Abstract
OBJECTIVE:
Our objective was to identify patterns of opioid use among pregnant women enrolled in RI Medicaid.
METHODS:
This study used linked RI Medicaid and RI Birth Certificate data from 01/01/2006 to 12/31/2016. We examined temporal trends of prescription opioid dispensings and identified risk factors associated with opioids use during pregnancy.
RESULTS:
Among 25,500 RI Medicaid enrolled pregnant women who delivered a live baby from 2008 to 2016, 1,914 (7.5%) received at least one prescription for an opioid medication during pregnancy, 810 (3.2%) were during the first trimester, 633 (2.5%) during the second trimester, and 866 (3.4%) during the third trimester. Of these, 213 (0.8%) women received 3 or more opioids during pregnancy. The prevalence of prescription opioids dispensed in pregnant women increased from 4.9% in 2008 to 9.6% in 2015 (β±SD: 0.66±0.28, P=0.05).
CONCLUSIONS:
Prescription opioid use during pregnancy has increased among women enrolled in RI Medicaid.
Keywords: opioid analgesics, pregnant women, RI Medicaid
INTRODUCTION
While gestational opioid use may be associated with increased risk of maternal, fetal, and neonatal complications,1,2 few studies regarding the opioid use in pregnant women exist. It is important to investigate the extent of opioid use during pregnancy to gain insight into the potential risk of maternal opioid exposure on mother and infant health. Therefore, we assessed the use of prescription opioids in a cohort of Medicaid-enrolled pregnant women in Rhode Island. This population is highly relevant as Rhode Island has been disproportionately affected by opioid overuse and addiction; it is among the top 10 states with the highest rate of opioid overdose deaths.3 In RI the number of substance-affected newborns and babies diagnosed with neonatal abstinence syndrome (NAS) has more than doubled from 44/10,000 in 2005 to 94/10,000 in 2016.4 Further, national research has shown that patients with opioid abuse or dependence are more likely to be insured through Medicaid than a private insurance company (76.1% vs. 42.8%).5 Covering 30% of the population, 25% of women of childbearing age, RI Medicaid is an important data source to investigate the impact of the opioid epidemic on pregnant women.
The goal of the study was to determine the prevalence of prescription opioid use among pregnant women enrolled in the Rhode Island (RI) Medicaid program with characterization of use over time, by types of, and intensity of opioid medication prescribed.
METHOD
Data Source
This retrospective cohort study evaluated linked RI Medicaid and RI Birth Certificate data from January 1, 2006 to December 31, 2016. Deidentified Medicaid medical information was provided by the RI Executive Office of Health & Human Service (EOHHS). Data were comprised of eligibility, medical, and prescription drug records for healthcare services from inpatient hospitals, outpatient clinics, emergency rooms, and pharmacies. Demographic information for enrolled members include age, gender, race, and location of residency. We validated the pregnancy (exposure) period, and ascertained pregnancy outcomes using birth certificate data provided by the RI Department of Health (RIDOH), which collects the data within 24 to 48 hours after delivery. Data were linked by RI EOHHS and RIDOH, then subsequently deidentified for third-party research purposes. This study was approved by the Institutional Review Board at the RIDOH and the University of Rhode Island.
Study Cohort
We included mothers enrolled in RI Medicaid who had a live birth occurring between July 01, 2006 and December 31, 2016. Mothers who had a diagnosis of cancer pre- or post- delivery or were dispensed opioids (methadone or buprenorphine) for Opioid Use Disorder (OUD) during pregnancy were excluded from the study.
Exposure Window
Gestational age was derived through ultrasound examination of mothers and included in RIDOH birth certificate data. The conception date was estimated by subtracting the infant’s gestational age, derived through ultrasound examination of mothers. The first 6 months prior to the conception date was evaluated for baseline opioid utilization. The pregnancy window included the period from conception date through delivery date. Prescription opioid exposure was evaluated 14 days prior to the conception date through delivery date to encompass the possible residual effects of opioid use during the preconception period. Opioid use was assessed between January 01, 2008 and December 31, 2015 to compare the full year utilization rate.
Prescription Opioid Exposure
Prescription opioid exposure during pregnancy was obtained through pharmacy records using Therapeutic Class Code for filled prescription opioids, and using the number of days for which the medication was supplied to determine if exposure occurred during the pregnancy window. Prescription opioids considered in this study included hydrocodone, oxycodone, codeine, morphine, and tramadol, which were the most commonly prescribed opioids for RI Medicaid-covered pregnant women.
Opioid exposure was defined as any one receipt of a prescription opioid dispensed during the pregnancy exposure window; including opioids dispensed before the exposure window with days supply extending to cover at least 1 day within the exposure window. Average days of supply and daily doses were calculated. The daily doses for each opioid prescription were converted to Morphine Milligram Equilibrium (MME) using the Centers for Disease Control (CDC) conversion Table (2016 version). Further investigation was conducted to determine the pattern of prescription opioids filled in three pregnancy trimesters.
Comparison Group
To identify the risk factors associated with opioid use during pregnancy, we selected a comparison group that included women without any opioid dispensings during pregnancy.
Baseline Characteristics
Maternal characteristics at baseline included: age, race, substance use and abuse, tobacco use, alcohol use, preexisting conditions, pain conditions, psychiatric medications use, and opioid use at baseline. The operational definitions for the medical covariates, including substance use and abuse, tobacco use, and alcohol use, are described in Appendix Table 1.
Statistical Analyses
Descriptive analyses of prescription opioid dispensings, temporal trends, and corresponding demographic and clinical characteristics were conducted. Continuous variables were presented as mean ± standard deviation (SD) and compared using a student t test. Categorical variables were presented as frequency (%) and compared using a chi-square test or Fisher exact test depending upon the sample size in each level. A multivariate logistic regression model was developed to identify significant risk factors associated with opioid use during pregnancy. Statistical significance was set up at p≤ 0.05. All statistical analyses were conducted using SAS 9.4 (Cary, NC).
RESULTS
Of the total included 25,500 pregnancies, 1,914 (7.5%) received a total of 4,046 opioid prescriptions at any time during their pregnancy; 810 (3.2%) received a prescription for an opioid medication during their first trimester; 633 (2.5%) during their second trimester; 866 (3.4%) during their third trimester; 315 (1.2%) received prescriptions in two trimesters, and 80 (0.3%) in all three trimesters. Only 213 (0.8%) pregnancies were identified with dispensings of more than 3 prescriptions for opioid medication.
Among those with opioid use during the 6-month period preceding pregnancy, the leading documented pain-related indications included: abdominal pain (27%), back pain (30%), or headache (18%). Among those with opioid use during the pregnancy period the leading documented pain-related indications included: antepartum conditions or complications (66%), abdominal pain (38%), back pain (36%), or headache (24%).
Figure 1 shows the temporal trend of prescription opioid use during pregnancy from January 01, 2008 to December 31, 2015. The percentage of pregnancies with a dispensed opioid increased significantly from 4.9% in 2008 to 11.1% in 2015 (Slope β ± SE: 0.9 ± 0.2, p = 0.01), with sharp increases occurring in 2011 (145% from the previous year) and 2012 (43% from the previous year).
Figure 1.

Percentage of Pregnancies Dispensed Prescription Opioids for Pain. Rhode Island Medicaid: 2008–2015
The most commonly dispensed opioids were hydrocodone, oxycodone, codeine, and tramadol, respectively (Figures 2a,2b). Hydrocodone was the most frequently dispensed opioid for all years (35–61% of prescription opioids) except 2008, for which oxycodone use was more prevalent (50%). Four types of opioids (hydrocodone, oxycodone, codeine and tramadol) accounted for nearly all prescription opioid use between 2011–2015, while use of other opioid types including morphine and hydromorphone dissipated after 2010.
Figure 2a.

Percent of Prescriptions Per Year for Opioid Types Prescribed for Pregnant Women in RI Medicaid.
Figure 2b.
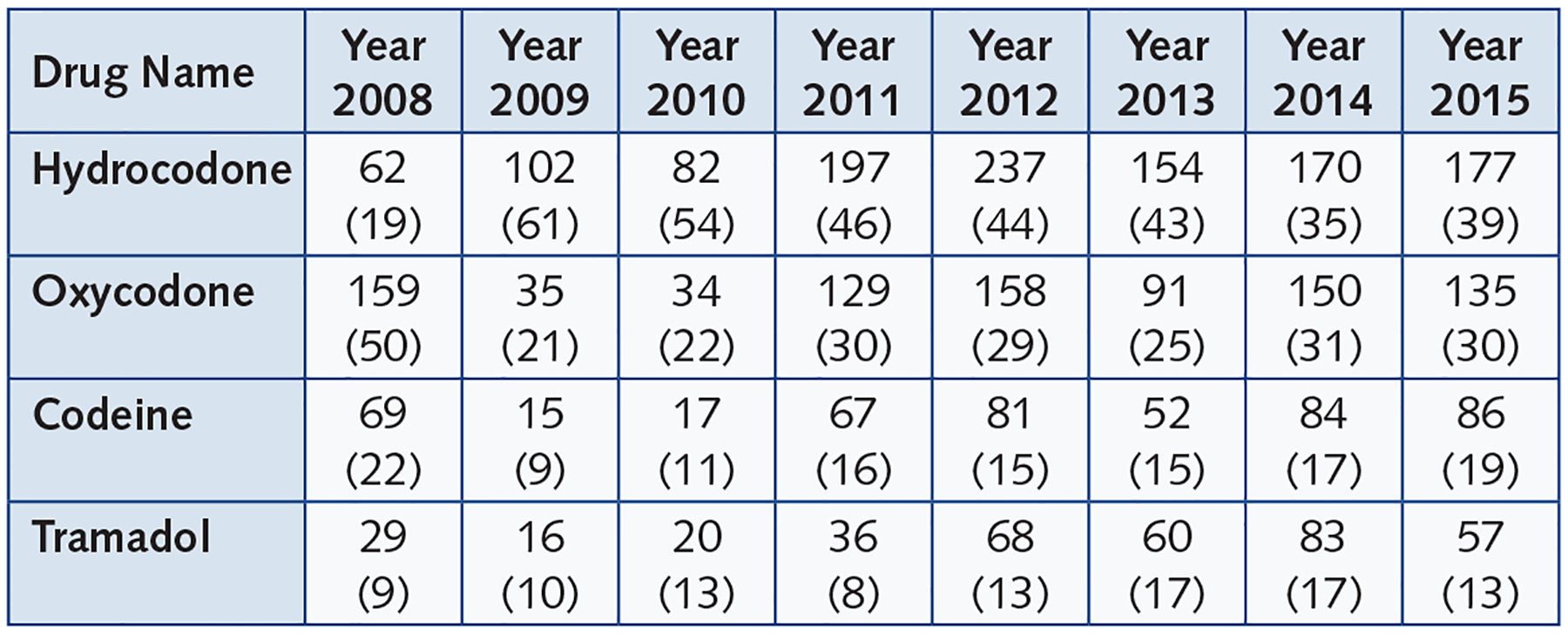
Frequencies and Percentages of Opioid Types by Years
The average days of supply for prescription opioids dispensed was 9.3 days ± 9.4 (mean±SD). A statistically significant trend in change in days supply from 2008 to 2015 was not observed. The average days of supply and MME for four commonly dispensed opioids are listed in Table 1.
Table 1.
Morphine Milligram Equivalents (MME) and Supply Days for Opioids Prescribed in RI Women.
| Drug Name | Average Supply Days Median (25th percentile, 75th percentile) | Average MME Median (25th percentile, 75th percentile) |
|---|---|---|
| Hydrocodone | 5 (3, 10) | 25 (20, 38) |
| Oxycodone | 5 (3, 13) | 45 (30, 71) |
| Codeine | 3 (2, 5) | 27 (18, 45) |
| Tramadol | 10 (6, 22) | 20 (15, 32) |
Demographic and clinical characteristics were assessed during the 6-month baseline window and compared between women with and without opioid dispensing during pregnancy (Table 2). Women with opioid dispensings during pregnancy were observed to be younger (<18: 41% vs 34%, p<.0001), of white race (64% vs 52%, p <.0001), had higher rates of tobacco (6.2% vs 2.2%, p<.0001), alcohol (7% vs 1%, p<.001), or cocaine use (0.4% vs 0.07%, p<.0001), a higher rate of chronic pain diagnoses (e.g., fibromyalgia, migraines and back pain), have more comorbid psychiatric conditions (e.g., depression or anxiety), and increased use of psychiatric medications, including benzodiazepines, antidepressants, and antipsychotics.
Table 2.
Comparison of baseline characteristics of pregnant women in RI Medicaid. N=25,500.
| Characteristics | Exposed to Opioids during pregnancy N = 1,914 | Unexposed to Opioids during pregnancy N = 23,586 | P Value |
|---|---|---|---|
| Maternal Age at delivery | |||
| Mean ± SD | 25.6 ± 8.3 | 25.9 ± 6.3 | 0.06 |
| 18–24 | 387 (20) | 6,914 (29) | |
| ≥35 | 107 (5.6) | 1,459 (6) | |
| Race, N(%) | |||
| White | 1,228 (64) | 12,277 (52) | <0.0001 |
| Other | 565 (29.5) | 2,214 (9.4) | |
| Substance use and abuse | |||
| Cocaine | 8 (0.4) | 16 (0.07) | <0.0001 |
| Tobacco use, N(%) | 118 (6.2) | 518 (2.2) | <0.0001 |
| Alcohol use, N(%) | 135 (7) | 331 (1.4) | <0.001 |
| Maternal preexisting condition, N(%) | |||
| Multiple gestation | 29 (3.2) | 214 (2.3) | 0.31 |
| Anxiety | 50 (2.6) | 296 (1.3) | <0.0001 |
| Pain conditions | |||
| Fibromyalgia | 39 (2) | 117 (0.5) | <0.0001 |
| Back pain | 231 (12) | 955 (4) | <0.0001 |
| Psychiatric medications | |||
| Anxiolytics | 106 (5.5) | 355 (1.5) | <0.0001 |
| Stimulants | 4 (0.2) | 57 (0.24) | 0.78 |
| Opioid use at baseline | 311 (16) | 773 (3) | <0.0001 |
Table 3 presents the results of multivariable logistic regression analysis. The significant risk factors that were associated with maternal opioid exposure during pregnancy included: mother’s age at the delivery (18–24 or 25–34 years old), white race, cocaine use, tobacco use, alcohol use, migraine, low back pain, opioid use prior to pregnancy, and the year of delivery after 2011. The model fits well (Hosmer and Lemeshow Goodness-of-Fit Test: P=0.95) with C Statistics at 0.72.
Table 3.
Significant Risk Factors for Maternal Opioid Use during Pregnancy. Results of Multivariable Logistic Regression Analysis
| Characteristics | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Mother Race White vs Black | 1.65 | 1.32 | 2.06 | <0.0001 |
| Cocaine use | 3.10 | 1.15 | 8.36 | 0.025 |
| Tobacco use | 1.44 | 1.12 | 1.86 | 0.005 |
| Alcohol Use | 6.38 | 5.02 | 8.10 | <0.0001 |
| Migraines | 1.51 | 1.22 | 1.88 | 0.0002 |
| Low back pain | 1.86 | 1.52 | 2.29 | <0.0001 |
| Benzodiazepine Use | 1.60 | 1.19 | 2.14 | 0.0019 |
| Opioid use prior to pregnancy | 4.40 | 3.64 | 5.32 | <0.0001 |
| Mother Age < 18 vs 18–24 | 0.26 | 0.13 | 0.54 | 0.0002 |
| Mother Age 25–34 vs 18–24 | 1.45 | 1.25 | 1.69 | <0.0001 |
| Mother Age ≥ 35 vs 18–24 | 1.22 | 0.94 | 1.59 | 0.14 |
| Delivery Year | ||||
| 2014 vs 2008 | 14.24 | 6.81 | 29.76 | <0.0001 |
| 2012 vs 2008 | 2.64 | 2.10 | 3.31 | <0.0001 |
| 2009 vs 2008 | 1.03 | 0.77 | 1.37 | 0.8 |
DISCUSSION
From 2008 to 2015, approximately 7.5% of RI Medicaid covered women received at least one opioid prescription during their pregnancy, with 1 in 6 receiving opioid medication for two trimesters, which raises concern given the risks of longer-term opioid use in pregnancy. The increased risk for neonatal abstinence syndrome or specific cardiovascular and central nervous system defects has been reported in previous studies.1,2
Although recent studies showed that opioid use in pregnant women decreased from 14.9% in 2005 to 12.9% in 2011 within the US,5,6 we observed an increased dispensing of opioids to pregnant women in RI Medicaid that more than doubled from 5% in 2008 to 11% in 2015, with a sharp rise in 2010 and 2011. The overall increase in the dispensing of prescription opioids directly led to an increase in dispensing of combination products with hydrocodone, which was dispensed more than all other prescription opioids. Based on CDC reports in 2010, approximately 16,651 opioid overdose deaths involved oxycodone, hydrocodone, or methadone.3 To control for the dramatic increase in opioid abuse and overdose deaths, the Drug Enforcement Administration (DEA) designated hydrocodone combination products from Schedule III to a more stringently controlled Schedule II category of drug in 2014.7 However, our results showed that hydrocodone was still the most widely dispensed opioid in pregnant women on Medicaid in RI in 2014 and 2015.
Opioids most commonly dispensed to RI pregnant women were hydrocodone, oxycodone, codeine, and tramadol, respectively. In other studies using national claims data, codeine was a more commonly dispensed opioid in pregnant women than oxycodone.6,8 However, oxycodone was more widely dispensed to pregnant women in RI than codeine. The dispensed prescriptions of hydrocodone and oxycodone almost tripled from 2010 to 2011 (from 82 to 197 for hydrocodone and 34 to 129 for oxycodone), and increased more than four-fold for codeine (from 17 to 67). Prescriptions for hydrocodone also increased throughout 2011 and 2012, while oxycodone and codeine saw only a relatively small increases during this period. After 2012, the dispensing rates for all four commonly used opioids appeared to level off.
Previous studies have demonstrated the risk of teratogenesis for some opioids, especially when used in the first trimester of pregnancy.1,9 Codeine and hydrocodone used early in pregnancy have been associated with greater risk of cardiac septal defects, hypoplastic left heart syndrome, spina bifida, and gastroschisis.1 Oxycodone has the potential to cause more dependence and overdose. Surprisingly, tramadol, classified pregnancy category C by the FDA because of its potential teratogenic effects suggested by animal studies and schedule IV by the DEA resulting from its abuse potential, was also prescribed to pregnant women in RI during the study period.
Implications for Practice and/or Policy
Pregnant women can experience a variety of pain syndromes (e.g., back pain, abdominal pain, and migraines) during pregnancy.10 Prevalence of low back or pelvic pain was 71.7% in pregnancies,11 while migraines were present in 35% of pregnancies.12 Providers often face difficult choices when prescribing safe and effective pain control medications during pregnancy. Non-steroidal anti-inflammatory drugs are a poor alternative, as they also pose potential harm to both mother and fetus.13 Careful consideration of the potential benefits and harms of perinatal opioid use is of particular importance. While opioids are often prescribed, they can have adverse effects on the fetus.1,2
According to the CDC data, RI has a disproportionately high rate of opioid overdose deaths, (N=320, 31.0% in 2017), which is ninth in the nation.3 Rhode Island dispensed the highest total Morphine Milligram Equivalents (MME) in 2016 (2,623.7 mg/person), more than two-fold higher than the average total MME in the US.14 Medicaid enrollees were also noted to have higher rates of prescription opioid use (22%) compared to commercially insured patients in the general population (9%).15 Our findings that the rates of opioid use significantly increased from 2008 to 2015 confirmed that RI Medicaid-covered pregnant women are at a high risk of opioid use. In March 2017 and July 2018, RI updated the regulations for Pain Management, Opioid Use, and the Registration of Distributors of Controlled Substances.16 The new version of regulations placed stricter controls on prescribing opioids, including dose limits for initial prescriptions. We expect to see decreased rates of opioid use in RI in the future.
In some instances the use of opioids for the treatment of pain occurring during pregnancy is clinically justifiable. For an opioid prescription to be written and given to a patient, a prescriber-patient relationship and ‘medical need’ for the medication is required. In a statement on Opioid Use During Pregnancy, the American College of Obstetricians and Gynecologists (ACOG) recommended that, “Opioids should only be used for treatment of pain when alternatives are not appropriate or effective…”17 Yet the available literature regarding the safety of opioid use during pregnancy is mixed and would be better informed by additional and larger cohort studies. A review of the evidence regarding the short-term and long-term risks of opioid use during pregnancy reported inconsistent results for studies of fetal development, preterm birth, and birth defects overall.18 Many of the studies included in the review involved the use of codeine, which was less frequently utilized in our population. The FDA labels for oxycodone and hydrocodone note that no well-controlled studies in pregnant women are available, highlighting an important gap in evidence.19 The new CDC guideline for prescribing opioids for chronic pain issued in 2016 suggested that physicians prescribe non-opioid analgesics or opioids with the lowest dose.20 With the new CDC guideline and RI regulation for opioid prescribing,16,20 we expect to see the lower prescribing rate of opioids in RI pregnant women.
Limitations
Our study has several limitations. First, the use of illicit drugs, including illicit prescription opioids, was not captured since the prevalence of illicit drug use is not available in the Medicaid claims data. Second, our data source only collects prescriptions filled by patients in pharmacies located in RI. Any illicit drug use or opioids filled in pharmacies outside of RI are not accounted for. Third, expected opioid use was estimated based on pharmacy claims. Actual patient utilization may deviate from the pharmacy dispensing. Fourth, the study is based on the RI Medicaid data. Rates of opioid use have substantial geographical variations and are significantly different between Medicaid enrollees and commercially insured patients.14,15 Therefore, the study findings can only be generalizable to RI Medicaid enrollees. Lastly, the study only included women with pregnancies resulting in a live birth. If opioids are associated with therapeutic or spontaneous terminations of pregnancies, total opioid use may be underestimated. Nevertheless, Medicaid pharmacy claims data is seen as the gold standard of drug exposure compared to outpatient medical records or self-reported drug use information.
In conclusion, our findings of increasing rates of prescription opioids, filled by pregnant women enrolled in RI Medicaid, calls for a comprehensive safety assessment of opioids and their long-term effects on the developing fetus to help inform clinical practice.
Acknowledgments
We sincerely appreciate William Arias, Ellen Amore, and Samara Viner-Brown from the Center for Health Data & Analysis, Rhode Island Department of Health, and Christine Leveille in DXC Technology, Rhode Island Human Services Data Warehouse, for data linkage, data cleaning, and data preparation.
Financial disclosures
Dr. Xuerong Wen was supported by Oh-Zopfi Pilot Project Award Program, Rhode Island Women & Infants Hospital (2017–2018), RI INBRE Early Career Development Award (P20GM103430, 2018–2019), and the NIH/NICHD R15 award (R15HD097588, 2019–2022). Dr. Stephen Kogut is partially supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix Table 1.
Data Sources and Operational Definitions for Covariates.
| Variables | Data Sources | Operational Definitions |
|---|---|---|
| Maternal Age | RI DOH Birth Certificates | Age at delivery |
| Race | RI DOH Birth Certificates | White, Black, and Others |
| Marijuana | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Cocaine | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Tobacco use | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Alcohol use | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Multiple gestation | RI DOH Birth Certificates | |
| HIV infection | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Depression | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Anxiety | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Fibromyalgia | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Migraine | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Back pain | RI Medicaid Inpatient/Outpatient Claims | ICD-9 Diagnosis Code |
| Anxiolytics Use | RI Medicaid Pharmacy Claims | Therapeutic Class Code |
| Antidepressants Use | RI Medicaid Pharmacy Claims | Therapeutic Class Code |
| Antipsychotics Use | RI Medicaid Pharmacy Claims | Therapeutic Class Code |
| Stimulants Use | RI Medicaid Pharmacy Claims | Therapeutic Class Code |
| Opioid use at baseline | RI Medicaid Pharmacy Claims | Therapeutic Class Code |
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The views expressed herein are those of the authors and do not necessarily reflect the views of the RI Department of Health.
Contributor Information
XUERONG WEN, Health Outcomes, Department of Pharmacy Practice, College of Pharmacy, University of Rhode Island..
NICHOLAS BELVISO, Health Outcomes, Department of Pharmacy Practice, College of Pharmacy, University of Rhode Island..
REBECCA LEBEAU, Executive Office of Human & Health Services, Rhode Island..
JEFF BRATBERG, Health Outcomes, Department of Pharmacy Practice, College of Pharmacy, University of Rhode Island..
BRANDI COTTON, College of Nursing, University of Rhode Island..
KRISTINA E. WARD, Health Outcomes, Department of Pharmacy Practice, College of Pharmacy, University of Rhode Island..
DEBRA ERICKSON-OWENS, College of Nursing, University of Rhode Island..
STEPHEN KOGUT, Health Outcomes, Department of Pharmacy Practice, College of Pharmacy, University of Rhode Island..
References
- 1.Broussard CS, Rasmussen SA, Reefhuis J, Friedman JM, Jann MW, Riehle-Colarusso T, Honein MA. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1–11. [DOI] [PubMed] [Google Scholar]
- 2.Zagon IS, Wu Y, McLaughlin PJ. Opioid growth factor and organ development in rat and human embryos. Brain Res. 1999;839:313–22. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. CDC Drug Overdose Death Data, Opioid Overdose; 2018. https://www.cdc.gov/drugoverdose/data/statedeaths.html.Accessed November 27, 2017. [Google Scholar]
- 4.RI Department of Health. Neonatal Abstinence Syndrome. 2016. http://health.ri.gov/data/neonatalabstinencesyndrome. Accessed January 4, 2019.
- 5.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158–65. [DOI] [PubMed] [Google Scholar]
- 6.Bateman BT, Hernandez-Diaz S, Rathmell JP, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology. 2014;120:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drug Enforcement Administration. Diversion Control Division, U.S. Department of Justice. 2014. https://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0822.htm. Accessed August 1, 2018.
- 8.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman KJ, Flyer DC, Goldblatt A, Kreidberg MB. Exogenous hormones and other drug exposures of children with congenital heart disease. Am J Epidemiol. 1979;109:433–9. [DOI] [PubMed] [Google Scholar]
- 10.Ray-Griffith SL, Wendel MP, Stowe ZN, and Magann EF. Chronic pain during pregnancy: a review of the literature. Int J Womens Health. 2018;10:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mogren IM, Pohjanen AI. Low back pain and pelvic pain during pregnancy: prevalence and risk factors. Spine (Phila Pa 1976). 2005;30:983–991. [DOI] [PubMed] [Google Scholar]
- 12.Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia. 1997;17:765–769. [DOI] [PubMed] [Google Scholar]
- 13.Antonucci R, Zaffanello M, Puxeddu E, Porcella A, Cuzzolin L, Pilloni MD, et al. Use of non-steroidal anti-inflammatory drugs in pregnancy: impact on the fetus and newborn. Curr Drug Metab. 2012;13:474–90. [DOI] [PubMed] [Google Scholar]
- 14.Piper BJ, Shah DT, Simoyan OM, McCall KL, Nichols SD. Trends in Medical Use of Opioids in the U.S., 2006–2016. Am J Prev Med. 2018;54:652–660. doi: 10.1016/j.amepre.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Brennan Braden J, et al. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RI Department of Health. Safe Opioid Prescribing. http://www.health.ri.gov/healthcare/medicine/about/safeopioidprescribing/. Accessed January 3, 2019.
- 17.American College of Obstetricians and Gynecologists. ACOG Statement on Opioid Use During Pregnancy. https://www.acog.org/About-ACOG/News-Room/Statements/2016/ACOG-Statement-on-Opioid-Use-During-Pregnancy. May 26, 2016. Accessed 07/31/2018.
- 18.Yazdy MM, Desai RJ, Brogly SB. Prescription opioids in pregnancy and birth outcomes: A review of the literature. J Pediatr Genet. 2015;4:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Drug Administration. Guidance for Industry: Establishing Pregnancy Exposure Registries. August 2002. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatory-information/guidances/ucm071639.pdf. Accessed 07/31/2018.
- 20.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain. https://www.cdc.gov/drugover-dose/prescribing/guideline.html. Accessed 04/12/2019.


