to be.
Level of Evidence
Level IV, diagnostic study.
Introduction
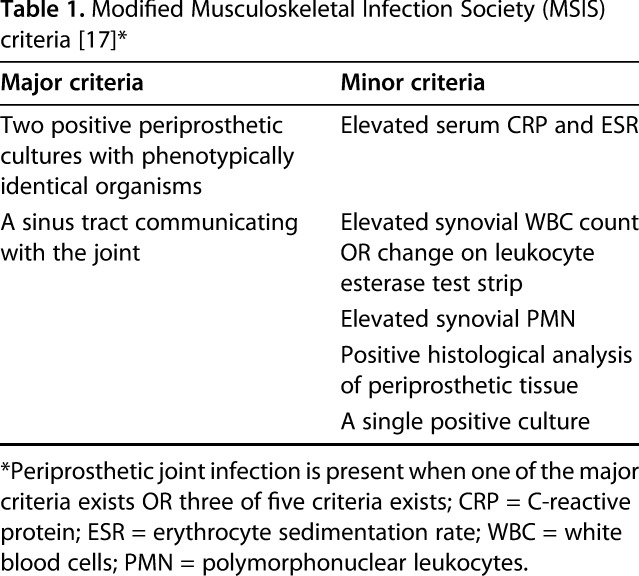
Periprosthetic joint infection (PJI) remains a major devastating complication after primary total joint arthroplasty (TJA) that is associated with serious morbidity and risk of death, as well as substantial cost to both patients and the healthcare system [1]. It also represents a diagnostic challenge that relies on multiple clinical and laboratory criteria that may not readily available in a timely and consistent manner. The Musculoskeletal Infection Society (MSIS) has established a set of evidence-based criteria to reliably diagnose periprosthetic joint infection (PJI) [17]. The use of these criteria in PJI diagnosis has been well-described and widely adopted [17]. Furthermore, in the absence of a single test to determine the successful control of a PJI, a Delphi-based international consensus definition was established (referred to as “Delphi criteria” here) to allow a more-systematic way of comparing treatments and their effectiveness [9].
Recently, there has been increasing interest in several biomarkers that can be identified locally in the synovial fluid of an infected prosthetic joint to diagnose PJI [2, 4–8, 12]. Among these, the alpha-defensin-1 (AD-1) assay test appears most promising, with high sensitivity and specificity for detecting PJI in multiple studies [2, 4–8]. In addition, the AD-1 test correlated accurately with the MSIS classification for PJI diagnosis, and incorporating the test as a standard tool in primary PJI diagnosis based on its accuracy and simplicity has been recommended [2]. Furthermore, the updated MSIS criteria for PJI definition listed the AD-1 test as one of the minor criteria, reflecting the growing evidence supporting its use [3, 19, 21].
However, most of these studies assessed the utility of AD-1 in the setting of PJI diagnosis. An even more challenging clinical scenario is ruling out persistent infection in patients who have antibiotic-containing cement spacers after resection arthroplasty and before definitive reimplantation. There is currently a substantial need for a test that can determine whether infection has been controlled before reimplantation in these two-stage revisions [12, 14, 16]. Based on its utility in diagnosing acute infections, some surgeons have expanded their use of the AD-1 test in the setting of reimplantation, despite the lack of evidence to support its usefulness in confirming infection eradication during the second stage of two-stage revisions. To the best of our knowledge, there have been no studies that specifically evaluated this role of AD-1.
Therefore, we asked: (1) Can the AD-1 test determine whether infection has been controlled using the MSIS Delphi criteria as a surrogate for infection eradication during two-stage revision for PJI treatment with a spacer? (2) How does the performance of the AD-1 test compare with the MSIS criteria?
Patients and Methods
Study Design and Setting
This was an institutional review board-approved, multicenter, retrospective cases series that reviewed patients who underwent a two-stage revision arthroplasty for the treatment of periprosthetic hip and knee joint infections from May 2014 to July 2016.
Participants/study subjects
Patients were included in this study if they had a previously confirmed PJI and received a cement spacer, underwent the second stage, had MSIS criteria data (Table 1) and synovial fluid AD-1 results available, and had a minimum followup of 1 year. We decided to publish our 1-year data as the results showed very poor performance of the AD-1 test in the short-term, which was not expected to change at the 2-year mark. We were not able to determine for all study sites how many had the test but did not meet all the criteria and so could not be studied, but we were able to identify a total of 69 patients (43 knees, 26 hips) who met all criteria. The sample included 44 men and 25 women who had a mean age of 67 years (range, 50–90 years) and mean BMI of 31 kg/m2 (range, 18.5–56 kg/m2). During the period in question, indications for use of AD-1 varied by surgeon; however, during that time, in general if a surgeon ordered it as part of the initial workup, the test would have been repeated before the second-stage reimplantation procedure. In all patients, joint aspiration samples were obtained intraoperatively and shipped overnight to a certified lab for AD-1 immunoassay.
Table 1.
Modified Musculoskeletal Infection Society (MSIS) criteria [17]*
Variables, Outcome Measures, Data Sources, and Bias
We collected results of the AD-1 test and data pertaining to MSIS criteria at the reimplantation stage for each patient. Next, we assessed the presence or absence of persistent PJI after the second stage revision for each patient using the previously described Delphi criteria (Table 2) [9] at 1 year postoperatively to determine the status of every patient with regard to persistent infection. Per the Delphi criteria, persistent PJI here was defined as the presence of any of the following: a nonhealing wound with fistula, drainage, or pain; infection recurrence caused by the same organism strain; subsequent surgical intervention for infection after reimplantation surgery; or occurrence of PJI-related mortality (by causes such as sepsis or necrotizing fasciitis). The performance of the AD-1 test was compared with the final patient outcome reflected by the Delphi criteria. Since this role of AD-1 has not been previously validated, we also compared the performance of the MSIS criteria with the Delphi criteria as a control analysis.
Table 2.
The Delphi criteria for diagnosis of persistent periprosthetic joint infection (PJI) following a septic revision procedure

Statistical Analysis, Study Size
To assess the validity of AD-1 as a diagnostic tool for infection eradication at 1 year, we calculated the sensitivity, specificity, positive and negative predictive values, accuracy, area under the receiver operating curve (AUC) with 95% confidence intervals (CIs) using the Delphi persistent PJI criteria as the reference standard. In addition, we calculated the Concordance index (c-index) and its Wald 95% CI with receiver operating characteristic (ROC) curve in relation to the Delphi criteria using AD-1 then MSIS criteria. We compared the two c-indices of AD-1 and MSIS using the DeLong nonparametric approach. The analysis was performed using SAS software Version 9.4 (SAS Institute, Cary, NC, USA).
Results
AD-1 Performance Against Delphi Criteria
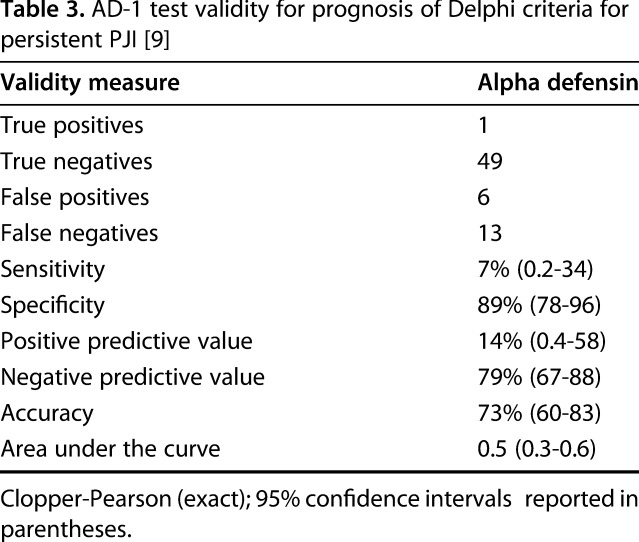
At 1 year, the AD-1 test showed poor sensitivity (7%; 95% CI, 0.2–40), and low specificity (89%; 95% CI, 78–96) in its ability to identify infection eradication. A total of 14 patients had persistent PJI in the cohort at 1 year; the AD-1 test predicted only one persistent PJI. In addition, AD-1 yielded a false positive in another six patients at the time of implantation, none of which met the Delphi criteria for persistent PJI at 1 year. Overall, the test also showed poor accuracy (73%; 95% CI, 60–83; AUC, 0.5; 95% CI, 0.3–0.6) (Table 3).
Table 3.
AD-1 test validity for prognosis of Delphi criteria for persistent PJI [9]
AD-1 Performance Against MSIS Criteria
The MSIS criteria also showed poor ability to correlate with persistent PJI at 1 year. In all 14 patients who demonstrated recurrent PJI at 1 year, the MSIS criteria did not predict any persistent PJI, although the criteria returned two false positive results.
Other Findings
The c-index for AD-1 versus Delphi criteria was 0.519 (95% CI, 0.44–0.60), and the c-index for MSIS criteria versus Delphi criteria was 0.518 (95% CI, 0.49–0.54), suggesting the weak predictive abilities of these models (Table 4). The contrast estimate between MSIS criteria and AD-1 were not different from one other at -0.001 (95% CI%, -0.09 to 0.09; p = 0.99) (Fig. 1).
Table 4.
2 x 2 contingency table for MSIS vs Delphi criteria for persistent PJI [17]
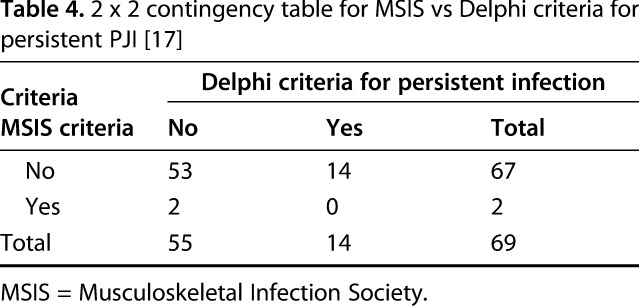
Fig. 1.
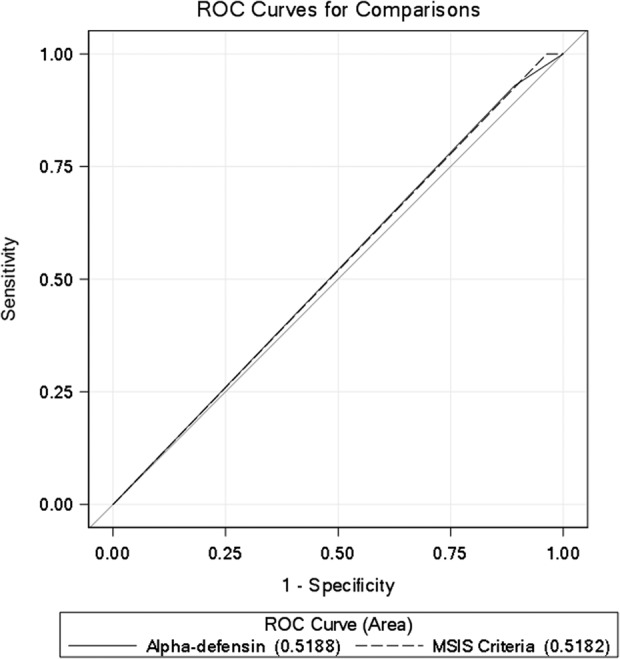
Receiver operator curves (ROC) for the two models: alpha-defensin for predicting Delphi criteria for periprosthetic joint infection (PJI), and the Musculoskeletal Infection Society (MSIS) criteria for predicting Delphi criteria for PJI.
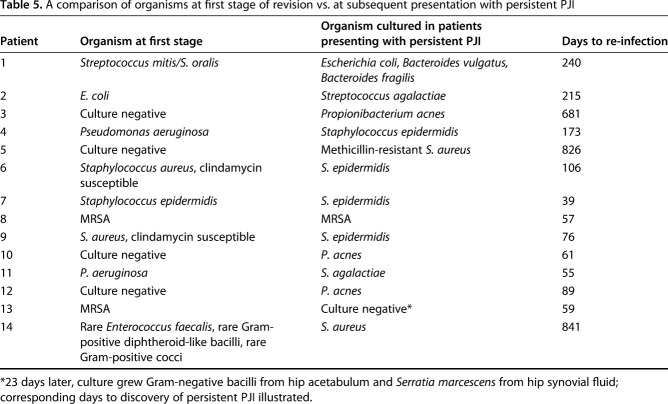
In the 14 patients who had persistent PJI at 1 year, we compared cultured organisms isolated during the first stage of the index revision with those isolated at the time of infection recurrence to determine whether these were persistent or new infections. When we compared initial cultures from the first stage of the procedure, only two patients (14.3%) had the same organisms when they presented with persistent PJI. Seven patients (50%) developed other organisms, four patients with previously negative cultures had organisms isolated at the time of infection recurrence, and one patient had a culture negative infection recurrence that later grew an organism different from the index revision (Table 5).
Table 5.
A comparison of organisms at first stage of revision vs. at subsequent presentation with persistent PJI
Discussion
There is currently no consensus regarding the best test to determine whether an infection has been controlled in patients with cement spacers who are undergoing two-stage arthroplasty revision for PJI. The AD-1 test has demonstrated promising results in establishing a primary PJI diagnosis [2, 4–8]; however, it is unclear whether it has a role in ruling out infection in this revision setting. To the best of our knowledge, our study is the first to evaluate the validity of AD-1 as a tool to evaluate whether a positive AD-1 test performed during the reimplantation phase of a two-stage revision is associated with persistent PJI. Our results suggest that AD-1 may have little or no role in this setting, as it demonstrated poor sensitivity, overall accuracy, and concordance index with Delphi criteria for determining if PJI has been controlled. It is important to note, though, that the MSIS criteria also performed poorly when compared with the Delphi criteria that we evaluated as a control analysis, since the role of AD-1 in this setting has not been yet validated.
Our study had several limitations. Through a chart review, we were unable to identify a clear set of indications in common use for the AD-1 test at initial revision; however, we believe that it was used fairly consistently during the second stage among those patients who received it at the initial revision. Related to this, we could not confirm with certainty the number who had the test but who did not meet all the study inclusion criteria, making it possible—if not likely—that some patients who received it were not included in this report because of loss to followup. However, if anything, that would possibly make the test appear better than it actually is, since patients who are lost to followup generally are not doing as well as those who are accounted for, and so in this instance, this limitation does not appear to us to be disqualifying. The small sample size in addition to its heterogenous nature (that is, multiple procedures, surgical techniques, surgeons at different institutions) may also affect the accuracy of the findings. Even so, while larger studies might deliver more-precise estimates of the test’s properties in this setting, we felt it important to report our findings, given how concerning they are. The nonblinded comparison of AD-1 and MSIS criteria with the Delphi criteria may introduce assessment bias, which was mitigated by the objective nature of these measures. However, none of the MSIS criteria results were determined by the reviewer, but rather by trained microbiologists and pathologists. Another limitation is that the AD-1 performance was compared with the Delphi criteria at 1-year followup (rather than at 2 years); if anything, this would tend to make the test appear better in our report than it actually is. We chose to report our results at 1 year because they were already poor at that time; this is unlikely to improve (and may in fact worsen) with longer surveillance periods, which could result in patients who tested negative for infection presenting with symptoms and signs of PJI in the future. However, since the test performed very poorly at 1 year, we decided to report the findings that we did not believe will be any different (and may probably be worse) at 2 years of followup.
In most of the patients who had persistent PJI at 1 year, the intraoperative cultures showed a different organism from the one at primary diagnosis (Table 4), when the AD-1 test was used. This finding is in concordance with multiple previous studies that demonstrated the potential role of new infecting organisms leading to the recurrence of PJI [10, 13, 22]. It is possible that AD-1 utility is confounded by the recurrence of infection with a different organism in relation to the primary infection. Therefore, this may represent the status of certain patients being more prone to recurrent PJI rather than a true inability of the AD-1 test to detect infection eradication when performed at the second stage. Future research for the utility of AD-1 in this setting should also investigate this relationship.
In a previous study by Deirmengian et al. [8], the authors compared the median AD-1 levels for various bacterial Gram-type, species, virulence, oral pathogenicity, and source joint. The authors demonstrated that AD-1 showed consistent results with a wide spectrum of organisms as reflected by the absence of differences in medians and interquartile ranges (IQR) of AD-1 levels for various categories of organisms, including Gram-positive, 4.7 (IQR, 3.6–5.3); Gram-negative, 4.8 (IQR, 4.2–5.3); yeast, 4.1 (IQR, 2.2–5.1); virulent, 4.7 (IQR, 3.8–5.2); less virulent, 4.8 (IQR, 3.6–5.4); and oral pathogens, 4.5 (IQR, 3.2–5.2). These results are not in concordance with the present study in which the causative organisms of persistent PJI were different from the cause of the index PJI. Additionally, the AD-1 test had more false positives in our cohorts than true positives, which demonstrated that in this setting, it may have been triggered by other factors unrelated to infections, including the locally reactive inflammatory process from the cement spacers [20], further confounding its results. Other biomarkers may serve as better predictive tools than AD-1. In a study with similar design to the present one, Kheir et al. [11] investigated the use of leukocyte esterase (LE) strip test at the reimplantation of two-stage revision in patients who had cement spacers to predict recurrent PJI according to the Delphi criteria. Although the LE test also had low sensitivity (26.3%), it had a specificity of 100%, which was higher when compared with the MSIS criteria (87.3%). On this basis, the authors in that study concluded that it can be encouraging to use this test to rule out infection. Another biomarker that demonstrated potential as a diagnostic tool in PJI and for determining the appropriate timing for reimplantation is serum D-dimer, as shown by Shahi et al. [18] In their analysis, serum D-dimer had higher sensitivity and specificity than both serum ESR and CRP (89% and 93% versus 73% and 78% for ESR and 79% and 80% for CRP).
Several earlier reports demonstrated higher sensitivity and accuracy of AD-1; all reported on its use as a tool to establish the primary PJI diagnosis. Bingham et al. [2] reported that the sensitivity for the AD-1 assay was 100% (95% CI, 79%–100%) and specificity was 95% (95% CI, 83%–99%), and that it outperformed a myriad of other laboratory tests to establish PJI diagnosis, including cell count, culture, erythrocyte sedimentation rate, and C-reactive protein. In another study by Deirmengian et al. [5], the test accurately correlated with the MSIS classification for patients who were diagnosed with PJI, demonstrating a sensitivity of 100%. Multiple other studies have also shown that AD-1 alone or in combination with other biomarkers should be a standard tool for PJI diagnosis. However, the current studies do not provide evidence regarding the utility and validity of AD-1 as a tool in patients who received cement spacers and who supposedly became infection-free before the second stage or reimplantation. Although theoretically the presence of a cement spacer may suggest the presence of a local proinflammatory response that can confound the results of AD-1 in these settings, other studies have shown that AD-1 accuracy was not affected when used to diagnose primary PJI in patients with active inflammatory conditions. Therefore, our study is the first to try to answer the question examining the performance of AD-1 in a true clinical setting.
In conclusion, despite the utility of AD-1 in previous studies as a diagnostic tool for PJI [2, 4–8, 15], we found that it was not useful in identifying persistent infection 1 year after reimplantation in patients who underwent two-stage reimplantation after placement of a cement spacer. Future larger, prospective, and preferably longer-term studies are needed to confirm our results.
Footnotes
One of the authors certifies that he (CAH), or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from KCI (San Antonio, TX, USA); an amount of less than USD 10,000 from Zimmer Biomet (Warsaw, IN, USA); an amount of less than USD 10,000 from Covance (Princeton, NJ, USA); an amount of less than USD 10,000 from Pfizer (New York, NY, USA); an amount of less than USD 10,000 from TenNor Therapeutics Limited (Suzhou Industrial Park, China); and has received research support funding from Stryker (Kalamazoo, MI, USA), Myoscience (Fresno, CA, USA), CD Diagnostics (Claymont, DE, USA), KCI (San Antonio, TX, USA), OREF (Rosemont, IL, USA), Pacira (Parsippany, NJ, USA), 3M (Maplewood, MN, USA), Cymedica (Scottsdale, AZ, USA), Ferring Pharmaceuticals (Parsippany, NJ, USA), and Orthofix, Inc. (Lewisville, TX, USA), outside of this work.
One of the authors certifies that he (JP), or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Zimmer Biomet (Warsaw, IN, USA); an amount of less than USD 10,000 from ConvaTec (Skillman, NJ, USA); an amount of USD 10,000 from TissueGene (Rockville, MD, USA); an amount of less than USD 10,000 from CeramTec (Plochingen, Germany); and an amount of less than USD 10,000 from Ethicon (Somerville, NJ, USA), and has stock options with Parvizi Surgical Innovations (Philadelphia, PA, USA); Hip Innovation Technology (Plantation, FL, USA); CD Diagnostics (Wynnewood, PA, USA); CorenTec (Seoul, Korea); Alphaeon (Irvine, CA, USA); Joint Purification Systems (Solana Beach, CA, USA); Ceribell (Mountain View, CA, USA); MedAP (Cape Town, South Africa); Cross Current Business Intelligence (Doylestown, PA, USA); Invisible Sentinel (Philadelphia, PA, USA); Physician Recommended Nutriceuticals (Plymouth Meeting, PA, USA); Intellijoint (Waterloo, Ontario, Canada); and MicroGenDx (Tallahassee, FL, USA).He has received royalties in the amount of less than USD 10,000 from Corentec (Seoul, Korea); an amount of less than USD 10,000 from Datatrace (Houston, TX, USA); an amount of less than USD 10,000 from Elsevier (Philadelphia, PA, USA); an amount of less than USD 10,000 from Wolters-Kluwer (Philadelphia, PA, USA); an amount of less than USD 10,000 from Slack Inc (Thorofare, NJ, USA); and an amount of less than USD 10,000 from Jaypee (New Delhi, India); has a board membership with the Journal of Arthroplasty; Journal of Bone and Joint Surgery; Bone and Joint Journal; Eastern Orthopaedic Association (Towson, MD, USA); Muller Foundation (Baltimore, MD, USA); and United Healthcare (Hopkins, MN, USA).
One of the authors certifies that he (PP), or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from Stryker (Kalamazoo, MI, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Cleveland Clinic, Cleveland, Ohio, USA.
References
- 1.Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord . 2012;5:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res . 2014;472:4006–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T. How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin Orthop Relat Res . 2017;475:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Saint Vincent B, Migaud H, Senneville E, Loiez C, Pasquier G, Girard J, Putman S. Diagnostic accuracy of the alpha defensin lateral flow device (Synovasure) for periprosthetic infections in microbiologically complex situations: A study of 42 cases in a French referral centre. Orthop Traumatol Surg Res . 2018;104:427–431. [DOI] [PubMed] [Google Scholar]
- 5.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE, Parvizi J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res . 2015;473:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am . 2014;96:1439–45. [DOI] [PubMed] [Google Scholar]
- 7.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res . 2014;472:3254–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res . 2015;473:2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res . 2013;471:2374–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br . 2000;82:689–694. [DOI] [PubMed] [Google Scholar]
- 11.Kheir MM, Ackerman CT, Tan TL, Benazzo A, Tischler EH, Parvizi J. Leukocyte esterase strip test can predict subsequent failure following reimplantation in patients with periprosthetic joint infection. J Arthroplasty. 2017;32:1976–1979. [DOI] [PubMed] [Google Scholar]
- 12.Kheir MM, Tan TL, George J, Higuera CA, Maltenfort MG, Parvizi J. Development and evaluation of a prognostic calculator for the surgical treatment of periprosthetic joint infection. J Arthroplasty. 2018;33:2986-2992. [DOI] [PubMed] [Google Scholar]
- 13.Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two-staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res . 2005;441:243–249. [DOI] [PubMed] [Google Scholar]
- 14.Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res . 2010;468:2060–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz SM, Lau EC, Son M-S, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J. Arthroplasty. 2018;33:3238-3245. [DOI] [PubMed] [Google Scholar]
- 16.Mortazavi SMJ, Vegari D, Ho A, Zmistowski B, Parvizi J. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res . 2011;469:3049–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res . 2011;469:2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am . 2017;99:1419–1427. [DOI] [PubMed] [Google Scholar]
- 19.Shahi A, Parvizi J, Kazarian GS, Higuera C, Frangiamore S, Bingham J, Beauchamp C, Valle C Della, Deirmengian C. The Alpha-defensin test for periprosthetic joint infections is not affected by prior antibiotic administration. Clin Orthop Relat Res . 2016;474:1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh G, Deutloff N, Maertens N, Meyer H, Awiszus F, Feuerstein B, Roessner A, Lohmann CH. Articulating polymethylmethacrylate (PMMA) spacers may have an immunomodulating effect on synovial tissue. Bone Joint J . 2016;98–B:1062–1068. [DOI] [PubMed] [Google Scholar]
- 21.Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW. The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection: a systematic review and meta-analysis. J Bone Joint Surg Am . 2016;98:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zmistowski B, Tetreault MW, Alijanipour P, Chen AF, Della Valle CJ, Parvizi J. Recurrent periprosthetic joint infection. J Arthroplasty. 2013;28:1486–1489. [DOI] [PubMed] [Google Scholar]