Abstract
Objective
To assess the prevalence of potential drug-drug interactions (pDDIs) among polypharmacy patients in Jordan using Lexicomp®. Additionally, this study aims to categorize and rate the identified pDDIs according to interaction risk, severity, and reliability.
Methods
A descriptive cross-sectional study was conducted at six different hospitals representing different public health sectors in Jordan (ministry of health, royal medical services, and university-affiliated hospitals). Polypharmacy patients from outpatient clinics (e.g., cardiology,& and internal medicine) were identified, recruited, and interviewed by clinical pharmacists. pDDIs were assessed using the Lexicomp® mobile application and classified according to interaction risk rating, severity, and reliability rating. Furthermore, the prevalence of pDDIs across chronic medical conditions was assessed. P-value <0.05 was considered as significant.
Results
A total of 801 patients with polypharmacy were identified. The average number of drugs per patient was 6.6 ± 1.96, with an average of 4.2 ± 3.0 pDDIs per patient. Potential drug-drug interactions were detected in 769 patients (96%), with a total of 3359 interactions. Blood pressure lowering agents were involved in 39.9% of the pDDIs. Cardiovascular system drugs contributed to the largest share of pDDIs (46.6%). While diuretics had the major share of interactions among cardiovascular system drugs (16.2%), drugs used in diabetes had the highest share across all groups (17.1%). The majority of pDDIs were of “C” risk rating with a moderate interaction severity, whilst 1.6% of pDDIs could have been avoided in the first place as the concurrent administration of these agents is contraindicated (i.e., risk rating X). Patients with cardiovascular diseases, diabetes, chronic obstructive pulmonary disease, gout, and chronic kidney disease were associated with the highest number of potential drug-drug interactions.
Conclusion
Our study showed that 96% of polypharmacy patients at outpatient clinics have at least one pDDI. Almost half of the detected interactions involved cardiovascular medications. The majority of these pDDIs had moderate severity, with no more than 10% of the interactions requiring therapy modification.
Keywords: Polypharmacy, Drug-drug interactions, Lexicomp®, Medication management
1. Introduction
Given the rising tide of patients living with multiple comorbidities, chronic co-prescription of several drugs (i.e., polypharmacy) is becoming more prevalent (Maher et al., 2014). The exact definition of polypharmacy can vary; however, the most commonly used definition is the simultaneous use of five or more medications (Masnoon et al., 2017). The combination of these medications is usually recommended by guidelines to manage and control chronic conditions. However, the use of multiple medications simultaneously is associated with undesirable outcomes, primarily due to drug-drug interactions (DDIs) (Maher et al., 2014, Mallet et al., 2007). DDIs are defined as changes in the drug’s effect due to the concurrent addition of another drug for the same or different diseases (Hines and Murphy, 2011). In DDIs, the physiological response caused by a combination of two or more drugs is different from that induced by the use of each drug alone (Hines & Murphy, 2011). DDIs can result from pharmacokinetic or pharmacodynamic mechanisms (Mistry et al., 2017). Pharmacokinetically, a drug can increase the concertation (e.g., leading to toxicity) or decrease the therapeutic effect or concentration of another drug (Corrie and Hardman, 2011). Pharmacodynamically, the pharmacological effect can be modified at the molecular level (Corrie and Hardman, 2011).
The severity of DDIs can vary from minor and undetectable to severe enough to negatively affect health and significantly increase treatment costs (Olsen and Sletvold, 2018). DDIs can occur in any practice setting; however, they are highly prevalent and well studied in hospitalized patients (Mousavi and Ghanbari, 2017). In the past decade, there has been a growing prevalence of potential drug-drug interactions (pDDIs) among outpatients (Aljadani and Aseeri, 2018, Bucher et al., 2016, Eljaaly et al., 2019, Ismail et al., 2018, Létinier et al., 2019). The prevalence of pDDIs varies from 16% to 91% in different studies due to variability in the study population, design, setting, and drug interaction screening tools used in each study (Al-Qerem et al., 2018, Ismail et al., 2018, Mistry et al., 2017, Patel et al., 2014).
DDIs in outpatients can account for over 38% of ADRs (Mirošević Skvrce et al., 2011) and account for 1.1% of hospital admissions (Dechanont et al., 2014). Several factors were found to be associated with a high prevalence of pDDIs such as patients’ age, gender, education, co-morbidities, and number of prescribed medications (Al-Qerem et al., 2018, Ismail et al., 2018, Mistry et al., 2017, Olsen and Sletvold, 2018, Patel et al., 2014). In some countries, the prevalence of pDDIs among outpatients can be associated with insufficient use of DDI screening tools in outpatient clinics and pharmacies, in addition to the inadequate documentation of medical and medication history (Chatsisvili et al., 2010).
Although several studies have evaluated the prevalence and severity of pDDIs, studies which evaluate the prevalence of pDDIs among adult polypharmacy outpatients are limited. A recent study reported that 91% of elderly patients in Jordan had at least one pDDI (Al-Qerem et al., 2018). However, there are no published large-scale multi-center studies which evaluate the prevalence of pDDIs among adult polypharmacy patients at outpatient settings in Jordan. Therefore, this study aims to assess the prevalence and predictors of pDDIs among adult polypharmacy patients at different hospital settings in Jordan. Further, this study aims to categorize and rate the identified pDDIs according to interaction risk, severity, and reliability.
2. Methods
2.1. Study design
In this descriptive cross-sectional study, patients at six different hospitals in Jordan were interviewed. These hospitals represent different health sectors in Jordan: public, royal medical services, and university-affiliated hospitals. This study was approved by the institutional review boards of the university hospitals, the royal medical services, and the ministry of health.
2.2. Sample
Patients from outpatient clinics (e.g., cardiology and internal medicine) were recruited and interviewed by clinical pharmacists. The inclusion criteria were: patients ≥18 years old, had at least one chronic medical condition, and taking at least five medications (i.e., polypharmacy) including all routes of administration (i.e.topical, inhaled, as needed, over the counter, etc). Patients with moderate to severe cognitive impairment or those who did not speak Arabic or English languages were excluded from the study. Patients who met the inclusion/exclusion criteria were given a brief explanation about the study objectives, after which they signed informed consent forms.
2.3. Data analysis
Potential drug-drug interactions (pDDIs) were assessed using the Lexicomp® mobile application and classified according to interaction risk rating, severity, and reliability rating (Table 1). Lexi-interact is considered one of the best performing DDI screening programs. Several previous studies have assessed the performance of Lexi-interact as a DDI screening software (Aljadani and Aseeri, 2018, Al-Qerem et al., 2018, Smithburger et al., 2010). Lexi-interact was reported to be highly specific (80–90%) and sensitive (87–100%) software in most of these studies (Barrons, 2004, Kheshti et al., 2016, Roblek et al., 2015).
Table 1.
Lexicomp® drug-drug interactions rating scales and corresponding categories and definitions.
| Rating Scales | Categories |
|---|---|
| Risk Rating: indicates the level of urgency and the actions necessary to respond to an interaction. | A: No known interaction |
| No evidence to support pharmacodynamic or pharmacokinetic interactions. | |
| B: No Action Needed Evidence demonstrate that two drugs may interact with each other, but there is little to no clinical data to support it. | |
| C: Monitor Therapy | |
| Evidence suggest that the two drugs may interact with each other in a clinically significant manner. The benefits of concomitant use of these two medications usually outweigh the risks. An appropriate monitoring plan should be implemented to avoid potential negative outcomes. | |
| D: Consider Therapy Modification | |
| Evidence suggests that the two medications may interact with each other in a clinically significant manner. Specific actions must be taken to minimize the toxicity resulting from concomitant use of the medications. | |
| X: Avoid Combination | |
| The interaction of the two drugs is of clinical significance. The risks of concomitant use of these drugs usually outweigh the benefits and generally contraindicated. | |
| Severity Rating: indicated the magnitude of an interaction outcome | Major: the interaction is possibly life-threatening or may cause permanent damage |
| Moderate: the patient’s condition may deteriorate due to the interaction. Additional care may be required. | |
| Minor: an interaction that is inconvenient, but otherwise not medically detrimental. | |
| Reliability Rating: indicates the quantity and nature of documentation for an interaction. | Poor; Fair; Good; Excellent. |
Adapted from: https://www.wolterskluwercdi.com/facts-comparisons-online/user-guide/tools-interactions/ accessed Feb 1st, 2019.
Lexicomp® can be easily accessed and downloaded on smartphones and tablets. pDDIs are defined as the possible drug-drug interactions that may theoretically occur during the concurrent use of two or more drugs (Hines et al., 2012). Finally, medications that were detected to have potential drug-drug interactions were categorized according to the Anatomical Therapeutic Chemical classification system (ATC; World Health Organization, 2019).
Descriptive statistics were used to analyze the data, and the results are presented as percentages and frequencies. Univariable analyses were performed to evaluate the effect of covariates (patients’ characteristics and medical conditions were analyzed as categorical variables, while age and body mass index were analyzed as continuous variables) on the occurrence of pDDIs. In order to control for the confounding variables, variables showing association in the univariable analyses (p < 0.2) were included in the multivariable linear regression analysis (using the stepwise method). All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 25.
3. Results
3.1. Characteristics of the study population
A total of 801 patients with polypharmacy were identified in this study. The mean age of the recruited patients was 58.5 years, and 59.7% of the patients were females (Table 2). The average number of drugs per patient was 6.6 ± 1.96 with an average of 4.2 ± 3.0 pDDIs per patient (Table 2). The majority of patients were hypertensive (87.5%) or diabetic (67.5%; Table 2). Potential drug-drug interactions were detected in 769 patients (96%) with a total of 3359 interactions.
Table 2.
Patients’ demographics and clinical characteristics (N = 801).
| Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 323 (40.3%) |
| Female | 478 (59.7%) |
| Marital Status | |
| Single | 39 (4.9%) |
| Married | 669 (83.5%) |
| Divorced/widowed | 93 (11.6%) |
| Education | |
| Up to secondary level | 601 (75.1%) |
| Post-secondary level | 200 (24.9%) |
| Occupation | |
| Unemployed | 605 (75.5%) |
| Employed (healthcare related profession) | 16 (2%) |
| Employed (non-healthcare related) | 180 (22.5%) |
| SmokingStatus | |
| Non-smoker | 701 (87.5%) |
| Smoker | 100 (12.5%) |
| Clinical Conditions | |
| Hypertension | 701 (87.5%) |
| Diabetes | 541 (67.5%) |
| Ischemic Heart Disease | 330 (41.3%) |
| Dyslipidemia | 431 (53.8%) |
| Heart Failure | 126 (15.7%) |
| Hyperthyroidism | 10 (1.3%) |
| Hypothyroidism | 55 (6.9%) |
| Chronic Kidney Disease | 59 (7.4%) |
| Liver Disease | 6 (15.5%) |
| Asthma | 124 (15.5%) |
| Chronic Obstructive Pulmonary Disease | 19 (2.4%) |
| Rheumatoid Arthritis | 24 (3%) |
| Gout | 50 (6.2%) |
| Depression | 5 (0.6%) |
|
Mean (Std. dev.) |
|
| Age | 58.5 (10.7) |
| Body Mass Index | 30.7 (6.0) |
| Number of medications per patient | 6.6 (1.96) |
| Number of drug-drug interactions per patient | 4.2 (3.0) |
3.2. pDDI prevalence and severity
Overall, there were 116 different medications involved in the detected 3359 pDDIs. According to the ATC classification system, these 116 medications are divided into nine main groups (i.e., first level) and 34 subgroups (i.e., second level). The majority of the detected pDDIs were related to the use of cardiovascular system drugs (46.6%; Table 3). While diuretics had the greatest share of interactions among cardiovascular system drugs (16.2%), drugs used in diabetes had the highest share across all groups (17.1%; Table 3).
Table 3.
The number of medications detected to have pDDIs and their corresponding ATC classes and codes (n = 6718).
| First Level ATC Classification n (%) |
ATC Classification (Second Level) |
Frequency (%) |
|---|---|---|
| Alimentary Tract and Metabolism (A) n = 1714 (25.5%) |
Drugs for Acid Related Disorders (A02) | 149 (2.2%) |
| Drugs for Functional Gastrointestinal Disorders (A03) | 16 (0.2%) | |
| Drugs for Constipation (A06) | 2 (<0.1%) | |
| Antidiarrheals (A07) | 5 (0.1%) | |
| Drugs Used in Diabetes (A10) | 1147 (17.1%) | |
| Vitamins (A11) | 112 (1.7%) | |
| Minerals (A12) | 283 (4.2%) | |
| Blood and Blood Forming Organs (B) n = 1069 (15.9%) |
Antithrombotic Agents (B01) | 1027 (15.3%) |
| Antianemic Preparations (B03) | 42 (0.6%) | |
| Cardiovascular System (C) n = 3130 (46.6%) |
Cardiac Therapy (C01) | 155 (2.3%) |
| Antihypertensives (C02) | 24 (0.4%) | |
| Diuretics (C03) | 1093 (16.2%) | |
| Peripheral Vasodilators (C04) | 12 (0.2%) | |
| Beta Blocking Agents (C07) | 549 (8.2%) | |
| Calcium Channel Blockers (C08) | 204 (3%) | |
| Agents Acting on The Renin-Angiotensin System (C09) | 811 (12.1%) | |
| Lipid Modifying Agents (C10) | 282 (4.2%) | |
| Genito Urinary System and Sex Hormones (G) | Urologicals (G04) | 51 (0.8%) |
| Systemic Hormonal Preparations (H) n = 93 (1.4%) |
Corticosteroid for Systemic Use (H02) | 34 (0.5%) |
| Thyroid Therapy (H03) | 59 (0.9%) | |
| Antineoplastic and Immunomodulating Agents (L) | Immunosuppressants (L04) | 35 (0.5%) |
| Musculo-skeletal System (M) n = 210 (3.1%) |
Anti-inflammatory and Antirheumatic Products (M01) | 39 (0.6%) |
| Muscle Relaxants (M03) | 9 (0.1%) | |
| Antigout Preparations (M04) | 84 (1.3%) | |
| Drugs for Treatment of Bone Diseases (M05) | 78 (1.2%) | |
| Nervous System (N) n = 138 (2%) |
Analgesics (N02) | 7 (0.1%) |
| Antiepileptic (N03) | 79 (1.2%) | |
| Anti-Parkinson (N04) | 12 (0.2%) | |
| Psycholeptics (N05) | 3 (<0.1%) | |
| Psychoanaleptics (N06) | 28 (0.4%) | |
| Other Nervous System Drugs (N07) | 9 (0.1%) | |
| Respiratory System (R) N = 278 (4.1%) |
Nasal Preparations (R01) | 38 (0.6%) |
| Drugs for Obstructive Airway Diseases (R03) | 218 (3.2%) | |
| Antihistamines for Systemic Use (R06) | 22 (0.3%) | |
The majority of pDDIs were of “C” risk rating with a moderate interaction severity (Table 4). These potential interactions require an appropriate monitoring plan to detect negative effects and dose adjustments when necessary. Moreover, 8.4% of the potential interactions were of “D” risk rating indicating potential interactions of clinical significance. Furthermore, 53 out of the 3359 (1.6%) potential drug-drug interactions should have been avoided in the first place as the concurrent administration of these agents is contraindicated (i.e., risk rating X; Table 4).
Table 4.
Prevalence, risk rating, severity, and reliability rating for the detected pDDIs (n = 3359).
| Interaction Risk n(%) |
Interaction Severity n (%) |
Reliability (n%) |
|||
|---|---|---|---|---|---|
| Poor | Fair | Good | Excellent | ||
| B [n = 412 (12.3%)] | Minor = 318 (77.2%) | 1 (0.3%) | 233 (73.3%) | 19 (6%) | 65 (20.4%) |
| Moderate = 94 (22.8%) | 0 | 40 (42.6%) | 54 (57.4%) | 0 | |
| C [n = 2613 (77.8%)] | Major = 52 (2%) | 1 (1.9%) | 49 (94.2%) | 2 (3.9%) | 0 |
| Moderate = 2559 (97.9%) | 172 (6.7%) | 1677 (65.5%) | 626 (24.5%) | 84 (3.3%) | |
| Minor = 2 (0.1%) | 0 | 1 (50%) | 0 | 1 (50%) | |
| D [n = 281 (8.4%)] | Major = 175 (62.3%) | 0 | 115 (65.7%) | 44 (25.2%) | 16 (9.1%) |
| Moderate = 94 (33.5%) | 0 | 67 (71.3%) | 17 (18.1%) | 10 (10.6%) | |
| Minor = 12 (4.3%) | 0 | 12 (100%) | 0 | 0 | |
| X [n = 53 (1.6%)] | Major = 51 (96.2%) | 0 | 19 (37.2%) | 3 (5.9%) | 29 (56.9%) |
| Moderate = 2 (3.8%) | 0 | 1 (50%) | 0 | 1 (50%) | |
Interaction severity (n, %): minor (332, 9.9%); moderate (2749, 81.8%); major (278, 8.3%).
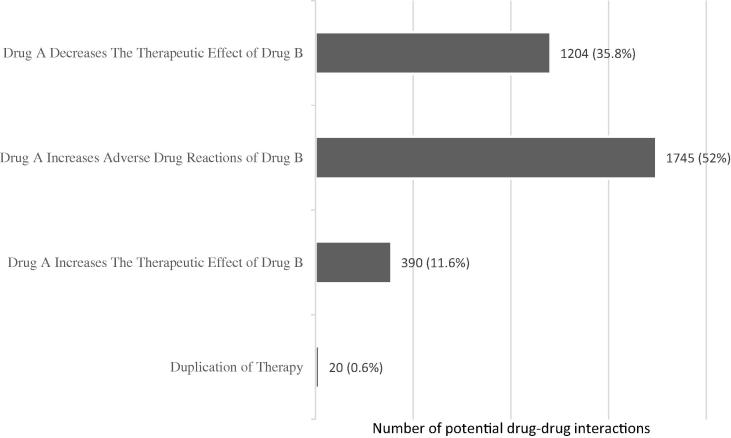
Over 50% of the detected potential interactions could result in one agent (i.e., drug) increasing the adverse drug reactions of the second agent (Fig. 1), 35.8% could result in one agent decreasing the therapeutic effect of the second agent, and 11.6% could result in one agent increasing the therapeutic effect of the second agent. In less than 1% of the interactions detected, the two medications were a duplication of therapy (e.g., two Angiotensin Converting Enzyme Inhibitors simultaneously consumed).
Fig. 1.
Predicted impact of pDDIs on the clinical outcome.
3.3. Factors associated with potential DDIs
The univariable analysis showed that the number of pDDIs was significantly associated with age (p value <0.001; Table 5) and with clinical conditions such as hypertension, diabetes and dyslipidemia (p value <0.001; Table 5). Meanwhile, the number of pDDIs was found to be negatively associated with education and with asthma (p value <0.001; Table 5). On the other hand, some demographic variables (e.g., gender, marital status, and BMI) and clinical conditions (e.g., hyperthyroidism, liver disease, and depression) were not significantly associated with the number of pDDIs.
Table 5.
Univariable and multivariable linear regression analysis for pDDIs.
| Variables | Univariable |
Multivariable |
||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient B | 95% CI | p value | Std β | Coefficient B | 95% CI | p value | Std β | |
| Age | 0.048 | 0.028-0.067 | < 0.001 | 0.171 | – | – | – | – |
| Education | −0.288 | −0.445 to −0.130 | < 0.001 | −0.126 | −0.212 | −0.359 to −0.065 | 0.005 | −0.093 |
| BMI | 0.023 | −0.012 to 0.058 | 0.197 | 0.046 | – | – | – | – |
| Hypertension | 1.914 | 1.299-2.529 | < 0.001 | 0.211 | 0.857 | 0.239–1.475 | 0.007 | 0.094 |
| Diabetes | 1.054 | 0.615-1.492 | < 0.001 | 0.165 | 0.867 | 0.439–1.295 | < 0.001 | 0.136 |
| IHD | 1.164 | 0.749–1.579 | < 0.001 | 0.191 | 0.610 | 0.185–1.036 | 0.005 | 0.101 |
| Dyslipidemia | 1.141 | 0.732–1.551 | < 0.001 | 0.190 | 0.432 | 0.009–0.855 | 0.045 | 0.072 |
| Heart Failure | 1.734 | 1.176–2.293 | < 0.001 | 0.211 | 1.346 | 0.794–1.898 | < 0.001 | 0.164 |
| CKD | 2.244 | 1.463–3.025 | < 0.001 | 0.196 | 1.863 | 1.108–2.617 | < 0.001 | 0.162 |
| Asthma | −0.934 | −1.502 to −0.359 | 0.001 | −0.112 | – | – | – | – |
| COPD | 1.475 | 0.112–2.838 | 0.034 | 0.075 | 2.076 | 0.792–3.361 | 0.002 | 0.104 |
| Gout | 1.679 | 0.828–2.531 | < 0.001 | 0.136 | 1.262 | 0.442–2.082 | 0.003 | 0.103 |
Std: standard; IHD: ischemic heart disease; BMI; body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease.
The multivariable linear regression indicated that patients with chronic obstructive pulmonary disease (p = 0.002), gout (p = 0.003), heart failure and chronic kidney disease (p <0.001) were highly associated with the number of pDDIs (Table 5). Meanwhile, a negative association remained evident between education and the number of pDDIs (Table 5). This means that patients with higher levels of education (i.e., post-secondary) are at a lower risk of developing multiple pDDIs.
4. Discussion
DDIs are a major public health concern which usually complicate the clinical management of patients especially elderly polypharmacy patients with multi-morbidity. This study was conducted to detect the prevalence and severity of pDDIs among adult polypharmacy outpatients at different hospitals in Jordan.
4.1. pDDIs prevalence and severity
In this study, 96% of the adult polypharmacy patients had at least one pDDIs (mean 4.2 ± 3.0 interactions per patient). This is in agreement with Al-Qerem et al., 2018, who reported a 95.6% prevalence of pDDIs in elderly polypharmacy patients in Jordan. The prevalence of pDDIs in these studies conducted in Jordan is high compared with results reported by outpatient studies from other countries (16-91%) (Aljadani and Aseeri, 2018, Ismail et al., 2018, Mistry et al., 2017, Olsen and Sletvold, 2018, Patel et al., 2014). The high prevalence in this study could be associated with polypharmacy. Studies have reported a significant association between the number of medications and pDDIs (Albadr et al., 2014, Johnell and Klarin, 2007, Olsen and Sletvold, 2018).
The majority of pDDIs reported in this study were of “C” risk rating (77.8%), while pDDIs with higher risk ratings (D and X) accounted for 10% of the total pDDIs. These findings are comparable to other studies which have reported risk rating of pDDIs (Andersson et al., 2018, Dirin et al., 2014, Doubova et al., 2007, Jazbar et al., 2018). Moreover, the majority of pDDIs were of moderate interaction severity (81.8%), while major severity interactions accounted for 8.3% of total pDDIs. Previous studies reported major potential interactions ranging from 5.3% to 32% (Aljadani and Aseeri, 2018, Al-Qerem et al., 2018, Chelkeba et al., 2013, Mistry et al., 2017, Venturini et al., 2011). This variation in results could be due to the use of different DDIs screening tools (Kannan et al., 2016, Kheshti et al., 2016) and the frequent updates on pDDIs databases.
It is fortunate that the majority of pDDIs identified in this study were of “C” risk rating with moderate severity. Therefore, they are not expected to have serious or fatal outcomes. However, it is important that these interactions are detected, documented, monitored and discussed with patients.
4.2. Factors associated with potential DDIs
In this study, the most common drugs associated with pDDIs were drugs used in diabetes (17.1%), diuretics (16.2%), antithrombotic agents (15.3%), and agents acting on the renin-angiotensin system (12.1%). In concordance, the multivariate linear regression showed a significant association between pDDIs and co-morbidities treated with these drugs (i.e., hypertension, diabetes, ischemic heart disease and heart failure). Most of our results, except for results on diuretics, are comparable with another study from Jordan (Al-Qerem et al., 2018). In a study by Al-Qerem et al. (2018), agents acting on the renin-angiotensin system had the highest frequency of cardiovascular system drugs contributing to pDDIs. Meanwhile, in other studies, nervous system drugs and drugs for acid related disorders were more common compared to our study (Chelkeba et al., 2013, Farooqui et al., 2018, Létinier et al., 2019, Olsen and Sletvold, 2018).
The association between co-morbidities and pDDIs has been described in several studies (Lin et al., 2011, Patel et al., 2014, Subramanian et al., 2018). The current study also found a negative association between education level and the number of pDDIs. Patients with high education levels were found to have less pDDIs. This finding has not been described as a predictor for pDDI in the literature. However, studies suggest that patients with low education have a higher probability of polypharmacy, and which indirectly suggests an association with an increased number of pDDIs (Haider et al., 2009).
In Jordan, the lack of patient centered practice and lack of effective communication between healthcare providers and patients may result in such Drug Related Problems (DRPs) (Yasein et al.,2017). Also, the inadequate coordination between health care providers in different health sectors (i.e., collaborative or coordinated care) and multiple prescribers could explain the high prevalence of pDDIs in Jordan (Al-Qerem et al., 2018). Moreover, the lack of medication review services in Jordan may contribute to preventable DDIs and DRPs. Given the aforementioned explanations, Jordanian patients are highly susceptible of a ‘prescribing cascade’ that starts with an ADR misinterpreted as a new medical condition and ends with the initiation of a new unnecessary medication that will ultimately result in more DRPs and endure a considerable cost to the healthcare system (Rochon and Gurwitz, 1997; Arabyat et al., 2019).
Therefore, more intensive and organized management of patients with polypharmacy is recommended. An effective medication review should be accomplished each time a new medication is prescribed to avoid significant DDIs. Additionally, drug alternatives should be considered where medication benefits can be achieved while avoiding DDIs. This can be accomplished by the efficient implementation of clinical pharmacy services in hospitals and outpatient clinics. Clinical pharmacists are the most qualified healthcare experts for the selection of proper drug combinations which minimize DDIs and ADRs (Al-Hajje et al., 2012). Finally, better communication between different health care providers in different health institutions is needed to improve polypharmacy patient care and management.
5. Conclusion
Despite the reported advances in health care systems, patients with polypharmacy are still at elevated risk for DDIs with significant clinical impact. Our study showed that 96% of polypharmacy patients at outpatient clinics have at least one potential DDI. Almost half of the detected interactions were involved with cardiovascular medications. The majority of these potential interactions had moderate severity, with no more than 10% of the interactions requiring therapy modification.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This project was supported by the Scientific Research Funds (project number: 104/2012) at the Ministry of Higher Education and Scientific Research Support Fund, Amman, Jordan.
Footnotes
Peer review under responsibility of King Saud University.
References
- Albadr Y., Bohassan A.K., Ming L.C., Khan T.M. An exploratory study investigating the potential drug–drug interactions in internal medicine department, Alahsa, Saudi Arabia. J. Pharm. Health Care Sci. 2014;5(4):237–241. [Google Scholar]
- Al-Hajje A., Atoui F., Awada S., Rachidi S., Zein S., Salameh P. Drug-related problems identified by clinical pharmacist's students and pharmacist's interventions. Ann. Pharm. Fr. 2012;70(3):169–176. doi: 10.1016/j.pharma.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Aljadani R., Aseeri M. Prevalence of drug–drug interactions in geriatric patients at an ambulatory care pharmacy in a tertiary care teaching hospital. BMC Res. Notes. 2018;11(1):234. doi: 10.1186/s13104-018-3342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qerem W., Jarrar Y.B., Al-Sheikh I., ElMaadani A. The prevalence of drug-drug interactions and polypharmacy among elderly patients in Jordan. Mortality. 2018;15:16. [Google Scholar]
- Andersson M.L., Böttiger Y., Kockum H., Eiermann B. High prevalence of Drug-Drug interactions in primary health care is caused by prescriptions from other healthcare units. Basic Clin. Pharmacol. Toxicol. 2018;122(5):512–516. doi: 10.1111/bcpt.12939. [DOI] [PubMed] [Google Scholar]
- Arabyat R., Nusair M., Al-Azzam S., Alzoubi K. Analysis of prevalence, risk factors, and potential costs of unnecessary drug therapy in patients with chronic diseases at the outpatient setting. Expert Review of Pharmacoeconomics & Outcomes Research. 2019 doi: 10.1080/14737167.2019.1612243. [DOI] [PubMed] [Google Scholar]
- Barrons R. Evaluation of personal digital assistant software for drug interactions. Am. J. Health Syst. Pharm. 2004;61(4):380–385. doi: 10.1093/ajhp/61.4.380. [DOI] [PubMed] [Google Scholar]
- Bucher H.C., Achermann R., Stohler N., Meier C.R. Surveillance of physicians causing potential drug-drug interactions in ambulatory care: a pilot study in Switzerland. PLoS ONE. 2016;11(1):e0147606. doi: 10.1371/journal.pone.0147606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatsisvili A., Sapounidis I., Pavlidou G., Zoumpouridou E., Karakousis V., Spanakis M., Niopas I. Potential drug–drug interactions in prescriptions dispensed in community pharmacies in Greece. Pharm. World Sci. 2010;32(2):187–193. doi: 10.1007/s11096-010-9365-1. [DOI] [PubMed] [Google Scholar]
- Chelkeba L., Alemseged F., Bedada W. Assessment of potential drug-drug interactions among outpatients receiving cardiovascular medications at jimma university specialized hospital, south west Ethiopia. Int. J. Basic Clin. Pharmacol. 2013;2(2):144. [Google Scholar]
- Corrie K., Hardman J.G. Mechanisms of drug interactions: Pharmacodynamics and pharmacokinetics. Anaesth. Intens. Care Med. 2011;12(4):156–159. [Google Scholar]
- Dechanont S., Maphanta S., Butthum B., Kongkaew C. Hospital admissions/visits associated with drug–drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014;23(5):489–497. doi: 10.1002/pds.3592. [DOI] [PubMed] [Google Scholar]
- Dirin M.M., Mousavi S., Afshari A.R., Tabrizian K., Ashrafi M.H. Potential drug-drug interactions in prescriptions dispensed in community and hospital pharmacies in east of Iran. J. Res. Pharm. Pract. 2014;3(3):104–107. doi: 10.4103/2279-042X.141118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubova S.V., Reyes-Morales H., del Pilar Torres-Arreola L., Suárez-Ortega M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico city. BMC Health Serv. Res. 2007;7(1):147. doi: 10.1186/1472-6963-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaaly K., Alshehri S., Bhattacharjee S., Al-Tawfiq J.A., Patanwala A.E. Contraindicated drug–drug interactions associated with oral antimicrobial agents prescribed in the ambulatory care setting in the United States. Clin. Microbiol. Infect. 2019;25(5):620–622. doi: 10.1016/j.cmi.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Farooqui R., Hoor T., Karim N., Muneer M. Potential drug-drug interactions among patients prescriptions collected from medicine out-patient setting. Pak. J. Med. Sci. 2018;34(1):144–148. doi: 10.12669/pjms.341.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S.I., Johnell K., Weitoft G.R., Thorslund M., Fastbom J. The influence of educational level on polypharmacy and inappropriate drug use: a register-based study of more than 600,000 older people. J. Am. Geriatr. Soc. 2009;57(1):62–69. doi: 10.1111/j.1532-5415.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- Hines L.E., Malone D.C., Murphy J.E. Recommendations for generating, evaluating, and implementing drug-drug interaction evidence. Pharmacotherapy. 2012;32(4):304–313. doi: 10.1002/j.1875-9114.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- Hines L.E., Murphy J.E. Potentially harmful drug–drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011;9(6):364–377. doi: 10.1016/j.amjopharm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Ismail M. Potential drug-drug interactions in outpatient department of a tertiary care hospital in Pakistan: A cross-sectional study. BMC Health Serv. Res. 2018;18(1):762. doi: 10.1186/s12913-018-3579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbar J., Locatelli I., Horvat N., Kos M. Clinically relevant potential drug–drug interactions among outpatients: A nationwide database study. Res. Social Adm. Pharm. 2018;14(6):572–580. doi: 10.1016/j.sapharm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Johnell K., Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly. Drug Saf. 2007;30(10):911–918. doi: 10.2165/00002018-200730100-00009. [DOI] [PubMed] [Google Scholar]
- Kannan B., Nagella A.B., Sathia Prabhu A., Sasidharan G.M., Ramesh A.S., Madhugiri V. Incidence of potential drug-drug interactions in a limited and stereotyped prescription setting - comparison of two free online pharmacopoeias. Cureus. 2016;8(11):e886. doi: 10.7759/cureus.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheshti R., Aalipour M., Namazi S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J. Res. Pharm. Pract. 2016;5(4):257–263. doi: 10.4103/2279-042X.192461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létinier L. Risk of drug-drug interactions in out-hospital drug dispensings in France: results from the DRUG-drug interaction prevalence study. Front. Pharmacol. 2019;10:265. doi: 10.3389/fphar.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Wang C., Bai C. Polypharmacy, aging and potential drug-drug interactions in outpatients in Taiwan. Drugs Aging. 2011;28(3):219–225. doi: 10.2165/11586870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Maher R.L., Hanlon J., Hajjar E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug. Saf. 2014;13(1):57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L., Spinewine A., Huang A. The challenge of managing drug interactions in elderly people. The Lancet. 2007;370(9582):185–191. doi: 10.1016/S0140-6736(07)61092-7. [DOI] [PubMed] [Google Scholar]
- Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirošević Skvrce N., Macolić Šarinić V., Mucalo I., Krnić D., Božina N., Tomić S. Adverse drug reactions caused by drug-drug interactions reported to croatian agency for medicinal products and medical devices: A retrospective observational study. Croat. Med. J. 2011;52(5):604–614. doi: 10.3325/cmj.2011.52.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry M., Gor A., Ganguly B. Potential drug-drug interactions among prescribed drugs in paediatric outpatients department of a tertiary care teaching hospital. J. Young Pharm. 2017;9(3):371. [Google Scholar]
- Mousavi S., Ghanbari G. Potential drug-drug interactions among hospitalized patients in a developing country. Caspian J. Intern. Med. 2017;8(4):282–288. doi: 10.22088/cjim.8.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R.M., Sletvold H. Potential drug-to-drug interactions: A cross-sectional study among older patients discharged from hospital to home care. Saf. Health. 2018;4(1):8. [Google Scholar]
- Patel P.S., Rana D.A., Suthar J.V., Malhotra S.D., Patel V.J. A study of potential adverse drug-drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J. Basic Clin. Pharm. 2014;5(2):44–48. doi: 10.4103/0976-0105.134983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblek T., Vaupotic T., Mrhar A., Lainscak M. Drug-drug interaction software in clinical practice: a systematic review. Eur. J. Clin. Pharmacol. 2015;71(2):131–142. doi: 10.1007/s00228-014-1786-7. [DOI] [PubMed] [Google Scholar]
- Rochon P.A., Gurwitz J.H. Optimising drug treatment for elderly people: the prescribing cascade. BMJ (Clin. Res. Ed.) 1997;315(7115):1096–1099. doi: 10.1136/bmj.315.7115.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithburger P.L., Kane-Gill S.L., Seybert A.L. Drug-drug interactions in cardiac and cardiothoracic intensive care units. Drug Saf. 2010;33(10):879–888. doi: 10.2165/11532340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Subramanian A., Adhimoolam M., Kannan S. Study of drug-drug interactions among the hypertensive patients in a tertiary care teaching hospital. Perspect. Clin. Res. 2018;9(1):9–14. doi: 10.4103/picr.PICR_145_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini C.D. Gender differences, polypharmacy, and potential pharmacological interactions in the elderly. Clinics. 2011;66(11):1867–1872. doi: 10.1590/S1807-59322011001100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2019. The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses. (No. 22nd). Available from https://www.whocc.no/filearchive/publications/2019_guidelines_web.pdf.
- Yasein N.A., Shakhatreh F.M., Shroukh W.A., Farah M.S., Jaber R.M. A comparison between patients’ and residents’ perceptions of patient centeredness and communication skills among physicians working at Jordan university hospital. Open J. Nurs. 2017;7(06):698. [Google Scholar]