Abstract
A number of psychiatric disorders, including anxiety, schizophrenia, Parkinson’s disease, depression and others CNS diseases are known to induce defects in the function of neural pathways sustained by the neurotransmitters, like dopamine and serotonin. N-arylpiperazine moiety is important for CNS-activity, particularly for serotonergic and dopaminergic activity. In the scientific literature there are many examples of coumarin-piperazine derivatives, particularly with arylpiperazines linked to a coumarin system via an alkyl liner, which can modulate serotonin, dopamine and adrenergic receptors. Numerous studies have revealed that the inclusion of a piperazine moiety could occasionally provide unexpected improvements in the bioactivity of various biologically active compounds. The piperazine analogs have been shown to have a potent antimicrobial activity and they can also act as BACE-1 inhibitors. On the other hand, arylpiperazines linked to coumarin derivatives have been shown to have antiproliferative activity against leukemia, lung, colon, breast, and prostate tumors. Recently, it has been reported that coumarin-piperazine derivatives exhibit a Fneuroprotective effect by their antioxidant and anti-inflammatory activities and they also show activity as acetylcholinesterase inhibitors and antifilarial activity. In this work we provide a summary of the latest advances in coumarin-related chemistry relevant for biological activity.
Keywords: Coumarin, Arylpiperazinyl moiety, Biological activity
1. Introduction
From the historical perspective the scientific interest in coumarin derivatives began in early 1900 but the discovery of their various biological effects, including antimicrobial, antioxidant, anti-inflammatory, anti-arrhythmic, spasmolytic and antiviral activity has resulted in a dramatic increase in publications on this subject in the 1950s (Ellinger, 1908, Ito et al., 1950, Ito et al., 1951a, Ito et al., 1951b, Simonis and Wenzel, 1900). This fact is well illustrated by statistical data, as currently more than eleven thousand publications related to coumarins can be found in the PubMed database. In the 1950s, there were exactly 937 publications on the topic of coumarins, then this number dropped to 280 in the 1960s and has been steadily growing since then, effectively doubling the number of studies on coumarins every decade. In in previous decade there were 2914 publications on coumarins, and in the last decade the interest in coumarin derivatives resulted in over 5000 publications in PubMed.
Coumarin derivatives from natural and synthetic sources are useful in a number of fields and are commonly used in medicine, often in the form of glycosides. Plants from the families of Rutaceae, Rubiceae, Asteraceae, Apiaceae, Oleaceae, Fabaceae, Solanaceae and Hippocastanaceae, as well as bacteria of Aspergillus and Streptomyces strains are the most inspiring and the richest source of leading structures containing the coumarin system. Numerous efforts, including the isolation and purification of naturally occurring coumarins from a variety of plants as well as laboratory synthesis of coumarin derivatives with novel structures and properties, have been focusing on the research and development of coumarins as potential drugs. So far a number of coumarins such as warfarin, acenocoumarol, armillarisin A, carbochromen and hymecromone have been approved for therapeutic purposes in clinic (Abate et al., 2001, Božič-Mijovski, 2019, Dávila-Fajardo et al., 2019, Opherk et al., 1990). The most important group of commonly used drugs based on coumarin derivatives are vitamin K antagonists such as warfarin, phenprocoumon or acenocoumarol, used as anticoagulants (Gierlak and Kuch, 2010, Salvo et al., 2017). Active compounds in these drugs are derivatives of coumarin and are used mainly in the treatment of venous and arterial thrombosis, as well as in ischemic strokes. These compounds do not directly affect blood coagulation but modify the metabolism of vitamin K, which is necessary in the regulation of the biosynthesis of coagulation factors. Psoralen, a compound structurally related to coumarin is used in PUVA (psoralen and ultraviolet A therapy) in various skin-related conditions, such as psoriasis and (to a lesser extent) eczema and albinism (Archier et al., 2012, Ibbotson, 2018).
Further studies have shown that coumarins' pharmacological and biochemical properties depend on the pattern of substitutions, including the therapeutic applications, and can beneficially affect toxicity (Hoult and Paya, 1996, Kulkarni et al., 2006). It is interesting to note that the presence of a hydroxyl group and amine moieties is crucial for many biologically-active coumarins. A good example of this action, may be the fact that the introduction of additional O-alkyl or O-aminoalkyl groups to the coumarin ring, makes them effective against certain Gram-negative, Gram-positive bacterial strains and increases their antifungal potential (Kayser and Kolodziej, 1999, Montagner et al., 2008, Trykowska Konc et al., 2011).
Piperazine (hexahydropyrazine diethylenediamine) is an organic, heterocyclic compound with a six-membered ring containing two nitrogen heteroatoms at the C-1 and C-4 positions. The scienctific literature abounds in examples of combinations of piperazine with various heterocyclic moieties. The piperazine skeleton is found in many biologically active compounds and piperazine derivatives belong to a very wide class of chemical compounds with important pharmacological properties. Piperazine derivatives were initially used as anthelmintics (Martin, 1997). Currently, many market drugs contain a piperazinyl ring as an integral part of the structure and these include, but are not limited to, compounds with action as antianginals (ranolazine), antidepressants (buspirone), antihistamines (hydoxyzine), antiserotonergics (EGIS-7625), antipsychotics (flupentixol) or urological (sildenafil). Combining coumarin with an amino fragment in the form of piperazine, also results in a drastic improvement in the biological properties of such compounds relative to unsubstituted coumarins. Effects of coumarin - piperazine compounds on the central nervous system are documented, similarly to their antibacterial, antitumor, antioxidant or antiviral activity. These mentioned properties will be discussed in detail in the following subsections.
2. Biological effects of coumarins containing the piperazinyl moiety
2.1. Coumarin-piperazine derivatives as serotonin and dopamine receptor agents
Both natural and synthetic coumarin derivatives can be used in the therapy of neurodegenerative mental disorders such as Alzheimer's and Parkinson's disease, schizophrenia, epilepsy or depression. The medical interest in coumarins is related to their effects on the central nervous system, in particular on the serotoninergic and dopaminergic systems. Due to the fact, that serotonin (5-HT) and dopamine (DA) receptors are involved in the mechanisms of many neurological and psychiatric disorders, research on chemicals that affect these systems is a huge branch of drug chemistry (Jaber et al., 1996, Shih-Hsien et al., 2014).
Natural coumarins such as scopoletin isolated from the entire plant Polygala sabulosa and psoralen isolated from Psoralea corylifolia seeds have antidepressant activity that is a result of the activation of serononergic neurotransmission and dopaminergic receptors (Capra et al., 2010, Xu et al., 2008). Also, scoparone from Artemisia scoparia, as well as licopyranocoumarin and glycyrurol, isolated from a Glycyrrhiza sp., showed neuroprotection by reducing L-DOPA induced cytotoxicity in PC12 cells or inhibiting MPP+-induced neuronal PC12D cell death (Fujimaki et al., 2014, Yang et al., 2009).
First literature reports on the action of N-phenylpiperazinyl derivatives of coumarin appeared in 1998. The authors described four compounds containing N-arylpiperazinyl moiety witch is very important for CNS-activity, especially for serotonergic and dopaminergic activity, Fig. 1 (Teran et al., 1998).
Fig. 1.
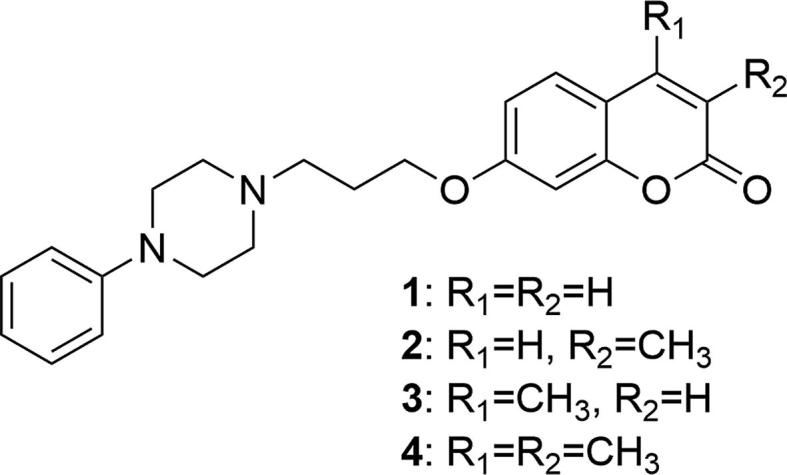
Coumarin derivatives synthesized by the Santana’s group in 1998 (Teran et al., 1998).
The affinities of propoxycoumarins connected to the N-arylpiperazinyl fragment (1–4) to 5-HT1A and D2A, D3 receptors were evaluated using [3H]-OH-DPAT and [3H]-raclopride, respectively, as reference compounds and were in the nanomolar range. The authors showed that 7-[3-(4-phenyl-1-piperazinyl)propoxy]coumarins possess high affinity to the above-mentioned receptors. They additionally showed that modifications of the coumarin ring itself also affect the affinity for serotonin and dopamine receptors. Introduction of the methyl group in the C-3 position of 7-hydroxycoumarin (3) gave 6–7 times better results for the 5-HT1A receptor (Ki = 0.79 ± 0.08) and 2–4 times better results for the D3 receptor (Ki = 18.9 ± 6.0) than the unsubstituted (1) (Ki5-HT1A = 5.61 ± 0.07; KiD3 = 49.7 ± 14.0) or compounds substituted in the C-4 (2) (Ki5-HT1A = 5.54 ± 0.02; KiD3 = 73.8 ± 9.9) and C-3, 4 positions (4) (Ki5-HT1A = 5.31 ± 0.35; KiD3 = 44.7 ± 15.0). The compound with the methyl group at the 4-position (2) had a lower affinity to the D3 receptor but showed an interesting mixed profile to the 5-HT1A/D2A receptors (Ki5-HT1A = 5.54 ± 0.02; KiD2A = 3.7 ± 2.6). The activity of compound 3 for the 5-HT1A receptor was stronger than both reference compounds and it also showed moderate selectivity for this receptor over the D2A and D3 receptors (Ki5-HT1A = 0.79 ± 0.08; KiD2A = 10.8 ± 2.2; KiD3 = 18.9 ± 6.0). Next, the synthesis and receptor binding assay of analogues of 4-methyl-7-[3-(4-phenyl-1-piperazinyl)propoxy]coumarin (2) were also described (Santana et al., 2002).
Additional modifications, including the introduction of -NO2 and -OCH3 substituents at the para position of the phenyl ring at the piperazine, replacement of the methyl group at the C-4 position of the coumarin ring with -OSO2Ph, -C6H11, -OMe and -OSO2Me groups or shifting the 3-(4-phenyl-1-piperazinyl) propoxy substituent from positions C-7 to C-6 of the coumarin ring, were also introduced and analyzed, Fig. 2. All structural modifications (derivatives 5–16) displayed lower affinity to 5-HT1A and D2A receptors than the reference compound 2.
Fig. 2.
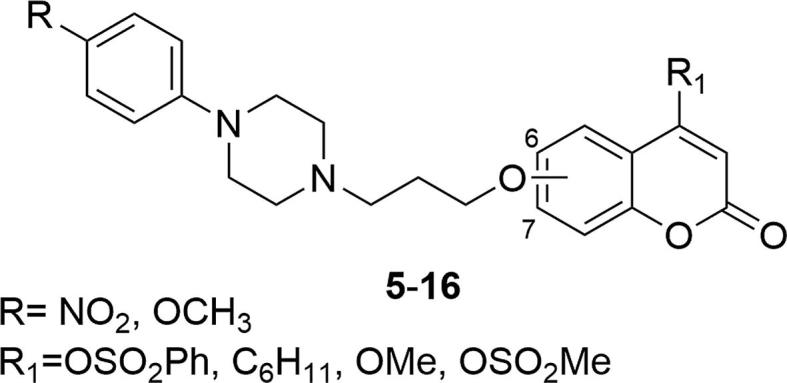
Coumarin derivatives synthesized by the Santana’s group in 2002 (Santana et al., 2002).
Based on previous results, consecutive analogues of 4-methyl-7-[3-(4-phenyl-1-piperazinyl)propoxy]coumarin (2) were designed and their effect on α1A, D2 and 5-HT2A receptors was studied with the haloperidol, as the reference substance, Fig. 3 (Gonzalez-Gomez et al., 2003). The influence of the substituent on the phenyl ring at piperazine on the affinity for the receptors was demonstrated.
Fig. 3.
Coumarin derivatives synthesized by the Santana’s group in 2003 (Gonzalez-Gomez et al., 2003).
All compounds showed high affinity to α1A the receptor. The highest affinity to α1A and D2 receptor was due to 4-methyl-7-[3-(4-(2-methoxyphenyl)phenyl-1-piperazinyl)propoxy] coumarin (18) (pA2 = 9.07 ± 0.10, pKiD2 = 7.93 ± 0.07) having a methyl group at the C-4 position of the coumarin ring and an o-methoxy phenyl ring at the piperazine moiety. The conversion of the 4-methyl group to 4-methoxy in the coumarin ring reduced the activity relative to all three receptors. Similarly, after the removal of the methoxy group from the phenylene ring at the piperazine or its conversion to a heterocyclic ring, the affinity was lower. Based on the results the authors concluded that the activity towards the above-mentioned receptors depends primarily on the type of substituent in the N-4 position of the piperazine ring and is as follows: o-methoxyphenyl group > phenyl group > pyrimidine group > pyridine group. In turn, the substitution of the coumarin ring at C-4 position only affects the affinity for the serotonin receptor.
The authors of the next work decided to validate the multireceptor affinity of described above 4-methyl-7-[3-(4-phenyl-1-piperazinyl)propoxy]coumarin (2) and 4-methyl-7-[3-(4-(2- methoxyphenyl)phenyl-1-piperazinyl)propoxy]coumarin (18) profile approach to antipsychotics, characterized by high affinity to D2, D3, 5-HT1A, and 5-HT2A receptors (Gonzalez-Gomez et al., 2003). Structural modifications included replacing the methyl group at the 4-position of the coumarin ring with other alkyl groups. It was evaluated that, in terms of affinity to the D2, 5-HT1A and 5-HT2A receptors, the presence of a methyl group at this position is the most preferred, followed by other groups in the following order: n-propyl > ethyl > isopropyl > cyclopropyl. In addition, the methyl group or chlorine was also introduced at the C-5 or C-6 position of the coumarin ring, and the results showed that such change does not affect the affinity, whereas the introduction of these substituents into position C-8 may increase the affinity to D2, 5-HT1A and 5-HT2A receptors. On the other hand, the introduction of fluoro- or methoxy- substituents into the phenyl ring on the piperazine, or replacing the phenyl ring with the heterocyclic system resulted in a significant reduction in the activity of the tested derivatives. The effect of the length of the alkyl linker between the coumarin and piperazinyl moieties using chains of three to five carbon atoms was also investigated. It was shown that the compounds with the highest affinity had four-carbon linkers (Chen et al., 2013). From among several dozen of new derivatives, 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)-4-methylcoumarin (25) and 7-(4-(4-(6-fluorobenzo[d]-isoxazol-3-yl)-piperidin-1-yl)butoxy)-4-methyl-8-chlorocoumarin (26) were selected, as the most promising systems that could form the basis of a new class of schizophrenic drugs, Fig. 4. These compounds showed high affinity to dopamine D2 and D3 (KiD2 = 1.4 ± 0.16, KiD3 = 4.8 ± 0.6 for 25; KiD2 = 2.6 ± 0.3, KiD3 = 4.3 ± 0.5 for 26) and serotonin 5-HT1A and 5-HT2A receptors (Ki5-HT1A = 0.012 ± 0.001, Ki5-HT2A = 8.0 ± 0.9 for 25; Ki5-HT1A = 3.3 ± 0.4, Ki5-HT2A = 0.3 ± 0.03 for 26), compared with clozapine and risperidone (KiD2 = 128.7 ± 1.9, KiD3 = 239.8 ± 29.6, Ki5-HT1A = 141.6 ± 1.6, Ki5-HT2A = 11.6 ± 1.3 for clozapine and KiD2 = 3.7 ± 0.3, KiD3 = 9.7 ± 0.9, Ki5-HT1A = 180 ± 15, Ki5-HT2A = 0.18 ± 0.02 for risperidone). In addition, they showed low affinity for the 5-HT2C and H1 receptors (Ki5-HT2C = 995.5 ± 100.3, KiH1 > 10000 for 25; Ki5-HT2C = 1700.7 ± 180.5, KiH1 = 1125.3 ± 126.3 for 26) as well as hERG channels (IC50 = 31 ± 3.6 for 25 and 1591 ± 193.8 for 26) which translates into a reduction in the risk of obesity, which is associated with chronic treatment. Compound 26 also inhibited MK-80 induced hyperactivity, conditioned avoidance response without noticeable catalepsy and apomorphine induction during climb at the highest dose tested, in animal models. Compound 26 showed low acute toxicity and a good safety profile, even at the highest dose tested (LD50 > 2000 mg/kg).
Fig. 4.
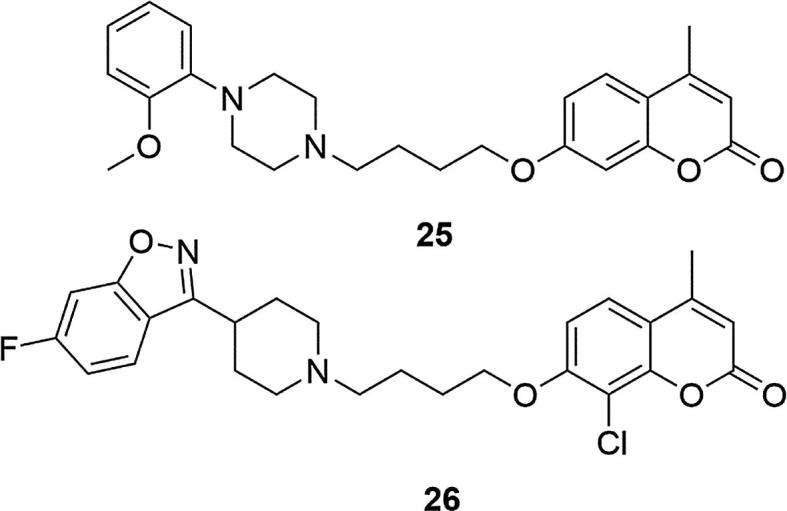
Structures of 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)-4-methylcoumarin (25) and 7-(4-(4-(6-fluoro benzo[d]-isoxazol-3-yl)-piperidin-1-yl)butoxy)-4-methyl-8-chlorocoumarin (26).
Subsequent modifications of 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)-4-methylcoumarin (25) confirmed that for 7-hydroxycoumarin the highest D2, 5-HT1A and 5-HT2A receptors affinity was obtained using 2-methoxphenylpyierazine, a 4-carbon alkyl chain and a methyl group at the C-4 position of the coumarin ring. Changing the substituent position in phenylpiperazine, changing the substituent itself, replacing the phenyl ring with a heterocyclic moiety, introducing a methyl group or chlorine in the C-8 or C-6 position of the coumarin ring or changing the length of the alkyl chain in the molecule, resulted in a decrease in activity. It was confirmed that combination of substituents at the C-3 and C-4 positions in ring derivatives is preferred when the new ring is a 5-membered ring. The introduction of an additional 6-membered ring reduced D2, 5-HT1A and 5-HT2A receptor activity. As a result of the above research, 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)-2,3-dihydrocyclo-penta[c]coumarin (27) was successfully selected as compound with high affinity to D2, 5-HT1A and 5-HT2A receptors (KiD2 = 5.6 ± 0.6, Ki5-HT1A = 0.2 ± 0.01, Ki5-HT2A = 3.9 ± 0.4) compared with risperidone and clozapine and low affinity to H1 receptors (KiH1 = 699.1 ± 78.5 for 27, KiH1 = 6.8 ± 0.8,] for clozapine, KiH1 = 22.9 ± 3.2 for risperidone), Fig. 5, (Chen et al., 2014). As with compound 26, the acute toxicity of derivative 27 was determined in terms of LD50 values. Analogous results were obtained, the tested compound showed good safety profile, even at the highest dose tested (LD50 > 2000 mg/kg). It has been recognized that derivatives of this type may reduce catalepsy in patients with schizophrenia at the same time reducing the risk of weight gain associated with such treatment.
Fig. 5.
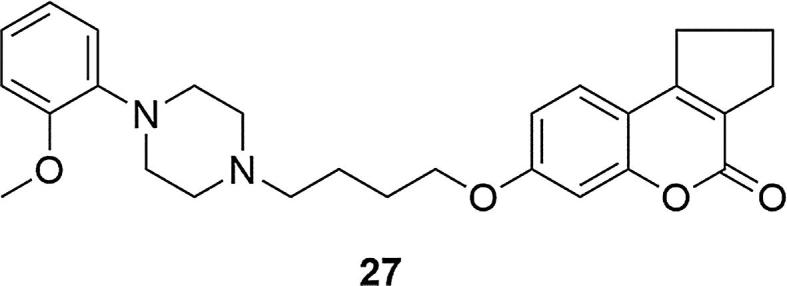
Structure of 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)-2,3-dihydrocyclo-penta[c]coumarin (27).
Another coumarin analogue, enasculin (7-methoxy-6-((3-[4-(2-methoxyphenyl)-1-piperazinyl]propoxy)-3,4-dimethylcoumarin (28)) also showed high affinity for serotoninergic 5-HT1A and 5-HT7, adrenergic α1 and dopaminergic D2 and D3 receptors (IC50(5-HT1A) = 8, IC50(5-HT7) = 2.4, IC50(α1A) = 4.7, IC50(D2) = 16.6, IC50(D3) = 27.7 [nM]), Fig. 6. In different animal models, enasculin demonstrated improved learning and memory, neuroprotective effects in the NMDA (N-methyl-D-aspartate) toxicity model and neurotrophic effects in primary cultured rat brain cells. By means of toxicological tests, ensaculin has been found to have low toxicity to target organs and it was well tolerated at daily doses up to 20 mg in clinical trials. It is recognized that thanks to this unique pharmacological profile, enasculin may have potential as a treatment for dementia, acting on various transmitter systems (Hoerr and Noeldner, 2002, Skalicka-Wozniak et al., 2016).
Fig. 6.
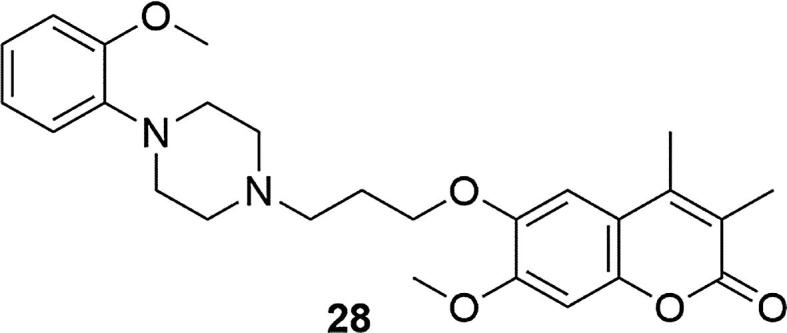
Structure of 7-methoxy-6-((3-[4-(2-methoxyphenyl)-1 piperazinyl]propoxy)-3,4-dimethylcoumarin (ensaculin) (28).
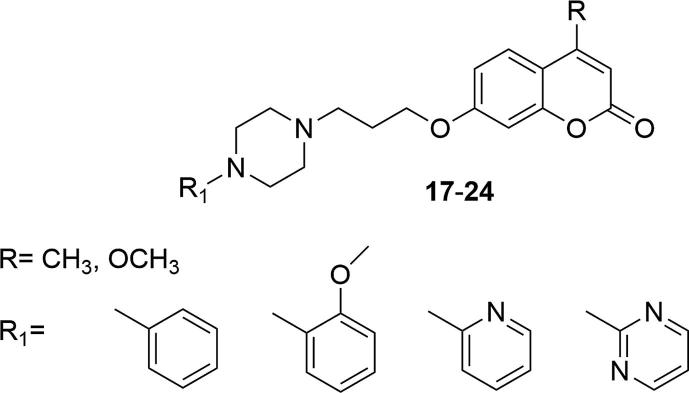
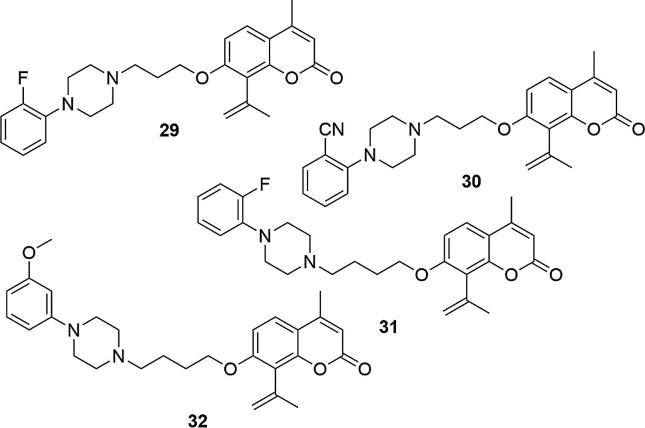
Chen's and Santana's strategy was continued by the Ostrowska group. The design of new series of biologically-active compounds was still focused on 7-hydroxy-4-methylcoumarin as the leading structure, but additionally an acetyl group was introduced in the C-8 position of the coumarin ring. The acetyl moiety with the ability to form hydrogen bonds, was more potent to bind additional residues in the 5-HT1A receptor pocket, compared to compounds with methyl or chlorine group in C-8 position. As previously, the action on 5-HT1A and 5-HT2A serotonin receptors has also been tested for compounds with structural differences in the arylpiperazine moiety and different link lengths between the piperazinyl ring and coumarin (Ostrowska et al., 2017a, Ostrowska et al., 2017b, Ostrowska et al., 2018, Żołek et al., 2019). Biological investigations were carried out on 5-HT1A/2A receptors, which are known as important factors in the pathogenesis and treatment of depression. It has been found that substituents in the ortho or meta position in the phenyl ring of the piperazine moiety have the decisive effect on affinity for 5-HT1A receptors, regardless of their chemical nature. On the other hand only minimal differences in affinity were obtained when using either a three or four carbon linker. Four derivatives showed affinity for the 5-HT1A receptor at the serotonin level used as the reference compound (Ki5-HT1A = 1.3 ± 0.1, 1.0 ± 0.1, 1.0 ± 0.1, 0.8 ± 0.009, for 29–32, respectively; Ki5-HT1A = 1.3 ± 0.1 for serotonin), Fig. 7. In functional tests an antagonistic profile was obtained, which is considered as good starting point for optimizing and designing new antipsychotic drugs (Ostrowska et al., 2017a, Ostrowska et al., 2017b).
Fig. 7.
Coumarin derivatives synthesized by the Ostrowska’s group (Ostrowska et al., 2017a, Ostrowska et al., 2017b).
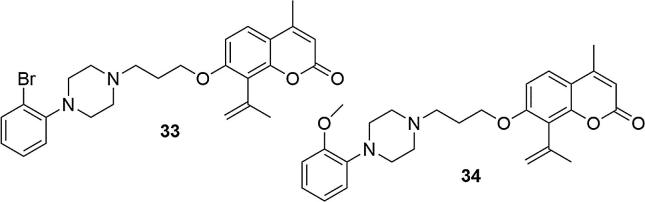
Similar results were obtained for 8-acetyl-7-(3-[4-(2-bromophenyl)piperazin-1-yl]propoxy)-4-methylcoumarin (33) and 8-acetyl-7-(3-[4-(2-methoxyphenyl)piperazin-1-yl]propoxy)-4-methylcoumarin (34), that were also tested for affinity to 5-HT1A and 5-HT2A receptors, Fig. 8, (Ostrowska et al., 2018). The compounds showed very high, nanomolar affinity at the serotonin level to 5-HT1A receptor (Ki5-HT1A = 0.5 ± 0.05, 0.6 ± 0.05 for 33–34, respectively; Ki5-HT1A = 1.4 ± 0.1 for serotonin), and moderate affinity to the 5-HT2A receptor (Ki5-HT2A = 7.0 ± 0.5, 8.0 ± 0.7 for 33–34, respectively; Ki5-HT2A = 2.0 ± 0.1 for mianserin). The [35S] GTPϒS binding assay showed that these compounds acted as potent antagonists of the 5-HT1A receptors but the results of in vivo studies did not show any antidepressant activity of 33 and 34 compounds in mice.
Fig. 8.
Structures of 8-acetyl-7-{3-[4-(2-bromophenyl)piperazin-1-yl]propoxy}-4-methylcoumarin (33) and 8-acetyl-7-{3-[4-(2-methoxyphenyl) piperazin-1-yl] propoxy}-4-methylcoumarin (34).
Additional theoretical calculations and experiments have shown that the theoretical values of the drug similarity parameters of the tested compounds, such as lipophilicity, topological polar surface area and BBB (the blood–brain barrier) permeation characteristics are in the range of standard CNS drug candidates. All tested coumarins can be metabolized by cytochrome P450 to aldehydes and hydroxyl derivatives and also showed moderate to strong binding to human serum albumin (Żołek et al, 2019).
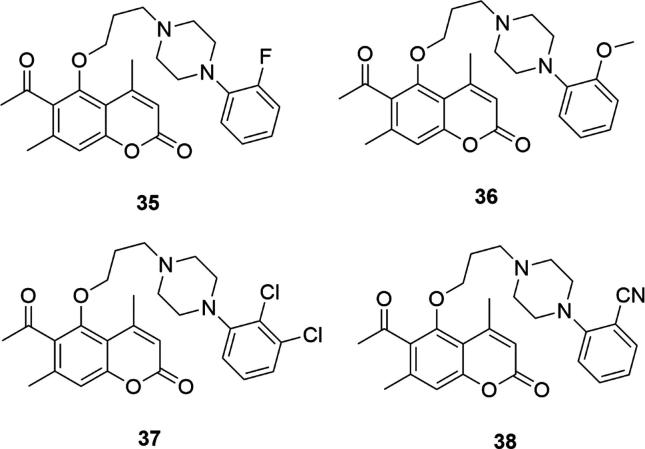
All of the above considerations and structures 1–34 relate to arylpiperazinyl derivatives of 7 or 6-hydroxycoumarin. In the scientific literature, however, there are also examples of arylpiperazines of 5-hydroxycoumarin acting on the central nervous system. A series of derivatives of 5-hydroxy-4,7-dimethylcoumarin and 6-acetyl-5-hydroxy-4,7-dimethylcoumarin attached to N-arylpiperazine moiety via a propoxy or butoxy chain was tested for their affinity to 5-HT1A and 5-HT2A receptors as potential antipsychotic agents (Ostrowska et al., 2017a, Ostrowska et al., 2017b, Żołek et al., 2018). As in the previous series, it turned out that the highest affinity to 5-HT1A receptors can be attributed to compounds containing an acetyl group in the immediate vicinity of the piperazinyl group (position C-6, relative to the C-5 position for propoxy/butoxy phenylpiperazine). The highest, subnanomolar affinity for this receptor was found in compounds containing substituents at the 1, 2 or simultaneously positions 1 and 2 of the phenyl ring at the piperazine - 6-acetyl-4,7-dimethyl-5-(3-[4-(2-fluorophenyl)piperazin-1-yl propoxy)coumarin (35) (Ki5-HT1A = 0.3 ± 0.05), 6-acetyl-4,7-dimethyl-5-(3-[4-(3-methoxyphenyl)-piperazin-1-yl]propoxy)coumarin (36) (Ki5-HT1A = 0.4 ± 0.02), 6-acetyl-4,7-dimethyl-5-(3-[4-(2,3-dichlorophenyl)piperazin-1yl]propoxy) coumarin (37) (Ki5-HT1A = 0.4 ± 0.03), and 6-acetyl-4,7-dimethyl-5-(3-[4-(2cyanophenyl) piperazin-1-yl]-propoxy)coumarin (38) (Ki5-HT1A = 0.3 ± 0.04), Fig. 9, (Ostrowska et al., 2017a, Ostrowska et al., 2017b). Replacing the piperazine ring with morpholine or introducing an additional heterocyclic ring reduced their activities. In addition, the most active compounds, acting at the serotonin level, had a three-carbon linker between the piperazinyl and coumarin moiety.
Fig. 9.
Coumarin derivatives synthesized by the Ostrowska’s group (Ostrowska et al., 2017a, Ostrowska et al., 2017b).
Computational methods allowed to determine the TPSA (topological polar surface area) values of all tested compounds, which were in the 55.15–86.78 Å range, which means that they are capable of penetrating the blood–brain barrier, important for ligands targeting brain receptors. Fluorescence quenching spectroscopy studies found a moderate degree of binding of all investigated coumarin derivatives to HSA, which is a major transport vehicle for a variety of biologically-active compounds (Żołek et al, 2018).
In 2006, Garino's group showed that substituted phenyl-piperazine coupled to coumarin core demonstrate improved β-secretase inhibitors activity compared to naphthyl counterparts (Garino et al., 2006). The β-secretase plays an important role in the synthesis of pathogenic amyloid-β in Alzheimer's disease because its two enzymes (BACE-1 and BACE-2) are considered as ideal targets for therapeutic intervention. Three of the synthesized compounds showed a good activity against BACE-1 in fluorescent resonance energy transfer assays. The most potent inhibitor 39 was much more active than its corresponding naphthyl analogue (IC50 = 0.093 for 39; IC50 = 1.7 [µM] for naphthyl compound). It was a derivative of coumarin with a methoxy substituent in the C-7 position, a methylene linker in the C-4 position, connecting coumarin with amide moiety and Boc substituent on the nitrogen atom of the piperazine ring, Fig. 10. Change of connection location of the coumarine moiety with amide group from position C-4 to C-3 decreased activity. The same effect was observed when the Boc moiety was substituted with -H, benzyl or -SO2-2-thiophene groups.
Fig. 10.
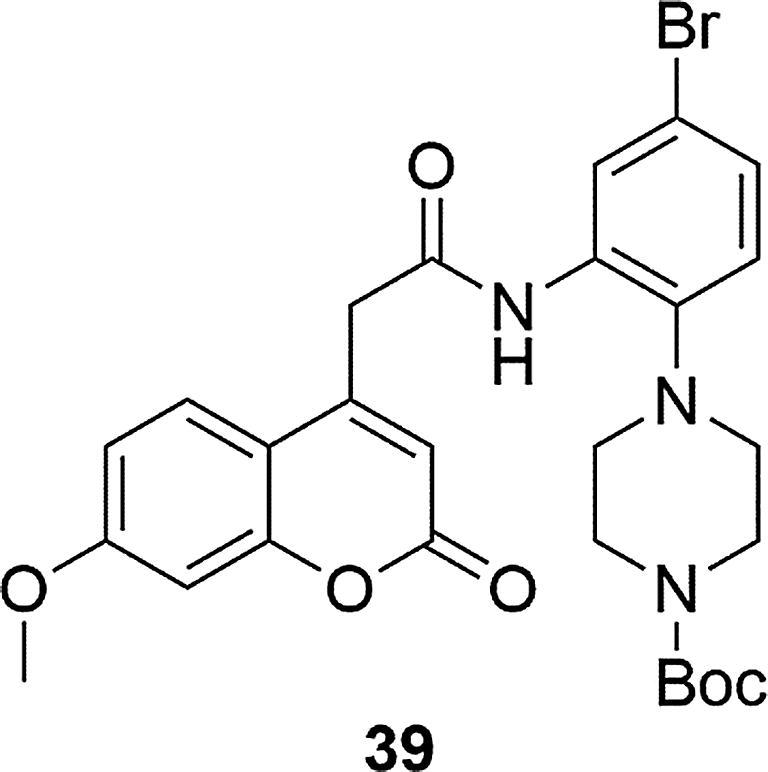
Coumarin derivative synthesized by the Garino’s group (Garino et al., 2006).
2.2. Coumarin-piperazine derivatives as acetylcholinesterase inhibitors agents
Acetylcholinesterase (AChE) plays a key role in accelerating aggregation of β-amyloid peptides and deposition in fibers in chronic and progressive neurodegradative problems such as Alzheimer's disease. AChE inhibition, enhancement of cholinergic neurotransmission and inhibition of β-amyloid peptide formation is considered an effective strategy for the treatment of Alzheimer's disease (Alvarez et al., 1995, Carreiras and Marco, 2004, Mori, 2000, Selkoe and Podlisny, 2002).
Natural scopoletin and its glycoside, scopolin, extracted from twigs of Vaccinium oldhami Miquel showed activity as an AChE inhibitor, similar to 4-methylumbelliferone (Lee et al., 2004, Orhan et al., 2008). The activity of scopoletin in vivo experiments was at the same level as that produced by the clinical agent galathamin and it demonstrated the ability to increase extracellular AChE concentration in rat brain (Loizzo et al., 2008, Rollinger et al., 2004).
Several studies have demonstrated that coumarin-derived AChE inhibitors interact mainly with the peripheral AchE anionic site and the amino functional moiety (including benzylamine phenylpiperazine or anilino group). This fragment linked to the coumarin ring with an appropriate linker can interacts with the AChE catalytic site (Anand et al., 2012). Ensaculine, a piperazine-coumarin derivative, has demonstrated the ability to slow down and prevent the neurodegradation processes associated with Alzheimer's disease (Hoerr and Noeldner, 2002, Weinstock, 1999). Studies have shown neuroprotective effects of ensaculine in the NMDA toxicity model, neurotrophic effects in primary cultured rat brain cells and memory-enhancing effects in paradigms of passive and conditioned avoidance in rodents (Hoerr and Noeldner, 2002).
Ensaculine was characterized as a weak NMDA receptor-mediated channel blocker and had high affinity for both serotonergic 5-HT1A and 5-HT7, dopaminergic D2 and D3 receptors as well as adrenergic α1 and receptors. This compound contains a 6-hydroxycoumarin, a methoxy group in the C-7 position and two methyl groups at the C-4 and C-3 positions of the ring and coumarin system with 2-methoxyphenylpiperazine combines a three-carbon linker, Fig. 6. It is believed that due to its unique pharmacodynamic profile, ensaculine may have potential as an anti-dementia agent that acts on various transmitter systems. Based on the structure of ensaculine, many coumarin-piperazine compounds have been designed over the years and their potential use as acetylcholinesterase inhibitors has been studied. In 2013 a series of 4-hydroxycoumarin derivatives was obtained in excess of piperazine, followed by substitution with aromatic acetamides (Modh et al., 2013). The inhibitory potency of human AChE enzyme of synthesized compounds were tested, however, the compounds showed moderate activity, slightly lower than the level of action of ensaculine, which was the reference compound (IC50 = 4.9 ± 0.03 µM). The most active derivative had a methoxy group in the meta position of the phenyl ring at the piperazine, Fig. 11. A change in the position of this moiety in the ring or conversion to a methyl, nitro or chlorine group resulted in each case in the reduction of activity.
Fig. 11.
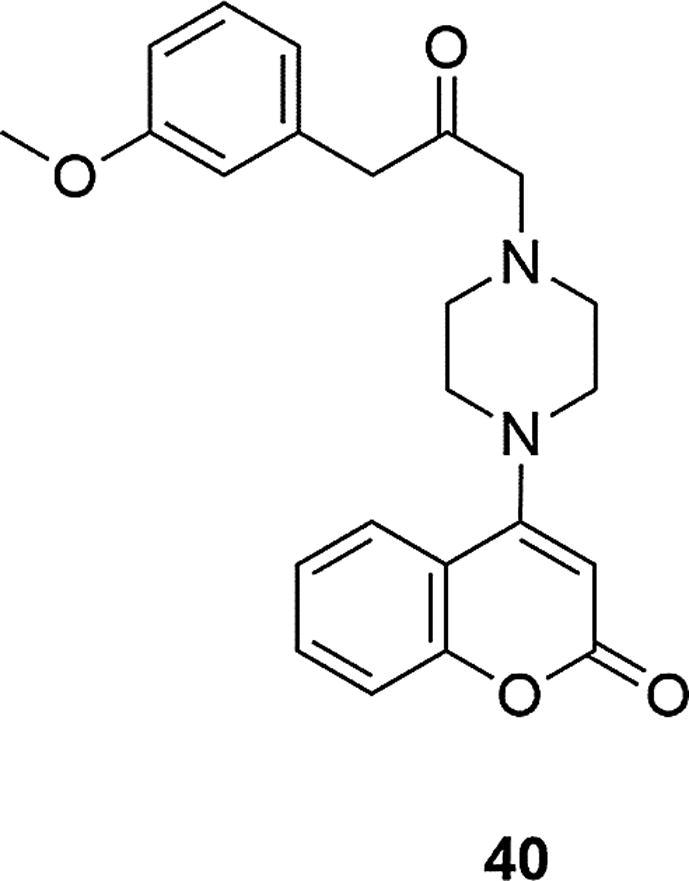
Coumarin derivative synthesized by the Modh’s group (Modh et al, 2013).
In addition, molecular docking studies to the receptor have been performed for this derivative (Modh et al., 2013). Based on this data, it was determined that the key condition for derivatives of 4-hydroxycoumarin to exhibit high AChE inhibition was the presence of an amine functional group on the alkyl side chain (Razavi et al., 2013). Therefore, further studies have focused on systems containing N-phenylpiperazine and N-benzylpiperidine linked with an alkoxyamide to the coumarin moiety and their binding to the enzyme. The derivative containing the N-(1-benzylpiperidin-4-yl)acetamide moiety showed a good activity, compared to donepezil, a standard AChE inhibitor (IC50 = 1.2 µM and IC50 = 0.014 µM for dopenezil). Compounds with a phenylpiperazine system also showed significant AChE activity. Among them, 4-(2-(4-(4-fluorophenyl)piperazin-1-yl)-2-oxoethoxy)coumarin (41) containing the fluorine substituent at the C-4 position of the phenyl ring on the piperazine and one-carbon linker, displayed the highest affinity (IC50 = 2.1 µM) and good selectivity for AChE with respect to BChE, Fig. 12. Similar results were also found for derivatives containing the phenylpiperazine fragment or additionally the 3,4-dichloropiperazine. Changing the fluorine substitution position, lengthening the linker or changing the substituent to a hydroxyl group resulted in a decrease in activity. Docking studies for the above-mentioned derivatives have not been carried out.
Fig. 12.
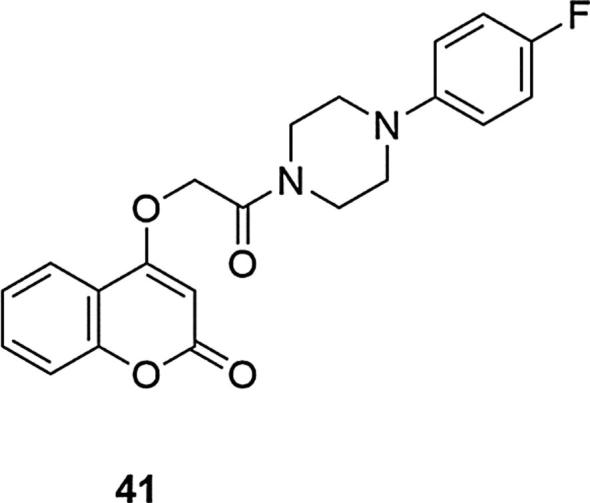
Coumarin derivative synthesized by the Razavi’s group (Razavi et al., 2013).
Subsequent studies on coumarin derivatives containing a piperazinyl ring and having significant inhibitory activity AChE allowed to identify N-(3-chloro-4-((4-ethylpiperazin-1-yl)methyl)phenyl)-6-nitrocoumarin-3-carboxamide (42) as a compound which displayed remarkable activity inhibitors, much stronger than Huperzine A, which was used as a reference substance (IC50 = 34, IC50 = 647.6 nM for 42 and Huperzine A, respectively), Fig. 13, (Yao et al., 2016). Studies on this compound with flow cytometry, western blot assay and molecular docking suggested that 42 could induce cytoprotective autophagy to attenuate H2O2-induced cell death in human neuroblastoma cells. Molecular docking and molecular dynamics simulations revealed the interaction mode of compound 42 with AChE and further supported 42 as a potent selective AChE inhibitor. The authors found that such findings could be the core of a new approach to the design of compounds with neuroprotective properties of AChE, and hence, become an important part in the therapy of Alzheimer's disease.
Fig. 13.
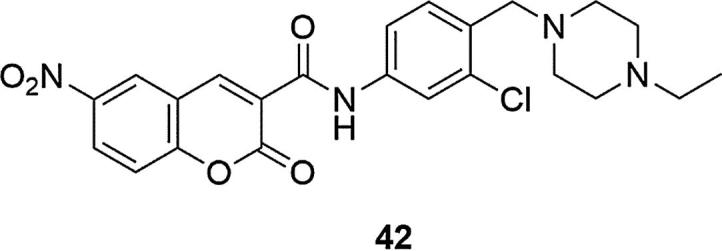
Coumarin derivative synthesized by the Yao’s group (Yao et al., 2016).
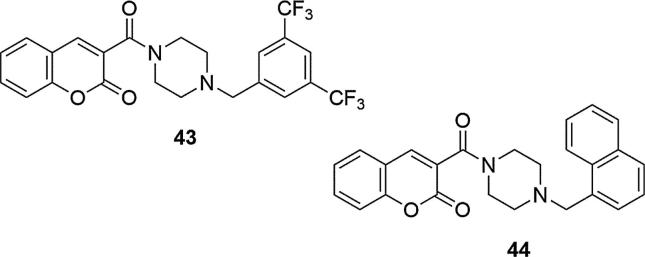
It has also been found that in this type of systems conversion of the piperazine ring to morpholine or primary aliphatic amine causes loss of inhibitors activities against AChE. In addition, the most beneficial inhibotory effect was noted for systems containing a chlorine moiety in the phenyl ring at the piperazine. Chlorine conversion to a trifluoromethyl or hydrogen group reduced their biological activity, similar to replacing the ethyl substituent at the N-4 piperazine position with a methyl group. The trifluoromethyl group, however, proved to be, next to the naphthalene group, crucial for another series of analogs that were synthesized based on the hybridization of coumarin and piperazine pharmacophores (Zhang and Jiang, 2018). Two compounds with high AChE inhibitory potential with no observed cytotoxicity against neuroblastoma cell line (SH-SY5Y) at 100 μM, relative to donepezil, have been identified from these systems. These were 3-(4-(3,5-bis(trifluoromethyl)benzyl)piperazine-1-carbonylcoumarin (43) and 3-(4-(naphthalen-1-ylmethyl)piperazine-1-carbonylcoumarin (44) (IC50 = 2.42, IC50 = 8.78, IC50 = 0.03 µM for 43, 44 and donepezil, respectively), Fig. 14. The use of one, instead of two trifluoromethyl substituents in the systems studied, resulted in a significant reduction in activity, as was their conversion into methyl, fluoro, cyano or ester groups in various positions in the phenyl ring at the piperazine. In addition, compound 44 with the naphthalene moiety showed slightly weaker AChE inhibition than 43 but it showed relatively high selectivity towards AChE with respect to BChE (9.8 times).
Fig. 14.
Coumarin derivatives synthesized by the Zhang’s group (Zhang and Yiang, 2018).
A probable mode of binding of those derivatives with the catalytic AChE domain (PDB: 4EY7) was examined by molecular docking studies, which revealed compounds 43 and 44 as dual binding site AChE inhibitors (Zhang and Yiang, 2018).
2.3. Coumarin-piperazine derivatives as antibacterial and antifungal agents
The treatment of infectious diseases has become a major medical problem over the last decades due to the emergence of multi-drug resistant gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). Therefore, it is extremely important to search for new agents that could overcome the increasing resistance of bacterial pathogens to the currently used drugs (Coleman, 2004). There are numerous examples of coumarins with antibacterial effects, including novobiocin or its analogues such as clorobiocin or RU 79115 (Maxwell, 1993, Musicki et al., 2000). Compounds of this type inhibit ATPase activity of DNA gyrase by competing with ATP for binding to the B subunit of the enzyme. It has been shown that the combination of a coumarin fragment with quinolones, other naturally occurring bacterial DNA gyrase inhibitors, may bring additional benefits for the design of drugs with lower toxicity, better solubility and increased activity in relations to gram-negative bacteria (Schio et al., 2001).
Antibacterial activity of N-[2-(coumarin-3-yl)ethyl]piperazinyl quinolones have been first shown in 2008 by the Emami’s group (Emami et al., 2008). The compounds were designed as analogs of ciprofloxacin, norfloxacin and enoxacin, modifying their structure by inserting new substituents into the piperazine ring located at the C-7 quinolones position. A series of quinolone-based compounds with coumarin core has been studied using strains of gram-negative and gram-positive bacteria, including MRSA. The results were compared with parent quinolones, ciprofloxacin, norfloxacin and enoxacin (MIC values (minimum inhibitory concentration) [µg/mL] for enoxacin = 0.39 (Staphylococcus aureus ATCC 6538), 0.78 (MRSA I and II), 0.098 (Staphylococcus epidermidis ATCC 12228) 0.19 (Bacillus subtilis ATCC 6633), 0.098 (Esterichia coli ATCC 8739), 0.049 (Klebsiella pneumoniae ATCC 10031) and 1.56 (Pseudomonas aeruginosa ATCC 9027)). N-[2-(coumarin-3-yl)-2-oxoethyl]ciprofloxacin derivative (45) was shown to have comparable or higher activity to the tested strains than the reference compounds (MIC values [µg/mL] for 45 = 0.19 (Staphylococcus aureus ATCC 6538), 0.39 (MRSA I and II), 0.049 (Staphylococcus epidermidis ATCC 12,228 and Bacillus subtilis ATCC 6633), 0.013 (Esterichia coli ATCC 8739), 0.003 (Klebsiella pneumoniae ATCC 10031) and 0.39 (Pseudomonas aeruginosa ATCC 9027)), Fig. 15.
Fig.15.
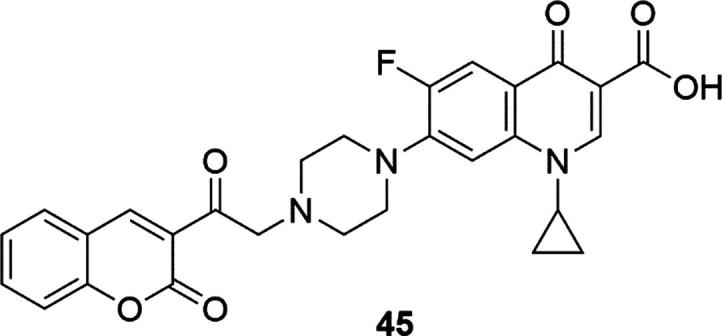
Coumarin derivative synthesized by the Emami’s group (Emami et al., 2008).
The presence of 2-oxo-ethyl coupling between the coumarin and the piperazine ring as well as cyclopropyl in the N-1 position of the quinolone ring had the greatest influence on the antimicrobial activity. Conversion of the cyclopropyl to ethyl group or exchange of the linker between the piperazine and coumarin core to ketone, oxime, O-methyloxime or O-benzyloxime, resulted in a decrease in activity.
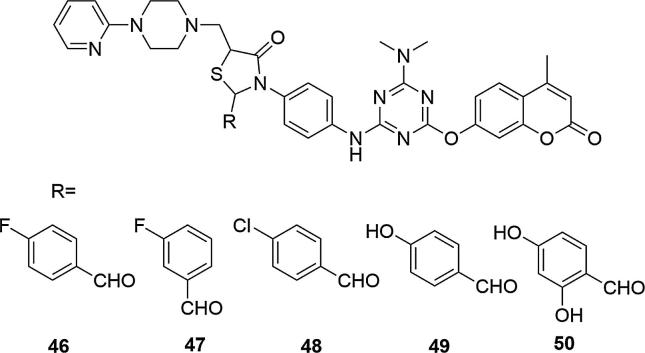
Another group of coumarin derivatives with antibacterial activity, containing the piperazinyl fragment are thiazolidinones combined with 1-pyridin-2-yl-piperazine (Patel et al., 2012a, Patel et al., 2012b, Patel et al., 2012c). The highest activity against gram-positive (S. aureus and B. cereus) and gram-negative bacteria (E. coli, P. aeruginosa, K. pneumoniae, S. typhi, P. vulgaris and S. flexneria) relative to ciprofloxacin, showed compounds containing electron withdrawing (chloro or fluoro) moieties on aryl ring at thiazolidinone group: 3-[4-[4-dimethylamino-6-(4-methyl-2-oxo-2H-chromen-7-yloxy)-[1,3,5]triazin-2-ylamino]-phenyl]-2-(4-fluorophenyl)-5-(4-pyridin-2-yl-piperazin-1-ylmethyl) -thiazolidin-4-one (46), 3-[4-[4-dimethylamino-6-(4-methyl-2-oxo-2H-chromen-7-yloxy)[1,3,5]triazin-2-ylamino]-phenyl]-2-(3-fluorophenyl)-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-thiazolidin-4-one (47) and 2-(4-chloro-phenyl)-3-[4-[4-dimethylamino-6-(4-methyl-2-oxo-2H-chromen-7-yloxy)-[1,3,5]triazin-2-ylamino]-phenyl]-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-thiazolidin-4-one (48). MIC values [µg/mL] for 46 were respectively: 28 (S. aureus), 23 (B. cereus), 20 (E. coli), 26 (P. aeruginosa), 18 (K. pneumoniae), 19 (S. typhi), 22 (P. vulgaris), 21 (S. flexneria) and for ciprofloxacin: 30 (S. aureus), 31 (B. cereus), 32 (E. coli), 33 (P. aeruginosa), 33 (K. pneumoniae), 30 (S. typhi), 31 (P. vulgaris), 32 (S. flexneria) (see Fig. 16).
Fig. 16.
Coumarin derivatives synthesized by the Patel’s group (Patel et al., 2012a, Patel et al., 2012b, Patel et al., 2012c).
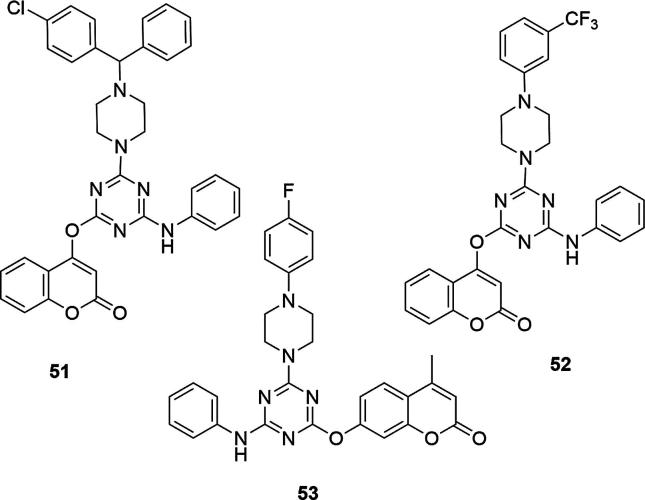
The presence of the hydroxyl group on aryl ring at thiazolidinone group also increased the activity of the obtained compounds, such as 3-[4-[4-dimethylamino-6-(4-methyl-2-oxo-2H-chromen-7-yloxy)-[1,3,5]triazin-2-ylamino]-phenyl]-2-(4-hydroxyphenyl)-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-thiazolidin-4-one (49) and 2-(2,4-dihydroxy-phenyl)-3-[4-[4-dimethylamino-6-(4-methyl-2-oxo-2H-chromen-7-yloxy)-[1,3,5]triazin-2-ylamino]-phenyl]-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-thiazolidin-4-one (50), Fig. 17. The activity of the compounds decreased sharply if the chloro, fluoro or hydroxy groups were replaced with methyl, methoxy, nitro or bromo moiety. In addition, compounds 46 and 49 have shown antifungal activity against the strains used: MIC [µg/mL] values for 46 were: 29 (A. niger), 21 (A. fumigatus), 26 (A. clavatus) and 27 (C. albicans), at the level of ketoconazole, used as the reference compound (MIC values [µg/mL] for ketoconazole were: 30 (A. niger), 29 (A. fumigatus), 31 (A. clavatus) and 33 (C. albicans). In the next stage of the study, similar results were obtained for s-triazinyl piperazines and piperidines derivatives, bearing 4-hydroxycoumarin or 7-hydroxy-4-methylcoumarin moieties, Fig. 17, (Patel et al., 2012a, Patel et al., 2012b, Patel et al., 2012c). Studies have shown that halogenated benzhydryl compound 51 containing 4-hydroxycoumarin combined with piperazine was the most active inhibitor of the S. aureus strain, a gram-positive bacteria (MIC = 22 µg/mL). After adding the additional trifluoromethyl group to the C-3 position of the piperazine ring connected to the 4-hydroxycoumarin (52) or adding the additional 4-fluoropiperazine to the ring connected to the 7-hydroxy-4-methylcoumarin fragment (53) the activity against S. aureus was also very high.
Fig. 17.
Coumarin derivatives synthesized by the Patel’s group (Patel et al., 2012a, Patel et al., 2012b, Patel et al., 2012c).
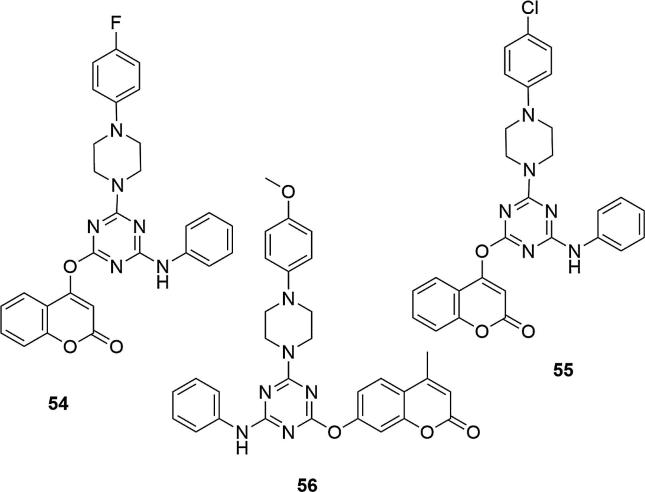
The presence of fluorine and methoxy groups on the phenyl ring on the piperazine caused an increase in the activity of compounds containing both 7-hydroxy-4-methylcoumarin (53) and 4-hydroxycoumarin (54) relative to the E. coli strains. In contrast, fluorine or chloro substituents at the C-4 position of the piperazine ring with 4-hydroxycoumarin systems (55, 56) had very good activity against P. aeruginosa, as the methoxy substituents on the 7-hydroxy-4-methylcoumarin systems (56), Fig. 18.
Fig. 18.
Coumarin derivatives synthesized by the Patel’s group (Patel et al., 2012a, Patel et al., 2012b, Patel et al., 2012c).
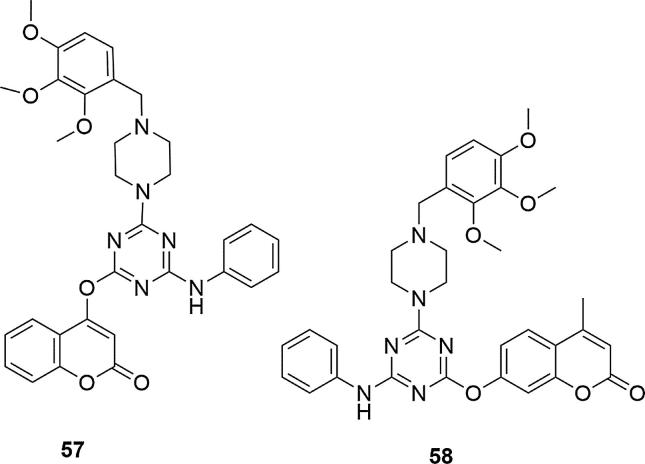
Compounds with three methoxy groups on the phenyl ring on the piperazine showed high activity on the tested fungal strains A. niger and C. albicans MTCC 183, relative to ketoconazole, which was the reference compound (MIC values [µg/mL] for 57 = 22 (A. niger MTCC 282), 23 (C. albicans MTCC 183); for 58 = 24 (A. niger MTCC 282), 24 (C. albicans MTCC 183); for ketoconazole 30 (A. niger MTCC 282), 33 (C. albicans MTCC 183), Fig. 19.
Fig. 19.
Coumarin derivatives synthesized by the Patel’s group (Patel et al., 2012a, Patel et al., 2012b, Patel et al., 2012c).
Compounds containing chlorine groups on the phenyl ring of the piperazine scaffolds attached to 4-hydroxycoumarin or 7-hydroxy-4-methylcoumarin, bearing s-triazine moiety displayed strong inhibition against gram-negative E. coli and P. aeruginosa bacteria strains and A. niger and C. albicans fungal strains ((Patel et al., 2012a, Patel et al., 2012b, Patel et al., 2012c). The presence of chlorine significantly increased the activity of compounds, which increased with the amount of chlorine atoms introduced into the system. It was also found, that the compounds having a fragment of 7-hydroxy-4-methyl coumarin in the skeleton have a more favorable microbiological effect than those having a 4-hydroxycoumarin fragment, with the exception of systems with N-acetylpiperazine.
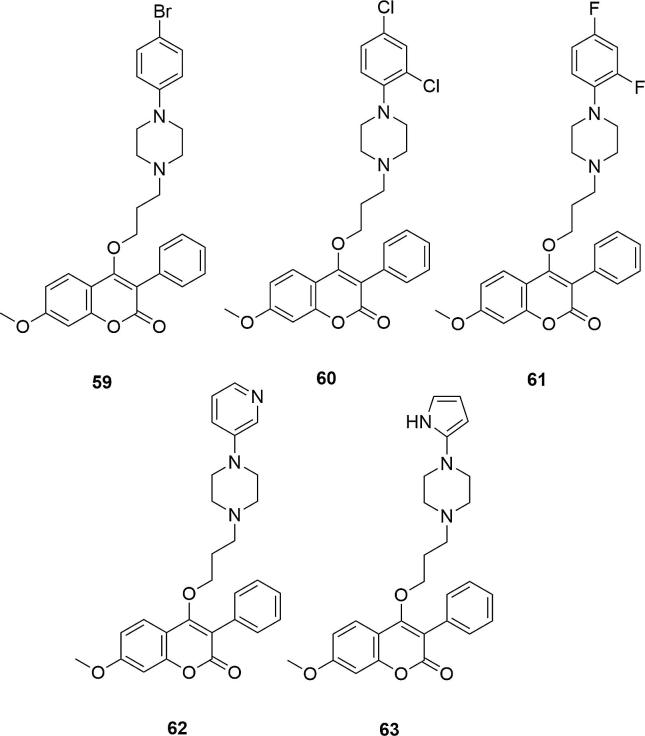
These results were confirmed by microbiological tests over the extended 4-hydroxycoumarin system, enriched with the phenyl group in the C-3 position of the coumarin ring and the methoxy group in the C-7 position of this ring [45]. 4-[3-[4-(4-bromobenzyl)-piperazin-1-yl]-propoxy]-7-methoxy-3-phenylcoumarin (59), 4-[3-[4-(2,4-dichlorobenzyl)-piperazin-1-yl]-propoxy]-7-methoxy-3-phenylcoumarin (60) and 4-[3-[4-(2,4-difluorobenzyl)-piperazin-1-yl]-propoxy]-7-methoxy-3-phenylcoumarin (61), with the bromine, chlorine or fluorine atoms at the C-2 or C-4 positions of the phenyl ring on piperazine favored the premier activity against K. pneumonia and E. coli (Zone of inhibition (in mm) for 59 = 2.89 (K. pneumonia) and 2.00 (E. coli); for 60 = 8.82 (K. pneumonia) and 7.02 (E. coli); for streptomycin = 3.24 (K. pneumonia) and 3.06 (E. coli), Fig. 20. Compounds having an additional heterocyclic system on the piperazine ring (62, 63) also showed excellent activity against all tested bacteria strains (E. coli, Proteus Vulgaris, Proteus mirabilis, Klebsiella pneumonia, Entero aerogenes, Bacillus subtilis, Bacillus megaterium, Bacillus pumilus, S. aureus, St. Pyrogens) (Mandala et al., 2013).
Fig. 20.
Coumarin derivatives synthesized by the Mandala’s group (Mandala et al., 2013).
Results of biological tests were in agreement with structure-based docking studies using two crystal structures of oxidoreductases (PDB: 1XDQ and 3QLS), showing a relatively strong binding of selected compounds. Compound 60 turned out to be a good oxidoreductase inhibitor possessing both anti-bacterial and antifungal activity.
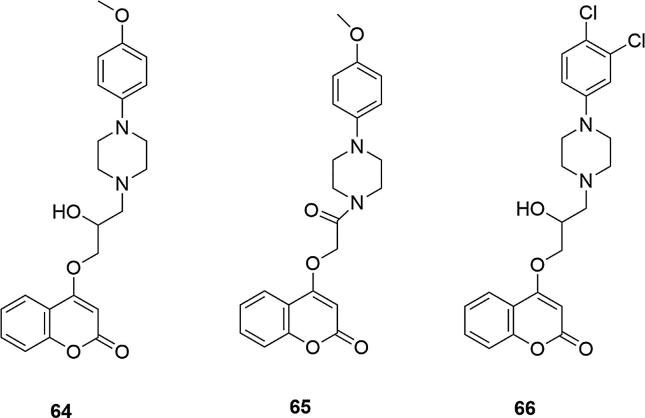
Studies on 4-hydroxycoumarin derivatives were also carried out using the 2-hydroxypropyl and 2-oxoethoxyl linkers between the coumarin and the piperazinyl fragment (Wang et al., 2014). Two series of new compounds were synthesized in this manner, and their antibacterial activities against Staphylococcus aureus, Bacillus subtilis, Staphylococcus aureus Enoyl-ACP-reductase (SaFabI), Escherichia coli and Pseudomonas aeruginosa were studied. It was shown that the compounds were very active against gram-positive bacteria and inactive against gram-negative bacteria, relative to penicillin G, which was the reference compound. The strongest action against B. subtilis and S. aureus at the level of penicillin G were displayed by derivatives containing a methoxy group in the para position of the phenyl ring of piperazine: 4-(2-hydroxy-3-(4-(4-methoxyphenyl)piperazin-1-yl)propoxy)coumarin (64) and 4-(2-(4-(4-methoxy phenyl)piperazin-1-yl)-2-oxoethoxy)coumarin (65), for both series of compounds (MIC values [µg/mL] for 64 = 0.236 (B. subtilis), 0.355 (S. aureus); for 65 = 0.861 (B. subtilis), 0.327 (S. aureus); for penicylin G = 0.752 (B. subtilis), 1.685 (S. aureus)). The activity decreased if the methyl group was in the ortho or meta positions of this ring. Also, derivatives containing dichloro substituent in the meta and para position were shown to be active, and their other positions in the phenyl ring, caused a decrease in activity (66) (MIC values [µg/mL] for 66 = 0.339 (B. subtilis), >50 (S. aureus)), Fig. 21. Introduction of fluoride into the molecule caused a decrease in activity each time, regardless of the linker between the piperazinyl and coumarin moiety.
Fig. 21.
Coumarin derivatives synthesized by the Wang’s group (Wang et al., 2014).
To assess the cytotoxicity of the investigated derivatives, hemolysis and cytotoxicity tests were also performed. Compounds with a strong biological activity showed low haemolytic activity and low toxicity (IC50 values [µM] for 64 = 192.42; for 65 = 191.53, for penicylin = 185.72). To determine the potential binding modes, molecular docking of compound 64 in S. aureus Enoyl-ACPreductase active site was performed. Analysis of the binding conformation of this derivative showed stabilization by hydrogen bonding interaction with Lys164 and Ile20 (Wang et al., 2014).
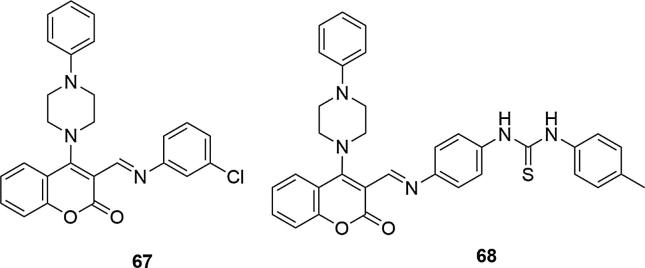
Another series of derivatives relied on coumarin-piperazine combination with thiourea and Schiff Base moiety. Most synthesized and tested compounds have shown promising efficacy against various microorganisms, such as bacteria (Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae), including mycobacteria (Mycobacterium tuberculosis) as well as fungal strains (Aspergillus niger, Aspergillus fumigates, Aspergillus clavatus, Candida albicans) (Lakum et al., 2015). The first group of derivatives were coumarin Schiff bases with N-phenylpiperazine in C-4 position of the coumarin ring, the second group was additionally enriched with thiourea fragment, Fig. 22. In both groups of compounds, derivatives containing methyl, chlorine or hydroxyl substituents, were the most active. Even better results were obtained for the group of compounds containing an additional thiourea system, which showed that its presence significantly influences the antimicrobial activity of coumarin derivatives. The two most effective derivatives containing chlorine and methyl substituents were identified, namely (E)-3-[[(3-chlorophenyl)imino]methyl]-4-(4-phenylpiperazin-1-yl)coumarin (67) and 1-[4-[((1E)-(2-oxo-4-(4-phenylpiperazin-1-yl)-2Hchromen-3-yl)methylene)amino]phenyl]-3-(p-tolyl)thiourea (68). Both compounds showed high activity against specific bacteria and fungi and, as well, compared to ciprofloxacin and ketoconazole, used as reference compounds (MIC values [µg/mL] for 67 = 20 (Staphylococcus aureus), 31 (Bacillus subtilis), 21 (Escherichia coli), 25 (Pseudomonas aeruginosa), 10 (Klebsiella pneumoniae), 26 (Aspergillus niger), 21 (Aspergillus fumigates), 19 (Aspergillus clavatus), 32 (Candida albicans); for 68 = 17 (Staphylococcus aureus), 30 (Bacillus subtilis), 25 (Escherichia coli), 30 (Pseudomonas aeruginosa), 21 (Klebsiella pneumoniae), 25 (Aspergillus niger), 23 (Aspergillus fumigates), 27 (Aspergillus clavatus), 29 (Candida albicans); for ciprofloxacin = 22 (Staphylococcus aureus), 38 (Bacillus subtilis), 27 (Escherichia coli), 32 (Pseudomonas aeruginosa), 19 (Klebsiella pneumoniae); for ketoconazole = 30 (Aspergillus niger), 29 (Aspergillus fumigates), 31 (Aspergillus clavatus), 33 (Candida albicans)).
Fig. 22.
Coumarin derivatives synthesized by the Lakum’s group (Lakum et al., 2015).
2.4. Coumarin-piperazine derivatives with cytotoxic activity
In 2011, the N1-(coumarin-7-yl) series of amidrazones incorporating N-piperazines was synthesized and tested for antitumor activity by conducting cell viability assays, using MTT method with K562 human leukemia cells, MCF7 and ZR-75-1 breast cancer cells as well as HL60 leukemia cells (Mustafa et al., 2011). The type of substituent in the N-4 piperazine position was shown to have a key influence on cytotoxic activity. The highest activity for all cells tested was found for 7-[2-[1-(4-(1-benzyl-2-ethyl-4-nitro-1H-imidazol-5-yl)piperazin-1-yl)-2-oxo propylidene]hydrazinyl]-4-methylcoumarin (69) with N-(1-benzyl-2-ethyl-4-nitroimidazol-5-yl) substituent, with the doxorubicin used as a reference substance (% survival for 69 = 43.3 ± 5.1 (MCF-7), 52.7 ± 6.1 (K562); IC50 (half maximal inhibitory concentration) [µg/mL] for 69 = 20.2 ± 3.7 (MCF-7), >100 (ZR-75–1), 9.2 ± 2.8 (K562), >100 (HL60); IC50 for doxorubicin = 0.31 ± 0.01 (MCF-7), 1.0 ± 0.01 (ZR-75–1), 3.54 ± 0.54 (K562), 0.09 ± 0.005 (HL60), Fig. 23. It was shown that for MCF-7 breast cancer cells the presence of a nucleophilic component in the N-4 position of the piperazine ring was important, but the activity decreased after replacing N-(1-benzyl-2-ethyl-4-nitroimidazol-5-yl) with N-CO2Et or N-(2-pyrimidyl) moiety. In contrast, for human K562 leukemia cells only compound 69, of the whole pool of tested derivatives, showed cytotoxic activity. Each studied change in the N-4 position on the piperazine ring resulted in the loss of antitumor activity.
Fig. 23.
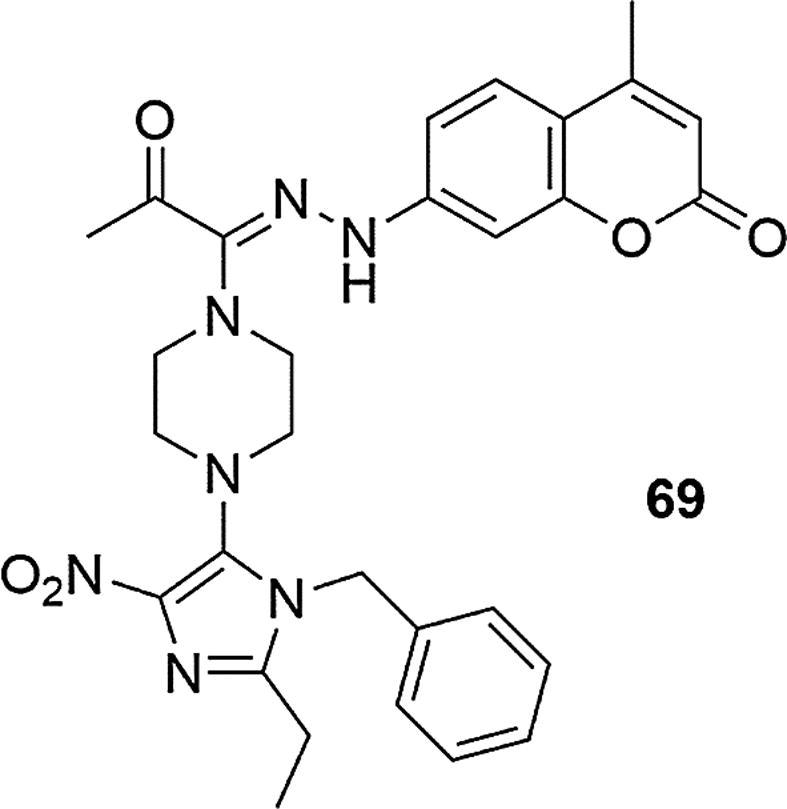
Coumarin derivative synthesized by the Mustafa’s group (Mustafa et al., 2011).
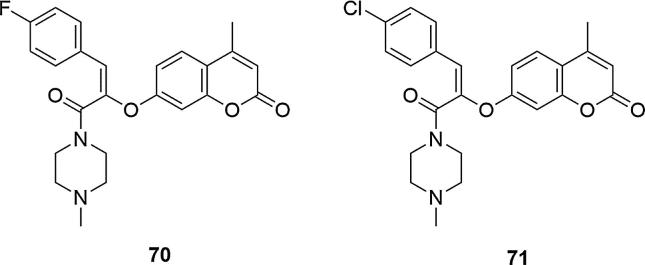
Cytotoxic activity against human breast cancer cells MCF-7, human cervical cancer cells HeLa and non-small cell lung cancer cells NCI-H226, has also been demonstrated for piperazinyl-prop-1-en-2-yloxy-coumarins derivatives (Nikalje et al., 2017). Compounds 7-(1-(4-fluorophenyl)-3-(4-methylpiperazin-1-yl)-3-oxoprop-1-en-2-yloxy)-4-methylcoumarin (70) and 7-(1-(4-chlorophenyl)-3-(4-methylpiperazin-1-yl)-3-oxoprop-1-en-2-yloxy)-4-methyl coumarin (71) showed strong inhibitory activity against MCF-7 and HeLa compared to a standard drug adriamycin and moderate inhibition activity on the human lung cell NCI-H226 (GI50 (the concentration exhibiting 50% inhibition of the growth as compared to the for growth of control) for 70 < 0.1 (MCF-7), 0.77 (NCI-H226), < 0.1 (HeLa); for 71 < 0.1 (MCF-7), < 0.1 (NCI-H226), < 0.1 (HeLa); for adriamycin < 0.1 (MCF-7), <0.1 (NCI-H226), < 0.1 (HeLa), Fig. 24.
Fig. 24.
Coumarin derivatives synthesized by the Nikalje’s group (Nikalje et al., 2017).
It was showed that compounds having electron withdrawing groups in the structure, such as para-fluoro or para-chloro, had activity at levels of adriamycin. On the other hand, derivatives containing an unsupported phenyl ring showed significantly less cytotoxicity, as well as compounds with electron-donating substituents, such para-methoxy group. The substitution of phenyl into a heterocyclic system such as thiophene also reduced the cytotoxicity of the derivatives against the tumor cells under investigation.
Predictions of ADME properties using theoretical calculations showed a good human oral absorption of the examined systems. In the molecular docking studies these compounds also showed good binding affinity to topoisomerase-II and the results were consistent with the observed values of GI50. In general, a good correlation was observed between in vitro cytotoxic activity and the study of docking of synthesized compounds. Compound 70 showed important interactions near the active site with key catalytic residues of topoisomerase II (Nikalje et al., 2017).
The series of 7-hydroxycoumarin-containing derivatives of urea-piperazine and thioureapiperazine as the elements of the structure that induces antitumor activity were also studied in the direction of cytotoxic activity (Hua et al., 2015). 5-Fluorouracil (5-FU) and cisplatin were used as a positive control and the inhibitory activity of the compounds was tested was tested against nine tumor lines: non-small Lung lines cancer cell line A549, Human large cell lung cancer cell line NCI-H460, Human liver cancer cell line Bel-7404, Human bladder cancer cell line T24, Human liver cancer cell line HepG2, Human cervical cancer cell line HeLa, Human colon cancer cell line Hct-116, Human gastric cancer cell line MGC-803 Human lung adenocarcinoma cell line SPCA-2 and Human normal umbilical vein endothelial cel HUVEC line (Huang et al., 2017). The piperazine system in the 4-methyl-7-hydroxycoumarin scaffold has been shown to induce increased cytotoxic activity, similar to the combination urea/thioureapiperazine moieties and the systems with urea linker were definitely more active. The presence of the electron-withdrawing moiety in position C-3 of the benzene ring also played an important role since the presence of electron-donating groups in this place resulted in a reduction of activity several times. The most preferred combinations of moieties constituted electron-withdrawing substituents in the meta and para positions of the phenyl ring, and the derivative exhibiting the best range of inhibition (72) had fluorine in the meta position and bromine in the para position of this ring (IC50 [µg/mL] for 72 = 9.78 ± 1.29 (HepG2), 18.5 ± 1.26 (A549), 16.87 ± 1.02 (Bel-7404), 10.42 ± 1.89 (SPCA-2), 2.13 ± 0.75 (MGC-803), 8.99 ± 0.91 (HeLa), 9.68 ± 1.32 (Hct-116), 1.86 ± 0.73 (NCI-H460), 8.34 ± 0.95 (T24), >100 (HUVEC); for 5-FU = 31.98 ± 0.56 (HepG2), 36.34 ± 0.57 (A549), 40.21 ± 1.98 (Bel-7404), 58.92 ± 8.02 (SPCA-2), 45.76 ± 1.29 (MGC-803), 21.46 ± 1.15 (HeLa), 11.46 ± 0.77 (Hct-116), 45.44 ± 0.94 (NCI-H460), 37.56 ± 0.49 (T24), 25.12 ± 1.03 (HUVEC); for cisplatin = 10.12 ± 0.71 (HepG2), 13.48 ± 0.37 (A549), 14.72 ± 0.66 (Bel-7404), 15.83 ± 0.54 (SPCA-2), 8.50 ± 0.58 (MGC-803), 40.46 ± 0.83 (HeLa), 10.43 ± 0.47 (Hct-116), 20.36 ± 0.50 (NCI-H460), 30.05 ± 0.39 (T24), 9.68 ± 1.15 (HUVEC)), Fig. 25. In summary, it was shown that the compound 72 had better inhibitory activity on tumor cells than commercial anti-cancer drug, cisplatin, significantly lower cytotoxicity in normal HUVEC cells as well as good selectivity. Other combinations of these substituents or compounds having m-Cl and p-F or m-Cl and p-CH3 substituents showed significantly lower activity.
Fig. 25.
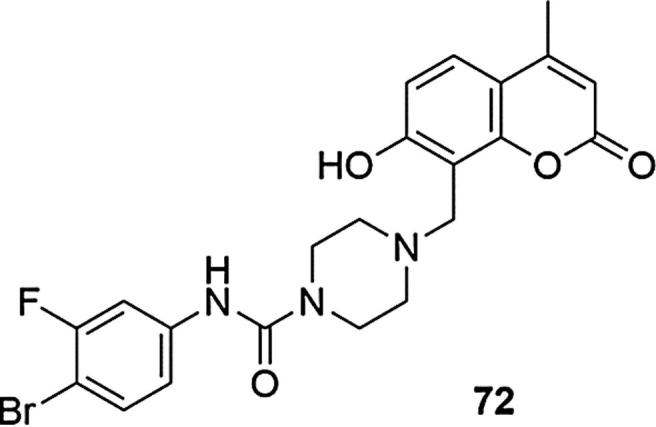
Coumarin derivative synthesized by the Huang’s group (Huang et al., 2017).
2.5. Coumarin-piperazine derivatives with a different biological effect
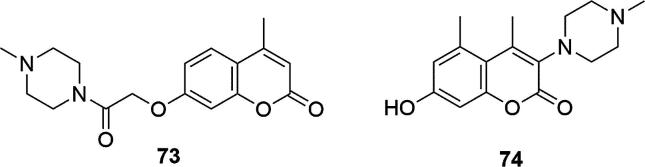
Lymphatic filariosis is a serious disease affecting millions of people around the world, especially on Indian subcontinent and in Africa (Ottesen et al., 1997). So far, there is no single therapeutic agent capable of combating it and agents that combine the structural features of two classes of antifilarial agents to obtain a system with microfilaricidal and macrofilaricidal capability in one are still in the development phase. Diethylcarbamazine and ivermectin are two important antifilarial agents, but both show many side effects (Fan, 1992). In 1993, it was shown that benzopyrone in combination with diethylcarbamazine reduced the fillarial manifestations in symptomatic patients (Casley-Smith et al., 1993). Due to the fact that diethylcarbamazine, as a piperazine derivative, has proven anthelmintic activity, numerous benzopyrone-piperazine derivatives have been tested against various pillar infections in rodents (Tripathi et al., 1997). One of such systems 7-O-[4-methylpiperazine-1-(2-acetyl)]-coumarin (73) showed activity against adulticidal action against rodent filariid - L. carinii in cotton rats, Fig. 26. Compound 73 was also evaluated against infection with Brugia malayi in Mastomys coucha and jird (Meriones unguiculatus) and have shown promising macrofilaricidal action against rodent filariid Litomosoides carinii in cotton rats (Tripathi et al., 2003). It revealed adulticidal and microfilaricidal activity along with high sterilization effect on the female worms in the Brugia malayi in Mastomys coucha and jird (Meriones unguiculatus) system, interfered with the establishment of infective larvae (L3)-induced infection and inhibited protease activity of B. malayi (73 in dose of 300 mg/kg, oral (p.o.) after5 days showed 53.6% adulticidal and 46.0% microfilaricidal activity along with 46.3% sterilization effect on the female worms and interfered with the establishment of infective larvae (L3)-induced infection to an extent of 50%; at dose of 1 mM 73 inhibited protease activity of B. malayi to 82%).
Fig. 26.
Coumarin derivative synthesized by the Tripathi’s and Li’s groups (Li et al., 2010, Tripathi et al., 2003).
In diseases such as multiple sclerosis or asthma, the activation of the chemokine receptor 4 (CCR4) by a chemokine-like factor 1 (CKLF1) plays an important role (Li et al., 2010). In 2010, Li’s group selected, in screening tests, 5,7-dihydroxy-4-methyl-3-(4-methylpiperazin-1-yl)coumarin (74), that reduced the asthmatic pathologic changes in human tissue CKLF1-transfected mice, blocked cytotoxicity induced by CKLF1 and ameliorated pathological changes via inhibition of the NF-κB signal pathway (IC50 = 2.12 × 10−8 M), Fig. 26. It is also worth mentioning that the derivative of compound 74 with the methoxy group in the C-5 position of the coumarin ring -7-hydroxy-5-methoxy-4-methyl-3-(4-methylpiperazin-1-yl)coumarin showed neuroprotective activity, which is a very important factor in the treatment of ischemic brain injury (proliferation 100.7 ± 5.4 relative to control proliferation 100 ± 3.4, in damaged pheochromocytoma PC12 cells in rats) (Sun et al., 2013). This compound attenuated damage in the cell line derived from the pheochromocytoma and rat adrenal gland caused by oxygen deficiency in vitro. Furthermore, this compound showed neuroprotection in the middle cerebral artery occlusion rats. He worked by suppressing neuronal loss and neuropathological changes in the cortex and hippocampus, improving neurological function and reducing infarct size and brain-water content.
3. Conclusion
As shown in this study, coumarins have a wide range of biological activities, and many of them occur in traditional medicinal plants that are still used around the world. Numerous efforts, including the isolation and purification of naturally occurring coumarins from a variety of plants as well as laboratory synthesis of coumarin derivatives with novel structures and properties, have been focusing on the research and development of coumarins as potential drugs. We hope that our summary shows unequivocally that piperazine derivatives, and coumarin-piperazine derivatives in particular, have been of interest to synthetic and medicinal chemists for many years due to their structural and therapeutic diversity. Coumarin-piperazinyl derivatives have been shown to possessed activity as antidepressants, anticancer, antibacterial agents and as α-adrenergic antagonists. In addition, scientific literature describes coumarin-piperazine systems that exhibit a neuroprotective effect by their antioxidant and anti-inflammatory activities. Due to the potential widespread use of these privileged structures with enhanced bioactivities, they can be treated as scaffolds to develop new leading structures and, as a result, can be considered as future therapeutic agents for use in a serious global disorders. Even though the number of investigations on coumarins in 2018 showed a small decline with respect to 2017, we are in the decade of an unprecedented rise of coumarin-related studies and we hope that our review will serve as an outline for future, exciting research in this field.
Founding resource
This work was supported by the Medical University of Warsaw/University of Warsaw grant, Faculty of Pharmacy, grant No. FW24/NUW1/19.
Declaration of Competing Interest
Author declares no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abate A., Dimartino V., Spina P., Costa P.L., Lombardo C., Santini A., Del Piano M., Alimonti P. Hymecromone in the treatment of motor disorders of the bile ducts: a multicenter, double-blind, placebo-controlled clinical study. Drugs. Exp. Clin. Res. 2001;27:223–231. [PubMed] [Google Scholar]
- Alvarez A., Bronfman F., Perez C.A., Vicente M., Garrido J., Inestrosa N.C. Acetylcholinesterase, a senile plaque component, affects the fibrillogenesis of amyloid-beta-peptides. Neurosci. Lett. 1995;201:49–52. doi: 10.1016/0304-3940(94)12127-c. [DOI] [PubMed] [Google Scholar]
- Anand P., Singh B., Singh N.A. Review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012;20:1175–1180. doi: 10.1016/j.bmc.2011.12.042. [DOI] [PubMed] [Google Scholar]
- Archier E., Devaux S., Castela E., Gallini A., Aubin F., Le Maître M., Aractingi S., Bachelez H., Cribier B., Joly P., Jullien D., Misery L., Paul C., Ortonne J.P., Richard M.A. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012;26(Suppl 3):11–21. doi: 10.1111/j.1468-3083.2012.04519.x. [DOI] [PubMed] [Google Scholar]
- Božič-Mijovski M. Advances in monitoring anticoagulant therapy. Adv. Clin. Chem. 2019;90:197–213. doi: 10.1016/bs.acc.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Carreiras M.C., Marco J.L. Recent approaches to novel anti-Alzheimer therapy. Curr. Pharm. Des. 2004;10:3167–3175. doi: 10.2174/1381612043383421. [DOI] [PubMed] [Google Scholar]
- Casley-Smith J.R., Jamal S., Casley-Smith J.R. Reduction of filaritic lymphoedema and elephantiasis by 5,6-benzo-5-pyrone(coumarin), and the effect of diethylcarbamazine (DEC) Ann. Trop. Med. Parasit. 1993;87:247–258. doi: 10.1080/00034983.1993.11812763. [DOI] [PubMed] [Google Scholar]
- Capra J.C., Cunha M.P., Machado D.G., Zomkowski A.D., Mendes B.G., Santos A.R., Pizzolatti M.G., Rodrigues A.L. Antidepressant-like effect ofscopoletin, a coumarin isolated from Polygala sabulosa (Polygalaceae) inmice: evidence for the involvement of monoaminergic systems. Eur. J. Pharmacol. 2010;643:232–238. doi: 10.1016/j.ejphar.2010.06.043. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang S., Xu X., Liu X., Yu M., Zhao S., Liu S., Qiu Y., Zhang T., Liu B.F., Zhang G. Synthesis and biological investigation of coumarin piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics. J. Med. Chem. 2013;56:4671–4690. doi: 10.1021/jm400408r. [DOI] [PubMed] [Google Scholar]
- Chen Y., Lan Y., Wang S., Zhang H., Xu X., Liu X., Yu M., Liu B.F., Zhang G. Synthesis and evaluation of new coumarin derivatives as potential atypical antipsychotics. Eur. J. Med. Chem. 2014;74:427–439. doi: 10.1016/j.ejmech.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Coleman K. Recent advances in the treatment of gram-positive infections. Drug Discov. Today Ther. Strateg. 2004;1:455–460. [Google Scholar]
- Dávila-Fajardo C.L., Díaz-Villamarín X., Antúnez-Rodríguez A., Fernández-Gómez A.E., García-Navas P., Martínez-González L.J., Dávila-Fajardo J.A., Barrera J.C. Pharmacogenetics in the treatment of cardiovascular diseases and its current progres regarding implementation in the clinical routine. Genes (Basel). 2019;10:261–286. doi: 10.3390/genes10040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger A. The pharmacodynamic characterization of coumarin. Arch. Exp. Pathol. Phar. Supplement. 1908:150–163. [Google Scholar]
- Emami S., Foroumadi A., Faramarzi M.A., Samadi N. Synthesis and antibacterial activity of quinolone-based compounds containing a coumarin moiety. Arch. Pharm. Chem. Life Sci. 2008;341:42–48. doi: 10.1002/ardp.200700090. [DOI] [PubMed] [Google Scholar]
- Fan P.C. Diethylcarbamazine treatment of bancroftian and Malayan filariasis with emphasis on side effects. Ann. Trop. Med. Parasit. 1992;86:399–405. doi: 10.1080/00034983.1992.11812684. [DOI] [PubMed] [Google Scholar]
- Fujimaki T., Saiki S., Tashiro E., Yamada D., Kitagawa M., Hattori N., Imoto M. Identification of licopyranocoumarin and glycyrurol from herbal medicinesas neuroprotective compounds for Parkinson’s disease. PLoS One. 2014;9:100395–100412. doi: 10.1371/journal.pone.0100395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garino C., Pietrancosta N., Laras Y., Moret V., Rolland A., Quelever G., Kraus J.L. BACE-1 inhibitory activities of new substituted phenyl-piperazine coupled to various heterocycles: chromene, coumarin and quinoline. Bioorg. Med. Chem. Lett. 2006;16:1995–1999. doi: 10.1016/j.bmcl.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Gierlak, W., Kuch, M., 2010. How to use cardiac drugs in everyday practice? In: Czelej, Dłużniewski, M., (Eds.), Anticoagulants in cardiology - Vitamin K antagonists. chapter 6.
- Gonzalez-Gomez J.C., Santana L., Uriarte E., Brea J., Villazon M., Loza M.I., De Luca M., Rivas M.E., Montenegro G.Y., Fontenla J.A. Bioorg. Med. Chem. Lett. 2003;13:175–178. doi: 10.1016/s0960-894x(02)00933-2. [DOI] [PubMed] [Google Scholar]
- Hua S.X., Huang R.Z., Ye M.Y., Pan Y.M., Yao G.Y., Zhang Y., Wang H.S. Design, synthesis and in vitro evaluation of novel ursolic acid derivatives as potential anticancer agents. Eur. J. Med. Chem. 2015;95:435–452. doi: 10.1016/j.ejmech.2015.03.051. [DOI] [PubMed] [Google Scholar]
- Huang R.Z., Hua S.X., Wang C.Y., Pan Y.M., Qin J.M., Ding Z.Y., Zhang Y., Wang H.S. 4-Methylumbelliferones analogues as anticancer agents: synthesis and in cell pharmacological studies. Anti-Cancer Agents Me. 2017;17:576–589. doi: 10.2174/1871520616666160926113109. [DOI] [PubMed] [Google Scholar]
- Hoerr R., Noeldner M. Ensaculin (KA-672HCl): a multitransmitter approachto dementia treatment. CNS Drug Rev. 2002;8:143–158. doi: 10.1111/j.1527-3458.2002.tb00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult J.R.S., Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen. Pharmacol. Vasc. S. 1996;27:713–722. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- Ibbotson S. Drug and chemical induced photosensitivity from a clinical perspective. Photochem. Photobiol. Sci. 2018;5:1885–1903. doi: 10.1039/c8pp00011e. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kitagawa H., Hiramoti T. Research on coumarin derivatives for medicinal purpose. 3. Sedative and hypnotic actions. Yakugaku Zasshi. 1951;71:686–692. [Google Scholar]
- Ito Y., Kitagawa H., Suzuki Y. Research on coumarin derivatives for medicinal purpose. 2. The toxicity of coumarin, 2-thiocoumarin ans 4-methylcoumarin. Yakugaku Zasshi. 1951;71:596–601. [Google Scholar]
- Ito Y., Kitagawa H., Tamaoki B. Coumarin derivatives for medical purpose.1. Anthelmintic action. Yakugaku Zasshi. 1950;70:730–733. [Google Scholar]
- Jaber M., Robinson S.W., Missale C., Caron M.G. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Kayser O., Kolodziej H. Antibacterial activity of simple coumarins: structural requirements for biological activity. Z. Naturforsch. C. 1999;54:169–174. doi: 10.1515/znc-1999-3-405. [DOI] [PubMed] [Google Scholar]
- Kulkarni M.V., Kulkarni G.M., Lin C.H., Sun C.M. Recent advances in coumarins and 1-azacoumarins as versatile biodynamic agents. Curr. Med. Chem. 2006;13:2795–2818. doi: 10.2174/092986706778521968. [DOI] [PubMed] [Google Scholar]
- Lakum H.P., Shah D.R., Chikhalia K.H. The Novel derivatives of 3-(iminomethyl)-2H-chromen-2-one with thiourea and piperazine structural motive: rationale, synthesis, antimicrobialand anti-TB evaluation. Lett. Drug Des. Discov. 2015;12:324–341. [Google Scholar]
- Lee J.H., Lee K.T., Yang J.H., Baek N.I., Kim D.K. Acetylcholinesteraseinhibitors from the twigs of Vaccinium oldhami Miquel. Arch. Pharm. Res. 2004;27:53–56. doi: 10.1007/BF02980046. [DOI] [PubMed] [Google Scholar]
- Li G., Wang D., Sun M., Li G., Hu J., Zhang Y., Yuan Y., Ji H., Chen N., Liu G. Discovery and optimization of novel 3-piperazinylcoumarin antagonist of chemokine-like factor 1 with oral antiasthma activity in mice. J. Med. Chem. 2010;53:1741–1754. doi: 10.1021/jm901652p. [DOI] [PubMed] [Google Scholar]
- Loizzo M.R., Tundis R., Menichini F., Menichini F. Natural products and theirderivatives as cholinesterase inhibitors in the treatment ofneurodegenerative disorders: an update. Curr. Med. Chem. 2008;15:1209–1228. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- Mandala D., Valeru A., Pochampalli J., Vankadari S.R., Tigulla P., Gatla R., Thampu R. Synthesis, antimicrobial activity, and molecular modeling of novel 4-(3-(4-benzyl piperazin-1-yl)propoxy)-7-methoxy-3-substituted phenyl-2H-chromen-2-one. Med. Chem. Res. 2013;22:5481–5489. [Google Scholar]
- Martin R.J. Modes of action of anthelmintic drugs. Vet. J. 1997;154:11–34. doi: 10.1016/s1090-0233(05)80005-x. [DOI] [PubMed] [Google Scholar]
- Maxwell A. The interaction between coumarin drugs and DNA gyrase. Mol. Microbiol. 1993;9:681–689. doi: 10.1111/j.1365-2958.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Modh R.P., Kumar S.P., Jasrai Y.T., Chikhalia K.H. Design, synthesis, biological evaluation, and molecular modeling of coumarin-piperazine derivatives as acetylcholinesterase inhibitors. Arch. Pharm. Chem. Life Sci. 2013;346:793–804. doi: 10.1002/ardp.201300242. [DOI] [PubMed] [Google Scholar]
- Montagner C., de Souza S.M., Groposoa C., Delle Monache F., Smania E.F., Smania A., Jr. Antifungal activity of coumarins. Z. Naturforsch. C. 2008;63:21–28. doi: 10.1515/znc-2008-1-205. [DOI] [PubMed] [Google Scholar]
- Mori H. Untangling Alzheimer's disease from fibrous lesions of neurofibrillary tangles and senile plaques. Neuropathology. 2000;20:55–60. doi: 10.1046/j.1440-1789.2000.00317.x. [DOI] [PubMed] [Google Scholar]
- Musicki B., Periers A.M., Laurin P., Ferroud D., Benedetti Y., Lachaut S., Chatreaux F., Haesslein J.L., Iltis A., Pierre C., Khider J., Tessot N., Airault M., Demassey J., Dupuis-Hamelin C., Lassaigne P., Bonnefoy A., Vicat P., Klicha M. Improved antibacterial activities of coumarin antibiotics bearing 50,50-dialkylnoviose: biological activity of RU79115. Bioorg. Med. Chem. Lett. 2000;10:1695–1699. doi: 10.1016/s0960-894x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- Mustafa M.S., El-Abadelah M.M., Zihlif M.A., Naffa R.G., Mubarak M.S. Synthesis, and antitumor activity of some N1-(coumarin-7-yl) amidrazones and aelated congeners. Molecules. 2011;16:4305–4317. doi: 10.3390/molecules16054305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikalje A.P.G., Tiwari S.V., Tupe J.G., Vyas V.K., Qureshl G. Ultrasound assisted-synthesis and biological evaluation of piperazinylprop-1-en-2-yloxy-2H-chromen-2-ones as cytotoxic agents. Lett. Drug Des. Discov. 2017;14:1195–1205. [Google Scholar]
- Opherk, D., Schuler, G., Waas, W., Dietz, R., Kubler, W., 1990. Intravenous carbochromen: a potent and effective drug for estimation of coronary dilatory capacity. 11, 342–347. DOI: 10.1093/oxfordjournals.eurheartj.a059708. [DOI] [PubMed]
- Orhan I., Tosun F., Sener B. Coumarin, anthraquinone and stilbenederivatives with anticholinesterase activity. Z. Naturforsch. C. 2008;63:366–370. doi: 10.1515/znc-2008-5-610. [DOI] [PubMed] [Google Scholar]
- Ostrowska K., Grzeszczuk D., Głuch-Lutwin M., Gryboś A., Siwek A., Dobrzycki Ł., Trzaskowski B. Development of selective agents targeting serotonin 5HT1A receptors with subnanomolar activities based on a coumarin core. Med. Chem. Comm. 2017;8:1690–1696. doi: 10.1039/c7md00281e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowska K., Młodzikowska K., Głuch-Lutwin M., Gryboś A., Siwek A. Synthesis of a new series of aryl/heteroarylpiperazinyl derivatives of 8-acetyl-7-hydroxy-4-methylcoumarin with low nanomolar 5-HT1A affinities. Eur. J. Med. Chem. 2017;137:108–116. doi: 10.1016/j.ejmech.2017.05.047. [DOI] [PubMed] [Google Scholar]
- Ostrowska K., Grzeszczuk D., Głuch-Lutwin M., Gryboś A., Siwek A., Leśniak A., Sacharczuk M., Trzaskowski B. 5-HT1A and 5-HT2A receptors affinity, docking studies and pharmacological evaluation of a series of 8-acetyl-7-hydroxy-4-methylcoumarin derivatives. Bioorg. Med. Chem. 2018;26:527–535. doi: 10.1016/j.bmc.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Ottesen E.A., Duke B.O.L., Karam M., Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. B. World Health Organ. 1997;75:491–503. PMID:9509621 PMCID:PMC2487030. [PMC free article] [PubMed] [Google Scholar]
- Patel D., Kumari P., Patel N. Synthesis of 3-{4-[4-dimethylamino-6-(4-methyl-2-oxo-2Hchromen-7-yloxy)-[1,3,5]triazin-2-ylamino]-phenyl}-2-phenyl-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-thiazolidin-4-one and their biological evaluation. Med. Chem. Res. 2012;21:2926–2944. [Google Scholar]
- Patel D., Patel R., Kumari P., Patel N. Microwave-assisted synthesis of coumarin based 1,3,5-triazinyl piperazines and piperidines and their antimicrobial activities. Acta Polon. Pharm. Drug Res. 2012;69:879–891. [PubMed] [Google Scholar]
- Patel D., Patel R., Kumari P., Patel N. In vitro antimicrobial assessment of coumarin-based s-triazinyl piperazines. Med. Chem. Res. 2012;21:1611–1624. [Google Scholar]
- Razavi S.F., Khoobi M., Nadri H., Sakhteman A., Moradi A., Emami S., Foroumadi A., Shafiee A. Synthesis and evaluation of 4-substituted coumarins as novel acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2013;64:252–259. doi: 10.1016/j.ejmech.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Rollinger J.M., Hornick A., Langer T., Stuppner H., Prast H. Acetylcholinesterase inhibitory activity of scopolin and scopoletindiscovered by virtual screening of natural products. J. Med. Chem. 2004;47:6248–6254. doi: 10.1021/jm049655r. [DOI] [PubMed] [Google Scholar]
- Salvo F., Bezin J., Bosco-Levy P., Letinier L., Blin P., Pariente A., Moore N. Pharmacological treatments of cardiovascular diseases: evidence from real-life studies. Pharmacol. Res. 2017;118:43–52. doi: 10.1016/j.phrs.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Santana L., Uriarte E., Fall Y., Teijeira M., Teran C., García-Martínez E., Tolf B.R. Eur. J. Med. Chem. 2002;37:503–510. doi: 10.1016/s0223-5234(02)01357-0. [DOI] [PubMed] [Google Scholar]
- Schio L., Chatreaux F., Loyau V., Murer M., Ferreira A., Mauvais P., Bonnefoy A., Klicha M. Fine tuning of physico-chemical parameters to optimise a new series of novobiocin analogues. Bioorg. Med. Chem. Lett. 2001;11:1461–1464. doi: 10.1016/s0960-894x(01)00257-8. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J., Podlisny M.B. Deciphering the genetic basis of Alzheimer's disease. Annu. Rev. Genom. Hum. G. 2002;3:67–99. doi: 10.1146/annurev.genom.3.022502.103022. [DOI] [PubMed] [Google Scholar]
- Shih-Hsien L., Lan-Ting L., Yen K.Y. Serotonin and mental disorders: a concise review on molecular neuroimaging evidence. Clin. Psychopharmacol. Neurosci. 2014;12:196–202. doi: 10.9758/cpn.2014.12.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonis H., Wenzel G. Tribromine coumarin and some of its derivetives. Ber. Dtsch. Chem. Ges. 1900;33:421–425. [Google Scholar]
- Skalicka-Wozniak K., Orhan I.E., Cordell G.A., Nabavi S.M., Budzynska B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016;103:188–203. doi: 10.1016/j.phrs.2015.11.023. [DOI] [PubMed] [Google Scholar]
- Sun M., Hu J., Song X., Wu D., Kong L., Sun Y., Wang D., Wang Y., Chen N., Liu G. Coumarin derivatives protect against ischemic brain injury in rats. Eur. J. Med. Chem. 2013;67:39–53. doi: 10.1016/j.ejmech.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Teran C., Santana L., Uriarte E., Fall Y., Unelius L., Tolf B.R. Phenylpiperazine derivatives with strong affinity for 5-HT1A, D2A and D3 receptors. Bioorg. Med. Chem. Lett. 1998;8:3567–3570. doi: 10.1016/s0960-894x(98)00646-5. [DOI] [PubMed] [Google Scholar]
- Tripathi, R.P., Khan, A.R., Singh, S.N., Murthy, P.K., Chatterjee, R.K., Bhaduri, A.P., 1997.A process for the preparation of 4-alkyl-7-0 [N,N-di(mono)substituted acetamide-2-yl-2H -1-benzopyran-2-ones useful as filaricidal agent. Indian Patent Application. No. 1265/DEL/97/13/05/97.
- Tripathi R.P., Tiwari V.K., Misra-Bhattacharya S., Tyagi K., Srivastava V.M.L., Murthy P.K. 7-O-[4-methyl piperazine-1-(2-acetyl)]-2H-1-benzopyran-2-one: a novel antifilarial lead compound. Acta Trop. 2003;87:215–224. doi: 10.1016/s0001-706x(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Trykowska Konc J., Hejchman E., Kruszewska H., Wolska I., Maciejewska D. Synthesis and pharmacological activity of O-aminoalkyl derivatives of 7-hydroxycoumarin. Eur. J. Med. Chem. 2011;46:2252–2263. doi: 10.1016/j.ejmech.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Wang S.F., Yin Y., Wu X., Qiao F., Sha S., Lv P.C., Zhao J., Zhu H.L. Synthesis, molecular docking and biological evaluation of coumarin derivatives containing piperazine skeleton as potential antibacterial agents. Bioorg. Med. Chem. 2014;22:5727–5737. doi: 10.1016/j.bmc.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Selectivity of cholinesterase inhibition. CNS Drugs. 1999;12:307–323. [Google Scholar]
- Xu Q., Pan Y., Yi L.T., Li Y.C., Mo S.F., Jiang F.X., Qiao C.F., Xu H.X., Lu X.B., Kong L.D., Kung H.F. Antidepressant-like effects of psoralen isolated from theseeds of Psoralea corylifolia in the mouse forced swimming test. Biol. Pharm. Bull. 2008;31:1109–1114. doi: 10.1248/bpb.31.1109. [DOI] [PubMed] [Google Scholar]
- Yao D., Wang J., Wang G., Jiang Y., Shang L., Zhao Y., Huang J., Yang S., Wang J., Yu Y. Design, synthesis and biological evaluation of coumarin derivatives as novel acetylcholinesterase inhibitors that attenuate H2O2-induced apoptosis in SH-SY5Y cells. Bioorg. Chem. 2016;68:112–123. doi: 10.1016/j.bioorg.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Yang Y.J., Lee H.J., Huang H.S., Lee B.K., Choi H.S., Lim S.C., Lee C.K., Lee M.K. Effects of scoparone on dopamine biosynthesis and L-DOPA-inducedcytotoxicity in PC12 cells. J. Neurosci. Res. 2009;87:1929–1937. doi: 10.1002/jnr.22009. [DOI] [PubMed] [Google Scholar]
- Zhang J., Jiang C.S. Synthesis and evaluation of coumarin/piperazine hybrids as acetylcholinesterase inhibitors. Med. Chem. Res. 2018;27:1717–1727. [Google Scholar]
- Żołek T., Dömötör O., Ostrowska K., Enyedy E.A., Maciejewska D. Evaluation of blood-brain barrier penetration and examination of binding to human serum albumin of 7-O-arylpiperazinylcoumarins as potential antipsychotic agents. Bioorg. Chem. 2019;84:211–225. doi: 10.1016/j.bioorg.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Żołek T., Enyedy E.A., Ostrowska K., Pósa V., Maciejewska D. Drug likeness prediction of 5-hydroxy-substituted coumarins with high affinity to 5-HT1A and 5-HT2A receptors. Eur. J. Pharm. Sci. 2018;115:25–36. doi: 10.1016/j.ejps.2018.01.011. [DOI] [PubMed] [Google Scholar]