Abstract
TRIM72 is a membrane repair protein that protects against ischemia reperfusion (I/R) injury. We previously identified Cys144 (C144) on TRIM72 as a site of S-nitrosylation. To study the importance of C144, we generated a knock-in mouse with C144 mutated to a serine (TRIM72 C144S). We subjected ex vivo perfused mouse hearts to 20 min of ischemia followed by 90 min of reperfusion and observed less injury in TRIM72 C144S compared to WT hearts. Infarct size was smaller (54 vs 27% infarct size) and cardiac functional recovery (37 vs 62% RPP) was higher for the TRIM72 C144S mouse hearts. We also demonstrated that TRIM72 C144S hearts were protected against I/R injury using an in vivo LAD occlusion model. As TRIM72 has been reported to be released from muscle we tested whether C144 is involved in TRIM72 release. After I/R there was significantly less TRIM72 in the perfusate normalized to total released protein from the TRIM72 C144S compared to WT hearts, suggesting that C144 of TRIM72 regulates myocardial TRIM72 release during I/R injury. In addition to TRIM72’s protective role in I/R injury, TRIM72 has also been implicated in cardiac hypertrophy and insulin resistance, and secreted TRIM72 has recently been shown to impair insulin sensitivity. However, insulin sensitivity (measured by glucose and insulin tolerance) of TRIM72 C144S mice was not impaired. Further, whole body metabolism, as measured using metabolic cages, was not different in WT vs TRIM72 C144S mice and we did not observe enhanced cardiac hypertrophy in the TRIM72 C144S mice. In agreement, protein levels of the TRIM72 ubiquitination targets insulin receptor β, IRS1, and focal adhesion kinase were similar between WT and TRIM72 C144S hearts. Overall, these data indicate that mutation of TRIM72 C144 is protective during I/R and reduces myocardial TRIM72 release without impairing insulin sensitivity or enhancing the development of hypertrophy.
Introduction
The balance between cysteine oxidation and protein S-nitrosylation (SNO) plays an important role in the pathogenesis of myocardial ischemia-reperfusion (I/R) injury. Recent findings from our group and others suggest that enhanced protein SNO is cardioprotective1–6, in part by shielding critical cysteine residues from further oxidation during I/R injury. An increase in SNO due to treatment with SNO donors or preconditioning protects against cysteine oxidation1,2,4,7. We previously identified cysteine 144 (C144) on Tripartite motif-containing protein 72 (TRIM72, also known as mitsugumin 53 (MG53)) as a site of both oxidation and SNO1,8. TRIM72 is a membrane repair protein predominantly expressed in heart and skeletal muscle9, that functions as a scaffold to facilitate membrane repair9–11. I/R injury has been shown to reduce TRIM72 levels in the heart and this correlates with cell death, as does the genetic ablation of TRIM7212. Ischemic pre- and post-conditioning have also been shown to block the I/R-induced decrease in TRIM72 levels12,13, and our recent studies further suggest that TRIM72 can be SNO-modified at C144 during pre- and post-conditioning1,14. Additional cell culture experiments showed that mutating C144 of TRIM72 to a serine prevented the H2O2 induced reduction in TRIM72 protein levels and cell death1. Together, these results suggest that SNO and/or mutation of TRIM72 at C144 is cytoprotective in the context of I/R injury.
TRIM72 also plays a role in cellular metabolism. Overexpression of TRIM72 has been shown to increase insulin resistance and induce cardiac hypertrophy1,15–17, while the loss of TRIM72 is associated with the preservation of insulin sensitivity16,17. Several mechanisms have been proposed regarding the regulation of insulin signaling by TRIM72. Ubiquitination of insulin receptor substrate 1 (IRS1) by TRIM72 can induce the degradation of IRS116–18. Additionally, TRIM72 has been identified as a regulator of peroxisome proliferator activated receptor α (PPARα) expression15. Finally, it was recently reported that TRIM72 also impairs insulin sensitivity through actions as a cardiokine/myokine19. The secreted TRIM72 binds to the insulin receptor and decreases intracellular insulin signaling19. Since SNO and/or mutation of TRIM72 at C144 has been found to regulate intracellular levels of TRIM72, in this study we tested whether this residue may regulate TRIM72 release.
The aim of the current study was to examine whether modification of C144 of TRIM72 regulates I/R injury and whether it alters the cardiac release of TRIM72. Our previous studies suggested that a free thiol at cysteine at 144 of TRIM72 promotes I/R injury and that either SNO or mutation of this cysteine is cardioprotective. In the current study, we generated a knock-in mouse with TRIM72 C144 mutated to a serine (TRIM72 C144S) to test the role of C144 of TRIM72 in protection against myocardial I/R injury. TRIM72 C144S hearts were protected against ex vivo and in vivo models of I/R injury, as TRIM72 C144S hearts had enhanced functional recovery during reperfusion and developed smaller infarcts compared to WT hearts, and this was accompanied by a reduction in cardiac release of TRIM72 during reperfusion providing more TRIM72 for cardiac repair. In contrast to the results observed with overexpression of TRIM7215–17,20, we did not observe enhanced cardiac hypertrophy, impaired insulin sensitivity, or altered whole body metabolism in the TRIM72 C144S mouse. The results suggest that the C144 of TRIM72 may represent a viable target for enhancing the cardioprotective benefits of TRIM72 in the context of I/R injury, without altering insulin sensitivity or inducing cardiac hypertrophy.
Methods
Mice
The TRIM72 C144S Knock-in mouse line was generated using the TALEN method. TRIM72 C144S mice were then backcrossed on a C57BL6N (Taconic Farm) background. TALEN mRNAs were synthesized using the mMESSAGE mMACHINE In vitro Transcription Kit (ThermoFisher). This pair of mRNAs (20 ng/ul each) were comicroinjected with 100ng/μl of the donor oligos into the cytoplasm of zygotes collected from B6CBAF1/J mice (JAX Stock #100011). The sequence for the donor oligos was as follows: ATCATCTCTTTTAATTTGTCCCATAGACACAGCTTCCACAACAAAAGATGCAGCT GCAGGAGGCATCCATGCGCAAAGAGAAGACTGTAGCGGTGCTGGAGCATCAGCTGG TGGAGGTG, where the four bold letters indicate nucleotide changes for mediating the intended cysteine to serine change as well as silent mutations that are required for stopping TALENs from continuingly cutting the genomic sequence after the oligo was successfully introduced. Microinjected zygotes were cultured overnight in M16 medium (Milllipore) in a humidified 37°C incubator with 5% CO2. The next morning, embryos that had reached the 2-cell stage of development were implanted into the oviducts of pseudopregnant foster mothers. Offspring born to the foster mothers were genotyped by PCR (as described below) and Sanger sequencing for identifying founders with the desired knock-in mutation. Founder mice were subsequently backcrossed to C57BL6N background and then used for the current study. Mice were kept in a 12:12 hour light dark cycle and given food (standard chow) and water ad libitum. Mice were 2–6 months old in all experiments. Mice were treated in accordance with the Guide for the Care and Use of Laboratory Animals, and all experimentation was approved by the Institutional Animal Care and Use Committee of the National Heart, Lung and Blood Institute and Johns Hopkins University.
Genotype was determined by PCR with the following primer set: forward primer, 5’-GGAGAAGGAGCTAACATCAAGG-3’ and reverse primer, 5’-CTTCACGGTCCAGAGAACTTT-3’. These primers were used to amplify a 357 bp fragment which was then subjected to a restriction digest reaction (using NsiI enzyme). In the case of this reaction, the NsiI enzyme will not cut DNA containing the TRIM72 C144S mutation, but wild-type DNA will be cut. As such, wild-type DNA produces two bands (125 bp and 232 bp), heterozygous TRIM72 C144S DNA produces three bands (125 bp, 232 bp, and 357 bp), and homozygous TRIM72 C144S DNA produces a single band (357 bp).
Langendorff perfused heart
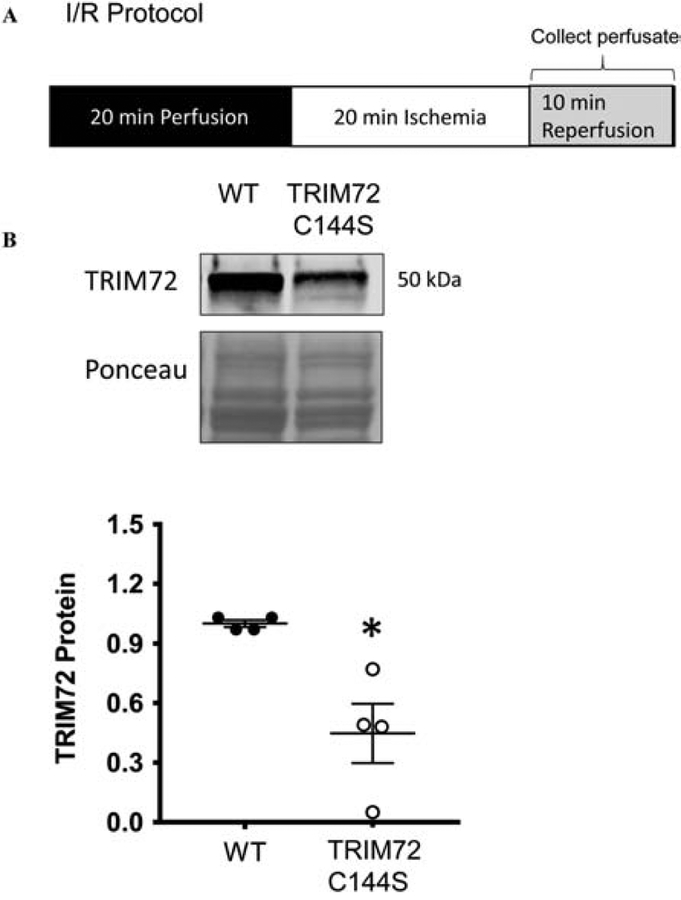
The Langendorff perfused-heart protocol was performed as described previously21. Briefly, after 20 min of equilibrium perfusion, Langendorff perfused hearts were subjected to 20 min of global, no-flow ischemia followed by 90 min (for assessment of functional recovery and infarct size) or 10 min (assessment of TRIM72 protein in heart perfusate) of reperfusion. Rate-pressure product (RPP), which is the product of left ventricular developed pressure (LVDP) and heart rate (HR) were assessed during the perfusion protocol using a latex balloon inserted into the left ventricle. To determine % RPP recovery we normalized the RPP during the reperfusion period to the preischemic RPP. At the end of the perfusion protocol hearts were stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC). Infarct size was determined from cross sectional slices of these stained hearts. To determine % infarct we normalized the infarct size to the total LV area. To assess TRIM72 in the perfusate, the collected perfusate was concentrated using 10 kDa filters (Amicon) and then run on a TGX gel (BioRad) with TRIM72 protein measured as described below. TRIM72 protein was normalized to Ponceau S stain.
In vivo left anterior descending coronary artery (LAD) occlusion surgical protocol
Mice were fully anesthetized with isoflurane (1%−1.5%), intubated, and connected to a rodent ventilator. A median sternotomy was performed to access and identify the LAD, which was surgically ligated with a 7–0 silk suture. A short segment of PE-10 silicon tubing was placed between the LAD and the 7–0 suture to cushion the artery against trauma. Mice were subjected to 45 minutes of LAD ischemia, followed by reperfusion for 24 hours. After 24 hours of reperfusion, transthoracic echocardiography was performed in conscious mice to assess post-ischemic myocardial function using a Vevo 2100 system with a 40-MHz linear transducer (FUJIFILM VisualSonics, Toronto, Canada) as described22. Mice were then anesthetized with a mixture of ketamine (Hofspira) and xylazine (Sigma-Aldrich) via intraperitoneal injection, and anticoagulated with heparin (Fresenvis Kabi USA). The heart was then rapidly excised and Langendorff-perfused with Krebs-Henseleit buffer for five minutes to clear blood, and the LAD was re-ligated at the same position as the day prior. A 3% Evans blue solution (0.1 mL) was then injected retrogradely into the aorta to delineate the area-at-risk. Hearts were then cut cross-sectionally into 2-mm-thick sections, which were incubated in 1.0% TTC for four minutes at 37°C to assess infarct within the area-at-risk. Each heart section was then weighed and imaged, and area-at-risk and infarct size were determined by a blinded investigator.
Angiotensin II treatment
Mice were treated with angiotensin II (1.5 mg/kg BW/day) or saline (vehicle) via Alzet osmotic minipump for 4 weeks. At the end of the treatment, echocardiography was performed with the Vevo2100 system and a 30-MHz linear transducer. Heart weight and tibia length were measured to assess cardiac hypertrophy.
Glucose and insulin tolerance test
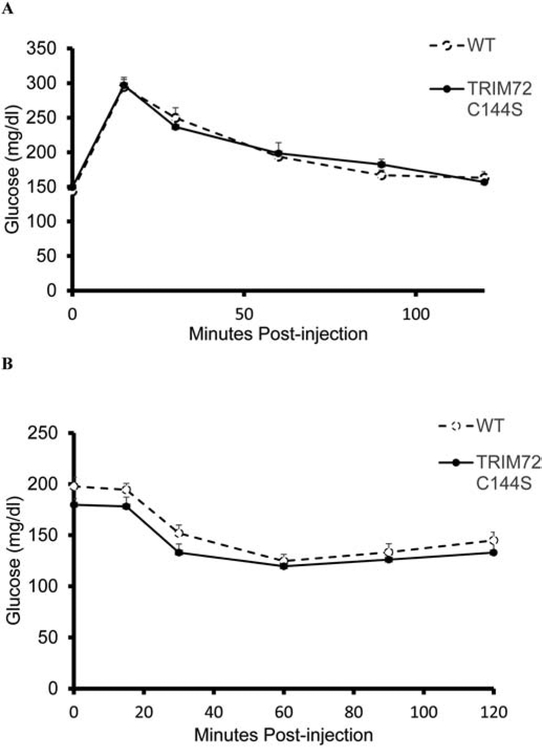
9–13 wk old male mice were fasted for 6 hr and then injected with either glucose (1.5 mg glucose/g lean body weight) to measure glucose tolerance or insulin (0.5 mU insulin/g lean body weight) to measure insulin tolerance. Blood glucose was measured at baseline and at 15, 30, 60, 90, and 120 minutes post injection.
Metabolic cages
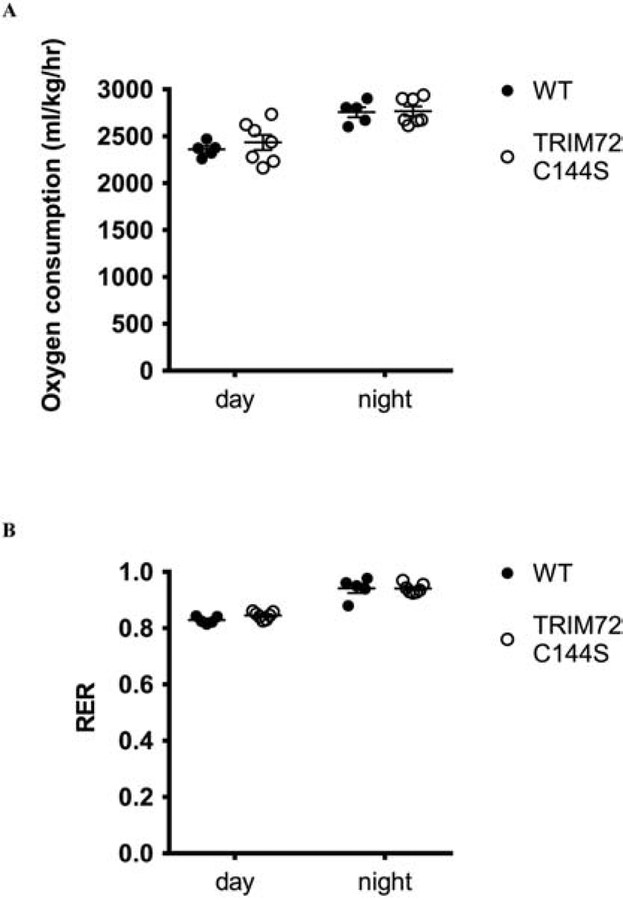
Whole body metabolism was assessed using the Oxymax Comprehensive Laboratory Animal Monitoring System (CLAMS). Male mice were acclimatized to the CLAMS for 24 hrs and then data was collected for 72 hrs.
Western blot
Standard western blot protocol was followed. Briefly, hearts were homogenized in RIPA buffer with 1 mM EDTA, 100 mM NEM, and Halt protease phosphatase inhibitor cocktail (Thermo Fisher cat no 78440). Following standard SDS PAGE using Criterion TGX gels (BioRad) and transfer, nitrocellulose membranes were incubated for 1 hr in 5% non-fat dry milk in TBST, incubated overnight in primary antibody in 5% BSA in TBST at 4°C, washed 4× 5 min in TBST, incubated for 1 hr in secondary antibody in 1% NFDM in TBST, and then washed 4× 5 min in TBST. Primary antibodies included insulin receptor β (Cell Signaling cat no 3025S), IRS1 (Millipore cat no 06248), focal adhesion kinase (FAK; Cell Signaling cat no 385S), TRIM72 (gift from Jianjie Ma’s lab), hydroxyacyl CoA dehydrogenase (HADH, Abcam cat no 110302), and GAPDH (Santa Cruz cat no sc-2035). Anti-rabbit (Santa Cruz cat no sc-2030), anti-mouse (Santa Cruz cat no sc-2031), and anti-goat (Santa Cruz cat no sc-2056) secondary antibodies were used.
Statistics
T-test, two-way ANOVA with Bonferroni posthoc test, or one-way ANOVA with repeated measures with Bonferroni posthoc test were performed as appropriate to determine statistical significance (p<0.05). We tested to see if the data showed a normal distribution and if normality was not found the Mann-Whitney test was performed instead of the t-test. The specific statistical test performed is listed in the figure and table legends. Data are presented as mean±SEM.
Results
TRIM72 C144S reduces myocardial I/R injury
Previous studies have shown that SNO is protective during myocardial I/R injury, and that TRIM72 C144 can be S-nitrosylated1. We have previously shown that C144 of TRIM72 is a target of SNO and oxidation1, and that mutation of TRIM72 at C144 is protective during oxidative stress. To further understand a role for this TRIM72 C144 mutation in cardioprotection, we generated a knock-in mouse in which C144 of TRIM72 was mutated to a serine (TRIM72 C144S). Langendorff perfused hearts were subjected to 20 min of aerobic perfusion followed by 20 min of global ischemia and 90 min of reperfusion. Injury was assessed by measuring post-ischemic recovery of cardiac function and infarct size (Fig 1). As shown in Figure 1, TRIM72 C144S hearts showed a significant improvement in recovery of function, as determined via rate-pressure product (37% in WT vs. 62% in TRIM72 C144S), and a significant reduction in infarct size by ~50% (54% in WT vs 27% in TRIM72 C144S) (Fig 1).
Figure 1. TRIM72 C144S protects against myocardial ischemia/reperfusion injury.
Hearts were Langendorff perfused and subjected to a A. 20 min ischemia and 90 min reperfusion protocol. B. Cardiac functional recovery and C. infarct size were measured. Values are mean ± SEM (WT n=5; TRIM72 C144S n=5); T-tests were performed to assess significance, * p<0.05 compared to WT
We also tested whether the TRIM72 C144S mice were protected against I/R injury using an in vivo model. The LAD was occluded for 45 min to induce ischemia followed by 24 hr of reperfusion (Fig 2A). Echocardiography performed after 24 hrs of reperfusion showed higher ejection fraction in TRIM72 C144S vs WT mice (Fig 2B). Consistent with the ex vivo perfused heart data we found that TRIM72 C144S hearts exhibited a smaller infarct size compared to WT hearts (Fig 2C). Evans blue was used to delineate the area at risk, which, was similar between groups (Fig 2D). These results show that the TRIM72 C144S mutation is protective against myocardial I/R injury.
Figure 2. TRIM72 C144S is protective in an in vivo model of LAD occlusion.
Mice were subjected to A. LAD occlusion and ischemia for 45 min followed by 24 hr reperfusion. B. Ejection fraction, C. infarct size, and D. area at risk (AAR) were measured. Values are mean ± SEM (WT n=12; TRIM72 C144S n=8); T-tests were performed to assess significance (*p<0.05 compared to WT).
TRIM72 release is reduced following myocardial I/R injury in TRIM72 C144S hearts
I/R injury has been shown to decrease TRIM72 levels in the heart which is blocked by ischemic pre- and post-conditioning12,13. Our recent studies indicate that TRIM72 can be SNO at C144 during pre- and post-conditioning and that modification on this residue regulates TRIM72 protein levels and ischemic injury1,14. Recently, it was reported that TRIM72 secretion is actively regulated and as a cardiokine/myokine regulates insulin signaling19. We therefore tested whether C144 plays a role in the release of TRIM72 following I/R injury. To test whether TRIM72 C144S ex vivo perfused hearts had altered TRIM72 release we measured TRIM72 protein levels in the heart perfusate during the first 10 min of the reperfusion period following 20 min of ischemia (Fig 3). There was significantly less TRIM72 (normalized to Ponceau S stain for total protein) in the heart perfusate from the TRIM72 C144S vs WT hearts, suggesting that less TRIM72 is released from the heart upon reperfusion following ischemia and also showing that C144 may be a key regulator of this release.
Figure 3. TRIM72 release is reduced in TRIM72 C144S in response to I/R injury.
Hearts were subjected to A. 20 min ischemia and 10 min reperfusion and perfusate was collected during the 10 min reperfusion period. B. TRIM72 protein was measured by western blot in the heart perfusate and normalized to the Ponceau S stain for total protein. Values are mean ± SEM (WT n=4; TRIM72 C144S n=4); Mann-Whitney test was performed to assess significance * p<0.05 compared to WT
Whole body insulin sensitivity is maintained in TRIM72 C144S mice
As recent studies19 have reported that TRIM72 is secreted and impairs insulin sensitivity we further examined whether insulin sensitivity is altered in the TRIM72 C144S mouse by examining whole body glucose and insulin sensitivity in TRIM72 C144S and WT mice. Whole-body glucose and insulin tolerance (Fig 4) as well as body composition were not different between WT and TRIM72 C144S mice. Body weights were similar at 9–13 wks of age and % fat mass and % lean mass were not significantly different (Table 1). This suggests that the TRIM72 C144S mutation does not affect whole body insulin sensitivity.
Figure 4. TRIM72 C144S does not impair whole body insulin sensitivity.
A. Glucose tolerance (WT n=12; TRIM72 C144S n=12) and B. insulin tolerance tests (WT n=10; TRIM72 C144S n=12) were performed on mice fasted for 6 hrs. Values are mean ± SEM; One-way ANOVA with repeated measures with Bonferroni posthoc test were performed to assess significance
Table 1.
Body composition of WT and TRIM72 C144S mice.
| WT | TRIM72 C144S | |
|---|---|---|
| Body weight (g) | 25.52±0.48 | 24.92±0.40 |
| Fat mass (g) | 3.66±0.85 | 2.88±0.20 |
| Lean mass (g) | 21.1±0.37 | 20.25±0.36 |
| % fat mass | 10.48±0.92 | 11.50±0.72 |
| % lean mass | 82.88±0.89 | 81.29±0.83 |
Body composition was measured in WT and TRIM72 C144S mice using an echoMRI. Values are mean ± SEM (WT n=17; TRIM72 C144S n=17); T-test and Mann-Whitney test were performed to assess significance
TRIM72 C144S does not affect whole body substrate utilization
We also examined whole body energy metabolism to determine if this was potentially altered in the TRIM72 C144S mice. WT and TRIM72 C144S mice were placed in metabolic cages and oxygen consumption and the respiratory exchange ratio (RER) were assessed. TRIM72 C144S and WT mice showed similar oxygen consumption rates (Fig 5A) and there was no significant difference in the RER (Fig 5B). As such, the TRIM72 C144S mutation does not appear to affect whole body energy metabolism.
Figure 5. TRIM72 C144S mice do not have altered oxygen consumption and RER.
A. Oxygen consumption and B. RER were measured in mice with metabolic cages. Values are mean ± SEM; (WT n=5; TRIM72 C144S n=7); T-tests were performed to assess significance
Expression of TRIM72 E3 ubiquitin ligase targets are not altered in TRIM72 C144S mouse hearts.
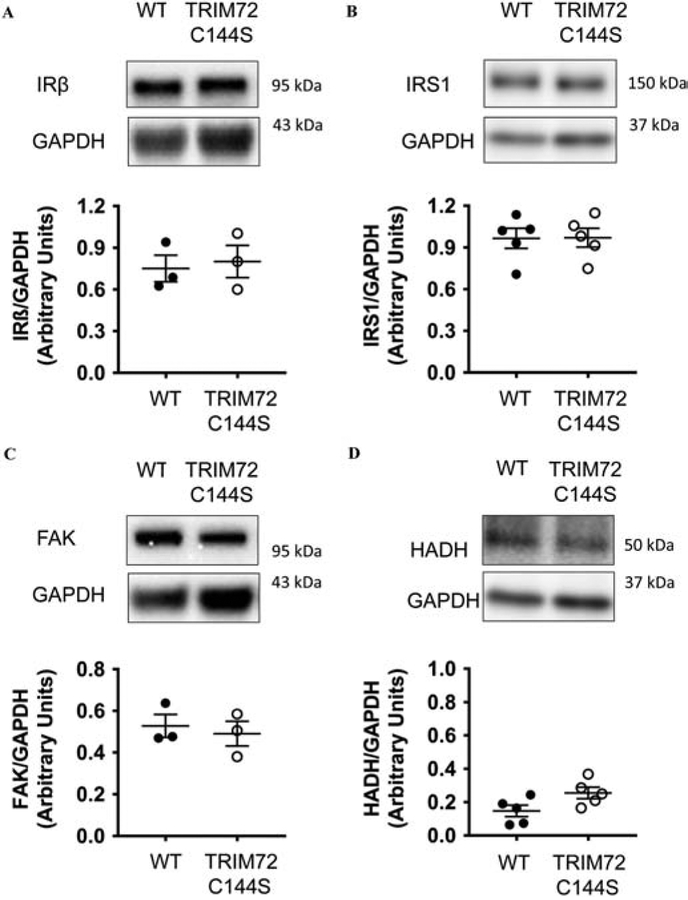
Recent studies have suggested that TRIM72 can regulate insulin sensitivity and development of cardiac hypertrophy15–17,19. Alterations in insulin sensitivity and metabolism could alter the response to I/R and these changes could contribute to the protection that we observed. In addition, the strategy of targeting C144 by SNO to promote cardioprotection could be problematic if this cysteine is important in modulating insulin sensitivity. We therefore tested whether mutation of C144 to serine might lead to altered insulin sensitivity. Recent studies suggest that TRIM72 regulates insulin sensitivity by targeting the insulin receptor and IRS115–17,19. Therefore, we examined insulin receptor β expression, but found no differences in expression between WT and TRIM72 C144S mouse hearts (Fig. 6A). We also examined IRS1 expression, but found no difference between TRIM72 C144S and WT hearts (Fig. 6B).
Figure 6. Expression of the E3 ubiquitin ligase targets of TRIM72 are not altered in TRIM72 C144S mouse hearts.
Protein expression of A. IRβ (WT n=3; TRIM72 C144S n=3), B. IRS1 (WT n=5; TRIM72 C144S n=5), C. FAK (WT n=3; TRIM72 C144S n=3) and D. HADH (WT n=5; TRIM72 C144S n=5) in WT and TRIM72 C144S mouse hearts. Values are mean ± SEM; T-tests were performed to assess significance
Focal adhesion kinase (FAK) is another E3 ubiquitin ligase target of TRIM7223, which has been shown to play a role in cardioprotection24,25. However, FAK expression was not different between WT and TRIM72 C144S mouse hearts (Fig. 6C). Finally, we indirectly assessed PPARα expression, which can be targeted by TRIM72 via transcriptional regulation to increase fatty acid metabolism15. Protein levels of HADH, an enzyme that is upregulated by PPARα, were not different between TRIM72 C144S and WT mouse hearts (Fig. 6D). These results suggest that alterations to insulin signaling and metabolism are not likely to contribute to the observed protection in TRIM72 C144S mice.
TRIM72 C144S does not induce cardiac hypertrophy
In addition to playing a role in membrane repair and insulin sensitivity, TRIM72 has also been identified as a regulator of cardiac hypertrophy15. We examined hearts from TRIM72 C144S mice for signs of cardiac hypertrophy but found no evidence of increased cardiac hypertrophy compared to WT mice (Fig 7). Cardiac function as measured by echocardiography was also not impaired in TRIM72 C144S compared to WT mice (Table 2).
Figure 7. Angiotensin II induced cardiac hypertrophy is not exacerbated in TRIM72 C144S mice.
A. Body weight (all groups n=6), B. heart weight (all groups n=6), C. heart weight/tibia length (all groups n=6), and D. ejection fraction (all groups n=5) of male WT mice treated with vehicle (WT+Veh) or Angiotensin II (WT+AngII) and male TRIM72 C144S mice treated with vehicle (TRIM72 C144S+Veh) or Angiotensin II (TRIM72 C144S+AngII). Two-way ANOVA with Bonferroni posthoc test were performed to assess significance, Values are mean ± SEM; * p<0.05 vs WT+Veh, > p<0.05 vs TRIM72 C144S+Veh
Table 2.
Cardiac Function of WT and TRIM72 C144S mice.
| WT | TRIM72 C144S | |
|---|---|---|
| Heart rate | 493.8±20.0 | 520.8±9.3 |
| Volume; s | 32.6±1.7 | 25.5±1.4* |
| Volume; d | 78.3±2.8 | 64.9±2.9* |
| EF | 58.3±0.9 | 60.7±0.8 |
| %FS | 30.5±0.6 | 32.1±0.5 |
| Diameter; s | 2.9±0.1 | 2.6±0.1* |
| Diameter; d | 4.2±0.1 | 3.9±0.1* |
| LVAW; d | 0.7±0.01 | 0.7±0.01 |
| LVAW; s | 1.1±0.02 | 1.1±0.01 |
| LVPW; d | 0.7±0.01 | 0.7±0.01 |
| LVPW; s | 1.1±0.01 | 1.1±0.02 |
| LV mass | 79.7±3.0 | 75.0±0.2 |
| LVOT mean gradient | 2.6±0.2 | 2.4±0.1 |
| LVOT mean velocity | 811.2±23.0 | 773.6±20.9 |
| mmHg peak gradient | 6.3±0.3 | 6.0±0.2 |
| mm/s Peak Velocity | 1255.0±27.9 | 1223.6±21.6 |
| PA mean gradient | 0.7±0.1 | 0.8±0.1 |
| PA mean velocity | 430.2±14.7 | 442.8±22.2 |
| PA peak gradient | 2.1±0.1 | 2.5±0.2 |
| PA peak Velocity | 731.2±15.7 | 784.6±37.3 |
Echocardiography was performed in WT and TRIM72 C144S male mice. Values are mean ± SEM (WT n=5; TRIM72 C144S n=5); T-test and Mann-Whitney test were performed to assess significance
p<0.05 vs WT
We considered that differences in hypertrophy may emerge following treatment with angiotensin II. WT and TRIM72 C144S mice were treated with Angiotensin II for 4 weeks and as expected, angiotensin II induced cardiac hypertrophy and a modest decline in ejection fraction in WT mice (Fig 7). However, angiotensin II treatment induced a similar degree of cardiac hypertrophy in TRIM72 C144S and WT hearts as measured by heart weigh/tibia length (Fig 7). These results suggest that the TRIM72 C144S mutation does not exacerbate the development of cardiac hypertrophy.
Discussion
A role for TRIM72 in membrane repair was reported a decade ago9 and from additional studies, it became apparent that TRIM72 is a key player in myocardial I/R injury13,26,27. Herein, we demonstrate for the first time that mutation of TRIM72 at C144 protects the mouse heart from ex vivo (Fig. 1) and in vivo (Fig. 2) models of I/R injury. Previous studies from our lab determined that TRIM72 can be SNO-modified at C144 in the context of ischemic pre- and postconditioning1. Furthermore, we demonstrated that SNO or mutation of C144 can protect TRIM72 from oxidation-induced degradation in the context of oxidative stress in HEK293 cells1.
In the current study, we sought to better understand the importance of C144 of TRIM72 in cardioprotection by generating a knock-in mouse with C144 mutated to a serine (TRIM72 C144S). Since the C144S mutation prevents SNO and more importantly oxidation, we expected that I/R injury would be reduced in TRIM72 C144S hearts. As hypothesized, TRIM72 C144S hearts exhibited better post-ischemic recovery of function and smaller infarcts compared to WT hearts using an ex vivo model of I/R injury (Fig 1). Similar results were also seen when we subjected the mice to LAD occlusion in vivo (Fig. 2). These exciting findings suggest that C144 may be a useful target for enhancing the cardioprotective capabilities of TRIM72. Surprisingly, we also observed that C144 regulates TRIM72 release and that TRIM72 release is reduced in TRIM72 C144S hearts vs WT following I/R injury (Fig 3). To address the possibility that the difference of TRIM72 in the perfusate is due to I/R injury induced differences in overall protein released between WT and TRIM72 C144S hearts, TRIM72 protein was normalized against total protein. However, it is still possible that the observed difference in TRIM72 release is a reflection of the degree of I/R induced cardiac injury making it at least partially more of a biomarker of injury as opposed to being directly regulated by the C144 residue. These data would be consistent with the hypothesis that the decrease in TRIM72 following I/R injury is due to release of TRIM72 and that a free C144 is needed to facilitate release of TRIM72. Taken together, these findings support a mechanism whereby the TRIM72 C144S mutant yields protective effects by keeping more TRIM72 inside of the cell and thus C144 may be a useful target for enhancing the cardioprotective capabilities of TRIM72.
The development of insulin resistance has been shown to occur in TRIM72 overexpressing mice by seven months of age16. In these transgenic mice, TRIM72 expression was negatively correlated with insulin signaling15,17. A correlation between TRIM72 expression and reduced IRS1 signaling has also been observed in C2C12 cells18. Additional mechanistic studies suggest that this reduction in insulin signaling results from the ubiquitination of IRS1 by TRIM72, leading to IRS1 degradation16,17. The regulation of insulin signaling by TRIM72 has also been reported in vivo, with TRIM72 knockout mice showing resistance to HFD-induced insulin resistance16,17. In addition, the actions of TRIM72 as a cardiomyokine/myokine have been implicated in the negative effects of TRIM72 on insulin resistance. Secreted TRIM72 binds to the insulin receptor decreasing insulin signaling19. Another recent study showed that TRIM72 can act as a transcription factor to increase PPARα expression, which regulates fatty acid oxidation and can impair insulin sensitivity15. Hearts from aged cardiac specific TRIM72 overexpressing mice have been reported to have elevated PPARα expression, fatty acid metabolism, and reduced sensitivity of the insulin signaling pathway to insulin stimulation15. Since these detrimental effects could offset any beneficial role for TRIM72 during I/R injury, we tested whether mutation of C144 to serine altered insulin sensitivity or cardiac hypertrophy.
We initially examined insulin and glucose sensitivity but we found no impairment in glucose tolerance, insulin sensitivity, or whole-body metabolism in TRIM72 C144S mice compared to WT at baseline (Fig. 4 and 5). Furthermore, we examined the expression of insulin receptor β, IRS1, and FAK, which are known to be targets of TRIM72 as an E3 ubiquitin ligase and found no difference in expression between WT and TRIM72 C144S hearts (Fig. 6). The expression of HADH, which is regulated by PPARα, was also not different (Fig. 6).
Finally, we examined cardiac hypertrophy in TRIM72 C144S mice and WT mice at baseline, but found no evidence of hypertrophy. Angiotensin II also failed to reveal a difference in cardiac hypertrophy between WT and TRIM72 C144S hearts (Fig. 7). Since TRIM72 overexpression has, in contrast, been reported to induce cardiac hypertrophy and insulin resistance15–17, these data suggest that the C144S mutation of TRIM72 provides cardioprotection during I/R injury without promoting insulin resistance or contributing to the development of cardiac hypertrophy.
Conclusions
In summary, this study identifies C144 of TRIM72 as a major protective target in myocardial I/R injury. TRIM72 C144S hearts were protected against I/R injury and this protection was accompanied by a reduction in the cardiac release of TRIM72 C144S during reperfusion. Importantly, the TRIM72 C144S mutation did not impair insulin sensitivity or promote the development of cardiac hypertrophy compared to WT mice. Overall, these results suggest that targeting C144 of TRIM72 may be an effective strategy for reducing ischemic injury in the heart, without having deleterious effects on insulin sensitivity or cardiac hypertrophy.
Highlights:
TRIM72 is a membrane repair protein that protects against I/R injury.
We generated a TRIM72 knock-in mouse with C144 mutated to a serine
TRIM72 C144S hearts were protected against ex vivo and in vivo models of I/R injury
TRIM72 C144S hearts showed no changes insulin sensitivity or cardiac hypertrophy.
Acknowledgements
We acknowledge support from National Institutes of Health Grants: ZIA HL002066 and ZIA HL006059 to EM; R01HL136496 to MK; and T32ES0741 to KC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kohr MJ, Evangelista AM, Ferlito M, Steenbergen C & Murphy E S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. Journal of molecular and cellular cardiology 69, 67–74, doi: 10.1016/j.yjmcc.2014.01.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohr MJ et al. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circulation research 108, 418–426, doi: 10.1161/CIRCRESAHA.110.232173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen TT et al. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. The Journal of biological chemistry 286, 40184–40192, doi: 10.1074/jbc.M111.243469 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Morgan M, Shen RF, Steenbergen C & Murphy E Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circulation research 101, 1155–1163, doi: 10.1161/CIRCRESAHA.107.155879 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Shao Q et al. Characterization of the sex-dependent myocardial S-nitrosothiol proteome. American journal of physiology. Heart and circulatory physiology 310, H505–515, doi: 10.1152/ajpheart.00681.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menazza S et al. Molecular Signature of Nitroso-Redox Balance in Idiopathic Dilated Cardiomyopathies. Journal of the American Heart Association 4, e002251, doi: 10.1161/JAHA.115.002251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohr MJ et al. Measurement of S-nitrosylation occupancy in the myocardium with cysteine-reactive tandem mass tags: short communication. Circulation research 111, 1308–1312, doi: 10.1161/CIRCRESAHA.112.271320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohr MJ et al. Characterization of potential S-nitrosylation sites in the myocardium. American journal of physiology. Heart and circulatory physiology 300, H1327–1335, doi: 10.1152/ajpheart.00997.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai C et al. MG53 nucleates assembly of cell membrane repair machinery. Nature cell biology 11, 56–64, doi: 10.1038/ncb1812 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai C et al. MG53 regulates membrane budding and exocytosis in muscle cells. The Journal of biological chemistry 284, 3314–3322, doi: 10.1074/jbc.M808866200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisleder N, Takeshima H & Ma J Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol 2, 225–226 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao CM et al. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation 121, 2565–2574 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y et al. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovascular research 91, 108–115, doi: 10.1093/cvr/cvr029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong G et al. Postconditioning leads to an increase in protein S-nitrosylation. Am J Physiol Heart Circ Physiol 306, H825–832, doi: 10.1152/ajpheart.00660.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor alpha. Circulation 131, 795–804, doi: 10.1161/CIRCULATIONAHA.114.012285 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Song R et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 494, 375–379, doi: 10.1038/nature11834 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Yi JS et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nature communications 4, 2354, doi: 10.1038/ncomms3354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CS et al. TRIM72 negatively regulates myogenesis via targeting insulin receptor substrate-1. Cell death and differentiation 17, 1254–1265, doi: 10.1038/cdd.2010.1 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Wu HK et al. Glucose-Sensitive Myokine/Cardiokine MG53 Regulates Systemic Insulin Response and Metabolic Homeostasis. Circulation 139, 901–914, doi: 10.1161/CIRCULATIONAHA.118.037216 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Ma H et al. Effect of metabolic syndrome on mitsugumin 53 expression and function. PloS one 10, e0124128, doi: 10.1371/journal.pone.0124128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiwata-Endo H et al. Role of a TRIM72 ADP-ribosylation cycle in myocardial injury and membrane repair. JCI Insight 3, doi: 10.1172/jci.insight.97898 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casin KM et al. S-Nitrosoglutathione Reductase Is Essential for Protecting the Female Heart From Ischemia-Reperfusion Injury. Circulation research 123, 1232–1243, doi: 10.1161/CIRCRESAHA.118.313956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen N, Yi JS, Park H, Lee JS & Ko YG Mitsugumin 53 (MG53) ligase ubiquitinates focal adhesion kinase during skeletal myogenesis. The Journal of biological chemistry 289, 3209–3216, doi: 10.1074/jbc.M113.525154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Z et al. Targeted focal adhesion kinase activation in cardiomyocytes protects the heart from ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 32, 924–933, doi: 10.1161/ATVBAHA.112.245134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perricone AJ, Bivona BJ, Jackson FR & Vander Heide RS Conditional knockout of myocyte focal adhesion kinase abrogates ischemic preconditioning in adult murine hearts. Journal of the American Heart Association 2, e000457, doi: 10.1161/JAHA.113.000457 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao CM et al. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation 121, 2565–2574, doi: 10.1161/CIRCULATIONAHA.110.954628 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Wang X et al. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circulation research 107, 76–83, doi: 10.1161/CIRCRESAHA.109.215822 (2010). [DOI] [PubMed] [Google Scholar]