Abstract
Background
The investigation of nonnarcotic drug regimens for postoperative pain management is important in addressing the opioid epidemic. NSAIDs can be a powerful adjunct in managing postoperative pain, but the possibility of delayed bone healing is a major concern for orthopaedic surgeons. Our recent retrospective study on ketorolac administration demonstrated that the NSAID is not associated with an increased risk of delayed union or nonunion after ankle fracture surgery.
Questions/purposes
To determine whether postoperative ketorolac (1) reduces opioid consumption, (2) improves VAS pain control, and (3) affects fracture healing after open reduction and internal fixation of ankle fractures.
Methods
Between August 2016 and December 2017, 128 patients undergoing open reduction and internal fixation of an acute ankle fracture were randomized before surgery via simple randomization to treatment with or without ketorolac. No patients changed treatment regimen groups or opted out of randomization. All other aspects of perioperative care were treated identically. A once-daily survey was distributed via email on postoperative Days 1 to 7. Unblinded participants were asked to report their daily opioid consumption, pain level, and sleep interference using the VAS, and pain frequency using a five-point Likert scale, and side effects with the VAS. For VAS pain, > 20 mm/100 mm on the VAS scale was required to be considered “improved.” In all, 83% (106 of 128) patients completed all seven postoperative surveys with 14 in the control group and eight in the ketorolac group lost to follow-up. Fifty-six patients were administered ketorolac with opioid medication (treatment group) and 50 were administered opioids alone (control group). Participants were comprised of 42% men (44), and 58% women (62); mean age was 48 years. The treating surgeon assessed clinical healing based on the patient's ability to ambulate comfortably at 12 weeks postoperatively. Radiographic healing was assessed by two fellowship-trained orthopaedic foot and ankle surgeons blinded to the patient’s name and time since surgery. The surgeons evaluated randomized standard ankle series (anteroposterior, mortise, and lateral) radiographs for resolution of each fracture line to determine fracture union, with delayed union being defined as fracture lines present on radiographs taken at 12-week postoperative visits. Intention-to-treat analysis was performed.
Results
Patients in the treatment group consumed a mean of 14 opioid pills, which was less than the mean of 19.3 opioids pills consumed by patients in the control group (p = 0.037). Patients with ketorolac had lower median VAS scores for pain (p < 0.035) postoperatively on postoperative Days 1 and 2 than did control patients. By contrast, patient-reported pain scores and scores for sleep did not convincingly show a benefit to the use of ketorolac. For patients whose ankle fractures healed at 12 weeks, there was no difference between the groups in terms of clinical healing (p = 0.575) and radiographic healing (p = 0.961).
Conclusions
In this randomized study, adding ketorolac to the postoperative drug regimen decreased the use of opioid medication after open reduction and internal fixation of ankle fractures in the early postoperative period, and there were mixed, small effects on pain reduction. This NSAID is a valuable tool in helping patients manage postoperative pain with less use of narcotic analgesia. However, our study was underpowered to determine the true safety of this drug in terms of fracture healing and side effects and these questions warrant higher-powered randomized study investigation.
Level of Evidence
Level I, therapeutic study.
Introduction
The balance between adequate narcotic prescribing patterns and appropriate postoperative pain management compels randomized prospective studies to investigate effective nonnarcotic postoperative drug regimens. Studies have found that patients who consume fewer opioids after orthopaedic fracture surgery report greater satisfaction and less pain than those who consume more opioids [2, 11, 19]. Although NSAIDs may be an adjunct analgesic, many orthopaedic surgeons have hesitated to use NSAIDs because of evidence that they may lead to delayed bone healing [8, 9, 22, 23, 37].
In this study, we chose ketorolac for perioperative management because of its analgesic effect, with 30 mg of ketorolac having potency equivalent to 12 mg of morphine [40]. Ketorolac has advantages over other NSAIDs because it is formulated for both injectable and oral use [29], with good oral bioavailability and a longer half-life. Since ketorolac’s activity is primarily analgesic, rather than antiinflammatory or antipyretic, we thought this factor would limit the inhibitory healing effects seen with other NSAIDs. Although ketorolac has a high side-effect profile, predominantly gastrotoxic, these effects are diminished when it is used for fewer than 5 days. We further thought that this 5-day duration would diminish osteoinhibition, which is seen with longer NSAID use. In a recent retrospective study, we reported that delayed healing and nonunion did not appear to be a concern in patients who took ketorolac compared with historical controls [27].
Although most therapeutic NSAID trials are powered for efficacy, some studies have evaluated bone healing across different NSAID formulas as a secondary endpoint. These studies found that ketorolac caused no delay [4, 21, 31], and patients who took ketorolac had better union rates than controls and patients who took other NSAIDs [3, 14, 21]. Although some studies suggest that ketorolac may cause delayed union after spinal fusion when high doses are used [17, 24, 32], other studies report statistically significant delay [13, 30]. McDonald et al. [27] investigated bone healing after a sustained course of postoperative ketorolac after foot and ankle surgery open reduction and internal fixation (ORIF) of ankle fractures and found no difference in bone healing when compared with a control group. However, additional study should be performed to support these findings.
We therefore sought to determine whether postoperative ketorolac (1) reduces opioid consumption, (2) improves VAS pain control, and (3) affects fracture healing after ORIF of ankle fractures.
Patients and Methods
Patient Selection
This study, which was conducted at a large, academically affiliated, private institution, prospectively enrolled patients undergoing ORIF of isolated lateral malleolar, bimalleolar, or trimalleolar ankle fractures. Institutional review board approval was obtained before study initiation. Inclusion criteria were patients older than 18 years undergoing ORIF in an outpatient setting by a fellowship-trained foot and ankle orthopaedic surgeon (JND, RJS, DIP, SMR, BSW), for one of the three aforementioned fracture types from August 2016 to December 2017. The study has been retrospectively registered at clinicaltrials.gov #NCT03727048. Fracture and fracture types were identified using AP, lateral, and mortise-view radiographs and confirmed clinically before surgery. Any patients with an open fracture, any concomitant ankle fracture or other fractures, allergies to any study medication, existing narcotic use, renal insufficiency as defined by the patient’s history and preoperative creatinine level, pregnancy, or hospital admittance were excluded from enrollment.
Patients who met the inclusion criteria and who chose to enroll were prospectively randomized via simple randomization to treatment with or without ketorolac in a parallel design (Fig. 1). There were no crossover or dropouts. Intention-to-treat analysis was performed. Overall, 72% patients (46 of 64) in the treatment group and 73% (47 of 64) in the control group were available for assessment of radiographic healing at 12 weeks. Fracture types were not stratified during the randomization process. A statistician (KN) used the R Statistical Computing Environment (R Foundation, Vienna, Austria) to generate a random number allocation sequence, which was given to the research staff member (KN) enrolling patients before the start of patient enrollment. This staff member informed each surgeon of participant enrollment and the intervention assignment after the surgeon had completed a preoperative holding room conversation with the patient. Surgeons needed to be informed before surgery because participants in the ketorolac group received intraoperative intravenous ketorolac, which was administered by the surgeon. Patients were blinded at enrollment and were first made aware which intervention they were randomized to when receiving their postoperative pain medication, typically 6 to 36 hours after surgery, at outside locations that did not participate in the study and were therefore unable to blind patients as to which medications were used. Patients in both groups were offered perioperative regional anesthesia (popliteal and saphenous nerve blocks) as per the protocol at our institution (Fig. 1). Those who refused the nerve block were excluded from the study. Those randomized to the treatment group were administered 30 mg of intravenous ketorolac intraoperatively, 20 tablets of 10-mg ketorolac with instructions to take one 10-mg tablet every 6 hours, and 30 tablets of 5/325 oxycodone-acetaminophen with instructions to take one or two tablets every 4 to 6 hours as needed for pain. Meanwhile, patients randomized to the control group were given 30 tablets of 5/325 oxycodone-acetaminophen with instructions to take one or two tablets every 4 to 6 hours as needed for pain. In both groups, patients were prescribed 81 mg of aspirin twice daily to prevent deep venous thrombosis. Outside of study intervention, both groups were treated identically.
Fig. 1.

Patients who met the inclusion criteria and chose to enroll were prospectively randomized via simple randomization to treatment with or without ketorolac in a parallel study design. Patients in both groups were offered perioperative regional anesthesia (popliteal and saphenous nerve blocks) as per the protocol at our institution. Patients who opted for general anesthesia without a regional block were excluded from pain and clinical outcome analyses.
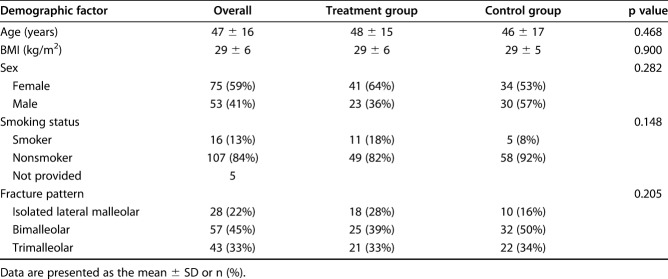
In all, 83% (106 of 128) completed all seven daily postoperative surveys. Fifty-six patients were administered ketorolac with opioid medication and 50 were given opioids alone. Among the participants, 42% were men (44 of 106) and 58% were women (62 of 106) with a mean age of 48 years (± 16) (Table 1). Baseline age, BMI, sex, smoking status, and fracture type were not different between the groups (Table 1). The number of patients with diabetes was too small to analyze; only four patients in the control group and five in the treatment group had diabetes. Fracture patterns also did not differ between the groups (p = 0.205) (Table 1). To eliminate the effect of regional anesthesia on postoperative narcotic consumption and pain levels, we excluded the few patients (n = 9) who opted to not receive a regional nerve block from our analyses of pain, and the number of opioid pills consumed. This left 97 patients for analysis, of whom 52 were given ketorolac with opioid medication (treatment group) and 45 were given opioids alone (control group).
Table 1.
Baseline demographics and fracture pattern (n = 128)
Operative Protocol
Five fellowship-trained orthopaedic foot and ankle surgeons (JND, RJS, DIP, SMR, BSW) performed the procedures in five different facilities at a single practice. Plate and screw constructs were used in a standard fashion to stabilize the fractures. Intraoperative stress tests were used to assess for syndesmotic and deltoid instability, which was stabilized as needed using a syndesmotic screw, suture button construct, or bone anchor [10, 28].
Postoperative Care
Patients underwent routine postoperative care including clinical and radiographic evaluation with AP, lateral, and mortise views of the ankle at a minimum of 6 and 12 weeks postoperatively. Typically, at 6 weeks, patients began using a weightbearing fracture boot based on a clinical examination and radiographic evidence of adequate fracture healing. Weightbearing was occasionally delayed for an additional 2 to 6 weeks at the surgeon’s discretion because of insufficient healing.
Study Outcomes
Our primary study outcome was reduction in narcotic consumption during the first 7 days after surgery. To obtain clinical and pain outcomes, we distributed a survey daily via email through the Research Electronic Data Capture (REDCap, Nashville, TN, USA) system on postoperative Days 1 through 7. Once daily, patients were asked to report opioid consumption and pain level, perceived frequency of pain using a five-point Likert scale (never, almost never, often, almost always, or always), pain interference with sleep, and side effects, including hypersensitivity, numbness, paresthesia, nausea or vomiting, and constipation via a 100-point VAS scale.
To evaluate the secondary outcome of healing, we separately assessed clinical and radiographic healing at the 12-week follow-up visit, which typically occurred 12 to 14 weeks postoperatively. Clinical healing was assessed based on the patient's ability to ambulate comfortably. In all, 89% (114 of 128) returned at approximately 12 weeks for an assessment of clinical healing. Patients were followed until all fractures had bony union. Of patients available for followup until union, there was no report of nonunion, but one patient was lost to followup. To evaluate radiographic healing, another secondary endpoint, two fellowship-trained orthopaedic foot and ankle surgeons (BSW, JND) were blinded to the patient’s name and time since surgery. The surgeons evaluated blinded radiographs for resolution of each fracture line to determine fracture union. Interrater reliability was evaluated by computing Cohen’s kappa, which was 0.876 for lateral malleolus, 0.859 for posterior malleolus, and 0.956 for medial malleolus fractures. Patients’ records were also reviewed for postoperative complications. Wound complications were defined as any usage of local wound care, antibiotics, or repeat surgery.
Statistical Analysis
A chi-square power analysis demonstrated that 108 patients would need to be enrolled to detect a medium-effect [7] difference in healing by 12 weeks between groups, with a power of 0.80 and a significance level of 0.05. To account for potential loss to followup, 128 patients were enrolled. Seventeen percent (22 of 128) of patients did not complete all seven daily postoperative surveys and were excluded from the analysis. Patients who opted for general anesthesia without a regional block were excluded from pain and clinical outcome analyses (Fig. 1).
A trained statistician (KN) who was blinded to the allocation process analyzed the data. An intention-to-treat analysis was performed and there was no crossover. No participants opted out of randomization. The normality of age and BMI data was evaluated by skewness and kurtosis. To test for differences in age and BMI between the randomized groups, we performed a t-test. To test for smoking status, sex, and fracture patterns between the two groups, we used a chi-square test. For each patient outcome, we used a random-intercept generalized linear mixed-effect model to test for differences between groups over time. Pairwise comparisons between groups for each day were made using least-square means with Tukey’s honestly significant difference test.
Results
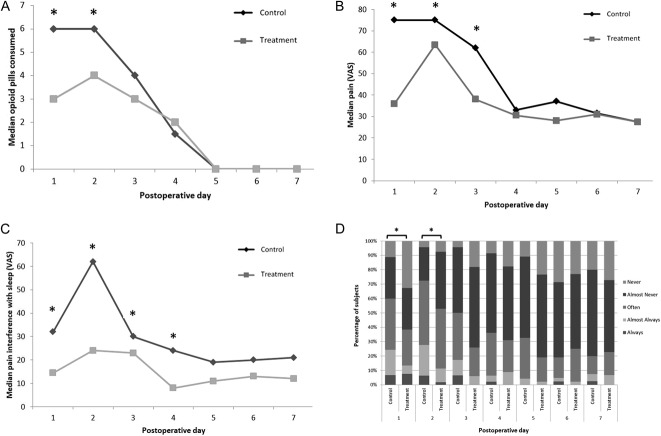
Opioid consumption was reduced in the patients randomized to ketorolac. Patients in the treatment group reported taking a mean of 14.0 ± 11.8 [95% CI 3.4 to 15.2] tablets of 5/325 oxycodone-acetaminophen in the first 7 days postoperatively, which was fewer than the mean number of tablets consumed by participants in the control group (19.3 ± 13.9 [95% CI 3.7 to 17.6]; p = 0.037). This equated to 21 morphine mg equivalents and 29 morphine mg equivalents consumed by patients in the treatment and control groups, respectively, within the first postoperative week [6]. During postoperative Days 1 and 2, patients who were given ketorolac consumed less oxycodone-acetaminophen than patients in the control group, respectively (Day 1: 3.0 ± 3.6 [95% CI 1.0 to 5.0] versus 5.5 ± 4.0 [95% CI 1.8 to 6.0]; p = 0.0308; Day 2: 4.0 ± 3.2 [95% CI 2 to 6]; p = 0.0031) (Fig. 2A).
Fig. 2 A-D.
(A) During postoperative Days 1 and 2, patients who were administered ketorolac consumed less oxycodone-acetaminophen than did those in the control group. Although patient-reported daily opioid consumption did not differ between the groups after Day 2, patients treated with ketorolac had (B) lower VAS pain scores (Days 1 to 3;), (C) better sleep (Days 1 to 4), and (D) lower frequency of pain (Days 1 to 3;) than patients in the control group. Asterisks indicate significant (p ≤ 0.05) differences between variables on that postoperative day.
By contrast, patient-reported pain scores and scores for sleep did not convincingly show a benefit from ketorolac. Although patient-reported daily opioid consumption did not differ after Day 2, patients treated with ketorolac had lower VAS pain scores (Days 1 to 3; Day 1: 42 ± 34.2 [95% CI 7.5 to 74.0] versus 75 ± 30.9 [95% CI 39.5 to 89.0]; p < 0.001; Day 2: 63 ± 25.6 [95% CI 40 to 74] versus 75 ± 26.2 [95% CI 59 to 91]; p = 0.031; Day 3: 37.5 ± 25.3 [95% CI 23.5 to 62.0] versus 62.0 ± 24.2 [95% CI 34.0 to 73.0]; p = 0.008), better sleep (Days 1 to 4; Day 1: 14 ± 28.3 [95% CI 2.5 to 38.0] versus 32 ± 33.3 [95% CI 10.0 to 74.0]; p = 0.002; Day 2: 23 ± 28.4 [95% CI 11.5 to 60.0] versus 62 ± 31.2 [95% CI 27.0 to 78.5]; p < 0.001; Day 3: 7.5 ± 21.7 [95% CI 0.3 to 20.5] versus 23 ± 22.6 [95% CI 10.0 to 40.0]; p = 0.01; Day 4 10 ± 20.1 [95% CI 2.8 to 21.5] versus 18 ± 21.1 [95% CI 8.0 to 29.0]; p = 0.04), and lower frequency of pain than participants in the control group (Days 1 to 3; Day 1: 1 ± 1.2 [95% CI 0 to 2] versus 2 ± 1.1 [95% CI 1 to 2]; p = 0.005; Day 2: 2 ± 0.8 [95% CI 1 to 2] versus 2 ± 0.9 [95% CI 1 to 3]; p = 0.01; Day 3: 1 ± 0.8 [95% CI 1 to 1.8] versus 1 ± 0.8 [95% CI 1 to 1.8]; p = 0.004) (Fig. 2B-D). Of note, VAS pain only reached a MCID clinically significant value of > 20 mm/100 m on Day 1. Further, patient-reported hypersensitivity (p = 0.003) and numbness (p = 0.031) measured with the VAS were lower in the ketorolac group than in the control group on Day 1, but there were no patient-reported differences in paresthesia (p = 0.813), nausea or vomiting (p = 0.198), or constipation (p = 0.273).
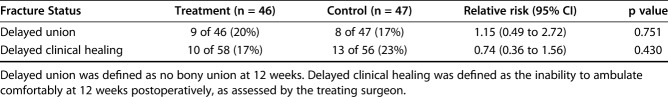
With the numbers available, there was no difference between the study groups in the proportion of patients who had united by 12 weeks after surgery, but this study was underpowered to assess this important endpoint. In all, 83% of patients (48 of 58) in the treatment group and 77% (43 of 56) in the control group had clinical healing within 12 weeks of surgical correction (RR = 0.74 [95% CI 0.36 to 1.56]; p = 0.430) (Table 2). Eighty percent of patients (37 of 46) in the treatment group and 83% of participants (39 of 47) in the control group had radiographic healing within 12 weeks, also with no difference between the groups (RR = 1.15 [95% CI 0.49 to 2.72]; p = 0.751).
Table 2.
Outcomes of delayed union and delayed clinical healing between treatment and control groups
Other Findings
During the study period, no deep venous thrombosis, pulmonary embolism, or cardiac events were recorded in the surgeons’ records or reported by patients. Further, no renal or gastrointestinal adverse events occurred in our cohort of patients; however, side effects could have been overlooked because laboratory data were not available. Six wound complications (all in the control group) occurred; five patients had local wound care and one underwent irrigation and débridement. The number of wound complications was insufficient for statistical analysis. Two patients (both in the treatment group) experienced extensive delayed union. Both patients fractures eventually united and required no further surgery.
Discussion
With the opioid epidemic receiving increasing attention, the orthopaedic community has critically examined its narcotic prescribing habits. Despite the advantages of NSAIDs as part of a multimodal pain management approach [15, 16, 35, 38], the risk of interference with osteogenesis and the potential for fracture nonunion have been concerns for orthopaedic surgeons [21, 34]. We have demonstrated that the addition of our ketorolac regimen to the postoperative drug course reduced pain and decreased the use of opioid medication, and should be considered an effective treatment regimen by clinicians, although we were unable to statistically determine ketorolac’s effect on fracture healing and side effects due to underpowering.
There are limitations to this study. First, participants and surgeons were not blinded owing to external limitations. A source of bias also exists because fracture types and patterns and fracture fixation methods were not stratified during the enrollment process. Patients could have misreported their opioid use, pain levels, and side effects, which could have led to an information bias. Second, although we did not detect any differences in fracture healing or adverse events between the study groups, statistical power to detect these differences in fracture was not achieved, which could have led to and information bias. In a larger study, if nonunion and adverse events were found to be a problem, the risks would far outweigh the analgesic benefit. We also acknowledge that the absence of CT scans to determine healing is a limitation of the study. However, CT scans are not routine for ankle fracture care and to limit patients’ radiation exposure, we did not CT scans to assess healing. Additionally, patients who were administered ketorolac were instructed about when to take it. Patients self-reported the number of opioids they consumed; therefore, adherence to the treatment regimen was not recorded. Although a limitation, this reflects actual practice. Self-reporting by patients may have also led to a recall bias. However, to limit this, we asked patients to complete the survey daily. The social stigma associated with opioid consumption may have also led to underreporting. Furthermore, all participants were instructed to not take any additional NSAIDs or acetaminophen in the postoperative period, but this, similar to true clinical practice, was not controlled. Also, patients were not asked about past acute opioid use or opioid addiction problems; therefore, we were unable to categorize patients as opioid-naïve and opioid-exposed. Opioid pill consumption after 1 week postoperatively was not recorded, but this, or a shorter endpoint, is not uncommon for tracking opioid use after ankle fracture surgery because this is the primary opioid consumption time period [5, 20, 33]. Additionally, the risks and benefits of administration of another drug class should be considered on an individual basis, as NSAIDS are associated with the risk of side effects that may not be worth the benefit of decreased narcotic consumption, especially in the elderly population, where polypharmacy is prevalent and consequential.
We found that patients randomized to the ketorolac group took somewhat less opioid medication than the control group (a difference of 5 opioid tablets), which is consistent with the result of previous studies [2, 11, 19]. The greatest reduction in the median opioid use was on postoperative Days 1 and 2, when the regional block tended to lose its efficacy and the risk of postoperative pain was at a maximum [12, 18]. This suggests that a multimodal approach with the addition of ketorolac can serve as a powerful adjunct to not only improve postoperative pain control in general but also help protect the patient from having extensive rebound pain, which is a well-documented complication of regional blockades [1, 12, 18, 39]. Although the addition of this NSAID has substantial benefits to ankle fracture patients in terms of pain management and narcotic consumption, we still must recommend that surgeons consider each patient on an individual basis, as the risks associated with ketorolac must always be taken into consideration, especially in elderly patients and those patients already on other medications known to have side effects in the gastrointestinal and renal systems.
We found a mixed result on postoperative pain; while the VAS pain scores were reduced at some time points, this was not true across the board, and the differences often were small (below the MCID for the VAS pain scale), and so likely imperceptible to many patients.
Within 12 weeks after ORIF, more than 80% of patients in both groups had evidence of clinical and radiographic fracture healing, with no difference between the groups. Of the patients whose fractures did not heal within 12 weeks postoperatively, the median time to radiographic healing was 17 weeks in both groups, which is consistent with the time to healing in previous studies [25, 26, 36]. There were no reports of nonunion in this cohort. It must be mentioned that although we demonstrated no differences in delayed unions or nonunions, our study was underpowered to determine the true safety of this drug in terms of fracture healing. Lastly, the safety of using ketorolac in conjunction with low-dose aspirin was not determined regarding this regimen’s relationship with the gastrointestinal and renal systems. This is important to elucidate because aspirin is routinely used for prophylaxis against deep venous thrombosis in patients undergoing foot and ankle surgery. No renal or gastrointestinal adverse events occurred in our small study; however, side effects could have been overlooked because laboratory data were not available or missed because they are relatively uncommon and our sample size was insufficient to detect them.
The addition of oral ketorolac to the postoperative drug regimen reduced pain during Day 1 and mixed, small effects on the other postoperative days, while decreasing opioid use in the first 2 days after ORIF of ankle fractures, although our study was underpowered to determine the true safety of this drug in terms of fracture healing and side effects. Better pain management during postoperative Days 1 and 2 is particularly important because patients on average consume the most opioids during this time. Based on these improved outcomes and the affordability of ketorolac, this drug is highly valuable for the treatment of ankle fractures. Future high-powered, randomized studies are needed to investigate interference with bone healing and safety.
Acknowledgments
None.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Rothman Orthopaedic Institute, Philadelphia, PA, USA.
References
- 1.Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth Analg . 2015;120:1114–1129. [DOI] [PubMed] [Google Scholar]
- 2.Bot AGJ, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res . 2014;472:2542–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br . 2003;85:700–705. [PubMed] [Google Scholar]
- 4.Cappello T, Nuelle JAV, Katsantonis N, Nauer RK, Lauing KL, Jagodzinski JE, Callaci JJ. Ketorolac administration does not delay early fracture healing in a juvenile rat model: a pilot study. J Pediatr Orthop . 2013;33:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen KP, Møller AM, Nielsen JK, Klausen TW, Sort R. The effects of anesthetic technique on postoperative opioid consumption in ankle fracture surgery. Clin J Pain. 2016;32:870–874. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention: U.S. Department of Health and Human Services. Calculating Total Daily Dose of Opioids For Safer Dosage . Available at: https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf. [Accessed May 6, 2018].
- 7.Cohen J. A power primer. Psychol Bull . 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 8.Cottrell J, O’Connor JP. Effect of non-steroidal anti-inflammatory drugs on bone healing. Pharm Basel Switz . 2010;3:1668–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br. J Anaesth. 1991;66:703–712. [DOI] [PubMed] [Google Scholar]
- 10.Davidovitch RI, Weil Y, Karia R, Forman J, Looze C, Liebergall M, Egol K. Intraoperative syndesmotic reduction: three-dimensional versus standard fluoroscopic imaging. J Bone Joint Surg Am . 2013;95:1838–1843. [DOI] [PubMed] [Google Scholar]
- 11.Dawson R, Spross JA, Jablonski ES, Hoyer DR, Sellers DE, Solomon MZ. Probing the paradox of patients’ satisfaction with inadequate pain management. J Pain Symptom Manage . 2002;23:211–220. [DOI] [PubMed] [Google Scholar]
- 12.Ding DY, Manoli A, Galos DK, Jain S, Tejwani NC. Continuous popliteal sciatic nerve block versus single injection nerve block for ankle fracture surgery: a prospective randomized comparative trial. J Orthop Trauma. 2015;29:393–398. [DOI] [PubMed] [Google Scholar]
- 13.Donohue D, Sanders D, Serrano-Riera R, Jordan C, Gaskins R, Sanders R, Sagi HC. Ketorolac administered in the recovery room for acute pain management does not affect healing rates of femoral and tibial fractures. J Orthop Trauma. 2016;30:479–482. [DOI] [PubMed] [Google Scholar]
- 14.Dorn U, Grethen C, Effenberger H, Berka H, Ramsauer T, Drekonja T. Indomethacin for prevention of heterotopic ossification after hip arthroplasty. A randomized comparison between 4 and 8 days of treatment. Acta Orthop Scand . 1998;69:107–110. [DOI] [PubMed] [Google Scholar]
- 15.Elia N, Lysakowski C, Tramèr MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103:1296–1304. [DOI] [PubMed] [Google Scholar]
- 16.Gillis JC, Brogden RN. Ketorolac. A reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997;53:139–188. [DOI] [PubMed] [Google Scholar]
- 17.Glassman SD, Rose SM, Dimar JR, Puno RM, Campbell MJ, Johnson JR. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834–838. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein RY, Montero N, Jain SK, Egol KA, Tejwani NC. Efficacy of popliteal block in postoperative pain control after ankle fracture fixation: a prospective randomized study. J Orthop Trauma. 2012;26:557–561. [DOI] [PubMed] [Google Scholar]
- 19.Helmerhorst GTT, Lindenhovius ALC, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43:1958–1961. [DOI] [PubMed] [Google Scholar]
- 20.Henningsen MJ, Sort R, Møller AM, Herling SF. Peripheral nerve block in ankle fracture surgery: a qualitative study of patients’ experiences. Anaesthesia. 2018;73:49–58. [DOI] [PubMed] [Google Scholar]
- 21.Jeffcoach DR, Sams VG, Lawson CM, Enderson BL, Smith ST, Kline H, Barlow PB, Wylie DR, Krumenacker LA, McMillen JC, Pyda J, Daley BJ, University of Tennessee Medical Center, Department of Surgery Nonsteroidal anti-inflammatory drugs’ impact on nonunion and infection rates in long-bone fractures. J Trauma Acute Care Surg . 2014;76:779–783. [DOI] [PubMed] [Google Scholar]
- 22.Kinsella J, Moffat AC, Patrick JA, Prentice JW, McArdle CS, Kenny GN. Ketorolac trometamol for postoperative analgesia after orthopaedic surgery. Br J Anaesth . 1992;69:19–22. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinidis I, Papageorgiou SN, Kyrgidis A, Tzellos T-G, Kouvelas D. Effect of non-steroidal anti-inflammatory drugs on bone turnover: an evidence-based review. Rev Recent Clin Trials. 2013;8:48–60. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Zhang Z, Cai Z. High-dose ketorolac affects adult spinal fusion: a meta-analysis of the effect of perioperative nonsteroidal anti-inflammatory drugs on spinal fusion. Spine. 2011;36:E461-468. [DOI] [PubMed] [Google Scholar]
- 25.Lindsjö U. Operative treatment of ankle fracture-dislocations. A follow-up study of 306/321 consecutive cases. Clin Orthop Relat Res . 1985:28–38. [PubMed] [Google Scholar]
- 26.Matson AP, Hamid KS, Adams SB. Predictors of time to union after operative fixation of closed ankle fractures. Foot Ankle Spec . 2017;10:308–314. [DOI] [PubMed] [Google Scholar]
- 27.McDonald E, Winters B, Nicholson K, Shakked R, Raikin S, Pedowitz DI, Daniel JN. Effect of postoperative ketorolac administration on bone healing in ankle fracture surgery. Foot Ankle Int. 2018. 2018;39:1135-1140. [DOI] [PubMed] [Google Scholar]
- 28.Michelson JD, Wright M, Blankstein M. Syndesmotic ankle fractures. J Orthop Trauma. 2018;32:10–14. [DOI] [PubMed] [Google Scholar]
- 29.Norman PH, Daley MD, Lindsey RW. Preemptive analgesic effects of ketorolac in ankle fracture surgery. Anesthesiology. 2001;94:599–603. [DOI] [PubMed] [Google Scholar]
- 30.Pradhan BB, Tatsumi RL, Gallina J, Kuhns CA, Wang JC, Dawson EG. Ketorolac and spinal fusion: does the perioperative use of ketorolac really inhibit spinal fusion? Spine. 2008;33:2079–2082. [DOI] [PubMed] [Google Scholar]
- 31.Reikeraas O, Engebretsen L. Effects of ketoralac tromethamine and indomethacin on primary and secondary bone healing. An experimental study in rats. Arch Orthop Trauma Surg . 1998;118:50–52. [DOI] [PubMed] [Google Scholar]
- 32.Reuben SS, Ablett D, Kaye R. High dose nonsteroidal anti-inflammatory drugs compromise spinal fusion. Can J Anaesth J Can Anesth . 2005;52:506–512. [DOI] [PubMed] [Google Scholar]
- 33.Sort R, Brorson S, Gögenur I, Møller AM. AnAnkle Trial study protocol: a randomised trial comparing pain profiles after peripheral nerve block or spinal anaesthesia for ankle fracture surgery. BMJ Open. 2017;7:e016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiro AS, Beil FT, Baranowsky A, Barvencik F, Schilling AF, Nguyen K, Khadem S, Seitz S, Rueger JM, Schinke T, Amling M. BMP-7-induced ectopic bone formation and fracture healing is impaired by systemic NSAID application in C57BL/6-mice. J Orthop Res Off Publ Orthop Res Soc . 2010;28:785–791. [DOI] [PubMed] [Google Scholar]
- 35.Straube S, Derry S, McQuay HJ, Moore RA. Effect of preoperative Cox-II-selective NSAIDs (coxibs) on postoperative outcomes: a systematic review of randomized studies. Acta Anaesthesiol Scand . 2005;49:601–613. [DOI] [PubMed] [Google Scholar]
- 36.Tejwani NC, Park JH, Egol KA. Supination external rotation ankle fractures: A simpler pattern with better outcomes. Indian J Orthop . 2015;49:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Esch RW, Kool MM, van As S. NSAIDs can have adverse effects on bone healing. Med Hypotheses. 2013;81:343–346. [DOI] [PubMed] [Google Scholar]
- 38.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg . 2017;152:691–697. [DOI] [PubMed] [Google Scholar]
- 39.Williams BA, Bottegal MT, Kentor ML, Irrgang JJ, Williams JP. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med . 2007;32:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yee JP, Koshiver JE, Albon C, Brown CR. Comparison of intramuscular ketorolac tromethamine and morphine sulfate for analgesia of pain after major surgery. Pharmacotherapy. 1986;6:253–61. [DOI] [PubMed] [Google Scholar]