Abstract
Background
Distinguishing osteomyelitis from soft-tissue infection of the foot is important because osteomyelitis is associated with more operations, amputation, and prolonged antibiotic exposure. Both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are routinely ordered inflammatory biomarkers for evaluating foot infection. When initial evaluation is inconclusive, advanced imaging is indicated, and high clinical or radiographic suspicion of osteomyelitis may indicate bone biopsy to identify organisms and antibiotic sensitivity. Although ESR and CRP levels are helpful for distinguishing osteomyelitis from soft-tissue infections in patients with diabetes-related foot infections, parameters regarding optimal cutoff values for those tests have not, to our knowledge, been defined.
Questions/purposes
(1) What are the optimal cutoff values for ESR and CRP to differentiate osteomyelitis from soft-tissue infection in patients with diabetes-related foot infection? (2) Can a diagnostic algorithm be derived to guide interpretation of ESR and CRP to improve recognition of osteomyelitis in the setting of diabetic foot infection?
Methods
The medical records of 1842 patients between 18 and 89 years of age treated at our institution between January 1, 2010 and February 6, 2017 for foot infection were reviewed. For inclusion, patients must have had a diagnosis of diabetes mellitus, moderate or severe infection, ESR and CRP values within 72 hours of admission, either advanced imaging (MRI or single-positron emission computed tomography/computed tomography [SPECT/CT]) or bone biopsy during admission and must not have had comorbidities that could affect ESR and CRP, such as autoimmune disorders. As such, 1489 patients were excluded, and 353 patients were included in the study. Osteomyelitis was diagnosed by positive bone culture or histopathology. Osteomyelitis was considered to be absent if there was a negative MRI or SPECT/CT result, or negative bone culture and histology findings if imaging was inconclusive. We identified 176 patients with osteomyelitis and 177 with soft-tissue infection. A blinded investigator performed the statistics. Optimal cutoffs of ESR and CRP were determined using receiver operative characteristic (ROC) analysis. A diagnostic algorithm was determined using epidemiologic principles of screening evaluations.
Results
An ESR of 60 mm/h and a CRP level of 7.9 mg/dL were determined to be the optimal cutoff points for predicting osteomyelitis based on results of the ROC analysis. The ESR threshold of 60 mm/h demonstrated a sensitivity of 74% (95% confidence interval [CI], 67–80) and specificity of 56% (95% CI, 48–63) for osteomyelitis, whereas the CRP threshold of 7.9 mg/dL had a sensitivity of 49% (95% CI, 41–57) and specificity of 80% (95% CI, 74–86). If the ESR is < 30 mm/h, the likelihood of osteomyelitis is low. However, if ESR is > 60 mm/h and CRP level is > 7.9 mg/dL, the likelihood of osteomyelitis is high, and treatment of suspected osteomyelitis should be strongly considered.
Conclusions
While ESR is better for ruling out osteomyelitis initially, CRP helps distinguish osteomyelitis from soft-tissue infection in patients with high ESR values. Further prospective studies addressing the prognostic value of ESR and CRP are needed, and a more comprehensive diagnostic algorithm should be developed to include other diagnostic tests such as probe-to-bone and imaging.
Level of Evidence
Level III, diagnostic study.
Introduction
Diabetic foot infections are a common cause of diabetes-related hospitalizations [4, 26]. Distinguishing osteomyelitis from soft-tissue infection is important because bone infections are associated with more operations, amputation procedures, and prolonged antibiotic exposure, which may increase antibiotic-related complications such as pathogen resistance, gastrointestinal complications, and acute kidney injury [2, 8, 20, 21, 29, 32]. An incorrect diagnosis of osteomyelitis in a patient with a soft-tissue infection can lead to treatments that may increase the risk of complications and may even result in unnecessary amputation. Conversely, late recognition of osteomyelitis of the foot can lead to spread of the infection, sepsis or septic shock, and amputation [16]. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are inflammatory biomarkers that are evaluated using readily available and relatively inexpensive laboratory tests. Previously reported cutoffs of ESR and CRP for distinguishing osteomyelitis and soft-tissue infection involving the foot in diabetic patients were evaluated separately in small study populations [6, 7, 9, 11, 17, 19, 21, 23, 30]. However, these studies did not propose detailed guidelines on how these tests should be interpreted. In addition, most of these studies did not consistently use results of culture or histopathology from bone biopsy as the gold standard evaluation for determining the presence of osteomyelitis, and no studies used both culture and histopathology results to identify the presence of osteomyelitis [6, 9, 19, 23]. To our knowledge, the current study is the largest study to examine ESR and CRP cutoffs to distinguish osteomyelitis from soft-tissue infections in diabetes and foot infections. Furthermore, this is the only study to propose a diagnostic algorithm to maximize the utility of both biomarkers for detecting osteomyelitis of the foot rather than simply defining individual cutoffs for ESR and CRP.
Therefore, we asked: (1) What are the optimal cutoff values for ESR and CRP to differentiate osteomyelitis from soft-tissue infection in patients with diabetes-related foot infection? (2) Can a diagnostic algorithm be derived using ESR and CRP cutoffs to guide interpretation and evaluation of inflammatory biomarkers to improve recognition of osteomyelitis in the setting of diabetic foot infection?
Patients and Methods
Study Design and Setting
After receiving institutional review board approval for the protocol of this study, we reviewed the medical records of patients hospitalized for moderate and severe foot infections that raised concerns of osteomyelitis at a safety net hospital between January 1, 2010 and February 6, 2017, the period that the senior investigator has been practicing at our institution. The presence of foot infection was defined based on criteria consistent with the Infectious Diseases Society of America and the International Working Group Diabetic Foot Infection Guidelines [15, 16]; that is, the clinical observation of purulence or at least two local signs or symptoms of inflammation. Initially, the medical records of 1842 patients between the ages of 18 and 89 years with moderate and severe foot infection were reviewed. In these patients, ESR and CRP are routinely ordered around the time of admission by hospitalists and emergency department staff at our institution. Indications for advanced imaging through MRI or single-positron emission computed tomography/computed tomography (SPECT/CT) include inconclusive clinical and plain radiographic findings. Based on clinical signs, probe-to-bone examination, plain radiographs and advanced imaging, bone biopsy may be indicated depending on whether there is a high index of suspicion for osteomyelitis to identify causative organisms and determine antibiotic sensitivities. However, patients with exposed bone may undergo bone biopsy without advanced imaging. Naturally, patients who did not have ESR or CRP values assessed within 72 hours of admission, pathology results or radiographic evaluation through MRI or SPECT/CT were excluded. However, we excluded patients with comorbidities that could affect baseline ESR and CRP levels, such as autoimmune diseases. After excluding nondiabetic patients based on the American Diabetes Association diagnostic criteria, 353 patients were included in the study [1]. The gold standard for diagnosis of osteomyelitis is bone biopsy. Ideally, every patient, regardless of MRI or SPECT/CT results, would undergo bone biopsy. However, as this study was performed retrospectively, this was not possible as bone biopsy is not standard of care for all foot infections.
Diagnostic Categorization
After we excluded 1489 patients for the reasons listed above, 353 patients were included in the study. Patients were separated into two groups, osteomyelitis or soft-tissue infection, based on a combination of bone culture, histopathology, and imaging results. We used bone biopsy results to confirm the diagnosis of osteomyelitis, and negative MRI, negative SPECT/CT, or negative results on bone biopsy to identify patients without a bone infection [3]. In this study, 88 patients had positive culture and histopathological changes consistent with osteomyelitis. Forty-one patients had positive histopathology without cultures results, and five patients had positive cultures without histopathology results reported. Twenty-two patients had positive culture with negative histopathology, and 21 had positive histopathology with negative culture. As long as bone biopsy culture or histopathology was positive, we categorized these patients as having osteomyelitis. As a result, 177 patients were included in the osteomyelitis group.
Patients with negative bone culture results and negative histopathology or negative MRI or SPECT/CT findings without bone biopsy results were considered not to have osteomyelitis and were included in the soft-tissue infection group. Specifically, 129 patients had negative MRI and/or negative SPECT/CT and did not undergo bone biopsy. However, 16 patients with negative MRI and/or negative SPECT/CT underwent bone biopsy based on clinical suspicion of osteomyelitis, but none of these patients had positive culture or histopathology. Seventeen of 19 patients with nonspecific MRI or SPECT/CT findings underwent bone biopsies, which were all negative for osteomyelitis. Two patients with nonspecific MRI underwent SPECT/CT scans that were negative and did not subsequently undergo bone biopsy. Five patients who had positive MRI or SPECT/CT findings underwent bone biopsies that were all negative. Seven patients who did not have MRI or SPECT/CT imaging underwent bone biopsy which demonstrated negative culture and histopathology. As a result, these 176 patients were categorized in the soft-tissue infection group.
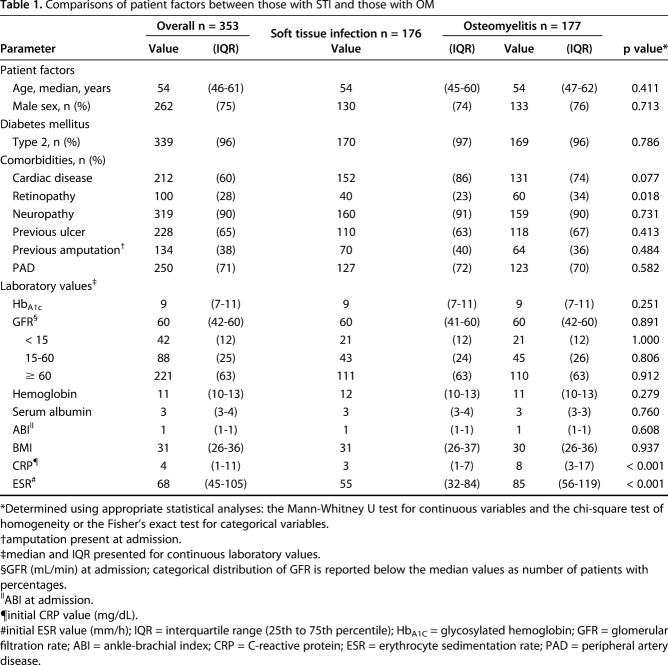
The median age of the study patients was 54 years (interquartile range [IQR], 46–61 years). Seventy-five percent were male, and approximately 96% had type II diabetes mellitus. No differences between the groups were identified regarding age (p = 0.411), sex (p = 0.713), or comorbidities, except that there was a lower prevalence of retinopathy (p = 0.018) in patients with soft-tissue infection than in those with osteomyelitis (Table 1).
Table 1.
Comparisons of patient factors between those with STI and those with OM
Statistical Analysis
After data collection was completed, a blinded investigator (JA) not involved in data collection was given a coded version of the data for statistical analysis. All continuous variables were tested for normality using a quantile-quantile plot, histogram, and Shapiro-Wilk analysis. Because most of these variables did not have a normal distribution, descriptive statistical analyses were used to determine the median values of continuous variables, with an IQR between the 25th to 75th quartiles, and frequencies and percentages of categorical variables. Continuous variables were compared between groups using either a Student t-test or Mann-Whitney U test, and categorical variables were analyzed using the Pearson χ2 test or Fisher exact test, when appropriate. Contingency tables were used to calculate descriptive epidemiologic measures (odds ratios [OR], sensitivity, and specificity). A receiver operating characteristic (ROC) curve analysis was used for both ESR and CRP to determine the performance of each test in detecting osteomyelitis. The ROC curves were compared through the DeLong test [5]. In addition, various cutoff levels of interest for ESR and CRP were evaluated to determine the relationship between increasing values of these inflammatory biomarkers and their sensitivity and specificity. Optimal threshold values for ESR and CRP, defined as those with the lowest rates of false results, were identified by selecting the thresholds with the maximum Youden’s J-statistic value and then confirming through the ROC analysis [34]. An alpha value of 0.05 was set for all statistical analyses. All statistical analyses were performed using the open source statistical program R version 3.3.1 (R Development Core Team, Vienna, Austria) [22].
Results
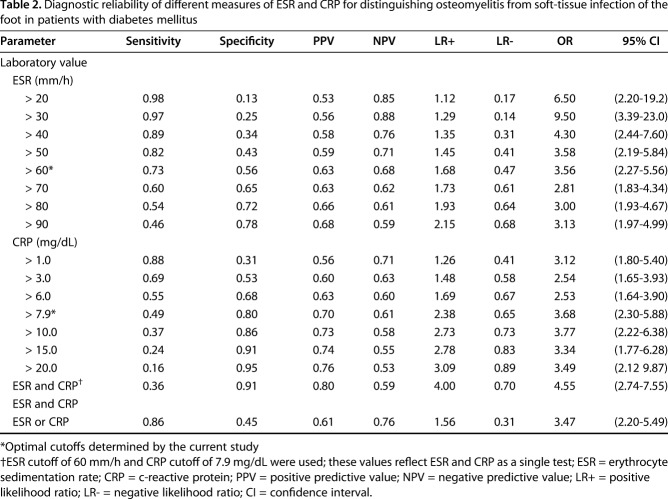
An ESR of 60 mm/h and CRP level of 7.9 mg/dL were determined to be the optimal cutoff points for predicting osteomyelitis. The ESR threshold of 60 mm/h demonstrated a sensitivity of 74% (95% CI, 66–78) and specificity of 56% (95% CI, 49–64) for osteomyelitis, whereas the CRP threshold of 7.9 mg/dL had a sensitivity of 49% (95% CI, 42–57) and specificity of 80% (95% CI, 73–86). Sensitivity and specificity were also calculated for combinations of ESR and CRP thresholds (Table 2). When combined in an “or” fashion, where a suprathreshold value of ESR or CRP is a positive result, and a subthreshold value of ESR and CRP is a negative result, the combination demonstrated high sensitivity (87% [95% CI, 81–92]) and low specificity (45% [95% CI, 37–53]). In contrast, when combining the tests in an “and” fashion, where suprathreshold values of both ESR and CRP are a positive result, and a subthreshold value of one or both tests is considered a negative result, the combination showed low sensitivity (36%; 95% CI, 29–44) but high specificity (91%; 95% CI, 86–95) (Fig. 1). The ESR threshold of 60 mm/h had a positive predictive value (PPV) of 63% and a negative predictive value (NPV) of 68%. In comparison, for the CRP threshold of 7.9 mg/dL, the PPV was 70% and the NPV was 61%. However, the highest NPV was seen with an ESR threshold of 30 mm/h (88%), and the highest PPV was seen with the “and” combination of the ESR threshold of 60 mm/h and CRP threshold of 7.9 mg/dL (Table 2). In addition, positive likelihood ratios (LR+) and negative likelihood ratios (LR-) followed the same pattern. An ESR threshold of 30 mm/h had the lowest LR- while the “and” combination of both the ESR and CRP thresholds showed the highest LR+. These results suggest that ESR < 30 mm/h had the lowest proportion of false negatives while the “and” combination of both ESR and CRP thresholds above the optimal cutoff values had the lowest proportion of false positives.
Table 2.
Diagnostic reliability of different measures of ESR and CRP for distinguishing osteomyelitis from soft-tissue infection of the foot in patients with diabetes mellitus
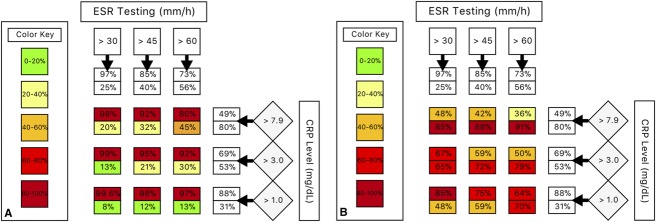
Fig. 1.
(A) Using an abnormal erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level as a positive indicator of the presence of osteomyelitis increases the overall sensitivity (top number) but reduces specificity (bottom number). Note that an ESR value of < 30 mm/h is highly sensitive, and testing CRP marginally increases the sensitivity. (B) Conversely, if both an abnormal ESR and an abnormal CRP value are considered to indicate the presence of osteomyelitis, this increases overall specificity but reduces sensitivity. Note that ESR > 60 mm/h and CRP level > 7.9 mg/dL have a specificity of 91%.
Our proposed algorithm for differentiating osteomyelitis from soft-tissue infection is as follows: when the ESR is > 60 mm/h and the CRP level is > 7.9 mg/dL, osteomyelitis is likely (sensitivity, 36%; specificity, 91%; PPV, 0.80; NPV, 0.59; LR+, 4.00; LR-, 0.70). When the ESR is < 30 mm/h, osteomyelitis is unlikely (LR-, 0.14). Because an ESR value > 30 mm/h is highly sensitive (97%; 95% CI, 94–99), CRP only marginally increases the sensitivity (ESR > 30 mm/h and CRP >7.9 mg/dL, sensitivity 98%; 95% CI, 95–99) (Fig. 1). Therefore, when the ESR is < 30 mm/h, there is little justification to consider it in combination with CRP. For patients with an ESR value between 30 and 60 mm/h, or when there is high clinical suspicion in the absence of elevated ESR and CRP values, clinicians should consider using imaging or another diagnostic evaluation methods (Fig. 2).
Fig. 2.
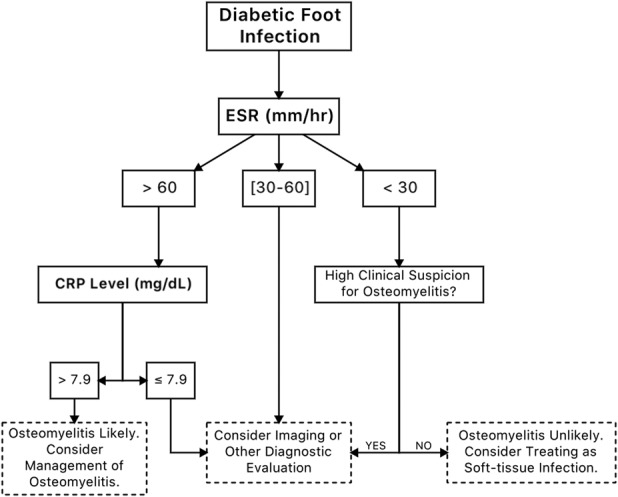
This shows a flowchart outlining the recommended approach to foot infections in patients with diabetes with possible underlying osteomyelitis using the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) based on the findings of the current study. In patients with an ESR < 30 mm/h, CRP testing offers marginal value for diagnosing osteomyelitis. In addition, CRP testing does not add value for patients with an ESR between 30 and 60 mm/h because a low CRP value does not increase likelihood of osteomyelitis, and a high CRP value does not decrease likelihood of osteomyelitis.
Discussion
The presence of foot osteomyelitis in patients with diabetes has major clinical implications because its presence affects treatment outcomes, complication risks, and therapy costs [27]. Failure to diagnose osteomyelitis may delay the onset of proper treatment and result in spread of the infection, potentially necessitating more surgeries, proximal amputation, and prolonged antibiotic exposure [2, 29]. Although several studies have examined the diagnostic value of ESR for osteomyelitis of the foot in patients with diabetes, few have been performed for CRP [7, 9, 19]. In addition, previous reports simply reported optimal cutoff values for ESR and/or CRP without a clear method of using both tests [7, 19]. Providing simple cutoff values are not as clinically valuable because they do not specify how physicians should approach certain situations, such as when ESR and CRP values conflict. To the best of our knowledge, this study is the first to describe a flowchart detailing the optimal usage of both ESR and CRP for differentiating osteomyelitis from soft-tissue infection of the foot in patients with diabetes. We found that ESR values less than 30 mm/h are not likely to be associated with osteomyelitis and that CRP only adds diagnostic value when ESR is greater than 60 mm/h (Fig. 2).
Optimal Cutoff Values for ESR and CRP to Diagnose Osteomyelitis
We found that both ESR and CRP have optimal cutoff values for differentiating osteomyelitis from soft-tissue infection. The optimal cutoff points for ESR and CRP in the current study were similar to those of Fleischer et al. [7]. In addition, that was the only study to evaluate ESR and CRP while also confirming the diagnosis of osteomyelitis through bone histology [7]. The conclusions of Fleischer et al. [7] were that (1) patients with ESR values greater than 60 mm/h and CRP values greater than 3.2 mg/dL should be quickly recognized as having an increased likelihood of having osteomyelitis, and (2) those with mild-to-moderate elevations of these markers likely have soft-tissue infection. Although the results of the current study corroborate their first conclusion about ESR and CRP values above the optimal threshold values, our results also suggest that ESR and CRP should only be used together in certain situations.
Diagnostic Algorithm
Our proposed clinical decision-making tool uses ESR and CRP in combination to make the diagnosis of osteomyelitis and uses ESR alone to exclude it (Fig. 2). A patient with an ESR greater than 60 mm/h should have their CRP level evaluated because it can add to the diagnostic value because an ESR greater than 60 mm/h has low specificity for osteomyelitis (56%), and the CRP threshold of 7.9 mg/dL has much-higher specificity. A CRP level greater than 7.9 mg/dL with a finding of ESR greater than 60 mm/h increases the specificity, making a diagnosis of osteomyelitis likely. However, a CRP level less than 7.9 mg/dL with an ESR greater than 60 mm/h does not suggest presence of osteomyelitis. In patients with a normal ESR (< 30 mm/h), osteomyelitis is unlikely. For values of ESR between 30 and 60 mm/h, the prevalence of osteomyelitis was roughly 50%, and we could not identify any CRP values that helped distinguish osteomyelitis from soft-tissue infection within this range of ESR values.
Limitations
This study had several limitations. Retrospective studies like this one may contain errors in the collection of data, disease definitions, and missing data. However, the key variables assessed in this study, such as the presence of diabetes, laboratory values, bone culture, and pathology findings, were given careful consideration and were confirmed with secondary reports, such as evaluating proportion of glycated hemoglobin for presence of diabetes and a review of patient history. No patients had missing values with regard to these key variables. However, we did not confirm all patient comorbidities such as searching for systolic ejection fraction to confirm reports of heart disease or evaluating antibody titers to confirm reports of autoimmune disease. Furthermore, we were not able to identify the rationale for physicians’ decisions to pursue various evaluations. It is difficult to determine if the results of CRP or ESR influenced the decision to get advanced imaging or perform a bone biopsy. However, we noted that advanced imaging and inflammatory markers were frequently ordered at the same time. Furthermore, the cutoff values for CRP and ESR are higher with osteomyelitis of the foot in patients with diabetes compared with other anatomic sites [10, 12, 13, 24, 28, 31, 33]. However, these measures are not applicable to the diabetic foot. Therefore, extrapolating biomarker cutoff points from other anatomic sites to the diagnosis of diabetic foot osteomyelitis has little use in guiding clinical decision making. The use of these values may lead to unwarranted, expensive, and/or invasive testing. In addition, neither ESR nor CRP is particularly useful for distinguishing osteomyelitis from soft-tissue infection of the foot in patients without diabetes [25]. In our experience, internists and emergency room physicians often rely on advanced imaging with a high index of suspicion for deep abscess or osteomyelitis regardless of ESR or CRP. As such, it is unlikely that these physicians were acutely aware of previous evidence to associate particular ESR values with osteomyelitis of the foot in patients with diabetes. Physicians do not often report their rationale for ordering specific tests, so it was not possible to conclusively determine if inflammatory markers influenced prescriptive behaviour. In addition, although we used the “gold standard” of bone biopsy as the confirmatory test for osteomyelitis based on clinical and imaging findings, the evaluation of histology and cultures is not entirely reliable or accurate. However, interobserver variability in the interpretation of bone histology has been reported to be high and could provide an inconsistent interpretation of histology specimens [18]. Lastly, all patients in this study were hospitalized for moderate and severe diabetic foot osteomyelitis, where the pretest probability of osteomyelitis was high (50%–67%). The study results may not be applicable to patients seen in outpatient settings, where the pretest probability of osteomyelitis is low (20%) [8, 14]. Therefore, the clinical algorithm presented in this study should be used with caution in patients with diabetes treated in the outpatient setting with mild infections of the foot.
Conclusions
Although ESR is better for excluding the diagnosis osteomyelitis initially, CRP helps distinguish osteomyelitis from soft-tissue infection in patients with high ESR values. Our proposed clinical algorithm can help clinicians to distinguish osteomyelitis from soft-tissue infection in patients with diabetic foot infection using thresholds for ESR and CRP (Fig. 2). Further studies addressing the prognostic value of ESR and CRP are needed, and a more comprehensive diagnostic algorithm that includes other diagnostic tests such as probe-to-bone and imaging should be developed. Preferably, the optimal study would be performed prospectively with each patient with diabetes-related foot infection evaluated undergoing the same clinical examination, laboratory testing, radiographic imaging and bone biopsy protocol. In addition to determining ESR and CRP levels, future studies may consider the location of the infection because there could be an effect on ESR and CRP levels.
Footnotes
Each author certifies that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13-S27. [DOI] [PubMed] [Google Scholar]
- 2.Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, Jeffcoate WJ, Lipsky BA, Senneville E, Teh J, Valk GD. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev. 2008; 24( Suppl 1):S145-161. [DOI] [PubMed] [Google Scholar]
- 3.Butalia S, Palda VA, Sargeant RJ, Detsky AS, Mourad O. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813. [DOI] [PubMed] [Google Scholar]
- 4.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure). National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568-577. [DOI] [PubMed] [Google Scholar]
- 5.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] [Google Scholar]
- 6.Ertugrul BM, Savk O, Ozturk B, Cobanoglu M, Oncu S, Sakarya S. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-312. [PubMed] [Google Scholar]
- 7.Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg. 2009;48:39-46. [DOI] [PubMed] [Google Scholar]
- 8.Grayson ML, Gibbons GW, Habershaw GM, Freeman DV, Pomposelli FB, Rosenblum BI, Levin E, Karchmer AW. Use of ampicillin/sulbactam versus imipenem/cilastatin in the treatment of limb-threatening foot infections in diabetic patients. Clin Infect Dis. 1994;18:683-693. [DOI] [PubMed] [Google Scholar]
- 9.Jeandrot A, Richard JL, Combescure C, Jourdan N, Finge S, Rodier M, Corbeau P, Sotto A, Lavigne JP. Serum procalcitonin and C-reactive protein concentrations to distinguish mildly infected from non-infected diabetic foot ulcers: a pilot study. Diabetologia. 2008;51:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang N, Ma YF, Jiang Y, Zhao XQ, Xie GP, Hu YJ, Qin CH, Yu B. Clinical Characteristics and Treatment of Extremity Chronic Osteomyelitis in Southern China: A Retrospective Analysis of 394 Consecutive Patients. Medicine (Baltimore). 2015;94:e1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaleta JL, Fleischli JW, Reilly CH. The diagnosis of osteomyelitis in diabetes using erythrocyte sedimentation rate: a pilot study. J Am Podiatr Med Assoc. 2001;91:445-450. [DOI] [PubMed] [Google Scholar]
- 12.Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6:311-315. [DOI] [PubMed] [Google Scholar]
- 13.Kim CJ, Song KH, Park WB, Kim ES, Park SW, Kim HB, Oh MD, Kim NJ. Microbiologically and clinically diagnosed vertebral osteomyelitis: impact of prior antibiotic exposure. Antimicrob Agents Chemother. 2012;56:2122-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract. 2009;83:347-352. [DOI] [PubMed] [Google Scholar]
- 15.Lipsky BA, Aragon-Sanchez J, Diggle M, Embil J, Kono S, Lavery L, Senneville E, Urbancic-Rovan V, Van Asten S, International Working Group on the Diabetic F, Peters EJ. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):45-74. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E, America IDSo. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-173. [DOI] [PubMed] [Google Scholar]
- 17.Malabu UH, Al-Rubeaan KA, Al-Derewish M. Diabetic foot osteomyelitis: usefulness of erythrocyte sedimentation rate in its diagnosis. West Afr J Med. 2007;26:113-116. [PubMed] [Google Scholar]
- 18.Meyr AJ. Reply: To PMID 21907594. J Foot Ankle Surg. 2013;52:693. [DOI] [PubMed] [Google Scholar]
- 19.Michail M, Jude E, Liaskos C, Karamagiolis S, Makrilakis K, Dimitroulis D, Michail O, Tentolouris N. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99. [DOI] [PubMed] [Google Scholar]
- 20.Mutluoglu M, Sivrioglu AK, Eroglu M, Uzun G, Turhan V, Ay H, Lipsky BA. The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scand J Infect Dis. 2013;45:497-503. [DOI] [PubMed] [Google Scholar]
- 21.Newman LG, Waller J, Palestro CJ, Schwartz M, Klein MJ, Hermann G, Harrington E, Harrington M, Roman SH, Stagnaro-Green A. Unsuspected osteomyelitis in diabetic foot ulcers. Diagnosis and monitoring by leukocyte scanning with indium in 111 oxyquinoline. JAMA. 1991;266:1246-1251. [DOI] [PubMed] [Google Scholar]
- 22.R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0. http://www.R-project.org. [Google Scholar]
- 23.Roberts K, Troiano M, Schoenhaus H. Diagnostic and prognostic value of erythrocyte sedimentation rate in contiguous osteomyelitis of the foot and ankle. J Foot Ankle Surg. 2007;46:230-237. [DOI] [PubMed] [Google Scholar]
- 24.Radtke K, Tetzlaff T, Vaske B, Ettinger M, Claassen L, Florkemeier T, Windhagen H, Lewinski G. Arthroplasty-center related retrospective analysis of risk factors for periprosthetic joint infection after primary and after revision total hip arthroplasty. Technol Health Care. 2016;24:721-728. [DOI] [PubMed] [Google Scholar]
- 25.Ryan EC, Ahn J, Wukich DK, Kim PJ, La Fontaine J, Lavery LA. Diagnostic utility of erythrocyte sedimentation rate and C-reactive protein in osteomyelitis of the foot in persons without diabetes. J Foot Ankle Surg. [Published online ahead of print January 23, 2019]. DOI: 10.1053/j.jfas.2018.09.025. [DOI] [PubMed]
- 26.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [DOI] [PubMed] [Google Scholar]
- 27.Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care. 2004;27:2129-2134. [DOI] [PubMed] [Google Scholar]
- 28.Tsai CE, Lee FT, Chang MC, Yu WK, Wang ST, Liu CL. Primary cervical osteomyelitis. J Chin Med Assoc. 2013;76:640-647. [DOI] [PubMed] [Google Scholar]
- 29.Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int. 2013;2013:385641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Victoria van Asten SA, Geradus Peters EJ, Xi Y, Lavery LA. The role of biomarkers to diagnose diabetic foot osteomyelitis. A meta-analysis. Curr Diabetes Rev. 2016;12:396-402. [DOI] [PubMed] [Google Scholar]
- 31.Wright EH, Khan U. Serum complement-reactive protein (CRP) trends following local and free-tissue reconstructions for traumatic injuries or chronic wounds of the lower limb. J Plast Reconstr Aesthet Surg. 2010;63:1519-1522. [DOI] [PubMed] [Google Scholar]
- 32.Wukich DK, Hobizal KB, Sambenedetto TL, Kirby K, Rosario BL. Outcomes of osteomyelitis in patients hospitalized with diabetic foot infections. Foot Ankle Int. 2016;37:1285-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon SH, Chung SK, Kim KJ, Kim HJ, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19:575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35. [DOI] [PubMed] [Google Scholar]