Abstract
Objective-
Cardiovascular disease (CVD) is a major public health problem. Among CVD’s risk factors, tobacco smoking is considered the single most preventable cause of death, with thrombosis being the main mechanism of CVD mortality in smokers. While tobacco smoking has been on the decline, the use of waterpipes/hookah has been rising, mainly due to the perception that they are “less harmful” than regular cigarettes. Strikingly, there are few studies on the negative effects of waterpipes on the cardiovascular system, and none regarding their direct contribution to thrombus formation.
Approach and results-
We employed a waterpipe whole body exposure protocol that mimics real-life human exposure scenarios, and investigated its effects, relative to clean air, on platelet function, hemostasis and thrombogenesis. We found that, waterpipe smoke (WPS) exposed mice exhibited both shortened thrombus occlusion and bleeding times. Further, our results show that platelets from WPS exposed mice are hyperactive, with enhanced agonist-induced aggregation, dense and α-granule secretion, αIIbβ3 integrin activation, phosphatidylserine expression and platelet spreading, when compared with clean air exposed platelets. Finally, at the molecular level, it was found that Akt and ERK phosphorylation are enhanced in the WPS, and in nicotine-treated platelets.
Conclusion-
Our findings demonstrate, for the first time, that WPS exposure directly modulates hemostasis and increases the risk of thrombosis, and that this is mediated, in part, via a state of platelet hyperactivity. The negative health impact of WPS/hookah, therefore, should not be underestimated. Moreover, this study should also help in raising public awareness of the toxic effects of waterpipe/hookah.
Keywords: Waterpipe, hookah, cardiovascular disease, platelets, thrombosis
Graphical Abstract
Introduction
Cardiovascular disease (CVD) is a major public health problem, not only in the US, but also all over the world, as it accounts for more than 17 million deaths worldwide1. In the US, CVD is the number one leading cause of deaths accounting for 610,000 deaths each year, mainly as a result of heart attack and stroke2. Among CVD risk factors, tobacco smoking is the single most preventable cause of death and it is responsible for 33% of CVD-linked deaths3. Although reports have been showing a decline in traditional cigarette smoking in the western world4, waterpipe (also known as hookah) tobacco smoking is an emerging trend in the US and Europe5 especially among the youth6–9. It is to be noted that waterpipes have been in existence for a millennium, and thought to have emerged in the North Western provinces of India, before they spread to Iran, and the Arab world10. One of the reasons for such a surge in the popularity of waterpipes is in part due to: the wide spread misperception that waterpipe smoke is “filtered” via its passage through water, and therefore is less harmful than cigarette smoke5. Further, the type of tobacco used in the waterpipe, called “ma’assel”, which is honeyed and available in numerous flavors is another appealing reason for smoking waterpipes. Other reasons for use include social acceptability and accessibility11, curiosity12, and peer pressure13. While waterpipe users and those in close proximity are exposed to many of the potentially dangerous toxicants, the health risks associated with its use- including its impact in the context of the cardiovascular system- continue to be under debate. This is unlike the effects of tobacco smoking on the cardiovascular system, which are well-established14–19. Thus, cigarette smoking predisposes to cardiovascular events and contributes significantly to cardiovascular related mortality and morbidity16, 20. In addition, smoking causes a prothrombotic state through disrupting the hemostatic balance21, enhanced aggregation22, amongst others23. However, whether waterpipe use/WPS exerts similar effects remains ill defined, but warrants investigation. It is to be noted that waterpipes’ toxicant/chemical profile overlaps with that of regular cigarettes24–26.
While studies on the impact of waterpipe usage on the cardiovascular system are limited, they have shown that it is associated with increased heart rate (HR) and blood pressure (BP)27, 28, endothelial dysfunction29, as well as oxidative stress30. These studies would suggest that waterpipe use/smoke would also increase the risk of thrombotic disease. Therefore, the current study examined the impact of waterpipes/hookah/WPS on platelet function, hemostasis and thrombogenesis, in mice, by employing a whole-body exposure method. Moreover, we employed the widely adopted “Beirut protocol”31 that resembles real-life waterpipe exposure scenarios. Our findings revealed that “short-term” WPS exposure triggers a hyperactive platelet state, both functionally and biochemically, that seems to increase the risk of thrombus formation, and to enhance hemostasis. Taken together, our data support the conclusion that waterpipes/WPS, contrary to many held beliefs, do exert negative health effects, and predispose users to thrombosis-related CVD.
Materials and Methods
The data that support the findings of this study are available from the corresponding authors upon reasonable requests.
Reagents and Materials
Thrombin was purchased from Chronolog Corporation (Havertown, PA), whereas ADP, nicotine and cotinine were from Sigma Aldrich (St. Louis, MO). Fluorescein isothiocyanate (FITC)-conjugated anti-P-selectin and FITC-conjugated Annexin V were purchased from Cell Signaling Technology, Inc (Danvers, MA). The JON/A antibody was obtained from Emfret analytics (Würzburg, Germany). The phycoerythrin-conjugated anti-CD69 and FITC-conjugated anti-CD45 were obtained from BD Biosciences (San Jose, CA). Stir bars and other disposables were purchased from Chrono-Log Corporation (Havertown, PA). Akt, phospho-Akt (Ser473), ERK and phospho-ERK antibodies were purchased from Cell signaling (Danvers, MA). The anti-PAR4 and anti-GPIIb-IIIa (αIIbβ3) antibodies were from Abcam (Cambridge, MA), whereas the anti-p85/PI3K antibody was from Proteintech (Rosemont, IL). The ELISA cotinine detection kit was purchased from Calbiotech (El Cajon, CA). The fibrinogen plasma detection/quantification ELISA kit was purchased form Innovative Research (Novi, MI).
Animals
C57BL/6J (10-week-old male) mice (referred to hereafter as C57BL/6) were purchased from the Jackson Laboratory (Bar Harbor, ME), and were housed in groups of 1 to 4 at 24°C, under 12/12 light/dark cycles, with access to water and food ad libitum. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Texas at El Paso.
Waterpipe Smoke (WPS) Whole Body Exposure Protocol
The exposure of mice to waterpipe smoke (WPS) utilized a whole-body exposure chamber (40cm × 30cm × 25cm, LxWxH), which was connected to a programmable waterpipe smoking machine, as described in a previous report32. The smoking machine delivered 171 puffs of 530 mL volume, 2.6 s puff duration, and 17 s interpuff interval according to the Beirut Method33. This method is based on the smoking behavior in real-life waterpipe/hookah settings (Hookah café), and the protocol itself has been used worldwide and in a host of different studies31, 34–43. The clay bowl of the waterpipe used in this study was loaded with 12g of commercially available flavored tobacco (Al Fakher - Double Apple Tobacco Trading, Ajman, UAE), whose ingredients include tobacco, glycerin, molasses and natural flavor with nicotine and tar. We also conducted exposures using a second tobacco brand, namely the Starbuzz (Dale Ave Stanton, CA), and performed specific platelet function experiments (i.e., aggregation and dense granule secretion). After covering the clay bowl with Aluminum foil, quick-light charcoal briquettes (Holland Hookah Charcoal) were used to burn the tobacco. The bowl of the waterpipe was filled with about 700 mL of tap water, prior to each exposure session. Along with the smoke puff delivered to the exposure chamber by the smoking machine, fresh/clean air was continuously pumped into the chamber, resulting in an air change rate of approximately 1.5 changes per hour. Carbon monoxide (CO) concentration (average levels were 925 ppm) was continuously monitored in the chamber using an electrochemical sensor (Bacharach Monoxor Plus) drawing a flow rate of 0.3 LPM through a 25 mm glass fiber filter (Pall Type A/E). The WPS mice were exposed, as described above, for one exposure session 60 min/day for seven days, and all experiments performed on the day of the last exposure session.
Cotinine Assay
The serum levels of cotinine, a metabolite of nicotine, were measured in both WPS and clean air exposed mice, using the Cotinine Direct ELISA kit as per the manufacturer’s instructions.
Tail Bleeding Time
After a complete cycle of exposure, we performed the tail bleeding time assay as we described before44, 45. Briefly, anesthesia was induced and WPS and clean air exposed mice were placed on a homeothermic blanket at 37°C, after that the tail was transected 5 mm from the tip using a sterile scalpel. After transection, the tail was immediately immersed in saline (37°C constant temperature), and the time to bleeding stoppage was measured. Bleeding stoppage was not considered complete until bleeding had stopped for 1 minute. If bleeding did not stop in 10 minutes, the experiment was stopped to prevent excessive blood loss from the mice, and 10 minutes was considered the cutoff bleeding time.
In vivo FeCl3 Carotid Artery Injury Induced Thrombosis Model
Thrombosis studies were performed as described previously44, 45. Briefly, WPS and clean air exposed mice (8–10 weeks old) were anesthetized with avertin (2.5%), and the left carotid artery was exposed and cleaned with normal saline (37°C), before baseline carotid artery blood flow was measured with Transonic Micro-Flowprobe (Transonic Systems Inc, Ithaca, NY). After stabilizing blood flow, 7.5% ferric chloride was applied to a filter paper disc (1-mm diameter) that was immediately placed on top of the artery for 3 minutes. Blood flow was continuously monitored for 20 minutes or until blood flow reached stable occlusion (no blood flow for 2 minutes). Data were recorded, and time to vessel occlusion was calculated as the difference in time between stable occlusion and removal of the filter paper (with ferric chloride). An occlusion time of 20 minutes was considered as the cutoff time for statistical analysis.
Peripheral Blood Cell/Platelet Counts
The hematology profile, including platelets and white blood cells, of the WPS/clean air exposed mice was performed on whole blood using a HEMAVET® 950FS Multi-species Hematology System from Erba® Diagnostics (Miami Lakes, FL).
Murine Platelet Rich Plasma Preparation
Mice were anesthetized before blood was collected from the heart, with 0.38% sodium citrate solution (Fisher Scientific, Hampton, NH) added to inhibit coagulation. Then blood was centrifuged (237g for 15 minutes) at room temperature, and the platelet-rich plasma was then collected. Platelets were counted with the HEMAVET® 950FS Multi-species Hematology System, and their count adjusted to 7×107 platelets/mL before each experiment.
Washed Platelet Preparation
Blood was collected form each mice as described above and were pooled in each group by diluted phosphate-buffered saline, pH 7.4, in 1:1 ratio, incubated with PGI2 (10 ng/mL; 5 minutes), and centrifuged at 237g for 10 minutes at room temperature. Platelet-rich plasma was recovered, and incubated with 0.37 U/mL apyrase, and 10 ng/mL PGI2, platelets were pelleted at 483×g for 10 minutes at room temperature. The pellets were resuspended in HEPES/Tyrode buffer (20 mmol/L HEPES/KOH, pH 6.5, 128 mmol/L NaCl, 2.8 mmol/L KCl, 1 mmol/L MgCl2, 0.4 mmol/L NaH2PO4, 12 mmol/L NaHCO3, 5 mmol/L D-glucose) supplemented with 1 mmol/L EGTA, 0.37 U/mL apyrase, and 10 ng/mL PGI2. Platelets were then washed and resuspended in HEPES/Tyrodes (pH 7.4). Platelets were counted using the HEMAVET® 950FS Multi-species Hematology System and adjusted to the indicated concentrations.
In vitro Platelet Aggregation
The platelet-rich plasma (PRP) from WPS exposed and clean air mice was activated with thrombin (0.1 U/mL) or ADP (5 μmol/L). Platelet aggregation was measured by the turbidometric method using a model 700 aggregometer (Chrono-Log Corporation, Havertown, PA). Each experiment was repeated at least 3 times with blood pooled from at least 3 different groups (i.e., at least 8 mice each) that were exposed to either WPS or clean air. The aggregation assay was conducted after exposure to the two different tobacco brands.
Dense Granule Release
Platelet-rich plasma (250 μL; 7×107/mL) were placed into siliconized cuvettes and stirred for 5 minutes at 37°C at 1200 rpm, followed by the addition of luciferase substrate/luciferase mixture (12.5 μL, Chrono-Log). PRP was then activated using the agonists thrombin (0.1 U/mL) or ADP (5 μmol/L). Release of ATP were measured using a model 700 aggregometer (Chrono-Log Corporation, Havertown, PA). This assay was conducted after exposure to the two different tobacco brands.
Flow Cytometric Analysis
Flow cytometric analysis was carried out as described44, 45. Briefly, washed platelets (2×107/mL) from clean air- or WPS–exposed mice were prepared as described above and stimulated with thrombin (0.1 U/ml) or ADP (5 μmol/L) for 5 minutes. Platelets were then fixed with 2% formaldehyde for 30 minutes at room temperature, followed by incubated with FITC-conjugated CD62P (P-selectin), Annexin V or phycoerythrin-conjugated rat anti-mouse JON/A antibodies at room temperature for 30 minutes in the dark. Finally, platelets were diluted 2.5-fold with HEPES/Tyrode buffer (pH 7.4). The samples were transferred to FACS-tubes and fluorescent intensities were measured using a BD Accuri C6 flow cytometer and analyzed using CFlow Plus (BD Biosciences, Franklin Lakes, NJ). Each experiment was repeated at least 3 times with blood pooled from at least 3 different groups (at least 8 mice each) that were exposed to either clean air or WPS.
Platelet Spreading
Platelet spreading assay was carried out as we described before46, 47. Briefly, sterile glass coverslips were coated with 0.2 μg/mL of fibrinogen for 30 minutes at room temperature. Washed platelets were placed onto these fibrinogen-coated coverslips before they were fixed with 3.7% (vol/vol) formaldehyde for 15 minutes and quenched with 50 mmol/L ammonium chloride. Cells were rinsed with PBS and incubated with tetramethylrhodamine-conjugated phalloidin (1 μg/mL) in 10% fetal bovine serum/PBS with 0.2% saponin. Coverslips were mounted and examined and imaged using a Leica DMi8 inverted widefield fluorescence microscope with integrated high-precision focus drive. Images were processed using LAS X Wizard imaging software. Objective lenses used were a ×63/×100 numeric aperture. Type A immersion oil (Fryer) was used for the ×63 objective. This experiment was repeated at least 3 times, with blood pooled from a group of 8 mice each time. Comparison is based on difference in the level of spreading.
Immunoblotting
Immunoblot was carried out as described before44, 48. Briefly, clean air– or WPS–exposed washed platelets were stimulated with thrombin (0.1 U/ml) for 3 minutes followed by lysis with 1X sample buffer. Next, proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to Immobilon-P PVDF membranes (Bio-Rad, Hercules, CA). Membranes were then probed with the primary antibodies (ERK, pERK, Akt, and pAkt) and visualized with horseradish peroxidase–labeled antirabbit or antimouse immunoglobulin G as required. The antibody binding was detected using enhanced chemiluminescence substrate (Thermo Scientific, Rockford, IL). Images were obtained with ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA), and quantified using the Image Lab Software 6.0.1 (Bio-Rad, Hercules, CA). For expression levels of GPIIb-IIIa, PI3K (p85) and PAR4, platelet proteins were separated by SDS-PAGE and transferred to Immobilon-P PVDF membranes (Bio-Rad, Hercules, CA). They were then probed with anti-GPIIb-IIIa (αIIbβ3), anti-PI3K/p85 or anti- PAR4 primary antibodies and visualized with an appropriate alkaline phosphatase–coupled secondary antibody using enhanced chemifluorescent substrate (Thermo Scientific, Rockford, IL).
Mouse Treatment with Nicotine and Cotinine
Mice were injected with 1 μM cotinine (a “real-life” dose selected based on waterpipe literature)49–52 or vehicle once daily for one week. Experiments (specifically platelet aggregation and dense granule secretion) on cotinine treated animals were performed on the day of the last injection. As for nicotine, mice were injected with 1 mg/kg nicotine, as previously used in the literature53, and the blood collected after one hour post injection, before Akt and ERK phosphorylation were assessed as described above.
Fibrinogen Assay
The plasma concentration of fibrinogen was determined using an ELISA kit as per the manufacturer’s instructions.
Statistical Analysis
All experiments were performed at least 3 times with blood pooled from 3 groups of at least 8 mice each time, as applicable. Data analysis was performed using GraphPad PRISM. V7 statistical software and presented as mean ± standard deviation/SD, but the normality and variance were not tested to determine whether the applied parametric tests were appropriate. The Mann-Whitney test was used for the evaluation of differences in mean occlusion and bleeding times, whereas the one-way ANOVA with Tukey’s multiple comparisons test as post hoc were used for the analysis of the flow cytometry data, as applicable (per number of groups). Significance was accepted at P <0.05, unless stated otherwise.
Results
Waterpipe/hookah exposure systemically delivers nicotine in exposed mice
As means of validating the WPS exposure protocol, the serum levels of the nicotine metabolite cotinine54 are typically measured in exposed animals and/or “tobacco users”. Hence, our results showed significant increases of cotinine levels in the WPS exposed mice (15.2 ± 0.72 ng/ml; Fig 1A), in serum obtained immediately after the conclusion of the exposures, whereas it was undetectable in that from the clean air controls. These levels overlap with those found in human waterpipe users (5.5–77.8 ng/ml)49–51, 55, 56. Our data support the notion that our WPS exposure protocol results in the systemic delivery of nicotine into the exposed mice.
Figure 1:
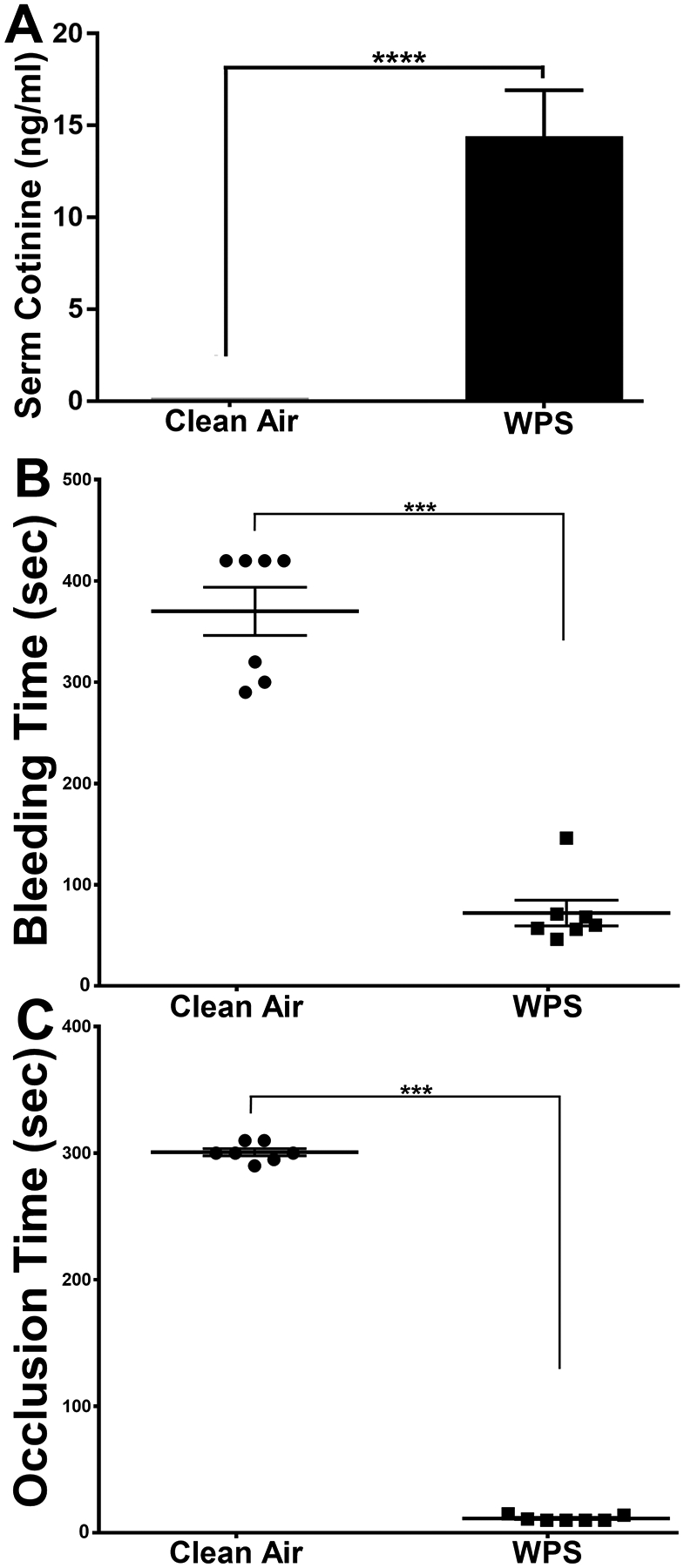
WPS exposure results in systemic delivery of nicotine (cotinine) and shortens the bleeding time in the tail bleeding time assay, and the time to occlusion in the ferric chloride in vivo thrombosis model. (A) The concentrations of cotinine were measured in serum from WPS and clean air exposed mice. Each bar represents the mean ± SD (n = 10; ****P<0.0001). (B) This data illustrates the results of tail bleeding time assay (as described in the “Methods”) comparing WPS and clean air exposed mice. Each point represents the tail bleeding time of a single animal (clean air, n=7; and WTS, n=7; ***P<0.001). (C) This data illustrates the results of the ferric chloride–induced thrombosis model (as described in the “Methods”) comparing WPS and clean air exposed mice time to occlusion. Each point represents the occlusion time of a single animal (clean air, n=7; and WPS, n=7; ***P<0.001). WPS indicates Waterpipe Smoke.
WPS Exposure Modulate Hemostasis and Thrombosis Development
Numerous studies showed that cigarette smoking causes disease states that are linked to thrombosis22, 57. On contrast, it is yet to be determined whether waterpipe smoking carries a similar effect. Therefore, we initially investigated the effects of waterpipe smoke (WPS) exposure on both hemostasis and thrombogenesis. As for its effect on hemostasis, the tail bleeding time assay revealed that the time to bleeding stoppage was significantly shortened in the WPS-exposed mice, when compared with those exposed to clean air, specifically 11.43 ± 2.15 seconds to 300.7 ± 7.32 seconds, respectively (Figure 1B). This line of evidence indicates a prothrombtic phenotype in the WPS-exposed mice. Thus, we next sought to examine if the WPS mice are more prone to thrombosis. Using the ferric chloride carotid artery injury–induced thrombosis model, we indeed found that the WPS-exposed mice had a shortened time for occlusion, in comparison with the controls, specifically, 72 ± 33.7 seconds to 370 ± 62.9 seconds, respectively (Figure 1C). These data document, for the first time, that WPS exposure directly modulates hemostasis and thrombus occlusion.
Acute WPS Exposure Does Not Affect Peripheral Blood Cell Counts
To exclude the possibility that changes in platelet number may have contributed to the hemostasis and thrombosis phenotype we observed, platelets from the WPS and clear air mice were counted. We did not observe any significant difference in the platelet count (Table 1) between the WPS-, and clean air-exposed mice, namely (556 ± 24.3 to 573 ± 31.2 (thousand/μL; P=0.40). These results indicate that under the present experimental conditions, the prothrombotic phenotype is independent of changes in platelet count. Moreover, we did not observe any detectable effects for WPS on the counts of white/peripheral blood cells (Table 1).
Table 1:
Blood was collected from the heart and counted as described in the Methods section. All counts are expressed as thousands per microliter, except for red blood cells, which are expressed as millions per microliter. Data are presented as mean ± SD. WPS indicates Waterpipe Smoke; MPV, mean platelet volume.
| Cell Type | WPS | Clean Air | P Values |
|---|---|---|---|
| Platelets | 556 ± 24.3 | 573 ± 31.2 | 0.40 |
| MPV | 4.8 ± 0.26 | 4.9 ± 0.1 | 0.54 |
| Red blood cells | 8.15 ± 0.85 | 8.05 ± 0.90 | 0.88 |
| Lymphocytes | 1.77 ± 0.05 | 1.83 ± 0.37 | 0.76 |
| Monocytes | 0.07 ± 0.01 | 0.10 ± 0.02 | 0.17 |
| Granulocytes | 2.29 ± 0.10 | 2.50 ± 0.30 | 0.3 |
| HCT | 40.4 ± 1.45 | 39.45 ± 2.11 | 0.72 |
WPS Exposure Enhances Agonist-Induced Platelet Aggregation
Previous studies have shown that cigarette smoking enhances platelet function58. Given that our data showed a robust prothrombotic phenotype in WPS-exposed mice, we hypothesize that this will derive, in part, from platelet hyperactivity as a result of WPS exposure. Thus, we sought to examine the effects of WPS exposure on agonist–induced platelet aggregation. It was found that platelets from WPS-exposed mice exhibited an enhanced platelets aggregation in comparison to those from clean air controls, in response to thrombin or ADP (Figure 2A & 2B; inset shows quantification of data). Together, the hyperactive platelet state is consistent with shortened bleeding and occlusion times phenotype observed in the WPS mice. Similar result was obtained when the same experiment was repeated with a different waterpipe tobacco brand (see Methods for details; Figure 2C & 2D; inset shows quantification of data).
Figure 2:
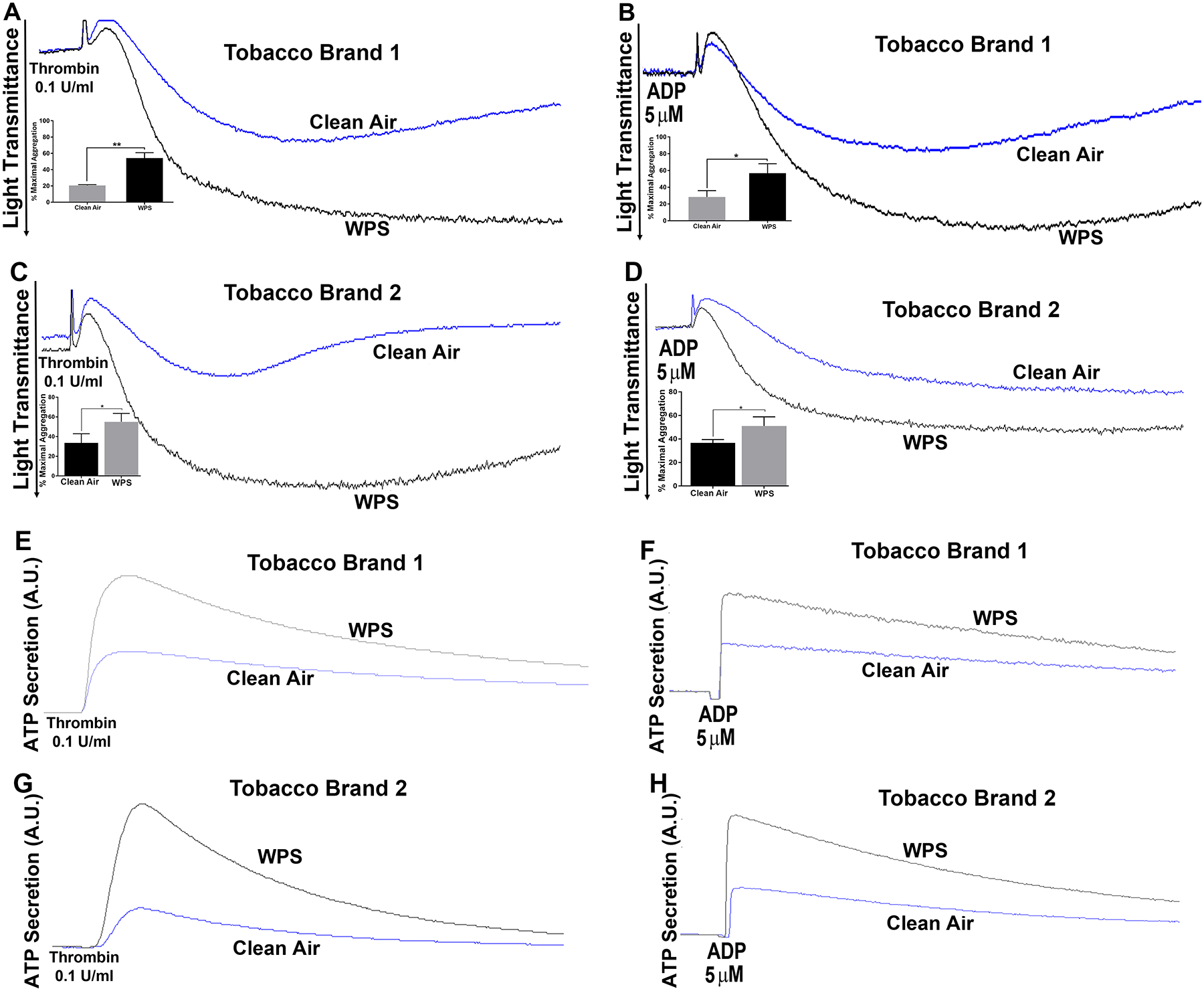
Platelet aggregation and dense granule secretion are enhanced in WPS exposed mice, using two different brands. Platelets from WPS (two separate brands) and clean air exposed mice were stimulated with 0.1 U/ml thrombin, or 5 μmol/L ADP, before their aggregation (A-D) and dense granule secretion (E-H) responses were measured in a lumi-aggregometer. Platelets were incubated with luciferase/luciferin (12.5 μL) for the dense granules measurements. The experiment was repeated 3 times, with blood pooled from at least 8 mice each time (*P <0.05; **P <0.01). WPS indicates Waterpipe Smoke.
WPS Exposure Enhances Agonist-Induced Platelet Secretion
We next sought to investigate the impact of WPS exposure on agonist-induced platelet secretion, given the importance of the platelet granule release in amplifying the initial platelet response59, 60. Our results demonstrated dense granule/ATP secretion is enhanced in the WPS platelets, in response to thrombin or ADP, even with exposure to a different tobacco brand (Figure 2E–2H), which is consistent with the aggregaion data. Similarly, platelets from mice exposed to WPS had significantly higher expression of P-selectin on their surface in response to 0.1 U/ml of the agonist thrombin or 5 μM ADP (Figure 3A & 3B). These findings indicate that whole-body exposure to WPS enhances both dense and α-granule secretion, which is in line with the notion that these platelets are hyperactive.
Figure 3:
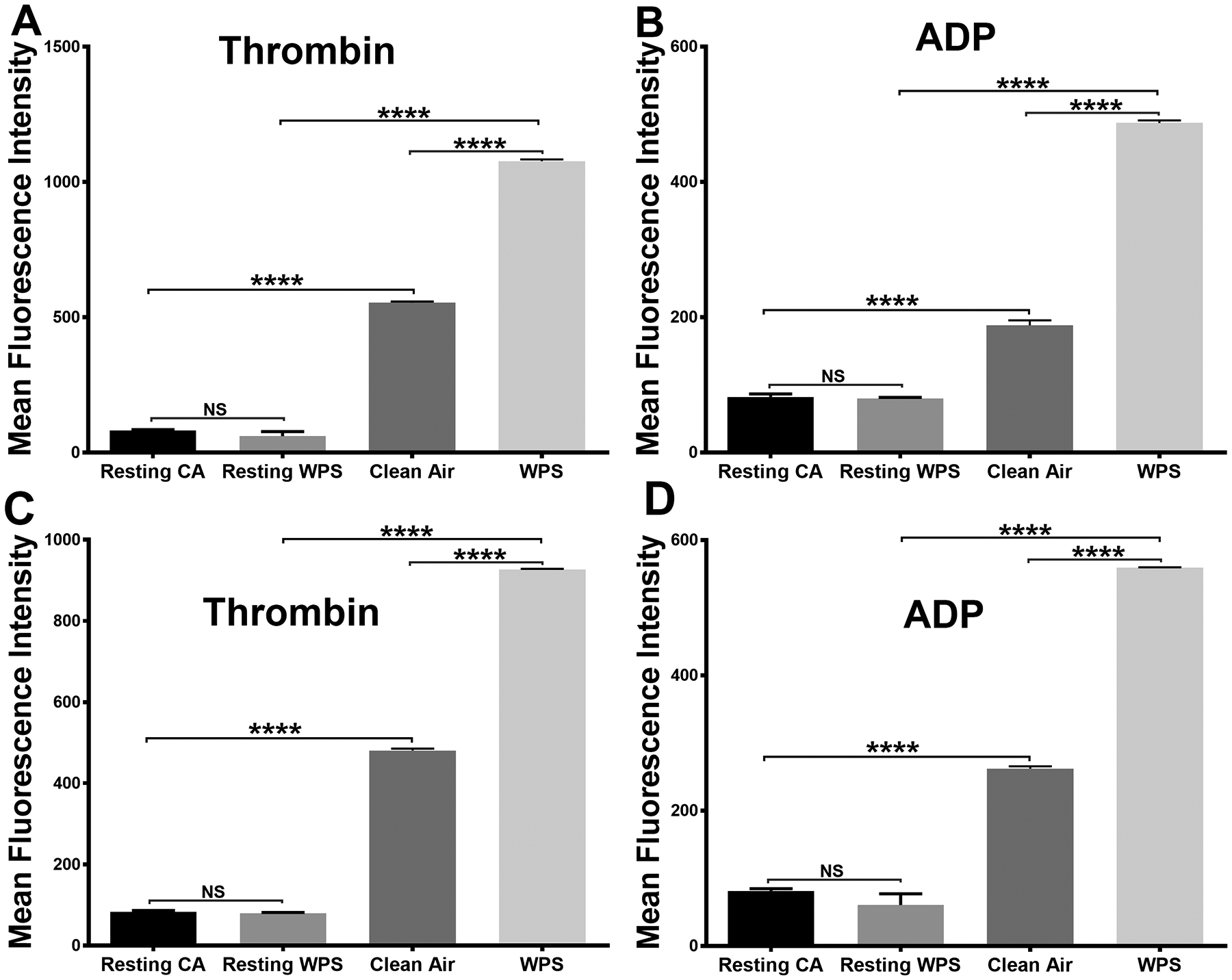
Platelets alpha granule secretion and integrin GPIIb-IIIa activation are increased in WPS exposed mice. Platelets from WPS and clean air exposed mice were prepared and washed. (A, B) Platelets were incubated with fluorescein isothiocyanate–conjugated CD62P antibody (for α granules), and the fluorescent intensities were measured by flow cytometry after stimulation with 0.1 U/ml thrombin or 5 μmol/L ADP. (C, D) Platelets were incubated with fluorescein isothiocyanate–conjugated JON/A antibody, and the fluorescent intensities were measured by flow cytometry after stimulation with 0.1 U/ml thrombin or 5 μmol/L ADP. Average mean fluorescence intensities shown (****P < 0.0001; NS. nonsignificant). Each experiment was repeated 3 times, with blood pooled from at least 8 mice each time. WPS indicates Waterpipe Smoke.
WPS Exposure Enhances Agonist-Induced Integrin αIIbβ3 Activation
In light of the notion that WPS exposure is responsible for the hyperactive platelet phenotype, including enhanced aggregation, we next determined if the integrin GPIIb-IIIa (αIIbβ3) activation would also be potentiated. Indeed, our data revealed that activation of the αIIbβ3 integrin is enhanced in the WPS platelets, in contrast with the clean air controls, in response to 0.1 U/ml thrombin or 5 μM ADP (Figure 3C & 3D). These results are consistent with the enhanced aggregation response in the WPS-exposed platelets.
WPS Exposure Enhances Agonist-Induced Phosphatidylserine Expression
Phosphatidylserine (PS) exposure provides the essential platelets “platform” upon which the assembly of coagulation factor complexes takes place61. Therefore, it is important to assess any changes in PS expression that may result from WPS exposure. Our data showed that PS expression was significantly higher in the WPS-exposed platelets, compared to those from the clean air-exposed mice, upon stimulation with 0.1 U/ml thrombin or 5 μM ADP (Figure 4A & 4B).
Figure 4:
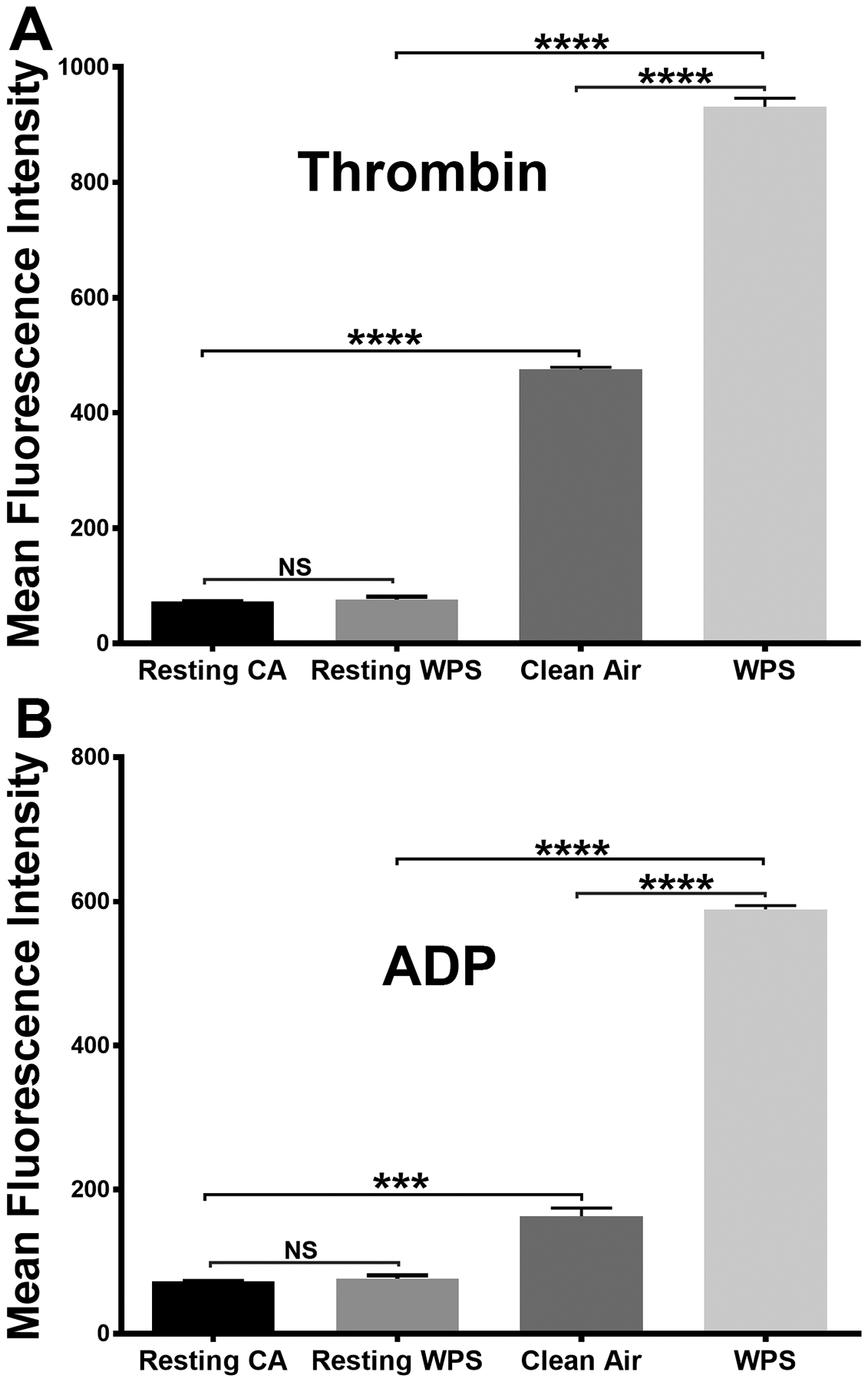
Platelet phosphatidylserine (PS) exposure is enhanced in WPS exposed mice. Platelets from WPS and clean air exposed mice were prepared and washed. Platelets were incubated with fluorescein isothiocyanate-conjugated Annexin V antibody and the fluorescent intensities were measured by flow cytometry after stimulation with 0.1 U/ml thrombin (A) or 5 μmol/L ADP (B). Average mean fluorescence intensities shown (****P < 0.0001; NS. nonsignificant). Each experiment was repeated 3 times, with blood pooled from at least 8 mice each time. WPS indicates Waterpipe Smoke.
Taken together, our data thus far support the notion that exposure of platelets to WPS produces a hyperactive state, in comparison to clean air. This hyperactive state (enhanced function) was in the form of enhanced aggregation, secretion, integrin activation, and phosphatidylserine exposure.
WPS Exposure Enhances Platelet Spreading
Platelet spreading is a vital aspect of hemostatic plug formation and thrombosis62. To this end, and upon vessel injury, platelet activation mediated via a host of receptor-induced cascades, triggers rapid reorganization of the actin cytoskeleton, thereby resulting in platelet rounding, and adhesion to the surface. Consequently, platelet morphological changes occur, e.g. filopodia and lamellipodia formation, which ultimately strengthens contact with the surface as well as to other platelets. As for the impact of WPS on spreading, filopodia and lamellipidia formation were found to be higher/potentiated in the WPS-exposed platelets, compared to those from the clean air-exposed mice, upon stimulation with 0.1 U/ml thrombin (Figure 5). This finding further supports the notion that WPS results in a hyper-state of platelet activity.
Figure 5:
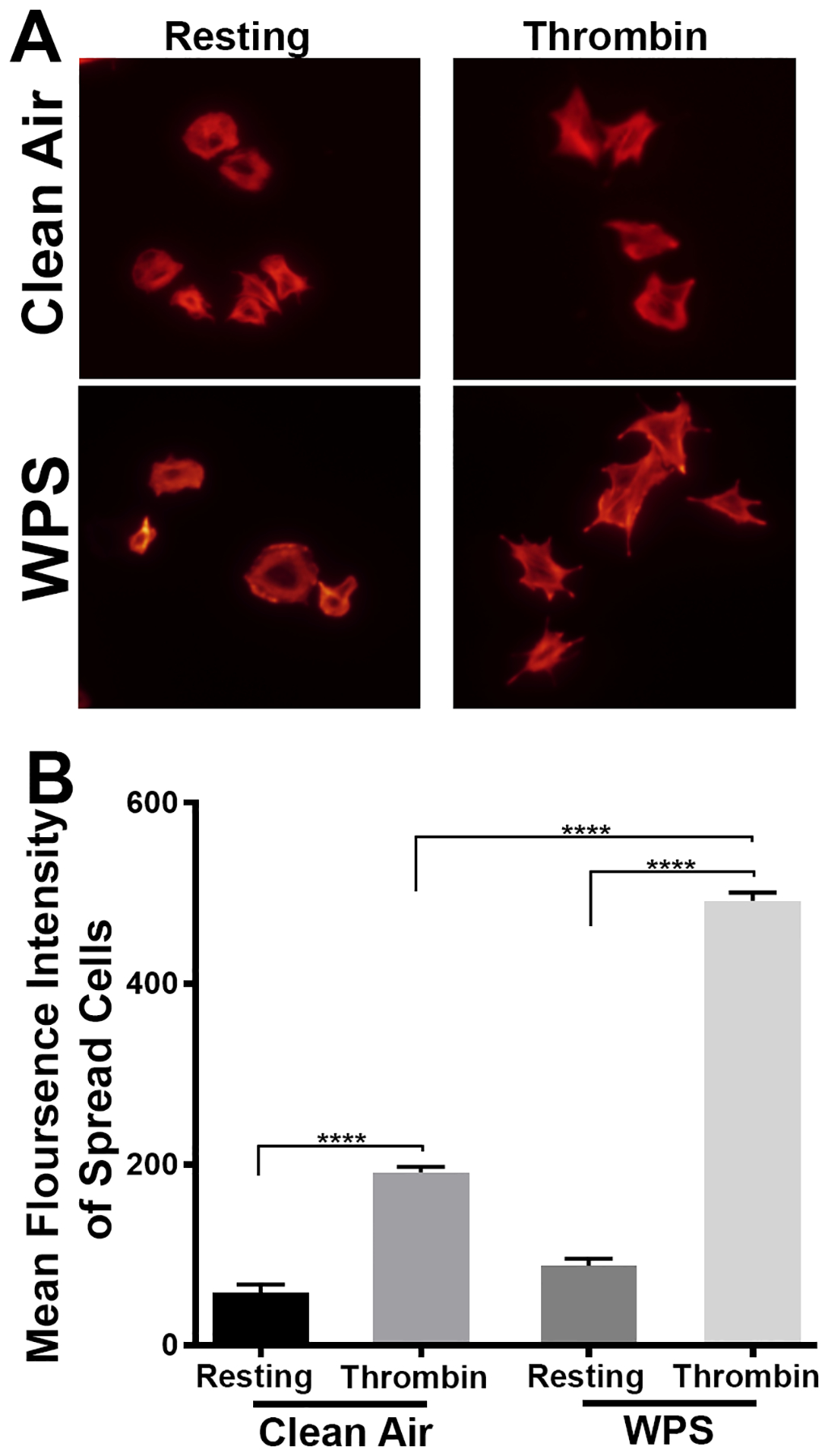
Platelet spreading is enhanced in WPS exposed mice. Platelets from WPS and clean air exposed mice were allowed to adhere to fibrinogen-coated coverslips for 5 minutes after stimulation with thrombin (0.1 U/mL). (A) Tetramethylrhodamine-conjugated phalloidin was used to stain for F-actin and imaged using Leica DMi8 inverted widefield fluorescence microscope with integrated high precision focus drive. Images were processed using LAS X Wizard imaging software. (B) Quantification of the spreading data. Data are representative of 3 independent experiments. Each experiment was repeated at least 3 times, with blood pooled from a group of 8 mice each time. WPS indicates Waterpipe Smoke.
WPS Exposure and Nicotine Treatment Enhance Agonist-Induced Akt and ERK Phosphorylation
A critical signaling mechanism in platelet function and thrombus formation is the phosphorylation of Akt and/or ERK proteins63–66. Therefore, we investigated whether WPS exposure would enhance phosphorylation of Akt and/or ERK in platelets. Our data showed that Akt and ERK phosphorylation are indeed enhanced in WPS-exposed platelets, relative to controls after stimulation with 0.1 U/ml thrombin (Figure 6A; data quantification is shown in Figure 6B). These results provide biochemical evidence and indicate that Akt and/or ERK are an essential component in the WPS-mediated modulation of platelets toward a hyperactive state. We next determined whether nicotine, the WPS chemical, has the capacity to trigger Akt and/or ERK activation. Indeed, platelets from mice that were injected with nicotine (1 mg/kg IV) exhibited enhancement of Akt and ERK phosphorylation in response to 5 μM ADP (Figure 6C; data quantification is shown in Figure 6D); which is consistent with previous reports67–69. This data suggests that nicotine plays a role- “at least in part”- in the priming of platelets into a hyperactive state.
Figure 6:
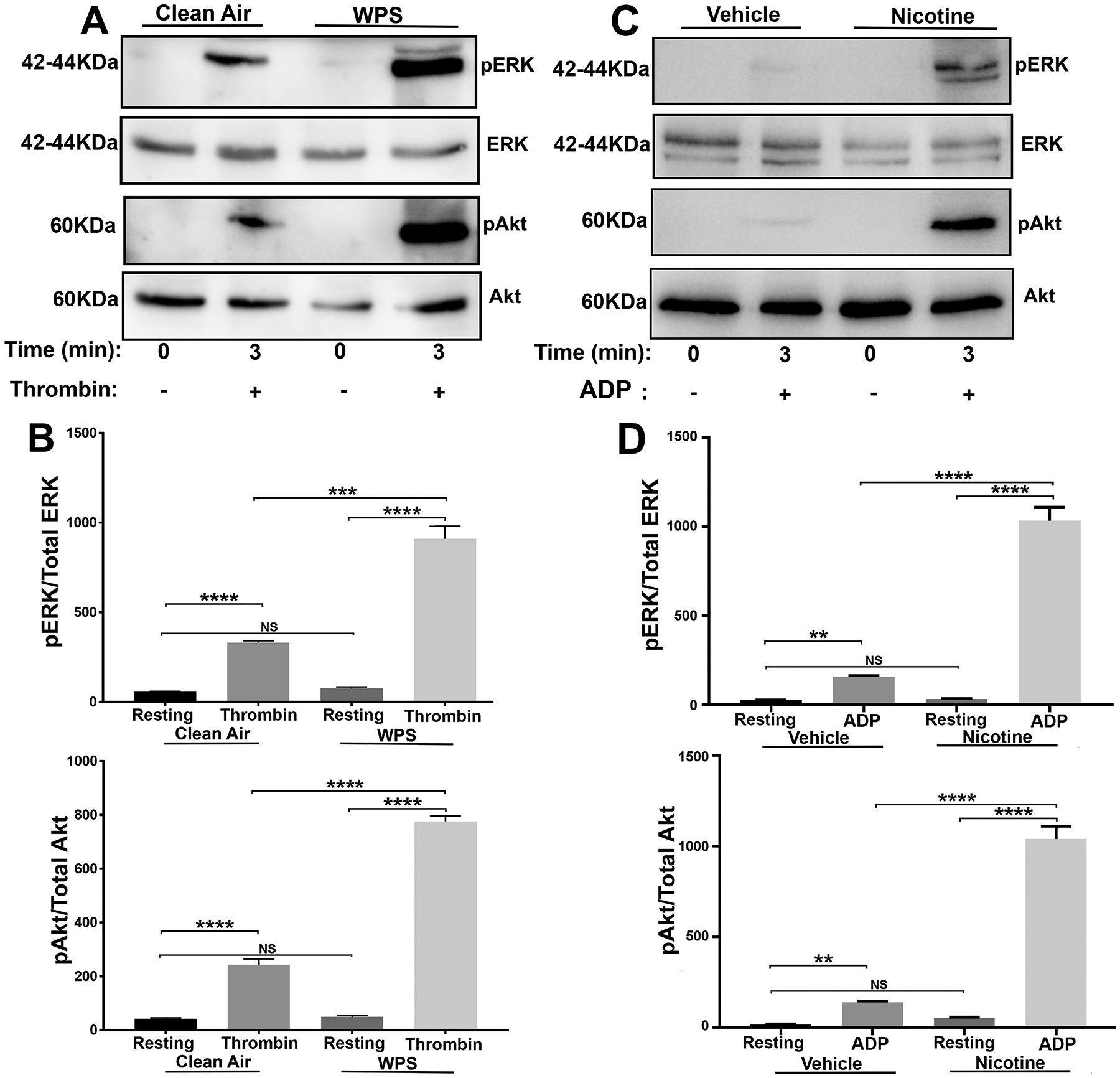
Platelet Akt and ERK activation (phosphorylation) are enhanced in WPS exposed and nicotine treated mice. Platelets from WPS and clean air exposed mice as well as from nicotine and vehicle injected mice were prepared and washed. Platelets were stimulated with 0.1 U/ml thrombin (A) or 5 μmol/L ADP (C) for 3 minutes, and proteins were lysed using 1X sample buffer. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis before being subjected to immunoblotting with anti-Akt, anti-pAkt (Ser473), anti-ERK, and anti-pERK antibodies. (B. D) Data quantification of agonist-induced ERK and Akt phospgorylation (****P < 0.0001; ***P < 0.001; NS. nonsignificant). Each experiment was repeated at least 3 times, with blood pooled from a group of 8 mice each time. WPS indicates Waterpipe Smoke.
WPS Exposure Does Not Modulate Plasma Fibrinogen Levels
To determine if the coagulation system is impacted by WPS, we sought to investigate if there is any change in the plasma concentration of fibrinogen. Our results showed that the plasma concentration of fibrinogen was not affected in WPS exposed mice in comparison to clean air, at least under the present experimental conditions (Figure 7A).
Figure 7:
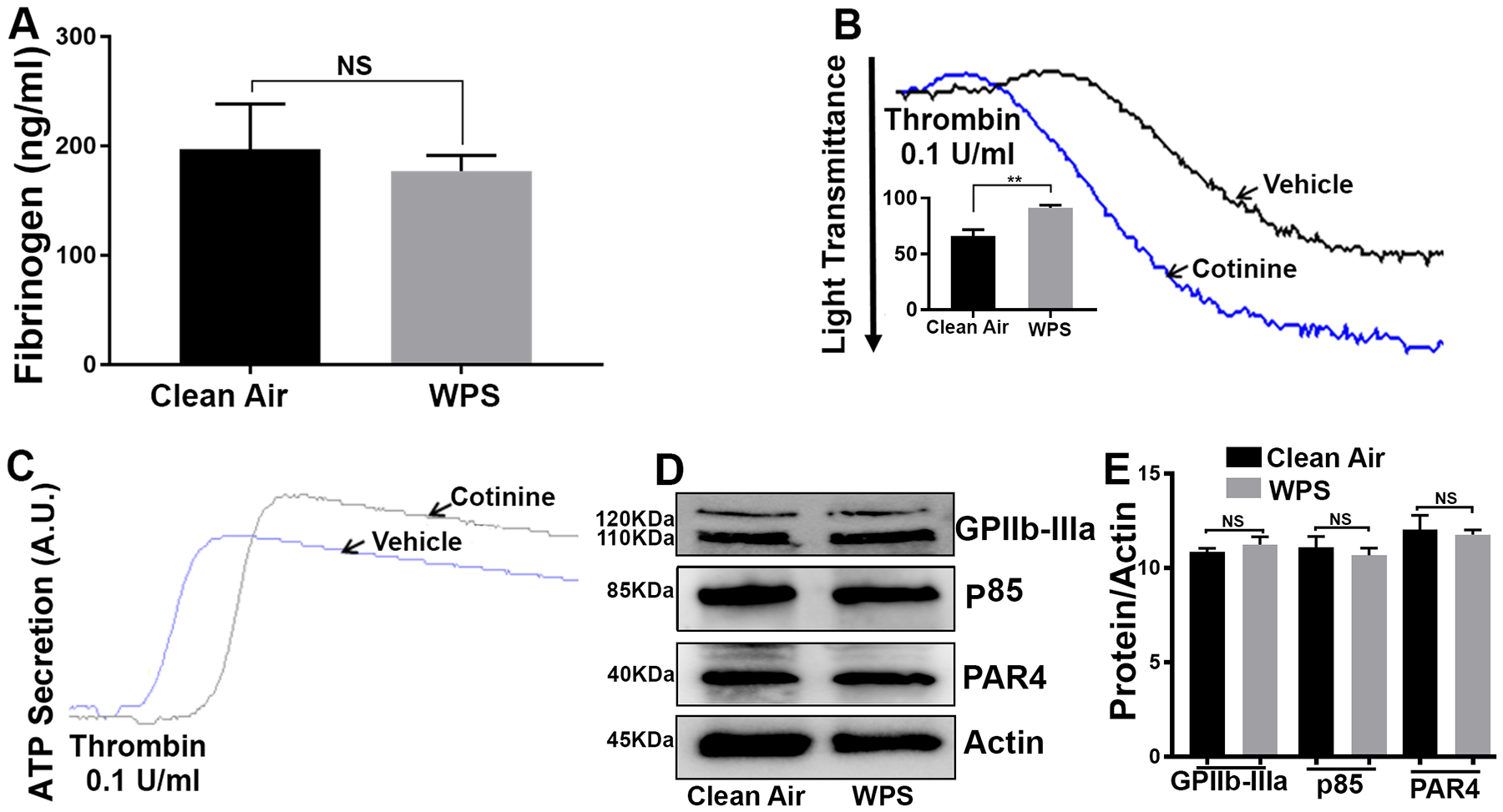
WPS does not affect plasma fibrinogen or protein expression levels of GPIIb-IIIa, PAR4 or p85 (PI3K), whereas continine enhances aggregation and dense granule secretion. Plasma was collected from WPS and clean air controls before the levels of fibrinogen were measured (A); data is presented as mean ± SD (n = 5). Mice were injected with 1 μM cotinine or vehicle, once daily for 1 week before platelets were harvested, and stimulated with thrombin (0.1 U/ml), and aggregation (B) and ATP release (C) were monitored using Lumi-aggregometer. (D) Platelets from WPS and clean air exposed mice were prepared and washed. Proteins were lysed using 1X sample buffer, and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis before being subjected to immunoblotting with anti-GPIIb-IIIa (αIIbβ3), anti-PAR4 or anti-p85/PI3K antibodies; (E) data quantification of GPIIb-IIIa,PAR4 and p85/PI3K expression; NS: nonsignificant. Each experiment was repeated at least 3 times, with blood pooled from a group of 8 mice each time. WPS indicates Waterpipe Smoke.
Cotinine Enhance Platelets Aggregation and Secretion
Previous studies suggested that cotinine, a main metabolite of nicotine (with a long/er half-life) can induce thrombus formation70. Therefore, we sought to investigate the effect of cotinine on platelets function (aggregation and secretion ex vivo). Our data revealed that cotinine (1 μM injection for one week) did in fact enhance thrombin-induced platelet aggregation and dense granule secretion, in comparison to the vehicle control (Figure 7B & 7C). These data indicate that cotinine is a likely key contributor to WPS’s “negative” effects on platelets.
WPS Exposure Does Not Modulate Expression of GPIIb-IIIa, PAR4 or PI3K.
We next sought to investigate of the aforementioned effects of WPS may involve changes in the expression levels of some of platelet proteins, namely GPIIb-IIIa (αIIbβ3), PAR4 and PI3K/p85. To this end, we did not observe any detectable changes in the expression levels of PAR4 or PI3K/p85, between WPS and clean air platelets (Figure 7D; data quantification is shown in Figure 7E). These data suggest that, at least under the current experimental conditions, short-term exposure to WPS does not alter expression of platelet proteins.
Discussion & Conclusion
In this study, we investigated the effect of exposure to hookah/waterpipe smoke (WPS) on platelet function, hemostasis, and thrombogenesis. Similar to cigarette smoking, our results indicates that, even when the exposure to WPS is short-term, namely in this case seven/7 days long, mice are under an increased risk for thrombosis with a shortened time for physiological hemostasis, which seems to derive from a hyperactive platelet function phenotype.
Many studies have undisputedly established that cigarette smoking is a risk factor for cardiovascular disease, including unstable angina71, 72, myocardial infarction73, and stroke74. On the other hand, despite its widespread use and being a rising trend in the west5–9- driven by its perceived “less harm”- studies on the effects of waterpipe smoking/WPS on cardiovascular disease are limited. Moreover, the direct consequences of WPS exposure in the context of platelets and thrombosis are yet to be investigated. Waterpipes carry a toxic profile that is thought to be comparable or to even exceed that of cigarette smoking32, 75–77, which would suggest that they have “similar” negative health effects. In fact, according to some studies, the smoke emitted from a single waterpipe tobacco smoking episode contains eight times the CO, three times the nitric oxides, an average of 11.5 times the acrolien, an average of 18.2 times the formaldehyde, and up to 245 times the polycyclic aromatic hydrocarbons (PAHs)26, 41, 77, 78, relative to a single regular tobacco cigarette.
Based on the aforementioned considerations, and limitations of previous studies, we sought to “uncover” the effects of WPS on platelet function by employing a “novel” whole body exposure protocol that simulates real life exposure scenarios31. An important aspect of our system is the fact that it involves minimal animal handling, with the animals unrestrained and less stressed. Thus, the animal model we used in our study (mice) should provide translational results that are clinically applicable to humans, especially in the context of tobacco exposure79, 80, especially since the number of puffs, puff interval, etc., are based on human exposure data. Of note, this model gives us flexibility in mimicking many aspects of human exposure that cannot be directly obtained from humans, due to ethical considerations and other challenges81. Nonetheless, one limitation is the fact that the exposure parameters (number of puffs, puffs interval, etc.) do not consider the concentration of the smoke relative to the size of a mouse.
The primary aim of this study was to determine the effects of short-term WPS exposure on platelet activation, and the genesis of thrombosis. Our findings revealed- for the first time- that short term exposure to WPS significantly reduced the bleeding time in exposed mice, and increased their tendency to carotid artery thrombosis. These findings provide evidence that WPS is not safe as the common misperception implies, even with a short-term exposure of only seven/7 days. In addition, our data are consistent with other reports that short-term use of waterpipes disrupts normal cardiovascular function82–89, and that exposure to other forms of tobacco impacts hemostasis and thrombosis45, 90. Notably, patients who have a history of cardiovascular disease (e.g. atherosclerotic disease) are probably at a higher risk of having negative outcomes from waterpipe use.
We next investigated the mechanism by which enhanced hemostasis and thrombosis may have manifested in the WPS exposed mice. Importantly, since it has been shown that an increase in platelets activity plays a major role in the pathogenesis of acute myocardial infarction (MI)91 and acute stroke92–94, and since thrombosis is the main mechanism of smoking related cardiovascular mortality21, this notion was investigated herein. Our results demonstrated that agonist-induced platelet aggregation, secretion (dense and α-granules), integrin activation, phosphatidylserine expression, and platelet spreading were all enhanced as a result of WPS exposure. Moreover, similar enhancement of platelet aggregation and dense granule secretion was observed when mice were exposed to a different waterpipe/hookah tobacco brand (see Methods for details). It is noteworthy that one study in humans reported a significant increase in TXB2 levels - a metabolite of the biologically active TXA2- after a single waterpipe smoking session95. This increase in TXB2 levels would suggest an increase in platelet activity96, which is consistent with our findings. Together, these findings support our initial hypothesis that WPS exposure (similar to cigarette smoking) modulates platelets to a state of hyperactivity, which eventually leads to the prothrombotic phenotype observed in the WPS-exposed mice. Notably, there were no changes in platelet count under our exposure protocol, which indicates that short-term (7 days) exposure to WPS does not result in any change in platelet formation. This data is consistent with a report that showed insignificant difference in platelet count in mice exposed to waterpipe smoke97. However, whether platelet number would change if waterpipe exposure is repeated over longer periods of time, as was also reported previously with waterpipe exposure98, remains to be determined.
Given the “functional” evidence we have obtained thus far, we next examined whether platelet activation-mediated downstream signaling, namely Akt and ERK activation, would also be impacted by WPS. Our data showed an increase in the levels of phosphorylated Akt and ERK in the WPS exposed platelets in comparison to the clean air controls. Interestingly, we previously observed similar results with exposure to e-cigarette44. This might suggest overlap between waterpipes and e-cigarettes with regard to the mechanism by which they enhance platelet activation, which is presumably through nicotine. The latter notion is consistent with previous findings that nicotine upregulates Akt and ERK pathways67–69, a notion we were able to verify under our experimental conditions, i.e., platelets from nicotine treated mice showed an increase in phosphorylation of Akt and ERK. Given the short half life of nicotine (6–7 minutes), one cannot exclude the fact that one of its metabolites could be responsible for the enhanced Akt and ERK activation.
As far as what toxic ingredient(s) in waterpipe smoke that might be responsible for the hyperactive state of platelets, several toxicants have been found including nicotine/cotinine41, 78, 99–101, aldehydes26, 101, particulate matter102 as well as furanic and phenolic compounds76, 103. To this end, we found that treatment with cotinine- one of nicotine’s main metabolites- is capable of priming platelets into a hyperactive state. These data support the notion that cotinine is a key toxic waterpipe ingredient, and is a likely contributor to their prothrombotic phenotype, as well as to their harmful effects on the cardiovascular system. It is noteworthy that the amounts of these toxicants vary between waterpipe compared with cigarette smoke (per cigarette/and per pack/day) due to different heating process and charcoal combustion104–107. Although, the effect of each one of these chemicals on platelets is not fully studied, particulate matter108, 109 and the aldehyde acrolein90 were indeed found to enhance platelet function. Of note, waterpipes may in fact result in higher levels of nicotine given the longer duration of a session, and which involves 100–200 times the volume of smoke from a single cigarette. This also applies to other toxins in tobacco110. In regards to carbon monoxide (CO), which is one of the main toxic ingredients of WPS, there is a debate regarding its impact on hemostasis and platelets function. On the one hand, studies reported an inhibitory effect on hemostasis and platelets function, whereas on the other hand, separate studies reported an enhancing effect on hemostasis and platelets function111. This controversy may be related to the variability in the exposure protocols, as well as the methods used. Nonetheless, CO may have contributed to the negative health effects we observed with WPS.
We also examined whether the WPS phenotype observed thus far may involve modulation of coagulation, or changes in the basal expression of receptors and/or signaling molecules/proteins critically involved in platelet activation. Our data showed no detectable differences in the plasma levels of the clotting factor fibrinogen between the WPS and control mice. In terms of changes in the basal expression levels of proteins, our data revealed that there was no difference in the levels of the GPIIb-IIIa, PAR4 and p85 (PI3K) proteins between the control and the WPS platelets. However, one cannot exclude the possibility that other aspects of coagulation and/or that the expression of other proteins may have changed. It is also possible that different real-life exposure scenarios may lead to changes in the plasma levels of fibrinogen and/or the expression levels of platelet proteins. Of note, higher fibrinogen plasma levels were reported in long term waterpipe smokers112, and it was previously shown that the levels of the P2Y12 receptor protein do change in megakaryoblasts and other cells113, in response to nicotine.
Notably, it is well documented that regular cigarette smoking and (more recently) e-cigarettes enhance platelet function and increase the risk of thrombosis44, 114, and our present studies show “similar” negative effects from WPS. However, comparative studies regarding differences in the magnitude or potency of each form of tobacco are lacking, but are clearly warranted to better understand and appreciate the relative negative health risk associated with each form.
The present study did not address the role of sex in the negative health consequences of waterpipe, as it employed exclusively male mice. The main purpose of this experimental design is/was to minimize the variability that would be caused by hormonal fluctuations associated with the female’s reproductive cycle115. Moreover, it is highly likely that “non-platelet” factors are contributing to the prothrombotic phenotype associated with WPS/hookah. Both of these issues will be the subject of future investigations.
In summary, by employing a validated animal exposure model, we are the first to investigate the impact of short-term whole-body WPS/waterpipe exposure on platelet function, homeostasis, and thrombosis. We document that WPS increases the risk of thrombosis, due to platelet hyperactivity. In conclusion, our findings provide evidence to argue against the common/currently held beliefs regarding the perceived safety of waterpipe smoking, which is expected to raise awareness regarding the negative health consequences of this increasingly popular form of tobacco.
Supplementary Material
Highlights.
Whole body exposure to hookah/waterpipe smoke/WPS modulates physiological hemostasis and increases the risk of thrombotic disease.
WPS enhances a host of platelet functional responses, namely aggregation, secretion, PS exposure, GPIIb-IIIa activation, and spreading, as well as Akt and ERK phosphorylation.
These findings are expected to help clinicians educate their patients and the public regarding the evidence that waterpipes are apparently not as safe as one might think, as well as regarding the their negative cardiovascular health effects.
Grants/Sources of Funding
Research reported in this publication was supported by the National Institute Of Environmental Health Sciences and the National Heart, Lung, And Blood Institute, of the National Institutes of Health under Awards Number R21ES029345 and R01HL145053. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by startup funds provided by the School of Pharmacy, The University of Texas at El Paso (to Khasawneh and Alshbool).
Nonstandard Abbreviations and Acronyms
- CVD
Cardiovascular disease
- WPS
Waterpipe smoke
- HR
Heart rate
- BP
Blood pressure
- ADP
Adenosine diphosphate
- CO
Carbon monoxide
- GPCRs
G-protein coupled receptors
- ERK
Extracellular signal regulated kinases
- AKT
Protein kinase B
- MPV
Mean platelet volume
- HCT
Hematocrit
- PS
Phosphatidylserine
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors
Reference
- 1.Rinkūnienė E, Petrulionienė Ž, Dženkevičiūtė V, Gimžauskaitė S, Mainelis A, Puronaitė R, Jucevičienė A, Gargalskaitė U, Laucevičius A. Trends in cigarette smoking among middle-aged lithuanian subjects participating in the primary prevention program between 2009 and 2016. Medicina. 2019;55:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Heart disease facts. 2017
- 3.Health UsDo, Staff HS, Prevention NCfCD, Smoking HPOo, Control CfD, Prevention. How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: A report of the surgeon general. US Government Printing Office; 2010. [PubMed] [Google Scholar]
- 4.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults - united states, 2016. MMWR. Morbidity and mortality weekly report 2018;67:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jukema J, Bagnasco D, Jukema R. Waterpipe smoking: Not necessarily less hazardous than cigarette smoking. Netherlands Heart Journal. 2014;22:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jawad M, Charide R, Waziry R, Darzi A, Ballout RA, Akl EA. The prevalence and trends of waterpipe tobacco smoking: A systematic review. PloS one. 2018;13:e0192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salloum RG, Thrasher JF, Getz KR, Barnett TE, Asfar T, Maziak W. Patterns of waterpipe tobacco smoking among u.S. Young adults, 2013–2014. American journal of preventive medicine. 2017;52:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Primack BA, Freedman-Doan P, Sidani JE, Rosen D, Shensa A, James AE, Wallace J. Sustained waterpipe tobacco smoking and trends over time. American journal of preventive medicine. 2015;49:859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orth B, Merkel C. [the decline of cigarette smoking among adolescents and young adults in germany and the rising relevance of waterpipes, ecigarettes and ehookahs]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2018;61:1377–1387 [DOI] [PubMed] [Google Scholar]
- 10.Jukema JB, Bagnasco DE, Jukema RA. Waterpipe smoking: Not necessarily less hazardous than cigarette smoking: Possible consequences for (cardiovascular) disease. Neth Heart J. 2014;22:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin TT, Amr MAM, Zaza BO, Kaliyadan F. Predictors of waterpipe smoking among secondary school adolescents in al hassa, saudi arabia. International journal of behavioral medicine. 2012;19:324–335 [DOI] [PubMed] [Google Scholar]
- 12.Baheiraei A, Sighaldeh SS, Ebadi A, Kelishadi R, Majdzadeh SR. Psycho-social needs impact on hookah smoking initiation among women: A qualitative study from iran. International journal of preventive medicine. 2015;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelishadi R, reza Mokhtari M, Tavasoli AA, Khosravi A, Ahangar-Nazari I, Sabet B, Kazemi A, Amini A. Determinants of tobacco use among youths in isfahan, iran. International journal of public health. 2007;52:173–179 [DOI] [PubMed] [Google Scholar]
- 14.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. Journal of the American College of Cardiology. 2004;43:1731–1737 [DOI] [PubMed] [Google Scholar]
- 15.Messner B, Bernhard D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:509–515 [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL. Cigarette smoking and cardiovascular disease: Pathophysiology and implications for treatment. Progress in Cardiovascular Diseases.46:91–111 [DOI] [PubMed] [Google Scholar]
- 17.How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: A report of the surgeon general. Atlanta (GA); 2010. [PubMed] [Google Scholar]
- 18.Bullen C Impact of tobacco smoking and smoking cessation on cardiovascular risk and disease. Expert review of cardiovascular therapy. 2008;6:883–895 [DOI] [PubMed] [Google Scholar]
- 19.Michael Pittilo R Cigarette smoking, endothelial injury and cardiovascular disease. International journal of experimental pathology. 2000;81:219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ. Impact of electronic cigarettes on the cardiovascular system. Journal of the American Heart Association. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1460–1467 [DOI] [PubMed] [Google Scholar]
- 22.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. Journal of the American College of Cardiology. 2004;43:1731–1737 [DOI] [PubMed] [Google Scholar]
- 23.Barua RS, Sy F, Srikanth S, Huang G, Javed U, Buhari C, Margosan D, Ambrose JA. Effects of cigarette smoke exposure on clot dynamics and fibrin structure: An ex vivo investigation. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:75–79 [DOI] [PubMed] [Google Scholar]
- 24.El-Nachef W, Hammond S. Exhaled carbon monoxide with waterpipe use in us students. JAMA. 2008;299:36–38 [DOI] [PubMed] [Google Scholar]
- 25.Elsayed Y, Dalibalta S, Abu-Farha N. Chemical analysis and potential health risks of hookah charcoal. The Science of the total environment. 2016;569–570:262–268 [DOI] [PubMed] [Google Scholar]
- 26.Al Rashidi M, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2008;46:3546–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.OA-A. A-O. The acute effect of shisha smoking on oxygen saturation level and heart rate. Med Princ Pract. 2012;21:588–590 [Google Scholar]
- 28.Blank MD, Cobb CO, Kilgalen B, Austin J, Weaver MF, Shihadeh A, Eissenberg T. Acute effects of waterpipe tobacco smoking: A double-blind, placebo-control study. Drug and alcohol dependence. 2011;116:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diab OA, Abdelrahim EM, Esmail M. Effect of water pipe tobacco smoking on plasma high sensitivity c reactive protein level and endothelial function compared to cigarette smoking. The Egyptian Heart Journal. 2015;67:233–241 [Google Scholar]
- 30.Haddad L, Kelly DL, Weglicki LS, Barnett TE, Ferrell AV, Ghadban R. A systematic review of effects of waterpipe smoking on cardiovascular and respiratory health outcomes. Tobacco use insights. 2016;9:13–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shihadeh A, Azar S, Antonios C, Haddad A. Towards a topographical model of narghile water-pipe cafe smoking: A pilot study in a high socioeconomic status neighborhood of beirut, lebanon. Pharmacology, biochemistry, and behavior. 2004;79:75–82 [DOI] [PubMed] [Google Scholar]
- 32.Khabour OF, Alzoubi KH, Bani-Ahmad M, Dodin A, Eissenberg T, Shihadeh A. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhalation Toxicology. 2012;24:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katurji M, Daher N, Sheheitli H, Saleh R, Shihadeh A. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhalation Toxicology. 2010;22:1101–1109 [DOI] [PubMed] [Google Scholar]
- 34.Katurji M, Daher N, Sheheitli H, Saleh R, Shihadeh A. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhalation toxicology. 2010;22:1101–1109 [DOI] [PubMed] [Google Scholar]
- 35.Shihadeh A, Azar S. A closed-loop control “playback” smoking machine for generating mainstream smoke aerosols. J Aerosol Med. 2006;19:137–147 [DOI] [PubMed] [Google Scholar]
- 36.Khabour OF, Alzoubi KH, Bani-Ahmad M, Dodin A, Eissenberg T, Shihadeh A. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhal Toxicol. 2012;24:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan NA, Sundar IK, Rahman I. Strain- and sex-dependent pulmonary toxicity of waterpipe smoke in mouse. Physiol Rep. 2018;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khabour OF, Alzoubi KH, Al-Sawalha N, Ahmad MB, Shihadeh A, Eissenberg T. The effect of chronic exposure to waterpipe tobacco smoke on airway inflammation in mice. Life Sci. 2018;200:110–114 [DOI] [PubMed] [Google Scholar]
- 39.Ali BH, Al Balushi KA, Ashique M, Shalaby A, Al Kindi MA, Adham SA, Karaca T, Beegam S, Yuvaraju P, Nemmar A. Chronic water-pipe smoke exposure induces injurious effects to reproductive system in male mice. Front Physiol. 2017;8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemmar A, Al Hemeiri A, Al Hammadi N, Yuvaraju P, Beegam S, Yasin J, Elwasila M, Ali BH, Adeghate E. Early pulmonary events of nose-only water pipe (shisha) smoking exposure in mice. Physiol Rep. 2015;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2005;43:655–661 [DOI] [PubMed] [Google Scholar]
- 42.Rababa’h AM, Sultan BB, Alzoubi KH, Khabour OF, Ababneh MA. Exposure to waterpipe smoke induces renal functional and oxidative biomarkers variations in mice. Inhal Toxicol. 2016;28:508–513 [DOI] [PubMed] [Google Scholar]
- 43.Rababa’h AM, Bsoul RW, Alkhatatbeh MJ, Alzoubi KH, Khabour OF. Waterpipe tobacco smoke distresses cardiovascular biomarkers in mice: Alterations in protein expression of metalloproteinases, endothelin and myeloperoxidase. Inhal Toxicol. 2019:1–8 [DOI] [PubMed] [Google Scholar]
- 44.Qasim H, Karim ZA, Silva-Espinoza JC, Khasawneh FT, Rivera JO, Ellis CC, Bauer SL, Almeida IC, Alshbool FZ. Short-term e-cigarette exposure increases the risk of thrombogenesis and enhances platelet function in mice. Journal of the American Heart Association. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karim ZA, Alshbool FZ, Vemana HP, Adhami N, Dhall S, Espinosa EV, Martins-Green M, Khasawneh FT. Third-hand smoke: Impact on hemostasis and thrombogenesis. Journal of cardiovascular pharmacology. 2015;66:177–182 [DOI] [PubMed] [Google Scholar]
- 46.Choi W, Karim ZA, Whiteheart SW. Arf6 plays an early role in platelet activation by collagen and convulxin. Blood. 2006;107:3145–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qasim H, Karim ZA, Hernandez KR, Lozano D, Khasawneh FT, Alshbool FZ. Arhgef1 plays a vital role in platelet function and thrombogenesis. Journal of the American Heart Association. 2019;8:e011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karim ZA, Alshbool FZ, Vemana HP, Conlon C, Druey KM, Khasawneh FT. Cxcl12 regulates platelet activation via the regulator of g-protein signaling 16. Biochimica et biophysica acta. 2016;1863:314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kassem NOF, Kassem NO, Liles S, Jackson SR, Posis AIB, Chatfield DA, Hovell MF. Levels of urine cotinine from hookah smoking and exposure to hookah tobacco secondhand smoke in hookah lounges and homes. Int J High Risk Behav Addict. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon KA, Rule AM, Magid HS, Ferguson JM, Susan J, Sun Z, Torrey C, Abubaker S, Levshin V, Carkoglu A, Radwan GN, El-Rabbat M, Cohen JE, Strickland P, Breysse PN, Navas-Acien A. Biomarkers of secondhand smoke exposure in waterpipe tobacco venue employees in istanbul, moscow, and cairo. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2018;20:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nosratzehi T, Arbabi-Kalati F, Alijani E, Tajdari H. Comparison of cotinine salivary levels in hookah smokers, passive smokers, and non-smokers. Addict Health. 2015;7:184–191 [PMC free article] [PubMed] [Google Scholar]
- 52.Kassem NO, Daffa RM, Liles S, Jackson SR, Kassem NO, Younis MA, Mehta S, Chen M, Jacob P 3rd, Carmella SG, Chatfield DA, Benowitz NL, Matt GE, Hecht SS, Hovell MF. Children’s exposure to secondhand and thirdhand smoke carcinogens and toxicants in homes of hookah smokers. Nicotine Tob Res. 2014;16:961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ljungberg LU, Persson K, Eriksson AC, Green H, Whiss PA. Effects of nicotine, its metabolites and tobacco extracts on human platelet function in vitro. Toxicol In Vitro. 2013;27:932–938 [DOI] [PubMed] [Google Scholar]
- 54.Benowitz NL, Hukkanen J, Jacob P, 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macaron C, Macaron Z, Maalouf MT, Macaron N, Moore A. Urinary cotinine in narguila or chicha tobacco smokers. J Med Liban. 1997;45:19–20 [PubMed] [Google Scholar]
- 56.Levine H, Berman T, Goldsmith R, Goen T, Spungen J, Novack L, Amitai Y, Shohat T, Grotto I. Exposure to tobacco smoke based on urinary cotinine levels among israeli smoking and nonsmoking adults: A cross-sectional analysis of the first israeli human biomonitoring study. BMC Public Health. 2013;13:1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levine PH. An acute effect of cigarette smoking on platelet function. A possible link between smoking and arterial thrombosis. Circulation. 1973;48:619–623 [DOI] [PubMed] [Google Scholar]
- 58.Takajo Y, Ikeda H, Haramaki N, Murohara T, Imaizumi T. Augmented oxidative stress of platelets in chronic smokers. Mechanisms of impaired platelet-derived nitric oxide bioactivity and augmented platelet aggregability. Journal of the American College of Cardiology. 2001;38:1320–1327 [DOI] [PubMed] [Google Scholar]
- 59.Dawood BB, Wilde J, Watson SP. Reference curves for aggregation and atp secretion to aid diagnose of platelet-based bleeding disorders: Effect of inhibition of adp and thromboxane a(2) pathways. Platelets. 2007;18:329–345 [DOI] [PubMed] [Google Scholar]
- 60.Storrie B, Whiteheart SW. Editorial: Platelet secretion. Platelets. 2017;28:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao C, Xie R, Yu C, Wang Q, Shi F, Yao C, Xie R, Zhou J, Gilbert GE, Shi J. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thrombosis and haemostasis. 2012;107:681–689 [DOI] [PubMed] [Google Scholar]
- 62.Hawiger J. Formation and regulation of platelet and fibrin hemostatic plug. Hum Pathol. 1987;18:111–122 [DOI] [PubMed] [Google Scholar]
- 63.Senis YA, Sangrar W, Zirngibl RA, Craig AW, Lee DH, Greer PA. Fps/fes and fer non-receptor protein-tyrosine kinases regulate collagen- and adp-induced platelet aggregation. Journal of thrombosis and haemostasis: JTH. 2003;1:1062–1070 [DOI] [PubMed] [Google Scholar]
- 64.Stojanovic A, Marjanovic JA, Brovkovych VM, Peng X, Hay N, Skidgel RA, Du X. A phosphoinositide 3-kinase-akt-nitric oxide-cgmp signaling pathway in stimulating platelet secretion and aggregation. The Journal of biological chemistry. 2006;281:16333–16339 [DOI] [PubMed] [Google Scholar]
- 65.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase-mediated and erk-mediated thromboxane a2 generation are essential for vwf/gpib-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking akt2. The Journal of clinical investigation. 2004;113:441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. The Journal of clinical investigation. 2003;111:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic creb and erk signaling in c57bl/6j mice. Journal of neurochemistry. 2003;84:1431–1441 [DOI] [PubMed] [Google Scholar]
- 69.Dajas-Bailador FA, Soliakov L, Wonnacott S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase a, in sh-sy5y cells and hippocampal neurones. Journal of neurochemistry. 2002;80:520–530 [DOI] [PubMed] [Google Scholar]
- 70.Sastry BV, Gujrati VR. Activation of paf-acetylhydrolase by nicotine and cotinine and their possible involvement in arterial thrombosis. Annals of the New York Academy of Sciences. 1994;714:312–314 [DOI] [PubMed] [Google Scholar]
- 71.Daly LE, Mulcahy R, Graham IM, Hickey N. Long term effect on mortality of stopping smoking after unstable angina and myocardial infarction. Br Med J (Clin Res Ed). 1983;287:324–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merry AH, Boer JM, Schouten LJ, Feskens EJ, Verschuren WM, Gorgels AP, van den Brandt PA. Smoking, alcohol consumption, physical activity, and family history and the risks of acute myocardial infarction and unstable angina pectoris: A prospective cohort study. BMC cardiovascular disorders. 2011;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart diseasea systematic review. JAMA. 2003;290:86–97 [DOI] [PubMed] [Google Scholar]
- 74.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. Bmj. 1989;298:789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sepetdjian E, Shihadeh A, Saliba NA. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2008;46:1582–1590 [DOI] [PubMed] [Google Scholar]
- 76.Sepetdjian E, Abdul Halim R, Salman R, Jaroudi E, Shihadeh A, Saliba NA. Phenolic compounds in particles of mainstream waterpipe smoke. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2013;15:1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Primack BA, Carroll MV, Weiss PM, Shihadeh AL, Shensa A, Farley ST, Fine MJ, Eissenberg T, Nayak S. Systematic review and meta-analysis of inhaled toxicants from waterpipe and cigarette smoking. Public health reports (Washington, D.C. : 1974). 2016;131:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shihadeh A Investigation of mainstream smoke aerosol of the argileh water pipe. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2003;41:143–152 [DOI] [PubMed] [Google Scholar]
- 79.Morissette MC, Lamontagne M, Berube JC, Gaschler G, Williams A, Yauk C, Couture C, Laviolette M, Hogg JC, Timens W, Halappanavar S, Stampfli MR, Bosse Y. Impact of cigarette smoke on the human and mouse lungs: A gene-expression comparison study. PloS one. 2014;9:e92498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Churg A, Wright JL. Animal models of cigarette smoke-induced chronic obstructive lung disease. Contributions to microbiology. 2007;14:113–125 [DOI] [PubMed] [Google Scholar]
- 81.Moolchan ET, Mermelstein R. Research on tobacco use among teenagers: Ethical challenges. Journal of adolescent health. 2002;30:409–417 [DOI] [PubMed] [Google Scholar]
- 82.Hakim F, Hellou E, Goldbart A, Katz R, Bentur Y, Bentur L. The acute effects of water-pipe smoking on the cardiorespiratory system. CHEST.139:775–781 [DOI] [PubMed] [Google Scholar]
- 83.Shaikh RB, Vijayaraghavan N, Sulaiman AS, Kazi S, Shafi MS. The acute effects of waterpipe smoking on the cardiovascular and respiratory systems. Journal of preventive medicine and hygiene. 2008;49:101–107 [PubMed] [Google Scholar]
- 84.Kadhum M, Jaffery A, Haq A, Bacon J, Madden B. Measuring the acute cardiovascular effects of shisha smoking: A cross-sectional study. JRSM Open. 2014;5:2054270414531127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Azar RR, Frangieh AH, Mroué J, Bassila L, Kasty M, Hage G, Kadri Z. Acute effects of waterpipe smoking on blood pressure and heart rate: A real-life trial. Inhalation Toxicology. 2016;28:339–342 [DOI] [PubMed] [Google Scholar]
- 86.Al-Kubati M, Al-Kubati AS, al’Absi M, Fiser B. The short-term effect of water-pipe smoking on the baroreflex control of heart rate in normotensives. Auton Neurosci. 2006;126–127:146–149 [DOI] [PubMed] [Google Scholar]
- 87.Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: Direct comparison of toxicant exposure. American journal of preventive medicine. 2009;37:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hawari FI, Obeidat NA, Ayub H, Ghonimat I, Eissenberg T, Dawahrah S, Beano H. The acute effects of waterpipe smoking on lung function and exercise capacity in a pilot study of healthy participants. Inhalation toxicology. 2013;25:492–497 [DOI] [PubMed] [Google Scholar]
- 89.Layoun N, Saleh N, Barbour B, Awada S, Rachidi S, Al-Hajje A, Bawab W, Waked M, Salameh P. Waterpipe effects on pulmonary function and cardiovascular indices: A comparison to cigarette smoking in real life situation. Inhalation Toxicology. 2014;26:620–627 [DOI] [PubMed] [Google Scholar]
- 90.Sithu SD, Srivastava S, Siddiqui MA, Vladykovskaya E, Riggs DW, Conklin DJ, Haberzettl P, O’Toole TE, Bhatnagar A, D’Souza SE. Exposure to acrolein by inhalation causes platelet activation. Toxicology and applied pharmacology. 2010;248:100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tousoulis D, Paroutoglou IP, Papageorgiou N, Charakida M, Stefanadis C. Recent therapeutic approaches to platelet activation in coronary artery disease. Pharmacology & therapeutics. 2010;127:108–120 [DOI] [PubMed] [Google Scholar]
- 92.Mulley GP, Heptinstall S, Taylor PM, Mitchell JR. Adp-induced platelet release reaction in acute stroke. Thrombosis and haemostasis. 1983;50:524–526 [PubMed] [Google Scholar]
- 93.Cevik O, Adiguzel Z, Baykal AT, Somay G, Sener A. The apoptotic actions of platelets in acute ischemic stroke. Molecular biology reports. 2013;40:6721–6727 [DOI] [PubMed] [Google Scholar]
- 94.McCabe DJ, Harrison P, Mackie IJ, Sidhu PS, Purdy G, Lawrie AS, Watt H, Brown MM, Machin SJ. Platelet degranulation and monocyte-platelet complex formation are increased in the acute and convalescent phases after ischaemic stroke or transient ischaemic attack. British journal of haematology. 2004;125:777–787 [DOI] [PubMed] [Google Scholar]
- 95.Wolfram RM, Chehne F, Oguogho A, Sinzinger H. Narghile (water pipe) smoking influences platelet function and (iso-)eicosanoids. Life sciences. 2003;74:47–53 [DOI] [PubMed] [Google Scholar]
- 96.Catella F, Healy D, Lawson JA, FitzGerald GA. 11-dehydrothromboxane b2: A quantitative index of thromboxane a2 formation in the human circulation. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5861–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nemmar A, Yuvaraju P, Beegam S, John A, Raza H, Ali BH. Cardiovascular effects of nose-only water-pipe smoking exposure in mice. American Journal of Physiology-Heart and Circulatory Physiology. 2013;305:H740–H746 [DOI] [PubMed] [Google Scholar]
- 98.Nemmar A, Al-Salam S, Yuvaraju P, Beegam S, Yasin J, Ali BH. Chronic exposure to water-pipe smoke induces cardiovascular dysfunction in mice. American Journal of Physiology-Heart and Circulatory Physiology. 2017;312:H329–H339 [DOI] [PubMed] [Google Scholar]
- 99.Schubert J, Hahn J, Dettbarn G, Seidel A, Luch A, Schulz TG. Mainstream smoke of the waterpipe: Does this environmental matrix reveal as significant source of toxic compounds? Toxicology Letters. 2011;205:279–284 [DOI] [PubMed] [Google Scholar]
- 100.Jacob P 3rd, Abu Raddaha AH, Dempsey D, Havel C, Peng M, Yu L, Benowitz NL. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a water pipe. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:2345–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shihadeh A, Salman R, Jaroudi E, Saliba N, Sepetdjian E, Blank MD, Cobb CO, Eissenberg T. Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing co, no, pah, volatile aldehydes, “tar” and nicotine yields. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2012;50:1494–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monn C, Kindler P, Meile A, Brändli O. Ultrafine particle emissions from waterpipes. Tobacco Control. 2007;16:390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schubert J, Bewersdorff J, Luch A, Schulz TG. Waterpipe smoke: A considerable source of human exposure against furanic compounds. Analytica chimica acta. 2012;709:105–112 [DOI] [PubMed] [Google Scholar]
- 104.Chaouachi K. Hookah (shisha, narghile) smoking and environmental tobacco smoke (ets). A critical review of the relevant literature and the public health consequences. International journal of environmental research and public health. 2009;6:798–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shihadeh A, Eissenberg T, Rammah M, Salman R, Jaroudi E, El-Sabban M. Comparison of tobacco-containing and tobacco-free waterpipe products: Effects on human alveolar cells. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2014;16:496–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calafat AM, Polzin GM, Saylor J, Richter P, Ashley DL, Watson CH. Determination of tar, nicotine, and carbon monoxide yields in the mainstream smoke of selected international cigarettes. Tobacco Control. 2004;13:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moldoveanu SC, Coleman W III, Wilkins JM. Determination of polycyclic aromatic hydrocarbons in exhaled cigarette smoke. Beiträge zur Tabakforschung International/Contributions to Tobacco Research. 2008;23:85 [Google Scholar]
- 108.Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an il-6-dependent pathway. The Journal of clinical investigation. 2007;117:2952–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilson DW, Aung HH, Lame MW, Plummer L, Pinkerton KE, Ham W, Kleeman M, Norris JW, Tablin F. Exposure of mice to concentrated ambient particulate matter results in platelet and systemic cytokine activation. Inhalation toxicology. 2010;22:267–276 [DOI] [PubMed] [Google Scholar]
- 110.Cobb C, Ward KD, Maziak W, Shihadeh AL, Eissenberg T. Waterpipe tobacco smoking: An emerging health crisis in the united states. Am J Health Behav. 2010;34:275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nielsen VG, Pretorius E. Carbon monoxide: Anticoagulant or procoagulant? Thromb Res. 2014;133:315–321 [DOI] [PubMed] [Google Scholar]
- 112.Seyyed Hashem Sezavar MAA, MSc; Homayoun Sadeghi Bazargani,MD. A comparative study of plasma fibrinogen among hookah smokers, cigarette smokers and non-smokers Iranian Heart Journal. 2004;5: 48–54 [Google Scholar]
- 113.Shanker G, Kontos JL, Eckman DM, Wesley-Farrington D, Sane DC. Nicotine upregulates the expression of p2y12 on vascular cells and megakaryoblasts. J Thromb Thrombolysis. 2006;22:213–220 [DOI] [PubMed] [Google Scholar]
- 114.Davis JW, Shelton L, Eigenberg DA, Hignite CE, Watanabe IS. Effects of tobacco and non-tobacco cigarette smoking on endothelium and platelets. Clin Pharmacol Ther. 1985;37:529–533 [DOI] [PubMed] [Google Scholar]
- 115.Fields RD. Nih policy: Mandate goes too far. Nature. 2014;510:340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


