Abstract
Early overnutrition disrupts leptin sensitivity and the development of hypothalamic pathways involved in the regulation of metabolism and feeding behavior. While previous studies have largely focused on the development of neuronal projections, few studies have examined the impact of early nutrition on hypothalamic synaptic physiology. In this study we characterized the synaptic development of proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARH), their sensitivity to leptin, and the impact of early overnutrition on the development of these neurons. Electrophysiology recordings were performed in mouse ARH brain slices containing POMC-EGFP neurons from postnatal age (P) 7–9 through adulthood. We determined that pre- and postsynaptic components of inhibitory inputs increased throughout the first three weeks of the postnatal period, which coincided with a decreased membrane potential in POMC neurons. We then examined whether chronic postnatal overnutrition (CPO) altered these synaptic connections. CPO mice exhibited increased body weight and circulating leptin levels, as described previously. POMC neurons in CPO mice had an increase in post-synaptic inhibitory currents compared to controls at 2 weeks of age, but this effect reversed by the third week. In control mice we observed heterogenous effects of leptin on POMC neurons in early life that transitioned to predominantly stimulatory actions in adulthood. However, postnatal overfeeding resulted in POMC neurons becoming leptin-resistant which persisted into adulthood. These studies suggest that postnatal overfeeding alters the postsynaptic development of POMC neurons and induces long-lasting leptin resistance in ARH-POMC neurons.
Keywords: Development, proopiomelanocortin, leptin, overnutrition, hypothalamus, arcuate
1. Introduction
Changes in early nutritional environment can result in lifelong changes to metabolic systems and are likely a contributing factor to the rising rates of obesity [1, 2]. These changes also increase the susceptibility to develop obesity-associated comorbidities such as diabetes, cardiovascular disease, and mental health issues [3]. Studies in maternal obesity, undernutrition, and overnutrition models have demonstrated that changes in nutritional environment may increase susceptibility to obesity by compromising the proper development of hypothalamic pathways regulating appetite [4–6].
One region critical for appetite and body-weight regulation is the arcuate nucleus of the hypothalamus (ARH). Projections from ARH neurons begin reaching target nuclei in the hypothalamus within the first few weeks of life, and thus changes in nutrition during this period may impact their development [7–9]. A key population of ARH neurons involved in the control of food intake and metabolic regulation are the anorexigenic proopiomelanocortin (POMC) neurons [10–13]. These neurons respond to external stimuli such as glucose and endocrine hormones and project to other hypothalamic sites involved with metabolic regulation and ingestive behavior such as the dorsomedial hypothalamus (DMH), lateral hypothalamus (LHA) and paraventricular nucleus of the hypothalamus (PVH), as well as creating interconnections within the ARH [14, 15].
Early overnutrition caused by manipulation of litter size, termed chronic postnatal overnutrition (CPO), results in increased body weight, hyperleptinemia, increased susceptibility to a high-fat diet (HFD), and leptin resistance later in life [16, 17]. CPO also disrupts the development of ARH neuronal projections throughout the brain [18]. This appears to be, in part, due to a reduced ability of POMC neurons to respond to the adipokine hormone leptin, which is critical for the regulation of POMC and other ARH neuronal projections [12, 13]. POMC neurons in the ARH express the leptin receptor on their somas and dendrites and leptin signaling at these sites plays a crucial role in the axonal outgrowth of POMC neurons [12, 13]. One mechanism by which leptin activates POMC neurons is through phosphorylations of the STAT3 signalling pathway, which is attenuated in early overnutrition models [16]. However, leptin also impacts the electrical activity of POMC neurons.
Leptin receptor activation of POMC neurons depolarizes the membrane through the activation of transient receptor potential (TRPC) channels, which occurs independent of STAT3 [19, 20]. Additionally, leptin can inhibit presynaptic GABAergic neurons, reducing the inhibitory tone onto POMC neurons [21]. One population of GABAergic neurons that synapse onto POMC neurons are the orexigenic neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons [22]. Nutritional changes during development impact the synaptic development of both glutamatergic and GABAergic inputs onto NPY/AgRP neurons, as well as KATP channel expression [23]. The development of leptin signaling onto NPY neurons has been characterized, and interestingly, these neurons undergo a developmental leptin ‘switch’ as leptin stimulates these neurons during early development and, through increased expression of KATP channels, leptin inhibits NPY neurons later in development and into adulthood [24].
While much is appreciated in regard to the synaptic physiology of leptin signaling on POMC neurons and the development of their regulatory counterparts, the development of inhibitory inputs onto POMC neurons and leptin responsitivity of POMC neurons is unknown [24]. Additionaly, while it is known that changes in developmental nutrition can impact the development of NPY neurons, it is not known whether early overnutrition impacts the development of inhibitory inputs onto POMC neurons [23]. Here we explore the synaptic development of inhibitory inputs onto POMC neurons, their response to leptin, and the impact of overnutrition on the electrophysiological functioning of this system.
2. Materials and methods
2.1. Animals
All animal procedures and experiments were approved by the Oregon National Primate Research Center Animal Institutional Care and Use Committee (IACUC) in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Guide). Male POMC-EGFP transgenic mice on a C57BL/6 background were bred in-house (JAX stock #009593, The Jackson Laboratory). All mice were group-housed at 22–24°C, under a 12/12 hr light/dark cycle, with food (Purina, LabDiet 5001) and water available ad libitum. A chronic postnatal overnutrition model was generated by culling litters of 7–9 pups to three pups at postnatal age 3. For induction of diet-induced obesity (DIO), mice were placed on a 60% high-fat diet (HFD) fat 6–8 weeks of age for 12–16 weeks (D12492, Research Diets). POMC-EGFP mice on a C57BL/6 background at various developmental ages and adults between 12–16 weeks were used for these studies (JAX stock #009593, The Jackson Laboratory) [22]. For fasting experiments, mice were weaned from the dam at P21–23 before the dark cycle and subjected to a 16-hr overnight fast leading up to the experiment. For electrophysiology and ELISA experiments, mice of various ages were anesthetized in a chamber with isoflurane before sacrifice by decapitation between zeitgeber time (ZT) 5 and ZT 7.
2.2. Brain slice electrophysiology
The forebrain was removed and placed for 1 min in cold (0–4°C) artificial cerebrospinal fluid (aCSF) sucrose solution composed of (mM): 2 KCl, 1 MgCl2, 1.25 NaH2PO4, 10 HEPES, 26 NaHCO3, 1 CaCl2, 2 MgSO4, 10 glucose, 208 sucrose, 0.7 ascorbic acid, and aerated using 95% O2/5% CO2. The forebrain region containing the ARH was blocked (rostral-caudal) and mounted in a vibrating microtome (Leica VT-1000S). Brains were sectioned with a sapphire knife (Delaware Diamond Knives) yielding roughly three slices (250-μm) per mouse. A naïve slice without previous exposure to leptin or synaptic blockers was used for each recording. Slices were transferred and maintained at room temperature in a six well-dish containing a recording aCSF solution composed of (mM): 124 NaCl, 5 KCl, 2.6 NaH2PO4, 10 HEPES, 26 NaHCO3, 2 CaCl2, MgSO4, 10 dextrose, and bubbled using 95% 02/5% C02. Sucrose was used to adjust the osmolarity to 310–314 mOsm. For recordings, brain slices were transferred to a perfusion chamber containing aCSF maintained at 34–37°C. POMC-EGFP-tagged neurons were visualized using an upright microscope (Zeiss Axoskop 2). Recording electrodes were back-filled with experiment-specific internal solutions as follows (mM): Current-clamp; 125 K-gluconate, 2 KCl, 5 HEPES, 10 EGTA, 5 MgATP, 0.25 NaGTP. Voltage-clamp IPSC; 140 CsCl, 5 MgCl2, 1 BAPTA, 10 HEPES, 5 MgATP, and 0.25 Na2GTP. All internal solutions were brought to pH 7.3 using KOH (voltage-clamp) or CsOH (IPSC), and had a final osmolarity of 301–304 mOsm. Neurons were recorded from the lateral ARH, away from the median eminence and third ventricle. Patch electrodes with a resistance of 3–5MΩ were guided to neurons using differential interference contrast (DIC) optics (Hammamatsu). Patch-clamp recordings were made with Axopatch 700B (Molecular Devices), a Digidata 1322A digitizer (Molecular Devices), and Clampex 10 recording software. Only neurons with holding currents not exceeding 100pA at VH= −60mV (holding voltage for voltage-clamp recordings) for the 10-min control period (input resistance > 150 MΩ) were studied further. For voltage-clamp recordings the N-methyl-d-aspartate (NMDA) receptor antagonist (DL)-2-amino-5-phosphonovaleric acid (APV; 50 μM), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonist (CNQX; 10 μM) and sodium-channel blocker tetrodotoxin (TTX; 1 μM) were bath applied for 10-min following the 10 min control period to isolate miniature inhibitory post-synaptic currents (mIPSCs). After 10 min bath application of synaptic blockers in voltage-clamp experiments, leptin (100 nM) was co-administered via bath applicitation for 15-min. In current-clamp recordings, leptin was bath applied to the slice for 15 min following the control period. Series resistance was monitored throughout the recording, and neurons were not considered for further analysis if resistance exceeded 24MΩ or drifted >25%. Series resistance did not differ between control (aCSF) and treatment. Current-clamp recordings were made at resting membrane potentials, and current injections were not used to hold the membrane at set potentials. All membrane potentials reported were corrected for junction potential (13 mV). All compounds were obtained from Tocris Cookson or Sigma Aldrich.
2.3. Enzyme-linked immunosorbent assay (ELISA)
To measure circulating leptin concentrations, trunk blood was obtained between ZT 5 and ZT 7 by decapitating and pipetting blood into a collection tube. Samples were incubated at room temperature for 20 min before centrifugation at 988 g for 10 min. Serum was then collected and transferred to a fresh sample tube. Samples were frozen at −20°C until all samples needed to run the assay were collected. Samples were ran in duplicate at a 1:40 dilution according to manufacturer’s instructions (Mouse Leptin DuoSet DY498/DY009, R&D Systems) [25].
2.4. Statistical analysis
For electrophysiology experiments, statistical comparison of drug effect between groups was made using one-way ANOVA with Tukey’s post hoc analysis for parametric data, except when data was only compared to one group in which case Dunnett’s post hoc was used. The appropriate non-parametric tests were used when data did not meet the statistical assumptions for parametric analysis (see results). For experiments using a two-way ANOVA a Bonferroni post hoc test was used. N values are presented as number of neurons recorded. The Kolmogrov-Smirnov (KS) test was used to determine the significance of drug effect within individual neurons (Mini Analysis, Synaptosoft). For all experiments, data are presented as mean ± standard error of the mean (SEM) and statistics were calculated using RStudio and Prism7 software (Graphpad).
3. Results
3.1. The frequency and amplitude of mIPSCs onto ARH-POMC-EGFP neurons develop throughout the first three weeks of postnatal development
We determined the pre- and postsynaptic development of inhibitory inputs onto POMC-EGFP neurons during several stages of development by measurement of miniature (m) inhibitory post-synaptic currents (IPSCs). To measure mIPSCs, recordings were performed in the presence of the AMPA receptor antagonist CNQX (10 μM) and the NMDA receptor antagonist APV (50 μM) to block excitatory glutamatergic inputs. Additionally, we added the sodium channel blocker tetrodotoxin (TTX; 1 μM) to the external solution to block action potential firing, allowing us to capture changes occurring specifically at the synapse. First we measured the frequency of mIPSCs which is associated with the presynaptic release of GABA. mIPSC frequency greatly increased between postnatal day (P) 7–9 and P21–23, at which point they matched the frequency seen in the adult (one-way ANOVA (F(3,43) = 4.64, p = 0.007; Figure 1A,B). This data is consistent with previous studies characterizing inputs onto ARH neurons during the third week of development and adulthood [26].
Figure 1. Development of inhibitory inputs onto ARH-POMC-EGFP neurons.
(A) Representative trace of mIPSCs in POMC-EGFP neurons from various age groups. (B) Average mIPSC frequency (Hz) in POMC-EGFP neurons at P7–9 (n = 9), P13–15 (n = 9), P21–23 (n = 13), and adult (n = 16). (C) Average mIPSC amplitude (pA) in POMC-EGFP neurons at P7–9, P13–15, P21–23, and adult. (D) I-V curve of a voltage ramp in POMC-EGFP neurons with CsCl internal solution. (E) Average maximal inward current (pA) at a 0 mV a depolarization in age groups from panel (D). (Error bars indicate ± SEM; one-way ANOVA, Tukey’s post-hoc; groups sharing the same letter are not significantly different).
We next examined changes in mIPSC amplitude in POMC-EGFP neurons throughout development, which is the amount of current coming into the cell during each event and influenced by postsynaptic components such as receptor expression, kinetics, and membrane capacitance [27]. We observed that the basal amplitude of mIPSCs increased between P7–9 and P21–23 (one-way ANOVA (F(3,40) = 3.34, p = 0.003; Figure 1A,C).
To explore the postsynaptic component further, we ran I-V curves using a 10mV ramp protocol from −90 to 0 mV using our CsCl internal solution. This allowed us to determine the maximum amount of current that can enter the neuron in a highly depolarized state. Similar to our observations in Figure 1C, we found that the maximal inward current significantly increased between P7–9 and P21–23 where it reached adult-like levels, with reversal potentials of P7–9: −41.8 mV, P13–15: −25.9 mV, P21–23: −26.1 mV, adult: −29.2 mV. The maximal current at P21–23 remained into adulthood (one-way ANOVA, F(3,33) = 7.99, p < 0.001; Figure 1D,E). Thus, there is a postsynaptic component to the development of inhibitory inputs onto POMC neurons, possibly due to changes in receptor expression or functionality throughout development.
3.2. Resting membrane potential of ARH-POMC-EGFP neurons hyperpolarizes throughout development and into adulthood
To determine whether this developmental increase in inhibitory inputs impacted the functional output of POMC-EGFP neurons, we switched to the current clamp configuration, which allowed us to measure the functional output of the neuron such as changes in action potential firing and membrane potential. In line with the increase in inhibitory tone that we observed, the resting membrane potential of POMC-EGFP neurons hyperpolarized through postnatal development and into adulthood (one-way ANOVA, F(3,82) = 5.79, p = 0.001; Figure 2A,B). Additionally, we observed a significant difference in baseline action potential firing with a notable decrease in adult versus P13–15 mice (one-way ANOVA, Kruskal-Wallis non-parametreic, p < 0.05; Figure 2C). Coinciding with the decrease in membrane potential, we observed a significant increase in quiescent neurons throughout development (Chi-Squared, X2 (2, 83) 7.98, p = 0.047; Figure 2D), which supports our finding that the number and strength of inhibitory inputs onto POMC neurons is increased throughout development.
Figure 2. Development of the membrane potential in ARH-POMC-EGFP neurons.
(A) Representative traces from current clamp recordings at various ages. (B) Average basal membrane potential (mV) in POMC-EGFP neurons at P7–9 (n = 15), P13–15 (n = 21), P21–23 (n = 24), and adult (n = 26). (C) Average action potential frequency (Hz) in POMC-EGFP neurons at P7–9, P13–15, P21–23, and adult. (D) Percent of quiescent neurons in each age group. (Error bars indicate ± SEM; *p < 0.05, one-way ANOVA, Tukey’s post-hoc; groups sharing the same letter are not significantly different).
3.3. Chronic postnatal overnutrition alters post- but not presynaptic development of inhibitory inputs onto ARH-POMC-EGFP neurons
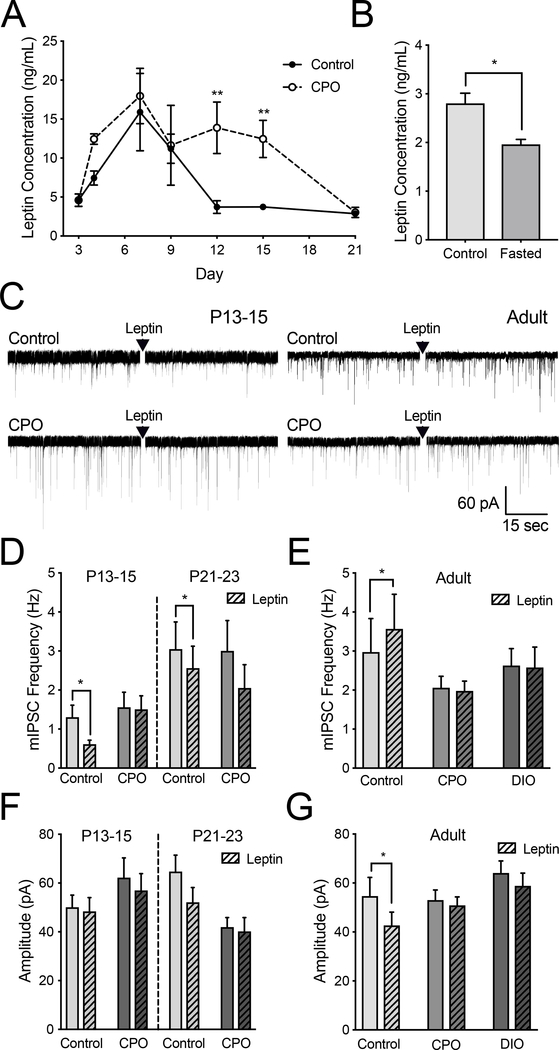
To test the impact of early overnutrition on the synaptic development of POMC neurons, we utilized the chronic postnatal overnutrition (CPO) model. In this model litters are reduced to three pups at P3, providing increased access to the lactating dam. CPO pups became heavier than control counterparts by P7 and this lasted throughout the preweaning period (two-way ANOVA, Bonferroni post hoc, F(6, 136) = 15.61, p < 0.001, Figure 3A). To determine whether CPO had an impact on the development of inhibitory inputs onto POMC-EGFP neurons, we measured the frequency of mIPSCs and found no significant effect at P7–9, P13–15, or P21–23 compared to controls (two-way ANOVA, F(2, 52) = 0.002, p = 0.998; Figure 3C). We added an adult diet-induced obesity (DIO) group to compare to CPO mice. We did not see a difference in mIPSC frequency between adult control, CPO, and DIO mice (one-way ANOVA, F(2, 44) = 0.29, p = 0.75; Figure 4C). However, when we looked at the amplitude of mIPSCs to determine whether there was an impact on the postsynaptic strength of these inputs, we found P13–15 CPO mice had a significantly higher mIPSC amplitude compared to control, an effect which reversed in the opposite direction by P21–23 (two-way ANOVA, treatment-effect, F (2,55) = 10.27, p < 0.001; Figure 3B,D). By adulthood there was no difference in mIPSC amplitude between control, CPO, and DIO mice (one-way ANOVA, F(2, 42) = 0.58, p = 0.56; Figure 4E)
Figure 3. Impact of CPO effects on inhibitory inputs and membrane potential in ARH-POMC-EGFP mice.
(A) Developmental body weight of control (n = 7–15) and CPO (n = 6–9) mice (B) Representative trace comparing mIPSC frequency in control and CPO mice at P13–15. (C) Average frequency (Hz) of mIPSCs at P7–9 (Control: n = 9; CPO n = 6), P13–15 (Control: n = 9; CPO n = 8), P21–23 (Control: n = 13; CPO n = 15), and adult (Control: n = 16; CPO n = 16) mice. (D) Average amplitude (pA) of mIPSCs at P7–9 (Control: n = 9; CPO n = 6), P13–15 (Control: n = 9; CPO n = 8), and P21–23 (Control: n = 12; CPO n = 20). (E) Representative trace of a current-clamp recording comparing membrane potential of adult control and CPO mice. (F) Average membrane potential (mV) in P13–15 (Control: n = 25; CPO n = 16), P21–23 (Control: n = 27; CPO n = 13), and adult (Control: n = 26; CPO n = 12; DIO n = 22) mice. (Error bars indicate ± SEM; *p < 0.05, ** p < 0.01, ***p < 0.001).
Figure 4. Alteration of leptin signaling on mIPSCs in CPO and DIO mice.
(A) Serum leptin concentration in control (n = 5–10) and CPO (n = 3–5) mice throughout the first two weeks of development (two-way ANOVA, Bonferroni post hoc). (B) Average mIPSC frequency (Hz) after bath application of leptin (100 nM) in P13–15 control (n = 12) and CPO (n = 11; left) and P21–23 control (n = 12) and CPO (n = 11; right) mice. (C) Average mIPSC frequency (Hz) after bath application of leptin (100 nM) in POMC-EGFP neurons from adult control (n = 12), CPO (n = 17), and DIO (n = 17) mice. (D) Average mIPSC amplitude (pA) in POMC-EGFP neurons from P13–15 (left) and P21–23 (right) control and CPO before and after bath application of leptin (100 nM). (E) Average mIPSC amplitude (pA) in control and CPO mice from panel (C) before and after bath application of leptin (100 nM). (Error bars indicate ± SEM; *p < 0.05).(F) Average mIPSC amplitude (pA) in POMC-EGFP neurons from P13–15 (left) and P21–23 (right) control and CPO before and after bath application of leptin (100 nM). (G) Average mIPSC amplitude (pA) in control and CPO mice from panel (C) before and after bath application of leptin (100 nM). (Error bars indicate ± SEM; *p < .05).
Although CPO altered the amplitude of mIPSCs at P13–15 and P21–23, there was no resulting impact of CPO on the membrane potential at P13–15 (student t-test, t(39) = 0.69, p = 0.50) or P21–23 (student t-test, t(38) = 0.14, p = 0.89) when compared to controls (Figure 3F). This suggests that although more inhibitory current is flowing into the cell, this increase is not sufficient to change the resting state of these neurons. We also measured the membrane potential in adult mice, adding a DIO group to compare to the effects of CPO, which also led to a null finding (one-way ANOVA; F(2, 57) = 0.35; p = 0.32; Figure 3E,F).
3.4. CPO attenuates pre- and postsynaptic effects of leptin on mIPSCs in ARH-POMC-EGFP neurons
Leptin plays a significant role in the development of hypothalamic feeding circuits and there is a surge in circulating leptin between roughly P7 and P12 [28, 29]. In control mice circulating leptin was significantly elevated at P7 and P9 when compared to P3 (one-way ANOVA, F(6,35) = 3.37, p < 0.0001, Dunnett’s post-hoc; Figure 4A). We observed similar timing for the initiation of the leptin surge in CPO mice, but leptin levels remained elevated in CPO mice up to six days longer than control mice (two-way ANOVA, Bonferroni post hoc, F(5, 50) = 2.77, p = 0.03, Figure 4A). Because P3 was the day of culling, this group was not included when comparing control to CPO circulating leptin levels and is presented as a baseline value for CPO in the graph (Figure 4A). To ensure the reliability and consistency of our data with that in the literature, we also compared fed versus fasted circulating leptin at P21 as circulating leptin levels are known to decrease in fasted mice (t(10) = 2.70, p = 0.02; Figure 4B) [30]. We next determined if CPO altered leptin effects on inhibitory inputs onto POMC neurons. For these experiments we measured mIPSCs before and after a 15 min bath application of leptin. At P13–15 and P21–23, leptin significantly decreased mIPSC frequency in control mice (paired t-test, P13–15: t(6) = 2.82, p = 0.03; P21–23: t(11) = 2.51, p = 0.03), an effect that was lost in CPO mice (Figure 4C). Leptin also did not have an effect on mIPSC amplitude in P13–15 and P21–23 mice (Figure 4E).
To determine whether the attenuating effect of CPO on leptin’s ability to increase mIPSC frequency persisted into adulthood we measured mIPSCs in adult control, CPO and DIO mice. As previously reported, in adult control mice leptin significantly increased mIPSC frequency (t(11) 4.09, p = 0.002) [21]. However, mIPSC frequency was unchanged in response to leptin in both CPO and DIO mice (CPO: t(32) = 0.41, p = 0.68; DIO: t(32) = 0.74, p = 0.47; Figure 4C). Neither leptin or CPO treatment had any impact on mIPSC amplitudes at P13–15 or P21–23 (Figure 4D). By adulthood, leptin decreased mIPSC amplitude in control (paired t-test, t(11) = 2.32, p = 0.04), but not CPO or DIO mice. This suggests that leptin’s ability to decrease the strength of inhibitory inputs onto POMC neurons is blunted by CPO and DIO (paired t-test, CPO: t(16) = 1.17, p = 0.26; DIO: t(16) = 2.04, p = 0.06; Figure 4E).
3.5. Leptin has heterogeneous effects on ARH-POMC-EGFP neurons during early development
We demonstrated that increases in mIPSC frequency during development resulted in the hyperpolarization of POMC neurons. Since leptin has divergent effects on mIPSCs, which is dependent on age and nutritional status, we next sought to determine the effect of leptin on membrane potential during development and how this leptin response may be impacted in CPO offspring. At P7–9, 45% of neurons depolarized with an average increase of 1.7 ± 0.5 mV from the basal membrane potential, while 55% of neurons hyperpolarized by −3.2 ± 1.2 mV, with a non-significant combined net change (Figure 5A,B). At P13–15 43% of neurons depolarized (4.0 ± 0.9 mV), while 55% of neurons hyperpolarized by −1.5 ± 0.4 mV (Figure 5C,D). By P21–23, 79% of neurons depolarized (5.5 ± 0.9 mV) from baseline and only 21% of neurons hyperpolarized (−2.7 ± 1.1 mV) with a significant net change of 4.6 ± 1.2 mV (t(12) = 3.69, p = 0.003; Figure 5E,F). By adulthood, 94% of neurons depolarized in response to leptin (one neuron did not respond) with an increase of 4.8 ± 0.9 mV from the basal membrane potential (t(16) = 4.89, p < 0.001; Figure 5G,H). Additionally, the net effect of leptin was significantly greater in adult than mice at ages P7–9 and P13–15 (one-way ANOVA, F(3,56) = 5.73, p = 0.002, Dunnett’s post-hoc; data not shown). Linear regression was run in each group to determine whether leptin’s effect on membrane potential was correlated with the resting membrane potential to address the hypothesis that leptin is less likely to depolarize a cell with a high resting membrane potential. This proved to be false for P7–9 (R2 = 0.13), P13–15 (R2 = 0.13), and adult (R2 = 0.01), but true for P21–23 mice (R2 = 0.46, p = 0.001, Figure 5B,D,F,H).
Figure 5. Leptin effects on membrane potential of ARH-POMC-EGFP neurons throughout postnatal development.
(A) Representative traces of current clamp recordings from POMC-EGFP neurons in control P7–9 (n = 9) and (C) P13–15 mice (n = 9) in which leptin either hyperpolarized (top) or depolarized (bottom) the membrane potential (mV). (B) Scatter-plot and average comparison of leptin (100 nM) induced changes in the membrane potential of POMC-EGFP neurons compared against the basal membrane potential with linear regression analysis in P7–9 and (D) P13–15 mice. (E) Representative traces of current clamp recordings from POMC-EGFP neurons in control P21–23 (n = 9) and (G) adult mice (n = 13) in which leptin depolarized the membrane potential (mV). (F) Scatter-plot and average comparison as described in (B) in P21–23 and (H) adult mice. (Error bars indicate ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001).
3.6. CPO induces leptin resistance in ARH-POMC-EGFP neurons
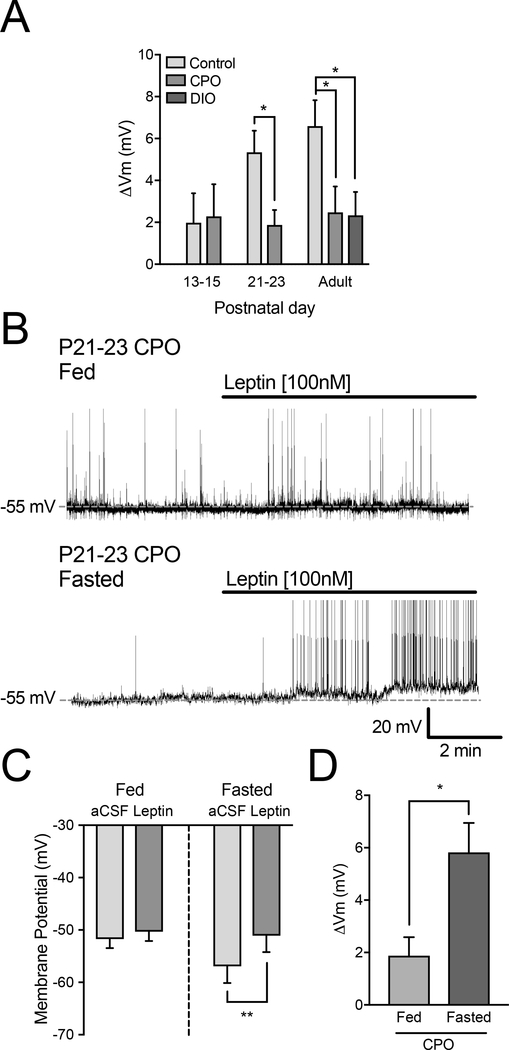
After we determined the leptin responsiveness of POMC neurons during development, we investigated whether this is altered in CPO mice. Given that leptin had a modest net effect on POMC-EGFP neurons at P13–15 and leptin receptor expression in POMC neurons is low at this age (Figure 5C,D), it was not surprising that CPO did not alter leptin responsiveness at that age (Figure 6A) [24]. However, by P21–23 POMC-EGFP neurons in CPO mice were significantly less responsive to leptin than controls (Figure 6A). This decrease in leptin responsiveness in CPO mice was perpetuated into adulthood and displayed a surprising similarity to the reduced leptin responsivity of POMC-EGFP neurons in adult DIO mice (one-way ANOVA, F(2,31) = 4.29, p = 0.023; Dunnett’s adjusted p = 0.025; Figure 6A).
Figure 6. Changes in leptin effects on membrane potential in CPO and DIO mice.
(A) Average mV change in response to leptin in P7–9 (Control: n = 12), P13–15 (Control: n = 16, CPO: n = 8), P21–23 (Control: n = 16, CPO: n = 11), adult (Control: n = 14, CPO: n = 9, DIO: n = 12) mice. (B) Representative traces of current clamp recordings from POMC-EGFP neurons in P21–23 CPO fed and fasted mice. (C) Comparison of leptin (100 nM) induced changes in the membrane potential of POMC-EGFP neurons in P21–23 CPO fed (left) and fasted (right) mice. (D) Leptin induced change in membrane potential in fed versus fasted P21–23 CPO mice (n = 7; Error bars indicate ± SEM; *p < 0.05).
Previous studies have demonstrated that fasting decreases leptin resistance via lowering circulating leptin levels and increasing leptin receptor expression in the brain [31–33]. Therefore, we tested whether an overnight fast at the time of weaning (P21–23) would alter leptin effects on POMC-EGFP neurons. We recorded the membrane potential in fasted P21–23 CPO mice and while there was no difference in basal membrane potential, we demonstrated that an overnight fast restored leptin effects on membrane potential (fed: t(10) = 1.82, p = 0.11; fasted: t(6) = 5.19, p = 0.002; Figure 6B,C) and leptin effects were significantly larger when compared to the leptin response in fed CPO mice (t(16) = 3.14, p = 0.006; Figure 6D).
4. Discussion
Studies characterizing the electrophysiological development of ARH-POMC neurons, and the impact of early changes in nutrition on this development, are limited [26]. Here we identified the postsynaptic development of inhibitory signals and leptin responsivity in POMC neurons. We also demonstrated that early overnutrition disrupts this development and promotes early-onset leptin resistance in POMC neurons.
First, we showed that the number of inhibitory inputs as well as the amplitude of the postsynaptic currents increase during development and reached adult-like maturity by P21–23. The former is consistent with previous studies demonstrating that GABAergic inputs from NPY neurons develop onto POMC neurons during this period [26]. The increase in amplitude of mIPSCs, however, is suggestive of postsynaptic maturation of the POMC neuron, perhaps through changing receptor expression or kinetics [34–36]. In support of this, we also saw that the maximal inward current from a voltage ramp increased with age. As expected, this overarching increase in inhibitory tone coincided with the hyperpolarization of the membrane potential during development and an increase in the number of quiescent POMC neurons [26]. Given that the characterization of inhibitory inputs onto POMC neurons at three weeks of age is largely similar to findings in the adult, these results suggests that the second and third week of postnatal life are a critical time for the synaptic development of inhibitory signals onto the POMC neuron.
Next we investigated whether early overnutrition during this critical developmental period altered these inhibitory inputs. In control mice, we observed a steady increase in the amplitude of inhibitory inputs. Interestingly, CPO offspring had a significant increase in mIPSC amplitude at P13–15 and reverted to significantly smaller currents by P21–23. Although intriguing, the functionality of this altered pattern in mIPSC amplitude requires further investigation as increases in inhibitory currents did not coincide with a decrease in resting membrane potential. It is possible that this change in amplitude primes the neuron to be more responsive to other inhibitory signals during development or that CPO causes a shift in synaptic development, since this increase in amplitude coincides with what we observed in P21–23 control mice and both groups returned to similar levels during adulthood. Understanding that these changes coincided with the same ages in which CPO alters circulating leptin and leptin’s ability to activate STAT3 phosphorylation, we investigated the link between circulating leptin levels and inhibitory inputs onto the POMC neuron [16]. Leptin undergoes a surge that peaks early in the second week of postnatal development, during which circulating levels of leptin increase up to 10-fold before returning back to basal levels [28, 37]. Here we demonstrated that early overnutrition extends the leptin surge, with leptin levels decreasing by P21 [16]. Because this age range coincided with the altered development of post-synaptic inihibitory currents in CPO mice, we asked whether leptin modulates post-synaptic inhibitory currents in POMC neurons and if CPO alters this effect.
In our configuration leptin induced a significant increase in inhibitory frequency on POMC neurons in adult control mice. While this observation presents itself as counterintuitive given the excitatory actions of leptin on ARH-POMC neurons, this phenomenon is due to a negative feedback loop [38]. Previous studies show that excitation of POMC neurons can induce dendritic release of glutamate and αMSH, which in turn stimulates presynaptic GABAergic neurons that synapse back onto POMC neurons [22, 38].
Interestingly, the effect of leptin on mIPSC frequency and amplitude in POMC neurons was attenuated in adult CPO mice, an effect we also observed in DIO mice. To determine whether this resulted in functional changes of leptin actions, we switched to the current clamp configuration and measured the membrane potential. We characterized the actions of leptin on the membrane potential of POMC neurons throughout development, as this has not been established. Leptin had heterogeneous effects on the membrane potential of POMC neurons until P21–23, where the majority of POMC neurons were depolarized by leptin. As demonstrated by Baquero et. al., less than 20% of POMC neurons express the leptin receptor during the first two weeks of life and it is not until the third week in which this number dramatically increases to over 80% [24]. Unlike POMC neurons, the majority of NPY neurons express the leptin receptor throughout these developmental ages [24]. Thus the heterogeneity of leptin responses on the membrane potential may be due in part to leptin exciting presynaptic NPY neurons, which release GABA and NPY onto POMC neurons [22, 39]. Although leptin inhibits NPY neurons in adulthood, for the first two weeks of life leptin excites the majority of NPY neurons and this is due to increased expression of KATP channels causing a leptin ‘switch’ around P21–23, which may also contribute to this heterogeneity [24]. POMC neurons also express KATP channels, but in our recordings KATP channels are likely inactivated due to the high concentrations of glucose in our aCSF [40]. However, previous reports do show that leptin is able to activate KATP channels on NPY neurons in the presence of high glucose [24]. Additionally, the development of leptin signaling in POMC neurons is likely independent of KATP channels because leptin signaling is mediated by TRPC5 in POMC neurons [19, 41].
Althought ARH-NPY neurons have inhibitory inputs onto POMC neurons, another possible source of these inhibitory inputs are VGAT and leptin receptor containing neurons in the DMH, which innervate the ARH and have inhibitory inputs onto both POMC and NPY/AgRP neurons [42]. NPY release may also contribute to the overall inhibitory tone onto POMC neurons as POMC neurons receive inhibitory NPY-containing inputs, and through Y1-receptor mediated activation of GIRK channels, NPY robustly hyperpolarizes POMC neurons [39, 43, 44]. Further studies are needed to identify exactly which inhibitory inputs are disrupted by CPO.
We next measured leptin responsivity of POMC neurons in CPO mice. As mentioned, by the second week of life there are few leptin receptors on POMC neurons and consistent with this we did not see an effect of CPO on leptin sensitivity [24]. However, by P21 the net effect of leptin on the membrane potential of POMC neurons was greatly diminished. This effect lasted into adulthood and looked similar to what was observed in DIO mice, despite the CPO adult mice never consuming a HFD. Thus similar to DIO mice, POMC neurons in CPO mice display early-onset leptin resistance, potentially leading to decreased activation of POMC neurons and presumably a net decrease in satiety signaling that may play part in the weight gain observed in the CPO model.
The exact mechanism responsible for the physiological changes observed in CPO mice is yet to be determined, but here we demonstrated that changes in post-synaptic leptin signaling in POMC neurons is likely a contributing factor. We were able to acutely restore leptin actions on POMC neurons in CPO mice by a 16-hr fast at time of weaning, which is known to increase leptin receptor expression and improve overall leptin sensitivity [31] Overnutrition could also be attenuating leptin receptor signaling cascades [16]. Leptin activation of the JAK2-PI3K-PLC signaling pathway attributes to the activation of TRPC5, which results in a large calcium influx [41]. Whether disruption of TRPC5 expression or the JAK2-PI3K-PLC pathway contributes to CPO induced leptin resistance in POMC neurons is an avenue to be explored in future studies.
Taken together, these results show that (1) GABAergic inputs slowly develop onto POMC neurons throughout the preweaning period (Figure 7; top), (2) leptin has hetereogeneous effects on POMC neurons until these GABAergic inputs are well developed (Figure 7; top), (3) early overnutrition disrupts the postsynaptic development of POMC neurons and causes leptin resistance via a postsynaptic mechanism (Figure 7; bottom), and (4) fasting acutely rescues CPO induces leptin resistance in POMC neurons (Figure 7; bottom). Given that CPO effects last into adulthood, this study suggests that nutritional environments during development are able to program metabolic circuits at the level of the synapse for later in life. Future studies are needed to determine the source of CPO-altered inhibitory inputs, the exact mechanism by which POMC neurons become leptin resistant, and if any interventions are able to reverse these effects long-term.
Figure 7. Representative model demonstrating proposed development of of inhibitory inputs onto ARH-POMC-EGFP neurons and the impact of CPO.
Early development (top) shows few inhibitory inputs onto POMC-EGFP neurons and mixed effects of leptin on the membrane potential, while during late development (bottom) there are more inhibitory inputs onto POMC-EGFP neurons with leptin increasing the presynaptic release of GABA, while decreasing the amplitude of the postsynaptic current. Leptin’s overall effects are excitatory during late development and into adulthood. In early development (top), CPO increases the amplitude of postsynaptic inhibitory currents, while during late development (bottom) CPO decreases the amplitude of these currents, and the effect of leptin on these curents.
Acknowledgements
We give thanks to Cadence True and Charles Roberts for helping with the editing of this manuscript.
Source of funding
This work was supported by P51 OD011092 for operation of the Oregon National Primate Research Center.
Footnotes
Declarations of interest
The authors declare no competing financial interests.
References
- [1].Hales CN, Barker DJ, Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis, Diabetologia, 35 (1992) 595–601. [DOI] [PubMed] [Google Scholar]
- [2].Ogden CL, Carroll MD, Fryar CD, Flegal KM, Prevalence of Obesity Among Adults and Youth: United States, 2011–2014, NCHS Data Brief, DOI (2015) 1–8. [PubMed] [Google Scholar]
- [3].Biro FM, Wien M, Childhood obesity and adult morbidities, Am J Clin Nutr, 91 (2010) 1499S–1505S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bouret SG, Draper SJ, Simerly RB, Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice, The Journal of neuroscience : the official journal of the Society for Neuroscience, 24 (2004) 2797–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW, Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring, PLoS One, 4 (2009) e5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB, Hypothalamic neural projections are permanently disrupted in diet-induced obese rats, Cell metabolism, 7 (2008) 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Myers MG Jr., Olson DP, Central nervous system control of metabolism, Nature, 491 (2012) 357–363. [DOI] [PubMed] [Google Scholar]
- [8].Nilsson I, Johansen JE, Schalling M, Hokfelt T, Fetissov SO, Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse, Brain Res Dev Brain Res, 155 (2005) 147–154. [DOI] [PubMed] [Google Scholar]
- [9].Grove KL, Allen S, Grayson BE, Smith MS, Postnatal development of the hypothalamic neuropeptide Y system, Neuroscience, 116 (2003) 393–406. [DOI] [PubMed] [Google Scholar]
- [10].Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B, Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus, The Journal of neuroscience : the official journal of the Society for Neuroscience, 18 (1998) 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hu J, Jiang L, Low MJ, Rui L, Glucose rapidly induces different forms of excitatory synaptic plasticity in hypothalamic POMC neurons, PLoS One, 9 (2014) e105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ha S, Baver S, Huo L, Gata A, Hairston J, Huntoon N, Li W, Zhang T, Benecchi EJ, Ericsson M, Hentges ST, Bjorbaek C, Somato-dendritic localization and signaling by leptin receptors in hypothalamic POMC and AgRP neurons, PLoS One, 8 (2013) e77622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bouret SG, Draper SJ, Simerly RB, Trophic action of leptin on hypothalamic neurons that regulate feeding, Science, 304 (2004) 108–110. [DOI] [PubMed] [Google Scholar]
- [14].Gao Q, Horvath TL, Neurobiology of feeding and energy expenditure, Annu Rev Neurosci, 30 (2007) 367–398. [DOI] [PubMed] [Google Scholar]
- [15].Williams KW, Elmquist JK, From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior, Nat Neurosci, 15 (2012) 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Glavas MM, Kirigiti MA, Xiao XQ, Enriori PJ, Fisher SK, Evans AE, Grayson BE, Cowley MA, Smith MS, Grove KL, Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet, Endocrinology, 151 (2010) 1598–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen H, Simar D, Morris MJ, Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment, PLoS One, 4 (2009) e6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Caron E, Ciofi P, Prevot V, Bouret SG, Alteration in neonatal nutrition causes perturbations in hypothalamic neural circuits controlling reproductive function, The Journal of neuroscience : the official journal of the Society for Neuroscience, 32 (2012) 11486–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qiu J, Wagner EJ, Ronnekleiv OK, Kelly MJ, Insulin and leptin excite anorexigenic pro-opiomelanocortin neurones via activation of TRPC5 channels, Journal of neuroendocrinology, 30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jobst EE, Enriori PJ, Cowley MA, The electrophysiology of feeding circuits, Trends Endocrinol Metab, 15 (2004) 488–499. [DOI] [PubMed] [Google Scholar]
- [21].Lee DK, Jeong JH, Chun SK, Chua S Jr., Jo YH, Interplay between glucose and leptin signalling determines the strength of GABAergic synapses at POMC neurons, Nature communications, 6 (2015) 6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ, Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus, Nature, 411 (2001) 480–484. [DOI] [PubMed] [Google Scholar]
- [23].Juan De Solis A, Baquero AF, Bennett CM, Grove KL, Zeltser LM, Postnatal undernutrition delays a key step in the maturation of hypothalamic feeding circuits, Mol Metab, 5 (2016) 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baquero AF, de Solis AJ, Lindsley SR, Kirigiti MA, Smith MS, Cowley MA, Zeltser LM, Grove KL, Developmental switch of leptin signaling in arcuate nucleus neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience, 34 (2014) 9982–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Imai A, Satoi K, Fujimoto E, Sato K, Inducing maternal inflammation promotes leptin production in offspring but does not improve allergic symptoms in a mouse model of allergic rhinitis, Heliyon, 3 (2017) e00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Newton AJ, Hess S, Paeger L, Vogt MC, Fleming Lascano J, Nillni EA, Bruning JC, Kloppenburg P, Xu AW, AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice, Endocrinology, 154 (2013) 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hille B, Ionic Channels of Excitable Membranes, 2nd Edition ed., Sinauer Associates, Sunderland, MA, 1992. [Google Scholar]
- [28].Ahima RS, Prabakaran D, Flier JS, Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function, J Clin Invest, 101 (1998) 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pinsky M, Rauch M, Abbas A, Sharabi-Nov A, Tamir S, Gutman R, Long-lived weight-reduced alphaMUPA mice show higher and longer maternal-dependent postnatal leptin surge, PLoS One, 12 (2017) e0188658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hardie LJ, Rayner DV, Holmes S, Trayhurn P, Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA, Biochem Biophys Res Commun, 223 (1996) 660–665. [DOI] [PubMed] [Google Scholar]
- [31].Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW, Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting, Diabetes, 47 (1998) 538–543. [DOI] [PubMed] [Google Scholar]
- [32].Mizuno TM, Bergen H, Funabashi T, Kleopoulos SP, Zhong YG, Bauman WA, Mobbs CV, Obese gene expression: reduction by fasting and stimulation by insulin and glucose in lean mice, and persistent elevation in acquired (diet-induced) and genetic (yellow agouti) obesity, Proc Natl Acad Sci U S A, 93 (1996) 3434–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS, Role of leptin in the neuroendocrine response to fasting, Nature, 382 (1996) 250–252. [DOI] [PubMed] [Google Scholar]
- [34].Kilman V, van Rossum MC, Turrigiano GG, Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses, The Journal of neuroscience : the official journal of the Society for Neuroscience, 22 (2002) 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Del Castillo J, Katz B, Quantal components of the end-plate potential, Journal of Physiology, 124 (1954) 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fatt P, Katz B, Spontaneous subthreshold activity at motor nerve endings, The Journal of physiology, 117 (1952) 109–128. [PMC free article] [PubMed] [Google Scholar]
- [37].Nozhenko Y, Asnani-Kishnani M, Rodriguez AM, Palou A, Milk Leptin Surge and Biological Rhythms of Leptin and Other Regulatory Proteins in Breastmilk, PLoS One, 10 (2015) e0145376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee DK, Jeong JH, Chun SK, Chua S Jr., Jo YH, Interplay between glucose and leptin signalling determines the strength of GABAergic synapses at POMC neurons, Nature Communication, 6 (2015) 6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM, Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice, Neuron, 41 (2004) 711–722. [DOI] [PubMed] [Google Scholar]
- [40].Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ, Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels, Endocrinology, 144 (2003) 1331–1340. [DOI] [PubMed] [Google Scholar]
- [41].Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ, Leptin excites proopiomelanocortin neurons via activation of TRPC channels, The Journal of neuroscience : the official journal of the Society for Neuroscience, 30 (2010) 1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, Tannous BA, Myers MG Jr., Andermann ML, Krashes MJ, Lowell BB, Dynamic GABAergic afferent modulation of AgRP neurons, Nat Neurosci, 19 (2016) 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Csiffary A, Gorcs TJ, Palkovits M, Neuropeptide Y innervation of ACTH-immunoreactive neurons in the arcuate nucleus of rats: a correlated light and electron microscopic double immunolabeling study, Brain research, 506 (1990) 215–222. [DOI] [PubMed] [Google Scholar]
- [44].Horvath TL, Naftolin F, Kalra SP, Leranth C, Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis, Endocrinology, 131 (1992) 2461–2467. [DOI] [PubMed] [Google Scholar]