Abstract
Background
Implant loosening is a common cause of reoperation after THA. Plain radiographs have been the default modality to evaluate loosening, although radiographs provide a relatively insensitive assessment of integration; cross-sectional modalities may provide a more detailed evaluation but traditionally have suffered from metal-related artifacts. We sought to determine whether MRI is capable of reliably detecting operatively confirmed component loosening in patients after hip arthroplasty.
Questions/purposes
(1) Is assessing implant integration using MRI (with multiacquisition variable resonance image combination, [MAVRIC]) repeatable between readers? (2) What is the sensitivity and specificity of MRI with MAVRIC to evaluate component loosening, using intraoperative assessment as a gold standard? (3) How does the sensitivity and specificity of MRI with MAVRIC for surgically confirmed component loosening compare with those of radiographs?
Methods
Between 2012 and 2017, 2582 THAs underwent revision at one institution. Of those, 219 had a preoperative MRI with MAVRIC. During that period, the most common indication for obtaining an MRI was evaluation of potential adverse local tissue reaction. The surgeons’ decision to proceed with revision was based on their overall assessment of clinical, imaging, and laboratory findings, with MRI findings cited as contributing to the decision to revise commonly occurring in the setting of recalled implants. Of the THAs that underwent MRI, 212 were included in this study, while seven were excluded due to equivocal operative notes (5) and excessively poor quality MRI (2). MRI was performed at 1.5T using a standardized arthroplasty imaging protocol, including MARS (metal artifact reduction sequencing) and MAVRIC techniques. Two independent musculoskeletal fellowship-trained readers (one with 26 and one with 5 years of experience) blinded to operative findings scored a subset of 57 hips for implant integration based on Gruen zone and component loosening (defined as complete circumferential loss of integration around a component) to evaluate interobserver reliability. A third investigator blinded to imaging findings reviewed operative notes for details on the surgeon’s assessment of intraoperative loosening.
Results
Gwet’s agreement coefficients (AC) were used to describe interobserver agreement; these are similar to Cohen’s kappa but are more resistant to certain paradoxes, such as unexpectedly low values in the setting of very high or low trait prevalence, or good agreement between readers on marginal counts. Almost perfect interobserver agreement (AC2 = 0.81–1.0) was demonstrated for all acetabular zones and all femoral Gruen zones on MRI, while perfect (AC1 = 1.0) agreement was demonstrated for the overall assessment of acetabular component loosening and near perfect agreement was shown for the assessment of femoral component loosening (AC1 = 0.98). MRI demonstrated a sensitivity and specificity of 83% (95% CI, 65–96) and 98% (95% CI, 97–100), respectively, for acetabular component loosening and 75% (95% CI, 55–94) and 100% (95% CI, 100–100), respectively, for femoral component loosening. Radiographs demonstrated a sensitivity and specificity of 26% (95% CI, 12–47) and 100% (95% CI, 96–100), respectively, for acetabular component loosening and 20% (95% CI, 9–47) and 100% (95% CI, 100–100), respectively, for femoral component loosening.
Conclusion
MRI may provide a repeatable assessment of implant integration and demonstrated greater sensitivity than radiographs for surgically confirmed implant loosening in patients undergoing revision THA at a single institution. Additional multi-institutional studies may provide more insight into the generalizability of these findings.
Level of Evidence
Level III, diagnostic study.
Introduction
MRI has proven to be an effective, noninvasive method of diagnosing pathologic conditions in patients with a variety of metallic implants; however, evaluating the immediate periprosthetic region, including the bone-implant interface, has been challenging [26, 29]. Improving our approaches in this area is important given the frequency with which pathology affects the tissues immediately surrounding the implant. Component loosening, which may occur as a purely mechanical process as well as in the setting of bulky circumferential osteolysis, is a common cause of THA revision surgery, accounting for most revision THAs in several studies [4, 12, 24, 25, 27].
While serial radiography has traditionally been the default method to assess component loosening [27], radiographs provide a relatively insensitive assessment of implant integration, in which a soft tissue fibrous interface can form between the metallic implant and trabecular bone [7, 27, 28]. Cross-sectional modalities such as CT and MRI provide better tomographic visualization of the implant interfaces but have suffered in the past from limitations arising from artifacts related to the metallic components [5, 8]. Application of metal artifact reduction sequencing to conventional MRI pulse sequences, as well as the use of three-dimensional multispectral imaging (3-DMSI) techniques such as multiacquisition variable resonance image combination selective (MAVRIC-SL) have been shown to reduce metal artifacts and improve visualization of the immediate periprosthetic region in patients with metallic implants, therefore facilitating assessment of implant integration and component loosening [1, 2, 13, 14, 19, 20].
Although these techniques have allowed improved assessment of the bone-component interface, the diagnostic accuracy of MRI for the assessment of loosening remains unclear. Therefore, we asked (1) Is assessing implant integration using MRI with MAVRIC repeatable between readers? (2) What is the sensitivity and specificity of MRI with MAVRIC to evaluate component loosening, using intraoperative assessment as a gold standard? (3) How does the sensitivity and specificity of MRI with MAVRIC for surgically confirmed component loosening compare with those of radiographs?
Patients and Methods
Study Design and Setting
Following institutional review board approval, we retrospectively identified patients who underwent preoperative imaging and subsequent revision THA between January 2012 and January 2017 at a tertiary care orthopaedic hospital in New York City. Ongoing data collection and scoring was performed from March 2016 through February 2018, with a final statistical analysis being performed at the end of the data-collection period.
Participants
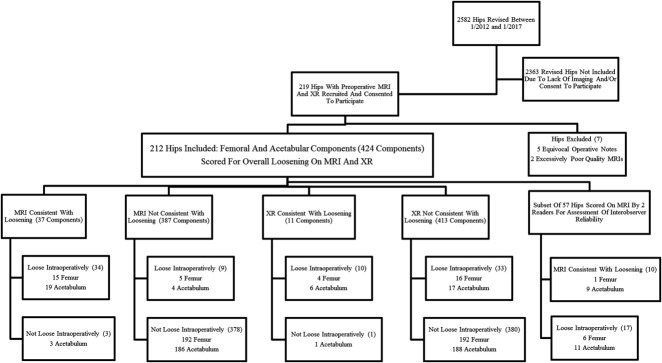
Patients who had undergone revision THA after preoperative imaging evaluation with radiographs and MRI with MAVRIC-SL were retrospectively identified using a longitudinal implant database. The need to obtain informed consent was waived for this retrospective Health Insurance Portability and Accounting Act-compliant study. A total of 219 hips that were operated on between January 2012 and January 2017 met the criteria for study inclusion. We considered all implant types and bearing constructs as eligible, regardless of implant composition or design. Of the hips meeting the inclusion criteria, seven were excluded because of equivocal operative notes (5) or excessively poor image quality (2). A total of 212 hip arthroplasties were included; 424 components (212 femoral and 212 acetabular) were therefore evaluated (Fig. 1).
Fig. 1.
The overall study design is depicted.
Demographics, Description of Study Population
A total of 212 hip arthroplasties from 206 patients (mean age, 65 ± 12 years; range, 24-91 years; 55% female) were included in the study. The median time between implantation and revision surgery was 6 years (interquartile range [IQR], 3-11 years), and the median time between MRI and revision was 60 days (IQR, 24-120 days) (Table 1). Bearing constructs included meta-on-metal (n = 56, 26%), metal-on-polyethylene (n = 107, 51%), ceramic-on-metal (n = 2, 1%), ceramic-on-ceramic (n = 7, 3%), and ceramic-on-polyethylene (n = 40, 19%) articulations. Twenty-six (12%) implants incorporated cemented femoral stems. Both conventional THA and resurfacing THA were included (Table 1).
Imaging Methods
Preoperative radiographic evaluation consisted of a standard AP view of the pelvis and frog and/or crosstable lateral views of the affected hip. MRI was performed on a 1.5T clinical scanner (General Electric Healthcare, Waukesha, WI, USA), using a cardiac or small body coil and the institution’s routine clinical THA imaging protocol (Table 2), including coronal MAVRIC inversion recovery, and MAVRIC proton density (PD)-weighted images, in addition to high-resolution axial, sagittal, and coronal PD-weighted fast spin echo (FSE) images. Metal artifact reduction sequencing (MARS) parameter modifications were applied to the conventional PD FSE images to reduce the effects of metal susceptibility artifacts. This imaging algorithm provides good spatial resolution as afforded by the FSE images and excellent suppression of metal susceptibility artifacts because of inclusion of the MAVRIC-SL pulse sequences.
Indications for MR Imaging and Revision Arthroplasty
Between January 2012 and January 2017, 2582 THAs underwent revision at one institution; of those, 219 (8%) had a preoperative MRI including MAVRIC. MR imaging evaluation after THA is commonly performed at our institution in patients presenting with pain and negative/equivocal radiographs, as well as in patients with suspected soft tissue pathology. During the study period, the most common indication for obtaining an MRI was suspected adverse local tissue reaction (ALTR) in patients with metal-on-metal (MOM) bearings or modular dual taper stems, followed by unexplained pain and suspected loosening, while the most common indication for revision was ALTR, followed by loosening, and polymeric wear/osteolysis. The most frequent histopathologic finding in patients with loosening was a chronic tissue reaction without identifiable implant material (17 of 42 [40%]), followed by ALTR (13 of 42 [31%]), and polymeric wear/osteolysis (10 of 42 [24%]) (Table 3). The decision to revise was commonly based on a combination of clinical, imaging, and laboratory findings. Although documentation of the role of MRI in the decision to revise was inconsistent and therefore not quantified, operative notes often referenced MRI findings; this was especially common in patients with suspected ALTR. In comparing the indications for MRI versus the indications for revision in these 212 patients, unexplained pain was a common indication for MRI (44 [21%]), although it was a less common indication for revision (16 [8%]), suggesting that MRI may have helped pinpoint a diagnosis in several patients, contributing to the decision to proceed with revision (Table 3). We acknowledge this as a source of selection bias in this study.
Variables, Outcome Measures, Data Sources, and Bias
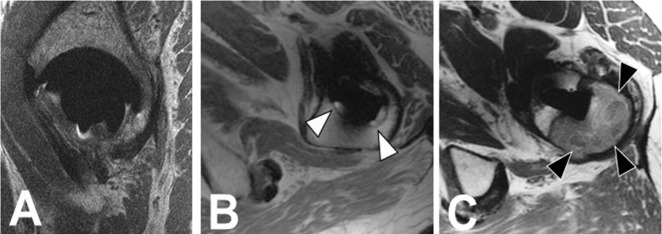
To evaluate the interrater reliability, two fellowship-trained musculoskeletal radiologists specializing in MRI with daily clinical experience in interpretation of arthroplasty MRI (HGP, with 26 years of experience, and AJB, with 5 years of experience) who were blinded to operative findings independently scored a subset of 57 hips. MR images were evaluated with respect to osseous implant integration by anatomic region, using the standard femoral Gruen zones. The periacetabular region was divided into anterior, middle, posterior, and superior segments. The type of bone loss was graded on a scale of 0-2, with 0 reflecting no loss of integration, 1 reflecting fibrous membrane formation, and 2 reflecting osteolysis. Fibrous membrane formation was defined as a thin rim of linear iso- to hyperintense osseous resorption with sclerotic margins along the implant-bone interface, the MR imaging analog of the “radiolucent line” typically described on radiographs in the setting of mechanical loosening. Osteolysis was defined as bulky, more lobular osseous resorption, typically isointense, as commonly seen in the setting of polymeric wear (Fig. 2). The interpreting radiologists (HGP, AJB) use these definitions regularly in their standard clinical interpretations, and therefore no pretraining was undertaken to establish a consensus. Additionally, the acetabular and femoral components were deemed integrated (0) or loose (1) based on the extent of osseous resorption, with the imaging definition of loose being complete, circumferential loss of osseous incorporation surrounding a given component. All three imaging planes were assessed. After confirming interrater reliability, a single reader (AJB) evaluated overall acetabular and femoral loosening for the remainder of the 212 hips on MRI, as well as on radiographs for the entire cohort of 212 hips; there was a delay of 6 months between the initial MRI scoring and scoring of the radiographs, which were evaluated in a blinded fashion with respect to MRI findings, so as not to bias the reader. Scoring of radiographic loosening was based on the same imaging criteria as for MR loosening (complete, circumferential loss of incorporation along either component).
Fig. 2 A-C.
Using MR imaging, we graded osseous integration as (A, sagittal PD FSE) 0, no osseous resorption; (B, axial PD FSE, white arrowheads); 1, fibrous membrane formation; and (C, axial PD FSE, black arrowheads) 2, osteolysis.
Assessment of Component Loosening at Revision Surgery
Intraoperative assessment of component loosening was determined via a retrospective evaluation of operative notes by an independent investigator (JB) who was blinded to the MRI findings. Operative notes generally included a preoperative working diagnosis, a postoperative diagnosis, and a narrative account of the procedure. Each of these components was evaluated to attempt to gain an accurate assessment of whether the surgeon had detected component loosening at the time of revision surgery. As previously mentioned, patients with equivocal operative notes were excluded from the study (Fig. 1). Information recorded included the presence or absence of loosening of either component, postoperative determination of the reason for implant failure, implant type, and whether the femoral component was cemented. Because failure of component incorporation and mechanical loosening of a previously well-incorporated component were indistinguishable by both imaging and operative criteria, the term loosening is used to encompass both processes in this study, although the authors acknowledge the distinction between the two.
Statistical Analysis and Study Size
Gwet’s agreement coefficients (AC1 for binary and AC2 for ordinal grades) with corresponding 95% confidence intervals (CIs) were calculated as a measure of interrater reliability of our MRI assessments in a subset of 57 hips [10]. Raw agreement was also calculated to account for situations in which Gwet’s AC was incalculable (such as when only one grade was observed between both raters). Similar to the commonly used Cohen’s kappa, Gwet’s ACs are measures of agreement that are adjusted for the degree of agreement that would be expected solely by chance. However, Gwet’s ACs are more resistant to certain paradoxes (such as unexpectedly low values when there is very high or very low trait prevalence or there is good agreement between raters on marginal counts) than kappa coefficients. The strength of agreement was interpreted as follows: < 0.00 = poor, 0.00–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, and 0.81–1.00 = almost perfect [3, 22].We calculated the diagnostic accuracy of x-ray and MRI readings using operative notes as the reference standard. Sensitivities, specificities, positive predictive values, and negative predictive values are reported as point estimates with 95% percentile cluster bootstrap CIs calculated from 1000 resamples. To evaluate the difference in sensitivity and specificity between MRI and x-ray, we specified a marginal logistic regression model using generalized estimating equations that adjusted for patients with bilateral hips. Significance was set at p < 0.05. All statistical analyses were performed with SAS Version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Interrater Reliability for MRI Assessment of Loosening
Interrater reliability demonstrated near-perfect agreement for MRI assessment of loosening in every region surrounding the acetabular component: Anterior AC2 = 0.87 (95% CI, 0.76–0.99), Middle AC2 = 0.82 (95% CI, 0.69–0.95), Posterior AC2 = 0.90 (95% CI, 0.80–1.0), and Superior AC2 = 0.88 (95% CI, 0.76–1.0) (see Appendix, Supplemental Digital Content 1, http://links.lww.com/CORR/A185). The overall reader agreement for acetabular component loosening on MRI was perfect (AC1 = 1.0). Along the femoral component, perfect or near-perfect agreement was found in all Gruen zones. Perfect agreement was found in Gruen zones 3, 4, 11, and 12 (AC2 = 1.0 and/or 1.0, raw agreement). Overall reader agreement for femoral component loosening was near perfect, with AC1 = 0.98 (95% CI, 0.95–1.0) (see Appendix, Supplemental Digital Content 1, http://links.lww.com/CORR/A185).
Sensitivity and Specificity of MRI for Component Loosening
Intraoperatively, 43 loose components were detected, 20 femoral and 23 acetabular; while the remainder (381) were fixed (Table 4). MRI assessment of component loosening demonstrated a sensitivity of 83% (95% CI, 62–93) and a specificity of 98% (95% CI, 95–100), for acetabular component loosening using intraoperative assessment as the gold standard. Sensitivity 75% (95% CI, 55–94) and specificity was100% (95% CI, 100–100) for femoral component loosening. For acetabular component loosening, the positive predictive value was 86% (95% CI, 65–96) and the negative predictive value was 98% (95% CI, 95–99). The positive and negative predictive values were 100% (95% CI, 100–100) and 98% (95% CI, 95–100), respectively, for femoral component loosening (Table 5).
Comparing Sensitivity and Specificity of MRI to that of Radiographs
Radiographic evaluation of loosening using intraoperative assessment as a gold standard demonstrated a sensitivity and specificity of 26% (95% CI, 12–47) and 100% (95% CI, 96–100), respectively, for acetabular component loosening and 20% (95% CI, 9–47) and 100% (95% CI, 100–100), respectively, for femoral component loosening at the time of revision surgery in patients with failed THA. For acetabular component loosening, the positive predictive value was 86% (95% CI, 42–98) and the negative predictive value was 92% (95% CI, 87–95). The positive and negative predictive values were 100% (95% CI, 100–100) and 92% (95% CI, 89–96), respectively, for femoral component loosening (Table 5).
A formal statistical comparison of differences in the sensitivity and specificity between radiographs and MRI demonstrated MRI to be 57% (95% CI, 37–77; p < 0.001) more sensitive than radiographs for detecting acetabular component loosening, but there was no difference between radiographs and MRI in terms of specificity (Table 5). Because of the lack of false positives in patients with femoral components, the issue of quasicomplete separation prevented the logistic model from converging; therefore, a formal statistical comparison between radiographs and MRI for femoral component loosening could not be performed.
Discussion
MRI is effective in diagnosing a variety of failure modes in patients undergoing THA, although assessment of the bone-implant interface has historically proven challenging [6, 11, 17], limiting the evaluation of implant integration and component loosening, which are frequent reasons for arthroplasty revision. In the current cohort, MRI incorporating metal reduction techniques demonstrated high repeatability between observers, as well as overall good sensitivity and specificity for detecting operative loosening.
Limitations
This study had a number of limitations. Our cohort was comprised of 212 hips that underwent both preoperative MRI and revision arthroplasty. During the 5-year study period, a total of 2582 revision THAs were performed, while more than 6000 MRIs were performed in patients with THAs. The study cohort therefore suffers from an element of selection bias, in that not all patients undergoing revision had a preoperative MRI and not all patients who had an MRI underwent revision. MRI is commonly ordered as a problem-solving modality in patients with unexplained pain and negative radiographs at our institution, while revision surgery is obviously typically indicated for more serious pathology. In many instances within this study population, the MRI likely confirmed/detected pathology that contributed to the decision to proceed with revision, contributing to selection bias. Additionally, during the study period, ALTR in patients with MOM and modular dual-taper stems was a more frequent indication for both MRI and revision than currently. This may affect the composition of the cohort and therefore affect generalizability; the incidence of revision due to loosening within our cohort was overall lower than that which has often been reported in the evidence, which is often greater than 50%. Studies have suggested that during the timeframe of our investigation, revisions due to ALTR increased greatly in number, and the lower incidence of operatively confirmed loosening within the total cohort (43 of 212 [20%]) versus the initial subset of 57 patients (17 [30%]) seems to support this, suggesting that this shift in revision indications may have been relatively widespread [23, 24, 27].
Additionally, lack of standardized language in the operative reports made the interpretation of our results difficult in certain hips, although overtly equivocal operative reports were excluded from the study. A lack of well-defined criteria for defining intraoperative component loosening may have resulted in a degree of disagreement between operative and imaging assessment of loosening. For example, one operative report of a femoral component deemed loose intraoperatively stated that a small amount of fibrous tissue was removed and the femoral component could be extracted manually, while the report of a second femoral component deemed loose intraoperatively described the use of a burr to remove bone ingrowth around the proximal stem, followed by the use of an osteotome to remove additional bone around the stem. Although the final postoperative diagnosis in both hips was recorded as femoral component loosening, the description of the extraction of the second implant appears to suggest that at least some osseous integration was present at the time of surgery, which would result in a discrepancy with the imaging definition of loosening, which requires circumferential loss of osseous integration. Going forward, a more objective system of assessing component loosening intraoperatively would be beneficial to define and maintain a consistent gold standard.
Third, the range of time between MRI and revision surgery was 3 to 481 days (median, 60 days). The lag between imaging and revision when there was a longer delay could potentially have allowed for the evolution of pathology, possibly leading to discrepancies between imaging and operative findings. Subsequent evaluation of time from imaging to revision in concordant versus discordant cases demonstrated an overall shorter delay in the discordant cases (median, 22 days) versus the concordant cases (median, 63 days), suggesting that the magnitude of delay in general did play a role in diagnostic accuracy in the current cohort (see Appendix, Supplemental Digital Content 2, http://links.lww.com/CORR/A186).
Finally MRI was performed at a single institution specializing in orthopaedics that performs a high volume of MRI in patients after THA, using well-established advanced arthroplasty imaging protocols. Clinically, the two radiologists (HGP, AJB) read almost exclusively MRI, and interpret a large portion of the institution’s arthroplasty MRI. This may limit the generalizability of these results when a given institution’s MRI protocol deviates substantially from that used in this study, as well as when the reader is less experienced in interpreting MRI in the presence of metal. However, even without advanced sequences such as MAVRIC, thoughtful sequencing with parameter modifications can yield improvements in image quality, and ultimately, the critical factors in the accurate interpretation of any imaging study are familiarity with the appearance of normal versus pathologic imaging findings, practice in interpretation, and feedback regarding interpretation.
Interrater Reliability for MRI Assessment of Loosening
MRI around metal implants is often challenging, particularly along the implant-bone interface; however, applying metal artifact reduction sequencing and 3DMSI techniques improves the ability to directly visualize this region [2, 9, 13, 18]. Using an imaging algorithm incorporating metal artifact reduction sequencing and MAVRIC techniques, we found that the interobserver reliability was near perfect to perfect for zonal assessment of osseous integration and for overall assessment of component loosening within this cohort. To the authors knowledge, few similar studies exist. A study of 48 hips revised between 2010 and 2013 reported a sensitivity and specificity of 93% and 95% for MRI assessment of aseptic loosening; however, MRI diagnosis was established by consensus read, and interobserver reliability was not presented [15]. Further investigation is necessary to determine whether our results can be replicated outside of our institution; multi-institutional collaborations would likely be beneficial in this regard.
Sensitivity and Specificity of MRI for Component Loosening
MRI showed overall good diagnostic accuracy for prediction of operative component loosening (Fig. 3). As mentioned above, few similar studies exist; the previously cited study reported a similarly high sensitivity and specificity, though interobserver reliability was not calculated [15]. Within our cohort, a number of discordant cases were identified and reviewed individually to gain insight into the possible cause of each discrepancy. Cases in which MRI was not concordant with operative findings occurred most frequently in the setting of cemented femoral components (Fig. 4), bulky osteolysis, and greater-than-typical susceptibility artifacts. Additionally, the lack of a standardized definition of operative component loosening may have resulted in discordance with the imaging definition of loosening, which required complete loss of integration for a component to be deemed loose (Fig. 5). Discordance also tended to occur in patients on whom relatively older scanning techniques had been used. This possibly reflected an overall improvement in image quality over time that was related to the evolution of metal suppression techniques, particularly 3DMSI sequences, which now permit isotropic acquisition with high through-plane (1-2 mm) resolution [16]. No discrepancies were observed in patients with scans performed later than 2015. As above, further investigation would be useful in determining whether our results are generalizable to other imaging centers, particularly those in which MAVRIC or similar advanced metal reduction sequencing not available.
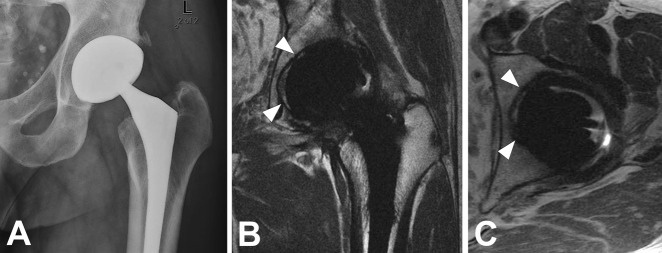
Fig. 3 A-B.
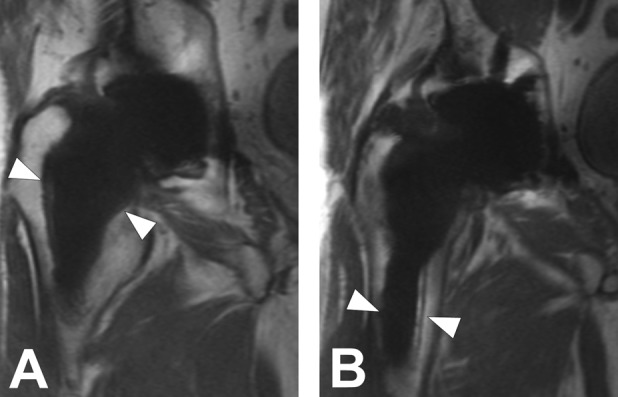
(A, B) Coronal MAVRIC-SL PD images in a 74-year-old man with painful THA demonstrate fibrous membrane formation surrounding the femoral component, the extent of which was compatible with component loosening, which was confirmed operatively.
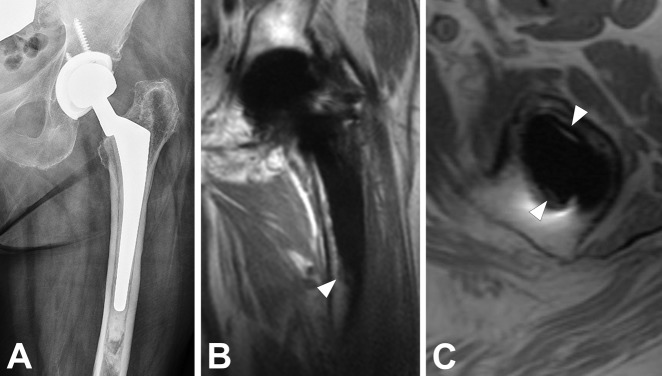
Fig. 4 A-C.
(A) Radiograph of an 84-year-old woman with painful THA suggests areas of lucency surrounding the cemented femoral stem. (B) Coronal MAVRIC-SL PD and (C) axial PD FSE images demonstrate focal areas of apparent resorption; however, areas of residual integration were suspected, and the component was therefore not deemed loose on imaging. On revision, however, the stem was deemed loose and was easily removed from the cement mantle.
Fig. 5 A-B.
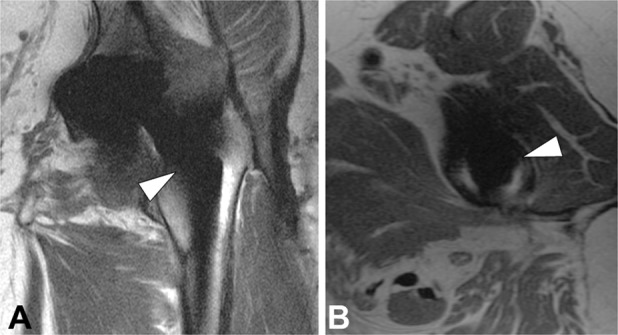
(A) Coronal and (B) axial PD FSE images in a 63-year-old woman with painful THA demonstrate areas of minimal incomplete fibrous membrane formation surrounding the femoral stem. Because osseous resorption appeared incomplete, the component was not deemed frankly loose on MRI, although the component was deemed loose intraoperatively. Further scrutiny of the patient’s operative report revealed the use of a surgical drill and osteotomes to remove osseous ingrowth surrounding the stem before component removal, emphasizing the somewhat subjective nature of the intraoperative definition of component loosening.
Comparing Sensitivity and Specificity of MRI to That of Radiographs
MRI provided a more-sensitive evaluation of implant integration than conventional radiographs in our cohort (Fig. 6), without the use of ionizing radiation. Although the relatively high cost of MRI prohibits global use as a first-line imaging test, MRI may disclose the cause of unexplained pain, including the status of implant integration, in patients with normal radiographs. MRI has also been shown to be capable of showing other types of implant-related pathologic conditions, such as synovial processes including polymeric wear and adverse local tissue reaction, as well as other types of periarticular soft tissue pathology, such as iliopsoas impingement and abductor dehiscence [1, 21].
Fig. 6 A-C.
(A) Radiographs of a 52-year-old man who underwent THA demonstrate minimal evidence of lucency at the superolateral dome, and the results were interpreted as negative for loosening. (B) Coronal and (C) axial PD FSE images demonstrate fibrous membrane formation surrounding the acetabular component, the extent of which was consistent with component loosening, which was confirmed at the time of revision surgery.
Potential for Future Research
With this study, we hope to demonstrate that MRI can potentially be used to assess component loosening after THA. The authors acknowledge the various limitations of our study, and hope that these may be addressed in future studies. Establishing generalizability of our findings to imaging centers and readers with less experience in metal imaging would likely best be achieved via multi-institutional collaborations. Additionally, with currently ongoing longitudinal prospective studies of MRI for detection of failure modes in patients after THA, we hope to lessen the degree of selection bias going forward.
Conclusions
MRI may be a noninvasive and accurate means of assessing implant integration in most patients who undergo THA, with an overall high sensitivity and specificity for predicting intraoperative loosening at the time of revision surgery within the setting of an experienced musculoskeletal radiology practice using established advanced metal imaging protocols. Future investigation will be necessary to establish the generalizability of these findings. Additionally, certain scenarios appeared to result in decreased sensitivity for determining component loosening; cemented femoral stems proved particularly difficult to assess because of limited contrast at the metal-cement interface. Hips with greater-than-typical susceptibility artifacts, as well as bulky osteolysis, also presented some challenges in assessing implant integration. Remaining aware of these pitfalls may aid the interpreting radiologist in evaluating such scans, in which a degree of extra vigilance may be warranted. These initial results demonstrated the efficacy of MRI for evaluating component loosening in patients who undergo THA, and ongoing prospective studies will provide a more uniformly standardized assessment of implant integration, using both operative assessment and noninvasive MRI in patients undergoing THA.
Acknowledgments
We thank Mauro Miranda BA, for assistance in recruiting patients and obtaining operative records.
Footnotes
One or more of the authors (HGP, MFK, AJB) has received funding from NIH/NIAMS Grant RO1AR064840 (HGP, MFK). The institution of the authors receives research support from General Electric Healthcare.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Berkowitz JL, Potter HG. Advanced MRI techniques for the hip joint: Focus on the postoperative hip. AJR Am J Roentgenol. 2017;209:534-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SJ, Koch KM, Hargreaves BA, Stevens KJ, Gold GE. Metal artifact reduction with mavric sl at 3-T MRI in patients with hip arthroplasty. AJR Am J Roentgenol. 2015;204:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cicchetti DV, Feinstein A.R. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol 1990;43:551-558. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Pardos A, Garcia-Rey E, Garcia-Cimbrelo E. Total hip arthroplasty with use of the cementless zweymuller alloclassic system: A concise follow-up, at a minimum of 25 years, of a previous report. J Bone Joint Surg Am. 2017;99:1927-1931. [DOI] [PubMed] [Google Scholar]
- 5.Filli L, Luechinger R, Frauenfelder T, Beck S, Guggenberger R, Farshad-Amacker N, Andreisek G. Metal-induced artifacts in computed tomography and magnetic resonance imaging: Comparison of a biodegradable magnesium alloy versus titanium and stainless steel controls. Skeletal Radiol. 2015;44:849-856. [DOI] [PubMed] [Google Scholar]
- 6.Fritz J, Lurie B, Miller TT, Potter HG. MR imaging of hip arthroplasty implants. Radiographics. 2014;34:E106-132. [DOI] [PubMed] [Google Scholar]
- 7.Goldring SR, Schiller AL, Roelke M, Rourke CM, O'Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am. 1983;65:575-584. [PubMed] [Google Scholar]
- 8.Gupta A, Subhas N, Primak AN, Nittka M, Liu K. Metal artifact reduction: Standard and advanced magnetic resonance and computed tomography techniques. Radiol Clin North Am. 2015;53:531-547. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez LB, Do BH, Gold GE, Hargreaves BA, Koch KM, Worters PW, Stevens KJ. MR imaging near metallic implants using MAVRIC SL: Initial clinical experience at 3T. Acad Radiol. 2015;22:370-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwet KL. Handbook of inter-rater reliability. The definitive guide to measuring the extent of agreement among raters. 2 ed. Gaithersburg, MD: Advanced Analytics, LLC; 2010. [Google Scholar]
- 11.Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE. Metal-induced artifacts in MRI. AJR Am J Roentgenol. 2011;197:547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311. [PubMed] [Google Scholar]
- 13.Hayter CL, Koff MF, Potter HG. Magnetic resonance imaging of the postoperative hip. J Magn Reson Imaging. 2012;35:1013-1025. [DOI] [PubMed] [Google Scholar]
- 14.Hayter CL, Koff MF, Shah P, Koch KM, Miller TT, Potter HG. MRI after arthroplasty: Comparison of MAVRIC and conventional fast spin-echo techniques. AJR Am J Roentgenol. 2011;197:W405-411. [DOI] [PubMed] [Google Scholar]
- 15.He C, Lu Y, Jiang M, Feng J, Wang Y, Liu Z. Clinical value of optimized magnetic resonance imaging for evaluation of patients with painful hip arthroplasty. Chin Med J (Engl). 2014;127:3876-3880. [PubMed] [Google Scholar]
- 16.Kaushik SS, Marszalkowski C, Koch KM. External calibration of the spectral coverage for three-dimensional multispectral MRI. Magn Reson Med. 2016;76:1494-1503. [DOI] [PubMed] [Google Scholar]
- 17.Koch KM, Hargreaves BA, Pauly KB, Chen W, Gold GE, King KF. Magnetic resonance imaging near metal implants. J Magn Reson Imaging. 2010;32:773-787. [DOI] [PubMed] [Google Scholar]
- 18.Koch KM, Koff MF, Shah PH, Kanwischer A, Gui D, Potter HG. Flexible longitudinal magnetization contrast in spectrally overlapped 3d-MSI metal artifact reduction sequences: Technical considerations and clinical impact. Magn Reson Med. 2014. [DOI] [PubMed] [Google Scholar]
- 19.Koff MF, Burge AJ, Koch KM, Potter HG. Imaging near orthopedic hardware. J Magn Reson Imaging. 2017;46:24-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koff MF, Shah P, Koch KM, Potter HG. Quantifying image distortion of orthopedic materials in magnetic resonance imaging. J Magn Reson Imaging. 2013;38:610-618. [DOI] [PubMed] [Google Scholar]
- 21.Lachiewicz PF, Kauk JR. Anterior iliopsoas impingement and tendinitis after total hip arthroplasty. J Am Acad Orthop Surg. 2009;17:337-344. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch G.G. The measurement of observer agreement for categorical data. . Biometrics. 1977;33:159-174. [PubMed] [Google Scholar]
- 23.Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty -- a changing paradigm. J Arthroplasty. 2014;29:1285-1288. [DOI] [PubMed] [Google Scholar]
- 24.Mulcahy H, Chew FS. Current concepts of hip arthroplasty for radiologists: Part 2, revisions and complications. AJR Am J Roentgenol. 2012;199:570-580. [DOI] [PubMed] [Google Scholar]
- 25.Ollivere B, Wimhurst JA, Clark IM, Donell ST. Current concepts in osteolysis. J Bone Joint Surg Br. 2012;94:10-15. [DOI] [PubMed] [Google Scholar]
- 26.Potter HG, Foo LF. Magnetic resonance imaging of joint arthroplasty. Orthop Clin North Am. 2006;37:361-373, vi-vii. [DOI] [PubMed] [Google Scholar]
- 27.Ulrich SD, Seyler TM, Bennett D, Delanois RE, Saleh KJ, Thongtrangan I, Kuskowski M, Cheng EY, Sharkey PF, Parvizi J, Stiehl JB, Mont MA. Total hip arthroplasties: What are the reasons for revision? Int Orthop. 2008;32:597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urban RM, Jacobs JJ, Gilbert JL, Galante JO. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am. 1994;76:1345-1359. [DOI] [PubMed] [Google Scholar]
- 29.Zou YF, Chu B, Wang CB, Hu ZY. Evaluation of MR issues for the latest standard brands of orthopedic metal implants: Plates and screws. Eur J Radiol. 2015;84:450-457. [DOI] [PubMed] [Google Scholar]