Abstract
PURPOSE
In this study, we aimed to investigate trends in the age-standardized and age-specific incidence rates in two distinct regions (the northern and southern areas) of South Africa covered by a population-based cancer registry. In addition, trends in coverage of the cervical cancer screening program were assessed using routine health service data.
METHODS
Occurrences (topography C53.0-C53.9) for the period 1998-2012 were extracted from a cancer registry database from which basic descriptive statistics and frequencies were analyzed for all variables using CanReg4. Trends over time were estimated using a direct standardization method and world standard population as a reference. Screening coverage annualized figures for women age ≥ 30 years by sub–health district were extracted from the District Health Information System.
RESULTS
In the northern area, annual age-standardized incidence rates per 100,000 women increased from 24.0 (95% CI, 21.1 to 27.0) in 1998-2002 to 39.0 (95% CI, 35.6 to 42.5) in 2008-2012, with a screening coverage rate of 15% by 2012. In contrast, no increase was observed in incidence in the southern area, with rates of 20.0 (95% CI, 18.5 to 21.4) in 1998-2002 and 18.8 (95% CI, 16.2 to 21.4) in 2008-2012, and the southern area had a higher screening coverage of 41% in 2012. Overall, the percentage distribution of stage at diagnosis showed that 28.5% of occurrences were diagnosed at disease stages I and II and 35%, at III and IV; 36% had with missing stage information (2003-2012). In 77% of occurrences, a histologically verified diagnosis was made, compared with only 12.3% by cytology.
CONCLUSION
This study has demonstrated an almost two-fold increase in the incidence rate in the northern area but little change in the southern area of the cancer registry.
INTRODUCTION
Globally, cervical cancer accounts for 6.6% of all cancers reported in women and is ranked as the fourth highest cause of both incidence and mortality.1 There were an estimated 570,000 new occurrences and 311,000 deaths from cervical cancer worldwide in 2018. Of this global burden, cervical cancer accounts for 90% of deaths in women living in low- and middle-income countries (LMICs), with the highest burden borne by countries in sub-Saharan Africa. This contrasts with the much lower incidence rates of 10.4 per 100,000 women in developed countries. During the past few decades, cervical cancer incidence and mortality rates have been in decline in many populations worldwide. Aside from screening (where available), these declines have been ascribed to factors linked to either increasing average socioeconomic levels or a diminishing risk of persistent infection with high-risk human papillomavirus (HPV) or other sexually transmitted diseases. Cervical cancer treatment takes approximately 18% of a health facility’s budget, yet it is preventable and can be diagnosed early through screening using various methods—with the Papanicolau (Pap) test as the gold standard.2
Cervical cancer is the second most common cancer among women in South Africa after breast cancer.3 The National Cancer Registry reported age-standardized incidence rates (ASRs) of 26.2 and 29.1 per 100,000 for black African women in 2008 and 2012, respectively. The population-based Eastern Cape Cancer Registry (ECCR) that records incident cancer occurrences among a population living in a defined rural area also reported an increase in cervical cancer ASRs from 22.0 to 29.4 per 100,000 women during the same period.4
South Africa adopted a cytology-based screening program for cervical cancer in 1999 as part of its National Cancer Control Policy.5 Women attending the public-sector services would be entitled to three free Pap smears per lifetime starting at the age of 30 years or older, with a 10-year interval between each smear unless the smears reveal dysplastic or atypical cellular changes. The goal of this screening program was to screen 70% of the target population within 10 years of implementation. However, small-scale screening evaluation studies identified problems with the implementation of the program. These problems were associated with level of recipients’ knowledge about screening, health system implementation challenges, and limited population coverage.6 Findings of other studies conducted in the Western Cape,7,8 the Free State,8 and KwaZulu-Natal9 also revealed fragmented services with little or no follow-up for women with positive smears. These studies also highlighted the unequal distribution of resources, which was worse in rural clinics.9
It has been demonstrated that women with HIV/AIDS have significantly higher rates of cervical cancer than the general population.10 However, there have been mixed findings about the effect of antiretroviral therapy (ART)/highly active ART. Unlike other AIDS-defining cancers, the incidence of cervical cancer does not seem to have diminished after the advent of ART.11 Conversely, a global systematic review showed that women with HIV who receive ART have a lower prevalence of high-risk HPV and experience a reduction in the incidence of invasive cervical cancer.12 Additional studies with larger sample sizes and cohort designs were encouraged.11
It is unclear whether the observed increase in cervical cancer incidence in this study population is related to increased availability of free screening or other factors. This study was designed to investigate trends in the age-standardized and age-specific incidence rates in two distinct regions (the northern and southern areas) covered by the ECCR. In addition, trends in coverage of the cervical cancer screening program were assessed using routine health service data.
METHODS
Study Population
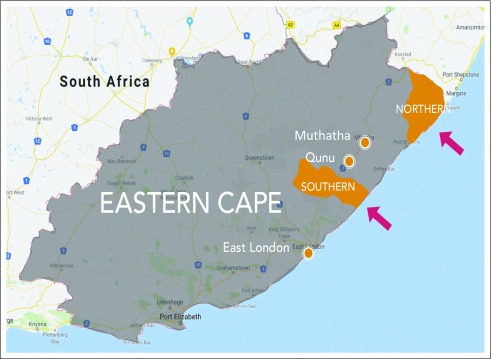
The ECCR collects data for the population living in two distinct rural areas in the former Transkei region of the Eastern Cape Province of South Africa, originally selected for monitoring esophageal cancer in four magisterial areas (Fig 1). In 1998, the surveillance scope was expanded to include eight magisterial areas, of which three (Bizana, Flagstaff, and Lusikisiki) form the northern part in the Alfred Nzo Health District and five (Butterworth, Centane/Kentani, Idutywa, Nqamakwe, and Willowvale) form the southern part in the Amathole Health District.
FIG 1.
Map of South Africa showing cancer registration area in the Eastern Cape Province.
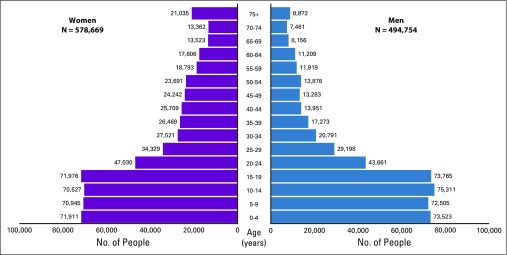
The ECCR covers a population of just more than 1 million; 54% are women, and 46% are men, according to the population census (2011).13 ECCR data account for 16% of the population residing in the Eastern Cape Province, and the age-sex composition in the population pyramid is typical of a rural setting (Fig 2). South Africa is an upper-middle income country but experiences high unemployment, poor economic growth, and very high wealth inequalities. The study population included communities with higher-than-average proportions living below the poverty line14 and the highest rates of HIV,15 and AIDS accounted for 31.5% of deaths experienced in the province.16
FIG 2.
Estimated average annual population of eight magisterial areas for the period of 2008-2012.
Data Collection Method
The ECCR is one of two rural population-based cancer registries in Africa with important features for maintaining data quality, as described previously.4,17 Both active and passive case-finding methods are used to collect data. Medical records, including pathology reports, are viewed, and cancer information is abstracted manually for each patient diagnosed using a structured data collection tool. Identified duplicates are managed accurately and deleted when the patient information is consolidated. A customized cancer registry data management program, CanReg4 (International Agency for Research on Cancer, Lyon, France) is used.18
Statistical Analysis
Basic descriptive statistics and frequencies were analyzed for all variables using CanReg4. Analysis of the collected data provided occurrences reported by age, year, and magisterial areas. The average annual population at risk was calculated for the three 5-year periods (1998-2002, 2003-2007, and 2008-2012). The 1996, 2001, and 2011 censuses13 provided age-specific counts of the population. The annual rates of change (by age, sex, and magisterial area) between these years were used to prepare annual and 5-year period estimates. A direct method, as described by Boyle and Parkin,19 was adopted to calculate ASRs per 100,000 person-years. The world standard population was used as the reference population.20 Weighted standard errors were calculated to provide 95% CIs for the ASRs.
Topography C53.0-C53.9 according to International Classification of Diseases—Oncology (ICD-O)21 for the period 1998-2012 were extracted from the ECCR database. The annual cervical cancer incidence trends were calculated for the years 1998-2012, a 15-year period encompassing the initiation of the national cervical cancer free cytology screening program in South Africa. Information was checked on the age and stage distribution of incident occurrences (stages I and II v III and IV) to assess the proportion of occurrences diagnosed early in the progression of the disease. The proportion of occurrences with pathologically verified diagnoses was calculated to check the proportion of cytology-identified versus histology-verified occurrences in particular. Subanalyses were conducted for the distinct northern and southern areas of the region covered by the registry.
Screening Coverage Data and Analysis
The District Health Information System (DHIS) collects monthly facility-based data from all public-sector clinics and hospitals across the country. From 2007 on, a data element was added on the basis of the number of women older than age 30 years who had a Pap test in the past month. An annualized indicator for screening coverage calculated using the DHIS population estimate is reported. DHIS data were obtained from the National Department of Health, and annualized figures of screening coverage for women age ≥ 30 years were extracted for the Mbashe, Mnquma, and Qaukeni Health subdistricts. With small boundary variations, the eight magisterial areas can be mapped directly onto the three health subdistricts. The coverage indicator in the DHIS has been calculated by taking the number of women age ≥ 30 years who were screened divided by the number of women in the target population, divided by 10 to account for a cervical cytology–based screening policy that aimed to screen once every 10 years.
Approved ethics.
The South African Medical Research Council Ethics Committee approved the study proposal; Protocol ID No. EC014-10/2014 was assigned on February 23, 2015. The Eastern Cape Health Research Committee approval No. EC RP52-33 was designated on May 12, 2015.
RESULTS
A total of 1,808 new occurrences were reported; 63% were from the northern area of the registry, and 37% were from the southern area. Table 1 lists the basis of diagnosis and the stage of the cervical cancer by area and period. Overall, histologically verified diagnoses ranged between 64.7% and 73.6%, whereas cytologically verified diagnoses ranged between 7.9% and 12.5%; clinically diagnosed occurrences ranged between 27.4% and 14.0%. Histologic verification by areas ranged between 65.8% and 71.3% in the northern area and between 63.6% and 77.7% in the southern area; respective regional cytologic diagnoses ranged between 5.8% and 13.6% and 10.2% and 10.4%. Occurrences without additional confirmatory tests in the northern and southern areas ranged between 28.4% and 15.1% and between 26.3% and 11.9%, respectively.
TABLE 1.
Basis of Diagnosis and Stage at Diagnosis of Cervical Cancer Occurences by Area and Period, Eastern Cape Cancer Registry
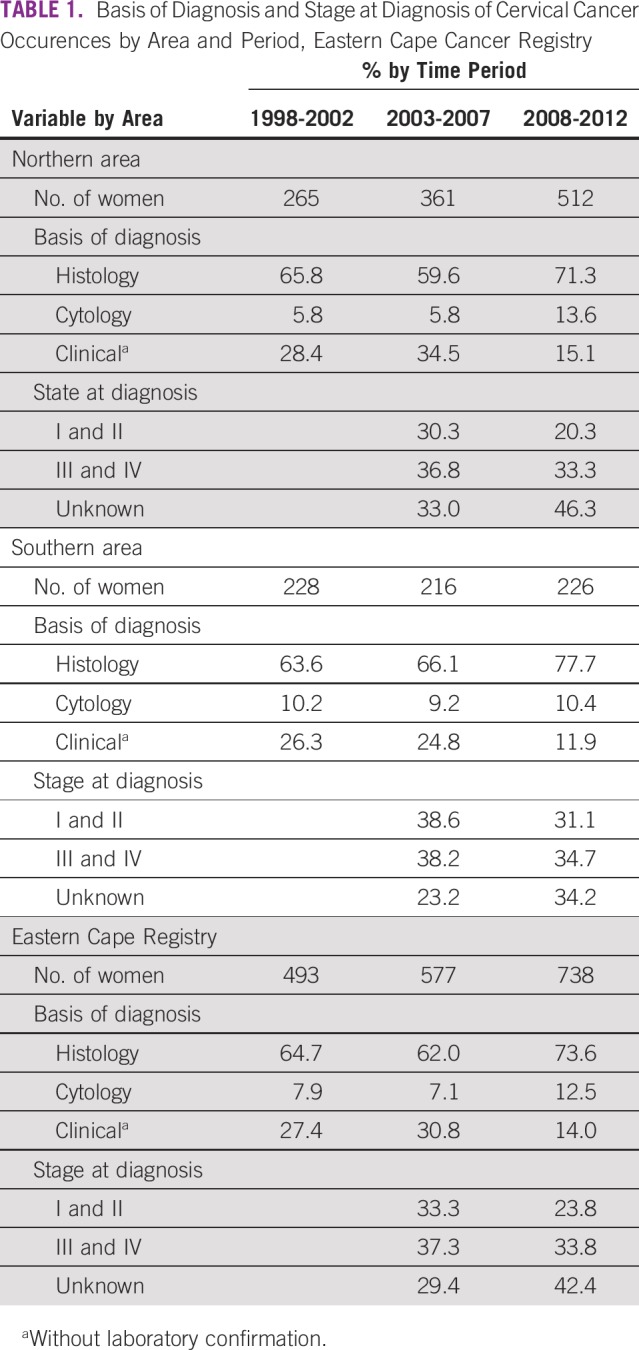
Information about the stage of disease at diagnosis was collected by the ECCR from 2003 onward, and the results for the two periods (2003-2007 and 2008-2012) are presented in Table 1. During 2003-2007, 29.4% of the occurrences had missing stage information, and this increased to almost half (42.4%) in 2008-2012. The proportion with missing stage information differed; in the northern area, it ranged between 33.0% and 46.3%, whereas it ranged between 23.2% and 34.2% in the southern area. Stage III was the most common stage at diagnosis, and its rate increased from 23.7% in 2003-2007% to 25.7% in 2008-2012.
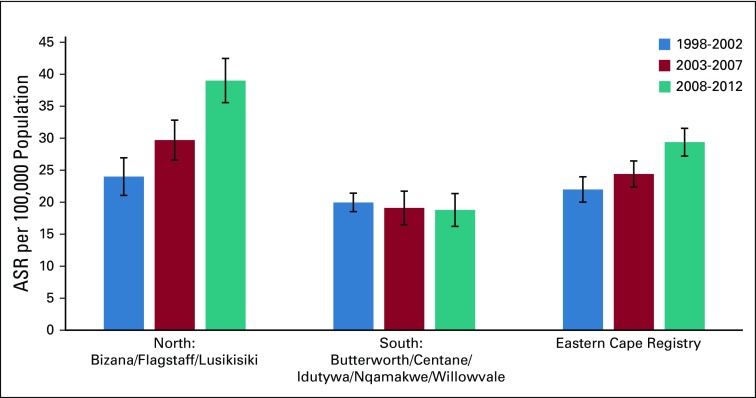
ASRs per 100,000 of women are reported for three periods by area in Figure 3. Overall ASRs per 100,000 women were 22.0 (95% CI, 20.0 to 24.0) in 1998-2002, 24.4 (95% CI, 22.4 to 26.4) in 2003-2007, and 29.2 (95% CI, 27.3 to 31.6) in 2008-2012. Although the ASR in the entire region showed a progressive increase, there was a slight decrease in the southern area during this period. ASRs per 100,000 women were 20.0 (95% CI, 18.5 to 21.4) in 1998-2002, 19.1 (95% CI, 16.5 to 21.7) in 2003-2007, and 18.8 (95% CI, 16.2 to 23.4) in 2008-2012. These differences were not statically significant. In contrast, the ASRs in the northern area increased significantly from 24.0 (95% CI, 21.1 to 27.0) in 1998-2002 to 29.7 (95% CI, 26.6 to 32.8) in 2003-2007 and 39.0 (95% CI, 35.6 to 42.5) in 2008-2012.
FIG 3.
Age-standardized annual incidence rates for cervical cancer over time periods by area, Eastern Cape Cancer Registry. ASR, age-standardized rate.
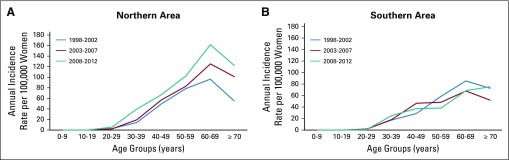
The ASRs are shown for the three periods in Figures 4A (northern) and 4B (southern). Incidence increased steadily by age, with a decrease at ≥ 70 years. In the northern area (Fig 4A), a distinct increase was observed during the third period (2008-2012); the increase occurred across all ages but was more marked in the 50-59 years and 60-69 years age groups. In the southern area (Fig 4 B), little difference was observed across the three periods.
FIG 4.
Age-specific annual incidence rates for cervical cancer over time periods by area, Eastern Cape Cancer Registry.
Screening Coverage Proportions
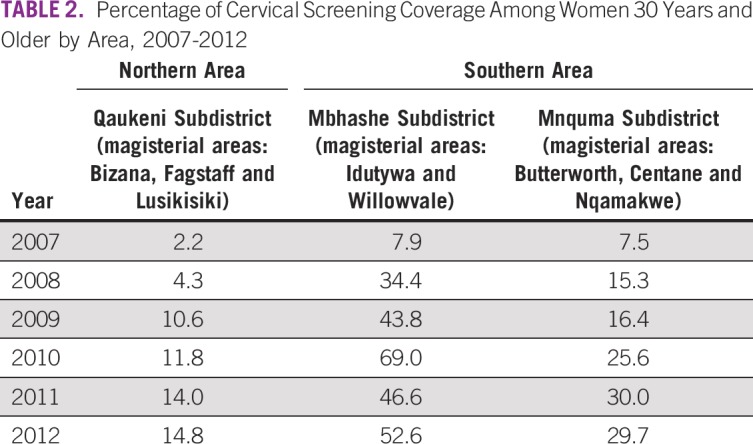
The cervical screening coverage results for women age ≥ 30 years are listed by health subdistrict and year in Table 2. Inclusion of these data in the routine information systems started in 2007 but had particularly low coverage in the northern area (2.2% in 2007 and 4.3% in 2008). An increase was observed from 2009 to 2012 to a maximum of only 14.8% in 2012. The southern area reported slightly better coverage of the screening program, with an average of 7.7% in 2007, an increase to 41.0% in 2012, and anomalously high coverage of 69.0% reported for Mbashe in 2010. Furthermore, this subdistrict had almost twice the screening percentage of the Mnquma subdistrict (52.3% v 29.7%) in 2012.
TABLE 2.
Percentage of Cervical Screening Coverage Among Women 30 Years and Older by Area, 2007-2012
DISCUSSION
Compared with the global average cervical cancer incidence rates of 6.9 per 100,000 women in 2012,22 the rates of 18.8 (95% CI, 16.2 to 21.4) and 39.0 (95% CI, 35.6 to 42.5) per 100,000 women reported in the study population (Fig 3) are unacceptably high. Incidence rates increased significantly over time in the northern area, contributing to the previously reported overall increase.4 Several factors could be associated with the increasing trends, including increased population-based screening and the high prevalence of HIV. Conversely, the incidence rates in the southern area showed a slight decrease, which was not statistically significant.
The proportion of patients with cytologically confirmed cervical cancer increased in the northern area from 5.8% to 13.6% in the final period, whereas the proportion in the southern area remained stable at approximately 10% across the whole study period (2007-2012). Data from the DHIS showed that screening coverage increased in both study areas, albeit to less than optimal levels (Table 2). It appears that the southern area experienced better coverage, reaching levels of 41% in 2012, compared with the northern area, which reached only 15%. Given the contrasting trends between screening and incidence, it appears unlikely that the increase in incidence observed in the northern area could be ascribed to increased access to the screening program.
The high cervical cancer incidence experienced in this population might be due to the high burden of HIV infection, which concurs with studies that were conducted in South Africa and Rwanda.23,24 Both studies observed a strong association between HIV and cervical cancer. Stein et al24 observed an increased risk of cervical cancer associated with HIV (odds ratio, 1.6; 95% CI, 1.3 to 2.0) in a case-control study conducted in South Africa, whereas Mpunga et al23 observed an even stronger association in Rwanda (odds ratio, 5.9; 95% CI, 3.8 to 9.2). Despite consideration of cervical cancer as an AIDS-defining cancer, conflicting trends have been observed. Early reports found no association between HIV and the incidence of cervical cancer.25 Late-stage HIV infection was associated with Pap test abnormalities at a younger age26, which also was observed in a study in South Africa.27 Both Moodley28 and the International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans reported a reduction of invasive cervical cancer incidence in an area of high HIV prevalence and noted that cervical cancer was not an AIDS-defining condition in the African setting.28, 29
South Africa is in the throes of an HIV/AIDS pandemic that has affected both areas of the ECCR. In 2012, the prevalence of HIV among pregnant women was 30.0% in the OR Tambo Health District (incorporating the northern area) and was 31.5% in Amathole Health District (incorporating the southern area).30 Since 2008, there has been a countrywide rollout of ART. Unfortunately, small-area data on the provision of ART are unavailable. However, health service data point toward the possibility of a quicker rollout in the northern area. The OR Tambo District reported that 86.2% of antenatal clients identified as HIV positive initiated ART compared with 61.9% in Amathole.30 The increased incidence of cervical cancer observed in the northern area does not appear to be associated with the screening program (Table 2) but may be associated with high HIV infection coupled by a quicker rollout of ART.
The Strategic Plan for the Prevention and Control of Non-Communicable Diseases identified the need to screen women with HIV infection for cervical cancer and other sexually transmitted diseases at a younger age and more frequently.31 Presentation of patients at late stages of the disease may indicate that the diagnosis was made by clinical signs and symptoms rather than by screening. The high incidence rates, together with a low proportion of cytologic confirmation, are indicative of a low reach of screening in this area. Other factors include poor infrastructure, which thus reduces the number of women effectively screened in South Africa7-9 and Botswana.9,32,33 Denny et al34 viewed the coverage as more important than frequency of screening. In their randomized controlled trial, they used three screen-and-treat strategies that included screening by visual inspection with acetic acid. However, only women in an urban area participated in this study, which was not extended to rural women who have limited access to facilities.
Other factors that may have contributed to the high incidence of cervical cancer in this area are health inequity and low health-seeking behavior35 with loss to follow-up after the initial screening test among women identified with abnormal cytology9 and unequal distribution of resources.6-9,36 In South Africa, better-equipped health facilities are those classified as secondary and tertiary hospitals in urban areas, and this location denies women living in rural areas an opportunity to receive medical attention in time and closer to home. Those in the study area with positive smears, for example, travel a distance of 100-600 km for better care and disease management at secondary and/or tertiary hospitals.
The study has several limitations. Many occurrences had missing staging information at diagnosis. This information is not part of mandatory variables collected by cancer registrars; rather, it is collected only when available during data abstraction. There were few occurrences with cytology as basis of diagnosis but a high percentage of histologically verified diagnoses, which was worrisome. Screening coverage proportions from the routine health data were based on aggregate data; the coverage proportions may be even lower than reported, and they do not distinguish the individuals who have been screened.
In conclusion, South Africa struggles with huge disparities in the health system capacity to address the growing burden of cervical cancer, especially among rural populations. The scarcity of trained specialists coupled with the absence of resources for a robust primary care structure result in late diagnoses with poor survival for cervical cancer—a condition that often is an incurable but preventable disease. The increased cervical cancer incidence experienced by the population in this study may portend increases associated with the widespread provision of ART and low screening reach. The reviewed screening policy that included women with HIV and other sexually transmitted diseases show the South African government’s commitment to addressing cervical cancer (Data Supplement). Unless innovative approaches to test for and treat this disease become available, serving a rural area will remain a major challenge. This study illustrates the contribution of a population-based cancer registry as an essential tool to inform cancer control efforts. Opportunities to strengthen and complement these data through data linkage with other information systems would facilitate additional research.
ACKNOWLEDGMENT
We thank M.D. Parkin, MD, for his advice in shaping this article at the inception phase as well as Siphokazi Dada and Nomfuneko Sithole for research support; Akhona Ncinitwa and Linda Mbuthini for technical support; collaborating hospitals, including data collectors, for cooperation during data collection; and National Department of Health for sharing District Health Information System information.
Footnotes
This study has been sponsored entirely by the South African Medical Research Council, and author C.D.S. is the President of the African Cancer Organisation for Research and Training on Cancer; neither institution provided financial support or influence on this work.
AUTHOR CONTRIBUTIONS
Conception and design: Nontuthuzelo I.M. Somdyala, Debbie Bradshaw, Muhammad A. Dhansay, Daniela C. Stefan
Collection and assembly of data: Nontuthuzelo I.M. Somdyala
Data analysis and interpretation: Nontuthuzelo I.M. Somdyala, Debbie Bradshaw
Manuscript writing: All authors
Final approval of manuscript: All authors
Agree to be accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniela C. Stefan
Employment: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Denny L. Cervical cancer in South Africa: An overview of current status and prevention strategies. Continuing Medical Education. 2010;28:70–73. [Google Scholar]
- 3.National Cancer Registry of South Africa . Cancer Incidence in South Africa. Johannesburg, South Africa, : National Cancer Registry of South Africa; 2012. [Google Scholar]
- 4.Somdyala NI, Parkin DM, Sithole N, et al. Trends in cancer incidence in rural Eastern Cape Province, South Africa, 1998-2012. Int J Cancer. 2015;136:E470–E474. doi: 10.1002/ijc.29224. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health . Cervical Cancer Prevention and Control Policy. Pretoria, South Africa: Department of Health; 1999. [Google Scholar]
- 6.Ramathuba DU, Ngambi D, Khoza LB, et al. Knowledge, attitudes and practices regarding cervical cancer prevention at Thulamela Municipality of Vhembe District in Limpopo Province. Afr J Prim Health Care Fam Med. 2016;8:e1–e7. doi: 10.4102/phcfm.v8i2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosavel M, Simon C, Oakar C, et al. Cervical cancer attitudes and beliefs: A Cape Town community responds on World Cancer Day. J Cancer Educ. 2009;24:114–119. doi: 10.1080/08858190902854590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronjé HS, Beyer E. Screening for cervical cancer in an African setting. Int J Gynaecol Obstet. 2007;98:168–171. doi: 10.1016/j.ijgo.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Sibiya MN, Grainger L. An assessment of the implementation of the provincial cervical screening programme in selected Primary Health Care Clinics in the Ilembe Region, KwaZulu-Natal. Curationis. 2007;30:48–55. doi: 10.4102/curationis.v30i1.1050. [DOI] [PubMed] [Google Scholar]
- 10.Denslow SA, Rositch AF, Firnhaber C, et al. Incidence and progression of cervical lesions in women with HIV: A systematic global review. Int J STD AIDS. 2014;25:163–177. doi: 10.1177/0956462413491735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries HJC, Steenbergen RDM. The effect of ART on cervical cancer precursor lesions. Lancet HIV. 2018;5:e6–e8. doi: 10.1016/S2352-3018(17)30189-3. [DOI] [PubMed] [Google Scholar]
- 12.Cobucci RN, Lima PH, de Souza PC, et al. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: A systematic review. J Infect Public Health. 2015;8:1–10. doi: 10.1016/j.jiph.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Statistics South Africa . Census 2011: Statistical Release p0301.4. Pretoria, South Africa: Statistics South Africa; 2012. [Google Scholar]
- 14.Statistics South Africa . Poverty Mapping in South Africa: Applying Small Area Estimation Techniques Using IES 2010/11 and Census 2011. Pretoria, South Africa: Statistics South Africa; 2018. [Google Scholar]
- 15.Department of Health . National Antenatal Sentinel HIV Prevalence Survey, South Africa. Pretoria, South Africa: Department of Health; 2013. [Google Scholar]
- 16.Msemburi W, Pillay-van Wyk V, Dorrington R, et al. Second National Burden of Disease Study for South Africa: Cause-of-Death Profile for South Africa, 1997-2010. Cape Town, South Africa: South African Medical Research Council; 2014. [Google Scholar]
- 17.Somdyala NI, Bradshaw D, Gelderblom WC, et al. Cancer incidence in a rural population of South Africa, 1998-2002. Int J Cancer. 2010;127:2420–2429. doi: 10.1002/ijc.25246. [DOI] [PubMed] [Google Scholar]
- 18.Cooke AP, Parkin DM, Ferlay J, editors. CanReg 4 Manual: Descriptive Epidemiology Production Unit. Lyon, France: International Agency for Research on Cancer; 2006. (eds) [Google Scholar]
- 19.Boyle P, Parkin D. Statistical methods for registries: Cancer Registration—Principles and Methods. Lyon, France: International Agency for Research Cancer; 1991. [PubMed] [Google Scholar]
- 20.Doll R. Comparison between registries. Age-standardized rates, in Waterhouse JAH, Muir CS, Correa P, et al (eds): Cancer Incidence in Five Continents, Vol. III (IARC Scientific Publications No. 15), Lyon, International Agency for Research on Cancer, 1976, pp 453–459. [Google Scholar]
- 21.Fritz A. International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 22.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 23.Mpunga T, Znaor A, Uwizeye FR, et al. A case-control study of HIV infection and cancer in the era of antiretroviral therapy in Rwanda. Int J Cancer. 2018;143:1348–1355. doi: 10.1002/ijc.31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein L, Urban MI, O’Connell D, et al. The spectrum of human immunodeficiency virus-associated cancers in a South African black population: Results from a case-control study, 1995-2004. Int J Cancer. 2008;122:2260–2265. doi: 10.1002/ijc.23391. [DOI] [PubMed] [Google Scholar]
- 25.Sitas F, Bezwoda WR, Levin V, et al. Association between human immunodeficiency virus type 1 infection and cancer in the black population of Johannesburg and Soweto, South Africa. Br J Cancer. 1997;75:1704–1707. doi: 10.1038/bjc.1997.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Agency for Research on Cancer . Monographs on the Evaluation of Carcinogenic Risks to Humans: Human Immunodeficiency Viruses and Human T-Cell Lymphotrophic Viruses. Lyon, France: International Agency for Research on Cancer; 1997. [Google Scholar]
- 27.Gaym A, Mashego M, Kharsany AB, et al. High prevalence of abnormal Pap smears among young women co-infected with HIV in rural South Africa: Implications for cervical cancer screening policies in high HIV prevalence populations. S Afr Med J. 2007;97:120–123. [PubMed] [Google Scholar]
- 28.Moodley M. Reduction in prevalence of invasive cervical cancer in KwaZulu-Natal, South Africa: Impact of the human immunodeficiency virus epidemic. Int J Gynecol Cancer. 2006;16:1036–1040. doi: 10.1111/j.1525-1438.2006.00588.x. [DOI] [PubMed] [Google Scholar]
- 29.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens: Part B—Biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 30.Massyn N, Day C, Peer N, et al. District Health Barometer 2013/14. Durban, South Africa: Health Systems Trust; 2014. [Google Scholar]
- 31. Department of Health: Strategic Plan for the Prevention and Control of Non-Communicable Diseases 2013-17. Pretoria, South Africa, Department of Health, 2013, pp 1-80.
- 32.Mingo AM, Panozzo CA, DiAngi YT, et al. Cervical cancer awareness and screening in Botswana. Int J Gynecol Cancer. 2012;22:638–644. doi: 10.1097/IGC.0b013e318249470a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith N, Moodley J, Hoffman M. Challenges to cervical cancer screening in the Western Cape province. S Afr Med J. 2003;93:32–35. [PubMed] [Google Scholar]
- 34.Denny L, Kuhn L, Hu CC, et al. Human papillomavirus-based cervical cancer prevention: Long-term results of a randomized screening trial. J Natl Cancer Inst. 2010;102:1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 35.Botha MH, Richter KL. Cervical cancer prevention in South Africa: HPV vaccination and screening both essential to achieve and maintain a reduction in incidence. S Afr Med J. 2015;105:33–34. doi: 10.7196/samj.9233. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann A, Schneider A. Therapeutic human papillomavirus vaccination. Therapy. 2008;5:339–348. [Google Scholar]