Abstract
Background:
Previous reviews and meta-analyses, which predominantly focused on patients treated before 2000, have reported conflicting evidence about the association between hospital/surgeon volume and rectal cancer outcomes. Given advances in rectal cancer resection such as total mesorectal excision, it is essential to determine if volume plays a role in rectal cancer outcomes among patients treated since 2000.
Objective:
Determine if there is an association between hospital/surgeon volume and rectal cancer surgery outcomes among patients treated since 2000.
Data sources:
We searched PubMed and Embase for articles published between January 2000 and 29 December 2017.
Study selection:
Articles published between January 2000 and 29 December 2017 that analyzed the association between hospital/surgeon volume and rectal cancer outcomes.
Study selection:
Rectal cancer resection.
Main outcome measures:
The outcomes of this study were surgical morbidity, post-operative mortality, surgical margin positivity, permanent colostomy rates, recurrence, and overall survival.
Results:
While 2,845 articles were retrieved and assessed by the search strategy, 21 were met the inclusion and exclusion criteria. There was a significant protective association between higher hospital volume and surgical morbidity [Odds Ratio = 0.80 (0.70, 0.93); I2=35%], permanent colostomy [Odds Ratio = 0.51 (0.29, 0.92); I2=34%] and post-operative mortality [Odds Ratio = 0.67 (0.50, 0.90); I2=41%]. Stratified analysis showed variation in significance between hospital volume and rectal surgery outcomes by geographic location. Hospital and surgeon volume were not significantly associated with overall survival. The articles included in this analysis were high quality according to the Newcastle Ottawa scale. Funnel plots suggested that the potential for publication bias was low.
Limitations:
Variations in volume definitions across the studies limits inference about the appropriate minimum volume threshold value associated with better outcomes.
Conclusion:
Among patients diagnosed since 2000, higher hospital volume has a significant protective effect on rectal cancer surgery outcomes.
Introduction
Rectal cancer is expected to account for approximately 43,000 newly diagnosed cancer cases in the United States in 20181. Currently, the advanced rectal cancer standard of care is a multimodal approach which entails neoadjuvant therapy and surgery2,3. Surgical excision of the rectum is complex because of its proximity to genitourinary organs, and the bony confines of the pelvis which present challenges to achieving good oncologic outcomes while minimizing morbidity4,5. Even though advances in rectal cancer management, such as total mesorectal excision (TME), have improved oncologic and quality of life outcomes for rectal cancer patients6–10, the average 5-year survival rate is only 66%11. Determining factors that affect rectal cancer surgery outcomes is essential to improving morbidity and mortality in rectal cancer patients.
In particular, it has been postulated that surgeons and hospitals that treat a high volume of rectal cancer patients have better rectal cancer surgery outcomes12,13. High volume subspecialty trained surgeons have better outcomes based on their training, volume and experience, while high volume hospitals achieve superior outcomes based on available resources and multidisciplinary care13. Nevertheless, previous reviews analyzing the association between hospital volume and rectal cancer surgery outcomes have been inconsistent13–15. These reviews included studies that had patients treated for rectal cancer from 1990 through the early 2000s13–15. Given the widespread use of technically complex TME and advances in rectal cancer management such as sphincter preserving surgery and neoadjuvant therapy since 2000, it is essential to evaluate the effect of surgeon and hospital volume on patient outcomes based on current practice. Hence, the purpose of this meta-analysis is to estimate the strength of the association between hospital/surgeon volume and outcomes in rectal cancer patients who received surgery since 2000.
Materials and Methods
Search strategy
Boolean logic was used to retrieve relevant PubMed and Embase English articles published from 1 January 2000 to 29 December 2017 using the following keywords; (“colorectal cancer” or (“rectal/rectum cancer”) and “surgery” and (hospital volume” or “surgeon volume” or “hospital caseload” or “surgeon caseload” or “hospital workload” or “surgeon workload” or “surgical volume” or “surgical caseload” or “surgical workload”) and (“treatment outcomes” or “treatment failure” or “adverse” or “surgical complications” or “intraoperative complications” or “postoperative complications” or “stoma” or “quality of healthcare” or “length of stay” or “recurrence” or “mortality” or “survival”). Relevant articles were retrieved from references found from PubMed and Embase articles.
Article titles and abstracts that were identified from the literature using the above search strategy were uploaded to Endnote; no duplicates were found. The eligibility of research articles was assessed by four reviewers (CC, JS, NDV and MC). Two reviewers were involved in the data abstraction process (CC and NDV). Disagreements pertaining to the eligibility of articles or data abstraction was resolved via discussion.
Inclusion and exclusion criteria
This systematic literature review included studies that reported results based on original data analyzing the association between hospital or surgeon volume and rectal cancer outcomes in patients treated since 2000. We included articles that included patients with cancer of the rectum or rectosigmoid junction; this information was based on the International Classification of Diseases, 9th Revision (ICD-9-M) codes or tumor location information. The articles had to have information about rectal cancer surgery, hospital or surgeon volume and patient outcomes after surgery. Studies that delineated between colon and rectal cancers were included in the analysis. Articles that were based on single institutions or had one hospital/surgeon volume level were excluded from the study since they did not compare outcomes across hospital/surgeon volume levels. Only English language peer-reviewed literature found in PubMed or Embase were reviewed to reduce bias since authors were unable to translate the Chinese articles. For further information, some authors were contacted.
Measures and outcomes
Hospital or surgeon volume were the primary exposures of interest. Hospital volume was defined as the mean/number of rectal and/or rectosigmoid resections (i.e. low anterior resection and abdominoperineal resection) per year or over the study period in a specific hospital. Surgeon volume was defined as either the mean/number of resections performed by a surgeon per year or over the study period. Hospital and surgeon volume categorizations were based on the definitions from the original articles. The outcomes of interest in this study were: surgical morbidity, post-operative mortality, surgical margin positivity, permanent colostomy rates, recurrence, and overall survival. Surgical morbidity included conditions such as anastomic leakage, abscess, iatrogenic complications, bleeding, peritonitis, stoma necrosis, stoma fistula, and wound dehiscence; the definition of surgical morbidity varied across the studies. Post-operative mortality was defined as death within 30 days of surgery. Follow-up time after rectal cancer surgery for articles that reported overall survival was defined as 1, 3 or 5 years.
Analysis
An evidence grid (Table 1) was constructed to characterize study population characteristics (age, cancer stage, type of surgery), sample size, study type and study results. Statistical significance from the articles was reported for effect sizes regardless of level of significance; in the presence of both bivariate and multivariate analyses, we reported multivariate effect sizes.
Table 1.
Description of included studies
| Patient characteristics |
||||||||
|---|---|---|---|---|---|---|---|---|
| Study (Dates) |
Study Type, Data source |
Hospital, n Surgeon, n Patient, n |
Location (Country/ State) |
Age (years)a /M:F |
Stage | Surgery type | Volume definition | Adjusted Results |
| Ptok 2007 39; (2000–2001) |
Prospective cohort, study data (voluntary) |
Patients: 1557 | Germany | Median 66 26–92) / 1.6:1 |
UICC stage I = 16% II= 35% III =48% |
LAR = 39% APR = 61% |
Hospital volume Number of annual surgeries Low <10, Medium 10–19, High ≥20 |
Hospital HR (95%CI) [ref = High] Local recurrence rate: Low 1.01 (0.83–1.52) Medium 1.39 (1.06–1.81)* |
| Yasunaga 2009 33; (2006–2007) |
Retrospective cohort; Web based registration system (voluntary) |
Hospital: 371 Patients: 2285 |
Japan | Mean = 67 (SD=11) / 1.8:1 |
Stage 0 = 2% I = 21% II = 28% III = 37% IV = 12% |
HAR = 20% LAR = 55% APR = 19% Hartman = 6% |
Hospital volume Number of 2005 surgeries Low ≤ 9 High ≥10 Surgeon volume Number of cumulative surgeries Very low ≤49 Low 50–99 High 100–499 Very High ≥500 |
OR (95%CI) [ref =Low] Intraoperative blood loss: Hospital: High 1.09 (0.81–1.48) Surgeon: Low: 1.02 (0.73–1.42) Surgeon: High: 0.93 (0.67–1.28) Surgeon: Very High: 0.67 (0.46–0.99)* RR ratio (95%CI)(ref =Very low) Postoperative complications: Hospital: High: 1.00 (0.75–1.34) Surgeon: Low: 0.92 (0.64–1.33) Surgeon: High: 0.94 (0.69–1.28) Surgeon: Very High: 0.93 (0.65–1.36) Length of stay: Hospital: High 1.41 (1.23–1.62)* Surgeon: Low 0.99 (0.83–1.17) Surgeon: High 0.99 (0.85–1.16) Surgeon: Very High 1.09 (0.87–1.36) |
| Gort 2010 28; (2001–2005) |
Retrospective cohort; North East cancer registry |
Hospital: 16 Patients: 819 |
North East Netherlands | <70 = 58% ≥70 = 42% / 1.6:1 |
TNM Stage I = 32% II = 30% III = 38% |
LAR = 40% APR = 42% Hartmann = 18% |
Hospital volume Mean annual surgeries Low <20, Medium 20–40 High ≥40 Surgeon volume Mean annual surgeries Low <5, Medium 5–10, High ≥10 |
Hospital OR (95%CI) [ref = Low]
Complications: Medium: 0.79 (0.52–1.20); High: 0.65 (0.44–0.96)* Surgeon HR (95%CI) [ref = Low] Overall Survival (3 years): Medium: 1.02 (0.73–1.42); High: 0.70 (0.47–1.03) |
| Elferink 2010 26; (2001–2006) |
Retrospective cohort; Netherlands cancer registry |
Hospital: 97 Patients: 16039 |
Netherlands | <60 = 26% 60–74 = 43% 75+= 30% / 1.4:1 |
Clinical Stage T0/IS-M0 = <1% T1-M0 = 9% T2/T3-M0 = 59% T4-M0 = 10% T/Nany-M1 = 17% Missing = 5% |
LAR = Included APR = Included Other = Included |
Hospital volume Number of annual surgeries Low <25 Medium 25–50 High >50 |
Hospital OR (95%CI) [ref = Low] Postoperative Mortality: Medium: 0.70 (0.44–1.14) High: 0.40 (0.19–0.84)* RER (95%CI) [ref = Low] Overall survival (2 years): Medium: 0.95 (0.88–1.04) High: 1.02 (0.91–1.14) |
| Manchon-Walsh 2011 24; (2005 & 2007) |
Retrospective cohort; Hospital data (Catalonian Hospital Discharge Minimum Basic Data Set) |
Hospital: 51 Patients: 1831 |
Spain/ Catalonia |
Median =70 (29–92) / 1.9:1 |
TNM Stage 0 = <1%; I = 12%; II = 28%; III = 43%; IV = 7%; Missing = 8% |
LAR = 73% APR = 21% Hartmann = 5% Missing = 2% |
Hospital volume Number of annual surgeries Low ≤ 11 Medium 12–30 High ≥30 |
OR (95%CI) [ref=Low] Postoperative complications: Medium: 0.65 (0.49–0.88)* High: 0.74 (0.56–0.99)* Postoperative Mortality: Medium: 0.93 (0.38–2.31) High: 1.02 (0.42–2.46) |
| Kolfschoten 2011 25; (2010) |
Retrospective cohort; Dutch Surgical Colorectal Audit |
Hospital: 90 Patients: 2419 |
Netherlands | <70 = 59% 70–80 = 30% 80+ = 11% / 1.6:1 |
TNM Stage I = 30%; II = 27%; III = 32%; IV = 10%; X = 1% |
LAR = 67% APR = 30% Other = 3% |
Hospital volume Number of annual surgeries Low <50, High 50–100 |
Hospital SMR (95%CI)
Postoperative Mortality: Low: 1.00 (0.72–1.38) High: 1.0 (0.63–1.59) |
| Yun 201235; (2001–2005) |
Retrospective cohort; Korean cancer registry |
Hospital:>180 Patients: 19028 |
South Korea | Compositeⱡⱡ | Compositeⱡⱡ | Compositeⱡⱡ |
Hospital volume Mean 5 year surgeries Low <23, High ≥23 |
Hospital HR (95%CI) [ref = High] Overall survival (5 years): Low: 1.39 (1.27–1.52)* |
| Comber 2012 40; (2007) | Retrospective cohort; National cancer registry | Hospital: 49 Surgeon: 86 Patients: 457 |
UK/ Ireland | 30–49 = 11% 50–69 = 51% 70+ = 34% Missing=4%/NRⱡ |
NRⱡ | NRⱡ |
Hospital volume Number of annual surgeries Low <10, Medium 10–19, High ≥20 Surgeon volume Continuous |
Hospital OR (95%CI) [ref = High] Overall survival (1 year): Hospital: 1.03 (1.00–1.06)* Surgeon: 0.97 (0.93–1.01) |
| Richardson 2013 36; (2002–2006) |
Retrospective cohort; Nova Scotia Cancer Registry | Hospital: 10 Surgeon: 51 Patients: 466 |
Canada/ Nova Scotia | Mean=66 (27–94) / 1.9:1 |
AJCC stage I = 31% II= 27% III =35% IV = 7% |
LAR = Included APR = Included Hartman= Included Other= Included |
Hospital and surgeon volume Mean annual surgeries Hospital and surgeon volume: based on volume distribution (actual definitions not reported) |
OR (95%CI)(ref = High) Permanent colostomy: Hospital: Medium: 2.02 (0.90–4.57) Hospital: Low: 2.10 (0.85–5.20) Surgeon: Low: 1.23 (0.58–2.62) RR ratio (95%CI)(ref = High) Inappropriate colostomy: Medium: 3.32 (1.08–10.27)*; Hospital: Low: 5.03 (1.44–17.54)* Surgeon: Low: 1.50 (0.54–4.16) |
| Richardson 2013 37; (2002–2006) |
Retrospective cohort; Nova Scotia Cancer Registry and surgeon survey (voluntary) | Surgeon: 25 Patients: 377 |
Canada/ Nova Scotia | Mean=67 (27–96) / 1.8:1 |
AJCC stage
I = 31% II = 28% III =35% IV = 7% |
NRⱡ |
Surgeon volume 2002–2006 mean surgeries based on volume distribution (actual definitions not reported) |
Surgeon OR (95%CI) [ref = Low] Permanent colostomy: High: 0.49 (0.25–0.97)* TME: High: 3.59 (2.21–5.83)*1 12 nodes examined: High: 0.95 (0.55–1.64)* HR (95%CI) [ref = Low] Local recurrence: High: 0.54 (0.29–0.99)* 1 Disease specific survival: High: 0.71 (0.42–1.02) Overall survival: High: 0.67 (0.43–1.02) |
| van Erning 2013 27; (2008–2011) |
Retrospective cohort; Eindhoven cancer registry | Hospital: 10 Patients: 1721 |
Southern Netherlands | <60 = 26% 60–69 = 31% 70–79 = 32% ≥80 = 8% / 1.7:1 |
AJCC T stage (%) T1 = 9%; T2 = 32%; T3 = 52% T4 = 7% AJCC N stage (%) N0= 65%; N1= 23%; N2= 12% AJCC M stage (%) M0 = 92%; M1 = 8% |
LAR = Included APR = Included |
Hospital volume Number of annual surgeries Low <130 High ≥130 |
Hospital OR (95%CI)[ref = <130] Overall survival (3 years): High: 1.0 (0.7–1.4) |
| Leonard 2014 38; (2006–2010) |
Retrospective cohort; Belgian cancer registry (BCR); (exclude PROCARE results) | Hospital: 108 Patient: 5869 |
Belgium | NRⱡ | NRⱡ | NRⱡ |
Hospital volume Continuous |
Hospital HR (95%CI) Postoperative mortality: 0.99 (0.98–1.00)*2 Overall survival (5-years): 0.99 (0.99–1.00)* |
| Ortiz 2015 22; (2006–2013) |
Prospective cohort; Rectal Cancer Project of the Spanish Society of Surgeons (voluntary) | Hospital: 84 Patients: 9809 |
Spain | <65 =39%, 65–80=48% >80=13% / 1.5:1 |
AJCC T stage (%) T0 = 11%; T1 = 7%; T2 = 26%; T3 = 49%; pT4 = 6% AJCC N stage (%) Nx-0= 67%; N1–2= 33% AJCC M stage (%) pM0 = 90%; pM02 = 10% |
LAR = 74% APR = 26% |
Hospital volume Median annual surgeries Very low 11 Low 12–23 Medium 24–35 Very high ≥36 |
Hospital OR (95%CI) [ref=Low] Postoperative mortality: Low 1.852 (0.710, 5.881) High 1.700 (0.649, 5.248) Very high 1.309 (0.483, 4.238) |
| Aquina 2016 31; (2000–2011) | Retrospective cohort; New York Statewide Planning and Research Cooperative System | Hospitals: 206 Surgeons: 849 Patients: 7798 |
US/ New York | 18–65 = 50% 65–79 = 38% ≥80= 12% / 1.4:1 |
NRⱡ | LAR = 64% APR = 35% |
Hospital and Surgeon volume Mean surgeries/period Hospital: Low <25, High ≥25 Surgeon: Low <10, High ≥10 Categories1 LVS/LVH, HVH only, HVS only, HVS/HVH |
OR (95%CI) [ref = LVS/LVH] Non-restorative protectomy: HVH only: 0.86 (0.70–1.05); HVS only: 0.84 (0.60–1.17); HVS/HVH: 0.65 (0.48–0.89)* Postoperative mortality: HVH only: 1.06 (0.52–2.15); HVS only: 0.72 (0.32–1.62); HVS/HVH: 0.43 (0.21–0.87)* |
| Baek 2016 32; (2000–2011) | Retrospective cohort; California Office of Statewide Health Planning and Development | Hospital: 321 Patients: 7187 |
US/ California | <65 = 38% ≥65 = 49% Missing= 13% / 1.7:1 |
NRⱡ | LAR = Included APR = Included |
Hospital volume Number of 20006–2007 surgeries Low 1–5, Medium 6–10, High 11–24 |
Hospital OR (95%CI) [Low] Sphincter preserving surgery: Medium: 1.14 (0.99–1.29) High: 1.63 (1.40–1.89)* Postoperative mortality: Medium: 0.46 (0.27–0.78)* High: 0.45 (0.24–0.84)* |
| Yeo 2016 20; (2000–2013) | Retrospective cohort; New York Statewide Planning and Research Cooperative System | Surgeons: 1860 Patients: 14833 |
US/New York | <65 = 48% 65–75 = 26% ≥75= 26% / 1.2:1 |
NRⱡ | LAR = 74% APR = 26% |
Surgeon volume
Low cumulative; 0–23 High cumulative; ≥24 Low annual; 0–4 High annual; ≥5 Categories2 LC/LA, LC/HA, HC/LA and HC/HA |
Surgeon HC/HA OR (95%CI) [ref=LC/LA] Major events: 0.82 (0.67–0.99)* Prolonged LOS: 0.74 (0.65–0.85)* Surgical complications: 0.71 (0.60–0.83)* Anastomic leak: 1.04 (0.88–1.24) Nonroutine discharges: 0.89 (0.79–1.00) High charges: 0.73 (0.63–0.86)* 30-day readmission: 0.92 (0.80–1.05) Reoperation: 0.98 (0.82–1.17) |
| Bos 2016 29; (2005–2012) | Retrospective cohort; Netherlands cancer registry | Hospital: 95 Patients: 20481 |
Netherlands | <60 = 24% 60–69 = 32% 70–79= 31% ≥80 = 13% / 1.5:1 |
AJCC T stage (%) T1 = 11%; T2 = 33%; T3 = 25%; T4 = 28% AJCC N stage (%) N0= 67%; N1= 22%; N2= 11% |
NRⱡ |
Hospital volume Number of annual surgeries Low <20 Medium 20–39 High ≥40 |
Hospital OR (95%CI) [ref = High] Anastomic leakage: Low: 1.03 (0.79–1.34) Medium: 0.97 (0.83–1.15) Postoperative Mortality: Low: 1.42 (1.09–1.84)* Medium: 1.12 (0.92–1.36) Hospital HR (95%CI)(ref = High) Overall survival (5 years): Low: 0.98 (0.91–1.07) Medium: 1.00 (0.95–1.06) |
| Ortiz 2016 23; (2006–2010) |
Prospective cohort; Rectal Cancer Project of the Spanish Society of Surgeons (voluntary) | Hospital: 36 Patients: 2910 |
Spain | <65 =37%, 65–80=49% >80=14% / 1.4:1 |
AJCC T stage (%) T0 = 8%; T1 = 10%; T2 = 28%; T3 = 49%; T4 = 5% AJCC N stage (%) pN0-ypN0= 68%; pN1-pN2= 32% |
LAR = 69% APR = 23% Hartmann = 8% |
Hospital volume Number of annual surgeries Low 12–23 Medium 24–35 High ≥36 |
Hospital OR (95%CI) [ref=Low] Overall survival (5 years): Medium: 0.858 (0.653, 1.126) High: 0.727 (0.556, 0.951)* Local recurrence: Medium: 1.098 (0.630, 1.916) High:0.835 (0.480, 1.452) Metastasis: Medium: 0.951 (0.683, 1.324) High: 0.727 (0.636, 1.217) |
| Ortiz 2016 21; (2006–2013) |
Prospective cohort; Rectal Cancer Project of the Spanish Society of Surgeons (voluntary) | Hospital: 84 Patients: 7231 |
Spain | <65 =41%, 65–80=47% >80=12% / 1.9:1 |
AJCC T stage (%) T0 = 11%; T1 = 8%; T2 = 26%; T3 = 50%; T4 = 6% AJCC N stage (%) Nx-0= 67%; N1–2= 33% AJCC M stage (%) M0= 90%;M1= 10% |
LAR = 100% |
Hospital volume Median number of annual surgeries Very low 11 Low 12–23 High 24–35 Very high ≥36 |
Hospital OR (95%CI) [ref=Very low] Anastomic leakage: Low: 0.836 (0.492, 1.449); High: 0.833 (0.485, 1.455); Very high: 0.852 (0.487, 1.518) |
| Gietelink 2016 30; (2011–2012) | Retrospective cohort; Dutch Surgical Colorectal Audit | Hospital: 91 Patients: 5161 |
Netherlands | <75= 72–74% >75= 26–28% / 1.7:1 |
AJCC T stage (%) T1-T2 = 32%; T3 = 54%; T4 = 9% |
LAR = 67% APR = 30% Other = 12% |
Hospital volume Mean annual surgeries Low <20 High ≥20 |
Hospital OR (95%CI) [ref = High] CRM involvement: Low: 1.54 (1.12–2.11)* |
| Lorimer 2017 34; (2004–2014) |
Retrospective cohort; National Cancer Database | Hospital: 1179 Patients: 27532 |
US | Mean = 59 (18–90) / 1.7:1 |
Stage II/III only AJCC T stage (%) T1 = <1%; T2 = 5%; T3 = 87%; T4 = 8% AJCC N stage (%) N0= 46%; N1= 46%; N2= 8% |
NRⱡ |
Hospital volume Mean annual surgeries Low <2.2 Very low 2.2–4.37 High 4.37–7.82 Very high >7.82 |
Hospital OR (95%CI) [ref =Very high] Pathological complete response: Low: 0.66 (0.58–0.74)*; Very low: 0.96 (0.86–1.08); High: 0.93 (0.83–1.04) Positive surgical margins: Low: 1.45 (1.25–1.70)*; Very low: 1.32 (1.13–1.54); High: 0.96 (0.82–1.13) |
Key: = Significant p-value<0.0005
NR =Not reported in the article
Composite = Cannot delineate between rectal and other cancer with regard to information for this variable
High volume hospital (HVH), Low volume hospital (LVH), High volume surgeon (HVS), Low volume surgeon (HVS)
High cumulative volume surgeon (HC), Low cumulative volume surgeon (LC), Low annual volume surgeon (HA), Low annual volume surgeon (LA); Odds ratio (OR); Hazard ratio (HR); Relative risk ratio (RR); Relative excess risks (RER); Standard mortality ratio (SMR); High anterior resection (HAR; Low anterior resection (LAR); Abdominoperineal resection (LAR); American Joint Committee on Cancer (AJCC); Union for International Cancer Control’s (UICC), Complete resection margin (CRM)
Review Manager 516 was used to perform the meta-analysis. A meta-analysis was performed when more than two studies reported on an outcome. A random effects model was used to perform a meta-analysis using statistically adjusted data from the included studies17. The meta-analysis used the natural logarithm of adjusted odds ratios that were extracted from the original articles, while the natural logarithm of standard errors was derived from the extracted confidence intervals. We stratified the analyses by the following factors: study location, type of outcome (i.e. surgical morbidity was stratified by articles reporting anastomic leak only versus studies that include anastomic leaks and other type of complications) and low volume definitions (≤ 11/ >11 rectal cancer resections per year). Articles were classified into ≤ 11 low volume definitions if low hospital volume was defined as less than or equal to eleven surgeries per year while the rest were classified into ≥11 rectal surgeries per year; this cutoff was based on the hospital volume distribution of the articles in the paper. Heterogeneity between studies was assessed using the I2 statistic18. Risk of bias was assessed using the Newcastle Ottawa scale for observational studies19; this was assessed by 4 reviewers (CC, JS, NDV and MC). Funnel plots were used to evaluate publication bias.
Results
Description of Included Studies
The search strategy yielded 2,845 potentially relevant articles from PubMed (n=2,745) and Embase (n=100) (Figure 1). Of the 2,866 articles that were screened for eligibility based on the title, 2,820 were excluded, and an additional 121 articles were excluded after reading the abstract. There were 21 additional articles that were retrieved from the references of the remaining eligible articles (n=24); hence a total of 35 full articles were read to determine eligibility. Upon reading the full articles, 14 more articles were excluded because they did not meet the inclusion and exclusion criteria. Hence, a total of 2120–40 articles were included in the meta-analysis.
Figure 1.
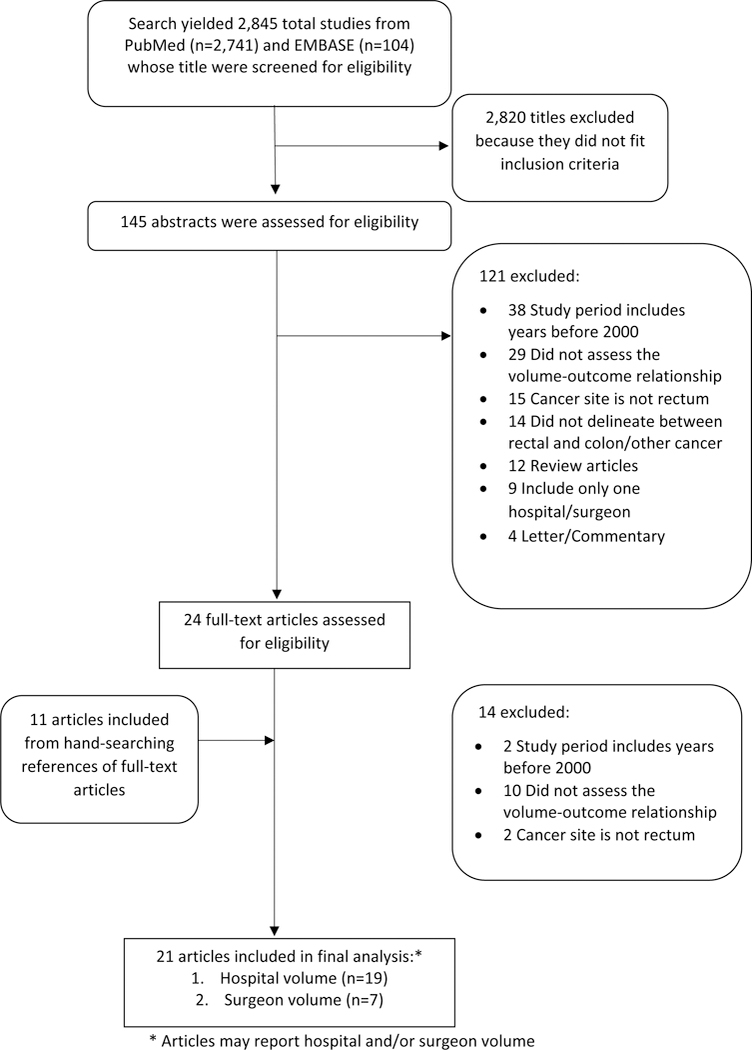
Flow diagram of search strategy.
Table 1 describes the characteristics of the studies that were included in the meta-analysis. Thirteen were from Europe21–23,25–30, six studies were from the Northern America20,31,32,34,36,37 and two were from Asia33,35. Only four studies were based on prospective cohort data21–23,39; two other studies were based on study populations derived from voluntary inclusion33,37. Population based datasets, such as cancer registries or state health records, were used in the remaining 15 studies20,24–32,34,35,37,38,40. The mean patient age ranged from 59 to 67 years33,34,36,37,39 and there were more male versus female rectal cancer patients. Only five articles included rectosigmoid tumors20,24,29,35,38, while five articles did not report on the inclusion of rectosigmoid tumors25,28,30,33,34 and 11 articles did not include rectosigmoid tumors21–23,26,27,31,32,36,37,39,40. The types of surgeries reported in the decreasing order in the majority of articles were low anterior resection and abdominoperineal resection.
Risk of bias assessment
Based on the Newcastle Ottawa scale, the studies included were generally high quality studies (Figure 2). All the articles had an adequate selection of non-exposed cohorts, demonstrated that the outcome was not present before the beginning of the study and had study populations that were generally representative of rectal cancer patient demographic and disease stage. Of the 21 studies, only three studies did not have adequate follow-up time or had minimal loss to follow-up27,29,38. Even though all the studies included in the meta-analysis adjusted for potential confounders, the type of variables that were adjusted for varied across the studies. In particular, three studies did not adjust for cancer stage31,32,35, 11 studies did not adjust for neoadjuvant treatment20,24,26,28,30–33,38–40 and nine studies did not adjust for either type of surgery or urgency of surgery21,27,34–39. An evaluation of the funnel plots showed symmetry, suggesting that the potential of publication bias was limited (see Appendix).
Figure 2.
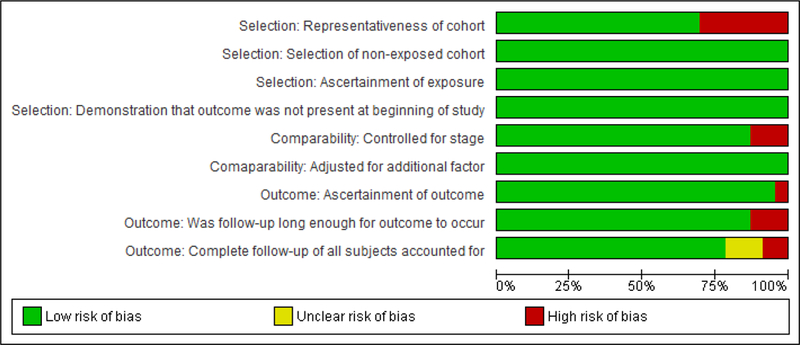
Newcastle Ottawa Risk of Bias Assessment Summary
Quantitative synthesis
Surgical morbidity
Higher hospital volume was significantly associated with decreased surgical morbidity [OR = 0.80; (0.70, 0.93); I2=35%] in rectal cancer patients who received surgery since 2000 (Figure 3). Similar results were obtained after excluding the Yasunaga et al.33 article to reduce heterogeneity because there is no standard neoadjuvant chemoradiation for rectal cancer and variation in types of rectal resection in Japan. Stratified analysis revealed a marginally significant association between higher hospital volume and surgical morbidity in five studies from non-USA countries [OR = 0.85 (0.72, 1.00); I2=18%]. Yeo et al.20 did a study in the USA that suggested that higher hospital volume is significantly associated with decreased surgical morbidity [OR = 0.71 (0.60, 0.83)]. Furthermore, hospital volume was significantly associated with surgical morbidity in studies that defined low volume as greater than 11 rectal cancer resections [OR = 0.77 (0.62, 0.97); I2=56%] compared to those that defined low volume as less than or equal to 11 rectal cancer resections [OR = 0.86 (0.70, 1.04); I2=5%]. Stratified analysis by the nature of the surgical morbidity also showed that studies that incorporated anastomic leakage and other complications, such as peritonitis and bleeding, had a significant association with hospital volume [OR = 0.76 (0.65, 0.90); I2=36%]. However, Bos et al.29 and Ortiz et al.21 who only looked at the association between hospital volume and anastomic leakage did not report significant results (Table 1).
Figure 3.
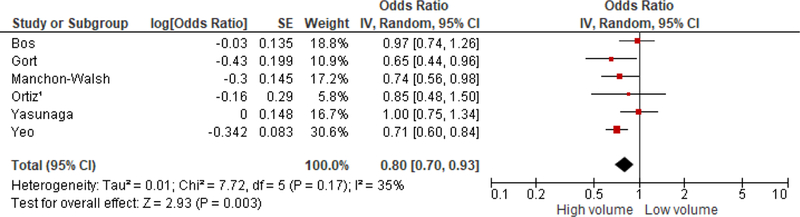
Association between hospital volume and surgical morbidity
Pathological surgical margins, Permanent colostomy and Recurrence
Among the two studies that assessed pathological margins, Gietelink et al.30 and Lorimer et al.34 suggested that lower volume versus higher hospital volume was significantly associated with circumferential resection margins [OR = 1.54 (1.12, 2.11)] and positive surgical margins [OR = 1.45 (1.25, 1.70)], respectively. In addition, higher volume hospitals were 49% less likely to perform surgery with permanent colostomy compared to low volume hospitals [OR = 0.51 (0.29, 0.92); I2=34%]. Among the two studies that assessed recurrence, higher hospital volume was not significantly associated with recurrence in either study [Ortiz et al.23: OR = 0.84 (0.48, 1.45); Ptok et al.39: OR = 0.99 (0.51, 1.91)].
Post-operative mortality
Higher hospital volume had a significantly protective association with post-operative mortality, however, these studies were moderately heterogeneous [OR = 0.67 (0.50, 0.90); I2=41%] (Figure 4). The Leonard et al.38 study which measured hospital volume continuously was excluded from this analysis because including it in the analysis introduced significant heterogeneity. The Leonard et al.38 study reported borderline significant associations between hospital volume and post-operative mortality. Higher hospital volume was significantly associated with decreased post-operative mortality in the USA [Baek et al.32: OR = 0.45 (0.24, 0.84); Aquina et al.31: OR = 0.43 (0.21, 0.88)]. Nevertheless, hospital volume was not associated with post-operative mortality in studies from non-USA countries. Similar to the association between hospital volume and surgical morbidity, hospital volume was significantly associated with post-operative mortality in >11 low volume definitions studies [OR = 0.56 (0.38, 0.83); I2=38] but not significant in >11 low volume definitions studies [OR = 0.76 (0.39, 1.50); I2=48%].
Figure 4.
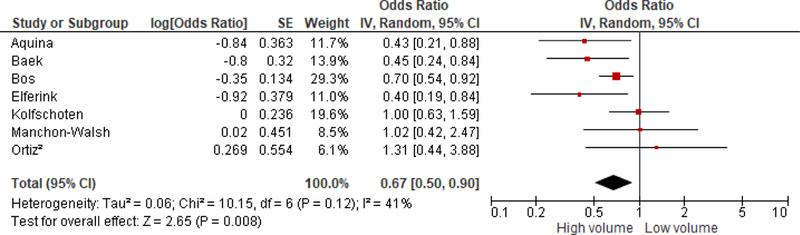
Association between hospital volume and post-operative mortality
Overall survival
Overall, survival appears marginally associated with hospital volume [OR = 0.95 (0.91, 1.00); I2=92%] (Figure 5); this stratified analysis suggested significant heterogeneity. There was no significant association between hospital volume and overall survival in >11 low volume definition studies [OR = 0.92 (0.80, 1.05); I2=92%]. Analysis of the association between hospital volume and overall survival by follow-up time differed; hospital volume was significantly associated with overall survival within 5 years [OR = 1.03 (1.01, 1.05); I2=0%], while this association was not significant and had significant heterogeneity if follow-up time was more than 5 years [OR = 0.87 (0.74, 1.02); I2=94%].
Figure 5.
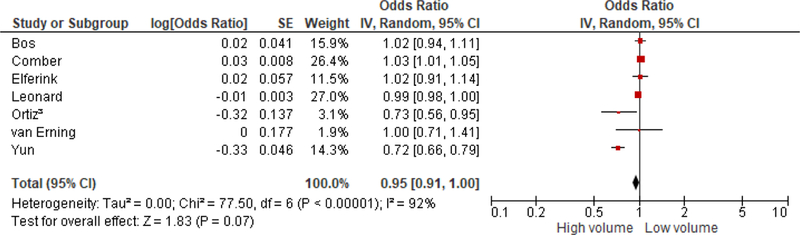
Association between hospital volume and overall survival
Similarly, surgeon volume was not significantly associated with overall survival [OR = 0.82 (0.62, 1.08); I2=63%]; there was significant heterogeneity in this analysis. Richardson et al.37 [OR = 0.67 (0.43, 1.02)], Comber et al.40 [OR = 0.97 (0.93, 1.01)] and Gort et al.28 [OR = 0.70 (0.47, 1.03)] reported no significant association between surgeon volume and overall survival.
Discussion and Conclusion
The results of this study are similar to what has been published by the Consortium for Optimizing the Treatment of Rectal Cancer (OSTRiCh)4. The results of this study suggest that high hospital volume is associated with lower odds of surgical morbidity, permanent colostomy and post-operative mortality. Surgeon volume was not significantly associated with overall survival.
Generally, the included studies had low heterogeneity. The similarity of these results to previously published meta-analyses is a strength of this study13,15. Furthermore, all the studies were sufficiently powered to analyze the association of interest, the potential for publication bias was low and the patients were representatives of rectal cancer patients. The inclusion of high quality studies with low risk of bias is another strength of the study.
A limitation in this analysis was that the definitions of hospital and surgeon volume were heterogeneous across the studies; some studies used continuous variables38,40 and the cutoff values in studies with categorical definitions of volume differed20–37,39 (Table 1). This introduced bias in the meta-analysis; nevertheless, the low heterogeneity in most of the analyses suggest that its impact may be minimal. In addition, there were variations in the studies based on data source, data period, geographic location, tumor location, neoadjuvant treatment and surgical procedures used. However, the use of stratified analysis was able to illuminate the volume-outcome association across some strata. Only eight of the studies accounted for clustering by surgeon or hospital20–23,31,33,38,40. Even though most studies adjusted for some potential confounders, most of them did not adjust for all confounders, which is probably due to limitations in data availability.
The significance of these associations differed across strata. In particular, while the volume-outcome relationship remained significant in USA based studies20,31,32,34, this was not the case in non-USA based studies21,22,24–29,33,35,36,38,40. This is not surprising since most non-USA locations, especially in Europe, have centralized rectal cancer management centers while the USA does not; USA based articles generally had ≤11 low volume definitions (annual hospital volume between 5 and 11 surgeries) while non-USA based articles reported >11 low volume definitions (annual hospital volume greater than or equal to 20 surgeries) (Table 1). This suggests that centralization of rectal cancer management could result in better rectal cancer care management and ultimately improve outcomes in the USA. Baek et al.32 indicated a significant association between non-mandated regionalization and improved outcomes in rectal cancer patients in New York, strengthening the argument for regionalization of rectal cancer surgery, which has also been shown in relation to other high risk procedures like esophagectomy and pancreatic surgery41.
The association between volume and surgical morbidity, mortality, and overall survival was not significant in studies that had ≤ 11 low volume definitions while the aforementioned associations were significant in studies that had >11 low volume definitions (Table 2). This result suggests that there is a threshold volume that confers better outcomes; this is similar to what has been published previously on other high risk cancer resections42. Nevertheless, the variations in defining high versus low volume across the studies makes it difficult to infer the appropriate minimum threshold values that confer better outcomes.
The variation in significance between hospital volume and surgical morbidity type (anastomic leakage versus other surgical morbidity) suggests that high volume may be beneficial in preventing specific complications. Similar to what has been previously published13,15,43, the significant association between hospital volume and <5 year overall survival suggests that volume does have a significant impact on short-term outcomes. However, this is in contrast to what was published in another review. A systemic literature review concluded that volume was not associated with rectal cancer outcomes14 while two meta-analyses13,15 reported the opposite, study types may also explain this variation. The differences in results may also be due to variations in factors, such as the populations of the studies, study period and types of rectal cancer resections received in those populations. Given that most rectal cancer recurrences occur within 5 years of diagnosis, it is not surprising that volume is not significantly associated with overall survival ≥5 years. The high heterogeneity between studies analyzing hospital volume and 5-year survival limit the inferences that can be made about this association, hence there is a need for further research in this area of inquiry.
The results of this study contribute to the body of knowledge that indicate that high hospital volume is associated with better outcomes among rectal cancer patients treated since 2000. Future research should determine how hospital and surgeon characteristics contribute to better outcomes in rectal cancer patients who receive surgery. In conclusion, as rectal cancer treatment becomes more complex, initiatives to reduce variation in outcomes by hospital and surgeon volume in countries such as the US are essential.
Acknowledgments
Source of support: No financial support was received to perform this project.
Appendix
Figure A1:
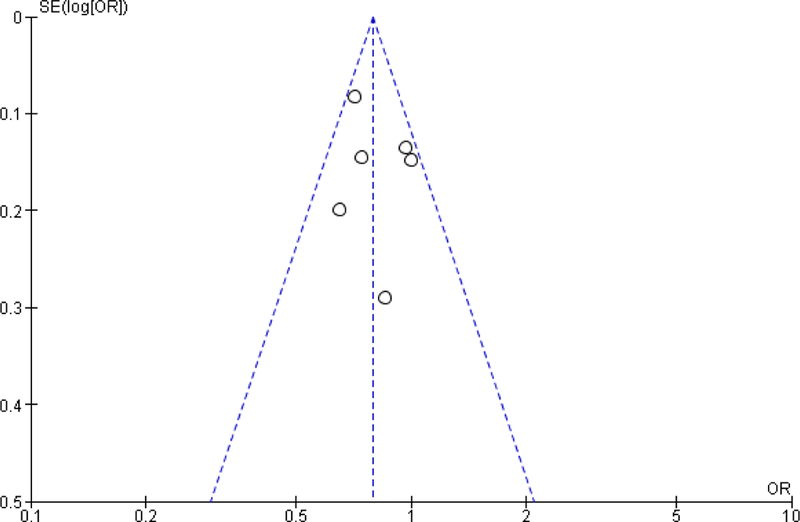
Funnel plot of association between hospital volume and surgical morbidity
Figure A2:
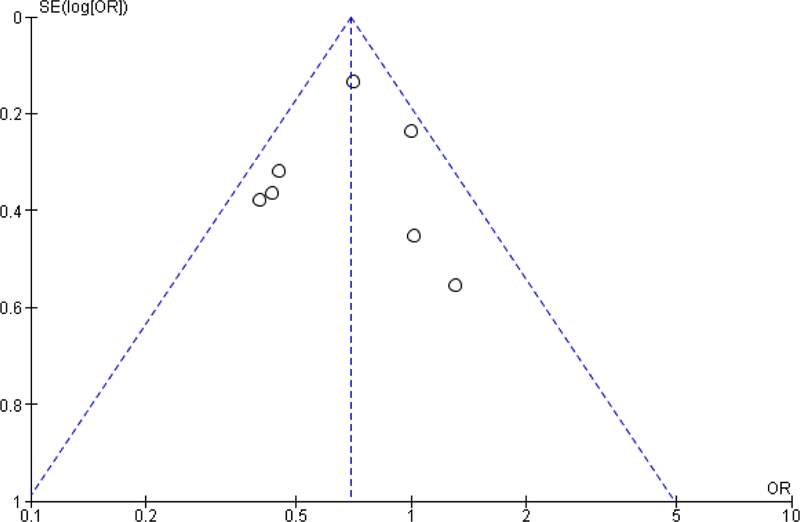
Funnel plot of association between hospital volume and postoperative mortality
Figure A3:
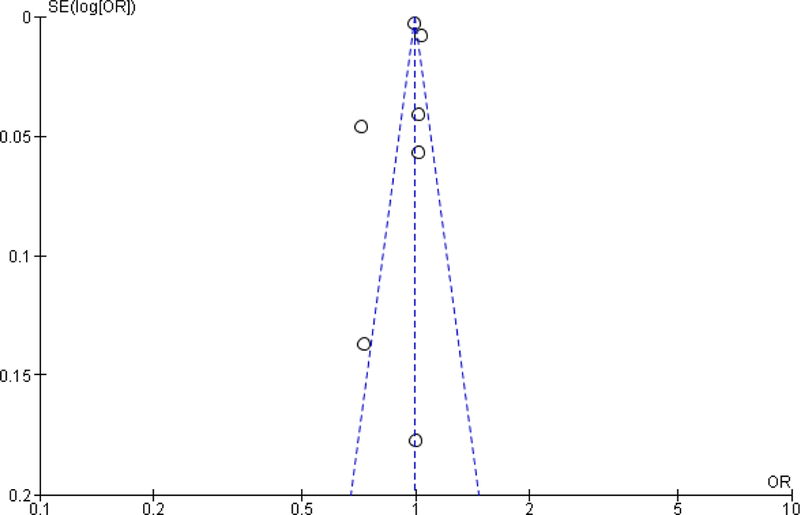
Funnel plot of association between hospital volume and overall survival
Footnotes
Manuscript Category: Colon and rectal surgery
Disclaimers: This manuscript is original and neither published, accepted, or submitted for publication elsewhere.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. Journal of the National Cancer Institute. 2001;93(8):583–596. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. New England Journal of Medicine. 2004;351(17):1731–1740. [DOI] [PubMed] [Google Scholar]
- 4.Abbas M, Chang G, Read T, et al. Optimizing rectal cancer management: analysis of current evidence. Diseases of the Colon & Rectum. 2014;57(2):252–259. [DOI] [PubMed] [Google Scholar]
- 5.Church JM, Gibbs P, Chao MW, Tjandra JJ. Optimizing the outcome for patients with rectal cancer. Diseases of the colon & rectum. 2003;46(3):389–402. [DOI] [PubMed] [Google Scholar]
- 6.Heald R, Ryall R. Recurrence and survival after total mesorectal excision for rectal cancer. The Lancet. 1986;327(8496):1479–1482. [DOI] [PubMed] [Google Scholar]
- 7.Allal AS, Bieri S, Pelloni A, et al. Sphincter-sparing surgery after preoperative radiotherapy for low rectal cancers: feasibility, oncologic results and quality of life outcomes. British journal of cancer. 2000;82(6):1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marijnen CA, Van De Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. Journal of Clinical Oncology. 2005;23(9):1847–1858. [DOI] [PubMed] [Google Scholar]
- 9.Vironen JH, Kairaluoma M, Aalto A-M, Kellokumpu IH. Impact of functional results on quality of life after rectal cancer surgery. Diseases of the colon & rectum. 2006;49(5):568–578. [DOI] [PubMed] [Google Scholar]
- 10.Pollett WG, Nicholls R. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Annals of surgery. 1983;198(2):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. Journal of Clinical Oncology. 2005;23(25):6126–6131. [DOI] [PubMed] [Google Scholar]
- 12.Rogers SO Jr, Wolf RE, Zaslavsky AM, Wright WE, Ayanian JZ. Relation of surgeon and hospital volume to processes and outcomes of colorectal cancer surgery. Annals of surgery. 2006;244(6):1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archampong D, Borowski D, Wille‐Jørgensen P, Iversen LH. Workload and surgeon s specialty for outcome after colorectal cancer surgery. The Cochrane Library. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salz T, Sandler RS. The effect of hospital and surgeon volume on outcomes for rectal cancer surgery. Clinical Gastroenterology and Hepatology. 2008;6(11):1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo YR, Phan K, Morris DL, Liauw W. Systematic review and a meta-analysis of hospital and surgeon volume/outcome relationships in colorectal cancer surgery. J Gastrointest Oncol. 2017;8(3):534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Review Manager (RevMan)[Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Center TCC. [Google Scholar]
- 17.Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009;62(Pt 1):97–128. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 20.Yeo HL, Abelson JS, Mao J, O’Mahoney PR, Milsom JW, Sedrakyan A. Surgeon Annual and Cumulative Volumes Predict Early Postoperative Outcomes after Rectal Cancer Resection. Ann Surg. 2017;265(1):151–157. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz H, Biondo S, Codina A, et al. [Hospital variation in anastomotic leakage after rectal cancer surgery in the Spanish Association of Surgeons project: The contribution of hospital volume]. Cir Esp. 2016;94(4):213–220. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz H, Biondo S, Codina A, et al. Hospital variability in postoperative mortality after rectal cancer surgery in the Spanish Association of Surgeons project: The impact of hospital volume. Cir Esp. 2016;94(1):22–30. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz H CA, Ciga MÁ, Biondo S, Enríquez-Navascués JM, Espín E, García-Granero E, Roig JV. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery in the Spanish Rectal Cancer Project. Cir Esp. 2016;94(8):442–452. [DOI] [PubMed] [Google Scholar]
- 24.Manchon-Walsh P, Borras J, Espinas J, Aliste L. Variability in the quality of rectal cancer care in public hospitals in Catalonia (Spain): clinical audit as a basis for action. European Journal of Surgical Oncology (EJSO). 2011;37(4):325–333. [DOI] [PubMed] [Google Scholar]
- 25.Kolfschoten NE, Marang van de Mheen PJ, Gooiker GA, et al. Variation in case-mix between hospitals treating colorectal cancer patients in the Netherlands. Eur J Surg Oncol. 2011;37(11):956–963. [DOI] [PubMed] [Google Scholar]
- 26.Elferink M, Krijnen P, Wouters M, et al. Variation in treatment and outcome of patients with rectal cancer by region, hospital type and volume in the Netherlands. European Journal of Surgical Oncology (EJSO). 2010;36:S74–S82. [DOI] [PubMed] [Google Scholar]
- 27.Van Erning F, van Steenbergen L, van den Broek W, Rutten H, Lemmens V. No difference between lowest and highest volume hospitals in outcome after colorectal cancer surgery in the southern Netherlands. European Journal of Surgical Oncology (EJSO). 2013;39(11):1199–1206. [DOI] [PubMed] [Google Scholar]
- 28.Gort M, Otter R, Plukker JT, Broekhuis M, Klazinga NS. Actionable indicators for short and long term outcomes in rectal cancer. Eur J Cancer. 2010;46(10):1808–1814. [DOI] [PubMed] [Google Scholar]
- 29.Bos AC, van Erning FN, Elferink MA, et al. No Difference in Overall Survival Between Hospital Volumes for Patients With Colorectal Cancer in The Netherlands. Dis Colon Rectum. 2016;59(10):943–952. [DOI] [PubMed] [Google Scholar]
- 30.Gietelink L, Henneman D, van Leersum NJ, et al. The Influence of Hospital Volume on Circumferential Resection Margin Involvement: Results of the Dutch Surgical Colorectal Audit. Ann Surg. 2016;263(4):745–750. [DOI] [PubMed] [Google Scholar]
- 31.Aquina CT, Probst CP, Becerra AZ, et al. High volume improves outcomes: the argument for centralization of rectal cancer surgery. Surgery. 2016;159(3):736–748. [DOI] [PubMed] [Google Scholar]
- 32.Baek J-H, Alrubaie A, Guzman EA, et al. The association of hospital volume with rectal cancer surgery outcomes. International journal of colorectal disease. 2013;28(2):191–196. [DOI] [PubMed] [Google Scholar]
- 33.Yasunaga H, Matsuyama Y, Ohe K, Japan Surgical S. Volume-outcome relationship in rectal cancer surgery: a new perspective. Surg Today. 2009;39(8):663–668. [DOI] [PubMed] [Google Scholar]
- 34.Lorimer PD, Motz BM, Kirks RC, et al. Pathologic Complete Response Rates After Neoadjuvant Treatment in Rectal Cancer: An Analysis of the National Cancer Database. Ann Surg Oncol. 2017;24(8):2095–2103. [DOI] [PubMed] [Google Scholar]
- 35.Yun Y, Kim Y, Min Y, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Annals of oncology. 2012:mds101. [DOI] [PubMed] [Google Scholar]
- 36.Richardson DP, Porter GA, Johnson PM. Population-based use of sphincter-preserving surgery in patients with rectal cancer: is there room for improvement? Dis Colon Rectum. 2013;56(6):704–710. [DOI] [PubMed] [Google Scholar]
- 37.Richardson DP, Porter GA, Johnson PM. Surgeon knowledge contributes to the relationship between surgeon volume and patient outcomes in rectal cancer. Ann Surg. 2013;257(2):295–301. [DOI] [PubMed] [Google Scholar]
- 38.Leonard D, Penninckx F, Kartheuser A, Laenen A, Van Eycken E, Procare. Effect of hospital volume on quality of care and outcome after rectal cancer surgery. Br J Surg. 2014;101(11):1475–1482. [DOI] [PubMed] [Google Scholar]
- 39.Ptok H, Marusch F, Kuhn R, Gastinger I, Lippert H. Influence of hospital volume on the frequency of abdominoperineal resection and long-term oncological outcomes in low rectal cancer. Eur J Surg Oncol. 2007;33(7):854–861. [DOI] [PubMed] [Google Scholar]
- 40.Comber H, Sharp L, Timmons A, Keane FB. Quality of rectal cancer surgery and its relationship to surgeon and hospital caseload: a population-based study. Colorectal Dis. 2012;14(10):e692–700. [DOI] [PubMed] [Google Scholar]
- 41.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135(6):569–575. [DOI] [PubMed] [Google Scholar]
- 42.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–2127. [DOI] [PubMed] [Google Scholar]
- 43.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236(5):583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]


