Abstract
Background
Despite the promising clinical results of autologous osteochondral transplantation in the treatment of osteochondral lesions of the talus, the occurrence of knee donor-site morbidity remains a concern. However, the proportion of patients experiencing donor-site morbidity is not well established because of important variations in estimates drawn by heterogeneous studies with loss to followup, often made at short-term (< 1 year). Therefore, both a meta-analysis of studies that assumed no patients lost to followup had donor-site morbidity and assumed all patients lost to followup had donor-site morbidity may help to estimate the true risk of donor-site morbidity.
Questions/purposes
To evaluate the proportion of patients who developed knee donor-site morbidity after autologous osteochondral transplantation for osteochondral lesion of the talus, by (1) meta-analysis of the proportion of patients experiencing donor-site morbidity in the best-case scenario as reported, in which no patients lost to followup were assumed to have donor-site morbidity and (2) meta-analysis of the percentage of patients who had donor-site morbidity in the worst-case scenario, in which all patients lost to followup were assumed to have donor-site morbidity and (3) present the characteristics of studies associated with the reporting of donor-site morbidity.
Methods
A systematic search of the PubMed, Embase and The Cochrane Library databases was performed from their inception to October 2017 according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The inclusion criteria were clinical studies that reported knee donor-site morbidity after autologous osteochondral transplantation for osteochondral lesion of the talus, mean followup ≥ 1 year, full-text studies published in a peer-review journal and written in English. Quality of evidence (Case Series Quality Appraisal Checklist), sample size, mean patient age, study design, mean followup time, and observed rate of knee donor-site morbidity were evaluated. Twenty-six studies with 915 ankles (904 patients) were included in the systematic review and meta-analysis. Approximately half of the included studies were of small cohort (n < 30, 12 studies), and 12 of 26 studies did not report at all on loss to followup. In the studies that reported loss to followup (14 of 26), a total of 32 patients (35 ankles) were reported lost. Random-effects models were used to estimate the risk of donor-site morbidity as between-study heterogeneity was determined to be high in both meta-analyses that assumed that no patients lost to followup experienced donor-site morbidity (I2 = 82.1%) and the one that assumed all patients lost to followup experienced donor-site morbidity (I2 = 88.7%). Multivariable metaregression was used to estimate the association between study characteristics and the observed proportion of patients who experienced of donor-site morbidity. If there was evidence of an association between a study characteristic and proportion, a subgroup analysis was performed.
Results
The estimated proportion of donor-site morbidity was 6.7% (95% confidence interval [CI], 2.8–11.8), assuming that no patients lost to followup experienced donor-site morbidity and 10.8% (95% CI, 4.8–18.3) assuming that all patients lost to followup experienced donor-site morbidity after a mean followup of 43.8 ± 24.7 months (range, 15.9–120 months). There was a negative association between study sample size and proportion of donor-site morbidity (β = -0.26; 95% CI, -0.39 to -0.12; p < 0.001 assuming that no patients lost to followup experienced donor-site morbidity and β = -0.31; 95% CI, -0.48 to -0.13; p < 0.001 assuming that all patients lost to followup experienced donor-site morbidity); that is, as study size increased, the proportion of patients reported with donor-site morbidity decreased. In larger studies (n ≥ 30), the estimated percentage of donor-site morbidity was 2.8% (95% CI, 1.2%–5.0%; I2 = 47.6%) assuming that no patients lost to followup experienced donor-site morbidity, and 5.0% (95% CI, 2.1%–9.0%; I2 = 74.5%) assuming all patients lost to followup experienced donor-site morbidity. High between-study heterogeneity (differences in methodology) could not be completely explained by variability in study sample size, mean patient age, design, or mean followup time, and may be attributable to other factors such as inconsistent definitions of donor-site morbidity.
Conclusions
The estimated proportion of donor-site morbidity after autologous osteochondral transplantation for osteochondral lesion of the talus ranged from 6.7% to 10.8% in the current meta-analysis. However, subgroup analysis demonstrated that larger studies (n ≥ 30) estimated a lower donor-site morbidity risk (< 5.0%) than smaller studies (n < 30). This estimate should be interpreted in light of the fact that nearly half of the included studies did not report on loss to followup, and so their estimates of donor-site morbidity may be low. In addition, high between-study heterogeneity and the inclusion of predominantly retrospective studies with small sample sizes likely contributed to estimates that suffered from a high risk of bias, probably in favor of the surgical treatment being studied.
Level of Evidence
Level IV, therapeutic study.
Introduction
Autologous osteochondral transplantation is an established treatment for large or cystic osteochondral talar lesions. Autologous osteochondral transplantation is a replacement procedure that substitutes the talar lesion with cylindrical autograft of viable hyaline cartilage and subchondral bone, typically harvested from the ipsilateral knee [19, 25]. Clinical evidence has demonstrated functional improvement after autologous osteochondral transplantation, with 90% of professional athletes returning to preinjury activity levels [9, 19] and greater than 90% of patients returning to preinjury activity in the general population [16, 31]. However, the lack of loss to followup reported in these studies is a limitation that may have resulted in overestimation of the functional improvement after autologous osteochondral transplantation.
Graft harvesting can cause the release of proinflammatory cytokines that can induce pain postoperatively and, therefore, donor-site morbidity is a concerning complication for autologous osteochondral transplantation as it can be symptomatic and painful [17, 24]. Large differences in the published proportions of patients experiencing donor-site morbidity after knee-to-talus autologous osteochondral transplantation procedures have been reported, ranging from 0% to 54.5% in studies of short- to medium-term followup (< 5 years) [8, 10, 11, 16, 20, 27, 31, 35, 36]. These studies often do not report the proportion of patients who are lost to followup, and many of these papers report only short-term followup; these important limitations may underestimate the risk of donor-site morbidity. Therefore, a meta-analysis that includes both a best- and worst-case analysis can help us to better understand the risk of donor-site morbidity.
The purpose of this systematic review and meta-analysis was to evaluate the proportion of knee donor-site morbidity after autologous osteochondral transplantation for osteochondral lesions of the talus by (1) meta-analysis of the percentages of donor-site morbidity in the best-case scenario as reported, in which no patients lost to followup were assumed to have donor-site morbidity and (2) meta-analysis of the proportions of donor-site morbidity in the worst-case scenario, in which all patients lost to followup were assumed to have donor-site morbidity and (3) present the characteristics of studies associated with the reporting of donor-site morbidity.
Materials and Methods
Search Strategy
Two reviewers (YS, DS) performed a systematic review of the PubMed, Embase and Cochrane Library databases from their inception through October 2017, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. The search terms were: (osteochondral autologous transplantation OR osteochondral autologous transplantation system OR osteochondral autograft OR osteochondral transfer OR osteochondral transplant OR mosaicplasty OR OATS) AND (talus OR talar OR ankle) AND (osteochondral lesion OR osteochondritis dissecans OR cartilage OR chondral OR osteochondral OR transchondral OR OCL OR OCD) (see Appendix, Supplemental Digital Content 1, http://links.lww.com/CORR/A172). We also screened for possible inclusion the references of all articles identified by this search. The inclusion criteria were clinical studies that reported knee donor-site morbidity after autologous osteochondral transplantation for osteochondral lesion of the talus, mean followup ≥ 1 year, full-text studies published in a peer-review journal and written in English. The exclusion criteria were review studies, case reports, cadaver studies and animal studies.
Two independent reviewers (YS, DS) screened the titles, abstracts and full-text articles of all searched studies by applying the aforementioned criteria. Any disagreements were resolved by consensus, and if disagreement persisted, a senior author was consulted.
Assessment of Evidence
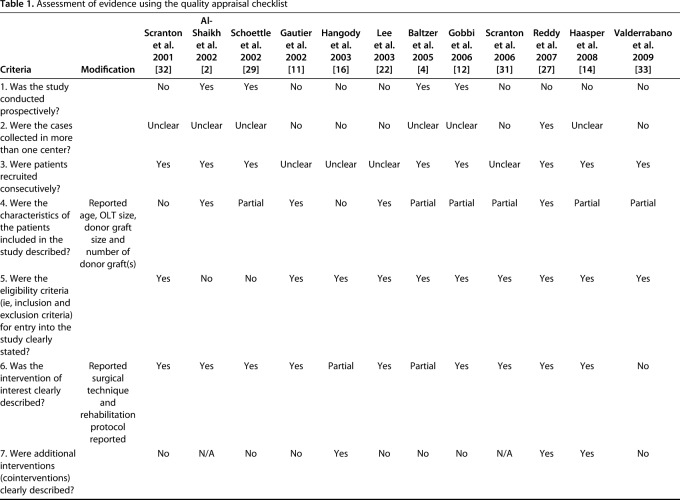
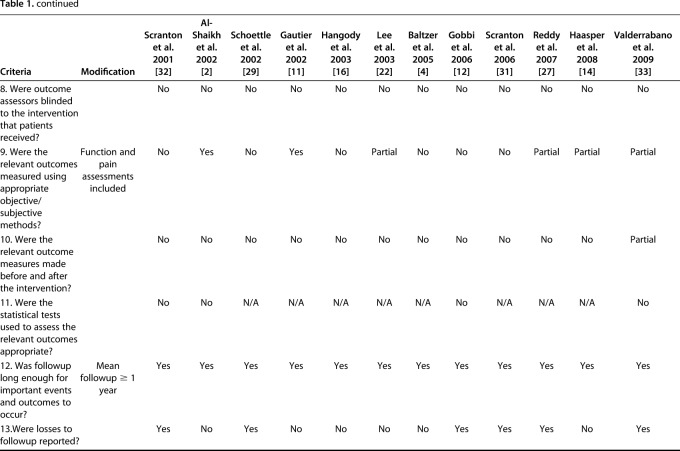
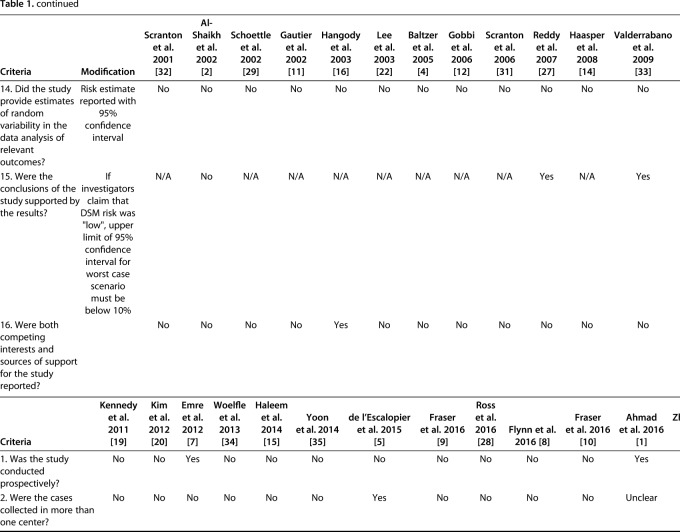
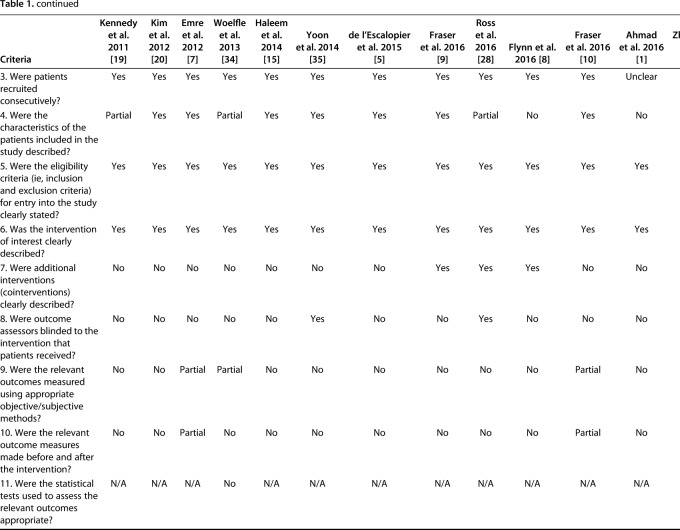
The quality of evidence was assessed using a previously published case series quality checklist [13] and modified to exclude four components that were inapplicable to the included studies; this generated a checklist scale out of 16. Prospective studies were strictly defined as studies that followed a similar cohort over time; studies from “prospectively maintained” databases were classified as retrospective regardless of how the authors of those studies identified them.
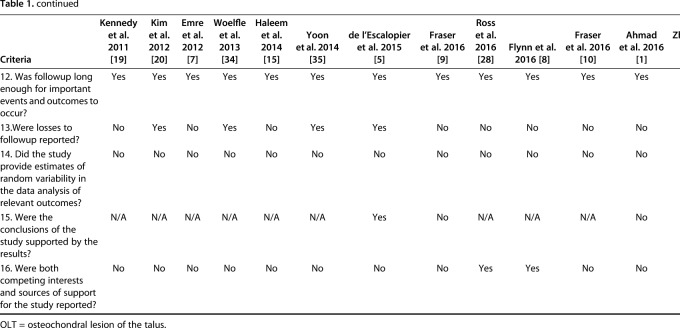
Two independent reviewers (DS, KF) determined the case series quality appraisal checklist for each study. If any discrepancy occurred, a third author (YS) evaluated the available data and a consensus was reached. The checklist was answered with either “yes,” “no,” “partial,” or “unclear.” We summarized the case series quality appraisal checklist of all included studies (Table 1). The mean total number of responses of either yes or partial per individual study was 6.4 (4 to 10) out of the 16 checklist items.
Table 1.
Assessment of evidence using the quality appraisal checklist
Study Outcomes of Interest
We defined donor-site morbidity as any disability specifically at the knee donor-site after autograft extraction for talar osteochondral transplantation. When reported by symptom, pain was the most common donor-site morbidity (median [Q1, Q3], 58.3% [20.8, 100]) and this was followed by stiffness. Other symptoms included instability, crepitus, discomfort, and effusion. The primary outcome of interest was to estimate the rate of donor-site morbidity as reported, in which no patients lost to followup were assumed to have donor-site morbidity.
The secondary outcomes of interest were a best-case and a worst-case analysis of donor-site morbidity, and the factors associated with a study reporting donor-site morbidity events. Factors such as loss to followup reported, short-term followup, and differing study characteristic differences can overestimate or underestimate the rate of donor-site morbidity and, therefore, it is important to account for these limitations to estimate of the rate of donor-site morbidity. This between-study ambiguity can be accounted by the inclusion of both best-case scenario analysis which assumed no patients lost to followup had donor-site morbidity and worst-case scenario analysis that assumed all patients lost to followup had donor-site morbidity. A noteworthy limitation is that in studies that reported no donor-site morbidity and no loss to followup, the assumption was made that no donor-site morbidity occurred despite the possibility that patient(s) lost to followup could have experienced donor-site morbidity.
Data Evaluation
Data collected from eligible studies included total sample size (number of ankles and number of patients), number of donor-site morbidities, number of pain events, number of knee stiffness events, mean patient age at the time of surgery, study design (prospective versus retrospective case series), and mean followup time. Surgical procedure characteristics, including lesion size, donor-site for the grafts, diameter and number of the grafts needed, were collected for descriptive purposes.
Heterogeneity
Between-study heterogeneity was tested by form of heterogeneity using the χ2 test and quantified using the I2 statistic by subgroup and overall. I2 values < 25% were considered low, 25%-75% were classified as moderate, and > 75% were considered high. Random-effects models were employed given high heterogeneity in both meta-analyses that assumed that no patients lost to followup experienced donor-site morbidity (I2 = 82.1%) and all patients lost to followup experienced donor-site morbidity (I2 = 88.7%). Heterogeneity within subgroups was moderate to high. In the subgroup analysis that assumed no patients lost to followup experienced donor-site morbidity, the heterogeneity in larger studies was moderate (n ≥ 30, I2 = 47.6%), and this was also present in smaller studies (n < 30, I2 = 74.9%). In the subgroup analysis that assumed all patients lost to followup experienced donor-site morbidity, the heterogeneity in larger studies was high (n ≥ 30, I2 = 74.5%) and this also present in smaller studies (n < 30, I2 = 83.4%). Multivariable metaregression revealed that 41.1% and 22.9% of observed heterogeneity could be accounted for by study characteristics (sample size, mean age, design, and mean followup time) in both analyses that assumed none and all patients lost to followup experienced donor-site morbidity.
Publication Bias
We did not assess for publication bias as typical detection methods have been found to be deficient when applied to meta-analyses of rare events [18, 30].
Statistical Analysis
Random-effects models were employed to estimate the proportion of knee-to-talus autologous osteochondral transplantation procedures that experienced donor-site morbidity, either assuming that no patients lost to followup experienced donor-site morbidity or all patients lost to followup experienced donor-site morbidity. It is important for both best- and worst-case analyses to be performed, since the clinical evidence consists mainly of retrospective, short-term studies with considerable loss to followup. Therefore, an overestimation or underestimation of the knee donor-site morbidity after autologous osteochondral transplantation for osteochondral talar lesions may be present. We pooled the proportion of knees that experienced donor-site morbidity via the restricted maximum-likelihood method after the Freeman-Tukey double-arcsine transformation. We used multivariable metaregression to assess the association of sample size (n ≥ 30 versus < 30), mean patient age, prospective versus retrospective study design, and mean followup time with the Freeman-Tukey double-arcsine transformed outcome proportions. If there was evidence of an association between a predictor and transformed outcome proportion, we performed a subgroup analysis. All analyses were performed using the metafor and meta packages implemented in R software version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
Study Characteristics and Patients Demographics
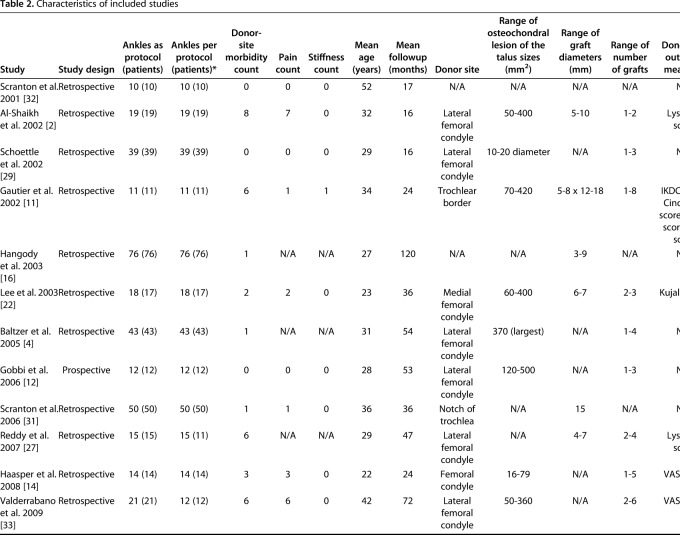
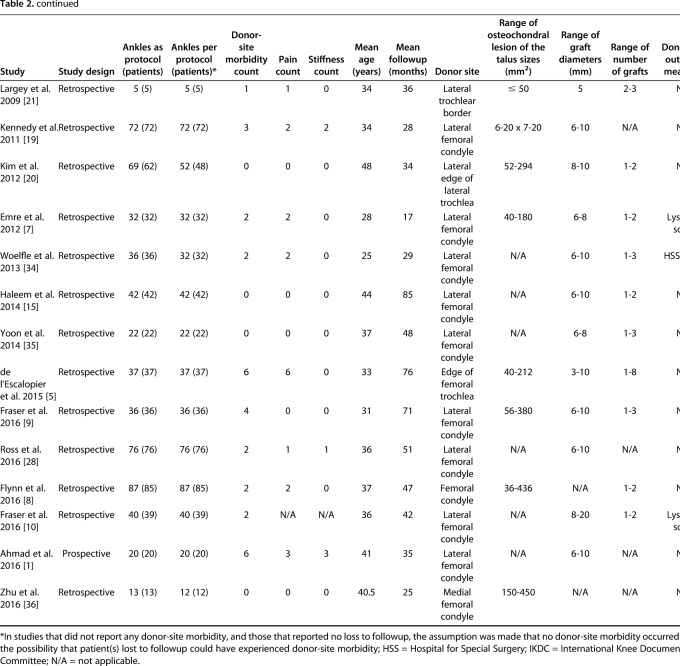
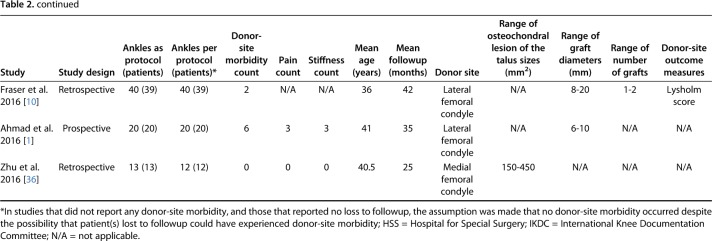
The search yielded 1329 studies, of which 26 studies met the inclusion and exclusion criteria for the current review (Fig. 1) [1, 2, 4, 5, 7, 8, 9, 10, 11, 12, 14, 15, 16, 19, 20, 21, 22, 27, 28, 29, 31, 32, 33, 34, 35, 36]. The 26 included studies were published between 2001 and 2016 (Table 2). A total of 904 patients (915 ankles) underwent knee-to-ankle AOT. The mean patient age was 34.2 ± 7.4 years (range, 22 years to 52 years) and the mean followup was 43.8 ± 24.7 months (range, 15.9 months to 120 months). The donor-site used for autologous osteochondral transplantation was the either femoral condyle or femoral trochlea. Twelve studies reported loss to followup and in these studies, 32 patients (35 ankles) were reported in total; however, since 12 studies did not comment on loss to followup, it is likely that loss to followup is much greater.
Fig. 1.
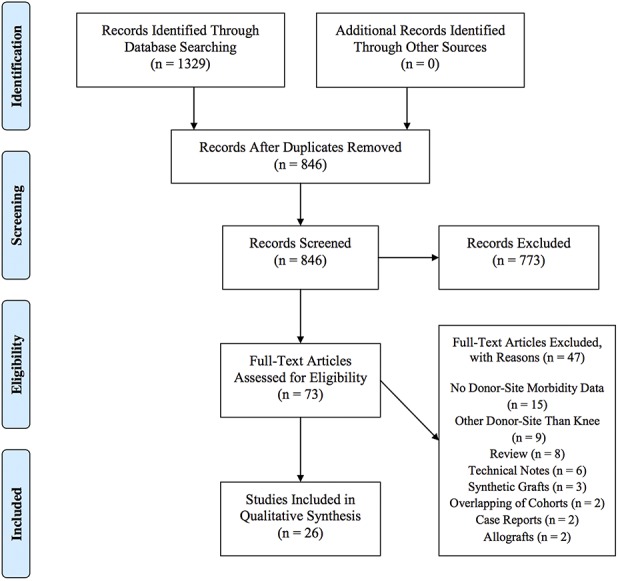
The PRISMA Flow Diagram is shown here.
Table 2.
Characteristics of included studies
Surgical Data
The donor-sites used for harvesting osteochondral grafts were reported in 24 of included studies, and all of them were from the ipsilateral knee. The donor-sites included the lateral femoral condyle or lateral edge of the trochlea (19 studies), the medial femoral condyle (two studies) and the notch of the femoral trochlea (one study). Nineteen studies reported the number of grafts, with a weighted mean graft number of 1.7. Eighteen studies reported graft sizes, with a weighted mean diameter of grafts 9.9 mm. In four studies, biosynthetic plugs were used to backfill the knee donor-sites.
Outcome Measures of the Knee
To analyze the donor knee status, two studies reported pre- and postoperative knee outcome measures, and seven studies reported only postoperative outcome measures. Seven different scoring systems were used in nine studies, and the Lysholm score was the most common scoring system. Four studies evaluated the knee donor-sites using radiological tools to assess the condition of donor-site cartilage and bone; of these, only one reported magnetic resonance observation of cartilage repair tissue (MOCART) score [10]. Fraser et al. [10] reported that the mean MOCART score after backfill of a biosynthetic plug into the knee donor-site was 60.0 at a mean followup of 41.8 months, but they found no correlation between the MOCART score and Lysholm score.
Results
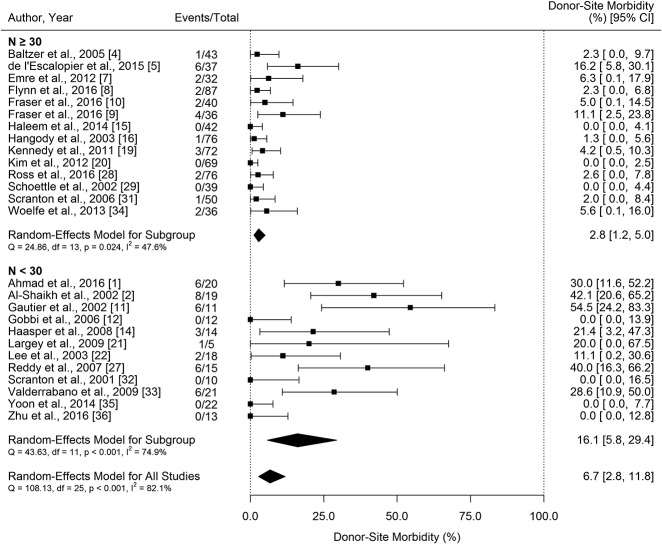
Best-case Analysis of Knee Donor-site Morbidity
If no patients lost to followup had donor-site morbidity, the estimated proportion of patients experiencing donor-site morbidity was 6.7% (95% confidence interval [CI], 2.8%–11.8%) (Fig. 2).
Fig. 2.
Forest plots of knee donor-site morbidity autologous osteochondral transplantation assuming none of the patients lost to followup experienced donor-site morbidity are shown here; CI = confidence interval.
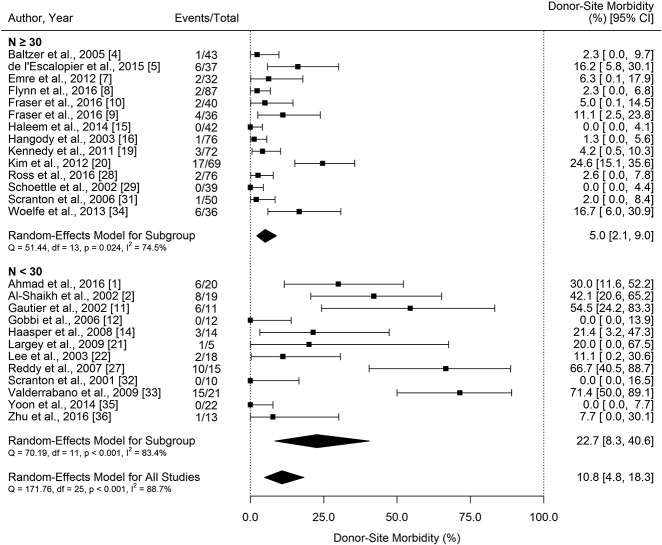
Worst-case Analysis of Knee Donor-Site Morbidity
If all patients lost to followup experienced donor-site morbidity, the estimated rate of donor-site morbidity rose to 10.8% (95% CI, 4.8%–18.3%) (Fig. 3). It must be noted in this worst-case analysis, that the assumption was made that no donor-site morbidity occurred in studies that did not report loss to followup. It is, of course possible—and perhaps likely—that the studies that did not comment on loss to followup experienced some loss to followup, and that some of the patients lost to followup had donor-site morbidity. That being so, the estimates of donor-site morbidity made in our worst-case analysis may in fact themselves underestimate the extent and severity of the problem. We note that 14 of the 26 included studies reported loss to followup and of these studies, a total of 32 patients (35 ankles) were reported lost to followup; 12 studies did not comment on loss to followup.
Fig. 3.
Forest plots of knee donor-site morbidity autologous osteochondral transplantation assuming all patients lost to followup experienced donor-site morbidity are shown here; CI = confidence interval.
Study Characteristics Associated with Reporting of Donor-Site Morbidity
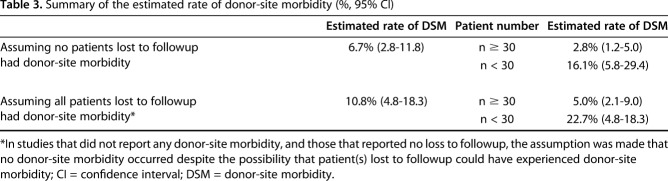
There was evidence of a negative association between study sample size and rate of donor-site morbidity (β = -0.26; 95% CI, -0.39 to -0.12; p < 0.001 assuming that no patients lost to followup experienced donor-site morbidity and β = -0.31; 95% CI, -0.48 to -0.13; p < 0.001 assuming that all patients lost to followup experienced donor-site morbidity); that is, as study size increased, the proportion of patients reported with donor-site morbidity decreased. Because of this, a subgroup analysis was performed by sample size. Assuming that no patients lost to followup experienced donor-site morbidity, the estimated rate of donor-site morbidity was 2.8% (95% CI, 1.2–5.%) in larger studies (n ≥ 30), and 16.1% (95% CI, 5.8–29.4) in smaller studies (n < 30) (Fig. 2). With the assumption that all patients lost to followup experienced donor-site morbidity, the estimated rate of donor-site morbidity was 5.0% (95% CI, 2.1–9.0) in larger studies (n ≥ 30, Fig. 2), and 22.7% (95% CI, 8.3–40.6) in smaller studies (n < 30) (Fig. 3). It is important to restate that an added assumption was made that no donor-site morbidity occurred in studies that did not report loss to followup and also did not experience donor-site morbidity, despite the possibility that patient(s) lost to followup could have experienced donor-site morbidity. We summarized the estimated proportion of donor-site morbidity (Table 3).
Table 3.
Summary of the estimated rate of donor-site morbidity (%, 95% CI)
Discussion
Autologous osteochondral transplantation of the talus has generally been considered a successful procedure. However, donor-site morbidity is a concerning complication because it can cause symptoms in an otherwise asymptomatic joint. The current published percentages of donor-site morbidity after knee-to-talus autologous osteochondral transplantation procedures have varied, ranging from 0% to 54.5%. [8, 10, 11, 15, 20, 27, 31, 35, 36]. Further confusing matters is that studies on talar osteochondral autografts have not consistently reported loss to followup, and often presented their findings only at short-term followup. These shortcomings in the context of retrospective research tend to inflate the apparent benefits of surgical treatments. We therefore sought to pool the data in a meta-analysis and also to provide both a best- and worst-case analysis of patients reported as missing, with the goal of arriving at a more precise estimate of the risk of donor-site morbidity.
The most important limitation in this meta-analysis was that it was very difficult to assess accurately loss to followup in the studies we included. A total of 14 of the 26 included studies reported loss to followup, and of these studies, a total of 32 patients (35 ankles) were reported as lost. However, that still left 12 studies that did not comment on loss to followup; for our worst-case analysis, we assumed no loss to followup occurred in these studies. However, this assumption is unlikely to be accurate, meaning that estimates of donor-site morbidity may even be worse than estimated in our worst-case analysis. This could have resulted in an underestimation of knee-to-talus donor-site morbidity, as patients could have been lost in these series, and these patients may have experienced donor-site morbidity. The inclusion of retrospective studies in the meta-analysis adds both selection and assessor bias, as underestimation of donor-site morbidity often is present in these study designs, such that they may have de-emphasized the identification and reporting of important complications; this may result in the reported incidence of donor-site morbidity being lower than the actual incidence in patients undergoing autologous osteochondral transplantation from the knee to talus. The included studies contained small sample sizes. Uncommon complications often do not arise in small studies, sometimes resulting in erroneous or overstated claims of safety. The mean followup was 44 months (range, 16–120 months) and can be categorized as belonging to the short- to medium-term followup (< 5 years); one expects that an increase in donor-site morbidity may become evident in time if the harvesting of cartilage results in arthritic changes to the donor sites. If that does not occur, it is likely that estimates of morbidity would be stable at the mean followup in this meta-analysis. In fact, no included studies showed an increase of knee donor-site morbidity throughout the followup. Observation of high between-study heterogeneity unexplainable by variability in sample size, patient age, study design, or followup time was also present.
Other than knee pain, several other symptoms were reported, including stiffness, instability, crepitus, discomfort, and effusion. The current review found that no studies described the definition of knee donor-site morbidity, and the lack of definition has confounded the accuracy of the analysis. Knee function assessment using patient-reported outcome scores was performed in nine studies, but there was large variability because seven different scoring systems were used. In the remaining studies, only descriptions of knee symptoms were reported. In addition, only two studies evaluated both pre- and postoperative knee function. These findings may further emphasize the caveat that donor-site morbidity may be underreported in this meta-analysis. The establishment of a clear and unambiguous definition of donor-site morbidity would be helpful going forward. Future studies may opt to employ the most common knee donor-site morbidity function assessment to date, the Lysholm score.
The proportion of donor-site morbidity ranged from 6.7% (as reported) to 10.8% (worst-case analysis) and as for reasons already mentioned, it is possible that our worst-case analysis is itself optimistic. A 2016 systematic review by Andrade et al. [3] reported a 16.9% proportion of knee donor-site morbidity for knee-to-ankle autologous osteochondral transplantation. However, that systematic review only included 10 studies and the pooling methodology was unclear. The current meta-analysis included more than double the number of studies coupled with a transparent and extremely rigorous pooling method.
In a systematic review by de Windt et al. [6], only 28% of the included studies reported a correlation between MRI findings and clinical outcomes experienced after knee cartilage repair [6]. In the current systematic review, no correlation was found in the only study that investigated the correlation between MRI and clinical outcomes at the knee donor site using the MOCART score [10]. In light of these findings, it is understandable that factors that are difficult to detect on MRI (inflammation, increased vascular penetration and nerve growth) can influence knee donor-site morbidity [24]. In addition, because the MOCART score was originally developed as a standardized evaluation tool for cartilage grafts and not clinical outcome, future research aiming at clinical sensitivity of advanced MRI evaluation techniques could be important.
Interestingly, metaregression identified a negative association between study sample size and donor-site morbidity. Limiting the sample size to at least 30 knees and assuming that no patients lost to followup experienced donor-site morbidity, the estimated percentage of donor-site morbidity after autologous osteochondral transplantation in this meta-analysis was 2.8%. Still, a future meta-analysis including larger prospective studies with more complete followup will be important if we are to be able to estimate accurately the risk of donor-site morbidity; longer followup is especially important, as symptoms of donor-site morbidity may increase over time. However, there are mixed results with several studies showing improvement of knee donor-site morbidity over time [10, 26], but in light of the low quality of these studies further well-designed studies are needed.
In conclusion, the estimated proportion of patients experiencing knee-to-talus donor-site morbidity ranged from 6.7% to 10.8% at short- to medium-term followup (< 5 years) in this meta-analysis. However, subgroup analysis demonstrated that larger studies (n ≥ 30) estimated a lower donor-site morbidity risk (< 5.0%) than smaller studies (n < 30). This estimate should be interpreted considering the fact that nearly half of the included studies did not report on loss to followup, and so their estimates of donor-site morbidity may be low. In addition, high between-study heterogeneity and the inclusion of predominantly retrospective studies with small sample sizes likely contributed to estimates that suffered from a high risk of bias, probably in favor of the surgical treatment being studied. A future meta-analysis that includes larger prospective studies with longer-term and more complete followup is needed.
Acknowledgments
None.
Footnotes
Only one of the authors (JGK) has received, during the study period, funding from The Ohnell Family Foundation, and Mr. and Mrs. Michael J. Levitt.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This investigation was performed at NYU Langone Health, New York, NY, USA.
References
- 1.Ahmad J, Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent or large talar osteochondral lesions. Foot Ankle Int . 2016;37:40-50. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shaikh RA, Chou LB, Mann JA, Dreeben SM, Prieskorn D. Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int. 2002;23:381-389. [DOI] [PubMed] [Google Scholar]
- 3.Andrade R, Vasta S, Pereira R, Pereira H, Papalia R, Karahan M, Oliveira JM, Reis RL, Espregueira-Mendes J. Knee donor-site morbidity after mosaicplasty - a systematic review. J Exp Orthop . 2016;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltzer AW, Arnold JP. Bone-cartilage transplantation from the ipsilateral knee for chondral lesions of the talus. Arthroscopy. 2005;21:159-166. [DOI] [PubMed] [Google Scholar]
- 5.de l'Escalopier N, Barbier O, Mainard D, Mayer J, Ollat D, Versier G. Outcomes of talar dome osteochondral defect repair using osteocartilaginous autografts: 37 cases of Mosaicplasty®. Orthop Traumatol Surg Res. 2015;101:97-102. [DOI] [PubMed] [Google Scholar]
- 6.de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, Saris DB. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41:1695-702. [DOI] [PubMed] [Google Scholar]
- 7.Emre TY, Ege T, Cift HT, Demircioğlu DT, Seyhan B, Uzun M. Open mosaicplasty in osteochondral lesions of the talus: a prospective study. J Foot Ankle Surg. 2012;51:556-560. [DOI] [PubMed] [Google Scholar]
- 8.Flynn S, Ross KA, Hannon CP, Yasui Y, Newman H, Murawski CD, Deyer TW, Do HT, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int . 2016;37:363-372. [DOI] [PubMed] [Google Scholar]
- 9.Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24:1272-1279. [DOI] [PubMed] [Google Scholar]
- 10.Fraser EJ, Savage-Elliott I, Yasui Y, Ackermann J, Watson G, Ross KA, Deyer T, Kennedy JG. Clinical and MRI donor site outcomes following autologous osteochondral transplantation for talar osteochondral lesions. Foot Ankle Int. 2016;37:968-976. [DOI] [PubMed] [Google Scholar]
- 11.Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84:237-244. [DOI] [PubMed] [Google Scholar]
- 12.Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22:1085-1092. [DOI] [PubMed] [Google Scholar]
- 13.Guo B, Moga C, Harstall C, Schopflocher A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. 2016;69:199–207. e92. [DOI] [PubMed] [Google Scholar]
- 14.Haasper C, Zelle BA, Knobloch K, Jagodzinski M, Citak M, Lotz J, Krettek C, Zeichen J. No mid-term difference in mosaicplasty in previously treated versus previously untreated patients with osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2008;128:499-504. [DOI] [PubMed] [Google Scholar]
- 15.Haleem AM, Ross KA, Smyth NA, Duke GL, Deyer TW, Do HT, Kennedy JG. Double-plug autologous osteochondral transplantation shows equal functional outcomes compared with single-plug procedures in lesions of the talar dome: a minimum 5-year clinical follow-up. Am J Sports Med . 2014;42:1888-1895. [DOI] [PubMed] [Google Scholar]
- 16.Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(A Suppl)2:25-32. [DOI] [PubMed] [Google Scholar]
- 17.Hennerbichler A, Rosenberger R, Arora R, Hennerbichler D. Biochemical, biomechanical and histological properties of osteoarthritic porcine knee cartilage: implications for osteochondral transplantation. Arch Orthop Trauma Surg. 2008;128:61-70. [DOI] [PubMed] [Google Scholar]
- 18.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897-903. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy JG, Murawski CD. The treatment of osteochondral lesions of the talus with autologous osteochondral transplantation and bone marrow aspirate concentrate: surgical technique. Cartilage. 2011;2:327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YS, Park EH, Kim YC, Koh YG, Lee JW. Factors associated with the clinical outcomes of the osteochondral autograft transfer system in osteochondral lesions of the talus: second-look arthroscopic evaluation. Am J Sports Med. 2012;40:2709-2719. [DOI] [PubMed] [Google Scholar]
- 21.Largey A, Faure P, Hebrard W, Hamoui M, Canovas F. Osteochondral transfer using a transmalleolar approach for arthroscopic management of talus posteromedial lesions. Orthop Traumatol Surg Res. 2009;95:537-542. [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, Chao KH, Huang GS, Wu SS. Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int. 2003;24:815-822. [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-94. [DOI] [PubMed] [Google Scholar]
- 24.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390-398. [DOI] [PubMed] [Google Scholar]
- 25.Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am . 2013;95:1045-1054. [DOI] [PubMed] [Google Scholar]
- 26.Paul J, Sagstetter A, Kriner M, Imhoff AB, Spang J, Hinterwimmer S. Donor-site morbidity after osteochondral autologous transplantation for lesions of the talus. J Bone Joint Surg Am. 2009;91:1683-1688. [DOI] [PubMed] [Google Scholar]
- 27.Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80-85. [DOI] [PubMed] [Google Scholar]
- 28.Ross AW, Murawski CD, Fraser EJ, Ross KA, Do HT, Deyer TW, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus: does previous bone marrow stimulation negatively affect clinical outcome? Arthroscopy. 2016;32:1377-1383. [DOI] [PubMed] [Google Scholar]
- 29.Schoettle PB, Imhoff AB. Autologous osteochondral transplantation for talar lesions. Orthop and Traumatol. 2002;10:113-129. [Google Scholar]
- 30.Schwarzer G, Antes G, Schumacher M. Inflation of type I error rate in two statistical tests for the detection of publication bias in meta-analyses with binary outcomes. Stat Med. 2002;21:2465-2477. [DOI] [PubMed] [Google Scholar]
- 31.Scranton PE, Jr, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88:614-619. [DOI] [PubMed] [Google Scholar]
- 32.Scranton PE, Jr, McDermott JE. Treatment of type V osteochondral lesions of the talus with ipsilateral knee osteochondral autografts. Foot Ankle Int . 2001;22:380-384. [DOI] [PubMed] [Google Scholar]
- 33.Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med . 2009;37(Suppl 1):105S-111S. [DOI] [PubMed] [Google Scholar]
- 34.Woelfle JV, Reichel H, Javaheripour-Otto K, Nelitz M. Clinical outcome and magnetic resonance imaging after osteochondral autologous transplantation in osteochondritis dissecans of the talus. Foot Ankle Int. 2013;34:173-179. [DOI] [PubMed] [Google Scholar]
- 35.Yoon HS, Park YJ, Lee M, Choi WJ, Lee JW. Osteochondral autologous transplantation is superior to repeat arthroscopy for the treatment of osteochondral lesions of the talus after failed primary arthroscopic treatment. Am J Sports Med. 2014;42:1896-1903. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Xu X. Osteochondral autograft transfer combined with cancellous allografts for large cystic osteochondral defect of the talus. Foot Ankle Int. 2016;37:1113-1118. [DOI] [PubMed] [Google Scholar]