Abstract
The heart is lined by a single layer of mesothelial cells called the epicardium that provides important cellular contributions for embryonic heart formation. The epicardium harbors a population of progenitor cells that undergo epithelial-to-mesenchymal transition (EMT) displaying characteristic conversion of planar epithelial cells into multipolar and invasive mesenchymal cells prior to differentiating into non-myocyte cardiac lineages such as vascular smooth muscle cells, pericytes, and fibroblasts. The epicardium is also a source of paracrine cues that are essential for fetal cardiac growth, coronary vessel patterning, and regenerative heart repair. Although the epicardium becomes dormant after birth, cardiac injury reactivates developmental gene programs that stimulate EMT; however, it is not clear how the epicardium contributes to disease progression or repair in the adult. In this review, we will summarize the molecular mechanisms that control epicardium-derived progenitor cell migration, and the functional contributions of the epicardium to heart formation and cardiomyopathy. Future perspectives will be presented to highlight emerging therapeutic strategies aimed at harnessing the regenerative potential of the fetal epicardium for cardiac repair.
Subject Terms: Cell Biology/Structural Biology, Cell Signaling/Signal Transduction, Developmental Biology, Growth Factors/Cytokines, Myocardial Regeneration
Keywords: Epicardium, Cardiac Development, Fibrosis, Paracrine Signaling, Cardiac Repair and Regeneration
Introduction
Cardiovascular disease caused by ischemic injury remains the leading cause of death worldwide1. Millions of cardiomyocytes (CMs) are lost to myocardial infarction (MI), which rapidly triggers an innate immune response to clear dead or dying cells and activate collagen-producing myofibroblasts to remodel the extracellular environment2. Cardiac muscle damage is irreversible as adult mammalian CMs do not re-enter the cell cycle to replace ischemic tissue3. Over the last decade, several strategies have been employed to promote cardiac repair including stimulation of CM cell cycle re-entry, delivery of stem cells or stem cell-derived CMs, modulation of tissue repair using cell-free therapeutics, and applying viral vectors and bioengineered patches to deliver pro-regenerative factors3–5. However, it is becoming apparent that cardiac repair will require active contributions from numerous cardiac cell types.
The epicardium is an evolutionarily conserved layer of mesothelium covering the outermost cell layer of the vertebrate heart. During fetal development, the epicardium serves as a progenitor source, contributing multipotent cells that gives rise to cardiac mesenchyme. However, the role of the epicardium is not limited to de novo formation of non-myocyte lineages. This mesothelial layer also fosters a paracrine milieu critical for myocardial growth and coronary vessel patterning. As the heart matures, the epicardium undergoes a period of dormancy, functioning as a simple barrier between the myocardium and the pericardial cavity. However, recent studies in zebrafish and mice have elegantly shown that cardiac injury reanimates the epicardium and its derivatives to modulate tissue repair6. This review will summarize the dynamic roles of the epicardial lineage during heart development with a focus on developmental programs that may be harnessed to facilitate cardiac repair.
The Epicardium: From origin to a progenitor source
Formation of a functioning heart requires the assembly and integration of multiple progenitor cell populations that arise from embryonic structures such as the cardiogenic mesoderm, the cardiac neural crest, and the proepicardium (PE)7. The PE is an evolutionarily conserved and transient cluster of progenitor cells located at the venous pole of the fetal heart which has been described in many vertebrate species ranging from lampreys to humans8–13. PE-derived cells exhibit a migratory event at embryonic day (E)9.5 in mice and Hamburger-Hamilton stage 17–18 in chick, translocating from the PE and towards the atrioventricular canal (in chick) of the nascent heart. Here, PE-derived cells form a single cell layer of mesothelium, called the epicardium, that lines the outermost layer of the heart11, 12, 14. Here, PE-derived cells form a single cell layer of mesothelium, called the epicardium, that lines the outermost layer of the heart11, 14. Initially, the epicardium exhibits epithelial-like properties, serving as a barrier between the primitive myocardium and the pericardial space. However, a subset of epicardial cells delaminate from the epicardial sheet and invade the myocardium generating a majority of vascular smooth muscle cells (SMCs) and cardiac fibroblasts in the heart15. Thus, the epicardium is critical for providing support cells that contribute to myocardial integrity.
Epicardial Epithelial-to-Mesenchymal Transition
Epicardial-derived progenitor cells (EPDCs) arise from the epicardial layer through an epithelial-to-mesenchymal transition (EMT) event that initiates after Hamburger-Hamilton stage 18 in chick and approximately E12.5 in mice16, 17. In human, expression of EMT markers have been observed in the expanded epicardium surrounding the ventricles at fetal stage 3 (equivalent to E17.5–18.5 in mouse)8. EMT is an evolutionarily conserved process by which epithelial cells lose their apical-basal polarity and cell-cell adhesions, and acquire migratory and invasive characteristics akin to multipotent mesenchymal stem cells18. Progenitor cells that emerge from EMT often give rise to new cell lineages during organogenesis. Early studies in chick embryos employed dyes or retroviral vectors to label epicardial cells, which demonstrated the incorporation of EPDCs in cardiac mesenchyme and coronary vasculature19–21. Epicardial EMT and epicardial-derived cell fates (Figure 1) were subsequently described in mice using genetic fate-mapping approaches that indelibly label all progeny of Cre recombinase-expressing cells with beta-galactosidase (LacZ) or a fluorescent lineage reporter. This technology utilizes specific regulatory sequences to drive the expression of Cre in the epicardium of transgenic mice. Importantly, while it appears that no single gene exclusively marks the epicardium, several gene regulatory sequences have been used to trace epicardial cells and mesothelium-derived cells found in other tissues, including Scx, Sema3d, Gata5, Tbx18, Tcf21 and Wt1. The use of tamoxifen-inducible Cre alleles (Cre recombinase fused to a mutant estrogen ligand-binding domain or CreERT2) has also provided temporal control to study the timing of epicardial EMT and to fate map descendants of the epicardium22. Tables 1 and 2 summarize the current genetic animal models available and the cells and tissues labeled by each marker.
Figure 1. Epicardium During Cardiac Development.
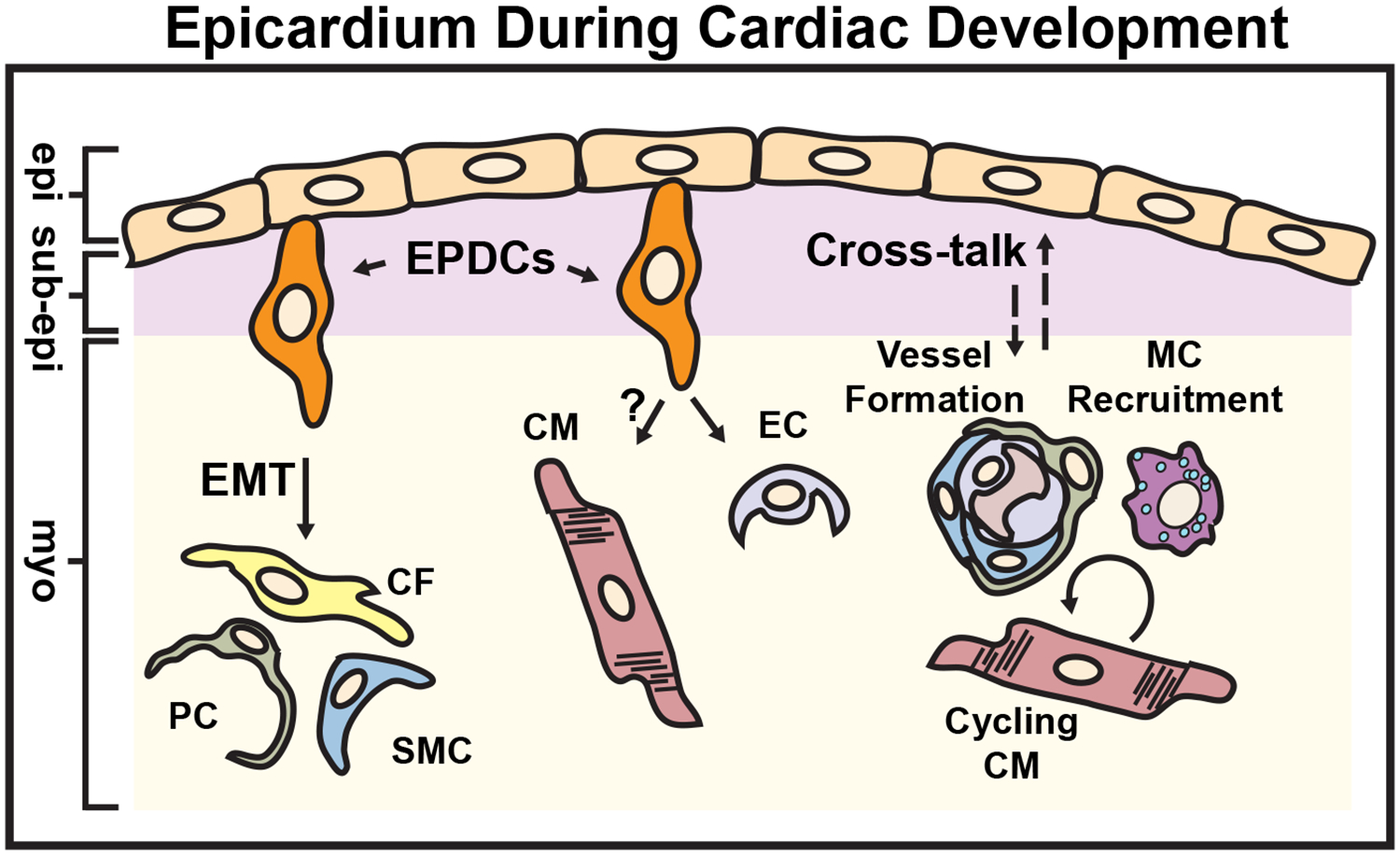
Epicardium-derived progenitor cells (EPDCs) emerge from the fetal epicardium through the process of epithelial-to-mesenchymal transition (EMT) and contribute to various cardiac lineages such as cardiac fibroblasts (CFs), smooth muscle cells (SMCs) and pericytes (PCs). The ability of EPDCs to differentiate into endothelial cells (ECs) in vivo is limited, and CMs are not generally thought to derive from EPDCs. In addition to cellular contributions, the epicardium participates in reciprocal paracrine signaling (dashed arrows) to stimulate CM proliferation, macrophage (MC) recruitment and coronary vessel growth and maturation. epi = epicardium, sub-epi = sub-epicardium, myo = myocardium. (Illustration credit: Ben Smith)
Table 1. Epicardial Lineage Tracing Strategies.
Genetic Cre-loxP-based lineage-tracing strategies have been employed to fate map epicardial-derived cells in mice. Although an epicardium-specific reporter does not exist, a number of epicardium-enriched Cre lines have been described: Wt123, Tcf2131, 163, 164, 171, Tbx1870, 71, cGata5 (chicken Gata5 enhancer)79, Mesothelin (Msln)173, Raldh2 (Aldh1a2)27, 93, Podoplanin (Pdpn)174, Gpm6a160, Scleraxis (Scx)82, Sema3d (Sema3d)82, G2-Gata4 (Gata4 enhancer)83 and cytokeratin175. Among these markers, the most common transgenic mouse lines have been engineered to express Cre recombinase under the control of the Wt115, 65, Tcf2159, Tbx1869, cGata579, Sema3d82, or Scx82 promoters. To indelibly label epicardium-derived progenitor cells, a given Cre line is often bred into a reporter mouse strain (i.e. RosaLacz, RosaeGFP). Fate mapping has demonstrated co-localization of labeled epicardial cells with markers of smooth muscle cells15, 32, 69, cardiac fibroblasts32, 64, 65, 69, 82, valvular interstitial cells64, 65, pericytes43, endothelial cells15, 82, 83 and cardiomyocytes15, 65, 69, 82. In general, the epicardium predominately gives rise to cardiac fibroblasts and vascular mural cells, and to a lesser extent, endothelial cells and cardiomyocytes70, 81.
| Transgenic model | Organism | Description | Cardiac cell fates mapped |
|---|---|---|---|
| G2-Gata4Cre; G2-Gata4LacZ [83] | mouse | Constitutive, transgenic line expressing the conserved CR2 enhancer of Gata4 | EP, EC, SMC83 |
| cGata5Cre [79] | mouse | Constitutive, transgenic line expressing chicken Gata5 promoter fused to Cre | EP, SMC, CF79 |
| Wt1CreERT2 [15] | mouse | Inducible, CreERT2 knock-in into the Wt1 locus | EP, SMC, EC, CM, CF, VIC and PC (TAM E9.5 – E14.5)15, 43, 64, 80 AC (Re)124, 162, Mes (Re)103 |
| Wt1Cre [65] | mouse | Constitutive, BAC transgenic – IRES/EGFP-Cre inserted downstream of the Wt1 translation stop site | EP, CF, VIC and CM65 CFs (Re)60, 113 |
| Wt1GFPCre [15] | mouse | Constitutive, GFP-Cre fusion knock-in into the Wt1 locus | EP, CM15 |
| Tbx18CreERT2 [115] | mouse | Inducible, CreERT2 knock-in into the Tbx18 locus | PC and SMC115 |
| Tbx18Cre [69] | mouse | Constitutive, Cre knock-in into the endogenous Tbx18 locus | EP, SMC, PC, CF, and CM69, 76 CFs (Re)112 |
| Tcf21iCre [59] | mouse | Inducible, MerCreMer knock-in into the Tcf21 locus | EP, CF, SMC (TAM E10.5, E14.5)32 and CF (Re)105 |
| Tcf21Lacz [163, 164] | mouse | Constitutive knock-in into the Tcf21 locus | EP and CF32 |
| tcf21:CreER [111] | zebrafish | Inducible, BAC transgenic – CreERT2 knock-in into the tcf21 locus | EP and SMC; EP and PV (Re)111 |
| tcf21:dsRed [111] | zebrafish | Constitutive | EP, PV, and SMC; Mes and PV (Re)111 |
| ScxGFPCre [82, 165] | mouse | Constitutive | EP, EC, SMC, and CM82 VIC165 |
| Sema3DGFPCre [82] | mouse | Constitutive | EP, EC, SMC, and CM82 |
EP = epicardial cells, SMC = smooth muscle cells, EC = endothelial cells, CM = cardiomyocytes, CF = cardiac fibroblasts, PC = Pericytes, PV = perivascular, AC = adipocytes, Mes = mesenchymal cells, VIC = valve interstitial cells, TAM = tamoxifen, (Re) = reactivation/regeneration
Table 2. Labeling of Cre lines in non-cardiac tissue.
In addition to expression of Wt1, Tbx18, Gata5, Tcf21, Scx, and Sema3d in the pro-epicardium and epicardium, these genes are localized to various non-cardiac tissues.
| Gene | Non-cardiac tissue labeling |
|---|---|
| WT1 | adrenal gland & kidney23, gut166, liver167, lung168, diaphragm169, adipose tissue170 |
| TBX18 | skeletal muscle, brain, retina, lymph nodes, skin, sino-atrial node, adipose tissue, bone marrow115 |
| GATA5 | septum transversum, liver, kidney79 |
| TCF21 | lung, kidney, gonads, spleen, gut, adrenal gland, skeletal muscle59, 163, 171 |
| SCX | tendons and ligaments172 |
| SEMA3D | pharyngeal arch endoderm82 |
Molecular Regulation of Epicardial EMT
Transcription factors (TFs) mediate transdifferentiation of epithelial cells into a mesenchymal phenotype through the repression of genes encoding epithelial adhesion molecules (E-cadherin, claudins, and occludens) and activation of mesenchymal genes (N-cadherin, collagens and fibronectin) necessary for ECM production and migration18. Here, we will highlight known transcriptional regulators, as well as upstream growth factor responsive pathways, implicated in epicardial EMT (Figure 2).
Figure 2. Molecular Control of Epicardial EMT.
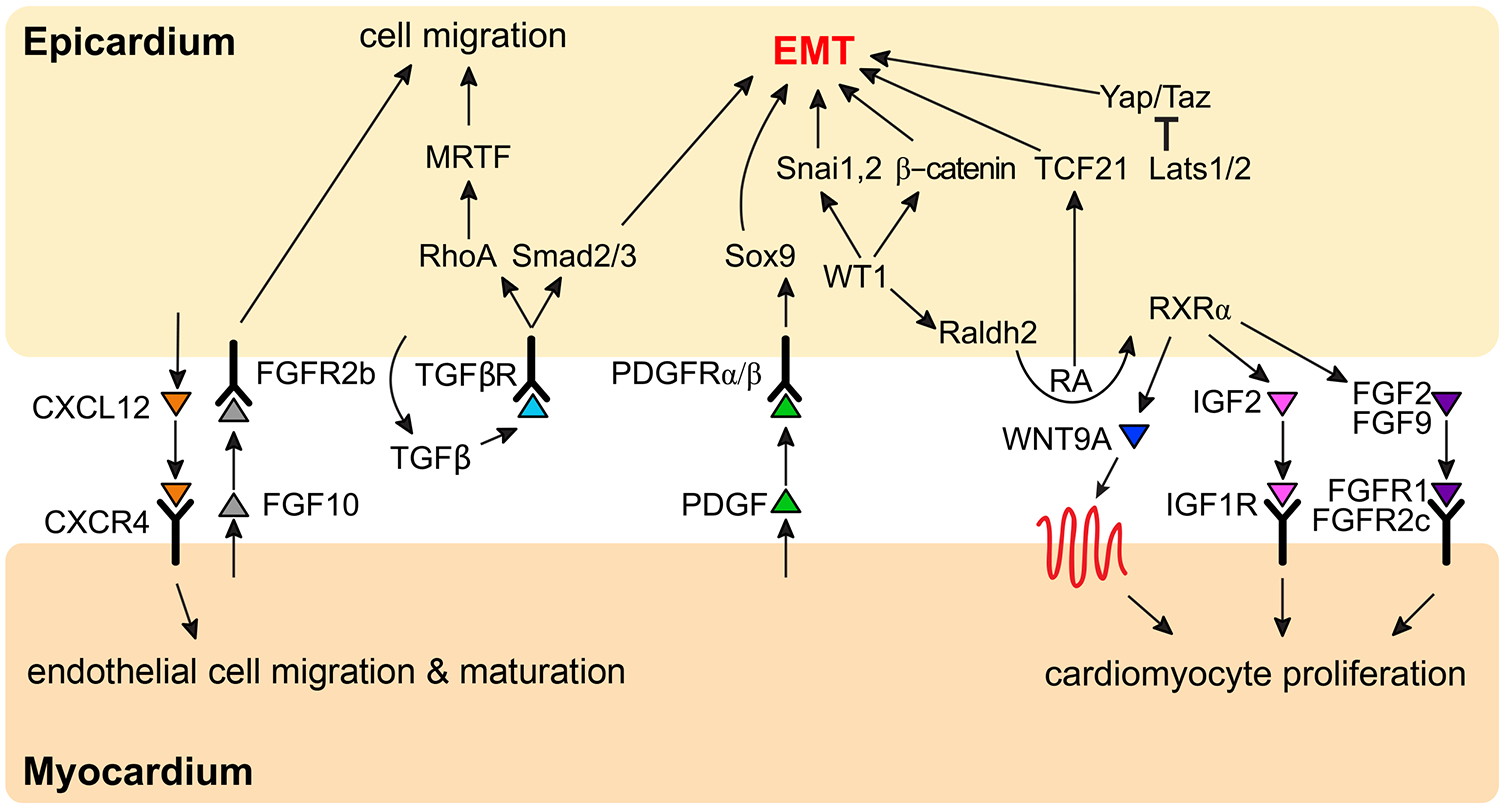
Intrinsic and extrinsic molecular programs regulate epicardial epithelial-to-mesenchymal transition in mice, which include the regulation of transcription factors and molecular signaling. Cytokine mediated signaling between the epicardium and cardiomyocytes is reported to stimulate heart growth, and epicardium-derived signals support the growing coronary vasculature.
Wilms Tumor-1 (Wt1) is a zinc finger TF that is highly expressed in the epicardium during heart development and downregulated concomitant with epicardial EMT23, 24. Global or conditional deletion of Wt1 in mice results in embryonic lethality during mid to late gestation and hearts display defective epicardial EMT and EPDC invasion, which leads to myocardial hypoplasia and abnormal coronary vascular plexus coverage25, 26. These defects may be attributed to altered transcription as WT1 regulates numerous targets during EMT26–29, including activation of Snai1 which represses the expression of epithelial Cdh1 (E-cadherin)25. Wt1 has also been shown to act upstream of canonical Wnt/β-catenin and retinoic acid (RA) signaling, both critical for epicardial biology and EMT17. Consequently, loss of β-catenin in the epicardium disrupts adherens junctions and cell polarity, ultimately impairing EPDC migration and coronary artery formation17, 30.
Tcf21 (Pod1/Epicardin/Capsulin) is a class II basic helix-loop-helix TF highly expressed in the PE and epicardium31. Tcf21-depleted Xenopus embryos fail to form a cohesive sheet of mesothelial cells lining the heart, suggesting that Tcf21 is critical for PE migration and proper formation of the epicardium13. In mice, Tcf21 regulates epicardial EMT as evidenced by downregulation of Snai1, Sox9, and Zeb1 in Tcf21 null epicardial explants32. The exact mechanisms linking Tcf21-dependent transcription to epicardial homeostasis and EMT remain unclear, although interactions with transcriptional co-repressors and direct binding to E-box consensus sequences (CAnnTG) may contribute to these processes13, 33.
Regulation of EMT and motility gene programs by Hippo signaling and myocardin-related transcription factors (MRTFs)/Serum Response Factor (SRF) are required for the invasion of EPDCs into the myocardium. Yap (Yap1) and Taz (Wwtr1) are evolutionary conserved co-factors negatively targeted by the Hippo pathway to restrain organ size34. In addition to regulating contact inhibition, cell proliferation and stem cell self-renewal, Yap and Taz also mediate EMT in various contexts34. Conditional deletion of Yap and Taz using the Wt1CreERT2 allele diminishes the expression of multiple EMT associated TFs including Snai1, Snai2, Twist1, Wt1 and Tbx1835. Interestingly, the Hippo pathway must be tightly controlled in the epicardium as Yap/Taz-deficient (active Hippo) or Lats1/2-deficient (inactive Hippo) murine EDPCs exhibit defects in cell migration and fibroblast differentiation35, 36.
SRF is a TF that is essential for the formation of the PE and epicardial EMT37, 38. SRF binds to a DNA motif called a CArG box [CC(A/T)6GG], activating gene programs related to actin cytoskeletal rearrangements, cell adhesion and migration, and vascular cell commitment37–40. Nuclear SRF in epicardial cells corresponds with increased levels of smooth muscle genes, whereas expression of dominant negative SRF inhibits smooth muscle formation38. SRF transcriptional activity is greatly enhanced by interactions with the MRTF family of signal responsive co-factors41. MRTFs are tethered in the cytoplasm via interactions with monomeric actin, which conceals a nuclear localization signal42. Mechanical stretch or pathways that activate Rho-kinase and actin polymerization lead to nuclear accumulation of MRTFs and activation of SRF-dependent gene programs42. MRTF deletion using the WtCreERT2 allele results in decreased migration of EPDCs during developmental EMT coupled with delayed maturation of the coronary plexus43. These findings highlight the importance of signal responsive transcriptional regulation in epicardial EMT and EPDC differentiation. Generally, EPDCs display heterogeneous expression of epicardial TFs in both the mouse and chick epicardium44; thus, the diverse epicardium-derived lineages described in this review may arise in part due to intrinsic differences in EPDCs that modulate the response to paracrine cues.
Growth Factor-Dependent Regulation of Epicardial EMT
Transforming growth factor-β (TGFβ) is among the most well-studied upstream regulators of EMT and ECM production45. TGFβ1–3 are all expressed in the mouse epicardium by E12.546 and targeted deletion of type I TGFβ receptor Alk5 using the chicken Gata5 enhancer (cGata5)-Cre perturbs epicardial EMT, leading to myocardial hypoplasia, and attrition of SMCs and microvascular structures47. Likewise, global deletion of type III TGFβ receptor (Tgfbr3−/− mouse model) abrogates the coronary plexus, which is attributed to dysregulated nuclear factor kappa-light-chain enhancer of activated B cells signaling in the epicardium and defects in bone morphogenetic protein 2-mediated EPDC motility48, 49. Mouse and human epicardial explants stimulated with TGFβ1 and/or TGFβ2 lose epithelial markers (Tight junction protein Zonula occludens-1 and Cytokeratin) and upregulate SMC markers (α-smooth muscle actin, Transgelin or SM22, Calponin, and Caldesmon) indicative of EMT50, 51. TGFβ2 stimulation also amplifies epicardial motility by indirectly stimulating expression of hyaluronan synthase 2 (Has2) and the production of the ECM component hyaluronan (HA)52.
Platelet-derived growth factor (PDGF) signaling is ubiquitous during mouse cardiogenesis and regulates critical processes such as cardiac neural crest cell recruitment to the outflow tract, coronary vessel maturation and myocardium development53–55. PDGF receptor (PDGFR)α and PDGFRβ are receptor tyrosine kinases that play critical roles in regulating epicardial EMT and EPDC specification56. Epicardial cells null for Pdgfra/b are unresponsive to pro-EMT stimuli (TGFβ, fibroblast growth factor-2, and PDGF-BB) and express low levels of EMT TFs (Snai1 and Sox9) and mesenchymal genes56. Defects in EMT can be rescued by forced overexpression Sox9 in PDGFR mutant cells, suggesting that Sox9 is downstream of PDGFRα/β signaling56. Conditional or global deletion of each receptor separately or together, using cGata5-Cre or WT1CreERT2 alleles, limits EPDC motility by disrupting phosphoinositide 3-kinase-mediated regulation of actin polymerization56–58.
Epicardium-Derived Interstitial Lineages
Various cardiac interstitial cell lineages, including fibroblasts, vascular mural cells, and to a limited extent, endothelial cells (ECs), arise from the epicardium (Figure 1).
Cardiac fibroblasts
Lineage tracing and reporter mouse lines such as Tcf21MerCreMer/+;Rosa26EGFP59, Collagen1a1(Col1a1)-GFP60, 61 and PDGFRαGFP/+62 facilitate the identification of resident cardiac fibroblasts originating from EPDCs63, which are especially populous in regions near the sinoatrial node, atrioventricular cushions and valves, and annulus fibrosis64–67. Notably, Tcf21 controls the specification of EPDCs to SMC or fibroblast lineages during murine embryonic development68. Tcf21 null hearts have fewer Col1a1-GFP+ fibroblasts while the number of SMCs remain unaltered32. In contrast, activation of Tcf21 through exogenous RA to embryonic chick hearts reduces the number of SMCs44. In addition to Tcf21, upstream cues such as PDGF signaling can influence cardiac fibroblast cell fate. PDGFR isoforms display overlapping expression patterns in the early stages of epicardial formation but become distinct by E13.5 in murine models56. Pdgfra loss-of-function impairs EPDC differentiation into cardiac fibroblasts while conditional deletion of Pdgfrb impacts the vascular SMC lineage56. These findings suggest that PDGFRα and PDGFRβ regulate distinct epicardium-derived developmental trajectories.
Vascular mural cells
Vascular SMCs lining the coronary arteries were first demonstrated to arise from PE-derived cells in avian models19. These findings were later substantiated in mice using Cre-dependent tracing of cells originating from Tcf2156, Wt115 and Tbx1869-expressing progenitors. Tbx18 belongs to a large family of T-box TFs that regulate a variety of developmental processes in vertebrates and invertebrates and is enriched in the PE, epicardium, subepicardial mesenchyme, septum transversum, and a subset of the myocardium70–72. Tbx18 is dispensable for epicardial development and EMT; however, loss of Tbx18 results coronary vessel maturation defects70, 73. Forced expression of Tbx18 prevents SRF-dependent activation of smooth muscle reporters73. Conversely, conditional misexpression of Tbx18VP16 (fusion of the Herpes simplex virus encoded protein VP16 to the C-terminus of Tbx18) results in hypoplastic hearts and ectopic smooth muscle differentiation mediated by Notch3 and TGFβ signaling74, 75. Mature SMCs (Smooth muscle myosin heavy chain, also known as MYH11+) are reported to arise via the differentiation of Tbx18 lineage-derived pericytes76, which are stellate-like cells that encapsulate the microvasculature and preserve vascular integrity. Epicardium-derived cardiac pericytes, as defined by expression of PDGFRβ and chondroitin sulfate proteoglycan 4 (also known neuron-glial antigen 2), are required for the stability and growth of the embryonic coronary plexus43, 77. Indeed, EMT deficiency associated with genetic deletion of MRTFs in the epicardium depletes the heart of cardiac pericytes, leading to reduced vascular integrity and sub-epicardial hemorrhage43.
Vascular endothelial cells
Coronary ECs are predominately derived from non-epicardial sources78. Lineage tracing studies in mice have excluded epicardial-derived ECs from Tbx18, c-Gata5, or Tcf21 lineage-derived EPDCs32, 69, 79. However, Wt1-derived epicardial cells have been shown to give rise to ECs in the developing chick embryo21 as well as EPDCs fate mapped from the Wt1CreERT2 lineage after administration of tamoxifen at E10.5 or early postnatal time points in murine models15, 80. However, the contribution of the epicardium towards the EC fate has been challenged by reports of endogenous Wt1 expression in mature endothelium beginning at E12.5 and persisting throughout postnatal mouse development81. Scleraxis (Scx) and Sema3D positive epicardial cells form rare populations of ECs validated by in vivo fate mapping and in vitro cultures82. While the Sema3DGFPCre, ScxCre, Wt1CreERT2, and G2-Gata4Cre epicardial Cre lines82, 83 may sporadically label the endothelium, the majority of cardiac ECs are derived from the sinus venosus and endocardium77.
The Epicardium-Derived Secretome
The previous sections highlight the complexity of epicardial EMT and epicardium-derived cell fate determination, which are controlled by both cell autonomous and cell non-autonomous mechanisms. The following sections will discuss some of the intercellular signaling cascades underlying paracrine regulation of CM and vascular development and homeostasis (See Figure 2).
Epicardium-Cardiomyocyte Crosstalk
In addition to directly differentiating into various cell lineages, the epicardium also supports CM proliferation and embryonic heart growth by providing a cocktail of secreted mitogens. Fibroblast growth factors (FGFs) FGF1, 2, 4, 6, 9, and 16 are all expressed by the fetal epicardium84, 85. In particular, both endocardial and epicardial FGF9 signals regulate CM proliferation via activation of fibroblast growth factor receptors (FGFR) FGFR1c and FGFR2c84. In contrast, FGF10 secreted from the myocardium promotes EPDC invasion and EPDC-to-fibroblast conversion by signaling through FGFR2b expressed on epicardial cells86. The mitogenic activity of CMs can be stimulated by the secretion of insulin growth factor-2 (IGF2) from epicardial cells in vitro, a phenomenon that is recapitulated through the pro-proliferative signals emanating from the developing epicardium in vivo87, 88. In fact, global deletion of Igf2, or conditional deletion of its receptor Igfr1 in Nkx2.5 expressing CMs, results in severe cardiac defects in the form of ventricular non-compaction88, 89.
RA signaling is critical for proper cardiac morphogenesis90, 91 and can induce the expression of epicardial-restricted TFs such as Wt1 and Tcf2144. RA regulates transcription by binding to nuclear retinoic acid receptors which form heterodimers with retinoid X receptor (RXR)92. RXRα−/− mice display normal cardiac mouse development up to E11.5; however, thereafter fail to undergo proper myocardial compaction and become severely hypoplastic90, 91. RA signaling stimulates epicardial secretion of trophic signals such as WNT9A, FGF2, FGF9 and IGF2 to promote myocardial growth and proper coronary vessel maturation79, 84, 87, 88. RA is synthesized by the oxidation of retinaldehyde by retinaldehyde dehydrogenases (Raldh 1, 2 and 3)92, which are highly expressed in the fetal mouse epicardium at E12.593. Global deletion of Raldh2 results in embryonic lethality around E9.5 with a single dilated cardiac chamber94. Remarkably, this phenotype can be partially rescued by maternal supplementation of exogenous RA94.
Epicardium-Endothelial Cell Crosstalk
The epicardium is also an important source of chemokines that regulate coronary vessel patterning. Prevention of pro-epicardial outgrowth, and genetic ablation of epicardial cells with diphtheria toxin results in disorganized coronary vasculature and reduced recruitment of sub-epicardial cardiac macrophages95, 96. Wt1 knockout cells also express lower levels of vascular growth factors such as angiopoietin-1 and vascular endothelial growth factor A (VEGF-A)26, and Wt1 is needed to suppress the production of pro-inflammatory cytokines chemokine ligand 5 and C-X-C motif chemokine ligand (CXCL) 10 in epicardial cells28. Thus, the epicardium serves as an essential secretory hub to maintain vascular and inflammatory cell stability in the fetal heart. ECs also communicate with the developing epicardium by facilitating the induction of EMT and differentiation towards mural cell lineages via activation of PDGFRβ97. Pericyte recruitment to the microvasculature is coordinated in part by the expression of the Notch ligand Jagged-1 in ECs activating Notch3 receptors on epicardial-derived pericytes76. CXC chemokines and receptors (CXCR), such as the CXCL12-CXCR4 axis has been shown to play an essential role in coronary artery maturation98. Cxcr4 in zebrafish is expressed in angiogenic ECs and mature coronary arteries lined by mural cells, whereas cxcr4 deletion results in disorganized angiogenic sprouts98. In contrast, Cxcl12 is expressed in the epicardium and epicardial-derived mural cells in both zebrafish and mouse models98, 99. Although localized expression of CXCL12 is sufficient to increase capillary density through the recruitment of CXCR4+ ECs, global myocardial overexpression disrupts vascular formation98. These findings indicate that discrete gradients of chemotactic cues are required during the intricate process of coronary vessel formation.
Overall, these data support a critical cell non-autonomous role for the epicardium in the fetal heart. Thus, the role of epicardial EMT appears to be two-fold: 1) Contribution of multipotent progenitors and 2) Secretion of growth factors necessary for embryonic heart formation (Figure 1 and 2). Harnessing fetal epicardium programs that build the developing heart may have therapeutic implications for the stimulation of scarless cardiac repair through regenerative medicine, which is discussed below.
Overview of Cardiac Repair and Regeneration
Advances in emergency medicine and cardiology have led to an abundance of patients surviving a MI. However, these successes have revealed deficiencies in supporting cardiac repair after an initial injury, which results in a growing population living with chronic heart disease. Adult mammalian CMs are postmitotic with a limited capacity for self-renewal following acute insult or over periods of chronic disease100. Therefore, progressive loss of CMs via apoptotic or necrotic cell death leads to the replacement of damaged contractile tissue with a fibrotic scar, irreversible cardiac remodeling and organ failure2. Recent studies have highlighted that CM renewal can be enhanced through molecular signaling pathways converging on the induction of critical cell cycle regulators (e.g. Cyclin A2), growth factors (Neuregulin1, IGF1/2), and intrinsic signaling pathways (e.g. Hippo)3. Therefore, understanding endogenous cardiac repair mechanisms is an emerging topic of intense investigation that may lead to new regenerative medicine strategies.
Reactivation of the Adult Epicardium
Fetal epicardial gene activation after myocardial injury is thought to represent a conserved cardiac repair mechanism101. Wt1, Tbx18, Raldh1 and Raldh2 transcripts are all transiently upregulated between 1 to 5 days after MI in mice, particularly surrounding the valves and atria, and wane in expression by 2 to 4 weeks after injury102, 103. Fetal epicardial gene induction correlates with increased proliferation and pronounced epicardial thickening, as documented in both adult mice and canine models102, 103. Unlike the fetal epicardium, which undergoes EMT and generates cardiac fibroblasts and vascular mural cells, lineage tracing experiments reveal minimal contribution of the epicardium to new adult fibroblasts or to collateral formation (Figure 3). In contrast, the adult epicardium is thought to serve as a source of paracrine signals with limited regenerative potential103. Thus, an emerging area of investigation includes developing methods to harness epicardial-derived developmental programs to promote cardiac repair and regeneration in adult hearts. The remainder of this review will highlight what is currently known regarding the role of the epicardium in cardiac regeneration and disease, and how we may take advantage of EPDC plasticity and signaling to promote efficient cardiac repair and remodeling and advance regenerative based medicine for the treatment of human cardiac disease.
Figure 3. Resident Epicardium-Derived Fibroblasts Contribute to Fibrosis Following Myocardial Infarction.
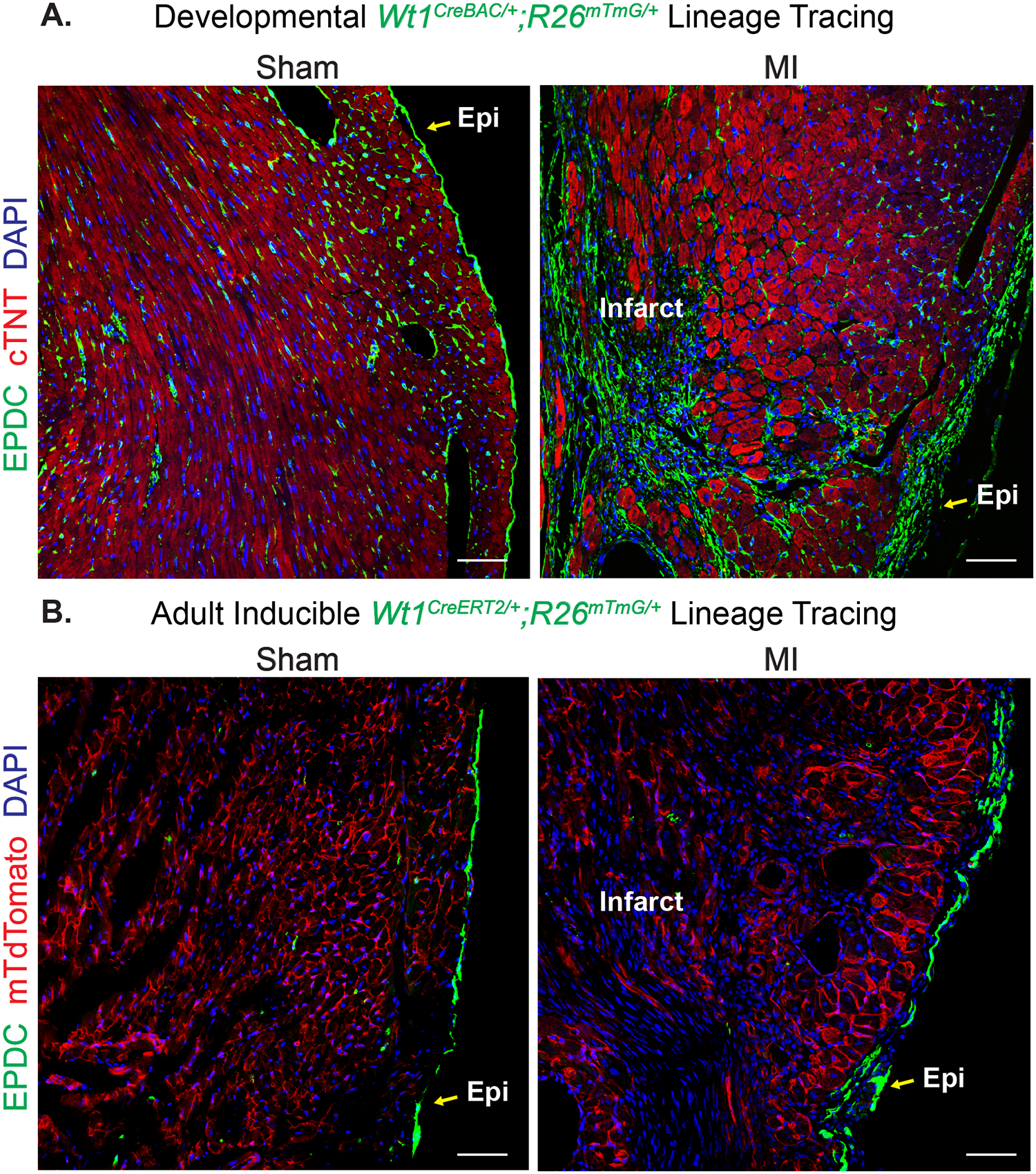
Evaluation of epicardium-derived cell distribution in left ventricles of mice subjected to sham or myocardial infarction (MI). Epicardial cells are visualized (GFP+=green) by crossing constitutive (Wt1CreBAC/+) or tamoxifen-inducible (Wt1CreERT2/+) Cre lines to the Rosa26mTmG Cre-dependent fluorescent lineage reporter mouse line. (A) Epicardial cells of developmental origin (Wt1CreBAC/+; Rosa26mTmG/+) were observed on the epicardial surface (Epi), and in both perivascular and interstitial areas of the left ventricle of sham-operated hearts. Upon cardiac injury, pre-existing GFP+ cells contribute to epicardial thickening and the formation of a cellularized scar. Green= GFP+ EPDCs, Red = cardiac troponin T or cTNT, Blue = DAPI to visualize nuclei. (B) When tamoxifen was administered to 8-week old Wt1CreERT2/+; Rosa26mTmG/+ mice, GFP+ cells were observed primarily on the epicardial surface of the left ventricle in sham operated animals. After MI, adult Wt1-lineage derived cells increase in number at the epicardial border surrounding the borderzone region of the left ventricle, but they do not directly contribute to scar formation. Green= GFP+ EPDCs, Red = membrane tethered tdTomato (non-recombined cells), Blue = DAPI to visualize nuclei. Scale bars for (A) and (B) = 50μm.
Pre-existing Fibroblasts Contribute to Cardiac Fibrosis
The developmental origins of a majority of cardiac fibroblasts in the adult heart can be traced back to the fetal epicardium32, 56. These fibroblasts make up ~20% of the total composition of cells in the mature mammalian heart104, however, mechanical and/or ischemic stress leading to the loss of CMs and vasculature can increase the total numbers and volume of fibroblasts following cardiac injury. Resident cardiac fibroblasts labeled with Col1a1-GFP, PDGFRα and Tcf21 transgenic alleles display the ability to proliferate after injury and give rise to a majority of cells in the fibrotic scar32, 105. Myofibroblasts, labeled by Periostin (Postn), emerge from Tcf21 lineage-traced epicardium-derived fibroblasts when mice are subjected to MI or left ventricle pressure overload and are a major source of ECM105, 106. Alterations in fibronectin (FN), mitogen-activated protein kinase, or Wnt/β-catenin signaling in the Tcf21 lineage significantly alters fibroblast to myofibroblast conversion in response to cardiac stress107–109. Interestingly, it was proposed that subpopulations of myofibroblasts re-express Tcf21 following cardiac injury105, which may indicate that a subset of myofibroblasts revert towards quiescence and/or senescence in chronic stages of disease. Although pre-existing resident cardiac fibroblasts originating from the epicardium during development are the primary source of cardiac scar tissue during ischemic and non-ischemic remodeling, nascent EPDCs and resident or infiltrating inflammatory cells have also been observed within the scar tissue, albeit to a lesser extent60, 110 (Figure 3). Tcf21, Tbx18 and Wt1 serve as markers of both developmental and injury-induced epicardial-derived fibroblasts in zebrafish and mammalian adult hearts60, 105, 110–112. In both the human and adult mouse heart, fibroblasts expressing Wt1, Tbx18 and Tcf21 proteins are observed within interstitial fibrosis following ischemic injury110. In contrast, perivascular fibrosis was primarily composed of Tcf21+ fibroblasts, but not Wt1+ or Tbx18+ cells, potentially indicating heterogeneity within epicardial derived fibroblast lineages. While this study depended on the endogenous protein labeling of epicardial markers, it is interesting to speculate that spatial diversity of epicardial-derived lineages may contribute to functional differences in cardiac repair and remodeling.
In addition to the Tcf21 and Postn lineages, robust investment of Wt1 lineage fibroblasts have been observed in the scar after cardiac injury. Tamoxifen-inducible tracing via Wt1-directed CreERT2 in the adult mouse heart reveals that EPDCs have enriched expression of Snai1, Snai2 and Smad1 and display a propensity towards secretion of pro-angiogenic factors as compared to non-EPDCs following MI102, 103. Re-activated Wt1-derived cells acquire a phenotype consistent with fibroblast and myofibroblast lineages as evidenced by the expression of FN, fibroblast-specific protein 1, collagen type III alpha 1, and smooth muscle markers, but are generally devoid of endothelial or cardiomyogenic cell markers103. Although the contribution of new Wt1+ cells towards various intramyocardial fates is limited in the adult, the Wt1 lineage originating during development is the major source of mesenchymal cells within the heart during stress (Figure 3). The constitutive Wt1-Cre line labels >90% of the fibroblasts present in the left ventricular free wall at baseline and following trans-aortic constriction surgery (TAC) or MI60, 113. Additionally, 96% of the Wt1-Cre lineage cells expressed the ECM gene Col1a160. Inhibition of embryonic Wt1-Cre lineage mobilization via deletion of Srf or Mrtfs led to a significant reduction in the number of epicardium-derived fibroblasts colonizing the post-MI infarct114. Interestingly, the WT1 lineage depleted scar was populated with PDGFRα+ cells, apparently from an alternative source114.
In complementary lineage tracing experiments, resident fibroblast composition within the uninjured heart is ~75% Tbx18+ and 90% Wt1+ 60, 112, 113. Tbx18+ / Thy1 cell surface antigen+ fibroblasts are dispersed throughout the heart and express markers of fibroblasts, SMCs and cardiac pericytes112, whereas Wt1-lineage cells are primarily localized to the ventricular septum and left ventricle free wall60, 113. Although injury typically increases the total number of fibroblasts in the heart due to proliferation, the percentage of either Tbx18 or Wt1-derived fibroblasts were unaltered following TAC60, 112. This data may be interpreted to indicate resident fibroblasts may arise from the heterogeneous compilation of various developmental epicardial lineages.
Mesenchymal cell derivatives such as cardiac pericytes have also been implicated in fibrotic diseases. However, Tbx18-lineage derived cardiac pericytes do not exhibit mesenchymal cell characteristics in vivo after TAC or prolonged aging in mice115. Due to these inconsistencies, further investigation is required to rule out a potential role of pericytes in adult cardiac repair processes, particularly in vascular remodeling during ischemic events. Taken together, these studies also conclude that epicardial activation does not contribute to the formation of de novo fibroblasts during adult pressure overload or following MI. Overall, understanding the cellular origin and heterogeneity of cardiac mesenchymal cells may offer critical insights for ameliorating the proliferation and/or differentiation of pathological myofibroblasts during the progression of heart disease.
The Epicardium Modulates Inflammation
Acute inflammation is a compensatory response that clears tissue of dead cells; however, chronic inflammation can increase cell death, formation of fibrosis and cardiac dysfunction116. Recent reports suggest that cellular and paracrine inflammatory processes are in part coordinated by the re-activated epicardium95, 117. CD45+ hematopoietic cells emerge during fetal development alongside Wt1+ EPDCs, and both become activated and migrate towards the subepicardium upon cardiac injury118. Resident cardiac macrophages with reparative characteristics (M2 macrophages) are observed in the healthy epicardium, but are significantly depleted in models of aging related cardiac dysfunction119. Wt1b is an early epicardial response gene that overlaps with expression of pro-regenerative macrophages in zebrafish120 implicating the requirement of the epicardium in the recruitment of cardiac macrophages as observed during development95. Indeed, epicardial deficiency in YAP/TAZ signaling exacerbates cardiac inflammation after MI due to the limited recruitment of immunosuppressive regulatory T cells and depressed secretion of inflammatory cytokine interferon-γ from the adult mouse epicardium121. Attempts to modulate epicardial activation have harnessed the activity of CCAAT/enhancer binding proteins, a group of TFs that bind to enhancer elements controlling epicardial gene expression117. Adenoviral delivery of CCAAT/enhancer binding protein following ischemia/reperfusion (IR) injury in mice enhances activation of Wt1 and Raldh2, blunting cardiac damage and the initiation of fibrosis, presumably through decreased acute neutrophil infiltration117. These studies emphasize the importance of epicardium reactivation in inflammatory signaling and the promise of harnessing epicardial cell biology for cardiac repair.
Epicardium Contribution to Subepicardial Adipose Tissue
The human heart is surrounded by a layer fat called epicardial adipose tissue (EAT) that lies beneath the epicardium. The extent of EAT has prognostic value and correlates with various pathological conditions such as coronary artery disease and ischemic remodeling122. While a better understanding of the developmental source and molecular mechanisms that control EAT accumulation may have therapeutic implications, the study of EAT biology has been hampered by its paucity in experimental model organisms. Advances in genetic lineage tracing in mice have recently revealed that EAT originates from the epicardium in a process mediated by the metabolic regulator peroxisome proliferator-activated receptor-γ123. Furthermore, delivery of IGF-1 to mice augments epicardial cell conversion into adipocytes in experimental MI124.
Importantly, disorders such as arrhythmogenic right ventricular cardiomyopathy are characterized by fibro-fatty tissue deposition that replaces functional heart muscle and markedly disturbs cardiac conduction in human patients125–127. Recent evidence suggests that mutations in the Plakophilin-2 gene are causative in arrhythmogenic right ventricular cardiomyopathy through disruption of the CM desmosome128. In addition, Plakophilin-2 may modulate epicardial cell migration and initiate an adipogenic program in epicardial cells, potentially relevant due to the epicardium-to-endocardium gradient of fibrofatty tissue deposition129. Defining the cellular and molecular avenues leading to fibrosis and the formation of EAT may thus lead to new clinical treatment options to prevent progression of arrhythmogenic cardiomyopathies. Collectively, reactivation of the adult heart due to cardiac disease encourages cell contributions and paracrine signals from the epicardium to take part in fibrotic, adipogenic, and vasculature remodeling, CM growth and cell death, and inflammatory signaling (Figure 4). Harnessing the regenerative power of the fetal epicardium at the expense of insult-responsive pathological programs holds promise for the advancement of new cardiac restoration strategies, as described in regenerative models below.
Figure 4. Epicardium-Derived Cells in the Cardiac Injury Response.
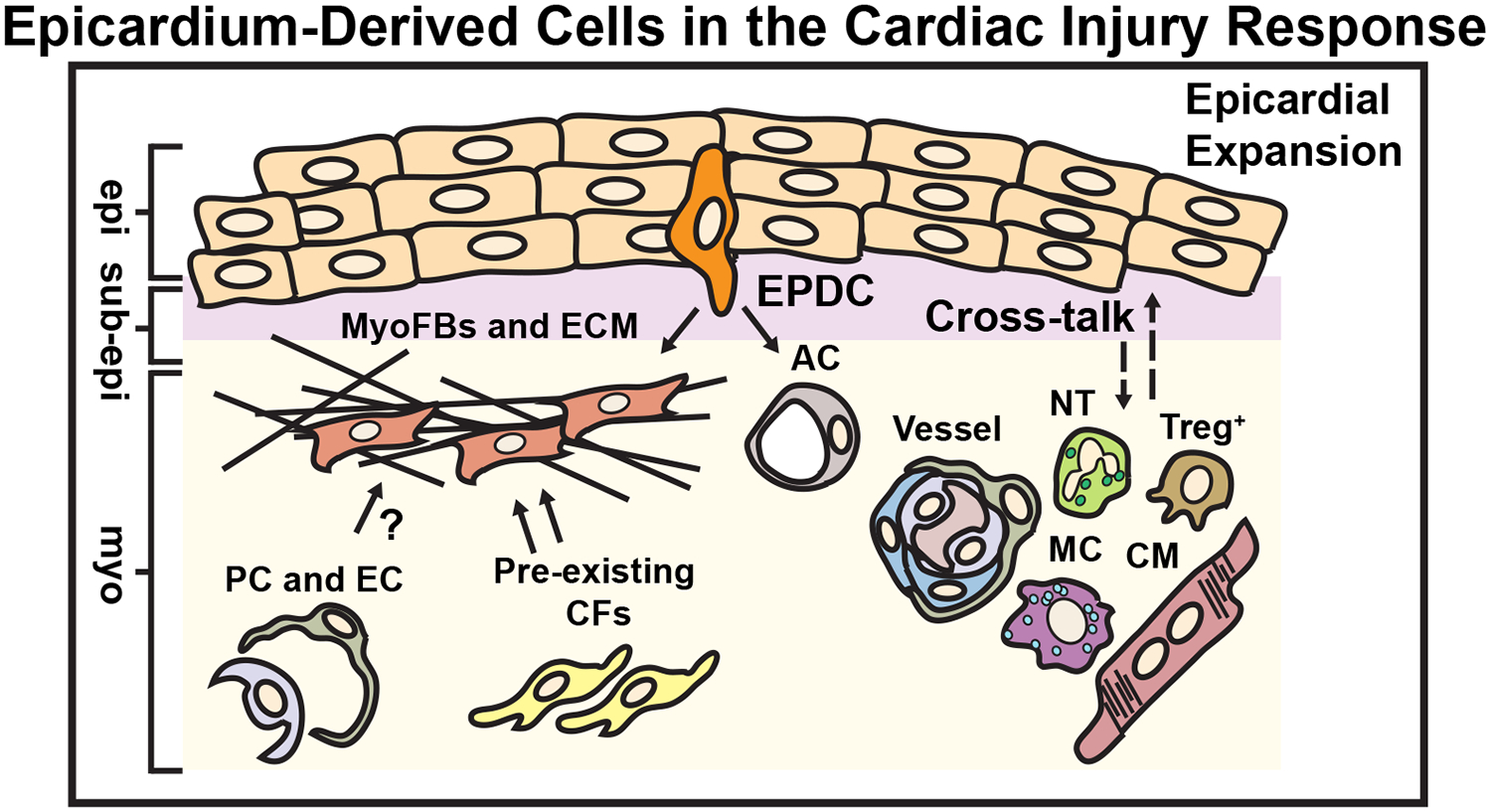
Pre-existing epicardium-derived resident cardiac fibroblasts are the primary source of MyoFbs that generate scar tissue during pressure overload or ischemic remodeling through the secretion ECM. Epicardium-derived pericytes (PC) or endothelial cells (EC) have also been reported to exhibit mesenchymal cell characteristics and produce ECM in response to injury. The adult epicardium is reactivated and expands in response to cardiac injury. A small proportion of epicardium-derived progenitor cells (EPDCs) undergo epithelial-to-mesenchymal transition (EMT) to become myofibroblasts (MyoFbs) that secrete extracellular matrix (ECM). In limited cases, EPDCs can become adipocytes (AC). The activated epicardium also promotes the recruitment of inflammatory cells (Neutrophils or NT, T-regulatory cells or Treg+, and Macrophages or MC) and stabilizes coronary vasculature through the secretion of chemokines (dashed arrows). The epicardium may also promote cardiomyocyte (CM) survival and/or cell growth. epi = epicardium, sub-epi = sub-epicardium, myo = myocardium. (Illustration credit: Ben Smith)
Mechanisms of Cardiac Regeneration
Many adult amphibians and fish possess an incredible capacity to completely regenerate damaged tissue, including the heart101. Tissue repair in axolotl and zebrafish is initiated by the migration of multipotent mesenchymal cells to the wound and the formation a regenerative blastema6. Particularly in zebrafish hearts, surgical removal of ~10–15% of the cardiac apex results in the formation of a reversible fibrin clot followed by the remarkable restoration of cardiac muscle via the proliferation of pre-existing CMs130. Zebrafish heart regeneration occurs efficiently due to four essential processes: 1) efficient CM proliferation, 2) endocardium activation, 3) re-activation of epicardial fetal gene expression, and 4) efficient resolution of fibrosis130, 131. In contrast, the adult mammalian heart is unable to regenerate following injury; however, neonatal mammals retain the capacity to regenerate cardiac tissue following apical resection, cryoinjury, or MI when the injury is performed prior to 7 days after birth, which is within the window when pre-existing CM proliferation can be stimulated132, 133. Non-myocytes, including epicardial cells, are reported to play critical roles in cardiac regeneration by regulating inflammation, stimulating CM proliferation and modulating ECM turnover134.
The ECM Landscape in Regeneration
ECM modulates the fate and function of EPDCs in the embryo or during regenerative cardiac repair; and likewise, epicardial cells provide a unique extracellular milieu that mediate cardiac healing. In the healthy adult mouse heart, clusters of 2 or more Wt1+ cells are encased within ECM components such as FN, Collagen IV and HA, which help to define an epicardial-specific niche118. In zebrafish, the epicardium supports CM recruitment to sites of injury via the secretion of fn1 and fn1b, which presumably bind the FN receptor (integrinb3) on CMs and promote motility required for regeneration135. In addition to FN, HA can also promote regeneration. HA signaling through the hyaluronan-mediated motility receptor (Hmmr) is induced 3 days post resection of the zebrafish heart; blocking Hmmr or inhibiting HA synthesis suppresses CM proliferation and exacerbates scar formation136. Importantly, inhibition of the HA-Hmmr axis perturbs cell-to-cell anchoring via focal adhesion kinase, which limits the expression of EMT genes snail1a, snail1b, snail2, and twist1136. Collectively, these data demonstrate that epicardial ECM production and EMT are essential in processes of adult cardiac regeneration.
Paracrine Regulation of Zebrafish Cardiac Regeneration
Intercellular signaling between the myocardium, epicardium, fibroblasts and endocardium coordinate regenerative processes in the adult zebrafish heart. PDGF, FGF, IGF, and VEGF ligands influence CM growth and proliferation in development and are proposed to promote CM dedifferentiation and the upregulation of cell cycle regulators during regeneration137–140. Disruption of the epicardium can lead to a marked reduction in regenerative programs consistent with the requirement of cell non-autonomous and epicardial-secreted factors in CM proliferation. Based on initial microarray analysis of the early zebrafish cardiac wound, pdgfa and pdgfb ligands are both highly expressed in regenerating tissue141. Administration of PDGF-BB is sufficient to induce CM proliferation, whereas PDGF inhibition prevents CM DNA synthesis in culture141. Zebrafish hearts exhibit an upregulation of classical EMT regulators (snai2 and twist1b) that correlate with induction of the mural cell marker gene, Pdgfrβ, while Pdgfrα, a fibroblast marker, was unchanged140. Inhibition of PDGFR following injury limits epicardial proliferation in vivo and further suppresses the expression of EMT (snai2) and mural cell (acta2) markers141. Notably, PDGFR inhibition leads to impaired coronary vessel formation signifying the requirement of PDGF signaling in the process of revascularization and regeneration in the zebrafish heart140.
FGF signaling functions downstream of RA signaling and is necessary for fin repair and cardiac regeneration in zebrafish138, 142. Global deletion of fgfr1 results in a reduction of Tbx18+ EPDCs in the ventricle and correlates with increased incidence of CM hypoplasia142. Following acute injury, fgfr2 and fgfr4 are increased in EPDCs surrounding the atria whereas fgfr4 is expressed adjacent to the wound and up to 30-days post resection138. Suppression of FGFR signaling in the epicardium disrupts EMT and neovascularization of damaged tissue138.
IGF was discovered as a potent mitogen during cardiac development87. Activated igf2b is observed in the endocardium and epicardial surface and surrounding apical wounds of the adult injured zebrafish heart117, 139. Limiting igf2 or igf1r suppresses DNA synthesis in CMs following resection139. Further examination of CM proliferation using the gata4:EGFP reporter confirms that IGF signaling is required for the proliferation of subepicardial CMs during wound repair139. Notch receptor (notch1a, notch2, notch3) expression is upregulated in both the epicardium and endocardium; Notch activity is required for regenerative replacement of CMs, but is dispensable for the formation of primitive endothelial tubes in the wound area143. The epicardium is also a source of soluble factors that control CM and vasculature regeneration through the secretion of vegfaa137. Interestingly, PDGF, FGF and VEGF ligands interact with Neuropilin transmembrane receptors, which are activated in response to cryoinjury in both endocardial and epicardial cells144. Loss-of-function of neuropilin1a significantly impairs Wt1-induced epicardial reactivation and EMT following cryoinjury144.
Of note, the epicardium itself has a regenerative capacity. Deletion of the epicardium in the adult zebrafish resection model significantly reduces CM proliferation and regeneration134. Hedgehog signaling, expressed primarily in the bulbus arteriosus (analogous to the mouse outflow tract), is required for epicardial activation and regrowth134. Indeed, removal of the bulbous arteriosus impairs epicardial activation and migration towards the ventricle, as well as cardiac regeneration after injury, an outcome that can be reversed after implantation of Sonic hedgehog soaked beads in the ventricle134. Overall, studies directed at identifying the instructive cues to support the replenishment and recruitment of epicardial cells may lead to novel therapeutic strategies to enhance epicardium-mediated cardiac repair.
Epicardial-Derived Lineages in Zebrafish Regeneration
In zebrafish, tcf21 is highly expressed in epicardial precursors that give rise to perivascular cells during development and regeneration111. Genetic ablation of tcf21+ EPDCs results in prolonged fibrin clot deposition and collagen expression negatively impacting CM turnover and revascularization after injury134. However, even in cases where 80% of Tcf21+ cells are depleted from the epicardium, Tcf21+ cells re-populate the surface of the heart within 60 days post amputation indicating the endogenous reparative capacity of the epicardium134. Changes in mechanical tension have been recently shown to facilitate regeneration of the epicardium following ablation145. Utilizing a strategy to deplete tcf21 in zebrafish134, the migratory edge of the regenerating epicardium was populated by hypertrophic and multinucleated leader cells, which subsequently underwent apoptosis allowing for the persistence of mononucleated epicardial cells in regenerative tissue145. This study also indicated that mechanical signaling can regulate epicardial regeneration such as through modification of cellular behavior including cell death, nuclear polarity and migration; all factors that may significantly alter cellular processes of differentiation and/or secretion during cardiac repair.
Pan-epicardial reporters wt1a and wt1b have further revealed that the epicardium contributes to fibroblast plasticity in zebrafish heart regeneration131. The wt1b lineage reporter labels cells present at the epicardial border that contribute to fibroblastic collagen1a2+ and postn+ cells in response to cryoinjury131. Additionally, wt1b+;postn+ cells revert towards a quiescent state as evidenced by the noticeable downregulation of ECM genes, a process that appears to be conducive to CM proliferation131. This transient fibrosis mediated by cardiac fibroblast plasticity should be further examined in order to identify mechanisms that might lead to resolution of fibrosis during cardiac remodeling in adult mammalian models. In summary, the combination of in vivo and in vitro studies presented here contribute to the understanding of EPDC responsiveness and secretion of growth factors that impact potential downstream signaling programs associated with enhanced cardiac regeneration (Figure 5).
Figure 5. Epicardium in Cardiac Regeneration.
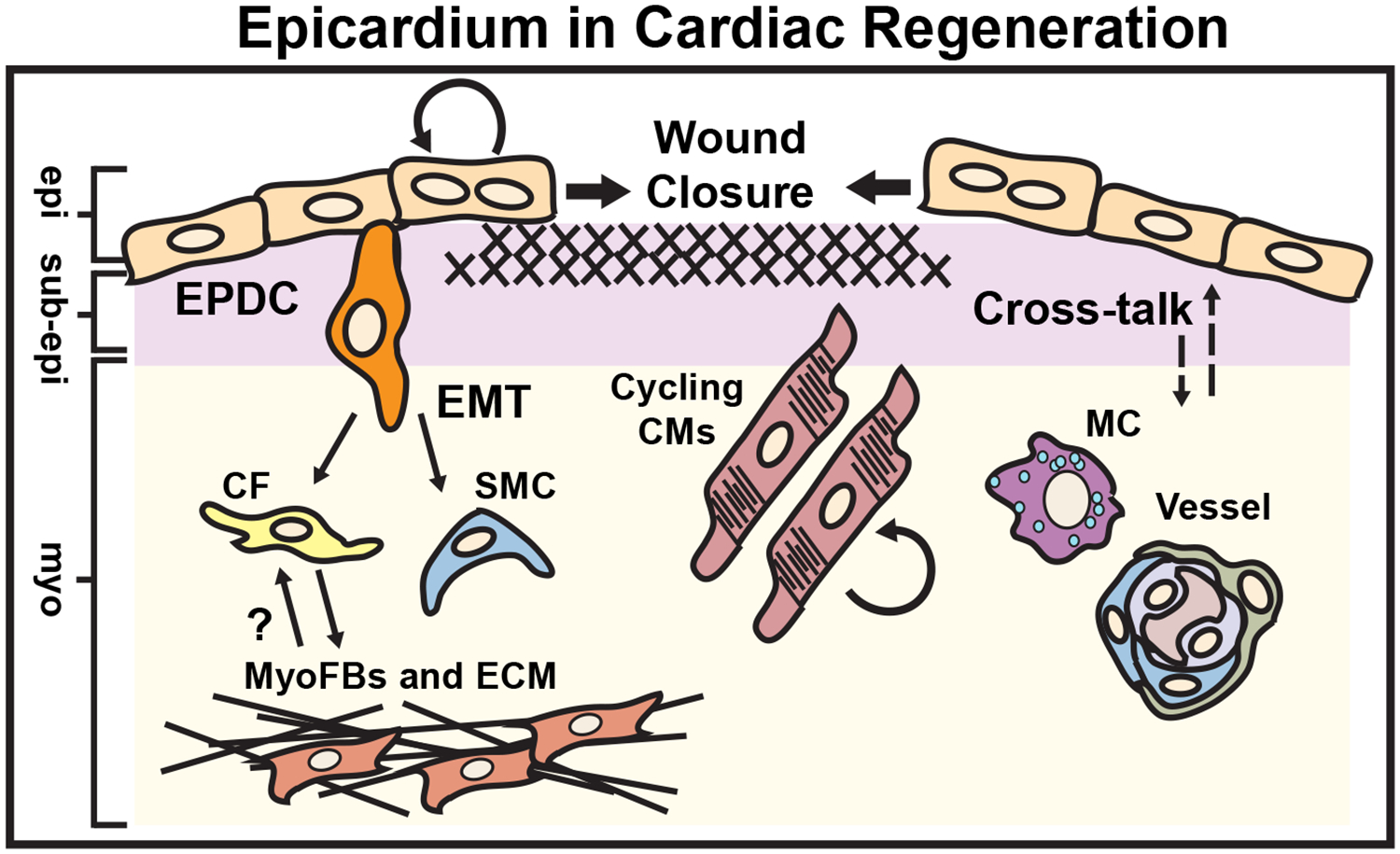
Cardiac regeneration occurs in response to a number of stimuli including apical resection, neonatal myocardial infarction, cryoablation or targeted ablation of the epicardium in both zebrafish and the early postnatal mammalian heart. The epicardium actively regenerates through the migration of “leader” binucleated epicardial cells initiating wound closure, and contributes to the formation of perivascular cells (smooth muscle cells or SMCs and fibroblasts or Fbs). Epicardium-derived fibroblasts have been shown to acquire myofibroblast phenotypes (MyoFb and ECM), which may be reversible during the resolution phase of the scar. The epicardium promotes macrophage (MC) recruitment, neovascularization as well as cardiomyocyte (CM) cell cycle re-entry via secretion of mitogens (dashed arrows). epi = epicardium, sub-epi = sub-epicardium, myo = myocardium. (Illustration credit: Ben Smith)
Mechanisms of Postnatal Heart Regeneration
Neonatal mammalian CMs demonstrate preserved cell cycle activity underlying the endogenous regenerative capacity of the mouse heart in the first week of life132. Apical resection (~15% of the ventricle) or permanent ligation induced-MI at postnatal day 1–2 results in complete regeneration by three weeks post injury due to increased CM proliferation132. Like zebrafish, acute upregulation of Wt1 and EMT marker genes are detected following apical resection and cryo-injury, as well as permanent coronary artery ligation in the neonatal mouse heart132, 146. More recently, Tbx18+ cell accumulation was observed in the neonatal heart following apical resection; however, Tbx18 lineage-traced cells display minimal differentiation potential147. Despite the limited regenerative capacity of the adult EPDCs, recent studies have been aimed at discovering molecular and cellular programs that enhance and/or extend regeneration beyond the early postnatal window by preventing CM cell cycle arrest and limiting fibroblast activation. Previous reports in development show that FGF10 is critical for CM maturation86, and although FGF10 treatment promoted the expansion of epicardial cells in the neonatal heart, FGF treatment did not significantly enhance cardiac regeneration148. However, pre-treatment of neonatal cardiac tissue with Thymosinβ4, which has been previously shown to enhance revascularization and cardiomyogenesis in the adult149, 150, stimulated regeneration following resection at 7 days post birth, mediated in part by the enhanced migration and differentiation of Wt1+ cells in the wound151. In addition to defining factors that have effective and combinatorial influence on CM proliferation and the prevention of fibrosis, stimulation of neoangiogenesis during neonatal cardiac regeneration has been largely overlooked. We previously described the importance of the positional cues stemming from the epicardium that can regulate the formation of the coronary vasculature98, 99. Similar to studies observed during cardiac development, efficient neonatal regeneration is prevented in models exhibiting Cxcl12 deletion152. Exogenous CXCL12 administration facilitated arterial cell re-organization and collateral artery formation 14 days post-MI in the adult mouse heart, although adult cardiac physiology was not evaluated in this study152.
Recruitment of inflammatory cells may also play a critical role in stimulation of angiogenesis and scar resolution in neonatal heart regeneration. Clodronate liposome-mediated depletion of monocytes and macrophages during the postnatal regenerative window augments fibrotic remodeling and impairs neovascularization in both peri-infarct and distal regions153. Transcriptional analysis of macrophages between postnatal day 1 and day 21 suggests that loss of regenerative potential with time is associated with macrophage polarization and expression of anti-inflammatory factors153. However, these studies suggest that in addition to immune cells, a combination of cardiac cells, including ECs and epicardial cells, likely contribute secretory factors required for cardiac repair. Here, we presented some of the factors that may be harnessed to prolong the postnatal regenerative window, which may be adapted to induce cycling of adult CMs and/or stimulation of angiogenic processes that are needed to promote repair in human diseased hearts.
Future Perspectives
In this review, we have discussed the gene programs that regulate cardiac growth and tissue repair through both cell autonomous and cell non-autonomous functions of the epicardium. Our broad analysis describes the study of epicardial-secreted factors such as PDGFs, IGFs or Thymosin-β4, and the modulation of inflammatory factors that might enhance epicardial mediated cardiac repair56, 88, 150. Additionally, the development of therapeutic strategies that re-direct EPDCs towards pro-regenerative cells types at the expense of fibroblast and adipocyte differentiation could complement current regenerative medicine approaches that are largely based on stem and induced pluripotent cell delivery.
Enhancement of endogenous cardiac repair via the isolation, expansion and transplantation of progenitor cells derived from bone marrow and cardiac niches have seen limited success. Methods to derive primary EPDCs from zebrafish, mouse and human hearts have been validated and may lead to the creation of novel progenitor cell sources for therapies in the future51, 154, 155. Adult human EPDCs recapitulate EMT-like processes of fetal human EPDCs, including the ability to migrate and differentiate into mural cell lineages154. However, the promise of cell transplantation for regenerative purposes can be hampered by immune rejection if the donor is not autologous to the recipient. Recent advances in human induced pluripotent stem cell technologies have demonstrated the feasibility of differentiating pluripotent cells into epicardial cells and their derivatives through the modulation of bone morphogenic factor, Wnt, FGF and RA signaling pathways156. Co-culture of human embryonic stem cell derived epicardial cells (hESC-EPDCs)with hESC-derived CMs (hESC-CMs) was shown to promote cardiac contractility and CM maturation in engineered heart tissues157. Furthermore, co-transplantation of hESC-EPDCs and hESC-CMs into infarcted rat hearts enhances engraftment, improves systolic function and augments revascularization compared to hESC-CMs alone157, making promising strides towards therapeutic practice.
Recent developments in evaluating epicardial activation and lineage commitment in various cardiac disease models indicate that cardiac regeneration is enhanced by facilitating cross talk between the epicardium and ECs or CMs. Replicating endogenous cross talk was accomplished via epicardial cell conditioned media delivery to the surface of the heart in a three-dimensional collagen patch, which promotes CM maturation and improves cardiac function after MI158. Furthermore, this study identified Follistatin-like 1 as the primary and potent epicardial cardiogenic factor to enhance cardiac function and repair after patch implementation158. Follistatin-like 1 treatment results in reduced fibrosis, improved re-vascularization and increased CM proliferation in both adult mouse and porcine pre-clinical models indicating an applicability of epicardial-derived paracrine factors in future clinical practices158. Cell-free therapeutics, such as synthetic modified RNA and small molecules can promote the expression of pro-regenerative genes in multiple cell types including the epicardium124, 149. Delivery of a modified RNA expressing VEGF-A activates EPDCs and promotes their migration and differentiation towards EC fate after cardiac injury159. Therefore, as mentioned throughout this review, knowledge of upstream growth factor signaling can be utilized to enhance epicardial cell expansion in vitro or the generation of novel therapeutic strategies for clinical practice.
Finally, important questions remain regarding epicardial cell heterogeneity, which may complicate strategies to harness the epicardium for cardiac repair or may lead to identification of rare or unique EPDCs that are particularly amenable to pro-regenerative approaches. Transcriptional profiling of the epicardium using bulk and single cell RNA-sequencing platforms (RNA-seq or scRNA-seq, respectively) can prove useful in identifying genes and pathways that respond to cardiac injury. RNA-seq performed on laser captured epicardial tissue identified a number of markers that distinguish non-injured and injured epicardial cells160. In zebrafish, the Tcf21 lineage appears to be comprised of a number of distinct epicardium-derived cell populations161. This study reveals the complex heterogeneity within the Tcf21-lineage and encourages the use of single cell transcriptomics to study progenitor cell biology effectively in various model organisms and patient samples. A more comprehensive analysis of epicardial cell contributions to normal development, congenital cardiomyopathy, and heart failure, and may reveal unanticipated mechanisms that can be harnessed to improve cardiac regeneration and repair.
Sources of Funding
Generous grant funding was provided to P. Quijada (NIHT32HL066988, NIHF32HL134206, and the American Heart Association 19CDA34590003); M.A. Trembley (T32HL07572, NIHT32HL066988, NIHT32GM068411, the American Heart Association 16PRE3049000, and the Howard Hughes Medical Institute Med-Into-Grad program); and E.M. Small (NIHR01-HL133761, NIHR01-HL136179, NIHR01-HL120919, NIHR01-HL144867, NIHUL1-TR002001, NYSTEM-C32566GG).
Non-Standard Abbreviations
- αSMA
alpha smooth muscle actin
- CCL
CC chemokine ligand
- CM
cardiomyocyte
- Col1a1
collagen1a1
- CXCL
chemokine receptor ligand
- EAT
epicardial adipose tissue
- EC
endothelial cell
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- EPDC
epicardium-derived progenitor cell
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- HA
hyaluronan or hyaluronic acid
- IGF
insulin growth factor
- IGFR
insulin growth factor receptor
- MI
myocardial infarction
- MRTF
myocardin-related transcription factor
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PE
proepicardium
- Postn
periostin
- RA
retinoic acid
- Raldh
aldehyde dehydrogenase
- RXR
retinoid x receptor
- Scx
scleraxis
- SMC
smooth muscle cell
- SRF
serum-response factor
- TAM
tamoxifen
- Tbx18
T-box protein 18
- TF
transcription factor
- TGFβ
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- VIC
valve interstitial cell
- WT1
wilms tumor-1
- YAP
yes associated protein 1
Footnotes
Disclosures
None.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Burchfield JS, Xie M and Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heallen TR, Kadow ZA, Kim JH, Wang J and Martin JF. Stimulating Cardiogenesis as a Treatment for Heart Failure. Circulation research. 2019;124:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vagnozzi RJ, Molkentin JD and Houser SR. New Myocyte Formation in the Adult Heart: Endogenous Sources and Therapeutic Implications. Circulation research. 2018;123:159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forte E, Furtado MB and Rosenthal N. The interstitium in cardiac repair: role of the immune-stromal cell interplay. Nat Rev Cardiol. 2018;15:601–616. [DOI] [PubMed] [Google Scholar]
- 6.Cao J and Poss KD. The epicardium as a hub for heart regeneration. Nat Rev Cardiol. 2018;15:631–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meilhac SM, Lescroart F, Blanpain C and Buckingham ME. Cardiac cell lineages that form the heart. Cold Spring Harb Perspect Med. 2015;5:a026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risebro CA, Vieira JM, Klotz L and Riley PR. Characterisation of the human embryonic and foetal epicardium during heart development. Development. 2015;142:3630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pombal MA, Carmona R, Megias M, Ruiz A, Perez-Pomares JM and Munoz-Chapuli R. Epicardial development in lamprey supports an evolutionary origin of the vertebrate epicardium from an ancestral pronephric external glomerulus. Evol Dev. 2008;10:210–6. [DOI] [PubMed] [Google Scholar]
- 10.Serluca FC. Development of the proepicardial organ in the zebrafish. Developmental biology. 2008;315:18–27. [DOI] [PubMed] [Google Scholar]
- 11.Nahirney PC, Mikawa T and Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;227:511–23. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B, von Gise A, Ma Q, Rivera-Feliciano J and Pu WT. Nkx2–5- and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochem Biophys Res Commun. 2008;375:450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tandon P, Miteva YV, Kuchenbrod LM, Cristea IM and Conlon FL. Tcf21 regulates the specification and maturation of proepicardial cells. Development. 2013;140:2409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers LS, Lalani S, Runyan RB and Camenisch TD. Differential growth and multicellular villi direct proepicardial translocation to the developing mouse heart. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:145–52. [DOI] [PubMed] [Google Scholar]
- 15.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR and Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona R, Guadix JA, Cano E, Ruiz-Villalba A, Portillo-Sanchez V, Perez-Pomares JM and Munoz-Chapuli R. The embryonic epicardium: an essential element of cardiac development. J Cell Mol Med. 2010;14:2066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamora M, Manner J and Ruiz-Lozano P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamouille S, Xu J and Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nature reviews Molecular cell biology. 2014;15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikawa T and Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Developmental biology. 1996;174:221–32. [DOI] [PubMed] [Google Scholar]
- 20.Dettman RW, Denetclaw W Jr., Ordahl CP and Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Developmental biology. 1998;193:169–81. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R and Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs). Developmental biology. 2002;247:307–26. [DOI] [PubMed] [Google Scholar]
- 22.Braitsch CM and Yutzey KE. Transcriptional Control of Cell Lineage Development in Epicardium-Derived Cells. J Dev Biol. 2013;1:92–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore AW, McInnes L, Kreidberg J, Hastie ND and Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–57. [DOI] [PubMed] [Google Scholar]
- 24.Carmona R, Gonzalez-Iriarte M, Perez-Pomares JM and Munoz-Chapuli R. Localization of the Wilm’s tumour protein WT1 in avian embryos. Cell and tissue research. 2001;303:173–86. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, Velecela V, Hall E, Reichmann J, Devenney PS, Hohenstein P, Hosen N, Hill RE, Munoz-Chapuli R and Hastie ND. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nature genetics. 2010;42:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Gise A, Zhou B, Honor LB, Ma Q, Petryk A and Pu WT. WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Developmental biology. 2011;356:421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guadix JA, Ruiz-Villalba A, Lettice L, Velecela V, Munoz-Chapuli R, Hastie ND, Perez-Pomares JM and Martinez-Estrada OM. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development. 2011;138:1093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velecela V, Lettice LA, Chau YY, Slight J, Berry RL, Thornburn A, Gunst QD, van den Hoff M, Reina M, Martinez FO, Hastie ND and Martinez-Estrada OM. WT1 regulates the expression of inhibitory chemokines during heart development. Human molecular genetics. 2013;22:5083–95. [DOI] [PubMed] [Google Scholar]
- 29.Norden J, Grieskamp T, Lausch E, van Wijk B, van den Hoff MJ, Englert C, Petry M, Mommersteeg MT, Christoffels VM, Niederreither K and Kispert A. Wt1 and retinoic acid signaling in the subcoelomic mesenchyme control the development of the pleuropericardial membranes and the sinus horns. Circulation research. 2010;106:1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu M, Smith CL, Hall JA, Lee I, Luby-Phelps K and Tallquist MD. Epicardial spindle orientation controls cell entry into the myocardium. Dev Cell. 2010;19:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Richardson JA and Olson EN. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mechanisms of development. 1998;73:23–32. [DOI] [PubMed] [Google Scholar]
- 32.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN and Tallquist MD. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funato N, Ohyama K, Kuroda T and Nakamura M. Basic helix-loop-helix transcription factor epicardin/capsulin/Pod-1 suppresses differentiation by negative regulation of transcription. The Journal of biological chemistry. 2003;278:7486–93. [DOI] [PubMed] [Google Scholar]
- 34.Piccolo S, Dupont S and Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–312. [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Ramesh S, Cibi DM, Yun LS, Li J, Li L, Manderfield LJ, Olson EN, Epstein JA and Singh MK. Hippo Signaling Mediators Yap and Taz Are Required in the Epicardium for Coronary Vasculature Development. Cell reports. 2016;15:1384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Hill MC, Zhang M, Martin TJ, Morikawa Y, Wang S, Moise AR, Wythe JD and Martin JF. Hippo Signaling Plays an Essential Role in Cell State Transitions during Cardiac Fibroblast Development. Dev Cell. 2018;45:153–169 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landerholm TE, Dong XR, Lu J, Belaguli NS, Schwartz RJ and Majesky MW. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development. 1999;126:2053–62. [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, Nagata K, Inagaki M and Majesky MW. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Developmental biology. 2001;240:404–18. [DOI] [PubMed] [Google Scholar]
- 39.Small EM. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. Journal of cardiovascular translational research. 2012;5:794–804. [DOI] [PubMed] [Google Scholar]
- 40.Nelson TJ, Duncan SA and Misra RP. Conserved enhancer in the serum response factor promoter controls expression during early coronary vasculogenesis. Circulation research. 2004;94:1059–66. [DOI] [PubMed] [Google Scholar]
- 41.Pipes GC, Creemers EE and Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes & development. 2006;20:1545–56. [DOI] [PubMed] [Google Scholar]
- 42.Olson EN and Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nature reviews Molecular cell biology. 2010;11:353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trembley MA, Velasquez LS, de Mesy Bentley KL and Small EM. Myocardin-related transcription factors control the motility of epicardium-derived cells and the maturation of coronary vessels. Development. 2015;142:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braitsch CM, Combs MD, Quaggin SE and Yutzey KE. Pod1/Tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium-derived cells into smooth muscle in the developing heart. Developmental biology. 2012;368:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Lamouille S and Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell research. 2009;19:156–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molin DG, Bartram U, Van der Heiden K, Van Iperen L, Speer CP, Hierck BP, Poelmann RE and Gittenberger-de-Groot AC. Expression patterns of Tgfbeta1–3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;227:431–44. [DOI] [PubMed] [Google Scholar]
- 47.Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P and Kaartinen V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Developmental biology. 2008;322:208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill CR, Sanchez NS, Love JD, Arrieta JA, Hong CC, Brown CB, Austin AF and Barnett JV. BMP2 signals loss of epithelial character in epicardial cells but requires the Type III TGFbeta receptor to promote invasion. Cellular signalling. 2012;24:1012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark CR, Robinson JY, Sanchez NS, Townsend TA, Arrieta JA, Merryman WD, Trykall DZ, Olivey HE, Hong CC and Barnett JV. Common pathways regulate Type III TGFbeta receptor-dependent cell invasion in epicardial and endocardial cells. Cellular signalling. 2016;28:688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Compton LA, Potash DA, Mundell NA and Barnett JV. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:82–93. [DOI] [PubMed] [Google Scholar]
- 51.Trembley MA, Velasquez LS and Small EM. Epicardial Outgrowth Culture Assay and Ex Vivo Assessment of Epicardial-derived Cell Migration. Journal of visualized experiments : JoVE. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craig EA, Austin AF, Vaillancourt RR, Barnett JV and Camenisch TD. TGFbeta2-mediated production of hyaluronan is important for the induction of epicardial cell differentiation and invasion. Experimental cell research. 2010;316:3397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bloomekatz J, Singh R, Prall OW, Dunn AC, Vaughan M, Loo CS, Harvey RP and Yelon D. Platelet-derived growth factor (PDGF) signaling directs cardiomyocyte movement toward the midline during heart tube assembly. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tallquist MD and Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development. 2003;130:507–18. [DOI] [PubMed] [Google Scholar]
- 55.Van Den Akker NM, Lie-Venema H, Maas S, Eralp I, DeRuiter MC, Poelmann RE and Gittenberger-De Groot AC. Platelet-derived growth factors in the developing avian heart and maturating coronary vasculature. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:1579–88. [DOI] [PubMed] [Google Scholar]
- 56.Smith CL, Baek ST, Sung CY and Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circulation research. 2011;108:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT and Tallquist MD. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circulation research. 2008;103:1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bax NA, Bleyl SB, Gallini R, Wisse LJ, Hunter J, Van Oorschot AA, Mahtab EA, Lie-Venema H, Goumans MJ, Betsholtz C and Gittenberger-de Groot AC. Cardiac malformations in Pdgfralpha mutant embryos are associated with increased expression of WT1 and Nkx2.5 in the second heart field. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:2307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Acharya A, Baek ST, Banfi S, Eskiocak B and Tallquist MD. Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis. 2011;49:870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J and Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. The Journal of clinical investigation. 2014;124:2921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin SL, Kisseleva T, Brenner DA and Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamilton TG, Klinghoffer RA, Corrin PD and Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lighthouse JK and Small EM. Transcriptional control of cardiac fibroblast plasticity. J Mol Cell Cardiol. 2016;91:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou B, von Gise A, Ma Q, Hu YW and Pu WT. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Developmental biology. 2010;338:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, Dettman RW and Burch JB. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Developmental biology. 2012;366:111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eralp I, Lie-Venema H, Bax NA, Wijffels MC, Van Der Laarse A, Deruiter MC, Bogers AJ, Van Den Akker NM, Gourdie RG, Schalij MJ, Poelmann RE and Gittenberger-De Groot AC. Epicardium-derived cells are important for correct development of the Purkinje fibers in the avian heart. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2006;288:1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG and Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circulation research. 1998;82:1043–52. [DOI] [PubMed] [Google Scholar]
- 68.Miyagishi M, Hatta M, Ohshima T, Ishida J, Fujii R, Nakajima T and Fukamizu A. Cell type-dependent transactivation or repression of mesoderm-restricted basic helix-loop-helix protein, POD-1/Capsulin. Mol Cell Biochem. 2000;205:141–7. [DOI] [PubMed] [Google Scholar]
- 69.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J and Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C and Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–9; discussion E9–10. [DOI] [PubMed] [Google Scholar]
- 71.Christoffels VM, Mommersteeg MT, Trowe MO, Prall OW, de Gier-de Vries C, Soufan AT, Bussen M, Schuster-Gossler K, Harvey RP, Moorman AF and Kispert A. Formation of the venous pole of the heart from an Nkx2–5-negative precursor population requires Tbx18. Circulation research. 2006;98:1555–63. [DOI] [PubMed] [Google Scholar]
- 72.Stennard FA and Harvey RP. T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development. 2005;132:4897–910. [DOI] [PubMed] [Google Scholar]
- 73.Wu SP, Dong XR, Regan JN, Su C and Majesky MW. Tbx18 regulates development of the epicardium and coronary vessels. Developmental biology. 2013;383:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greulich F, Farin HF, Schuster-Gossler K and Kispert A. Tbx18 function in epicardial development. Cardiovascular research. 2012;96:476–83. [DOI] [PubMed] [Google Scholar]
- 75.Grieskamp T, Rudat C, Ludtke TH, Norden J and Kispert A. Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circulation research. 2011;108:813–23. [DOI] [PubMed] [Google Scholar]
- 76.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, Kofler N, Kitajewski J, Weissman I and Red-Horse K. Pericytes are progenitors for coronary artery smooth muscle. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Red-Horse K, Ueno H, Weissman IL and Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian X, Pu WT and Zhou B. Cellular origin and developmental program of coronary angiogenesis. Circulation research. 2015;116:515–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Izpisua Belmonte JC, Chien KR and Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou B and Pu WT. Genetic Cre-loxP assessment of epicardial cell fate using Wt1-driven Cre alleles. Circulation research. 2012;111:e276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudat C and Kispert A. Wt1 and epicardial fate mapping. Circulation research. 2012;111:165–9. [DOI] [PubMed] [Google Scholar]
- 82.Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA and Tabin CJ. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell. 2012;22:639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cano E, Carmona R, Ruiz-Villalba A, Rojas A, Chau YY, Wagner KD, Wagner N, Hastie ND, Munoz-Chapuli R and Perez-Pomares JM. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio-venous connections. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J and Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. [DOI] [PubMed] [Google Scholar]
- 85.Pennisi DJ and Mikawa T. Normal patterning of the coronary capillary plexus is dependent on the correct transmural gradient of FGF expression in the myocardium. Developmental biology. 2005;279:378–90. [DOI] [PubMed] [Google Scholar]
- 86.Vega-Hernandez M, Kovacs A, De Langhe S and Ornitz DM. FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development. 2011;138:3331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen H, Cavallero S, Estrada KD, Sandovici I, Kumar SR, Makita T, Lien CL, Constancia M and Sucov HM. Extracardiac control of embryonic cardiomyocyte proliferation and ventricular wall expansion. Cardiovascular research. 2015;105:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li P, Cavallero S, Gu Y, Chen TH, Hughes J, Hassan AB, Bruning JC, Pashmforoush M and Sucov HM. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138:1795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang K, Shen H, Gan P, Cavallero S, Kumar SR, Lien CL and Sucov HM. Differential roles of insulin like growth factor 1 receptor and insulin receptor during embryonic heart development. BMC Dev Biol. 2019;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P and Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. [DOI] [PubMed] [Google Scholar]
- 91.Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR and Evans RM. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes & development. 1994;8:1007–18. [DOI] [PubMed] [Google Scholar]
- 92.Cunningham TJ and Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature reviews Molecular cell biology. 2015;16:110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Drager UC and Rosenthal N. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Developmental biology. 1998;199:55–71. [DOI] [PubMed] [Google Scholar]
- 94.Niederreither K, Subbarayan V, Dolle P and Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature genetics. 1999;21:444–8. [DOI] [PubMed] [Google Scholar]
- 95.Stevens SM, Gise A, VanDusen N, Zhou B and Pu WT. Epicardium is required for cardiac seeding by yolk sac macrophages, precursors of resident macrophages of the adult heart. Developmental biology. 2016;413:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manner J, Schlueter J and Brand T. Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:1454–63. [DOI] [PubMed] [Google Scholar]
- 97.Hellstrom M, Kalen M, Lindahl P, Abramsson A and Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. [DOI] [PubMed] [Google Scholar]
- 98.Harrison MR, Bussmann J, Huang Y, Zhao L, Osorio A, Burns CG, Burns CE, Sucov HM, Siekmann AF and Lien CL. Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish. Dev Cell. 2015;33:442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cavallero S, Shen H, Yi C, Lien CL, Kumar SR and Sucov HM. CXCL12 Signaling Is Essential for Maturation of the Ventricular Coronary Endothelial Plexus and Establishment of Functional Coronary Circulation. Dev Cell. 2015;33:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S and Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uygur A and Lee RT. Mechanisms of Cardiac Regeneration. Dev Cell. 2016;36:362–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Wijk B, Gunst QD, Moorman AF and van den Hoff MJ. Cardiac regeneration from activated epicardium. PLoS One. 2012;7:e44692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX and Pu WT. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. The Journal of clinical investigation. 2011;121:1894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA and Tallquist MD. Revisiting Cardiac Cellular Composition. Circulation research. 2016;118:400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC JL, Aronow BJ, Tallquist MD and Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature communications. 2016;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC and Molkentin JD. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. The Journal of clinical investigation. 2018;128:2127–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valiente-Alandi I, Potter SJ, Salvador AM, Schafer AE, Schips T, Carrillo-Salinas F, Gibson AM, Nieman ML, Perkins C, Sargent MA, Huo J, Lorenz JN, DeFalco T, Molkentin JD, Alcaide P and Blaxall BC. Inhibiting Fibronectin Attenuates Fibrosis and Improves Cardiac Function in a Model of Heart Failure. Circulation. 2018;138:1236–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiang FL, Fang M and Yutzey KE. Loss of beta-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nature communications. 2017;8:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, Gunaje J, Otsu K and Davis J. Fibroblast-Specific Genetic Manipulation of p38 Mitogen-Activated Protein Kinase In Vivo Reveals Its Central Regulatory Role in Fibrosis. Circulation. 2017;136:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD and Yutzey KE. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J Mol Cell Cardiol. 2013;65:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y and Poss KD. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138:2895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K and Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circulation research. 2014;115:625–35. [DOI] [PubMed] [Google Scholar]
- 113.Moore-Morris T, Cattaneo P, Guimaraes-Camboa N, Bogomolovas J, Cedenilla M, Banerjee I, Ricote M, Kisseleva T, Zhang L, Gu Y, Dalton ND, Peterson KL, Chen J, Puceat M and Evans SM. Infarct Fibroblasts Do Not Derive From Bone Marrow Lineages. Circulation research. 2018;122:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quijada P, Misra A, Velasquez LS, Burke RM, Lighthouse JK, Mickelsen DM, Dirkx RA Jr. and Small EM. Pre-existing fibroblasts of epicardial origin are the primary source of pathological fibrosis in cardiac ischemia and aging. J Mol Cell Cardiol. 2019;129:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J and Evans SM. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell. 2017;20:345–359 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J, Wang H and Li J. Inflammation and Inflammatory Cells in Myocardial Infarction and Reperfusion Injury: A Double-Edged Sword. Clinical Medicine Insights Cardiology. 2016;10:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, Bassel-Duby R and Olson EN. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338:1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Balmer GM, Bollini S, Dube KN, Martinez-Barbera JP, Williams O and Riley PR. Dynamic haematopoietic cell contribution to the developing and adult epicardium. Nature communications. 2014;5:4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pinto AR, Godwin JW, Chandran A, Hersey L, Ilinykh A, Debuque R, Wang L and Rosenthal NA. Age-related changes in tissue macrophages precede cardiac functional impairment. Aging. 2014;6:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanz-Morejon A, Garcia-Redondo AB, Reuter H, Marques IJ, Bates T, Galardi-Castilla M, Grosse A, Manig S, Langa X, Ernst A, Piragyte I, Botos MA, Gonzalez-Rosa JM, Ruiz-Ortega M, Briones AM, Salaices M, Englert C and Mercader N. Wilms Tumor 1b Expression Defines a Pro-regenerative Macrophage Subtype and Is Required for Organ Regeneration in the Zebrafish. Cell reports. 2019;28:1296–1306 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramjee V, Li D, Manderfield LJ, Liu F, Engleka KA, Aghajanian H, Rodell CB, Lu W, Ho V, Wang T, Li L, Singh A, Cibi DM, Burdick JA, Singh MK, Jain R and Epstein JA. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. The Journal of clinical investigation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patel VB, Shah S, Verma S and Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart failure reviews. 2017;22:889–902. [DOI] [PubMed] [Google Scholar]
- 123.Yamaguchi Y, Cavallero S, Patterson M, Shen H, Xu J, Kumar SR and Sucov HM. Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARgamma activation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zangi L, Oliveira MS, Ye LY, Ma Q, Sultana N, Hadas Y, Chepurko E, Spater D, Zhou B, Chew WL, Ebina W, Abrial M, Wang QD, Pu WT and Chien KR. Insulin-Like Growth Factor 1 Receptor-Dependent Pathway Drives Epicardial Adipose Tissue Formation After Myocardial Injury. Circulation. 2017;135:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Agullo-Pascual E, Cerrone M and Delmar M. Arrhythmogenic cardiomyopathy and Brugada syndrome: diseases of the connexome. FEBS Lett. 2014;588:1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Delmar M and McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circulation research. 2010;107:700–14. [DOI] [PubMed] [Google Scholar]
- 127.Iyer VR and Chin AJ. Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D). Am J Med Genet C Semin Med Genet. 2013;163C:185–97. [DOI] [PubMed] [Google Scholar]
- 128.Corrado D, Basso C and Judge DP. Arrhythmogenic Cardiomyopathy. Circulation research. 2017;121:784–802. [DOI] [PubMed] [Google Scholar]
- 129.Matthes SA, Taffet S and Delmar M. Plakophilin-2 and the migration, differentiation and transformation of cells derived from the epicardium of neonatal rat hearts. Cell Commun Adhes. 2011;18:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lien CL, Harrison MR, Tuan TL and Starnes VA. Heart repair and regeneration: recent insights from zebrafish studies. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sanchez-Iranzo H, Galardi-Castilla M, Sanz-Morejon A, Gonzalez-Rosa JM, Costa R, Ernst A, Sainz de Aja J, Langa X and Mercader N. Transient fibrosis resolves via fibroblast inactivation in the regenerating zebrafish heart. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:4188–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN and Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lam NT and Sadek HA. Neonatal Heart Regeneration. Circulation. 2018;138:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang J, Cao J, Dickson AL and Poss KD. Epicardial regeneration is guided by cardiac outflow tract and Hedgehog signalling. Nature. 2015;522:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang J, Karra R, Dickson AL and Poss KD. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Developmental biology. 2013;382:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Missinato MA, Tobita K, Romano N, Carroll JA and Tsang M. Extracellular component hyaluronic acid and its receptor Hmmr are required for epicardial EMT during heart regeneration. Cardiovascular research. 2015;107:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karra R, Foglia MJ, Choi WY, Belliveau C, DeBenedittis P and Poss KD. Vegfaa instructs cardiac muscle hyperplasia in adult zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:8805–8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG and Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–19. [DOI] [PubMed] [Google Scholar]
- 139.Huang Y, Harrison MR, Osorio A, Kim J, Baugh A, Duan C, Sucov HM and Lien CL. Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. PLoS One. 2013;8:e67266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim J, Wu Q, Zhang Y, Wiens KM, Huang Y, Rubin N, Shimada H, Handin RI, Chao MY, Tuan TL, Starnes VA and Lien CL. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lien CL, Schebesta M, Makino S, Weber GJ and Keating MT. Gene expression analysis of zebrafish heart regeneration. PLoS Biol. 2006;4:e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wills AA, Holdway JE, Major RJ and Poss KD. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 2008;135:183–92. [DOI] [PubMed] [Google Scholar]
- 143.Zhao L, Borikova AL, Ben-Yair R, Guner-Ataman B, MacRae CA, Lee RT, Burns CG and Burns CE. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lowe V, Wisniewski L, Sayers J, Evans I, Frankel P, Mercader-Huber N, Zachary IC and Pellet-Many C. Neuropilin 1 mediates epicardial activation and revascularization in the regenerating zebrafish heart. Development. 2019;146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cao J, Wang J, Jackman CP, Cox AH, Trembley MA, Balowski JJ, Cox BD, De Simone A, Dickson AL, Di Talia S, Small EM, Kiehart DP, Bursac N and Poss KD. Tension Creates an Endoreplication Wavefront that Leads Regeneration of Epicardial Tissue. Dev Cell. 2017;42:600–615 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mizutani M, Wu JC and Nusse R. Fibrosis of the Neonatal Mouse Heart After Cryoinjury Is Accompanied by Wnt Signaling Activation and Epicardial-to-Mesenchymal Transition. Journal of the American Heart Association. 2016;5:e002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cai W, Tan J, Yan J, Zhang L, Cai X, Wang H, Liu F, Ye M and Cai CL. Limited Regeneration Potential with Minimal Epicardial Progenitor Conversions in the Neonatal Mouse Heart after Injury. Cell reports. 2019;28:190–201 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rubin N, Darehzereshki A, Bellusci S, Kaartinen V and Ling Lien C. FGF10 Signaling Enhances Epicardial Cell Expansion during Neonatal Mouse Heart Repair. J Cardiovasc Dis Diagn. 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT and Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR and Riley PR. Thymosin beta-4 is essential for coronary vessel development and promotes neovascularization via adult epicardium. Annals of the New York Academy of Sciences. 2007;1112:171–88. [DOI] [PubMed] [Google Scholar]
- 151.Rui L, Yu N, Hong L, Feng H, Chunyong H, Jian M, Zhe Z and Shengshou H. Extending the time window of mammalian heart regeneration by thymosin beta 4. J Cell Mol Med. 2014;18:2417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Das S, Goldstone AB, Wang H, Farry J, D’Amato G, Paulsen MJ, Eskandari A, Hironaka CE, Phansalkar R, Sharma B, Rhee S, Shamskhou EA, Agalliu D, de Jesus Perez V, Woo YJ and Red-Horse K. A Unique Collateral Artery Development Program Promotes Neonatal Heart Regeneration. Cell. 2019;176:1128–1142 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA and Olson EN. Macrophages are required for neonatal heart regeneration. The Journal of clinical investigation. 2014;124:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moerkamp AT, Lodder K, van Herwaarden T, Dronkers E, Dingenouts CK, Tengstrom FC, van Brakel TJ, Goumans MJ and Smits AM. Human fetal and adult epicardial-derived cells: a novel model to study their activation. Stem cell research & therapy. 2016;7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cao J and Poss KD. Explant culture of adult zebrafish hearts for epicardial regeneration studies. Nature protocols. 2016;11:872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Witty AD, Mihic A, Tam RY, Fisher SA, Mikryukov A, Shoichet MS, Li RK, Kattman SJ and Keller G. Generation of the epicardial lineage from human pluripotent stem cells. Nature biotechnology. 2014;32:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bargehr J, Ong LP, Colzani M, Davaapil H, Hofsteen P, Bhandari S, Gambardella L, Le Novere N, Iyer D, Sampaziotis F, Weinberger F, Bertero A, Leonard A, Bernard WG, Martinson A, Figg N, Regnier M, Bennett MR, Murry CE and Sinha S. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nature biotechnology. 2019;37:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, Tian X, Liu Q, Wang A, Matsuura Y, Bushway P, Cai W, Savchenko A, Mahmoudi M, Schneider MD, van den Hoff MJ, Butte MJ, Yang PC, Walsh K, Zhou B, Bernstein D, Mercola M and Ruiz-Lozano P. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Spater D, Xu H, Tabebordbar M, Gorbatov R, Sena B, Nahrendorf M, Briscoe DM, Li RA, Wagers AJ, Rossi DJ, Pu WT and Chien KR. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nature biotechnology. 2013;31:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bochmann L, Sarathchandra P, Mori F, Lara-Pezzi E, Lazzaro D and Rosenthal N. Revealing new mouse epicardial cell markers through transcriptomics. PLoS One. 2010;5:e11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cao J, Navis A, Cox BD, Dickson AL, Gemberling M, Karra R, Bagnat M and Poss KD. Single epicardial cell transcriptome sequencing identifies Caveolin 1 as an essential factor in zebrafish heart regeneration. Development. 2016;143:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu Q, Huang X, Oh JH, Lin RZ, Duan S, Yu Y, Yang R, Qiu J, Melero-Martin JM, Pu WT and Zhou B. Epicardium-to-fat transition in injured heart. Cell research. 2014;24:1367–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Quaggin SE, Vanden Heuvel GB and Igarashi P. Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mechanisms of development. 1998;71:37–48. [DOI] [PubMed] [Google Scholar]
- 164.Lu J, Chang P, Richardson JA, Gan L, Weiler H and Olson EN. The basic helix-loop-helix transcription factor capsulin controls spleen organogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levay AK, Peacock JD, Lu Y, Koch M, Hinton RB, Jr., Kadler KE and Lincoln J. Scleraxis is required for cell lineage differentiation and extracellular matrix remodeling during murine heart valve formation in vivo. Circulation research. 2008;103:948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wilm B, Ipenberg A, Hastie ND, Burch JB and Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–28. [DOI] [PubMed] [Google Scholar]
- 167.Asahina K, Zhou B, Pu WT and Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dixit R, Ai X and Fine A. Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development. 2013;140:4398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Paris ND, Coles GL and Ackerman KG. Wt1 and beta-catenin cooperatively regulate diaphragm development in the mouse. Developmental biology. 2015;407:40–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A and Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nature cell biology. 2014;16:367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M and Rossant J. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–83. [DOI] [PubMed] [Google Scholar]
- 172.Pryce BA, Brent AE, Murchison ND, Tabin CJ and Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:1677–82. [DOI] [PubMed] [Google Scholar]
- 173.Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR, Jr. and Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nature cell biology. 2012;14:1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mahtab EA, Wijffels MC, Van Den Akker NM, Hahurij ND, Lie-Venema H, Wisse LJ, Deruiter MC, Uhrin P, Zaujec J, Binder BR, Schalij MJ, Poelmann RE and Gittenberger-De Groot AC. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:847–57. [DOI] [PubMed] [Google Scholar]
- 175.Vrancken Peeters MP, Mentink MM, Poelmann RE and Gittenberger-de Groot AC. Cytokeratins as a marker for epicardial formation in the quail embryo. Anat Embryol (Berl). 1995;191:503–8. [DOI] [PubMed] [Google Scholar]


