SUMMARY
Frequent mutation of PI3K/AKT/mTOR signaling pathway genes in human cancers has stimulated large investments in targeted drugs but clinical successes are rare. As a result, many cancers with high PI3K pathway activity such as triple-negative breast cancer (TNBC) are treated primarily with chemotherapy. By systematically analyzing responses of TNBC cells to a diverse collection of PI3K pathway inhibitors, we find that one drug, Torin2, is unusually effective because it inhibits both mTOR and PI3K-like kinases (PIKKs). In contrast to mTOR-selective inhibitors, Torin2 exploits dependencies on several kinases for S-phase progression and for cell cycle checkpoints, thereby causing accumulation of single-stranded DNA and death by replication catastrophe or mitotic failure. Thus, Torin2 and its chemical analogs represent a mechanistically distinct class of PI3K pathway inhibitors that are uniquely cytotoxic to TNBC cells. This insight could be translated therapeutically by further developing Torin2 analogs or combinations of existing mTOR and PIKK inhibitors.
Graphical Abstract

eTOC
In tumors with high PI3K pathway activity, such as triple-negative breast cancer (TNBC), selective inhibitors of PI3K/AKT/mTOR pathway kinases exhibit limited efficacy as single agents. To identify possible co-targeting opportunities, we systematically analyzed responses to a compendium of PI3K/AKT/mTOR inhibitors with varying degrees of polyselectivity. We found that preclinical drugs that inhibit both mTOR and PI3K-like kinases are more potent and effective than most clinical-grade PI3K pathway inhibitors. These polyselective mTOR inhibitors warrant further investigation as a new strategy for targeting TNBC.
INTRODUCTION
Triple-negative breast cancers (TNBCs) are high-grade, invasive mammary ductal carcinomas defined by absence of estrogen/progesterone receptor expression and HER2-amplification (Foulkes et al., 2010). TNBCs are managed primarily with chemotherapy, but early disease relapse and poor overall survival underscore the need for new treatments (Foulkes et al., 2010). TNBCs have the highest inferred PI3K/AKT/mTOR signaling activity of all breast cancer subtypes, providing a rationale for use of PI3K pathway inhibitors (Cancer Genome Atlas Network, 2012). High PI3K pathway activity in TNBC results both from activating mutations in PIK3CA, which encodes the catalytic subunit of a phosphoinositide kinase, and from reduced expression of PTEN and INPP4B, which encode phosphoinositide phosphatases (Cancer Genome Atlas Network, 2012). PI3K pathway kinases have been targeted with >40 small-molecule drugs but clinical responses to date have been modest (Janku et al., 2018). PI3K pathway drugs are non-selective for transformed cells, which also limits their therapeutic index (Chia et al., 2015). Isoform-selective PI3K inhibitors are better tolerated but only sporadically active in solid tumors, possibly due to insufficient pathway inhibition or feedback mechanisms that cause pathway reactivation (Elkabets et al., 2013; Schwartz et al., 2015).
One way to overcome such limitations is to combine PI3K pathway inhibitors with other drugs or exploit the polyselectivity of existing molecules (André et al., 2019; Knight et al., 2010). Whereas many PI3K pathway drugs inhibit several PI3K isoforms and/or mTORC1/2, some also inhibit PI3K-like kinases (PIKKs) due to structurally similar ATP binding sites. For example, the PI3K/mTOR inhibitor dactolisib (NVP-BEZ235) and the mTORC1/2 inhibitors Torin1 and Torin2 also inhibit ATR, ATM, and/or DNA-PK (Liu et al., 2010, 2013; Toledo et al., 2011). PIKK inhibition may be particularly relevant to TNBC because of frequent alterations in TP53, MYC, and CCNE1, which cause increased dependency on PIKKs due to defective DNA damage checkpoints and increased replication stress (Cancer Genome Atlas Network, 2012; Lin et al., 2017).
In this paper, we analyzed the responses of TNBC cells with an activated PI3K pathway to a diverse collection of PI3K pathway inhibitors. We found that most drugs neither fully block proliferation nor cause cell death. In contrast, the preclinical compound Torin2 induced apoptosis in all cell lines tested. To determine which of the four high-affinity targets of Torin2 is responsible, we used a chemical genetic approach involving dose-response studies in multiple cell lines, analysis of chemical analogs and pharmacological reconstitution with mixtures of selective inhibitors. Phenotypes were determined using single-cell, time-course and pulse-labeling assays. These studies show that inhibition of both mTORC1/2 and PIKKs is responsible for the high activity of Torin2 in TNBC. Thus, co-targeting the two classes of kinases with a single polyselective drug or a mixture of selective compounds makes it possible to exploit replicative and checkpoint vulnerabilities unique to triple-negative breast cancers to promote selective cell killing.
RESULTS
Torin2 produces strong cytotoxic and anti-proliferative effects in TNBC cells
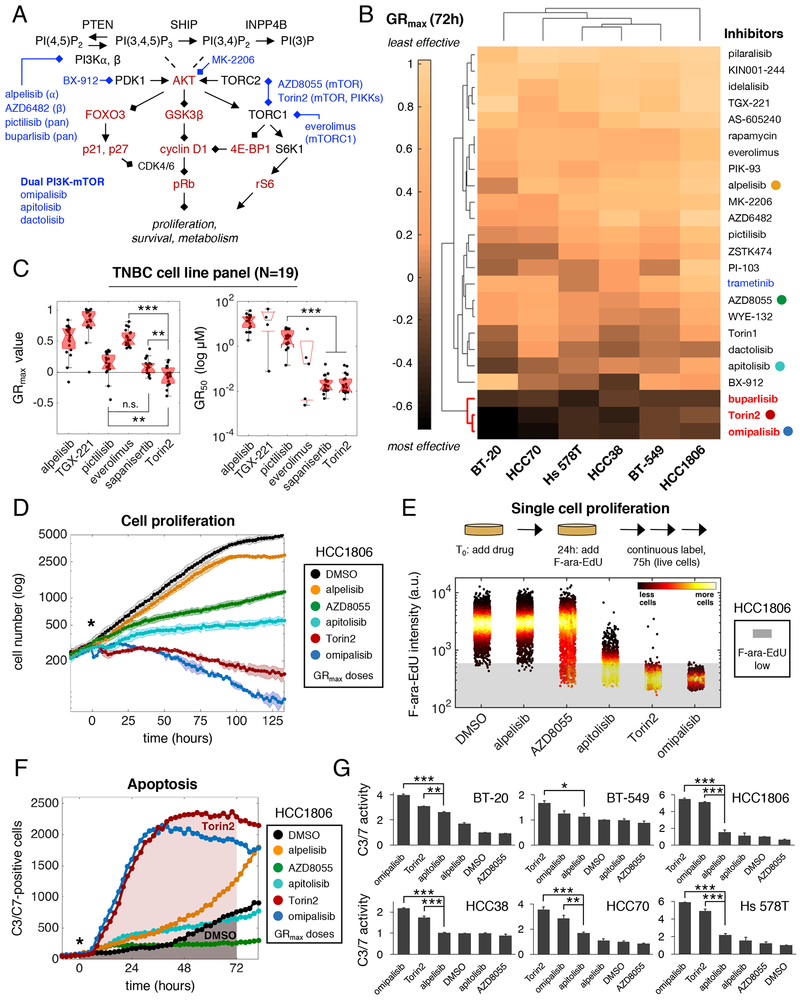
Breast cancer cell lines are classified as basal or luminal based on gene expression (Neve et al., 2006). We performed western blotting on 46 cell lines and found that those with basal-like gene expression had uniformly low levels of PTEN and/or INPP4B (Figure S1A, Table S1); five such lines were selected for compound screening (HCC1806, Hs 578T, HCC38, BT-549 and HCC70). A sixth basal line (BT-20) with an activating PIK3CA mutation (H1047R) but normal levels of PTEN and INPP4B was also screened. All six lines lack hormone receptor expression and HER2 amplification and are homozygous for TP53 mutations (Table S2). We assembled a diverse panel of 23 PI3K pathway inhibitors that included clinically approved drugs, investigational agents and tool compounds; the MEK inhibitor trametinib served as a MAPK pathway-specific comparator (Figure 1A; Table S3). Sub-confluent cultures were treated for 72h with each drug at nine doses and viable cells counted by microscopy at time t=0 and 72h (Figure S1B). Dose-response data were analyzed using growth rate inhibition (GR) metrics, which quantify drug sensitivity while correcting for differences in cell division times (Hafner et al., 2016): GR50 is analogous to IC50 and measures potency, while GRmax is analogous to Emax and measures maximal drug effect (Figure S1C). A value of GRmax <0 indicates net cell loss, a value of zero represents no change in viable cell number, and a value >0 indicates net cell gain. By convention, GRmax = 1 in control cultures, which were treated with DMSO only.
Figure 1. Torin2 produces strong cytotoxic and anti-proliferative effects in TNBC cells.
A. Schematic of the PI3K pathway with select drugs (blue) and measured proteins (red). B. Clustergram heatmap of mean GRmax values for 24 drugs in six TNBC cell lines; N=3 experiments. Dark shading denotes GRmax<0 (i.e., cytotoxicity). Red: highly effective drugs; blue: MAPK inhibitor. Colored circles mark drugs analyzed in panels D-G. C. GRmax, GR50 values in 19 TNBC cell lines. **P<0.01, ***P<0.001, and n.s. (not significant) by Mann-Whitney U test. D. Growth curves for HCC1806 NLS-mCherry cells. “*” denotes start of treatment. Data points/shading depict mean±SD of 3 replicates in a representative time-lapse experiment; N=3 experiments. E. Mean nuclear F-ara-EdU intensity values in HCC1806 cells after treatment for ~100h; N=3 experiments, representative data shown. “a.u.” indicates arbitrary units. Shading indicates unlabeled cells. F. C3/C7-positive cell counts over time as detected by live-cell imaging of HCC1806 cells. Shading indicates AUC for 72h of drug exposure. G. C3/C7 activity (i.e., mean fold-change in AUC±SEM for drug vs. DMSO); N=2 experiments. *P<0.05, **P<0.01, ***P<0.001 by one-way ANOVA and Tukey’s test (selected comparisons shown). Drugs were used at GRmax doses (1–3.2μM) in D-G, with exceptions as noted in STAR methods. See also Figure S1, Tables S1–3.
Highly effective responses (i.e., negative GRmax values) were rare across the 144 drug-cell line combinations examined (Figure 1B), but three drugs were broadly cytotoxic: the phase 3 PI3K p110 pan-isoform inhibitor buparlisib, the phase 1 PI3K/mTOR inhibitor omipalisib and the preclinical mTOR/PIKK inhibitor Torin2. Omipalisib and Torin2 were also highly potent, with nanomolar GR50 values (Figure S1D). Torin2 is the least studied molecule of the three, but we found it to be more cytotoxic in 19 basal-like cell lines than sapanisertib, an mTORC1/2 inhibitor presently in clinical trials (Figure 1C). Torin2 exhibited similar efficacy to sapanisertib in luminal breast cancer cell lines (N=7), and caused little or no cytotoxicity in non-malignant mammary epithelial cells (N=2) (Figure S1E). Thus, Torin2 may represent an improved way to target TNBC.
To identify factors influencing drug response, we assayed proliferation and apoptosis in live cells. To enable a principled comparison, compounds were used at the lowest concentration eliciting a GRmax response (i.e., the ‘GRmax dose’). We imaged HCC1806 cells expressing NLS-mCherry continuously for >5 days (~4.5 cell divisions; division time Td=28h) in the presence of DMSO or several drugs whose GRmax values span a broad range. Drugs with positive GRmax values, such as alpelisib (GRmax=0.92), AZD8055 (GRmax=0.56) and apitolisib (GRmax=0.37), reduced viable cell number but did not fully block proliferation (Figure 1D). Conversely, drugs with negative GRmax values such as Torin2 (GRmax=−0.16) and omipalisib (GRmax=−0.29) steadily reduced cell number. At a 10-fold lower dose (0.32μM) of Torin2 or omipalisib, there was still no increase in cell number over time, consistent with complete cytostasis or a balance between cell division and death (Figure S1F).
As another measure of proliferation, we exposed HCC1806 cells to drugs for 24h and then added F-ara-EdU, a non-toxic thymidine analog that is incorporated into newly-synthesized DNA, for an additional 75h (2.7xTd) (Neef and Luedtke, 2011). GRmax doses of alpelisib, AZD8055, and apitolisib were only partly effective at inhibiting F-ara-EdU labeling, resulting in 0.7%, 6.3%, and 65.4% unlabeled cells; in contrast, Torin2 and omipalisib each inhibited labeling of >99% of viable cells (Figures 1E, S1G). Thus, the low GRmax values of Torin2 and omipalisib are associated with fewer cells that actively synthesize DNA during drug exposure.
To assay apoptosis, we exposed six cell lines to drugs in the presence of a fluorogenic substrate of executioner caspases 3 and 7 (C3/C7) and measured substrate cleavage by imaging (Figure S1H). We then calculated the fold-change in the area under the fluorescence curve (AUC) between 0 and 72h relative to a DMSO control (Figures 1F–G). Drugs such as AZD8055 induced no detectable C3/C7 activity in any cell line. In contrast, Torin2 and omipalisib increased C3/C7 activity by an average of ~3.5-fold across all six lines. Overall, these data show that low GRmax values for Torin2 and omipalisib in TNBC cells result from effective inhibition of proliferation and induction of apoptosis.
PI3K/AKT/mTOR inhibitors impede progression of S phase
To evaluate the effects of Torin2 on signaling, we exposed TNBC cells to GRmax doses of drugs for 24h and then quantified the levels or localization of nine proteins/phosphoproteins by immunofluorescence microscopy (Figures 1A, 2A, S2A–C). AKT activity was inferred from (i) phosphorylation at T308 and S473, (ii) phosphorylation of GSK3β at S9 and (iii) nuclear localization of FoxO3. The activities of mTORC1, mTORC2, and S6K1 were assayed by phospho-4E-BP1 T37/46, phospho-AKT S473 and phospho-S6 S235/236. We also measured levels of cyclin D1, p21 and p27, which are regulated by the PI3K pathway. Based on changes in these proteins/phosphoproteins, Torin2 and omipalisib were found to be the most effective PI3K pathway inhibitors (Figures 2A, S2D). To assay the “output” of the PI3K pathway, we quantified CDK-dependent phosphorylation of retinoblastoma protein on S807/811 (p-pRb). When mitogenic signaling is low, CDK activity is also low, pRb is dephosphorylated and E2F is inhibited, causing G1 arrest (Duronio and Xiong, 2013). In HCC1806 cells, exposure to Torin2 or omipalisib for t=Td increased the percentage of p-pRb-low cells from 5% to 64% and 81%, compared to 6-42% for six other PI3K pathway drugs (Figures 2B, S2E). Thus, low GRmax values for Torin2 and omipalisib correlate with greater suppression of PI3K signaling and lower levels of p-pRb (Liang and Slingerland, 2003).
Figure 2. PI3K/AKT/mTOR inhibitors impede progression of S phase.
Drugs were used at GRmax doses (1–3.2μM) throughout, with exceptions as noted in STAR methods. A. Clustergram heatmaps rank the activity of PI3K pathway drugs based on levels and/or localization of nine proteins/phosphoproteins at 24h. Data normalized to DMSO; N≥2 experiments. B. Mean nuclear intensity values of p-pRb S807/811 in HCC1806 cells at t=Td (28h). C. Cell cycle distributions at t=Td for BT-20 (48h), HCC70 (45h) and HCC1806 (28h) based on analysis of DNA vs EdU content. Colors: G0/1 (black), S phase (red), S-phase non-replicating (SNR; yellow), and G2/M (blue). Drugs are ordered from least to most effective (top to bottom) based on viable cell counts. D. Top row: DNA vs EdU content at t=Td. Data points for DMSO appear in background (grey); colors indicate different cell cycle stages as in C. Middle, bottom rows: DNA vs mean nuclear intensity of geminin or cyclin A2. Colors indicate different cell cycle stages determined by DNA and EdU content values measured in the same cells. E. Quantification of total nuclear EdU content in gated S-phase cells (red cells, top row, panel D; SNR cells excluded). Al:alpelisib, A8:AZD8055, D:DMSO, M:MK-2206, O:omipalisib, R:rapamycin, T2:Torin2. ***, P<0.001 for each drug vs DMSO (Mann-Whitney U test). F. Changes in intracellular polar metabolites after exposure of BT-549 cells to drugs for t=0.15xTd (6h). Nucleotides and precursors are labeled. See also Figures S2–3, Table S4.
To determine if Torin2 inhibits cell cycle progression at G1/S to a greater extent than other PI3K pathway drugs, we stained DNA with Hoechst and pulse-labeled S-phase cells with EdU (Figure S3A). Cell lines with different division times were compared by exposing cells to drugs for t=Td and then labeling with EdU for 0.025xTd (40-70min). EdU-positive cells were scored as being in S phase and EdU-negative cells scored as being in G0/G1 or G2/M based on DNA content. EdU-negative cells with DNA content intermediate between G0/G1 and G2/M cells were scored as S-phase-non-replicating (SNR) cells (Shi et al., 2001).
Exposure of BT-20 cells to 0.3xGRmax dose (1μM) of Torin2 or omipalisib for t=Td (48h) increased the G1 fraction to ~85% (from 55% in DMSO control) and reduced the S-phase fraction to 0% (from 33% in DMSO control; Figure 2C). In contrast, GRmax doses of drugs less effective than Torin2 at suppressing PI3K pathway activity (i.e., alpelisib, rapamycin) increased the G0/G1 fraction to a lesser degree (76–79%) and incompletely reduced the S-phase fraction (to 13–15%). Thus, in PIK3CA-mutant TNBC cells, the high activities of Torin2 and omipalisib are associated with increased blockade of cells at G1/S and elimination of S-phase cells.
In contrast, exposure of other cell lines to Torin2 or omipalisib, even at doses that blocked proliferation, did not eliminate S-phase cells (Figures 2C, S3B). For example, 26-41% of HCC70 cells were in S phase after exposure to Torin2 or omipalisib for t=Td (45h). To determine if this phenotype arises because Torin2 and other PI3K pathway drugs impede progression of cells through S phase, we quantified the amount of EdU incorporated into DNA during pulse labeling. To identify S-phase cells independent of DNA synthesis, we measured levels of expression of the APC/C substrates geminin and cyclin A2 by immunofluorescence. In HCC70 cells, multiple PI3K pathway drugs reduced EdU content in geminin- and cyclin A2-positive cells (Figures 2D–E). Torin2 suppressed DNA synthesis to the greatest degree and caused accumulation of SNR cells (~9% of all cells). In HCC1806 cells, Torin2 elicited even stronger inhibition of S phase, with accumulation of >20% SNR cells (Figures 2D–E, S3C–D). These SNR cells exhibited decreased levels of geminin, cyclin A2 and p-pRb, consistent with cell cycle exit (Figures 2D, S3E). PI3K pathway drugs also reduced EdU content in BT-549 and Hs 578T cells, with Torin2 again having the strongest effect (Figures 2E, S3F–G). The same was true of BT-20 cells exposed to drugs for less time (t=0.125-0.3xTd; 6-14h), substantially earlier than the G0/G1 arrest that occurred by t=Td (Figures S3H–I). Thus, Torin2 and other PI3K pathway drugs not only inhibit the cell cycle at G1/S, but also interfere with progression of S phase.
The PI3K pathway is known to play a role in nucleotide metabolism (Wang et al., 2009). We therefore hypothesized that exposure of TNBC cells to PI3K pathway drugs impedes S phase by decreasing levels of DNA precursors (Juvekar et al., 2016a). To test this idea, we performed targeted metabolomic profiling by mass spectrometry following a short exposure of BT-549 cells to one of three mTOR inhibitors (rapamycin, AZD8055, or Torin2) or DMSO for t=0.15xTd (6h). Drug-exposed cells exhibited changes in levels of multiple purine and pyrimidine deoxyribonucleotides and their precursors, consistent with inhibition of both de novo synthesis and salvage pathways (Figure 2F, Table S4). Torin2 elicited the largest number of changes, but all three drugs elicited higher levels of cytidine and cytosine, key substrates in the salvage of pyrimidines. The drugs also increased levels of deoxythymidine monophosphate (dTMP) and decreased levels of deoxythymidine diphosphate (dTDP), consistent with reduced activity of deoxythymidylate kinase, an enzyme that acts downstream of both de novo pyrimidine synthesis and salvage pathways. Thus, it is likely that Torin2 and other drugs we tested inhibit S-phase progression at least in part by depleting TNBC cells of nucleotides required for DNA replication.
Torin2 causes substantial cell killing during S/G2
To better understand how Torin2 acts on cells in different cell cycle stages, we performed time-lapse imaging of asynchronous HCC1806 cells stably expressing H2B-mTurquoise and mVenus-hGeminin(1-110) (Sakaue-Sawano et al., 2008). Cells were exposed to DMSO or 0.3xGRmax dose (1μM) of omipalisib or Torin2 and then followed for 48h (N=340 single cells tracked and analyzed; Figure 3A). H2B-mTurquoise was used to identify nuclei, monitor cell division and score cell death by nuclear fragmentation. Cell cycle stage was determined by the fluorescence intensity level of mVenus, which is low in G0/G1 and high in S/G2. In the presence of DMSO, single cells divided every 24.2±0.8h, consistent with measured population doubling times, and only ~6% died (Figure 3A). Exposure to omipalisib caused death of 38% of cells (N=43/112) and blocked progression of 55% (N=61/112); the remainder of cells were incompletely blocked (Figures 3A–B). Torin2 was more cytotoxic, killing 51% of cells (N=62/122) and blocking progression of 43% (N=53/122).
Figure 3: Torin2 causes substantial cell killing during S/G2.
A. Time-lapse imaging of asynchronous HCC1806 cells expressing H2B:mTurquoise and mVenus:hGeminin(1-110). mTurquoise was used to score cell division and death; mVenus intensity levels were used to identify cell cycle stage, as illustrated for a representative DMSO-exposed cell. Heatmaps show the progression of 340 cells through the cell cycle after exposure to DMSO or a 0.3xGRmax dose (1μM) of omipalisib or Torin2 for 48h. Colors denote cell cycle stages and cell fates (see legend). B. Frequency of deaths and cell cycle block (left) and distribution by cell cycle stage (right). C. Frequencies of response classes leading to G1 death/block, based on cell cycle stage at the time of drug exposure. Class 1: a cell in G1 is killed/blocked in the same G1; class 2: a cell in S/G2 divides but the daughter cell is killed/blocked in the following G1; class 3: similar to class 2, except the parental cell starts in G1. D. Phenotypic responses caused by drug exposure. Shown are representative traces of mVenus fluorescence intensity vs time for single cells treated with omipalisib (blue) or Torin2 (red). The trace for a DMSO-exposed cell is shown in the background (grey) for comparison.
Confirming data obtained from fixed cells, live-cell imaging showed that Torin2 and omipalisib are active during both G1 and S/G2 phases of the cell cycle. In the presence of omipalisib, similar numbers of cells died in S/G2 (44%; N=19/43) as in G1 (56% of cells; N=24/43) (Figures 3A–B). In contrast, 8-fold more cells exposed to Torin2 died in S/G2 (89% of cells, N=55/62) than in G1 (11%, N=7/62). Among surviving cells, Torin2 also imposed a stronger S/G2 block than omipalisib: 30% of blocked cells (N=16/53) were in S/G2 after exposure to Torin2 compared to only 2% (N=1/61) for omipalisib. The strong effects of Torin2 on S/G2 cells caused the sequence of events leading to G1 block or death to differ between the two drugs. For example, the majority of omipalisib-treated cells that died in G1 (66.7%, N=16/24) or remained blocked there (65%, N=39/60) were in S/G2 at the time of drug exposure; they then proceeded through mitosis to the following G1 where they either died or arrested (G1 “class 2” death or block; Figures 3C–D). In contrast, G1 class 2 deaths (57.1%, N=4/7) and block (40.5%, N=15/37) were less frequent in the presence of Torin2 because the majority of cells in S/G2 at the time of initial drug exposure never reached mitosis. We conclude that both Torin2 and omipalisib are active on cells in G1 and S/G2, but that exposure to Torin2 causes substantially greater arrest and death of S/G2 cells than omipalisib.
Torin2 causes replication catastrophe
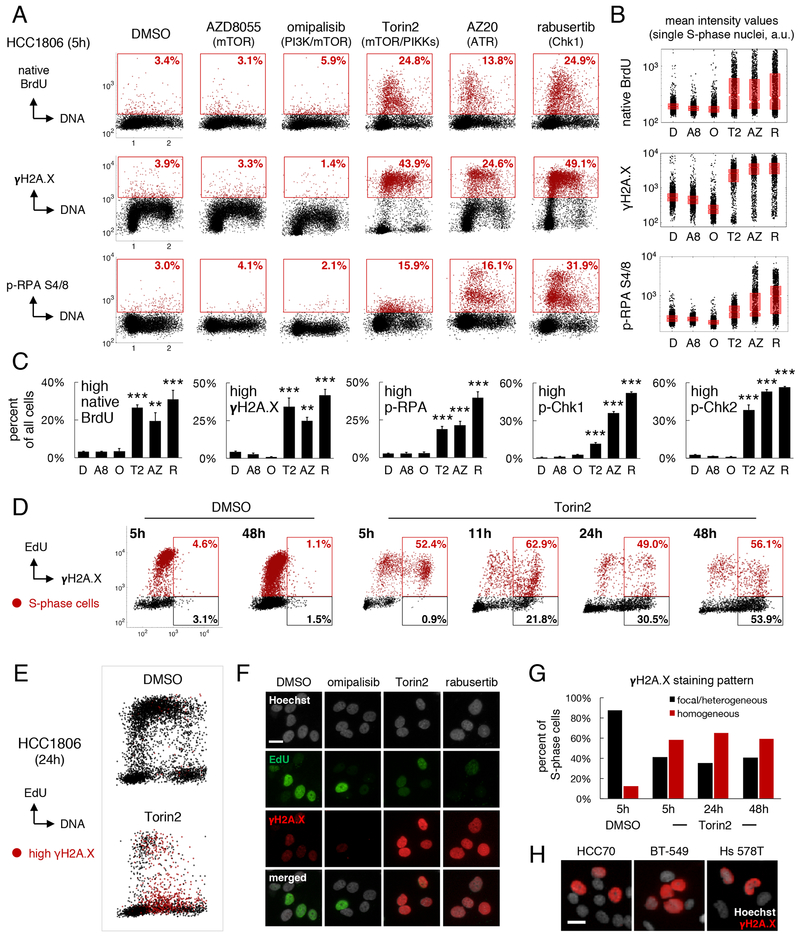
Impaired S-phase progression by nucleotide insufficiency or other genotoxic insults is a cardinal manifestation of “replication stress” (Zeman and Cimprich, 2014). Stalled replication forks cause accumulation of ssDNA because the activities of DNA polymerases and replicative helicases become uncoupled; excess ssDNA can then consume replication factors and cause DNA breakage and death by “replication catastrophe” (Toledo et al., 2013). To determine if exposure to Torin2 causes accumulation of ssDNA, HCC1806 cells were labeled with BrdU for t=Td, treated with GRmax doses of drug for t=0.2xTd (5h) and immunostained with anti-BrdU antibodies under non-denaturing (“native”) conditions. Exposure to Torin2, but not to omipalisib or AZD8055, increased the fraction of cells with elevated ssDNA ~8-fold (Figures 4A, labeled red; 4C) and mean nuclear BrdU intensity in gated S-phase cells ~2-fold (Figure 4B). Similar increases were observed with AZ20 and rabusertib, selective inhibitors of ATR kinase and its substrate kinase Chk1, which act together to stabilize stalled replication forks and suppress origin firing (Zeman and Cimprich, 2014). Exposure of HCC1806 cells to Torin2, AZ20 and rabusertib also increased levels of phosphorylated H2A.X S139 (γH2A.X), a marker of DNA damage, and of phosphorylated RPA S4/8 (p-RPA), a ssDNA-binding protein (Figures 4A–C). Similar effects were seen in HCC70 cells but after longer periods of drug exposure (~0.5xTd, 24h) (Figures S4A–B). In HCC1806 cells, these responses were associated with activated intra-S-phase checkpoint signaling, as evidenced by a ~15-fold increase in the fraction of cells with elevated phosphorylated Chk1 S317 (p-Chk1) and Chk2 T68 (p-Chk2) (Figures 4C, S4C). In contrast, neither AZD8055 nor omipalisib caused any detectable increase in ssDNA, γH2A.X, p-RPA, p-Chk1 or p-Chk2, despite inhibiting DNA synthesis. Thus, Torin2 is unique among the PI3K pathway drugs we tested in causing accumulation of ssDNA, DNA damage and increased checkpoint signaling in TNBC cells.
Figure 4: Torin2 causes replication catastrophe.
HCC1806 cells were exposed to 0.3-1xGRmax drug doses (1–3.2μM) throughout. A. DNA content vs. intensities of native BrdU, γH2A.X or p-RPA after drug exposure for ~0.2xTd (5h). Cells with high levels are gated (red). Percentages are of all cells. B. Quantification of nuclear intensity values in gated S-phase cells. Boxplots show median and 25th/75th percentiles. D:DMSO, A8:AZD8055, O:omipalisib, T2:Torin2, AZ:AZ20, R:rabusertib. C. Mean percent±SEM of cells with high levels of the indicated markers; N=3 experiments. **P<0.01, ***P<0.001 vs DMSO by one-way ANOVA and Dunnett’s test. D. Mean nuclear intensity of EdU vs γH2A.X after treatment with DMSO or 1μM Torin2 for the indicated times. EdU/γH2A.X double-positive cells (red gate) and EdU-negative/γH2A.X-positive cells (black gate) are quantified as a percent of all EdU-positive (red) or EdU-negative (black) cells. E. DNA vs mean EdU intensity at 24h post-treatment with DMSO or 1μM Torin2. Cells with γH2A.X >103 a.u. are red. F. Pan-nuclear γH2A.X staining pattern in S-phase/SNR cells at 24h post-treatment with 1μM of drugs. G. Percent of S-phase cells with pan-nuclear γH2A.X (red) vs focal (black) staining after exposure to DMSO or 1μM Torin2. H. γH2A.X staining pattern in various TNBC cell lines after treatment with 1μM Torin2. Scale bars in F, H=20μm. See also Figure S4.
To determine whether increased ssDNA and DNA damage precede accumulation of SNR cells and death, HCC1806 cells were exposed to Torin2 at 0.3xGRmax dose (1μM) for 0.2–1.7xTd (5–48h). At 5, 11, 24 or 48h, cells were pulse labeled with EdU for 0.025xTd (40min) and counterstained with anti-γH2A.X antibodies. Between 5 and 48h of drug exposure, ~50–60% of EdU-positive cells were positive for γH2A.X, a >10–fold increase over cells exposed to DMSO for 5h (Figure 4D). Over the same time period, the fraction of EdU-negative cells that were γH2A.X-positive increased from 1% to 54%; these cells had DNA content values characteristic of SNR cells (Figure 4E). Torin2 exposure was similar to rabusertib exposure in generating in SNR cells with homogeneous, pan-nuclear γH2A.X staining, thereby demonstrating severe genotoxic stress and commitment to apoptosis (de Feraudy et al., 2010) (Figure 4F). No such staining pattern was observed after cells were exposed to omipalisib. Overall, more than 50% of S-phase HCC1806 cells exposed to Torin2 for 5–48h exhibited pan-nuclear γH2A.X staining, and similar effects were observed in other TNBC cell lines (Figures 4G–H). Thus, ssDNA and DNA damage induced by Torin2 precede the appearance of SNR cells exhibiting hallmarks of replication catastrophe.
The activity of Torin2 results from combined inhibition of mTORC1/2 and PIKKs
To test the hypothesis that the activity of Torin2 on TNBC cells results from its unique polypharmacology, we attempted to reconstitute Torin2-induced phenotypes in HCC1806 and HCC70 cells by combining selective inhibitors of mTOR and PIKKs. The combination of AZD8055 and rabusertib most closely matched the activity of Torin2 across a range of doses (Figures 5A, S5A), possibly because inhibition of Chk1 mimics the inhibition of multiple PIKKs (Buisson et al., 2015). AZD8055 combined with either rabusertib or AZ20 was more effective (by GRmax) than inhibiting mTORC1/2 alone and more potent (by GR50) than inhibiting PIKKs alone. In a complementary approach, we compared AZD8055 to nine Torin2 analogs (Figure S5B) and to Torin1, which inhibits mTORC1/2 and DNA-PK but not ATR or ATM (Liu et al., 2010). Most Torin2 analogs were less potent than AZD8055 and Torin1, but were more effective (i.e., negative GRmax values; Figures 5B–C).
Figure 5: The activity of Torin2 results from combined antagonism of mTORC1/2 and PIKKs.
A-B. 72h dose-response curves. “Mixture” denotes equimolar concentrations of AZD8055/rabusertib; “analogs” indicate Torin2 chemical analogs. C. Mean±SEM of GRmax, GR50 values for drugs in B; N=3 experiments. D. Levels of p-AKT, p-4E-BP1 and γH2A.X in HCC1806 cells at t=0.5xTd (14h) of drug exposure vs GR values (72h) for 0.032–3.2μM of drugs in B. Black regression lines show drugs/doses whose GR values correlate with decreased mTORC1/2 signaling (left, middle) or increased γH2A.X (right). Red lines show drugs/doses discontinuous with the regression. E. DNA vs. nuclear γH2A.X or p-pRb levels in HCC1806 cells at 14h of exposure to DMSO or numbered drugs in D. Gates indicate cells with high/low levels; percentages are of all cells. F. Mean percent±SEM of data in E; N=3 experiments. **P<0.01, ***P<0.001 by one-way ANOVA and Dunnett’s test. G. Nuclear γH2A.X staining pattern in HCC1806 cells after exposure to Torin2 analogs for 14h. Numbers as in D-F. Scale bar=20μm. See also Figure S5.
To dissect the relative contribution of mTORC1/2 and PIKK inhibition to the activity of Torin2 analogs, we exposed HCC1806 and HCC70 cells to a 100-fold concentration range of each drug for t=0.33–0.5xTd (14h) and assayed activity against mTORC1/2 by immunofluorescence for p-AKT S473 and p-4E-BP1 T37/46. We found that the magnitude of mTORC1/2 inhibition at 14h correlated well with GR values at 72h for multiple drugs and analogs, including Torin1 and AZD8055 (Figures 5D, S5C, black regression lines). However, at low levels of mTORC1/2 signaling activity, the GR values for Torin2 (red circles), QL-VI-86 (#5), QL-V-107 (#6), QL-IV-100 (#7) and QL-VIII-58 (#8) were substantially lower, resulting in a discontinuous relationship between mTORC1/2 inhibition and GR values (Figures 5D, S5C, red lines/boxes). Thus, mTORC1/2 inhibition alone does not fully account for the high activity of these drugs. This same subset of drugs caused elevated pan-nuclear staining of γH2A.X in S-phase cells, consistent with inhibition of PIKKs (Figures 5D–G, S5C–E). In HCC1806 cells, these drugs also elicited an increase in the fraction of p-pRb-low cells in G1, S and G2/M phases, indicative of arrest at multiple points in the cell cycle (Figures 5E, black lines beneath scatterplots, 5F). We conclude that Torin2 and its analogs exhibit more or less of two distinct cellular activities. Whereas all compounds inhibit mTORC1/2 and cause growth inhibition, a subset of compounds including Torin2 also inhibit PIKKs to cause DNA damage and cytotoxicity. The most effective drugs are those with the strongest combined activity.
Combined mTORC1/2 and PIKK inhibition produces benefit by co-targeting pathways required in S phase
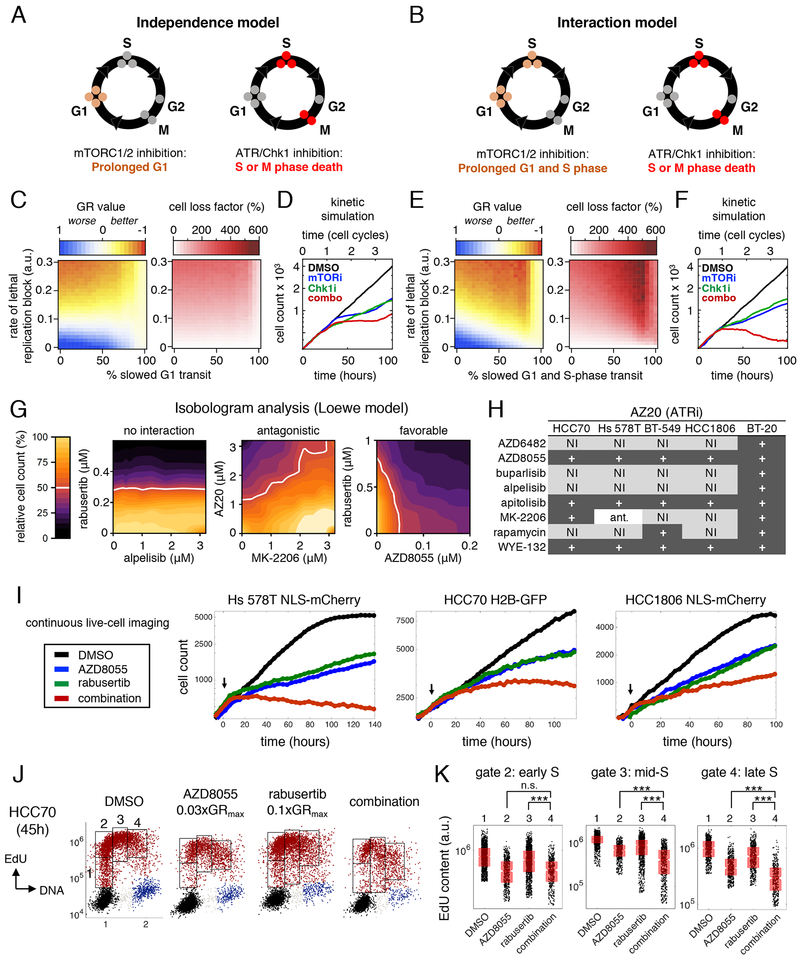
The high activity of Torin2 and its analogs is unexpected. In principle, G0/G1 arrest caused by mTORC1/2 inhibition should antagonize cell death in S phase (or mitosis, M) from PIKK inhibition (Johnson et al., 1999; King et al., 2015). To better understand why Torin2 and its analogs are so effective, we developed two cell cycle models based on discrete time-step Monte Carlo simulations. In these models, single cells progressed through G1, S, G2 and M “compartments” at rates matching experimental data. Following M phase, each mother cell was replaced by two G1 daughters. In the “independence model,” mTORC1/2 inhibition slowed transit only through G1, while in the “interaction model,” mTORC1/2 inhibition slowed transit through both G1 and S phase (Figures 6A–B). In both models, ATR/Chk1 inhibition caused replication block and S- or M-phase lethality. GR values and total cell killing (“cell loss factor”) were then calculated for simulations with different degrees of mTORC1/2 and ATR/Chk1 inhibition.
Figure 6: Combined mTORC1/2 and PIKK inhibition produces benefit by co-targeting pathways required in S phase.
A-B. Schematics of the independence and interaction models. C-D. Simulated GR values (left), cell loss factor/cytotoxicity (middle) and growth curves (right) for the independence model, in which mTORC1/2 inhibition prolongs G1 and ATR/Chk1 inhibition causes lethality in S-phase or mitosis, as a consequence of replication block. E-F. Identical simulations as in C-D, but for the interaction model, in which mTORC1/2 inhibition prolongs both G1 and S-phase. G. Examples of no interaction, antagonism or favorable drug interaction by isobologram analysis. White line indicates 50% reduced viable cell count. H. Summary of interactions between PI3K pathway drugs and the ATR inhibitor AZ20. “+” indicates additive or greater benefit by Loewe criteria, “N.I.” indicates no interaction/independence, and “ant.” indicates antagonism. I. Live-cell imaging of cells treated with sub-GRmax doses of AZD8055 and/or the Chk1 inhibitor rabusertib. Doses for each cell line (AZD8055, rabusertib): Hs 578T (0.1μM, 0.5μM); HCC70 (0.032μM, 0.32μM); HCC1806 (0.320μM, 0.32μM). J. DNA content vs EdU content at time=Td (45h) for HCC70 cells treated with 0.1μM AZD8055 and/or 0.32μM rabusertib. Cells at S phase entry and in early, mid- and late S phase are gated (1-4). K. EdU content in S-phase cells in gates 2-4 in J. ***P<0.001 by Mann-Whitney U test; n.s.: not significant. See also Figure S6.
When the rate of cell killing caused by ATR/Chk1 inhibition was low (between 0 and 0.15 in Figure 6C), the independence model predicted greater cytostasis with increased mTORC1/2 inhibition. However, with higher cytotoxicity (values of 0.2 to 0.3), blocking mTORC1/2 had an antagonistic effect. A kinetic simulation of submaximal doses of an ATR/Chk1 inhibitor combined with an mTORC1/2 inhibitor demonstrated only a modest effect on viable cell number (Figure 6D). In contrast, the interaction model predicted substantially decreased GR values and increased cell killing across a wide range of activities of ATR/Chk1 and mTORC1/2 inhibitors (Figure 6E). Antagonism between mTORC1/2 and ATR/Chk1 inhibitors was observed only at the highest levels of mTORC1/2 inhibition, which markedly prolonged G1. Kinetic simulations of submaximal drug activities acting in combination yielded growth curves with negative slopes, denoting cytotoxicity (Figure 6F). Thus, the interaction model demonstrates that mTORC1/2 inhibitors causing incomplete G1/S block can be beneficially combined with ATR/Chk1 inhibitors if the two drugs increase the probability of S- or M-phase cell killing. The cytotoxic effects of Torin2 and its analogs are thus likely to depend on the sensitivity of TNBC cells to both mTORC1/2 and PIKK inhibition during S phase.
To test experimentally whether combinations of PI3K/AKT/mTOR and ATR/Chk1 inhibitors are beneficial or antagonistic, we performed isobologram analysis on two-way dose-response landscapes for 16 different combinations—eight PI3K/AKT/mTOR inhibitors versus two ATR/Chk1 inhibitors—in five TNBC cell lines. Each of the 80 landscapes involved 100 different dose ratios. Using Loewe criteria (Tallarida, 2011), we classified combinations as exhibiting independence or favorable/antagonistic interactions (Figure 6G). In total, 46 out of 80 combinations exhibited pharmacological interaction, of which 45 (98%) were at least additive and only one was antagonistic (Figures 6H, S6A). As predicted by modeling, favorable drug interactions gave rise to increased cell killing at submaximal concentrations of PI3K pathway inhibitors that only partly suppress mTORC1/2 signaling and incompletely block proliferation (Figure 6I, S6B–C). In HCC70 cells, for example, low doses of AZD8055 (0.03xGRmax, 0.1μM) were sufficient to delay progression of S phase; when combined with low doses of rabusertib (0.1xGRmax, 0.32μM), we observed significantly greater suppression of EdU incorporation in mid- and late S-phase cells compared to either drug alone (Figures 6J–K). Thus, as predicted by the interaction model, several PI3K pathway and ATR/Chk1 inhibitors can be beneficially combined in TNBC cell lines.
Low doses of mTORC1/2 and ATR/Chk1 inhibitors in combination cause increased ssDNA in mitotic prophase and death
Exposure of HCC70 cells to low doses of AZD8055 and rabusertib in combination strongly suppressed DNA synthesis but did not cause the accumulation of SNR cells observed in other lines (i.e., HCC1806). We therefore asked whether low dose combinations increase cell killing by a mechanism distinct from replication catastrophe, such as by causing cells with incompletely-replicated DNA to inappropriately enter mitosis (King et al., 2015). To investigate this possibility, we studied early mitotic (prophase) cells in asynchronous drug-treated cultures, as identified by positive staining for phospho-Histone H3 S10 (p-HH3) and uncondensed chromatin (visualized by Hoechst staining). To detect ssDNA, cells were labeled with BrdU for t=Td and stained with anti-BrdU antibodies under native conditions (Figure 7A). We found that exposure of HCC70 cells to the combination of 0.03xGRmax AZD8055 (0.1μM) and 0.1xGRmax rabusertib (0.32μM) for t=0.5xTd (24h) produced higher fractions of BrdU-positive prophase cells and higher nuclear BrdU intensity levels than either drug alone or than a 0.3xGRmax dose (1μM) of Torin2 (Figures 7B–D). Thus, the low-dose combination causes increased mitotic entry of HCC70 cells with ssDNA.
Figure 7. Low doses of mTORC1/2 and ATR/Chk1 inhibitors in combination cause increased ssDNA in mitotic prophase and death.
“Combination” indicates 0.1μM AZD8055 and 0.32μM rabusertib. Data in A-F are from HCC70 cells after t=0.5xTd (24h) of drug exposure. A. Representative images of nuclei stained for Hoechst, p-HH3 and native BrdU; scale bar=20μM. B. Mean percent±SEM of native BrdU/p-HH3 double-positive cells; N=3 experiments. C. Native BrdU levels in single nuclei; boxplots show the median, 25th/75th percentiles. D. Representative scatterplots quantifying p-HH3/native BrdU double-positive cells (red); percentages are of p-HH3-positive (prophase) cells. E. Maximum intensity projections of confocal image stacks of metaphase cells; scale bar=10μm. F. Mean percent±SEM of metaphases with the indicated aberrations; N=2 experiments, 188 metaphases. G. Time-lapse imaging of HCC70 H2B-GFP cells showing aberrant chromatin condensation in cells treated with the combination, followed by abnormal metaphase (arrowhead) and death. Numbers denote hours:minutes; scale bar=10μm. H. Percent of failed mitoses after 15 or 30h of drug exposure (top) and stage of all failed mitoses (bottom). N ~200 cells at each timepoint. *P<0.05, **P<0.01, ***P<0.001, and n.s. (not significant) by ANOVA and Tukey’s test (panels B, F; selected comparisons shown) and Mann-Whitney U-test (C). See also Figure S7.
To determine the fates of these cells, we stained chromosomes, centromeres, spindle fibers, and centrosomes using Hoechst and anti-CREST, anti-β-tubulin, and anti-γ-tubulin antibodies, following drug exposure for t=0.5xTd (24h). Whereas AZD8055 alone decreased the total number of mitotic figures per high-powered field, rabusertib and the low-dose combination increased mitotic cell counts by 1.7-fold and 2.6-fold, respectively (N=672 fields; P<0.001 for combination vs rabusertib by one-way ANOVA and Tukey’s test) (Figure S7A). The combination also increased the percentage of mitotic cells in metaphase to 80%, compared to 28% for DMSO, 36% for AZD8055 alone and 58% for rabusertib alone (N=569 mitotic cells; P<0.01 for combination vs rabusertib) (Figure S7B). More than 90% of metaphase cells treated with AZD8055 plus rabusertib exhibited fragmented and/or misaligned chromosomes (N=188 metaphase cells; P<0.01 for combination vs rabusertib for severe misalignment and P<0.05 for chromosome fragmentation) (Figures 7E–F). Thus, cells with high levels of ssDNA are delayed in metaphase with incorrectly aligned or damaged chromosomes.
The interdependency of these events was made more obvious by time-lapse imaging of cells expressing H2B-GFP. Drug-induced abnormalities in chromatin condensation visible in prophase frequently led to aberrant metaphases (N=198 and 222 cells tracked through mitosis at 15 and 30h, respectively) (Figure 7G). Mitotic death ensued after varying lengths of time without evidence of progression to anaphase. Although the rate of mitotic entry was lower in cells treated with the low-dose combination versus rabusertib alone (0.8% vs 1.7% of cells per hour after 30h of drug exposure; Figure S7C), nearly 80% of cells entering mitosis in the presence of the drug combination underwent mitotic catastrophe (a 2.2-fold increase over rabusertib alone) (Figure 7H). Of all drug-treated cells that died during mitosis, >97% failed between prophase and metaphase. We conclude that low doses of mTORC1/2 and Chk1 inhibitors in combination can cause high rates of mitotic failure in some TNBC cell lines.
DISCUSSION
The frequency of mutations in PI3K pathway genes in human cancers has motivated development of numerous small-molecule kinase inhibitors (Janku et al., 2018), few of which are effective in patients. We find that the preclinical compound Torin2 is unusually active in blocking proliferation and inducing death of PI3K-activated TNBC cells. In contrast, Torin2 is only partially cytostatic in non-transformed mammary epithelial cells. Torin2 was developed as an mTORC1/2 inhibitor, but reconstitution experiments and analysis of chemical analogs both show that its activity in TNBC results from combined inhibition of mTOR and PIKKs. In multiple TNBC lines, combinations of drugs targeting mTORC1/2 and ATR or Chk1 also show favorable effects. Thus, the polyselectivity of Torin2 is essential for its high activity in TNBC.
The benefits of combining mTORC1/2 and ATR/Chk1 inhibitors are unexpected because these two drug classes primarily affect successive cell cycle states. PI3K/AKT/mTOR signaling canonically regulates the G1/S transition (Liang and Slingerland, 2003) while ATR/Chk1 is required during S phase and at the G2/M checkpoint (Zeman and Cimprich, 2014). Prior studies have shown that combinations of chemotherapies or chemotherapy plus radiation are antagonistic when successive cell cycle stages are affected (Johnson et al., 1999; Sui et al., 2004). This is intuitive, because cells blocked in G1 cannot proceed into later phases of the cell cycle where cytotoxicity occurs. However, in TNBC cells we find that PI3K pathway drugs also disrupt progression of S phase. Modeling and experimentation together suggest that combinations of mTORC1/2 and PIKK inhibitors can produce benefit under conditions in which mTORC1/2 inhibitors incompletely block cells at G1/S and yet slow the rate of replication in S phase. Thus, the relative insensitivity of TNBC cells to the anti-proliferative effects of mTORC1/2 inhibitors at G1/S is a liability for monotherapy, but an opportunity for combination therapies that exploit vulnerabilities in S phase. The strong effects of Torin2 on S-phase cells appear to result from a felicitous combination of reduced rates of DNA synthesis due to mTORC1/2 inhibition and reduced replication fork stability due to PIKK inhibition. The accumulation of ssDNA and DNA damage caused by exposure to Torin2 consequently results in death of TNBC cells in S phase or subsequent mitosis.
We find that reduced rates of DNA synthesis following exposure to PI3K pathway inhibitors are associated with altered levels of nucleotides and their precursors. Short exposure of TNBC cells to mTOR inhibitors causes increased levels of cytidine and cytosine, known substrates for pyrimidine salvage pathways, as well as changes in the levels of dTDP and dTMP consistent with reduced activity of deoxythymidylate kinase, an enzyme required for pyrimidine synthesis. Prior studies have found that PI3K pathway activity promotes metabolic flux through the non-oxidative arm of the pentose phosphate pathway to generate ribose 5-phosphate and phosphoribosylpyrophosphate (PRPP), which are required for purine and pyrimidine synthesis (Juvekar et al., 2016; Wang et al., 2009). Moreover, mTORC1 regulates activity of CAD, which catalyzes de novo pyrimidine synthesis, and expression of MTHFD2, which increases de novo purine biosynthesis (Ben-Sahra et al., 2013, 2016). In addition to causing metabolic derangements, mTOR inhibitors may also impede S phase progression by reducing translation of proteins required for DNA replication and repair (Silvera et al., 2017).
Dependencies on ATR/Chk1 signaling for S-phase progression are also likely to be multifactorial in origin (Zeman and Cimprich, 2014). Oncogenes commonly amplified in TNBC such as MYC and CCNE1 cause excessive origin firing and conflicts between replication and transcription, resulting in stalled replication forks (Macheret and Halazonetis, 2018). Due to uncoupling of replicative helicases and DNA polymerases, stalled forks generate ssDNA and activate ATR/Chk1 signaling (Zeman and Cimprich, 2014). In the presence of replication stress, ATR/Chk1 inhibition causes accumulation of ssDNA, consumption of replication factors, DNA damage and “replication catastrophe” (Toledo et al., 2013). Loss-of-function mutations in TP53 may also contribute to this outcome, since absence of the DNA damage checkpoint at G1/S renders cells more dependent on ATR/Chk1 signaling for the function of alternative cell cycle checkpoints to maintain genomic integrity (Ma et al., 2011). By inhibiting both mTORC1/2 and PIKKs, Torin2 effectively exploits S-phase and checkpoint vulnerabilities in TNBC to promote tumor cell killing.
Translational prospects
The foremost challenge in targeting the PI3K pathway for anti-cancer therapy is achieving anti-proliferative effects and cell killing sufficient to block tumor growth at doses tolerated by patients. The PI3K/mTOR inhibitor omipalisib is emblematic of this challenge: omipalisib produces substantial growth inhibition and apoptosis in TNBC cell lines, but the maximum tolerated dose in patients fails to fully inhibit intratumoral AKT and has only modest anti-tumor activity (Munster et al., 2016). To improve the therapeutic index, recent efforts have focused on development of selective inhibitors of PI3K p110 isoforms (α, β, δ, γ), but these drugs often lack sufficient activity in solid tumors as monotherapy (Costa et al., 2015; Elkabets et al., 2013; Schwartz et al., 2015). Thus, the polyselectivity of Torin2-like compounds appears promising in that it eliminates the requirement for strong inhibition of mitogenic signaling and G1/S arrest to produce efficacy. We have also shown that certain PI3K pathway inhibitors can be used at relatively low doses in combination with other drugs to exploit replicative and/or checkpoint vulnerabilities and produce cytotoxicity. Thus, new combinatorial strategies inspired by Torin2 may not only be more effective, but also have a greater therapeutic index in patients.
STAR METHODS
LEAD CONTACT AND MATRIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Peter Sorger (peter_sorger@hms.harvard.edu).
Materials Availability Statement
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines
The media and culture conditions for all breast cancer and non-transformed mammary epithelial cell lines used in this study are described below. All cell lines were isolated from females, to the best of our knowledge. STR profiling was performed to confirm cell line identity.
| Cell line | RRID | Growth medium (FBS: heat-inactivated fetal bovine serum; P/S: penicillin/streptomycin) |
Temp | CO2 |
|---|---|---|---|---|
| 184-B5 | CVCL_4688 | MEBM (Lonza/Clonetics; MEGM Kit #CC-3150) + 1ng/ml cholera toxin (unfiltered) | 37°C | 5% |
| AU-565 | CVCL_1074 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| BT-20 | CVCL_0178 | EMEM + 10% FBS + 1% P/S | 37°C | 5% |
| BT-474 | CVCL_0179 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| BT-483 | CVCL_2319 | RPMI-1640 + 20% FBS + 1% P/S + 0.01mg/ml bovine insulin | 37°C | 5% |
| BT-549 | CVCL_1092 | RPMI-1640 + 10% FBS + 1% P/S + 0.023IU/ml insulin | 37°C | 5% |
| CAL-120 | CVCL_1104 | DMEM + 10% FBS + 1% P/S | 37°C | 5% |
| CAL-51 | CVCL_1110 | DMEM + 20% FBS + 1% P/S | 37°C | 5% |
| CAL-85-1 | CVCL_1114 | DMEM + 10% FBS + 1% P/S | 37°C | 5% |
| CAMA-1 | CVCL_1115 | EMEM + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1143 | CVCL_1245 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1395 | CVCL_1249 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1419 | CVCL_1251 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1428 | CVCL_1252 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1500 | CVCL_1254 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1569 | CVCL_1255 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1806 | CVCL_1258 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1937 | CVCL_0290 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC1954 | CVCL_1259 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC202 | CVCL_2062 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC38 | CVCL_1267 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| HCC70 | CVCL_1270 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| Hs 578T | CVCL_0332 | DMEM + 10% FBS + 1% P/S | 37°C | 5% |
| hTERT-HME1 | CVCL_3383 | MEBM + Lonza CC-3150 kit | 37°C | 5% |
| MCF-10A | CVCL_0598 | 1:1 DMEM and Ham’s F12 + 20ng/ml human epidermal growth factor + 100ng/ml cholera toxin + 0.01mg/ml bovine insulin + 500ng/ml hydrocortisone, 95% + 5% horse serum | 37°C | 5% |
| MCF-10F | CVCL_3633 | 1:1 DMEM and Ham’s F12 + 20ng/ml human epidermal growth factor + 100ng/ml cholera toxin + 0.01mg/ml bovine insulin + 500ng/ml hydrocortisone, 95% + 5% horse serum | 37°C | 5% |
| MCF-12A | CVCL_3744 | 1:1 DMEM and Ham’s F12 + 20ng/ml human epidermal growth factor + 100ng/ml cholera toxin + 0.01mg/ml bovine insulin + 500ng/ml hydrocortisone, 95% + 5% horse serum | 37°C | 5% |
| MCF-7 | CVCL_0031 | DMEM + 10% FBS + 1% P/S | 37°C | 5% |
| MDA-MB-134-VI | CVCL_0617 | Leibovitz’s L-15 + 20% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| MDA-MB-157 | CVCL_0618 | Leibovitz’s L-15 +10% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| MDA-MB-175-VII | CVCL_1400 | Leibovitz’s L-15 +10% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| MDA-MB-231 | CVCL_0062 | DMEM (or L-15) + 10% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| MDA-MB-361 | CVCL_0620 | Leibovitz’s L-15 + 20% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| MDA-MB-415 | CVCL_0621 | Leibovitz’s L-15 + 15% FBS (not heat-inactivated) + 1% P/S + 2mM L-glutamine + 10mg/l insulin | 37°C | 0% |
| MDA-MB-436 | CVCL_0623 | Leibovitz’s L-15 + 10% FBS (not heat-inactivated) + 1% P/S + 10mg/l insulin | 37°C | 0% |
| MDA-MB-453 | CVCL_0418 | Leibovitz’s L-15 + 10% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| MDA-MB-468 | CVCL_0419 | Leibovitz’s L-15 + 10% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| SK-BR-3 | CVCL_0033 | McCoy’s 5a + 10% FBS + 1% P/S | 37°C | 0% |
| SUM102PT | CVCL_3421 | Ham’s F-12 + 1% P/S + 5 μg/ml insulin + 1ug/ml hydrocortisone + 10ng/mL epidermal growth factor + 5mM ethanolamine + 10mM HEPES + 5ug/mL transferrin + 10nM T3 + 50nM sodium selenite + 1g/L BSA | 37°C | 5% |
| SUM1315MO2 | CVCL_5589 | Ham’s F-12 + 5% FBS + 1% P/S + 10ng/ml human epidermal growth factor + 5μg/ml insulin + 10 mM HEPES | 37°C | 5% |
| SUM149PT | CVCL_3422 | Ham’s F-12 + 5% FBS + 1% P/S + 1ug/ml hydrocortisone + 5μg/ml insulin + 10mM HEPES | 37°C | 5% |
| SUM159PT | CVCL_5423 | Ham’s F-12 + 5% FBS + 1% P/S + 1ng/ml hydrocortisone + 5μg/ml insulin + 10mM HEPES | 37°C | 5% |
| SUM225CWN | CVCL_5593 | Ham’s F-12 + 5% FBS + 1% P/S + 5μg/ml insulin + 1ug/ml hydrocortisone + 10mM HEPES | 37°C | 5% |
| SUM44PE | CVCL_3424 | Ham’s F-12 + 1% P/S + 5μg/ml insulin + 1ug/ml hydrocortisone + 5mM ethanolamine + 10mM HEPES + 5ug/mL transferrin + 10nM T3 + 50nM sodium selenite + 1g/L BSA | 37°C | 5% |
| SUM52PE | CVCL_3425 | Ham’s F-12 + 5% FBS + 1% P/S + 5 μg/ml insulin + 1ug/ml hydrocortisone + 10mM HEPES | 37°C | 5% |
| T-47D | CVCL_0553 | RPMI-1640 + 10% FBS + 1% P/S + 0.2units/ml bovine insulin | 37°C | 5% |
| UACC-812 | CVCL_1781 | Leibovitz’s L-15 + 20% FBS (not heat-inactivated) + 1% P/S + 2mM L-glutamine + 20ng/ml human EGF | 37°C | 0% |
| UACC-893 | CVCL_1782 | Leibovitz’s L-15 + 10% FBS (not heat-inactivated) + 1% P/S | 37°C | 0% |
| ZR-75-1 | CVCL_0588 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
| ZR-75-30 | CVCL_1661 | RPMI-1640 + 10% FBS + 1% P/S | 37°C | 5% |
Cell line generation
HCC70 H2B-GFP cells were established via lentiviral transduction (Addgene, 25999) followed by fluorescence-activated cell sorting (FACS) sorting for GFP-positive cells (Beronja et al., 2010). HCC1806 cells expressing H2B-mTurquoise and mVenus-hGeminin(1-110) were established via sequential lentiviral transduction of CSII-pEF-H2B-mTurquoise and CSII-pEF-mVenus-geminin(1-110), followed by (FACS) for mTurquoise/mVenus double-positive cells (Sakaue-Sawano et al., 2008; Spencer et al., 2013). The generation of Hs 578T NLS-mCherry and HCC1806 NLS-mCherry cell lines, which also express a F3aN400-Venus construct not utilized here, was previously described (Sampattavanich et al., 2018). The media and culture conditions for genetically-engineered cell lines were identical to the parental cell lines.
METHOD DETAILS
Western blotting
To determine protein levels under unstimulated conditions, cells grown to approximately 75% confluency under the conditions described above were serum starved overnight and then lysed with Mammalian Protein Extraction Buffer (M-PER; Thermo Scientific, 78501) supplemented with protease inhibitor cocktail (Sigma-Aldrich, P2714), 1mM sodium orthovanadate (Sigma-Aldrich, S6508), 5mM sodium pyrophosphate (Sigma-Aldrich, 221368), 50μM oxophenylarsine (EMD Biosciences, 521000) and 10mM bpV (phen) (EMD Biosciences, 203695). Two independent protein lysates were prepared from SK-BR-3 cells, and MDA-MB-231 cells were cultured in two different types of media (=total 48 samples for 46 different cell lines). Lysed cells were scraped off the plate, collected in microcentrifuge tubes and incubated on ice for 30min. Membranes and cell debris were sedimented by centrifugation at 20,000xg for 10min at 4°C. Supernatants were pooled, aliquoted and stored at −80°C. Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific, 23225). Protein electrophoresis was performed using E-PAGE pre-cast 8% gels with 48 wells (Thermo Fisher Scientific, EP04808). Separated protein was transferred to Immobilon-FL PVDF membranes (EMD Millipore, IPFL00005) using the Criterion blotting system (Bio-Rad). Membranes were blocked for 1h at room temperature (RT) with Odyssey blocking buffer (OBB; Li-COR 927-40000), incubated in primary antibodies in OBB overnight at 4°C, washed for 30min in PBS plus 0.1% Tween-20 (PBST), incubated in secondary antibody in OBB for 1h at RT, and washed again for 30min in PBST. Anti-PTEN (Cell Signaling Technologies, 9188) and anti-INPP4B (Epitomics, 2512-1) primary antibodies were each used at 1:1000 dilution. Detection was performed using anti-rabbit IRDye 800CW pre-adsorbed secondary antibody (Rockland, 613-431-028) at 1:20,000 dilution. The fluorescence intensity of bands was quantified using the Odyssey system (Li-COR).
Evaluation of drug sensitivity
All compounds except Torin2 analogs were obtained from MedChem Express. Stock solutions were prepared at a concentration of 10mM in DMSO and stored at −30°C. Torin2 analogs (QL-VIII-58, QL-V-107, QL-IV-100, QL-VI-86, QL-XII-47, QL-V-73, QL-X-138, QL-XII-108, QL-XII-61) were obtained from the laboratory of Nathanael Gray (Dana-Farber Cancer Institute) as 10mM stock solutions in DMSO. PI3K pathway drugs were arrayed in master library plates and pin-transferred to cells in Cell Carrier 384 well plates (Perkin Elmer, 6007550). Torin2 analogs were delivered to cells in Cell Carrier 384 well plates using the D300 digital dispenser and accompanying software (Hewlett Packard). Cells were plated at a density of 1,000-2,000 cells per well using the Multidrop Combi (Thermo Scientific) and treatments were performed on the following day approximately 18-24h after plating. Inhibitors were administered at 9 different concentrations in at least technical triplicate and DMSO alone was used as a control. To determine cell number at the start (time = 0) and end (t = 72h) of treatment, cells were arrayed in two different plates with the time = 0 plate receiving no treatment and the 72h plate receiving DMSO control and active drug. Viable cells were identified using 15μL of staining solution, comprising 1:5,000 LIVE/DEAD Far Red Dead Cell Stain (Invitrogen, L10120), 1:10,000 Hoechst 33342 trihydrochloride trihydrate 10mg/mL solution in water (Invitrogen, H3570) and 1:10 OptiPrep (Sigma-Aldrich, D1556) in PBS. Cells were incubated in staining solution for 30min at 37°C with 5% CO2 and then fixed with 20μL fixing solution, comprising 1:20 37% formaldehyde (Sigma-Aldrich, F1635) and 1:5 OptiPrep in PBS, for 30min at RT. To achieve accurate cell counts, no aspiration of well contents was performed prior to fixing cells. Following fixation, all reagents were aspirated and replaced with 60μL of PBS using an EL406 Washer Dispenser (BioTek). Cells were imaged using the Operetta High-Content Imaging System (Perkin Elmer). Six fields of view spanning each well in its entirety were acquired using the 10x high-NA objective lens and appropriate excitation/emission filters for Hoechst and LIVE/DEAD Far Red cell stains. The total viable cell count per well was determined using Columbus image analysis software (Perkin Elmer), as described below.
Drug dosing
When possible, GRmax doses of drugs were used to facilitate a principled comparison of drug activities. However, for certain experiments, decreased drug doses were used due to excessive cell death or growth inhibition that resulted in insufficient cells for analysis (e.g. DNA synthesis in surviving cell fractions; EdU content in post-treatment S-phase fractions, etc.). For the F-ara-EdU data presented in Figure 1E, Torin2 and omipalisib were used at 0.3xGRmax (1μM). For the signaling data presented in Figure 2A, Torin2 and omipalisib were used at 0.3xGRmax (1μM) in HCC1806 cells. For the cell cycle data presented in Figure 2C, Torin2 and omipalisib were used at 0.3xGRmax dose (BT-20: 1μM; HCC70: 0.32–1μM; HCC1806: 1μM). For the scatterplot data from HCC70 cells appearing in the middle and bottom rows of panels in Figure 2D, Torin2 and omipalisib were used at 0.1xGRmax dose (0.1–0.32μM). For the analysis of EdU content presented in Figure 2E, drug doses for HCC70 and HCC1806 cells were as described for Figure 2C. For Hs 578T cells, Torin2 and omipalisib were used at 0.3xGRmax dose (1μM). For BT-549 cells, AZD8055 was used at 0.3xGRmax dose (0.32μM) and omipalisib and Torin2 were used at 0.03xGRmax dose (0.1μM). For metabolomics profiling data presented in figure 2F, GRmax doses of rapamycin and AZD8055 were used (1μM) and compared to a 0.3xGRmax dose of Torin2 (also 1μM).
Immunofluorescence microscopy
Cells were plated in Cell Carrier 384 well plates at either 1,000 (Hs 578T) or 2,000 (BT-20, BT-549, HCC1806, HCC38, HCC70) cells per well, treated with active drug or DMSO using the D300 digital dispenser, and fixed at various timepoints using 4% formaldehyde in PBS for 30min at RT. Cells were washed twice with PBS for 5min each at RT using the EL406 automated plate washer, permeabilized with 0.25% Triton X-100 (Bio-Rad, 1610407) in PBS for 15min, washed twice again for 5min each with PBS and blocked with OBB for at least 1h at RT. Cells were incubated with primary antibody diluted in OBB overnight at 4°C. The following morning, cells were washed three times with PBST for 5min each and then stained with secondary antibodies diluted 1:2,000 in OBB for 1h at RT. Secondary antibodies varied for different experiments but included Alexa Fluor 647 donkey anti-rabbit (Invitrogen, A31573), Alexa Fluor 647 donkey anti-mouse (Invitrogen, A31571), Alexa Fluor 647 goat anti-human (Invitrogen, A21445), Alexa Fluor 488 donkey anti-rabbit (Invitrogen, A21206), Alexa Fluor 488 goat anti-mouse (Invitrogen, A11001), and Alexa Fluor 568 goat anti-rabbit (Invitrogen, A11011). Cells were washed twice with PBST and once with PBS for 5min each prior to staining with Hoechst 33342 (Thermo Scientific, 1:10,000 in PBS) for 30min at RT. To facilitate segmentation of signal intensities specifically in the nucleus, cytoplasm, or whole cell in certain experiments, cells were simultaneously stained with 1:5000 Cellomics Whole Cell Stain (Thermo Scientific, 8403502) in PBS. Cells were washed twice with PBS, sealed with foil, and imaged using the Operetta High-Content Imaging System, the ImageXpress Micro Confocal High-Content Imaging System (Molecular Devices) or the IN Cell Analyzer 6000 (GE Healthcare Life Sciences) using the appropriate excitation and emission filters, depending on the specific assay.
Primary antibodies and dilutions
The following primary antibodies were obtained from Cell Signaling Technology (catalog number, dilution in OBB): rabbit anti-phospho-AKT T308 (13038, 1:400), rabbit anti-phospho-AKT S473 (4060, 1:200), rabbit anti-phospho-Chk1 S317 (12302, 1:800), rabbit anti-phospho-Chk2 T68 (2661, 1:100), rabbit anti-phospho-4E-BP1 T37/46 (2855, 1:200), rabbit anti-phospho-S6 S235/236 (4858, 1:1000), rabbit anti-phospho-H2A.X S139 (“γH2A.X”) (9718, 1:400), rabbit anti-phospho-Histone H3 S10 (3377, 1:800), mouse anti-β-tubulin (86298, 1:400), rabbit anti-β-tubulin (2128, 1:400), mouse anti-BrdU (Bu20a) (5292, 1:200), rabbit anti-phospho-GSK3B S9 (5558, 1:400), rabbit anti-FOXO3 (2497, 1:200), rabbit anti-p21 (2947, 1:400), rabbit anti-p27 (3686, 1:400), and rabbit anti-phospho-Rb S807/811 (8516, 1:800). The following primary antibodies were obtained from Abcam (catalog number, dilution in OBB): rabbit anti-cyclin D1 (ab134175, 1:100), rabbit anti-geminin (ab195047, 1:100), and rabbit anti-cyclin A2 (ab181591, 1:500). Rabbit anti-phospho-RPA S4/8 antibodies were obtained from Bethyl (A300-245A, 1:400), rabbit anti-γ-tubulin antibodies from Sigma (T5192, 1:400), and Human Antibody against Centromere (CREST) antibodies from ImmunoVision (HCT-0100, 1:1000).
Single-stranded DNA (ssDNA) and cell cycle analysis
To measure ssDNA, newly-synthesized DNA in proliferating cells was first labeled with 10μM BrdU (Sigma, B9285) in growth media for a time equal to the measured cell line division time (Td) in culture. Drug treatments were administered to labeled cells at Td using the D300 digital dispenser, and cells were fixed at various timepoints using 4% formaldehyde. BrdU was detected under non-denaturing (“native”) conditions by standard immunofluorescence microscopy, as described above. Cell cycle analysis was performed after treating cells with active drug or DMSO-only control for time=Td, which was 28h for HCC1806 cells, 32h for Hs 578T cells, 40h for BT-549 cells, 45h for both HCC70 and HCC38 cells and 48h for BT-20 cells. Active S-phase cells were labeled with 10μM EdU (Thermo Fisher Scientific, C10637) in growth media for 0.025xTd in an incubator. Cells were then fixed with 4% formaldehyde in PBS for 30min at RT. Incorporated EdU was detected using the Click-iT EdU Plus Alexa Fluor 488 imaging kit (Thermo Fisher Scientific, C10637), per the manufacturer’s instructions. Following the Click-iT reaction, cells were counterstained with 5μg/mL Hoechst dye and imaged using the Operetta or ImageXpress Micro Confocal High-Content Imaging System. In certain experiments, EdU-labeled cells were counterstained with anti-geminin, anti-cyclin A2, anti-phospho-pRb or anti-phospho-H2A.X antibodies overnight following the Click-iT reaction and prior to Hoechst staining. Continuous labeling of S-phase cells was performed using 1μM F-ara-EdU (Sigma, T511293) for 48 or 75h.
Live-cell microscopy
Time-lapse imaging of proliferation and apoptosis in live cells was performed using the Incucyte Zoom live-cell analysis system (Essen Bioscience) inside of an incubator maintained at 37°C and 5% CO2. Cells were plated at low density in 384-well plates (Corning, 3712) and images were taken of whole wells every 30-120min using the 4x lens. Drugs were administered during logarithmic-phase growth. For proliferation assays, stable cell lines with nuclear-expressing fluorophores (HCC70 H2B-GFP, Hs 578T NLS-mCherry, HCC1806 NLS-mCherry) were used to quantify live cell number at regular time intervals. Apoptotic cells were identified in wild-type cell lines using the Incucyte caspase 3/7 (C3/7) apoptosis assay reagent (Essen Bioscience, 4440), which was added to growth media at the recommended final concentration (5μM) several hours in advance of drug treatment. For each experimental replicate, the number of apoptotic cells was measured for each drug, dose, timepoint and cell line in technical triplicate.
For live-cell studies of mitosis, HCC70 H2B-GFP cells were plated at 2,000 cells per well in Cell Carrier 384-well plates. Plates were kept in a standard tissue culture incubator maintained at 37°C and 5% CO2 for approximately 48h before cells were treated with drugs using the D300 digital dispenser. Starting at either 15 or 30h after drug treatment, cells were imaged every 5-10 minutes for 5-7h in a humidified chamber maintained at 37°C and 5% CO2 using the 40x/0.95 NA lens of the IN Cell Analyzer 6000 microscope. The rate of mitotic entry and the percentage and stage of aberrations were visually scored for individual cells followed from prophase through cytokinesis in time-lapse image stacks.
To analyze the effects of drugs on cells in different stages of the cell cycle, HCC1806 cells expressing H2B-mTurquoise and mVenus-hGeminin(1-110) were plated at 4,000 cells per well in 96-well plates (Ibidi, 89626), kept in a standard tissue culture incubator overnight, treated with drugs the next morning using the D300 digital dispenser and then imaged every 12min for 48h in a humidified chamber maintained at 37°C and 5% CO2 using the 20x/0.75NA lens of the ImageXpress Micro high-content imaging system with binning 2x2. To minimize the effect of phototoxicity, cells were imaged at minimal laser intensity and exposure times necessary to achieve approximately 8-fold actual signal intensity versus background for each fluorophore. At the end of the experiment, cells were stained with Hoechst and LIVE/DEAD stain to identify and quantify viable cells. To assess for possible phototoxicity, cell counts for DMSO-only control wells on the same plate that did not undergo imaging were compared to cell counts for DMSO-only control wells that underwent time-lapse imaging. To validate environmental conditions such as temperature, humidity, and CO2, viable cells in an identically-treated plate kept in a standard tissue culture incubator set at 37°C and 5% CO2 for 48h (i.e., the duration of the experiment) were also compared by Hoechst and LIVE/DEAD co-staining.
Extraction of polar metabolites
Measurement of intracellular polar metabolite levels was performed to detect drug-induced changes in metabolic activity including de novo nucleotide biosynthesis and salvage pathways. BT-549 cells were selected for profiling following acute drug exposure (i.e., 6h) because inhibition of DNA synthesis was observed in S-phase cells (i.e., decreased EdU incorporation) prior to any detectable changes in cell cycle distribution, which can strongly bias metabolite levels. Cells in complete media were plated in 10cm2 dishes at 2.5x106 cells per dish and cultured at 37°C and 5% CO2. Approximately two days later when cells were 75-80% confluent, the media was replaced with 10mL of fresh media containing rapamycin, AZD8055 or Torin2, or an equivalent volume of DMSO. After 6h of treatment, the 10cm2 dishes were placed on dry ice and the media was aspirated and replaced with ice cold 80% (v/v) methanol. Dishes were kept at −80°C for 20min prior to using a cell scraper to produce a cell lysate/methanol mixture. The mixture for each sample was collected in a 15mL Eppendorf tube and cellular debris and protein were pelleted by centrifugation at 1,800xg for 5min at 4°C. The methanol supernatant containing polar metabolites was transferred to a new 15mL tube on dry ice and the cell debris/protein pellets were resuspended two additional times with methanol, centrifuged and re-pelleted. Recovered methanol supernatants for each sample were pooled and then divided equally into four 1.5mL tubes on dry ice. Methanol was evaporated using a SpeedVac (no applied heat) and the resulting polar metabolite pellets were stored at −80°C.
Metabolomics profiling by targeted mass spectrometry
Metabolites were re-suspended in 20mL HPLC-grade water for mass spectrometry. 5-7μL were injected and analyzed using a hybrid 5500 QTRAP triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFPLC system (Shimadzu) via selected reaction monitoring (SRM) of a total of 262 endogenous water-soluble metabolites for steady-state analyses of samples. Some metabolites were targeted in both positive and negative ion mode for a total of 298 SRM transitions using positive/negative ion polarity switching. ESI voltage was +4950V in positive ion mode and −4500V in negative ion mode. The dwell time was 3msec per SRM transition, and the total cycle time was 1.55sec. Approximately 10-14 data points were acquired per detected metabolite. Samples were delivered to the mass spectrometer via hydrophilic interaction chromatography (HILIC) using a 4.6mm i.d. x 10cm Amide XBridge column (Waters) at 400 μL/min. Buffer A was comprised of 20mM ammonium hydroxide/20mM ammonium acetate (pH=9.0) in 95:5 water:acetonitrile and Buffer B was HPLC grade acetonitrile. Gradients were run starting from 85% buffer B (in buffer A) to 42% B from 0-5min; 42% B to 0% B from 5-16min; 0% B was held from 16-24min; 0% B to 85% B from 24-25min; 85% B was held for 7min to re-equilibrate the column. Peak areas from the total ion current for each metabolite SRM transition were integrated using MultiQuant v2.1 software (AB/SCIEX).
Isobolograms and analysis of drug interactions
Viable cell counts were determined after exposure to PI3K pathway and ATR/Chk1 inhibitors administered in combinations over two-dimensional gradients. Dose ranges typically spanned at least one order of magnitude and were selected based on the GR50 values for each single agent. Continuous interpolations were constructed over the 2D response surface, which were then plotted as contour maps that highlight contours of equal inhibitory effect, or isoboles (Interpolation function and ContourPlot functions in Mathematica v11). As described by the Loewe additivity model, drug combinations were classified as “additive” based on the concept of dose equivalence, which manifests as diagonal isoboles that intersect both axes. Combinations were classified as “antagonistic” when isoboles were observed to deviate away from either axis, indicating that the drugs used together were less effective than either drug used alone. Combinations were classified as having “no interaction” when the activity of each drug appeared to be unaffected by the second drug, indicating independent action and manifested by linear isoboles. Examples of each case are shown in Figure 6G.
Cell cycle model and simulation of combinatorial drug effect
The effects of mTORC1/2 or ATR/Chk1 inhibition individually or in combination were simulated by a discrete time-step Monte Carlo simulation. This simulation followed the progress of individual cells through the G1, S, G2, and M phases of the cell cycle, with each cell possessing a cell-state variable that increments with cell cycle progression. When a cell completed M phase, the mother cell was replaced by two daughter cells at the start of G1. Simulations were initialized with a population of 300 cells distributed throughout the cell cycle (i.e., asynchronous) based on experimentally measured cell cycle distributions for TNBC mTORC1/2 inhibition slowed each cell’s progress through G1 (independence model) or through both G1 and S phases (interaction model) by imposing a lower probability per time-step that the cell-state variable will increase. ATR/Chk1 inhibition imposed on each S-phase cell a specific rate of lethal replication block at each time-step. In light of prior knowledge that the effects of ATR/Chk1 inhibition during replication can ultimately cause death of cells in S phase (replication catastrophe) or in mitosis (mitotic catastrophe), simulations did not discriminate between whether the moment of death arises in S phase or in mitosis. In the interaction model, slowing of cells through S phase by mTORC1/2 inhibition was found to elicit an increase in the accumulated probability of lethal damage caused by ATR/Chk1 inhibition.
QUANTIFICATION AND STATISTICAL ANALYSIS
Details regarding the number of experimental replicates, specific statistical tests used and the level of significance are described in the primary and supplementary figure legends. Significant differences are also indicated on the primary and supplemental figures.
Viable cell counts
Images of TNBC cells co-stained with Hoechst and LIVE/DEAD stains were analyzed using Columbus image data storage and analysis system (Perkin Elmer) in order to quantify the total number of viable cells per well. Nuclear segmentation was performed using the Hoechst fluorescence intensity. For example, the number of HCC1806 cell nuclei in a particular well was determined using the following segmentation routine: Module: ‘Find Nuclei’; Channel: ‘Hoechst’; Method: M; diameter 28μm; splitting coefficient: 0.45; common threshold: 0.05. Because of differences in nuclear size and morphology, optimal segmentation routines varied for each TNBC cell line and were determined in an empirical fashion. After identification of all nuclei in a well, additional features were extracted from images to exclude over/under segmented nuclei, doublets and non-viable cells from the total cell counts. Over/under segmented nuclei and doublets were of atypical size and shape compared to appropriately segmented nuclei. Non-viable cells were characterized by positive LIVE/DEAD staining and/or small pyknotic nuclei with extremely bright Hoechst signal. Collectively, the image analysis features that best identified these characteristics included nuclear area and roundness (module: ‘Calculate Morphology Properties’); nuclear Hoechst intensity (module: ‘Calculate Intensity Properties’); nuclear LIVE/DEAD stain intensity (module: ‘Calculate Intensity Properties’); Hoechst texture features (module: ‘Calculate Texture Properties’; SER Features, spot; scale: 4px; normalization by: unnormalized); and LIVE/DEAD texture features (module: ‘Calculate Texture Properties’; SER Features; scale: 6px; normalization by: kernel). These extracted features were then used to set filters (i.e., gates) in the module ‘Select Population.’ In order to exclude extreme outliers representing non-viable or incorrectly segmented cells, filter settings were tuned interactively for each experimental replicate by visually comparing images of cells in DMSO-treated wells against images of cells in active drug-treated wells, and by using histograms produced by Columbus to visualize gates applied to the distribution of measured/calculated values for each feature. Identical gates were applied to all wells on each imaged plate.
Normalized growth rate inhibition (GR) metrics
Viable cell counts at time=0 and time=72h as determined in Columbus were then exported and used to compute GR values and GR metrics with the freely available online GR calculator (http://www.grcalculator.org/grcalculator/), which is based on previously published methods (Hafner et al., 2016). Dose response curves of GR values were produced using MATLAB (MathWorks).
Quantification of immunofluorescence images
Immunofluorescence intensities in the entire cell or relevant subcellular compartment were measured in Columbus. Masks for the nucleus, cytoplasm and entire cell were generated by thresholding the Hoechst and the Whole Cell Stain channels. Custom masks were generated for each cell line due to variation in cell morphology. To quantify changes in the levels of proteins/phosphoproteins after treatment with DMSO or active drug, the relevant mask (as determined both by prior knowledge and by the observed staining pattern for each antibody) was applied to microscopy images and the fluorescence intensity was measured in that specific region of each cell. Levels of phospho-AKT (T308 and S473), phospho-4E-BP1 and phospho-GSK3β in single cells were measured using a whole-cell mask. Phospho-S6 levels were measured using a cytoplasmic mask. Signals for BrdU, EdU, F-ara-EdU, cyclin A2, cyclin D1, FoxO3, geminin, p21, p27, p-Chk1, p-Chk2, p-H2A.X, p-Histone H3, p-pRb and p-RPA levels were measured using a nuclear mask. For generation of PI3K signaling heatmaps, background-subtracted fluorescence intensity measurements were first normalized to values for DMSO. The PI3K signaling index was computed by adding the mean DMSO-normalized values of each of nine different proteins/phosphoproteins (scale 0-9, with 9 indicating no difference from DMSO). For signals that increase with PI3K pathway suppression (nuclear FOXO3, nuclear p21, and nuclear p27), the inverse value was used when computing the index. “Percent high” for any measured protein/post-translational modification was determined by applying an identical threshold to the entire distribution of values determined for cells treated with DMSO or active drug (see Figure 4A). Total DNA content and EdU content per nucleus were calculated by multiplying the average nuclear fluorescence intensity by the nuclear area. All raw data were exported from Columbus; visualization, plotting and statistical analysis of the data were performed in MATLAB. Specific gating strategies are illustrated in individual figure panels; experimental replicates and statistical analyses are described in figure legends. Cells with mitotic aberrations were quantified based on visual inspection of individual microscopy images.
Cell cycle analysis and cell cycle stage-specific immunofluorescence values
After using Columbus to apply a nuclear mask to individual cells, average Hoechst and EdU intensity values were extracted and multiplied by the nuclear area to determine the DNA content and EdU content, respectively, on a single-cell level basis. The two-parameter plot of nuclear DNA content versus EdU content readily separated cells into three groups representing G1, S and G2/M cells (see Figure S3A). S-phase-non-replicating (SNR) cells were identified based on DNA content intermediate to G1 and G2/M and no EdU signal. The percentages of cells in different cell cycle stages were determined in MATLAB by gating different groups of cells by DNA content (X) and EdU content (Y). In each experiment, specific values for gates were first determined by using data for cells treated with DMSO only and then applied to data for drug-treated cells. For analysis of immunofluorescence intensities in specific subpopulations of cells defined by cell cycle stage (e.g., Figure 2D), nuclear intensity values from cells labeled with Hoechst/EdU and immunostained for a nuclear marker of interest were first plotted in MATLAB using the 3-way scatterplot function to preserve relationships among the three measured intensity values in single cells. Cells in different cell cycle stages were then gated as described above. The entire distribution of immunofluorescence intensity values for cells in each cell cycle stage was then replotted in relation to DNA content.
Live-cell analysis of proliferation/apoptosis
To determine population growth trajectories, total cell number in an entire well imaged using the 4x lens was determined using Incucyte Zoom software (Essen Bioscience). Cell number was calculated by identifying nuclei positive for GFP (HCC70) or mCherry (Hs 578T, HCC1806) in microscopy images, based on a background-subtracted fluorescence intensity threshold. C3/7-positive cells were similarly identified based on a fluorescence intensity threshold in the GFP channel. For each of three technical replicates in each independent experiment, C3/7 counts over time were plotted and area under the curve (AUC) calculations were determined in MATLAB and averaged. The mean fold-change value was then calculated as the ratio of the average AUC for drug versus the average for DMSO. Plotted mean fold-change and standard error values were determined using data from independent experiments.
Live-cell analysis of drug effect on the cell cycle
Semi-automated tracking of individual HCC1806 cells expressing H2B-mTurquoise and mVenus-hGeminin(1-110) was performed using p53CinemaManual 2.0, a MATLAB-based software package freely available at: https://github.com/balvahal/p53CinemaManual/releases/tag/v2.0.0. Individual cells were tracked in time-lapse movies using nuclear mTurquoise; mVenus fluorescence intensities were then extracted from each tracked cell nucleus. Cell cycle analysis was performed by analyzing plots of mVenus intensity versus time (see annotated trace of representative single cell in DMSO, Figure 3A). Each individual trace was manually reviewed and compared to DMSO control to ascertain the timing of cell cycle stage transitions. Data for each cell and each condition were then compiled into matrices, which were used to generate heatmaps. Cell divisions were scored by direct visualization of chromosome segregation followed by appearance of daughter cell nuclei. Cell deaths were scored by the appearance of nuclear fragmentation (karyorrhexis).
Analysis of steady-state polar metabolites
Metabolite peak intensities for drug-treated samples were compared to peak intensities for samples treated with DMSO (N=3 independent experiments) using MetaboAnalyst 4.0 (MA) (https://www.metaboanalyst.ca/MetaboAnalyst/faces/home.xhtml). Prior to uploading the data, an initial processing step was performed to exclude metabolites with two or more missing values in either the DMSO or active treatment group. For rapamycin, AZD8055, and Torin2, 34 (11.2%), 35 (11.6%), and 34 (11.2%) of 303 metabolites, respectively, were excluded for this reason. For a small number of excluded metabolites (4/34 for rapamycin, 8/35 for AZD8055, and 9/34 for Torin2), three numerical values were available for DMSO but only 0 or 1 for active treatment, suggesting that treated samples had undetectable levels by mass spectrometry due to low abundance. Because the fold-change values for these metabolites were incalculable but in certain cases could be large, these metabolites and their raw values are listed in separate tables in Table S4. To facilitate downstream analysis in MA, single missing values in either treatment group were imputed by taking the average of the two measured values. This step was performed prior to analyzing the data in MA because the software performs missing value estimation for each metabolite using all of the sample data rather than the data for individual treatment groups. In certain instances, this method was observed to obscure strong changes in metabolite levels induced by drug treatment. Once the data were uploaded to MA, no additional filtering was applied. Sample data were quantile-normalized, and then the metabolite data were log-transformed. No scaling was performed. The effects of data normalization and transformation were visualized using diagnostic plots. Fold-change values were calculated as the ratio between group means, using absolute values of metabolite data prior to log-transformation. T-tests were performed to assess the statistical significance of fold-change values. Volcano plots were generated using fold-change values and p-values from T-tests to identify metabolites with the largest and most significant changes after drug treatment.
DATA AVAILABILITY
The datasets generated during this study are freely available as detailed below:
-
Evaluation of the sensitivity of six TNBC cell lines to 23 PI3K pathway drugs or trametinib (MEK inhibitor). Data summarized in Figures 1B, S1D.
Measured GR values (72h): PENDING
Calculated GR metrics (72h): PENDING
-
Evaluation of the sensitivity of Torin2 compared to the PI3K pathway drugs alpelisib, TGX-221, pictilisib, everolimus, and sapanisertib in a collection of breast cancer cell lines (N=28). Data summarized in Figures 1C, S1E.
Measured GR values (72h): http://lincs.hms.harvard.edu/db/datasets/20343/
Calculated GR metrics (72h): http://lincs.hms.harvard.edu/db/datasets/20344/
-
Evaluation of the sensitivity of HCC1806 and HCC70 cells to Torin1, Torin2, AZD8055 or nine Torin2 analogs. Data summarized in Figures 5B–C.
Measured GR values (72h): PENDING
Calculated GR metrics (72h): PENDING
Targeted metabolomics profiling data (i.e., peak intensity measurements) of BT-549 cells after exposure of to DMSO, rapamycin (1μM), AZD8055 (1μM), or Torin2 (1μM) for 6 hours. Data summarized in Figure 2F. PENDING
Supplementary Material
Figure S1: High-content imaging and GR metrics reveal that Torin2 is highly active in TNBC cells, Related to Figure 1. This figure provides additional data on PI3K pathway dysregulation in breast cancer cell line models, imaging methods used to determine viable cell counts, GR metrics and interpretation, drug screening results in breast cancer cell lines, and phenotyping of proliferation and apoptosis.
Figure S2: PI3K/AKT/mTOR inhibitors variably suppress mitogenic signals in TNBC cells, Related to Figure 2. This figure provides examples of how changes in protein abundance were measured by quantitative immunofluorescence microscopy, as well as actual measurements that were used to generate the signaling heatmaps in Figure 2. Signaling index values and quantification of p-pRb levels are also provided.
Figure S3: PI3K/AKT/mTOR inhibitors impede progression of S phase, Related to Figure 2. This figure shows how cell cycle distributions were determined by co-staining with Hoechst/EdU, and provides actual data on cell cycle distributions and EdU content for additional cell lines and conditions.
Figure S4: Torin2 increases replication stress in TNBC cells, Related to Figure 4. This figure shows additional analyses of replication stress markers in HCC70 and HCC1806 cells.
Figure S5: Torin2 analogs have two distinct activities in HCC70 cells, Related to Figure 5. This figure provides additional data on pharmacological reconstitution experiments and the effects of Torin2 analogs in a second cell line.
Figure S6: Combinations of mTORC1/2 and PIKK inhibitors produce benefit at low doses that only partially suppress mTOR signaling, related to Figure 6. This figure provides additional data on drug interaction analysis performed in breast cancer cell lines as well as data on the signaling effects of submaximal doses of AZD8055 in two cell lines.
Figure S7: Low doses of an mTORC1/2 inhibitor and a Chk1 inhibitor in combination perturb mitotic entry and progression, Related to Figure 7. This figure provides additional data on the effects of the low-dose combination of AZD8055 and rabusertib on HCC70 cells during mitosis.
Table S1: Classification of breast cancer and non-transformed mammary epithelial cell lines used in this study, related to Figure 1.
Table S2: Molecular characteristics of the six TNBC/basal-like cell lines selected for quantitative analysis of responses to PI3K pathway inhibitors, related to Figure 1.
Table S3: Drug compounds used in this study, related to Figure 1.
Table S4: Results of metabolomics analysis, related to Figure 2. Upregulated and downregulated metabolites following 6 hours of exposure of BT-549 cells to three different mTOR inhibitors.
HIGHLIGHTS.
Torin2 causes death of TNBC cells by inhibiting both mTOR and PI3K-like kinases
Live-cell imaging shows that Torin2 exploits vulnerabilities during DNA replication
Combined inhibition of mTORC1/2 and Chk1 with selective drugs mimics Torin2
Computational models confirm the importance of mTOR inhibition in S phase
ACKNOWLEDGMENTS
This work was funded by NIH/NCI grants HL127365 (to PKS, NG) and CA225088 (to PKS, SC), the DFCI Leadership Council (SC), Conquer Cancer Foundation of ASCO (SC), and T32 CA 9172-4 (SC). Mass spectrometry was supported in part by CA120964 (JA) and CA006516 (JA). We thank U Matulonis for her support, and M Hafner, J Reyes, M Yuan and A Chen for their assistance with experiments, analysis and preparation of the manuscript.
DECLARATION OF INTERESTS
Peter K. Sorger is a member of the SAB or Board of Directors of Merrimack Pharmaceuticals, Glencoe Software, Applied Biomath and RareCyte Inc and has equity in these companies. In the last five years the Sorger lab has received research funding from Novartis and Merck. Sorger declares that none of these relationships are directly or indirectly related to the content of this manuscript. Nathanael Gray and Qingsong Liu are co-inventors of Torin2, which is protected by the patent “Modulators of mTOR Complexes,” European Patent Office EP2350053B1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al. (2019). Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med 380, 1929–1940. [DOI] [PubMed] [Google Scholar]
- Ben-Sahra I, Howell JJ, Asara JM, and Manning BD (2013). Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, and Manning BD (2016). mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronja S, Livshits G, Williams S, and Fuchs E (2010). Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat. Med 16, 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini G, Balko JM, Mayer IA, Sanders ME, and Gianni L (2016). Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol 13, 674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson R, Boisvert JL, Benes CH, and Zou L (2015). Distinct but Concerted Roles of ATR, DNA-PK, and Chk1 in Countering Replication Stress during S Phase. Mol. Cell 59, 1011–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012). Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S, Gandhi S, Joy AA, Edwards S, Gorr M, Hopkins S, Kondejewski J, Ayoub JP, Califaretti N, Rayson D, et al. (2015). Novel agents and associated toxicities of inhibitors of the pi3k/Akt/mtor pathway for the treatment of breast cancer. Curr. Oncol 22, 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, Huang A, Wang Y, Nishtala M, Hall B, et al. (2015). Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer. Cancer Cell 27, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio RJ, and Xiong Y (2013). Signaling pathways that control cell proliferation. Cold Spring Harb. Perspect. Biol 5, a008904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. (2010). Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, et al. (2013). mTORC1 Inhibition Is Required for Sensitivity to PI3K p110α Inhibitors in PIK3CA-Mutant Breast Cancer. Sci. Transl. Med 5, 196ra99–ra196ra99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Feraudy S, Revet I, Bezrookove V, Feeney L, and Cleaver JE (2010). A minority of foci or pan-nuclear apoptotic staining of γH2AX in the S phase after UV damage contain DNA double-strand breaks. Proceedings of the National Academy of Sciences 107, 6870–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, and Reis-Filho JS (2010). Triple-negative breast cancer. N. Engl. J. Med 363, 1938–1948. [DOI] [PubMed] [Google Scholar]
- Hafner M, Niepel M, Chung M, and Sorger PK (2016). Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat. Methods 13, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F, Yap TA, and Meric-Bernstam F (2018). Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol 15, 273–291. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Young KK, and Fan W (1999). Antagonistic interplay between antimitotic and G1-S arresting agents observed in experimental combination therapy. Clin. Cancer Res 5, 2559–2565. [PubMed] [Google Scholar]
- Juvekar A, Hu H, Yadegarynia S, Lyssiotis CA, Ullas S, Lien EC, Bellinger G, Son J, Hok RC, Seth P, et al. (2016). Phosphoinositide 3-kinase inhibitors induce DNA damage through nucleoside depletion. Proc. Natl. Acad. Sci. U. S. A 113, E4338–E4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Diaz HB, McNeely S, Barnard D, Dempsey J, Blosser W, Beckmann R, Barda D, and Marshall MS (2015). LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms. Mol. Cancer Ther 14, 2004–2013. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Lin H, and Shokat KM (2010). Targeting the cancer kinome through polypharmacology. Nat. Rev. Cancer 10, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, and Slingerland JM (2003). Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2, 339–345. [PubMed] [Google Scholar]
- Lin AB, McNeely SC, and Beckmann RP (2017). Achieving Precision Death with Cell-Cycle Inhibitors that Target DNA Replication and Repair. Clin. Cancer Res 23, 3232–3240. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, Hur W, Zhang J, Sim T, Sabatini DM, et al. (2010). Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J. Med. Chem 53, 7146–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Xu C, Kirubakaran S, Zhang X, Hur W, Liu Y, Kwiatkowski NP, Wang J, Westover KD, Gao P, et al. (2013). Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res. 73, 2574–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CX, Janetka JW, and Piwnica-Worms H (2011). Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol. Med 17, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheret M, and Halazonetis TD (2018). Intragenic origins due to short G1 phases underlie oncogene-induced DNA replication stress. Nature 555, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster P, Aggarwal R, Hong D, Schellens JHM, van der Noll R, Specht J, Witteveen PO, Werner TL, Dees EC, Bergsland E, et al. (2016). First-in-Human Phase I Study of GSK2126458, an Oral Pan-Class I Phosphatidylinositol-3-Kinase Inhibitor, in Patients with Advanced Solid Tumor Malignancies. Clin. Cancer Res 22, 1932–1939. [DOI] [PubMed] [Google Scholar]
- Neef AB, and Luedtke NW (2011). Dynamic metabolic labeling of DNA in vivo with arabinosyl nucleosides. Proc. Natl. Acad. Sci. U. S. A 108, 20404–20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe J-P, Tong F, et al. (2006). A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. (2000). Molecular portraits of human breast tumours. Nature 406, 747–752. [DOI] [PubMed] [Google Scholar]
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, et al. (2008). Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498. [DOI] [PubMed] [Google Scholar]
- Sampattavanich S, Steiert B, Kramer BA, Gyori BM, Albeck JG, and Sorger PK (2018). Encoding Growth Factor Identity in the Temporal Dynamics of FOXO3 under the Combinatorial Control of ERK and AKT Kinases. Cell Syst 6, 664–678. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, Will M, Yellen P, de Stanchina E, Baselga J, et al. (2015). Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3β. Cancer Cell 27, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Azuma A, Sampath D, Li Y-X, Huang P, and Plunkett W (2001). S-Phase Arrest by Nucleoside Analogues and Abrogation of Survival without Cell Cycle Progression by 7-Hydroxystaurosporine. Cancer Res. 61, 1065–1072. [PubMed] [Google Scholar]
- Silvera D, Ernlund A, Arju R, Connolly E, Volta V, Wang J, and Schneider RJ (2017). mTORC1 and -2 Coordinate Transcriptional and Translational Reprogramming in Resistance to DNA Damage and Replicative Stress in Breast Cancer Cells. Mol. Cell. Biol 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SL, Cappell SD, Tsai F-C, Overton KW, Wang CL, and Meyer T (2013). The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui M, Dziadyk JM, Zhu X, and Fan W (2004). Cell cycle-dependent antagonistic interactions between paclitaxel and gamma-radiation in combination therapy. Clin. Cancer Res 10, 4848–4857. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ (2011). Quantitative methods for assessing drug synergism. Genes Cancer 2, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, Oyarzabal J, Pastor J, Bischoff JR, and Fernandez-Capetillo O (2011). A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat. Struct. Mol. Biol 18, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Altmeyer M, Rask M-B, Lukas C, Larsen DH, Povlsen LK, Bekker-Jensen S, Mailand N, Bartek J, and Lukas J (2013). ATR Prohibits Replication Catastrophe by Preventing Global Exhaustion of RPA. Cell 155, 1088–1103. [DOI] [PubMed] [Google Scholar]
- Wang W, Fridman A, Blackledge W, Connelly S, Wilson IA, Pilz RB, and Boss GR (2009). The phosphatidylinositol 3-kinase/akt cassette regulates purine nucleotide synthesis. J. Biol. Chem 284, 3521–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Wiederschain D, Maira S-M, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao Y-M, and Lengauer C (2008). PTEN-deficient cancers depend on PIK3CB. Proc. Natl. Acad. Sci. U. S. A 105, 13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman MK, and Cimprich KA (2014). Causes and consequences of replication stress. Nat. Cell Biol 16, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: High-content imaging and GR metrics reveal that Torin2 is highly active in TNBC cells, Related to Figure 1. This figure provides additional data on PI3K pathway dysregulation in breast cancer cell line models, imaging methods used to determine viable cell counts, GR metrics and interpretation, drug screening results in breast cancer cell lines, and phenotyping of proliferation and apoptosis.
Figure S2: PI3K/AKT/mTOR inhibitors variably suppress mitogenic signals in TNBC cells, Related to Figure 2. This figure provides examples of how changes in protein abundance were measured by quantitative immunofluorescence microscopy, as well as actual measurements that were used to generate the signaling heatmaps in Figure 2. Signaling index values and quantification of p-pRb levels are also provided.
Figure S3: PI3K/AKT/mTOR inhibitors impede progression of S phase, Related to Figure 2. This figure shows how cell cycle distributions were determined by co-staining with Hoechst/EdU, and provides actual data on cell cycle distributions and EdU content for additional cell lines and conditions.
Figure S4: Torin2 increases replication stress in TNBC cells, Related to Figure 4. This figure shows additional analyses of replication stress markers in HCC70 and HCC1806 cells.
Figure S5: Torin2 analogs have two distinct activities in HCC70 cells, Related to Figure 5. This figure provides additional data on pharmacological reconstitution experiments and the effects of Torin2 analogs in a second cell line.
Figure S6: Combinations of mTORC1/2 and PIKK inhibitors produce benefit at low doses that only partially suppress mTOR signaling, related to Figure 6. This figure provides additional data on drug interaction analysis performed in breast cancer cell lines as well as data on the signaling effects of submaximal doses of AZD8055 in two cell lines.
Figure S7: Low doses of an mTORC1/2 inhibitor and a Chk1 inhibitor in combination perturb mitotic entry and progression, Related to Figure 7. This figure provides additional data on the effects of the low-dose combination of AZD8055 and rabusertib on HCC70 cells during mitosis.
Table S1: Classification of breast cancer and non-transformed mammary epithelial cell lines used in this study, related to Figure 1.
Table S2: Molecular characteristics of the six TNBC/basal-like cell lines selected for quantitative analysis of responses to PI3K pathway inhibitors, related to Figure 1.
Table S3: Drug compounds used in this study, related to Figure 1.
Table S4: Results of metabolomics analysis, related to Figure 2. Upregulated and downregulated metabolites following 6 hours of exposure of BT-549 cells to three different mTOR inhibitors.
Data Availability Statement
The datasets generated during this study are freely available as detailed below:
-
Evaluation of the sensitivity of six TNBC cell lines to 23 PI3K pathway drugs or trametinib (MEK inhibitor). Data summarized in Figures 1B, S1D.
Measured GR values (72h): PENDING
Calculated GR metrics (72h): PENDING
-
Evaluation of the sensitivity of Torin2 compared to the PI3K pathway drugs alpelisib, TGX-221, pictilisib, everolimus, and sapanisertib in a collection of breast cancer cell lines (N=28). Data summarized in Figures 1C, S1E.
Measured GR values (72h): http://lincs.hms.harvard.edu/db/datasets/20343/
Calculated GR metrics (72h): http://lincs.hms.harvard.edu/db/datasets/20344/
-
Evaluation of the sensitivity of HCC1806 and HCC70 cells to Torin1, Torin2, AZD8055 or nine Torin2 analogs. Data summarized in Figures 5B–C.
Measured GR values (72h): PENDING
Calculated GR metrics (72h): PENDING
Targeted metabolomics profiling data (i.e., peak intensity measurements) of BT-549 cells after exposure of to DMSO, rapamycin (1μM), AZD8055 (1μM), or Torin2 (1μM) for 6 hours. Data summarized in Figure 2F. PENDING