Abstract
Background
A physiological hallmark of patients with type 2 diabetes mellitus (T2DM) is β cell dysfunction. Despite adequate treatment, it is an irreversible process that follows disease progression. Therefore, the development of novel therapies that restore β cell function is of utmost importance.
Methods
This study aims to unveil the mechanistic action of mesenchymal stem cells (MSCs) by investigating its impact on isolated human T2DM islets ex vivo and in vivo.
Findings
We propose that MSCs can attenuate β cell dysfunction by reversing β cell dedifferentiation in an IL-1Ra-mediated manner. In response to the elevated expression of proinflammatory cytokines in human T2DM islet cells, we observed that MSCs was activated to secret IL-1R antagonist (IL-1Ra) which acted on the inflammed islets and reversed β cell dedifferentiation, suggesting a crosstalk between MSCs and human T2DM islets. The co-transplantation of MSCs with human T2DM islets in diabetic SCID mice and intravenous infusion of MSCs in db/db mice revealed the reversal of β cell dedifferentiation and improved glycaemic control in the latter.
Interpretation
This evidence highlights the potential of MSCs in future cell-based therapies regarding the amelioration of β cell dysfunction.
Keywords: Mesenchymal stem cells, Type 2 diabetes mellitus, β cell dysfunction, Inflammation, β cell dedifferentiation
Research in context.
Evidence before this study
β cell dysfunction is a physiological hallmark of patients with type 2 diabetes mellitus (T2DM). One key mechanism for β cell dysfunction is β cell dedifferentiation, which eventually causes the loss of maturity and insulin secretion ability of β cells. T2DM is an inflammation-related disease and islet inflammation impairs β cell function. Presently, MSCs represent an important paradigm of cell-based therapy for a range of inflammation- and immunity-related diseases due to their superior anti-inflammation and immunomodulatory abilities. However, the impact and mechanistic action of MSCs on human T2DM islets remain elusive.
Added value of this study
In the present study, we demonstrated that β cell dysfunction in human T2DM islets was ameliorated following MSCs treatment both ex vivo and in vivo. We found that elevated expression of IL-1β and TNF-α in human T2DM islets can activate MSCs to secrete IL-1Ra, a requirement for MSCs to restore the function of hT2DM islets. This functional improvement of human T2DM islets by MSCs is due to the reversal of β cell dedifferentiation.
Implications of all the available evidence
This study demonstrated MSCs’ beneficial effect on improving the β cell function by reversing β cell dedifferentiation in human T2DM islets. This finding implies that MSCs administration can be a potential approach to hinder or reverse β cell dedifferentiation and hence may offer a therapeutic strategy for the protection or restoration of β cell function in T2DM patients.
Alt-text: Unlabelled box
1. Introduction
There is an increasing number of people affected by type 2 diabetes mellitus (T2DM), which places a significant socioeconomic burden on both the individual and health care systems globally. Altogether, an estimated 425 million people are affected by diabetes mellitus worldwide [1], with over 90% of patients comprised of T2DM. β cell dysfunction is a hallmark of T2DM that follows the disease progression [2], which interestingly, appears to be especially prominent in East Asian populations [3]. Unfortunately, there is a lack of appropriate therapies in mitigating β cell dysfunction.
Although the causes of β cell dysfunction are multifactorial [4], inflammation, especially the local inflammation in the islets, is thought to play a critical role in the pathogenesis of β cell defects [5], [6], [7], [8], [9], [10]. Glucotoxicity, lipotoxicity, oxidative and amyloid stress are prevailing contributors in T2DM islet dysfunction through direct and indirect inflammatory responses [6,[11], [12], [13], [14], [15]]. Accordingly, therapies that target inflammation have demonstrated improvement in β cell function. For instance, the administration of an IL-1 antagonist was shown to reduce HbA1c in T2DM patients [16]. Diacerein, which decreases both IL-1β and TNF-α demonstrated improved insulin secretion of T2DM patients [17,18]. Moreover, the COX-2 inhibitor Celecoxib was shown to improve insulin secretion of human islets [19]. Together, these studies suggest that targeting the inflammatory response is critical in mitigating β cell dysfunction.
One strategy in modulating excessive inflammation involves the use of mesenchymal stem cells (MSCs). Previous clinical trials have explored MSCs as a potential cell-based therapy in treating various diseases with promising results that demonstrate their superior anti-inflammatory, immunomodulatory and angiogenic properties [20], [21], [22], [23]. Although several clinical trials have shown that MSCs can reduce hyperglycaemia by increasing insulin secretion in T2DM patients [24], [25], [26], [27], [28], [29], the mechanistic action of MSCs on human T2DM islets remains unknown.
The present study utilizes human umbilical cord MSCs and purified human T2DM (hT2DM) islets to unveil its parallel relationship. We report that MSCs can mitigate the β cell dysfunction of hT2DM islets by reversing β cell dedifferentiation through a crosstalk between the two, a phenomenon that is absent in human non-diabetic (hND) islets. This crosstalk mediates MSCs-secreted IL-1Ra, reducing the overall inflammatory response in hT2DM islets. In vivo studies revealed the beneficial effect of MSCs on hT2DM islets, which further highlights its translational potential in treating β cell dysfunction.
2. Materials and methods
2.1. Human islets isolation and culture
Human pancreata were obtained between Dec. 2016 to Dec. 2018 from 17 T2DM and 12 ND organ donors with informed consent for research. Organ donor information was obtained and displayed in the Table 1. The protocol of this study was approved by the Medical Ethics Committee of the Tianjin First Central Hospital (No.:2016N066KY). hND or hT2DM islets were isolated by Collagenase NB1 (SERVA, Heidelberg, Germany) and Neutral Protease NB (SERVA, Heidelberg, Germany) digestion followed by continuous density purification. High purity islets (>90%) were collected and cultured on CMRL-1066 medium (Corning, Manassas, VA, USA), supplemented with 10% Human Serum Albumin (Baxter, Vienna, Austria), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in 5% CO2.
Table 1.
Donor information.
| Donor Characteristics | Age (y) | BMI (kg/m2) | HbA1c (%) | Male (%) | Female (%) |
|---|---|---|---|---|---|
| ND (N = 12) | 45.90±8.69 | 25.33±4.78 | 5.38±0.20 | 91.67% | 8.33% |
| T2DM (N = 17) | 52.53±9.27 | 25.34±2.50 | 7.76±1.45 | 76.47% | 23.53% |
| p value | 0.0783 | 0.9933 | 0.0002 |
2.2. Human umbilical cord MSCs isolation
Human umbilical cord tissues were obtained during Dec. 2016 to Dec. 2018 from healthy post-natal females with informed consent for research. The Warton Jelly was cut into 1–3 mm3 pieces and cultured in Human MSC Serum-Free Medium (TBD, Tianjin, China) with 100 U/mL penicillin and 100 μg/mL streptomycin. MSCs that were positive for the mesenchymal markers CD45, CD90, CD73, CD105 (>95%) and negative for hematopoietic markers CD34 and CD45 (<5%) at passage 3–6 were selected for experimental use.
2.3. Coculture of islets and MSCs
500 hND or hT2DM islets were placed in the upper transwell insert with a 0.4 μm pore size (Corning, Manassas, VA, USA) and 5 × 104 MSCs pre-seeded in the bottom well were cocultured for 24 h prior to further analyses.
2.4. Insulin secretion assay
10 hND or hT2DM islets were pre-treated in a low-glucose (1.67 mM) Krebs-Ringer bicarbonate buffer (KRB; supplemented with 0.5% BSA) for 1 h, followed by an 1 h treatment with 1 mL low-glucose KRB solution and 1 mL high-glucose KRB solution (16.7 mM). Insulin concentration at low and high glucose was measured by ELISA (Mercodia, Uppsala, Sweden). Insulin secretion was measured and expressed as the glucose stimulated index (GSI; insulin concentration at high glucose/insulin concentration at low glucose). GSI of control group was arbitrarily set to 1, and that of treatment groups were expressed as fold change compared with that of the control group.
2.5. Neutralization of IL-1Ra
In hT2DM islet and MSCs coculture system, anti-IL-1Ra antibody (Abcam, Cambridge, UK) at a concentration of 500 ng/mL was added to neutralize IL-1Ra for 24 h.
2.6. Knockdown of IL-1Ra in MSCs
Recombinant lentivirus containing shRNAs targeting IL-1Ra (GCCCGTCAGCCTCACCAATAT, GGTACCCATTGAGCCTCATGC, and GCCTGTTCCCATTCTTGCATG) or a scramble sequence (shNC: TTCTCCGAACGTGTCACGT) (GenePharma, Shanghai, China) were used to infect MSCs at 40% confluence according to the manufacturer's recommended protocol (http://www.genepharma.com/public/upload/1495416183.pdf). Puromycin resistant cells with positive GFP expression were harvested for qPCR to determine IL-1Ra expression.
2.7. Stimulation of MSCs
500 hND or hT2DM islets were cultured in CMRL-1066 medium for 24 h, and then the culture medium of islets was collected as conditioned media (hND-CM, or hT2DM-CM). At roughly 80% confluency, MSCs were either cultured in CMRL-1066 medium, islet-conditioned media, or cocultured with islets for 24 h, followed by qPCR analyses.
MSCs at ~80% confluence were either treated with 2.5/5/10 ng/mL IL-1β, 25/50/100 ng/mL TNF-α, 25/50/100 ng/mL, IL-6 for 6 h and 12 h. MSCs and culture supernatants were harvested and analysed by qPCR and ELISA (R&D, Minneapolis, MN, USA), respectively.
2.8. RNA extraction, RT-PCR and qPCR
RNA extraction and cDNA synthesis was performed using the RNeasy Mini Kit (QIAGEN, Dusseldorf, Germany) and PrimeScript RT reagent Kit with GDNA Eraser (Takara, Kohoku-cho, Kusatsu, Japan) respectively. Quantitative real-time qPCR was measured with SYBR Premix ExTaq II (Takara, Kohoku-cho, Kusatsu, Japan) using LightCycler96 System (Roche, Basel, Switzerland). Relative mRNA expression of different treatments was calculated by the 2−△△CT method. Relative mRNA expression between hND and T2DM islets was calculated by 2−△CT. The primers sequences are shown in Table S1.
2.9. MSCs and hT2DM islets co-transplantation
All mice were fed normal chow and maintained on a 12-hour light–dark cycle (lights on at 7:00 AM). The Nankai University Institutional Animal Care and Utilization Committee approved all experiments. SCID mice (8–10 weeks) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China) and administrated with streptozotocin (STZ, 150 mg kg−1; S0130, Sigma) by intraperitoneal injection. Seven days after injection, mice exhibiting hyperglycemia (>20 mM) were selected for use in subsequent experiments. MSCs and isolated hT2DM islets were cotransplanted to the kidney capsule of diabetic SCID mice (1500 IEQ+1 × 106 MSCs/mouse). 2 weeks after transplantation, the islet graft and kidney were harvested for immunohistochemical analyses.
2.10. MSCs treatment of db/db mice
C57BL/KsJ-db/db mice (male) and their respective controls were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). 1 × 106 MSCs in 0.2 mL PBS were injected to each mouse in the MSCs treatment group (db/db MSC, n = 9) via tail vein at 7 weeks and 9 weeks of age, respectively. Injection of equal volume of PBS was applied for the mice in control group (db/db Ctrl, n = 8). Blood glucose level, weight, and food intake were monitored every week. The mice were sacrificed at 11-weeks of age after an oral glucose tolerance test (OGTT). Pancreata were harvested for further immunohistology analysis. For OGTT, the mice were fasted for 18 h and subsequently administered with a glucose solution (1 g/kg) by intragastric gavage. Blood glucose levels were determined at 0 (baseline), 15, 30, 60, 90 and 120 min and the area under the curve (AUC) was calculated.
2.11. Immunofluorescence staining and image quantifications
Human islets and mice pancreatic tissues were fixed in 4% paraformaldehyde, embedded with paraffin and sectioned (3 µm). After deparaffinization, sections were treated with EDTA antigen retrieval solution (Solarbio, Beijing, China) in a microwave oven, washed, permeabilized and blocked. This was followed by incubation of primary antibodies and secondary antibodies. Immunohistochemical staining was performed using insulin (1:200, Abcam, Cambridge, MA, USA), glucagon (1:200, Abcam, Cambridge, MA, USA), NKX6.1 (1:500, Novus, Carpinteria, CA, USA), ALDH1A3 (1:500, Novus, Carpinteria, CA, USA), PDX1 (1:50, Cell Signaling Technology, Danvers, MA, USA), FOXO1 (1:100, LifeSpan BioSciences, Seattle, WA, USA) primary antibodies, along with FITC AffiniPure Goat Anti-Rabbit IgG H&L (1:200, Jackson Immunoresearch Laboratories and Molecular Probes, West Grove, PA, USA), TRITC AffiniPure Goat Anti-Rabbit IgG H&L (1:100, Jackson Immunoresearch Laboratories and Molecular Probes, West Grove, PA, USA), Alexa Fluor 488 AffiniPure Goat Anti-Guinea pig IgG H&L (1:200, Abcam, Cambridge, MA, USA) secondary antibodies. Counterstaining was performed with DAPI (Vector, Burlingame, CA, USA).
Sections were counterstained with DAPI (Vector, Burlingame, CA, USA). Pannoramic MIDI and Pannoramic Viewer (3DHistech) were used to scan stained slides and capture images. Two observers (T.L. and Y.L.) performed the quantification in a blinded fashion using the CytoNuclear count function of the Image Pro-Plus software (Media Cybernetics, Silver Spring, Maryland). The mean total cell counts per islets were 109.7 ± 33.83 (mean ± SD). The cell counts of each cell type were normalized as the cell percentage in each islet by the formula: mean β cell percentage per islet = average (β cell number/total islet cell number) X 100%. Islets containing at least one positive stained protein of interest were scored. Only cells that had a clearly labelled nucleus, positive cells for each marker or demonstrated colocalization of different markers were included. At least 3 random microscopic fields per sample and 5 random islets per microscopic field were scored.
2.12. Statistical analysis
Data processing were performed using GraphPad Prism v7.0. Two-tailed Student's t-test was used for data analysis and p< 0.05 were considered statistically significant. Quantitative data were shown as mean ± SEM when n<10, and Turkey's box-whisker when n>10.
3. Results
3.1. MSCs improve β cell function of hT2DM islets, but not hND islets
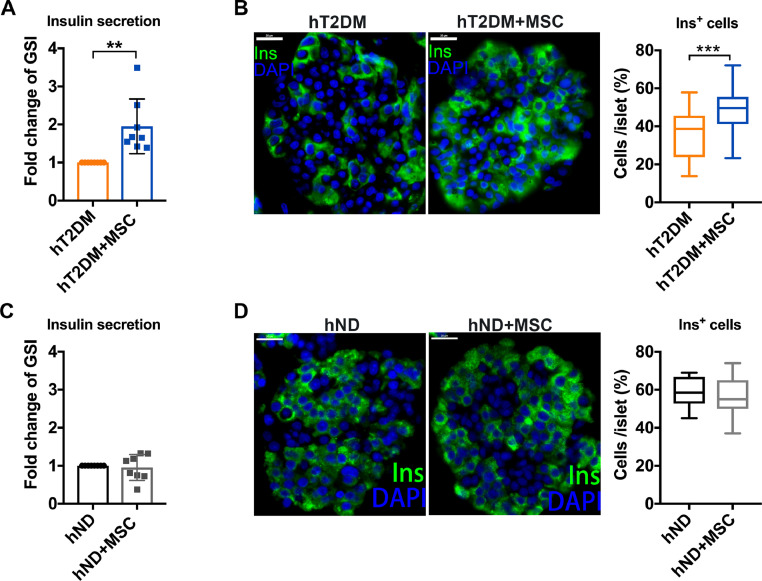
To determine the effect of MSCs on hT2DM and hND islets, islet cells were cocultured with MSCs for 24 h in a transwell device. The MSCs coculture demonstrated a significant elevation in the glucose stimulated index (GSI) of hT2DM islets (p<0.01, Fig. 1A), but not in the hND islets (Fig. 1C). Likewise, immunofluorescence staining demonstrated a significant elevation of insulin positive cells (Ins+ cells) by MSCs coculture (p<0.001, Fig. 1B), whereas hND islets did not (Fig. 1D). Using a FDA/PI assay, the MSCs coculture did not significantly improve the survival of hT2DM islets within 24 h (Figure S1), ruling out potential confounding variables associated with cell viability. Furthermore, when two non-MSC cells (HEK293T and HL7702) were cocultured with hT2DM islets, GSI did not show improvement (Figure S2). This reiterates the notion that the observed functional improvement of hT2DM islets is specific to MSCs.
Fig. 1.
MSCs coculture improves β cell function of hT2DM islets. (A)Glucose stimulated index (GSI) of hT2DM islets cocultured with or without MSCs coculture. GSI of control group was arbitrarily set to 1, and that of treatment group was expressed as fold change compared with that of control group. Data were shown as mean±SEM of GSI fold change. n = 8. (B) Immunofluorescence with Insulin (Ins, green) and DAPI (blue) of hT2DM islets with or without MSCs coculture. Scale bars = 20 μm. Quantification of Ins+cells per islet is shown as mean±SEM in at least 45 islets from 3 donors (at least 15 islets per donor). (C)GSI of hND islets cocultured with or without MSCs coculture. Data were shown as mean±SEM of GSI fold change. n = 8. (D) Immunofluorescence with Insulin (Ins, green) and DAPI (blue) of hT2DM islets with or without MSCs coculture. Scale bars = 20 μm. Quantification of Ins+cells per islet is shown as mean±SEM of at least 45 islets from 3 donors (at least 15 islets per donor). **p < 0.01, ***p < 0.001.
3.2. hT2DM islets, but not hND islets, stimulate MSCs to secrete IL-1Ra
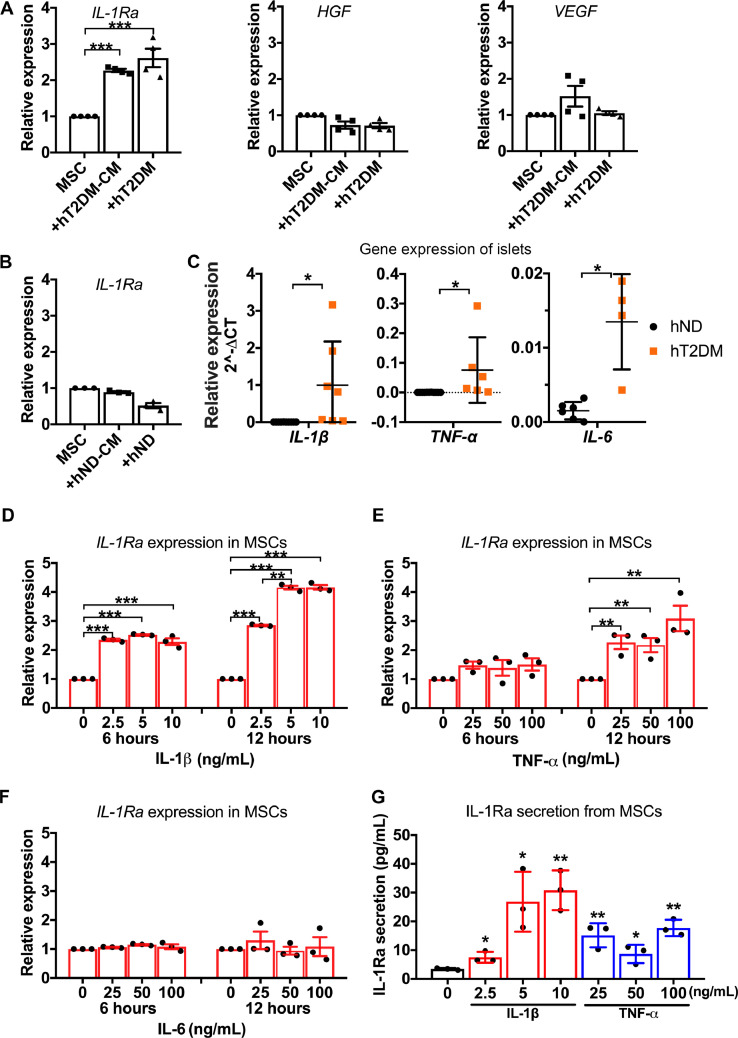
With the absence of direct contact between MSCs and hT2DM islets, we hypothesized that MSCs secrete soluble factors that are trafficked through the transwell device, mediating MSCs’ effect on hT2DM islets. Thus we compared the gene expressions of several factors including hepatocyte growth factor (HGF) [30], vascular endothelial growth factor (VEGF) [31], and IL-1Ra [32], all of which have been previously reported as factors secreted by MSCs. Only IL-1Ra was significantly induced by hT2DM islets or their conditioned media in comparison to unstimulated-MSCs (Fig. 2A). Unlike hT2DM islets, hND islets did not induce the expression of IL-1Ra in MSCs (Fig. 2B).
Fig. 2.
Human T2DM islets, but not ND islets, stimulate MSCs to secrete IL-1Ra. (A) Relative mRNA expression of IL-1Ra, VEGF, HGF in MSCs with the treatment of hT2DM islets conditioned medium (+hT2DM-CM), coculture with hT2DM islets (+hT2DM). Data were shown as mean±SEM of 4 independent experiments with islets from 2 donors. (B) Relative mRNA expression of IL-1Ra in MSCs with treatment of hND islets conditioned medium (+hND-CM), or coculture with hND islets (+hND). Data were shown as mean±SEM of 4 independent experiments with islets from 2 donors. (C)IL-1β, TNF-α,andIL-6 expression in hT2DM islets in comparison to hND islets (n = 6 for hND, n = 4–7 for hT2DM). (D-F)IL-1Ra expression in MSCs treated with IL-1β (D), TNF-α (E), or IL-6 (F) for 6 h or 12 h. (G) IL-1Ra secretion from MSCs treated with IL-1β or TNF-α for 12 h. n = 3. *p<0.05, **p<0.01, ***p<0.001.
3.3. IL-1β and TNF-α, highly expressed in hT2DM islets, activate MSCs to secrete IL-1Ra
We found that IL-1β expression was significantly higher in hT2DM islets relative to hND islets (Fig. 2C). Though less prominent, TNF-α and IL-6 also exhibited significantly higher expression in hT2DM islets in comparison to hND islets (Fig. 2C). To determine the role of these inflammatory cytokines in activating MSCs, we exposed MSCs to either IL-1β, TNF-α, or IL-6 for 6 h and 12 h. We observed that the IL-1β treatment significantly induced IL-1Ra expression in MSCs (Fig. 2D) at 6 h and 12 h. TNF-α slightly provoked IL-1Ra expression at 6 h but prolonged treatment with TNF-α (ie. 12 h) demonstrated significant activation of IL-1Ra (Fig. 2E). IL-1Ra expression from MSCs was not significantly elevated following IL-6 exposure (Fig. 2F). ELISA further confirmed that a 12-hour treatment of IL-1β and TNF-α enhanced IL-1Ra secretion from MSCs (Fig. 2G).
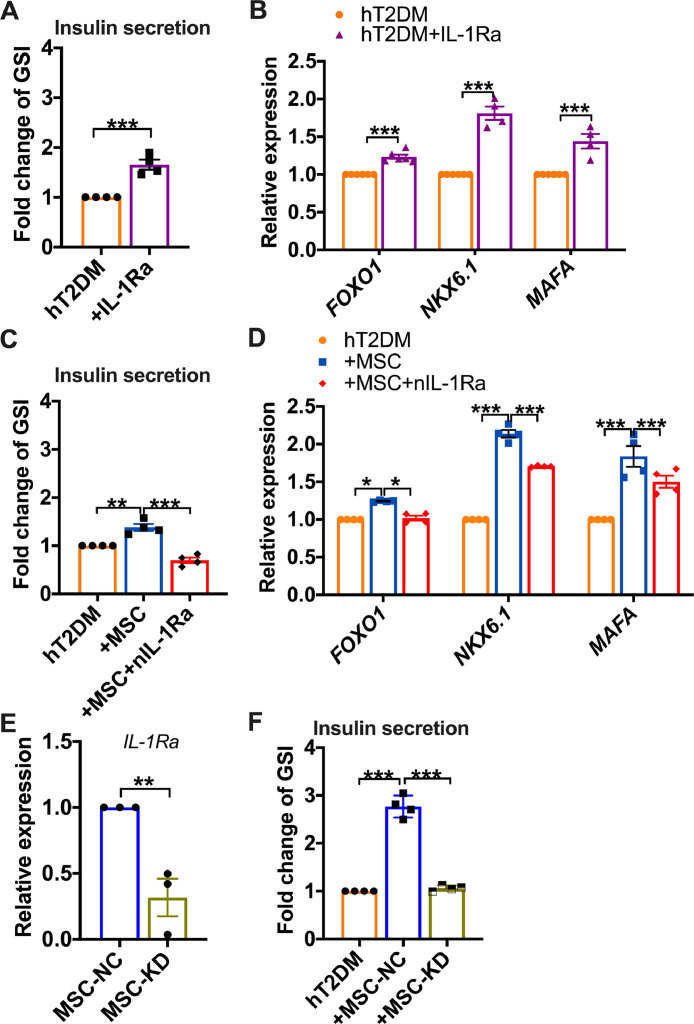
3.4. IL-1Ra mediates the functional improvement of hT2DM islets by MSCs
Using IL-1Ra to treat hT2DM islets, we found that IL-1Ra treatment significantly elevated GSI (p<0.01, Fig. 3A) and increased the gene expression of FOXO1, NKX6.1, and MAFA of hT2DM islets (Fig. 3B). To confirm this effect, we used a neutralizing antibody (nIL-1Ra) to block the function of IL-1Ra. As predicted, nIL-1Ra abrogated GSI improvement (p<0.05, Fig. 3C) and nullified the elevated expression of β cell functional genes in hT2DM islets by MSCs (Fig. 3D). Previous GSI-improvement was also reversed when shRNAs were used to specifically knock down IL-1Ra expression in MSCs (p<0.01, Figs. 3E and 3F). These results demonstrate that MSCs ameliorated the β cell dysfunction in an IL-1Ra dependent manner.
Fig. 3.
IL-1Ra mediates the functional improvement of human T2DM islets by MSCs. (A) GSI fold change of hT2DM islets with or without IL-1Ra treatment (1000 ng/mL) for 24 h. (B) Relative mRNA expression of FOXO1, NKX6.1, and MAFA in hT2DM islets with or without IL-1Ra treatment. (C) GSI fold change of hT2DM islets treated with MSCs coculture, MSCs coculture in the presence of neutralizing anti-IL-1Ra (nIL-1Ra, 500 ng/mL). (D) Relative mRNA expression of FOXO1, NKX6.1, MAFA in hT2DM islets treated with MSCs coculture, or MSCs coculture in the presence of neutralizing anti-IL-1Ra (nIL-1Ra, 500 ng /mL). (E) Relative mRNA expression of IL-1Ra in MSCs with IL-1Ra knockdown (MSC-KD) or control cells (MSC—NC). (F) GSI fold change of hT2DM islets cocultured with MSCs with negative control (+MSC—NC) or with IL-1Ra knockdown (+MSC-KD),or cultured alone. GSI of control group (hT2DM) was arbitrarily set to 1, and that of treatment groups were expressed as fold change compared with that of the control group. Data were shown as mean±SEM of 4 independent experiments with islets from 2 donors. *p<0.05, **p<0.01, ***p<0.001.
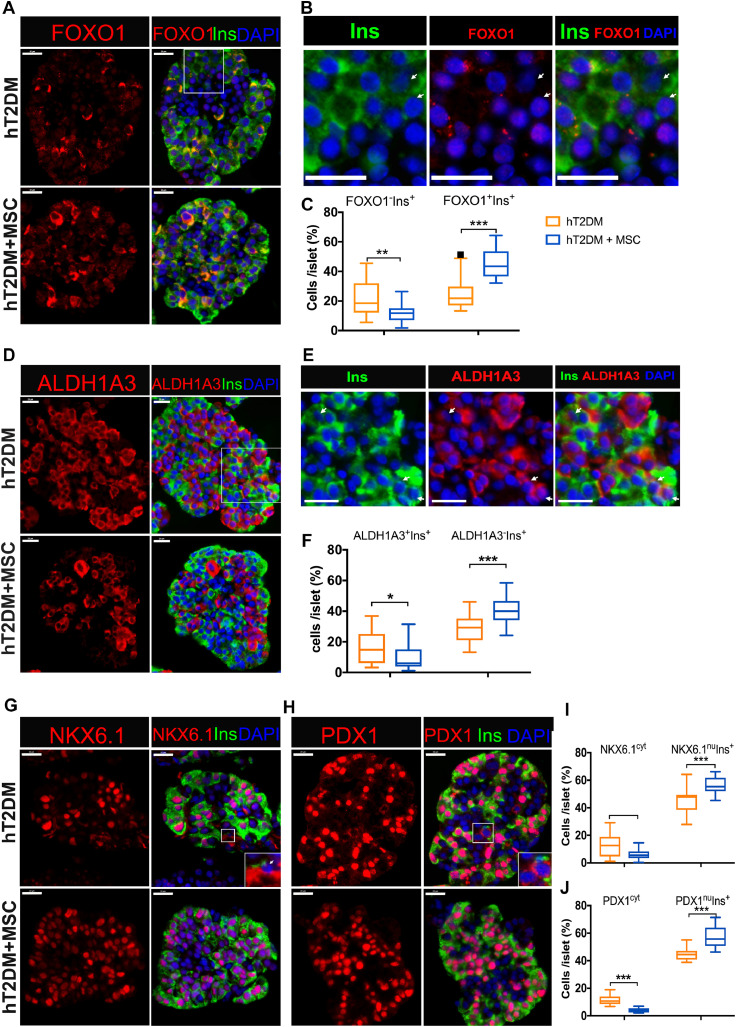
3.5. MSCs reverse β cell dedifferentiation in hT2DM islets
Current literature suggests that β cell dedifferentiation plays a central role in β cell dysfunction [13,[33], [34], [35], [36]]. Immunofluorescence staining revealed that the percentage of dedifferentiated β cells in hT2DM islets cocultured with MSCs was significantly reduced. This is evidenced by a decreased percentage of FOXO1−Ins+ cells (Fig. 4A-C) and ALDH1A3+Ins+cells (Fig. 4D-F), as well as a decreased percentage of cells with cytoplasmic expression of NKX6.1 (NKX6.1Cyt) (Fig. 4G&I) or with cytoplasmic expression of PDX1 (PDX1cyt) cells (Fig. 4H&J). In parallel, we observed a significant increase of the well-differentiated β cells (FOXO1+Ins+, ALDH1A3−Ins+, NKX6.1nuIns+, PDX1nuIns+) in MSCs cocultured islets (Fig. 4). This indicates that the MSCs coculture reversed β cell dedifferentiation in hT2DM islets.
Fig. 4.
MSCs reverse β cell dedifferentiation of hT2DM islets. (A) Immunofluorescence of FOXO1 (red), Insulin (Ins, green) and DAPI (blue) in hT2DM islets treated with MSCs coculture. (B) The amplified images of the white rectangle in (A), with white arrows showing the FOXO1−Ins+ cells. (C) FOXO1+Ins+ and FOXO1−Ins− cells percentage per islet. (D) Immunofluorescence with ALDH1A3 (red), Insulin (Ins, green) and DAPI (blue) hT2DM islets treated with MSCs coculture. (E) The amplified images of the white rectangle in (A), with white arrows showing the ALDH1A3+Ins+ cells. (F) ALDH1A3+Ins+ and ALDH1A−Ins+ cells percentage per islet. (G&H) Immunofluorescence with NKX6.1 (red) (G) or PDX1 (red) (H), Insulin (Ins, green) and DAPI (blue) of hT2DM islets with or without MSCs coculture. (I) Quantification of NKX6.1cyt and NKX6.1nuIns+ cells percentage per islet. (J) Quantification of PDX1cyt and PDX1nuIns+ cells percentage per islet. Scale bars=20 μm. Data were shown as mean±SEM of 45–60 islets from 3–4 donors (at least 15 islets per donor). cyt: cytoplasmic expression, nu: nucleic expression, *p<0.05, **p<0.01, ***p<0.001.
3.6. Co-transplantation of MSCs reverses the β dedifferentiation of with human T2DM islets
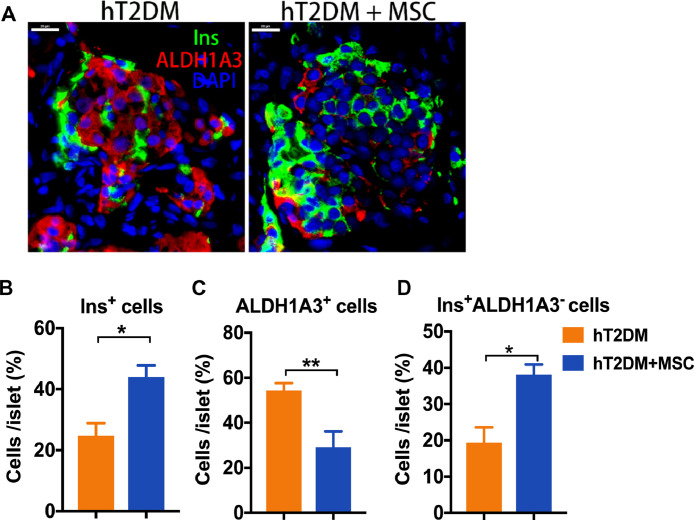
To determine whether the MSCs-mediated reversal of β cell dedifferentiation is true in hT2DM islets in vivo, we co-transplanted hT2DM islets with MSCs to the kidney capsule of diabetic SCID mice (STZ induced). Immunostaining of the islet grafts demonstrated a reduction in ALDH1A3+ and ALDH1A3+Ins+ cells in the MSCs co-transplantation group in comparison to the control group (Fig. 5). These results validated that the MSCs can reverse the β cell dedifferentiation of the hT2DM islets in vivo.
Fig. 5.
Cotransplantation with MSCs to SCID mice reversed β cell dedifferentiation of human T2DM islets. hT2DM islets were transplanted to STZ-induced diabetic SCID mice with or without MSCs cotransplantation. Immunofluorescence of ALDH1A3 (red), Insulin (Ins, green) and DAPI (blue) in hT2DM islet grafts of the two groups (A). Ins+, ALDH1A3+ and ALDH1A3−Ins+ cells percentage per islet were shown as mean±SEM(B-D). *p<0.05, **p<0.01, ***p<0.001.
3.7. MSCs transplantation reverses β cell dedifferentiation of db/db mice
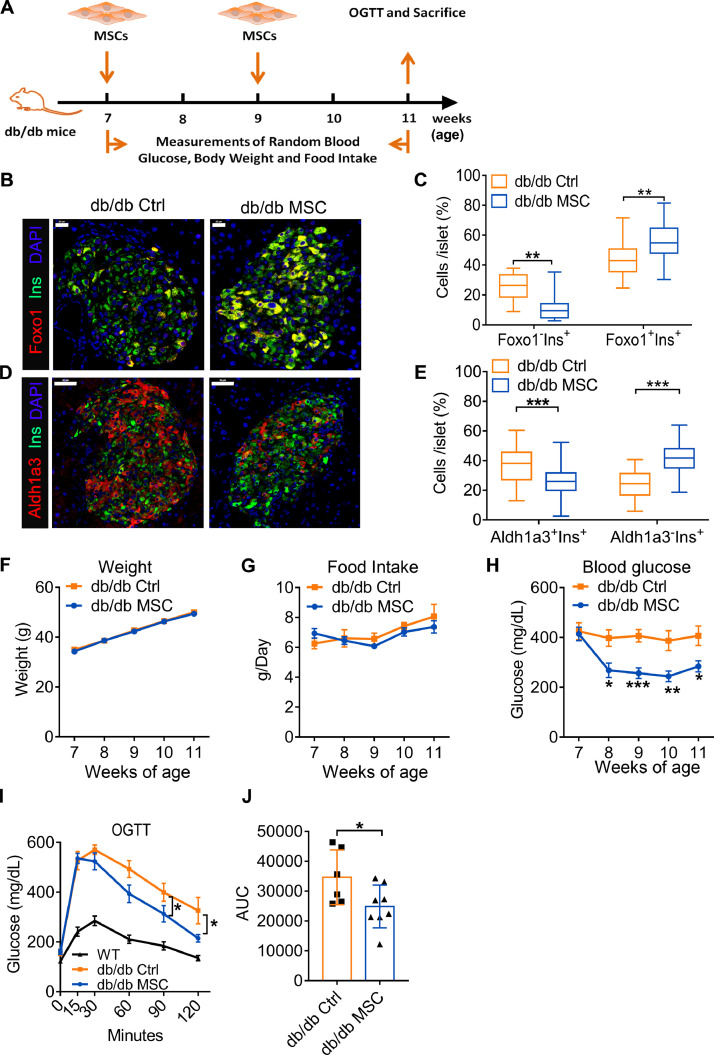
We administered MSCs to db/db mice, a well-established mouse model of hT2DM [37] and β cell dedifferentiation [38]. Two doses of MSCs were administered to db/db mice at the age of 7 weeks and 9 weeks using a tail vein injection and equal volume of PBS as a control treatment (Fig. 6A). Immunofluorescent analysis of mice pancreata demonstrated that MSCs transplantation reversed the β cell dedifferentiation of db/db mice in vivo. This was evidenced by a significantly decreased percentage of dedifferentiated β cells (Foxo1−Ins+ and Aldh1a3+Ins+cells) but increased percentage of well-differentiated β cells (Foxo1+Ins+ and Aldh1a3−Ins+ cells) in the MSCs treated group with respect to the control group (p<0.001, Fig. 6B-E).
Fig. 6.
MSCs transplantation reversed β cell dedifferentiation and improved β cell function of db/db mice. (A) Study design of the MSCs transplantation in db/db mice. (B) Immunofluorescence with Insulin (Ins, green), Foxo1 (red) and DAPI (blue) in db/db mice islets of the MSCs treatment group and the control group 4 weeks after treatment. Scale bars=20 μm. (C) Quantification of the percentage of Foxo1−Ins+ and Foxo1+Ins+ cells per islet (n = 5 per group). (D) Immunofluorescence with Insulin (Ins, green), Aldh1a3 (red) and DAPI (blue) of db/db mouse islets with MSCs treatment group and control group 4 weeks after treatment. Scale bars=20 μm. (E) Quantification of the percentage of Aldh1a3+Ins+ and Aldh1a3−Ins+cells per islet (n = 5 per group). (F) Weight of the db/db mice of the MSCs treatment group and control group (n = 8 per group). (G) Daily food intake of the db/db mice of the MSCs treatment group and control group (n = 8 per group). (H) Non-fasting glucose measurement of db/db mice in the MSCs treatment group and control group (n = 8 per group). (I) OGTT of the db/db mice of the MSCs treatment group and control group 4 weeks after the treatment. (J) AUC of the OGTT curve of the db/db mice of the MSCs treatment group and control group 4 weeks after the treatment (n = 6–8 per group). Data were shown as mean±SEM. *p<0.05, **p<0.01, ***p<0.001.
Consistent with reversal of MSCs-mediated β cell dedifferentiation in db/db mice, islet function was improved in mice with MSCs treatment in comparison to control mice. However, weight gain (Fig. 6F) and daily food intake (Fig. 6G) showed no significant difference between the two groups. In comparison to the control group, MSCs transplantation significantly decreased the non-fasting blood glucose one week after the first injection (p<0.05, Fig. 6H). OGTT results demonstrate that the blood glucose in the MSCs treated group was significantly lower than the control group at 90 min and 120 min (p<0.05, Fig. 6I) and the glucose tolerance was significantly reduced in the MSCs treated group (p<0.01, Fig. 6J). These results indicate improvement in islet function of db/db mice following MSCs treatment, further evidencing the reversal of β cell dedifferentiation by MSCs.
4. Discussion
The present study demonstrated that β cell dysfunction in hT2DM islets was ameliorated following MSCs treatment both ex vivo and in vivo. This effect can be attributed to the reversal of β cell dedifferentiation in hT2DM islets, a key mechanism behind β cell dysfunction in T2DM patients. Our study provides direct evidence for the improved β cell function by MSCs treatment, a finding that aligns with prior clinical trials that reported increased insulin secretion following MSCs treatment in T2DM patients [[24], [25], [26], [27], [28],39].
Presently, MSCs represent an important paradigm of cell-based therapy for a range of diseases due to their wide tissue regeneration potential [20], [21], [22]. Yet, its mechanistic role remains unclear. Our study investigated the differential impact of MSCs on diseased or healthy human islets. We found that MSCs improved the function of hT2DM islets but not the hND islets, which can be attributed to hT2DM, but not hND, islets’ ability to stimulate MSCs to secret IL-1Ra. This result supports the notion that MSCs can respond to different physiological conditions and act accordingly [40].
In islet cells, the expression of proinflammatory cytokines can be induced by the destruction of IAPP [41], hyperglycaemia [11,42] and free fatty acid stimulation [43]. The proinflammatory cytokines can cause β cell dysfunction [44] as well as dedifferentiation [45]. hT2DM islets display greater inflammation in comparison to hND islets, evidenced by an increased proinflammatory cytokines expression. In our study, we demonstrated that elevated expression of IL-1β and TNF-α can activate MSCs to secrete IL-1Ra, a requirement for MSCs to restore the function of hT2DM islets. Moreover, IL-1Ra has been shown to inhibit the IL-1 signaling transduction pathway, subsequently preventing damage caused by intermediates such as COX-2 [19] and NF-kB [46,47]. IL-1Ra was also found to reduce the endogenous production of IL-1β in hT2DM islets, possibly due to a feedback loop blockade in IL-1β signallng [48]. Amongst numerous previously reported effects, this study demonstrates that MSCs can diminish endogenous IL-1β production in hT2DM islets to decrease islet damage, reverse β cell dedifferentiation, and more importantly, this process is mediated by the secretion of IL-1Ra. In our study, IL-1Ra inhibition by neutralizing antibody or depletion by shRNAs abolished the protective effects of MSCs on human T2DM islets. These data convincingly indicated the key role of IL-1Ra in meditating the beneficial effect of MSCs on improving the function of human T2DM islets. In addition, IL-1Ra can directly block the IL-1 signaling in β cells, which have been proven expressing the highest level of IL-1R1 [43], and hence prevent the impairment caused by IL-1β. IL-1Ra can also regulate the phenotype switch of macrophages [49]. A previous study in T2DM mice has shown that MSCs infusion improved the β cell function through promoting the phenotype switch of macrophages in the diabetic mice islets [50]. Therefore it is possible that macrophages may also be involved in restoring the homeostasis of human T2DM islets.
Apart from the islet destruction, physiological IL-1β may also have a role in effects in glycaemic control. Previous evidence has demonstrated that low levels of IL-1β can amplify glucose-induced insulin secretion [51,52]. In contrast, over suppression of IL-1 signaling may also impair glucose homeostasis and increase the risk of infection [53,54]. Therefore, the proposed crosstalk between MSCs and T2DM islets ensures tight regulation in IL-1Ra secretion, thus controlling the IL-1β homeostasis.
Numerous studies have established that β cell dedifferentiation results in β cell dysfunction in T2DM patients [33,55,56]. Stressed β cells can revert from their mature state back to a dedifferentiated state, resulting in reduced or absent insulin production [13,34,57]. By demonstrating the reversibility of human β cell dedifferentiation, we propose that MSCs administration can be a potential approach to hinder or reverse this process. Furthermore, cell dedifferentiation has been observed in a number of cells such as neural cells [58], chondrocytes [59] and cancer cells [60,61]. In all cases, local inflammation plays a key role in inducing the dedifferentiation of these cells. Therefore, cell dedifferentiation may contribute to the pathogenesis of a wide range of diseases or compromise the therapeutic efficacy of existing therapies. With the proposed role of MSCs in reversing β cell dedifferentiation, our study may offer insight into future studies attempting to develop MSCs-based therapies in other diseases.
Declaration of Competing Interest
No conflict of interest to disclose.
Acknowledgments
Acknowledgments
We thank Xiao Han (Nanjing Medical University, Nanjing, China) and Tao Yang (People's Hospital of Jiangsu Province, Nanjing, China) for providing constructive suggestions in manuscript preparation.
Funding sources
This work was supported by grants from the National Natural Science Foundation of China (81870535), Key projects of Tianjin Natural Science Foundation (18JCZDJC33100), National key research and development program (2016YFC1305104), and Foundation of State Key Laboratory of Medicinal Chemical Biology (Nankai University) (2018016). The funders did not participate in study design, data collection, data analysis, interpretation, or writing of the report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.102615.
Contributor Information
Shusen Wang, Email: shusen@vip.163.com.
Zhongyang Shen, Email: zhongyangshen@vip.sina.com.
Appendix. Supplementary materials
References
- 1.IDF . 8th edn. International Diabetes Federation; Brussels, Belgium: 2017. IDF Diabetes Atlas.http://www.diabetesatlas.org2017 [Google Scholar]
- 2.Taniguchi A., Fukushima M., Sakai M., Nagata I., Doi K., Nagasaka S. Insulin secretion, insulin sensitivity, and glucose effectiveness in nonobese individuals with varying degrees of glucose tolerance. Diabetes care. 2000;23(1):127–128. doi: 10.2337/diacare.23.1.127. [DOI] [PubMed] [Google Scholar]
- 3.Ma R.C., Chan J.C. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castiglione F., Tieri P., De Graaf A., Franceschi C., Lio P., Van Ommen B. The onset of type 2 diabetes: proposal for a multi-scale model. JMIR Res Protoc. 2013;2(2):e44. doi: 10.2196/resprot.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi K., Nagai R. Islet inflammation in type 2 diabetes and physiology. The Journal of clinical investigation. 2017;127(1):14–23. doi: 10.1172/JCI88877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schludi B., Moin A.S.M., Montemurro C., Gurlo T., Matveyenko A.V., Kirakossian D. Islet inflammation and ductal proliferation may be linked to increased pancreatitis risk in type 2 diabetes. JCI insight. 2017;2(13) doi: 10.1172/jci.insight.92282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sepehri Z., Kiani Z., Afshari M., Kohan F., Dalvand A., Ghavami S. Inflammasomes and type 2 diabetes: An updated systematic review. Immunology letters. 2017;192:97–103. doi: 10.1016/j.imlet.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Sung K.C., Ryu S., Sung J.W., Kim Y.B., Won Y.S., Cho D.S. Inflammation in the Prediction of Type 2 Diabetes and Hypertension in Healthy Adults. Archives of medical research. 2017 doi: 10.1016/j.arcmed.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Akbari M., Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26(3):685–698. doi: 10.1007/s10787-018-0458-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 12.Nackiewicz D., Dan M., He W., Kim R., Salmi A., Rutti S. TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair beta cell insulin gene expression via IL-1 and IL-6. Diabetologia. 2014;57(8):1645–1654. doi: 10.1007/s00125-014-3249-1. [DOI] [PubMed] [Google Scholar]
- 13.Bensellam M., Jonas J.C., Laybutt D.R. Mechanisms of beta-cell dedifferentiation in diabetes: recent findings and future research directions. The Journal of endocrinology. 2018;236(2):R109–RR43. doi: 10.1530/JOE-17-0516. [DOI] [PubMed] [Google Scholar]
- 14.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rother K.I. Diabetes treatment–bridging the divide. The New England journal of medicine. 2007;356(15):1499–1501. doi: 10.1056/NEJMp078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen C.M., Faulenbach M., Vaag A., Ehses J.A., Donath M.Y., Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes care. 2009;32(9):1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos-Zavala M.G., Gonzalez-Ortiz M., Martinez-Abundis E., Robles-Cervantes J.A., Gonzalez-Lopez R., Santiago-Hernandez N.J. Effect of diacerein on insulin secretion and metabolic control in drug-naive patients with type 2 diabetes: a randomized clinical trial. Diabetes care. 2011;34(7):1591–1594. doi: 10.2337/dc11-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar M.M., Martinez-Abundis E., Preciado-Marquez R.O., Gonzalez-Ortiz M. Effect of diacerein as an add-on to metformin in patients with type 2 diabetes mellitus and inadequate glycemic control. Archives of endocrinology and metabolism. 2017;61(2):188–192. doi: 10.1590/2359-3997000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G., Liang R., Liu T., Wang L., Zou J., Liu N. Opposing effects of IL-1beta/COX-2/PGE2 pathway loop on islets in type 2 diabetes mellitus. Endocr J. 2019 doi: 10.1507/endocrj.EJ19-0015. [DOI] [PubMed] [Google Scholar]
- 20.Qi K., Li N., Zhang Z., Melino G. Tissue regeneration: The crosstalk between mesenchymal stem cells and immune response. Cellular immunology. 2018;326:86–93. doi: 10.1016/j.cellimm.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z., Hao H., Tong C., Cheng Y., Liu J., Pang Y. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem cells. 2016;34(3):627–639. doi: 10.1002/stem.2238. [DOI] [PubMed] [Google Scholar]
- 22.English K. Mesenchymal stem cells to promote islet transplant survival. Current opinion in organ transplantation. 2016;21(6):568–573. doi: 10.1097/MOT.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 23.Gamble A., Pawlick R., Pepper A.R., Bruni A., Adesida A., Senior P.A. Improved islet recovery and efficacy through co-culture and co-transplantation of islets with human adipose-derived mesenchymal stem cells. PloS one. 2018;13(11) doi: 10.1371/journal.pone.0206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J., Wang Y., Gong H., Yu C., Guo C., Wang F. Long term effect and safety of Wharton's jelly-derived mesenchymal stem cells on type 2 diabetes. Experimental and therapeutic medicine. 2016;12(3):1857–1866. doi: 10.3892/etm.2016.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X., Zheng P., Wang X., Dai G., Cheng H., Zhang Z. A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem cell research & therapy. 2014;5(2):57. doi: 10.1186/scrt446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai J., Wu Z., Xu X., Liao L., Chen J., Huang L. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes care. 2016;39(1):149–157. doi: 10.2337/dc15-0171. [DOI] [PubMed] [Google Scholar]
- 27.Estrada E.J., Valacchi F., Nicora E., Brieva S., Esteve C., Echevarria L. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell transplantation. 2008;17(12):1295–1304. doi: 10.3727/096368908787648119. [DOI] [PubMed] [Google Scholar]
- 28.Bhansali A., Upreti V., Khandelwal N., Marwaha N., Gupta V., Sachdeva N. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem cells and development. 2009;18(10):1407–1416. doi: 10.1089/scd.2009.0164. [DOI] [PubMed] [Google Scholar]
- 29.Skyler J.S., Fonseca V.A., Segal K.R., Rosenstock J., Investigators M.-D. Allogeneic Mesenchymal Precursor Cells in Type 2 Diabetes: A Randomized, Placebo-Controlled, Dose-Escalation Safety and Tolerability Pilot Study. Diabetes care. 2015;38(9):1742–1749. doi: 10.2337/dc14-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang H.K., Kim P.H., Cho H.M., Yum S.Y., Choi Y.J., Son Y. Inducible HGF-secreting Human Umbilical Cord Blood-derived MSCs Produced via TALEN-mediated Genome Editing Promoted Angiogenesis. Molecular therapy: the journal of the American Society of Gene Therapy. 2016;24(9):1644–1654. doi: 10.1038/mt.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge Q., Zhang H., Hou J., Wan L., Cheng W., Wang X. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol Med Rep. 2018;17(1):1667–1675. doi: 10.3892/mmr.2017.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volarevic V., Al-Qahtani A., Arsenijevic N., Pajovic S., Lukic M.L. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 2010;43(4):255–263. doi: 10.3109/08916930903305641. [DOI] [PubMed] [Google Scholar]
- 33.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weir G.C., Aguayo-Mazzucato C., Bonner-Weir S. beta-cell dedifferentiation in diabetes is important, but what is it? Islets. 2013;5(5):233–237. doi: 10.4161/isl.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Accili D., Talchai S.C., Kim-Muller J.Y., Cinti F., Ishida E., Ordelheide A.M. When beta-cells fail: lessons from dedifferentiation. Diabetes, obesity & metabolism. 2016;18(Suppl 1):117–122. doi: 10.1111/dom.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brereton M.F., Rohm M., Ashcroft F.M. beta-Cell dysfunction in diabetes: a crisis of identity? Diabetes, obesity & metabolism. 2016;18(Suppl 1):102–109. doi: 10.1111/dom.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke S.J., Batdorf H.M., Burk D.H., Noland R.C., Eder A.E., Boulos M.S. db/db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. Journal of diabetes research. 2017;2017 doi: 10.1155/2017/8503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida E., Kim-Muller J.Y., Accili D. Pair Feeding, but Not Insulin, Phloridzin, or Rosiglitazone Treatment, Curtails Markers of beta-Cell Dedifferentiation in db/db Mice. Diabetes. 2017;66(8):2092–2101. doi: 10.2337/db16-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong D., Zhuang X., Wang D., Qu H., Jiang Y., Li X. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin Lab. 2014;60(12):1969–1976. doi: 10.7754/clin.lab.2014.140305. [DOI] [PubMed] [Google Scholar]
- 40.Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Hui Q., Asadi A., Park Y.J., Kieffer T.J., Ao Z., Warnock G.L. Amyloid formation disrupts the balance between interleukin-1beta and interleukin-1 receptor antagonist in human islets. Molecular metabolism. 2017;6(8):833–844. doi: 10.1016/j.molmet.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H.I., Spinas G.A. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. The Journal of clinical investigation. 2002;110(6):851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boni-Schnetzler M., Boller S., Debray S., Bouzakri K., Meier D.T., Prazak R. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150(12):5218–5229. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 44.Yeung T.Y., Seeberger K.L., Kin T., Adesida A., Jomha N., Shapiro A.M. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PloS one. 2012;7(5):e38189. doi: 10.1371/journal.pone.0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordmann T.M., Dror E., Schulze F., Traub S., Berishvili E., Barbieux C. The Role of Inflammation in beta-cell Dedifferentiation. Scientific reports. 2017;7(1):6285. doi: 10.1038/s41598-017-06731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melloul D. Role of NF-kappaB in beta-cell death. Biochemical Society transactions. 2008;36(Pt 3):334–339. doi: 10.1042/BST0360334. [DOI] [PubMed] [Google Scholar]
- 47.Peng H., Olsen G., Tamura Y., Noguchi H., Matsumoto S., Levy M.F. Inhibition of inflammatory cytokine-induced response in human islet cells by withaferin A. Transplantation proceedings. 2010;42(6):2058–2061. doi: 10.1016/j.transproceed.2010.05.131. [DOI] [PubMed] [Google Scholar]
- 48.Boni-Schnetzler M., Thorne J., Parnaud G., Marselli L., Ehses J.A., Kerr-Conte J. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. The Journal of clinical endocrinology and metabolism. 2008;93(10):4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortiz L.A., Dutreil M., Fattman C., Pandey A.C., Torres G., Go K. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin Y., Hao H., Cheng Y., Zang L., Liu J., Gao J. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell death & disease. 2018;9(7):760. doi: 10.1038/s41419-018-0801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair G.G., Liu J.S., Russ H.A., Tran S., Saxton M.S., Chen R. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat Cell Biol. 2019;21(2):263–274. doi: 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donath M.Y., Boni-Schnetzler M., Ellingsgaard H., Halban P.A., Ehses J.A. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends in endocrinology and metabolism: TEM. 2010;21(5):261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Everett B.M., Donath M.Y., Pradhan A.D., Thuren T., Pais P., Nicolau J.C. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J Am Coll Cardiol. 2018;71(21):2392–2401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 55.Akirav E., Kushner J.A., Herold K.C. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57(11):2883–2888. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brereton M.F., Iberl M., Shimomura K., Zhang Q., Adriaenssens A.E., Proks P. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nature communications. 2014;5:4639. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szabat M., Lynn F.C., Hoffman B.G., Kieffer T.J., Allan D.W., Johnson J.D. Maintenance of beta-cell maturity and plasticity in the adult pancreas: developmental biology concepts in adult physiology. Diabetes. 2012;61(6):1365–1371. doi: 10.2337/db11-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J., Hao X., Yin M.X., Lu Y., Jin Y., Xu J. Prevention of medulla neuron dedifferentiation by Nerfin-1 requires inhibition of Notch activity. Development. 2017;144(8):1510–1517. doi: 10.1242/dev.141341. [DOI] [PubMed] [Google Scholar]
- 59.Hong E.H., Song J.Y., Lee S.J., Park I.C., Um H.D., Park J.K. Low-dose gamma-radiation inhibits IL-1beta-induced dedifferentiation and inflammation of articular chondrocytes via blockage of catenin signaling. IUBMB life. 2014;66(2):128–137. doi: 10.1002/iub.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landsberg J., Kohlmeyer J., Renn M., Bald T., Rogava M., Cron M. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490(7420):412–416. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 61.Mehta A., Kim Y.J., Robert L., Tsoi J., Comin-Anduix B., Berent-Maoz B. Immunotherapy Resistance by Inflammation-Induced Dedifferentiation. Cancer Discov. 2018;8(8):935–943. doi: 10.1158/2159-8290.CD-17-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.