ABSTRACT
Although the role of B cells in sepsis immunoregulation has become an interesting topic, there is lack of data on the role of B cell function regulators in prediction of multiorgan dysfunction syndrome (MODS). The aim of this study was to evaluate the prognostic value of A Proliferation Inducing Ligand (APRIL) and soluble Transmembrane Activator and CAML Interactor Protein (sTACI), the main B cell function regulators, in prediction of MODS development within the first 48 h after admission to intensive care unit, among septic patients. We included 112 patients with sepsis, treated at Clinic for Infectious Diseases and Emergency Center, Clinical Center of Vojvodina, Novi Sad, Serbia. Plasma concentrations of APRIL and sTACI were determined at the admission and potential development of MODS was confirmed in the first 48 h. Concentrations of APRIL (p = 0.003) and sTACI (p<0.001) were higher in patients who developed MODS (n = 30). ROC curve analysis showed that AUC for sTACI (AUC = 0.764) was greater than that for procalcitonin (AUC = 0.719) and APRIL (AUC = 0.673) in MODS development prediction. Multivariate regression analysis showed that sTACI, as an anti-inflammatory biomarker stimulating the apoptosis of B cells, was the only independent predictor of MODS, beside SOFA score. Elevated level of sTACI could be the alarm for the increased B cell apoptosis and development of immune paralysis. Including these biomarkers into predictive scores specific for septic patients may potentially improve their sensitivity and specificity. Measurement of their concentrations dynamics could contribute to better assessment of sepsis evolution and timely introduction of immunomodulatory therapy.
KEYWORDS: biomarkers, multiple organ failure, prognosis, sepsis, Transmembrane activator and CAML interactor protein, tumor necrosis factor ligand superfamily member 13
Sepsis is the leading cause of death in intensive care units (ICU). Despite all the medical procedures undertaken, mortality due to sepsis remains high [1,2].
Predicting potential development of multiorgan dysfunction syndrome (MODS) before it becomes clinically manifest is essential for timely initialization of therapy. Therefore, experts in the field continue to investigate the biomarkers predictors of MODS development within the first few hours after admission to the ICU [3,4].
It is known that sepsis pathogenesis implies secretion of large number of pro- and anti-inflammatory mediators, refferred to as the cytokine storm. According to the literature, these mediators have different diagnostic and prognostic values [5–7]. Whether pro- or anti-inflammatory cytokines dominate in an individual patient, depends on various factors, including age, comorbidities and assumably genetic predisposition [6,8]. To the best of our knowledge, prognostic value of cytokines regulating B-cell function, as well as their role in the pathogenesis of sepsis, have not been clearly characterized.
Members of the tumor necrosis factor alfa (TNF-α) superfamily, A Proliferation Inducing Ligand (APRIL) and Transmembrane Activator and CAML Interactor Protein (TACI), have been recognized as the main regulators of B-cell homeostasis [9–11]. Therefore, they have been studied in the autoimmune and malignant diseases [12–14], while there are not much available data on their role in bacterial infections. Roderburg et al. were the first to examine the role of APRIL in sepsis, in the aspect of mortality prediction. However, the role of APRIL in the prediction of early development of MODS has not been evaluated to the date [15]. However, there is no available data on the concentrations of soluble TACI (sTACI) in septic patients.
APRIL stimulates B-cell proliferation and is thought to be essential in regulating their homeostasis. It is responsible for growth stimulation, differentiation and regulation of apoptosis of B-cells [9,10]. Taken into account the role of humoral immunity in the pathogenesis of sepsis [10], as well as the proven role of APRIL in homeostasis of B-cells [9,11], we hypothesized that the concentration of this biomarker could serve as the valuable predictor of the sepsis course and outcome.
TACI receptor is expressed in all developmental stages of B-cells after leaving the bone marrow. The highest level of expression is found on so-called mature innate-like B-cells: marginal zone B-lymphocytes [9,16] and B1-lymphocytes [10], that implies their essential role in the immune response to T-cell independent antigens. Mutations in the gene for TACI receptor have been identified in the patients with different types of immunodeficiency [17]. In addition to its transmembrane form, TACI receptor circulates as a soluble form, playing the same role in the circulation. Prior research has shown that concentrations of sTACI receptor strongly correlate to the concentrations of transmembrane form of the receptor [18]. Since the concentrations of the soluble form of the receptor are easily determined using ELISA (enzyme-linked immunosorbent assay) test, this biomarker could become important in everyday clinical practice.
The aim of this study is to determine the prognostic value of APRIL and sTACI receptors in the prediction of MODS development, within the first 48 hours after the admission to the ICU.
In this study we included 140 patients admitted to the Clinic for Infectious Diseases and Department for Anaesthesiology and Reanimation of the Clinical Center of Vojvodina, with diagnosis of sepsis, defined according to the criteria of The Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3) [50]. According to Sepsis-3 definition, criteria for diagnosis of sepsis are acute increase of SOFA score for 2 or more points, caused by infection. SOFA score represents nummerical scoring system used for the assessment of the function of six organ systems- central nervous, cardiovascular, respiratory, renal, liver and coagulation- by scoring from 0 to 4 (0 is given for preserved function, 1 is minor organ impairment, while 2 or more points mean the dysfunction of an organ system). MODS is defined as the dysfunction of at least two organ systems that were given scores 2 or more using SOFA scoring system.
Initially, all the patients with SOFA score 2 and higher were included in the study (n = 140). Patients with signs of MODS development present at the admission were excluded from further analysis (n = 28). The development of MODS was monitored in the rest of 112 patients (Figure 1).
Figure 1.
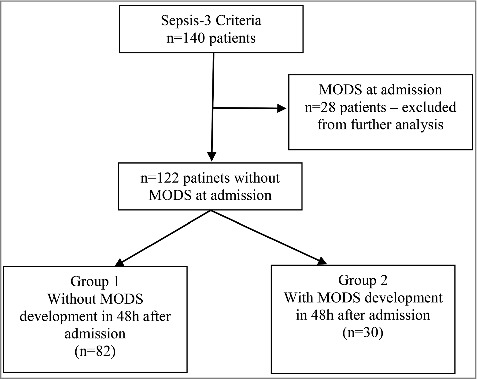
Patients selection criteria.
One hundred- fourty patients presented with the diagnosis of sepsis and had routine laboratory parameters determined within the first hour after admission (complete blood count (CBC), glucose, urea, creatinine, electrolyte levels, bilirubin level and concentrations of C reactive protein (CRP) and procalcitonin (PCT)), as well as predictive APACHE II (Acute Physiology and Chronic Health Evaluation II) and SOFA score (Sequential Organ Failure Assessment). Concentrations of C-reactive protein (CRP) were determined using immunoturbidimetric assay (ABX Micros) and expressed in mg/L. Concentrations of procalcitonin (PCT) were determined using automatic nalysed (mini Vidas), the lower limit of detection was set at 0.05 ng/L.
Twenty-eight patients presented with dysfunction of two or more organs at the admission (in two or more organ systems, SOFA score was equal to or higher than 2) so these patients were excluded from the study.
Plasma samples of the rest 112 patients were frozen on -70C and concentration of APRIL and sTACI were determined later, using ELISA tests, by commercially available R&D Systems kits (Human APRIL/TNFSF13 DuoSet ELISA R&D and Human TACI/TNFRSF13B DuoSet ELISA R&D), according to the technical guide of the producer. Concentrations of APRIL were expressed in ng/ml, and BAFF and sTACI in pg/ml. Tests we used were sandwich enzyme immunoassays for the quantitative measurement of APRIL and sTACI in human serum, plasma or cell supernatants, with detection limits of 0.94–60.0 ng/ml for APRIL and 93.6–6000.0 pg/ml for sTACI.
In the following period we monitored the state of patients and confirmed the development of MODS in the first 48 hours after the admission. MODS was defined as the development of two or more organs dysfunction (acute increase of ≥2 SOFA points per each organ system) in the first 48 h of the admission to the ICU.
Source and cause of infection were confirmed by isolation from body fluid culture (blood culture, urine, sputum culture derived from forced sputum production, tracheal aspiration, bronchoalveolar lavage, cerebrospinal fluid culture) or using SEPTIFAST method or radiology methods (Chest rail or CT scan).
Exclusion criteria were: patients with systemic inflammation caused by some other condition like burns or pancreatitis, polytraumatized patients, patients in haemorrhagic shock, patients with malignancy, autoimmune disorder, liver or cardiac failure, pregnant women, patients younger than 18 and patients who have not signed informed consent.
The study was approved by ethical committee of the Clinical Center of Vojvodina. All the participants have signed the informed consent (the patient himself, or the family)
Data were analyzed using SPSS v. 21.0 software. Categorical variables were expressed numerically and as a percentage, while mean values of continuous variables were given as arithmetic means and standard deviations or median and interquartile range, as appropriate according to the distribution. Non-parametric approaches were used, since most continuous variables were skewed. Differences between groups were evaluated using Mann-Whitney U test for continuous variables and using χ2 test for categorical variables. For all the variables that were statistically significant, we constructed Receiver Operating Characteristic (ROC) curves and measured areas under curve (AUC). For the purpose of defining independent predictors of MODS development, we performed multivariate regression analysis using binary logistic regression model. We constructed regression model with the inclusion of the MODS development in the first 48 hours after admission as the dependent variable. For the independent variables we picked those parameters that showed predictive value using univariate analysis. In the case of multicollinearity between parameters, we included in the model the parameter that showed greater predictive value using univariate analysis. Significance (p) was set at the value of 0.05.
We included overall of 112 patients diagnosed with sepsis in the study, hospitalized in the Clinic for Infectious Diseases and Department for Anaesthesiology and Reanimation, Clinical Center of Vojvodina. The state of each patient was monitored and eventual development of MODS was proven within the first 48 hours after the admission.
In 30 (26.8%) patients we confirmed MODS development, while in 82 (73.2%) patients there were no signs of MODS in the first 48 hours after admission to intensive care unit. Demographic and clinical data on the patients are summarized in Table 1. Among patients who developed MODS within 48 hours, 14 of them had 2-organ failure, 11 of them had 3-organ failure, and 5 of them had 4-organ failure. Structure of the organ failure among patients who developed MODS 48 h after admission was following: kidney (creatinin >170 µmol/l) – 22 patients, liver (bilirubin >32 µmol/l) – 13 patients, coagulation (platelets <100 × 106/L) – 12 patients, cardiovascular system (Dopamin or Dobutamin therapy) – 10 patients, central nervous system (GCS < 13) – 19 patients, respiratory insufficiency (pO2/FiO2 < 300 mmHg) – 5 patients.
Table 1.
Demographic and clinical features of patients.
| MODS development in first 48 h |
|||
| Variable |
Present n = 30 |
Absent n = 82 |
p |
| Age (x±SD) (Min-max) | 65.47 ± 12.26 (35–87) | 59.35 ± 16.54 (18–85) | 0.068 * |
| Gender – n (%) | |||
| male | 17 (56.7%) | 46 (56.1%) | 0.957** |
| Female | 13 (43.3%) | 36 (43.9%) | |
| APACHEII (x±SD) | 18.30 ± 3.40 | 11.90 ± 5.18 | <0.001** |
| SOFA (x±SD) | 7.33 ± 1.97 | 3.95 ± 1.79 | <0.001* |
| Bloodstream infection (Blood culture or SEPTIFAST confirmed) | 8/30 (26.7%) | 22/82 (26.8%) | |
| S. aureus | 1 | 3 | |
| S. pneumoniae | 2 | 4 | |
| Coagulasa negative Staphilococcus | 0 | 2 | |
| Enterococcus spp. | 0 | 1 | |
| Enterobacter spp. | 1 | 0 | |
| N. meningitidis | 0 | 3 | |
| K. pneumoniae | 1 | 3 | |
| Acinetobacter | 2 | 1 | |
| E. coli | 1 | 5 | |
| SEPSIS SOURCE | |||
| Lungs | 5 | 14 | |
| Urinary tract | 9 | 27 | |
| Abdomen | 11 | 15 | |
| Central nervous system | 3 | 10 | |
| Soft tissue | 1 | 12 | |
| Other | 1 | 4 | |
p values calculated using unpaired t test (normal distribution).
p values calculated using chi-square test.
Observed groups of patients (MODS development present or absent) showed no statistically significant difference in age and gender.
Baseline APACHE II and SOFA scores were significantly higher in patients who developed MODS, compared to those who did not develop MODS within the first 48 hours after admission.
The cause of infection was confirmed by blood culture or SEPTIFAST method in 30 (26.8%) patients. Data on the isolated pathogens are summarized in Table 1.
Baseline levels of relevant laboratory parameters obtained at the admission are shown in Table 2. Among routine laboratory parameters procalcitonin (PCT) was shown to be important predictor of MODS development, while white blood cell (WBC) count, CRP concentration, fibrinogen, platelet count and INR failed to predict MODS development (Table 2). In addition to PCT, APRIL and sTACI were significant predictors of MODS development (Table 2 and Figure 2).
Table 2.
Baseline levels of laboratory parameters.
| MODS development in first 48 h |
|||
| Variable |
Present n = 30 |
Absent n = 82 |
p* |
| WBC [x109/L] | 11.0 (8.4–16.2) | 15.3 (8.1–20.5) | 0.267 |
| CRP [mg/L] | 205.8 (126.7–284.0) | 232.6 (179.6–281.4) | 0.289 |
| PCT [ng/L] | 73.4 (14.9–146.9) | 12.0 (3.4–29.1) | <0.001 |
| Fibrinogen [g/L] | 4.8 (3.9–5.7) | 5.5 (4.5–6.3) | 0.069 |
| Plt [x109/L] | 141.5 (106.0–192.0) | 188.0 (118.0–273.0) | 0.067 |
| INR | 1.3 (1.1–2.1) | 1.3 (1.1–1.6) | 0.392 |
| APRIL [ng/mL] | 10.0 (7.0–13.5) | 6.5 (4.7–10.0) | 0.005 |
| sTACI [pg/mL] | 887.5 (600.0–1425.0) | 275.0 (150.0–700.0) | <0.001 |
p values calculated using Mann-Whitney U test (skewed distribution), data presented as median (IQR).
Figure 2.
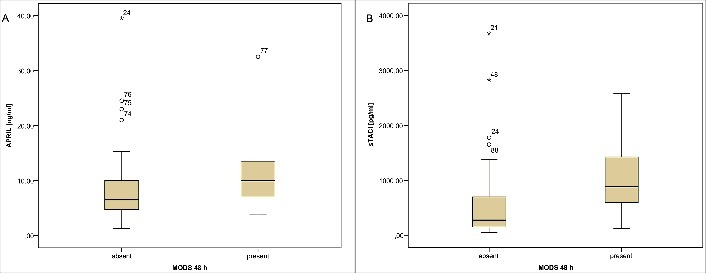
APRIL (a) and sTACI (b) concentrations in the means of MODS development within the first 48 h after admission. Abbreviations: APRIL – A Proliferation Inducing Ligand; sTACI – Soluble Transmembrane Activator and CAML Interactor Protein; MODS – Multiorgan Dysfunction Syndrome.
For the purpose of quantification and comparison of the discriminative value of separate parameters in prediction of MODS development within the first 48 hours after admission to the ICU, we constructed ROC curves for parameters that were classified as significant predictors of MODS development (Table 3 and Figure 3). The most valuable in the prediction of MODS development within the first 48 hours after the admission to the ICU, were APACHE II (AUC = 0.869, p<0.001) and SOFA scores (AUC = 0.888, p<0.001). Although there were no significant differences among individual biomarkers, the highest predictive value was observed with sTACI (AUC = 0.764, p<0.001), that had higher precitive value than routinely used CRP and PCT. An important marker, in addition to sTACI, was APRIL (AUC = 0.673, p<0.001), which was somewhat less significant than procalcitonin (AUC = 0.719, p<0.001) (Table 3 and Figure 3). When comparing significance between AUC ROC of observed variables, both scores showed significantly higher AUC than any other single biomarker (AUCSOFA vs AUCPCT p = 0.004, AUCSOFA vs AUCAPRIL p<0.001, AUCSOFA vs AUCsTACI p = 0.04, AUCAPACHEII vs AUCPCT p = 0.012, AUCAPACHEII vs AUCAPRIL p = 0.002). However, AUCAPACHEII was not significantly higher than AUCsTACI (p = 0.07). There was no significant difference among AUC ROC value sof separate biomarkers razlike (AUCsTACI vs AUCAPRIL p = 0.178, AUCsTACI vs AUCPCT p = 0.618, AUCAPRIL vs AUCPCT p = 0.554).
Table 3.
ROC curve analysis.
| |
AUC |
SE |
95% CI |
p |
Cut-off |
Sens |
Spec |
| APACHE II | 0.869 | 0.0329 | 0.792–0.925 | <0.001 | 14 | 90.00% | 75.61% |
| SOFA | 0.888 | 0.0323 | 0.815–0.940 | <0.001 | 5 | 76.67% | 80.49% |
| PCT | 0.719 | 0.0552 | 0.630–0.803 | <0.001 | 34.6 | 70.00% | 79.27% |
| APRIL | 0.673 | 0.0572 | 0.580–0.761 | <0.001 | 8.25 | 66.7% | 67.07% |
| sTACI | 0.764 | 0.0501 | 0.674–0.839 | <0.001 | 550 | 76.67% | 67.07% |
Figure 3.
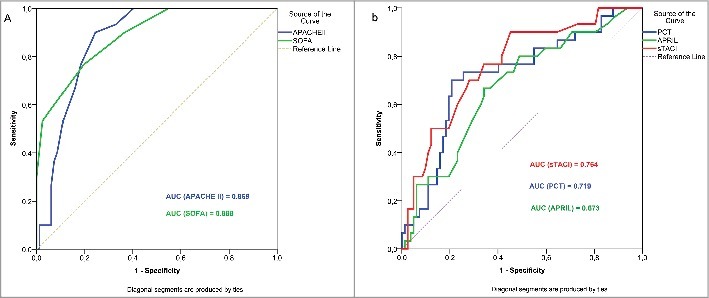
ROC curve analysis comparing the prognostic values of APACHE II and SOFA scores for prediction of MODS development (a), and ROC curve analysis comparing the prognostic values of APRIL, sTACI and procalcitonin for prediction of MODS development (b). Abbreviations: APRIL – A Proliferation Inducing Ligand; sTACI – Soluble Transmembrane Activator and CAML Interactor Protein; MODS – Multiorgan Dysfunction Syndrome; PCT – procalcitonin; AUC – Area Under the Curve.
In order to assess the predictive role of all the observed parameters we performed multivariate analysis using binary logistic regression model. In the model we included parameters that showed the greatest predictive value using previously described methods. Taken into account high correlation between APACHE II and SOFA scores (Spearman ρ = 0.811), we could not include both scores in the regression model, so we picked SOFA score, due to its higher predictive value. Along with the SOFA score, we included PCT, sTACI and APRIL as independent variables.
Model was statistically significant χ2 (4, N = 112) = 62.252, p<0.001. It successfully differentiated patients that developed MODS from those who did not. Model explained 42.6–62.0% of variance in the means of MODS development and classified correctly 84.8% of cases. Introducing the observed parameters in the regression model, only two independent variables (SOFA score p<0.001 and sTACI p = 0.004) gave the unique contribution to the model. The most important predictor remained SOFA score, which significance ratio was 2.247, compared to sTACI's significance ratio of 1.001 (Table 4).
Table 4.
Binary logistic regression model for the prediction of MODS development within first 48 h after admission.
| Variable |
B |
S.E. |
Wald |
df |
Sig. |
Exp(B) |
|
| Step 1a | SOFA | 0.809 | 0.170 | 22.566 | 1 | <0.001 | 2.247 |
| APRIL | 0.030 | 0.058 | 0.273 | 1 | 0.601 | 1.031 | |
| sTACI | 0.001 | <0.001 | 8.178 | 1 | 0.004 | 1.001 | |
| PCT | 0.004 | 0.003 | 1.643 | 1 | 0.201 | 1.004 | |
| Constant | -7.192 | 1.344 | 28.623 | 1 | <0.001 | 0.001 | |
Variables entered on step 1: SOFA, APRIL, TACI, PCT.
The significant predictors of MODS development in our study were APACHE II and SOFA scores, as well as PCT and concentrations of APRIL and sTACI receptor. According to the AUC analysis, the most significant predictors of MODS development within the first 48 hours after admission to the ICU were predictive APACHE II (AUC = 0.869) and SOFA scores (AUC = 0.888).
Among the individual biomarkers, the most valuable predictor was sTACI with AUC = 0.764, while areas under curves for APRIL (AUC = 0.673) and PCT (AUC = 0.719) were smaller. Using multivariate regression analysis, sTACI was the only independent predictor of MODS development within the first 48 hours after admission to the ICU, in addition to the SOFA score.
Although several studies presented CRP as the predictor of MODS development [19] and lethal outcome, majority of the studies [20–23], as well as review articles [24] are concordant with our results, that did not confirm prognostic relevance of baseline levels of CRP in sepsis [20–24].
New definition of sepsis puts the development of organ failure as the parameter for establishment of diagnosis into focus. Theories connected with the organ failure development are various, with leading role attributed to the hypoperfusion and conversion to anaerobic metabolism. The level of lactates is thus one of the major indicators of tissue damage [25,26]. However, recent studies point to the multifactorial etiology of organ failure development in sepsis, with important role being attributed to the interaction of immuneinflammatory, nervous, endocrine system, as well as behavioral and emotional status. Many authors have emphasized the unregulated immune response as dominant cause of MODS development [27,28]. Inflammatory immune reaction can provoke direct tissue toxicity and contribute to the organ failure. Activating intrising inflammatory cells in distant organs, different than the primary organ affected, leads to the development of multiorgan dysfunction and formation of positive interreaction (inflammatory mediators released into the circulation cause cytokine storm and further organ failure) [27,28]. Research on the single organ dysfunction are concordant with abovementioned pathophysiological theories of sepsis-induced organ failure. Namely, Gomez and Kellum reported the dominant role of inflammation per se in the sepsis induced acute kidney injury, more than hypoperfusion itself [29]. In addition, sepsis-induced brain dysfunction is described as the consequence of hypoperfusiona and neuroinflammation [30,31]. In addition to the hypoperfusion in the liver dysfunction, the role of inflammatory response and metabolic changes that decrease the potency of biotransformation processes has been described [32]. Therefore, it comes as no surprise that markers of immune response represent important predictive factors in MODS development in septic patients – APRIL, secreted by the antigen presenting cells in the first line of defense, and its soluble receptor, TACI.
Although the role of APRIL in bacterial infections has not been cleared to the date, our results are not surprising, since it plays the key role in B-cell regulation [10,11] while B-cells have an important role in immune response to bacterial infections [33–36]. In the research on APRIL concentrations in autoimmune and malignant diseases increased concentrations of this biomarker are shown to correlate with the degree of B-cell activation [14,37–39]. Studies showed that the main role of APRIL is in induction of B-cell proliferation and regulation of the B-cells apoptosis [9,11]. This feature is used for the synthesis of new drugs in the treatment of autoimmune diseases and lymphomas, which block the function of APRIL, induce the apoptosis of B-cells and that way decrease the number of circulating B-cells [9,40]. APRIL is secreted by antigen-presenting cells that are the first line defence from infection. Recent study of Roderburg et al. showed that APRIL may serve as a valuable predictor of sepsis outcome, defined as survival or lethal [15]. Concentrations of this molecule seem to increase during bacterial infections and may serve as a useful biomarker of MODS development, since those concentrations indirectly determine the degree of initial activation of innate immunity and further coordination of innate and adaptive immunity. To the date, there are no published data on the role of APRIL in MODS development prediction.
Nevertheless, concentrations of soluble TACI receptor have shown better predictive capacity than APRIL, as well as the other evaluated sepsis predictors. Pathophysiological basis of obtained results is partly explained by the fact that TACI is the receptor that binds APRIL with the highest affinity [41]. After binding with transmembrane form of TACI receptor, APRIL expresses its protective role on B-cells, preventing their apoptosis. Soluble TACI receptor in blood has the same function as transmembrane form. Binding circulating APRIL before it binds to transmembrane receptor, sTACI is considered to be the main negative regulator of B-cell count [10,18,42]. Our results show that the concentrations of sTACI are better predictor of negative course of the sepsis than APRIL concentrations, and indirectly point to the potential development of depletion of the circulating B-cell count and entering the “state of immune paralysis”, that leads to the lethal outcome. Earlier post mortem studies on patients who died of sepsis, showed an important depletion of all the lines of white cells, first of all lymphocytes that are more important for the development of immune paralysis than apoptosis of neutrophils and other immune cells [43]. In addition, it is shown that treatment modalities preventing apoptosis of all the white cell lines, including B-cells, increase the survival rate [33,43–45]. Apoptosis of the cells is recognized as one of the basic processes in MODS development in sepsis. Patients with increased apoptosis at the admission, presented with greater chances for later development of MODS [46–48]. Our results show that sTACI concentration is the most important individual predictor of the negative outcome, which confirms the urge for development of therapeutic modalities that would block the process of B-cell apoptosis and contribute to the improvement of sepsis outcome. We assume that monitoring of APRIL and sTACI concentrations dynamic would be of an even higher significance, because it would enable individualization of the sepsis treatment. Additionally, identifying the state of immune paralysis versus proinflammatory response in a single patient could contribute to clinical decision on introducing immunomodulatory therapy [49]. Future research on the role of sTACI as the biomarker in MODS development is necessary, since there are indices that it may be more confident in prediction than PCT, the only biomarker routinely used nowadays.
Actual scoring systems do not have the satisfactory predictive value, bearing in mind specificity of the septic patients’ population in the ICU or triage dispensaries, as well as the complex pathogenesis of sepsis [24]. In order to assess the risk accurately, we are in demand for further research that would introduce new variables, and thus enable construction and validation of the new specific scoring system for septic patients. In the light of new theories about sepsis pathogenesis, that accentuate the dominance of anti-inflammatory response in patients with worse prognosis, construction of the new scoring system could contribute to better assessment of clinical course of sepsis and making on time clinical decision to introduce immunomodulators in addition to antibioitics and supportive therapy. In addition, commercializing the test might be a reasonable option, if further research confirm it to be more indicative of MODS development than procalcitonin.
Funding Statement
This work was supported by the Provincial Secretariat for Science and Technological Development (114-451-745/2015-01).
Abbreviations
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- APRIL
A Proliferation Inducing Ligand
- AUC
area under curve
- CNS
central nervous system
- CRP
C-reactive protein
- ICU
intensive care units
- ELISA
enzyme-linked immunosorbent assay
- INR
International Normalised Ratio
- MODS
multiorgan dysfunction syndrome
- PCT
procalcitonin
- Plt
Platelits
- ROC
Receiver Operating Characteristic curve
- SOFA
Sequential Organ Failure Assessment
- sTACI
soluble Transmembrane Activator and CAML Interactor Protein
- TACI
Transmembrane Activator and CAML Interactor Protein
- TNF-α
tumor necrosis factor alfa
- WBC
white blood cell
Conflict of interests
All the authors listed deny any financial or non-financial conflict of interests.
Acknowledgments
Funding for the work was received from the project: Non-invasive markers in diagnosis and prognosis of critical illness, Provincial secretariat for science and technological development, Republic of Serbia, Autonomous province of Vojvodina, grant number 114-451-745/2015-01.
References
- [1].Stoller J, Halpin L, Weis M, et al.. Epidemiology of severe sepsis: 2008-2012. J Crit Care 2016;31:58–62. [DOI] [PubMed] [Google Scholar]
- [2].Maye FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014;35:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Esper AM, Martin GS. Extending international sepsis epidemiology: the impact of organ dysfunction. Crit Care 2009;13(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Charles P-E, Gibot S. Predicting outcome in patients with sepsis: new biomarkers for old expectations. Crit Care 2014;18:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Skirecki T, Borkowska-Zielińska U, Złotorowicz M, Hoser G. Sepsis immunopathology: Perspectives of monitoring and modulation of the immune disturbances. Arch. Immunol. Ther. Exp. (Warsz) 2012;60:123–35. [DOI] [PubMed] [Google Scholar]
- [6].Cho S, Choi J. Biomarkers of Sepsis. Infect Chemother 2014;46:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deutschman CS, Tracey KJ. Sepsis: Current dogma and new perspectives. Immunity 2014;40:463–75. [DOI] [PubMed] [Google Scholar]
- [8].Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T. Host innate immune responses to sepsis. Virulence 2014;5:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009;9:491–502. [DOI] [PubMed] [Google Scholar]
- [10].Vincent FB, Saulep-Easton D, Figgett WA, et al.. The BAFF/APRIL system: Emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev 2013;24:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Matthes T, Dunand-sauthier I, Krause K. Production of the plasma-cell survival factor APRIL peaks in myeloid precursor cells from human bone marrow Production of the plasma-cell survival factor APRIL peaks in myeloid precursor cells from human bone marrow. Blood 2011;118:1838–45. [DOI] [PubMed] [Google Scholar]
- [12].Koyama T, Tsukamoto H, Miyagi Y, et al.. Raised serum APRIL levels in patients with systemic lupus erythematosus. Ann Rheum Dis 2005;64:1065–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vallerskog T, Heimbürger M, Gunnarsson I, et al.. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther 2006;8:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lemancewicz D, Bolkun L, Jablonska E, et al.. Evaluation of TNF superfamily molecules in multiple myeloma patients: Correlation with biological and clinical features. Leuk Res 2013;37:1089–93. [DOI] [PubMed] [Google Scholar]
- [15].Roderburg C, Koch A, Tacke F, et al.. Serum concentrations of A Proliferation-Inducing Ligand (APRIL) are elevated in sepsis and predict mortality in critically ill patients. J Crit Care 2013;28(5):882.e1–11. [DOI] [PubMed] [Google Scholar]
- [16].Goenka R, Scholz JL, Sindhava VJ, Cancro MP. New roles for the BLyS/BAFF family in antigen-experienced B cell niches. Cytokine Growth Factor Rev 2014;25:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Salzer U, Chapel HM, Webster AD, et al.. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet 2005;37:820–828. [DOI] [PubMed] [Google Scholar]
- [18].Hoffmann FS, Kuhn P-H, Laurent SA, et al.. The Immunoregulator Soluble TACI Is Released by ADAM10 and Reflects B Cell Activation in Autoimmunity. J Immunol 2014;194:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lobo SMA, Lobo FRM, Bota DP. C-Reactive Protein Levels Correlate With Mortality and Organ Failure in Critically Ill Patients *. Chest 2003;123:2043–9. [DOI] [PubMed] [Google Scholar]
- [20].Suberviola B, Castellanos-Ortega A, González-Castro A, et al.. Prognostic value of procalcitonin, C-reactive protein and leukocytes in septic shock. Med Intensiva (English Ed) 2012;36:177–84. [DOI] [PubMed] [Google Scholar]
- [21].Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care 2011;26:54–64. [DOI] [PubMed] [Google Scholar]
- [22].Jensen JU, Heslet L, Jensen TH et al.. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med 2006;34:2596–602. [DOI] [PubMed] [Google Scholar]
- [23].Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother 2011;66:33–40. [DOI] [PubMed] [Google Scholar]
- [24].Pierrakos C, Vincent J-L. Sepsis biomarkers: a review. Crit Care 2010;14:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang B, Chen G, Cao Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J Crit Care 2015;30(2):271–5. [DOI] [PubMed] [Google Scholar]
- [26].Dellinger RP, Levy MM, Rhodes A, et al.. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- [27].Rossaint J, Zarbock A. Pathogenesis of multiple organ failure in spesis. Crircal Reviews in Immunology No Title. 2015;35(4):277–91. [DOI] [PubMed] [Google Scholar]
- [28].Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. 2008;26:711–5. [DOI] [PubMed] [Google Scholar]
- [29].Gomes H, Kellum JA. Sepsis induced acute kidney injury. Curr Opin Crit Care 2016;22(6):546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zampieri FG, Park M, Machado FS, et al.. Sepsis-associated encephalopathy: not just delirium. Clinics 2011;66(10):1825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Adam N, Kandelman S, Mantz J, et al.. Sepsis-induced brain dysfunction. Expert Rev Anti Infect Ther 2013;11(2):211–21. [DOI] [PubMed] [Google Scholar]
- [32].Nesseler N, Launey Y, Aninat C, et al.. Clinical review: The liver in sepsis. Criti Care 2012;16:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].de Pablo R, Monserrat J, Prieto A, et al.. Role of Circulating Lymphocytes in Patients with Sepsis. Biomed Research International 2014;671087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kelly-Scumpia KM, Scumpia PO, Weinstein JS, et al.. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med 2011;208:1673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vaughan AT, Roghanian A, Cragg MS. B cells–masters of the immunoverse. Int J Biochem Cell Biol 2011;43:280–5. [DOI] [PubMed] [Google Scholar]
- [36].Anolik JH, Looney RJ, Lund FE, et al.. Insights into the heterogeneity of human B cells: Diverse functions, roles in autoimmunity, and use as therapeutic targets. Immunol Res 2009;45:144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bolkun L, Lemancewicz D, Jablonska E, et al.. BAFF and APRIL as TNF superfamily molecules and angiogenesis parallel progression of human multiple myeloma. Ann Hematol 2014;93:635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Treamtrakanpon W, Tantivitayakul P, Benjachat T, et al.. APRIL, a proliferation-inducing ligand, as a potential marker of lupus nephritis. Arthritis Res Ther 2012;14:R252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Salazar-Camarena DC, Ortiz-Lazareno PC, Cruz A, et al.. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus 2016;25(6):582–92. [DOI] [PubMed] [Google Scholar]
- [40].Haiat S, Billard C, Quiney C, et al.. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology 2006;118:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Warnatz K, Salzer U, Rizzi M, et al.. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci U S A 2009;106:13945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yu G, Boone T, Delaney J, et al.. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol 2000;1:252–6. [DOI] [PubMed] [Google Scholar]
- [43].Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013;13:862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen X, Yin Y, Zheng J. Sepsis and immune response. World J Emerg Med 2011;2(2):88–92. [PMC free article] [PubMed] [Google Scholar]
- [45].Huttunen R, Aittoniemi J. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J Infect 2011;63:407–19. [DOI] [PubMed] [Google Scholar]
- [46].Exline MC, Crouser E. Mitochondrial Mechanisms of Sepsis-Induced Organ failure. Front Biosi 2014;13:5030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Power C, Fanning N, Redmond HP. Cellular apoptosis and organ injury in sepsis: a review. Shock 2002;18(3):197–211. [DOI] [PubMed] [Google Scholar]
- [48].Hofer S, Brenner T, Bopp C, et al.. Cell death serum biomarkers are early predictors for survival in severe septic patients with hepatic dysfunction. Crit Care 2009;13:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence 2014;5:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Opal SM, Rubenfeld GD, Poll T Van Der, et al.. Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


