Abstract
Background
The F-box protein S-phase kinase-associated protein 2 (Skp2) is overexpressed and correlated with poor prognosis in human malignancies, including colorectal cancer (CRC).
Methods
A natural product library was used for natural compound screening through glycolysis analysis. The expression of Skp2 in CRCs and the inhibitory effect of dioscin on glycolysis were examined through methods of immunoblot, immunofluorescence, immunohistochemical staining, anchorage-dependent and -independent growth assays, EdU incorporation assay, ubiquitination analysis, co-immunoprecipitation assay, CRISPR-Cas9-based gene knockout, and xenograft experiment.
Findings
We demonstrated that Skp2 was highly expressed in CRC tissues and cell lines. Knockout of Skp2 inhibited HK2 and glycolysis and decreased CRC cell growth in vitro and in vivo. We screened 88 commercially available natural products and found that dioscin, a natural steroid saponin derived from several plants, significantly inhibited glycolysis in CRC cells. Dioscin decreased the protein level of Skp2 by shortening the half-life of Skp2. Further study showed that dioscin attenuated Skp2 phosphorylation on S72 and promoted the interaction between Skp2 and Cdh1, which eventually enhanced Skp2 lysine 48 (K48)-linked polyubiquitination and degradation. Depletion of Cdh1 impaired dioscin-induced Skp2 reduction, rescued HK2 expression, and glycolysis in CRC cells. Finally, dioscin delayed the in vivo tumor growth, promoted Skp2 ubiquitination, and inhibited Skp2 expression in a mouse xenograft model.
Interpretation
This study suggests that in addition to pharmacological inactivation of Skp2, enhancement of ubiquitination-dependent Skp2 turnover is a promising approach for cancer treatment.
Keywords: S-phase kinase-associated protein 2 (Skp2), Hexokinase 2 (HK2), Dioscin, Ubiquitination, Cdh1
Abbreviations: CRC, colorectal cancer; Skp2, S-phase kinase-associated protein 2; HK2, Hexokinase 2; APC, anaphase-promoting complex; SCF, Skp1-Cullin-1-F-box; WCE, whole cell extract; SDS, sodium-deoxycholate; EdU, 5-ethynyl-2′-deoxyuridine; co-IP, co-immunoprecipitation; IHC, immunohistochemistry; IB, immunoblotting; shRNAs, short hairpin RNAs; sgRNAs, single-guide RNAs
Research in Context.
Evidence before this study
Colorectal cancer is the third most common cancer worldwide. Previous studies have shown that Skp2 is overexpressed and positively correlated with poor prognosis in human malignancies. Suppression of Skp2 reduced tumorigenic properties of various cancer cells, including colorectal cancer. The E3 ligase activity of Skp2 plays a crucial role in tumorigenesis via either inducing protein degradation through lysine 48 (K48)-linked polyubiquitination chains or activating signaling transduction by lysine 63 (K63)-linked polyubiquitination chains. Thus, Skp2 could be a potential drug target for human cancer therapy based on enzyme activity. Currently, the synthetic anti-cancer agents provide limited clinical benefits due to high toxicity and unwanted side effects. Natural products are a good source of compounds with unique chemical structures that are effective and less toxic. Therefore, targeting Skp2 by natural compounds is of great therapeutic significance in colorectal cancer.
Added value of this study
We demonstrated that Skp2 is highly expressed in CRC tissues and cell lines. Knockout of Skp2 inhibited HK2 and glycolysis, and decreased CRC cell growth in vitro and in vivo. By screening a natural product library through glycolysis analysis, we found that dioscin, a natural steroid saponin derived from several plants, inhibited glycolysis significantly in CRC cells. Dioscin decreased the protein level of Skp2 by shortening the half-life of Skp2. Further study showed that dioscin attenuated Skp2 phosphorylation on S72 and promoted the interaction between Skp2 and Cdh1, which eventually enhanced Skp2 lysine 48 (K48)-linked polyubiquitination and degradation. Depletion of Cdh1 impaired dioscin-induced Skp2 reduction, rescued HK2 expression, and glycolysis in CRC cells. Finally, dioscin delayed the in vivo tumor growth, promoted Skp2 ubiquitination, and inhibited Skp2 expression in a mouse xenograft model.
Implications of all the available evidence
The cumulative data suggest that in addition to pharmacological inactivation of Skp2, enhancement of ubiquitination-dependent Skp2 turnover is a promising approach for cancer treatment.
Alt-text: Unlabelled box
1. Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, causing approximately 9.2% of cancer-related deaths [1,2]. Even after surgery, which represents the mainstay of treatment for early-stage of CRC, patients are often diagnosed with distant metastases. Currently, fluorouracil (5-FU) based systemic chemotherapy significantly improves the overall survival of advanced CRC patients. However, for those patients who have inherent resistance to chemotherapeutic agents, or acquired resistance with unknown mechanisms, chemotherapy still often fails [3], [4], [5], [6]. Therefore, a better understanding of the mechanisms of colorectal tumorigenesis, or identification of pivotal targets toward the development of novel strategies with lower toxicity will have a high clinical impact.
The F-box protein S-phase kinase-associated protein 2 (Skp2) is an essential subunit of the Skp1-Cullin-1-F-box (SCF) ubiquitin E3 ligase complex. Skp2 harbors the E3 ligase activity, which is required for substrate recognition of the SCF complex [7]. Previous studies have shown that Skp2 is overexpressed and positively correlated with poor prognosis in human breast cancer [8], prostate cancer [9], and nasopharyngeal carcinoma [10]. By disturbing the stability of tumor suppressors, such as p27 [11], p21 [12], and p57 [13] et al., Skp2 promotes cell cycle progression, angiogenesis, metastasis, survival, and confers tumor cell chemoresistance [14], [15], [16], [17]. Moreover, Skp2 was demonstrated to exhibit cross-talk with other oncogenic pathways in human malignancies, including mTOR, ERK1/2, PI3K/Akt, and IGF-1 signaling [14]. However, little is known about the biological role of Skp2 in the tumorigenesis of human colorectal cancer, and its functions in glycolysis regulation.
In this study, we investigate the biological function of Skp2 in CRC and identified dioscin, a natural steroid saponin, as an Skp2 inhibitor for use in CRC therapy. We examine the anti-tumor effect of dioscin in CRC cells both in vitro and in vivo, and elucidate its mechanism of action.
2. Materials and methods
2.1. Reagents and antibodies
Chemical reagents, such as DMSO, NaCl, SDS, and Tris base for buffer preparation were obtained from Sigma-Aldrich (St. Louis, MO). The FBS, cell culture media, and antibiotics were purchased from Invitrogen (Grand Island, NY). Antibodies against Skp2 (#2652, IB:1:2000, IHC: 1:100), HK1 (#2024, IB: 1:2000), HK2 (#2867, IB: 1:2000, IHC:1:200), p-Akt (#4060, IB: 1:1000), Akt (#4691, IB: 1:2000), p-S6 (#4858, IB: 1:4000), p-Histone H3 (Ser10) (#53348, IF: 1:200), ubiquitin (#3936, IB: 1:1000), β-actin (#4970, IB: 1:5000), p27 (#3686, IB: 1:1000), anti-rabbit IgG HRP (#7074), and anti-mouse IgG HRP (#7076) were obtained from Cell Signaling Technology, Inc. (Beverly, MA). HK2 (LS‑C404653) antibody for IHC staining was obtained from LifeSpan BioSciences, Inc. Antibodies against Ki67 (ab16667, IHC: 1: 250) and donkey anti-rabbit IgG H&L (Alexa Fluor® 488) (ab150073, IF: 1:800) were purchased from Abcam (Cambridge, UK).
2.2. Cell lines and cell culture
Human colorectal cancer cell lines, including DLD1, HCT116, SW480, HT29, HCT8, and SW620 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Human normal colon epithelial cell FHC was obtained from ATCC. All cells were maintained at 37°C in a humidified incubator with 5% CO2 according to the ATCC protocols. The cells were cytogenetically tested and authenticated before being frozen.
2.3. Protein preparation and western blotting
Whole cell extract (WCE) was prepared with RIPA buffer (20 mM NAP, pH7.4, 150 mM NaCl, 1% Triton, 0.5% Sodium-deoxycholate, and 0.1% SDS) supplemented with protease inhibitors. BCA assay (#23228, Pierce, Rockford, IL) was used for protein concentration following the standard procedures. Western blotting was performed as previously described [18]. Briefly, WCE was boiled with loading buffer at 95 °C for 5 min and subjected to SDS-PAGE followed by electrotransfer to the PVDF membrane. The membrane was blocked with 5% non-fat milk and incubated with primary antibody at 4 °C overnight. Anti-rabbit IgG HRP and anti-mouse IgG HRP were used as second antibodies. The target protein was visualized using the ECL substrate (#32106, Thermo Fisher Scientific).
2.4. Immunofluorescence
Immunofluorescence was performed as previously described [19]. Briefly, HT29 cells were seeded in chamber slides and treated with various concentrations of compound for 24 h. Cells were fixed in ice-cold methanol for 20 min at 4 °C. After a PBS wash, the fixed cells were incubated with 5% BSA at room temperature for 1 h and hybridized with p-Histone H3 Ser10 antibody in a humidified chamber overnight at 4 °C. Alexa Fluor 488 dye-labeled anti-rabbit IgG was used as the secondary antibody.
2.5. Immunohistochemical staining (IHC)
This study was approved by the Institute Research Ethics Committees of Xiangya Hospital, Central South University. Human colorectal cancer tissues and the paired adjacent tissues were obtained from the Departments of Pathology at Xiangya Hospital with written informed consent (n = 63). The tissues were fixed, embedded, and subjected to IHC analysis as described previously [20]. Briefly, after incubating at 65 °C for 1 h, the tissue slides were submersed into sodium citrate buffer (10 mM, pH 6.0) and boiled for 10 min, followed by incubation in 3% H2O2 for 10 min. Tissue slides were blocked with 50% goat serum albumin at room temperature for 1 h and incubated with the primary antibody in a humidified chamber overnight in a cold room. Tissue slides were washed with PBS and hybridized with the secondary antibody at room temperature for 45 min. Hematoxylin was used for counterstaining.
2.6. MTS assays
Human colorectal cancer cells were seeded in 96-well plates (2 × 103/well) and treated with different doses of compound or DMSO control. Cell viability was examined at various time points using the MTS reagent (G3581, Promega, Madison, WI).
2.7. 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay
The stable colorectal cancer cells were seeded into chamber slides at a density of 1 × 104 cells and cultured overnight. Cells were then incubated with 10 μM of EdU (C10339, Thermo Fisher) and subjected to EdU incorporation analysis according to the manufacturer's instructions.
2.8. Anchorage-independent cell growth assay
Colorectal cancer cells (8 × 103 per well) were counted and seeded into 6-well plates with 0.3% Basal Medium Eagle agar containing 10% FBS and cultured. The cultures were maintained at 37 °C in a 5% CO2 incubator for 2 weeks. Colonies were counted under a microscope, as previously described [21].
2.9. Natural compound screening
The Natural Product Library (Cat. No. L1400-01/02), which contains 88 compounds of interest, was a product of Selleck Chemicals (Houston, TX). HT29 cells were seeded in a 48-well plate and treated with a single dose of 2 μM natural products or DMSO (control) for 12 h. Cell culture medium was collected and subjected to glucose and lactate analysis at the Laboratory of Xiangya Hospital (Changsha, China). The relative glucose consumption and lactate production rate were normalized by protein concentration. Tested compounds are listed in Table S1.
2.10. Transient transfection and generation of silencing stable cell lines
Lipofectamine 2000 (#11668–019, Invitrogen, Carlsbad, CA) was used for transient transfection according to the instructions provided. Two different single-guide RNAs (sgRNAs) were used to generate CRISPR-Cas9-based Skp2 knockout constructs (sgSkp2#1forward, 5′- AAGACTTTGTGATTGTCCGC-3′, reverse, 5′- CGGGACAATCACAAAGTCTT −3′, sgSkp2#2forward, 5′- GCAACGTTGCTACTCAGGTC −3′, reverse, 5′- GACCTGAGTAGCAACGTTGC-3′). The Skp2 stable knockout signal clone was generated by transient transfection of sgSkp2 plasmids followed by selection with 1 μg/mL puromycin for 3 weeks.
The Cdh1 siRNA (Cat. A-003877–17–0005) and shRNA (Cat. V3SH11240-228718527) were purchased from GE Horizon Discovery. Lentivirus-mediated Cdh1 knockdown stable cell generation was performed as described previously [22]. Briefly, the shCdh1 lentivirus plasmid, psPAX2, and pMD2.G were co-transfected into 293T cells. The virus-containing supernatant was collected and filtered through a 0.45 μm filter at 48 h after transfection and infected with CRC cells together with 6 μg/mL polybrene. Cells were selected by 1 μg/mL puromycin for 3 days. The primer for Skp2 qRT-PCR analysis is forward sequence: GATGTGACTGGTCGGTTGCTGT, reverse sequence: GAGTTCGATAGGTCCATGTGCTG.
2.11. Glucose uptake and lactate production
Glycolysis measurement was performed, as described previously [23]. Briefly, colorectal cancer cells were seeded in 6-well plates (5 × 105) and maintained in the incubator overnight. The cells were treated with different doses of dioscin or DMSO control for 10 h. The cell culture medium was harvested and subjected to glycolysis analysis. Glucose and lactate levels were measured (Automatic Biochemical Analyzer; 7170A, HITACHI, Tokyo, Japan) at the Laboratory of Xiangya Hospital (Changsha, China). Protein concentration was determined by BCA protein assay to normalize the relative glucose consumption and lactate production rate.
2.12. Ubiquitination analysis
Ubiquitination analysis was performed, as described previously [17]. Briefly, cell lysates were prepared using the modified RIPA buffer (20 mM NAP, pH7.4, 150 mM NaCl, 1% Triton, 0.5% Sodium-deoxycholate, and 1% SDS) supplied with 10 mM N-Ethylmaleimide (NEM) and protease inhibitors. After sonication for 30 s, the supernatant was boiled at 95 °C for 15 min, followed by diluted with RIPA buffer containing 0.1% SDS and centrifuged at 16,000 × g for 15 min at 4 °C. The supernatant was incubated with anti-Skp2 antibody and 30 μL protein A-Sepharose beads overnight in a cold room. After extensive washing and centrifuge, the binding proteins were eluted by boiling with 2 × SDS sample loading buffer at 95 °C for 5 min, Skp2 ubiquitination was determined by western blotting analysis.
2.13. In vivo tumor growth assay
The in vivo animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Xiangya Hospital, Central South University (Changsha, China). The xenograft mouse model was generated by s.c.injection of colorectal cancer cells (2 × 106) into the right flank of 6-week-old athymic nude mice (n = 6). Tumor size was measured with calipers every two days. For compound treatment, mice were administrated with dioscin (10 mg/kg every two days) by i.p. injection when the tumor volume reached around 100 mm3, while the control group was administrated with the vehicle. Tumor volume was measured with calipers every two days and determined according to the following formula: length × width × width/2.
2.14. Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad 5.0, San Diego, CA, USA). The quantitative data are expressed as mean ± sd, the difference was evaluated using the Student's t-test or ANOVA. A probability value of p < 0.05 was used as the criterion for statistical significance.
3. Results
3.1. Skp2 is required for the maintenance of tumorigenic properties in human colorectal cancer cells
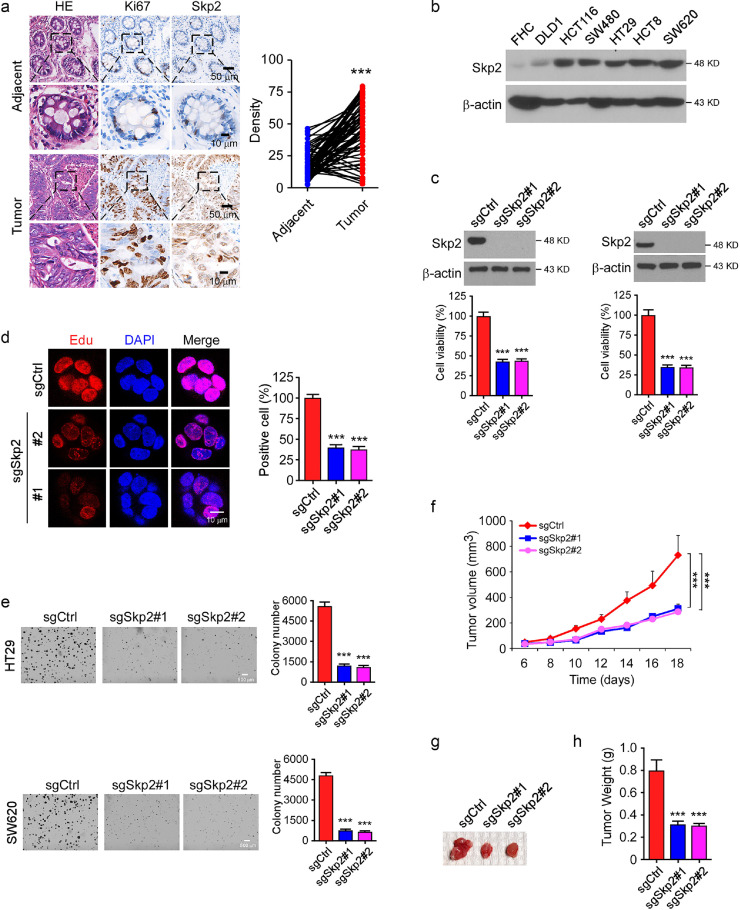
To determine the role of Skp2 in human colorectal cancer (CRC), immunohistochemical (IHC) staining was performed to examine the protein level of Skp2 in colorectal cancer tissues. As shown in Fig. 1a, Skp2 was upregulated in CRC tissues as compared to the paired adjacent tissues. The immunoblotting (IB) data indicated that Skp2 was highly expressed in human CRC cell lines, whereas the normal colon epithelial cell FHC exhibited a lower protein level by comparison (Fig. 1b). Based on these observations, we hypothesized that Skp2 might affect the tumorigenic properties in CRC. Thus, we constructed Skp2 stable knockout HCT116 and HT29 cells. MTS results showed that depletion of Skp2 significantly decreased cell viability in both SW620 and HT29 cells (Fig. 1c). The EdU incorporation assay further demonstrated that knockout of Skp2 reduced cell proliferation in HT29 cells (Fig. 1d). Furthermore, the efficacy of colony formation in soft agar was attenuated significantly in Skp2 deficient cells as expected (Fig. 1e). To determine the role of Skp2 in the tumorigenesis of CRC in vivo, we constructed a xenograft mouse model using SW620-sgCtrl and SW620-sgSkp2 stable cells. Our data showed that depletion of Skp2 delayed in vivo tumor development significantly (Fig. 1f–h). These results suggest that blocking Skp2 expression reduces the tumorigenic properties of CRC cells.
Fig. 1.
Skp2 is required for the maintaining of tumorigenic properties of colorectal cancer (CRC) cells. (a) Left, the representative staining images of CRC specimens and adjacent tissues; Right, quantification of the staining intensity using Image-Pro-PLUS (v.6) and Image J (NIH) computer software. ***p < 0.001. (b) Immunoblot (IB) analysis of Skp2 expression in human CRC cells and normal epithelial cells. (c) Top, IB analysis of Skp2 expression in HT29 sgCtrl and sgSkp2 cells (left) or SW620 sgCtrl and sgSkp2 cells (right). Bottom, MTS assay was performed to determine cell viability of HT29 sgCtrl and sgSkp2 cells (left) or SW620 sgCtrl and sgSkp2 cells (right). ***p < 0.001. (d) EdU incorporation assay analysis of cell proliferation in HT29 sgCtrl and sgSkp2 cells. ***p < 0.001. (e) Soft agar assay examination of the anchorage-independent cell growth of HT29 sgCtrl and sgSkp2 cells (top) or SW620 sgCtrl and sgSkp2 cells (bottom). ***p < 0.001. (f–h) Average tumor volume (f), representative photographed xenograft tumors (g), and average tumor weight (h) of SW620 sgCtrl and sgSkp2 xenograft tumors. n = 6 mice per group. ***p < 0.001.
3.2. Skp2 is required for aerobic glycolysis in CRC cells
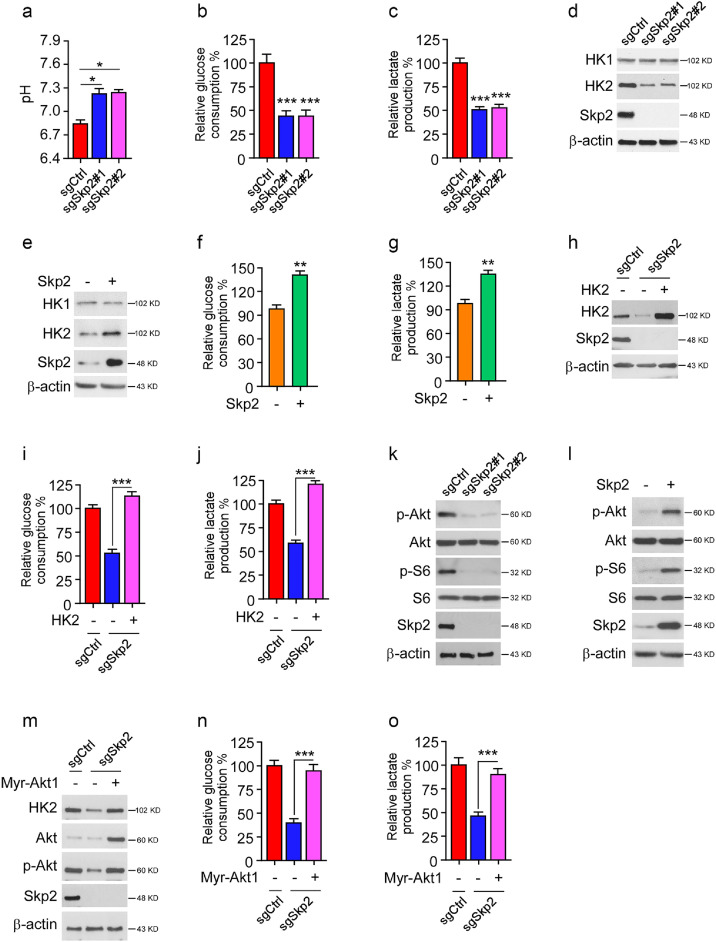
As cell culture media of sgCtrl cells turned yellow much faster than that of sgSkp2 stable cells, we hypothesized that this phenotype might be due to lactate acidosis. Indeed, depletion of Skp2 in HT29 cells impaired the capability to decrease medium pH values in sgCtrl cells (Fig. 2a). Moreover, glucose consumption and lactate production were significantly reduced in Skp2 deficient HT29 cells (Fig. 2b and 2c). Because these metabolic changes resembled aerobic glycolysis, we further identified that Hexokinase 2 (HK2), but not HK1, was markedly decreased in Skp2 stable knockout cells (Fig. 2d). Consistently, ectopic overexpression of Skp2 promoted HK2 protein level, glucose consumption, and lactate production (Fig. 2e–g) in FHC epithelial cells. We then restored HK2 expression in Skp2 deficient HT29 cells to investigate whether it was required for Skp2-mediated aerobic glycolysis. Overexpression of HK2 rescued glucose consumption and lactate production in sgSkp2 stable cells (Fig. 2h–j). Skp2 is reportedly required for Akt K63-linked ubiquitination and activation, and the upregulation of Akt signaling promotes HK2 expression and glycolysis. Thus, we examined whether the decrease of HK2 in Skp2 knockout cells is related to Akt deactivation. The IB results showed that depletion of Skp2 impaired the phosphorylation of Akt and downstream kinase S6 in HT29 cells (Fig. 2k). In contrast, overexpression of Skp2 promoted the activation of Akt and S6 in FHC cells (Fig. 2l). Moreover, constitutively activated Akt1 (Myr-Akt1) upregulated HK2 protein level and rescued glucose consumption and lactate production in Skp2 stable knockout HT29 cells (Fig. 2m–o). These results indicate that Skp2-mediated glycolysis in CRC cells is partly dependent on the Skp2-Akt-HK2 axis.
Fig. 2.
Skp2 promotes glycolysis in CRC cells. (a) pH value of cell culture medium from HT29 sgCtrl and sgSkp2 cells. *p < 0.05. (b and c) Relative glucose consumption (b) and lactate production (c) in HT29 sgCtrl and sgSkp2 cells. ***p < 0.001. (d) IB analysis of HK1, HK2, and Skp2 protein levels in HT29 sgCtrl and sgSkp2 cells. (e–g) FHC cells were transfected with Skp2 or vector control, whole cell lysates (WCE) were subjected to IB analysis (e), the cell culture medium was subject to glucose consumption (f) and lactate production (g) analysis. **p < 0.01. (h) WCE from HT29 sgCtrl and HK2 transient transfected HT29 sgSkp2 cells were subjected to IB analysis with the indicated antibodies. (i and j) Cell culture medium from (h) was subject to glucose consumption (i) and lactate production (j) analysis. ***p < 0.001. (k) WCE from HT29 sgCtrl and sgSkp2 cells were subjected to IB analysis with the indicated antibodies. (l) FHC cells were transfected with Skp2 or vector control, WCE was subjected to IB analysis. (m) WCE from HT29 sgCtrl and Myr-Akt1 transient transfected HT29 sgSkp2 cells were subjected to IB analysis with the indicated antibodies. (n and o) Cell culture medium from (m) was subject to glucose consumption (n) and lactate production (o) analysis. ***p < 0.001.
3.3. Dioscin inhibits glycolysis in CRC cells
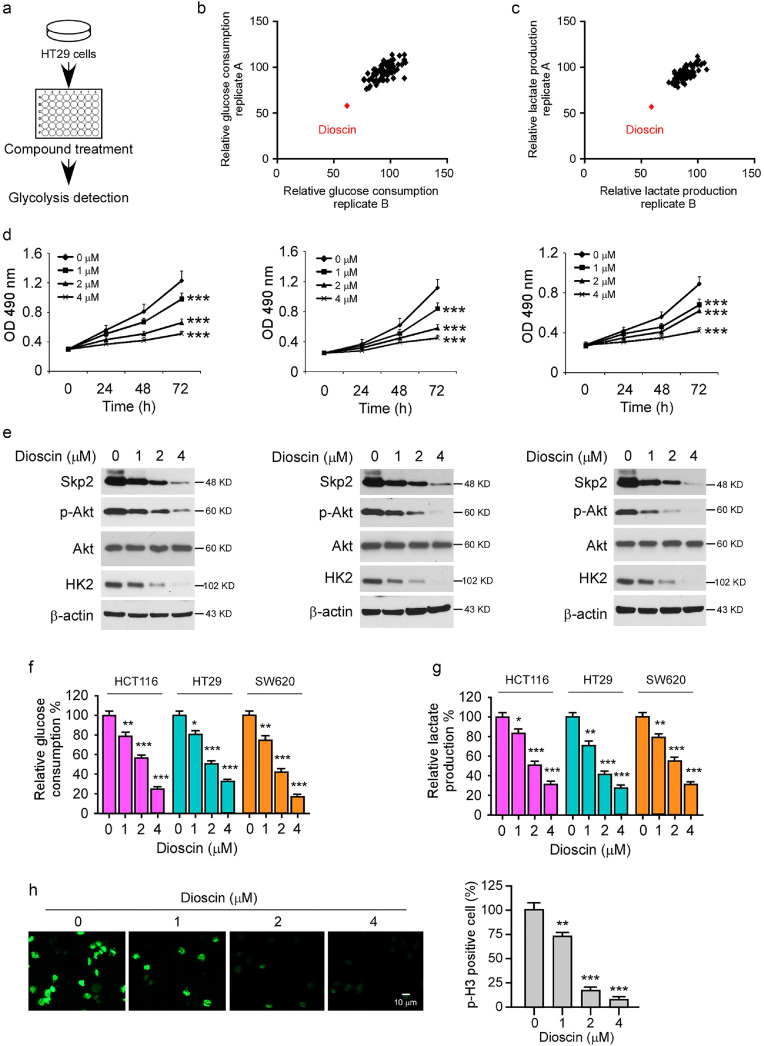
To discover natural compounds (Table S1) that can specifically suppress Skp2 signaling and glycolysis, we screened a library of 88 natural products that inhibit glucose consumption and lactate production of CRC cells. The results showed that only dioscin decreased both glucose consumption and lactate production by over 30% at the concentration of 2 μM (Fig. 3a–c) on HT29 cells. Therefore, dioscin is our focus for further study. To validate the inhibitory effect of dioscin, we treated CRC cells at various concentrations. As Fig. 3d demonstrated, dioscin suppressed cell viability in a dose- and time-dependent manner. Moreover, the IB results indicated that dioscin decreased the protein level of Skp2, p-Akt, and HK2 in HCT116, HT29, and SW620 cells (Fig. 3e), suggesting that dioscin might be an inhibitor of Skp2 signaling. Furthermore, dioscin impaired glycolysis dose-dependently, because the glucose uptake and lactate production efficacy were attenuated significantly after dioscin treatment (Fig. 3f and g). Aerobic glycolysis is required for maintaining cancer cell growth, so we examined the effect of dioscin on phosphorylation of Histone H3 Ser10, a marker of cell proliferation. The immunofluorescence (IF) results showed that dioscin inhibited the phosphorylation of Histone H3 Ser10 significantly (Fig. 3h), which is consistent with the MTS data and further confirms that dioscin exhibits an anti-tumor effect on CRC cells.
Fig. 3.
Dioscin suppresses Skp2 and glycolysis in CRC cells. (a) Schematic workflow of the natural compound screening. (b and c) The inhibitory efficacy of screened compounds on glucose consumption (b) and lactate production (c) of HT29 cells. (d and e) HCT116 (left), HT29 (middle), and SW620 cells (right) were treated with DMSO or dioscin and subjected to MTS assay (d) or IB analysis (e). ***p < 0.001. (f and g) CRC cells were treated with DMSO or dioscin and subjected to glucose consumption (f) and lactate production (g) analysis. *p < 0.05, **p < 0.01, ***p < 0.001. (h) HT29 cells were treated with DMSO or dioscin and subjected to immunofluorescence analysis with a p-H3 S10 antibody. **p < 0.01, ***p < 0.001.
3.4. Dioscin promotes Skp2 ubiquitination and degradation
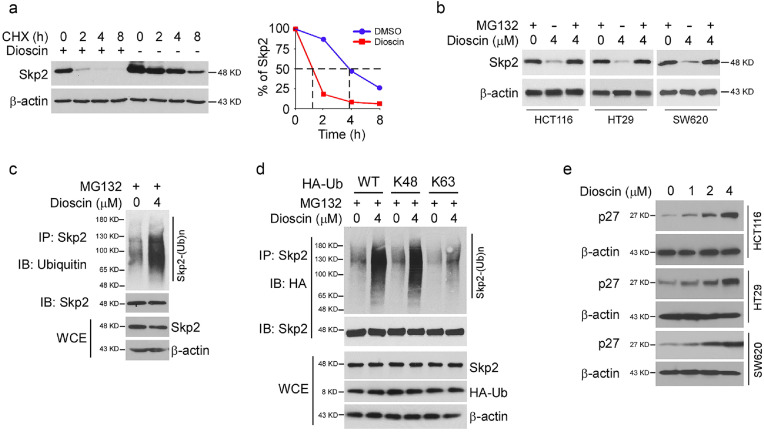
Because dioscin decreased Skp2 protein levels and glycolysis dose-dependently (Fig. 3e), we first characterize the mechanism that regulates Skp2 turnover. Dioscin shortened the half-life of endogenous Skp2 from 4 h to 1 h in the presence of cycloheximide (CHX) (Fig. 4a), whereas the mRNA had no significant difference (Fig. S1), suggesting that exposure to dioscin caused protein instability. Indeed, treatment with proteasome inhibitor, MG132, rescued dioscin-induced reduction of Skp2 in CRC cells (Fig. 4b), which further implied that dioscin promoted Skp2 degradation. To determine whether Skp2 destruction in CRC cells is increased upon dioscin treatment, we determined endogenous Skp2 poly-ubiquitination in vivo. As shown in Fig. 4c, dioscin enhanced Skp2 poly-ubiquitination markedly in HT29 cells. Moreover, using the ubiquitin mutants, HA-Ub K48 (lysine 48 only) and HA-Ub K63 (lysine 63 only), we demonstrated that dioscin-induced Skp2 ubiquitination was K48-linked (Fig. 4d). To examine the relationship between Skp2 activity and destruction, we further determined the protein level of p27, the downstream target of E3 ligase Skp2. The IB results showed that dioscin promoted p27 protein level dose-dependently (Fig. 4e) in HCT116, HT29, and SW620 cells. These results indicate that dioscin decreases Skp2 protein levels in a ubiquitination-dependent manner.
Fig. 4.
Dioscin promotes Skp2 ubiquitination and degradation in CRC cells. (a) HT29 cells were treated with DMSO or 4 μM dioscin for 24 h, followed by cycloheximide (CHX) treated for various time points, WCE was collected and subjected to IB analysis. (b) CRC cells were treated with DMSO or dioscin, followed by 20 μM MG132 treatment for 8 h, WCE was collected and subjected to IB analysis. (c) HT29 cells were treated with DMSO or dioscin for 24 h, MG132 was added to the cell culture medium and maintained for 6 h. WCE were subjected to in vivo ubiquitination assay. (d) HT29 cells were transfected with HA-Ub WT or mutants for 24 h, followed by DMSO or dioscin treated for another 24 h. MG132 was added to the cell culture medium and maintained for 6 h. WCE were subjected to in vivo ubiquitination assay. (e) CRC cells were treated with DMSO or dioscin for 24 h, WCE was subjected to IB analysis.
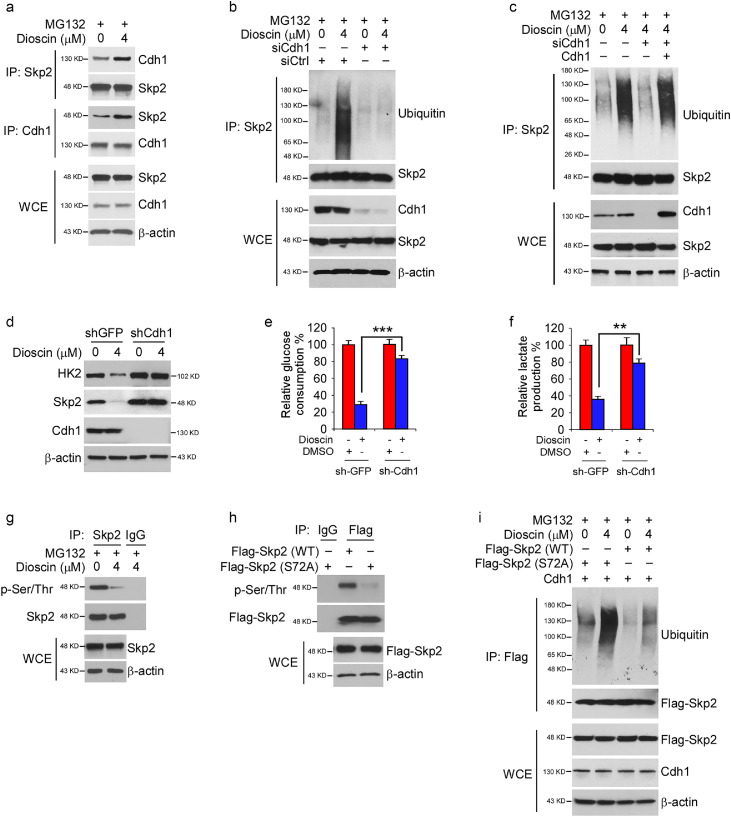
3.5. Cdh1 is required for dioscin-induced Skp2 destruction in CRC cells
The tumor suppressor APC/CCdh1 plays a crucial role in the regulation of Skp2 stability [24,25]. We determined whether Cdh1 is required for dioscin-induced Skp2 degradation in CRC cells. The endogenous binding between Skp2 and Cdh1 was identified via reciprocal co-immunoprecipitation (co-IP) in HT29 cells. Moreover, this interaction was enhanced by dioscin treatment (Fig. 5a). Dioscin promoted Skp2 ubiquitination in siCtrl transfected cells, whereas knockdown of Cdh1 impaired dioscin-induced Skp2 ubiquitination (Fig. 5b). Moreover, overexpression of Cdh1 in Cdh1 knockdown cells restored dioscin-induced Skp2 ubiquitination (Fig. 5c), indicating that Cdh1 is required for dioscin-induced Skp2 destruction. Stable knockdown of Cdh1 in HT29 cells increased Skp2 and HK2 protein level and compromised dioscin-induced reduction of Skp2 and HK2 (Fig. 5d). The efficacy of dioscin-mediated glycolysis suppression was attenuated significantly in Cdh1 deficient cells (Fig. 5e and f). Previous studies show that phosphorylation of S72 promotes Skp2 stability and suppresses the proteasome-mediated degradation [26,27]. The IB data showed that treatment with dioscin decreased Skp2 Ser/Thr-phosphorylation (Fig. 5g). To validate whether S72 is phosphorylated, we constructed the S72A (Ser-to Ala) mutant and found that Skp2 Ser/Thr-phosphorylation was markedly decreased (Fig. 5h), which suggests that Skp2 S72 is one of the primary phosphorylation sites in CRC cells. To determine whether Skp2 S72 phosphorylation is required for maintaining protein stability, we compared the poly-ubiquitination of Skp2 S72A with Skp2 WT. The in vivo ubiquitination assay showed that dioscin-induced Skp2 ubiquitination was enhanced in the Skp2 S72A mutant (Fig. 5i). To determine whether dioscin decreased Skp2 Ser/Thr-phosphorylation is dependent on Akt signaling, we ectopically overexpressed constitutively activated Akt1, HA-Myr-Akt1, in CRC cells. The data showed that overexpression of Akt1 compromised dioscin-induced Skp2 Ser/Thr-phosphorylation suppression (Fig. S2a). Furthermore, overexpression Akt1 promoted phosphorylation of WT Skp2, but not that of the Skp2 S72A mutant (Fig. S2b). These results indicated that Akt promoted Skp2 phosphorylation on S72, and this residue was one of the major phosphorylation sites which was inhibited by dioscin and rescued by Akt kinase. In addition, the interaction between Akt and Skp2 was unaffected by dioscin treatment (Fig. S2c), indicating that the decrease of Skp2 phosphorylation on S72 was not caused by attenuation of interaction. Collectively, our data suggest that the suppression of Skp2 phosphorylation is required for Cdh1-mediated Skp2 destruction in dioscin-treated CRC cells.
Fig. 5.
Cdh1 is required for dioscin-induced Skp2 ubiquitination. (a) HT29 cells were treated with dioscin for 24 h, WCE was subjected to co-immunoprecipitation (co-IP) analysis. (b) HT29 cells were transfected with siCtrl or siCdh1 and treated with DMSO or dioscin for 24 h, followed by MG132 treatment for another 6 h, WCE was collected and subjected to in vivo ubiquitination assay. (c) HT29 cells were transfected with siCtrl or siCdh1 for 24 h. The siCdh1 transfected cells were overexpressed with Cdh1 and treated with DMSO or dioscin for 24 h, followed by MG132 treatment for another 6 h. WCE were subjected to in vivo ubiquitination assay. (d–f) HT29 cells stable expression of shGFP or shCdh1 were treated with DMSO or dioscin for 24 h, WCE was subjected to IB analysis (d). Glucose consumption (e), and lactate production (f) were examined in the cell culture medium. **p < 0.01, ***p < 0.001. (g) HT29 cells were treated with DMSO or dioscin for 24 h, WCE was collected and subjected to co-IP assay, followed by IB analysis. (h) HT29 cells were transfected with Flag-Skp2 WT and Flag-Skp2 (S72A) mutant for 48 h, WCE was subjected to co-IP assay followed by IB analysis. (i) HT29 cells were transfected with the constructs as indicated for 24 h, cells were then treated with DMSO or dioscin for 24 h, followed by MG132 treatment for 6 h, WCE was subjected to in vivo ubiquitination assay.
3.6. Dioscin inhibits tumor growth in vivo
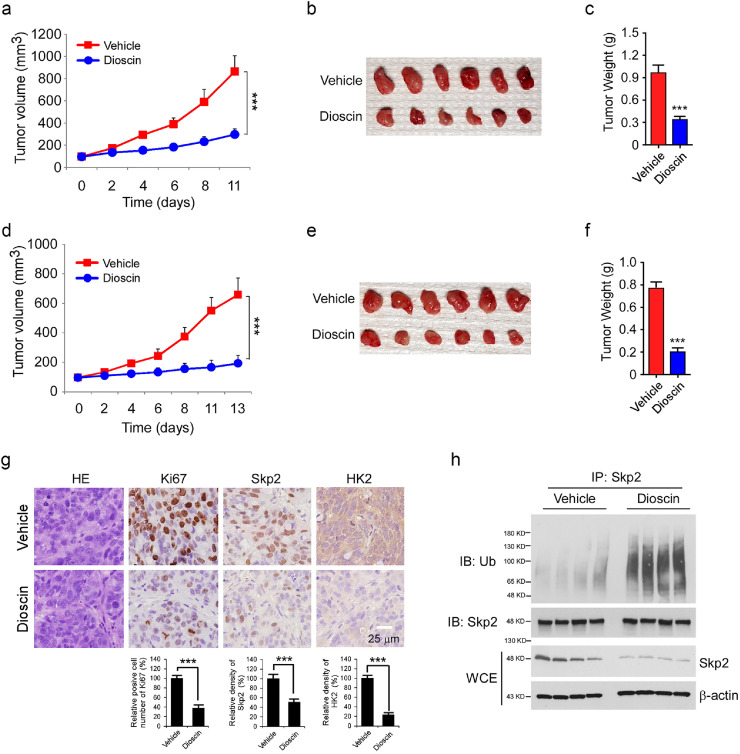
We next determined the anti-tumor effect of dioscin using an in vivo nude mouse model. HT29 and SW620 cells were injected (s.c.) into the right flank of female athymic nude mice. After the tumor volume reached around 100 mm3, the tumor-bearing mice were treated with dioscin or vehicle control. The results showed that in the HT29 xenograft model, the average tumor size of the vehicle-treated group reached 867 ± 143 mm3, whereas the dioscin-treated group was only 297 ± 51 mm3 (Fig. 6a). Dioscin exhibited a similar inhibitory efficacy on SW620 xenograft tumors, as the tumor volume of the vehicle- and dioscin-treated groups were 659 ± 113 mm3 and 194 ± 53 mm3, respectively (Fig. 6d). Moreover, the average tumor weight of both HT29 (Fig. 6b and c) and SW620 (Fig. 6e and f) xenograft tumors with dioscin treatment was significantly reduced compared to that of the vehicle-treated groups. Mice tolerated the treatment with dioscin well without noticeable body weight loss (Fig. S3a and S3b). The immunohistochemical staining (IHC) results showed that dioscin-treated HT29 and SW620 xenograft tumors exhibited significant attenuation of Ki67 expression, correlated with the reduction of Skp2 and HK2 protein level (Fig. 6g, Fig. S3c). We next determined whether Skp2 ubiquitination is upregulated in compound-treated xenograft tumors. Indeed, the ubiquitination of Skp2 was higher in dioscin-treated tumors than that in vehicle-treated tumors (Fig. 6h, Fig. S3d). These findings indicate that dioscin inhibits CRC tumor growth in vivo. This may occur through the induction of Skp2 ubiquitination and suppression of HK2 in tumor tissues.
Fig. 6.
Dioscin suppresses xenograft tumor growth in vivo. (a–c) HT29 cells were injected into nude mice and treated with vehicle control or dioscin when tumor volume reached 100 mm3. Tumor volume was measured by caliper (a), tumor mass was photographed (b), and weight (c). ***p < 0.001. (d–f) SW620 cells were injected into nude mice and treated with vehicle control or dioscin when tumor volume reached 100 mm3. Tumor volume was measured by caliper (d), tumor mass was photographed (e), and weight (f). ***p < 0.001. (g) Immunohistochemistry (IHC) staining analysis of Ki67, Skp2, and HK2 in HT29 xenograft tumors. ***p < 0.001. (h) WCE from HT29 xenograft tumors were subjected to in vivo ubiquitination analysis. Scale bar, 25 μm.
4. Discussion
Overexpression of Skp2 is frequently observed in human malignancies [28]. Although p27 is a critical substrate of Skp2, many additional targets of Skp2 have been identified, including Akt [8], LKB1 [29], NBS1 [30], amongst others. The E3 ligase activity of Skp2 plays a crucial role in tumorigenesis via either induced protein degradation through lysine 48 (K48)-linked polyubiquitination chain or activating signaling transduction by lysine 63 (K63)-linked polyubiquitination chain. Thus, Skp2 could be a potential drug target for human cancer therapy based on enzyme activity. Early studies show that Skp2 is required for tumorigenesis in mouse models in the context of BCR-ABL overexpression [31], p53/PTEN-deficient [32], or pRB inactivation [33]. Pharmacological inactivation of Skp2 ubiquitin ligase by small-molecules restricts cancer stem cell traits and cancer progression in multiple cancer models [34], [35], [36]. In this study, we identify that the natural compound dioscin suppresses Skp2 expression via the promotion of Skp2 ubiquitination and degradation in a Cdh1 dependent manner. Dioscin decreased Skp2 protein level and reduced glycolysis in CRC cells both in vitro and in vivo. Moreover, several natural compounds such as curcumin [37], [38], [39], saurolactam [40], rottlerin [41], and Longikaurin A [42] have been reported to inhibit the expression of Skp2 and decrease the malignant phenotype of human cancer cells. These results support the notion that Skp2 is a promising drug target for human cancers.
Currently, synthetic anti-cancer agents only provide limited clinical benefits due to high toxicity and unwanted side effects. Natural products are a good source of compounds with unique chemical structures that are effective and less toxic. Dioscin, a natural steroid saponin derived from several plants, exhibits significant anti-tumor activity towards several types of cancer, including lung [43], prostate [44], breast [45], liver [46], and colorectal [47] cancer. Attenuation of kinase activity, inhibition of angiogenesis and metastasis, suppression of transcription factor activity, and induction of apoptosis were demonstrated to be the underlying mechanisms of dioscin-mediated anti-tumor activity [48]. However, very little is known about the effects of dioscin on the regulation of glycolysis in human CRC cells. In this study, we identify that dioscin exhibits a strong inhibitory effect on glucose consumption and lactate production using natural compound screening in CRC cells. We unexpectedly find that this process is dependent on dioscin-induced Skp2 ubiquitination and degradation. Early reports demonstrate that the regulation of Skp2 turnover is mediated by the anaphase-promoting complex (APC), in association with its substrate-specific factor Cdh1 [24,25]. Moreover, mutation or dysfunction of Cdh1 is implicated in the tumorigenesis of human CRC [49,50]. Our data show that dioscin suppresses Skp2 phosphorylation on S72 and promotes the interaction between Skp2 and Cdh1, thus enhancing Cdh1-mediated Skp2 destruction, and eventually decreasing HK2 protein level and glycolysis in CRC cells.
Accumulating evidence indicates that Skp2 plays a critical role in CRC development. Amplification or higher copy numbers of Skp2 is associated with increased CRC risk [51]. G9a-mediated methylation promotes Skp2-mediated FOXO1 degradation and cell proliferation in CRC [52]. Combined Menin and EGFR inhibitors synergize to inhibit CRC via EGFR-independent and calcium-mediated repression of Skp2 transcription [53]. Moreover, NDRG2 inhibits Skp2 and results in the induction and stabilization of p21 and p27, which facilitates CRC differentiation [54]. Additionally, bortezomib suppresses CRC through the promotion of Skp2 degradation in CRC cells [55]. Our data show that Skp2 is required for the maintenance of CRC cell growth both in vitro and in vivo. Depletion of Skp2 decreases cell viability, proliferation, colony formation, and in vivo tumor formation of CRC cells. Furthermore, we demonstrate that Skp2 promotes glycolysis in CRC cells via upregulation of HK2 protein level. Ectopic overexpression of HK2 rescued Skp2 deficiency-induced glycolysis suppression. Although the glucose uptake of mammalian cells is controlled by the glucose transporter, Gluts [56], reduction of HK2 protein level might impair the glucose metabolism in mitochondria, which eventually decreases the glucose uptake rate due to the accumulation of glucose in cells. Also, HK2 mitochondrial localization is regulated by Akt signaling [17,57]. Knockout of Skp2 might suppress Akt activity, which in turn increases the cytosolic distribution of HK2 and reduces the mitochondrial associated HK2 simultaneously. Moreover, the accumulation of glucose 6-phosphate (G6P) in mitochondria might lead to negative regulation of HK2 kinase activity through a negative feedback mechanism [58,59], which might cause the downregulation total quantity of HK2 in mitochondria. Thus, restoration of HK2 in Skp2 knockout cells rescued glycolysis. Lin et al. have demonstrated that Skp2 is required for aerobic glycolysis in breast cancer by the promotion of Glut1 expression. Skp2 induced K63-linked Akt ubiquitination and conferred herceptin resistance [8]. Our data show that knockout of Skp2 decreases Akt signaling, and transfection of Myr-Akt1 restores HK2 expression and glycolysis in CRC cells, indicating that Akt activity is also required for Skp2-mediated HK2 and glycolysis regulation.
In summary, we demonstrate that Skp2 promotes HK2 expression and glycolysis, and knockout of Skp2 reduces tumorigenic properties of human CRC cells. We identify that dioscin inhibits Skp2 S72 phosphorylation and eventually enhances Skp2 ubiquitination and degradation in a Cdh1 dependent manner. Beyond pharmacological inactivation of Skp2 E3 ligase activity, our discovery reveals that the suppression of Skp2 protein levels, by the inducement of ubiquitination and degradation, is a compelling therapeutic strategy that deserves further study for cancer prevention and treatment.
Funding sources
This work was supported by the National Natural Science Foundation of China (No. 81572280, No. 81904262, and No. 81972837), the Natural Science Foundation of Hunan Province (2018JJ3787, 2018JJ2604, 2019JJ40420, 2019JJ50682), and the Natural Science Foundation of Changsha Science and Technology Bureau (kq1701018). The funding sponsors played no role in study design, data collection, data analysis, interpretation, writing of the report, and the decision to submit the paper for publication.
Declaration of Competing Interest
The authors have declared no conflicts of interest.
Acknowledgments
The authors are grateful to John Angles and Shiyu Chen at The State University of New York, for editing the manuscript and providing critical comments.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.11.031.
Contributor Information
Li Zhou, Email: zhouli723@csu.edu.cn.
Wei Li, Email: weililx@csu.edu.cn.
Feng Gao, Email: gf0731@csu.edu.cn.
Haidan Liu, Email: haidanliu@csu.edu.cn.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Keum N., Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019 doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 3.Nagtegaal I.D., Knijn N., Hugen N., Marshall H.C., Sugihara K., Tot T. Tumor deposits in colorectal cancer: improving the value of modern Staging-A systematic review and meta-analysis. J Clin Oncol. 2017;35(10):1119–1127. doi: 10.1200/JCO.2016.68.9091. [DOI] [PubMed] [Google Scholar]
- 4.Petrelli F., Zaniboni A., Ghidini A., Ghidini M., Turati L., Pizzo C. Timing of adjuvant chemotherapy and survival in colorectal, gastric, and pancreatic cancer. a systematic review and meta-analysis. Cancers (Basel). 2019;11(4) doi: 10.3390/cancers11040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong W., To K.K.W. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cell Mol Life Sci. 2019;76(17):3383–3406. doi: 10.1007/s00018-019-03134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szczepaniak A., Fichna J. Organometallic compounds and metal complexes in current and future treatments of inflammatory bowel disease and colorectal cancer-a critical review. Biomolecules. 2019;9(9) doi: 10.3390/biom9090398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng N., Zhou Q., Wang Z., Wei W. Recent advances in SCF ubiquitin ligase complex: clinical implications. Biochim Biophys Acta. 2016;1866(1):12–22. doi: 10.1016/j.bbcan.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan C.H., Li C.F., Yang W.L., Gao Y., Lee S.W., Feng Z. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149(5):1098–1111. doi: 10.1016/j.cell.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G., Ayala G., De Marzo A., Tian W., Frolov A., Wheeler T.M. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res. 2002;8(11):3419–3426. [PubMed] [Google Scholar]
- 10.Wang J., Huang Y., Guan Z., Zhang J.L., Su H.K., Zhang W. E3-ligase Skp2 predicts poor prognosis and maintains cancer stem cell pool in nasopharyngeal carcinoma. Oncotarget. 2014;5(14):5591–5601. doi: 10.18632/oncotarget.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsvetkov L.M., Yeh K.H., Lee S.J., Sun H., Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9(12):661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 12.Yu Z.K., Gervais J.L., Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A. 1998;95(19):11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamura T., Hara T., Kotoshiba S., Yada M., Ishida N., Imaki H. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A. 2003;100(18):10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Fukushima H., Inuzuka H., Wan L., Liu P., Gao D. Skp2 is a promising therapeutic target in breast cancer. Front Oncol. 2012;1(57) doi: 10.3389/fonc.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao Z., Huang S. E3 ubiquitin ligase Skp2 as an attractive target in cancer therapy. Front Biosci (Landmark Ed) 2015;20:474–490. doi: 10.2741/4320. [DOI] [PubMed] [Google Scholar]
- 16.Su K.J., Yu Y.L. Downregulation of SHIP2 by hepatitis B virus X promotes the metastasis and chemoresistance of hepatocellular carcinoma through SKP2. Cancers (Basel) 2019;11(8) doi: 10.3390/cancers11081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X., Wang R., Zhang Y., Zhou L., Wang W., Liu H. Skp2-mediated ubiquitination and mitochondrial localization of Akt drive tumor growth and chemoresistance to cisplatin. Oncogene. 2019 doi: 10.1038/s41388-019-0955-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L., Li M., Yu X., Gao F., Li W. Repression of hexokinases II-Mediated glycolysis contributes to piperlongumine-induced tumor suppression in non-small cell lung cancer cells. Int J Biol Sci. 2019;15(4):826–837. doi: 10.7150/ijbs.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X., Liang Q., Liu W., Zhou L., Li W., Liu H. Deguelin, an aurora B kinase inhibitor, exhibits potent anti-tumor effect in human esophageal squamous cell carcinoma. EBioMedicine. 2017;26:100–111. doi: 10.1016/j.ebiom.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Li W., Yu X., Gao F., Duan Z., Ma X. EZH2-mediated Puma gene repression regulates non-small cell lung cancer cell proliferation and cisplatin-induced apoptosis. Oncotarget. 2016;7(35):56338–56354. doi: 10.18632/oncotarget.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W., Yu X., Xia Z., Yu X., Xie L., Ma X. Repression of Noxa by Bmi1 contributes to deguelin-induced apoptosis in non-small cell lung cancer cells. J Cell Mol Med. 2018;22(12):6213–6227. doi: 10.1111/jcmm.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X., Li W., Xia Z., Xie L., Ma X., Liang Q. Targeting MCL-1 sensitizes human esophageal squamous cell carcinoma cells to cisplatin-induced apoptosis. BMC Cancer. 2017;17(1):449. doi: 10.1186/s12885-017-3442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Gao F., Ma X., Wang R., Dong X., Wang W. Deguelin inhibits non-small cell lung cancer via down-regulating hexokinases II-mediated glycolysis. Oncotarget. 2017;8(20):32586–32599. doi: 10.18632/oncotarget.15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei W., Ayad N.G., Wan Y., Zhang G.J., Kirschner M.W., Kaelin W.G., Jr. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428(6979):194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 25.Bashir T., Dorrello N.V., Amador V., Guardavaccaro D., Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428(6979):190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 26.Lin H.K., Wang G., Chen Z., Teruya-Feldstein J., Liu Y., Chan C.H. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11(4):420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao D., Inuzuka H., Tseng A., Chin R.Y., Toker A., Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11(4):397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Zhang T., Zhang S., Shan J. Prognostic values of F-box members in breast cancer: an online database analysis and literature review. Biosci Rep. 2019;39(1) doi: 10.1042/BSR20180949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S.W., Li C.F., Jin G., Cai Z., Han F., Chan C.H. Skp2-dependent ubiquitination and activation of LKB1 is essential for cancer cell survival under energy stress. Mol Cell. 2015;57(6):1022–1033. doi: 10.1016/j.molcel.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J., Zhang X., Zhang L., Wu C.Y., Rezaeian A.H., Chan C.H. Skp2 E3 ligase integrates ATM activation and homologous recombination repair by ubiquitinating NBS1. Mol Cell. 2012;46(3):351–361. doi: 10.1016/j.molcel.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A., Bumm T.G., Corbin A.S., O'Hare T., Loriaux M., VanDyke J. Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood. 2008;112(5):1960–1970. doi: 10.1182/blood-2007-09-113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H.K., Chen Z., Wang G., Nardella C., Lee S.W., Chan C.H. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464(7287):374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Bauzon F., Ji P., Xu X., Sun D., Locker J. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/- mice. Nat Genet. 2010;42(1):83–88. doi: 10.1038/ng.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan C.H., Morrow J.K., Li C.F., Gao Y., Jin G., Moten A. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013;154(3):556–568. doi: 10.1016/j.cell.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Q., Xie W., Kuhn D.J., Voorhees P.M., Lopez-Girona A., Mendy D. Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111(9):4690–4699. doi: 10.1182/blood-2007-09-112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soucy T.A., Smith P.G., Milhollen M.A., Berger A.J., Gavin J.M., Adhikari S. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 37.Khan A.Q., Siveen K.S., Prabhu K.S., Kuttikrishnan S., Akhtar S., Shaar A. Curcumin-Mediated degradation of S-Phase kinase protein 2 induces cytotoxic effects in human papillomavirus-positive and negative squamous carcinoma cells. Front Oncol. 2018;8:399. doi: 10.3389/fonc.2018.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su J., Zhou X., Wang L., Yin X., Wang Z. Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. Am J Cancer Res. 2016;6(9):1949–1962. [PMC free article] [PubMed] [Google Scholar]
- 39.Feng S., Wang Y., Zhang R., Yang G., Liang Z., Wang Z. Curcumin exerts its antitumor activity through regulation of miR-7/Skp2/p21 in nasopharyngeal carcinoma cells. Onco Targets Ther. 2017;10:2377–2388. doi: 10.2147/OTT.S130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z., Liu H., Li B., Zhang Y., Piao C. Saurolactam inhibits proliferation, migration, and invasion of human osteosarcoma cells. Cell Biochem Biophys. 2015;72(3):719–726. doi: 10.1007/s12013-015-0523-x. [DOI] [PubMed] [Google Scholar]
- 41.Su J., Wang L., Yin X., Zhao Z., Hou Y., Ye X. Rottlerin exhibits anti-cancer effect through inactivation of s phase kinase-associated protein 2 in pancreatic cancer cells. Am J Cancer Res. 2016;6(10):2178–2191. [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Y.J., Bai H.Y., Li Z.H., Zou J., Chen J.W., Zheng F. Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells. Cell Death Dis. 2014;5:e1137. doi: 10.1038/cddis.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Fu M., Liu J., Yang Y., Yu Y., Li J. Inhibition of tumor metastasis by targeted daunorubicin and dioscin codelivery liposomes modified with PFV for the treatment of non-small-cell lung cancer. Int J Nanomedicine. 2019;14:4071–4090. doi: 10.2147/IJN.S194304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao X., Xu L., Yin L., Han X., Qi Y., Xu Y. Dioscin induces prostate cancer cell apoptosis through activation of estrogen receptor-beta. Cell Death Dis. 2017;8(8):e2989. doi: 10.1038/cddis.2017.391. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Wang C., Huo X., Wang L., Meng Q., Liu Z., Liu Q. Dioscin strengthens the efficiency of adriamycin in MCF-7 and MCF-7/ADR cells through autophagy induction: more than just down-regulation of MDR1. Sci Rep. 2016;6:28403. doi: 10.1038/srep28403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao Z., Han X., Chen D., Xu Y., Xu L., Yin L. Potent effects of dioscin against hepatocellular carcinoma through regulating TP53-induced glycolysis and apoptosis regulator (TIGAR)-mediated apoptosis, autophagy, and DNA damage. Br J Pharmacol. 2019;176(7):919–937. doi: 10.1111/bph.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S., Cheng B., Hou L., Huang L., Cui Y., Xu D. Dioscin inhibits colon cancer cells' growth by reactive oxygen species-mediated mitochondrial dysfunction and p38 and JNK pathways. Anticancer Drugs. 2018;29(3):234–242. doi: 10.1097/CAD.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 48.Tao X., Yin L., Xu L., Peng J. Dioscin: a diverse acting natural compound with therapeutic potential in metabolic diseases, cancer, inflammation and infections. Pharmacol Res. 2018;137:259–269. doi: 10.1016/j.phrs.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Richards F.M., McKee S.A., Rajpar M.H., Cole T.R., Evans D.G., Jankowski J.A. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum Mol Genet. 1999;8(4):607–610. doi: 10.1093/hmg/8.4.607. [DOI] [PubMed] [Google Scholar]
- 50.Ow S.G.W., Tan K.T., Yang H., Yap H.L., Sapari N.S.B., Ong P.Y. Next generation sequencing reveals novel mutations in mismatch repair genes and other cancer predisposition genes in asian patients with suspected lynch syndrome. Clin Colorectal Cancer. 2019 doi: 10.1016/j.clcc.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Bi H., Liu Y., Tian T., Xia T., Pu R., Zhang Y. A propensity score-adjusted analysis of the effects of ubiquitin E3 ligase copy number variation in peripheral blood leukocytes on colorectal cancer risk. J Cancer. 2019;10(14):3291–3302. doi: 10.7150/jca.29872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chae Y.C., Kim J.Y., Park J.W., Kim K.B., Oh H., Lee K.H. FOXO1 degradation via G9a-mediated methylation promotes cell proliferation in colon cancer. Nucleic Acids Res. 2019;47(4):1692–1705. doi: 10.1093/nar/gky1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katona B.W., Glynn R.A., Paulosky K.E., Feng Z., Davis C.I., Ma J. Combined Menin and EGFR inhibitors synergize to suppress colorectal cancer via EGFR-Independent and calcium-mediated repression of SKP2 transcription. Cancer Res. 2019;79(9):2195–2207. doi: 10.1158/0008-5472.CAN-18-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen L., Qu X., Li H., Xu C., Wei M., Wang Q. NDRG2 facilitates colorectal cancer differentiation through the regulation of Skp2-p21/p27 axis. Oncogene. 2018;37(13):1759–1774. doi: 10.1038/s41388-017-0118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uddin S., Ahmed M., Bavi P., El-Sayed R., Al-Sanea N., AbdulJabbar A. Bortezomib (Velcade) induces p27Kip1 expression through S-phase kinase protein 2 degradation in colorectal cancer. Cancer Res. 2008;68(9):3379–3388. doi: 10.1158/0008-5472.CAN-07-6109. [DOI] [PubMed] [Google Scholar]
- 56.Adekola K., Rosen S.T., Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24(6):650–654. doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts D.J., Tan-Sah V.P., Smith J.M., Miyamoto S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem. 2013;288(33):23798–23806. doi: 10.1074/jbc.M113.482026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedersen P.L., Mathupala S., Rempel A., Geschwind J.F., Ko Y.H. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta. 2002;1555(1–3):14–20. doi: 10.1016/s0005-2728(02)00248-7. [DOI] [PubMed] [Google Scholar]
- 59.Tan V.P., Miyamoto S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy. 2015;11(6):963–964. doi: 10.1080/15548627.2015.1042195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.