Graphical abstract
Keywords: Buccal film, Proloc, Rabbits, Release, Eudragit, In vivo
Abstract
Administration of almotriptan as an oral therapy is largely limited because of poor aqueous solubility and rather low bioavailability. The aim of present investigation was to formulate oral mucoadhesive film of almotriptan to improve the drug delivery and desired therapeutic effects. Placebo films (F1-F8) were prepared by varying the concentrations of Proloc 15 (7.5-15% w/v) and Eudragit RL 100/RS 100 (15-30% w/v) polymers. Physicomechanical and pharmaceutical characteristics of drug loaded films (FA1-FA4) were examined. Selected FA4 film was evaluated in vivo by assessing the pharmacokinetic profile and compared with oral therapy in rabbits. FA1-FA4 films exhibited excellent physicomechanical properties and rapid hydration. A biphasic and considerably greater drug release (p < 0.05) was observed in FA3 and FA4 films contain higher amount of hydrophilic polymer. The rate of permeation of almotriptan was found to be significantly higher in FA4 than FA3 film (p < 0.005). Fourier transform infrared spectral scan indicates no incompatibility exists between the drug and polymers used. Differential scanning calorimetry thermogram represents the evidence of almotriptan amorphization and molecular dispersion of it in the film. Scanning electron microscopy images shows that FA4 possess good morphological features and hence suitable for use in the buccal application. In vivo data demonstrated rapid and efficient absorption (p < 0.005) of almotriptan with greater AUC0-12 (>2 folds, p < 0.0001) by FA4 film as compared to oral (control). In general, the data established the potential of FA4 film to improve the therapeutic delivery of almotriptan and offers a promising option in migraine therapy.
1. Introduction
Migraine is one of the most frequent neurological conditions affecting more than 1 billion population worldwide (Woldeamanuel and Cowan, 2017). This episodic disorder is the 3rd most widespread illness in the world and also ranks the 6th position in the list of most disabling illness (Steiner et al., 2013, Steiner et al., 2015). Typically, migraine is a harsh throbbing persistent pain generally last for long period (4–72 h), together with devastating and disabling symptoms accompanied by disturbed vision, nausea, vomiting and dizziness (Schwedt, 2014). Undeniably, this progressive disorder is one of the major public health issues and is actively associated with considerable personal suffering, depression, diminishes wellbeing, temporary disability and has a serious impact on the socioeconomic burden (Raggi et al., 2012). In addition, migraine is comorbid with disorders like, anxiety, allergies, chronic pain, epilepsy and a family history are considered as a prominent risk factors (Antonaci et al., 2011). Furthermore, the prevalence of this chronic condition differs with age and sex, wherein the females are three times more prone to such attack than males.
Existing treatment for migraine involves acute (abortive), preventive and behavioral therapy. Various categories of drugs are used in the acute and preventive therapy of this multifactorial neurovascular disorder. Literature suggests that drugs like antiepileptic, antidepressants, beta blockers are used as preventive while NSAIDs, analgesics, corticosteroids and triptans are indicated in acute migraine. The history of migraine pharmacotherapy indicates that both ergotamine and dihydroergotamine have been widely used since 1900s (Bigal and Tepper, 2003). Better understanding on the neurobiology of migraine leads to the emergence of certain selective and specific serotonin receptor (5-HT1B/1D) agonists namely triptans (Tepper et al., 2002). The development of triptans is a major revolution in the management of acute migraine pharmacotherapy. More than a few triptans have been launched into the market which are meant for oral therapy and possess distinct advantages over ergots. Marketed triptans include almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan and zolmitriptan (Bhambri et al., 2015). However, the excitement dwindled into disappointment as the therapeutic efficacy of most of the triptans are primarily limited by their poor oral bioavailability, high variations in absorption, contraindicated in patients having ischemic heart disease or coronary artery vasospasm and very high rate of recurrence of head ache (Tfelt-Hansen et al., 2000). Triptans are classified as class I and II based on their potency and onset of action. Class I oral triptans are molecules (almotriptan, eletriptan, sumatriptan, rizatriptan, zolmitriptan) which exhibits higher potency and quicker onset of action while class II drugs (naratriptan and frovatriptan) shows lower potency and slower onset of action (Pringsheim and Gawel, 2002). However, the pharmacokinetic properties of all triptans are found to be different which in turn varies their clinical efficacy, safety and tolerability.
Second generation triptans were evolved with the objective of enhancing the oral bioavailability, which also yielded with limited clinical advantages. Almotriptan, a class I and second generation oral triptan having highly specific and selective 5-HT1B/1D agonist characteristics further demonstrates high potency and nanomolar affinity. Due to improved safety and tolerability profile, almotriptan has been currently considered as the best possible choice in acute therapy of moderate to severe migraine attacks (Antonaci et al., 2016). Consequently, almotriptan has become the first triptan to be approved by the US FDA in 2009 for the use in adolescents (Lewis, 2010). Though the bioavailability of almotriptan is around 70%, the time to reach peak plasma concentration (Tmax) is quite high (2–4 h) among various triptan derivatives (Johnston and Rapoport, 2010). Thus, the clinical efficacy of this drug could be attained either by developing suitable formulations or delivering it through alternative routes. Few attempts have been already made to improve almotriptan delivery by formulating orally disintegrating tablets (Alladi and Damodharan, 2012) or delivering it through nasal route (Abbas and Marihal, 2014, Ahmed Kassem, 2016, Pradhan et al., 2009). Recently, intranasal brain targeting of mucoadhesive in situ gel formulation containing almotriptan loaded solid lipid nanoparticles was reported (Youssef et al., 2018). Attempt has also been made to improve the systemic delivery of almotriptan through the skin using physical enhancement method namely iontophoresis (Calatayud-Pascual et al., 2011). Overall, the results from these studies suggest moderate improvement in almotriptan efficacy.
Mucoadhesive buccal delivery systems have gained much attention in the last decade because the oral mucosal membrane is more permeable and provides rapid permeation of pharmaceutical actives into the systemic circulation. The buccal mucosal delivery is currently considered as the best suitable alternative for oral route as it surmounts most of the issues associated with oral therapy. The drug transported through the buccal mucosa reaches the blood capillaries, exert pharmacological activity and avoids first pass effect (Padula et al., 2018). The buccal delivery system has already attained commercial status and few molecules including buprenorphine, fentanyl and naloxone are commercially available. Indeed, this delivery route has achieved greater patient acceptance primarily due to its ease of application and termination of therapy in case of unwanted effects (Montenegro-Nicolini and Morales, 2017). Moreover, this route is optimal for the delivery of potent drug substances which are aimed for acute therapy with quick onset of action as well as for prolonged effect. A recent literature review indicates that extensive studies have been carried out on buccal drug delivery in the last decade, which also established the prospective of buccal films to improve the bioavailability of various drugs (Al-Dhubiab et al., 2016, Elagamy et al., 2019, El Sharawy et al., 2017, Jay et al., 2002, Kumria et al., 2016, Zaman et al., 2018). In this context, the clinical situation in migraine episode demands immediate delivery of almotriptan to offer quick onset of action as well as prolonged/sustained delivery, which is feasible with the buccal therapy. Moreover, the physicochemical properties of almotriptan such as low molecular weight (335 Da), high log P (1.6) and low dose (6.25 mg) are ideal for the buccal delivery (Gras et al., 2002). Taking into consideration all the aspects of buccal route and the physicochemical properties of almotriptan, the therapeutic delivery of this drug could be improved through the buccal route, while overcoming the biopharmaceutical issues. A systematic review of literature from various database sources suggests that there has been no investigation so far carried out for the buccal delivery of almotriptan. Therefore, the aim of this study was to develop mucoadhesive buccal film contain almotriptan and evaluate its feasibility as an alternative pharmacotherapy of migraine. Mucoadhesive buccal film with adequate mechanical strength was fabricated using a combination of Eudragit and Proloc polymers. These polymers were selected based on the solubility, permeability, and pH independent swelling characteristics of Eudragit as well as bioadhesive property of Proloc, which is ideal for a buccal film (Nair et al., 2018a, Thakral et al., 2013). Primary investigations were carried out to optimize the film composition using Proloc 15 and Eudragit RL 100/RS 100 polymers. Best formulation was further loaded with almotriptan and evaluated for physicomechanical and pharmaceutical characteristics. In vivo pharmacokinetics studies were performed in rabbits and compared with oral administration.
2. Materials and methods
2.1. Materials
Almotriptan (Emcure Pharmaceuticals limited, Ahmedabad, India), Proloc 15 (Henkel Corporation, Rocky Hill, Connecticut, USA), Eudragit RL 100 and Eudragit RS 100 (Evonik, Darmstadt, Germany) have been provided as gratis samples. Propylene glycol, polyvinylpyrrolidone, polyethylene glycol 400 (PEG 400), methanol, ethyl cellulose, acetone, isopropyl alcohol, dibutyl phthalate and polyvinyl pyrrolidone were commercially purchased (Sigma Aldrich, St. Louis, MO, USA). All other reagents utilized in the experimental investigation were of analytical grade.
2.2. Analysis of almotriptan
Estimation of almotriptan in various samples was analyzed by Shimadzu Prominence high performance liquid chromatography (HPLC, Tokyo, Japan) system furnished with degasser unit, pump, auto injector and column oven. A monolithic C18 HPLC column, Chromolith® Speed Rod with a 25 μl sample loop coupled to UV–Vis detector (MD-4010) with CMB 20A Shimadzu corporation software was used for analysis. Chromatographic separation of almotriptan as well as rizaptriptan benzoate (internal standard) was carried using methanol and water (30:70), pH was adjusted to pH 3 with orthophosphoric acid. Mobile phase was allowed at a flow rate of 1.0 ml/min with an injection volume of 25 µl. The isocratic elution was done at 25 °C and was monitored using UV detector at 227 nm (Lavudu et al., 2013). Linear regression analysis indicates good linearity in the almotriptan concentration of 20–600 ng/ml (r2 = 0.9982). The limit of quantitation and limit of detection of the developed method is 7.95 ng/ml and 2.62 ng/ml, respectively. The coefficient of variation and accuracy ranged 1.06–5.84% and −1.64 to −8.86, respectively. Drug level in plasma was analyzed after precipitation of proteins using equal ratio of acetonitrile and 2 propanol. The recovery of almotriptan and internal standard from plasma was 94.25 ± 2.3% and 92.51 ± 3.94%, respectively.
2.3. Optimization of film composition
Mucoadhesive buccal films were developed using conventional solvent casting technique. The types and composition of film forming polymers and plasticizers tested for the preparation of placebo films (F1-F8) is compiled in Table 1. In short, the required quantity of Proloc 15 was added gradually to the adequate amount of water with continuous mixing by a magnetic stirrer (MLH-2; Remi, Mumbai, India) for 15 min to obtain polymeric dispersion (Table 1). Prepared Proloc dispersion was then added to Eudragit dispersion prepared in ethyl alcohol (70% v/v) and thoroughly mixed by vortexing. Then, PEG 400 or propylene glycol was added gradually in drop wise and mixed for 30 min to obtain uniform dispersion. The viscous solution obtained was further sonicated (Qsonica, LLC, Misonix, USA) for 30 min for deaeration. The polymer-drug solution (~10 ml) was later transferred to the petri dishes (90 mm diameter) and dried in a hot air oven at 40 °C.
Table 1.
Compositions of placebo films.
| Batch code | Proloc 15 (% w/v) | Eudragit RL 100 (% w/v) | Eudragit RS 100 (% w/v) | PEG 400 (% w/v) | PG (% w/v) | Film characteristics |
|---|---|---|---|---|---|---|
| F1 | 7.5 | 30 | – | 2.5 | 2.5 | Hard film |
| F2 | 10 | 25 | – | 2.5 | 2.5 | Soft, homogenous, sticky film |
| F3 | 12.5 | 20 | – | 2.5 | 2.5 | Soft, homogenous, peelable film |
| F4 | 15 | 15 | – | 2.5 | 2.5 | Minor cracking observed |
| F5 | 7.5 | – | 30 | 2.5 | 2.5 | Hard film |
| F6 | 10 | – | 25 | 2.5 | 2.5 | Soft and sticky film |
| F7 | 12.5 | – | 20 | 2.5 | 2.5 | Soft, homogenous, sticky film |
| F8 | 15 | – | 15 | 2.5 | 2.5 | Soft, homogenous, peelable film |
PEG: polyethylene glycol; PG: propylene glycol.
2.4. Preparation of almotriptan loaded films
Optimized placebo formulations (F3 and F8) were incorporated with almotriptan to obtain matrix films (Table 2). Almotriptan loaded films were prepared with the same experimental protocol used for the preparation of placebo formulations. Films were developed with drug to obtain two different concentrations of almotriptan like 6.25 mg/cm2 and 12.58 mg/cm2 (Table 2). The films were cut into appropriate size, packed in aluminum foil and stored in desiccator at room temperature (25 ± 1 °C). The backing membrane for the buccal film was formulated using ethyl cellulose solution (5% w/v) and dibutyl phthalate (2% v/v) (Kumria et al., 2016). It was allowed to dry, and the film developed was later adhered to the drug loaded film using polyvinyl pyrrolidone (5% w/v).
Table 2.
Compositions of almotriptan loaded mucoadhesive buccal films.
| Batch code | Drug (% w/v) | Proloc 15 (% w/v) | Eudragit RL 100 (% w/v) | Eudragit RS 100 (% w/v) | PEG 400 (% w/v) | PG (% w/v) |
|---|---|---|---|---|---|---|
| FA1 | 4 | 12.5 | 20 | – | 2.5 | 2.5 |
| FA2 | 8 | 12.5 | 20 | – | 2.5 | 2.5 |
| FA3 | 4 | 15 | – | 15 | 2.5 | 2.5 |
| FA4 | 8 | 15 | – | 15 | 2.5 | 2.5 |
PEG: polyethylene glycol; PG: propylene glycol.
2.5. Evaluation of almotriptan buccal films
Developed films were examined to assess the various physico-mechanical characteristics including color, clarity, flexibility, pliability and uniformity. The color and transparency were recorded against dark and white background against light. The softness and flexibility were checked by physical touch with the prepared buccal films.
2.5.1. Thickness and pH
The thickness of the films was determined at 5 different sites to ascertain thickness uniformity by means of digital micrometer. The pH of the films was assessed by taking suitable size (1 cm × 1 cm) and permitted to swell in petri dishes containing minimum quantity (5 ml) of deionized water for 30 min. The swollen films were taken out and the excess surface moisture was wiped with the edge of Whatman No.1 filter paper and the pH of the film was recorded using surface electrodes (Orion 4 Star Benchtop, Thermo Fischer Scientific Inc., Waltham, MA).
2.5.2. Drug content
The uniform distribution of almotriptan was assessed by estimating the drug content in each film. Three film units (1 cm × 1 cm) were cut from six randomly selected films in each formulation. Accurately weighed films were individually immersed in mobile phase for 30 min under continuous stirring on a water bath (37 ± 1 °C) for 6 h. The solutions were filtered through a 0.2 μm syringe membrane (Millipore, Bedford, MA), diluted suitably and estimated using HPLC.
2.5.3. Folding endurance
Six films of specific dimension, each measuring 4 cm × 4 cm were cut from particular formulation and folded at the same place repeatedly making an angle of 180°. The number of times the strip could be folded without breaking was noted as the value for film endurance test.
2.5.4. Mucoadhesive properties
The oral mucosal membrane of white rabbits (2.5–3.0 kg) was excised and the adhering connective tissue was scraped off using scalpel and scissors. The mucoadhesive strength of the films was carried out by employing a texture analyzer (Stable Micro systems, Surrey, UK). The buccal membrane of rabbit was examined for its biological integrity before affixing to the static platform of the texture analyzer. Around 2 ml of simulated saliva (pH 6.2) was added to the static assembly which was holding the buccal membrane to wet the tissue during contact time. The composition of simulated saliva used in the study consists of 12 mM of potassium di hydrogen phosphate, 40 mM of sodium chloride and 1.5 mM of calcium chloride and the pH was calibrated to 6.2 using sodium hydroxide (Nair et al., 2013). The film strips of suitable size (1 cm × 1 cm) was secure to the probe of texture analyzer with the help of double-sided adhesive tape. The probe of texture analyzer was slide down to contact the membrane at a probe speed of 0.5 mm/s for a period of 60 s at fixed applied force (1N) (Kumria et al., 2016). Mucoadhesive strength is determined as the force in dynes needed to separate the film from adhering rabbit mucosal epithelium.
2.5.5. Percentage hydration
Films of particular size (1 cm × 1 cm) were cut by using sharp blade and weighed. Samples of the films were kept on a pre-weighed stainless-steel wire mesh and allowed to immerse separately in 25 ml of phosphate buffer saline (pH 6.8) maintained at 37 ± 1 °C by means of thermostat. At specific time intervals (1–5 h), the films were taken out, excess moisture on its surface was wiped off using Whatman No.1 filter paper. The swollen films were weighed again and the percentage hydration of the films was computed by the given equation (Kumria et al., 2014).
Where W1 is the weight of the film at zero time and W2 is the weight of the swollen film after certain time ‘t’.
2.6. Drug release
The in vitro release of almotriptan from FA1-FA4 films was performed by paddle over disc method using USPXXIV Type 2 apparatus (Electro Lab TDC 50, Mumbai, India) as per the product performance methodology described in USP, Ph. Eur. and FDA (Jug et al., 2018). Six films selected from individual formulation and each measuring specified size (2 cm × 1 cm) were cut and adhered to the glass slide. The glass slide was placed in the dissolution jar such that the drug containing surface of the film was visible towards the medium. Simulated saliva (900 ml; pH 6.2) was the medium used for the dissolution experiment, the temperature was set to 37 ± 0.5 °C and the paddle was rotated at 50 rpm. Samples withdrawn at various time intervals were filtered using syringe membrane filter (0.2 μm, Millipore, Bedford, MA, USA) and readily estimated by HPLC. The drug release data were fitted into established mathematical release kinetics models such as zero order, first order, Higuchi, Korsmeyer-Peppas, Weibull and Hixon-Crowell using KinetDS 3.0 software to predict the drug release pattern of formulated buccal films.
2.7. Ex vivo permeation
The rate and extend of mucosal permeation of almotriptan from FA3 and FA4 films across the rabbit buccal mucosa was determined on a Franz diffusion cell. The excised membrane was fixed on the diffusion cell such that the smooth and polished buccal surface facing towards the donor compartment. The prepared films were cut into small sections (0.6 cm2) and positioned on the surface of the buccal epithelium. Simulated saliva (pH 6.2; 5 ml) was filled in the receptor compartment. The media was stirred at 500 rpm by means of a 2 mm magnetic bead and the temperature was set to 37 ± 0.5 °C. Aliquots (1 ml) of the samples were drawn at specific time periods for each formulation, at 13,416g centrifuged (R-83; Remi, Mumbai, India) for 10 min and analyzed by HPLC.
2.8. Fourier transform infrared (FTIR) spectroscopy
The infrared spectra of almotriptan, buccal film (FA4), physical mixture and placebo film were recorded on FTIR spectrophotometer (FT/IR-6100, Jasco, Tokyo, Japan). An intimate mixture of samples was prepared by grinding with anhydrous potassium bromide powder at 1:5 (sample: KBr) ratio and the pellets were prepared using a hydraulic press (5000–10,000 psi). The spectral scan of the discs were drawn in transmittance mode between the ranges of 400–4000 cm−1.
2.9. Differential scanning calorimetry (DSC)
The thermal properties of almotriptan, buccal film (FA4), and placebo film was analyzed using DSC instrument (DSC 60, Shimadzu Corp., Japan). An accurately weighed (5 mg) quantity of test samples were put in an aluminum pans and crimped nonhermetically while an empty pan at the same experimental condition was used as reference during analysis. The thermal scan of drug, polymer and film were performed between 50 and 300 °C and operated at a heating rate of 10 °C/min in an inert nitrogen atmosphere.
2.10. Surface morphology
The morphology and surface characteristics of FA4 film was studied by scanning electron microscopy (SEM). The micrographs of FA4 film were taken by fixing it on an aluminum stud by employing double sided adhesive carbon tape. The formulation was coated with gold–palladium by using the “POLORON”E5100 SEM coating system in neutral environment of argon maintained under vacuum. The images were recorded at HT-15 kV accelerating voltage employing Nova NanoSEM 450 (FEI Ltd., USA) 26–27 mm working distance and a probe current of 3 × 10−10 A.
2.11. In vivo evaluation
In vivo studies of FA4 film was done in male rabbits weighing 2.5–3.0 kg. Animals were housed separately in a ventilated room in standard cages that was maintained at 20–24 °C under a 12- h light/12-h dark cycle with free access to diet and water. Animals used for the experiment were fasted overnight and provided with water ad libitum. The guidelines of Institutional Animal ethical committee (IAEC/SSP/19/PR-042) was strictly followed throughout the in vivo investigations. Animals used in the study were anaesthetized by administering ketamine (40 mg/kg) and xylazine (5 mg/kg) (Nair et al., 2018b) through intramuscular route. The buccal film measuring 1 cm × 1 cm was wetted with a small quantity of water (30 µl) and attached to the buccal mucosa of the rabbits. The period of application of FA4 film was 3 h. An oral suspension of almotriptan equivalent to 12.58 mg was given to animals belong to control group. At pre-determined point of times (0.5, 1, 2, 4, 8 and 12 h), aliquots of blood samples (500 µl) were drawn from the marginal ear vein of the rabbits. The samples were analyzed using the previously described HPLC method.
2.12. Data analysis
The steady state flux was calculated from the slope of the graph plotted between the cumulative amount of drug permeated across the membrane versus time. Paired t-test was performed by Graph pad Prism (version 8, Graph-Pad Software, Inc., La Jolla, CA, USA). During data analysis a P value of less than 0.05 was used to determine the level of statistical significance.
3. Results and discussion
3.1. Preparation of buccal films
Buccal films generally consist of mucoadhesive as well as film forming polymers. Initial studies were conducted by preparing eight placebo films (F1-F8) with varying quantities of mucoadhesive and film forming polymers (Table 1). Proloc 15 possess good mucoadhesive/film forming properties and could be easily manipulated to obtain the desired drug release profiles (Nair et al., 2018a). Two water insoluble film forming polymers namely, Eudragit RL 100 and Eudragit RS 100 were evaluated for their effect on various physicochemical characteristics, drug release and permeation from almotriptan loaded films. These polymers have already demonstrated their potential in developing mucoadhesive buccal films with various drugs (Al-Dhubiab et al., 2016, Kumria et al., 2014, Nair et al., 2018a). The concentrations of Proloc 15 (7.5–15% w/v) and Eudragits (15–30% w/v) were varied to optimize mucoadhesive buccal film composition for clinical use. Two plasticizers (PEG 400 and propylene glycol) of similar concentration (2.5% w/v) were utilized as these agents shown better plasticity when combined, rather than using each alone. The characteristics of placebo films are outlined in Table 1. It is evident from Table 1 that the concentrations of polymer influenced the film formation. Formulations F3 [Proloc 15 (12.5% w/v) and Eudragit RL 100 (20% w/v)] and F8 [Proloc 15 (15% w/v) and Eudragit RS 100 (20% w/v)] showed suitable film characteristics and was soft, homogenous and peelable. Hence, formulations F3 and F8 were selected to incorporate almotriptan and films FA1, FA2, FA3 and FA4 were prepared. The composition of FA1-FA4 films are summarized in Table 2. Drug loading was done at two varied concentrations (4% w/v and 8% w/v) to evaluate its influence on physico-mechanical properties.
3.2. Characterization
The drug loaded buccal films (FA1-FA4) were dry, transparent, flexible, peelable, tack free and homogenous. The observed physico-mechanical properties of FA1-FA4 films are summarized in Table 3. Maintaining uniform thickness of the buccal film is critical to establish the homogenous dispersion of the drug in the film and for satisfactory mucoadhesion (Nair et al., 2013). The measured thickness of FA1-FA4 films were approximately 1 mm (Table 3) which implies their usefulness for buccal application with least discomfort to the patients. However, marginal increase in thickness was observed in FA1-FA4 films (0.94–1.12 mm) as compared to placebo films (F3 and F8; 0.82–0.88 mm). The pH of FA1-FA4 films was measured to ensure the suitability for the buccal application. The pH of FA1-FA4 films was found to be around neutral (pH 6–7.5) and close to the buccal pH of 6.78 (Aframian et al., 2006). Hence, the films are not expected to induce any sensitivity reaction or irritation to the buccal mucosa (Table 3). It was noticed that the incorporation of almotriptan has caused minor reduction in the pH of FA1-FA4 as compared to placebo films (Table 3).
Table 3.
Physicomechanical properties of prepared mucoadhesive buccal films.
| Batch code | Thickness (mm) | pH | Folding endurance | Drug content (%) | Mucoadhesive strength (N) |
|---|---|---|---|---|---|
| F3 | 0.88 ± 0.08 | 7.7 ± 0.4 | 315 ± 35 | – | 6.9 ± 0.7 |
| F8 | 0.82 ± 0.19 | 7.5 ± 0.5 | 360 ± 44 | – | 7.4 ± 0.5 |
| FA1 | 0.98 ± 0.11 | 7.1 ± 0.4 | 326 ± 38 | 93.5 ± 2.2 | 6.8 ± 0.3 |
| FA2 | 1.12 ± 0.32 | 6.8 ± 0.3 | 334 ± 45 | 95.1 ± 4.3 | 7.0 ± 0.2 |
| FA3 | 0.94 ± 0.15 | 6.7 ± 0.4 | 365 ± 37 | 94.8 ± 2.2 | 7.5 ± 0.6 |
| FA4 | 1.05 ± 0.26 | 6.5 ± 0.5 | 368 ± 32 | 96.4 ± 2.9 | 7.7 ± 0.5 |
All values are expressed as mean ± S.D; n = 6.
Flexibility and mechanical strength of prepared films were evaluated by measuring the folding endurance. The data observed in Table 3 suggests that FA1-FA4 films possess adequate folding endurance (>300) and is mechanically strong which in turn would resist breaking or tearing during application. It is also evident from Table 3 that folding endurance value of films is not influenced by the amount of drug or polymer, in the present investigational settings.
Content uniformity is another critical quality control parameter to be evaluated in any pharmaceutical preparations as it confirms the availability of drugs. It can be seen from Table 3 that the drug content does not vary among the films and is >90% in all the prepared films. These comparable values suggest that the change in polymer or drug load have no effect on drug content, in the present experimental conditions. The low standard deviation observed in Table 3 also denotes that the drug was uniformly dispersed within the film (Nair et al., 2017).
Mucoadhesion is a primary prerequisite for the effective buccal delivery since the poor adhesion may frequently results in either spitting or ingestion of the film. Therefore, mucoadhesive properties is a crucial parameter that indicates the performance of the buccal films especially in formulation development. The measurement of mucoadhesive strength provides binding ability of polymer included in the adhesive system to the buccal epithelia during application (Nair et al., 2013). The results in Table 3 suggests that the prepared placebo as well as drug loaded films possess adequate mucoadhesion (>6.5 N), probably contributed by Proloc 15. The polymer, Proloc 15 is a physical mixture constituted of a waxy maize starch (85% w/w) and carbomer (15% w/w) which exhibits an excellent mucoadhesive property. The adhesive property of Proloc 15 is mainly due to the carbomer which can potentially associate with the glycoprotein of the mucus and hence localized to a particular site (Nair et al., 2018a). Table 3 also indicates that film components have influenced the mucoadhesive strength. Greater mucoadhesion (approximately 7.5 N) was observed when the Proloc content was high (15% w/v). This observation is in agreement with earlier studies where it was demonstrated that an increase in the mucoadhesive polymer content generally leads to higher mucoadhesive strength (Kumria et al., 2014, Salehi and Boddohi, 2017). Overall, the physicomechanical properties of the FA1-FA4 films were comparable (Table 4).
Table 4.
Mean pharmacokinetic parameters of almotriptan in plasma following the buccal application of mucoadhesive buccal film (FA4) and control (oral solution of almotriptan equivalent to 12.58 mg) in rabbits.
| Parameter | Buccal film (FA4) | Control |
|---|---|---|
| Tmax (h) | 1 | 1 |
| Cmax (ng/ml) | 401.77 ± 85.27 | 215.55 ± 38.79 |
| AUC0-12 (ng·h/ml) | 2380.99 ± 486.51 | 962.51 ± 102.34 |
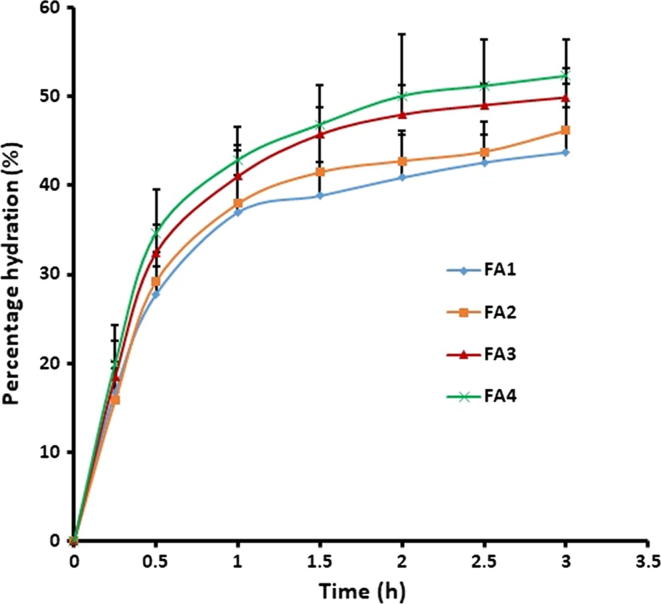
Degree of swelling is crucial for the bioadhesive polymer to relax and generate a macromolecular network of certain sizes and in addition to enhance the mobilization of the polymer chains so as to promote the interpenetration between polymer and mucin (Boateng and Okeke, 2019). In general, the swelling of film depends on film composition, polymer matrix characteristics and the type of polymers used (Avachat et al., 2013). When film is applied to the buccal epithelium, diffusion of the water molecules to the polymer matrix takes place leading to hydration and swelling. The magnitude of film hydration influences the film adhesion as well as the drug release. Water uptake can cause relaxation of the initially stretched, entangled or twisted polymer chains resulting in exposing of all available bioadhesive binding sites for bonding (Asane et al., 2008). The percentage hydration was calculated by evaluating the swelling property of the films. Fig. 1 indicates that the percentage hydration pattern of all the films (FA1-FA4) were slightly different, although statistically insignificant. In all the cases, the rate of hydration was quick in first hour as noticed by a sharp curve during this initial phase and was followed by a state of comparatively gradual swelling until 3 h, which also signifies that the hydration is reaching saturation. In general, the percentage hydration decreases after reaching saturation due to distortion of polymer molecule and/or erosion of the polymer matrix (Al-Dhubiab et al., 2016). Greater initial hydration (approximately 40%) recommends the capability of FA1-FA4 films to hydrate and adhere with the buccal mucosa. The rapid hydration detected in FA1-FA4 films is mostly because of the hydrophilic nature of the polymer (Proloc 15). Further, the films exhibited sufficient robustness and endurance till the end of 3 h and no deformity or erosion was observed.
Fig. 1.
The percentage hydration pattern of the prepared buccal films determined using 1 cm × 1 cm of the film. Data were expressed as mean ± SD (n = 6).
3.3. Drug release
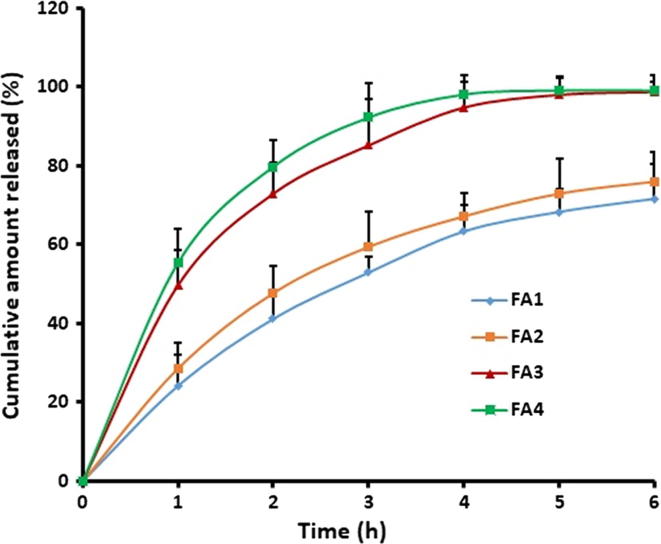
The release of drug from mucoadhesive film to the surface of buccal mucosa is necessary for its efficient transport through the buccal epithelium. In general, the solubility of drug/polymer and the diffusion coefficient of drug in the polymer are the primary factors that influence the release of drug from the polymer matrix (Fu and Kao, 2010). It was proposed that an in vitro-in vivo correlation established between in vitro dissolution/release model can successfully serve as a surrogate for human bioequivalence studies of buccal formulations (Jug et al., 2018). Therefore, assessment of drug release from developed buccal films was carried out by evaluating the influence of the amount of Proloc 15 and Eudragits (RL 100 and RS 100) on the almotriptan release from FA1-FA4 films. Fig. 2 illustrates the percentage of almotriptan released from FA1-FA4 buccal films. It is evident from Fig. 2 that the drug release profiles of the FA1 and FA2 films are recognizable from FA3 and FA4 films, suggesting the formulation components have influenced the almotriptan release significantly (p < 0.05). Considerably higher as well as rapid drug release was observed with FA3 and FA4 films, while it was slow in FA1 and FA2 films. The drug release profiles of FA3 and FA4 films exhibited a biphasic release pattern with > 40% of drug being released in initial hour (1 h) followed by a slow and steady release and was nearly complete in 4 h (Fig. 2). On the other hand, in FA1 and FA2 films, the drug release in 1 h and 6 h were approximately 25% and 75%, respectively. The observed difference in drug release profile could be due to the variation in the polymer composition used in the current study and can be corroborated to the percentage hydration pattern observed in Fig. 1. In case of FA3 and FA4 films, the higher content of hydrophilic polymer (Proloc-15% w/v) and lower amount of water insoluble polymer (Eudragit RS 100–15% w/v) would have favored the easy diffusion of drug molecules from the films. In contrast, FA1 and FA2 films had lower amount of Proloc (12.5% w/v) and higher amount of Eudragit RL 100 (20% w/v) led to slow diffusion and release of almotriptan. These results are in accordance with previous investigations wherein it was demonstrated that inclusion of hydrophobic polymers like Eudragit slows down the release of the drug from the bioadhesive films (De Caro et al., 2019, Di Prima et al., 2019). Separately, comparable drug release profiles of FA1 and FA2 suggests that the variation in the amount of drug did not affect the release rate.
Fig. 2.
Comparison of the cumulative percentage of almotriptan released from different buccal films. In vitro drug release study was carried out by placing the film (2 cm × 1 cm) in an USP apparatus (paddle over disc) using simulated saliva as dissolution medium. Data were expressed as mean ± SD (n = 6).
The rapid drug release observed with FA3 and FA4 films during the initial period could be beneficial since it provides larger amount of drug at the mucosal surface for diffusion across buccal epithelium for immediate achievement of desired therapeutic drug concentration. Additionally, an ideal formulation for migraine therapy is expected to provide an immediate release followed by sustained drug release for predetermined time period. The rapid achievement of minimum effective concentration in the plasma can contribute an immediate clinical response to patient and thereby the desired anti-migraine effects. The drug release was slow and partial from the FA1 and FA2 films, therefore excluded from further studies.
Drug release kinetics of FA3 and FA4 films was also assessed by various models to determine the underlying release mechanism. Various parameters were assessed to select the ideal mathematical model among various good fits (Shah et al., 2019). Based on regression coefficient calculation, FA3 and FA4 films shown highest r2 value of 0.9918 and 0.9841, respectively with Weibull model kinetics. The sum of squares of residuals (SSR) and Fischer Ratio (F) are two additional parameters used to reduce the error in predicting the release mechanism. A minimum value of these parameters indicates a good fit of the model to the data and minimum deviation from the actual data. The minimum values of SSR i.e. 13.1507 and 4.0696 for FA3 and FA4 films, respectively and F value of 2.6301 and 0.8139, respectively were found for Weibull model. Therefore, it is confirmed that the release of almotriptan from FA3 and FA4 films was Weibull Diffusion controlled mechanism.
3.4. Ex vivo permeation
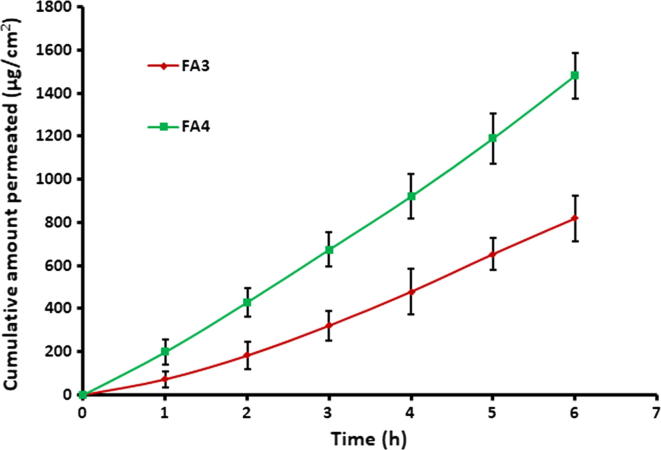
Permeation studies employing biological membranes is an important practical approach to anticipate the absorption and the kinetics of drug molecules. Drug transport across the biological membrane is mediated by the combined effects of the physicochemical properties of the drug and the physiological characteristics of the barrier membrane (Kumria et al., 2018). The almotriptan permeation from FA3 and FA4 films was investigated in an ex vivo environment using the rabbit mucosa which has non-keratinized mucosal lining resembling more closely to the human buccal mucosa (Sohi et al., 2010). The amount of almotriptan incorporated in the films was calculated based on its standard human dose (Nair et al., 2018b). The amount of drug penetrated through the rabbit cheek mucosal membrane and entered the receptor compartment at different time interval was measured and is displayed in Fig. 3. Typical permeation profiles were exhibited by both FA3 and FA4 films signifies the capability of prepared films to transport almotriptan into and across the buccal mucosal epithelium. Adequate swelling with sufficient mucoadhesion provided by Proloc would have caused diffusion of drug molecules through the buccal membrane. Nevertheless, the rate of drug transport was significantly more in FA4 than FA3 films (p < 0.005), as the amount of almotriptan permeated in each time intervals was higher in FA4. The greater almotriptan permeation observed in FA4 is due to the higher drug concentration (8% w/v) as compared to FA3 (4% w/v). In addition, both FA3 and FA4 films shown short lag time of 0.17 ± 0.05 h and 0.42 ± 0.14 h, respectively. The steady state flux values for FA3 and FA4 films were calculated as 139.88 ± 17.81 µg/cm2/h and 247.09 ± 26.27 µg/cm2/h (p < 0.0001), respectively. The cumulative amount of drug delivered at the end of 6 h of the study period was recorded to be 819.63 ± 46.45 µg/cm2 and 1481.51 ± 106.90 µg/cm2 for FA3 and FA4 films, respectively. Since FA4 film showed higher flux value, it was selected for further studies.
Fig. 3.
Comparison of ex vivo permeation profile of almotriptan from FA3 and FA4 buccal films using the rabbit buccal mucosa for a period of 6 h in simulated saliva (pH 6.2) at 37 ± 0.5 °C. Data were expressed as mean ± SD (n = 6).
3.5. FTIR
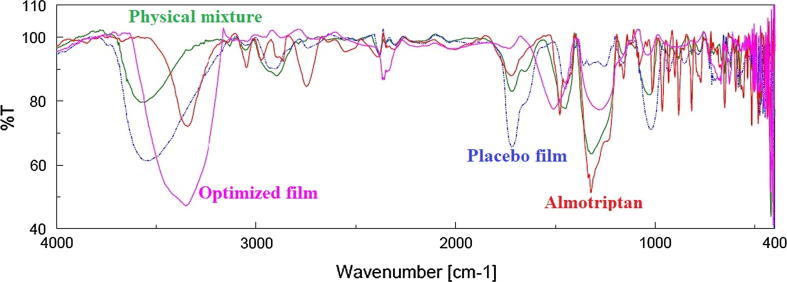
The FTIR spectrum of almotriptan, buccal film (FA4), physical mixture and placebo film are shown in Fig. 4. The fundamental peaks correspond to the major functional groups of almotriptan are observed in the structure. The peak at 3350 cm−1 assigns to the N—H stretching of secondary amine, 1480 cm−1 is due to C C stretching while peak at 1324 cm−1 is due to C—H bending (Dungarwal and Patil, 2016). The spectra of physical mixture show all characteristic peaks of drug and polymer. The spectra of FA4 also exhibited all the principle peaks present in pure drug indicates no incompatibility between the drug and polymer used in the formulations.
Fig. 4.
FTIR spectra of almotriptan, buccal film (FA4), physical mixture and placebo film.
3.6. DSC
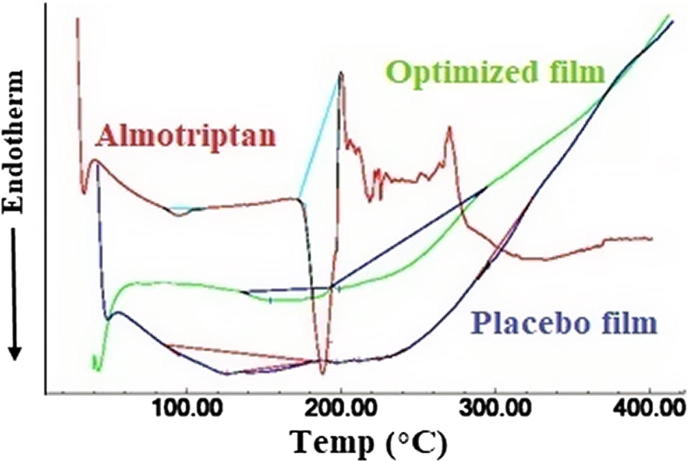
DSC technique was used to describe the physical state of drug as well as the changes in thermodynamic properties that might have happened inside the film. The change in process like melting and solid phase transformations indicated by endothermic or exothermic peaks were observed. The thermograms of almotriptan, placebo film and FA4 film is illustrated in Fig. 5. The melting point and crystalline nature of almotriptan was indicated by a sharp endothermic peak observed at 188 °C (Abbas and Marihal, 2014). In the thermogram of FA4 film no characteristic peak was observed at 188 °C, suggesting drug is molecularly dispersed in the film. Similarly no characteristics peaks were noticed in the placebo films.
Fig. 5.
Differential scanning calorimetric curves of almotriptan, optimized film (FA4) and placebo film.
3.7. SEM
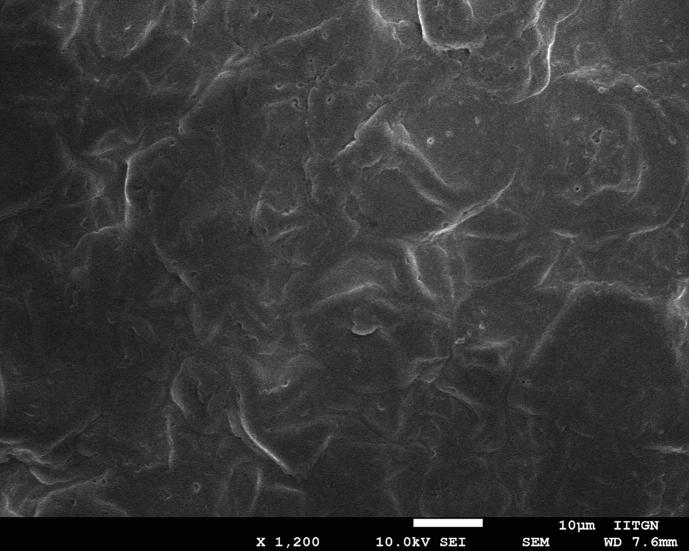
The surface characteristics of the selected buccal film (FA4) was inspected utilizing the high-resolution images provided by SEM. It is noticeable from the representative micrograph (Fig. 6) that the buccal film (FA4) appears to be tortuous and slightly irregular in texture. Few minor pores are also seen on the film which could help in water permeability. However, no visible cracks were noticed in the film matrix of the prepared films. Overall, the micrograph illustrates that external features of drug entrapped film is good and can be used for buccal application.
Fig. 6.
Scanning electron microscopy image of buccal film (FA4).
3.8. In vivo evaluation
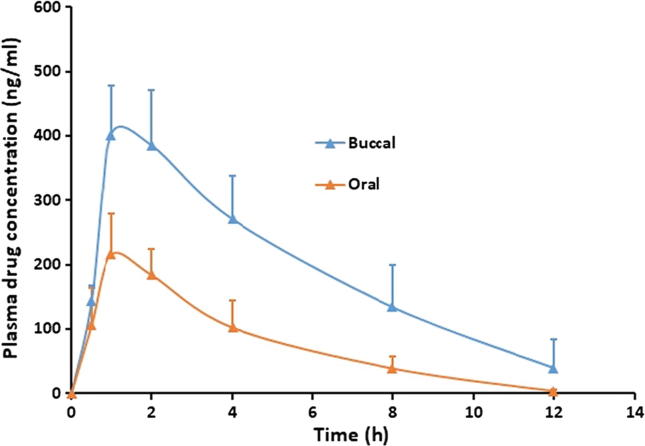
In vivo study in suitable animal models can provide a better understanding about the performance of prepared films in real situation. Therefore, in the final stage of the investigation, the pharmacokinetic evaluation of almotriptan from FA4 film was performed and compared with its oral counterpart. The evaluation was done in rabbit model since it is considered primary choice used in evaluating mucoadhesive films (Jay et al., 2002, Rana and Murthy, 2013). The dose of almotriptan used in both routes was fixed (12.58 mg) to estimate the relative bioavailability. Various pharmacokinetic parameters namely area under the plasma drug concentration time profile (AUC0-12), maximum concentration of drug (Cmax) and Tmax were determined by applying a non-compartmental method. Fig. 7 correlates the plasma profiles of FA4 film after buccal application and oral administration of control. The plasma profiles of almotriptan in buccal and oral route were found to be very distinct, indicating that the absorption of almotriptan is mainly affected by the route of administration. It is obvious from Fig. 7 that the absorption of almotriptan is fast in both routes and amount of drug found in plasma at 30 min (buccal; 143.46 ± 21.49 ng/ml, oral; 105.95 ± 58.21 ng/ml) were comparable. At 1 h, the drug concentration further increased in both routes and attained Cmax, however was significantly different (p < 0.005). The estimated Cmax, Tmax and AUC0-12 values are mentioned in Table 4. The higher Cmax value (approximately 2 folds) observed in FA4 film shows greater absorption of almotriptan suggesting better therapeutic delivery in buccal route as compared to oral. The greater absorption of almotriptan noticed in buccal therapy could be corroborated to the higher drug release observed from the FA4 film, which would have eventually diffused across the buccal membrane, without much resistance. Followed by Cmax, the drug plasma concentration declines rapidly in both routes, probably owing to the short biological half-life (3–4 h) of almotriptan. The mean AUC0-12 value observed in the buccal route was >2 folds higher (p < 0.0001) than the oral route. Table 4 implies superior bioavailability through the buccal route achieving up to 200% enhancement in comparison to oral. Overall, the current findings demonstrate that plasma drug concentration has achieved higher when the drug is delivered through the buccal route as compared to its oral counterpart.
Fig. 7.
Comparison of the plasma profiles of almotriptan following the buccal application of mucoadhesive buccal film (FA4) and control (oral suspension of almotriptan equivalent to 12.58 mg) in rabbits. Data were expressed as mean ± SD (n = 6).
4. Conclusion
Preliminary investigations were done to optimize the film composition by changing the amount of polymers and prepared placebo films (F1-F8). Drug was loaded in the selected placebo films and FA1-FA4 films with two different drug concentrations (4% w/v and 8% w/v) were prepared and evaluated. Physicomechanical properties, mucoadhesion and percentage hydration exhibited by FA1-FA4 films were found ideal for the buccal application. Promising results in drug release and permeation studies signified the feasibility of the buccal delivery of almotriptan. In vivo data demonstrate the improvement in therapeutic delivery of almotriptan through the buccal route as evidenced by higher Cmax and AUC0-12, compared to oral administration. In nutshell, this study demonstrated the benefit of trans-mucosal administration of almotriptan for the pharmacotherapeutic management of migraine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the Deanship of Scientific Research, King Faisal University for the financial support of research project (No. 170116). Also the authors acknowledge Mr. Tameem Al-Yahian, College of Clinical Pharmacy for his support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas Z., Marihal S. Gellan gum-based mucoadhesive microspheres of almotriptan for nasal administration: Formulation optimization using factorial design, characterization, and in vitro evaluation. J. Pharm. Bioallied. Sci. 2014;6(4):267–277. doi: 10.4103/0975-7406.142959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aframian D.J., Davidowitz T., Benoliel R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006;12(4):420–423. doi: 10.1111/j.1601-0825.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- Ahmed Kassem A. Formulation approaches of triptans for management of migraine. Curr. Drug Deliv. 2016;13(6):882–898. doi: 10.2174/1567201813666160425112600. [DOI] [PubMed] [Google Scholar]
- Al-Dhubiab B.E., Nair A.B., Kumria R., Attimarad M., Harsha S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016;23(7):2154–2162. doi: 10.3109/10717544.2014.948644. [DOI] [PubMed] [Google Scholar]
- Alladi A., Damodharan N. Formulation, taste masking and evaluation of almotriptan oral disintegrating tablets. Int. J. Pharm. Sci. Res. 2012;3(10):3940–3946. [Google Scholar]
- Antonaci F., Ghiotto N., Wu S., Pucci E., Costa A. Recent advances in migraine therapy. Springerplus. 2016;5:637. doi: 10.1186/s40064-016-2211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonaci F., Nappi G., Galli F., Manzoni G.C., Calabresi P., Costa A. Migraine and psychiatric comorbidity: a review of clinical findings. J. Headache Pain. 2011;12(2):115–125. doi: 10.1007/s10194-010-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asane G.S., Nirmal S.A., Rasal K.B., Naik A.A., Mahadik M.S., Rao Y.M. Polymers for mucoadhesive drug delivery system: A current status. Drug Dev. Ind. Pharm. 2008;34(11):1246–1266. doi: 10.1080/03639040802026012. [DOI] [PubMed] [Google Scholar]
- Avachat A.M., Gujar K.N., Wagh K.V. Development and evaluation of tamarind seed xyloglucan-based mucoadhesive buccal films of rizatriptan benzoate. Carbohydr. Polym. 2013;91(2):537–542. doi: 10.1016/j.carbpol.2012.08.062. [DOI] [PubMed] [Google Scholar]
- Bhambri R., Mardekian J., Liu L.Z., Schweizer E., Ramos E. A review of the pharmacoeconomics of eletriptan for the acute treatment of migraine. Int. J. Gen. Med. 2015;8:27–36. doi: 10.2147/IJGM.S73673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal M.E., Tepper S.J. Ergotamine and dihydroergotamine: a review. Curr. Pain Headache Rep. 2003;7(1):55–62. doi: 10.1007/s11916-003-0011-7. [DOI] [PubMed] [Google Scholar]
- Boateng J., Okeke O. Evaluation of clay-functionalized wafers and films for nicotine replacement therapy via buccal mucosa. Pharmaceutics. 2019;11(3):104. doi: 10.3390/pharmaceutics11030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calatayud-Pascual M.A., Balaguer-Fernández C., Serna-Jiménez C.E., Del Rio-Sancho S., Femenía-Font A., Merino V., López-Castellano A. Effect of iontophoresis on in vitro transdermal absorption of almotriptan. Int. J. Pharm. 2011;416(1):189–194. doi: 10.1016/j.ijpharm.2011.06.039. [DOI] [PubMed] [Google Scholar]
- De Caro V., Murgia D., Seidita F., Bologna E., Alotta G., Zingales M., Campisi G. Enhanced in situ availability of aphanizomenon flos-aquae constituents entrapped in buccal films for the treatment of oxidative stress-related oral diseases: biomechanical characterization and in vitro/ex vivo evaluation. Pharmaceutics. 2019;11(1):35. doi: 10.3390/pharmaceutics11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prima G., Conigliaro A., De Caro V. Mucoadhesive polymeric films to enhance barbaloin penetration into buccal mucosa: a novel approach to chemoprevention. AAPS PharmSciTech. 2019;20(1):18. doi: 10.1208/s12249-018-1202-1. [DOI] [PubMed] [Google Scholar]
- Dungarwal U.N., Patil S.B. Development of orodispersible tablets of taste masked rizatriptan benzoate using hydroxypropyl β cyclodextrin. J. Pharm. Investig. 2016;46(6):537–545. [Google Scholar]
- El Sharawy A.M., Shukr M.H., Elshafeey A.H. Formulation and optimization of duloxetine hydrochloride buccal films: in vitro and in vivo evaluation. Drug Deliv. 2017;24(1):1762–1769. doi: 10.1080/10717544.2017.1402216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elagamy H.I., Essa E.A., Nouh A., El Maghraby G.M. Development and evaluation of rapidly dissolving buccal films of naftopidil: in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2019;45(10):1695–1706. doi: 10.1080/03639045.2019.1656734. [DOI] [PubMed] [Google Scholar]
- Fu Y., Kao W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010;7(4):429–444. doi: 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras J., Llenas J., Jansat J.M., Jáuregui J., Cabarrocas X., Palacios J.M. Almotriptan, a new anti-migraine agent: a review. CNS Drug Rev. 2002;8(3):217–234. doi: 10.1111/j.1527-3458.2002.tb00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay S., Fountain W., Cui Z., Mumper R.J. Transmucosal delivery of testosterone in rabbits using novel bi-layer mucoadhesive wax-film composite disks. J. Pharm. Sci. 2002;91(9):2016–2025. doi: 10.1002/jps.10198. [DOI] [PubMed] [Google Scholar]
- Johnston M.M., Rapoport A.M. Triptans for the management of migraine. Drugs. 2010;70(12):1505–1518. doi: 10.2165/11537990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Jug M., Hafner A., Lovrić J., Kregar M.L., Pepić I., Vanić Ž., Cetina-Čižmek B., Filipović-Grčić J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018;147:350–366. doi: 10.1016/j.jpba.2017.06.072. [DOI] [PubMed] [Google Scholar]
- Kumria R., Al-Dhubiab B.E., Shah J., Nair A.B. Formulation and evaluation of chitosan-based buccal bioadhesive films of zolmitriptan. J. Pharm. Innov. 2018;13(2):133–143. [Google Scholar]
- Kumria R., Nair A.B., Al-Dhubiab B.E. Loratidine buccal films for allergic rhinitis: development and evaluation. Drug Dev. Ind. Pharm. 2014;40(5):625–631. doi: 10.3109/03639045.2014.884125. [DOI] [PubMed] [Google Scholar]
- Kumria R., Nair A.B., Goomber G., Gupta S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016;23(2):471–478. doi: 10.3109/10717544.2014.920058. [DOI] [PubMed] [Google Scholar]
- Lavudu P., Rani A.P., Divya C., Sekharan C.B. High performance liquid chromatographic analysis of almotriptan malate in bulk and tablets. Adv. Pharm. Bull. 2013;3(1):183–188. doi: 10.5681/apb.2013.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.W. Almotriptan for the acute treatment of adolescent migraine. Expert Opin. Pharmacother. 2010;11(14):2431–2436. doi: 10.1517/14656566.2010.517747. [DOI] [PubMed] [Google Scholar]
- Montenegro-Nicolini M., Morales J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech. 2017;18(1):3–14. doi: 10.1208/s12249-016-0525-z. [DOI] [PubMed] [Google Scholar]
- Nair A.B., Al-Dhubiab B.E., Shah J., Vimal P., Attimarad M., Harsha S. Development and evaluation of palonosetron loaded mucoadhesive buccal films. J. Drug Deliv. Sci. Tech. 2018;47:351–358. [Google Scholar]
- Nair A., Morsy M.A., Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018;79(8):373–382. doi: 10.1002/ddr.21461. [DOI] [PubMed] [Google Scholar]
- Nair A.B., Al-ghannam A.A., Al-Dhubiab B.E., Hasan A.A. Mucoadhesive film embedded with acyclovir loaded biopolymeric nanoparticles: in vitro studies. J Young Pharm. 2017;9(1):100–105. [Google Scholar]
- Nair A.B., Kumria R., Harsha S., Attimarad M., Al-Dhubiab B.E., Alhaider I.A. In vitro techniques to evaluate buccal films. J. Control. Release. 2013;166:10–21. doi: 10.1016/j.jconrel.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Padula C., Pescina S., Nicoli S., Santi P. New insights on the mechanism of fatty acids as buccal permeation enhancers. Pharmaceutics. 2018;10(4):201. doi: 10.3390/pharmaceutics10040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan V., Gaikwad R., Samad A., Prabhakar B. Formulation and evaluation of almotriptan malate nasal drops. Indian J. Pharm. Sci. 2009;71(6):727–729. [Google Scholar]
- Pringsheim T., Gawel M. Triptans: are they all the same? Curr. Pain Headache Rep. 2002;6(2):140–146. doi: 10.1007/s11916-002-0010-0. [DOI] [PubMed] [Google Scholar]
- Raggi A., Giovannetti A.M., Quintas R., D’Amico D., Cieza A., Sabariego C., Bickenbach J.E., Leonardi M. A systematic review of the psychosocial difficulties relevant to patients with migraine. J. Headache Pain. 2012;13(8):595–606. doi: 10.1007/s10194-012-0482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana P., Murthy R.S. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: a potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013;20(5):224–235. doi: 10.3109/10717544.2013.779331. [DOI] [PubMed] [Google Scholar]
- Salehi S., Boddohi S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog. Biomater. 2017;6(4):175–187. doi: 10.1007/s40204-017-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J. Chronic migraine. BMJ. 2014;348:1416. doi: 10.1136/bmj.g1416. [DOI] [PubMed] [Google Scholar]
- Shah H., Nair A.B., Shah J., Bharadia P., Al-Dhubiab B.E. Proniosomal gel for transdermal delivery of lornoxicam: optimization using factorial design and in vivo evaluation in rats. Daru. 2019;27(1):59–70. doi: 10.1007/s40199-019-00242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohi H., Ahuja A., Ahmad F.J., Khar R.K. Critical evaluation of permeation enhancers for oral mucosal drug delivery. Drug Dev. Ind. Pharm. 2010;36(3):254–282. doi: 10.1080/03639040903117348. [DOI] [PubMed] [Google Scholar]
- Steiner T.J., Birbeck G.L., Jensen R.H., Katsarava Z., Stovner L.J., Martelletti P. Headache disorders are third cause of disability worldwide. J. Headache Pain. 2015;16:58. doi: 10.1186/s10194-015-0544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner T.J., Stovner L.J., Birbeck G.L. Migraine: the seventh disabler. J. Headache Pain. 2013;14:1. doi: 10.1186/1129-2377-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper S.J., Rapoport A.M., Sheftell F.D. Mechanisms of action of the 5-HT1B/1D receptor agonists. Arch. Neurol. 2002;59(7):1084–1088. doi: 10.1001/archneur.59.7.1084. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P., De Vries P., Saxena P.R. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60(6):1259–1287. doi: 10.2165/00003495-200060060-00003. [DOI] [PubMed] [Google Scholar]
- Thakral S., Thakral N.K., Majumdar D.K. Eudragit®: a technology evaluation. Exp. Opin. Drug Deliv. 2013;10(1):131–149. doi: 10.1517/17425247.2013.736962. [DOI] [PubMed] [Google Scholar]
- Woldeamanuel Y.W., Cowan R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017;372:307–315. doi: 10.1016/j.jns.2016.11.071. [DOI] [PubMed] [Google Scholar]
- Youssef N.A., Kassem A.A., Farid R.M., Ismail F.A., El-Massik M.A., Boraie N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018;548(1):609–624. doi: 10.1016/j.ijpharm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Zaman M., Hanif M., Shaheryar Z.A. Development of Tizanidine HCl-Meloxicam loaded mucoadhesive buccal films: In-vitro and in-vivo evaluation. PloS One. 2018;13(3):e0194410. doi: 10.1371/journal.pone.0194410. [DOI] [PMC free article] [PubMed] [Google Scholar]