Abstract
Doxorubicin-induced cardiotoxicity in childhood cancer survivors is a growing problem. The population of patients at risk for cardiovascular disease is steadily increasing, as five-year survival rates for all types of childhood cancers continue to improve. Doxorubicin affects the developing heart differently from the adult heart and in a subset of exposed patients, childhood exposure leads to late, irreversible cardiomyopathy. Notably, the prevalence of late-onset toxicity is increasing in parallel with improved survival. By the year 2020, it is estimated that there will be 500,000 childhood cancer survivors and over 50,000 of them will suffer from doxorubicin-induced cardiotoxicity.
The majority of the research to-date, concentrated on childhood cancer survivors, has focused mostly on clinical outcomes through well-designed epidemiological and retrospective cohort studies. Preclinical studies have elucidated many of the cellular mechanisms that elicit acute toxicity in cardiomyocytes. However, more research is needed in the areas of early- and late-onset cardiotoxicity and more importantly improving the scientific understanding of how other cells present in the cardiac milieu are impacted by doxorubicin exposure.
The overall goal of this review is to succinctly summarize the major clinical and preclinical studies focused on doxorubicin-induced cardiotoxicity. As the prevalence of patients affected by doxorubicin exposure continues to increase, it is imperative that the major gaps in existing research are identified and subsequently utilized to develop appropriate research priorities for the coming years. Well-designed preclinical research models will enhance our understanding of the pathophysiology of doxorubicin-induced cardiotoxicity and directly lead to better diagnosis, treatment, and prevention.
Introduction
Doxorubicin (DOX) is an effective and essential anticancer agent that is an indispensable treatment component for a majority of childhood cancer patients. However, use of DOX places the patient at risk for many complications in the near and far into the future. Most notably, patients can develop a cardiomyopathy leading to congestive heart failure. Currently, the heart failure that develops in these patients is irreversible and refractory to treatment strategies used for heart failure resulting from more common etiologies, such as persistent hypertension and myocardial infarction. The limited data available suggests that the mortality rate for DOX-exposed patients that have advanced to heart failure is close to 50% (49).
Despite numerous studies, there is no consensus on the pathological etiology of DOX-induced cardiotoxicity. Without a clear picture of the molecular processes at play in key cell types present in myocardial tissue and vasculature, early detection methods are lacking. Often, heart failure is diagnosed once it has become symptomatic; a time point that is frequently too late. The current gold standard for monitoring cardiac function is left ventricular ejection fraction (LVEF) measured via echocardiogram. More recent studies have highlighted the promise of other modalities, such as cardiac MRI and strain imaging. Despite recent efforts to perfect the use of new detection methods, depressed left ventricular (LV) function monitored by echocardiography remains the gold standard. Unfortunately, early cardiac changes induced by DOX exposure may include abnormalities such as, heart failure with preserved ejection fraction, and are not easily detected through a drop in LVEF. Thus, the next step in detection is finding a biomarker of DOX-induced cardiotoxicity that is both sensitive and specific. With a particular focus on the unique considerations of DOX exposure in the pediatric population (Table 1), this overview will describe current clinical monitoring guidelines, what we know about the pathological mechanisms, and create a foundation for future research priorities based on the gaps in existing clinical and preclinical research.
Table 1.
Comparison of Pediatric and Adult DOX-Induced Cardiac Complications
| Pediatric | Adult |
|---|---|
| Low acute toxicity incidence | High acute toxicity incidence |
| Minimal to no pre-existing CVD | Pre-existing CVD |
| Minimal to no pre-existing comorbidities | Pre-existing comorbidities |
| Decades-long latent period | Unknown latent period |
| Myocardial tissue primed for apoptosis (263) | Myocardial tissue resistant to apoptosis (263) |
| Under-going cardiac myocyte maturation | Mature myocyte population |
CVD, cardiovascular disease
Doxorubicin and the Heart
Anthracycline chemotherapies, such as DOX, have been used to treat various cancers since the 1960s. By 1970, two “Letters to the Editor” of The Lancet (34) had been published, warning physicians of the cardiac complications encountered with anthracycline treatment. Together, the two letters referenced eight articles that had been published between the years of 1967 and 1969 (26, 33, 184, 186, 187, 189, 191, 296). Studies varied on the type of cancer being treated, patient age, chemotherapeutic regimen (dose, cumulative dose, and accompanying drugs), and onset/type of cardiac event. The common denominator in all of these cases though was the use of an anthracycline.
Doxorubicin
DOX is one of the most commonly used anthracyclines in both adult and pediatric populations. The progenitor, daunorubicin, was first isolated from Streptomyces peucetius var. caesius for use as an antibiotic in the 1950s. It was not until the 1960s that the drug class was first utilized as a chemotherapeutic agent. To date, over 2,000 analogues have been developed, but only a handful have been approved by the FDA for clinical treatment. DOX remains the most extensively used entity among all anthracyclines, and is therefore the focus of this review (294). While it is effective against many types of cancer, it is most often associated with treatment of leukemia, Hodgkin’s and non-Hodgkin’s lymphomas, a variety of sarcomas, and breast cancers.
The mechanism of action against tumor cells is distinct from the known mechanisms responsible for the cardiotoxic effects. While there are many actions of DOX, it is most notable as a DNA intercalator and for generating reactive oxygen species (ROS) in several distinct cellular mechanisms. DOX electrostatically binds to the minor groove of DNA (335). In addition to interfering with DNA and macromolecule synthesis, DOX traps topoisomerase II during its normal enzymatic activity of nicking DNA and thereby creates DNA double strand breaks (15, 32). An immediate consequence of exposure is upregulation of p53, a response to DNA damage that ultimately leads to programed cell death (36,240). Very high DOX doses are needed to cause oxidative damage in tumor cells (202). Even though these drugs were discovered in the 1960s, new antitumor mechanisms of action (MOA) are still being elucidated. In 2012, a mechanism was identified wherein DOX induces ceramide synthesis, an activator of the transcription factor CREB3L1. Once CREB3L1 is activated, it regulates genes involved in inhibition of cellular proliferation (68). Additionally, in 2013, it was found that DOX could compete with and evict histones, including H2AX- a key component of the DNA damage repair signaling pathway. This study concluded that the histone eviction could alter the entire transcriptome, indicating a possible mechanism for the chronic cardiotoxicity (235).
Recent studies have shown that there might be overlap in the anti-tumor and cardiotoxic mechanisms. Pang et al. showed that the histone eviction by DOX was tissue-specific, with differential regulation of proinflammatory genes in the heart (235). Zhang et al. showed that cardiomyocyte-specific deletion of topoisomerase IIb attenuated the DNA damage and transcriptome changes that lead to defects in mitochondrial biogenesis. Overall, the mice were protected from progressive heart failure (356). These are two examples of crossover from the anti-oncogenic effects to the cardiotoxic effects.
Cardiotoxicity
The National Cancer Institute’s definition of cardiotoxicity is “toxicity that affects the heart.” This straightforward, but somewhat ambiguous, definition does not clearly articulate the nuances of this condition. Broadly, cardiotoxic exposures cause electrophysiological dysfunction or muscle damage to the heart. Clinically, it is not so clear-cut. As will be discussed in depth later, there is no strong consensus on the best way to detect and monitor DOX-induced cardiotoxicity. Also confounding the topic is the fact that DOX-induced cardiotoxicity can have myriad clinical manifestations, some of which are partially dependent on the time of onset. Since endomyocardial biopsy is invasive and rarely performed, DOX-induced cardiotoxicity is most often measured by decreases in the LVEF. The LVEF is the measurement of blood the heart pumps with each contraction and is used as an indicator of systolic health. However, the clinical utility of this standard in monitoring DOX-induced cardiotoxicity is often questioned. Groups have proposed different criteria for what magnitude in reduction in LVEF constitutes cardiomyopathy or heart failure. For example, while an LVEF of 50–70% is considered normal by most groups, the American Heart Association defines a reduced LVEF as <40%, while the American College of Cardiology defines an LVEF of <30% as severely dysfunctional. In addition, LVEF is often preserved in diastolic heart failure and thus relying solely on LVEF for identification of survivors with significant heart dysfunction may overlook a substantial fraction of at-risk patients. While LVEF can show changes before the on-set of symptoms in some patients, it may fail to detect changes early enough in the pathologic progression to facilitate successful intervention strategies.
As mentioned previously, there are different times of onset for DOX-induced cardiotoxicity that are often broken into three categories. Acute DOX-induced cardiotoxicity is most often defined as dysfunction with onset during the course of treatment. Manifestations most commonly involve arrhythmias and EKG changes, such as sinus tachycardia, premature supraventricular and ventricular complexes, reduced QRS amplitude, QT interval prolongation, and nonspecific ST-T changes (294, 328). Patients may also suffer from chest pain caused by myopericarditis. Less frequently, acute myocardial ischemia occurs. The incidence of acute DOX-induced cardiotoxicity in the adult patient population is 11% (49). Typically, most acute cardiotoxic effects are reversible and thought to be the result of myocardial edema. Subacute toxicity develops within the first year after cessation of treatment. It is rare compared to the acute and chronic toxicities. Subacute toxicity is only differentiated from acute cardiotoxicity by time of onset; clinical manifestations are similar. (119). Chronic DOX-induced cardiotoxicity can become evident anywhere from one year after the completion of therapy to decades later. While the most troubling outcome of chronic cardiotoxicity is congestive heart failure, that is not the only risk. Chronic DOX-induced cardiotoxicity can manifest as coronary artery disease, stroke, valvular disease, and cardiac mortality (170). Each category of cardiotoxicity may or may not have the same disease mechanism. Answering that question will allow us to develop better techniques and guidelines for detecting all forms of DOX-induced cardiotoxicity. Most importantly, the distinct molecular and cellular mechanisms that mediate the varied deleterious cardiovascular outcomes are not likely to be addressed by ongoing epidemiology studies. Thus, a robust preclinical research effort with novel models is desperately needed to move the field forward and lessen the long-term burden in survivors.
Comparing cardiotoxic responses in adult and pediatric cancer patients
Historically, DOX exposure has been assumed to have similar acute cellular effects in the heart, regardless of age. However, there is mounting evidence that highlights the significant clinical differences between pediatric and adult DOX-induced cardiotoxicity. For example, with the recognition that lifetime cumulative dose exposure of DOX is directly linked to the risk of developing acute cardiotoxicity, newer pediatric treatment regimens limit DOX exposure and acute cardiotoxicity is rarely seen. In contrast, acute cardiotoxicity is encountered with a much higher frequency in adult patients due to a higher frequency of existing cardiac conditions at the time of exposure and the concomitant use of other agents that synergistically increase cardiac damage when used with DOX (i.e., trastuzumab). These observed clinical differences suggest distinct molecular and cellular effects that could influence everything from mechanisms of acute damage, injury repair, and pathologic progression to overt cardiac disease. Because of these age-related differences, clinical management and preclinical studies must consider unique challenges faced by the pediatric population.
Because the age at treatment in children is so much earlier, pediatric cancer survivors who do develop cardiac dysfunction are often afflicted in their 30s and 40s. These patients are acquiring a life-threatening and debilitating disease at a much younger age that will affect their quality of life and increase the cost of care (42). Additionally, survival after a diagnosis of dilated cardiomyopathy drops to less than 50% at 10 years (307). Clinical management, including detection, treatment, and long-term care will likely differ in this population as well. There is no evidence-based consensus on the best method to detect DOX-induced cardiotoxicity. In the United States, there are expert-consensus follow-up guidelines crafted bi-annually by Children’s Oncology Group. Because cardiac complications are frequently not detected until the manifestation of symptoms, these screening guidelines are designed to identify patients who are on the path to heart failure, before it becomes irreversible. While still being investigated, it is thought that DOX-induced congestive heart failure is refractory to standard pharmacological treatment used for heart failure caused by other etiologies (11). Another aspect of patient care is education and the importance of long-term follow up must be impressed upon all cancer survivors. As one study found, a quarter of all survivors who reached 35 without a serious medical condition, developed one within the next 10 years (12).
While there are extensive clinical efforts to study the long-term cardiac outcomes of childhood cancer survivors, there is a marked absence of preclinical research focused specifically on the unique challenges faced by the pediatric population. To understand the differences of the disease pathology, a more suitable model for basic science research is also required. As stated by Lindsey et al., a sufficient animal model must “account for … clinical outcomes and the events … in the collective myocardial cell types” (170). Differences between the childhood cancer survivors and adult cancer survivors are significant enough to warrant separate investigations of disease and guidelines for treatment, diagnosis, and care. For more information on anthracycline cardiotoxicity in adults, see Anthracycline-Induced Cardiomyopathy in Adults (298).
Key Points
An abundance of clinical, epidemiological, and preclinical data demonstrates that DOX is cardiotoxic.
Even though DOX has been extensively used since the 1970s, the anticancer and cardiotoxic mechanisms are still being discovered and they are not necessarily mutually exclusive.
DOX is widely used in pediatric cancers because of its effectiveness as an anticancer agent
Earlier age of treatment exposure to DOX is associated with a higher risk of late development of clinically significant cardiac disease and may indicate a unique mechanism of action.
Cell-specific effects of doxorubicin
Current research points to cardiac myocyte damage as the focus of DOX pathophysiology. Most proposed mechanisms of damage result in cardiac myocyte apoptosis. However, acute myocyte death cannot explain the progressive onset of chronic cardiotoxicity. Additionally, as discussed in this paper, DOX has multiple mechanisms of action that will differentially effect different cell types. DOX was chosen as an anti-cancer agent because it was thought to target actively proliferating cancer cells via DNA intercalation. However, while cardiac myocytes are terminally differentiated, other cell types of the heart are not. To reach a full understanding of DOX cardiotoxicity, more work must be done to understand the interaction between DOX and cell-specific physiology. The following two sections summarize what is known about DOX’s effect on myocytes and non-myocyte cells of the heart.
Cardiac Myocytes
Because acute DOX exposure often causes contractile dysfunction, the effect of DOX on the cardiac myocyte, the major contractile cell of the heart, has been extensively studied. DOX has previously been shown to elicit disturbances in mitochondrial function, transcription regulation, and DNA and macromolecule synthesis. Numerous processes for each of these disturbances have been proposed and a common initial event for each is the production of reactive oxygen species (ROS). However, DOX can affect other cellular pathways, unrelated to ROS. Below is a summary of the various pathologic processes induced in cardiac myocytes after exposure to DOX.
ROS Production
Due to their limited capacity to scavenge free radicals, cardiac myocytes are more prone to ROS-induced damage, possibly explaining why the heart is more susceptible to DOX-induced toxicity than other organs (74). ROS can be generated via different complexes in the cytoplasm or mitochondria of the cell. The location of ROS generation has different effects on the myocyte, but with the common result of apoptosis. In addition, DOX exposure increases oxidative metabolism in cardiac myocytes, which leads to an increase in ROS production (289). One study showed that DOX exposure in cardiac myocytes elevated ROS levels. Surprisingly, ROS levels remained elevated for up to 5 weeks after exposure had ceased (358). A different study investigated heat shock protein (HSP)-27 and heat shock factor (HSF)-1, their role in redox cycling, and the effect on DOX-induced cardiotoxicity. HSP-27 protects the heart from cardiotoxicity and Turakia et al. hypothesized that HSP-27 can indirectly protect iron-regulatory protein (IRP-1) from DOX-induced superoxide radicals (312). In subsequent sections, additional methods of ROS formation and the cell’s response will be explored.
Mitochondrial toxicity
DOX-induced reactive oxygen species can be generated in the mitochondria and result in extensive damage to the mitochondrial membrane. DOX interacts with the NADPH dehydrogenase in complex I, forming a semiquinone radical. The radical can then react with the abundant oxygen to form superoxide radicals (65). The radicals are then perpetuated through redox cycling to form hydrogen peroxide (H2O2) and hydroxyl radicals (73). Additionally, DOX is concentrated to the inner mitochondrial membrane by binding to cardiolipin (105). The near-irreversible binding obstructs cardiolipin from interacting with the proteins of the electron transport chain. Without cardiolipin, many of these proteins cannot function properly, thereby inducing even more ROS production (269). In preclinical trials, increased superoxide dismutase 2 (MnSOD) expression and heme oxygenase (HO-1) have been shown to protect mitochondria from oxidative damage, decrease apoptosis, and preserve LV function (54,63,151). MnSOD relieves oxidative stress by converting superoxides to H2O2. HO-1 converts heme into bilirubin and CO, both anti-inflammatory and anti-apoptotic entities, while also removing excess oxygen molecules at risk of radicalization. Overexpression of the enzyme glutathione peroxidase (Gpx1), found in the cytosol and mitochondria, attenuates complex I inhibition and mitochondrial respiration defects. Gpx1 exerts its protective effect by reducing H2O2 (347). These studies serve to highlight the role oxidative of stress after DOX exposure.
Another source of damage in the mitochondria comes from the shift to anaerobic metabolism from aerobic metabolism (48). As DOX suppresses mitochondrial metabolism, it induces apoptosis. In addition to ROS-induced dysregulation of the electron transport chain, Wallace et al. showed that increased mitochondrial DNA (mtDNA) adducts were associated with increased ROS production from DOX exposure (358). The evidence that CO inhalation by mice can reverse these changes by stimulating metabolism and biogenesis genes in the mitochondria shows that DOX can affect mitochondrial as well as nuclear DNA (242,290). CO inhalation, in combination with heme oxygenase overexpression leads to an increase in NF-E2 related factor (Nrf 2), a transcription factor of mitochondrial biogenesis genes. Furthermore, CO inhalation increased MnSOD, an antioxidant that is part of a positive feedback loop for increasing Nrf 2 expression. Because mitochondria produce 90% of the myocyte’s ATP (324), even if excess ROS species do not induce mitochondria to release pro-apoptotic signals, dysfunctional mitochondria are overtly detrimental to the cell.
NOS/NAPDH
The isoforms of nitric oxide synthase (NOS) and the cofactor NADPH are another source of ROS in the cell. NO can react with superoxide radicals to produce peroxynitrites that can then damage DNA and lead to apoptosis. It is well supported, that while present, nNOS plays a minimal role in ROS production in the heart. Neither mRNA nor protein levels are altered with DOX exposure (87, 176). There is no consensus as to the relative impacts of eNOS and iNOS generation of DOX-induced ROS. Studies have reported conflicting results of eNOS and iNOS mRNA and protein expression in response to DOX. Whether increased or decreased iNOS is protective or deleterious, most studies found that DOX-induced increases in eNOS w detrimental (54), (212) (220) (176). A mechanism for the role of eNOS was elucidated and found to be reliant on NADPH. DOX could bind to the reductase domain of eNOS to produce ROS, supporting the role of eNOS in DOX pathology (321).
Very limited amounts of ROS have been observed by NADPH reduction without the aid of NOS enzymatic activity. While enzyme-free NAPDH production of radicals is negligible, NADPH oxidase (NOX)-assisted production can affect DOX-induced cardiotoxicity. NOX inhibitors used in vitro improved cell survival after treatment (102) and gp91phox (NOX catalytic domain) knockout mice were resistant to cardiotoxicity from chronic DOX treatments (69). NOX-deficient mice show a decrease in ROS production tied to decreased apoptosis, decreased interstitial fibrosis, and an attenuation in cardiac dysfunction (357). Clinically, Wojnowski et al. found NOX single nucleotide polymorphisms (SNP) responsible for DOX-induced cardiotoxicity susceptibility. The study even found patients with SNPs specific for acute cardiotoxicity and separate SNPs for chronic cardiotoxicity (345).
Iron-Complex
The interaction of DOX and iron was identified in the 1980s (194). The DOX-Fe complex can undergo redox cycling to propagate production of H2O2, superoxide radicals, and hydroxyl radicals in the cytoplasm (203). The alcohol metabolite of DOX, doxorubicinol (doxol), can also undergo redox cycling with free iron to create ROS, in addition to interacting directly with proteins involved in iron transport and storage (348). This, along with the fact that cellular free iron is not abundant enough to produce ROS levels that result in cardiac injury (202), leads to the idea that DOX-Fe complexes have multiple mechanisms of damage. IRP-1 and −2 are transcription factors, regulating the expression of transferrin receptor 1 and ferritin. Upon interaction with the doxol-Fe complex, the stabilization of transferrin mRNA by IRP-1 is enhanced. The concomitant decrease in ferrin translation leads to an increased uptake of free iron into the cell (204, 348). The changes in iron metabolism could amplify the damage of DOX by increasing intracellular stores of iron. Alterations in the redox pathway could augment the DOX-induced cardiotoxicity. Hfe (hemochromatosis gene) codes for a protein that regulates the interaction between transferrin and its receptor on the cell surface. Mutations in this gene cause hereditary hemochromatosis, a condition where iron accumulates at toxic levels in the body. The role of iron in DOX-induced cardiotoxicity is evident in Hfe(−/−) mice treated with DOX. Knockout mice had increased liver damage, mitochondrial damage in the heart, and increased mortality compared to their wild type counterparts (207). Additionally, rats on iron-rich diets for 10 to 14 weeks while being treated with DOX fared worse compared to regular diet animals as evidenced by more severe myocyte injury, greater weight loss, and greater annexin uptake (236). Lastly, DOX-Fe complexes potentially interact with the negatively charged membrane causing lipid peroxidation (145).
A notable point brought up in the review by Octavia et al., is the variability of total body iron and the inclination toward iron overload in patients undergoing chemotherapy, bone marrow transplants, and transfusions (228). Based on this knowledge and the role Hfe and iron play, Octavia recommends screening for Hfe mutations and measuring total body iron before implementing DOX-containing regimens.
Exploring this topic further, a recent review article attempted to combine the results of 52 different observational and interventional clinical studies. Variations in study types, dosing, and vitamin supplementations made it impossible to conclude anything from the observational studies. In the interventional studies, five addressed patients with hematological cancers and vitamin E and/or C supplementation. Of the three studies that used vitamin E supplementation, two found that vitamin E alone or in combination with vitamin C could not prevent the decline that occurs in levels of these vitamins with chemotherapy. Only one study found that the decline could be prevented with administration of alpha-tocopherol, beta-carotene, and vitamin C when administered before and during treatment (159). In addition to already being prone to ROS damage, cardiac myocytes may have a smaller pool of antioxidants to draw upon once chemotherapy begins. Understanding why total antioxidant capacity decreases could help explain why simple supplementation does seem to alleviate it.
DOX can generate ROS via many different mechanisms. As has been shown, ROS can cause damage by inducing mitochondrial dysfunction, variable gene regulation, and often result in programmed cell death. What is not so clear is the exact role ROS plays in the pathological process of DOX-induced cardiotoxicity. If ROS damage were the only or primary cause of injury to the myocyte, we would expect antioxidants to be more successful at preventing cardiomyopathy than recent studies have shown. Monohydroxyethylrutoside (monoHER), a flavonoid, was investigated for its antioxidant properties. Dosing is crucial to attenuating short-term toxicity, but longer doses needed to prevent chronic cardiotoxicity may actually exacerbate DOX-induced toxicity (40, 41). Studies using vitamin E have shown that it does not protect the heart or prevent mitochondrial dysfunction (28, 30). Berthiaum et al. theorized that vitamin E did not have access to cellular organelles such as mitochondria, to impart their protective properties. Berthiaum et al. enriched the cardiac membrane with alpha-tocopherol before treating rats for 7 weeks with 2 mg/kg doses of DOX. While the vitamin E content of the cardiac mitochondrial membranes and oxidized cardiac proteins were increased, cardiac histopathology was equal to non-enriched, treated mice and mitochondrial dysfunction was still evident (28). More work must be done to understand the role of ROS in the overall pathology of DOX-induced cardiotoxicity.
mtDNA Lesions
Numerous studies have clearly shown that DOX damages the mitochondria of cardiomyocytes. Putatively, DOX is able to enter the negatively charged mitochondrial membrane as a result of its positive charge (165). Once exposed to DOX, there are significant morphological changes that occur in mitochondria in response (327). In the mitochondria, DOX is able to generate ROS through multiple mechanisms, including the formation of a semiquinone (65) and reactions with iron (209). DOX interacts with iron, and the resulting Fe3+-DOX complex can bind to cardiolipin, a phospholipid that is critical to respiratory chain function (104,105,150,223). These changes are able to acutely impair respiratory chain function (116) as well as cause mtDNA damage (4, 163, 275).
mtDNA may be damaged through a variety of different mechanisms. Direct intercalation of mtDNA (4, 344), DOX metabolites binding to DNA (3), ROS (224), and inhibition of topoisomerase II (303) have been reported as potential causes. mtDNA damage in response to DOX has been found in the heart of humans and in the heart and kidneys of rats (4, 162–164). Other organs may potentially be involved, such as the lung and liver, as they may present with gradual functional decline clinically, like the heart and kidney (195, 221, 278). mtDNA primarily encodes thirteen proteins that participate in the respiratory chain or are components of ATP synthase. As mtDNA mutations persist throughout life, they may serve as the molecular marker associated with dose memory in the generation of late stage effects of DOX-induced cardiotoxicity (165).
Relative to those encoded by nuclear DNA, mtDNA-encoded proteins’ expression decreased in the hearts of humans and rats (162, 163). Further, in rats, these mutations accumulate with age, well beyond the time period of DOX exposure (163). These changes cannot be easily explained by the intracardial half-life of DOX, as the persistence of DOX and DOX metabolites is short lived (223) compared to the time scale for accumulating mtDNA deletions (162). mtDNA damage is a typical consequence of aging and is implicated in a variety of disease states (29). Further, it is important to note that mtDNA damage in general can produce a late-onset cardiomyopathy (160). Thus, acute exposure to DOX may produce DNA damage that propagates over time, which may later manifest as late-onset cardiomyopathy. The mtDNA mutator and the mtDNA deleter mouse models further evidence this. Both models develop late-onset cardiomyopathy (156, 311, 313).
In summary, DOX is a potent mitochondrial toxin that affects the heart through alteration of the respiratory chain and by damaging nDNA and mtDNA. The damage caused by DOX can then affect the mtDNA encoding free radical scavenging systems and/or respiratory chain proteins (the main products of mtDNA). Dysfunction of these proteins may cause a cycle of ROS mediated damage that continually damages more mtDNA, such that typical aging can unveil a deficiency in mitochondrial function. This process can produce clinically evident dilated cardiomyopathy, much like other mitochondrial diseases.
Neuregulin and ERBB signaling
Neuregulins (NRG) are transmembrane proteins of four varying isotypes (NRG1, 2, 3, and 4). Various proteins act on NRG1 to cleave it, including metalloproteinases, ADAM17, and ADAM19 (249). The active peptide is generated from the amino-terminal active domain, an activity modulated by protein kinase C (81). These effects are mediated through the ErbB2/ErbB4 receptors in the heart, which are activated by NRG1. Activation of ErbB2/ErbB4 heterodimers is mediated by the binding of the active peptide of NRG, after it is secreted from endothelial cells. The effects of ErbB2/ErbB4 signaling include differentiation of cardiomyocytes and the maintenance of typical heart architecture (322). Defects in either ventricular ErbB2 or ErbB4 are therefore able to manifest as a DCM (60, 93, 232). Further, in patients exposed to both trastuzumab (an antineoplastic agent that is an antibody antagonist directed against ErbB2) and DOX, the deficient NRG1 signaling is able to sensitize the heart to DOX-induced cardiotoxicity (244). This clinical observation highlights the potential for NRG1 and ErbB2/ErbB4 signaling to be important to the development of late onset DOX-induced cardiotoxicity.
Much research has shown that changes in the neuregulin-ErbB pathway are important in the development of cardiotoxicity in response to DOX therapy. In rats, acute DOX-induced cardiotoxicity can be modulated through the neuregulin-ErbB pathway (265). The combination treatment with DOX and trastuzumab results in enhanced myofibrillar disarray in rat ventricular myocytes. Treatment with NRG-1β decreased the myofibrillar disarray, relative to DOX alone. The effect of neuregulin on DOX exposure also prevents DOX-induced cardiomyocyte apoptosis (88). Further, heterozygous knockout of the NRG1 gene in mice exacerbates heart failure caused by DOX (92). HSP90 stabilizes ErbB2 protein following DOX exposure to protect cardiomyocytes against myocyte apoptosis (177). There is also evidence that acute DOX-induced cardiotoxicity is associated with upregulation of miR-146a, which has an inhibitory effect on the neuregulin-ErbB pathway (128). Through inhibition of miR-146a, Erb4 is upregulated and acute toxicity is reduced. These studies suggest that changes in expression, protein interactions, and miRNA can all modulate the neuregulin-ErbB pathway and generate significantly different outcomes in the development of cardiotoxicity.
The potential roles of the neuregulin-ErB pathway in preventing cardiotoxicity have also been investigated. In a recent publication, ErbB2 over-expression was found to up-regulate antioxidant enzymes, reduce basal ROS, and protect against cardiotoxicity (20). Elevated catalase and glutathione peroxidase occurs in the heart as a result of ErbB2 upregulation, due to signaling by c-Abl (cytoplasmic Abelson) and Arg (Abelson-related gene) in mouse heart tissue. Overall, these changes can reduce apoptosis in response to treatment with DOX. Further, NRG1 is able to protect against DOX-induced apoptosis through an Akt-dependent pathway in mice (10). It is important to note that activation of this pathway has pro-neoplastic potential. However, modifications to NRG1 may alleviate this concern, as bivalent neuregulin may not possess these pro-neoplastic effects, but retain the cardioprotective effects (139). Therefore, the neuregulin-ErbB pathway provides multimodal means to induce cardioprotection against acute DOX-induced toxicity.
Key Points
Cardiomyocyte damage is mediated through ROS production, mitochondrial dysfunction, mitochondrial DNA damage, and the ErbB-neuregulin pathway.
These acute changes induce apoptosis and mediate the effects of acute toxicity.
It is currently not well understood how cardiomyocyte damage can lead to the long-term toxicity and cardiac dysfunction observed in adult childhood cancer survivors.
Alterations in mtDNA and ROS, namely iron balance and nitric oxide, can produce different long-term outcomes in animals.
Other Cell Types
While most research has focused on the cardiac myocyte, endothelial and cardiac progenitor cells have also been investigated for the effect DOX might have on vascular health and the heart’s regenerative capacity. As studies into the disease mechanism continues, other cell types of the heart will have to be studied to understand the whole picture (Fig. 1).
Figure 1.
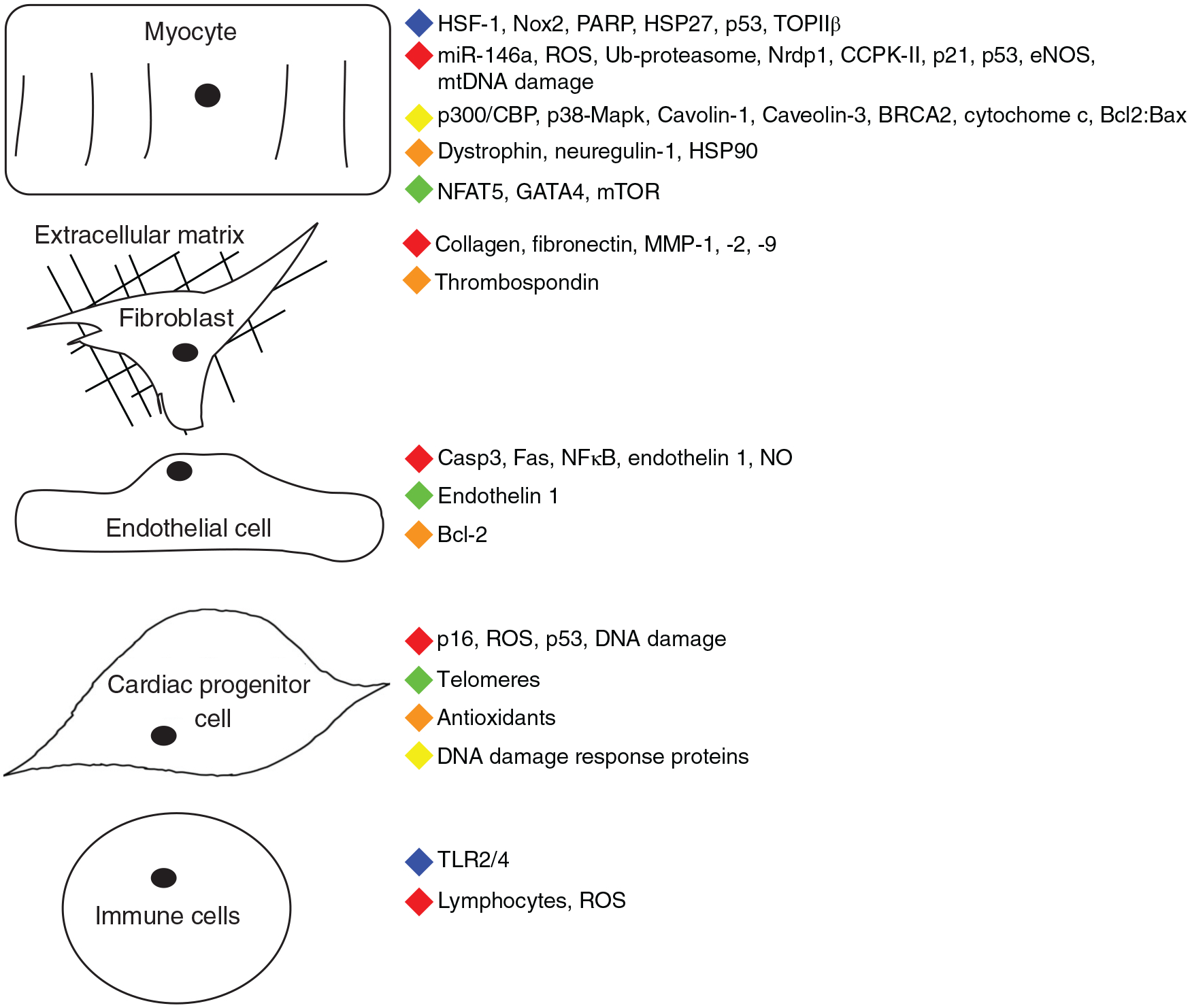
Summary of myocardial cellular effects and the injury response induced by doxorubicin. Color key—blue: absence abrogates cardiac dysfunction or protects from apoptosis. Green: Inhibition of pathway or downregulation of expression. Yellow: Mediates apoptotic response. Red: Expression increases or enhances cardiac dysfunction. Orange: Absence enhances cardiac dysfunction. (Interestingly, p53 both increases in expression, while global knockdown abrogates the cardiotoxic response.) Adapted from Lindsey et al. (170).
Endothelial cells
Knowledge citing the damage DOX inflicts on the vasculature has been known as early as 1991. A short article from 1991 in the British Journal of Cancer listing chronic complications of survivors mentioned seven patients who had died from stroke before 43 years of age (211). Since then, larger epidemiological studies have confirmed the increased incidence of stroke in survivors (52, 217). Survivors are also at a 10 times greater risk of coronary artery disease (12). Hypertension can be both a risk factor for these complications and a complication itself (83, 98). Recent studies by Mulrooney and colleagues have clearly shown that survivors of childhood cancer have decreased vessel distensibility, suggesting an underlying pathologic dysfunction of endothelium (214–216). Preclinical studies have begun to elucidate the effects of DOX on endothelial cells and the resulting vascular dysfunction. The following section reviews the current literature on DOX and endothelial cells.
Apoptosis occurs in endothelial cells as well as cardiomyocytes. This has been shown in both in vitro and in vivo studies of treated rats and cells with DOX. Increases in the expression of different apoptotic genes in both cell types indicates that DOX can directly cause apoptosis in both, although via different signaling pathways. Bcl-2 is decreased in both cell types, but an increase in Bax is seen only in cardiac myocytes, leading to a higher Bax/Bcl-2 ratio. In addition, the number of cells undergoing apoptosis decreased with a caspase inhibitor in endothelial cells, but not with a neutralizing anti-FasL antibody (346). While the outcome is similar, the pathways in each cell type varies and should be investigated further to determine novel, individual therapies for prevention of injury in both. In addition to these differences, ischemia-reperfusion injury induces the release of pro-apoptotic mediators from endothelial cells leading to cardiomyocyte apoptosis (267). A similar mechanism could be at play in DOX-induced cardiotoxicity, leading to not only endothelial dysfunction, but downstream myocyte injury as well.
Male rats dosed with increasing cumulative amounts of DOX have increased plasma levels of endothelin-1 and cardiac tissue nitric oxide. There was no increase in circulating nitric oxide. These increases were not dose dependent. However, increases in lactate dehydrogenase and creatine phosphokinase were dose-dependent, indicating a greater injury response with increasing doses of DOX. At the highest cumulative dose (25 mg/kg), the NO level actually began to decrease. Sayed-Ahmed et al. theorized that NO levels had reached a point where they were now being actively transformed into peroxynitrite via a superoxide radical formed by DOX (266). Peroxynitrite has been shown in many studies to play a role in cardiomyopathies (110). Endothelin-1 in the plasma has been shown to increase in heart failure and cardiomyopathies, including DOX-induced cardiomyopathies (181). While endothelin-1 and NO are released by the endothelial cells, it is believed the free radicals that create superoxides target the myocytes as they contain all three NOS isoforms. This enables the cells to cycle the DOX-redox reaction and increase superoxide while decreasing NO (314). Studies in humans have shown a rapid decline in endothelial cell function via attenuated vasodilation. Changes were seen as quickly as 30 min after DOX administration with a concomitant decrease in serum nitrate levels (77).
While congestive heart failure resulting from the cardiotoxicity is a serious issue, we must not forget the health outcomes of endothelial damage that are also associated with DOX-induced toxicity, or that damage to these cells may mediate damage elsewhere in the heart. Moreover, damage to vascular endothelium may result in significant morbidity in childhood cancer survivors who have decades of survival ahead of them in which subtle cellular damage can mediate pathological decline of the cardiovascular system.
Cardiac progenitor cells
Cardiac progenitor cells (CPC) and their efficacy in treating heart disease is a contentious topic. However, regardless of their potential therapeutic use in the clinic, there is great interest in the role they may play in the pathophysiology of DOX-induced cardiotoxicity.
Utilizing the nuclear testing of the cold war era, Bergman et al., were able to deduce the peak turnover rate of adult cardiomyocytes was 1%. This number slowly decreases to 0.45% over one’s life span. Interestingly, a few patients included in the study suffered from a cardiac pathology (without hypertrophy or dilatation), but these patients did not show an increase in cell turnover (23). This would seem to indicate that while these cells are involved in the homeostatic processes of the heart, they are not necessarily activated in response to myocardial pathology nor reverse pathology of the heart.
Perhaps though, damage to these cells is the cause of the pathology. CPCs, treated in vitro with DOX, had an increased rate of apoptosis and CPC growth was adversely affected. Similar to myocytes, CPCs were thought to be damaged by reactive oxygen species. Not only was there a decline in the activity of various antioxidants, but telomere lengths were shortened (without an impairment of telomerase activity). Along with the upregulation of ataxia-telangiectasia mutated kinase and phosphorylated p53, these processes lead to the inhibition of growth and survival in the CPCs (66). Similar findings were also seen in studies investigating the effect of DOX on endothelial progenitor cells (EPC). EPCs were treated with levels of DOX not meant to cause apoptosis; the goal of the study was to determine the drugs effect on senescence, not cell death. While DOX treatment increased p16INK4A, a marker of senescence, it was in a cytoplasmic, perinuclear pattern, as opposed to the typical nuclear localization. Pretreatment with p38 and JNK inhibitor individually attenuated the increase in SA-b-gal activity (another marker of senescence). The arrest of the cell cycle at G2 instead of G1 indicates that the senescence is not mediated via P16INK4A, but via the MAPKs JNK and p38 (280). Even though the level of DOX was not enough to cause apoptosis, there were still adverse effects on the function of the EPCs. This study suggests that no dose of DOX is safe. The effect DOX has on human CPCs was corroborated in 2013. Tissue samples were taken from patients who developed congestive heart failure after DOX exposure, patients who died during DOX exposure from other causes, and subjects that did not die from any cardiovascular disease. Exposed tissue showed a higher number of senescent and γ-H2AX positive (DNA damage repair signal) CPCs. Signs of senescence and DNA damage are also present in CPCs of exposed tissue in the absence of heart failure. Human CPCs were also isolated from unexposed myocardium collected during bypass or mitral valve surgery. In vitro, cells showed an increase in apoptotic signals and a decrease in cell cycle proteins (243). As seen with other cell types of the heart, CPCs are damaged via exposure to DOX. However, the injury to these cells cause the heart to lose what minimal regenerative properties it has. While CPCs make up a small population of the heart, their damage can be propagated far beyond their numbers.
For this reason, there have been numerous studies, clinical and preclinical, attempting to harvest stem cells, expand the population, and then re-plant them in the diseased heart. Anversa et al. (66), isolated and expanded CPC populations in vitro, and then re-planted them into DOX treated rats. CPC administration was able to rescue the DOX toxicity phenotype. Improvements in cardiac function and morphology were seen in LV developed pressure, the rate of developed pressure and relaxation, chamber diameter and volume, and wall thickness. For each parameter, the treatment with CPC almost restored the values to control status. However, there is still some question as to the longevity of transplanted stem cells. In multiple studies, cells injected after DOX treatment could either not be identified at all or only transiently (6, 14, 272). These studies included injected bone marrow cells, mesenchymal stem cells (MSC), and fetal cardiomyocytes. Aupperle et al. saw an increase in MMPs, TIMPs, and collagens after MSC injection, but did not investigate cardiac function (14). Scorsin et al. did not identify the fetal cardiomyocytes after injection, but did demonstrate an improvement in cardiac function over DOX-treated animals without cell injections (272). While the role of cardiac progenitor cells in DOX-induced cardiotoxicity is debatable, their role in the prevention or treatment of cardiotoxicity warrants further research.
Immune response
Immune cells, such as macrophages, neutrophils, and lymphocytes have largely been overlooked in DOX-induced cardiotoxicity research. As seen in all cell types investigated so far, DOX can have a direct effect upon exposure in vitro. In fact, human neutrophils treated with DOX exhibited increased ROS production and impairment of protein kinase C (PKC) signaling, a pathway involved in lymphocyte and macrophage activation (268, 297). Even without a direct effect, immune cells most likely play a role in the pathophysiology of the disease as they receive signals from the injured myocytes, endothelial cells, and cardiac progenitor cells. One study of 11 patients diagnosed with cardiotoxicity (via endomyocardial biopsy) showed that 56% of the patients had myocarditis by the Dallas criteria (94). Histology indicated that the predominant infiltrate were T lymphocytes. The samples also showed focal areas of replacement fibrosis. An increase in dendritic cells (macrophages and T helper cells, but not cytotoxic T cells) was also shown in spontaneous hypertensive rats given 1 mg/kg DOX for up to 12 weeks. The mechanism responsible for initiating the immune responses was diminished by pretreatment with dexrazoxane (354).
Toll-like receptors (TLR) play a well-researched role in inflammatory signal propagation and activation of the innate immune system. For their association with heart disease (86,113,200,349) and activation under oxidative stress, TLR2 and TLR4 specifically, have been investigated after DOX exposure. When TLR2 was knocked out in mice administered with 20 mg/kg DOX, left ventricular end diastolic dimension was attenuated and fractional shortening was preserved. Pro-inflammatory cytokines and markers of apoptosis were also decreased in TLR2 knockout mice (226). TLR4 plays a role in multiple anti-cancer drug cardiotoxicities. Its deficiency in mice treated with DOX or trastuzumab improved cardiac function and showed it was a key part of pathophysiological mechanisms in these drug-induced cardiomyopathies (254, 352).
As an activator of TLR2, heat shock protein (Hsp) 27 was overexpressed in mice receiving a onetime dose of 25 mg/kg DOX. Hsp27 overexpression protected mice from cardiac dysfunction. Changes seen in the myocyte mitochondria of DOX-treated wild type mice, such as apoptosis, morphological changes, and protein carbonylation, were not observed in mice overexpressing Hsp27. Additionally, Hsp70 and heme oxygenase-1 increased in transgenic mice after DOX treatment (178).
Together these studies show that an immune response is activated and that immune cells play a central role in the disease mechanism. However, many of these studies were performed with a single, large dose of DOX. A more clinically relevant approach might be to investigate the immune response with multiple, smaller doses. In addition to better mimicking the clinical administration, this would also allow researchers to evaluate the chronic effects of DOX. Ultimately, understanding any role these cells play will open up new avenues of intervention and/or prevention.
Cardiac fibroblasts, ECM, and MMPs
Bernaba et al. examined 10 cardiac tissue samples from cancer survivors suffering late-onset, DOX-induced cardiomyopathy. While necrosis and myocytolysis were absent in the samples, all 10 patients exhibited interstitial fibrosis. Six patients also demonstrated replacement fibrosis (25). Despite the fibrotic response of the myocardium after DOX exposure, little research has been done to characterize the effect of DOX on fibroblasts, a key cell in extracellular matrix production. One study investigating cell cycle arrest by DNA-damaging agents used a human foreskin fibroblast model. Brief treatment with DOX blocked the cell cycle at S phase for up to 15 days. However, treatment caused cell death more often than cellular senescence (255). In addition to cellular toxicity, DOX has been shown to inhibit fibroblast proliferation and migration in vitro (138).
Investigation of the changes in matrix metalloproteinases (MMPs) in response to DOX has been more extensive. Dysregulation in MMPs has long been known to be an early mechanism contributing to the development of heart failure in various cardiac injuries. Acute application of DOX in mice and pig models have demonstrated an increase in MMP-2 and MMP-9 mRNA and protein levels (103,152). mRNA levels increased in mice as early days 1 and 2, respectively, with a decrease in LV dimensions and weight. Christianson et al. also showed an increase in MMP-1 mRNA and protein in an acute cardiotoxicity model. Reactive oxygen species have been implicated in the activation of MMPs, and are known to be created by DOX-Fe interactions. The p39 MAPK, ERK/JNK, and NADPH oxidase pathways are all mediators of the MMP-2 and MMP-9 response in DOX-induced cardiotoxicity. Inhibition of NAPDH oxidase leads to an attenuation of the increase in MMP-2 (281). In addition to an increase in mRNA and protein levels, MMP activation is increased in acute DOX exposure. This increase is also seen at early time points in the heart and aorta (16). Chronic DOX induces increases in plasma and tissue levels of MMP-2 and −9 at 4 and 8 weeks, respectively (137).
The most commonly implicated MMPs of the pro-fibrotic pathways initiated after injury are MMPs 2, 7, 9, and 14. However, MMP-3 may play a role in the apoptotic effects of DOX. MMP-3 can cleave the Fas ligand from cell surfaces, effectively preventing the Fas-induced apoptotic pathway. In neuronal cells, Tissue Inhibitor of MMP (TIMP)-3 is necessary for DOX-induced cell death (342). Inhibition of TIMP-3 could be an avenue of therapeutic intervention. Interestingly MMP-3 and TIMP-3 are also seen to be differentially regulated after DOX exposure (14).
Key Points
Aside from cardiomyocytes, immune responses, endothelial dysfunction, and cardiac fibroblasts are implicated in DOX-induced cardiotoxicity. The relationship between DOX and CPCs has focused more on therapeutic capabilities, but the role of CPCs in pathology should also be further investigated.
Inhibition of TLR2, TLR4, and Hsp27 represent interesting pathways that could be modulated to mitigate the cardiotoxicity of DOX.
There is a paucity of research on endothelial dysfunction in the context of DOX exposure, but clinical observations suggest endothelial damage may be an important contributor to DOX-induced cardiovascular diseases.
Cardiac fibroblasts play an essential role in cardiac remodeling and appear to be affected by DOX.
Clinical Management
Evidence from the clinical literature unequivocally links doxorubicin exposure in childhood with an elevated risk of cardiovascular complications later in life. Table 2 summarizes the major clinical studies that provide the foundational evidence for this widely accepted conclusion. In contrast, the clinical management of DOX-induced cardiotoxicity is not straightforward. Large clinical studies in areas such as detection, prevention, and treatment are lacking and there is no definitive consensus on best practices. The following section will sort through some of the issues in each of these areas.
Table 2.
Summary of Key Clinical Trials That Have Unequivocally Linked Anthracycline (DOX) Exposure with Late Cardiac Complications
| Date | Author | Journal | Title | Key result |
|---|---|---|---|---|
| 2012 | Lipshultz SE | Pediatrics | Continuous Versus Bolus Infusion of Doxorubicin in Children with ALL: Long-term Cardiac Outcomes (173) | Continuous infusion of DOX did not offer long-term cardioprotection or event-free survival compared to bolus infusion. |
| 2010 | Ruelen RC | JAMA | Long-term cause-specific mortality among survivors of childhood cancer (253) | 11 × greater mortality in patients at 25 yr fu and 3 × at 45 yr fu; excess mortality at 25 and 45 yr fu primarily due to second primary cancers and circulatory diseases. |
| 2010 | Lipshultz SE | Lancet Oncol | Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukemia: long-term follow-up of a prospective, randomized, multicenter trial (175) | The iron chelator Dexrazoxane was shown to be cardioprotective when given simultaneously with DOX. Follow-up completed five years after drug/protectant exposures. Importantly, no impact on overall survival was observed. |
| 2007 | Mertens AC | Pediatr Blood Cancer | Cause of mortality in 5-year survivors of childhood cancer (198) | Standardized mortality rate for childhood cancer survivors was 6.9 compared to siblings. |
| 2006 | Oeffinger KC | NEJM | Chronic health conditions in adult survivors of childhood cancer (229) | Survivors had RR of 8.2 for severe or life-threatening conditions. |
| 2001 | Moller TR | J Clin Oncol | Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries (210) | Accidents and diseases of the respiratory tract, heart, CNS, including cerebrovascular disease, caused 69% of all noncancer deaths. |
| 2001 | Mertens AC | J Clin Oncol | Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study (199) | Significant excesses in mortality risk associated with treatment-related complications exist up to 25 years after the initial cancer diagnosis. |
| 1999 | Green DM | J Clin Oncol | Cancer and Cardiac Mortality Among 15-Year Survivors of Cancer Diagnosed During Childhood or Adolescence (106) | Excess cardiac mortality rates; Doxorubicin exposure was a clear risk factor for male survivors. |
| 1991 | Lipshultz SE | NEJM | Late Cardiac Effects of Doxorubicin Therapy for Acute Lymphoblastic Leukemia in Childhood (171) | DOX causes late cardiotoxicity in children exposed during cancer treatment. The only significant predictive factor was a higher cumulative dose. |
Diagnosis and monitoring
To treat any negative effects of DOX treatment, we must first be able to detect the problems. Sensitive and specific monitoring techniques are also needed to make important decisions regarding the continuation of anthracyclines in cancer patients. Currently, the common clinical practice is to take serial measurements, including baseline, of the left ventricular ejection fraction via echocardiogram. Optimally, diagnosis and monitoring will consist of a combination of imaging and biomarkers, such as troponins and brain natriuretic peptide (BNP).
Imaging
Currently, the most commonly used modality for monitoring DOX-induced cardiotoxicity is echocardiogram (echo). Most guidelines recommend monitoring the LVEF via echo at various points before, during, and after chemotherapeutic treatments. Results are used to determine the use or discontinuation of cardiotoxic drugs for treatment, as well as, if initiation of therapy for heart disease is necessary. In addition to determining the most accurate tools for measuring LVEF, bigger questions still loom about monitoring these at-risk patients. Some studies have questioned the utility of using LVEF as an index of systolic function (70, 264), and others still question if we should be monitoring diastolic function instead of systolic function, or a combination (299). There is also the added complication of the pediatric setting; not all methods are suitable for pediatric patients. Additionally, best measurements in the developing heart may differ from the adult population. The following section will give a brief description of the various imaging modalities and explore some of the advantages and disadvantages of each one.
Echocardiograms are relatively inexpensive compared to other detection techniques. They are noninvasive, readily available, and do not expose patients to x-rays, radioactivity, or contrast dyes (142). Echo can be used to visualize functional manifestations of other diseases (109, 148), in addition to measuring LVEF for cardiac dysfunction. It can be used to evaluate the larger vessels or combined with Doppler to assess regurgitation. As Jiji et al. explain in their review, this is significant because cardiac injury from chemotherapy has many outcomes and patients need to be monitored for more than just decreases in their LVEF (142). Echo, along with other modalities, can be combined with stress/exercise testing. Stress testing can uncover systolic and diastolic dysfunction that is not seen at rest (38,56,111), even in asymptomatic patients. An important consideration with echo is the variability that comes from obtaining and interpreting the images (43). Even the simplest modality, 2D echo, is not straightforward. There are multiple ways to measure and then calculate indices of function (19).
New technologies have built on the versatility of echocardiogram. In addition to using contrast to more accurately measure cardiac parameters and reduce interobserver discrepancies (126,130), 3D and 4D (real time) can also be used to improve accuracy (140). However, these modalities and the ability to interpret them are not as widely available as 2D echocardiography. More recently, the use of strain measurements via echo are being investigated as a way to monitor cardiac dysfunction. Strain has been shown to pick up changes that predict cardiotoxicity development, when no indications were observed in LVEF measurements (264). Many clinical studies have evaluated strain analysis in other diseases (17, 55, 155). While a few studies have been done showing detection of subclinical cardiac dysfunction related to cancer treatments (115,143), larger studies and more studies focused on pediatrics are still needed (193).
Related to echo is Tissue Doppler Imaging (TDI), a system that uses the shift in frequency of ultrasound signals being reflected off of moving tissue. Traditionally, Doppler is used to assess blood flow, but can be used to assess tissue instead by measuring high amplitude, low frequency signals (125). TDI can also measure strain and strain rates, possibly detecting subclinical cardiotoxicity (95). There are many limitations with tissue Doppler, such as the direction of movement detected and differentiating between active and passive motion. However, tissue Doppler is ideal for diastolic function in that preload does not influence early diastolic motion. With further testing, tissue Doppler could be an efficient, noninvasive method for monitoring cardiotoxicity via diastolic changes (146, 147).
Multigated acquisition (MUGA), also known as equilibrium radionuclide angiography (ERNA), uses a gamma camera to detect a radioactive tracer. Images of the heart can be used to create a movie and calculate the ejection fraction. In adults, MUGA measurements are highly reproducible measurements with low interobserver variability (67). As in 2D echo, MUGA could identify at risk patients (who need to discontinue treatment) before they become symptomatic (208). Similar to 2D echo, MUGA can be done at rest or with stress (exercise) and also evaluate diastolic dysfunction. Stress testing, done in any modality, is used to look for areas of the heart that are not pumping as vigorously and therefore may be receiving less oxygen or the heart muscle is already damaged. In combination with MUGA, stress testing can greatly increase the sensitivity of the test but at the cost of a lowered specificity (196). An early study that set the standard for use of MUGA showed that while reproducibility of EF measurements in healthy patients was decent using traditional echo, it was better using MUGA (320,334) and is considered, by some, the gold standard for detecting DOX-induced cardiotoxicity. Multiple studies showed changes in diastolic function via MUGA before there was a measurable drop in LVEF (157, 166, 218, 259). However, repeat MUGA in pediatric patients to obtain serial measurements may not be an appropriate way to monitor function (57, 146, 239).
In addition to being the gold standard for assessing LV function (59), cardiac magnetic resonance imaging (known as CMR) is approved for screening of DOX-induced cardiotoxicity, but due to cost and availability, is not commonly used. CMR has been evaluated for use with many cardiac diseases, including ones that are risk factors for DOX-induced cardiotoxicity. In most cases, it can pick up abnormalities from these diseases that are not detected with 2D echo (246, 333). Additionally, CMR can detect edema, which is present in most cases of acute DOX-induced cardiotoxicity. Similar to other modalities, it can also detect subclinical changes before LV dysfunction is apparent (2, 13, 353). Another advantage of CMR is that is can be used in obese patients that do not have ideal acoustic windows, a common problem in cancer survivors (343). CMR can be used with T1- and T2-weighted imaging and focal myocardial delayed enhancement (134,183,338), but needs to be further characterized in larger studies in DOX-induced cardiotoxicity.
An interesting approach to cardiotoxicity monitoring is apoptosis imaging. Labeled annexin A5 can be followed using echo, CMR, or single-photon emission computed tomography (SPECT). Studies in rats have shown a dose-dependent increase in annexin uptake via echo (21, 201). Iron stores in the heart can also be evaluated with T2-weighted CMR. A 2011 study by Dash et al., showed signal loss due to iron content that was confirmed by apoptosis markers in vivo, and indicated apoptosis in vitro earlier than a TUNEL assay (64). With any method used, physicians must be wary of the symptoms that are similar to heart failure, but actually related to the hazards of the cancer and its treatment (80).
Similar to previous and following sections in this paper, most studies do not focus on chronic cardiotoxicity or the pediatric setting. It is possible that changes in chronic cardiotoxicity may be too slow and are not detected easily with visual monitoring. Detection of the early signs of chronic cardiotoxicity may be more reliant on biomarkers in combination with visual imaging.
Biomarkers
Similar to the goals of many other research projects, clinical and basic science researchers strive to find a biomarker that can be measured in the blood or urine and predict acute and chronic DOX-induced cardiotoxicity. While important information can be learned from the various imaging techniques described above, cardiac dysfunction can only be observed after structural changes have already been initiated. The gold standard of endomyocardial biopsy can show histological changes in the myocytes before a drop in LVEF is apparent (82). A sensitive and specific biomarker would allow clinicians to predict cardiotoxicity before it has had major deleterious effects.
Early attempts at discovering a biomarker focused on atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) due to previous work associating circulating levels with cardiac dysfunction and CHF (44, 213). However, multiple papers with contradicting results have been published in the last two and a half decades. Most studies focus on BNP, as ANP had been shown to increase in one study (118) and not change in another (230). Sample populations of only 8 and 13, respectively, limit both studies. While BNP is almost universally considered a positive marker for cardiac dysfunction, there is some confusion as to whether its elevation applies to systolic and/or diastolic dysfunction (118, 291). In addition, the timing of BNP measurements in relation to chemotherapy treatment is important, as it is often transiently elevated during the acute exposure phase. Alternatively, more recent studies have investigated n-terminal pro-BNP (NT-proBNP). Similar to BNP, a positive correlation was found between increased NT-proBNP levels and indices of cardiac dysfunction (261,316). Again, there were similar discrepancies about whether NT-proBNP levels corresponded to systolic and/or diastolic dysfunction, or either (264).
Cardiac troponins T and I (cTnT and cTnI) are part of the complex necessary for cardiac muscle contraction, and thus, are a common biomarker for any injury that results in cardiomyocyte damage or death. While specific and sensitive for cardiac myocyte damage, cTns are not specific for damage mechanisms or etiology. cTns can be used to predict mortality in heart failure patients. It is no surprise then that its relevance has also been investigated in association with DOX-induced cardiotoxicity. cTnT levels in a rat model were elevated in the serum with only minimal changes in the myocyte histology, indicating that it may be a useful marker of toxicity before major pathological changes have occurred (123). While studies showed a positive correlation between Tns and decreased LVEF (45–47, 264) in adults treated with DOX, notably fewer studies examining the association in the pediatric population have been conducted (174). However, neither BNP nor troponins have been associated with chronic DOX-induced cardiotoxicity.
Other biomarkers that were investigated in the course of these studies were TNF-α, galectin-3, IL-6, GDF-15, MPO, PIGF, and sFlt-1 (158, 248, 316). MPO (myeloperoxidase) is the only one with evidence that it may be predictive of DOX-induced cardiotoxicity. A new approach has been to look at gene changes rather than protein levels. In 2012, Todorova et al. used a rat model to compare peripheral blood mononuclear cells (PBMC) and cardiac cells. They found similar expression changes in multiple pathways after DOX treatment, specifically oxidative stress and protein ubiquitination (304). The PBMCs could possibly be used as a surrogate for the condition of the heart and be obtained with a simple blood draw. Similar work in human embryonic stem cells revealed transcriptional profiles unique to cells treated with DOX. Importantly, these profile changes were sustained through the recovery period of the cells, unlike increases in cTnT (127). While serum levels of BNP and troponin may indicate acute toxicity, genetic and transcriptional changes may prove to be significant chronic biomarkers.
Prevention
Optimally, clinicians will be preventing DOX-induced cardiotoxicity rather than treating the resulting heart failure. In addition to the cardioprotectants already utilized, to be discussed later on, there are many experimental prophylactics being tested in laboratories and clinics. Another avenue of research is the effect of exercise before, during, and possibly after, DOX treatment.
Exercise
The association between exercise and cardiovascular health has long been known, even though the mechanisms are still being elucidated. Studies have shown, that not only is exercise preventative of cardiovascular diseases (24), but also protective in individuals that suffer a cardiovascular event (277). While short-term and long-term exercise regimens have both been proven to be beneficial, there is a dose-dependent (in kcal/week) increase in cardioprotection from exercise (234). Most likely, the benefits of exercise on the heart are multi-fold, including reduced blood pressure (241), weight loss and improved lipid profile (309), and increased protection against reactive oxygen species. While exercise plays an important role in endothelial vascular function, it is the effects of exercise on oxidative stress that has garnered the most attention. For this reason, many studies have investigated the effects of exercise on DOX cardiotoxicity.
One study showed that preconditioning (involuntary treadmill and voluntary wheel running) in male Sprague-Dawley rats attenuated the decline in contractile function seen in the sedentary DOX-treated animals. β/α-MHC ratio, which is increased during times of oxidative stress and correlated to cardiac function (154), were also measured in these rats. As expected, DOX treatment increased the β/α-MHC ratio and was mirrored in the cardiac dysfunction of these animals. Along with improved cardiac function, the preconditioned animals also exhibited a preservation of the β/α-MHC ratio. An interesting mechanism for the attenuation of cardiotoxicity from DOX-induced cardiotoxicity was pursued in a paper by Jensen et al. (141). The study showed that preconditioning (treadmill and wheel running) decreased the DOX accumulation in cardiac tissue. Jensen et al. reported that after 5 days DOX was no longer detectable in preconditioned rats, whereas it was still present at day 7 in sedentary treated animals. The group theorized that the difference may be due to the increased extrusion of DOX from cardiomyocytes via an increase in the ATP-binding cassette transporters. The group also concluded that more work would need to be done to consider other possibilities, such as altered drug metabolism or decreased influx of the drug in preconditioned animals. Preconditioning has also been shown to protect vascular dysfunction after DOX treatment. Interestingly, the protection was bestowed on the smooth muscle cells of the aorta, and not the endothelial cells (97). This was evaluated by measuring the endothelial-dependent and -independent vasorelaxation responses. Exercise may be acting in multiple ways to confer vascular protection. Exercise can boost NO levels, eNOS mRNA and protein, and the antioxidants superoxide dismutase and glutathione peroxidase (99,135,295,337). Numerous studies have begun to show the protective effect of exercise preconditioning against DOX.
Another avenue of research has explored the effect of exercise during DOX treatment. It was shown that even in voluntary wheel running, which was significantly less in some models under DOX treatment, the activity still provided a protective effect. Ex vivo parameters of cardiac function were preserved, along with β/α-myosin heavy chain ratios (131, 132). A similar study concluded with similar findings in addition to showing a role for increased antioxidants and decreased apoptotic pathways (51). Specifically, there was a decrease in caspase-3 activity and an increase in glutathione peroxidase, respectively, in the low intensity exercise animals treated with DOX. One study compared the results of modest exercise training during treatment with resveratrol-supplemented chow diet and found that the resveratrol improved cardiac function beyond the exercise regimen (71). The study linked the improvement in cardiac function to improved calcium trafficking and mitochondrial function. Resveratrol attenuated the decrease in SERCA2a and the increase in mitofuscin-1 and −2, respectively. Reseveratrol was shown to attenuate the drop in blood pressure and heart rate seen in rats treated with DOX. Additionally, it abrogated the increase in plasma lactate dehydrogenase, creatine phosphokinase, and the decrease in the total plasma antioxidant capacity (300). More recently, reduced DOX accumulation in myocardial tissue in the context of exercise was also shown by Wang and colleagues, but importantly in a relevant pediatric mouse model (336). One final consideration in the use of exercise as protection against cardiotoxicity is the extent to which cancer patients, especially pediatric, and patients undergoing chemotherapy, can adhere to a sustained exercise regimen. This is where resveratrol may play an important role as a cardioprotectant.
Experimental prophylactics
Aside from dexrazoxane, there are many promising potential prophylactics against chronic DOX-induced cardiomyopathy. These require additional investigation in the clinical setting to be truly applicable to patient care. However, they may help elucidate the potential pathophysiological processes that underlie the disease.
Carvedilol:
Carvedilol is a β-blocker with affinities for the β1, β2, and α1 adrenergic receptor subtypes. Blockade of these receptors may lead to improved outcomes and symptoms in the management of myocardial infarction, hypertension, and congestive heart failure. The α1 adrenergic receptor blockade allows it to function as an arterial vasodilator. Further, it has potent antioxidant properties that are unrelated to its role as an adrenergic receptor antagonist. As an antioxidant, it has the ability to reduce lipid peroxidation in myocardial cell membranes, inhibit neutrophil oxygen release, and preserve endogenous antioxidant systems (vitamin E and glutathione) (75). The antioxidant effects of carvedilol itself are approximately 10 times greater than vitamin E, whereas the metabolites of carvedilol may be 50 to 100 times greater than carvedilol (50). It is thought that these combined effects allow carvedilol to potentially be effective in ameliorating DOX-induced cardiotoxicity (222).
In various animal studies, carvedilol has been shown to abrogate the effects of acute DOX-induced toxicity (192, 231, 262, 282). This effect may be uniquely due to the antioxidant activity of carvedilol, as other drugs within the same class are not able to produce this effect (192). However, it is important to note here that antioxidants alone are not sufficient to abrogate the acute effects of DOX, as demonstrated by the failure of vitamin E, n-acetylcysteine, and other antioxidants in affecting the acute toxicity of DOX (170). There have been no animal studies investigating the effects of carvedilol in chronic DOX-induced toxicity. However, there are ongoing clinical investigations evaluating carvedilol’s efficacy in preventing acute DOX-induced toxicity in the pediatric population.
The effects of carvedilol in preventing DOX-induced cardiomyopathy in patients ages 6 to 12 with ALL and normal left ventricular systolic function were examined in a randomized control trial (79). Patients receiving the chemotherapy regimen, including DOX, and the addition of carvedilol were monitored for up to 29 days after remission reduction. Carvedilol was found to be beneficial in ameliorating the effects of DOX. Other clinical trials examining the effects of carvedilol in older populations were also performed and found similar beneficial effects on a longer timeline (144). Similarly, nebivolol, another β-blocker with antioxidant and vasodilatory properties, was found to be effective in ameliorating DOX-induced cardiotoxicity (149).
ACE-Inhibitor and Angiotensin Receptor Blockers:
Angiotensin II (Ang II) is a key effector of the reninangiotensin system (RAS), which has a myriad of effects on the cardiovascular system. These effects may alter hemodynamics to ameliorate congestive heart failure (CHF) and hypertension (180). In the context of prophylaxis for chronic DOX-induced cardiotoxicity, Ang II plays a role in mediating cardiac remodeling and fibrosis (340). Fibroblasts are converted to myofibroblasts (myoFb), a transformed fibroblast-like cell, which expresses α-SMA and angiotensin converting enzyme (ACE) and is contractile. The myoFb plays an integral role in generating Ang II that can act as a paracrine and autocrine messenger. These processes underlie the mal-adaptive process of cardiac remodeling in various disease processes, including myocardial infarction and hypertrophic cardiomyopathy. It is for this reason that angiotensin receptor 1 (AT1) and ACE blockade by angiotensin receptor blockers (ARBs) or angiotensin converting enzyme-inhibitors (ACE-I) are suspected to be beneficial as prophylaxis in chronic DOX-induced cardiomyopathy.
As RAS has been an attractive target for drugs in the management of various disease states associated with fibrosis and cardiac remodeling, basic research has explored the potential utility in the management of acute DOX-induced cardiotoxicity. In these studies, various ACE-Is, used as prophylaxis, have been found to be effective in reducing acute toxicity (37, 257, 305). It has also been found that these drugs do not alter the anti-tumor activity of DOX in rats with xenografted human ovarian carcinoma cell line A2780 (257). Further, mice that have a knockout of the AT1 receptor benefit from a similar reduction of cardiotoxicity, suggesting that Ang II signaling plays a pivotal role in the development of acute cardiotoxicity (305). Like many potential prophylactic agents, there has been little to no investigation on the effect of ACE-I and ARB on the chronic cardiotoxicity of DOX in animal models.
The combined effects of ACE-I and carvedilol on acute DOX-induced toxicity were examined in a randomized, controlled clinical trial (35). The reason a combination was chosen over testing the individual effects of each drug was due to the additive beneficial effects in patients with left ventricular systolic dysfunction (LVSD). The combined treatment was found to be able to ameliorate LVSD in a 6-month period. No studies examining a longer timeline, where the effects of chronic DOX-induced cardiotoxicity may be examined, were performed on this combination. In addition, no studies examined the efficacy of this treatment combination in the pediatric population.
Coenzyme Q10:
CoQ is an endogenous lipid synthesized by all aerobic organisms. In humans, cells generally rely on CoQ10 synthesized de novo. The mechanisms that regulate CoQ synthesis are poorly understood, but some evidence suggests the involvement of certain nuclear receptors in mice: retinoid X receptor alpha (RXRα) and liver X receptor alpha (LXR-α) (22). CoQ10 is most abundant in the heart and its deficiency has been associated with an assortment of disease states, including heart failure. In the mitochondria, CoQ is essential for ATP generation. By functioning as an electron carrier between complexes I and II/III, it is able to transfer protons across the inner mitochondrial membrane. It does this by cycling between oxidized and reduced forms. CoQ has been implicated in modulating the mitochondrial permeability transition pore (237). CoQ10 has also been found to play a role in regulating the cellular redox state. This is done through two mechanisms: first, a CoQ-dependent NADH oxidase that utilizes cytosolic NADH as an electron source (58), regulating the cytosolic ratio of NAD +/NADH and, second, the ubisemiquinone radical (QH*) can generate superoxide anion radicals with molecular oxygen, leading to H2O2 formation. In addition to these mitochondrial and redox effects, the reduced form of CoQ10, ubiquinol (CoQH2), is a potent antioxidant (2).
The mitochondria of cardiomyocytes have this CoQ-dependent NADH oxidase which utilizes cytosolic NADH as an electron source (224, 252). DOX is able to react with this NADH oxidase, which reduces it into the semiquinone form of DOX (65, 73, 101, 225). Further, these semiquinones can then undergo autooxidation that ultimately leads to the formation of DOX agylcones (101, 150). These aglycones displace CoQ10, as evidenced by an acute increase in plasma CoQ10 in patients treated with DOX (78). Following this, the DOX aglycones are then able to serve as electron acceptors from complexes I and II. However, they do not preserve the function of CoQ and do not donate electrons to complex III. Instead, they transfer electrons to molecular oxygen, resulting in the formation of superoxide radicals (101). This increases the oxidative stress of the cell, leading to damage of DNA and other cellular components. Further, DOX is capable of inhibiting mitochondrial biosynthesis of CoQ10 (85). Therefore, a CoQ10 deficiency may exist in the myocardium and increasing plasma levels of CoQ10 before or during chemotherapy may be beneficial in preventing cardiotoxicity; supplemental CoQ10 given prophylactically during exposure to DOX may ameliorate cardiotoxicity.
Basic science research aimed at investigating the efficacy of CoQ10 in preventing the changes induced by DOX has been performed in a variety of models. CoQ10 supplementation has been found to reverse electrocardiographic abnormalities that result from acute DOX exposure in rabbits (84). DOX-induced mitochondrial DNA deletions were found less in mice that were given prophylactic CoQ10 treatment (4). Other studies have investigated the effect CoQ10 has on the cytotoxicity of DOX in tumor cells and suggested that there is little, if any, effect (107, 276). All this evidence suggests that CoQ10 and related analogs may have benefit in the prevention of chronic DOX-induced cardiomyopathy.
A clinical trial was performed to investigate the protective effect of CoQ10 on the acute effects of DOX exposure in the pediatric population (133). The study found CoQ10 to be protective against decreases in cardiac function associated with DOX exposure. This study included a total population of 20 patients, with 10 in the CoQ10 therapy group and 10 in the standard chemotherapy group. No patients developed heart failure in either group.
Limitations of Clinical Trials:
Currently, all clinical trials were performed to investigate current drugs that are used in the management of congestive heart failure or myocardial infarction. In addition, there have been no longitudinal trials to investigate the long-term (10- to 30-year period) development of cardiomyopathy after DOX exposure in the pediatric population. Even if these potential prophylactic agents show promise in preventing acute phase cardiotoxicity, there is no guarantee that the late phase of cardiotoxicity that childhood cancer survivors experience can be rectified. Further, all current clinical trials performed on DOX prophylaxis, barring studies on dexrazoxane, do not have large enough populations to obtain data regarding risk reduction or guide clinical management of these patients. A further analysis of clinical trials can be obtained in the Cochrane review (279).
Treatment
Currently, the majority of research on DOX focuses on understanding the pathological mechanisms of DOX-induced cardiotoxicity to prevent acute and chronic sequelae. However, DOX has been used in pediatric cancers for over four decades and is currently used in close to 50% of all pediatric cancers diagnosed in the United States (89, 317). In short, this means that there is and will be a large population of childhood cancer survivors and at least up to 25% will develop CHF 30 years posttreatment (217, 318). It is important that we focus not only on preventing cardiotoxicity, but also on treating those already suffering from DOX-induced cardiotoxicity.
Comprehensive care for childhood cancer survivors is complicated by a lack of global guidelines defining monitoring for late effects, including cardiac dysfunction. Cardiac care is further complicated by lack of a specific clinical manifestations of DOX-induced cardiotoxicity. This section will address treatment issues related to chronic DOX-induced cardiotoxicity resulting in CHF. Similar to heart failure of other etiologies, DOX-induced CHF (DOX-CHF), is categorized into classes and stages by the New York Heart Association (NYHA) and the American College of Cardiology/American Heart Association, respectively. The 1- and 2-year mortality rates for patients categorized as class II-IV are 40% and 60%, respectively (1). Depending on the patient’s risk factors and comorbidities, ACE-I and ARBs should be prescribed. Additionally, β-blockers, loop diuretics, and statins may also be prescribed (350). Most importantly, patients should be seen regularly by a cardiologist familiar with complications of DOX treatment. As always, DOX-CHF is further complicated by studies that have suggested it is refractory to standard HF treatment (39, 100, 167).
In addition to recognizing the risk factors and related comorbidities to heart failure, physicians following the care of these patients must also be cognizant of the risk factors related to DOX-induced cardiotoxicity. Most obviously, patients with previous or concomitant heart disease, such as coronary artery disease, myocardial infarction, and hypertension, are at an increased risk. Extremes of age at treatment (90,319), female sex (172, 355), mediastinal radiation (197, 205, 247), and the cumulative dose are all risk factors for the development of DOX-induced cardiotoxicity (114). Certain chemotherapeutic drugs used in conjunction with DOX also increase a patient’s risk. Cyclophosphamide (167, 205), paclitaxel (72, 96), and trastuzumab (53, 256) have all been found to increase risk.
Ideally, long-term survivorship would be handled by a multidisciplinary team familiar with the risks associated with these patients. Currently, few comprehensive care management programs for these patients exist. Not only are they beneficial to the patient, but they are also cost-effective (42). Guidelines would dictate the necessary monitoring to identify late-term sequelae at the earliest time point possible. One study found that chemotherapy-exposed patients with an asymptomatic drop in LVEF were not routinely prescribed medications such as ACE-I, ARB, β-Blocker, or even referred to a cardiologist (351). A problem unique to the pediatric setting in long-term care is “aging out” of the system. Many patients are lost to follow up either when they are no longer seen by their pediatric physicians or no longer covered by their guardian’s insurance. Without a comprehensive, long-term structure in place for adequate monitoring and care, many patients will be left dealing with late-effect morbidities on their own.
Key Points
Earlier detection techniques are needed and will probably consist of a combination of imaging and biomarkers.
Promising prophylactics need larger clinical trials to determine efficacy and preclinical testing to understand the mechanism of action.
Treatment strategies currently consist of the standard care used in heart failure patients and management of comorbidities.
Clinical Cardioprotectant Strategies
After lung cancer, heart failure has the worst 5-year adjusted mortality (288). In 1993, the Framingham study found the 1-year mortality rate to be 20% to 30% and the 5-year mortality rate to be 45% to 60% (169). A more recent study published in 2003, found comparable rates of mortality at one year (112). While sudden death in CHF patients has declined with improved treatment regimens, the 1- and 5-year mortality ratios have remained mostly stable (179). These data suffice to point out that congestive heart failure is a disease with significant mortality. Congestive heart failure is one outcome of DOX-induced toxicity. In the general population, risk for CHF increases with age, and therefore the majority of the patients are diagnosed after the age of 50. However, pediatric patients treated with DOX can develop CHF in their 20s, 30s, and 40s. Considering the mortality, it is important to not only identify and treat patients in the early stages of cardiac dysfunction, but just as important to prevent the initiating injury in future patients.
Preventative measures currently used in the clinic are dexrazoxane and liposomal DOX formulations. There is no published data on the protective efficacy of dexrazoxane or liposomal DOX in late-onset DOX cardiotoxicity in the adult or pediatric populations. Another debated topic that needs clarification is the method of DOX administration. Is there an optimal dosing regimen? Can dosing regimen alone prevent chronic DOX-induced cardiotoxicity? The sections below will address the current knowledge in this area and additional concerns that need more investigation.
Dexrazoxane
Dexrazoxane and razoxane, stereoisomers, were discovered in 1972 by the clinical pharmacologist Kurt Hellman. While both were investigated as anti-cancer agents, with some success (332), dexrazoxane is more commonly used for its cardioprotective properties. Dexrazoxane’s myelosuppression proved to be problematic at the higher doses needed to combat tumor cells, this is not so for the lower doses utilized in cardioprotection (61). The American Society of Oncological Chemotherapy and Radiotherapy expert panel has made minimal updates to their guidelines to the use of dexrazoxane. Currently, they recommend its use only after the cumulative dose of DOX has exceeded 300 mg/m2 and further treatment with DOX is thought to be beneficial (120). The conclusion was also reached that there was insufficient evidence to recommend use in the pediatric setting. The limited use of dexrazoxane is recommended because there is no conclusive study on whether or not dexrazoxane effects the efficacy of DOX.
The mechanism of action for dexrazoxane is thought to be through its capabilities as an iron-chelator. By binding Fe3+, dexrazoxane can prevent the reduction of DOX in addition to inhibiting lipid peroxidation and ATPase inactivation (326). The active form of dexrazoxane is produced intracellularly after going through multiple intermediates and hydrolysis until both rings are open (190,273). The active form is biologically available almost immediately (270, 271). More recent work has shown that dexrazoxane can also inhibit topoisomerase II (117, 190). An analogue of dexrazoxane that could chelate iron, but not inhibit topoisomerase II, did not protect against chronic DOX in rats (190). Additional studies demonstrate that dexrazoxane attenuates depletion of mtDNA and the increase in mtDNA lesions, and decreases in the mtDNA-encoded COX II subunit (161). Even more compelling, was a study by Lyu et al. that evaluated the role of topoisomerase II in DOX-induced cardiotoxicity and dexrazoxane pretreatment. Dexrazoxane attenuated the DOX-induced γ-H2AX signal and interfered with the topoisomerase II cleavage complex formation, in addition to inducing the rapid degradation of topoisomerase II (182). Abolishment of the damage caused by DOX was also seen in TopoII(−/−) knockout mice, defining a role for topoisomerase II in DOX-induced cardiotoxicity and dexrazoxane cardioprotection.
Most preclinical studies of dexrazoxane have measured cardiotoxicity in the acute setting by evaluating the histopathology of samples. In most cases, the addition of dexrazoxane to treatment results in either minimal, less severe lesions or no lesions at all, whereas DOX alone animals had multiple cardiac lesions (121, 122, 124). Most clinical trials used advanced or metastatic breast cancer patients. While we are still lacking in long-term studies, the use of dexrazoxane as a cardioprotectant has been established by multiple studies (285, 286, 292). In addition to evaluating cardiac function by LVEF changes and biopsy, many of these studies were able to show that patients could tolerate a higher cumulative dose of DOX and greater number of cycles of treatment when pretreated with dexrazoxane. Early studies also revealed an increased myelosuppression resulting in severe leukopenia and thrombocytopenia when dexrazoxane and DOX were used in combination (285, 329). A phase I study compared a 96-h dexrazoxane infusion to the standard administration method. While similar plasma levels were reached, it still has to be seen if equal cardioprotection is granted (302).
Given the high incidence of abnormal findings in pediatrics patients’ echocardiograms after treatment with DOX (172), Trachtenberg et al. performed a review of all studies examining pediatric clinical trials involving dexrazoxane. However, their main finding was that more studies are needed (308). Dexrazoxane appeared promising in studies with shorter follow up periods (174), but in longer follow up periods no difference was observed in event-free survival between ALL patients treated with and without dexrazoxane (175). There are also other issues to be pursued to reach a conclusive answer, such as whether or not dexrazoxane interferes with DOX’s efficacy or if its use increases the chance for secondary malignant neoplasms. While many studies report similar rates of progression-free and overall survival (238, 325), some studies have reported a decrease in the response rate of certain cancers (293). The question of secondary neoplasms is a little more settled. Only one study has shown an increase in risk with dexrazoxane (301), while three more recent studies showed no association between secondary malignancies and dexrazoxane (18, 260, 331)
Other iron-chelating molecules have been investigated for use as a cardioprotectant. Deferiprone, the only orally active iron chelator currently available, inhibited acute cardiotoxicity measured via EKG changes (9), but did not alleviate chronic DOX-induced cardiotoxicity in a rabbit model (245). Pyridoxol 2-chlorobenzoyl hydrazine, another iron chelator, showed no effects on cardiotoxicity in vitro or in vivo (287). The lack of effectiveness of these iron chelators lends credence to the theory that it is dexrazoxane’s activity as a topoisomerase II inhibitor that protects the heart from DOX-induced toxicity.
Liposomal doxorubicin
Liposomal DOX has been approved by the Food & Drug Administration (FDA) for use in ovarian cancers, AIDS-related Kaposi sarcoma, and multiple myeloma. However, use of all three is limited to times when at least one other treatment type has failed (5). Recent and ongoing clinical trials are evaluating its efficacy, tolerability, and toxicity in various cancers, including cancers of the blood. Liposomal DOX decreases cardiotoxicity by altering the tissue distribution of the drug (323). The liposome cannot leave the blood vessels as easily in the heart and other organs with tight junctions. As Gabizon stated, tumor vessels are a more prone to “leak” due to “large inter-endothelial fenestrations, discontinuous basement membranes, and a high rate of trans-endothelial transport” (91). Due to fenestrated vessels, there is also more accumulation of liposomal DOX in the liver and spleen, as well as tumors (339). Concentration in tumors can also be increased by lymphatic “dead ends” that have no functional drainage (76, 185). Huang et al. showed an increase in tumor interstitial fluid when liposomal DOX was administered to mice, compared to DOX (129). However, the release of DOX from the liposome is not as well understood (91). The increased concentration in the liver may prove problematic. A study investigating liposomal DOX in mice found a transient reduction in macrophage numbers and activity (62).
Shortly after the use of liposomal drug delivery for chemotherapeutics was realized (108), the benefits were found to be short lived. Liposomes are targeted by the phagocytes of the reticuloendothelial system for swift removal, leading to a decreased bioavailability (306, 341). The solution to this setback was to coat the liposomes to camouflage it from the immune system. Methoxy polyethylene glycol (PEG) covalently bound to the surface of the liposome decreased the antigenicity and increased the half-life (8, 31, 153, 274).
Some clinical studies reported an increase in the incidence of palmar-plantar erthrodysesthesia (PPE), with one mentioning desquamation of the hands and feet (250). However, a meta-analysis including 2220 patients did not find a significant difference in occurrences of PPE in liposomal and conventional DOX-treated patients (251). Most studies found decreases in myelosuppression, alopecia, neutropenia, thrombocytopenia, and nausea, in addition to a decrease in acute cardiotoxicity (188, 227, 310). Cardiotoxicity was evaluated by various methods in each study, including biopsy, left ventricular ejection fraction, and troponin levels. A greater cumulative dose than conventional DOX could be reached in most studies, but the maximal peak dose that was tolerated was highly variable for the liposomal DOX (339). A study using endomyocardial biopsy only labeled one sample as grade 1 (cumulative dose of 750 mg/m2), while multiple samples from patients receiving over 500 mg/m2 of liposomal DOX received a grade 0 in pathologic scoring (258). Overall, the meta-analysis mentioned above, reviewed an improved outcome in the incidence of cardiotoxicity without impeding tumor efficacy (251).
However, the results of the meta-analysis do not address the time of follow-up in these patients. Many studies were done with a small number of patients, and few in pediatric patients. Despite the amount of data, more are still needed to address the benefit of liposomal DOX in pediatric cancer survivors and in relation to its protection against chronic DOX-induced cardiotoxicity (7).
Bolus versus continuous infusion
Clinicians have been searching for the best method of DOX administration since it was revealed that DOX could cause cardiotoxicity. The effect of cumulative dose on the incidence of acute and chronic cardiotoxicity and the need for a maximal dose was established in the 1970s (167, 206, 330). It was ascertained early on that the antitumor effects and cardiotoxic effects of the drug were separate, one dependent on cumulative dose and the other on peak plasma (Cmax), respectively (219, 283). After suggesting a maximal cumulative dose, research began to define an appropriate peak plasma level. One of the first preclinical studies compared drug delivery, survival, and toxicity in mice treated with either one 15 mg/kg injection or four 3.75 mg/kg injections over four days (233). While there was an equal amount of the drug in the tumor and spleen with each treatment, the heart actually showed decreased levels of drug with the multiple injections. The multiple injections also resulted in increased survival and decreased toxicity.
In patients, it was found that a divided dose was better received than a large bolus and that the longer the infusion rate the greater the decrease in cardiotoxicity (168, 315). An early study by Muggia et al. did not show a cardioprotective effect when comparing a single bolus injection to a 6-h infusion. However, the study was small and the infusion time may not have been long enough (284). Concurrent and later studies used 24-, 48-, and 96-h infusions. An increase in infusion time was associated with acute toxicity more so than the total dose (168). None of these studies showed a decrease in tumor response with variable infusion times. Drug infusions are not without their disadvantages. Catheters are prone to infection and an indwelling catheter must be used if the infusion is greater than 96 h. Depending on the length of the infusion, a longer duration could also mean hospitalization. Many studies have found that the incidence of stomatitis increased with increasing infusion duration.
Most studies described were either in adult breast cancer or sarcoma patients. The minimal clinical trials performed in the pediatric population have not reached a consensus on dosage regimen. Using simulation and a leukemic cell line, Ishisaka et al. showed that the anti-tumor effect of DOX was actually dependent on Cmax and not the cumulative dose. However, they also showed that the cytotoxicity was less influenced by Cmax than the cardiotoxicity and that there was still a clinical advantage to reducing the Cmax during treatment administration (136). One retrospective study reported that treatment in pediatric patients resulted in decreased cardiotoxicity and mortality when DOX was administered over 72 h every 3 weeks as opposed to over 24 h every 3 weeks (27). A more recent study found that there was no difference in the incidence of cardiotoxicity between a bolus injection given every 3 weeks versus a 48-h infusion given every 3 weeks (173). The main difference could be the follow-up time between the studies. The first study by Berrak had a mean follow-up time of 2.5 years while the second study by Lipshultz et al. had a mean follow-up time of 8 years. Possibly, the continuous infusion can slow down the onset of cardiac dysfunction but not prevent it completely. If there is a difference in the recommended dosage regimen from adults to children, understanding the why could illuminate more of the mechanism behind DOX cardiotoxicity.
Key Points
The common therapeutics used to address pathological cardiac remodeling in hypertension may be useful in the treatment of cardiac remodeling related to DOX.
However, no beneficial strategies have been uncovered in specifically addressing the pathological cardiac remodeling found in DOX.
The clinical trials performed on all agents that have been considered for cardioprotectant therapy have not followed up patients long enough to demonstrate efficacy in preventing the late onset cardiomyopathy.
Conclusion
Doxorubicin-induced cardiotoxicity is a growing problem for the increasing population of pediatric cancer survivors. With increased survival, the problem has shifted from an acute condition to a chronic one. The consequences of a potentially debilitating or lethal cardiac morbidity urge an increased awareness and scholarship in this area. Key areas that need to be addressed include diagnostic methods, an increased understanding of disease mechanisms and the role of all cardiac cells, preventive measures, and treatment of patients already afflicted.
Due to the separate clinical presentations of pediatric cancer survivors and adult cancer survivors, it is important to individually address each of these areas for each population. With substantial clinical and preclinical studies, prescreening for cancer patients to determine risk of DOX-induced cardiotoxicity may be possible. Additionally, research investigating the effect of DOX on different cell types will enhance efforts to create a preventive pharmacologic. Information learned in preclinical studies has impact beyond DOX-induced cardiotoxicity. Understanding could apply to other cardiotoxic drugs, newly developed anticancer agents currently in the drug development pipeline, and lead to improved screening methods for developing drugs. A true multidisciplinary approach is required to attain the knowledge needed to address prevention, diagnosis, and treatment.
Didactic Synopsis.
Major teaching points
- Anthracyclines have been unequivocally linked to cardiovascular disease later in life.
- Acute cardiotoxicity, which occurs during the first year of treatment, has a low incidence in the pediatric population due to dosing regimens that limit total cumulative dose delivered and infrequent pre-existing cardiovascular disease in children.
- Chronic cardiotoxicity can take decades to develop and the pathological mechanisms are not fully understood.
- Doxorubicin has multiple mechanisms of action and the effect depends on the cell type.
- Superoxide production and disruption of the electron transport chain leads to mitochondrial dysfunction.
- Doxorubicin intercalates DNA and inhibits topoisomerase II leading to DNA damage and the DNA damage response.
Early detection of cardiac damage that indicates initiation of a progressive process leading to chronic cardiotoxicity is needed. Ejection fraction determined by echocardiography is not sensitive enough and a molecular or more sensitive imaging biomarker is urgently needed.
Acknowledgements
We would like to thank members of the Aune Lab for their participation in the preparation of this review and for their helpful comments. Members participating include Thomas Andrews and Logan Davis.
References
- 1.SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 325: 293–302, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 109: 2411–2416, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Abdella BR, F J. A chemical perspective on the anthracycline antitumor antibiotics. Environ Health Perspect 64: 4, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi K, Fujiura Y, Mayumi F, Nozuhara A, Sugiu Y, Sakanashi T, Hidaka T, Toshima H. A deletion of mitochondrial DNA in murine doxorubicin-induced cardiotoxicity. Biochem Biophys Res Commun 195: 945–951, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Administration USFaF. Doxorubicin Hydrochloride Liposome Injection Availability [July 24, 2015, 2015].
- 6.Agbulut O, Menot M-L, Li Z, Marotte F, Paulin D, Hagège AA, Chomienne C, Samuel J-L, Menasché P. Temporal patterns of bone marrow cell differentiation following transplantation in doxorubicin-induced cardiomyopathy. Cardiovasc Res 58: 451–459, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Alberts DS, Muggia FM, Carmichael J, Winer EP, Jahanzeb M, Venook AP, Skubitz KM, Rivera E, Sparano JA, DiBella NJ, Stewart SJ, Kavanagh JJ, Gabizon AA. Efficacy and safety of liposomal anthracyclines in phase I/II clinical trials. Semin Oncol 31: 53–90, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta 1066: 29–36, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Ammar el SM, Said SA, Suddek GM, El-Damarawy SL. Amelioration of doxorubicin-induced cardiotoxicity by deferiprone in rats. Can J Physiol Pharmacol 89: 269–276, 2011. [DOI] [PubMed] [Google Scholar]
- 10.An T, Zhang Y, Huang Y, Zhang R, Yin S, Guo X, Wang Y, Zou C, Wei B, Lv R, Zhou Q, Zhang J. Neuregulin-1 protects against doxorubicin-induced apoptosis in cardiomyocytes through an Akt-dependent pathway. Physiol Res 62: 379–385, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Armenian SH, Gelehrter SK, Chow EJ. Strategies to prevent anthracycline-related Congestive heart failure in survivors of childhood cancer. Cardiol Res Pract 2012: 713294, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, Sklar CA, Robison LL, Oeffinger KC. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol 32: 1218–1227, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 28: 1242–1249, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Aupperle H, Garbade J, Schubert A, Barten M, Dhein S, Schoon HA, Mohr FW. Effects of autologous stem cells on immunohistochemical patterns and gene expression of metalloproteinases and their tissue inhibitors in doxorubicin cardiomyopathy in a rabbit model. Vet Pathol 44: 494–503, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Azarova AM, Lyu YL, Lin C-P, Tsai Y-C, Lau JY-N, Wang JC, Liu LF. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc Natl Acad Sci 104: 11014–11019, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai P, Mabley JG, Liaudet L, Virag L, Szabo C, Pacher P. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol Rep 11: 505–508, 2004. [PubMed] [Google Scholar]
- 17.Bansal M, Jeffriess L, Leano R, Mundy J, Marwick TH. Assessment of myocardial viability at dobutamine echocardiography by deformation analysis using tissue velocity and speckle-tracking. JACC Cardiovasc Imaging 3: 121–131, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, Clavell LA, Larsen EC, Moghrabi A, Samson Y, Schorin MA, Cohen HJ, Lipshultz SE, Sallan SE, Silverman LB. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol 26: 1106–1111, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 21: 1387–1396, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Belmonte F, Das S, Sysa-Shah P, Sivakumaran V, Stanley B, Guo X, Paolocci N, Aon MA, Nagane M, Kuppusamy P, Steenbergen C, Gabrielson KL. ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity. Am J Physiol Heart Circ Physiol 309: H1271–H1280, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennink RJ, van den Hoff MJ, van Hemert FJ, de Bruin KM, Spijkerboer AL, Vanderheyden JL, Steinmetz N, van Eck-Smit BL. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med 45: 842–848, 2004. [PubMed] [Google Scholar]
- 22.Bentinger M, Tekle M, Dallner G, Brismar K, Gustafsson JA, Steffensen KR, Catrina SB. Influence of liver-X-receptor on tissue cholesterol, coenzyme Q and dolichol content. Mol Membr Biol 29: 299–308, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Bergman O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary artery disease. Am J Epidemiol 132: 612–628, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol 19: 308–311, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Bernard J. Actualites hematologiques 1967. [Google Scholar]
- 27.Berrak SG, Ewer MS, Jaffe N, Pearson P, Ried H, Zietz HA, Benjamin RS. Doxorubicin cardiotoxicity in children: Reduced incidence of cardiac dysfunction associated with continuous-infusion schedules. Oncol Rep 8: 611–614, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Berthiaume JM, Oliveira PJ, Fariss MW, Wallace KB. Dietary vitamin E decreases doxorubicin-induced oxidative stress without preventing mitochondrial dysfunction. Cardiovasc Toxicol 5: 257–267, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Biala AK, Dhingra R, Kirshenbaum L. Mitochondrial dynamics: Orchestrating the journey to advanced age. J Mol Cell Cardiol 83: 37–43, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Bjelogrlic SK, Radic J, Jovic V, Radulovic S. Activity of d,l-alpha-tocopherol (vitamin E) against cardiotoxicity induced by doxorubicin and doxorubicin with cyclophosphamide in mice. Basic Clin Pharmacol Toxicol 97: 311–319, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Blume G, Cevc G. Liposomes for the sustained drug release in vivo. Biochim Biophys Acta 1029: 91–97, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Bodley A, Liu LF, Israel M, Seshadri R, Koseki Y, Giuliani FC, Kirschenbaum S, Silber R, Potmesil M. DNA topoisomerase II-mediated interaction of doxorubicin and daunorubicin congeners with DNA. Cancer Res 49: 5969–5978, 1989. [PubMed] [Google Scholar]
- 33.Bonadonna G, Monfardini S, Guindani A. [Therapy with daunomycin in chronic lymphoproliferative diseases]. Tumori 54: 465–481, 1968. [DOI] [PubMed] [Google Scholar]
- 34.Bonadonna G, Monfardini S, Marmont AM, Damasio E, Rossi F. Cardiac toxicity of daunorubicin. Lancet 1: 837, 1969. [PubMed] [Google Scholar]
- 35.Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, Morales-Ruiz M, Perea RJ, Monzo M, Esteve J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (prevention of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 61: 2355–2362, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Bottini A, Berruti A, Bersiga A, Brizzi MP, Brunelli A, Gorzegno G, DiMarco B, Aguggini S, Bolsi G, Cirillo F, Filippini L, Betri E, Bertoli G, Alquati P, Dogliotti L. p53 but not bcl-2 immunostaining is predictive of poor clinical complete response to primary chemotherapy in breast cancer patients. Clin Cancer Res 6: 2751–2758, 2000. [PubMed] [Google Scholar]
- 37.Boucek RJ Jr., Steele A, Miracle A, Atkinson J. Effects of angiotensin-converting enzyme inhibitor on delayed-onset doxorubicin-induced cardiotoxicity. Cardiovasc Toxicol 3: 319–329, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Bountioukos M, Doorduijn JK, Roelandt JR, Vourvouri EC, Bax JJ, Schinkel AF, Kertai MD, Sonneveld P, Poldermans D. Repetitive dobutamine stress echocardiography for the prediction of anthracycline cardiotoxicity. Eur J Echocardiogr 4: 300–305, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med 65: 823–832, 1978. [DOI] [PubMed] [Google Scholar]
- 40.Bruynzeel AM, Abou El Hassan MA, Schalkwijk C, Berkhof J, Bast A, Niessen HW, van der Vijgh WJ. Anti-inflammatory agents and mono-HER protect against DOX-induced cardiotoxicity and accumulation of CML in mice. Br J Cancer 96: 937–943, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruynzeel AM, Vormer-Bonne S, Bast A, Niessen HW, van der Vijgh WJ. Long-term effects of 7-monohydroxyethylrutoside (monoHER) on DOX-induced cardiotoxicity in mice. Cancer Chemother Pharmacol 60: 509–514, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Bublik N, Alvarez JA, Lipshultz SE. Pediatric cardiomyopathy as a chronic disease: A perspective on comprehensive care programs. Prog Pediatr Cardiol 25: 103–111, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bull SC, Main ML, Stevens GR, Goldman JH, Constable SA, Becher H. Cardiac toxicity screening by echocardiography in healthy volunteers: A study of the effects of diurnal variation and use of a core laboratory on the reproducibility of left ventricular function measurement. Echocardiography 28: 502–507, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Burnett JC Jr., Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science 231: 1145–1147, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109: 2749–2754, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, Cinieri S, Martinelli G, Fiorentini C, Cipolla CM. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol 13: 710–715, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 36: 517–522, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho RA, Sousa RP, Cadete VJ, Lopaschuk GD, Palmeira CM, Bjork JA, Wallace KB. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology 270: 92–98, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology 115: 155–162, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng J, Kamiya K, Kodama I. Carvedilol: Molecular and cellular basis for its multifaceted therapeutic potential. Cardiovasc Drug Rev 19: 152–171, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Chicco AJ, Hydock DS, Schneider CM, Hayward R. Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol 100: 519–527, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Chow EJ, Chen Y, Hudson MM, Feijen EAM, Kremer LC, Border WL, Green DM, Meacham LR, Mulrooney DA, Ness KK, Oeffinger KC, Ronckers CM, Sklar CA, Stovall M, van der Pal HJ, van Dijk IWEM, van Leeuwen FE, Weathers RE, Robison LL, Armstrong GT, Yasui Y. Prediction of ischemic heart Disease and stroke in survivors of childhood cancer. J Clin Oncol 36: 44–52, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cochet A, Quilichini G, Dygai-Cochet I, Touzery C, Toubeau M, Berriolo-Riedinger A, Coudert B, Cottin Y, Fumoleau P, Brunotte F. Baseline diastolic dysfunction as a predictive factor of trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer. Breast Cancer Res Treat 130: 845–854, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Cole MP, Chaiswing L, Oberley TD, Edelmann SE, Piascik MT, Lin SM, Kiningham KK, St Clair DK. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc Res 69: 186–197, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Correia E, Rodrigues B, Santos LF, Moreira D, Gama P, Cabral C, Santos O. Longitudinal left ventricular strain in hypertrophic cardiomyopathy: Correlation with nonsustained ventricular tachycardia. Echocardiography 28: 709–714, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Cottin Y, L’Huillier I, Casasnovas O, Geoffroy C, Caillot D, Zeller M, Solary E, Guy H, Wolf JE. Dobutamine stress echocardiography identifies anthracycline cardiotoxicity. Eur J Echocardiogr 1: 180–183, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Cottin Y, Touzery C, Coudert B, Gilles A, Walker P, Massing JL, Toubeau M, Riedinger-Berriolo A, Caillot D, Louis P, et al. Impairment of diastolic function during short-term anthracycline chemotherapy. Br Heart J 73: 61–64, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crane FL, Sun IL, Clark MG, Grebing C, Low H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta 811: 233–264, 1985. [DOI] [PubMed] [Google Scholar]
- 59.Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation 82: 154–163, 1990. [DOI] [PubMed] [Google Scholar]
- 60.Crone SA, Zhao Y-Y, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, R J Jr, Chien KR, Lee K-F. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8: 459–465, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Curran CF, Narang PK, Reynolds RD. Toxicity profile of dexrazoxane (Zinecard, ICRF-187, ADR-529, NSC-169780), a modulator of doxorubicin cardiotoxicity. Cancer Treat Rev 18: 241–252, 1991. [DOI] [PubMed] [Google Scholar]
- 62.Daemen T, Hofstede G, Ten Kate MT, Bakker-Woudenberg IA, Scherphof GL. Liposomal doxorubicin-induced toxicity: Depletion and impairment of phagocytic activity of liver macrophages. Int J Cancer 61: 716–721, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Daosukho C, Ittarat W, Lin SM, Sawyer DB, Kiningham K, Lien YC, St Clair DK. Induction of manganese superoxide dismutase (MnSOD) mediates cardioprotective effect of tamoxifen (TAM). J Mol Cell Cardiol 39: 792–803, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Dash R, Chung J, Chan T, Yamada M, Barral J, Nishimura D, Yang PC, Simpson PC. A molecular MRI probe to detect treatment of cardiac apoptosis in vivo. Magn Reson Med 66: 1152–1162, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261: 3060–3067, 1986. [PubMed] [Google Scholar]
- 66.De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F, Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121: 276–292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Geus-Oei LF, Mavinkurve-Groothuis AM, Bellersen L, Gotthardt M, Oyen WJ, Kapusta L, van Laarhoven HW. Scintigraphic techniques for early detection of cancer treatment-induced cardiotoxicity. J Nuclear Med 52: 560–571, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Denard B, Lee C, Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. eLife 1: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng S, Kruger A, Kleschyov AL, Kalinowski L, Daiber A, Wojnowski L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic Biol Med 42: 466–473, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol 97: 318–326, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Dolinsky VW, Rogan KJ, Sung MM, Zordoky BN, Haykowsky MJ, Young ME, Jones LW, Dyck JR. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol Metab 305: E243–E253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dombernowsky P, Gehl J, Boesgaard M, Paaske T, Jensen BV. Doxorubicin and paclitaxel, a highly active combination in the treatment of metastatic breast cancer. Semin Oncol 23: 23–27, 1996. [PubMed] [Google Scholar]
- 73.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 261: 3068–3074, 1986. [PubMed] [Google Scholar]
- 74.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: Alterations produced by doxorubicin. J Clin Invest 65: 128–135, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dulin B, Abraham WT. Pharmacology of carvedilol. Am J Cardiol 93: 3B–6B, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Duncan R. Drug-polymer conjugates: Potential for improved chemotherapy. Anticancer Drugs 3: 175–210, 1992. [DOI] [PubMed] [Google Scholar]
- 77.Duquaine D, Hirsch GA, Chakrabarti A, Han Z, Kehrer C, Brook R, Joseph J, Schott A, Kalyanaraman B, Vasquez-Vivar J, Rajagopalan S. Rapid-onset endothelial dysfunction with adriamycin: Evidence for a dysfunctional nitric oxide synthase. Vasc Med 8: 101–107, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Eaton S, Skinner R, Hale JP, Pourfarzam M, Roberts A, Price L, Bartlett K. Plasma coenzyme Q(10) in children and adolescents undergoing doxorubicin therapy. Clin Chim Acta 302: 1–9, 2000. [DOI] [PubMed] [Google Scholar]
- 79.El-Shitany NA, Tolba OA, El-Shanshory MR, El-Hawary EE. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail 18: 607–613, 2012. [DOI] [PubMed] [Google Scholar]
- 80.Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand J-L, Brutsaert DL, Condorelli G, Hansen A, Heymans S, Hill JA, Hirsch E, Hilfiker-Kleiner D, Janssens S, de Jong S, Neubauer G, Pieske B, Ponikowski P, Pirmohamed M, Rauchhaus M, Sawyer D, Sugden PH, Wojta J, Zannad F, Shah AM. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 13: 1–10, 2011. [DOI] [PubMed] [Google Scholar]
- 81.Esper RM, Loeb JA. Neurotrophins induce neuregulin release through protein kinase Cδ activation. J Biol Chem 284: 26251–26260, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ewer MS, Ali MK, Mackay B, Wallace S, Valdivieso M, Legha SS, Benjamin RS, Haynie TP. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J Clin Oncol 2: 112–117, 1984. [DOI] [PubMed] [Google Scholar]
- 83.Faber J, Wingerter A, Neu MA, Henninger N, Eckerle S, Munzel T, Lackner KJ, Beutel ME, Blettner M, Rathmann W, Peters A, Meisinger C, Linkohr B, Neuhauser H, Kaatsch P, Spix C, Schneider A, Merzenich H, Panova-Noeva M, Prochaska JH, Wild PS. Burden of cardiovascular risk factors and cardiovascular disease in childhood cancer survivors: Data from the German CVSS-study. Eur Heart J 39: 1555–1562, 2018. [DOI] [PubMed] [Google Scholar]
- 84.Folkers K, Choe JY, Combs AB. Rescue by coenzyme Q10 from electrocardiographic abnormalities caused by the toxicity of adriamycin in the rat. Proc Natl Acad Sci U S A 75: 5178–5180, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Folkers K, Liu M, Watanabe T, Porter TH. Inhibition by adriamycin of the mitochondrial biosynthesis of coenzyme Q10 and implication for the cardiotoxicity of adriamycin in cancer patients. Biochem Biophys Res Commun 77: 1536–1542, 1977. [DOI] [PubMed] [Google Scholar]
- 86.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 104: 271–280, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu J, Yamamoto K, Guan ZW, Kimura S, Iyanagi T. Human neuronal nitric oxide synthase can catalyze one-electron reduction of adriamycin: Role of flavin domain. Arch Biochem Biophys 427: 180–187, 2004. [DOI] [PubMed] [Google Scholar]
- 88.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 35: 1473–1479, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Fulbright JM, Huh W, Anderson P, Chandra J. Can anthracycline therapy for pediatric malignancies be less cardiotoxic? Curr Oncol Rep 12: 411–419, 2010. [DOI] [PubMed] [Google Scholar]
- 90.Fumoleau P, Roché H, Kerbrat P, Bonneterre J, Romestaing P, Fargeot P, Namer M, Monnier A, Montcuquet P, Goudier M-J, Luporsi E. Long-term cardiac toxicity after adjuvant epirubicin-based chemotherapy in early breast cancer: French Adjuvant Study Group Results. Ann Oncol 17: 85–92, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Gabizon AA. Stealth liposomes and tumor targeting: One step further in the quest for the magic bullet. Clin Cancer Res 7: 223–225, 2001. [PubMed] [Google Scholar]
- 92.Gabrielson K, Bedja D, Pin S, Tsao A, Gama L, Yuan B, Muratore N. Heat shock protein 90 and ErbB2 in the cardiac response to doxorubicin injury. Cancer Res 67: 1436–1441, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol 289: H1153–H1160, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Gaudin PB, Hruban RH, Beschorner WE, Kasper EK, Olson JL, Baughman KL, Hutchins GM. Myocarditis associated with doxorubicin cardiotoxicity. Am J Clin Pathol 100: 158–163, 1993. [DOI] [PubMed] [Google Scholar]
- 95.Geisberg CA, Sawyer DB. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr Hypertens Rep 12: 404–410, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gianni L, Munzone E, Capri G, Fulfaro F, Tarenzi E, Villani F, Spreafico C, Laffranchi A, Caraceni A, Martini C, et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13: 2688–2699, 1995. [DOI] [PubMed] [Google Scholar]
- 97.Gibson NM, Greufe SE, Hydock DS, Hayward R. Doxorubicin-induced vascular dysfunction and its attenuation by exercise preconditioning. J Cardiovasc Pharmacol 62: 355–360, 2013. [DOI] [PubMed] [Google Scholar]
- 98.Gibson TM, Li Z, Green DM, Armstrong GT, Mulrooney DA, Srivastava D, Bhakta N, Ness KK, Hudson MM, Robison LL. Blood pressure Status in Adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev 26: 1705–1713, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gielen S, Sandri M, Erbs S, Adams V. Exercise-induced modulation of endothelial nitric oxide production. Curr Pharm Biotechol 12: 1375–1384, 2011. [DOI] [PubMed] [Google Scholar]
- 100.Gilladoga AC, Manuel C, Tan CT, Wollner N, Sternberg SS, Murphy ML. The cardiotoxicity of adriamycin and daunomycin in children. Cancer 37: 1070–1078, 1976. [DOI] [PubMed] [Google Scholar]
- 101.Gille L, Nohl H. Analyses of the molecular mechanism of adriamycin-induced cardiotoxicity. Free Radic Biol Med 23: 775–782, 1997. [DOI] [PubMed] [Google Scholar]
- 102.Gilleron M, Marechal X, Montaigne D, Franczak J, Neviere R, Lancel S. NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis. Biochem Biophys Res Commun 388: 727–731, 2009. [DOI] [PubMed] [Google Scholar]
- 103.Goetzenich AHN, Zernecke A, Weber C, Czarnotta T, Autschback R, Christianson S. Alteration of matrix metalloproteinases in selective left ventricular adriamycin-induced cardiomyopathy in the pig. J Heart Lung Transplant 28: 1087–1093, 2009. [DOI] [PubMed] [Google Scholar]
- 104.Goormaghtigh E, Huart P, Brasseur R, Ruysschaert J-M. Mechanism of inhibition of mitochondrial enzymatic complex I–III by adriamycin derivatives. Biochim Biophys Acta 861: 83–94, 1986. [DOI] [PubMed] [Google Scholar]
- 105.Goormaghtigh E, Huart P, Praet M, Brasseur R, Ruysschaert JM. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys Chem 35: 247–257, 1990. [DOI] [PubMed] [Google Scholar]
- 106.Green DM, Hyland A, Chung CS, Zevon MA, Hall BC. Cancer and cardiac mortality among 15-year survivors of cancer diagnosed during childhood or adolescence. J Clin Oncol 17: 3207–3215, 1999. [DOI] [PubMed] [Google Scholar]
- 107.Greenlee H, Shaw J, Lau YK, Naini A, Maurer M. Lack of effect of coenzyme q10 on doxorubicin cytotoxicity in breast cancer cell cultures. Integr Cancer Ther 11: 243–250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gregoriadis G, Wills EJ, Swain CP, Tavill AS. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet 1: 1313–1316, 1974. [DOI] [PubMed] [Google Scholar]
- 109.Guendouz S, Buicuic O, Kirsch M, Benaiem N, Poulard JE, Deux JF, Hittinger L, Peltier M, Damy T. Restrictive cardiomyopathy associated with left ventricle and left atria endocardial calcifications following chemotherapy. J Am Coll Cardiol 57: 1633, 2011. [DOI] [PubMed] [Google Scholar]
- 110.Guffierrez JA, Clark SG, Guiulumian AD, Fuchs LC. Superoxide anion contribute in impaired regulation of blood pressure by nitric oxide during the development of cardiomyopathy. J Pharmacol Exp Ther 282: 1643–1649, 1997. [PubMed] [Google Scholar]
- 111.Guimaraes-Filho F, Tan D, Braga J, Rodrigues A, Waib P, Matsubara B. Ventricular Systolic Reserve in Asymptomatic Children Previously Treated With Low Doses of Anthracyclines. Am J Cardiol 100: 1303–1306, 2007. [DOI] [PubMed] [Google Scholar]
- 112.Gustafsson F, Torp-Pedersen C, Brendorp B, Seibaek M, Burchardt H, Kober L. Long-term survival in patients hospitalized with congestive heart failure: Relation to preserved and reduced left ventricular systolic function. Eur Heart J 24: 863–870, 2003. [DOI] [PubMed] [Google Scholar]
- 113.Ha T, Li Y, Hua F, Ma J, Gao X, Kelley J, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res 68: 224–234, 2005. [DOI] [PubMed] [Google Scholar]
- 114.Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: Strategies for prevention and management. Future Cardiol 8: 647–670, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J 158: 294–301, 2009. [DOI] [PubMed] [Google Scholar]
- 116.Hasinoff BB. Inhibition and inactivation of NADH-cytochrome c reductase activity of bovine heart submitochondrial particles by the iron(III)-adriamycin complex. Biochem J 265: 865, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hasinoff BB, Herman EH. Dexrazoxane: How it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc Toxicol 7: 140–144, 2007. [DOI] [PubMed] [Google Scholar]
- 118.Hayakawa H, Komada Y, Hirayama M, Hori H, Ito M, Sakurai M. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol 37: 4–9, 2001. [DOI] [PubMed] [Google Scholar]
- 119.Hengel CL, Russell PA, Gould PA, Kaye DM. Subacute anthracycline cardiotoxicity. Heart Lung Circ 15: 59–61, 2006. [DOI] [PubMed] [Google Scholar]
- 120.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A III, von Hoff D, Schuchter LM. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J Clin Oncol 27: 127–145, 2009. [DOI] [PubMed] [Google Scholar]
- 121.Herman EH, Ferrans VJ. Reduction of chronic doxorubicin cardiotoxicity in dogs by pretreatment with (+/−)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187). Cancer Res 41: 3436–3440, 1981. [PubMed] [Google Scholar]
- 122.Herman EH, Ferrans VJ, Myers CE, Van Vleet JF. Comparison of the effectiveness of (+/−)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) and N-acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res 45: 276–281, 1985. [PubMed] [Google Scholar]
- 123.Herman EH, Lipshultz SE, Rifai N, Zhang J, Papoian T, Yu ZX, Takeda K, Ferrans VJ. Use of cardiac troponin T levels as an indicator of doxorubicin-induced cardiotoxicity. Cancer Res 58: 195–197, 1998. [PubMed] [Google Scholar]
- 124.Herman EH, Zhang J, Rifai N, Lipshultz SE, Hasinoff BB, Chadwick DP, Knapton A, Chai J, Ferrans VJ. The use of serum levels of cardiac troponin T to compare the protective activity of dexrazoxane against doxorubicin- and mitoxantrone-induced cardiotoxicity. Cancer Chemother Pharmacol 48: 297–304, 2001. [DOI] [PubMed] [Google Scholar]
- 125.Ho CY, Solomon SD. A clinician’s guide to tissue Doppler imaging. Circulation 113: e396–e398, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Hoffmann R, von Bardeleben S, ten Cate F, Borges AC, Kasprzak J, Firschke C, Lafitte S, Al-Saadi N, Kuntz-Hehner S, Engelhardt M, Becher H, Vanoverschelde JL. Assessment of systolic left ventricular function: A multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J 26: 607–616, 2005. [DOI] [PubMed] [Google Scholar]
- 127.Holmgren G, Synnergren J, Bogestål Y, Améen C, Åkesson K, Holmgren S, Lindahl A, Sartipy P. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology 328: 102–111, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Horie T, Ono K, Nishi H, Nagao K, Kinoshita M, Watanabe S, Kuwabara Y, Nakashima Y, Takanabe-Mori R, Nishi E, Hasegawa K, Kita T, Kimura T. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res 87: 656–664, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang SK, Lee KD, Hong K, Friend DS, Papahadjopoulos D. Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing mice. Cancer Res 52: 5135–5143, 1992. [PubMed] [Google Scholar]
- 130.Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Gray-burn PA. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: Comparison with cine magnetic resonance imaging. J Am Coll Cardiol 32: 1426–1432, 1998. [DOI] [PubMed] [Google Scholar]
- 131.Hydock DS, Lien CY, Jensen BT, Parry TL, Schneider CM, Hayward R. Rehabilitative exercise in a rat model of doxorubicin cardiotoxicity. Exp Biol Med 237: 1483–1492, 2012. [DOI] [PubMed] [Google Scholar]
- 132.Hydock DS, Wonders KY, Schneider CM, Hayward R. Voluntary wheel running in rats receiving doxorubicin: Effects on running activity and cardiac myosin heavy chain. Anticancer Res 29: 4401–4407, 2009. [PubMed] [Google Scholar]
- 133.Iarussi D, Auricchio U, Agretto A, Murano A, Giuliano M, Casale F, Indolfi P, Iacono A. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: Control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med 15(Suppl): s207–s212, 1994. [DOI] [PubMed] [Google Scholar]
- 134.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 52: 1574–1580, 2008. [DOI] [PubMed] [Google Scholar]
- 135.Ishibazawa A, Nagaoka T, Takahashi T, Yamamoto K, Kamiya A, Ando J, Yoshida A. Effects of shear stress on the gene expressions of endothelial nitric oxide synthase, endothelin-1, and thrombomodulin in human retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci 31: 8496–8504, 2011. [DOI] [PubMed] [Google Scholar]
- 136.Ishisaka T, Kishi S, Okura K, Horikoshi M, Yamashita T, Mitsuke Y, Shimizu H, Ueda T. A precise pharmacodynamic study showing the advantage of a marked reduction in cardiotoxicity in continuous infusion of doxorubicin. Leuk Lymphoma 47: 1599–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 137.Ivanova M, Dovinova I, Okruhlicova L, Tribulova N, Simoncikova P, Barte-kova M, Vlkovicova J, Barancik M. Chronic cardiotoxicity of doxorubicin involves activation of myocardial and circulating matrix metalloproteinases in rats. Acta Pharmacol Sin 33: 459–469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Izaguirre L, Pinilla I, Gonzalvo F, Pérez S, Honrubia FM. Effect of doxorubicin on fibroblast migration and proliferation. Ann Ophthalmol 35: 48–52, 2003. [Google Scholar]
- 139.Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, Gannon J, Macrae CA, Griffith LG, Lee RT. An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced proneoplastic potential. Circulation 128: 152–161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol 44: 878–886, 2004. [DOI] [PubMed] [Google Scholar]
- 141.Jensen BT, Lien CY, Hydock DS, Schneider CM, Hayward R. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J Cardiovasc Pharmacol 62: 263–269, 2013. [DOI] [PubMed] [Google Scholar]
- 142.Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol 19: 377–388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jurcut R, Wildiers H, Ganame J, D’Hooge J, De Backer J, Denys H, Paridaens R, Rademakers F, Voigt JU. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr 21: 1283–1289, 2008. [DOI] [PubMed] [Google Scholar]
- 144.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R, Ergin A. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 48: 2258–2262, 2006. [DOI] [PubMed] [Google Scholar]
- 145.Kappus H, Muliawan H, Scheulen ME. In vivo studies on adriamycin-induced lipid peroxidation and effects of ferrous ions. Dev Toxicol Environ Sci 8: 635–638, 1980. [PubMed] [Google Scholar]
- 146.Kapusta L, Groot-Loonen J, Thijssen JM, DeGraaf R, Daniels O. Regional cardiac wall motion abnormalities during and shortly after anthracyclines therapy. Med Pediatr Oncol 41: 426–435, 2003. [DOI] [PubMed] [Google Scholar]
- 147.Kapusta L, Thijssen JM, Groot-Loonen J, Antonius T, Mulder J, Daniels O. Tissue Doppler imaging in detection of myocardial dysfunction in survivors of childhood cancer treated with anthracyclines. Ultrasound Med Biol 26: 1099–1108, 2000. [DOI] [PubMed] [Google Scholar]
- 148.Karabay CY, Kocabay G, Kalayci A, Zehir R, Tanboga H. Mitral regurgitation due to papillary muscle dyssynchrony during trastuzumab treatment. Cardiology 117: 296–300, 2010. [DOI] [PubMed] [Google Scholar]
- 149.Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M, Kalay N, Dikilitas M, Yarlioglues M, Karaca H, Berk V, Ardic I, Ergin A, Lam YY. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A randomized control study. Int J Cardiol 167: 2306–2310, 2013. [DOI] [PubMed] [Google Scholar]
- 150.Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther 47: 219–231, 1990. [DOI] [PubMed] [Google Scholar]
- 151.Kim DS, Kim HR, Woo ER, Kwon DY, Kim MS, Chae SW, Chae HJ. Protective effect of calceolarioside on adriamycin-induced cardiomyocyte toxicity. Eur J Pharmacol 541: 24–32, 2006. [DOI] [PubMed] [Google Scholar]
- 152.Kizaki K, Ito R, Okada M, Yoshioka K, Uchide T, Temma K, Mutoh K, Uechi M, Hara Y. Enhanced gene expression of myocardial matrix metalloproteinases 2 and 9 after acute treatment with doxorubicin in mice. Pharmacol Res 53: 341–346, 2006. [DOI] [PubMed] [Google Scholar]
- 153.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS letters 268: 235–237, 1990. [DOI] [PubMed] [Google Scholar]
- 154.Korte FS, Herron TJ, Rovetto MJ, McDonald KS. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am J Physiol Heart Circ Physiol 289: H801–H812, 2005. [DOI] [PubMed] [Google Scholar]
- 155.Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging 3: 333–342, 2010. [DOI] [PubMed] [Google Scholar]
- 156.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, S R, Jobling WA, Morrow JD, Remmen HV, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005. [DOI] [PubMed] [Google Scholar]
- 157.Kusuoka H, Futaki S, Koretsune Y, Kitabatake A, Suga H, Kamada T, Inoue M. Alterations of intracellular calcium homeostasis and myocardial energetics in acute adriamycin-induced heart failure. J Cardiovasc Pharmacol 18: 437–444, 1991. [DOI] [PubMed] [Google Scholar]
- 158.Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr., Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 63: 809–816, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy: A systematic review. J Clin Oncol 22: 517–528, 2004. [DOI] [PubMed] [Google Scholar]
- 160.Lauritzen KH, Kleppa L, Aronsen JM, Eide L, Carlsen H, Haugen ØP, Sjaastad I, Klungland A, Rasmussen LJ, Attramadal H, Storm-Mathisen J, Bergersen LH. Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. Am J Physiol Heart Circ Physiol 309: H343–H449, 2015. [DOI] [PubMed] [Google Scholar]
- 161.Lebrecht D, Geist A, Ketelsen UP, Haberstroh J, Setzer B, Walker UA. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br J Pharmacol 151: 771–778, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lebrecht D, Kokkori A, Ketelsen U-P, Setzer B, Walker UA. Tissue-specific mtDNA lesions and radical-associated mitochondrial dysfunction in human hearts exposed to doxorubicin. J Pathol 207: 436–444, 2005. [DOI] [PubMed] [Google Scholar]
- 163.Lebrecht D, Setzer B, Ketelsen U-P, Haberstroh J, Walker UA. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation 108: 2423–2429, 2003. [DOI] [PubMed] [Google Scholar]
- 164.Lebrecht D, Setzer B, Rohrbach R, Walker UA. Mitochondrial DNA and its respiratory chain products are defective in doxorubicin nephrosis. Nephrol Dial Transplant 19: 329–336, 2004. [DOI] [PubMed] [Google Scholar]
- 165.Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol 7: 108–113, 2007. [DOI] [PubMed] [Google Scholar]
- 166.Lee KJ, Southee AE, Bautovich GJ, Freedman B, McLaughlin AF, Rossleigh MA, Hutton BF, Morris JG. Normalised radionuclide measures of left ventricular diastolic function. Eur J Nucl Med 15: 123–127, 1989. [DOI] [PubMed] [Google Scholar]
- 167.Lefrak EA, Pit’ha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32: 302–314, 1973. [DOI] [PubMed] [Google Scholar]
- 168.Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, Rasmussen SL, Blumenschein GR, Freireich EJ. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 96: 133–139, 1982. [DOI] [PubMed] [Google Scholar]
- 169.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347: 1397–1402, 2002. [DOI] [PubMed] [Google Scholar]
- 170.Lindsey ML LR, Parsons H, Andrewa TG, Aune GJ. The tell-tale heart: Molecular and cellular responses to childhood anthracycline exposure. Am J Physiol Heart Circ Physiol 307: H1379–H1389, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 324: 808–815, 1991. [DOI] [PubMed] [Google Scholar]
- 172.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 332: 1738–1743, 1995. [DOI] [PubMed] [Google Scholar]
- 173.Lipshultz SE, Miller TL, Lipsitz SR, Neuberg DS, Dahlberg SE, Colan SD, Silverman LB, Henkel JM, Franco VI, Cushman LL, Asselin BL, Clavell LA, Athale U, Michon B, Laverdiere C, Schorin MA, Larsen E, Usmani N, Sallan SE. Continuous versus bolus infusion of doxorubicin in children with ALL: Long-term cardiac outcomes. Pediatrics 130: 1003–1011, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 351: 145–153, 2004. [DOI] [PubMed] [Google Scholar]
- 175.Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, Barry EV, Asselin BL, Athale U, Clavell LA, Larsen E, Moghrabi A, Samson Y, Michon B, Schorin MA, Cohen HJ, Neuberg DS, Orav EJ, Colan SD. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: Long-term follow-up of a prospective, randomised, multi-centre trial. Lancet Oncol 11: 950–961, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu B, Li H, Qu H, Sun B. Nitric oxide synthase expressions in ADR-induced cardiomyopathy in rats. J Biochem Mol Biol 39: 759–765, 2006. [DOI] [PubMed] [Google Scholar]
- 177.Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, Ho KK, Manning WJ, Marchionni MA, Lorell BH, Morgan JP, Yan X. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol 289: H660–H666, 2005. [DOI] [PubMed] [Google Scholar]
- 178.Liu L, Zhang X, Qian B, Min X, Gao X, Li C, Cheng Y, Huang J. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail 9: 762–769, 2007. [DOI] [PubMed] [Google Scholar]
- 179.Loh JC, Creaser J, Rourke DA, Livingston N, Harrison TK, Vandenbogaart E, Moriguchi J, Hamilton MA, Tseng C-H, Fonarow GC, Horwich TB. Temporal Trends in Treatment and Outcomes for Advanced Heart Failure With Reduced Ejection Fraction From 1993–2010: Findings From a University Referral Center. Circ Heart Fail 6: 411–419, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 25: 1454–1470, 2004. [DOI] [PubMed] [Google Scholar]
- 181.Love MP, McMurray JJ. Endothelin in chronic heart failure: Current position and future prospects. Cardiovasc Res 31: 665–674, 1996. [DOI] [PubMed] [Google Scholar]
- 182.Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF. Topoisomerase IIbeta mediated DNA double-strand breaks: Implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 67: 8839–8846, 2007. [DOI] [PubMed] [Google Scholar]
- 183.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 111: 186–193, 2005. [DOI] [PubMed] [Google Scholar]
- 184.Macrez C, Marneffe-Lebrequier H, Ripault J, Clauvel JP, Jacquillat C, Weil M. [Cardiac complications observed during treatment with rubidomycin]. Pathol Biol 15: 949–953, 1967. [PubMed] [Google Scholar]
- 185.Maeda H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv Drug Deliv Rev 46: 169–185, 2001. [DOI] [PubMed] [Google Scholar]
- 186.Malpas JS, Scott RB. Daunorubicin in acute myelocytic leukaemia. Lancet 1: 469–470, 1969. [DOI] [PubMed] [Google Scholar]
- 187.Malpas JS, Scott RB. Rubidomycin in acute leukaemia in adults. Br Med J 3: 227–229, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marina NM, Cochrane D, Harney E, Zomorodi K, Blaney S, Winick N, Bernstein M, Link MP. Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: A pediatric oncology group study. Clin Cancer Res 8: 413–418, 2002. [PubMed] [Google Scholar]
- 189.Marmont AM, Damasio E, Rossi F. Internationles Symposion uber die Anwendung der Vinca-Alkaloide Velbe und Vinoristin 173, 1969. [Google Scholar]
- 190.Martin E, Thougaard AV, Grauslund M, Jensen PB, Bjorkling F, Hasinoff BB, Tjornelund J, Sehested M, Jensen LH. Evaluation of the topoisomerase II-inactive bisdioxopiperazine ICRF-161 as a protectant against doxorubicin-induced cardiomyopathy. Toxicology 255: 72–79, 2009. [DOI] [PubMed] [Google Scholar]
- 191.Mathe G, Hayat M, Schwarzenberg L, Amiel JL, Schneider M, Cattan A, Schlumberger JR, Jasmin C. Acute lymphoblastic leukaemia treated with a combination of prednisone, vincristine, and rubidomycin. Value of pathogen-free rooms. Lancet 2: 380–382, 1967. [DOI] [PubMed] [Google Scholar]
- 192.Matsui H, Morishima I, Numaguchi Y, Toki Y, Okumura K, Hayakawa T. Protective effects of carvedilol against doxorubicin-induced cardiomyopathy in rats. Life Sci 65: 1265–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 193.Mavinkurve-Groothuis AM, Groot-Loonen J, Marcus KA, Bellersen L, Feuth T, Bokkerink JP, Hoogerbrugge PM, de Korte C, Kapusta L. Myocardial strain and strain rate in monitoring subclinical heart failure in asymptomatic long-term survivors of childhood cancer. Ultrasound Med Biol 36: 1783–1791, 2010. [DOI] [PubMed] [Google Scholar]
- 194.May PM, Williams GK, Williams DR. Solution chemistry studies of adriamycin–iron complexes present in vivo. Eur J Cancer 16: 1275–1276, 1980. [DOI] [PubMed] [Google Scholar]
- 195.Mazzotta M, Giusti R, Iacono D, Lauro S, Marchetti P. Pulmonary fibrosis after pegylated liposomal doxorubicin in elderly patient with cutaneous angiosarcoma. 1–5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McKillop JH, Bristow MR, Goris ML, Billingham ME, Bockemuehl K. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am Heart J 106: 1048–1056, 1983. [DOI] [PubMed] [Google Scholar]
- 197.Merrill J, Greco FA, Zimbler H, Brereton HD, Lamberg JD, Pomeroy TC. Adriamycin and radiation: Synergistic cardiotoxicity. Ann Intern Med 82: 122–123, 1975. [DOI] [PubMed] [Google Scholar]
- 198.Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatr Blood Cancer 48: 723–726, 2007. [DOI] [PubMed] [Google Scholar]
- 199.Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME Jr., Ruccione K, Smithson WA, Robison LL. Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol 19: 3163–3172, 2001. [DOI] [PubMed] [Google Scholar]
- 200.Methe H, Kim JO, K S, W M, Nabauer M, Koglin J. Expansion of circulating Toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation 111: 2654–2661, 2005. [DOI] [PubMed] [Google Scholar]
- 201.Min PK, Lim S, Kang SJ, Hong SY, Hwang KC, Chung KH, Shim CY, Rim SJ, Chung N. Targeted ultrasound imaging of apoptosis with annexin a5 microbubbles in acute Doxorubicin-induced cardiotoxicity. J Cardiovasc Ultrasound 18: 91–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56: 185–229, 2004. [DOI] [PubMed] [Google Scholar]
- 203.Minotti G, Recalcati S, Menna P, Salvatorelli E, Corna G, Cairo G. Doxorubicin cardiotoxicity and the control of iron metabolism: Quinone-dependent and independent mechanisms. Meth Enzymol 378: 340–361, 2004. [DOI] [PubMed] [Google Scholar]
- 204.Minotti G, Recalcati S, Mordente A, Liberi G, Calafiore AM, Mancuso C, Preziosi P, Cairo G. The secondary alcohol metabolite of doxorubicin irreversibly inactivates aconitase/iron regulatory protein-1 in cytosolic fractions from human myocardium. FASEB J 12: 541–552, 1998. [DOI] [PubMed] [Google Scholar]
- 205.Minow RA, Benjamin RS, Gottlieb JA. Adriamycin (NSC 123127) cardiomyopathy. An overview with determination of risk factors. In: CANCER CHEMOTHERREP 1975, pp. 195–201. [Google Scholar]
- 206.Minow RA, Benjamin RS, Lee ET, Gottlieb JA. Adriamycin cardiomyopathy–risk factors. Cancer 39: 1397–1402, 1977. [DOI] [PubMed] [Google Scholar]
- 207.Miranda CJ, Makui H, Soares RJ, Bilodeau M, Mui J, Vali H, Bertrand R, Andrews NC, Santos MM. Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood 102: 2574–2580, 2003. [DOI] [PubMed] [Google Scholar]
- 208.Mitani I, Jain D, Joska TM, Burtness B, Zaret BL. Doxorubicin cardiotoxicity: Prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol 10: 132–139, 2003. [DOI] [PubMed] [Google Scholar]
- 209.Miura T, Muraoka S, Ogiso T. Adriamycin-Fe3+-induced inactivation of rat heart mitochondrial creatine kinase : Sensitivity to lipid peroxidation. Biol Pharm Bull 17: 1220–1223, 1994. [DOI] [PubMed] [Google Scholar]
- 210.Moller TR, Garwicz S, Barlow L, Winther JF, Glattre E, Olafsdottir G, Olsen JH, Perfekt R, Ritvanen A, Sankila R, Tulinius H. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol 19: 3173–3181, 2001. [DOI] [PubMed] [Google Scholar]
- 211.Morris Jones PH. The late effects of cancer therapy in childhood. Br J Cancer 64: 1–2, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296: H1466–H1483, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mukoyama M, Nakao K, Saito Y, Ogawa Y, Hosoda K, Suga S, Shirakami G, Jougasaki M, Imura H. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med 323: 757–758, 1990. [DOI] [PubMed] [Google Scholar]
- 214.Mulrooney DA, Blaes AH, Duprez D. Vascular injury in cancer survivors. J Cardiovasc Transl Res 5: 287–295, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mulrooney DA, Ness KK, Huang S, Solovey A, Hebbel RP, Neaton JD, Clohisy DR, Kelly AS, Neglia JP. Pilot study of vascular health in survivors of osteosarcoma. Pediatr Blood Cancer 60: 1703–1708, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mulrooney DA, Ness KK, Solovey A, Hebbel RP, Neaton JD, Peterson BA, Lee CK, Kelly AS, Neglia JP. Pilot study of vascular health in survivors of Hodgkin lymphoma. Pediatr Blood Cancer 59: 285–289, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339: b4606, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Muntinga HJ, van den Berg F, Knol HR, Niemeyer MG, Blanksma PK, Louwes H, van der Wall EE. Normal values and reproducibility of left ventricular filling parameters by radionuclide angiography. Int J Card Imaging 13: 165–171; discussion 173, 1997. [DOI] [PubMed] [Google Scholar]
- 219.Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: The role of lipid peroxidation in cardiac toxicity and tumor response. Science 197: 165–167, 1977. [DOI] [PubMed] [Google Scholar]
- 220.Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, Furutani E, Perez-Sanz TM, Graveline A, Janssens SP, Picard MH, Scherrer-Crosbie M, Bloch KD. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation 116: 506–514, 2007. [DOI] [PubMed] [Google Scholar]
- 221.Nevadunsky NS, Mbagwu C, Mizrahi N, Burton E, Goldberg GL. Pulmonary fibrosis after pegylated liposomal Doxorubicin in a patient with uterine papillary serous carcinoma. J Clin Oncol 31: e167–e169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nichols AJ, Gellai M, Ruffolo RR Jr. Studies on the mechanism of arterial vasodilation produced by the novel antihypertensive agent, carvedilol. Fundam Clin Pharmacol 5: 25–38, 1991. [DOI] [PubMed] [Google Scholar]
- 223.Nicolay K, Kruijff Bd. Effects of adriamycin on respiratory chain activities in mitochondria from rat liver, rat heart and bovine heart. Evidence for a preferential inhibition of complex III and IV. Biochim Biophys Acta 892: 320–330, 1987. [DOI] [PubMed] [Google Scholar]
- 224.Nohl H. Demonstration of the existence of an organo-specific NADH dehydrogenase in heart mitochondria. Eur J Biochem 169: 585–591, 1987. [DOI] [PubMed] [Google Scholar]
- 225.Nohl H. Identification of the site of adriamycin-activation in the heart cell. Biochem Pharmacol 37: 2633–2637, 1988. [DOI] [PubMed] [Google Scholar]
- 226.Nozaki N, Shishido T, Takeishi Y, Kubota I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation 110: 2869–2874, 2004. [DOI] [PubMed] [Google Scholar]
- 227.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAE-LYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15: 440–449, 2004. [DOI] [PubMed] [Google Scholar]
- 228.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52: 1213–1225, 2012. [DOI] [PubMed] [Google Scholar]
- 229.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355: 1572–1582, 2006. [DOI] [PubMed] [Google Scholar]
- 230.Okumura H, Iuchi K, Yoshida T, Nakamura S, Takeshima M, Takamatsu H, Ikeno A, Usuda K, Ishikawa T, Ohtake S, Matsuda T. Brain natriuretic peptide is a predictor of anthracycline-induced cardiotoxicity. Acta haematologica 104: 158–163, 2000. [DOI] [PubMed] [Google Scholar]
- 231.Oliveira PJ, Bjork JA, Santos MS, Leino RL, Froberg MK, Moreno AJ, Wallace KB. Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol 200: 159–168, 2004. [DOI] [PubMed] [Google Scholar]
- 232.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A 99: 8880–8885, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pacciarini MA, Barbieri B, Colombo T, Broggini M, Garattini S, Donelli MG. Distribution and antitumor activity of adriamycin given in a high-dose and a repeated low-dose schedule to mice. Cancer Treat Rep 62: 791–800, 1978. [PubMed] [Google Scholar]
- 234.Paffenbarger RSJ, Hyde RT, Wing AL, Chung-cheng H. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 314: 605–613, 1986. [DOI] [PubMed] [Google Scholar]
- 235.Pang B, Qiao X, Janssen L, Velds A, Groothius T, Kerkhoven R, Nieuw-land M, Ovaa H, Rottenberg S, van Tellingen O, Janssen J, Huijgens P, Zwart W, Neefjes J. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat Commun 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Panjrath GS, Patel V, Valdiviezo CI, Narula N, Narula J, Jain D. Potentiation of Doxorubicin cardiotoxicity by iron loading in a rodent model. J Am Coll Cardiol 49: 2457–2464, 2007. [DOI] [PubMed] [Google Scholar]
- 237.Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G, Brancato R, Capaccioli S. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem 278: 28220–28228, 2003. [DOI] [PubMed] [Google Scholar]
- 238.Pearlman M, Jendiroba D, Pagliaro L, Keyhani A, Liu B, Freireich EJ. Dexrazoxane in combination with anthracyclines lead to a synergistic cytotoxic response in acute myelogenous leukemia cell lines. Leukemia research 27: 617–626, 2003. [DOI] [PubMed] [Google Scholar]
- 239.Pelikan PC, Weisfeldt ML, Jacobus WE, Miceli MV, Bulkley BH, Gerstenblith G. Acute doxorubicin cardiotoxicity: Functional, metabolic, and morphologic alterations in the isolated, perfused rat heart. J Cardiovasc Pharmacol 8: 1058–1066, 1986. [PubMed] [Google Scholar]
- 240.Penault-Llorca F, Cayre A, Bouchet Mishellany F, Amat S, Feillel V, Le Bouedec G, Ferriere JP, De Latour M, Chollet P. Induction chemotherapy for breast carcinoma: Predictive markers and relation with outcome. Int J Oncol 22: 1319–1325, 2003. [PubMed] [Google Scholar]
- 241.Pescatello LS, Fargo AE, Leach CNJ, Scherzer HH. Short-term effect of dynamic exercise on arterial blood pressure. Circulation 83: 1557–1561, 1991. [DOI] [PubMed] [Google Scholar]
- 242.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103: 1232–1240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Piegari E, De Angelis A, Cappetta D, Russo R, Esposito G, Costantino S, Graiani G, Frati C, Prezioso L, Berrino L, Urbanek K, Quaini F, Rossi F. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res Cardiol 108: 334, 2013. [DOI] [PubMed] [Google Scholar]
- 244.Popat S, Smith IE. Therapy Insight: Anthracyclines and trastuzumab[mdash]the optimal management of cardiotoxic side effects. Nat Clin Prac Oncol 5: 324–335, 2008. [DOI] [PubMed] [Google Scholar]
- 245.Popelova O, Sterba M, Simunek T, Mazurova Y, Guncova I, Hroch M, Adamcova M, Gersl V. Deferiprone does not protect against chronic anthracycline cardiotoxicity in vivo. J Pharmacol Exp Ther 326: 259–269, 2008. [DOI] [PubMed] [Google Scholar]
- 246.Praga C, Beretta G, Vigo PL, Lenaz GR, Pollini C, Bonadonna G, Canetta R, Castellani R, Villa E, Gallagher CG, von Melchner H, Hayat M, Ribaud P, De Wasch G, Mattsson W, Heinz R, Waldner R, Kolaric K, Buehner R, Ten Bokkel-Huyninck W, Perevodchikova NI, Manziuk LA, Senn HJ, Mayr AC. Adriamycin cardiotoxicity: A survey of 1273 patients. Cancer Treat Rep 63: 827–834, 1979. [PubMed] [Google Scholar]
- 247.Prout MN, Richards MJ, Chung KJ, Joo P, Davis HL Jr. Adriamycin cardiotoxicity in children: Case reports, literature review, and risk factors. Cancer 39: 62–65, 1977. [DOI] [PubMed] [Google Scholar]
- 248.Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer-Crosbie M, Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem 61: 1164–1172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Qi B, Newcomer RG, Sang QX. ADAM19/adamalysin 19 structure, function, and role as a putative target in tumors and inflammatory diseases. Curr Pharm Des 15: 2336–2348, 2009. [DOI] [PubMed] [Google Scholar]
- 250.Quarello P, Berger M, Rivetti E, Galletto C, Masetti R, Manicone R, Barisone E, Pession A, Fagioli F. FLAG-liposomal doxorubicin (Myocet) regimen for refractory or relapsed acute leukemia pediatric patients. J Pediatr Hematol Oncol 34: 208–216, 2012. [DOI] [PubMed] [Google Scholar]
- 251.Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp Hematol Oncol 1: 10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rasmussen UF, Rasmussen HN. The NADH oxidase system (external) of muscle mitochondria and its role in the oxidation of cytoplasmic NADH. Biochem J 229: 631–641, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, Skinner R, Stevens MC, Hawkins MM. Long-term cause-specific mortality among survivors of childhood cancer. Jama 304: 172–179, 2010. [DOI] [PubMed] [Google Scholar]
- 254.Riad A, Bien S, Gratz M, Escher F, Westermann D, Heimesaat MM, Bereswill S, Krieg T, Felix SB, Schultheiss HP, Kroemer HK, Tschope C. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail 10: 233–243, 2008. [DOI] [PubMed] [Google Scholar]
- 255.Robles SJ, Buehler PW, Negrusz A, Adami GR. Permanent cell cycle arrest in asynchronously proliferating normal human fibroblasts treated with doxorubicin or etoposide but not camptothecin. Biochem Pharmacol 58: 675–685, 1999. [DOI] [PubMed] [Google Scholar]
- 256.Russo G, Cioffi G, Di Lenarda A, Tuccia F, Bovelli D, Di Tano G, Alunni G, Gori S, Faggiano P, Tarantini L. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern Emerg Med 7: 439–446, 2012. [DOI] [PubMed] [Google Scholar]
- 257.Sacco G, Bigioni M, Evangelista S, Goso C, Manzini S, Maggi CA. Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. Eur J Pharmacol 414: 71–78, 2001. [DOI] [PubMed] [Google Scholar]
- 258.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 11: 1029–1033, 2000. [DOI] [PubMed] [Google Scholar]
- 259.Salerno M. Multi-modality imaging of diastolic function. J Nucl Cardiol 17: 316–327, 2010. [DOI] [PubMed] [Google Scholar]
- 260.Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, Camitta BA. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: A report from the children’s oncology group. Leukemia 24: 355–370, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sandri MT, Salvatici M, Cardinale D, Zorzino L, Passerini R, Lentati P, Leon M, Civelli M, Martinelli G, Cipolla CM. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: A marker predictive of cardiac dysfunction? Clin Chem 51: 1405–1410, 2005. [DOI] [PubMed] [Google Scholar]
- 262.Santos DL, Moreno AJ, Leino RL, Froberg MK, Wallace KB. Carvedilol protects against doxorubicin-induced mitochondrial cardiomyopathy. Toxicol Appl Pharmacol 185: 218–227, 2002. [DOI] [PubMed] [Google Scholar]
- 263.Sarosiek KA, Fraser C, Muthalagu N, Bhola PD, Chang W, McBrayer SK, Cantlon A, Fisch S, Golomb-Mello G, Ryan JA, Deng J, Jian B, Corbett C, Goldenberg M, Madsen JR, Liao R, Walsh D, Sedivy J, Murphy DJ, Carrasco DR, Robinson S, Moslehi J, Letai A. Developmental regulation of mitochondrial apoptosis by c-Myc governs ageand tissue-specific sensitivity to cancer therapeutics. Cancer Cell 31: 142–156, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 107: 1375–1380, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: Potential mechanism for trastuzumab-induced cardiotoxicity. Circulation 105: 1551–1554, 2002. [DOI] [PubMed] [Google Scholar]
- 266.Sayed-Ahmed MM, Khattab MM, Gad MZ, Osman A. Increased plasma endothelin-1 and cardiac nitric oxide during doxorubicin-induced cardiomyopathy. Pharmacol Toxicol 89: 140–144, 2001. [DOI] [PubMed] [Google Scholar]
- 267.Scarabelli T, Stephanou A, Rayment N, Pasini E, Comini L, Curello S, Ferriari R, Knight R, Latchman D. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ishcemia/reperfusion injry. Circulation 104: 253–256, 2001. [DOI] [PubMed] [Google Scholar]
- 268.Schinetti ML, Rossini D, Bertelli A. Interaction of anthracycline antibiotics with human neutrophils: Superoxide production, free radical formation and intracellular penetration. J Cancer Res Clin Oncol 113: 15–19, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res 39: 257–288, 2000. [DOI] [PubMed] [Google Scholar]
- 270.Schroeder PE, Hasinoff BB. The doxorubicin-cardioprotective drug dexrazoxane undergoes metabolism in the rat to its metal ion-chelating form ADR-925. Cancer Chemother Pharmacol 50: 509–513, 2002. [DOI] [PubMed] [Google Scholar]
- 271.Schroeder PE, Hasinoff BB. Metabolism of the one-ring open metabolites of the cardioprotective drug dexrazoxane to its active metal-chelating form in the rat. Drug Metab Dispos 33: 1367–1372, 2005. [DOI] [PubMed] [Google Scholar]
- 272.Scorsin M, Hagege AA, Dolizy I, Marotte F, Mirochnik N, Copin H, Barnoux M, le Bert M, Samuel JL, Rappaport L, Menasche P. Can cellular transplantation improve function in doxorubicin-induced heart failure? Circulation 98: II151–II155; discussion II155–156, 1998. [PubMed] [Google Scholar]
- 273.Seifert CF, Nesser ME, Thompson DF. Dexrazoxane in the prevention of doxorubicin-induced cardiotoxicity. Ann Pharmacother 28: 1063–1072, 1994. [DOI] [PubMed] [Google Scholar]
- 274.Senior J, Delgado C, Fisher D, Tilcock C, Gregoriadis G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: Studies with poly(ethylene glycol)-coated vesicles. Biochim Biophys Acta 1062: 77–82, 1991. [DOI] [PubMed] [Google Scholar]
- 275.Serrano J, Palmeira CM, Kuehl DW, Wallace KB. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration1. Biochim Biophys Acta 1411: 201–205, 1999. [DOI] [PubMed] [Google Scholar]
- 276.Shaeffer J, El-Mahdi AM, Nichols RK. Coenzyme Q10 and adriamycin toxicity in mice. Res Commun Chem Pathol Pharmacol 29: 309–315, 1980. [PubMed] [Google Scholar]
- 277.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation 99: 963–972, 1999. [DOI] [PubMed] [Google Scholar]
- 278.Shivakumar P, Rani MU, Reddy AG, Anjaneyulu Y. A study on the toxic effects of doxorubicin on the histology of certain organs. Toxicol In Vitro 19: 241–244, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sieswerda E, van Dalen EC, Postma A, Cheuk DK, Caron HN, Kremer LC. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database Syst Rev CD008011, 2011. [DOI] [PubMed] [Google Scholar]
- 280.Spallarossa P, Altieri P, Barisione C, Passalacqua M, Aloi C, Fugazza G, Frassoni F, Podestà M, Canepa M, Ghigliotti G, Brunelli C. p38 MAPK and JNK antagonistically control senescence and cytoplasmic p16INK4A expression in doxorubicin-treated endothelial progenitor cells. PLoS ONE 5: e15583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A, Brunelli C, Barsotti A. Matrix metalloproteinase-2 and −9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res 69: 736–745, 2006. [DOI] [PubMed] [Google Scholar]
- 282.Spallarossa P, Garibaldi S, Altieri P, Fabbi P, Manca V, Nasti S, Rossettin P, Ghigliotti G, Ballestrero A, Patrone F, Barsotti A, Brunelli C. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol 37: 837–846, 2004. [DOI] [PubMed] [Google Scholar]
- 283.Speyer JL, Green MD, Bottino J, Wernz J, Blum RH, Muggia FM. A phase I/II study of 6-hour and 24-hour intravenous infusions of doxorubicin In: Anthracycline Antibiotics in Cancer Therapy, edited by Muggia F, Young C, and Carter S. Springer: The Netherlands, 1982, pp. 398–410. [Google Scholar]
- 284.Speyer JL, Green MD, Dubin N, Blum RH, Wernz JC, Roses D, Sanger J, Muggia FM. Prospective evaluation of cardiotoxicity during a six-hour doxorubicin infusion regimen in women with adenocarcinoma of the breast. Am J Med 78: 555–563, 1985. [DOI] [PubMed] [Google Scholar]
- 285.Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, Dubin N, Ferrans V, Stecy P, Zeleniuch-Jacquotte A, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. N Engl J Med 319: 745–752, 1988. [DOI] [PubMed] [Google Scholar]
- 286.Speyer JL, Green MD, Zeleniuch-Jacquotte A, Wernz JC, Rey M, Sanger J, Kramer E, Ferrans V, Hochster H, Meyers M, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol 10: 117–127, 1992. [DOI] [PubMed] [Google Scholar]
- 287.Sterba M, Popelova O, Simunek T, Mazurova Y, Potacova A, Adamcova M, Kaiserova H, Ponka P, Gersl V. Cardioprotective effects of a novel iron chelator, pyridoxal 2-chlorobenzoyl hydrazone, in the rabbit model of daunorubicin-induced cardiotoxicity. The J Pharmacol Exp Ther 319: 1336–1347, 2006. [DOI] [PubMed] [Google Scholar]
- 288.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 3: 315–322, 2001. [DOI] [PubMed] [Google Scholar]
- 289.Strigun A, Wahrheit J, Niklas J, Heinzle E, Noor F. Doxorubicin increases oxidative metabolism in HL-1 cardiomyocytes as shown by 13C metabolic flux analysis. Toxicol Sci 125: 595–606, 2012. [DOI] [PubMed] [Google Scholar]
- 290.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest 117: 3730–3741, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Suzuki T, Hayashi D, Yamazaki T, Mizuno T, Kanda Y, Komuro I, Kurabayashi M, Yamaoki K, Mitani K, Hirai H, Nagai R, Yazaki Y. Elevated B-type natriuretic peptide levels after anthracycline administration. Am Heart J 136: 362–363, 1998. [DOI] [PubMed] [Google Scholar]
- 292.Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol 15: 1333–1340, 1997. [DOI] [PubMed] [Google Scholar]
- 293.Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, Jones SE, Wadler S, Desai A, Vogel C, Speyer J, Mittelman A, Reddy S, Pendergrass K, Velez-Garcia E, Ewer MS, Bianchine JR, Gams RA. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 15: 1318–1332, 1997. [DOI] [PubMed] [Google Scholar]
- 294.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49: 330–352, 2007. [DOI] [PubMed] [Google Scholar]
- 295.Takeshita S, Inoue N, Ueyama T, Kawashima S, Yokoyama M. Shear stress enhances glutathione peroxidase expression in endothelial cells. Biochem Biophys Res Commun 273: 66–71, 2000. [DOI] [PubMed] [Google Scholar]
- 296.Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 20: 333–353, 1967. [DOI] [PubMed] [Google Scholar]
- 297.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J 376: 545–552, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tan TC, Neilan TG, Francis S, Plana JC, Scherrer-Crosbie M. Anthracycline-induced cardiomyopathy in adults. Compr Physiol 5: 1517–1540, 2015. [DOI] [PubMed] [Google Scholar]
- 299.Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, Jouannaud C, Blaise AM, Elaerts J, Nazeyrollas P. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: Early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr 7: 141–146, 2006. [DOI] [PubMed] [Google Scholar]
- 300.Tatlidede E, Sehirli O, Velioglu-Ogunc A, Cetinel S, Yegen BC, Yarat A, Suleymanoglu S, Sener G. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res 43: 195–205, 2009. [DOI] [PubMed] [Google Scholar]
- 301.Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL. Dexrazoxane-associated risk for acute myeloid leukemia/myelodys-plastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol 25: 493–500, 2007. [DOI] [PubMed] [Google Scholar]
- 302.Tetef ML, Synold TW, Chow W, Leong L, Margolin K, Morgan R, Raschko J, Shibata S, Somlo G, Yen Y, Groshen S, Johnson K, Lenz HJ, Gandara D, Doroshow JH. Phase I trial of 96-hour continuous infusion of dexrazoxane in patients with advanced malignancies. Clin Cancer Res 7: 1569–1576, 2001. [PubMed] [Google Scholar]
- 303.Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomeraseII. Science 226: 466–468, 1984. [DOI] [PubMed] [Google Scholar]
- 304.Todorova VK, Beggs ML, Delongchamp RR, Dhakal I, Makhoul I, Wei JY, Klimberg VS. Transcriptome profiling of peripheral blood cells identifies potential biomarkers for doxorubicin cardiotoxicity in a rat model. PLoS ONE 7: e48398, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Toko H, Oka T, Zou Y, Sakamoto M, Mizukami M, Sano M, Yamamoto R, Sugaya T, Komuro I. Angiotensin II type 1a receptor mediates doxorubicin-induced cardiomyopathy. Hypertens Res 25: 597–603, 2002. [DOI] [PubMed] [Google Scholar]
- 306.Torchilin VP. Drug targeting. Eur J Pharm Sci 11(Suppl 2): S81–S91, 2000. [DOI] [PubMed] [Google Scholar]
- 307.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. Jama 296: 1867–1876, 2006. [DOI] [PubMed] [Google Scholar]
- 308.Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, Lipshultz SE. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol 32: 342–353, 2011. [DOI] [PubMed] [Google Scholar]
- 309.Tran ZV, Weltman A. Differential effects of exercise on serum lipid and lipoprotein levels seen with changes in body weight. A meta-analysis. Jama 254: 919–924, 1985. [PubMed] [Google Scholar]
- 310.Treat J, Greenspan A, Forst D, Sanchez JA, Ferrans VJ, Potkul LA, Woolley PV, Rahman A. Antitumor activity of liposome-encapsulated doxorubicin in advanced breast cancer: Phase II study. J Natl Cancer Inst 82: 1706–1710, 1990. [DOI] [PubMed] [Google Scholar]
- 311.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson N-G. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004. [DOI] [PubMed] [Google Scholar]
- 312.Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, Ilangovan G. Doxorubicin-induced cardiotoxicity: Direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am J Physiol Heart Circ Physiol 293: H3111–H3121, 2007. [DOI] [PubMed] [Google Scholar]
- 313.Tyynismaa H, Mjosund KP, Wanrooij S, Lappalainen I, Ylikallio E, Jalanko A, Spelbrink JN, Paetau A, Suomalainen A. Mutant mitochondrial helicase Twinkle causes multiple mtDNA deletions and a late-onset mitochondrial disease in mice. Proc Natl Acad Sci U S A 102: 17687–17692, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ursell PC, Mayes M. Anatomic distribution of nitric oxide synthase in the heart. Int J Cardiol 50: 217–223, 1995. [DOI] [PubMed] [Google Scholar]
- 315.Valdivieso M, Burgess MA, Ewer MS, Mackay B, Wallace S, Benjamin RS, Ali MK, Bodey GP, Freireich EJ. Increased therapeutic index of weekly doxorubicin in the therapy of non-small cell lung cancer: A prospective, randomized study. J Clin Oncol 2: 207–214, 1984. [DOI] [PubMed] [Google Scholar]
- 316.van Boxtel W, Bulten BF, Mavinkurve-Groothuis AMC, Bellersen L, Mandigers CMPW, Joosten LAB, Kapusta L, de Geus-Oei LF, van Laarhoven HWM. New biomarkers for early detection of cardiotoxicity after treatment with docetaxel, doxorubicin and cyclophosphamide. Biomarkers 20: 143–148, 2015. [DOI] [PubMed] [Google Scholar]
- 317.van Dalen EC, Raphael MF, Caron HN, Kremer LC. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev Cd006647, 2009. [DOI] [PubMed] [Google Scholar]
- 318.van der Pal HJ, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, Sieswerda E, Oldenburger F, Koning CC, van Leeuwen FE, Caron HN, Kremer LC. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 30: 1429–1437, 2012. [DOI] [PubMed] [Google Scholar]
- 319.van Laar M, Feltbower RG, Gale CP, Bowen DT, Oliver SE, Glaser A. Cardiovascular sequelae in long-term survivors of young peoples’ cancer: A linked cohort study. Br J Cancer 110: 1338–1341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, Atkinson P, Deman P, Wackers FJ. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol 77: 843–850, 1996. [DOI] [PubMed] [Google Scholar]
- 321.Vasquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA Jr., Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 36: 11293–11297, 1997. [DOI] [PubMed] [Google Scholar]
- 322.Vasti C, Hertig CM. Neuregulin-1/erbB activities with focus on the susceptibility of the heart to anthracyclines. World J Cardiol 6: 653–662, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J Am Coll Cardiol 64: 938–945, 2014. [DOI] [PubMed] [Google Scholar]
- 324.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol 555: 1–13, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Venturini M, Michelotti A, Del Mastro L, Gallo L, Carnino F, Garrone O, Tibaldi C, Molea N, Bellina RC, Pronzato P, Cyrus P, Vinke J, Testore F, Guelfi M, Lionetto R, Bruzzi P, Conte PF, Rosso R. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. J Clin Oncol 14: 3112–3120, 1996. [DOI] [PubMed] [Google Scholar]
- 326.Vile GF, Winterbourn CC. dl-N,N′-dicarboxamidomethyl-N,N′-dicarboxymethyl-1,2-diaminopropane (ICRF-198) and d-1,2-bis(3,5-dioxopiperazine-1-yl)propane (ICRF-187) inhibition of Fe3+ reduction, lipid peroxidation, and CaATPase inactivation in heart microsomes exposed to adriamycin. Cancer Res 50: 2307–2310, 1990. [PubMed] [Google Scholar]
- 327.Vj F. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer Treat Rep 62: 955–961, 1978. [PubMed] [Google Scholar]
- 328.Volkova M, Russell R. Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr Cardiol Rev 7: 214–220, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Von Hoff DD, Howser D, Lewis BJ, Holcenberg J, Weiss RB, Young RC. Phase I study of ICRF-187 using a daily for 3 days schedule. Cancer Treat Rep 65: 249–252, 1981. [PubMed] [Google Scholar]
- 330.Von Hoff DD, Layard MW, Basa P, Davis HL Jr., Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91: 710–717, 1979. [DOI] [PubMed] [Google Scholar]
- 331.Vrooman LM, Neuberg DS, Stevenson KE, Asselin BL, Athale UH, Clavell L, Cole PD, Kelly KM, Larsen EC, Laverdiere C, Michon B, Schorin M, Schwartz CL, Cohen HJ, Lipshultz SE, Silverman LB, Sallan SE. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: A report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer 47: 1373–1379, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wadler S, Green MD, Muggia FM. Synergistic activity of doxorubicin and the bisdioxopiperazine (+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF 187) against the murine sarcoma S180 cell line. Cancer Res 46: 1176–1181, 1986. [PubMed] [Google Scholar]
- 333.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study. Lancet 361: 374–379, 2003. [DOI] [PubMed] [Google Scholar]
- 334.Walker J, Bhullar N, Fallah-Rad N, Lytwyn M, Golian M, Fang T, Summers AR, Singal PK, Barac I, Kirkpatrick ID, Jassal DS. Role of three-dimensional echocardiography in breast cancer: Comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol 28: 3429–3436, 2010. [DOI] [PubMed] [Google Scholar]
- 335.Wang AH, Gao YG, Liaw YC, Li YK. Formaldehyde cross-links daunorubicin and DNA efficiently: HPLC and X-ray diffraction studies. Biochemistry 30: 3812–3815, 1991. [DOI] [PubMed] [Google Scholar]
- 336.Wang F, Iskra B, Kleinerman E, Alvarez-Florez C, Andrews T, Shaw A, Chandra J, Schadler K, Aune GJ. Aerobic exercise during early murine doxorubicin exposure mitigates cardiac toxicity. J Pediatr Hematol Oncol 40: 208–215, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wang W, Ha CH, Jhun BS, Wong CM, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 115: 2971–2979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wassmuth R, Lentzsch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, Friedrich MG. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J 141: 1007–1013, 2001. [DOI] [PubMed] [Google Scholar]
- 339.Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form: Changing toxicity profiles. Drug Saf 24: 903–920, 2001. [DOI] [PubMed] [Google Scholar]
- 340.Weber KT. Extracellular matrix remodeling in heart failure: A role for de novo angiotensin II generation. Circulation 96: 4065–4082, 1997. [DOI] [PubMed] [Google Scholar]
- 341.Weinstein JN. Liposomes as drug carriers in cancer therapy. Cancer Treat Rep 68: 127–135, 1984. [PubMed] [Google Scholar]
- 342.Wetzel M, Rosenberg GA, Cunningham LA. Tissue inhibitor of metalloproteinases-3 and matrix metalloproteinase-3 regulate neuronal sensitivity to doxorubicin-induced apoptosis. Eur J Neurosci 18: 1050–1060, 2003. [DOI] [PubMed] [Google Scholar]
- 343.Wilson CL, Liu W, Yang JJ, Kang G, Ojha RP, Neale GA, Srivastava DK, Gurney JG, Hudson MM, Robison LL, Ness KK. Genetic and clinical factors associated with obesity among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort. Cancer 121: 2262–2270, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wiseman A, Attardi G. Reversible tenfod reduction in mitochondrial DNA content of human cells treated with ethidium bromide. Mol Gen Genet MGG 167: 51–63, 1978. [DOI] [PubMed] [Google Scholar]
- 345.Wojnowski L, Kulle B, Schirmer M, Schluter G, Schmidt A, Rosenberger A, Vonhof S, Bickeboller H, Toliat MR, Suk EK, Tzvetkov M, Kruger A, Seifert S, Kloess M, Hahn H, Loeffler M, Nurnberg P, Pfreundschuh M, Trumper L, Brockmoller J, Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation 112: 3754–3762, 2005. [DOI] [PubMed] [Google Scholar]
- 346.Wu S, Ko Y-S, Teng M-S, Ko Y-L, Hsu L-A, Hsueh C, Chou Y-Y, Liew C-C, Lee Y-S. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: In vitro and in vivo studies. J Mol Cell Cardiol 34: 1595–1607, 2002. [DOI] [PubMed] [Google Scholar]
- 347.Xiong Y, Liu X, Lee CP, Chua BH, Ho YS. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic Biol Med 41: 46–55, 2006. [DOI] [PubMed] [Google Scholar]
- 348.Xu X, Persson HL, Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol 68: 261–271, 2005. [DOI] [PubMed] [Google Scholar]
- 349.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104: 3103–3108, 2001. [DOI] [PubMed] [Google Scholar]
- 350.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart Failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 128: e240–e327, 2013. [DOI] [PubMed] [Google Scholar]
- 351.Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies: Are clinicians responding optimally? J Am Coll Cardiol 56: 1644–1650, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yousif NG, Al-amran FG. Novel Toll-like receptor-4 deficiency attenuates trastuzumab (Herceptin) induced cardiac injury in mice. BMC Cardiovasc Disord 11: 62–62, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zagrosek A, Abdel-Aty H, Boye P, Wassmuth R, Messroghli D, Utz W, Rudolph A, Bohl S, Dietz R, Schulz-Menger J. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging 2: 131–138, 2009. [DOI] [PubMed] [Google Scholar]
- 354.Zhang J, Herman EH, Ferrans VJ. Dendritic cells in the hearts of spontaneously hypertensive rats treated with doxorubicin with or without ICRF-187. Am J Pathol 142: 1916–1926, 1993. [PMC free article] [PubMed] [Google Scholar]
- 355.Zhang J, Knapton A, Lipshultz SE, Cochran TR, Hiraragi H, Herman EH. Sex-related differences in mast cell activity and doxorubicin toxicity: A study in spontaneously hypertensive rats. Toxicol Pathol 42: 361–375, 2014. [DOI] [PubMed] [Google Scholar]
- 356.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18: 1639–1642, 2012. [DOI] [PubMed] [Google Scholar]
- 357.Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, McDermott BJ, Grieve DJ. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res 70: 9287–9297, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett 121: 151–157, 2001. [DOI] [PubMed] [Google Scholar]


