Abstract
Background
Anatomic and surgical complexity make pelvic and sacral bone sarcoma resections challenging. Positive surgical margins are more likely to occur in patients with pelvic and sacral bone sarcomas than in those with extremity sarcomas and are associated with an increased likelihood of local recurrence. Intraoperative navigation techniques have been proposed to improve surgical accuracy in achieving negative margins, but available evidence is limited to experimental (laboratory) studies and small patient series. Only one small historically controlled study is available. Because we have experience with both approaches, we wanted to assess whether navigation improves our ability to achieve negative resection margins.
Questions/purposes
Are navigated resections for pelvic and sacral primary bone sarcomas better able to achieve adequate surgical margins than nonnavigated resections?
Methods
Thirty-six patients with pelvic or sacral sarcomas treated with intraoperative navigation were retrospectively compared with 34 patients undergoing resections without navigation. All patients underwent resections between 2000 and 2017 with the intention to achieve a wide margin. Patients in the navigation group underwent surgery between 2008 and 2017; during this period, all resections of pelvic and sacral primary bone sarcomas with the intention to achieve a wide margin were navigation-assisted by either CT fluoroscopy or intraoperative CT. Patients in the control group underwent surgery before 2008 (when navigation was unavailable at our institution), to avoid selection bias. We did not attempt to match patients to controls. Nonnavigated resections were performed by two senior orthopaedic surgeons (with 10 years and > 25 years of experience). Navigated resections were performed by a senior orthopaedic surgeon with much experience in surgical navigation. The primary outcome was the bone and soft-tissue surgical margin achieved, classified by a modified Enneking system. Wide margins (≥ 2 mm) and wide-contaminated margins, in which the tumor or its pseudocapsule was exposed intraoperatively but further tissue was removed to achieve wide margins, were considered adequate; marginal (0-2 mm) and intralesional margins were considered inadequate.
Results
Adequate bone margins were achieved in more patients in the navigated group than in the nonnavigation group (29 of 36 patients [81%] versus 17 of 34 [50%]; odds ratio, 4.14 [95% CI, 1.43-12.01]; p = 0.007). With the numbers available, we found no difference in our ability to achieve adequate soft-tissue margins between the navigation and nonnavigation group (18 of 36 patients [50%] versus 18 of 34 [54%]; odds ratio, 0.89 [95% CI, 0.35-2.27]; p = 0.995).
Conclusions
Intraoperative guidance techniques improved our ability to achieve negative bony margins when performing surgical resections in patients with pelvic and sacral primary bone sarcomas. Achieving adequate soft-tissue margins remains a challenge, and these margins do not appear to be influenced by navigation. Larger studies are needed to confirm our results, and longer followup of these patients is needed to determine if the use of navigation will improve survival or the risk of local recurrence.
Level of Evidence
Level III, therapeutic study.
Introduction
The aim of surgery to treat bone sarcomas is to completely excise the tumor with negative margins while preserving as much normal tissue as possible. Preserving muscle, bone, and neurovascular structures may improve the surgeon’s ability to achieve good reconstruction and reduce the likelihood of complications, thereby improving short- and long-term functional outcomes [1, 30]. Achieving wide surgical margins in the pelvis is challenging, and pelvic tumors are more likely to result in positive surgical margins than extremity tumors are [4, 12, 13, 24, 25]. Local recurrence occurs in 20%-40% of patients overall, and in those with positive margins, this number increases up to 70%, resulting in an increased risk of local recurrence and perhaps metastasis [4, 12, 13, 24, 25]. Neoadjuvant chemotherapy or radiotherapy may be used to increase the likelihood of adequate resection or treat positive surgical margins in some types of sarcomas, but it is considered less effective for treating chondrosarcomas and chordomas, which are the predominant tumor types in adults with pelvic and sacral sarcomas.
Intraoperative guidance techniques such as computer-assisted surgery may be useful in achieving wide margins during tumor resections, and thus may assist in improving oncologic outcomes. Computer-assisted surgery allows for three-dimensional preoperative planning of resection and reconstruction procedures. Intraoperatively, there is real-time feedback for the actual location and orientation, which allows for more precision [27, 30].
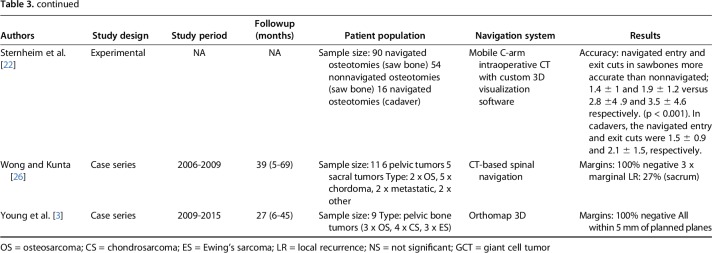
Available evidence about the putative benefits of computer-assisted surgery in resections for pelvic and sacral bone sarcomas are somewhat limited, consisting principally of experimental (laboratory) studies and small patient series [2, 5, 7, 16, 20, 22, 26, 30]. Only one small historically controlled study [19] comparing nine navigated resections for sacral and pelvic tumors with 12 nonnavigated resections is available. Although these studies have shown generally consistent results, with more accurate osteotomies, fewer intralesional resections, and increased benefits to the patient in terms of reduced operating time, less blood loss, and fewer complications [2, 5, 7, 16, 19, 20, 22, 26, 30], they either do not have a control group and/or have small cohorts. To our knowledge, there has been no large and well-performed, controlled study of navigated oncologic resections in the pelvis.
We therefore asked: are navigated pelvic and sacral primary bone sarcoma resections better able to achieve adequate surgical margins than nonnavigated resections?
Patients and Methods
We retrospectively studied patients undergoing resection of a primary pelvic or sacral bone sarcoma between 2000 and 2017. Our institutional review board reviewed and approved this study. All data were collected as part of routine patient followup examinations; therefore, the ethical review board waived the need to obtain individual informed consent.
Patients were identified in an intuitional database. Since 2008, computed-assisted surgery using CT fluoroscopy has been used for bone sarcoma resections at our institution; since 2015, intraoperative CT-based navigation has replaced CT fluoroscopy. Computer-assisted surgery was indicated for all patients presenting with a primary malignant pelvic or sacral bone sarcoma in a curative setting. Patients were eligible for inclusion if they had a pelvic or sacral primary malignant bone sarcoma and underwent resection with the intention to achieve a wide margin. Patients presenting with recurrent disease after a previous resection were excluded. Patients were included in the navigation group if computer-assisted surgery, either CT fluoroscopy or intraoperative CT-based surgery, was used with the intention to achieve a wide resection. Patients in the control group were selected from 2000 to 2008 because surgical navigation was not available at that time at our institution. One hundred four resections of primary pelvic and sacral bone sarcomas were performed. Twenty-four patients underwent debulking or intralesional curettage of low-grade chondrosarcomas and were excluded. In 10 patients—four who underwent intraoperative CT-based surgery and six who underwent CT fluoroscopy-based surgery—there were technical problems with the navigation software. These resections were performed without navigation and these patients were also excluded from the analysis. The remaining 70 patients were included in this study: 36 with surgical navigation and 34 without navigation (Fig. 1).
Fig. 1.
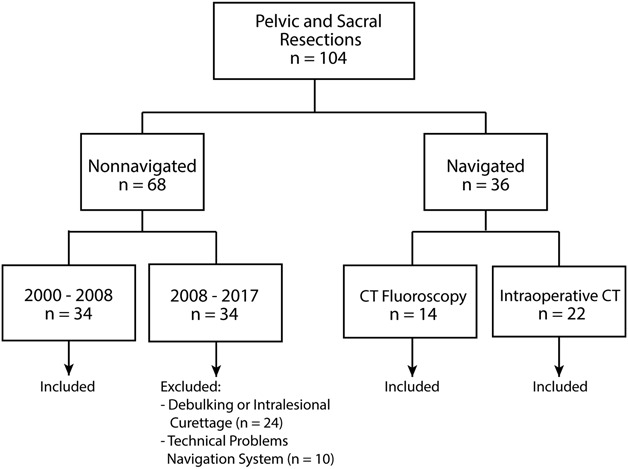
This flowchart shows how patients were included in our study.
Two senior orthopaedic oncologic surgeons (PDSD, who has 10 years of experience in pelvic surgery, and AHMT, who has more than 25 years of experience in pelvic surgery) performed the nonnavigated resections. The navigated resections were all performed by a single surgeon (PDSD) who is experienced in using this technique. He developed experience in surgical navigation first by practicing on sawbone models and cadavers. He performed more than 10 navigated spondylodesis procedures of the spine and resected at least 10 benign tumors of the pelvis and sacrum before performing navigated resection in patients with primary malignant bone sarcoma.
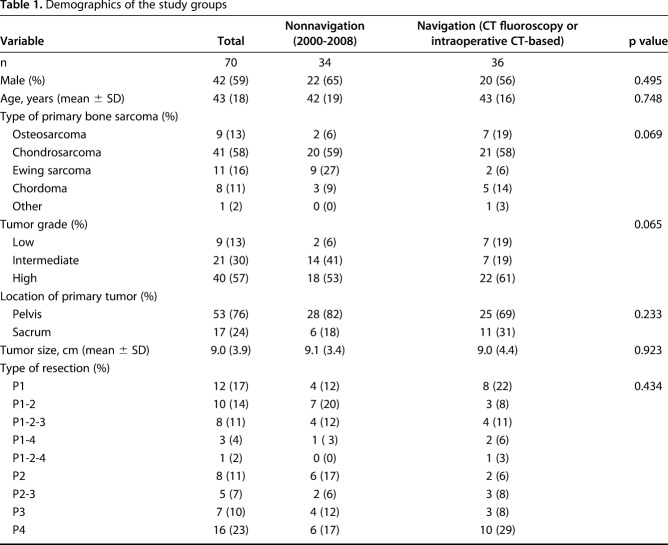
Data on patient demographics, tumor characteristics, (neo)adjuvant treatment, surgery details, and complications were extracted from the patients’ medical records. The mean age was 43 years ± 16.3 for patients in the navigation group versus 42 years ± 18.9 in the nonnavigation group (p = 0.748). No differences in the mean tumor size between the groups were observed (9.0 cm ± 4.4 for the navigation group versus 9.1 cm ± 3.4 for the nonnavigation group (p = 0.923). In the navigation group, the most prevalent type of sarcoma was chondrosarcoma (in 21 of 36 patients [58%]), followed by osteosarcoma (seven of 36 patients [19%]), chordoma (five of 36 patients [14%]), and Ewing’s sarcoma (two of 36 patients [6%]). In the nonnavigation group, the most prevalent type of sarcoma was chondrosarcoma (in 20 of 34 patients [59%]), followed by Ewing’s sarcoma (nine of 34 patients [27%]), chordoma (three of 34 patients [9%]), and osteosarcoma (two of 34 patients [6%]). No difference between the groups with regard to tumor type were observed (p = 0.069). The tumor grade also did not differ between the groups: 22 of the 36 patients in the navigation group [61%] had high-grade tumors, seven [19%] had intermediate-grade tumors, and seven [19%] had low-grade tumors; 18 of the 34 patients [53%] in the nonnavigation group had high-grade tumors, 14 [41%] had intermediate-grade tumors, and two [6%] had low-grade tumors (p = 0.065) (Table 1). In both groups, all patients presenting with an osteosarcoma or Ewing’s sarcoma received neoadjuvant chemotherapy. In the navigation group, four patients received radiation therapy, and in one, it was given preoperatively. In the nonnavigation group, seven patients received radiation therapy, and in one, it was given preoperatively. In all patients who had radiation, a dose of 54 or 55 Gy was administered. Resections were classified from P1 to P4 or a combination [9]. P1 resections involved the ilium; P2 resections involved the periacetabular regions, with or without involvement of the femur; P3 resections involved the pubis; and P4 resections involved the sacrum. No differences in the type of resection were observed between the groups (p = 0.434).
Table 1.
Demographics of the study groups
Preoperative Planning
All patients were treated with the intention to achieve a wide bone and soft-tissue resection margin. All patients underwent routine diagnostic workup, including biopsy (if indicated) and CT and MRI scanning with gadolinium. Treatment plans and decisions regarding (neo)adjuvant chemotherapy and/or radiation therapy were discussed at a multidisciplinary tumor board meeting for each patient. If navigation was used, preoperatively acquired CT and MR images were fused (Fig. 2A-2D), allowing for an accurate three-dimensional model of the tumor that correlated with the patient’s anatomy during surgery. Images were used before surgery to plan margins and, if needed, reconstruction.
Fig. 2.
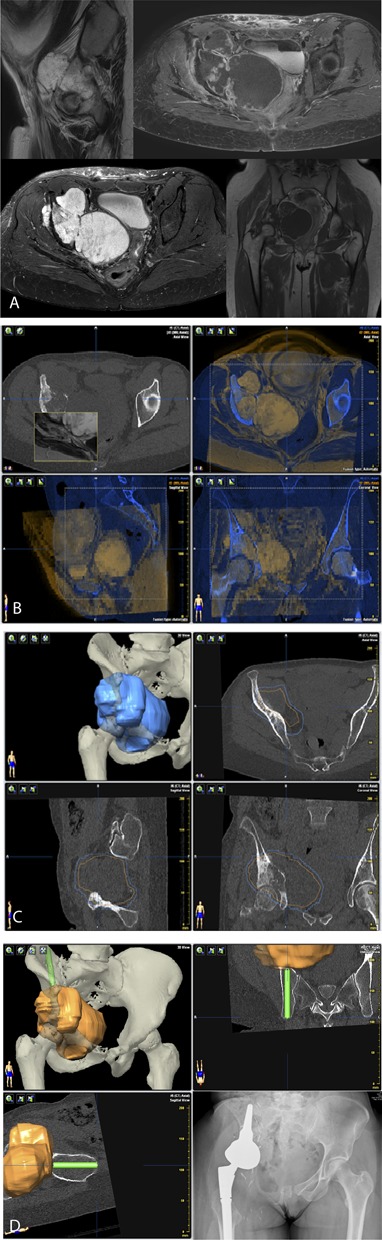
These images show preoperative surgical planning for tumor resection in a 34-year-old woman with a Grade II chondrosarcoma in the periacetabular region of the right side of the pelvis. (A) Preoperative MR images demonstrate a high-grade tumor in the right periacetabular region. (B) Computer screenshots of the navigation software show fusion of the MRI (orange) and CT scans (blue). (C) This screen image from the navigation system shows the mapped tumor in orange and the planned safety margin in blue, in three planes (clockwise from top left: three-dimensional reconstruction in the transverse, sagittal, and coronal planes). (D) This image shows the planning of a LUMiC® prosthesis (Implantcast, Buxtehude, Germany). The LUMiC® prosthesis is a modular device and consists of a separate cup and stem, both available in different sizes and with different coatings. An AP radiograph after navigation-assisted LUMiC® endoprosthetic pelvic reconstruction after resection is shown.
Intraoperative Navigation
Image-to-patient registration is the most-crucial step, in which the patient’s anatomy is linked to preoperatively acquired imaging data. There are three registration methods in general. The most common registration method is manual, in which important predefined anatomical landmarks on the preoperative image dataset are located as accurately as possible on the patient during surgery, using a probe or pen (paired points matching). Accuracy is further improved by collecting more points from the patient’s bone surface (surface matching) [26, 27].
Subsequent CT fluoroscopy matching allowed for semiautomatic registration. Fluoroscopic images (AP and lateral) taken intraoperatively were superimposed on the preoperative CT images. After manual image adjustment, they were displayed on the navigation monitor. Registration can also be performed automatically, in which matching is done using intraoperative CT-based navigation. The use of intraoperative CT improves the workflow and allows for intraoperative updates and, if needed, change of the plan [27, 30].
CT Fluoroscopy-based Navigation
For CT fluoroscopy navigation, we used a mobile C-arm (Philips BV, Eindhoven, the Netherlands) combined with a navigation platform (Curve™ Image Guided Surgery, Brainlab AG, Feldkirchen, Germany).
Intraoperative CT-based Navigation
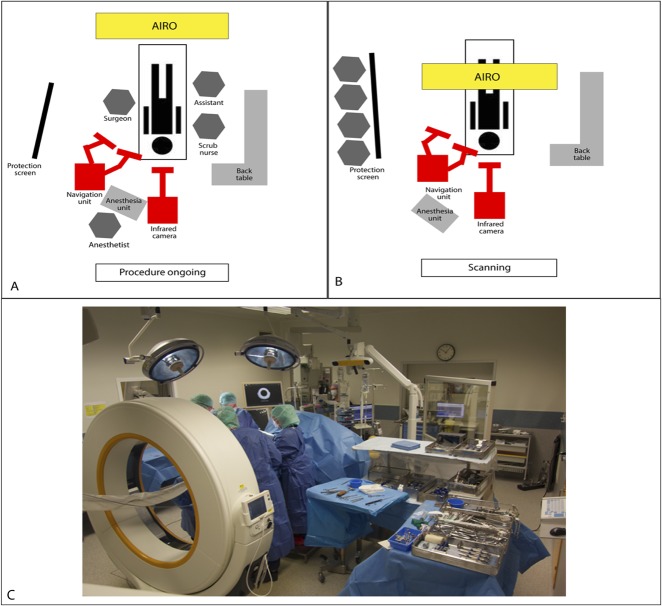
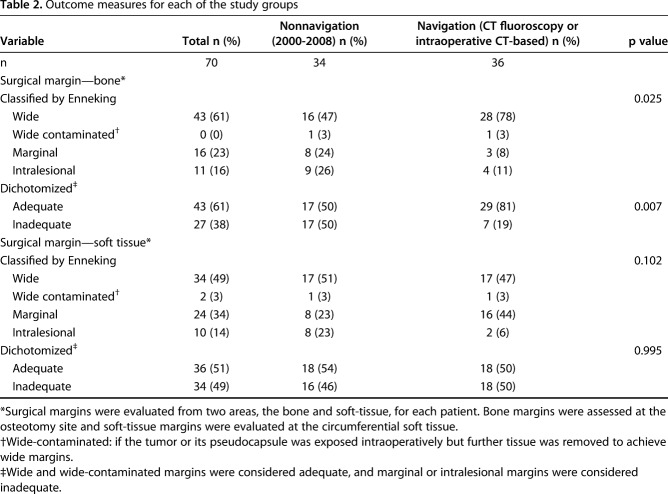
For intraoperative CT-based navigation, we used a mobile AIRO® CT scanner (Mobius Imaging, Ayer, MA, USA) with spinal navigation software (Curve™ Brainlab AG, Feldkirchen, Germany). The AIRO scanner consists of a mobile CT gantry with a radiography tube. It is designed to function in any operating room and can be moved using an electrical drive system. With a 105-cm diameter, the bore is extra-large. Surgery is performed on a radiolucent, carbon-fiber CT examination table (TRUMPF TruSystem 7500, Trumpf Inc., Farmington, CT, USA) that can be turned in any direction greater than 360°. The entire setup has a 1.5-m2 footprint (Fig. 3A-3C).
Fig. 3.
This figure shows the general set up of the AIRO® scanner in our operating room during surgery and intraoperative CT-based scanning. (A) This demonstrates the schematic setup of intraoperative CT-based navigation during the procedure. (B) This demonstrates the schematic setup of the intraoperative CT position during scanning. (C) This picture was made during a procedure in which intraoperative CT-based navigation was used.
Intraoperative Navigation and Surgical Resection
Standard surgical approaches were used, and soft-tissue dissection was performed. Next, stable attachment of a navigation tracking tree, with two 2.8-mm pins fixed to the iliac wing or a clamp to the spinous process, was performed. Image-to-patient registration was done using two-dimensional fluoroscopic images or intraoperative CT-based scanning (5-10 minutes). The operating room personnel stood behind a radiography protection screen, and an on-call radiology technician performed the scanning (Fig. 3B). The acquired imaging data were automatically transferred to DICOM and navigation software. A navigated pointer was used to validate the registration. A registration error of less than 1 mm was considered acceptable. All osteotomies were performed using a navigated oscillating saw or chisel (Fig. 4).
Fig. 4.
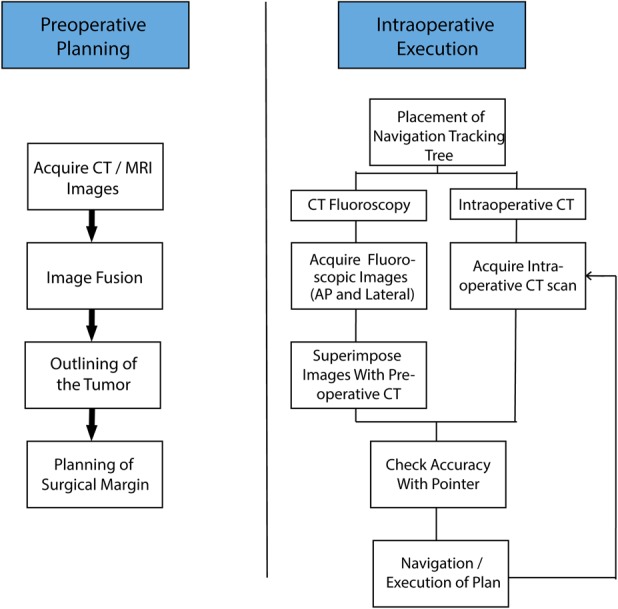
This shows the schematic workflow for computer-assisted orthopaedic surgery.
Resection planes of the nonnavigated resections were based on preoperative plain radiographs, CT images, and MR images. During the operation, we determined the site of the osteotomies using measurements from landmarks that were based on the preoperative imaging data.
After resection, the specimens were sent for a histopathological analysis, including assessment of the surgical margin. In the navigation group, 25 of the 36 patients [69%] underwent reconstruction after resection, of whom 12 had an endoprosthesis, eight had spongiosaplasty with the TSRH 3D® (Medtronic Sofamor Danek, Minneapolis, MN, USA), and five had reconstructions using an autograft or allograft. In the nonnavigation group, 28 of the 34 patients (82%) underwent reconstruction after resection, of whom 13 had an endoprosthesis, two underwent surgery using a TSRH 3D® with spongiosaplasty, and 13 underwent reconstruction using an autograft or allograft. The mean total blood loss, accurately measured by an anesthesiologist, was similar in both groups: 2270 ml ± 2160 in the navigation group versus 2740 ml ±1660 in the nonnavigation group (p = 0.308). Operating time, measured from the start of incision to closure of the wound, was also similar in both groups: 352 min ± 195 in the navigation group versus 333 min ± 126 in the nonnavigation group (p = 0.73).
Major complications were defined by the need for reintervention or a prolonged hospital stay. Fourteen of the 36 patients (39%) in the navigation group had major complications, mainly wound infections resulting in reintervention (10 patients; 71%). Other complications were neurapraxia in one patient (7%), acute renal failure in one (7%), iatrogenic fracture in one (7%), and two surgical sponges were left in the wound that resulted in reoperation in one (7%). None of these complications were thought to be caused by surgical navigation nor were they thought to be complications that could have been avoided using surgical navigation. They were all directly related to the tumor resection itself or reconstruction of the bone and soft-tissue defect. In the nonnavigation control group, 13 of the 34 patients (38%) had major complications; the most common were wound infections that indicated surgery (seven patients [54%]). Other complications consisted of neurapraxia in four patients (31%), excessive bleeding that was examined a second time the next day in one (8%), and screws in the sacral canal that impinged on the nerve roots in one patient (8%). The proportion of major complications was similar between the navigation and nonnavigation groups: 53% versus 47% (odds ratio [OR], 1.03 [95% CI, 0.39-2.69]; p = 0.955). Followup included imaging of the local site and chest every 3 months postoperatively for 2 years followed by every 6 months for 3 years.
Outcome Measures
Surgical margins were evaluated from two areas, the bone and soft tissue, for each patient. Margins were determined by a pathologist (AHGC) using surgical specimens. Bone margins were assessed at the osteotomy and soft-tissue margins at the circumferential soft tissue. All bone and soft-tissue surgical margins were histologically defined based on the worst margin according to a modified Enneking’s classification [10] into wide margins in patients with en bloc resection with a cuff of normal tissue of at least 2 mm, marginal margins in patients with viable tumor cells within 2 mm of the resection plane or a resection plane through the reactive zone, intralesional margins when tumor cells were present in the resection plane, and wide-contaminated if the tumor or its pseudocapsule was exposed intraoperatively but further tissue was removed to achieve wide margins. If the pathology report was inconclusive about either the bone or soft-tissue surgical margin or provided no information regarding the minimal margin, a senior pathologist who specializes in bone tumors (AHGC) reviewed the report and tissue slices and assigned a margin status. For analysis, wide and wide-contaminated margins were considered adequate, and marginal or intralesional margins were considered inadequate [3, 17, 21, 23].
Because we were primarily interested in how navigation affects the bony and soft-tissue surgical margins, we did not examine oncologic outcomes in this study.
Statistical Analysis
Patient characteristics were compared using the Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. ORs with corresponding 95% CIs are provided. A p value < 0.05 was considered significant. For all analyses, IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) was used.
Results
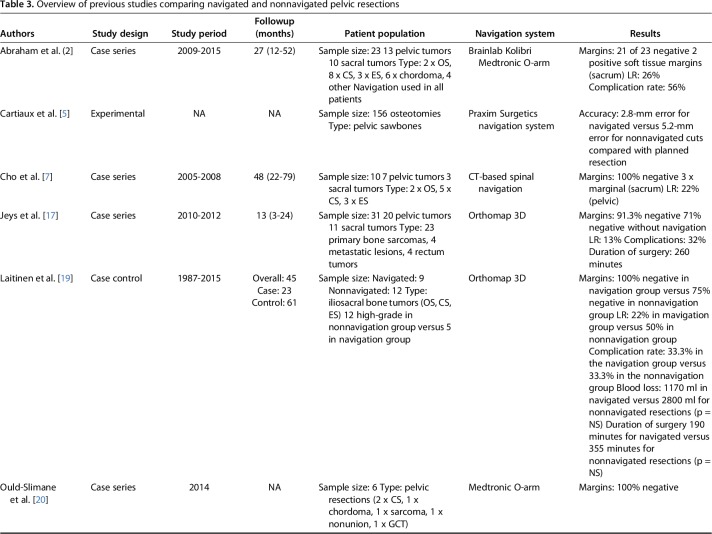
Adequate bone margins were achieved in a higher number of patients in the navigated group than in the nonnavigation group (28 of 36 patients [81%] versus 17 of 34 patients [50%]; OR, 4.14 [95% CI, 1.43-12.01]; p = 0.03). In the navigation group, the bone margin was wide in 28 of 36 patients (78%), wide-contaminated in one (3%), marginal in three (8%), and intralesional in four (11%). In the nonnavigation group, the bone margin was wide in 16 of 34 patients (47%), wide-contaminated in one (3%), marginal in eight (24%), and intralesional in nine (26%) (Table 2). With the numbers available, we found no difference in the ability to achieve adequate soft-tissue margins between the navigated and nonnavigation groups (18 of 36 patients [50%] versus 18 of 34 [54%]; OR, 0.89 [95% CI, 0.35-2.27]; p = 0.995). However, fewer intralesional soft-tissue margins were observed in the navigation group than in the nonnavigation group (two of 36 patients [6%] versus eight of 34 [23%]; OR, 0.19 [95% CI, 0.04-0.98]; p = 0.032) (Table 2). In the navigation group, seven inadequate margins were observed. In three patients, proximity to the nerves, which were not visible on preoperative images, made wide resection impossible. We might have prevented this by using better-quality imaging data and thinner slides. Furthermore, all three tumors originated in the sacrum. There was one wide-contaminated margin. A no-touch technique, in which a Gigli saw is used instead of a chisel or saw, might have prevented this.
Table 2.
Outcome measures for each of the study groups
Discussion
Pelvic and sacral bone sarcoma resections are challenging because of anatomic and surgical complexity. Inadequate surgical margins are more likely to result in inadequate surgical margins in the extremities, which is likely associated with a higher risk of local recurrence. This may have a major impact on the oncologic outcome, especially in patients with chondrosarcomas, for which surgical resection is frequently the preferred treatment option. Computer-aided surgery could assist in achieving higher surgical accuracy and thus improve the oncologic outcome. Available evidence, although promising, is limited to experimental (laboratory) studies and small patient series. Only one small historically controlled study [19] comparing nine navigated and 12 nonnavigated pelvic resections is available (Table 3). We showed that navigation improved our ability to achieve tumor-free bony resection margins in our patients compared with patients in whom navigation was not used, but no differences in the ability to achieve adequate soft-tissue margins were observed.
Table 3.
Overview of previous studies comparing navigated and nonnavigated pelvic resections
This study had a number of limitations. First, while we reported a relatively large cohort of patients who underwent navigated resection for pelvic and sacral primary bone sarcomas, the subgroups were small, and we cannot ensure that our finding of no difference between the groups in baseline characteristics such as tumor type, tumor grade, and type of pelvic resection does not reflect a Type II error.
Second, because we only included patients undergoing resection with the intention to achieve a wide margin, and because pelvic sarcomas are relatively rare, we did not enroll a large number of patients during the study period. Furthermore, different surgeons might disagree about whether a procedure will result in a wide margin. This might have resulted in a selection bias. However, a large cohort was presented, and we therefore feel that our study groups adequately represent the general population.
Third, the tumor location and type of reconstruction, if any, strongly influence the total blood loss, operating time, and proportion of complications. Because we only had data on the total operating time and blood loss, including those of reconstruction, we were not able to adequately assess differences in blood loss, complications, and operating time between the groups.
Fourth, we attempted to avoid selection bias by selecting control patients who underwent surgery when surgical navigation was not available at our institution. When surgical navigation was available, it was used for all patients presenting with a primary pelvic or sacral tumor in which the aim was to achieve a wide margin. In 10 patients, there were technical problems with the navigation software, which caused us to switch to nonnavigated resection. Twenty-four patients presenting with primary malignant tumors underwent debulking or intralesional curettage; these patients were excluded from this study. Because it is unlikely that a randomized study will be performed, we think that our group of patients treated with resection for similar tumor types before we began using navigation serves as a reasonable comparison.
Fifth, all nonnavigated resections were performed by two senior orthopaedic oncology surgeons who specialize in pelvic surgery. The potential differences in experience between the surgeons could have influenced the results. Most nonnavigated resections were performed by AHMT, who was at that time at the end of his learning curve and retired during the study period. All navigated resections were performed by one experienced senior orthopaedic surgeon (PDSD). Research by Farfalli et al. [11] shows that surgical time decreases as surgeons perform more navigated procedures. This learning curve might have influenced the operating time and therefore the results. However, this surgeon had much experience with surgical navigation for spondylodesis of the spine and benign tumor resections, and was familiar with the software before performing navigated resections for malignant bone sarcomas. Therefore, we feel that we minimized the risk of the learning curve because the surgeon was highly familiar with surgical navigation. Even though differences between the surgeons could have influenced the results, all resections were performed by experienced surgeons with more than 10 years of experience; therefore, we believe our study groups adequately represent the general population.
Sixth, in our study, wide and wide-contaminated margins were considered adequate. The definition of an adequate margin differs among tumor types and grades. For a low-grade chondrosarcoma, a marginal resection could be considered adequate, especially in the extremities, whereas for grade 2 or 3 chondrosarcomas, a 4-mm margin (or at least more than 2 mm) is advised to reduce the risk of local recurrence [23]. For a high-grade osteosarcoma, a wide margin (classified as > 2 mm) is prognostic for local recurrence-free survival [17]. Furthermore, in patients with Ewing’s sarcoma, the local recurrence rate and event-free survival in patients with or without distant metastasis is better after wide resection than after marginal or intralesional resections [3, 21]. Research showed that only wide surgical margins are associated with improved oncologic outcomes and less local recurrence of most tumor types. Wide-contaminated margins, in which the tumor or its pseudocapsule is exposed intraoperatively but further tissue is removed to achieve wide margins, were also considered adequate. The aim of our study was to assess our ability to achieve a wide margin using a certain technique; therefore, wide-contaminated margins were considered adequate. If the aim was to study the oncologic outcome and survival, a wide-contaminated margin should not have been considered adequate.
Finally, the short followup period for patients who underwent intraoperative CT-assisted resections and the wide variety of patient diagnoses, treatments, and ages inevitably resulted in missing information on clinical outcomes. However, the aim of this study was to assess our ability to achieve a negative margin in navigated and nonnavigated resections to treat pelvic and sacral primary bone sarcomas, and research showed that wide margins are associated with improved oncologic outcomes in most tumor types [3, 17, 21, 23]. For a true reflection of the oncologic outcome, long-term followup data are required for a range of tumor types and stages. Only then will we know the true effect of computer-assisted surgery.
The use of navigation during surgery resulted in a higher number of patients with adequate bony surgical margins. No differences in adequate soft-tissue margins were observed between the groups; however, we observed fewer intralesional soft-tissue margins with the use of navigation than without.
Computer-aided surgery for pelvic resections was first reported by Krettek et al. [18] and Hufner et al. [14]. They concluded that computer-aided surgery may increase accuracy in tumor resections involving anatomic and surgical complexity. Computer-aided surgery further evolved because of Wong et al. [28, 29], who described CT and MRI fusion in navigated tumor surgery. Two experimental studies showed improved bone cutting accuracy during pelvic resection using surgical navigation. Sternheim et al. [22] found that osteotomies using navigation within 5 mm of the planned cut resulted in a negative margin in more than 95% of the cuts. Cartiaux et al. [5] showed a mean location accuracy of 2.8 mm from the target plane using navigation and no intralesional cuts. These results imply that with a decrease in error, removal of less normal bone may be possible while achieving negative bony margins, which may translate to better reconstruction possibilities. Laitinen et al. [19] presented the first historically controlled study comparing nine navigated and 12 nonnavigated pelvic resections. In the navigation group, no intralesional margins and 22% local recurrence were observed, versus three intralesional margins (25%) and 50% local recurrence in the nonnavigation group. The authors also noted less intraoperative blood loss and reduced surgical time in the navigation group. Several studies used local recurrence as an endpoint (Table 3). However, surgical navigation is a relatively new technique; therefore, long-term followup data to assess oncologic outcomes are not available from any center we know of to date, which questions the strength of these conclusion. The use of navigation does not guide or help to achieve more adequate soft-tissue margins. However, better visualization during surgery could contribute to the lower proportion of intralesional soft-tissue margins observed in this study. Compromised soft-tissue margins are considered a poor independent prognostic factor, and most local recurrences occur in the soft tissue (margin) and not in osteotomy [7, 11, 15, 26].
We showed that computer-aided surgery improved our ability to achieve adequate margins during bony resections in patients with pelvic and sacral bone sarcomas. Achieving adequate soft-tissue margins remains a challenge, and we could not determine whether patients in whom sarcomas are resected using navigation will have reduced local recurrence rates or improved survival. Computer-aided surgery could also aid in pelvic reconstruction by facilitating allograft planning and three-dimensional printed endoprostheses, although we did not evaluate this in the current study [6, 8, 27]. Prospective multicenter studies with long-term followup are needed to further investigate the potential benefits of this promising technique.
Acknowledgments
We thank Professor Dr. A.H.M. Taminiau for his contribution to and expertise in the treatment of pelvic and sacral bone sarcoma.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Leiden University Medical Center, Leiden, the Netherlands.
References
- 1.Abed R, Grimer R. Surgical modalities in the treatment of bone sarcoma in children. Cancer Treat Rev. 2010;36:342-347. [DOI] [PubMed] [Google Scholar]
- 2.Abraham JA, Kenneally B, Amer K, Geller DS. Can Navigation-assisted Surgery Help Achieve Negative Margins in Resection of Pelvic and Sacral Tumors? Clin Orthop Relat Res. 2018;476:499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacci G, Longhi A, Briccoli A, Bertoni F, Versari M, Picci P. The role of surgical margins in treatment of Ewing's sarcoma family tumors: experience of a single institution with 512 patients treated with adjuvant and neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:766-772. [DOI] [PubMed] [Google Scholar]
- 4.Bus MPA, Campanacci DA, Albergo JI, Leithner A, van de Sande MAJ, Gaston CL, Caff G, Mettelsiefen J, Capanna R, Tunn PU, Jeys LM, Dijkstra PDS. Conventional Primary Central Chondrosarcoma of the Pelvis: Prognostic Factors and Outcome of Surgical Treatment in 162 Patients. J Bone Joint Surg Am. 2018;100:316-325. [DOI] [PubMed] [Google Scholar]
- 5.Cartiaux O, Paul L, Docquier PL, Raucent B, Dombre E, Banse X. Computer-assisted and robot-assisted technologies to improve bone-cutting accuracy when integrated with a freehand process using an oscillating saw. J Bone Joint Surg Am. 2010;92:2076-2082. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Xu L, Wang Y, Hao Y, Wang L. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput Methods Programs Biomed. 2016;125:66-78. [DOI] [PubMed] [Google Scholar]
- 7.Cho HS, Oh JH, Han I, Kim HS. The outcomes of navigation-assisted bone tumour surgery: minimum three-year follow-up. J Bone Joint Surg Br. 2012;94:1414-1420. [DOI] [PubMed] [Google Scholar]
- 8.Docquier PL, Paul L, Cartiaux O, Delloye C, Banse X. Computer-assisted resection and reconstruction of pelvic tumor sarcoma. Sarcoma. 2010;2010:125162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731-746. [PubMed] [Google Scholar]
- 10.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980:106-120. [PubMed] [Google Scholar]
- 11.Farfalli GL, Albergo JI, Ritacco LE, Ayerza MA, Milano FE, Aponte-Tinao LA. What Is the Expected Learning Curve in Computer-assisted Navigation for Bone Tumor Resection? Clin Orthop Relat Res. 2017;475:668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs B, Hoekzema N, Larson DR, Inwards CY, Sim FH. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop Relat Res. 2009;467:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han I, Lee YM, Cho HS, Oh JH, Lee SH, Kim HS. Outcome after surgical treatment of pelvic sarcomas. Clin Orthop Surg. 2010;2:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hufner T, Kfuri M, Jr., Galanski M, Bastian L, Loss M, Pohlemann T, Krettek C. New indications for computer-assisted surgery: tumor resection in the pelvis. Clin Orthop Relat Res. 2004:219-225. [DOI] [PubMed] [Google Scholar]
- 15.Jeon D-G, Song WS, Kong C-B, Cho WH, Cho SH, Lee JD, Lee S-Y. Role of surgical margin on local recurrence in high risk extremity osteosarcoma: a case-controlled study. Clin Ortho Surg. 2013;5:216-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeys L, Matharu GS, Nandra RS, Grimer RJ. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J. 2013;95-B:1417-1424. [DOI] [PubMed] [Google Scholar]
- 17.Jeys LM, Thorne CJ, Parry M, Gaston CLL, Sumathi VP, Grimer JR. A Novel System for the Surgical Staging of Primary High-grade Osteosarcoma: The Birmingham Classification. Clin Orthop Relat Res. 2017;475:842-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krettek C, Geerling J, Bastian L, Citak M, Rucker F, Kendoff D, Hufner T. Computer aided tumor resection in the pelvis. Injury. 2004;35 Suppl 1:S-A79-83. [DOI] [PubMed] [Google Scholar]
- 19.Laitinen MK, Parry MC, Albergo JI, Grimer RJ, Jeys LM. Is computer navigation when used in the surgery of iliosacral pelvic bone tumours safer for the patient? Bone Joint J. 2017;99-B:261-266. [DOI] [PubMed] [Google Scholar]
- 20.Ould-Slimane M, Thong P, Perez A, Roussignol X, Dujardin FH. The role of Intraoperative 3D navigation for pelvic bone tumor resection. Orthop Traumatol Surg Res. 2016;102:807-811. [DOI] [PubMed] [Google Scholar]
- 21.Ozaki T, Hillmann A, Hoffmann C, Rube C, Blasius S, Dunst J, Jurgens H, Winkelmann W. Significance of surgical margin on the prognosis of patients with Ewing's sarcoma. A report from the Cooperative Ewing's Sarcoma Study. Cancer. 1996;78:892-900. [DOI] [PubMed] [Google Scholar]
- 22.Sternheim A, Daly M, Qiu J, Weersink R, Chan H, Jaffray D, Irish JC, Ferguson PC, Wunder JS. Navigated pelvic osteotomy and tumor resection: a study assessing the accuracy and reproducibility of resection planes in Sawbones and cadavers. J Bone Joint Surg Am. 2015;97:40-46. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson JD, Laitinen MK, Parry MC, Sumathi V, Grimer RJ, Jeys LM. The role of surgical margins in chondrosarcoma. Eur J Surg Oncol. 2018;44:1412-1418. [DOI] [PubMed] [Google Scholar]
- 24.Sucato DJ, Rougraff B, McGrath BE, Sizinski J, Davis M, Papandonatos G, Green D, Szarzanowicz T, Mindell ER. Ewing's sarcoma of the pelvis. Long-term survival and functional outcome. Clin Orthop Relat Res. 2000:193-201. [PubMed] [Google Scholar]
- 25.Wirbel RJ, Schulte M, Maier B, Koschnik M, Mutschler WE. Chondrosarcoma of the pelvis: oncologic and functional outcome. Sarcoma. 2000;4:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong KC, Kumta SM. Computer-assisted tumor surgery in malignant bone tumors. Clin Orthop Relat Res. 2013;471:750-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong KC, Kumta SM. Use of Computer Navigation in Orthopedic Oncology. Curr Surg Rep. 2014;2:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong KC, Kumta SM, Antonio GE, Tse LF. Image fusion for computer-assisted bone tumor surgery. Clin Orthop Relat Res. 2008;466:2533-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br. 2007;89:943-947. [DOI] [PubMed] [Google Scholar]
- 30.Young PS, Bell SW, Mahendra A. The evolving role of computer-assisted navigation in musculoskeletal oncology. Bone Joint J. 2015;97-B:258-264. [DOI] [PubMed] [Google Scholar]