Abstract
Background and Purpose:
Serum cholesterol variability, independent of mean, has been associated with stroke, white matter hyperintensities on cranial MRI, and other cardiovascular events. We sought to assess the relationship between total serum cholesterol (TC) variability and cranial MRI findings of subclinical or covert vascular brain injury in a longitudinal, population-based cohort study of older adults.
Methods:
In the Cardiovascular Health Study, we assessed associations between intra-individual TC mean, trend, and variability over approximately five years with covert brain infarction (CBI) and white matter grade (WMG) on cranial MRI. Mean TC was calculated for each study participant from four annual TC measurements between two MRI scans. TC trend was calculated as the slope of the linear regression of the TC measurements, and TC variability was calculated as the standard deviation of the residuals from the linear regression. We evaluated the association of intra-individual TC variability with incident CBI and worsening WMG between two MRI scans in primary analyses and with prevalent CBI number and WMG on the follow-up MRI scan in secondary analyses.
Results:
Among participants who were eligible for the study and free of clinical stroke prior to the follow-up MRI, 17.9% of 1098 had incident CBI, and 27.8% of 1351 had worsening WMG on the follow-up MRI. Mean, trend, and variability of TC were not associated with these outcomes. TC variability, independent of mean and trend, was significantly associated with the number of CBI (β=0.009, 95% CI 0.003–0.016, p=0.004, N=1604) and was associated with WMG (β=0.009, 95% CI −0.0002–0.019, p=0.055, N=1602) on the follow-up MRI.
Conclusions:
Among older adults, TC variability was not associated with incident CBI or worsening WMG, but was associated with the number of prevalent CBI on cranial MRI. More work is needed to validate and to clarify the mechanisms underlying such associations.
Keywords: cerebral infarction, cerebral small vessel disease, cholesterol, MRI
Subject Terms: epidemiology, ischemic stroke, lipids and cholesterol
INTRODUCTION
Vascular brain injury is common in elderly populations, even among those without a history of transient ischemic attacks or strokes, manifesting primarily as subclinical or covert brain infarction (CBI) and white matter hyperintensities (WMH) on MRI.1 These findings cannot be considered silent or benign since they are associated with increased risk of subsequent overt, clinical events, including stroke, dementia, and death.2 Age and hypertension are the principal risk factors for covert vascular brain injury.3 Other vascular risk factors, including dyslipidemia and diabetes, have been less consistently associated with both CBI and WMH.3,4
Intra-individual variability in serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels were independent predictors of cardiovascular events, including stroke, in recent studies.5,6 An MRI sub-study of the PROSPER trial, which evaluated pravastatin vs placebo treatment in elderly participants with established vascular disease or those at high risk, demonstrated that higher LDL-C variability was associated with lower cerebral blood flow and greater WMH.7 The relationship between cholesterol variability and risk of developing CBI or WMH progression has not been assessed.
With cross-sectional analyses, we aimed to confirm and build upon previous work by evaluating the associations between intra-individual mean, trend, and variability of TC with CBI and WMH in the Cardiovascular Health Study (CHS), a longitudinal cohort study. We hypothesized that higher variability of TC levels, based on four annual measurements and independent of mean and trend, would be associated with greater risk of incident CBI and WMH progression comparing an initial and follow-up MRI and with a higher number of prevalent CBI and WMH burden on the follow-up MRI.
METHODS
Study population
We conducted analyses in the CHS, a population-based, longitudinal cohort study of coronary heart disease and stroke in adults 65 years and older.8 An initial cohort of 5,201 participants was recruited from communities in California, Maryland, North Carolina, and Pennsylvania beginning in study year 2 (1989–1990). An additional cohort of 687 predominantly African-American participants was added in year 5 (1992–1993), bringing the total number of participants to 5,888. The Institutional Review Boards at the University of Washington and each study site approved the study, and all CHS participants provided written informed consent.
Cholesterol measurements
As part of the CHS protocol, fasting plasma lipid analyses included measurement of TC (mg/dL) in years 2 and annually from years 5 to 11. HDL-C (mg/dL), and triglycerides (mg/dL) were measured in years 2 and 5, which allowed for LDL-C (mg/dL) calculation, according to the Friedewald equation, in these years.8 TC measurements were only available from one CHS clinic site in year 8 and were excluded from our analyses. LDL-C measurements were not available for CHS participants between study years 5 and 11.
MRI scans
The CHS protocol included two cranial MRI scans that occurred approximately in conjunction with the year 5 (1992–1993) and year 11 (1998–1999) visits. Two independent neuroradiologists reviewed scans in a standard fashion to identify number, size and location of brain infarcts, as well as the burden of WMH, graded on a semi-quantitative scale of 0 (least severe) to 9 (most severe).9,10 Brain infarcts were defined based on MRI signal characteristics and size ≥3 mm9 and described as subcortical or cortical. Lacunar infarcts were defined as subcortical infarcts 3 to 20 mm in size.11 For participants that completed both MRI scans, incident brain infarcts and worsening white matter grade (WMG) were determined. For incident infarcts, participants with prevalent infarcts on the initial MRI were excluded to ensure that infarcts detected on the follow-up MRI were new since the initial MRI, as described previously.9 For worsening WMG, the two scans were read side by side to assess for change in WMG, with readers blinded to the order of the scans, as detailed previously.10 Worsening WMG was defined as a one or more grade increase between the initial and follow-up MRI.
Covariates
Covariate data for primary and secondary analyses were collected from the last clinic visit prior to the initial MRI. These variables were age, sex, race, body mass index, systolic blood pressure, anti-hypertension medication use, diabetes, smoking status, myocardial infarction, congestive heart failure, and atrial fibrillation, as defined previously.8–10 Statin medication use during the time period between the two MRI scans was collected for sensitivity analyses.
Study Design
The exposures of interest for these cross-sectional analyses were intra-individual mean, trend, and variability of TC, based on four TC measurements between the initial and follow-up MRI scan. For all analyses, we excluded participants who had an overt, clinical stroke prior to the follow-up MRI. Primary outcomes for our study were any incident CBI or any worsening WMG on the follow-up MRI compared to the initial MRI. Participants with CBI on the initial MRI were excluded from the analysis of incident CBI. Secondary outcomes were the number of prevalent CBI and the actual WMG from the original reads on the follow-up MRI. The data and study materials will not be made available to other researchers for purpose of reproducing the results or replicating the procedure. The authors are not authorized to share CHS data. Information on CHS data requests can be found at https://chs-nhlbi.org.
Statistical analyses
To confirm that TC was a reasonable surrogate for LDL-C, we assessed the correlation of the two using 3 different measures: mean at year 2, mean at year 5, and mean of the difference between year 2 and 5. For the main analyses, each participant’s mean total serum cholesterol was calculated across four samples between the two MRI scans. If more than four total cholesterol measurements were completed, four were randomly selected. We calculated each participant’s trend as the slope of the linear regression of total cholesterol measures by clinic year and each participant’s variability as the standard deviation of the residuals from the linear regression, as in previous works from the CHS.12–14 An overview of the study design and analyses is shown in Figure 1.
Figure 1.
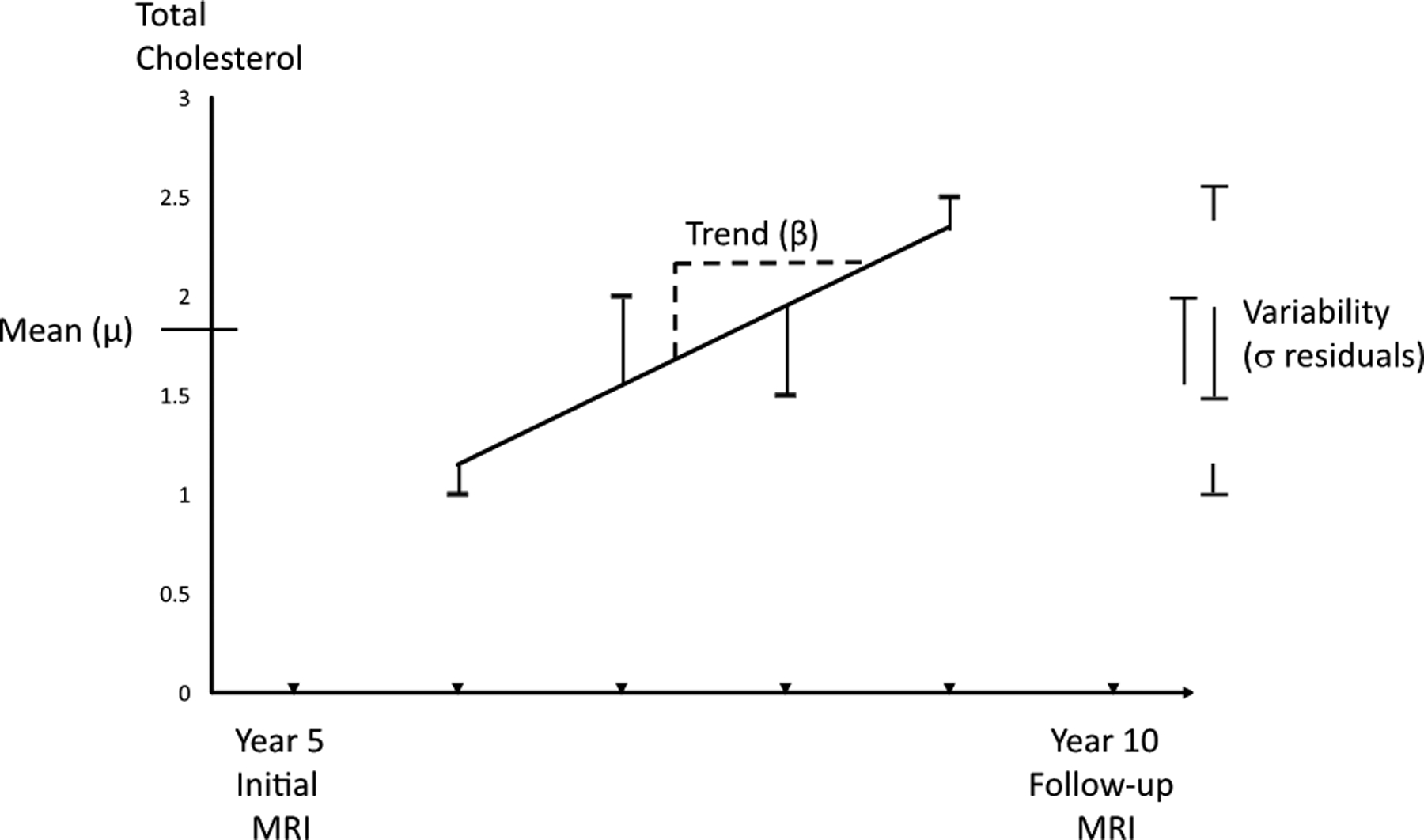
Intra-individual components of total cholesterol (TC) and study design. Intra-individual mean (μ) was defined as the mean of four TC measurements between study year 5 and year 10, around which times MRI scans were performed. Intra-individual trend over time, or slope, was defined as the beta coefficient (β) for the linear regression of the four measures. Intra-individual variability was defined as the standard deviation (σ) of the residuals from the linear regression of the four measures. Primary analyses involved changes from the initial to follow-up scans, and secondary analyses involved only findings on the follow-up scans. Covariate data were collected at the clinic visit prior to the initial magnetic resonance imaging (MRI) scans.
For primary analyses, participants were included if they underwent both brain MRIs and had at least four TC measurements between the two scans. We used relative risk (RR) regression to assess the relationship between intra-individual TC variability and the primary outcomes: incident CBI and worsening WMG comparing the initial and follow-up MRI scans. For secondary analyses, participants were additionally included if they underwent the follow-up MRI and completed at least four TC measures between year 5 and the follow-up MRI in the absence of an original MRI. We used linear regression to assess the relationship between intra-individual TC variability and the secondary outcomes: number prevalent CBI and WMG on the follow-up MRI scan. Minimally adjusted regression models included TC mean, TC trend, age, race, gender; fully adjusted models additionally included all covariates listed above. Models in the primary analyses were also adjusted for the time between MRI scans. In exploratory analyses, we examined the association of TC variability with the presence of CBI and of lacunar infarcts on the follow-up MRI. In sensitivity analyses, we repeated the primary and secondary analyses after excluding participants who used statin medications at any time during the study interval or whose statin use was unknown. Additionally, for participants with only three TC measurements completed between MRI scans, we imputed TC mean, TC trend and TC variability using multiple imputation and primary analyses were repeated. Robust standard errors were used for all regressions and a two-tailed p-value <0.05 was considered significant. Statistical analyses were conducted using Stata (version 12.1, StataCorp, College Station, Texas).
RESULTS
In the CHS, TC and calculated LDL-C measures were completed by 5098 participants at year 2 and 4678 participants at year 5. The Pearson’s correlation coefficient for TC and LDL-C measures from year 2 was 0.920 and from year 5 was 0.921. The difference between years 2 and 5 for TC and LDL-C was available for 3990 participants, and the correlation coefficient of these differences was 0.916. The correlation between total cholesterol variability, trend, and mean was low. The Spearman’s Rho is 0.075 for mean and trend, 0.264 for mean and variability, and −0.046 for trend and variability.
Figure 2 demonstrates CHS participants eligible for analyses of primary and secondary outcomes. Baseline characteristics for the 1473 participants eligible for the primary analyses and 1604 participants eligible for secondary analyses are detailed in Table 1. For the primary analyses of incident CBI, 1098 participants completed both MRI scans and did not have infarcts on the initial MRI, and 197 (17.9%) had incident CBI on the follow-up MRI. For the primary analyses of worsening WMG, 1351 participants had WMG readings on both MRI scans, and 375 (27.8%) demonstrated worsening WMG comparing the initial and follow-up MRI scans. The mean (SD) time between cranial MRI scans was 5.2 (0.5) years. For our secondary analyses of the follow-up MRI scans, 482 (30.0%) of 1604 participants had prevalent CBI present, with mean (SD) of 0.5 (0.9) infarcts, and 1105 (69.0%) of 1602 had WMG of 2 or more, with mean (SD) WMG of 2.5 (1.6). Of the 482 participants with prevalent CBI on the follow-up MRI, 411 (85.3%) had lacunar infarcts, and 71 (14.7%) had only non-lacunar infarcts.
Figure 2.
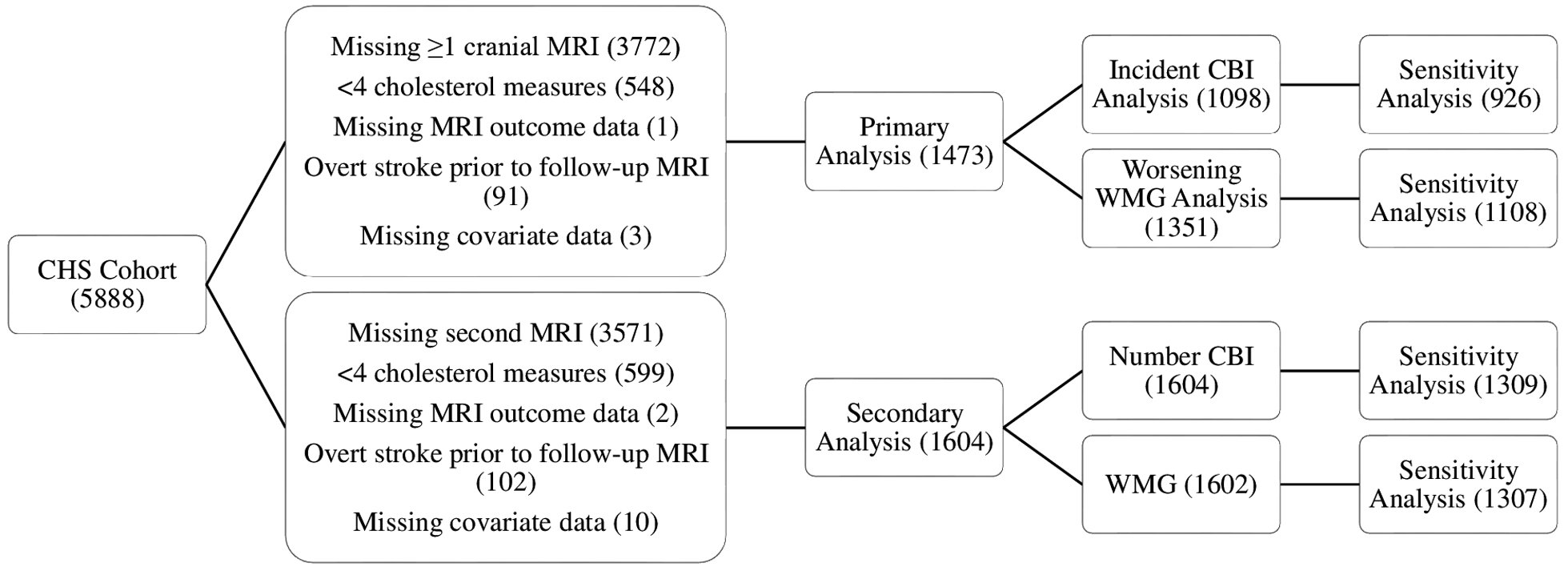
Participants in the Cardiovascular Health Study eligible for analyses of primary and secondary outcomes. Primary analyses involved changes from the initial to follow-up scans, and secondary analyses involved only findings on the follow-up scan. Sensitivity analyses excluded participants who used statin medications at any time during the study period or whose statin use was unknown.
Cardiovascular Health Study = CHS, MRI = magnetic resonance imaging, CBI = covert brain infarction, WMG = white matter grade
Table 1.
Baseline characteristics.
| Characteristic | Primary Analysis, mean (SD) or n (%) | Secondary Analysis, mean (SD) or n (%) |
|---|---|---|
| Eligible number of participants | 1473 | 1604 |
| Age (years, at initial MRI) | 73.8 (4.4) | 73.8 (4.4) |
| Sex (male) | 588 (39.9) | 640 (39.9) |
| Race (African-American) | 200 (13.6) | 234 (14.6) |
| Body mass index | 26.8 (4.2) | 26.9 (4.3) |
| Systolic blood pressure | 133.5 (19.9) | 133.9 (20.0) |
| Antihypertensive medication use | 642 (43.6) | 705 (44.0) |
| Smoking status | ||
| Never | 671 (45.6) | 726 (45.3) |
| Former | 680 (46.2) | 742 (46.3) |
| Current | 122 (8.3) | 136 (8.5) |
| Atrial fibrillation | 65 (4.4) | 70 (4.4) |
| Diabetes | 173 (11.7) | 196 (12.2) |
| Myocardial infarction | 104 (7.1) | 111 (6.9) |
| Heart Failure | 38 (2.6) | 43 (2.7) |
| CHS Sites | ||
| North Carolina | 360 (24.4) | 386 (24.1) |
| California | 362 (24.6) | 428 (26.7) |
| Maryland | 327 (22.2) | 346 (21.6) |
| Pennsylvania | 424 (28.8) | 444 (27.7) |
| Intra-individual components of four total cholesterol measurements | ||
| Mean (mg/dL) | 202.2 (33.1) | 202.4 (33.1) |
| Trend (mg/dL) | 0.05 (7.0) | 0.08 (7.0) |
| Variability (mg/dL) | 12.2 (8.5) | 12.3 (8.4) |
| Known statin use | 242 (16.4) | 266 (16.6) |
| Unknown statin use | 18 (1.2) | 29 (1.8) |
MRI = Magnetic Resonance Imaging, CHS = Cardiovascular Health Study, mg/dL = milligrams/deciliter
Results for models only adjusted for age, sex, and race did not differ substantially from fully adjusted models, so only the results of the fully adjusted models are presented. In primary analyses, mean, trend, and variability of TC were not associated with incident CBI or worsening WMG on the follow-up MRI after adjusting for covariates (Table 2). In the secondary analyses, TC variability, independent of mean and trend, was significantly associated with the number of prevalent CBI (β=0.009, 95% CI 0.003–0.016, p=0.004) on the follow-up MRI. TC variability was associated with WMG (β=0.009, 95% CI −0.0002–0.019, p=0.055) on the follow-up MRI, with the result not reaching statistical significance. In post-hoc exploratory analyses, TC variability, independent of mean and trend, was associated with the presence of any prevalent CBI (RR=1.012, 1.005–1.020, p=0.001) as well as prevalent lacunar infarcts on the follow-up MRI (RR=1.015, 1.006–1.023, p<0.001). Sensitivity analyses were completed with the exclusion of participants who used statin medications at any time during the study period or whose statin use history was unknown (Table 3). We found TC trend was associated with incident CBI (RR=1.028, 1.004–1.052, p=0.022), but not TC variability. However, TC variability was associated with the number of prevalent CBI (β=0.009, 0.002–0.016, p=0.016). After implementing multiple imputation for participants with three TC measurements available, TC mean, trend, and variability were still not associated with incident CBI or worsening WMG, despite the increase in the eligible number of participants from 1098 to 1395 for CBI analyses and from 1351 to 1709 for WMG analyses (data not shown).
Table 2.
Relationship between intra-individual mean, trend, and variability in total cholesterol with primary outcomes and secondary outcomes.
| Primary Outcomes | Incident CBI (N=1098) | Worsening WMG (N=1351) | ||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Mean | 0.999 (0.995, 1.004) | 0.736 | 0.998 (0.995, 1.000) | 0.075 |
| Trend | 1.012 (0.992, 1.032) | 0.256 | 1.009 (0.996, 1.021) | 0.166 |
| Variability | 1.003 (0.989, 1.018) | 0.661 | 1.004 (0.993, 1.014) | 0.509 |
| Secondary Outcomes | Number of CBI (N=1604) | WMG (N=1602) | ||
| β (95% CI) | P value | β (95% CI) | P value | |
| Mean | 0.001 (−0.001, 0.003) | 0.263 | −0.001 (−0.004, 0.001) | 0.373 |
| Trend | −0.003 (−0.011, 0.005) | 0.439 | 0.005 (−0.006, 0.016) | 0.391 |
| Variability | 0.009 (0.003, 0.016) | 0.004 | 0.009 (−0.0002, 0.019) | 0.055 |
For primary outcomes, relative risks (RR) and 95% confidence intervals (CI) were estimated from RR regression models. For secondary outcomes, beta coefficients (β) and 95% CI were estimated from linear regression models. Models adjusted for age, sex, race, body mass index, hypertension, diabetes, history of tobacco use, myocardial infarction, congestive heart failure, atrial fibrillation, and TC mean and trend. Time between MRI scans was adjusted for in primary analyses.
MRI = magnetic resonance imaging, CBI = covert brain infarction, WMG = white matter grade.
Table 3.
Relationship between intra-individual mean, trend, and variability in total cholesterol with primary and secondary outcomes after exclusion of participants who used statin medications during the study period or whose statin use was unknown.
| Primary Outcomes | Incident CBI (N=926) | Worsening WMG (N=1108) | ||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Mean | 0.998 (0.994, 1.003) | 0.418 | 0.997 (0.994, 1.000) | 0.071 |
| Trend | 1.028 (1.004, 1.052) | 0.022 | 1.005 (0.989, 1.021) | 0.539 |
| Variability | 1.010 (0.991, 1.030) | 0.308 | 1.003 (0.990, 1.016) | 0.657 |
| Secondary Outcomes | Number CBI (N=1309) | WMG (N=1307) | ||
| β (95% CI) | P value | β (95% CI) | P value | |
| Mean | 0.000 (−0.002, 0.002) | 0.976 | −0.002 (−0.005, 0.001) | 0.169 |
| Trend | 0.001 (−0.009, 0.011) | 0.870 | 0.005 (−0.009, 0.019) | 0.462 |
| Variability | 0.009 (0.002, 0.016) | 0.016 | 0.009 (−0.003, 0.020) | 0.161 |
See footnote for Table 2 for details of the analyses and abbreviations.
DISCUSSION
We did not find that TC variability was associated with incident CBI or worsening WMG in our primary analyses in this well characterized, population-based cohort of older adults who were free of transient ischemic attack or stroke. We did find that variability of TC was independently associated with prevalent CBI on the follow-up MRI, even after adjustment for mean and trend of TC. This association also remained significant after excluding those who used statin medications at any time during the study period or whose statin use was unknown.
Our study corroborates and builds upon findings from a prior cohort study6 and post-hoc analyses of statin treatment trials5,7 demonstrating an association between cholesterol variability with clinical stroke and MRI-defined WMH. A population-based study from the Korean National Health Insurance System cohort demonstrated that TC variability, independent of mean TC and use of lipid-lowering medical therapy, was associated with increased risk of overt, clinical stroke during a median follow-up of 8 years.6 In a post-hoc analysis of the Treating to New Targets (TNT) trial, Bangalore and colleagues reported an association between LDL-C variability and clinical stroke risk over a median follow-up of about 5 years.5 In contrast to these prior reports, we did not find an association between cholesterol variability and incident CBI. Our study demonstrates an association between TC variability and prevalent CBI, which persists even after exclusion of participants who used statins or whose statin use was unknown, a finding not previously reported. The relationship between TC variability and prevalent CBI slightly strengthened when restricting our outcome to lacunar infarcts, which represented the majority of CBI on cranial MRI. Smit and colleagues demonstrated a significant association between LDL-C variability and greater WMH in an MRI sub-study of the PROSPER trial among participants treated with pravastatin, but not among those enrolled in the control arm.7 We did not find an association between TC variability and worsening WMG in our primary analyses. We found that TC variability may be associated with WMG on the follow-up MRI in the CHS, although this association was not statistically significant and was no longer evident in sensitivity analyses when excluding participants who used statins or whose statin use was unknown.
The mechanisms underlying the association between TC variability with CBI or WMH are not well defined, but a few explanations have been proposed.6,7 Lipid variability may lead to alterations in atherosclerotic plaque cholesterol content, leading to plaque instability and rupture, increasing the risk for covert vascular brain injury. Statin treatment has been shown to reduce cholesterol variability in prior studies.5,7 Poor adherence to statin or other lipid-lowering therapy, or the use of other drugs that affect cholesterol levels, may increase TC variability and, in turn, increase the risk for CBI. Finally, TC variability may be an epiphenomenon of other systemic conditions or a general loss of homeostasis that increase the risk of CBI or WMH. The importance of homeostasis was proposed by Cannon in 192915 and recently restated by Smit and colleagues,7 “More than 85 years ago, Cannon hypothesized that loss of physiological homeostasis, for instance, through disease or the aging process, would lead to disturbances in intrinsic variability.” Clinical practitioners should be aware that intra-individual TC variability may increase risk of CBI and that increased variability may represent potential medication non-compliance. More work is needed to understand if the association between TC variability and CBI is causal and to evaluate treatment strategies to reduce fluctuations in TC.
The strengths of this study include the prospective cohort design of CHS, with standardized assessment of TC and LDL-C and of MRI findings of CBI and WMG. However, limitations also need to be considered. First, we were unable to directly assess the relationship between LDL-C variability with the outcomes of interest, which may have been more strongly associated with CBI and WMG, although correlations between TC and LDL-C were strong. Second, the annual cholesterol measures completed in CHS do not allow for assessment of the link between shorter-term TC variability, over weeks or months, and the MRI findings. Third, given the average time period between MRI scans was approximately 5 years, we are unable to assess the relationship between longer-term cholesterol variability and CBI or WMG. Finally, prior reports have demonstrated that participants who underwent both MRI scans were healthier than those who did not.9,10 Associations may have been different if all participants had completed both scans and been included in these analyses.
SUMMARY
In conclusion, intra-individual TC variability, independent of mean and trend, is associated with prevalent CBI on cranial MRI among elderly participants of a longitudinal cohort study, generally supporting the findings of prior studies. We were unable to extend these findings to incident CBI and worsening WMG, but these analyses may have been underpowered. More work is needed to validate such associations with these and other measures of both covert and overt vascular brain injury and to clarify the mechanisms underlying such associations.
FUNDING
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC15103, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
Psaty serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. The other authors report no conflicts of interest.
REFERENCES
- 1.Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, et al. Prevention of stroke in patients with silent cerebrovascular disease: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. [DOI] [PubMed] [Google Scholar]
- 2.Debette S, Schilling S, Duperron M-G, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 2014;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Breazna A, DeMicco DA, Wun C-C, Messerli FH, TNT Steering Committee and Investigators. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol. 2015;65:1539–1548. [DOI] [PubMed] [Google Scholar]
- 6.Kim MK, Han K, Kim H-S, Park Y-M, Kwon H-S, Yoon K-H, et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur Heart J. 2017;38:3560–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit RAJ, Trompet S, Sabayan B, Cessie S le, Grond J van der, Buchem MA, et al. Higher visit-to-visit low-density lipoprotein cholesterol variability is associated with lower cognitive performance, lower cerebral blood flow, and greater white matter hyperintensity load in older subjects. Circulation. 2016;134:212–221. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The cardiovascular health study: Design and rationale. Annals of Epidemiology. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 9.Longstreth WT, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth WT, Arnold AM, Beauchamp NJ, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth WT, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. [DOI] [PubMed] [Google Scholar]
- 12.Suchy-Dicey AM, Wallace ER, Mitchell SVE, Aguilar M, Gottesman RF, Rice K, et al. Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the Cardiovascular Health Study. Am J Hypertens. 2013;26:1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung LY, Bartz TM, Rice K, Floyd J, Psaty B, Gutierrez J, et al. Blood pressure and heart rate measures associated with increased risk of covert brain infarction and worsening leukoaraiosis in older adults. Arterioscler Thromb Vasc Biol. 2017;37:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floyd JS, Sitlani CM, Wiggins KL, Wallace E, Suchy-Dicey A, Abbasi SA, et al. Variation in resting heart rate over 4 years and the risks of myocardial infarction and death among older adults. Heart. 2015;101:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannon W Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]


