Abstract
Alternative-reinforcement-based treatments are among the most effective for reducing substance abuse. However, relapse often occurs when alternative reinforcement ends. Relapse following the loss of alternative reinforcement is called resurgence. An animal model has been used to study basic factors that may ultimately reduce resurgence, but uses drug unavailability (i.e., extinction) to reduce drug seeking. In humans, drug abstinence is thought to be a product of aversive consequences associated with drug use rather than extinction. This discrepancy is important because the environmental and neurobiological factors involved in relapse may differ between punished and extinguished behavior. Experiment 1 evaluated resurgence of previously-punished cocaine seeking. In Phase 1, rats earned cocaine for pressing levers. In Phase 2, cocaine remained available, but lever pressing also produced mild foot shocks while an alternative response produced food pellets for one group but not for another group. In Phase 3, alternative reinforcement and punishment were removed and resurgence of cocaine seeking occurred only in rats previously exposed to alternative reinforcement. In Experiment 2, resurgence was evaluated similarly, except that consequences of cocaine seeking (i.e. punishment and cocaine) remained available during Phase 3. Resurgence did not occur in either group during Experiment 2. The animal models of resurgence developed herein could increase translational utility and improve examination of the environmental and neurobiological factors underlying resurgence of drug seeking.
Keywords: Resurgence, Relapse, Punishment, Cocaine, Rats
1. Introduction
Alternative reinforcement techniques are among the most successful for the treatment of substance use disorders (SUDs; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). In such therapies, alternative reinforcers may be provided for maintaining abstinence and/or for engaging in behaviors unrelated to substance use. For example, in Contingency Management, patients earn vouchers for retail items by providing evidence of drug abstinence (e.g. drug-free urine specimen; Higgins & Silverman, 1999). In Community Reinforcement, participation in pro-social, non-drug related activities such as recreation, job procurement, and spending time with family are explicitly reinforced (Hunt & Azrin, 1973; Miller, Meyers, & Hiller-Sturmhofel, 2003). Previous work has also noted that alternative reinforcement is a common factor in successful abstinence from drug use in non-treatment environments (i.e. spontaneous autoremission; Burman, 1997; Klingemann, 1991). Alternative-reinforcement based strategies effectively reduce substance use while contingencies remain in place, but relapse often occurs when treatment is interrupted or concluded (McLellan, Lewis, O’Brien, & Kleber, 2000; Secades-Villa et al., 2011; Silverman, Chutuape, Bigelow, & Stitzer, 1999). Relapse induced by the loss of alternative reinforcement has been termed resurgence (Epstein, 1985) and represents a threat to otherwise effective strategies for reducing substance use. As such, a better understanding of the factors contributing to resurgence may be useful in designing more resilient alternative-reinforcement-based treatments for SUDs.
Resurgence of drug seeking is often studied in animals using a three-phase procedure (Craig, Nall, Madden, & Shahan, 2016; Frye et al., 2018; Nall, Craig, Browning, & Shahan, 2018; Quick, Pyszczynski, Colston, & Shahan, 2011; Shahan, Craig, & Sweeney., 2015). In Phase 1, animals are trained to perform a target response to earn drug reinforcement. Next, in Phase 2, drug seeking is extinguished such that target responses no longer produce drug access. At the same time, an alternative response is made available and produces access to an alternative non-drug reinforcer. Finally, in Phase 3, the alternative response is extinguished while the target response remains on extinction. Resurgence is evidenced by an increase in target responding following the removal of alternative reinforcement in Phase 3 (i.e. resurgence of drug seeking). This procedure has been used previously to demonstrate resurgence of cocaine (Nall et al., 2018; Quick et al., 2011; Shahan et al., 2015) and alcohol (Frye et al., 2018; Nall et al., 2018; Podlesnik, Jimenez-Gomez, & Shahan, 2006) seeking in rats, leading some to suggest that the animal model of resurgence may be useful for studying relapse following the loss of alternative reinforcement in human treatment settings (Marchant, Li, & Shaham, 2013; Peck & Ranaldi, 2014; Winterbauer & Bouton, 2010).
While traditional resurgence procedures have been useful for identifying factors that can modulate relapse, they typically use extinction to reduce drug seeking. However, the use of extinction in animal models of drug relapse has been criticized because it does not accurately reflect the reasons humans with SUDs pursue drug abstinence (Marchant, Li, et al., 2013; Panlilio, Thorndike, & Schindler, 2003). Individuals with SUDs most often refer to the aversive consequences of drug use as their reason for pursuing abstinence. This is true for individuals that stop taking drugs without treatment (e.g., Burman, 1997), and is often influential in the decision to enter treatment (e.g., Laudet, Savage, & Mahmood, 2002). Examples of aversive consequences of drug use might include loss of employment, family problems, financial strain, detriments to physical and mental health, and legal trouble (Burman, 1997; Laudet et al., 2002). Because aversive consequences play an important role in drug abstinence in humans, it may be important to simulate aversive consequences in animal models of relapse as well.
To more accurately simulate the suppression of drug seeking by aversive consequences in humans, more recent animal models of relapse have employed aversive consequences (i.e., most commonly using mild foot shock in rats) to suppress drug seeking. In these procedures, shock is delivered contingent upon drug seeking responses and ultimately results in a decrease in drug seeking behavior. Prior studies have used punishment to suppress drug seeking and observed relapse induced by contextual change (Campbell et al., 2019; Marchant et al., 2016, 2014; Marchant & Kaganovsky, 2015; Pelloux, Minier-Toribio, Hoots, Bossert, & Shaham, 2018), drug priming (Ducret et al., 2016; Panlilio et al., 2003; Panlilio, Thorndike, & Schindler, 2005), exposure to drug cues (Campbell et al., 2017; Economidou, Pelloux, Robbins, Dalley, & Everitt, 2009; Torres et al., 2017), and forced abstinence (Gancarz-Kausch, Adank, & Dietz, 2014; Krasnova et al., 2014; Pelloux, Murray, & Everitt, 2013), across a range of substances of abuse.
Evidence also indicates that the mechanisms underlying relapse may differ between models that use extinction or punishment to reduce drug seeking. For example, Panlilio, Thorndike, & Schindler (2005) found that administration of the benzodiazepine lorazepam reinstated remifentanil seeking (a short acting μ-opioid agonist with reinforcing properties similar to heroin; see Panlilio & Schindler, 2000) in rats whose responding was suppressed by punishment, but not by extinction. Further, Pelloux et al. (2018) found that inactivation of different sub-regions of the amygdala had opposite effects on relapse depending on the method used for response suppression. Thus, because punishment may better represent both the environmental and neurobiological conditions under which humans with SUDs reduce drug use, it is important to study relapse of drug seeking following suppression by punishment.
Resurgence effects following suppression by punishment may be of particular interest when investigating relapse of drug seeking, as alternative reinforcement is often used for treatment and plays an important role in spontaneous autoremission, as discussed above. Two recent studies have investigated resurgence of food seeking under punishment conditions. Nall, Rung, and Shahan (2019) found resurgence of previously-punished food seeking following the removal of alternative reinforcement. Another recent study by Fontes et al. (2018) found resurgence of a previously-extinguished target behavior following punishment of an alternative behavior. These findings suggest that resurgence may occur more generally when conditions of alternative reinforcement are worsened and that resurgence effects are not inherently extinction-based. While these studies are certainly useful for demonstrating the generality of resurgence effects beyond extinction conditions, their use of non-drug reinforcers limits their extension to relapse following treatment for SUDs.
Taken together, current evidence suggests that relapse of drug seeking can occur following suppression by punishment, and that the factors driving relapse may differ between procedures that use extinction or punishment to suppress drug seeking. Because of these potential differences in mechanism, and because aversive consequences are important for drug abstinence in humans, it is important to study relapse following punishment. Further, it may be particularly important to study resurgence of previously-punished drug seeking because of the prevalence and efficacy of alternative-reinforcement based treatments for SUDs. Thus, the goal of the present experiments was to develop a model for studying resurgence of cocaine seeking following punishment.
2. Experiment 1
Experiment 1 was designed to incorporate aversive consequences of drug use into the animal model of resurgence of drug seeking. In Phase 1, rats were trained to press a target lever to earn infusions of cocaine. In Phase 2, target responding continued to produce cocaine, but also produced intermittent foot shocks. Also during Phase 2, for an Alternative + Punishment group, food pellets could be earned for performing an alternative response. Finally, to test for resurgence of cocaine seeking, food pellet reinforcement was made unavailable for the alternative response. Because both alternative reinforcement and target punishment were removed during Phase 3, any increase in target responding could be due to the removal of punishment alone. Thus, the experiment also included a Punishment Control group for which target responding was reinforced and punished during Phase 2 as in the Alternative + Punishment group, but no alternative reinforcement was available. For the Punishment Control group in Phase 3, target reinforcement and punishment were discontinued. Thus, any difference between groups in target responding during Phase 3 should be due to the previous availability and then removal of alternative reinforcement for the Alternative + Punishment group (i.e. resurgence).
2.1. Materials and methods
2.1.1. Subjects
Ten experimentally naive male Long-Evans rats (Charles River, Portage, MI) served as subjects. Rats were 71–80 days old upon arrival and were restricted to 80% of their free-feeding weights following surgery (detailed below). Animal housing, care, and all procedures reported below were conducted in accordance with Utah State University’s Intuitional Animal Care and Use Committee and have been described in detail elsewhere (Nall et al., 2018).
2.1.2. Surgery
Prior to the start of the experiment, rats underwent jugular-catheterization surgery, described in detail elsewhere (Craig et al., 2016; Nall et al., 2018). In short, rats were anesthetized and an indwelling, back-mounted cannula (Plastics One, Roanoke, VA) was implanted and attached to a silastic catheter (SAI-Infusions, Lake Villa, IL) inserted into the right jugular vein. Following surgery, rats recovered for 5 days before undergoing food restriction.
2.1.3. Apparatus
Ten modular Med-Associates (St. Albans, VT) operant chambers measuring 30 cm × 24 cm × 21 cm were used. Chambers consisted of Plexiglas side walls, ceilings, and doors and were housed in sound- and light-attenuating cubicles. An aluminum response panel in the rear of the chamber contained 5 nose poke apertures that could be lighted yellow and were equipped to detect head entries. An aluminum response panel on the front wall contained two retractable levers with stimulus lights above them. A food aperture was centered on the front wall between the levers and was illuminated when delivering food (45-mg dustless pellets; Bio Serv, Flemington NJ). A house light near the ceiling on the front wall was used for general chamber illumination.
Chambers were also equipped for intravenous drug self-administration. A 60ml syringe was placed in a fixed-speed infusion pump (Med Associates) outside of the sound attenuating cubicle. Tygon tubing attached to the syringe was run inside the cubicle and attached to a swivel (Instech, Plymouth Meeting, PA) suspended above the ceiling of the chamber. From the swivel, another section of Tygon tubing was passed into the chamber inside a metal spring tether and attached to the rat’s back-mounted cannula. Rats were connected to the infusion apparatus at all times while in the chamber.
2.1.4. Drugs
Surgery was preceded by injections of an antibiotic (gentamicin, 2.0mg/kg, intraperitoneal) and an analgesic/anti-inflammatory (flunixin meglumine, 1.1mg/kg, subcutaneous), and anesthesia was induced and maintained using isoflurane. Cocaine hydrochloride (NIDA, USA) was dissolved in sterile 0.9% saline solution to a concentration of 2.56mg/ml. The dose of each infusion was determined daily based on individual body weights and achieved by changing the activation duration of a fixed-speed (0.0527ml/s) syringe pump. During the 5 days of recovery from surgery, subcutaneous injections of an analgesic/anti-inflammatory (flunixin meglumine, 1.1mg/kg, subcutaneous) were provided twice daily. Catheter patency was maintained by daily 0.2ml infusions of gentamicin heparinized saline solution (4mg/ml gentamicin, .04mg/ml heparin) throughout the experiment.
2.2. Procedure
2.2.1. Pellet training
Rats were first trained to consume food pellets from the food aperture. Levers were retracted and lights were not illuminated during pellet training except for the illumination of the food aperture when pellets were delivered response-independently every 60s, on average (Variable Time 60s schedule). Each food delivery was accompanied by a 3 s chamber blackout during which responses produced no consequences and all lights were extinguished except for the food aperture, which was illuminated for 3 s. This reinforcement schedule and all variable schedules below were constructed from Fleshler and Hoffman’s (1962) constant-probability distribution. All sessions throughout were 45min excluding chamber blackouts and reinforcer delivery times. Pellet training lasted 4 sessions.
2.2.2. Cocaine self-administration training
During Cocaine self-administration training and throughout the remainder of the experiment target and inactive levers were inserted at the beginning of each session and the stimulus light above the active lever was illuminated throughout the session except during chamber blackouts. Initially, each target lever press produced a 1mg/kg infusion of cocaine (Fixed Ratio [FR] 1 schedule). Each cocaine infusion throughout the experiment was followed by a tone and a 45s chamber blackout, during which all lights were extinguished and responses produced no consequences. As described previously (Nall et al., 2018), the reinforcement schedule was gradually thinned across sessions until rats were earning a cocaine infusion for every 20 responses, on average (Variable Ratio [VR] 20 schedule), and then the cocaine dose was gradually reduced across sessions to 0.32mg/kg/infusion. Throughout the experiment, responses to the inactive lever were recorded but had no consequence. Cocaine selfadministration training lasted approximately 50 sessions.
2.2.3. Phase 1: Baseline
Once rats reached the 0.32mg/kg/infusion condition, Phase 1 began. Reinforcement contingencies were identical to those at the end of the cocaine self-administration training phase described above. This phase lasted at least 5 sessions and until rats showed no downward trend in cocaine consumption over the last 3 sessions.
2.2.4. Phase 2: Punishment
Rats were divided into two groups matched on target response rate and cocaine consumption across the last 3 sessions of Phase 1. For both groups in Phase 2, target responding continued to produce cocaine infusions according to a VR 20 schedule, but each lever press also intermittently produced mild foot shock (probability = 0.5, 50ms, 0.5mA). For the Alternative + Punishment group (N = 5), the left-most nose poke aperture was illuminated, and entries into the aperture produced a food pellet according to a VI 15s schedule (the first response after an average of 15s was reinforced). Target and alternative responses were concurrently available throughout the punishment sessions and cocaine or food could be earned at any time except for during the timeout following cocaine infusions or food delivery. No alternative reinforcement was available for the Punishment Control group (N = 5). Phase 2 lasted 10 sessions.
2.2.5. Phase 3: Resurgence Test
All consequences were removed for all responses for both groups (i.e. no reinforcement or punishment was delivered) and resurgence of target responding was evaluated. Phase 3 lasted 5 sessions. Figure 1 displays a timeline and summary of the experimental conditions in place during Experiment 1.
Figure 1.
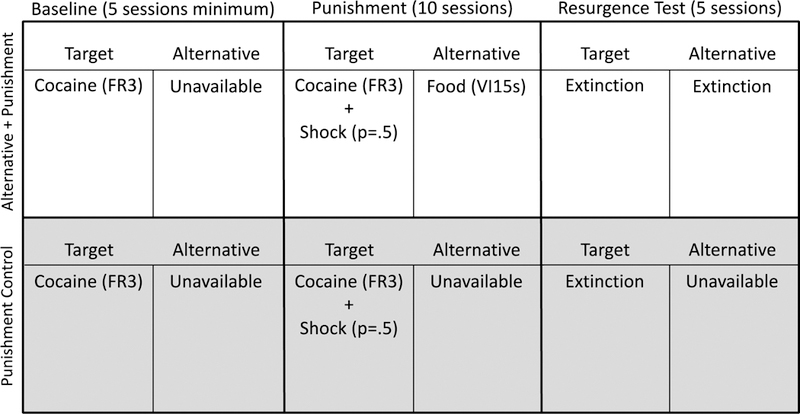
Timeline and summary of the conditions for each group during each phase of Experiment 1.
2.3. Experiment 1 data analysis
Time for reinforcer deliveries and chamber blackouts were excluded from session time in all rate measures reported below. All analyses were deemed significant at an α level of .05.
2.4. Experiment 1 results and summary
2.4.1. Phase 1: Baseline
Target response rates were similar between groups during the final three sessions of Phase 1 (see Table 1). This finding was confirmed by a one-way ANOVA conducted on the average of target response rates across the final three sessions of Phase 1 that revealed no significant effect of Group F(1,8) = .485, p = .506, η2 = .057. The amount of cocaine consumed was also similar between groups across the final three sessions of Phase 1 (see Table 1), as confirmed by a one-way ANOVA conducted on the average of obtained mg/kg across the final three sessions, F(1,8) = .336, p = .578, η2 = .040). Table 1 includes a summary of response rates, reinforcer rates, and cocaine consumption for both groups across phases of Experiment 1.
Table 1.
Mean (SEM) Response and Reinforcer Rates from each Phase of Experiment 1.
| Group | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Alternative + Punishment | Punishment Control | |||||
|
|
|
|||||
| Phase 1a | Phase 2b | Phase 3c | Phase 1a | Phase 2b | Phase 3c | |
|
| ||||||
| Target/Min | 4.44 | 0.20 | 1.49 | 4.89 | 0.93 | 0.88 |
| (1.35) | (0.08) | (0.31) | (1.31) | (0.51) | (0.45) | |
| Alt./Min | - | 59.63 | 9.30 | - | 0.10 | 0.03 |
| - | (12.57) | (0.43) | - | (0.04) | (0.01) | |
| Inactive/Min | 0.19 | 0.57 | 0.48 | 0.39 | 1.27 | 1.49 |
| (0.09) | (0.24) | (0.04) | (0.11) | (0.69) | (106) | |
| Infusions/Min | 0.26 | 0.004 | - | 0.24 | 0.05 | - |
| (0.06) | (0.004) | - | (0.06) | (0.03) | - | |
| Cocaine mg/kg | 3.20 | 0.06 | - | 3.95 | 0.70 | - |
| (0.91) | (0.06) | - | (0.91) | (0.46) | - | |
| Foods/Min | - | 3.44 | - | - | - | - |
| - | (0.22) | - | - | - | - | |
| Shocks/Min | - | 0.11 | - | - | 0.43 | - |
| - | (0.04) | - | - | (0.24) | - | |
Data averaged across the last three sessions of Phase 1 are shown,
Data from the last session of Phase 2 are shown,
Data from the first session of Phase 3 are shown.
2.4.2. Phase 2: Punishment of Cocaine Seeking
Figure 2A shows that target response rates decreased similarly across Phase 2 for both groups. A 2 × 10 (Group × Session) mixed-model ANOVA conducted on target response rates across all session of Phase 2 revealed a significant main effect of Session F(9,72) = 7.033,p < .001, ηp2 = .468, but no significant main effect of Group F(1,8) = .648, p = .444, ηp2 = 075, and no significant Group × Session interaction F(9,72) = 1.127, p = .356, ηp2 = 123, confirming that target responding decreased similarly across Phase 2 for both groups.
Figure 2.
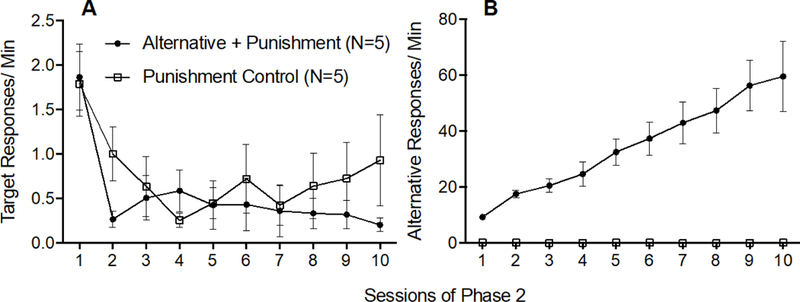
Target (A) and alternative (B) response rates across Phase 2 of Experiment 1. Error bars represent standard errors of the mean. Note difference in y-axes between panels A and B.
Figure 2B shows that alternative responding increased across Phase 2 in the Alternative + Punishment group, but not in the Punishment Control group. This finding was confirmed by a 2 × 10 (Group × Session) mixed-model ANOVA conducted on alternative response rates across all sessions of Phase 2 which revealed a significant Group x Session interaction F(9,72) = 12.169, p < .001, ηp2 = .603, a significant main effect of Session F(9,72) = 12.122, p < .001, ηp2 = 602, and a significant main effect of Group F(1,8) = 51.911, p < .001, ηp2 = 866.
2.4.3. Phase 3: Resurgence Test
The dotted data paths in Figure 3A show that target response rates increased (i.e., resurgence occurred) between the last session of Phase 2 and the first session of Phase 3 for only the Alternative + Punishment group. To confirm this finding, a 2 × 2 (Group × Phase) mixed-model ANOVA was conducted on target response rates during the last session of Phase 2 and the first session of Phase 3 and revealed a significant Group × Session interaction F(1,8) = 17.966, p = .003, ηp2 = 692, and a significant main effect of Session F(1,8) = 15.643, p = .004, ηp2 = 662, but no significant main effect of Group F(1,8) = .014, p = .909, ηp2 = 002. The solid data paths in Figure 3A show that target responding did not differ between groups across all sessions of Phase 3. A 2 × 5 (Group × Session) mixed-model ANOVA conducted on target response rates across all of Phase 3 revealed a significant main effect of Session F(4,32) = 3.343, p = .021, ηp2 = .295, but no significant Group × Session interaction F(4,32) = .154, p = .960, ηp2 = 019, and no significant main effect of Group F(1,8) = 1.201, p = .305, ηp2 = .131. Thus, target responding increased between Phases 2 and 3 for the Alternative + Punishment group alone, and then decreased across Phase 3 similarly for both groups.
Figure 3.
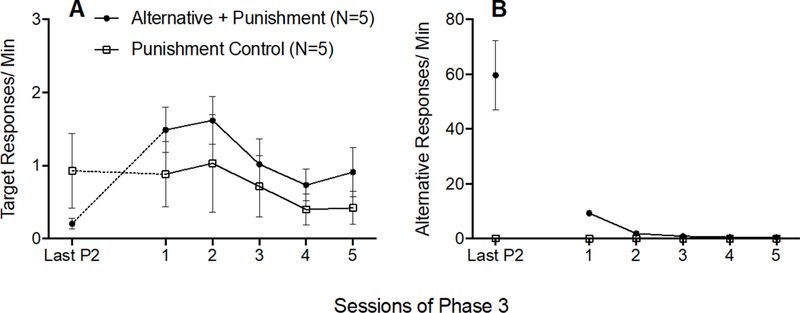
Target (A) and alternative (B) response rates across the last session of Phase 2 and all sessions of Phase 3 of Experiment 1. Error bars represent standard errors of the mean. Note difference in y-axes between panels A and B.
Figure 3B shows that alternative responding decreased across Phase 3 for the Alternative + Punishment group, and remained low for the Punishment control group. These findings were verified by a 2 × 5 (Group × Session) mixed-model ANOVA revealing a significant Group × Session interaction F(4,32) = 248.135,p < .001, ηp2 = 969, and significant main effects of Session F(4,32) = 246.580, p < .001, ηp2 = .969 and Group F(1,8) = 557.178, p < .001, ηp2 = .986.
Inactive lever response rates did not increase between the last session of Phase 2 and the first session of Phase 3 for either group, indicating that resurgence was the result of responding directed toward the lever that previously produced cocaine rather than a general increase in lever pressing (see Table 1). This result was verified by a 2 × 2 (Group × Phase) mixed-model ANOVA conducted on inactive responding on the last session of Phase 2 and the first session of Phase 3, which revealed no significant main effect of Session F(1,8) = .070, p = .798, ηp2 = 009, no significant main effect of Group F(1,8) = .961, p = .356, ηp2 = 107, and no significant Group × Session interaction F(1,8) = .419, p = .536, ηp2 = 050.
2.4.4. Summary
Resurgence of cocaine seeking following suppression by punishment occurred when alternative reinforcement was removed for the Alternative + Punishment group in Experiment 1. No increase in target responding was observed when the punishment and reinforcement contingencies were discontinued for the Punishment Control group during resurgence testing. Thus, the increase in drug seeking (i.e., target responding) between Phases 2 and 3 was due to the loss of alternative reinforcement and not the removal of the punishment contingency.
3. Experiment 2
The procedure developed in Experiment 1 evaluated resurgence induced by loss of alternative reinforcement in Phase 3 under conditions where cocaine-seeking responses had no consequences. This is advantageous for making comparisons to other resurgence procedures (Frye et al., 2018; Nall et al., 2018, 2019; Quick et al., 2011; Shahan et al., 2015) as well as other procedures that have examined relapse of previously-punished drug seeking (Marchant, Khuc, Pickens, Bonci, & Shaham, 2013; Nall et al., 2019; Panlilio et al., 2003; Pelloux et al., 2018). However, humans are not likely to experience extinction of drug seeking following treatment with alternative reinforcement (Marchant, Li, et al., 2013; Panlilio et al., 2005). Rather, when treatment ends, the individual retains the option to seek drugs and produce both the positive and negative consequences of doing so. Prior work has examined relapse of previously-punished behavior when either the positive (e.g., Panlilio et al., 2005) or negative (e.g., Cooper, Barnea-Ygael, Levy, Shaham, & Zangen, 2007) consequences of drug seeking remained in place, but not both. Thus, Experiment 2 was designed to assess resurgence of previously-punished cocaine seeking in rats while both reinforcement and punishment of drug seeking remained available during the Phase 3 test.
3.1. Material and method
3.2.1. Subjects.
Thirteen experimentally naive male Long-Evans rats served as subjects in Experiment 2. Housing, care, surgical procedures, apparatus, and drugs were identical to those detailed in Experiment 1. One rat in the Alternative + Punishment group with extremely high rates of target responding was identified as an outlier using Grubbs’ (Grubbs, 1969) method with a=.05, and thus removed from all analyses.
3.2. Procedure
Procedures for pellet training, cocaine self-administration training, and Phase 1: Baseline, were all identical to those described in Experiment 1 (see Section 2.2 above for details).
3.2.1. Phase 2: Punishment of Cocaine Seeking
Rats were divided into two groups matched on target response rate and cocaine consumption across the last 3 sessions of Phase 1. For the Alternative + Punishment group (N = 6), alternative responses produced food as described in Experiment 1 and target responses produced cocaine and shock as described in Experiment 1. For the Punishment Control group (N = 7), as described in Experiment 1, the target responding produced cocaine and shock but no alternative reinforcement was available. Phase 2 lasted 10 sessions.
3.2.2. Phase 3: Resurgence test
During Phase 3, alternative reinforcement was removed for the Alternative + Punishment group. All consequences for target responding remained in place for both groups. That is, target responding was reinforced with 0.32mg/kg infusions of cocaine according to a VR 20 schedule. Target responding also continued to produce intermittent mild foot shock as in Phase 2. Phase 3 lasted for 5 sessions. Figure 4 displays a timeline and summary of the experimental conditions in place during Experiment 2.
Figure 4.
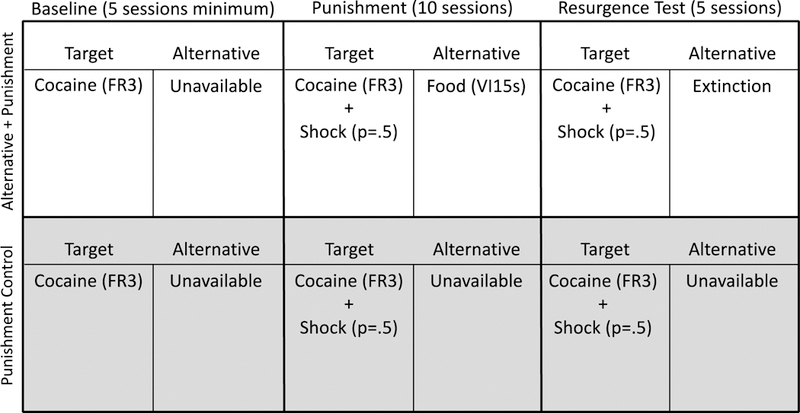
Timeline and summary of the conditions for each group during each phase of Experiment 2.
3.3. Experiment 2 data analysis
Primary data analyses were conducted as in Experiment 1.
3.4. Experiment 2 results and summary
3.4.1. Phase 1: Baseline
Target response rates were similar between groups during the final three sessions of Phase 1 (see Table 2). This finding was confirmed by a one-way ANOVA conducted on the average of target response rates across the final three sessions of Phase 1 that revealed no significant effect of Group F(1,12) = .011, p = .917, η2 = 001. The amount of cocaine consumed was also similar between groups across the final three sessions of Phase 1 (see Table 2), as confirmed by a one-way ANOVA conducted on the average of obtained mg/kg across the final three sessions which found no significant effect of Group F(1,12) = .063, p = .806, η2 = .006). Table 2 includes a summary of response rates, reinforcer rates, and cocaine consumption for both groups across phases of Experiment 2.
Table 2.
Mean (SEM) Response and Reinforcer Rates from each Phase of Experiment 2.
| Group | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Alternative + Punishment | Punishment Control | |||||
|
|
|
|||||
| Phase 1a | Phase 2b | Phase 3c | Phase 1a | Phase 2b | Phase 3c | |
|
| ||||||
| Target/Min | 6.65 | 0.11 | 0.52 | 6.77 | 1.30 | 1.21 |
| (0.84) | (0.04) | (0.10) | (0.93) | (0.56) | (0.78) | |
| Alt./Min | - | 51.84 | 12.17 | - | 0.17 | 0.07 |
| - | (14.58) | (4.04) | - | (0.10) | (0.04) | |
| Inactive/Min | 0.18 | 0.88 | 1.11 | 0.19 | 0.16 | 0.24 |
| (0.07) | (0.62) | (0.53) | (0.05) | (0.04) | (0.12) | |
| Infusions/Min | 0.33 | 0.00 | 0.02 | 0.34 | 0.07 | 0.06 |
| (0.04) | (0.00) | (0.01) | (0.05) | (0.03) | (0.04) | |
| Cocaine mg/kg | 4.73 | 0.00 | 0.27 | 4.93 | 1.01 | 0.82 |
| (0.60) | (0.00) | (0.10) | (0.70) | (0.50) | (0.57) | |
| Foods/Min | - | 3.34 | - | - | - | - |
| - | (0.21) | - | - | - | - | |
| Shocks/Min | - | 0.07 | 0.27 | - | 0.60 | 0.56 |
| - | (0.03) | (0.06) | - | (0.24) | (0.36) | |
Data averaged across the last three sessions of Phase 1 are shown,
Data from the last session of Phase 2 are shown,
Data from the first session of Phase 3 are shown.
3.4.2. Phase 2: Punishment
Figure 5A shows that target response rates decreased across Phase 2 for both groups and were lower for the Alternative + Punishment group than for the Punishment Control Group. A 2 × 10 (Group × Session) mixed-model ANOVA conducted on target response rates across all session of Phase 2 revealed a significant main effect of Session F(9,99) = 2.264, p = .024, ηp2 = .171, and a significant main effect of Group F(1,11) = 7.130, p = .022, ηp2 = 393, but no significant Group x Session interaction F(9,99) = .252, p = .985, ηp2 = .022. Thus, target response rates decreased at a similar rate across Phase 2 for both groups and target response rates were lower for the Alternative + Punishment group than for the Punishment Control group.
Figure 5.
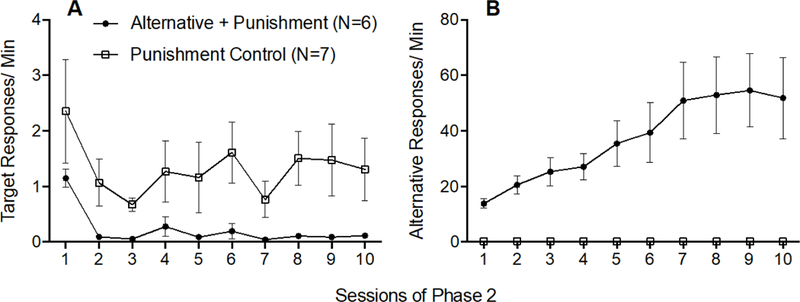
Target (A) and alternative (B) response rates across Phase 2 of Experiment 2. Error bars represent standard errors of the mean. Note difference in y-axes between panels A and B.
Figure 5B shows that alternative responding increased across Phase 2 in the Alternative + Punishment group, but not in the Punishment Control group. This finding was confirmed by a 2 × 10 (Group × Session) mixed-model ANOVA conducted on alternative response rates across all sessions of Phase 2 which revealed a significant Group × Session interaction F(9,99) = 7.289, p < .001, ηp2 = 399, a significant main effect of Session F(9,99) = 7.254, p < .001, ηp2 = 397, and a significant main effect of Group F(1,11) = 11.685, p = .006, ηp2 = 515.
3.4.3. Phase 3: Resurgence Test
Mean target response rates increased slightly only for the Alternative + Punishment group between the last session of Phase 2 and first session of Phase 3, but that effect was not statistically robust (see dotted data paths in Figure 6A). A 2 × 2 (Group × Phase) mixed-model ANOVA was conducted on target response rates during the last session of Phase 2 and the first session of Phase 3 and found no significant Group x Session interaction F(1,11) = 1.686, p = .221, ηp2 = .133, no significant main effect of Session F(1,11) = .824, p = .383, ηp2 = 070, and no significant main effect of Group F(1,11) = 1.399, p = .262, ηp2 = 113. The solid data paths in Figure 6A show that target responding did not differ between groups across all sessions of Phase 3. A 2 × 5 (Group × Session) mixed-model ANOVA conducted on target response rates across all of Phase 3 revealed no significant main effect of Session F(4,44) = .559, p = .694, ηp2 = .048, no significant main effect of Group F(1,12) = .006, p = .939, ηp2 = .001, and no significant Group × Session interaction F(4,44) = .579, p = .680, ηp2 = 050. Thus, the small increase in target response rates for the Alternative + Punishment group between the last session of Phase 2 and the first session of Phase 3 was not large enough to differ significantly from the Punishment Control group.
Figure 6.
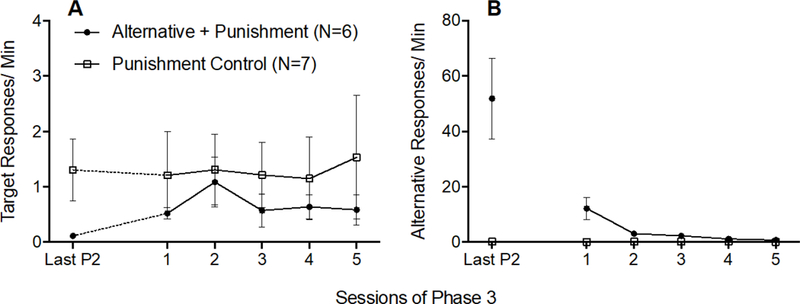
Target (A) and alternative (B) response rates across the last session of Phase 2 and all sessions of Phase 3 of Experiment 2. Error bars represent standard errors of the mean. Note difference in y-axes between panels A and B.
Figure 6B shows that alternative responding decreased across Phase 3 for the Alternative + Punishment group, and remained low for the Punishment Control group. These findings were verified by a 2 × 5 (Group × Session) mixed-model ANOVA revealing a significant Group × Session interaction F(4,44) = 12.545, p < .001, ηp2 = .533, and significant main effects of Session F(4,44) = 12.285, p < .001, ηp2 = .528 and Group F(1,11) = 19.888, p =.001, ηp2 = .644.
Inactive response rates did not increase between the last session of Phase 2 and the first session of Phase 3 for either group (see Table 2). This result was verified by a 2 × 2 (Group × Phase) mixed-model ANOVA conducted on inactive responding on the last session of Phase 2 and the first session of Phase 3, which revealed no significant main effect of Session F(1,11) = 2.961, p = .113, ηp2 = 212, no significant main effect of Group F(1,11) = 2.212, p = .165, ηp2 = .167, and no significant Group × Session interaction F(1,11) = .657, p = .435, ηp2 = .056.
Because resurgence appeared to be blunted in Experiment 2, and all other aspects of the experiments were similar besides the testing conditions in Phase 3, a comparison with the effect in Experiment 1 was warranted. Figure 7 shows target response rates during the last session of Phase 2 and first session of Phase 3 for each individual rat in the Alternative + Punishment groups from Experiment 1 and Experiment 2. Target responding increased for every rat in the Alternative + Punishment groups in both experiments when alternative reinforcement was discontinued during Phase 3, but the increases were much larger in Experiment 1. This finding was confirmed by a 2 × 2 (Experiment × Phase) mixed-model ANOVA conducted on target response rates in the last session of Phase 2 and the first session of Phase 3 for the Alternative + Punishment groups in Experiment 1 and Experiment 2. The ANOVA revealed a significant Experiment x Phase interaction F(1,8) = 9.787, p = .014, ηp2 = 550, and significant main effects of Experiment F(1,8) = 15.966, p = .004, ηp2 = 666, and Phase F(1,8) = 19.258, p = .002, ηp2 = .707. Follow up paired-sample t-tests indicated that target response rates increased between the last session of punishment and first session of resurgence testing in both experiments (Experiment 1, t = 4.277, p = .013; Experiment 2, t = 5.740, p = .002). Thus, mean target response rate increased between phases 2 and 3 of both experiments (i.e. resurgence occurred), and the increase in target responding was larger in Experiment 1 than in Experiment 2. Thus, in Experiment 2, target response rates did increase for rats in the Alternative + Punishment group when considered alone. However, that increase was not large enough to significantly differ from the Punishment Control group in the ANOVA conducted above.
Figure 7.
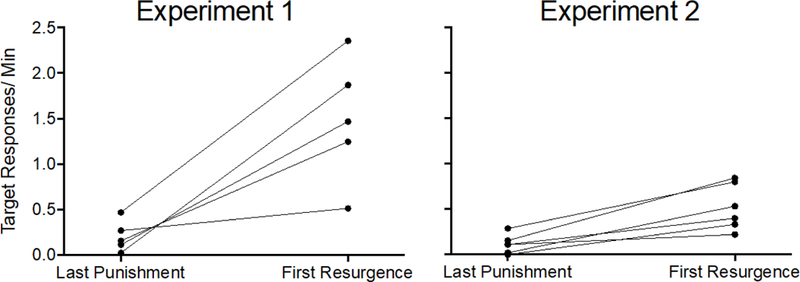
Target response rates for each individual in the Alternative + Punishment groups of Experiment 1 (left) and Experiment 2 (right) during the last session of Phase 2 and first session of Phase 3.
3.4.4. Summary
The goal of Experiment 2 was to evaluate resurgence while cocaine reinforcement and punishment remained available for the target behavior, as these conditions may be more analogous to the conditions present when humans with SUDs end alternative-reinforcement- based treatment. Though the dotted data paths in Figure 6A hint at a possible resurgence effect, the continued presence of punishment for cocaine seeking in Phase 3 considerably reduced the magnitude of the effect. Thus, the results indicate that under the current conditions, resurgence effects appear to be smaller when reinforcement and punishment remain in place for the target response during the resurgence test compared to the conditions for resurgence testing in Experiment 1 (i.e., target and alternative extinction, removal of punishment). Further implications will be discussed below.
4. General Discussion
The goal of the present experiments was to develop a model of resurgence of drug seeking following suppression by aversive consequences. In the first phase of Experiment 1, rats pressed levers to earn infusions of cocaine. In Phase 2, cocaine remained available, but lever pressing also produced mild intermittent foot shock. For the Alternative + Punishment group in Phase 2, nose poking produced food pellets (i.e. alternative reinforcement). No alternative reinforcement was available for the Punishment Control group. Finally, in Phase 3, all consequences were removed for both responses in both groups. That is, lever presses no longer produced shock or cocaine in either group and nose poking no longer produced food for the Alternative + Punishment group.
Resurgence of cocaine seeking was observed following the removal of alternative reinforcement for the Alternative + Punishment group in Experiment 1. Importantly, the removal of punishment alone in the Punishment Control group was not sufficient to produce relapse. Thus, the increase in cocaine seeking in the Alternative + Punishment group was due to the history of exposure to and then removal of alternative reinforcement (i.e. resurgence) and not the removal of punishment alone. These data are consistent with previous studies demonstrating a variety of relapse effects following suppression by punishment (Campbell et al., 2017; Ducret et al., 2016; Economidou et al., 2009; Krasnova et al., 2014; Marchant et al., 2016; Panlilio et al., 2003; Pelloux et al., 2018), with previous studies showing that the removal of non-drug alternative reinforcement can induce relapse of drug seeking following extinction (Craig, Browning, Nall, Marshall, & Shahan, 2017; Nall et al., 2018; Podlesnik et al., 2006), and with previous studies demonstrating resurgence of food seeking following suppression by punishment (Nall et al., 2019). The procedure developed in Experiment 1 represents an improvement in the face validity of the animal model of resurgence, better represents the environmental (and potentially neurobiological) factors involved in resurgence of drug seeking in humans, and allows for comparisons between extinction-based resurgence models and other punishment-based models of relapse that test under extinction conditions.
Previous work has examined relapse of previously-punished drug seeking when reinforcement or punishment was continued, but not both. For example, Panlilio et al. (2005) found greater reinstatement by drug-priming injections when remifentanil remained available than when it was unavailable following punishment of the remifentanil-seeking response. However, punishment was discontinued for both groups during the reinstatement test. Cooper et al. (2007) found greater reinstatement by noncontingent exposure to drug-paired cues when punishment was discontinued than when it remained in effect. However, reinforcement was discontinued for both groups during the reinstatement test. Thus, Experiment 2 was designed to assess resurgence while both the positive and negative consequences of drug seeking remained available following suppression of the drug-seeking response by punishment. Rats earned cocaine infusions during Phase 1 of Experiment 2. Next, cocaine seeking was reinforced and punished, and alternative reinforcement was made available for the Alternative + Punishment group but not the Punishment Control group. Finally, alternative reinforcement was removed for the Alternative + Punishment group and cocaine seeking continued to produce cocaine and punishment for both groups. An increase in target response rate occurred following the removal of alternative reinforcement for rats in the Alternative + Punishment group when considered alone, but that increase was not large enough to significantly differ from the Punishment Control group.
On the one hand, it is unsurprising that resurgence did not occur for rats in the Alternative + Punishment group during Experiment 2, as the continued presence of punishment should serve to reduce drug seeking compared to the extinction conditions present during testing in Experiment 1 (Cooper et al., 2007). On the other hand, one might have expected some resurgence as continued cocaine reinforcement should have served to increase target responding relative to the extinction conditions during testing in Experiment 1 (Panlilio et al., 2005). Thus, the reduced resurgence in Experiment 2 suggests that continued punishment was more effective at suppressing responding than continued reinforcement was at increasing responding. However, rates of target responding (and thus, shock) were higher for the Punishment Control group across Phases 2 and 3 (see Table 2), indicating that the parameters of shock used in Experiment 2 could permit higher rates of responding that those observed in the Alternative + Punishment group. Further, target responding remained stable during Phase 3 for both groups in Experiment 2, but decreased across Phase 3 for both groups in Experiment 1. This finding suggests that even though punishment suppressed resurgence in Experiment 2, the punishment schedule was permissive enough to allow relatively low and stable rates of cocaine self-administration to continue across 5 further sessions of punishment. Finally, target response rate did increase for the Alternative + Punishment group when alternative reinforcement was removed, albeit not to a level significantly different from the Punishment Control group (see Figure 6A). Taken together, these observations suggest that continued punishment of cocaine seeking reduced resurgence in Experiment 2 relative to the extinction conditions in place in Experiment 1, but that continued reinforcement maintained relatively low and stable rates of cocaine seeking for at least 5 sessions. These results indicate that continuation of both the positive and negative effects of drug seeking may play an important role in determining abstinence from drug use following treatment with alternative reinforcement.
Though resurgence of target responding in the Alternative + Punishment group was not significantly different from the Punishment Control group, rates of drug seeking were lower during Phase 2 punishment for the Alternative + Punishment group. This effect was not statistically significant during Experiment 1, but data for the Punishment Control group showed an increasing trend across the last six sessions of Phase 2 (see Figure 2A). Thus, if Phase 2 had been extended in Experiment 1, it is likely that the difference between groups would have been detectable. Further, the results of Experiment 2 and prior studies (e.g. Nall et al., 2019; Pelloux, Everitt, & Dickinson, 2007; Pelloux, Murray, & Everitt, 2015) provide evidence for the consistency of this effect. Thus, the reason alternative reinforcement further suppressed punished cocaine seeking in Experiment 2 but did not in Experiment 1 is most likely due to individual differences in sensitivity to punishment. The finding that availability of alternative reinforcement increases the efficacy of punishment may also be relevant for treatment of SUDs in humans. As discussed above, aversive consequences of substance use are thought to reduce drug seeking in natural environments and are often influential in decisions to enter treatment. Thus, treatments that include alternative reinforcement components or are based on alternative reinforcement should increase the efficacy of the natural punishment contingencies for substance use. Indeed, including alternative reinforcement in existing treatment approaches can increase treatment outcomes (e.g. García-Fernández et al., 2011) and alternative-reinforcement-based treatments are among the most effective for substance use disorders (Prendergast et al., 2006). Thus, the models developed here may be beneficial for investigations into the additional suppressive effects that alternative reinforcement may provide when available during punishment.
The procedures used here developed a basic experimental structure for studying resurgence of cocaine seeking following suppression by punishment. Future, larger N studies should ensure that these effects will generalize across sex and cocaine doses. In addition, future neurobiological and pharmacological studies similar to those conducted by Pelloux et al. (2018) and Panlilio et al. (2005) are necessary to determine if different underlying mechanisms are involved in relapse tested during extinction and relapse tested during continued reinforcement and punishment. The outcomes of these future studies could be instrumental in furthering a mechanistic understanding of relapse effects and for developing novel treatments to reduce relapse of drug seeking following treatment. Further, evidence suggests that individuals who spontaneously abstain from drug use often attribute their abstinence to the negative effects associated with drug use and the procurement of alternative reinforcement (Burman, 1997). Thus, the models developed herein could also contribute to a better understanding of abstinence and relapse outside of treatment.
5. Conclusion
The results of Experiment 1 suggest that loss of alternative reinforcement can induce relapse (i.e., resurgence) of cocaine seeking previously suppressed by punishment. The results of Experiment 2 showed that expected resurgence effects were suppressed when both punishment and cocaine reinforcement were produced by cocaine seeking during the resurgence test. Further manipulations of parameters of punishment, reinforcement, or both are necessary to determine if more robust resurgence can occur under these conditions. The models developed here improve the face validity of the animal model of resurgence of drug seeking and provide a basis for examining the factors underlying resurgence as well as those underlying relapse during continued reinforcement and punishment. As such, future work with these models should provide insights for a better mechanistic understanding of relapse effects and for development of novel treatments.
Public Significance Statement.
Drug abuse is characterized by chronic relapse to drug seeking following periods of abstinence. This study developed a novel behavioral procedure for a rat model of relapse to cocaine seeking that is better aligned with the human condition by modeling abstinence generated by experience of negative consequences and relapse induced by loss of a non-drug source of reward.
Acknowledgements
This work was supported by NIH grant R21DA038950 (TAS). Cocaine was kindly supplied by NIDA. The authors thank Kaitlyn O. Browning and Anthony N. Nist for their assistance in conducting this research.
This work was supported by NIH grant R21DA038950 (TAS). The authors thank NIDA for kindly supplying cocaine, and Kaitlyn O. Browning and Anthony N. Nist for their assistance in conducting this research. A portion of this manuscript was used in partial fulfillment of the degree of Ph. D. at Utah State University (RWN).
Footnotes
Disclosures
The authors have no competing interests to declare.
REFERENCES
- Burman S (1997). The challenge of sobriety: Natural recovery without treatment and self- help groups. Journal of Substance Abuse, 9(1), 41–61. 10.1016/S0899-3289(97)90005-5 [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Barker DJ, Nasser HM, Kaganovsky K, Dayas CV, & Marchant NJ (2017). Cue-Induced Food Seeking After Punishment Is Associated With Increased Fos Expression in the Lateral Hypothalamus and Basolateral and Medial Amygdala. Behavioral Neuroscience, 131(2), 155–167. 10.1037/bne0000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Flanagan JPM, Walker LC, Hill MKRI, Marchant XJ, & Lawrence AJ (2019). Anterior Insular Cortex is Critical for the Propensity to Relapse Following Punishment-Imposed Abstinence of Alcohol Seeking. 10.1523/JNEUROSCI.1596-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, & Zangen A (2007). A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology, 194(1), 117–125. 10.1007/s00213-007-0827-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AR, Browning KO, Nall RW, Marshall CM, & Shahan TA (2017). Resurgence and alternative-reinforcer magnitude. Journal of the Experimental Analysis of Behavior, 107(2), 218–233. 10.1002/jeab.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AR, Nall RW, Madden GJ, & Shahan TA (2016). Higher rate alternative non-drug reinforcement produces faster suppression of cocaine seeking but more resurgence when removed. Behavioural Brain Research, 306, 48–51. https://doi.org/10.1016Zj.bbr.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Puaud M, Lacoste J, Belin-Rauscent A, Fouyssac M, Dugast E, ... Belin D (2016). N-acetylcysteine Facilitates Self-Imposed Abstinence After Escalation of Cocaine Intake. Biological Psychiatry, 80(3), 226–234. https://doi.org/10.10167j.biopsych.2015.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, & Everitt BJ (2009). High Impulsivity Predicts Relapse to Cocaine-Seeking After Punishment-Induced Abstinence. Biological Psychiatry, 65(10), 851–856. https://doi.org/10.10167j.biopsych.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Epstein R (1985). Extinction-induced resurgence: Preliminary investigations and possible applications. The Psychological Record. Retrieved from https://link.springer.com/article/10.1007/BF03394918 [Google Scholar]
- Fleshler M, & Hoffman HS (1962). A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior, 5(4), 529–530. 10.1901/jeab.1962.5-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes RM, Todorov JC, & Shahan TA (2018). Punishment of an alternative behavior generates resurgence of a previously extinguished target behavior. Journal of the Experimental Analysis of Behavior, 110(2), 171–184. 10.1002/jeab.465 [DOI] [PubMed] [Google Scholar]
- Frye CCJ, Rung JM, Nall RW, Galizio A, Haynes JM, & Odum AL (2018). Continuous nicotine exposure does not affect resurgence of alcohol seeking in rats. PLoS ONE, 13(8), e0202230. 10.1371/journal.pone.0202230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz-Kausch AM, Adank DN, & Dietz DM (2014). Prolonged withdrawal following cocaine self-administration increases resistance to punishment in a cocaine binge. Scientific Reports, 4, 6876. 10.1038/srep06876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fernández G, Secades-Villa R, García-Rodríguez O, Sánchez-Hervás E, Fernández-Hermida JR, & Higgins ST (2011). Adding voucher-based incentives to community reinforcement approach improves outcomes during treatment for cocaine dependence. American Journal on Addictions, 20(5), 456–461. 10.1111/j.1521-0391.2011.00154.x [DOI] [PubMed] [Google Scholar]
- Grubbs FE (1969). American Society for Quality Procedures for Detecting Outlying Observations in Samples (Vol. 11). Retrieved from https://www.jstor.org/stable/pdf/1266761.pdf?acceptTC=true [Google Scholar]
- Higgins ST, & Silverman K (1999). Motivating behavior change among illicit-drug abusers: Research on contingency management interventions. Retrieved from http://doi.apa.org/psycinfo/1999-02363-000 [Google Scholar]
- Hunt GM, & Azrin NH (1973). A community-reinforcement approach to alcoholism. Behaviour Research and Therapy, 11(1), 91–104. 10.1016/0005-7967(73)90072-7 [DOI] [PubMed] [Google Scholar]
- Klingemann HK −. (1991). The motivation for change from problem alcohol and heroin use. British Journal of Addiction, 86(6), 727–744. 10.1111/j.1360-0443.1991.tb03099.x [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM,... Cadet JL (2014). Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology, 39(8), 2008–2016. 10.1038/npp.2014.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet AB, Savage R, & Mahmood D (2002). Pathways to long-term recovery: a preliminary investigation. Journal of Psychoactive Drugs, 34(3), 305–311. 10.1080/02791072.2002.10399968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, ... Shaham Y (2016). Role of Ventral Subiculum in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence. Journal of Neuroscience, 36(11), 3281–3294. 10.1523/JNEUROSCI.4299-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, & Kaganovsky K (2015). A critical role of nucleus accumbens dopamine D1- family receptors in renewal of alcohol seeking after punishment-imposed abstinence. Behavioral Neuroscience, 129(3), 281–291. 10.1037/bne0000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Khuc TN, Pickens CL, Bonci A, & Shaham Y (2013). Context-induced relapse to alcohol seeking after punishment in a rat model. Biological Psychiatry, 73(3), 256–262. 10.1016/_j.biopsych.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, & Shaham Y (2013). Recent developments in animal models of drug relapse. Current Opinion in Neurobiology, 23(4), 675–683. 10.1016/jxonb.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Rabei R, Kaganovsky K, Caprioli D, Bossert JM, Bonci A, & Shaham Y (2014). A Critical Role of Lateral Hypothalamus in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence. Journal of Neuroscience, 34(22), 7447–7457. 10.1523/JNEUR0SCI.0256-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, & Kleber HD (2000). Drug Dependence, a Chronic Medical Illness. Journal of the American Medical Association, 284(13), 1689. 10.1001/jama.284.13.1689 [DOI] [PubMed] [Google Scholar]
- Miller WR, Meyers RJ, & Hiller-Sturmhöfel S (2003). The community-reinforcement approach. Psychosocial Treatments, 49–59. 10.4324/9780203503508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall RW, Craig AR, Browning KO, & Shahan TA (2018). Longer treatment with alternative non-drug reinforcement fails to reduce resurgence of cocaine or alcohol seeking in rats. Behavioural Brain Research, 341, 54–62. https://doi.org/10.1016ZJ.BBR.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall RW, Rung JM, & Shahan TA (2019). Resurgence of a target behavior suppressed by a combination of punishment and alternative reinforcement. Behavioural Processes, 162, 177–183. 10.1016/_j.beproc.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, & Schindler CW (2000). Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology, 150(1), 61–66. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10867977 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, & Schindler CW (2003). Reinstatement of punishment-suppressed opioid self-administration in rats: An alternative model of relapse to drug abuse. Psychopharmacology, 168(1–2), 229–235. 10.1007/s00213-002-1193-0 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, & Schindler CW (2005). Lorazepam reinstates punishment- suppressed remifentanil self-administration in rats. Psychopharmacology, 179(2), 374–382. 10.1007/s00213-004-2040-2 [DOI] [PubMed] [Google Scholar]
- Peck JA, & Ranaldi R (2014). Drug abstinence: Exploring animal models and behavioral treatment strategies. Psychopharmacology, 231(10), 2045–2058. 10.1007/s00213-014-3517-2 [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, & Dickinson A (2007). Compulsive drug seeking by rats under punishment: Effects of drug taking history. Psychopharmacology, 194(1), 127–137. 10.1007/s00213-007-0805-0 [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Minier-Toribio A, Hoots JK, Bossert JM, & Shaham Y (2018). Opposite Effects of Basolateral Amygdala Inactivation on Context-Induced Relapse to Cocaine Seeking after Extinction versus Punishment. Neuroscience, 35(1), 51–59. 10.1523/JNEUROSCI.2521-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, & Everitt BJ (2013). Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. European Journal of Neuroscience, 35(7), 3018–3026. 10.1111/ejn.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, & Everitt BJ (2015). Differential vulnerability to the punishment of cocaine related behaviours: Effects of locus of punishment, cocaine taking history and alternative reinforcer availability. Psychopharmacology, 232(1), 125–134. 10.1007/s00213-014-3648-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, & Shahan TA (2006). Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behavioural Pharmacology, 17(4), 369–374. 10.1097/01.fbp.0000224385.09486.ba [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, & Roll J (2006). Contingency management for treatment of substance use disorders: A meta-analysis. Addiction, 101(11), 1546–1560. 10.1111/j.1360-0443.2006.01581.x [DOI] [PubMed] [Google Scholar]
- Quick SL, Pyszczynski AD, Colston KA, & Shahan TA (2011). Loss of alternative non-drug reinforcement induces relapse of cocaine-seeking in rats: role of dopamine D(1) receptors. Neuropsychopharmacology, 36(5), 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secades-Villa R, Garcia-Rodriguez O, Garcia-Fernandez G, Sanches-Hervas E, Fernandez- Hermida JR, & Higgins ST (2011). Community reinforcement approach plus vouchers among cocaine-dependent outpatients: twelve-month outcomes. Psychology of Addictive Behaviors, 25(1), 174–179. Retrieved from http://psycnet.apa.org/fulltext/2011-01108-001.html [DOI] [PubMed] [Google Scholar]
- Shahan TA, Craig AR, & Sweeney MM (2015). Resurgence of sucrose and cocaine seeking in free-feeding rats. Behavioural Brain Research, 279, 47–51. https://doi.org/10.1016Zj.bbr.2014.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, & Stitzer ML (1999). Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology, 146(2), 128–138. 10.1007/s002130051098 [DOI] [PubMed] [Google Scholar]
- Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, & Cadet JL (2017). Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behavioural Brain Research, 326, 265–271. https://doi.org/10.10167j.bbr.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, & Bouton ME (2010). Mechanisms of resurgence of an extinguished instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes, 36(3), 343–353. 10.1037/a0017365 [DOI] [PMC free article] [PubMed] [Google Scholar]


