Abstract
Background
Health systems and payers use patient-reported outcome measures (PROMs) to inform quality improvement and value-based payment models. Although it is known that psychosocial factors and priming influence PROMs, we sought to determine the effect of having patients complete functional tasks before completing the PROM questionnaire, which has not been extensively evaluated.
Questions/purposes
(1) Will QuickDASH scores change after patients complete the tasks on the questionnaire compared with baseline QuickDASH scores? (2) Will the change in QuickDASH score in an intervention (task completion) group be different than that of a control group? (3) Will a higher proportion of patients in the intervention group than those in the control group improve their QuickDASH scores by greater than a minimally clinically important difference (MCID) of 14 points?
Methods
During a 2-month period, 140 patients presented at our clinic with a hand or upper-extremity problem. We approached patients who spoke and read English and were 18 years old or older. One hundred thirty-two (94%) patients met the inclusion criteria and agreed to participate (mean ± SD age, 52 ± 17 years; 60 men [45%], 72 women [55%]; 112 in the intervention group [85%] and 20 in the control group [15%]). First, all patients who completed the QuickDASH PROM (at baseline) were recruited for participation. Intervention patients completed the functional tasks on the QuickDASH and completed a followup QuickDASH. Control patients were recruited and enrolled after the intervention group completed the study. Participants in the control group completed the QuickDASH at baseline and a followup QuickDASH 5 minutes after (the time required to complete the functional tasks). Paired and unpaired t-tests were used to evaluate the null hypotheses that (1) QuickDASH scores for the intervention group would not change after the tasks on the instrument were completed and (2) the change in QuickDASH score in the intervention group would not be different than that of the control group (p < 0.05). To evaluate the clinical importance of the change in score after tasks were completed, we recorded the number of patients with a change greater than an MCID of 14 points on the QuickDASH. Fisher’s exact test was used to evaluate the difference between groups in those reaching an MCID of 14.
Results
In the intervention group, the QuickDASH score decreased after the intervention (39 ± 24 versus 25 ± 19; mean difference, -14 points [95% CI, 12 to 16]; p < 0.001). The change in QuickDASH scores was greater in the intervention group than that in the control group (-14 ± 11 versus -2 ± 9 [95% CI, -17 to -7]; p < 0.001). A larger proportion of patients in the intervention group than in the control group demonstrated an improvement in QuickDASH scores greater than the 14-point MCID ([43 of 112 [38%] versus two of 20 [10%]; odds ratio, 5.4 [95% CI, 1 to 24%]; p = 0.019).
Conclusions
Reported disability can be reduced, thereby improving PROMs, if patients complete QuickDASH tasks before completing the questionnaire. Modifiable factors that influence PROM scores and the context in which scores are measured should be analyzed before PROMs are broadly implemented into reimbursement models and quality measures for orthopaedic surgery. Standardizing PROM administration can limit the influence of context, such as task completion, on outcome scores and should be used in value-based payment models.
Level of Evidence
Level II, therapeutic study.
Introduction
Patient-reported outcome measures (PROMs) are increasingly used in orthopaedic surgery to assess patient outcomes and improve quality of care. PROMs in orthopaedic surgery often measure symptom intensity and disability, in contrast to outcomes reported by a physician or objective data (measurable parameters such as range of motion). The DASH and QuickDASH are region-specific PROMs frequently used in hand and upper-extremity surgery. The DASH is a 30-item instrument developed to address a patient’s disability related to their upper extremity during the preceding week [15, 19]. The QuickDASH, a shorter 11-item instrument, was developed to reduce redundancy and improve the speed and efficiency of administration [2, 3].
PROMs vary in their design, collection, and characteristics, and are evaluated through psychometric testing to determine validity, reliability, and responsiveness to change. Evaluating the measurement properties of PROMs before implementation is necessary because it addresses the quality of data obtained with the tool and the conclusions that can be drawn [23]. These measurement domains, however, may not reflect a patient’s actual ability to complete the queried tasks or to actual disability. For example, if a patient has not completed a queried functional task, such as sweeping the floor, he or she may report disability based on an activity that he or she perceives to be similar in character. Not only are PROM scores subject to recall [26, 28] and availability bias [33], but the context in which PROM questions are asked may also influence the response. Psychosocial factors and priming have been found to have a notable effect on PROMs [4, 5, 8, 16, 17, 20, 21, 30, 32, 34], raising the possibility that the context in which PROMs are administered, specifically having patients conduct functional tasks, may influence PROM scores.
We therefore asked (1) Will QuickDASH scores change after patients complete the tasks on the questionnaire compared with baseline QuickDASH scores? (2) Will the change in QuickDASH score in an intervention (task completion) group be different than that of a control group? (3) Will a higher proportion of patients in the intervention group than those in the control group improve their QuickDASH scores by greater than a minimally clinically important difference (MCID) of 14 points?
Patients and Methods
Patient Selection
After institutional review board approval was obtained for this study, we enrolled patients from an outpatient hand and upper-extremity clinic at a suburban academic medical center. A research assistant (LMS) approached all new and returning patients presenting to the hand and upper-extremity clinic of the senior author (RNK). Inclusion criteria were age older than 18 years and the ability to speak and read English. Patients were excluded if they did not have complete questionnaires (that is, if the QuickDASH instrument had more than one missing item) [3]. One hundred forty patients were eligible for inclusion in the study. We excluded eight patients. One was younger than 18 years, four could not speak or read English, and three had not filled out the QuickDASH completely. No patients declined to participate. As per the clinic’s protocol, all patients filled out the QuickDASH questionnaire at registration before being seen or evaluated by the physician (baseline score). Patients meeting the inclusion criteria were approached by a member of the research team before their visit with the physician and provided consent.
Intervention Group
One hundred twelve patients were enrolled in the intervention group. Although the intervention group quickly reached the sample size determined by an a priori power analysis, we continued to recruit patients and collect data to strengthen the results. We recorded the patients’ demographics, hand dominance, and laterality of the affected upper extremity. Patients completed tasks as directed by the QuickDASH (items 1-6). The tasks included (1) opening a tight jar that was closed by the same member of the research team before each patient interview; (2) simulating washing a clinic wall with a sponge for 20 seconds; (3) carrying a shopping bag (weight, 3.3 kg) from the clinic room chair to the door and back for 20 steps; (4) simulating washing one’s back with a sponge for 20 seconds; (5) using a knife to cut a piece of Play-Doh into four pieces; and (6) hammering a piece of wood five times. The patient was not directed to use a specific extremity to complete each task. The research assistant (LMS) noted which extremity was used to complete the tasks. A followup QuickDASH questionnaire was administered after tasks were completed. QuickDASH scores range from 0 to 100; a score of 0 indicates no difficulty with tasks and a score of 100 indicates extreme limitation or an inability to perform tasks.
Control Group
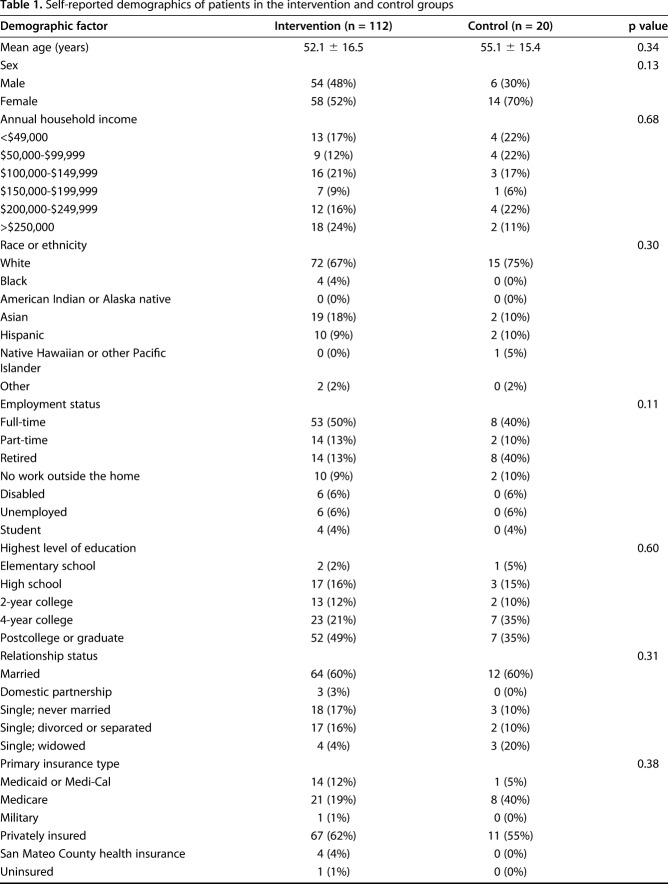
A control group was recruited and enrolled after the intervention group completed the study to strengthen and give context to the study. These patients were enrolled and approached in the same manner as those in the intervention group. Twenty patients were enrolled in the control group. We recorded these patients’ demographics, and patients completed a followup QuickDASH questionnaire 5 minutes after the baseline QuickDASH (no intervention). A 5-minute time interval was chosen because this was matched to the average time it took patients in the intervention group to complete the tasks. There were no demographic differences between patients in the intervention and control groups (Table 1).
Table 1.
Self-reported demographics of patients in the intervention and control groups
Data Calculation and Statistical Analysis
To detect a difference between baseline and followup QuickDASH scores, an a priori sample size estimate for the intervention group using an MCID of 14 identified that 64 patients were needed to provide 80% power, with a Cohen’s d of 0.65 (alpha = 0.05). To detect a difference between the change in score in the intervention group and that in the control group, an a priori sample size estimate using a mean change of 0 (from measurement theory, assuming that the change in QuickDASH score after waiting 5 minutes would be 0) and a mean change of 14.2 (from initial data) identified that 20 patients per group were needed to provide power of 80% (alpha = 0.05). We recorded data on demographics, the affected hand, and hand used for tasks (in the intervention group). Paired and unpaired t-tests were used to evaluate the null hypotheses that (1) QuickDASH scores in the intervention group would not change after completing the tasks on the instrument; and (2) the change in QuickDASH score in the intervention group would not be different than that in the control group. To evaluate the clinical importance of the change in score after completing tasks, we recorded the number of patients with an MCID greater than 14 [31]. Fisher’s exact test was used to evaluate the difference between groups in those reaching an MCID of 14.
Results
In the intervention group, the QuickDASH score decreased after the intervention (39 ± 24 versus 25±19; mean difference, -14 points [95% CI, 12 to 16]; p < 0.001) (Fig. 1).
Fig. 1.
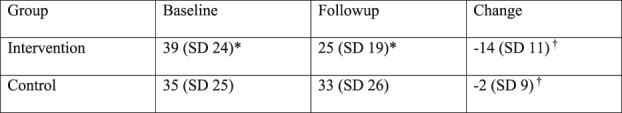
This figure shows the baseline, followup, and change in QuickDASH scores of the intervention and control groups. *indicates p < 0.05 between the baseline and followup scores within a group; †indicates p < 0.05 between the change in QuickDASH scores between groups.
The change in QuickDASH scores was greater in the intervention group (mean, -14.2) than that in the control group (-14 ± 11 versus -2 ± 9 [95% CI, -17 to -7]; p < 0.001).
A larger proportion of patients in the intervention group than the control group demonstrated an improvement in QuickDASH scores greater than the 14-point MCID (43 of 112 patients [38%] versus two of 20 patients [10%]; odds ratio, 5.4 [95% CI, 1% to 24%]; p = 0.019).
Discussion
The emphasis on patient-centered care and outcomes from the patient perspective has led to increased incorporation of PROMs into clinical care, quality improvement, and reimbursement models [6, 7, 9, 10, 12, 27, 28]. PROMs are initially evaluated through psychometric testing; however, these metrics may not accurately reflect patients’ disabilities. It is already known that psychosocial factors may influence PROM scores, but an understanding of the effect of the measurement context (such as the environment in which the test is administered) on PROM scores is also essential. We found that PROM scores can be improved by having patients complete the functional tasks queried before completing the PROMs. These results can impact clinical care because PROMs are being implemented into reimbursement models and quality measures. Methods to standardize PROM administration may limit the influence of context on these outcome scores.
Our study has some limitations. The primary limitation is the lack of randomization. The study was initially designed as an exploratory cohort study of the intervention group only. The authors added a control group after we completed collecting data for the intervention group because we thought this would strengthen the conclusion. Patients in both groups were recruited in the same manner, with the same inclusion and exclusion criteria, from the same clinic. Although we found no differences in the intervention and control groups, the sequential enrollment of patients may have led to a selection bias, and future studies should be randomized to mitigate selection bias. A second limitation is the 5-minute interval between the control group questionnaires, which might not be long enough. Five minutes was selected because this was the average time participants in the intervention group needed to complete the tasks.
Another limitation is that the MCID, defined as the minimal amount of change that is interpreted as important to a patient, should be regarded with caution [36]. The MCID for the QuickDASH ranged from 8 to 15.91 in prior studies, with variation primarily because of the method of calculation and population sampled [11, 22, 31]. All of these studies reported an MCID of or below 14, except one in which a post triangulation MCID of 15.91 was reported [11, 22, 31]. We selected an MCID of 14 as a cutoff to compare the score changes because the MCID reported in this study was calculated from the largest number of patients most similar to those in our study. If we had selected a threshold of 15.91, 10 of the score changes in the intervention group would have been above the MCID, and if we had selected a threshold of 8, 76 (68%) of the score changes in the intervention group would have been above the MCID. We also constructed the functional tasks to emulate tasks as they would be performed in a patient’s life (such as having patients carry a shopping bag and use a hammer); however, it was not feasible to have patients do the tasks in a clinical setting as they would do in daily life; for example, simulating washing one’s back in clinic does not perfectly emulate washing one’s back in the shower at home. We sought to emulate real life by allowing patients to conduct tasks the way they would outside a research setting, and therefore did not randomize patients to use a particular extremity (dominant or non-dominant, or injured or non-injured). This was a pragmatic approach that likely represents how the patient would complete a task in real life.
Additionally, and importantly, we did not capture information on psychosocial factors (such as depression and coping strategies), which have been demonstrated to affect outcome measures and may account for some of the discrepancy between impairment and disability [4, 5, 16, 17, 20, 21, 24, 30, 32, 34]. As an exploratory study, we did not conduct a multivariable linear regression analysis to identify factors that may influence a patient’s cognitive error and susceptibility to change. Future studies should measure and control for psychosocial factors, such as mood or anxiety levels, comorbidities, and diagnoses, and conduct multivariable linear regression analyses. We found that QuickDASH scores changed after patients completed functional tasks. The change in disability scores before and after tasks were completed raises concerns regarding construct validity. For example, which of these best measures a patient’s disability, and is this improvement durable or temporary? One method to control for the influence of task completion would be to standardize PROM administration to ensure that a patient’s disability is accurately evaluated. Although this could increase validity, it could also decrease feasibility.
Additionally, the implementation of initiatives linking PROMs with reimbursement may result in a Hawthorne effect because surgeons know not only that they are not only being observed, but also what is being measured, potentially allowing them to modify their behavior (through changing the context in which they measure PROMs) and practice toward the measures [35]. A possible explanation for the change in QuickDASH score after tasks were completed may be the correction of cognitive error by modifying or reversing patients’ maladaptive beliefs about the tasks. This explanation highlights the importance of addressing the patient’s entire health, such as psychosocial aspects of care. For example, prior work has demonstrated that interventions can address cognitive errors (such as cognitive behavioral therapy for chronic low back pain [18, 25]). While it is well known that psychosocial factors and behavioral economic principles such as priming affect outcome measures, these were not addressed by this study but warrant further investigation [4, 5, 8, 16, 17, 20, 21, 24, 30, 32, 34]. We found that the mean decrease in disability was greater in those who used their unaffected extremity for functional tasks than in those using their affected extremity (p = 0.005). Some patients may adapt to their condition by using their contralateral extremity to decrease their disability, while others with maladaptive coping mechanisms may not. We are not aware of studies that evaluate how simple actions, specifically completing functional tasks, affect PROM scores despite the growing use of PROMs to assess quality of care.
We found a difference in the change in QuickDASH score between the intervention and control groups, and a higher proportion of patients in the intervention group improved their scores greater than an MCID of 14. This puts the results into a clinical context and emphasizes the magnitude of the change. It also highlights the inherent discrepancy between disability (a subjective restriction in ability to perform an activity) and impairment (an objective loss of body function or structure). Having patients conduct specific tasks and place their disability in the context of their goals may minimize maladaptive cognitive errors, lead to less recall bias, and help mitigate the presence of an availability bias [33]. The magnitude of improvement because of a given treatment can be used to judge the efficacy of the treatment option and compare the treatment to alternatives. Our nonsurgical intervention improved QuickDASH scores with an effect size of 0.6, similar to effect sizes noted for carpal tunnel release (0.7) [13], arthroscopic acromioplasty (0.9) [13], and TKA (0.47-3.86) [14]. Although we did not evaluate the duration of the effect, the notion that completing functional tasks creates a response as clinically effective as surgery highlights the importance of the context in which PROMs are administered and should prompt further studies into how PROMs are deployed in diverse health care settings (for example, if PROM administration should be standardized). These results could inform health policy that uses PROMs (PRO Performance Measures) in value-based payment models [36]. For example, standardizing PROM administration could mitigate the effects of functional task completion on PROM scores and minimize the heterogeneity of the treatment effect.
Our results indicate that conducting quick functional tasks as queried on the QuickDASH instrument improves outcome scores. Future work to further elucidate the effect of circumstances on PROM scores should randomize patients and control for psychosocial factors to mitigate these potential biases. PROM scores should be assessed within the context in which they are measured, and quality measures, value-based payment models, and health policy should consider this phenomenon.
Acknowledgments
None.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.American Academy of Orthopaedic Surgery. Position Statement: Principles for musculoskeletal based patient reported outcome-performance measurement development. Available at: https://www.aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/position/1188%20Principles%20for%20Musculoskeletal%20Based%20Patient%20Reported%20Outcome-Performance%20Measurement%20Development.pdf. Accessed February 22, 2019.
- 2.Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the short form of the disabilities of the shoulder, arm and hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18:1043-1051. [DOI] [PubMed] [Google Scholar]
- 3.Beaton DE, Wright JG, Katz JN, Upper Extremity Collaborative Group. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87:1038-1046. [DOI] [PubMed] [Google Scholar]
- 4.Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ. Can preoperative patient-reported outcome measures be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res. 2017;475:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britteon P, Cullum N, Sutton M. Association between psychological health and wound complications after surgery. Br J Surg. 2017;104:769-776. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services. Comprehensive care for joint replacement payment model for acute care hospitals furnishing lower extremity joint replacement services. Available at: https://www.federalregister.gov/documents/2015/11/24/2015-29438/medicare-program-comprehensive-care-for-joint-replacement-payment-model-for-acute-care-hospitals. Accessed April 5, 2018. [PubMed]
- 8.Claessen MD, Jos JJ, Stoop N, Lubberts B, Ring D, Poolman RW. Influence on priming on patient-reported outcome measures: a randomized controlled trial. Psychosomatics. 2016;57:47-56. [DOI] [PubMed] [Google Scholar]
- 9.Davis AM, Perruccio AV, Canizares M, Hawker GA, Roos EM, Maillefert JF, Lohmander LS. Comparative, validity and responsiveness of the HOOS-PS and KOOS-PS to the WOMAC physical function subscale in total joint replacement for osteoarthritis. Osteoarthritis Cartilage. 2009;17:843-847. [DOI] [PubMed] [Google Scholar]
- 10.DeWalt DA, Rothrock N, Yount S, Stone AA, PROMIS Cooperative Group. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45:S12-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchigno F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero F. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther. 2014;44:30-39. [DOI] [PubMed] [Google Scholar]
- 12.Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11:304-314. [PMC free article] [PubMed] [Google Scholar]
- 13.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health Quality Ontario. Total knee replacement: an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5:1- 51. [PMC free article] [PubMed] [Google Scholar]
- 15.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29:602-608. [DOI] [PubMed] [Google Scholar]
- 16.London DA, Stepan JG, Boyer MI, Calfee RP. The impact of depression and pain catastrophization on initial presentation and treatment outcomes for atraumatic hand conditions. J Bone Joint Surg Am. 2014;96:806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDermid JC, Donner A, Richards RS, Roth JH. Patient versus injury factors as predictors of pain and disability six months after a distal radius fracture. J Clin Epidemiol. 2002;55:849-854. [DOI] [PubMed] [Google Scholar]
- 18.McBeth J, Prescott G, Scotland G, Lovell K, Keeley P, Hannaford P, McNamee P, Symmons DP, Woby S, Gkazinou C, Beasley M, Mcfarlane GJ. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Arch Int Med. 2012;172:48-57. [DOI] [PubMed] [Google Scholar]
- 19.McConnell S, Beaton DE, Bombardier C. The DASH outcome measure user’s manual. Toronto, Ontario: Institute for Work and Health; 1999. [Google Scholar]
- 20.Merrill RK, Zebala LP, Peters C, Qureshi SA, McAnany SJ. Impact of depression on patient-reported outcome measures after lumbar spine decompression. Spine (Phila Pa 1976). 2018;43:434-439. [DOI] [PubMed] [Google Scholar]
- 21.Miller JA, Derakhshan A, Lubelski D, Alvin MD, McGirt MJ, Benzel EC, Mroz TE. The impact of preoperative depression on quality of life outcomes after lumbar surgery. Spine J. 2015;15:58-64. [DOI] [PubMed] [Google Scholar]
- 22.Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18:920-926. [DOI] [PubMed] [Google Scholar]
- 23.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh Y, Drijkonigen T, Menendez ME, C FMAP, Ring D. The influence of psychological factors on the Michigan Hand Questionnaire. Hand (N Y). 2017;12:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostelo RW, van Tulder MW, Vlaeyen JW, Linton SJ, Morley SJ, Assendelft WJ. Behavioural treatment for chronic low back pain. Cochrane Database Syst Rev . 2005:CD002014. [DOI] [PubMed] [Google Scholar]
- 26.Redelmeier DA, Katz J, Kahneman D. Memories of colonscopy: a randomized trial. Pain. 2003;104:187-194. [DOI] [PubMed] [Google Scholar]
- 27.Reeve BB., Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D, PROMIS Cooperative Group. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007;45:S22-S31. [DOI] [PubMed] [Google Scholar]
- 28.Sanders C, Egger M, Donovan J, Tallon D, Frankel S. Reporting on quality of life in randomised controlled trials: bibliographic study. BMJ. 1998;317:1191-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmier JK, Halpern MT. Patient recall and recall bias of health state and health status. Expert Rev Pharmacoecon Outcomes Res. 2004;4:159-163. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz FH, Lange J. Factors that affect outcome following total joint arthroplasty: a review of the recent literature. Curr Rev Musculoskelet Med. 2017;10:346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-rated outcomes instruments. J Hand Surg Am. 2013;38:641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theologis AA, Ailon T, Scheer JK, Smith JS, Shaffrey CI, Bess S, Gupta M, Klineberg EO, Kebaish K, Schwab F, Lafage V, Burton D, Hart R, Ames CP, International Spine Study Group. Impact of preoperative depression on 2-year clinical outcomes following adult spinal deformity surgery: the importance of risk stratification based on type of psychological distress. J Neurosurg Spine. 2016;25:477-485. [DOI] [PubMed] [Google Scholar]
- 33.Tversky A, Kahneman D. Availability: a heuristic for judging frequency and probability. Cognitive Psychology. 1974;5:207-232. [Google Scholar]
- 34.Vranceanu AM, Jupiter JB, Mudgal CS, Ring D. Predictors of pain intensity and disability after minor hand surgery. J Hand Surg Am. 2010;35:956-960. [DOI] [PubMed] [Google Scholar]
- 35.Winegar AL, Moxham J, Erlinger TP, Bozic KJ. Value-based healthcare: measuring what matters-engaging surgeons to make measures meaningful and improve clinical practice. Clin Orthop Relat Res. 2018;476:1704-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther. 2012;20:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]