Abstract
A significant hindrance to effective treatment of addiction is identifying those most likely to relapse. Cocaine addiction is characterized by deficits in inhibitory control and elevated reactivity to cocaine cues, both hypothesized to be integral to development of addiction and propensity to relapse. It follows that reduction of both impulsivity and cue-reactivity following abstinence is protective against relapse, and that persistence of these factors increases vulnerability. Using functional magnetic resonance imaging, we examined neural activation patterns in dorsal and ventral striatum in abstinent cocaine dependent (CD) individuals (N = 20) and non-using controls (N = 19) as they performed a cocaine craving task. We also examined activations in nodes of the response inhibition circuit (RIC) as they performed an inhibition task. At the between-groups level, no differences in RIC or striatal activation were seen in former users, in contrast to previous investigations in current users, suggesting large-scale functional recovery with abstinence. However, at the individual participant-level, abstinent CD individuals displayed an association between cocaine cue-related neural activations in the right ventral striatum and compulsive cocaine craving scores. Compulsive craving scores were also negatively correlated with duration of abstinence. Further, there was an association between motor impulsivity scores and inhibition-related activations in the right inferior frontal gyrus and pre-supplementary motor area in abstinent CD individuals. Thus, while former users as a group did not show deficits in inhibitory function or cocaine-cue reactivity, participant-level results pointed to activation patterns in a minority of these individuals that likely contributes to enduring relapse vulnerability.
Keywords: Addiction, Drugs of abuse, Abstinence, fMRI, Recovery, Response inhibition, Impulsivity, Basal ganglia
1. Introduction
One in four people entering treatment for cocaine addiction will still be using on a weekly basis 5 years after treatment (Simpson et al., 2002). However, it is not well understood why the majority of abstainers show protracted resistance to relapse while a significant minority will continue to be at high-risk of recidivism over extended periods of time. A common refrain of treatment providers concerns the profound difficulties they face in identifying those individuals most likely to relapse. Understanding individual differences in recovering cocaine addicts that may contribute to relapse risk is a significant public health issue. Current theories of addiction posit that deficits in inhibitory control allied with increased compulsion to seek and take cocaine play an integral role in the development of addiction and the propensity to relapse (Everitt et al., 2008). Indeed, both the inability to inhibit behaviors with known negative consequences and the compulsion to seek and take drugs are represented in multiple diagnostic criteria for cocaine dependence (American Psychiatric Association, 2000).
Numerous behavioral studies have identified impulse control deficits in cocaine dependent (CD) individuals (Coffey et al., 2003; Fillmore and Rush, 2002; Monterosso et al., 2001; Verdejo-Garcia et al., 2007), but do not provide information on the neurobiological substrates of these deficits. Neuroimaging studies using classic Go/No-Go motor response inhibition tasks have consistently shown hypoactivity in the so-called cortical response inhibition circuit (RIC) when currently using cocaine addicts are compared to non-using controls. This hypoactivity is mainly seen in the right anterior cingulate cortex, right insula, right inferior parietal lobule and right middle frontal gyrus (Garavan et al., 2008; Hester and Garavan, 2004; Kaufman et al., 2003). It is thought that RIC hypo-activity may be related to the weakened impulse control associated with cocaine dependence, and it has been hypothesized that this decreased impulse control is partly responsible for the switch from single to daily drug usage (the acquisition stage) and the switch from controlled to habitual drug intake (Perry and Carroll, 2008). Indeed, Ersche et al. (2012) have shown that siblings of stimulant-dependent individuals also exhibit significantly decreased levels of inhibitory control compared to non-using controls, suggesting that deficits in inhibitory control may in fact be endophenotypic and predispose to substance dependence. Evidence supporting this view also comes from animal models of cocaine addiction which have shown that rats that score lower on measures of impulse control are more likely to escalate their initial drug-taking (Dalley et al., 2007) and ultimately to engage in compulsive drug intake (Belin and Everitt, 2008).
Along with deficits in inhibitory control, the compulsivity associated with drug-seeking is another defining characteristic of severe addiction. This compulsivity can be understood as perseverant actions that are inappropriate to the situation at hand, have no obvious relationship to the overall goals of the individual and which often result in undesirable consequences (Dalley et al., 2011). Everitt and Robbins (2005) forwarded the thesis that drug addiction is comprised of a switch from voluntary drug use to compulsive drug intake. This switch is believed to occur when previously neutral environmental stimuli become associated with drug use, forming conditioned stimuli (drug cues), which can then trigger drug-taking behavior. A proposed neurobiological mechanism for this transition is that striatal reactivity to drug cues switches from a predominantly ventral to a predominantly dorsal activation pattern and that this switch is mediated by dopaminergic innervation (Everitt et al., 2008; Everitt and Robbins, 2005; Pierce and Vanderschuren, 2010). Multiple animal studies have demonstrated that while the ventral striatum is involved with the acquisition of cue-controlled cocaine seeking, the appearance of habit-driven cocaine intake is dependent upon dorsal striatal control (Belin and Everitt, 2008; Ito et al., 2004; Murray et al., 2012; Vanderschuren et al., 2005). However, in humans, current cocaine dependent individuals continue to display ventral striatal activation to cocaine cues, suggesting a continued role for this subcortical region during acute addiction (Kuhn and Gallinat, 2011). Positron emission tomography (PET) studies have revealed increased dopamine levels in the dorsal striatum when human participants viewed cocaine cues (Volkow et al., 2006, 2008; Wong et al., 2006), findings also seen using functional MRI (fMRI) (Garavan et al., 2000). Activations in both the ventral and dorsal striatum have been correlated positively with subjective ratings of craving (Risinger et al., 2005). Further, fMRI studies have implicated dorsal striatal activation as a marker of addiction (Vollstadt-Klein et al., 2010) and relapse (Grusser et al., 2004; Prisciandaro et al., 2013). Therefore, hypoactivation of the RIC during inhibitory control and drug-cue related hyperactivation of the striatum both appear to be important biomarkers of cocaine addiction.
Although both these processes have been relatively well-characterized in current cocaine users, much less is known about how they function after cocaine cessation. In the only functional imaging study of craving in abstinence, that we are aware of, Potenza et al. (2012) utilized imagined situations of drug intake and saw correlations between subjective levels of craving and neural activations in the hippocampus, insula, and anterior and posterior cingulate in a cohort of recently abstinent CD individuals (mean = 22.3 weeks; SD = 3.0). Additionally, they saw increased neural activation in the striatum and multiple cortical and subcortical regions in patients during the drug imagery session when compared to non-using controls. These results suggest that the cue-induced striatal activation patterns seen in current cocaine users persist at least during the first few weeks of abstinence.
In contrast to the relative paucity of investigations looking at the neural correlates of craving in abstinence, a number of studies have assessed the functioning of the RIC during cocaine abstinence. Li et al. (2008) assessed response inhibition in abstinent CD males (n = 15) using a stop-signal task and found decreased activity in the rostral anterior cingulate in the abstinent CD group and theorized that this effect was responsible for inhibitory control deficits in CD individuals. The specific duration of abstinence was not reported in this study (although participants were at least 2 weeks post cessation), so one outstanding question is the length of abstinence that is required for the amelioration of cortical hypoactivity in the RIC. In Connolly et al. (2012), our research group employed a Go/No-Go motor response inhibition task to examine cortical activations in abstinent CD individuals who had attained either shorter (n = 9; average duration = 2.4 weeks) or longer (n = 9; average duration = 69 weeks) periods of abstinence. Both the short- and long-term abstinence groups displayed greater cortical activity than drug naïve controls when performing a successful motor response inhibition in multiple nodes of the canonical RIC. These findings were paralleled by the absence of behavioral differences between the abstinent groups and the non-using controls, a finding suggestive of recovery of inhibitory function through compensatory neural activations. In a recent follow-up fMRI study on response inhibition in a larger abstinent CD cohort, we found no evidence for between-group differences in activation of the RIC and no evidence for a behavioral deficit (Bell et al., 2014). Unlike the earlier Connolly study, hyperactivations of the RIC were not observed. A complementary event-related potential (ERP) study also showed an absence of behavioral and electrophysiological deficits in a separate cohort of abstinent drug abusers (Morie et al., 2014), and again, there was no evidence of hyperactivation. Thus, the finding of normalized inhibitory performance in tandem with an absence of the hypoactivations observed in previous investigations of current cocaine users likely reflects a period of major recovery within the RIC following drug cessation.
However, while it appears that at the group-level, individuals who are receiving treatment for cocaine dependence do not display the cortical hypoactivations in the RIC that have been identified in current CD individuals, it is unclear if there are more specific subject-level differences in RIC activation. Furthermore, it is unknown whether striatal hyperactivation to cocaine cues persists through more extended periods of abstinence and also whether there are subject-level differences in striatal activation to cocaine cues. Because persistent dysfunction within the neural circuitry underlying these two functions is theorized to disproportionately account for continued drug use, we believe that understanding the functioning of these regions after cocaine cessation at the subject-level is crucial to understanding relapse resistance. Indeed, supporting evidence shows that decreases in subjective levels of craving (Da Silveira et al., 2006; Heinz et al., 2006; Paliwal et al., 2008) and impulsivity (Aharonovich et al., 2006; Brewer et al., 2008; Moeller et al., 2001; Schmitz et al., 2009; Streeter et al., 2008; Winhusen et al., 2013) correlate with better drug treatment outcomes. Therefore, although at the group-level, recovering cocaine addicts may not display the neural activation deficits observed in current users, it could be that these deficits persist in certain high-risk individuals.
Here, we conducted a cross-sectional examination of CD individuals at varying durations of abstinence to investigate neural activation associated with inhibition and craving. We employed a multi-dimensional craving questionnaire to measure individual craving levels to the visual presentation of cocaine stimuli and a multi-dimensional impulsivity questionnaire. We hypothesized that a reduction in both subjective levels of craving and impulsivity would be associated with decreased dorsal striatal activation to cocaine cues as well as normalized RIC activation. We also hypothesized that ventral striatal activation in response to cocaine cues would be indicative of continued cocaine craving despite extended cocaine cessation. In line with our previous work, we fully expected that the cocaine abstinent group would not display the typical neural activation deficits observed in current cocaine users (Bell et al., 2011,2014; Morie et al., 2014). However, we postulated that a subset of the abstinent CD individuals would continue to display neural activation deficits related to cocaine-cue reactivity and inhibitory control that are independent of duration of abstinence. We hypothesized that a persistence of these neural activation deficits despite cocaine abstinence would be associated with persistently high craving scores and similarly high impulsivity scores, pointing collectively to an increased relapse-risk profile.
2. Methods
2.1. Participants
Twenty male abstinent CD patients were recruited from in-patient and out-patient addiction treatment centers located in New York City (Bronx County) and 19 male controls were recruited through internet advertisements. All 20 patients received a primary Axis I diagnosis of Cocaine Dependence. Fourteen of the patients were in-patients without access to drugs and alcohol and under constant supervision and mandatory drug-testing. Four of the patients were using out-patient facilities and were legally required to undergo random urine toxicology testing to monitor continued abstinence. Abstinence was also confirmed by a New York State accredited substance abuse counselor with whom the patient met on at least a weekly basis. Two of the patients were under no direct supervision but visited out-patient facilities for group-meetings or support. Individuals did not have an incentive to be dishonest about being abstinent as we were also conducting a study on current cocaine users and therefore possible recruits could enter either study without one study being more beneficial than the other. Patients were abstinent for an average of 44.9 weeks (Minimum = 1.9 weeks, Maximum = 574.2 weeks; SD = 127.1). Exclusion criteria for patients and controls were as follows: 1) Any major psychiatric illness; 2) Head trauma resulting in loss of consciousness for longer than 30 min; 3) Presence of any past or current brain pathology (two patients displayed clinically significant brain pathology and were not included in the cohort of 20 patients); 4) A diagnosis of HIV; 5) The presence of any contraindications to an MRI; 6) Age above 65 years and below 19 years; 7) Presence of WM hyper-intensities (one patient displayed clinically significant WM hyper-intensities and was not included in the cohort of 20 patients). Because of the high rates of comorbidity of alcohol and other drug abuse among the patient population, patients were not excluded if they had abused other drugs or alcohol prior to the onset of their cocaine abstinence (10 individuals had at least comorbid alcohol abuse and 5 individuals had comorbid heroin dependence). However, all 20 patients listed cocaine as their primary drug of choice. None of the patients were currently using any amount of alcohol or drugs. Years of self-reported drug use were recorded during the initial interviews. Controls were excluded if they had any history of drug or alcohol dependence and/or history of psychiatric illness. The study received Institutional Review Board approval at the Albert Einstein College of Medicine. All participants were screened for contraindications for MRI and signed an informed consent document administered by HIPAA-certified staff. Participants received a gift card worth $60 for successful completion of the experimental protocol. The study conformed to the principles outlined in the Declaration of Helsinki.
The comparison sample consisted of 19 controls (see Table 1). The patients and controls did not differ in age (47.8 ± 8.5, 42.2 ± 12.1, respectively, t(37) = 1.7, p = 0.10), but patients had fewer years of education (12.1 ± 1.9, 13.7 ± 1.9, respectively, t(37) = −2.66; p = 0.01).
Table 1.
Behavioral data.
| All patients versus All controls
| |||
|---|---|---|---|
| Demographics | Group
|
p | |
| CD (N = 20) | Controls (N = 19) | ||
| Age (years) | 47.8 (8.5) | 42.2 (12.1) | 0.10 |
| Years of education | 12.1 (1.9) | 13.7 (1.9) | 0.01a |
| WRAT | 30.2 (5.0) | 38.4 (5.6) | 0.000a |
| Race (Black/Hispanic/White) | 9/8/3 | 8/4/7 | 0.23b |
|
| |||
| Cocaine cue task | CD (N = 20) | Controls (N = 19) | |
|
| |||
| CCQ-Total | 119.9 (41.3) | 78.6 (24.3) | 0.001a |
| CCQ-F1 | 1.8 (0.9) | 1.2 (0.4) | 0.007a |
| CCQ-F2 | 2.7 (1.5) | 1.4 (0.8) | 0.003a |
| CCQ-F3 | 3.6 (1.5) | 1.8 (0.7) | 0.000a |
| CCQ-F4 | 2.8 (1.1) | 2.2 (0.9) | 0.09d |
|
| |||
| Response inhibition task | CD (N = 19) | Controls (N = 18) | |
|
| |||
| % Correct STOPS | 0.7 (0.2) | 0.8 (0.1) | 0.09 |
| Total STOPS | 42.3 (13.0) | 48.8 (13.1) | 0.14 |
| Total ERRORS | 19.1 (10.9) | 13.6 (9.8) | 0.11 |
| HITS RTc | 439.7 (54.9) | 399.1 (69.8) | 0.06 |
| ERRORS RTc | 366.9 (91.5) | 319.5 (97.3) | 0.14 |
| BRI-Total | 63.5 (13.4) | 53.9 (7.7) | 0.01a |
| BRI-Attention | 15.2 (4.7) | 12.6 (2.7) | 0.05a |
| BRI-Motor | 22.8 (5.5) | 20 (3.5) | 0.08d |
| BRI-NP | 25.5 (6.8) | 21.3 (4.1) | 0.03a |
Note:
Significant between patients and controls.
Pearson’s chi-square significance level.
RT = reaction time.
Significant at p ≤ 0.03 after exclusion of outlier control value.
2.2. Stimuli and tasks
2.2.1. Craving measurement
To measure the level of craving in response to drug cues, we used a subjective, self-report measure called the Cocaine Craving Questionnaire-Now (CCQ-N). The CCQ-N is a 45-question multifactorial self-report instrument that measures various aspects of craving in the present tense (Tiffany et al., 1993). The CCQ-N breaks down into four first-order factors that have been described as measuring the following; Factor 1 = Desire, Factor 2 = Lack of Self-Efficacy, Factor 3 = Compulsivity, Factor 4 = Relief (Heinz et al., 2006). Scores from the CCQ-N have been correlated with neurophysiological processes that may underlie compulsive cocaine craving. For example, it has been demonstrated that higher craving levels on the CCQ-N are correlated with increased dopamine levels (Volkow et al., 2006, 2008) and increased brain glucose metabolism (Volkow et al., 2010) in the dorsal striatum.
The CCQ-N was administered to all patients and controls in a private interview room before they performed any tasks in the scanner. The participants viewed 112 cocaine-related images on a computer monitor at least 24 h before completing the fMRI portion of the experiment to protect against habituation effects. The average time that elapsed between the two viewings was 8.4 days for all participants (Minimum = 1 day, Maximum = 86 days; SD = 14.9 days). The two groups did not differ significantly in the time between viewings. The cocaine images consisted of individuals engaged in cocaine use, paraphernalia for cocaine administration and pictures of cocaine itself. These images were collected from an extensive internet search and reflected normal and idealized interpretations of everyday cocaine use that would be experienced by the typical user. That is, these pictures did not depict a harsh environment containing negative imagery such as violence or unhealthy looking individuals or paraphernalia that might be seen in typical anti-drug advertisements. Each image was presented for a duration of 1800 ms and was separated by a blank screen presented for 200 ms. The total task time was 3.7 min. To ensure that participants were attending to the task, stimuli had a 12% chance of repeating for each block. Participants were instructed to press the spacebar only when they saw a stimulus repeat. As soon as all of the pictures were presented, participants were then instructed to fill out the CCQ-N. These same pictures were used in the subsequent fMRI experiment to assess the neural correlates of cocaine-cue reactivity.
2.2.2. Impulsivity measurement
Impulsivity was assessed through the self-administration of the Barratt Impulsiveness Scale (BIS-11) (Patton et al., 1995). This measure consists of 30 questions that have been used in multiple studies to assess personality traits associated with impulsivity in CD individuals (Coffey et al., 2003; Ersche et al., 2011; Moeller et al., 2005). There are three second-order factors in the BIS-11 consisting of Attentional impulsiveness, Motor impulsiveness and Non-planning impulsiveness (Patton et al., 1995). The BIS-11 has been correlated with cortical thickness (Schilling et al., 2012), structural integrity (Moeller et al., 2005) and neural functioning (Wittmann et al., 2011) of RIC regions and is therefore capable of capturing the neurophysiological characteristics of impulsivity. Participants filled out the BIS-11 after the successful completion of the MRI scanning session.
2.2.3. Cocaine cue task
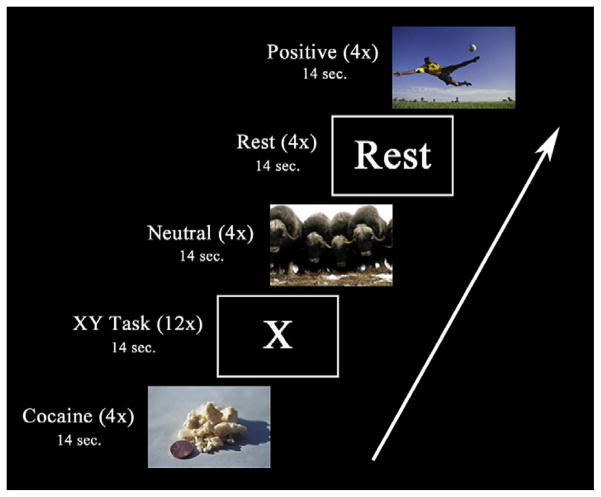
To invoke cocaine craving while in the MRI scanner, participants were shown the same cocaine-related pictures as described above. Stimuli were presented via VisuaStim digital goggles (Resonance Technology, Northridge, CA). A block design was utilized for this cocaine cue task with alternating blocks consisting of cocaine cues, positive images, neutral images and rest periods. The neutral images were taken from the International Affective Picture System (IAPS) (Lang et al., 2008) and were similar in complexity and color to the cocaine cue pictures. The IAPS is a large set of standardized photographs that are rated with regard to their tendency to evoke an emotional response in the viewer. A majority of the positive images were also obtained from the IAPS. However, due to the limited number of positive images in the IAPS set that were rated by men as high in both positive valence and arousal, we obtained additional images from outside the IAPS consisting of similar imagery. Positive images are included in the study design as a control for the cocaine images which are rated by CD men as high in positive valence and arousal (Moeller et al., 2009). Neither the neutral nor positive pictures had any images that could be construed to be related to drugs or drug use (e.g. there were no pictures containing cups which could be interpreted as containing alcohol). There were four separate runs for this task. Each run consisted of 12 stimuli blocks, 4 REST blocks and 12 distractor task blocks presented in a pseudorandom order (see Fig. 1). The stimulus blocks were comprised of four blocks each of cocaine cues and positive and neutrally valenced pictures. Each of the blocks contained seven distinct pictures for a total of 112 distinct pictures for each cue category. Each picture was presented for 1800 ms and followed by a blank screen presented for 200 ms. Pictures were preceded by a screen stating “Picture Task” for 2000 ms for the purpose of informing the participant that a cue category was about to begin. Total block length was 16 s. The order of category blocks and all of the pictures within the blocks were pseudo-randomized within each run. To ensure that participants were attending to the task, pictures had a 12% chance of repeating within each block presentation. Participants were instructed to press a button only when they saw a picture repeat. After each presentation of a cocaine, positive or neutral stimuli block, the participants completed either a Go/No-Go distracter task called the “XY” task (Fassbender et al., 2009; Hester et al., 2004), or encountered a REST block. The XY task consisted of a serial stream of alternating presentations of the letters X and Y. The stimuli were 5.08 and 6.35 cm in height for X and Y, respectively. Each letter presentation was 600 ms long and was separated by a blank screen presented for 400 ms. The participants were instructed to press a response button for each letter presentation but were to withhold their response if there was a back-to-back repeat presentation of a letter. There was a 12% chance of any of the letters repeating for each presentation of the XY task and the total task length was 14 s. The distracter task was meant to disrupt cognitive processes related to the preceding block of stimuli and help return participants to a neutral baseline prior to the next set of stimuli. Finally, there were four REST blocks presented pseudorandomly throughout each run that were 14 s in duration. These blocks consisted of the word “Rest” in white letters against a black background. REST blocks were presented so that they were not the first or last block shown to the participant. The total duration of the task was 29.44 min.
Fig. 1.
An example sequence of blocks during one run of the cocaine cue task. There were 12 pictorial stimulation blocks (4 each of cocaine, positive and neutral pictures), 12 XY Task blocks and 4 “Rest” blocks in a given run. Pictorial stimulation blocks were always followed by either an XY Task block or a “Rest” block. There were four such runs in total.
2.2.4. Response inhibition task
All participants also completed a Go/No-Go motor response inhibition task that consisted of a series of pictures depicting neutral scenes from the IAPS (Bell et al., 2014). From this set, 158 new, neutral pictures were chosen with a mean emotional valence and arousal of 5.2 and 3.5 respectively, on a scale from 1 to 9 based on normative ratings from the IAPS dataset (Lang et al., 2008). All stimuli subtended 8.6° horizontally × 6.5° vertically of visual angle. Stimuli were presented for 800 ms and were separated by a blank screen presented for 200 ms. Participants were instructed to quickly press a button at the onset of each stimulus (Go trials) and to withhold a response in instances when a stimulus was repeated (No-Go trials). Stimuli were presented pseudorandomly in three blocks with each block containing 180 trials. Within each block, 22 trials (12%) were No-Go trials. The high proportion of Go trials renders the quick button press to the occurrence of a stimulus to be the prepotent response. The withholding of a button press to stimulus repetition proves quite difficult for most individuals and therefore requires the recruitment of inhibitory control mechanisms.
2.3. Image acquisition
MRI scans were performed in a 3.0T Philips Achieva Quasar TX (Netherlands) at the Gruss Magnetic Resonance Research Center (MRRC) at the Albert Einstein College of Medicine. Functional scans for the cocaine cue task were acquired in four runs of 219 volumes utilizing a T2-weighted echo-planar sequence (TR/TE = 2000/20 ms, flip angle = 90°, 3 mm slice thickness, 240 mm FOV, 80 × 80 matrix, pixel size = 3.0 × 3.0 mm2, no gap). Forty-three axial slices were obtained parallel to the AC-PC plane. Functional scans for the inhibitory control task were acquired in three runs of 101 volumes utilizing a T2-weighted echo-planar sequence (TR/TE = 2000/20 ms, flip angle = 90°, 3 mm slice thickness, 240 mm FOV, 80 × 80 matrix, pixel size = 3.0 × 3.0 mm2, no gap). Forty-four axial slices were obtained parallel to the AC-PC plane. Structural images were acquired utilizing a T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) (TR/TE = 8.3/3.8 ms, flip angle = 8°, 220 slices, 1 mm slice thickness, 240 mm FOV, 240 × 187 matrix, voxel size = 1.0 × 1.0 mm2, no gap).
2.4. Image processing
The functional and anatomical data for both experiments were pre-processed and analyzed using Brain Voyager (QX 2.4, Maastricht, The Netherlands) running in the Windows XP environment. Functional scans were pre-processed by performing a 3D motion correction. Functional scans were excluded on a run-by-run basis if they displayed >3 mm of motion in a given plane. The T1-weighted anatomical slices were normalized into Talairach space and co-registered with the functional timecourses. The resulting volumetric time courses were then spatially smoothed using an 8 mm full-width at half-maximum (FWHM) Gaussian kernel.
2.5. Analysis strategy
2.5.1. Cocaine cue task
In this block design, cocaine (COC), positive (POS), neutral (NEU) and XY task blocks served as regressors of interest. The REST blocks were not modeled and served as the baseline measure. These regressors were convolved with a two-gamma-variate hemodynamic response function and subjected to a first-level analysis using a random effects general linear model (GLM).
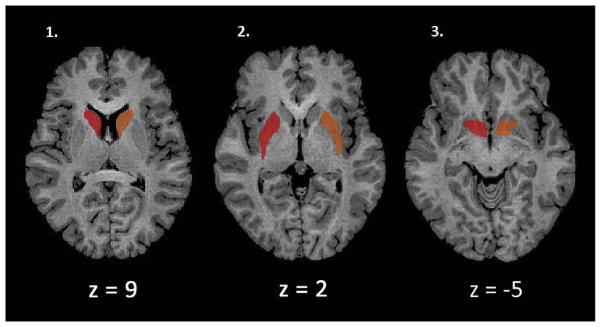
Because it has been hypothesized that an interaction between the dorsal and ventral striatum are responsible for cocaine dependence (Everitt et al., 2008; Everitt and Robbins, 2005; Pierce and Vanderschuren, 2010), we created six regions of interest (ROIs) consisting of the caudate, putamen and ventral striatum specific to either the left or right hemisphere that were independent of our dependent measures or group membership (See Fig. 2). The anatomically defined ROIs were created in BrainVoyager by using the “Draw with mouse” option on a standardized brain in Talairach space using a brain atlas for reference (Haines, 2007). The ventral striatum was defined as being inferior to the internal capsule and anterior to the anterior commissure while the dorsal striatum consisted of the remaining caudate and putamen (MacDonald et al., 2011).
Fig. 2.
The six ROIs utilized for the cocaine craving task are shown. Regions shown in red are right lateralized while regions in orange are left lateralized. 1) Left and right caudate 2) Left and right putamen and 3) Left and right ventral striatum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To examine if there were any differences in cocaine-cue related activation between patients and controls in the dorsal and ventral striatum, the activation for each ROI for each participant was calculated and compared with a Group (Patients versus Controls) × ROI (Left Caudate, Right Caudate, Left Putamen, Right Putamen, Left Ventral Striatum and Right Ventral Striatum) multivariate analysis of variance (MANOVA). This analysis was performed within IBM SPSS Statistics Version 20 (Armonk, NY) and utilized a contrast of COC > NEU.
To identify cocaine-cue related activation differences between patients and controls in any other regions of the brain, we conducted a whole-brain voxelwise between groups analysis (False Discovery Rate (FDR); q = 0.05) utilizing a t-test contrast of COC > NEU activation and a cluster threshold of at least four contiguous voxels.
A linear regression was utilized to investigate whether ROI activation in response to cocaine-cues was predictive of CCQ-N scores in patients. For this analysis, we extracted the mean beta-weights for each of the ROIs from each individual. Our activation measure was the contrast of COC > NEU. These values were entered as the dependent variable in a linear regression with CCQ-N Factor 1 (Desire), CCQ-N Factor 2 (Lack of Self-Efficacy), CCQ-N Factor 3 (Compulsivity), CCQ-N Factor 4 (Relief) (Heinz et al., 2006) and duration of abstinence as our independent variables. These independent variables were tested for multicollinearity and found to be acceptable as they all had tolerance levels well below 0.1.
This regression was conducted for each of the six basal ganglia ROIs in all 20 patients. Additionally, for any ROIs found to be a significant predictor of craving scores, we then conducted another linear regression utilizing scores from a contrast of POS > NEU as the dependent variable and included all of the same independent variables. This was conducted to determine if the results were specific to cocaine-related stimuli and not just a response to stimuli that are generally positively valenced and arousing.
2.5.2. Response inhibition task
In this event-related design, successful responses to Go trials (HITS), successful inhibitions to No-Go trials (STOPS) and unsuccessful inhibitions (ERRORS) served as regressors of interest. These regressors were convolved with a two-gamma-variate hemodynamic response function and subjected to a first-level analysis using a random effects general linear model (GLM). One control and one patient were dropped from the analysis because they did not complete the task properly.
The ROIs for this task were obtained from a previous examination of response inhibition in 27 abstinent CD individuals and 45 non-using controls utilizing the same Go/No-Go task (Bell et al., 2014). The ROIs were identified within a task-defined map based on a contrast of STOPS > Baseline. This procedure first involved identifying centers of mass based upon peak voxel activations and then confirming that the centers of mass fell within the canonical RIC. A review of the imaging literature on response inhibition processes utilizing similar motor response inhibition tasks identified the right middle and inferior frontal gyri, right inferior parietal lobule, bilateral insula and the midline cingulate and pre-SMA as canonical nodes of the RIC (Chen et al., 2009; Chevrier et al., 2007; Dodds et al., 2011; Fassbender et al., 2009, 2004; Garavan et al., 2006, 2008, 1999; Hampshire et al., 2010; Hester and Garavan, 2004; Kaufman et al., 2003; Konishi et al., 1999; Leung and Cai, 2007; Li et al., 2006; Xue et al., 2008). The peak activations within each of these regions were identified and then served as the centers of 13 mm3 (2197 voxels) cubic ROIs.
To examine if there were any differences in cortical activation between patients and controls in the RIC, the activation for each ROI for each participant was calculated and compared with a Group (Patients versus Controls) × ROI (Left Insula, Right Insula, Right Inferior Frontal Gyrus, Right Inferior Frontal Gyrus/Middle Frontal Gyrus, Right Inferior Parietal Lobule, Pre-Supplementary Motor Area/Cingulate, and Left Precentral Gyrus) MANOVA. This analysis was performed within IBM SPSS Statistics Version 20 (Armonk, NY) and utilized a contrast of STOPS > HITS.
Next, we conducted a whole-brain voxelwise comparison between patients and controls to investigate whether there were any activation differences between the two groups in any other regions of the brain. A voxelwise t-test was performed between groups (False Discovery Rate (FDR); q = 0.05) utilizing a t-test contrast of STOPS > HITS and a cluster threshold of at least four contiguous voxels.
A linear regression investigated whether BIS-11 factor scores were predictive of RIC activations in patients. For this analysis, we extracted the mean beta-weights for each of the ROIs based on the contrast of STOPS > HITS. These values were then entered as the dependent variables in separate linear regressions with BIS-11 Attentional impulsiveness, BIS-11 Motor impulsiveness, BIS-11 Non-planning impulsiveness, duration of abstinence and total number of STOPS as independent variables (Patton et al., 1995). These independent variables were tested for multi-collinearity and found to be acceptable as they all had tolerance levels well below 0.1. This regression was conducted for each of the seven ROIs. If impulsivity scores were found to be a significant predictor of ROI activation in the patient regression we then tested for a similar effect with similar regressors, excluding duration of abstinence, in the controls.
3. Results
3.1. Behavioral results
3.1.1. Craving scores (CCQ-N)
Patients had higher scores than controls on the Total Score (119.95 ± 41.3, 78.6 ± 24.3, respectively, t(37) = 3.8; p ≤ 0.001), Factor 1 (Desire) (1.8 ± 0.91, 1.2 ± 0.37, respectively, t(37) = 2.9; p ≤ 0.007), Factor 2 (Lack of Self-Efficacy) (2.7 ± 1.5, 1.4 ± 0.80, respectively, t(37) = 3.2; p ≤ 0.003), and Factor 3 (Compulsivity) (3.6 ± 1.5,1.8 ± 0.68, respectively, t(37) = 4.8; p ≤ 0.001) of the CCQ-N. Patients did not differ from controls on Factor 4 (Relief) of the CCQ-N (2.8 ± 1.1, 2.2 ± 0.92, respectively, t(37) = 1.7; p ≤ 0.09) although there was a trend towards significance (see Table 1.). However, the exclusion of a control value that was an outlier (>2.5 SD) resulted in a significant difference between patients and controls (2.8 ± 1.1, 2.1 ± 0.7, respectively, t(36) = 2.3; p ≤ 0.03). Higher scores on Factor 3 (Compulsivity) of the CCQ-N were negatively correlated with longer periods of cocaine abstinence (r = −0.48, p ≤ 0.03).
3.1.2. Impulsivity scores (BIS-11)
Patients had higher scores than controls on the Total Score of the BIS-11 (63.5 ± 13.4 versus 53.9 ± 7.7, t(35) = 2.6; p ≤ 0.01) and in terms of the Attentional impulsiveness (15.2 ± 4.7 versus 12.6 ± 2.7, t(35) = 2.0; p ≤ 0.05) and Non-planning impulsiveness (25.5 ± 6.8 versus 21.3 ± 4.1, t(35) = 2.3; p ≤ 0.03) factors of the BIS-11. Patients did not differ from controls on the Motor impulsiveness factor of the BIS-11 (22.8 ± 5.5 versus 20 ± 3.5, t(35) = 1.8; p ≤ 0.08) although there was a trend towards significance (see Table 1.). However, the exclusion of a control value that was an outlier (>2.5 SD) resulted in a significant difference between patients and controls (22.8 ± 5.5 versus 19.5 ± 2.8, t(34) = 2.2; p ≤ 0.03).
3.1.3. Response inhibition task
Patients did not differ from controls in the proportion of correct STOPS (0.69 ± 0.15 versus 0.78 ± 0.14, t(35) = −1.72; p ≤ 0.09), total number of STOPS (42.3 ± 13.0 versus 48.8 ± 13.1, t(35) = −1.51; p ≤ 0.14) or total number of ERRORS committed (19.1 ± 10.9 versus 13.6 ± 9.8, t(35) = 1.62; p ≤ 0.11). Patients also did not differ significantly from controls in reaction time for HITS (439.7 ± 54.9 versus 399.1 ± 69.8, t(35) = 1.97; p ≤ 0.06) or ERRORS (366.9 ± 91.5 versus 319.5 ± 97.3, t(35) = 1.52; p ≤ 0.14) (see Table 1).
3.2. Neuroimaging results
3.2.1. Abstinent patients versus controls
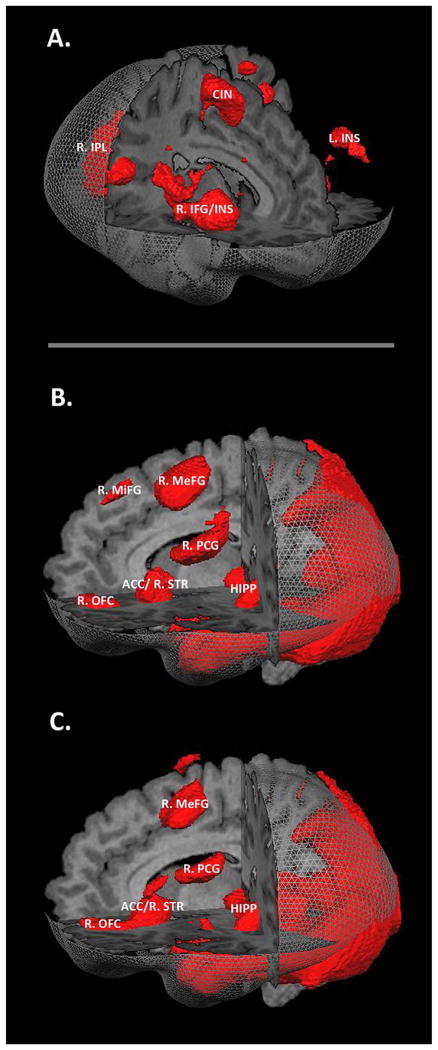
All participants, regardless of diagnosis, displayed robust neural activations when viewing COC and POS stimuli relative to the rest period (see Fig. 3B and C). These activations provide evidence that the evocative visual stimuli utilized in the experiment produced substantial neural activation patterns across all participants. In the ROI analysis for cocaine cue reactivity, abstinent patients did not differ from controls within any of the six pre-defined basal ganglia ROIs utilizing a threshold of p < 0.05 and a contrast of COC > NEU. There was no difference in the main effect of Group, F(1,37) = 0.68, p ≤ 0.67. In the whole-brain analysis for cocaine cue reactivity, no regions differed between patients and controls at a threshold of p < 0.05, corrected for multiple comparisons using the FDR method utilizing a contrast of COC > NEU.
Fig. 3.
Neural activations associated with the two tasks. A. Regions activated for all participants when performing the inhibitory control task utilizing a t-test contrast of STOPS > HITS (FDR corrected; q = 0.05). B. Regions activated for all participants when viewing COC stimuli utilizing a t-test contrast of COC > Baseline (FDR corrected; q = 0.01). C. Regions activated for all participants when viewing POS stimuli utilizing a t-test contrast of POS > Baseline (FDR corrected; q = 0.01). Abbreviations: R.IPL = right inferior parietal lobule, CIN = cingulate gyrus, L.INS = left insula, R.INS = right insula, R.IFG = right inferior frontal gyrus, R.MeFG = right medial frontal gyrus, R.MiFG = right middle frontal gyrus, R.PCG = right postcentral gyrus, HIPP = hippocampal formation, ACC = anterior cingulate cortex, R.STR = right striatum, R.OFG = right orbitofrontal gyrus.
We saw robust activation of the RIC circuit utilizing our inhibitory control task (see Fig. 3A). In the ROI analysis for inhibitory control, abstinent patients did not differ from controls within any of the seven pre-defined ROIs utilizing a threshold of p < 0.05 and a contrast of STOPS > HITS. There was no difference in the main effect of Group, F(1,35) = 1.09, p ≤ 0.40. In the whole-brain analysis for inhibitory control, no regions differed between patients and controls at a threshold of p < 0.05, corrected for multiple comparisons using the FDR method and utilizing a contrast of STOPS > HITS.
3.2.2. Abstinent patients
To examine if activations within the dorsal and ventral striatum were associated with cocaine craving or duration of abstinence, linear regressions were conducted utilizing the beta-weights from each of the pre-defined ROIs as the dependent variables. We saw that increased activation of the right ventral striatum when viewing cocaine stimuli was significantly associated with increased Compulsivity scores of the CCQ-N (β = 0.80, p = 0.01) (see Fig. 4). Utilizing a contrast of POS > NEU, patient’s right ventral striatum activation was not associated with Compulsivity scores (β = 0.46, p = 0.26). Duration of abstinence was not a significant predictor of striatal activation.
Fig. 4.
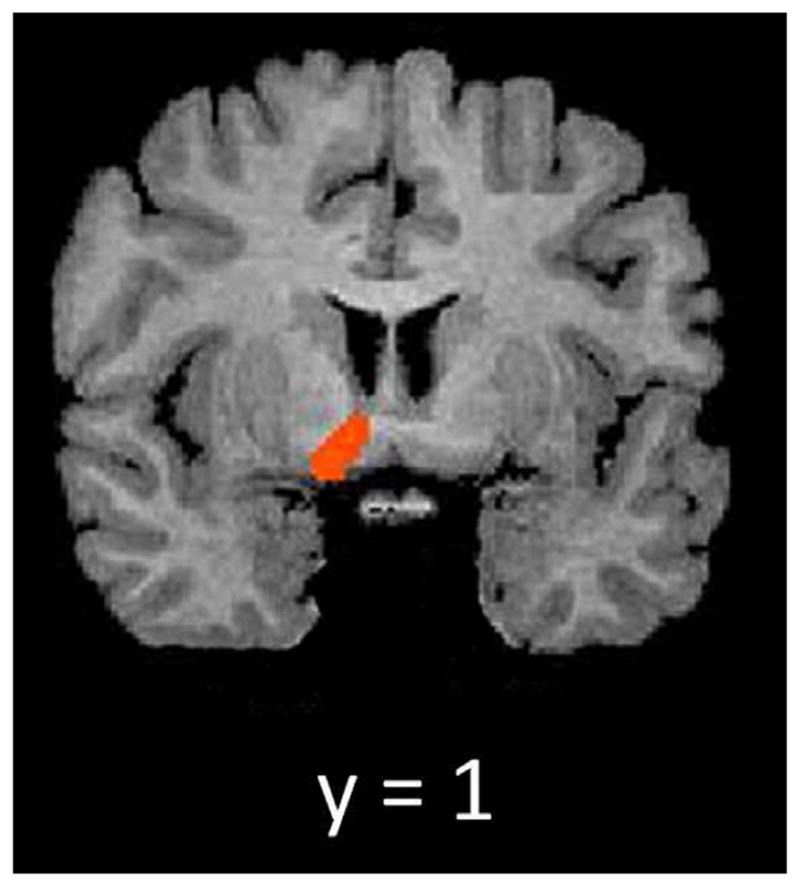
Activation map showing right ventral striatum activation when utilizing a t-test contrast of COC > NEU in the five abstinent CD individuals who had the highest scores on the compulsivity factor of the CCQ-N (FDR corrected; q = 0.05; mask restricted to ventral striatum).
We also examined whether scores on the BIS-11 were predictive of RIC activation during correct inhibitions. We found that increased activation of the right inferior frontal gyrus (rIFG) when inhibiting a response was predictive of decreased scores on the Attentional impulsiveness factor of the BIS-11 (β = −0.91, p = 0.01) and increased scores on the Motor impulsiveness factor of the BIS-11 (β = 0.81, p = 0.02) in abstinent patients (see Table 2). Controls did not exhibit the same relationship between rIFG activation and BIS-11 Attentional (β = 0.42, p = 0.12) or Motor impulsiveness scores (β = −0.01, p = 0.99). We also found that for patients, increased activation of the pre-supplementary motor area (pre-SMA) when inhibiting a response was predictive of decreased scores on the Attentional impulsiveness factor (β = −0.73, p = 0.02), increased scores on the Motor impulsiveness factor (β = 0.64, p = 0.03), and increased scores on the Non-planning impulsiveness factor (β = 0.65, p = 0.008) of the BIS-11 (see Table 2). Controls did not show a relationship between pre-SMA activation and scores on the Attentional (β = 0.89, p = 0.68) or the Non-planning impulsiveness factors of the BIS-11 (β = −0.42, p = 0.07). Controls did show a relationship between pre-SMA activation and scores on the Motor factor (β = 0.52, p = 0.03) of the BIS-11, but the results were driven by an outlier whose exclusion resulted in a non-significant outcome (β = 0.19, p = 0.45). Duration of abstinence was not a significant predictor of RIC activation.
Table 2.
Neuroimaging data.
| All patients
| |||
|---|---|---|---|
| Anatomical region | Predictor | β | p |
| 1. R. ventral striatum | CCQ-N F3 | 0.80 | 0.01 |
| 2. R.IFG | BIS-11 Att. | 30.91 | 0.01 |
| BIS-11 Motor | 0.81 | 0.02 | |
| 3. L. pre-SMA | BIS-11 Att. | 30.73 | 0.02 |
| BIS-11 Motor | 0.64 | 0.03 | |
| BIS-11 NP | 0.65 | 0.008 | |
4. Discussion
Many studies have provided evidence that decreased RIC activation and increased striatal activation to drug cues are both neuromarkers of cocaine dependence. This project sought to explore whether abstinent cocaine dependent individuals would show individual differences in neural activations associated with cocaine craving and impulsivity. The first point of note is that this investigation replicated our previous findings in a completely new cohort of abstinent CD participants, showing apparent recovery of inhibitory control processes (Bell et al., 2014), and it builds upon a series of recent studies that point to substantial recovery of functional and structural deficits associated with chronic prior cocaine dependence following relatively modest periods of abstinence (Bell et al., 2011; Connolly et al., 2013; Morie et al., 2014). Naturally, these findings are quite encouraging, suggesting that abstinence results in a relatively rapid recovery of function in most former addicts and they may go some way towards explaining why a fairly large majority of former addicts actually maintain abstinence after five years (see Simpson et al., 2002). Nonetheless, while significant differences in neural activation patterns between former addicts and controls were not evident at the group-level, as anticipated, individual participant-level differences were observable and these were exclusive to the abstinent CD cohort. Increased activation of the ventral striatum in response to the viewing of evocative cocaine cues was positively associated with “compulsivity” scores on the CCQ-N. Additionally, activation of the right IFG during successful response inhibitions was negatively associated with “attentional impulsiveness” scores and positively associated with “motor impulsiveness” scores on the BIS-11. Finally, we found that pre-SMA activation when inhibiting a response was negatively associated with “attentional impulsiveness” scores and positively associated with both “motor impulsiveness” and “non-planning impulsiveness” scores of the BIS-11. Since increased craving in response to drug cues (Da Silveira et al., 2006; Heinz et al., 2006; Paliwal et al., 2008) and increased impulsivity (Aharonovich et al., 2006; Moeller et al., 2001) are both behaviors indicative of cocaine dependence and relapse potential, we postulate that activations in these regions could well prove to be useful biomarkers of increased relapse risk. Obviously, it will require prospective longitudinal studies to firmly establish this.
It is theorized that instrumental behaviors are controlled by distinct systems referred to as the action–outcome (A–O) and the stimulus–response (S–R) systems respectively. The A–O system refers to behaviors that are conducted with outcome-expectancy, that is, behavior based upon an awareness of the outcome. In contrast, the S–R system refers to behaviors initiated by environmental stimuli with little regard for the outcome, or consequences thereof. Simply put, S–R behavior refers to actions commonly thought of as habits. The dorsal striatum is hypothesized to play a major role in habit formation and animal studies have clearly shown its contribution to S–R behavior. For example, Yin et al. (2006) showed that rats with a dorsolateral striatum lesion did not develop S–R behaviors when engaged in a lever-pressing task to acquire sucrose rewards, and instead continued to engage in A–O behavior, whereas rats with an intact dorsolateral striatum did go on to develop S–R behavioral patterns. Thorn et al. (2010) further elucidated the role of this region by showing that activity in dorsolateral striatal neurons in rats was positively correlated with increased habitual learning on a T-maze task. In humans, Tricomi et al. (2009) utilized a free-operant task that was initially rewarded on a variable-interval 10-s schedule and then devalued, showing that greater right putamen activation was associated with the appearance of habitual behavior (responding after devaluation). It should be noted that dorsolateral striatum is the hypothesized homolog of human putamen (Balleine and O’Doherty, 2010). Dorsal striatal activation has been observed in multiple human neuro-imaging studies of cue-reactivity (Prisciandaro et al., 2013; Volkow et al., 2006, 2008; Wong et al., 2006). The absence of dorsal striatum activation associated with cocaine craving in our sample may be indicative of a recovery of function where cocaine-cues have a reduced power to initiate habitual drug-seeking and drug-taking.
Right ventral striatum activation was identified as being positively associated with increased scores on the compulsivity factor of the CCQ-N. As above, it has been hypothesized that the dorsal striatum is responsible for habitual, compulsive cocaine-seeking and taking. On the other hand, animal studies have implicated the ventral striatum in the early stages of cocaine use where the drug is taken according to A–O expectations and not in a compulsive, habitual manner (Everitt and Robbins, 2013). However, a neuroimaging meta-analysis of drug-cue reactivity in drug-dependent humans identified right ventral striatal activation as a core region of cue reactivity to cocaine, as well as alcohol and nicotine cues (Kuhn and Gallinat, 2011). It is possible that while ventral striatal activation is indicative of increased craving, it is not responsible for cue-induced habitual drug-seeking and drug-taking. It could be that ventral striatal reactivity to cocaine-cues is indicative of individuals that are recovering from their cocaine addiction and are not as susceptible to cocaine craving as it does not induce automatic cocaine-seeking and taking behavior. However, ventral striatum activation could still be considered a risk factor for relapse as it indicates heightened cocaine-cue reactivity reflecting an increased desire for the drug. It may be the case that in the current cohort, we have a mixed sample that is in various stages of recovery with persistent ventral striatal activation to cocaine cues indicating greater relapse risk. It should be noted, however, that neither ventral nor dorsal striatal activations were associated with duration of abstinence.
It is worth pointing out that only Factor 3 (compulsivity) of the CCQ-N was associated with increased right ventral striatum activation. Some of the questions related to the factor of compulsivity on the CCQ-N are instructive here, asking for instance, whether the individual, if offered cocaine at that moment, “could control how much they use”, “turn down cocaine this minute”, or “stop [themselves] from using cocaine”. Rather unsurprisingly perhaps, a study of treatment-seeking CD individuals showed that lower scores on this factor were associated with longer retention periods in drug-treatment (Heinz et al., 2006), and it seems reasonable to suppose that higher scores on this compulsivity factor would be associated with relapse risk. Certainly, the questions nicely capture the compulsive aspects of cocaine dependence and so it is of significant interest to find here that lower scores on this factor were correlated with increased duration of abstinence. Therefore, ventral striatum activation being associated with increased scores on this particular factor lends credence to utilizing activation in this region in response to cocaine cues as a possible biomarker of relapse risk.
Our second significant finding is that for patients, increased rIFG activation was associated with decreased scores on the “attention impulsiveness” factor of the BIS-11 and increased scores on the “motor impulsiveness” factor of the BIS-11. The rIFG has been implicated in multiple studies examining inhibitory control (Fassbender et al., 2004; Garavan et al., 1999; Hester and Garavan, 2004; Hester et al., 2004; Simmonds et al., 2008). This region forms part of the fronto-parietal control network, responsible for initiating and adjusting control based on the circumstances of the situation (Dosenbach et al., 2008; Kelly et al., 2004). It follows that individuals who exhibit deficits in the functioning of this control system will have a more difficult time recognizing what needs to be inhibited and adjusting their behavior accordingly.
Our third significant finding is that for patients, increased pre-SMA activation was associated with decreased scores on the “attention impulsiveness” factor of the BIS-11, and increased scores on both the “motor impulsiveness” and “non-planning impulsiveness” factor of the BIS-11. This region has been identified in two separate meta-analyses of response inhibition experiments as being integral to inhibitory control (Simmonds et al., 2008; Swick et al., 2011). Furthermore, it has been shown that individuals with infarcts to the pre-SMA were specifically impaired in inhibiting responses (Picton et al., 2007). Therefore, neural activations in this region when performing an inhibitory control task can reasonably be assumed as essential to the inhibition of behavior.
The “motor impulsiveness” factor on the BIS-11 has been described as measuring the amount of spontaneous actions that an individual undertakes (Ersche et al., 2011) with higher scores indicating greater impulsiveness. Examples of questions on this subscale are, “I do things without thinking”, and “I act on the spur of the moment”. Of interest is that this factor has been negatively correlated with treatment dropout in a cohort of abstinent stimulant dependent users (Winhusen et al., 2013) and is indicative of more severe cocaine use (Moeller et al., 2001). Our results show that higher motor impulsivity is associated with increased neural activation within two critical nodes of the RIC, a finding that runs counter to our initial hypothesis where we predicted that higher impulsivity would be associated with reduced activation in nodes of the RIC, which would be consistent with the notion of unremitting chronic deficits in this circuit following abstinence in a minority of high risk individuals. How then to explain this rather counterintuitive finding? One reasonable proposition is that individuals who still suffer from strong impulsive tendencies are simply making greater efforts to engage inhibitory mechanisms since they are actively pursuing recovery, a matter of increased motivation. It is of note that we have previously observed hyper-activation in the RIC in a cohort of both short- and long-term abstinent cocaine users (Connolly et al., 2012), although in that study we did not relate RIC activations to impulsivity measures. Clearly, the direction of this association reduces the power of utilizing RIC activations alone to distinguish relapse risk since RIC activation is in fact “normalizing” with cocaine cessation and treatment services in most individuals. Therefore, increased RIC activation would have to be combined with increased motor impulsivity scores, along with ventral striatum activation to cocaine cues, to provide effective information regarding risk of relapse.
The aim of this paper was to identify whether abstinent cocaine dependent individuals show individual differences in neural activations related to cocaine craving and impulsivity. We found three significant regions where neural activations were predictive of both higher cocaine craving and impulsivity. Behavioral research has shown that higher craving and impulsivity scores are both correlated with worse treatment outcomes, including relapse (Aharonovich et al., 2006; Heinz et al., 2006; Moeller et al., 2001; Paliwal et al., 2008). Our own hypothesis is that a combination of these processes is responsible for an increased propensity to relapse. Most strikingly, the first-order factors that these cortical and subcortical regions are associated with (“compulsivity” and “motor impulsiveness”) have both been shown to be predictive of treatment success. It could be that individuals who display both neural control of craving by the ventral striatum and neural activations in the RIC associated with higher impulsivity are most susceptible to relapse and therefore might require more intensive treatment measures. An interesting finding is that duration of abstinence was not a significant predictor of either striatal or RIC neural activation. We hypothesize that there is a temporal variability in recovery from cocaine addiction. That is, some individuals may recover faster than others due to pre-existing differences or factors after cessation including access to therapy or other forms of support. Future studies could use a longitudinal experimental design to elucidate the role of abstinence duration in the amelioration of the cognitive deficits that are observed in current CD users.
There are specific limitations in the present study that need to be acknowledged. Since the same cocaine images were presented at two separate time points, there is a potential risk of visual habituation to the cocaine cues. However, the sheer number of unique cocaine-cue images presented (112), coupled with the extended period of time between viewings (mean = 8.4 days) mitigates any potential habituation effects in our view. Nonetheless, in retrospect, it would certainly have been preferable to use two entirely novel cocaine-cue stimulus sets. It should also be noted that the absence of a current user group in this study is a limitation, as we are unable to assess neuro-functional or performance deficits related to acute addiction. It could be argued that the version of the response inhibition task we used might not be as sensitive to deficits as those used in previous work in current users. However, a very recent electrophysiological study by our group employed precisely the same task in a large cohort (N = 27) of current cocaine users, showing substantial performance deficits in the task that were accompanied by clear deficits in classical inhibitory control components (N2, P3 and ERN) of the event-related potential (ERP) (Morie et al., 2014). Thus, as with a substantial previous literature, the current version of a standard response inhibition task is also highly sensitive to deficits in current users.
5. Conclusions
In contrast to current CD users, there were no between-group differences in activation of the RIC and striatum. However, at the subject-level, CD individuals displayed a positive association between neural activation in the right ventral striatum and “compulsive” cocaine craving scores. Furthermore, CD individuals also displayed a positive association between “motor impulsiveness” scores and neural activations in the rIFG and pre-SMA. Importantly, these findings occurred independently of duration of cocaine abstinence. We postulate that these results may be indicative of a recovery of the neural activation deficits observed in current CD individuals in most of the former users, while also illustrating how some persons display neural activation patterns associated with cocaine-cue reactivity and impulsivity. We hypothesize that this unique pattern of neural activations corresponding to cocaine cue reactivity and impulsivity after cocaine cessation represents a possible relapse risk phenotype.
Acknowledgments
Role of the funding source
This work was made possible through the extraordinary fund-raising efforts of the Women’s Division of the Albert Einstein College of Medicine which provided the initial funding for this project (to J.J. Foxe). The Cognitive Neurophysiology Laboratory at Einstein receives ongoing support from the Sheryl and Daniel R. Tishman Foundation. The Human Clinical Phenotyping Core, where the participants enrolled in this study were clinically evaluated, and the Translational Neuroimaging Core, where the neuroimaging was conducted, are both facilities of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (IDDRC) which is funded through a center grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P30 HD071593).
We thank The New York State Office of Alcoholism and Substance Abuse Service (OASAS), The Albert Einstein College of Medicine-Division of Substance Abuse (DOSA), Daytop Village Inc., David Gibson, Nancy Kuria-Taylor, Maria Tapia, Caroline Sullivan, Sarah Church, Alexandra Valentine, Juan Martinez, Marsha Dommel and the staff at the Bronx Addiction Treatment Center for all of their help in recruitment efforts. The work would simply not have been possible without the dedication of these individuals. We would like to express our sincere gratitude to the participants for giving their time to this effort.
Footnotes
Author contributions
JJF and RPB were responsible for initial study concept and design. RPB was responsible for participant recruitment, phenotyping and the coordination of data collection. RPB, JJF and HG contributed to data analysis and data interpretation. RPB wrote the first draft of the manuscript. JJF and HG provided extensive editorial input throughout the process, and critical revisions of the manuscript for important intellectual content. All authors critically reviewed the content of the paper and approved the final version for publication.
All authors of this paper declare no conflicts-of-interest, financial or otherwise, that could have biased their contributions to this work.
The senior author, Dr. Foxe, attests that all authors had access to the full dataset and to all stages of the analyses.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4 2000. [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacol – Off Publ Am Coll Neuropsychopharmacol. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114:159–168. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): a functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2014;82:143–150. doi: 10.1016/j.neuropharm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. NeuroImage. 2009;44:537–545. doi: 10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One. 2013;8:e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neuro-biology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121:45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silveira DX, Doering-Silveira E, Niel M, Jorge MR. Predicting craving among cocaine users. Addict Behav. 2006;31:2292–2297. doi: 10.1016/j.addbeh.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Morein-Zamir S, Robbins TW. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb Cortex. 2011;21:1155–1165. doi: 10.1093/cercor/bhq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain – J Neurol. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Muller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Hester R, Murphy K, Foxe JJ, Foxe DM, Garavan H. Pre-frontal and midline interactions mediating behavioural control. Eur J Neurosci. 2009;29:181–187. doi: 10.1111/j.1460-9568.2008.06557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuro-anatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haines DE. Neuroanatomy: an Atlas of Structure, Sections, and Systems. Lippincott Williams & Wilkins; North America: 2007. [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz AJ, Epstein DH, Schroeder JR, Singleton EG, Heishman SJ, Preston KL. Heroin and cocaine craving and use during treatment: measurement validation and potential relationships. J Subst Abuse Treat. 2006;31:355–364. doi: 10.1016/j.jsat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci – Off J Soc Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci – Off J Soc Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain – J Neurol. 1999;122 (Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci – Off J Soc Neurosci. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci – Off J Soc Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacol – Off Publ Am Coll Neuropsychopharmacol. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PA, MacDonald AA, Seergobin KN, Tamjeedi R, Ganjavi H, Provost JS, Monchi O. The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson’s disease: support from functional MRI. Brain – J Neurol. 2011;134:1447–1463. doi: 10.1093/brain/awr075. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacol – Off Publ Am Coll Neuropsychopharmacol. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Morie KP, De Sanctis P, Garavan H, Foxe JJ. Executive dysfunction and reward dysregulation: a high-density electrical mapping study in cocaine abusers. Neuropharmacology. 2014;85:397–407. doi: 10.1016/j.neuropharm.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): a high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2014;82:151–160. doi: 10.1016/j.neuropharm.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacol – Off Publ Am Coll Neuropsychopharmacol. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the now and brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013;131:44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kuhn S, Romanowski A, Schubert F, Kathmann N, Gallinat J. Cortical thickness correlates with impulsiveness in healthy adults. NeuroImage. 2012;59:824–830. doi: 10.1016/j.neuroimage.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Green CE, Lane SD, Steinberg JL, Swann AC, Moeller FG. Baseline neurocognitive profiles differentiate abstainers and non-abstainers in a cocaine clinical trial. J Addict Dis. 2009;28:250–257. doi: 10.1080/10550880903028502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Broome KM. A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 2002;59:538–544. doi: 10.1001/archpsyc.59.6.538. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacol – Off Publ Am Coll Neuropsychopharmacol. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Thorn CA, Atallah H, Howe M, Graybiel AM. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron. 2010;66:781–795. doi: 10.1016/j.neuron.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorso-lateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci – Off J Soc Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci – Off J Soc Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. NeuroImage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Telang F, Fowler JS, Pradhan K, Jayne M, Logan J, Goldstein RZ, Alia-Klein N, Wong C. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS One. 2010;5:e11509. doi: 10.1371/journal.pone.0011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Lewis D, Adinoff B, Brigham G, Kropp F, Donovan DM, Seamans CL, Hodgkins CC, Dicenzo JC, Botero CL, Jones DR, Somoza E. Impulsivity is associated with treatment non-completion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. J Subst Abuse Treat. 2013;44:541–547. doi: 10.1016/j.jsat.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Simmons AN, Flagan T, Lane SD, Wackermann J, Paulus MP. Neural substrates of time perception and impulsivity. Brain Res. 2011;1406:43–58. doi: 10.1016/j.brainres.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacol – Off Publ Am Coll Neuropsychopharmacol. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action–outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]