Abstract
Purpose:
To determine if extended PET acquisition times in the pelvis during PET/MRI increase detection rates of potentially metastatic lymph nodes for rectal cancer.
Methods:
Study was approved by the local institutional review board. 22 subjects with biopsy-proven rectal cancer imaged via simultaneous 3.0 T time-of-flight PET/MRI, with 7 subjects undergoing two separate PET/MRI examinations, for a total of 29 studies. Each examination included both a whole body PET/MRI and a dedicated pelvis PET/MRI with both 3 and 15 minute PET acquisitions of the pelvis. Three radiologists interpreted each examination with PET only, MRI only, then combined PET and MRI examination, using all available images. Additionally, the 3 minute and 15 minute PET acquisitions of the pelvis were reviewed separately by a single radiologist.
Results:
A total of 94 lymph nodes were identified as abnormal on PET imaging, all with MRI anatomic correlates. Of these, 37 (37/94, 39.4%) were seen only on the dedicated 15 minute acquisition. Fifty-seven (57/94) nodes measured 5 mm or less, including 29 (29/94, 30.9%) seen only on the 15 minute acquisition. Thirty-one (31/94) nodes measured 5.1–10 mm, including 8 (8/94, 8.5%) seen only on the 15 minute acquisition. Of the 17 subjects imaged for initial staging, 11 (11/18, 64.7%) were upstaged as a result of the increased PET acquisition time, 10 from N1 to N2, and 1 from N0 to N1.
Conclusion:
Longer PET acquisition times during PET/MRI for rectal cancer increases the number of FDG avid lymph nodes detected without increasing scan time.
Keywords: Rectal cancer, PET/MRI, Acquisition time
Introduction
Accurate T-staging of rectal cancer has been significantly improved in recent years through the use of rectal magnetic resonance imaging (MRI), which provides high resolution imaging of the primary tumor and its relationship with the muscularis propia, mesorectal fascia, and local organs [1,2,3,4,5]. Despite the success of MRI in T-staging, nodal disease detection and accuracy remain limited, with high lymph node size cutoffs (> 1 cm) providing high specificity but poor sensitivity and low size cutoffs (> 5 mm) inevitably trading improved sensitivity for lower specificity [6,7,8,9,10,11]. Although adding morphologic features to a 5 mm size cutoff improves sensitivity and specificity [6,7,8,11,12,13], a more sensitive, specific, and reproducible method of identifying nodal metastases is needed.
The addition of [18F]-Fluorodeoxyglucose (FDG) positron emission tomography with computed tomography (PET/CT) has been shown to improve specificity for nodal disease detection in rectal cancer compared to high resolution MRI, and the combination of MRI with PET/CT has been shown to improve accuracy of malignant lymph node detection compared to either of those modalities alone [14]. The addition of FDG PET/CT has also been shown to frequently alter therapeutic management during initial staging [11,15,16,17,18,19]. Multiple studies suggest that FDG PET/CT provides additional staging information in rectal cancer, including improved sensitivity for lymph node and distant metastatic disease detection [19,20,21,22,23,24].
Simultaneous FDG PET/MRI combines the benefits of rectal MRI with metabolic information from PET in a single scan that can be superimposed during image interpretation. Early studies evaluating the ability of FDG PET/MRI to accurately stage rectal cancer suggest FDG PET/MRI may be more accurate than CT, MRI, and FDG PET/CT [25,26]. For detection of abnormal lymph nodes less than 1 cm, FDG PET/MRI may be particularly helpful, given the low sensitivity of MRI alone for these lymph nodes [5].
To improve the accuracy of FDG PET/MRI in the detection of hypermetabolic lymph nodes, imaging protocols should be optimized. Lengthening the PET acquisition time to increase detection of potentially metastatic lymph nodes has been shown to improve detection rates for other cancers with PET/MRI [27]. This study was designed to determine if extending PET acquisition time in the pelvis during dedicated pelvic MRI increases the detection rate of potentially metastatic lymph nodes for rectal cancer without increasing scan time.
Materials and Methods
Institutional review board approval with waived informed consent was obtained for this retrospective investigation. At the authors’ home institution, the PET/MRI protocol for rectal cancer includes a whole body MRI with dedicated pelvic sequences, as well as both a 15 minute PET acquisition of the pelvis and a whole body PET acquisition composed of 3 minutes of PET imaging performed at each of 6 separate stations, one of them in the pelvis. The authors undertook a retrospective review of subjects scanned with this protocol for rectal cancer to evaluate if the 15 minute pelvic PET acquisition detected more potentially metastatic lymph nodes compared to the 3 minute pelvic PET acquisition.
A retrospective single institution search of the electronic medical record for PET/MRI studies performed in the setting of biopsy-proven rectal adenocarcinoma from January 1, 2015 to December 31, 2016 revealed a total of 29 PET/MRIs performed for 22 subjects (Table 1). Of 22 subjects, 17 were imaged for initial staging of rectal cancer, 5 for suspected recurrence, and 7 underwent a second PET/MRI examination for the purposes of restaging after chemotherapy and radiation therapy treatments. Subject characteristics are summarized in Table 1.
Table 1:
Subject characteristics (n = 29)
| Average Age (years): | 56.6±10.9 |
| Gender: | |
| Male: | 13 (13/29, 44.8%) |
| Female: | 16 (16/29, 55.2%) |
| FDG Dose (mCi): | 8.1±1.6 |
| Time from Injection to Imaging (min): | 72.8±22.3 |
Imaging protocol
Patients were injected with 300.81±59.2 MBq (8.13 +/− 1.6 mCi) of FDG. Images were acquired on a simultaneous 3.0T time-of-flight PET/MRI (Signa, GE Healthcare), and imaging was performed 72.8±22.3 min after injection. Two separate PET acquisitions were obtained during a single MRI acquisition: the first PET acquisition included a single bed position covering only the pelvis for 15 minutes. The second PET acquisition included a whole-body scan from the mid-thighs to the vertex, obtained for 3 minutes at each of six bed positions (18 minutes total). In the pelvis, all other parameters of the PET acquisitions besides time of acquisition were identical, including pixel size, voxel size, field of view, and area covered.
A whole body MRI as well as dedicated pelvic MRI sequences were obtained. The following MRI sequences were obtained of the pelvis:
Small field of view T2 (axial/coronal/sagittal): fast spin echo, flip angle = 125 degrees, slice thickness = 4.5 mm, number of slices per acquisition = 27, TE/TR = 129/7567 ms, NEX = 1.5, field of view (FOV) = 220 × 220 mm, acquisition matrix 448 × 256
Diffusion-weighted images (DWI): axial echo planar DWI, b values = 50 and 500 s/mm2, slice thickness = 6 mm, TE/TR = 54/14117 ms, matrix = 128 × 100
Dynamic contrast-enhanced (DCE) imaging with Differential Subsampling with Cartesian Ordering (DSCO) [28]: slice thickness = 2 mm, flip angle = 15 degrees, matrix = 512 × 512, TE1/TE2/TR = 2.0/4.1/5.6 ms, NEX = 0.7, parallel imaging acceleration factors of 2 (phase direction) × 2.5 (slice direction). Following a single injection (0.1 mmol/kg) of gadobutrol (Bayer Healthcare, Berlin, Germany), 17 phases were acquired every 11 s.
Post-contrast T1-weighted images (LAVA-FLEX): axial 3D spoiled gradient echo sequence using two-point Dixon LAVA flex, slice thickness = 3 mm, flip angle = 12 degrees, matrix size = 316 × 256, TE1/TE2/TR = 2.0/4.1/5.6 ms, NEX = 0.7.
A whole-body MRI was also obtained at six bed positions while completed the whole-body PET scan, with three minutes per bed position of PET acquisition, as previously described which included a post-gadolinium LAVA-FLEX, and coronal and axial single shot fast spin echo sequneces [27]. In the pelvis, attenuation correction was performed using a two point Dixon fat water separation algorithm, as supplied on the scanner [29].
Imaging interpretation
Three subspecialty-trained abdominal radiologists interpreted the PET/MRI examinations retrospectively, blinded to the previously reported results (JW, MO, TH). They were instructed to interpret each examination separately with PET images only, MR images only, then combined PET and MRI images, at separate sessions, separated by a minimum of one week. Lymph nodes were considered positive on PET if the reviewing radiologist considered them to demonstrate FDG uptake greater than background. Lymph nodes were considered positive on MRI if they demonstrated size greater than 5 mm in short axis dimension or abnormal morphology, e.g. spiculated borders or heterogeneous internal appearance [6]. The tumor stage (T-stage), number of abnormal lymph nodes, and nodal stage (N-stage) were recorded for each modality- PET alone, MR alone, and PET/MRI combined- using the American Joint Committee on Cancer (AJCC) TNM system [30].
Using the nodes detected by the three readers, the 3 minute and 15 minute PET acquisitions of the pelvis were separately reviewed by a fourth reader (CB), utilizing the corresponding high resolution T2-weighted MR sequence for anatomic localization. Visualized lymph nodes on MRI were again considered positive on PET if the reviewing radiologist considered them to demonstrate FDG uptake greater than background.
Statistical Analysis
Interreader variability calculations between the 3 reviewers were performed using Fleiss Kappa scores and interpreted using the Landis and Koch benchmark scale [31]. Statistical significance for interreader variability was calculated using Chi-Square test. Statistical analyses and calculations were performed by R (Vienna, Austria 2017) [32].
Results
Rates of lymph node identification on the 3 minute and 15 minute PET acquisitions are summarized in Table 2. Of the 29 studies, a total of 94 lymph nodes were identified as hypermetabolic on FDG-PET with a corresponding lymph node seen on MRI. Of these 94 lymph nodes, all 94 were seen on the 15 minute PET acquisition and 57 (55/94, 60.6%) were seen on the 3 minute PET acquisition, with 37 (37/94, 39.4%) lymph nodes seen only on the 15 minute PET acquisition (Figure 1).
Table 2:
Hypermetabolic lymph nodes identified on 3 minute and 15 minute PET/MRI acquisitions (n = 94)
| 3 minute acquisition | 15 minute acquisition | 15 minute acquisition only | |
|---|---|---|---|
| Total | 57 (57/94, 60.6%) | 94 (100%) | 37 (37/94, 39.4%) |
| 5 mm or smaller (57/94, 60.6%) |
28 (28/57, 49.1%) | 57 (100%) | 29 (29/57, 60.6%) |
| 5.1–10 mm (31/94, 34.1%) |
23 (23/31, 74.1%) | 31 (100%) | 8 (8/31, 25.8%) |
| > 10 mm (6/94, 6.4%) |
6 (100%) | 6 (100%) | 0 |
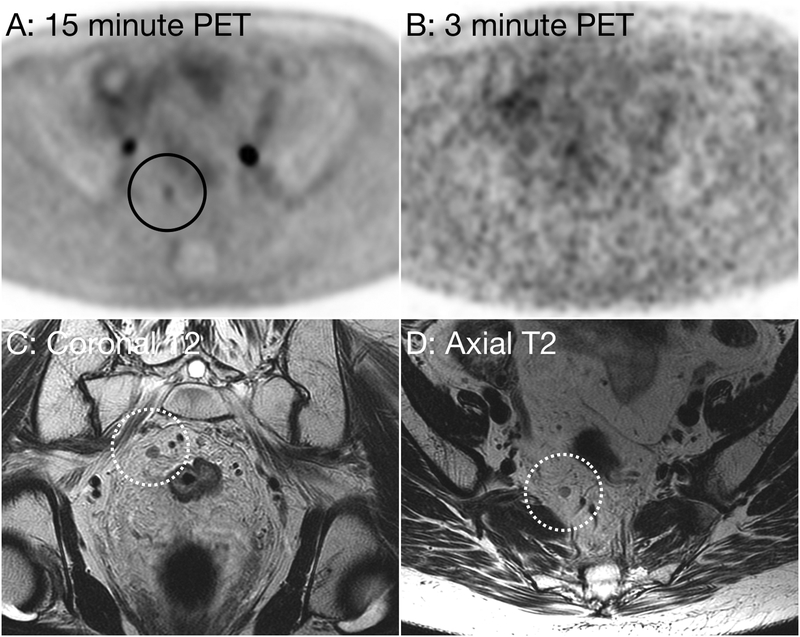
Figure 1:
53 year-old woman with metastatic rectal carcinoma. Axial PET image using a 15 minute acquisition time (Figure A, black circle) demonstrate a focus of abnormal hypermetabolism, suspicious for a metastatic lymph node. Axial PET image performed using a 3 minute acquisition time (B) does not demonstrate abnormal FDG uptake. Oblique coronal (D, dotted white circle) and oblique axial (C, dotted white circle) small field of view T2 weighted images demonstrate a right mesorectal lymph node measuring 4 mm in short axis without suspicious morphologic features.
When stratified by size, 57/94 (60.6%) abnormal lymph nodes were 5 mm or less, with 29/57 (50.8%) of these detected only on the 15 minute PET acquisition. 31/94 (32.9%) lymph nodes detected by PET were 5–10 mm, with 8/31 (25.8%) of these visible only on the 15 minute acquisition. Six lymph nodes greater than 10 mm were identified as hypermetabolic, with all 6 visible on both 3 minute and 15 minute PET acquisitions. 42% (37/88) of lymph nodes identified as abnormal on the 15 minute PET acquisition would not have been identified on either the 3 minute PET acquisition or the pelvic MRI.
Rates of upstaging disease between 3 minute and 15 minute PET acquisitions are summarized in Table 3. Of the 17 subjects imaged for initial staging, 11 (11/17, 64.7) were upstaged, 10 (10/17, 58.9%) from N1 to N2, and 1 (1/17, 5.9%) from N0 to N1. Of the 29 total studies performed, 17 were upstaged (17/29, 58.6%), 15 (15/29, 51.7%) from N1 to N2, and 2 (2/29, 6.9%) from N0 to N1.
Table 3:
Distribution of nodal disease staging based on 3 minute and 15 minute PET/MRI acquisitions during initial staging exams (n = 17) and for all studies (n = 29)
| Initial staging (n=17) | Initial staging (n=17) | All studies (n=29) | All studies (n=29) | |
|---|---|---|---|---|
| 3 minute acquisition | 15 minute acquisition | 3 minute acquisition | 15 minute acquisition | |
| N0 | 2 (2/17, 11.8%) | 1 (1/17, 5.9%) | 3 (3/29, 10.3%) | 1 (1/29, 3.4%) |
| N1 | 15 (15/17, 88.2%) | 6 (6/17, 35.3%) | 24 (24/29, 82.8%) | 11 (11/29, 37.9%) |
| N2 | 0 | 10 (10/17, 58.8%) | 2 (2/29, 6.9%) | 17 (17/29, 58.6%) |
Interreader variability calculations are summarized in Table 4. T-staging using PET/MRI and N-staging using PET alone demonstrated substantial agreement, with kappa scores of 0.649 and 0.619, respectively. N-staging with PET/MRI and T-staging with MRI alone demonstrated moderate agreement, with kappa scores of 0.487 and 0.532, respectively. T-staging with PET alone and N-staging with MRI alone demonstrated fair agreement, with kappa values of 0.296 and 0.386, respectively.
Table 4:
Interreader variability assessment of PET/MRI performed for rectal cancer in Kappa Values (Agreement grades using Landis and Koch benchmark scale).
| T Staging | N Staging | |
|---|---|---|
| PET/MRI | 0.65 | 0.49 |
| PET Alone | 0.30 | 0.62 |
| MRI Alone | 0.53 | 0.39 |
Discussion
We demonstrated that performing a 15 minute extended PET acquisition in the pelvis detected more FDG avid lymph nodes, detecting 40% more lymph nodes than a standard 3 minute PET acquisition. Using a 15 minute PET acquisition instead of a 3 minute acquisition resulted in nodal upstaging in over half of the patients. Our study also suggests that interreader variability is lower between PET/MRI readers compared to MRI alone.
Our results are concordant with previous work suggesting that extended PET acquisition times can increase detection of small lesions, for example in prostate cancer [27]. The explanation for increased detection with longer PET acquisitions is likely the improved emission counts for each voxel, which improves signal-to-noise ratio and makes abnormal lymph nodes easier for the radiologist to identify. The abnormal lymph nodes identified only on the 15 minute PET acquisition were likely present on the 3 minute acquisition but indistinguishable (or at least very difficult to distinguish from) noise on the PET images. In PET/CT, where typical z-axis field of views are 15 cm, most groups do not image for longer than 3 minutes at each bed position. Both clinically available PET/MRIs have a 25 cm z-axis field of view, which allows for nearly double the acquisition segment at each bed position without altering the overall scan time. Additionally, in PET/MRI, the MRI pulse sequences are the rate limiting step, meaning an increase in PET acquisition time does not actually increase overall PET/MRI scan time. In our protocol, we chose to image for 15 minutes at the rectal bed position due to the numerous bed specific MRI pulse sequences that are required for rectal cancer.
This study has multiple limitations. Firstly, our study has a small sample size, limited by PET/MRI being an emerging and novel technique in rectal cancer. Additionally, our study lacks correlation with pathologic staging. Given the small percentage of patients that had undergone surgery at the time of the study completion, comparison with pathology results was not yet feasible. It is certainly possible that increasing the PET acquisition time detects not just more abnormal lymph nodes but also more normal lymph nodes. For future investigation on this topic, a larger study population with post-surgical pathology as a gold standard should be considered.
Conclusion
Longer pelvis PET acquisitions during a simultaneous PET/MRI performed for rectal cancer detects an increased number of FDG avid pelvic lymph nodes and increases detection and upstaging of metastatic disease.
References
- 1.Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, Bourne MW, Williams GF. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999. Apr;211(1):215–22. [DOI] [PubMed] [Google Scholar]
- 2.Brown G, Daniels IR. Preoperative staging of rectal cancer: the MERCURY research project. Recent Results Cancer Res. 2005;165:58–74. [DOI] [PubMed] [Google Scholar]
- 3.Group MS. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572),779. doi: 10.1136/bmj.38938.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group MS. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243(1);132–9. doi: 10.1148/radiol.2431051925 [DOI] [PubMed] [Google Scholar]
- 5.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study BMJ. 2006; doi: 10.1136/bmj.38937646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high spatial resolution MR imaging with histopathologic comparison. Radiology. 2003;227(2);371–7. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52(1);78–83. doi: 10.1016/j.ejrad.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Koh DM, Brown G, Husband JE. Nodal staging in rectal cancer. Abdom Imaging. 2006;31(6);652–9. doi: 10.1007/s00261-006-9021-3. [DOI] [PubMed] [Google Scholar]
- 9.Lahaye MJ, Beets GL, Engelen SME, Kessels AGH, de Bruine AP, Kwee HWS, van Engelshoven JMA, van de Velde CJH, Beets-Tan RGH. Locally Advanced Rectal Cancer: MR Imaging for Restaging after Neoadjuvant Radiation Therapy with Concomitant Chemotherapy Part II. What Are the Criteria to Predict Involved Lymph Nodes? Radiology. 2009;252(1);81–91. [DOI] [PubMed] [Google Scholar]
- 10.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvemet with endoluminal US, CT, and MR Imaging- a meta-analysis. Radiology. 2004;232(3);773–83. [DOI] [PubMed] [Google Scholar]
- 11.Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Guterberlet M, Hunerbein M, Felix R, Wust P, Amthauer H. Comparison of CT, MRI, and FDG PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005:15(8);1658–66. doi: 10.1007/s00330-005-2658-4. [DOI] [PubMed] [Google Scholar]
- 12.Dworak O Morphology of lymph nodes in the resected rectum of patients with rectal carcinoma. Pathol Res Prac. 1991:187(8);1020–4. doi: 10.1016/s0344-0338(11)81075-7. [DOI] [PubMed] [Google Scholar]
- 13.Monig SP, Baldus SE, Zirbes TK, Schroder W, Lindemann DG, Dienes HP, Holscher AH. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999:6(6);579–81. [DOI] [PubMed] [Google Scholar]
- 14.Kim DJ, Kim JH, Ryu YH, Jeon TJ, Yu JS, Chung JJ. Nodal staging of rectal cancer: high-resolution pelvic MRI versus 18F-FDG PET/CT. J Comput Assist Tomogr. 2011:35(5);531–4. doi: 10.1097/RCT.0b013e318225720f. [DOI] [PubMed] [Google Scholar]
- 15.de Geus-Oei LF, van Laarhoven HW, Visser EP, Hermsen R, van Hoorn BA, Kamm YJ, Oyen WJ. Chemotherapy response evaluation with FDG PET in patients with colorectal cancer. Annals of Oncology. 2007:19(2);348–52. [DOI] [PubMed] [Google Scholar]
- 16.de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med. 2009:50(Suppl 1);43S–54S. [DOI] [PubMed] [Google Scholar]
- 17.Kunawudhi A, Sereeborwornthanasak K, Promteangtrong C, Siripongpreeda B, Vanprom S, Chotipanich C. Value of FDG PET/Contrast-Enhanced CT in Initial Staging of Colorectal Cancer – Comparison with Contrast-Enhanced CT. Asian Pac J Cancer Prev. 2016:17(8);4071–5. [PubMed] [Google Scholar]
- 18.Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of (18)F-FDG PET for Predicting Response to Neoadjuvant Therapy in Rectal Cancer: Systematic Review and Meta-Analysis. AJR. 2015:204(6);1261–8. doi: 10.2214/ajr.14.13210. [DOI] [PubMed] [Google Scholar]
- 19.Gearhart SL, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006:13(3);397–404. doi: 10.1245/aso.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Dewhurst C, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, Greene FL, Hindman NM, Jones B, Katz DS, Lalani T, Miller FH, Small WC, Sudakoff GS, Tulchinsky M, Yaghmai V, Yee J. ACR Appropriateness Criteria pretreatment staging of colorectal cancer. J Am Coll Radiol. 2012:9(11);775–81. doi: 10.1016/j.jacr.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Huh JW, Kwon SY, Lee JH, Kim HR. Comparison of restaging accuracy of repeat FDG PET/CT with pelvic MRI after preoperative chemoradiation in patients with rectal cancer. J Cancer Res Clin Oncol. 2015:141(2);353–9. doi: 10.1007/s00432-014-1815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partovi S, Kohan A, Rubbert C, Vercher-Conejero JL, Gaeta C, Yuh R, Zipp L, Herrmann KA, Robbin MR, Lee Z, Muzic RF Jr, Faulhaber P, Ros PR. Clinical oncologic aplications of PET/MRI: a new horizon. Am J Nucl Med Mol Imaging. 2014:4(2);202. [PMC free article] [PubMed] [Google Scholar]
- 23.Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011:17(7);828–34. doi: 10.3748/wjg.v17.i7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlemmer HP. PET/MRI imaging: current status and future direction. Cancer Imaging. 2015:15(1);O32. [Google Scholar]
- 25.Kang B, Lee JM, Song YS, Woo S, Hur BY, Jeon JH, Paeng JC. Added Value of Integrated Whole-Body PET/MRI for Evaluation of Colorectal Cancer: Comparison wWith Contrast-Enhanced MDCT. AJR Am J Roentgenol. 2016:206(1);W10–20. doi: 10.2214/AJR.14.13818. [DOI] [PubMed] [Google Scholar]
- 26.Jeong JH, Cho IH, Chun KA, Kong EJ, Kwon SD, Kim JH. Correlation Between Apparent Diffusion Coefficients and Standardized Uptake Values in Hybrid (18)F-FDG PET/MR: Preliminary Results in Rectal Cancer.. Nucl Med Mol Imaging. 2016:50(2);150–6. doi: 10.1007/s13139-015-0390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lake ST, Greene KL, Westphalen AC, Behr SC, Zagoria R, Small EJ, Carroll PR, Hope TA. Optimal MRI sequences for 68Ga-PSMA-11 PET/MRI in evaluation of biochemically recurrent prostate cancer. EJNMMI Res. 2017:7(1);77. doi: 10.1186/s13550-017-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hope TA. Petkovska I Saranathan M Hargreaves BA Vasanawala SS Combined parenchymal and vascular imaging: high spatiotemporal resolution arterial evaluation of hepatocellular carcinoma. J Magn Reson Imaging. 2016:43(4);859–65. [DOI] [PubMed] [Google Scholar]
- 29.Wollenweber SD, Ambwani S. Comparison of 4-class and continuous fat/water methods for whole-body, MR- based PET attenuation correction. IEEE Trans Nucl Sci. 2013;60(5):3391–98. [Google Scholar]
- 30.Edge SE, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed.: New York, NY.:Springer;2010. [Google Scholar]
- 31.Hallgren KA. Computing inter-rater reliability for observational data: an overview and tutorial. Tutorials in quantitative methods for psychology. 2012;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team. R: A language and environment for statistical computing R Foundation for Statisitical Computing. Vienna, Austria: 2017. URL https://www.R-project.org/. [Google Scholar]