Abstract
Background
The diagnosis of periprosthetic joint infection (PJI) after total shoulder arthroplasty (TSA) is challenging, especially in patients with Cutibacterium (formerly Propionibacterium) acnes infection. Despite the increasing number of patients with PJI of the shoulder, there are still no robust data regarding diagnostic tests in detecting shoulder PJI.
Questions/purposes
(1) What are the sensitivity, specificity, and negative- and positive-predictive values for the alpha-defensin enzyme-linked immunosorbent assay test in detecting PJI after TSA? (2) What are the diagnostic accuracies in detecting shoulder PJI for synovial alpha-defensin, leukocyte esterase Test, and serum C-reactive protein (CRP)?
Methods
All patients with painful TSA, who underwent joint aspiration to validate or exclude a PJI, between July 2015 and February 2018 were enrolled in this single-center study. Further indications for aspiration were as follows: planned revision arthroplasty, early loosening and clinical signs of infections, especially serum CRP elevation. A total of 121 patients were aspirated to exclude or verify a PJI, and 16 patients were excluded. In all, 105 patients with a mean age of 68 years (± 12 years) were included for analysis. Patients who underwent TSA were considered aseptic or septic according to the Musculoskeletal Infection Society criteria. Twenty-four patients had a PJI, and the remaining 81 patients were in the aseptic group. The microbiologic evaluation including polymicrobial infection showed C. (formerly P.) acnes in 15 patients (63%). Synovial fluid was then analyzed using microbiology cultures, alpha-defensin immunoassay, and leukocyte esterase. The specificity, sensitivity, and positive-predictive and negative-predictive values were calculated for each test.
Results
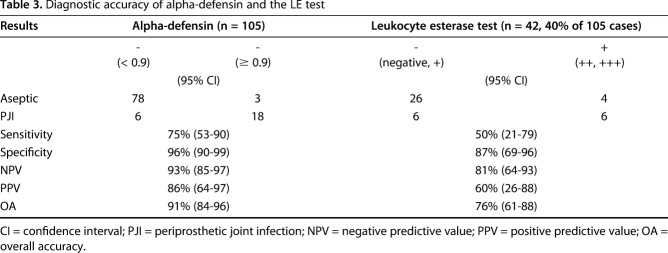
The overall accuracy for alpha-defensin was 91% (95% confidence interval [CI], 84.4–96); sensitivity was 75% (95% CI, 53–90), specificity was 96% (95% CI, 90–99), negative predictive value was 93% (95% CI, 85–97), and positive predictive value was 86% (95% CI, 64–97). In contrast, the overall accuracy for leukocyte esterase was 76% (95% CI, 61–88), sensitivity was 50% (95% CI, 21–79), specificity was 87% (95% CI, 69–96), positive predictive value 60% (95% CI, 26–88) and negative predictive value was 81% (95% CI, 64–93).
Conclusions
Summarizing the study results, the alpha-defensin ELISA and leukocyte esterase tests had less sensitivity in detecting shoulder PJI than previously reported TKA or THA results. The quality and low amount of joint fluid is the difficult part of the diagnostic. C. (formerly P.) acnes was the most common cause of PJI. Focusing on low-grade infections, alpha-defensin has shown its advantages in diagnosing PJI regardless pathogen virulence. Since the diagnostic of a PJI is always a synopsis of findings, the alpha-defensin and leukocyte esterase test can be used as adjunct diagnostic tool in patients with painful TSA. We propose further prospective studies to improve the diagnostic and confirm the results.
Level of Evidence
Level III, diagnostic study.
Introduction
Periprosthetic joint infection (PJI) after total shoulder arthroplasty (TSA) is a devastating complication that can jeopardize patient morbidity. As the number of TSAs has increased, there has been a corresponding rising trend in PJI risk [4, 9]. Adequate and early diagnosis and treatment can improve patients’ outcomes. However, the diagnosis of PJI is challenging and often delayed because there is no specific laboratory or clinical marker. Until now, there have been several studies available reporting on serum and synovial fluid markers with inconsistent or poor results. For instance, the determination of serum C-reactive protein (CRP) for the diagnosis of low-grade PJI showed poor results with a sensitivity of 42% [16]. Other serum markers such as interleukin-6 (IL-6) had even worse results with a sensitivity of 14% [21]. However, the detection of IL-6 in the synovial fluid had higher sensitivity (87%) and higher specificity (90%) but still less accuracy than other synovial fluid biomarkers. For instance, the detection of the alpha-defensin or leukocyte esterase test is already part of the diagnostic algorithm for the diagnosis of PJI after THA and TKA with overall high accuracy. The alpha-defensin enzyme-linked immunosorbent assay (ELISA) test has especially high accuracy regardless of the type of microbiologic specimen [1-3, 7, 11, 19].
This is particularly interesting in diagnosing a shoulder PJI with Cutibacterium because there is a delay in microbiologic cultures in up to 8 days and the fact that Cutibacterium is one of the dominant pathogens of PJI after TSA with incidences between 31% and 69% [5, 10, 12, 14, 17, 20].
For each alpha-defensin and leukocyte esterase test, there is only one study that has investigated the diagnostic accuracy of shoulder PJI with a small number of included patients [6, 13].
Therefore, this clinical investigation was performed to answer the following research questions: (1)What are the sensitivity, specificity, and negative- and positive-predictive values for the alpha-defensin enzyme-linked immunosorbent assay test in detecting PJI after TSA? (2) What are the diagnostic accuracies in detecting shoulder PJI for synovial alpha-defensin, leukocyte esterase test, and serum CRP?
Patients and Methods
We performed this retrospective study after approval from our institutional review board. All patients with painful TSA who underwent joint aspiration as part of our diagnostic protocol to confirm or exclude the presence of a PJI between July 2015 and February 2018 were enrolled in this single-center study. Generally, the indications for joint aspiration depend on clinical signs of infection such as soft tissue problems or history of wound healing disorders. Further aspects must be acknowledged such as elevated serum CRP, elevated white blood cell count, radiological signs such as loosening, osteolysis, or ossification. From our clinical point of view, painful TSA is the most common indication for joint aspiration. All patients who were scheduled for revision arthroplasty were also aspirated before revision surgery.
The following exclusion criteria were defined: early infection (< 4 weeks) and patients with an insufficient amount of synovial fluid. Patients who undergone antibiotic therapy in the last 2 weeks and patients with synchronous infections of the hip or knee were also excluded from the study. Patients who underwent TSA were classified as aseptic or septic according to the latest Musculoskeletal Infection Society (MSIS) criteria [15]. Indications for revision surgery were proof of PJI and aseptic reasons such as loosening, periprosthetic fracture or conversion to inverse TSA.
A total of 121 patients (upper extremity) were aspirated to exclude or verify a PJI. Ten out of 121 patients had a total elbow arthroplasty and were excluded. In two patients, alpha-defensin was not available, and they were also excluded. Four patients were excluded due to hip or knee synchronous infections.
A total of 105 patients fulfilled in the inclusion criteria and had an available alpha-defensin value. Of these, 11 patients had no CRP. The cell count was available in eight patients and 34 patients had polymorphonuclear leucocyte percentage (PMN%) According to our hospital protocol, the fluid taken from the aspiration was divided for testing in a specific order: microbiology, alpha-defensin, leukocyte esterase test, cell count and PMN%. Therefore, due to the limited number of fluids, not all tests could be performed. But all infected patients were classified based on major criteria (for example, fistula or two positive periprosthetic cultures with phenotypically identical organisms).
Joint Aspiration
All joint aspirations were performed in our aspiration room by an experienced orthopaedic surgeon. After skin disinfection and draping, shoulder aspiration was performed. We did not administer local anesthetics or saline solution. According to our hospital protocol, synovial fluid was then analyzed as follows: (1) there was a separate sterile tube for microbiology cultures; (2) 1 mL was sent for alpha-defensin immunoassay; and (3) leukocyte esterase activity was tested with a dipstick (Combur10 Test®; Roche Diagnostics, Florham Park, NJ, USA) and was sent to an independent laboratory of the University Hospital Schleswig-Holstein, Kiel, Germany. The department of microbiology in Kiel performed microbiologic cultures. The cultures were incubated for 14 days.
The aspirated synovial fluid was sent to an independent laboratory (Fenner, Hamburg, Germany) where the ELISA was conducted. The leukocyte esterase test was performed in the semisterile operating room as described by Günther et al. [8]. Patient data regarding blood samples (leukocytes, CRP), leukocyte esterase test, ELISA of alpha-defensin, and intraoperative findings including bacterial cultures and clinical symptoms were collected from medical records.
The alpha-defensin test was available in all patients. However, the leukocyte esterase test could only be analyzed in 40% of shoulders (42 of 105) because 35 were hemorrhagic and 28 had insufficient synovial fluid.
The study cohort consists of 105 patients with a mean age of 68 years (range, 36-88 years, SD = 12). There were 38 male (37%) and 67 female (63%) patients. The mean body mass index was 29 kg/m2 (range, 20–44 kg/m2; SD = 6). Though the overall proportion of men in the study is low, there were more men in the PJI group (Table 1). After preoperative evaluation by the multidisciplinary team, 24 patients were determined to have PJI. Five of 24 patients had a draining sinus, of which three had a polymicrobial infection. Two patients without microbiological proof had a draining sinus and one had high serum CRP 138 mg/L and high serum WBC 25/nL. The other patient had high serum CRP 37 mg/L, synovial cell count 9450 and PMN% 96. Intraoperative biopsies showed proof of C. (formerly P.) acnes.
Table 1.
Demographic data
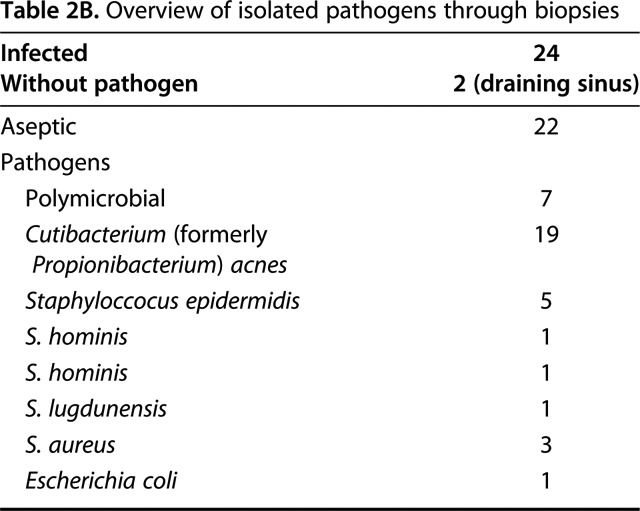
Not surprisingly our microbiological spectrum identified C. (formerly P.) acnes as the most common pathogen in PJI of the shoulder. In the aspiration group it was also part of polymicrobial infections and overall 15 patients (63%) showed C. (formerly P.) acnes as a pathogen (Table 2A). In the PJI group, C. (formerly P.) acnes was identified in 19 patients (79%) through intraoperative biopsies. 11 patients (46%) showed C. (formerly P.) acnes as a solitary pathogen (Table 2B).
Table 2A.
Overview of isolated pathogens
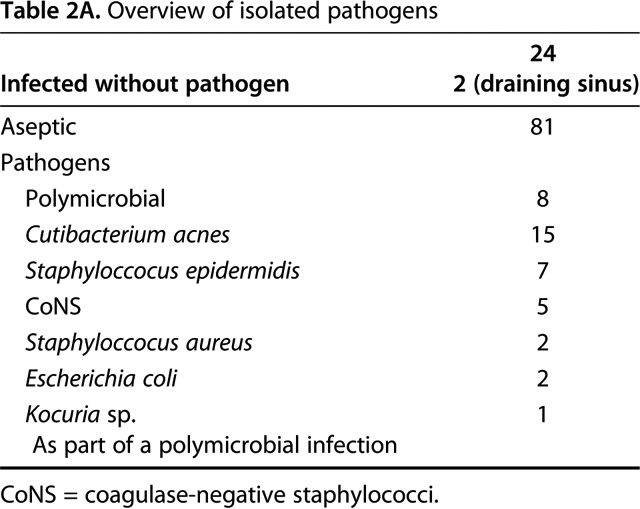
Table 2B.
Overview of isolated pathogens through biopsies
Histopathology samples were taken in 16 of 24 infections. Ten out of 16 showed histopathological signs of the infection type. Three were rated as particle-induced type. One was rated as endoprosthetic-associated arthrofibrosis type. Two were rated as indeterminate type [11].
Statistical Analysis
To statistically evaluate the performance of each test, the specificity, sensitivity, and positive predictive and negative predictive values were calculated. The receiver operating characteristic (ROC) for alpha-defensin was conducted (Fig.1). A cutoff value was established and the area under the curve (AUC) was calculated for serum CRP. Patients were divided into two groups, a PJI group and a nonPJI group according to the MSIS major criteria [15]. A 95% confidence interval (CI) was calculated for the mentioned values. The statistical analysis was performed with SAS 9.3 for Windows (SAS Institute Inc, Cary, NC, USA).
Fig. 1.
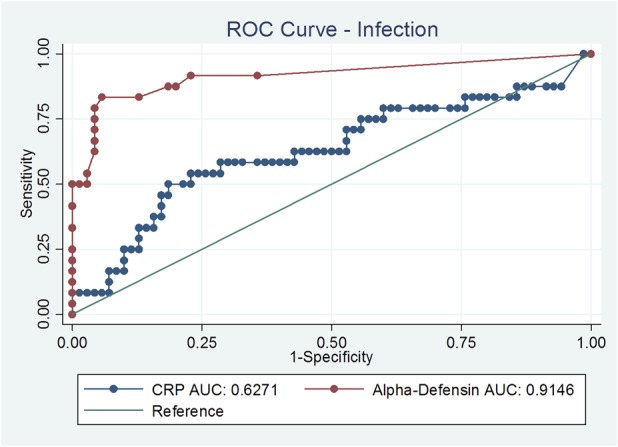
ROC curves of alpha defensin and serum CRP are shown here. The area under the curve (AUC) was 0.91 higher for the alpha-defensin test.
Results
The overall accuracy for alpha-defensin was 91% (95% CI, 84–96); sensitivity was 75% (95% CI, 53–90), specificity was 96% (95% CI, 90–99), negative predictive value was 93% (85–97), and positive predictive value was 86% (95% CI, 64–97) (Table 3). There were one false-positive and six false-negative results for the alpha-defensin ELISA test. Four of six patients with false-negative results had a draining sinus. One of two patients with metallosis had a positive alpha-defensin value with identification of coagulase-negative staphylococci. Interestingly, the other patient with metallosis had a true negative alpha-defensin test. Focusing on Cutibacterium, 11 of 15 patients (73%) were correctly identified using the alpha-defensin test. The remaining four patients (27%) had false-negative alpha-defensin results.
Table 3.
Diagnostic accuracy of alpha-defensin and the LE test
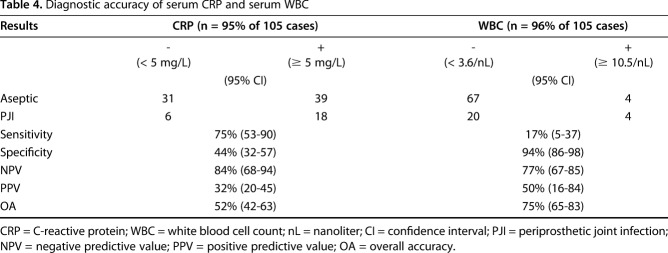
In contrast, the overall accuracy for leukocyte esterase was 76% (95% CI, 61–88), sensitivity was 50% (95% CI, 21–79), specificity was 87% (95% CI, 69–96), positive predictive value 60% (95% CI, 26–88) and negative predictive value was 81% (95% CI, 64–93). Interestingly, the leukocyte esterase test correctly classified a PJI with Cutibacterium-only in three of 15 patients (20%). In nine patients (60%), it was not possible to perform a leukocyte esterase test as a result of hemorrhagic fluid (n = 6) or insufficient synovial fluid (n = 3). Two false-negative results (13.3%) were found using the leukocyte esterase test in patients with Cutibacterium infection. Serum CRP and WBC levels had lower overall accuracies compared with alpha-defensin. In our study the serum CRP with a cutoff at 5 mg/L shows an accuracy of 75%. A cutoff with 10 mg/L, decreased the sensitivity to 62%. Serum WBC had a high specificity of 94% but a poor sensitivity of 17% (Table 4).
Table 4.
Diagnostic accuracy of serum CRP and serum WBC
Discussion
The diagnosis of a periprosthetic shoulder infection remains difficult and prolonged.
Clinical signs of infection might be missing, and patients often only present with pain or stiffness. General infection markers such as serum-CRP might be misleading and invasive methods as joint aspiration are hindered by the low amount of joint fluid. Acquiring a good, uncontaminated joint fluid sample seems to be a major problem in the diagnosis of a shoulder PJI. Due to our retrospective study design, we were unable to evaluate the lack of aspiration due to dry aspirations or hemorraghic samples. A prospective study might give more detailed information. We were able to show that alpha-defensin ELISA Test had the highest overall accuracy of all tested values. However, the accuracy is less accurate in comparison with patients with hip and knee PJIs as presented in the current evidence.
Our study had several limitations. Alpha-defensin has reasonable diagnostic accuracy for shoulder PJI, even in the setting of C. (formerly P.) acnes. However, it must be noted, that the short followup time interval could have affected our study results. Some of the patients currently categorized as infection-free may have an infection, which may decrease the overall accuracy of the alpha-defensin test. The second limitation of our study was that not all patients in the aseptic group underwent a surgical procedure. As a result, the number of PJIs might be higher because intraoperative biopsies for microbiologic or histologic testing during the revision procedure would detect more PJI patients. Third, we did not exclude patients with draining sinus or metallosis in the current study, which might also influence the overall accuracy.
In general, Frangiamore et al. [6] have reported higher overall accuracy in detecting PJI after TSA for the ELISA test. Besides this study’s results, which is based on a limited number of 11 PJI patients, no other studies are available to the best of our knowledge. Our study results showed a higher overall accuracy regarding the alpha-defensin ELISA test than the study cohort by Frangiamore et al. [6], although we did not exclude patients with a draining sinus or metallosis. Those factors can produce false-negative or false-positive results. In our study, four of six patients with false-negative results had a draining sinus. Excluding those patients, the overall accuracy would even be higher. It is obvious that patients with a draining sinus have a PJI and as such would not benefit from further testing with alpha-defensin or leukocyte esterase.
Focusing on leukocyte esterase test, several studies have already shown that the leukocyte esterase test has a very good diagnostic accuracy in hip and knee PJI [8, 11, 19]. However, in shoulder PJI only the study by Nelson et al. [13] has prospectively analyzed the leukocyte esterase strip test in 45 primary shoulder arthroplasty and 40 revision arthroplasty cases; the authors did not recommend the leukocyte esterase test as routine diagnostic tool in detecting shoulder PJI. The sensitivity of the leukocyte esterase test was also 50% in our cohort. It must be noted that a high amount of samples were indeterminate for the leukocyte esterase testing due to admixture of blood. This was also seen in the study by Nelson et al. [13] even after centrifugation. Also, poor results have been presented for serum laboratory parameters such as CRP or white blood cell count in predicting PJI. In our study, the serum CRP was defined with a cutoff at 5 mg/L and showed an accuracy of 75%. A cutoff with 10 mg/L, as suggested in the consensus classification decreases the sensitivity to 62%. This might be an aspect to consider in PJIs of the shoulder because most infections were caused by less virulent C. (formerly P.) acnes. As presented in recent studies, less virulent PJIs had been associated with low CRP values. Using only the serum CRP value as a diagnostic method, true PJIs would have never been detected in many cases.
In conclusion, the alpha-defensin ELISA and leukocyte esterase tests had less sensitivity in detecting shoulder PJI than previously reported TKA or THA results. The quality and low amount of joint fluid is the difficult part of the diagnostic. C. (formerly P.) acnes was the most common cause for PJI. Focusing on low-grade infections, alpha-defensin has shown its advantages in diagnosing PJI regardless of virulence of the pathogen. Since the diagnostic of a PJI is always a synopsis of findings, alpha-defensin and the leukocyte esterase test can be used as adjunct diagnostic tool in patients with painful TSA. We propose further prospective studies to improve the diagnostic and confirm the results.
Acknowledgments
None.
Footnotes
One of the authors (TG) reports personal fees in an amount more than USD 10,000 from Zimmer (Warsaw, IN, USA), personal fees in an amount more than USD 10,000 from Link (Hamburg, Germany), outside the submitted work. One of the authors (AZ) reports personal fees in an amount more than USD 10,000 and nonfinancial support from Zimmer and personal fees in an amount more than USD 10,000 from Link (Hamburg, Germany), outside the submitted work. One of the authors (MC) reports personal fees in an amount less than USD 10,000 from Waldemar Link (Hamburg, Germany), outside the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Helios ENDO-Klinik, Hamburg, Germany.
Niklas Unter Ecker and Alina Koniker contributed equally to the writing of this article.
References
- 1.Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T. How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin Orthop Relat Res . 2017;475:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid alpha-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am . 2014;96:1439–1445. [DOI] [PubMed] [Google Scholar]
- 3.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res . 2014;472:3254–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favard L. Revision of total shoulder arthroplasty. Orthop Traumatol Surg Res . 2013;99:S12–S21. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez Sampedro M, Piper KE, McDowell A, Patrick S, Mandrekar JN, Rouse MS, Steckelberg JM, Patel R. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis . 2009;64:138–145. [DOI] [PubMed] [Google Scholar]
- 6.Frangiamore SJ, Saleh A, Grosso MJ, Kovac MF, Higuera CA, Iannotti JP, Ricchetti ET. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg . 2015;24:1021–1027. [DOI] [PubMed] [Google Scholar]
- 7.Gehrke T, Lausmann C, Citak M, Bonanzinga T, Frommelt L, Zahar A. The accuracy of the alpha defensin lateral flow device for diagnosis of periprosthetic joint infection: comparison with a gold standard. J Bone Joint Surg Am . 2018;100:42–48. [DOI] [PubMed] [Google Scholar]
- 8.Günther D, Kokenge T, Jacobs O, Omar M, Krettek C, Gehrke T, Kendoff D, Haasper C. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38:2385–2390. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am . 2011;93:2249–2254. [DOI] [PubMed] [Google Scholar]
- 10.Klatte TO, Junghans K, Al-Khateeb H, Rueger JM, Gehrke T, Kendoff D, Neumann J. Single-stage revision for peri-prosthetic shoulder infection: outcomes and results. Bone Joint J . 2013;95:391–395. [DOI] [PubMed] [Google Scholar]
- 11.Lee YS, Koo K-H, Kim HJ, Tian S, Kim T-Y, Maltenfort MG, Chen AF. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am . 2017;99:2077–2084. [DOI] [PubMed] [Google Scholar]
- 12.Lutz M-F, Berthelot P, Fresard A, Cazorla C, Carricajo A, Vautrin A-C, Fessy M-H, Lucht F. Arthroplastic and osteosynthetic infections due to Propionibacterium acnes: a retrospective study of 52 cases, 1995–2002. Eur J Clin Microbiol Infect Dis . 2005;24:739–744. [DOI] [PubMed] [Google Scholar]
- 13.Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg . 2015;24:1421–1426. [DOI] [PubMed] [Google Scholar]
- 14.Nodzo SR, Boyle KK, Bhimani S, Duquin TR, Miller AO, Westrich GH. Propionibacterium acnes host inflammatory response during periprosthetic infection is joint specific. HSS J . 2017;13:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. [DOI] [PubMed] [Google Scholar]
- 16.Piper KE, Fernandez-Sampedro M, Steckelberg KE, Mandrekar JN, Karau MJ, Steckelberg JM, Berbari EF, Osmon DR, Hanssen AD, Lewallen DG, Cofield RH, Sperling JW, Sanchez-Sotelo J, Huddleston PM, Dekutoski MB, Yaszemski M, Currier B, Patel R. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One . 2010;5:e9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards J, Inacio MCS, Beckett M, Navarro RA, Singh A, Dillon MT, Sodl JF, Yian EH. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res . 2014;472:2809–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheehan E. CORR Insights®: The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res . 2015;473:204–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg . 2012;21:1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villacis D, Merriman JA, Yalamanchili R, Omid R, Itamura J, Rick Hatch GF. Serum interleukin-6 as a marker of periprosthetic shoulder infection. J Bone Joint Surg Am . 2014;96:41–45. [DOI] [PubMed] [Google Scholar]